Abstract
Objective
To perform a systematic review (SR) on the effectiveness of self-management interventions, in order to inform the European League Against Rheumatism Recommendations for its implementation in patients with inflammatory arthritis (IA).
Methods
The SR was conducted according to the Cochrane Handbook and included adults (≥18 years) with IA. The search strategy was run in Medline through PubMed, Embase, Cochrane Library, CINAHL Plus with Full Text, and PEDro. The assessment of risk of bias, data extraction and synthesis were performed by two reviewers independently. A narrative Summary of Findings was provided according to the Grading of Recommendations, Assessment, Development and Evaluation.
Results
From a total 1577 references, 57 were selected for a full-text review, and 32 studies fulfilled the inclusion criteria (19 randomised controlled trials (RCTs) and 13 SRs). The most studied self-management components were specific interactive disease education in ten RCTs, problem solving in nine RCTs, cognitive–behavioural therapy in eight RCTs, goal setting in six RCTs, patient education in five RCTs and response training in two RCTs. The most studied interventions were multicomponent or single exercise/physical activity in six SRs, psychosocial interventions in five SRs and education in two SRs. Overall, all these specific components and interventions of self-management have beneficial effects on IAs-related outcomes.
Conclusions
The findings confirm the beneficial effect of the self-management interventions in IA and the importance of their implementation. Further research should focus on the understanding that self-management is a complex intervention to allow the isolation of the effectiveness of its different components.
Keywords: arthritis, rheumatoid, arthritis, psoriatic, spondylitis, ankylosing, outcome assessment, health care, inflammation
Key messages.
What is already known about this subject?
Interventions which aim to strengthen self-management skills of people with inflammatory arthritis are complex.
What does this study add?
This systematic review underscores the need for a set of critical outcomes for self-management strategies.
It highlights the beneficial effects of different components of self-management, such as specific interactive disease education, problem solving, cognitive–behavioural therapy, goal setting, patient education, response training, and globally, multicomponent or single exercise/physical activity and psychosocial interventions on some patient outcomes, including self-efficacy.
However, evidence of the effectiveness of self-management in outcome results in patients with inflammatory arthritis is lacking.
How might this impact on clinical practice or further developments?
This systematic review highlights the importance of incorporating self-management interventions in routine clinical care.
Future research should explore which intervention components contribute most to achieving better critical outcomes.
Introduction
Inflammatory arthritis (IA), including rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS) and axial spondyloarthritis (ax-SpA) or unspecified polyarthritis (UA), is the chronic conditions with a pervasive impact on daily self-care and quality of life.1 An essential aspect of adjusting to IA is the ability to understand the disease and deal with the practical, physical and psychological impacts that come along with it.2 This goes beyond drug therapy and implies the recognition that the diagnosis of IA is life-changing, and that the ability to self-manage is crucial.3
Self-management, unlike the traditional medical model, emphasises the importance of interactive, collaborative care between the patient and the healthcare professional rather than one-way, passive care from expert to patient. Although several educational materials and resources may be available for self-management of IA, these are often underused and not always incorporated in the routine care. Time pressures, limited healthcare services, the perceived lack of evidence but also the lack of knowledge of healthcare professionals as to who is available and what resources are available to best address self-management aspects of care, are recognised obstacles to providing the necessary support in a sensitive and patient-centred manner.4
Several European Alliance of Associations for Rheumatology (EULAR) recommendations for the management of specific RMDs have highlighted the importance of self-management to achieve the desired effect of interventions.5–9 However, these recommendations do not orient clinicians and healthcare professionals on how to support patients to self-management, acquire self-management skills and make necessary behavioural changes.
In order to inform the task force responsible for the 2021 EULAR Recommendations for the implementation of self-management strategies in patients with IA, we performed a systematic review (SR) that aimed to identify the best evidence on the effectiveness of self-management interventions targeting IA and to describe their components.
Methods
This SR was conducted according to the Cochrane Handbook10 and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.11
The steering group of the EULAR task force (AM, ES, EN, AB and LC) established and followed the SR protocol, which was not registered, but is available on request. The outlined research questions, as approved by the entire task force at the first meeting, were: which self-management interventions are effective in IA? Which are the components of effective interventions? Who are the professionals who deliver these effective interventions? These questions were framed and structured according to the EULAR standardised operating procedures12 using the ‘Patients, Intervention, Comparator or Control, Outcome, Type of study format’.
Participants
A study was eligible for inclusion if the participants included were adults (≥18 years) with IA (specifically, RA, PsA, AS, ax-SpA or UA). To maximise precision, only studies in which patients were formally diagnosed with IA or who satisfied current disease criteria, were included.13–16 Studies focusing on the information regarding patients with other concomitant diseases, whether these were rheumatic or not, were excluded from the synthesis.
Interventions
With regards to eligible interventions, these had to be defined explicitly as ‘self-management’, in other words, the individual patient ability and competence regarding the management of symptoms, treatment, physical and psychosocial consequences and the lifestyle changes inherent in living with a chronic condition17; or they had to include at least one component from each of the following: biological, psychological and social management. Interventions must consist of disease information, medication management, management of the physical activity, disease-related problem solving, cognitive symptom management, management of emotions, communication skills and use of community resources.18 Additionally, these interventions should be promoted by, or result from interaction with a programme leader who is a health professional. They may be delivered face to face or online, with direct or indirect trained support provided.
