ABSTRACT
Diet is considered an important modifiable lifestyle factor capable of attenuating early cognitive changes in healthy older people. The inclusion of nuts in the diet has been investigated as a dietary strategy for maintenance of brain health across the lifespan. This review aimed to present up-to-date evidence regarding the association between nut intake and cognitive performance. Four databases (Ovid MEDLINE, Scopus, Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus, and Embase) were systematically searched from inception to April 2020. Eligible articles were interventional or observational studies in humans aged ≥18 y that measured the effects (or association) of nuts (almond, hazelnut, macadamia, pistachio, walnut, pecan, pine nut, Brazil nut, cashew, peanut) on cognitive outcomes. Out of the 2374 articles identified in the searches, 22 involving 43,793 participants met the criteria and were ultimately included in this review. Memory (immediate and delayed), attention, processing speed, executive function, and visual-spatial ability, as well as risk of mild cognitive impairment, were the outcomes investigated. Lack of consistency across the studies regarding study design, types of nut used, and cognitive outcomes measured resulted in inconsistent evidence that the regular consumption of mixed nuts has a protective effect on cognition in adults of different ages. Nonetheless, we observed that studies targeting populations with a higher risk of cognitive decline tended to find a more favorable outcome. Furthermore, homogeneous findings were observed in the studies that specifically addressed the association between walnut consumption and cognitive performance: out of the 6 studies, including 2 randomized controlled trials, only 1 did not find a positive association.
Keywords: nuts, diet, cognition, dementia, aging
Introduction
Global increase in life expectancy has resulted in an unprecedented increase in the prevalence of age-associated chronic diseases, such as cancer, diabetes, and cardiovascular disorders (1). The aging process leads to several underlying physiological changes. In the brain, the increased vulnerability to oxidative stress, chronic inflammation, and vascular impairment contributes to neuron and synapse loss, which may ultimately cause dementia (2). Although dementia is considered an abnormal consequence of aging, the condition currently affects 35.6 million people worldwide (3), and estimates project this number to double by 2030 and more than triple by 2050 (3).
Dementia is a progressive condition that leads to a drastic decline in different cognitive domains such as planning, processing speed, working memory, codification, and executive functions that require divided attention (4). As a result, the ability to perform daily activities is greatly compromised, which explains dementia as the leading cause of disability and dependency among older people worldwide (5). Considering that the pathological pathways underlying dementia may occur ≤30 y before symptom onset, strategies to reduce the risk of this disease are encouraged to take place early in life (3). It is believed that management of lifestyle-related risk factors such as physical inactivity, obesity, poor quality diet, and tobacco use throughout life may reduce the risk of dementia and optimize the trajectory of aging (6).
Diet is considered an important modifiable lifestyle factor capable of attenuating early cognitive changes in healthy older people. A comprehensive review of large observational studies (≥1000 participants) and clinical trials with follow-up of ≥6 mo examined the role of diet in age-associated cognitive decline and revealed that, overall, the consumption of long-chain ω-3 (n–3) fatty acids, B-vitamins (particularly folate), vitamin D, and antioxidants such as flavonoids are associated with lower rates of cognitive decline (7). Furthermore, strong evidence indicated that dietary patterns rich in foods with anti-inflammatory and antioxidant properties, such as the Mediterranean diet (MedDiet), the Dietary Approaches to Stop Hypertension (DASH) diet, and the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet are associated with slower rates of cognitive decline and reduced risk of dementia (7, 8). Amongst others, a common feature of these diets is the regular consumption of nuts (9, 10). Nuts have an optimal fatty acid profile, with a high concentration of monounsaturated and polyunsaturated fats and a low concentration of saturated fats. Furthermore, some nuts, particularly walnuts, are rich food sources of α-linolenic acid, a plant-based n–3 fatty acid. Additionally, nuts are substantial food sources of fiber, B-vitamins, minerals, and antioxidant compounds (11–14). Peanuts, although botanically classified as legumes, present with a similar nutrient profile as tree nuts and are therefore commonly included in this group (12). Research has demonstrated that the intake of nuts is associated with reduced cardiovascular risk (15–17) and improvement of glycemic control (18, 19). Given that these factors are tightly associated with the maintenance of neuronal function and brain health across the lifespan, it is hypothesized that their benefits are extended to improved cognitive performance in older people. This systematic review aims to present up-to-date evidence regarding the association between nut intake and cognitive performance. Considering the potential benefits of nut intake during different stages of life, this review includes studies involving adults aged ≥18 y with any health condition.
Methods
Study identification and eligibility
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (The PRISMA Statement) (20). The review was prospectively registered on a Systematic Literature Review registration website (PROSPERO Registration No. CRD42020188206). Research literature databases Ovid MEDLINE, Scopus, CINAHL Plus, and Embase were searched from database inception through to 6 April, 2020 using the following search terms: (Adult*) AND (Diet OR Nut* OR “Prunus dulcis” OR Almond* OR Anacardium OR Cashew* OR Corylus OR Hazelnut* OR Macadamia* OR Pistacia OR Pistachio* OR Juglans OR Walnut* OR Carya OR Pecan* OR Arachis OR Peanut* OR Pinus OR “Pine nut*” OR Bertholletia OR “Brazil nut*”) AND (Cognition OR “Cognition Disorders” OR Memory OR “Memory Disorders”). The search strategy is presented in Supplemental Table 1. Reference lists of selected studies and relevant review articles were manually searched to supplement the electronic search. Articles were eligible for inclusion if published in English, involved human participants (either healthy or with any medical condition), included an observational (cross-sectional or longitudinal) or interventional study design, quantified the consumption of ≥1 nut type, and assessed ≥1 cognitive outcome of interest: memory (immediate and delayed), attention, processing speed, executive function, visual-spatial ability, or risk of cognitive decline. Excluded articles were those that did not involve an observational or intervention study, assessed short-term nut intake (<3 wk in duration), did not quantify dietary nut intake, combined both nuts and other foods (e.g. seeds, fruits) or food components together for analysis, did not provide nut-specific outcome data, or measured outcomes that were unrelated to cognitive health.
Screening and data extraction
All resultant references were imported into a systematic review screening and data extraction software program (Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia), which was used to screen studies and identify those meeting the prespecified inclusion criteria. Duplicate articles were automatically identified and excluded by Covidence software. After the removal of duplicates, studies were screened by title and abstract independently by 2 of the listed authors (LET, EAM, EOC, EGC, NJK, and BRC) to determine their suitability for inclusion. Selected articles then underwent full-text screening, which was also conducted by 2 of the listed authors independently (LET, EAM, EOC, EGC, NJK, and BRC). Conflicts were resolved by discussion until consensus was reached. On completion of screening, the PRISMA flow chart was automatically generated by the Covidence program. Data were independently extracted from each article by all authors using a data collection table. Data collected included: first author, year of publication, country in which the study was conducted, study design, length of study, sample size, participant characteristics (age, health condition), nut intake (type, amount), nut intake of the comparator or control group, cognitive assessment conducted including the assessment tool utilized and cognitive outcome in nut eaters versus comparators/controls. Given that aging is strongly associated with cognitive performance, findings were presented according to age categories: young and middle-aged adults (≤60 y), middle-aged and older people (≥40 y), older people (≥60 y), and older people ≥70 y.
Quality assessment
The methodological quality of eligible studies were independently assessed by 2 authors (NJK and BRC) using the Quality Criteria Checklist tool of the Evidence Analysis Manual of the Academy of Nutrition and Dietetics (21). This tool rates primary research based on the relevance of the research (applicability to practice) and the scientific validity of the study. Studies were assessed as satisfying each of the 10 validity criteria questions using “Yes,” “No,” or “Unclear” responses. Studies receiving a “Yes” in response to ≥5 out of 10 questions (including questions 2, 3, 6, and 7) were designated “+/high quality,” studies receiving an “Unclear” response to questions 2, 3, 6, or 7 were considered “∅/neutral,” and studies receiving a “No” in response to 6 or more validity questions were considered “–/low quality.” Disagreements between author appraisals were resolved through collaborative discussion until consensus was reached.
