Abstract
Background:
Thigh muscle weakness after anterior cruciate ligament reconstruction (ACLR) can persist after returning to activity. While resistance training can improve muscle function, “nonfunctional” training methods are not optimal for inducing transfer of benefits to activities such as walking. Here, we tested the feasibility of a novel functional resistance training (FRT) approach to restore strength and function in an individual with ACLR.
Hypothesis:
FRT would improve knee strength and function after ACLR.
Study Design:
Case report.
Level of Evidence:
Level 5.
Methods:
A 15-year-old male patient volunteered for an 8-week intervention where he performed 30 minutes of treadmill walking, 3 times per week, while wearing a custom-designed knee brace that provided resistance to the thigh muscles of his ACLR leg. Thigh strength, gait mechanics, and corticospinal and spinal excitability were assessed before and immediately after the 8-week intervention. Voluntary muscle activation was evaluated immediately after the intervention.
Results:
Knee extensor and flexor strength increased in the ACLR leg from pre- to posttraining (130 to 225 N·m [+74%] and 44 to 88 N·m [+99%], respectively) and increases in between-limb extensor and flexor strength symmetry (45% to 92% [+74%] and 47% to 72% [+65%], respectively) were also noted. After the intervention, voluntary muscle activation in the ACLR leg was 72%, compared with the non-ACLR leg at 75%. Knee angle and moment during late stance phase decreased (ie, improved) in the ACLR leg and appeared more similar to the non-ACLR leg after FRT training (18° to 14° [−23.4] and 0.07 to −0.02 N·m·kg−1·m−1 [−122.8%], respectively). Corticospinal and spinal excitability in the ACLR leg decreased (3511 to 2511 [−28.5%] and 0.42 to 0.24 [−43.7%], respectively) from pre- to posttraining.
Conclusion:
A full 8 weeks of FRT that targeted both quadriceps and hamstring muscles lead to improvements in strength and gait, suggesting that FRT may constitute a promising and practical alternative to traditional methods of resistance training.
Clinical Relevance:
FRT may serve as a viable approach to improve knee strength and function after ACL reconstruction.
Keywords: ACL, rehabilitation, eddy current brake, robotics, H-reflex, TMS, isometric, kinematics, kinetics, biomechanics
Anterior cruciate ligament (ACL) injuries are common in young athletes participating in cutting and pivoting sports.24 ACL reconstruction (ACLR) remains the surgical treatment of choice for most individuals because of the resulting pain and knee instability. While ACLR restores knee stability and facilitates return to sports, quadriceps muscle weakness and activation deficits (ie, the inability to fully activate the quadriceps muscles during a maximal contraction) can linger in ACLR patients for months to years.56 Furthermore, biomechanical asymmetries that result from ACL injury and ACLR can be exacerbated by the persistent quadriceps weakness.
High-intensity resistance training can address persistent quadriceps dysfunction and ensuing biomechanical asymmetries in individuals with ACLR7,13,46,48,61; however, recovery has been reported to be incomplete even with such interventions.17,47,61 A key reason for incomplete recovery could be that interventions addressing strength and activation deficits are typically performed in a “nonfunctional” manner (eg, exercises performed in seated position), which is less than optimal for inducing transfer of benefits to functional activities such as walking because of practice specificity.2,27,52,60,74 Moreover, high-intensity exercise interventions cannot be introduced very early after the surgery because of the fear of potential graft failure, knee laxity, or patellar fracture.11,59 Thus, low-load progressive resistance training, when performed using a task-specific approach, could be a safe and effective alternative to combat knee strength deficits and gait asymmetries in individuals with ACLR. Furthermore, such task-specific resistance training (also known as functional resistance training [FRT]) may facilitate structural and physiological alterations of the corticospinal pathways and promote neuronal plasticity through principles of motor learning and experience-dependent neural plasticity.5,14,27,65,69,71 Thus, FRT may positively alter the excitability of spinal-reflexive and descending corticospinal pathways,4,22,62 which have been attributed as potential sources of persistent quadriceps weakness and activation deficits after ACLR.44,45 Thus, the implementation of FRT into a rehabilitation program could facilitate improved clinical and functional outcomes after ACLR; however, the feasibility of FRT to improve knee strength and function has not been verified to date in the ACLR population.
In this case study, we sought to determine whether FRT is a feasible approach to improve knee strength and activation, knee joint biomechanics, and corticospinal excitability in a patient after ACLR. We performed FRT using a novel resistive knee brace that is capable of providing scalable resistive torques to the quadriceps and hamstring muscles during walking.70,71 We hypothesized that incorporation of FRT in conjunction with standard ACLR rehabilitation would improve quadriceps strength and activation, gait mechanics, and corticospinal excitability.