Comparator or control
The comparator was placebo or usual care (standard care).
Context
There were no contextual constraints in this SR.
Outcomes
Concerning outcomes, the core concept in self-management is the realisation of self-efficacy; that is, confidence in oneself to carry out the required behaviour to acquire the desired goal.19 We accepted other patient-reported outcome measures that were quantitative measures of the impact of the disease, such as pain, functional disability, fatigue, emotional well-being, sleep, coping and physical well-being (eg, Visual Analogue Scale, Health Assessment Questionnaire, Functional Assessment of Chronic Illness Therapy, Rheumatoid Arthritis Impact of Disease).20 21 Health-related quality of life (eg, 36-Item Short Form Survey, EuroQol-5 Dimension).22 Self-efficacy should be measured by a validated tool (eg, General Self-Efficacy Scale or Stanford Self-Efficacy Scale].
Type of study
Eligible designs were only SR and randomised controlled trials (RCTs) or controlled clinical trials because they are the most robust designs and represent the highest evidence.
Search strategy and study selection
A search strategy was run in Medline through PubMed, Embase, Cochrane Library, CINAHL Plus with Full Text, and PEDro from 20 January 2020 to 24 January 2020. Studies published in English, French, Spanish, and Portuguese language, with no restriction of the publication date, were considered for inclusion. Details on complete search strategies are provided in online supplemental material S1.
rmdopen-2021-001647supp001.pdf (65.6KB, pdf)
All identified citations were uploaded into an EndNote VX7 (Clarivate Analytics, Pennsylvania, USA) library and the duplicates removed. Titles and abstracts were screened by two independent reviewers (ES and AM) to assess eligibility criteria. The full articles were retrieved for all studies that met or had insufficient information to assess these inclusion criteria, and two reviewers (ES and AM) independently examined them in detail. Any disagreements that arose between the reviewers were resolved through discussion or with a third reviewer (LC).
Assessment of risk of bias, data extraction and synthesis
Two reviewers (ES and AM) independently assessed the risk of bias of each included study using the AMSTAR2 for SR23 and the Cochrane Collaboration’s tool for RCT’s.24 Any disagreements between the reviewers were resolved through discussion, or with a third reviewer (LC).
Data were extracted from the selected reports by the same two independent reviewers (ES and AM), and disagreements were discussed until consensus was achieved, with the third reviewer (LC) involved whenever necessary. Authors of papers were contacted to request missing or additional data, where required. The overlap of original research studies included in SRs was rigorously checked to avoid double counting and expressed as percentage.
An overall assessment of the quality of the evidence for each comparison (intervention vs control) was performed using the Grading of Recommendations, Assessment, Development and Evaluation25 and Summary of Findings (SoF) tables were produced with the GRADEPro GDT software. A four-point rating scale was used to rate the quality of the evidence (high, moderate, low and very low), according to the following criteria: risk of bias, inconsistency, imprecision, indirectness and publication bias. A narrative SoF form was preferred due to the differences in metrics used by the included studies.
Results
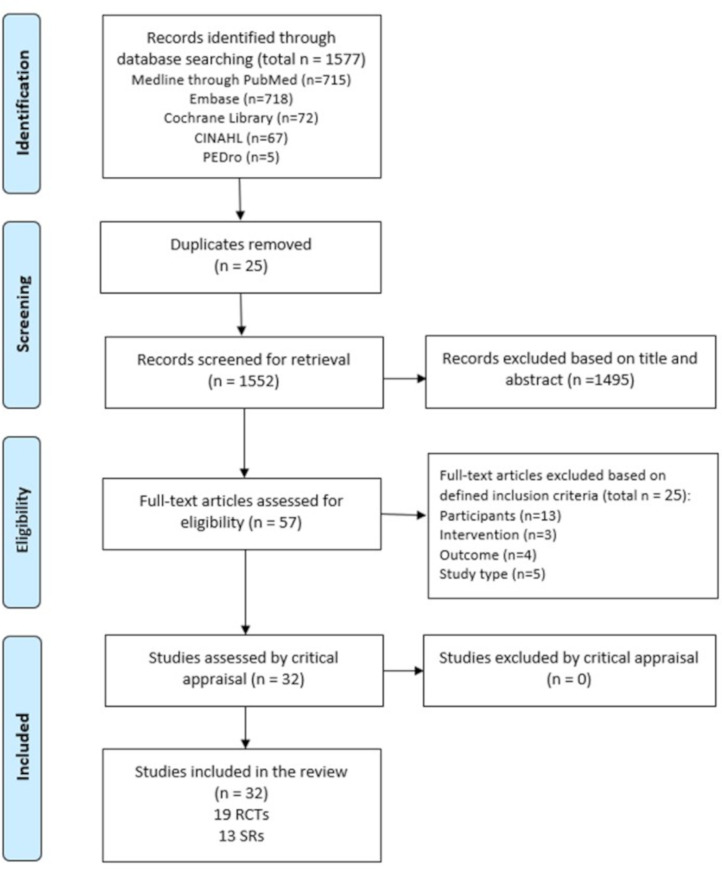
From a total of 1577 references, 57 were selected for a full-text review, and 32 studies fulfilled the inclusion criteria. Full-text studies that did not meet the inclusion criteria were excluded, and reasons for exclusion are provided in online supplemental material S2. Included studies were 19 RCTs and 13 SRs. As a result of the overlap, 91 RCTs (34.9%) were duplicated in the SRs. Only one author of the papers was contacted to request additional information. The results of the searches are shown in a flow diagram (figure 1).