Results
Study selection
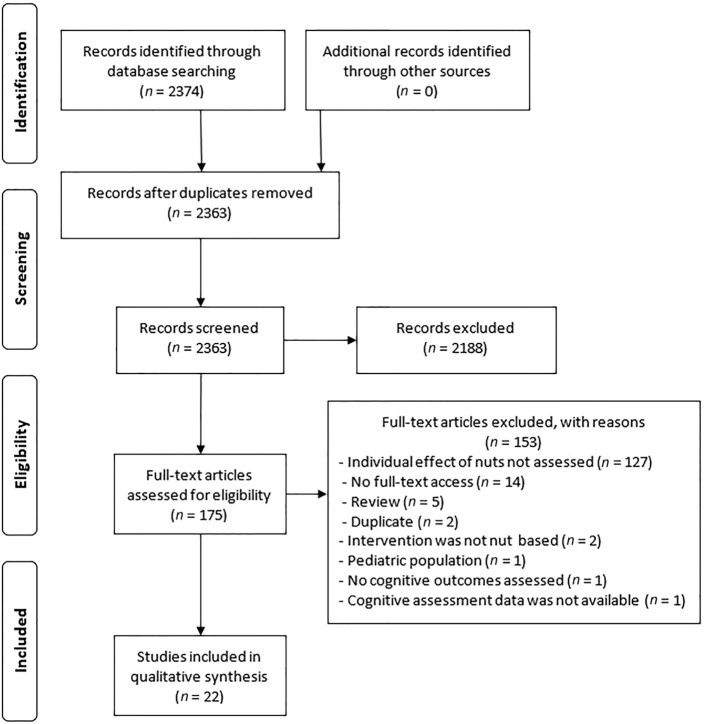
The initial database search returned 2374 articles. After the removal of duplicates, 2363 articles were subjected to initial screening for eligibility. This initial screening identified 2188 articles that did not meet the inclusion criteria. The remaining 175 articles were thoroughly assessed for eligibility, with 153 articles found to be ineligible according to predefined criteria. The main reason for exclusion was lack of information on nut consumption independently of other foods such as seeds (n = 127). After the exclusion of studies that did not meet the inclusion criteria, 22 articles involving 43,793 participants were ultimately included in this review (Figure 1).
FIGURE 1.
Flowchart of study selection process.
Study characteristics
This review included 7 cross-sectional studies (22–28), 5 prospective cohort studies (29–33), 2 case-control studies (34, 35), and 8 randomized controlled trials (RCTs) (Tables 1– 4). Six RCTs had a parallel-arm (36–41) and 2 had a crossover design (42, 43). The duration of intervention in the RCTs varied across the studies: 4 studies were conducted for 8–24 wk (36, 37, 42, 43), 1 RCT was conducted for 2 y (41), and the PREvencion con DIeta MEDiterranea (PREDIMED) trial, conducted in 2 different sites, was the longest study with an intervention period ranging from 3.6 to 6.5 y (38–40).
TABLE 1.
Nut consumption and cognitive performance in young and middle-aged adults (aged ≤60 y)
| Author, year, country | Study design | Study population | Nut intake (type, amount) | Comparison group | Cognitive measure | Findings |
|---|---|---|---|---|---|---|
| Arab & Ang, 2015, USA (22) | Cross-sectional | n = 5356 free-living (20–59 y) | Walnut (WWHC, WWON) | Nonconsumers | Simple reaction time test | WWHC: mean difference: –17.4 ms (β: –16.4; 95% CI: –21.4, –14.5; P = 0.031)WWON: mean difference: –10.5 ms (β: –10.5; 95% CI: –13.7, –9.3; P = 0.021) |
| Symbol digit substitution test | WWHC: mean difference: –0.35 s (β: –0.39; 95% CI: –0.71, –0.24; P = 0.011)WWON: mean difference: –0.31 s (β: –0.30; 95% CI: –0.70, –0.31; P = 0.011) | |||||
| Single digit learning test | WWHC: mean difference: –1.42 s (β: –2.38; 95% CI: –15.11, –0.39; P = 0.051)WWON: mean difference: –1.31 s (β: –2.21; 95% CI: –14.47, –0.51; P = 0.0011) | |||||
| Dhillon et al., 2017, USA (37) | RCT, parallel-arm (12 wk) | n = 86 overweight (18–60 y) | Almond, 15% daily energy (energy-restricted diet) | Nut-free diet (energy-restricted diet) | Immediate memoryImmediate attention | No differences between groupsNo differences between groups |
| Attention (delayed) | No differences between groups | |||||
| Delayed memory | No differences between groups | |||||
| Verbal list recognition test | No differences between groups | |||||
| Pribis et al., 2012, USA (42) | RCT, crossover (8 wk) | n = 47 college students (18–25 y) | Walnut within banana bread, 60 g/d | Placebo | Raven's Advanced Progressive Matrices | No differences between groups |
| Watson-Glaser Critical Thinking Appraisal | Difference: 11.2%; 95% CI: 2.9, 19.6; EF: 0.567; P = 0.009 | |||||
| Wechsler Memory Scale – Third Edition | No differences between groups |
Adjusted for age, gender, race, education, BMI, smoking, alcohol consumption, and physical activity. EF, Cohen's d effect size; RCT, randomized control trial; WWHC, walnuts with high certainty; WWON, walnuts with other nuts.
TABLE 4.
Nut consumption and cognitive performance in older people aged ≥70 y
| Author, year, country | Study design | Study population | Nut intake (type, amount) | Comparison group | Cognitive measure | Findings |
|---|---|---|---|---|---|---|
| Nurk et al., 2010, Norway (26) | Cross-sectional | n = 2031, free-living (70–74 y) | Total nuts | Nonconsumers | Kendrick Object Learning Test | No difference between groups1 |
| Trail making test A | No difference between groups1 | |||||
| Digit Symbol Test | No difference between groups1 | |||||
| Block design | No difference between groups1 | |||||
| MMSE | No difference between groups1 | |||||
| Controlled Oral Word Association Test | No difference between groups1 | |||||
| O'Brien et al., 2014, USA (32) | Prospective cohort (6 y) | n = 15,467 female nurses (≥70 y) | Total nuts | Average of 4 time points: nonconsumers x consumers ≥5 times/wk | Telephone interview for cognitive statusGlobal cognition | Mean difference: 0.21; 95% CI: –0.10, 0.52; P-trend = 0.022Mean difference: 0.08; 95% CI: 0.0, 0.15; P-trend = 0.0032 |
| Global cognition | Mean difference: 0.08; 95% CI: 0.0, 0.15; P-trend = 0.0032 | |||||
| Verbal memory | Mean difference: 0.09; 95% CI: 0.01, 0.17; P-trend = 0.0052 | |||||
| Change over time: quintiles of nut intake | Telephone interview for cognitive status (rate of decline) | No association2 | ||||
| Global cognition (rate of decline) | No association2 | |||||
| Verbal memory (rate of decline) | No association2 | |||||
| Walnuts | Average of 4 time points: nonconsumers x consumers 1 time/wk | Telephone interview for cognitive statusGlobal cognition | No association2No association2 | |||
| Verbal memory | No association2 | |||||
| Change over time: quintiles of nut intake | Telephone interview for cognitive status | No association2 | ||||
| Global cognition | No association2 | |||||
| Verbal memory | No association2 | |||||
| Samieri et al., 2013, USA (30) | Prospective cohort (6 y) | n = 16,058 free-living women (≥70 y) | Total nuts | Average of 4 time points: nonconsumers x consumers ≥5 times/wk | Global cognitionVerbal memory score | Mean difference: 0.02; 95% CI: –0.03, 0.06; P-trend = 0.023Mean difference: 0.01; 95% CI: –0.04, 0.06; P-trend = 0.053 |
| Change over time: quintiles of nut intake | Global cognitionVerbal memory score | No association3No association3 | ||||
| Wang et al., 2010, China (28) | Cross-sectional | n = 364 free-living (≥90 y) | Total nuts | Risk of MCI | No association4 |
Adjusted for sex, education, vitamin supplement use (multivitamins, folic acid, vitamins B, C, D, or E), smoking status, history of CVD, diabetes, intakes of dairy products, meat, fish, total fat, and protein.
Adjusted for age, education, time span between cognitive interviews, use of antidepressant medication, smoking status, physical activity, energy intake, alcohol intake, BMI, multivitamin use, history of diabetes, hypertension, hypercholesterolemia, and myocardial infarction.
Adjusted for age, treatment arm (in the original RCT), education, income, energy intake, physical activity, BMI, smoking status, diabetes, hypertension, hypercholesterolemia, hormone use, and depression.
Adjusted for gender, age, education, physical activity, blood pressure (systolic and diastolic), BMI, fasting plasma glucose, total cholesterol, triglycerides, HDL cholesterol and LDL cholesterol, smoking status, alcohol and tea consumption. MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; RCT, randomized controlled trial.
The average time span between the first and final cognitive assessments in the prospective cohort studies ranged from 3 to 6 y. Amongst the studies included in this review, 3 assessed only women in the Nurses’ Health Study (30, 32) or in the Women's Health Study (29). Regarding age groups, Arab and Ang (22) assessed both young adults (20–59 y) and older people (≥60 y). Two other studies included only participants younger than 60 y (37, 42), 9 combined middle-aged (≥40 y) and older people (24, 25, 27, 31, 34, 38–40, 43), 6 assessed only older people either above 60 or 65 y (23, 29, 33, 35, 36, 41), and 4 studies assessed only individuals above 70 y (26, 28, 30, 32). Free-living healthy populations were assessed in the majority of the studies; 2 studies examined overweight individuals (37, 43); 3 studies investigated subjects with mild cognitive impairment (MCI) (34–36), and the 4 studies conducted as part of the PREDIMED trial assessed participants with high cardiovascular risk (25, 38–40). Due to the heterogeneity in study designs, participant characteristics, dietary nut intakes, and outcome measurement techniques employed by studies included in this review, a meta-analysis of study results was not possible, therefore this review focuses on a narrative synthesis of study outcomes.