Methods
Case Description
An adolescent male patient (age = 15.1 years; body height = 197.8 cm; and body mass = 95.5 kg) presented with a complete ACL rupture and a grade II lateral collateral ligament sprain in the left (preferred kicking/dominant) leg. The noncontact injury occurred while sidestepping to evade a defender during a basketball game. The participant’s sporting background consisted of freshman-level high school basketball and water polo with no known prior history of lower-extremity injury, neurological issues (ie, headache, epilepsy, etc), uncontrolled illnesses (ie, diabetes, hypertension, etc), and/or medication (ie, antiseizure, antidepression, antianxiety, etc). Ten weeks after the injury, the participant began his preoperative rehabilitation (30 minutes/session, 2× per week for 4 weeks), which focused on restoring full range of motion, reducing effusion, and improving quadriceps/hip/gluteal strength. Fourteen weeks after the ACL rupture, the participant underwent pediatric reconstructive surgery with a left quadriceps tendon autograft and no reported complications. One week after the surgery, the participant began his standard postoperative rehabilitation (see Appendix, available in the online version of this article, for details of postoperative rehabilitation protocol).
A quadriceps tendon autograph procedure was selected for this particular patient, and agreed on, because of its versatility in the skeletally immature preservation of hamstring anatomy and function, reduced incidence of anterior knee pain and patellar fracture, and similarities in anterior knee stability and subjective outcomes compared with hamstring and patellar tendon grafts.6,50,64 Seven weeks after ACLR, the surgeon’s patient examination reported no infection, minimal joint effusion, disuse of crutches, no pain with walking or stairs, difficulties with squatting and ambulation without a slight limp, and decreased active and passive range of motion (ACLR: 0° to 120° and 0° to 130° [terminal extension achieved after 1- to 2-min warm-up], respectively; non-ACLR: 0° to 130° and 0° to 140°, respectively). After the surgeon’s approval, the participant and his mother met with the research team and were fully informed of the study details and commitment. After this, written informed consent/assent to participate in the research was obtained using documents approved by the University of Michigan Medical Institutional Review Board.
Study Protocol and Assessments
This investigation comprised pretesting (pre), an 8-week FRT intervention, and posttesting (post) immediately after the intervention (Figure 1). Pre- and posttesting sessions included a battery of assessments collected over the course of 1 week. Pre measurements included bilateral isometric strength of the quadriceps and hamstring muscle groups, sagittal-plane gait mechanics, corticospinal and spinal excitability of the quadriceps, and questionnaires to determine knee health–related quality of life. Posttesting sessions additionally included quadriceps muscle voluntary activation testing during isometric strength assessment—this testing was only performed during the posttesting session because of safety reasons.59 Each assessment lasted 1.5 to 3 hours and was performed approximately at the same time of day at pre and post time-points to account for any potential diurnal variations.63 In-depth descriptions on testing procedures, instrumentation, data reduction, and variable selection are fully detailed in the Appendix (available online).
Figure 1.
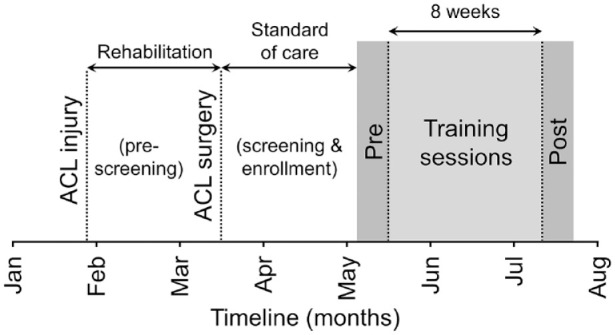
Schematic of the participant’s injury and general study timeline overview. ACL, anterior cruciate ligament.
Training Intervention
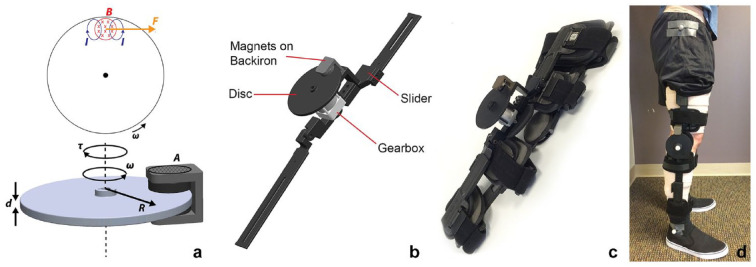
The FRT intervention included 3 training sessions per week (24 sessions total) lasting 1 hour per session, including training, setup and breakdown time, and rest periods. FRT was performed using a custom-designed resistive brace that was fitted with an eddy current brake to provide scalable resistive torques at the knee during walking.70 The details of this brace are provided elsewhere.70,71 Briefly, eddy current brakes are magnetic brakes that generate resistive torques when a conductive material/disc moves through a magnetic field. The equation governing the magnitude of torque is given by ,15,70,71 where t is the resistive torque, s is the conductivity of the disc, A is the area of the disc exposed to the magnetic field, d is the thickness of the disc, B is the magnetic field strength, R is the effective radius of the disc, and w is the angular velocity at which the disc rotates (Figure 2a). Thus, by simply changing the amount of magnet exposed over the disc using a linear slider (Figure 2b), we were able to adjust the resistive torque produced at the knee during training.
Figure 2.
(a) Schematic of torque generation in eddy current braking. As the disc rotates through a magnetic field (B) with angular velocity (w), eddy currents (I) form within the disc, which resists the motion of the disc by creating a force (F). The resulting torque (t) is proportional to the conductivity of the disc (s), the area of the disc exposed to the magnetic field (A), the thickness of the disc (d), the magnetic field strength (B), the effective radius of the disc (R), and the angular velocity at which the disc rotates (w). (b) Drawings showing the parts of eddy current braking device. The linear slider allows to change the resistance by changing the amount of disc exposed to the magnets and the gearbox helps in amplifying the angular velocity at which the disc rotates. (c) A picture showing the eddy current braking device attached to a commercially available adjustable knee brace. (d) A picture showing the brace applied to the leg of a human patient.