Figure 1.
Flow chart of the study selection and inclusion process.
rmdopen-2021-001647supp002.pdf (45.2KB, pdf)
Methodological quality
The critical appraisal results for each of the studies are summarised in online supplemental material S3. There was agreement among the reviewers to include all the studies that were appraised. Regarding SRs, most (n=9) had moderate quality, three had high quality and only one was of low quality. This lower quality was mainly due to problems of no explicit statement that the review methods were established prior to the conducting of the review, issues in selection of the study designs, insufficient search strategy, not providing a list of excluded studies and justifying the exclusions, not reporting on the sources of funding and not investigating the publication bias. The majority of the RCTs included were of moderate to high quality, except for one, that was low. In general, all RCTs had issues with allocation concealment and blinding of participants and outcomes, which might be expected given the nature of the intervention.
rmdopen-2021-001647supp003.pdf (73KB, pdf)
Characteristics of included studies and interventions
Study characteristics are detailed in online supplemental material S4. Regarding interventions, the most commonly studied among the 19 RCTs were specific interactive disease education (n=10),26–35 problem solving (n=9),26 28 32 33 35–40 cognitive–behavioural therapy (n=8),32 33 38–43 goal setting (n=6),26 32 35–38 40 patient education (n=5),35–37 41 44–46 and response training (n=2).32 34 Of note, several RCTs had addressed more than one intervention.
rmdopen-2021-001647supp004.pdf (320.2KB, pdf)
Of the 13 included SRs, the most studied interventions were multi-component or single exercise/physical activity (n=6),47–52 psychosocial interventions (n=5),47 53–56 education (n=2)57 58 and self-management (n=1).59 Studies were very heterogeneous from a methodological, clinical and even statistical point of view; thus, data pooling was not possible. Table 1 and online supplemental material S5 provide a summary on the effects of interventions per outcome.
Table 1.
Short version of GRADE Summary of Findings
| Interventions | Outcomes | Impact | Certainty of the evidence (GRADE) |
| Cognitive–behavioral therapy | Functional disability | Effective | ⨁⨁◯◯ LOW |
| Disease activity | Effective | ⨁⨁◯◯ LOW | |
| Impairment/disability | Effective | ⨁⨁⨁◯ MODERATE | |
| Anxiety/depression | Effective | ⨁⨁◯◯ LOW | |
| Psychophysiological complains | Effective | ⨁⨁◯◯ LOW | |
| Sleep problems | Effective | ⨁⨁◯◯ LOW | |
| Pain | Effective | ⨁⨁◯◯ LOW | |
| Self-efficacy/self-helplessness | Effective | ⨁⨁⨁◯ MODERATE | |
| Quality of life/health status/social support | Effective | ⨁⨁◯◯ LOW | |
| Healthcare use | No effect | ⨁⨁◯◯ LOW | |
| Fatigue | Effective | ⨁⨁⨁◯ MODERATE | |
| Response training | Functional disability | Effective | ⨁⨁⨁◯ MODERATE |
| Disease activity | Effective | ⨁⨁⨁◯ MODERATE | |
| Impairment/disability | Effective | ⨁⨁⨁◯ MODERATE | |
| Psychophysiological complains | No effect | ⨁⨁◯◯ LOW | |
| Pain | Effective | ⨁⨁⨁◯ MODERATE | |
| Self-Efficacy/self-helplessness | Effective | ⨁⨁⨁◯ MODERATE | |
| Quality of life/Health status/Social support | Effective | ⨁⨁⨁◯ MODERATE | |
| Fatigue | Effective | ⨁⨁⨁◯ MODERATE | |
| Specific interactive disease education | Knowledge | Effective | ⨁⨁⨁◯ MODERATE |
| Functional disability | Effective | ⨁⨁◯◯ LOW | |
| Disease activity | Effective | ⨁⨁◯◯ LOW | |
| Impairment/disability | Effective | ⨁⨁◯◯ LOW | |
| Anxiety/depression | No effect | ⨁⨁⨁◯ MODERATE | |
| Psychophysiological complains | No effect | ⨁⨁⨁◯ MODERATE | |
| Pain | Effective | ⨁⨁◯◯ LOW | |
| Self-efficacy/self-helplessness | Effective | ⨁⨁◯◯ LOW | |
| Quality of life/health status/social support | Effective | ⨁⨁◯◯ LOW | |