Quality assessment of included studies
Eleven of the 22 studies (50%) included in the current review were assessed to be of high methodological quality with the remaining 11 studies (50%) considered to be of neutral quality, as evaluated using the Quality Criteria Checklist tool of the Evidence Analysis Manual of the Academy of Nutrition and Dietetics (Table 5). Predominant threats to study validity included failure to describe the methods used to handle study withdrawals or loss to follow-up, inadequate use of blinding, inappropriate statistical analyses, and the likelihood of bias due to the study's funding. Twelve studies (55%) did not clearly specify the number of study withdrawals, discuss the characteristics of study dropouts (clinical trials), or disclose response rates (cohort, cross-sectional studies). Fifteen studies (68%) did not blind outcome assessors or specify whether data collectors or statisticians were blinded. Thirteen studies (59%) either did not provide a power calculation for the estimation of sample size, failed to adjust analyses for known confounders, or did not complete an intention to treat analysis. Clinical significance of findings was rarely considered. Ten studies (45%) were either funded by the nut industry or were conducted by authors who had received funds from the nut industry.
TABLE 5.
Quality criteria checklist: summary for publications included in the review on the association between nut consumption and cognitive performance in individuals aged ≥18 y
| Validity questions | Overall rating | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Arab & Ang, 2015 (22) | Y | Y | Y | U | U | Y | Y | Y | Y | U | + |
| Barbour et al., 2017 (43) | Y | Y | Y | Y | U | Y | Y | U | Y | U | + |
| Cardoso et al., 2016 (36) | Y | Y | Y | Y | Y | Y | U | U | Y | Y | ∅ |
| De Amicis et al., 2018 (23) | Y | U | Y | U | U | Y | Y | Y | Y | Y | ∅ |
| Dhillon et al., 2017 (37) | Y | Y | Y | Y | U | U | Y | Y | Y | U | ∅ |
| Dong et al., 2016 (24) | Y | Y | Y | Y | U | Y | U | Y | Y | Y | ∅ |
| Martínez-Lapiscina et al., 2013a (39) | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | + |
| Martínez-Lapiscina et al., 2013b (40) | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | + |
| Nooyens et al., 2011 (31) | Y | Y | U | U | U | Y | Y | U | Y | Y | ∅ |
| Nurk et al., 2010 (26) | Y | U | Y | U | U | Y | Y | U | Y | Y | ∅ |
| O'Brien et al., 2014 (32) | Y | Y | Y | U | U | Y | Y | U | Y | U | + |
| Pribis et al., 2012 (42) | Y | Y | Y | Y | Y | Y | Y | U | Y | U | + |
| Rabassa et al., 2020 (33) | Y | U | Y | U | U | Y | Y | U | Y | Y | ∅ |
| Salama et al., 2019 (27) | Y | U | U | N | U | U | U | U | N | Y | ∅ |
| Sala-Vila et al., 2020 (41) | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | + |
| Samieri et al., 2013a (29) | Y | Y | Y | U | U | Y | Y | Y | Y | Y | + |
| Samieri et al., 2013b (30) | Y | Y | Y | U | U | Y | Y | Y | Y | Y | + |
| Valls-Pedret et al., 2012 (25) | Y | Y | Y | Y | U | Y | Y | U | Y | U | + |
| Valls-Pedret et al., 2015 (38) | Y | Y | Y | Y | U | Y | Y | U | Y | U | + |
| Wang et al., 2010 (28) | Y | Y | U | U | U | Y | U | N | Y | Y | ∅ |
| Yuan et al., 2016 (34) | Y | Y | U | U | Y | Y | U | U | Y | Y | ∅ |
| Zhao et al., 2015 (35) | Y | Y | U | U | Y | Y | U | U | Y | Y | ∅ |
Validity Question Ratings: Y, Yes; N, No; U, Unclear.
Study validity questions assessed: 1. Was the research question clearly stated? 2. Was the selection of study subjects/patients free from bias? 3. Were study groups comparable? 4. Was the method of handling withdrawals described? 5. Was blinding used to prevent introduction of bias? 6. Were intervention/therapeutic regimens/exposure factor or procedure and any comparison(s) described in detail? Were intervening factors described? 7. Were outcomes clearly defined and the measurements valid and reliable? 8. Was the statistical analysis appropriate for the study design and type of outcome indicators? 9. Are conclusions supported by results with biases and limitations taken into consideration? 10. Is bias due to study's funding or sponsorship unlikely?
Overall study ratings:
+ (Positive) indicates that the report has clearly addressed issues of inclusion/exclusion, bias, generalizability, and data collection and analysis (answers to validity questions 2, 3, 6, and 7 plus ≥1 additional question are “Yes”).
– (Negative) indicates that these issues have not been adequately addressed (6 or more of the answers to the validity questions are “No”).
∅ (Neutral) indicates that the report is neither exceptionally strong nor exceptionally weak (answers to validity questions 2, 3, 6, or 7 are “Unclear”).
Types of nuts
The consumption of nuts without distinction of individual nut types was examined in 6 cross-sectional studies (23–28), 5 prospective cohort studies (29–33), and 2 case-controls (34, 35). When considering studies that reported the types of nuts, a total of 5 types of nut were used: walnut, peanut, almond, Brazil nut, and hazelnut. Walnuts were investigated in 2 cross-sectional studies (22, 25), 1 prospective cohort (32), and 2 RCTs: 1 consisted of a daily portion of banana bread containing walnuts (60 g/d) (42), and the other provided the equivalent of 15% of energy requirement, ranging from 30 to 60 g/d of walnuts (41). Intervention with high-oleic peanuts (84 g for men, 56 g for women, 6 d/wk) was investigated by Barbour et al. (43). Dhillon et al. (37) examined the long-term impact of the consumption of almonds (corresponding to 15% of the daily energy requirement) as part of an energy-restricted diet. The intervention protocol studied by Cardoso et al. (36) consisted of 1 Brazil nut a day (∼5 g). The study protocol of the PREDIMED trial comprised a daily mix of walnuts (15 g), hazelnuts (7 g), and almonds (7 g) as part of the MedDiet (25, 38–40).
Young and middle-aged adults
Three studies assessed participants aged ≤60 y (age range: 18–60 y), and they investigated either almonds or walnuts (Table 1). The consumption of an almond-enriched diet (corresponding to 15% of the energy requirement) did not result in better cognitive performance when compared with a nut-free diet after 12 wk (37). In the study by Pribis et al. (42), the consumption of 60 g of walnuts for 8 wk by college students (n = 47) was associated with better critical thinking abilities as measured by the Watson-Glaser Critical Thinking Appraisal (mean difference: 11.2%; Cohen's d effect size: 0.567; P = 0.009). However, no differences were observed for verbal reasoning (measured by Raven's Advanced Progressive Matrices) or memory (assessed by the Wechsler Memory Scale) when compared with the placebo group. Arab and Ang (22) assessed walnut consumption in US civilians aged 20–59 y participating in the NHANES. In that population, walnut consumption averaged 10.3 g/d. Based on their reported food sources of walnuts, participants were categorized as consumers of walnuts alone (walnuts with high certainty, WWHC) or consumers of walnuts as part of other different recipes or products (walnuts with other nuts, WWON). When compared with individuals who reported no consumption of nuts, walnut consumers presented better scores in the 3 cognitive tests: simple reaction time test (WWHC: mean difference: –17.4 ms; β: –16.4; 95% CI: –21.4, –14.5; P = 0.003; WWON: mean difference: –10.5 ms; β: –10.5; 95% CI: –13.7, –9.3; P = 0.002), symbol digit substitution test (WWHC: mean difference: –0.3 s; β: –0.4; 95% CI: –0.7, –0.2; P = 0.01; WWON: mean difference: –0.3 s; β: –0.3; 95% CI: –0.7, –0.3; P = 0.01), and single digit learning (WWHC: mean difference: –1.4 s; β: –2.4; 95% CI: –15.5, –0.4; P = 0.05; WWON: mean difference: –1.3 s; β: –2.2; 95% CI: –14.5, –0.5; P = 0.001). When tertiles of walnut consumption were examined, better outcomes were reported for all cognitive test scores among those in the highest tertile (P < 0.01).