During each session, the participant was fitted with 3 reflective markers (19-mm diameter) placed on the lateral side of the hip, knee, and ankle joints of the ACLR leg. The knee marker was outlined by pen and then removed to allow for the ACLR leg to be fitted with a custom-designed resistive leg brace (Figure 2, c and d).70,71 The axis of rotation of the brace was aligned with the previous placement of the knee marker. An additional 19 mm marker was placed on the axis of rotation of the brace to approximate the knee position while walking with the brace.
The training session commenced by first collecting a “baseline” trial where the participant was asked to walk normally on the treadmill for 60 seconds at 80% of his overground walking speed (determined weekly, prior to training). A camera (C920 Pro HD Webcam, Logitech) was used to track real-time sagittal kinematics via the reflective markers and obtain ensemble averages of his right hip and knee angles.39 The ensemble averaged trajectories were then scaled by 30% (130% of normal) during swing-phase to create a target template that was displayed in real-time in front of the participant for his target-match training.30,33-35,38 The stance phase was unaltered in the scaling of the template, as the foot was in contact with the ground (treadmill belt) in this phase and could not extend any further while in support of the body. An up-scaled version of the target template was used to ensure that the participant walked with an increased range of motion to work on achieving full knee extension and flexion, an essential element to combat quadriceps inhibition.10 This also ensured that the participant was adequately incentivized to increase effort and to maintain optimal spatiotemporal coordination.70,71 The slower than normal walking speed was used to allow the participant more time to learn the skill, promote a more symmetrical gait pattern, and allow greater loading on the leg muscles.26 Bidirectional brace resistance (0% to 100% exposure) was then manually set corresponding to a self-perceived difficulty of 5 to 7 using the OMNI scale.68 Target-match training consisted of six 5-minute walking trials with 1 minute rest between each trial, during which the participant walked on the treadmill with bidirectional resistance and matched the real-time target template. At each training session, close attention was paid to appropriately cue the participant in order to match the target-match template as close as possible. The cue involved bending the leg after toe-off by focusing on the hamstring muscles and extending the leg fully during the terminal swing by focusing on the quadriceps muscles.
Results
The participant tolerated the 8-week intervention well and completed the FRT protocol without any adverse effects. The participant had no complaints of pain or swelling during or after the intervention. The participant reported that the brace was comfortable and showed no visible gait deviations (eg, limping) during the training.
Knee Strength and Activation
The isometric knee extensor and flexor strength of the ACLR leg increased after the intervention (+73.8% and +98.8%, respectively), while knee extensor strength slightly decreased and flexor strength slightly increased (−14.8% and +15.8%, respectively) in the non-ACLR leg (Table 1). At posttraining, voluntary activation of the quadriceps muscle was similar between legs (Table 1). Limb symmetry indices of knee extensor and flexor strength also improved (+104.1% and +82.3%, respectively) from pre- to posttraining (Table 1).
Table 1.
Quadriceps and hamstrings isometric strength and voluntary activation in the healthy and ACLR leg during pre and post timepoints
| Pre | Post | |||||
|---|---|---|---|---|---|---|
| Non-ACLR | ACLR | LSI | Non-ACLR | ACLR | LSI | |
| Knee extensor peak torque (N·m) | 286.5 | 129.6 | 45.3 | 244.0 | 225.3 | 92.3 |
| Knee flexor peak torque (N·m) | 94.1 | 44.2 | 47.0 | 108.9 | 87.9 | 80.7 |
| Voluntary activation (%) | — | — | — | 75.2 | 71.6 | 95.2 |
ACLR, anterior cruciate ligament reconstruction; LSI, limb symmetry index; Post, subsequent to intervention; Pre, prior to intervention. Dashes indicate no values were recorded, as voluntary activation (%) was only measured after the intervention.
Gait Mechanics
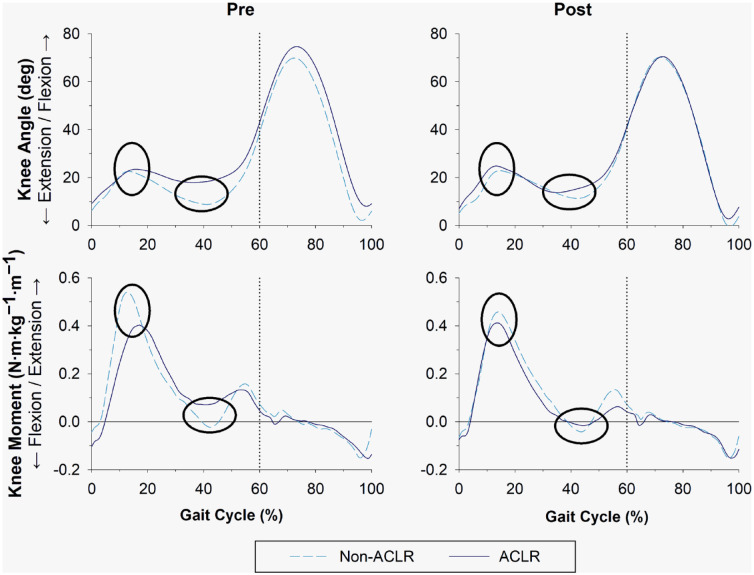
The knee angle excursion (computed from the entire gait cycle) of the ACLR leg increased slightly with training (66.6° to 67.6° [+1.6%]). There were small increases in peak knee flexion during early stance and peak knee extension during late stance (23.5° to 24.8° [+5.8%] and 18.0° to 13.8° [−23.4%], respectively) after the intervention. While there were only minor alterations in the knee moment during early stance (0.40 to 0.41 N·m·kg−1·m−1 [+2.2%]), the knee moment during late stance showed a larger change (changed from extension to flexion moment) and became more similar to the non-ACLR leg after the intervention (0.07 to −0.02 N·m·kg−1·m−1 [−122.8%]) (Figure 3).