| Fatigue | Effective | ⨁⨁◯◯ LOW | |
| Goal setting | Functional disability | Effective | ⨁⨁◯◯ LOW |
| Disease activity | Effective | ⨁⨁◯◯ LOW | |
| Impairment/disability | Effective | ⨁⨁⨁◯ MODERATE | |
| Anxiety/depression | Effective | ⨁⨁◯◯ LOW | |
| Psychophysiological complains | Effective | ⨁⨁◯◯ LOW | |
| Sleep problems | Effective | ⨁⨁⨁◯ MODERATE | |
| Pain | Effective | ⨁⨁◯◯ LOW | |
| Self-efficacy/self-helplessness | Effective | ⨁⨁◯◯ LOW | |
| Quality of life/health status/social support | Effective | ⨁⨁◯◯ LOW | |
| Fatigue | Effective | ⨁⨁◯◯ LOW | |
| Problem solving | Functional disability | Effective | ⨁⨁◯◯ LOW |
| Disease activity | Effective | ⨁⨁◯◯ LOW | |
| Impairment/disability | Effective | ⨁⨁◯◯ LOW | |
| Anxiety/depression | Effective | ⨁⨁◯◯ LOW | |
| Psychophysiological complains | Effective | ⨁⨁◯◯ LOW | |
| Sleep problems | Effective | ⨁⨁⨁◯ MODERATE | |
| Pain | Effective | ⨁⨁◯◯ LOW | |
| Self-efficacy/self-helplessness | Effective | ⨁⨁◯◯ LOW | |
| Quality of life/health status/social support | Effective | ⨁⨁◯◯ LOW | |
| Healthcare use | No effect | ⨁⨁⨁◯ MODERATE | |
| Fatigue | Effective | ⨁⨁◯◯ LOW | |
| Multicomponent or single exercise/physical activity interventions | Pain | Effective | ⨁⨁⨁◯ MODERATE |
| Functional disability | Effective | ⨁⨁◯◯ LOW | |
| Fatigue | Effective | ⨁⨁⨁⨁ HIGH | |
| Patient Global Assessment | Effective | ⨁⨁⨁◯ MODERATE | |
| BASDAI | Effective | ⨁⨁◯◯ LOW | |
| BASFI | Effective | ⨁⨁◯◯ LOW | |
| DAS-28 | No effect | ⨁◯◯◯ VERY LOW | |
| Psychosocial interventions | Pain | Effective | ⨁⨁⨁◯ MODERATE |
| Functional disability | Effective | ⨁⨁⨁◯ MODERATE | |
| Fatigue | Effective | ⨁⨁⨁◯ MODERATE | |
| Psychological status | Effective | ⨁⨁⨁◯ MODERATE | |
| Physical activity | Effective | ⨁⨁⨁◯ MODERATE | |
| Depression | Effective | ⨁⨁⨁◯ MODERATE | |
| Anxiety | Effective | ⨁⨁⨁◯ MODERATE | |
| Tender joints | Effective | ⨁⨁⨁◯ MODERATE | |
| Coping | Effective | ⨁⨁⨁◯ MODERATE | |
| Self-efficacy | Effective | ⨁⨁⨁◯ MODERATE | |
| DAS-28 | No effect | ⨁◯◯◯ VERY LOW | |
| Self-management interventions | Pain | Effective | ⨁⨁◯◯ LOW |
| Functional disability | No effect | ⨁◯◯◯ VERY LOW | |
| Educational interventions | Pain | Effective | ⨁⨁⨁◯ MODERATE |
| Fatigue | Effective | ⨁◯◯◯ VERY LOW | |
| Functional disability | Effective | ⨁⨁⨁◯ MODERATE | |
| Joint counts | Effective | ⨁⨁⨁◯ MODERATE | |
| Patient Global Assessment | Effective | ⨁⨁⨁◯ MODERATE | |
| Psychological status | Effective | ⨁⨁⨁◯ MODERATE | |
| Depression | Effective | ⨁⨁⨁◯ MODERATE | |
| Adherence | Effective | ⨁⨁⨁◯ MODERATE | |
| Self-efficacy | Effective | ⨁⨁⨁◯ MODERATE |
GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect; Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect; Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; DAS-28, Disease Activity Score-28; GRADE, Grading of Recommendations, Assessment, Development and Evaluation.
rmdopen-2021-001647supp005.pdf (156.2KB, pdf)
Discussion
This SR shows some beneficial effects of components of self-management, such as specific interactive disease education,26–35 problem solving,26 28 32 33 35–40 cognitive–behavioural therapy,32 33 38–43 goal setting,26 32 35–38 40 patient education35–37 41 44–46 and response training.32 34 Also, multicomponent or single exercise/physical activity,47–52 psychosocial interventions,47 53–56 education57 58 and self-management,59 in general, also corroborate this trend. Several other studies explored the effectiveness of self-management interventions in undifferentiated chronic diseases or other rheumatic diseases besides IA.60–64 Other outcomes, for example, disease activity, healthcare use, psychophysiological complaints, anxiety/depression and functional disability, were either controversial or had no positive effect.