Middle-aged and older people
Nine studies assessed both middle-aged and older people combined (age ≥40 y) (Table 2). The consumption of nuts was associated with better delayed memory (F = 4.87; P < 0.001) in community-dwelling Chinese, although no associations were observed for other cognitive domains (short-term memory, visuo-spatial and phonemic fluency abilities, language, executive function, attention, concentration, and working memory) (24). A cross-sectional analysis of free-living individuals in Egypt showed that the regular consumption of nuts decreased the risk of MCI (OR: 0.88; 95% CI: 0.80, 0.98; P = 0.02). Individuals with MCI presented with lower nut intake than cognitively healthy participants in 2 cross-sectional studies (24, 27), a difference that was not observed in a Chinese population (34). A cross-sectional analysis in the study of Nooyens et al. (31), conducted in a population from the Netherlands, identified that people in the highest quintile of nut intake presented better cognitive outcomes (global cognitive function, cognitive flexibility, memory, processing speed; all β: 0.05; all P < 0.05) than those in the lowest quintile, which was the equivalent to a difference of 5–8 y in age. However, a 5 y follow-up analysis in the same population revealed no association between nut intake and cognitive performance or incidence of MCI (31). In the study of Barbour et al. (43), the supplementation of high-oleic peanuts resulted in better performance in processing speed (mean difference: 0.2; Cohen's d effect size: 0.27; P = 0.047), verbal fluency (mean difference: 0.6; Cohen's d effect size: 0.46; P < 0.001), and executive function (mean difference: 0.5; Cohen's d effect size: 0.35; P = 0.016) tests, but not in memory, in comparison with a nut-free diet. Four studies conducted as part of the PREDIMED, the largest dietary intervention trial to assess the effects of the Mediterranean diet on cardiovascular disease (CVD) prevention, investigated the effects of nuts on cognition. Cross-sectional analysis from the PREDIMED conducted in Barcelona revealed a positive association between the consumption of walnuts, but not other nuts, and working memory, as assessed by the reverse digit span test (β: 1.2; 95% CI: 0.061, 2.322; P = 0.039) (25). The RCT conducted at the same study site (intervention period median: 4.1 y) showed that people in the MedDiet supplemented with a mix of nuts (MedDiet + nuts) group presented with better memory (nut group: mean change: 0.1; 95% CI: –0.04, 0.24; control group: mean change: –0.16; 95% CI: –0.32, –0.01; P difference < 0.05), but not frontal or global cognition, when compared with a control diet characterized by reduced fat intake (38). Data from a subgroup of the PREDIMED-Navarra, a recruitment site where a longer intervention was conducted (6.5 y) revealed no effect of MedDiet + nuts intervention on cognitive outcomes when compared with a low-fat diet (40). However, when data from the whole PREDIMED-Navarra cohort were considered in the analysis, the MedDiet + nuts arm presented better performance on the Mini-Mental State Examination (MMSE) (mean difference: 0.57; 95% CI: 0.11, 1.03; P = 0.0153) and clock drawing test (mean difference: 0.33; 95% CI: 0.003, 0.67; P = 0.0483) than the control low-fat diet (39).
TABLE 2.
Nut consumption and cognitive performance in middle-aged and older people (aged ≥40 y)
| Author, year, country | Study design | Study population | Nut intake (type, amount) | Comparison group | Cognitive measure | Findings |
|---|---|---|---|---|---|---|
| Barbour et al., 2017, Australia (43) | RCT, crossover | n = 61 overweight (50–75 y) | High-oleic peanuts (male: 84 g 6 times/wk, female: 56 g 6 times/wk) | Nut-free diet | Memory | No differences between groups |
| Processing speed | Mean difference: 0.2; SEM: 0.3; EF: 0.27; P = 0.047 | |||||
| Verbal fluency | Mean difference: 0.6; SEM: 0.1; EF: 0.46; P < 0.001 | |||||
| Executive function | Mean difference: 0.5; SEM: 0.2; EF: 0.35; P = 0.016 | |||||
| Dong et al., 2016, China (24) | Cross-sectional | n = 894 free-living (≥50 y) | Total nuts | MoCA (cut-off for MCI) | Cognitively healthy consumed more nuts than individuals with MCI | |
| MoCA total score | No significant association1 | |||||
| Delayed memory | F = 4.87, P < 0.0011 | |||||
| Visual-spatial ability | No significant association1 | |||||
| Name | No significant association1 | |||||
| Attention | No significant association1 | |||||
| Language | No significant association1 | |||||
| Abstraction | No significant association1 | |||||
| Orientation | No significant association1 | |||||
| Nooyens et al., 2011, the Netherlands (31) | Prospective cohort (5 y) | n = 2613, free-living (≥45 y) | Total nuts | Baseline: lowest quintile of nuts intake | Global cognitive functionCognitive flexibilityMemory | β: 0.05; P < 0.012β: 0.05; P < 0.012β: 0.05; P < 0.052 |
| Processing speed | β: 0.05; P < 0.052 | |||||
| Longitudinal: nut intake as continuous variable | Global cognitive function | No significant association2 | ||||
| Cognitive flexibility | No significant association2 | |||||
| Memory | No significant association2 | |||||
| Processing speed | No significant association2 | |||||
| Martínez-Lapiscina et al., 2013, Spain (Navarra city) (39) | RCT, parallel-arm (6.5 y) | n = 522 free-living high risk of CVD (55–80 y) | Nut mix (15 g walnuts, 7 g hazelnuts, 7 g almonds) + MedDiet (MedDiet + nuts) | Low-fat diet | MMSEClock drawing test | Mean difference: 0.57; 95% CI: 0.11, 1.03; P = 0.0153Mean difference: 0.33; 95% CI: 0.003, 0.67; P = 0.04833 |
| Martínez-Lapiscina et al., 2013, Spain (Navarra city) (40) | RCT, parallel-arm (6.5 y) | n = 268 free-living high risk of CVD (55–80 y) | Nut mix (15 g walnuts, 7 g hazelnuts, 7 g almonds) + MedDiet (MedDiet + nuts) | Low-fat diet | MMSEClock drawing testRey auditory verbal learning test – immediate | No difference between groups3No difference between groups3No difference between groups3 |
| Rey auditory verbal learning test – delay | No difference between groups3 | |||||
| Verbal paired associates | No difference between groups3 | |||||
| Rey-osterrieth complex figure – immediate | No difference between groups3 | |||||
| Rey-osterrieth complex figure – delay | No difference between groups3 | |||||
| Similarities | No difference between groups3 | |||||
| Trail making test-A | No difference between groups3 | |||||
| Trail making test-B | No difference between groups3 | |||||
| Digit (forward) | No difference between groups3 | |||||
| Digit (backward) | No difference between groups3 | |||||
| Semantic verbal fluency test – animals | No difference between groups3 | |||||
| Phonemic verbal fluency test – FAS | No difference between groups3 | |||||
| Boston Naming Test | No difference between groups3 | |||||
| Rey-osterrieth complex Figure – copy | No difference between groups3 | |||||
| Incidence of MCI | No difference between groups3 | |||||
| Salama et al., 2019, Egypt (27) | Cross-sectional | n = 186 free-living (40–65 y) | Total nuts | Nonconsumers | Risk of MCI | OR: 0.88; 95% CI: 0.80, 0.98; P = 0.02 |
| Valls-Pedret et al., 2012, Spain (Barcelona) (25) | Cross-sectional | n = 447 free-living high risk of CVD (55–80 y) | Total nuts and walnuts | Digit span test Wechsler Adult Intelligence Scale (working memory) | Walnuts: β: 1.191; 95% CI: 0.061, 2.322; P = 0.0394 | |
| MMSE | No association4 | |||||
| Rey auditory verbal learning test rate | No association4 | |||||
| Verbal paired associated test (Wechsler Memory Scale) | No association4 | |||||
| Verbal fluency test | No association4 | |||||
| Color Trail Test | No association4 | |||||
| Valls-Pedret et al., 2015, Spain (Barcelona) (38) | RCT, parallel-arm (3.6–4.2 y) | n = 334 free-living high risk of CVD (55–80 y) | Nut mix (15 g walnuts, 7 g hazelnuts, 7 g almonds) + MedDiet (MedDiet + nuts) | Low-fat diet | Memory composite | MedDiet + nuts: change: 0.1; 95% CI: –0.04, 0.24Control: change: –0.16; 95% CI: -0.32, –0.01; P-difference < 0.055 |
| Frontal cognition | No difference between groups5 | |||||
| Global cognition (average of 4 timepoints) | No difference between groups5 | |||||
| MMSE | No difference between groups5 | |||||
| Rey auditory verbal learning test rate | No difference between groups5 | |||||
| Verbal paired associated test (Wechsler Memory Scale) | No difference between groups5 | |||||
| Verbal fluency test | No difference between groups5 | |||||
| Digit span test Wechsler Adult Intelligence Scale (working memory) | No difference between groups5 | |||||
| Color Trail Test part 1 | No difference between groups5 | |||||
| Yuan et al., 2016, China (34) | Case-control | n = 276 (138 MCI, 138 age and sex-matched controls) (55–75 y) | Total nuts | Nut intake was not different between MCI and control groups (P = 0.523) |
Adjusted for age, gender, nationality, BMI, and education level.