Figure 3.
Sagittal-plane knee joint angle and internal moment (equal and opposite to an external moment) of the non-ACLR (dashed line) and ACLR (solid line) leg during the entire gait cycle (heel strike-to-heel strike) at pre and post timepoints. Dotted line represents the visual division between stance phase (0% to 60% of gait cycle) and swing phase (61% to 100% gait cycle). Ellipses represent the point at which gait data were evaluated: maximum knee angle and internal knee moment during early (loading response) stance, and minimum knee angle and internal knee moment during late (terminal) stance. ACLR, anterior cruciate ligament reconstruction.
Corticospinal and Spinal Excitability
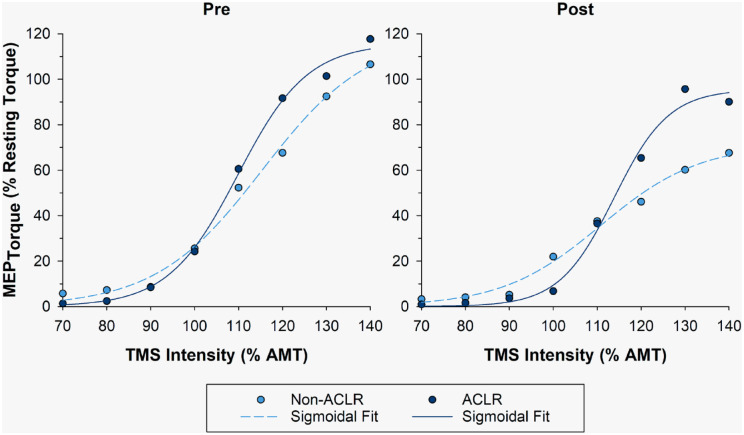
The corticospinal excitability (area under the recruitment curve) of the ACLR leg decreased after the intervention (−28.5%). The MEPmax (maximum motor evoked potential) and the slope (k) of the recruitment curve on both the ACLR and the non-ACLR legs also decreased with training (ACLR: −17.5% and −21.3%, respectively; non-ACLR: −40.4% and −7.1%, respectively) (Figure 4). There were minimal changes in the stimulus intensity leading to half-maximal MEP torque size (S50) on the ACLR leg with training (+3.6%). The normalized spinal excitability of the ACLR leg, as measured by the Hoffmann reflex to muscle response ratio (H:M ratio), also decreased after the intervention (0.42 to 0.24 [−43.7%]).
Figure 4.
Motor evoked torque recruitment curves of the non-ACLR (dashed line) and ACLR (solid line) leg at pre and post timepoints. Motor evoked torques were normalized to the peripherally evoked knee extensor torque responses elicited at 100% maximum stimulator output when the participant sat quietly on the chair (ie, resting torque). ACLR, anterior cruciate ligament reconstruction; AMT, active motor threshold; MEP, motor evoked potential; TMS, transcranial magnetic stimulation.
Knee Health Questionnaires
All clinical assessment scores improved from pre to post timepoints, many beyond a minimal clinically important difference (Table 2).
Table 2.
Self-reported knee health questionnaires reported at pre and post timepoints
| Clinical Assessment | Pre | Post |
|---|---|---|
| IKDC score | 49 | 54 |
| KOOS–Pain | 75 | 89 |
| KOOS–Symptoms | 61 | 79 |
| KOOS–ADL | 88 | 93 |
| KOOS–Sport/Recreation | 20 | 50 |
| KOOS–QOL | 50 | 54 |
ADL, Activities of Daily Living; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; Post, subsequent to intervention; Pre, prior to intervention; QOL, Quality of Life.
Discussion
The purpose of this case report was to assess whether FRT could serve as a feasible method to improve knee strength and function after ACLR. For this purpose, we recruited a young ACLR participant and trained him for 8 weeks (3 times per week) using a novel resistive knee brace that provided bidirectional resistance during treadmill walking. After the intervention, the participant showed substantial improvements in knee strength and limb symmetry indices, which were paralleled by reductions in corticospinal excitability and improvements in gait mechanics and patient-reported knee outcomes. There were also no adverse events during the training. These results suggest that FRT is a safe and feasible approach to improve knee strength and function and may serve as a promising adjuvant to traditional rehabilitation.