The presentation of the findings by individual components of the self-management interventions was driven primarily by the heterogeneity of the interventions, which did not allow ‘points of convergence’. As shown in table 1, there is no high certainty of the evidence on self-management interventions in IA, and most of the certainty of evidence is moderate or low. This is natural because self-management is a so-called ‘complex intervention’. In complex interventions, the efficacy of specific components is difficult to isolate.65 The majority of interventions included some sort of patient education, problem solving and cognitive–behavioural therapy which are to be expected in the context of self-management.
The interventions were delivered by a range of healthcare professionals including rheumatologists, nurses, psychologists, nutritionists, physiotherapists, occupational therapists, social workers and dieticians. Besides them, the multidisciplinary teams that delivered the interventions also included laypersons, pairs of lay leaders, counsellors and yoga teachers, although with a smaller participation.
No expert patients were involved in the delivery of education or interventions based on the published literature, which is in contrasts to what is being offered at least by some patient organisations. This observation was perhaps due to the setting of the studies (mainly hospitals (secondary care)), the year of the publication of the most long-standing studies which is still not sensitive to the growing patient research partners paradigm, and due to a pure research context of the study.
Surprisingly, only one study measured adherence as an outcome of patient education.46 Whereas several studies have examined adherence, only one RCT focused on the effect of self-management strategies (patient education) to improve it. This could be due to research bias or difficulties. The effect of patient education on adherence is positive, despite being based on a few patients and short time of follow-up.
Another issue that has not been the subject of this review, but that deserves attention, is the cost-effectiveness of the self-management interventions. Two of the excluded RCTs presented economic results and pointed out discrepancy in results. One,66 concluded that self-management programmes represent a cost-effective use of resources compared with usual care with a £20 000–£30 000/QALY gained and leads to lower healthcare costs and work absence. The other,67 suggested that although self-management improves the quality of life, it does so with a higher cost (Δ=€4211). This increased cost substantially reduced when medication costs were left out of the equation (Δ=€1863). Further economic studies are warranted to provide greater clarity on the subject.
In conclusion, several issues limit and make it difficult to state recommendations that can be made for the implementation of self-management interventions in IA. Well-structured self-management programmes are lacking or are poorly reported,68 and this is probably due to the articles’ word-count constraints. On the other hand, self-management behaviours are influenced by sociodemographic variables, health status and disease.69 This may lead to some components not having the same applicability between different contexts or countries. The multiform way of offering these interventions also makes it difficult to analyse their individual effectiveness because most of them are centred in hospitals, which is distorted by the very concept of self-management interventions. Professionals should look out for ‘new ways’ that are more adjusted and closer to the patient needs, such as internet programmes, which are proven to be effective in improving health status measures at 1 year.62 67 At last, there are even challenges in better defining which outcomes should be measured in self-management interventions.70 In the future, a formal outcomes core set should be established in self-management interventions.
Footnotes
Twitter: @EduardoJFSantos, @ElenaNikiUK, @carmona_loreto
Collaborators: Ailsa Bosworth (UK) ailsa@nras.org.uk; Elena Nikiphorou (UK) enikiphorou@gmail.com; Loreto Carmona (Spain) loreto.carmona@inmusc.eu; Andrea Marques (Portugal) andreamarques23@gmail.com; Eduardo Santos (Portugal) ejf.santos87@gmail.com; Claire Daien (France) cidaien@gmail.com; Codruta Zabalan (Romania) codruta.filip@gmail.com; George Fragoulis (Greece) geofragoul@yahoo.gr; Bente Appel Esbensen (Denmark) bente.appel.esbensen@regionh.dk; Annette de Thurah (Denmark) annethur@rm.dk; George Metsios (UK) g.metsios@wlv.ac.uk; Hans Biljsma (Netherlands) j.w.j.bijlsma@umcutrecht.nl; Hayley McBain (UK) hayley.mcbain.1@city.ac.uk; Pat Holmes (UK) patricia.karma22@gmail.com; Peter Boehm (Germany) peboehm@gmx.de; Ricardo Ferreira (Portugal) ferreira.rjo@gmail.com; Rikke Helene Moe (Norway) rikmoe@gmail.com; Sarah Ryan (UK) sarah.ryan@uhns.nhs.uk; Tanja Stamn (Austria) tanja.stamm@meduniwien.ac.at.
Contributors: All authors are members of the EULAR’s task force for the development of 2021 EULAR Recommendations for the implementation of self-management strategies in patients with inflammatory arthritis. AM and ES were the fellows. EN and AB were the convenors. LC was the methodologist. All authors have contributed to the work, read and finally approved the manuscript for submission.