Adjusted for age, sex, education, total energy intake (separate for energy from fat, energy from alcohol and energy from other sources), intake of other fruits, vegetables, legumes, and juices, serum
HDL cholesterol, systolic blood pressure, usage of blood pressure-lowering medication, waist circumference, coffee consumption, smoking, physical activity, vitality, mental health, and the baseline level of cognitive function (in the longitudinal data analyses).
Adjusted for sex, age, education, family history of cognitive impairment or dementia, APOE ɛ4 allele, hypertension, dyslipidemia, diabetes, smoking status, alcohol intake, BMI, physical activity, and total energy intake.
Adjusted for gender, age, education, BMI, smoking, APOE ɛ4 allele, physical activity, diabetes, hypertension, and hyperlipidemia.
Adjusted for sex, baseline age, years of education, marital status, APOE ε4 allele, ever smoking, baseline BMI, energy intake, physical activity, type 2 diabetes mellitus, hyperlipidemia, ratio of total cholesterol to HDL cholesterol, statin treatment, hypertension, use of anticholinergic drugs, time of follow-up, propensity score for group allocation. CVD, cardiovascular disease; EF, Cohen's d effect size; MCI, mild cognitive impairment; MedDiet, Mediterranean diet; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; RCT, randomized controlled trial.
Older people
Seven studies reported results exclusively for older people (age ≥60 y) (Table 3). The consumption of nuts was not different between people with MCI and healthy controls in a Chinese population (35). The consumption of 1 serving of nuts (30 g)/wk was associated with a reduced risk of cognitive impairment in an Italian sample of free-living older people (OR: 0.3; 95% CI: 0.13, 0.69; P = 0.005) (23). Another study conducted with community-dwelling Italians also showed that, in comparison to nonconsumers, the regular consumption of nuts (≥2.9g/d) was associated with a decreased risk of cognitive decline (OR: 0.78; 95% CI: 0.61, 0.99; P = 0.043) and better performance on MMSE (mean difference: 1.5; Cohen's d effect size: 0.47; P = 0.012) over a period of 3 y (33). In contrast, nut intake was not associated with cognitive status or changes in cognitive performance (measured as global cognitive function and verbal memory) over a period of 4 y in free-living American women whose average intake was 0.3 servings/d (29). In the study of Arab and Ang (22), individuals aged ≥60 y who reported consuming walnuts presented better performance in the story recall test, which assesses attention and delayed memory (WWHC: mean difference: 8.3; β: 7.1; 95% CI: 0.6, 13.7; P = 0.03; WWON: mean difference: 10.7; β: 8.1; 95% CI: 3.5, 12.7; P = 0.001), and in the digit-symbol substitution test, which measures processing speed, sustained attention, and working memory (WWHC: mean difference: 11.3; β: 7.3; 95% CI: 0.1, 14.6; P = 0.05; WWON: mean difference: 11.8; β: 4.8; 95% CI: 0.9, 8.7; P = 0.02). As observed for the younger group aged 20–59 y, better outcomes were seen for both cognitive test scores among those older people in the highest walnut intake tertile (P < 0.001). An RCT tested the effects of the daily consumption of walnuts (the equivalent to 15% daily energy requirement) for 2 y on cognitive performance in older people recruited in 2 sites: California, USA, and Barcelona, Spain. Overall, the intervention group did not show any difference in regards to cognitive outcomes (global cognition, perception, language, memory, frontal function) when compared with the nut-free diet group. However, when the 2 study sites were analyzed separately, the walnut group was reported to present with better global cognition and perception when compared with the nut-free diet group in the Spanish study population (41). Cardoso et al. (36) reported improvement in 2 out of 5 cognitive tests in older people with MCI after a 6-mo trial with Brazil nut (verbal fluency: Cohen's d effect size: 1.3; P = 0.007; constructional praxis: Cohen's d effect size: 1.0; P = 0.031) in comparison with a nut-free diet.
TABLE 3.
Nut consumption and cognitive performance in older people (aged ≥60 y).
| Author, year, country | Study design | Study population | Nut intake (type, amount) | Comparison group | Cognitive measure | Findings |
|---|---|---|---|---|---|---|
| Arab & Ang, 2015, USA (22) | Cross-sectional | n = 7337 free-living (≥60 y) | Walnut (WWHC, WWON) | Nonconsumers | Story recall test | WWHC: 8.3 (β: 7.09; 95% CI: 0.6, 13.6; P = 0.031)WWON: 10.7 (β: 8.11; 95% CI: 3.5, 12.7; P = 0.0011) |
| Digit-symbol substitution test | WWHC: 11.3 (β: 7.31; 95% CI: 0.09, 14.6; P = 0.051)WWON: 11.8 (β: 4.82; 95% CI: 0.89, 8.72; P = 0.021) | |||||
| Cardoso et al., 2016, Brazil (36) | RCT, parallel-arm (24 wk) | n = 20 MCI (≥60 y) | Brazil nut (1 kernel/d, ∼5 g) | Nut-free diet | Verbal fluencyConstructional praxis | EF: 1.33; P = 0.007EF: 1.01; P = 0.031 |
| Boston naming test | No difference between groups | |||||
| Word list learning test | No difference between groups | |||||
| Word list recall | No difference between groups | |||||
| CERAD total score | No difference between groups | |||||
| De Amicis et al., 2018, Italy (23) | Cross-sectional | n = 279 free-living (≥65 y) | Total nuts | Risk of MCI | OR: 0.3; 95% CI 0.1, 0.7; P = 0.005 | |
| Rabassa et al., 2020, Italy (33) | Prospective cohort (3 y) | n = 119 free-living (≥65 y) | Walnuts, almonds, hazelnuts, peanuts (combined) | Nonconsumers | MMSERisk of cognitive decline | Mean difference: 1.5; EF: 0.47; P = 0.012β: 0.25; 95% CI: 0.04, 0.46; P = 0.0182OR: 0.78; 95% CI: 0.61, 0.99; P = 0.0432 |
| Sala-Vila et al., 2020, USA and Spain (41) | RCT, parallel-arm (2 y) | n = 657 free-living (66–79 y) | Walnut, 15% daily energy (30–60 g/d) | Nut-free diet | Global cognition | No difference between groups (significant difference in the Barcelona site: mean difference: 0.07; P = 0.016)3 |
| Perception | No difference between groups (significant difference in the Barcelona site: mean difference: 0.2; P = 0.005)3 | |||||
| Language | No difference between groups3 | |||||
| Memory | No difference between groups3 | |||||
| Frontal function | No difference between groups3 | |||||
| Samieri et al., 2013, USA (29) | Prospective cohort (average 4 y) | n = 6174 free-living women (≥66 y) | Total nuts | Average of 3 time points: quintiles of nut intake | Global cognitive functionVerbal memory | No association4 No association4 |
| Change over time: quintiles of nut intake | Global cognitive function | No association4 | ||||
| Verbal memory | No association4 | |||||
| Zhao et al., 2015, China (35) | Case-control | n = 404 (98 MCI, 306 healthy controls) (60–90 y) | Total nuts | MoCA | Nut intake was not different between MCI and control groups (P > 0.05) |
Adjusted for age, gender, race, education, BMI, smoking, alcohol consumption, and physical activity.
Adjusted for sex, age, baseline score of cognitive function, depressive symptoms, education, BMI, physical activity, smoking status, energy intake, alcohol consumption, stroke, cardiovascular disease, hypertension, and diabetes.
Adjusted for age, gender, education, APOE ε4 allele, ever smoking, physical activity, BMI, diabetes, dyslipidemia, hypertension, Hamilton Depression Rating Scale score.
Adjusted for MedDiet score, treatment arm (in the original RCT), age, race, education, income, energy intake, physical activity, BMI, smoking status, diabetes, hypertension, hypercholesterolemia, hormone use, and depression. CERAD, Consortium to Establish a Registry for Alzheimer's Disease; EF, Cohen's d effect size; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; RCT, randomized controlled trial; WWHC, walnuts with high certainty; WWON, walnuts with other nuts.