After ACL surgery, restoration of lower-extremity strength (specifically in the quadriceps) is a top priority, as persistent weakness can lead to altered gait mechanics, degeneration of the knee joint, and eventually lowered quality of life.49,56 Previous authors have shown that FRT during treadmill walking can improve muscle activation and gait mechanics in healthy70 and poststroke71,75 individuals. However, to date, the benefits of FRT have not been tested in individuals with ACL injury or surgery. Here, we show for the first time that FRT can facilitate knee strength and function after ACLR. The participant in this study showed substantial improvements in knee extensor and flexor strength in the ACLR leg. The improvements in knee strength were also paralleled by improvements in limb symmetry indices (45.3% to 92.3% in knee extensors and 47.0% to 80.7% in knee flexors). The vast improvement in knee extensor symmetry after the intervention was due, in part, by a large increase in strength in the ACLR leg (+74%) and a slight decrease in strength in the non-ACLR leg (−15%). This observation may potentially be the result of less attention given to strengthening the non-ACLR leg during the intervention and/or a compensatory mechanism for the body to regain a higher level of symmetry between legs. We also found larger improvements in hamstring strength during the intervention, compared with quadriceps, but lower restoration of limb symmetry indices. The greater increase in hamstring strength could be explained, in part, by the specific demands of the bidirectional FRT device we implemented, which provided equal resistance to the quadriceps and hamstrings during gait. Thus, the hamstring muscles received greater training load as compared with the quadriceps muscles, resulting in a greater gain in the hamstring strength. However, unlike the quadriceps, the strength of the contralateral hamstring muscles also increased after the intervention, which resulted in lower limb symmetry index in the ACLR leg. Nonetheless, these improvements are noteworthy because standard postoperative rehabilitation programs have only reported small improvements in knee extensor and flexor strength (16% and 4%, respectively) and symmetry (12% and 10%, respectively).28 Furthermore, the participant showed high symmetry (95.2%) in voluntary activation of the quadriceps muscle after the intervention, although there were bilateral activation deficits. The observed symmetry values in knee strength and voluntary activation are typically considered to be normal and optimal for returning athletes to their sport. Thus, based on these results, we believe that FRT could serve as a valuable addition to the traditional ACL rehabilitation program.
During overground gait, improvements were seen in knee extension angle and internal flexion moment during stance, as well as an overall improvement in gait symmetry. Prior to the training intervention, the participant progressed through the gait cycle with less knee extension in the ACLR leg compared with his non-ACLR leg during midstance (ie, his knee stayed in a more flexed position). Because the participant showed the ability (with a 1- to 2-minute warm-up) to achieve full passive knee extension at his 7-week postoperative checkup, the increases seen in knee extension angle are most likely attributed to increased quadriceps strength. Less peak internal knee flexion moment was also seen in the participant’s ACLR leg, compared with his non-ACLR leg, coinciding with the lower knee extension angle. These discrepancies in knee angle and moment data were reduced after 8 weeks of FRT, subsequently improving overall gait symmetry at the posttraining timepoint. Considering that the average length of time at which gait returns to “normal” is suggested to occur at ~1 year postsurgery,72 our findings in this case study of near symmetrical gait at <4 months are encouraging and deserve further exploration.
Previous research has shown that altered corticospinal excitability contributes to the persistent quadriceps strength and activation deficits after ACLR.44,45 When examining the area under the curve and peak MEP of the recruitment curves of motor evoked torque responses, we saw a decrease in corticospinal excitability of the quadriceps muscles after the intervention. This observation is consistent with prior research studies that have shown a reduction in corticospinal excitability with resistance training in “healthy” individuals8,23; but was contradictory to other studies that have shown either no changes or an increase in corticospinal excitability after resistance training.16,25,43,73 The reduction in motor evoked torque responses for the same background activation after the training suggests that fewer motoneurons were activated (or recruited) to perform the task than were activated prior to the training.8 This could have been either due to a reduction in the magnitude of the descending volleys elicited by the transcranial magnetic stimulation or due to changes in the functional properties of circuitry within the spinal cord.8 The observed decrease in spinal excitability (H:M ratio) of the ACLR leg suggests that the observed changes in corticospinal excitability could have been primarily mediated at the spinal level.
Limitations
Several limitations should be noted when interpreting the results of this study. First, the findings presented within this article derive from a single participant; as such, generalized conclusions and practical takeaways are not possible at this time. As with any case study, the results only establish the safety and feasibility of the intervention. Second, while the results from our single participant were very promising, he may have simply been a “responder” to FRT and other individuals may not respond in a similar fashion (ie, “nonresponders”). Our participant was very self-motivated and focused throughout the intervention. In a larger cohort, however, there may be instances where participants could be less motivated (or are extrinsically motivated), physically or mentally exhausted from the day (ie, work, school, physical therapy, etc), or can only commit to a fewer number of training sessions within the 8-week intervention. Last, it is not clear to what extent FRT contributed to the improvements observed in this study, as the participant also received standard rehabilitation during the intervention period. Thus, it is likely that a good portion of the improvements could have been mediated by traditional rehabilitation that accompanied FRT. Further research on a larger cohort with appropriate control group is warranted to fully understand the true benefits of FRT.