Funding: This study was funded by the European League Against Rheumatism EULAR (Project (PARE-led) PAR028: EULAR Recommendations for the implementation of self-management strategies in patients with Inflammatory Arthritis.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study. All data relevant to the study are included in the article or uploaded as supplementary information. Data sharing not applicable as no datasets generated and/or analysed for this study. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.WHO . WHO scientific group on the burden of musculoskeletal conditions at the start of the new millennium. The burden of musculoskeletal conditions at the start of the new millenium: report of a WHO scientific group. Geneve; 2003. [Google Scholar]
- 2.Taylor SJC, Pinnock H, Epiphaniou E. Health services and delivery research. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions: prisms – practical systematic review of self-management support for long-term conditions. Southampton, UK: NIHR Journals Library; 2014. [PubMed] [Google Scholar]
- 3.Kvien TK, Balsa A, Betteridge N, et al. Considerations for improving quality of care of patients with rheumatoid arthritis and associated comorbidities. RMD Open 2020;6:e001211. 10.1136/rmdopen-2020-001211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kielmann T, Huby G, Powell A, et al. From support to boundary: a qualitative study of the border between self-care and professional care. Patient Educ Couns 2010;79:55–61. 10.1016/j.pec.2009.07.015 [DOI] [PubMed] [Google Scholar]
- 5.Baillet A, Gossec L, Carmona L, et al. Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: a EULAR initiative. Ann Rheum Dis 2016;75:965–73. 10.1136/annrheumdis-2016-209233 [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international Task force. Ann Rheum Dis 2016;75:3–15. 10.1136/annrheumdis-2015-207524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zangi HA, Ndosi M, Adams J, et al. EULAR recommendations for patient education for people with inflammatory arthritis. Ann Rheum Dis 2015;74:954–62. 10.1136/annrheumdis-2014-206807 [DOI] [PubMed] [Google Scholar]
- 8.Fernandes L, Hagen KB, Bijlsma JWJ, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 2013;72:1125–35. 10.1136/annrheumdis-2012-202745 [DOI] [PubMed] [Google Scholar]
- 9.Rausch Osthoff A-K, Niedermann K, Braun J, et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis 2018;77:1251–60. 10.1136/annrheumdis-2018-213585 [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Thomas J, Chandler J. Cochrane Handbook for systematic reviews of interventions version 6.0, 2019. Available: www.training.cochrane.org/handbook
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Heijde D, Aletaha D, Carmona L, et al. 2014 update of the EULAR standardised operating procedures for EULAR-endorsed recommendations. Ann Rheum Dis 2015;74:8–13. 10.1136/annrheumdis-2014-206350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 14.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 15.Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. 10.1002/art.21972 [DOI] [PubMed] [Google Scholar]
- 16.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 17.Barlow JH, Williams B, Wright CC. Patient education for people with arthritis in rural communities: the UK experience. Patient Educ Couns 2001;44:205–14. 10.1016/S0738-3991(00)00196-8 [DOI] [PubMed] [Google Scholar]
- 18.Holman H, Lorig K. Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public Health Rep 2004;119:239–43. 10.1016/j.phr.2004.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandura A. Self-Efficacy: the exercise of control. New York: Worth Publishers, 1997. [Google Scholar]
- 20.Gossec L, Dougados M, Rincheval N, et al. Elaboration of the preliminary rheumatoid arthritis impact of disease (raid) score: a EULAR initiative. Ann Rheum Dis 2009;68:1680–5. 10.1136/ard.2008.100271 [DOI] [PubMed] [Google Scholar]
- 21.Gossec L, Paternotte S, Aanerud GJ, et al. Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: a EULAR initiative. Ann Rheum Dis 2011;70:935–42. 10.1136/ard.2010.142901 [DOI] [PubMed] [Google Scholar]
- 22.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Vist GE, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Miedany Y, El Gaafary M, El Arousy N, et al. Arthritis education: the integration of patient-reported outcome measures and patient self-management. Clin Exp Rheumatol 2012;30:899–904. [PubMed] [Google Scholar]
- 27.Giraudet-Le Quintrec J-S, Mayoux-Benhamou A, Ravaud P, et al. Effect of a collective educational program for patients with rheumatoid arthritis: a prospective 12-month randomized controlled trial. J Rheumatol 2007;34:1684–91. [PubMed] [Google Scholar]
- 28.Lumley MA, Keefe FJ, Mosley-Williams A, et al. The effects of written emotional disclosure and coping skills training in rheumatoid arthritis: a randomized clinical trial. J Consult Clin Psychol 2014;82:644–58. 10.1037/a0036958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seneca T, Hauge EM, Maribo T. Comparable effect of partly supervised and self-administered exercise programme in early rheumatoid arthritis--a randomised, controlled trial. Dan Med J 2015;62:A5127. [PubMed] [Google Scholar]