Older people aged ≥70 y
Four studies investigated the association between the consumption of nuts and cognitive outcomes exclusively in older people aged ≥70 y (Table 4 ). A cross-sectional study did not find an association between nut intake (average intake: 4.6 g/d) and cognitive outcomes in a Norwegian population (26), and another cross-sectional analysis of community-dwelling Chinese revealed no association between nut intake and the risk of MCI (28). Two studies used prospective data from the Nurses’ Health Study cohort to investigate the association between nut intake and cognitive outcomes in women aged ≥70 y. O'Brien et al. (32) showed that higher intakes of nuts (measured as the frequency of 28 g servings of nuts consumed per week) were associated with better cognitive status over 6 y. When compared with individuals who reported not consuming nuts, mean score differences for participants who consumed 5 servings of nuts/wk were 0.21 (95% CI: –0.10, 0.52; P-trend = 0.02) in the telephone interview for cognitive status, 0.08 (95% CI: 0.00, 0.15; P-trend = 0.003) in the global cognition composite, and 0.09 (95% CI: 0.01, 0.17; P-trend = 0.005) in the verbal memory composite. Such differences were the equivalent to 2 y of cognitive aging. However, in the same study, nut intake was not associated with rates of cognitive decline over the 6 y follow-up. When walnuts were considered alone in the analysis, no significant association between cognitive status or rates of decline in cognitive function were observed (32). Similarly, Samieri et al. (30) found that higher quintiles of nut consumption were associated with better cognitive status over 4 y (global cognition: P-trend = 0.02; verbal memory: P-trend = 0.05), but no association was observed for changes in cognitive performance over the 4-y follow-up.
Discussion
This comprehensive systematic review was conducted to provide an insight into research exploring the notion of nut consumption as a strategy to slow age-associated cognitive decline. A previous review on this topic showed evidence that nut intake might be a useful tool to delay age-associated cognitive decline (44). Despite the limited clinical data included in that study in comparison to this present review, the authors reviewed experimental studies to provide mechanistic insight into the effects of nuts on brain function. Here, we systematically searched for studies that reported on adults aged ≥18 y, and given that aging is strongly associated with cognitive performance, findings were presented according to age categories. Memory (immediate and delayed), attention, processing speed, executive function, and visual-spatial ability, as well as risk of MCI, were the outcomes investigated. The findings compiled in this review do not provide consistent evidence that the regular consumption of mixed nuts has a protective effect on cognition in adults of different ages. Nonetheless, we observed that studies targeting populations with a higher risk of cognitive decline tended to find a more favorable outcome. Furthermore, research is indicative that the intake of walnuts, specifically, is associated with better cognitive performance in young, middle-aged, and older people. This review has also identified a lack of consistency across the studies regarding study design, types of nut consumed, and cognitive outcomes measured, which precludes further analyses or conclusions.
Given that cognitive changes as a normal process of aging start to occur in mid adulthood, it has been suggested that modulation of lifestyle-associated risks at midlife are of the utmost importance to decrease the risk of dementia (45). Nonetheless, research is limited when investigating the association between dietary strategies and cognitive function in young and middle-aged adults, as they mostly target older populations who present a higher risk of dementia. The current review identified only 3 studies that assessed solely individuals under 60 y, whereas 9 studies combined middle-aged and older people as the target population. On the other hand, a total of 11 studies investigated only older people, and 4 of them focused on assessing exclusively older people aged ≥70 y. Given that studies targeting similar age category populations presented with different designs, investigated different nut types in a range of concentrations, recruited participants with a range of health conditions and assessed a variety of cognitive outcomes, no age-related effects of nut intake can be inferred.
Although aging characterizes the most important risk factor for cognitive decline, dementia is a multifactorial and heterogeneous disorder that is predisposed by a combination of genetic and environmental factors such as educational attainment, lifestyle, and psychological factors (46, 47). The APOE gene, which encodes a protein involved in the transport of cholesterol and other fatty acids, is the strongest genetic risk factor for Alzheimer's disease (48). Research shows that carriers of the allele ɛ4 present with higher rates of cognitive impairment over the adult life course (48). The interaction between this genotype and the consumption of nuts was only explored in the study of Sala-Vila et al. (41), but no significant interaction was observed (P = 0.088). Even though other studies have adjusted their results for APOE status and given the high concentration of fatty acids in nuts, future studies should consider exploring the impact of nut-gene interaction on age-associated cognitive decline.
Modifiable risk factors such as CVD, metabolic syndrome, hypertension, obesity, type 2 diabetes, and education are suggested to account for one-third of Alzheimer's disease cases worldwide (46, 47). Besides the neuroprotective role of diet via modulation of most of these conditions, the link between diet and the aging brain can be summarized in 3 crucial mechanisms: regulation of blood flow, protecting against the formation of arterial plaques; reduction of oxidative stress and inflammation, protecting against neurodegeneration (49). Current evidence shows that nut consumption is associated with improved endothelial function (50), fasting glucose concentrations (18), risk of metabolic syndrome (51), and incidence of CVD (17, 52), although the effect on inflammatory markers seem to be dependent on the inflammatory status of the study population and design of the dietary intervention (type and amount of nuts) (50). These benefits of nut intake may be explained by their unique nutrition profile and bioactive compounds, such as the high content of unsaturated fatty acids that can influence glucose control; high concentration of PUFAs that have anti-inflammatory and vasculoprotective effects (53), besides being integral components of the neuronal membranes (54); and a high concentration of phytochemicals and micronutrients, which may reduce inflammation, oxidative stress, and endothelial function (13, 14, 55). Furthermore, the incorporation of nuts in the diet has been associated with dietary changes that improve diet quality (56, 57), which can have an indirect effect on cognitive function over time. In fact, a secondary analysis of the study by Sala-Vila et al. (41) revealed that the consumption of walnuts (corresponding to 15% daily energy) displaced the equivalent of 19% of the energy provided by other energy-containing foods in the diet. Furthermore, individuals consuming walnuts daily reported a lower intake of total carbohydrate, animal protein, saturated fatty acids, and sodium, and higher intake of PUFAs (including n–3 and n–6 fatty acids) and plant-based proteins (58).
However, the current review shows no consistent benefits when it comes to cognitive performance in adults and older people, which suggests that the relation between nut intake and cognition is perhaps more nuanced than previously hypothesized. Overall, out of the 7 cross-sectional analyses, 4 found a positive relation between the consumption of mixed nuts and cognitive performance (23–25, 27); nonetheless out of the 5 longitudinal analyses, only 1 demonstrated the consumption of mixed nuts to be protective against cognitive decline over time (33). On the other hand, the RCTs show more homogeneous findings: amongst the 8 RCTs included in this review, only 2 did not find a positive effect of nut intake on cognitive outcomes (Supplemental Table 2). Overall, we observed that positive outcomes were more likely in studies that targeted populations with lower educational attainment and higher cardiovascular risk, and therefore were at higher risk of cognitive decline. In this regard, the study population of the PREDIMED studies included participants at high cardiovascular risk (25, 38); in the trial conducted by Sala-Vila et al. (41), positive effects of the supplementation of walnuts were seen only in the Barcelona cohort, which presented with lower education attainment and higher rates of smokers. Education level was no higher than a low degree or junior high school in ∼65% of the participants of the studies by Dong et al. (24) and De Amicis et al. (23), whereas on average participants in the study by Rabassa et al. (33) presented with only 6 y of education. Furthermore, the only intervention study conducted with older people with MCI found positive effects of Brazil nut intake on cognitive performance (36). These findings imply that individuals at higher risk of cognitive decline may obtain the largest benefit from nut consumption, in alignment with other interventions aiming to reduce the risk of dementia (59).
A consistent beneficial effect was observed in the studies that explored the association between walnut consumption specifically and cognitive performance: of the 6 studies investigating walnut consumption (including 2 RCTs), only 1 did not find a positive association. Walnuts contain some unique nutritional properties including a high n–3:n–6 fatty acid ratio and an exceptional concentration of polyphenols, conferring superior antioxidant efficacy when compared with other nuts (60). These nutrients are hypothesized to contribute to the neuroprotective capacity of walnuts by mitigating neuroinflammation and oxidative stress (61, 62).
Despite the strong evidence indicating a protective role of MedDiet against cognitive impairment, hesitation remains when determining if the effects are due to the whole dietary pattern or its individual components (49). This review included 7 studies that assessed populations from Italy or Spain, which are known to have high adherence to MedDiet. The 2 studies conducted in Italy showed that nut intake was associated with a decreased risk of cognitive decline (23, 33), which was aligned with findings from the PREDIMED conducted in Spain (25, 38, 39). Interestingly, Sala-Vila et al. (41) investigated the effects of the supplementation of walnuts on cognitive performance in older people from Spain and the USA, and positive outcomes were only observed in the Spanish cohort. Findings from the studies conducted in non-Mediterranean countries, such as the USA, China, Australia, the Netherlands, Norway, Brazil, and Egypt presented less consistent findings in regard to cognitive function. Therefore, we may hypothesize that the benefit of nut intake on cognition is maximized when part of MedDiet, besides being more favorable to those who are at higher risk of cognitive impairment due to prior memory impairment, poor dietary habits, low educational status, and/or presence of cardiovascular risk factors (59). There may also be additional nondietary components of the complete Mediterranean lifestyle that confer protective benefits for cognitive health (physical activity, adequate sleep, and increased socialization) (63).