Conclusion
In summary, this case study confirms the feasibility of an 8-week FRT intervention to improve knee strength and function after ACLR. The participant showed substantial improvements in knee extensor and flexor strength (+73.8% and +98.8%, respectively) after the completion of the intervention in conjunction with standard rehabilitation. The symmetry in knee extensor strength and activation levels also reached the acceptable norms (<10% deficit) after the intervention. These improvements were also accompanied by improvements in gait mechanics and knee health–related quality of life; thereby, supporting the need for further research on FRT through a large-scale, controlled clinical trial.
Supplemental Material
Supplemental material, sj-docx-1-sph-10.1177_1941738120955184 for Functional Resistance Training to Improve Knee Strength and Function After Acute Anterior Cruciate Ligament Reconstruction: A Case Study by Scott R. Brown, Edward P. Washabaugh, Aviroop Dutt-Mazumder, Edward M. Wojtys, Riann M. Palmieri-Smith and Chandramouli Krishnan in Sports Health: A Multidisciplinary Approach
Acknowledgments
The authors would like to thank Mr William D. Ballew for his assistance with data collection and Mr Thomas E. Augenstein and Mr Steven A. Garcia for their critique of an earlier version of the manuscript.
Footnotes
The following author declared potential conflicts of interest: E.M.W. is a paid Editor-in-Chief for Sports Health. This work was supported by the National Institute of Child Health and Human Development of the National Institutes of Health (Grant No. R21 HD092614), the National Science Foundation Graduate Research Fellowship Program (Grant No. DGE 1256260), and the UM-BICI Collaboratory Initiative.
References
- 1. Ahn J, Hogan N. Walking is not like reaching: evidence from periodic mechanical perturbations. PLoS One. 2012;7:e31767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander NB, Galecki AT, Grenier ML, et al. Task-specific resistance training to improve the ability of activities of daily living-impaired older adults to rise from a bed and from a chair. J Am Geriatr Soc. 2001;49:1418-1427. [DOI] [PubMed] [Google Scholar]
- 3. Awiszus F. TMS and threshold hunting. Suppl Clin Neurophysiol. 2003;56:13-23. [DOI] [PubMed] [Google Scholar]
- 4. Bayona NA, Bitensky J, Salter K, Teasell R. The role of task-specific training in rehabilitation therapies. Top Stroke Rehabil. 2005;12:58-65. [DOI] [PubMed] [Google Scholar]
- 5. Beck S, Taube W, Gruber M, Amtage F, Gollhofer A, Schubert M. Task-specific changes in motor evoked potentials of lower limb muscles after different training interventions. Brain Res. 2007;1179:51-60. [DOI] [PubMed] [Google Scholar]
- 6. Belk JW, Kraeutler MJ, Marshall HA, Goodrich JA, McCarty EC. Quadriceps tendon autograft for primary anterior cruciate ligament reconstruction: a systematic review of comparative studies with minimum 2-year follow-up. Arthroscopy. 2018;34:1699-1707. [DOI] [PubMed] [Google Scholar]
- 7. Brasileiro JS, Pinto OM, Avila MA, Salvini TF. Functional and morphological changes in the quadriceps muscle induced by eccentric training after ACL reconstruction. Rev Bras Fisioter. 2011;15:284-290. [DOI] [PubMed] [Google Scholar]
- 8. Carroll TJ, Riek S, Carson RG. The sites of neural adaptation induced by resistance training in humans. J Physiol. 2002;544:641-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carson RG, Nelson BD, Buick AR, Carroll TJ, Kennedy NC, Cann RM. Characterizing changes in the excitability of corticospinal projections to proximal muscles of the upper limb. Brain Stimul. 2013;6:760-768. [DOI] [PubMed] [Google Scholar]
- 10. Cavanaugh JT, Powers M. ACL rehabilitation progression: where are we now? Curr Rev Musculoskelet Med. 2017;10:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coury HJ, Brasileiro JS, Salvini TF, Poletto PR, Carnaz L, Hansson GA. Change in knee kinematics during gait after eccentric isokinetic training for quadriceps in subjects submitted to anterior cruciate ligament reconstruction. Gait Posture. 2006;24:370-374. [DOI] [PubMed] [Google Scholar]
- 12. Erickson LC. The role of O-6 methylguanine DNA methyltransferase (MGMT) in drug resistance and strategies for its inhibition. Semin Cancer Biol. 1991;2:257-265. [PubMed] [Google Scholar]
- 13. Gerber JP, Marcus RL, Leland ED, Lastayo PC. The use of eccentrically biased resistance exercise to mitigate muscle impairments following anterior cruciate ligament reconstruction: a short review. Sports Health. 2009;1:31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gokeler A, Neuhaus D, Benjaminse A, Grooms DR, Baumeister J. Principles of motor learning to support neuroplasticity after ACL injury: implications for optimizing performance and reducing risk of second ACL injury. Sports Med. 2019;49:853-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gosline AH, Hayward V. Eddy current brakes for haptic interfaces: design, identification, and control. IEEE/ASME Trans Mechatron. 2008;13:669-677. [Google Scholar]
- 16. Hendy AM, Kidgell DJ. Anodal tDCS applied during strength training enhances motor cortical plasticity. Med Sci Sports Exerc. 2013;45:1721-1729. [DOI] [PubMed] [Google Scholar]