- 30.Shearn MA, Fireman BH. Stress management and mutual support groups in rheumatoid arthritis. Am J Med 1985;78:771–5. 10.1016/0002-9343(85)90282-7 [DOI] [PubMed] [Google Scholar]
- 31.Zuidema R, van Dulmen S, Nijhuis-van der Sanden M, et al. Efficacy of a web-based self-management enhancing program for patients with rheumatoid arthritis: explorative randomized controlled trial. J Med Internet Res 2019;21:e12463. 10.2196/12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barsky AJ, Ahern DK, Orav EJ, et al. A randomized trial of three psychosocial treatments for the symptoms of rheumatoid arthritis. Semin Arthritis Rheum 2010;40:222–32. 10.1016/j.semarthrit.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shigaki CL, Smarr KL, Siva C, et al. RAHelp: an online intervention for individuals with rheumatoid arthritis. Arthritis Care Res 2013;65:NA–81. 10.1002/acr.22042 [DOI] [PubMed] [Google Scholar]
- 34.Manning VL, Hurley MV, Scott DL, et al. Education, self-management, and upper extremity exercise training in people with rheumatoid arthritis: a randomized controlled trial. Arthritis Care Res 2014;66:217–27. 10.1002/acr.22102 [DOI] [PubMed] [Google Scholar]
- 35.Knittle K, De Gucht V, Hurkmans E, et al. Targeting motivation and self-regulation to increase physical activity among patients with rheumatoid arthritis: a randomised controlled trial. Clin Rheumatol 2015;34:231–8. 10.1007/s10067-013-2425-x [DOI] [PubMed] [Google Scholar]
- 36.Niedermann K, de Bie RA, Kubli R, et al. Effectiveness of individual resource-oriented joint protection education in people with rheumatoid arthritis. A randomized controlled trial. Patient Educ Couns 2011;82:42–8. 10.1016/j.pec.2010.02.014 [DOI] [PubMed] [Google Scholar]
- 37.Niedermann K, Buchi S, Ciurea A, et al. Six and 12 months' effects of individual joint protection education in people with rheumatoid arthritis: a randomized controlled trial. Scand J Occup Ther 2012;19:360–9. 10.3109/11038128.2011.611820 [DOI] [PubMed] [Google Scholar]
- 38.Hewlett S, Ambler N, Almeida C, et al. Self-Management of fatigue in rheumatoid arthritis: a randomised controlled trial of group cognitive-behavioural therapy. Ann Rheum Dis 2011;70:1060–7. 10.1136/ard.2010.144691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammond A, Bryan J, Hardy A. Effects of a modular behavioural arthritis education programme: a pragmatic parallel-group randomized controlled trial. Rheumatology 2008;47:1712–8. 10.1093/rheumatology/ken380 [DOI] [PubMed] [Google Scholar]
- 40.Evers AWM, Kraaimaat FW, van Riel PLCM, et al. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain 2002;100:141–53. 10.1016/S0304-3959(02)00274-9 [DOI] [PubMed] [Google Scholar]
- 41.van Lankveld W, van Helmond T, Näring G, et al. Partner participation in cognitive-behavioral self-management group treatment for patients with rheumatoid arthritis. J Rheumatol 2004;31:1738–45. [PubMed] [Google Scholar]
- 42.Basler HD, Rehfisch HP. Cognitive-Behavioral therapy in patients with ankylosing spondylitis in a German self-help organization. J Psychosom Res 1991;35:345–54. 10.1016/0022-3999(91)90089-7 [DOI] [PubMed] [Google Scholar]
- 43.Freeman K, Hammond A, Lincoln NB. Use of cognitive-behavioural arthritis education programmes in newly diagnosed rheumatoid arthritis. Clin Rehabil 2002;16:828–36. 10.1191/0269215502cr565oa [DOI] [PubMed] [Google Scholar]
- 44.Barlow JH, Pennington DC, Bishop PE. Patient education leaflets for people with rheumatoid arthritis: a controlled study. Psychol Health Med 1997;2:221–35. 10.1080/13548509708400580 [DOI] [Google Scholar]
- 45.Barlow JH, Wright CC. Knowledge in patients with rheumatoid arthritis: a longer term follow-up of a randomized controlled study of patient education leaflets. Br J Rheumatol 1998;37:373–6. 10.1093/rheumatology/37.4.373 [DOI] [PubMed] [Google Scholar]
- 46.Hill J, Bird H, Johnson S. Effect of patient education on adherence to drug treatment for rheumatoid arthritis: a randomised controlled trial. Ann Rheum Dis 2001;60:869–75. [PMC free article] [PubMed] [Google Scholar]
- 47.Cramp F, Hewlett S, Almeida C, et al. Non-Pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst Rev 2013;8:Cd008322. 10.1002/14651858.CD008322.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cramer H, Lauche R, Langhorst J, et al. Yoga for rheumatic diseases: a systematic review. Rheumatology 2013;52:2025–30. 10.1093/rheumatology/ket264 [DOI] [PubMed] [Google Scholar]
- 49.Dagfinrud H, Kvien TK, Hagen KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev 2008;1:Cd002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mudano AS, Tugwell P, Wells GA, et al. Tai chi for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2019;19. 10.1002/14651858.CD004849.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pécourneau V, Degboé Y, Barnetche T, et al. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil 2018;99:383–9. 10.1016/j.apmr.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 52.Lopes S, Costa S, Mesquita C, et al. [Home based and group based exercise programs in patients with ankylosing spondylitis: systematic review]. Acta Reumatol Port 2016;41:104–11. [PubMed] [Google Scholar]