The inclusion of studies that assessed young and middle-aged adults along with older people is a strength of this review, as well as the reporting of findings according to age categories. Furthermore, the current review encompasses 8 RCTs, which is the largest number of RCTs to date within a systematic review related to nut intake and cognitive outcomes. A limitation of the current review is the fact that several studies investigating the association between dietary patterns and cognition did not report a breakdown of food groups and were therefore ineligible for inclusion in this review. Furthermore, the most studied dietary patterns in this regard combine nuts with other foods in the same group: the Alternate Healthy Eating Index (64) combines nuts and legumes, whereas MedDiet combines nuts with legumes and beans as part of the same food category (65). Alternatively, the MIND diet uniquely specifies the consumption of nuts (9), but no research investigating this diet has reported food groups separately. Although we have attempted to group studies according to the age of the study populations, the use of different age cut-offs in the different studies resulted in overlaps between the age categories presented in this review. The majority of studies included in this review were of either a cross-sectional or cohort design, thus limiting the conclusions to associations between nut intake and cognitive function rather than causation. Some studies did not quantify nut intake using a validated FFQ, and a number of longitudinal studies only administered FFQs at 1 time rather than at multiple time points throughout the study, so changes in dietary nut intake were not captured. A further limitation of the current review was the variability in the tests used to assess cognition, and the heterogeneous nature of the study designs. Therefore, it is not possible to determine differences in efficacy between different types of nuts or the optimal amount to be consumed in order to maximize cognitive function.
Conclusions
The evidence summarized in the current review is inconclusive as to whether increasing nut consumption contributes to the maintenance of cognitive functions throughout life and reduces the risk of dementia. Nonetheless, it appears that the benefit of nut intake on cognition is more noticeable in individuals at higher risk of cognitive impairment. Furthermore, more consistent evidence indicates favorable effects of walnuts on cognition, although more studies are required to elucidate whether walnuts provide a superior advantage over other nuts.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—BRC: conceived the review; LET, EAM, EOC, EGC, NJK, and BRC: performed the systematic search, screened titles and abstracts, reviewed the full text of articles identified by the literature search, and performed the data extraction; NJK and BRC: performed quality assessment; LET, EAM, EOC, and EGC: wrote the first draft of the manuscript; NJK and BRC: checked the data extraction and edited the final draft of the manuscript; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
LET and NJK contributed equally to this work.
Abbreviations used: CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; MCI, mild cognitive impairment; MedDiet, Mediterranean diet; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; MMSE, Mini-Mental State Examination; n–3, long-chain ω-3; PREDIMED, PREvencion con DIeta MEDiterranea; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; WWHC, walnuts with high certainty; WWON, walnuts with other nuts.
Contributor Information
Lauren E Theodore, Deakin University, Institute for Physical Activity and Nutrition (IPAN), School of Exercise and Nutrition Sciences, Geelong, Victoria, Australia.
Nicole J Kellow, Department of Nutrition, Dietetics and Food, Monash University, Victoria, Australia.
Emily A McNeil, Deakin University, Institute for Physical Activity and Nutrition (IPAN), School of Exercise and Nutrition Sciences, Geelong, Victoria, Australia.
Evangeline O Close, Deakin University, Institute for Physical Activity and Nutrition (IPAN), School of Exercise and Nutrition Sciences, Geelong, Victoria, Australia.
Eliza G Coad, Deakin University, Institute for Physical Activity and Nutrition (IPAN), School of Exercise and Nutrition Sciences, Geelong, Victoria, Australia.
Barbara R Cardoso, Deakin University, Institute for Physical Activity and Nutrition (IPAN), School of Exercise and Nutrition Sciences, Geelong, Victoria, Australia; Department of Nutrition, Dietetics and Food, Monash University, Victoria, Australia.
References
- 1. World Health Organization . World Report on Ageing and Health. Luxembourg: World Health Organization; 2015. [Google Scholar]
- 2. Xia X, Jiang Q, McDermott J, Han J-DJ. Aging and Alzheimer's disease: comparison and associations from molecular to system level. Aging Cell. 2018;17(5):e12802–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO . Mental health. Dementia: a public health priority. Geneva: World Health Organization; 2018. [Google Scholar]
- 4. Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. [DOI] [PubMed] [Google Scholar]
- 5. Scheltens P, Blennow K, Breteler MMB, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer's disease. Lancet. 2016;388(10043):505–17. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization . Global Action Plan on the Public Health Response to Dementia 2017–2025. Geneva: World Health Organization; 2017. [Google Scholar]
- 7. Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17(11):1006–15. [DOI] [PubMed] [Google Scholar]
- 8. Cremonini AL, Caffa I, Cea M, Nencioni A, Odetti P, Monacelli F. Nutrients in the prevention of Alzheimer's disease. Oxidative Medicine and Cellular Longevity. 2019;2019:9874159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, Aggarwal NT. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ros E. Chapter 15—Contribution of nuts to the Mediterranean diet. In: Preedy VR, Watson RReds. The Mediterranean Diet (Second Edition). London: Academic Press; 2020. pp. 141–50. [Google Scholar]
- 11. Kornsteiner M, Wagner K-H, Elmadfa I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006;98(2):381–7. [Google Scholar]
- 12. Ros E, Tapsell LC, Sabate J. Nuts and berries for heart health. Curr Atheroscler Rep. 2010;12(6):397–406. [DOI] [PubMed] [Google Scholar]
- 13. Bolling BW, Chen CY, McKay DL, Blumberg JB. Tree nut phytochemicals: composition, antioxidant capacity, bioactivity, impact factors: a systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr Res Rev. 2011;24(2):244–75. [DOI] [PubMed] [Google Scholar]
- 14. Cardoso BR, Duarte GBS, Reis BZ, Cozzolino SMF. Brazil nuts: nutritional composition, health benefits and safety aspects. Food Res Int. 2017;100(Pt 2):9–18. [DOI] [PubMed] [Google Scholar]
- 15. Morgillo S, Hill AM, Coates AM. The effects of nut consumption on vascular function. Nutrients. 2019;11(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coates AM, Hill AM, Tan SY. Nuts and cardiovascular disease prevention. Curr Atheroscler Rep. 2018;20(10):48. [DOI] [PubMed] [Google Scholar]
- 17. Becerra-Tomás N, Paz-Graniel I, Kendall CWC, Kahleova H, Rahelić D, Sievenpiper JL, Salas-Salvadó J. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: a meta-analysis of prospective cohort studies. Nutr Rev. 2019;77(10):691–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim Y, Keogh JB, Clifton PM. Does nut consumption reduce mortality and/or risk of cardiometabolic disease? An updated review based on meta-analyses. IJERPH. 2019;16(24):4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viguiliouk E, Kendall CWC, Blanco Mejia S, Cozma AI, Ha V, Mirrahimi A, Jayalath VH, Augustin LSA, Chiavaroli L, Leiter LAet al. Effect of tree nuts on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled dietary trials. PLoS One. 2014;9(7):e103376–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG; for the PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Academy of Nutrition and Dietetics . Evidence Analysis Manual: steps in the Academy evidence analysis process. Chicago (IL), USA: Academy of Nutrition and Dietetics; 2016. [Google Scholar]
- 22. Arab L, Ang A. A cross sectional study of the association between walnut consumption and cognitive function among adult US populations represented in NHANES. J Nutr Health Aging. 2015;19(3):284–90. [DOI] [PubMed] [Google Scholar]
- 23. De Amicis R, Leone A, Foppiani A, Osio D, Lewandowski L, Giustizieri V, Cornelio P, Cornelio F, Fusari Imperatori S, Cappa SFet al. Mediterranean diet and cognitive status in free-living elderly: a cross-sectional study in northern Italy. J Am Coll Nutr. 2018;37(6):494–500. [DOI] [PubMed] [Google Scholar]
- 24. Dong L, Xiao R, Cai C, Xu Z, Wang S, Pan L, Yuan L. Diet, lifestyle and cognitive function in old Chinese adults. Arch Gerontol Geriatr. 2016;63:36–42. [DOI] [PubMed] [Google Scholar]