- 17. Hooper DM, Morrissey MC, Drechsler W, Morrissey D, King J. Open and closed kinetic chain exercises in the early period after anterior cruciate ligament reconstruction. Improvements in level walking, stair ascent, and stair descent. Am J Sports Med. 2001;29:167-174. [DOI] [PubMed] [Google Scholar]
- 18. Hopkins JT, Ingersoll CD, Krause BA, Edwards JE, Cordova ML. Effect of knee joint effusion on quadriceps and soleus motoneuron pool excitability. Med Sci Sports Exerc. 2001;33:123-126. [DOI] [PubMed] [Google Scholar]
- 19. Hopkins JT, Wagie NC. Intrasession and intersession reliability of the quadriceps Hoffmann reflex. Electromyogr Clin Neurophysiol. 2003;43:85-89. [PubMed] [Google Scholar]
- 20. Horstman AM, Beltman MJ, Gerrits KH, et al. Intrinsic muscle strength and voluntary activation of both lower limbs and functional performance after stroke. Clin Physiol Funct Imaging. 2008;28:251-261. [DOI] [PubMed] [Google Scholar]
- 21. Horstman AM, de Ruiter CJ, van Duijnhoven NT, Hopman MT, de Haan A. Changes in muscle contractile characteristics and jump height following 24 days of unilateral lower limb suspension. Eur J Appl Physiol. 2012;112:135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hubbard IJ, Parsons MW, Neilson C, Carey LM. Task-specific training: evidence for and translation to clinical practice. Occup Ther Int. 2009;16:175-189. [DOI] [PubMed] [Google Scholar]
- 23. Jensen JL, Marstrand PC, Nielsen JB. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J Appl Physiol (1985). 2005;99:1558-1568. [DOI] [PubMed] [Google Scholar]
- 24. Joseph AM, Collins CL, Henke NM, Yard EE, Fields SK, Comstock RD. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J Athl Train. 2013;48:810-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kidgell DJ, Bonanno DR, Frazer AK, Howatson G, Pearce AJ. Corticospinal responses following strength training: a systematic review and meta-analysis. Eur J Neurosci. 2017;46:2648-2661. [DOI] [PubMed] [Google Scholar]
- 26. Klarner T, Blouin JS, Carpenter MG, Lam T. Contributions to enhanced activity in rectus femoris in response to Lokomat-applied resistance. Exp Brain Res. 2013;225:1-10. [DOI] [PubMed] [Google Scholar]
- 27. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225-S239. [DOI] [PubMed] [Google Scholar]
- 28. Knezevic OM, Mirkov DM, Kadija M, Milovanovic D, Jaric S. Evaluation of isokinetic and isometric strength measures for monitoring muscle function recovery after anterior cruciate ligament reconstruction. J Strength Cond Res. 2014;28:1722-1731. [DOI] [PubMed] [Google Scholar]
- 29. Krishnan C. Effect of paired-pulse stimulus parameters on the two phases of short interval intracortical inhibition in the quadriceps muscle group. Restor Neurol Neurosci. 2019;37:363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krishnan C. Learning and interlimb transfer of new gait patterns are facilitated by distributed practice across days. Gait Posture. 2019;70:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krishnan C, Allen EJ, Williams GN. Torque-based triggering improves stimulus timing precision in activation tests. Muscle Nerve. 2009;40:130-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krishnan C, Dhaher Y. Corticospinal responses of quadriceps are abnormally coupled with hip adductors in chronic stroke survivors. Exp Neurol. 2012;233:400-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krishnan C, Dharia AK, Augenstein TE, et al. Learning new gait patterns is enhanced by specificity of training rather than progression of task difficulty. J Biomech. 2019;88:33-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krishnan C, Ranganathan R, Kantak SS, Dhaher YY, Rymer WZ. Active robotic training improves locomotor function in a stroke survivor. J Neuroeng Rehabil. 2012;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krishnan C, Ranganathan R, Tetarbe M. Interlimb transfer of motor skill learning during walking: no evidence for asymmetric transfer. Gait Posture. 2017;56:24-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krishnan C, Theuerkauf P. Effect of knee angle on quadriceps strength and activation after anterior cruciate ligament reconstruction. J Appl Physiol (1985). 2015;119:223-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krishnan C, Washabaugh EP, Dutt-Mazumder A, Brown SR, Wojtys EM, Palmieri-Smith RM. Conditioning brain responses to improve quadriceps function in an individual with anterior cruciate ligament reconstruction. Sports Health. 2019;11:306-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krishnan C, Washabaugh EP, Reid CE, Althoen MM, Ranganathan R. Learning new gait patterns: age-related differences in skill acquisition and interlimb transfer. Exp Gerontol. 2018;111:45-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krishnan C, Washabaugh EP, Seetharaman Y. A low cost real-time motion tracking approach using webcam technology. J Biomech. 2015;48:544-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krishnan C, Williams GN. Error associated with antagonist muscle activity in isometric knee strength testing. Eur J Appl Physiol. 2010;109:527-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011;29:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kukke SN, Paine RW, Chao CC, de Campos AC, Hallett M. Efficient and reliable characterization of the corticospinal system using transcranial magnetic stimulation. J Clin Neurophysiol. 2014;31:246-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Latella C, Kidgell DJ, Pearce AJ. Reduction in corticospinal inhibition in the trained and untrained limb following unilateral leg strength training. Eur J Appl Physiol. 2012;112:3097-3107. [DOI] [PubMed] [Google Scholar]