- 53.Astin JA, Beckner W, Soeken K, et al. Psychological interventions for rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum 2002;47:291–302. 10.1002/art.10416 [DOI] [PubMed] [Google Scholar]
- 54.DiRenzo D, Crespo-Bosque M, Gould N, et al. Systematic review and meta-analysis: Mindfulness-Based interventions for rheumatoid arthritis. Curr Rheumatol Rep 2018;20:75. 10.1007/s11926-018-0787-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knittle K, Maes S, de Gucht V. Psychological interventions for rheumatoid arthritis: examining the role of self-regulation with a systematic review and meta-analysis of randomized controlled trials. Arthritis Care Res 2010;62:1460–72. 10.1002/acr.20251 [DOI] [PubMed] [Google Scholar]
- 56.Dissanayake RK, Bertouch JV. Psychosocial interventions as adjunct therapy for patients with rheumatoid arthritis: a systematic review. Int J Rheum Dis 2010;13:324–34. 10.1111/j.1756-185X.2010.01563.x [DOI] [PubMed] [Google Scholar]
- 57.Carandang K, Pyatak EA, Vigen CLP. Systematic review of educational interventions for rheumatoid arthritis. Am J Occup Ther 2016;70:7006290020p1–12. 10.5014/ajot.2016.021386 [DOI] [PubMed] [Google Scholar]
- 58.Riemsma RP, Kirwan JR, Taal E. Patient education for adults with rheumatoid arthritis. Cochrane Database Syst Rev 2003;2:Cd003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du S, Yuan C, Xiao X, et al. Self-Management programs for chronic musculoskeletal pain conditions: a systematic review and meta-analysis. Patient Educ Couns 2011;85:e299–310. 10.1016/j.pec.2011.02.021 [DOI] [PubMed] [Google Scholar]
- 60.Solomon DH, Warsi A, Brown-Stevenson T, et al. Does self-management education benefit all populations with arthritis? A randomized controlled trial in a primary care physician network. J Rheumatol 2002;29:362–8. [PubMed] [Google Scholar]
- 61.Lorig K, Ritter PL, Plant K. A disease-specific self-help program compared with a generalized chronic disease self-help program for arthritis patients. Arthritis Rheum 2005;53:950–7. 10.1002/art.21604 [DOI] [PubMed] [Google Scholar]
- 62.Lorig KR, Ritter PL, Laurent DD, et al. The Internet-based arthritis self-management program: a one-year randomized trial for patients with arthritis or fibromyalgia. Arthritis Rheum 2008;59:1009–17. 10.1002/art.23817 [DOI] [PubMed] [Google Scholar]
- 63.Lambert SD, Beatty L, McElduff P, et al. A systematic review and meta-analysis of written self-administered psychosocial interventions among adults with a physical illness. Patient Educ Couns 2017;100:2200–17. 10.1016/j.pec.2017.06.039 [DOI] [PubMed] [Google Scholar]
- 64.Eisele A, Schagg D, Krämer LV, et al. Behaviour change techniques applied in interventions to enhance physical activity adherence in patients with chronic musculoskeletal conditions: a systematic review and meta-analysis. Patient Educ Couns 2019;102:25–36. 10.1016/j.pec.2018.09.018 [DOI] [PubMed] [Google Scholar]
- 65.Datta J, Petticrew M. Challenges to evaluating complex interventions: a content analysis of published papers. BMC Public Health 2013;13:568. 10.1186/1471-2458-13-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manning VL, Kaambwa B, Ratcliffe J, et al. Economic evaluation of a brief education, self-management and upper limb exercise training in people with rheumatoid arthritis (extra) programme: a trial-based analysis. Rheumatology 2015;54:302–9. 10.1093/rheumatology/keu319 [DOI] [PubMed] [Google Scholar]
- 67.Ferwerda M, van Beugen S, van Middendorp H, et al. Tailored, Therapist-Guided Internet-based cognitive behavioral therapy compared to care as usual for patients with rheumatoid arthritis: economic evaluation of a randomized controlled trial. J Med Internet Res 2018;20:e260. 10.2196/jmir.9997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keogh A, Tully MA, Matthews J, et al. A review of behaviour change theories and techniques used in group based self-management programmes for chronic low back pain and arthritis. Man Ther 2015;20:727–35. 10.1016/j.math.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 69.Chaleshgar Kordasiabi M, Akhlaghi M, Baghianimoghadam MH, et al. Self management behaviors in rheumatoid arthritis patients and associated factors in Tehran 2013. Glob J Health Sci 2015;8:156–67. 10.5539/gjhs.v8n3p156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banerjee A, Hendrick P, Bhattacharjee P, et al. A systematic review of outcome measures utilised to assess self-management in clinical trials in patients with chronic pain. Patient Educ Couns 2018;101:767–78. 10.1016/j.pec.2017.12.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-001647supp001.pdf (65.6KB, pdf)
rmdopen-2021-001647supp002.pdf (45.2KB, pdf)
rmdopen-2021-001647supp003.pdf (73KB, pdf)
rmdopen-2021-001647supp004.pdf (320.2KB, pdf)
rmdopen-2021-001647supp005.pdf (156.2KB, pdf)
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study. All data relevant to the study are included in the article or uploaded as supplementary information. Data sharing not applicable as no datasets generated and/or analysed for this study. All data relevant to the study are included in the article or uploaded as supplementary information.