- 25. Valls-Pedret C, Lamuela-Raventós RM, Medina-Remón A, Quintana M, Corella D, Pintó X, Martínez-González MA, Estruch R, Ros E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. JAD. 2012;29(4):773–82. [DOI] [PubMed] [Google Scholar]
- 26. Nurk E, Refsum H, Drevon CA, Tell GS, Nygaard HA, Engedal K, Smith AD. Cognitive performance among the elderly in relation to the intake of plant foods. The Hordaland Health Study. Br J Nutr. 2010;104(8):1190–201. [DOI] [PubMed] [Google Scholar]
- 27. Salama II, Salama SI, Elmosalami DM, Saleh RM, Rasmy H, Ibrahim MH, Kamel SA, Ganem MMF, Raslan HM. Risk factors associated with mild cognitive impairment among apparently healthy people and the role of microRNAs. Open Access Maced J Med Sci. 2019;7(19):3253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Z, Dong B, Zeng G, Li J, Wang W, Wang B, Yuan Q. Is there an association between mild cognitive impairment and dietary pattern in Chinese elderly? Results from a cross-sectional population study. BMC Public Health. 2010;10:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Samieri C, Grodstein F, Rosner BA, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Okereke OI. Mediterranean diet and cognitive function in older age. Epidemiology. 2013;24(4):490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Samieri C, Okereke OI, Devore EE, Grodstein F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr. 2013;143(4):493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nooyens AC, Bueno-de-Mesquita HB, van Boxtel MP, van Gelder BM, Verhagen H, Verschuren WM. Fruit and vegetable intake and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Br J Nutr. 2011;106(5):752–61. [DOI] [PubMed] [Google Scholar]
- 32. O'Brien J, Okereke O, Devore E, Rosner B, Breteler M, Grodstein F. Long-term intake of nuts in relation to cognitive function in older women. J Nutr Health Aging. 2014;18(5):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rabassa M, Zamora-Ros R, Palau-Rodriguez M, Tulipani S, Miñarro A, Bandinelli S, Ferrucci L, Cherubini A, Andres-Lacueva C. Habitual nut exposure, assessed by dietary and multiple urinary metabolomic markers, and cognitive decline in older adults: The InCHIANTI Study. Mol Nutr Food Res. 2020;64(2):1900532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yuan L, Liu J, Ma W, Dong L, Wang W, Che R, Xiao R. Dietary pattern and antioxidants in plasma and erythrocyte in patients with mild cognitive impairment from China. Nutrition. 2016;32(2):193–8. [DOI] [PubMed] [Google Scholar]
- 35. Zhao X, Yuan L, Feng L, Xi Y, Yu H, Ma W, Zhang D, Xiao R. Association of dietary intake and lifestyle pattern with mild cognitive impairment in the elderly. J Nutr Health Aging. 2015;19(2):164–8. [DOI] [PubMed] [Google Scholar]
- 36. Cardoso BR, Apolinario D, da Silva Bandeira V, Busse AL, Magaldi RM, Jacob-Filho W, Cozzolino SM. Effects of Brazil nut consumption on selenium status and cognitive performance in older adults with mild cognitive impairment: a randomized controlled pilot trial. Eur J Nutr. 2016;55(1):107–16. [DOI] [PubMed] [Google Scholar]
- 37. Dhillon J, Tan SY, Mattes RD. Effects of almond consumption on the post-lunch dip and long-term cognitive function in energy-restricted overweight and obese adults. Br J Nutr. 2017;117(3):395–402. [DOI] [PubMed] [Google Scholar]
- 38. Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martínez-González MÁ, Martínez-Lapiscina EH, Fitó M, Pérez-Heras A, Salas-Salvadó Jet al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med. 2015;175(7):1094–103. [DOI] [PubMed] [Google Scholar]
- 39. Martínez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvadó J, San Julián B, Sanchez-Tainta A, Ros E, Valls-Pedret C, Martinez-Gonzalez M. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013;84(12):1318–25. [DOI] [PubMed] [Google Scholar]
- 40. Martínez-Lapiscina EH, Clavero P, Toledo E, San Julián B, Sanchez-Tainta A, Corella D, Lamuela-Raventós RM, Martínez JA, Martínez-Gonzalez M. Virgin olive oil supplementation and long-term cognition: the PREDIMED-NAVARRA randomized, trial. J Nutr Health Aging. 2013;17(6):544–52. [DOI] [PubMed] [Google Scholar]
- 41. Sala-Vila A, Valls-Pedret C, Rajaram S, Coll-Padrós N, Cofán M, Serra-Mir M, Pérez-Heras AM, Roth I, Freitas-Simoes TM, Doménech Met al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts And Healthy Aging (WAHA) study: a randomized controlled trial. Am J Clin Nutr. 2020;111(3):590–600. [DOI] [PubMed] [Google Scholar]
- 42. Pribis P, Bailey RN, Russell AA, Kilsby MA, Hernandez M, Craig WJ, Grajales T, Shavlik DJ, Sabatè J. Effects of walnut consumption on cognitive performance in young adults. Br J Nutr. 2012;107(9):1393–401. [DOI] [PubMed] [Google Scholar]
- 43. Barbour JA, Howe PRC, Buckley JD, Bryan J, Coates AM. Cerebrovascular and cognitive benefits of high-oleic peanut consumption in healthy overweight middle-aged adults. Nutr Neurosci. 2017;20(10):555–62. [DOI] [PubMed] [Google Scholar]
- 44. Ros E, Sala-Vila A. Nuts and Brain Health. Health Benefits of Nuts and Dried Fruits. Boca Raton: CRC Press; 2020, 261–88. [Google Scholar]
- 45. Li X-Y, Zhang M, Xu W, Li J-Q, Cao X-P, Yu J-T, Tan L. Midlife modifiable risk factors for dementia: a systematic review and meta-analysis of 34 prospective cohort studies. CAR. 2019;16(14):1254–68. [DOI] [PubMed] [Google Scholar]
- 46. Monsuez J-J, Gesquière-Dando A, Rivera S. Cardiovascular prevention of cognitive decline. Cardiol Res Pract. 2011;2011:250970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Bruijn RF, Bos MJ, Portegies ML, Hofman A, Franco OH, Koudstaal PJ, Ikram MA. The potential for prevention of dementia across two decades: the prospective, population-based Rotterdam Study. BMC Med. 2015;13(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rawle MJ, Davis D, Bendayan R, Wong A, Kuh D, Richards M. Apolipoprotein-E (Apoe) ε4 and cognitive decline over the adult life course. Transl Psychiatry. 2018;8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr (Bethesda, Md). 2016;7(5):889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neale EP, Tapsell LC, Guan V, Batterham MJ. The effect of nut consumption on markers of inflammation and endothelial function: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2017;7(11):e016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Y, Zhang D-Z. Relationship between nut consumption and metabolic syndrome: a meta-analysis of observational studies. J Am Coll Nutr. 2019;38(6):499–505. [DOI] [PubMed] [Google Scholar]
- 52. Rusu ME, Mocan A, Ferreira I, Popa D-S. Health benefits of nut consumption in middle-aged and elderly population. Antioxidants. 2019;8(8):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ren J, Chung SH. Anti-inflammatory effect of alpha-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-kappaB and mitogen-activated protein kinase pathways. J Agric Food Chem. 2007;55(13):5073–80. [DOI] [PubMed] [Google Scholar]
- 54. Cutuli D. Functional and structural benefits induced by omega-3 polyunsaturated fatty acids during aging. CN. 2017;15(4):534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim Y, Keogh JB, Clifton PM. Benefits of nut consumption on insulin resistance and cardiovascular risk factors: multiple potential mechanisms of actions. Nutrients. 2017;9(11):1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fraser GE, Bennett HW, Jaceldo KB, Sabaté J. Effect on body weight of a free 76 Kilojoule (320 calorie) daily supplement of almonds for six months. J Am Coll Nutr. 2002;21(3):275–83. [DOI] [PubMed] [Google Scholar]
- 57. Jaceldo-Siegl K, Sabaté J, Rajaram S, Fraser GE. Long-term almond supplementation without advice on food replacement induces favourable nutrient modifications to the habitual diets of free-living individuals. Br J Nutr. 2004;92(3):533–40. [DOI] [PubMed] [Google Scholar]
- 58. Bitok E, Jaceldo-Siegl K, Rajaram S, Serra-Mir M, Roth I, Feitas-Simoes T, Ros E, Sabaté J. Favourable nutrient intake and displacement with long-term walnut supplementation among elderly: results of a randomised trial. Br J Nutr. 2017;118(3):201–9. [DOI] [PubMed] [Google Scholar]
- 59. Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653–66. [DOI] [PubMed] [Google Scholar]
- 60. Halvorsen BL, Carlsen MH, Phillips KM, Bøhn SK, Holte K, Jacobs DR Jr, Blomhoff R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am J Clin Nutr. 2006;84(1):95–135. [DOI] [PubMed] [Google Scholar]
- 61. Poulose SM, Miller MG, Shukitt-Hale B. Role of walnuts in maintaining brain health with age. J Nutr. 2014;144(4):561S–6S. [DOI] [PubMed] [Google Scholar]
- 62. Chauhan A, Chauhan V. Beneficial effects of walnuts on cognition and brain health. Nutrients. 2020;12(2):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bach-Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, Medina FX, Battino M, Belahsen R, Miranda Get al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12a):2274–84. [DOI] [PubMed] [Google Scholar]
- 64. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med. 2007;44(4):335–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.