- 44. Lepley AS, Ericksen HM, Sohn DH, Pietrosimone BG. Contributions of neural excitability and voluntary activation to quadriceps muscle strength following anterior cruciate ligament reconstruction. Knee. 2014;21:736-742. [DOI] [PubMed] [Google Scholar]
- 45. Lepley AS, Gribble PA, Thomas AC, Tevald MA, Sohn DH, Pietrosimone BG. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: a 6-month longitudinal investigation. Scand J Med Sci Sports. 2015;25:828-839. [DOI] [PubMed] [Google Scholar]
- 46. Lepley LK, Palmieri-Smith R. Effect of eccentric strengthening after anterior cruciate ligament reconstruction on quadriceps strength. J Sport Rehabil. 2013;22:150-156. [DOI] [PubMed] [Google Scholar]
- 47. Lepley LK, Wojtys EM, Palmieri-Smith RM. Combination of eccentric exercise and neuromuscular electrical stimulation to improve biomechanical limb symmetry after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2015;30:738-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lepley LK, Wojtys EM, Palmieri-Smith RM. Combination of eccentric exercise and neuromuscular electrical stimulation to improve quadriceps function post-ACL reconstruction. Knee. 2015;22:270-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2002;17:56-63. [DOI] [PubMed] [Google Scholar]
- 50. Lund B, Nielsen T, Fauno P, Christiansen SE, Lind M. Is quadriceps tendon a better graft choice than patellar tendon? A prospective randomized study. Arthroscopy. 2014;30:593-598. [DOI] [PubMed] [Google Scholar]
- 51. Madhavan S, Krishnan C, Jayaraman A, Rymer WZ, Stinear JW. Corticospinal tract integrity correlates with knee extensor weakness in chronic stroke survivors. Clin Neurophysiol. 2011;122:1588-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Manini T, Marko M, VanArnam T, et al. Efficacy of resistance and task-specific exercise in older adults who modify tasks of everyday life. J Gerontol A Biol Sci Med Sci. 2007;62:616-623. [DOI] [PubMed] [Google Scholar]
- 53. Mason J, Frazer A, Horvath DM, et al. Adaptations in corticospinal excitability and inhibition are not spatially confined to the agonist muscle following strength training. Eur J Appl Physiol. 2017;117:1359-1371. [DOI] [PubMed] [Google Scholar]
- 54. McLean SG, Fellin RE, Suedekum N, Calabrese G, Passerallo A, Joy S. Impact of fatigue on gender-based high-risk landing strategies. Med Sci Sports Exerc. 2007;39:502-514. [DOI] [PubMed] [Google Scholar]
- 55. Palmieri-Smith RM, Lepley LK. Quadriceps strength asymmetry after anterior cruciate ligament reconstruction alters knee joint biomechanics and functional performance at time of return to activity. Am J Sports Med. 2015;43:1662-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27:405-424. [DOI] [PubMed] [Google Scholar]
- 57. Palmieri RM, Ingersoll CD, Hoffman MA. The Hoffmann reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train. 2004;39:268-277. [PMC free article] [PubMed] [Google Scholar]
- 58. Patterson MR, Delahunt E, Sweeney KT, Caulfield B. An ambulatory method of identifying anterior cruciate ligament reconstructed gait patterns. Sensors (Basel). 2014;14:887-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone-patellar tendon-bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39:278-286. [DOI] [PubMed] [Google Scholar]
- 60. Proteau L, Marteniuk RG, Levesque L. A sensorimotor basis for motor learning: evidence indicating specificity of practice. Q J Exp Psychol A. 1992;44:557-575. [DOI] [PubMed] [Google Scholar]
- 61. Risberg MA, Moksnes H, Storevold A, Holm I, Snyder-Mackler L. Rehabilitation after anterior cruciate ligament injury influences joint loading during walking but not hopping. Br J Sports Med. 2009;43:423-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rossi F, Gianola S, Corvetti L. Regulation of intrinsic neuronal properties for axon growth and regeneration. Prog Neurobiol. 2007;81:1-28. [DOI] [PubMed] [Google Scholar]
- 63. Sedliak M, Finni T, Cheng S, Kraemer WJ, Hakkinen K. Effect of time-of-day-specific strength training on serum hormone concentrations and isometric strength in men. Chronobiol Int. 2007;24:1159-1177. [DOI] [PubMed] [Google Scholar]
- 64. Sheean AJ, Musahl V, Slone HS, et al. Quadriceps tendon autograft for arthroscopic knee ligament reconstruction: use it now, use it often. Br J Sports Med. 2018;52:698-701. [DOI] [PubMed] [Google Scholar]
- 65. Siddique U, Rahman S, Frazer AK, Pearce AJ, Howatson G, Kidgell DJ. Determining the sites of neural adaptations to resistance training: a systematic review and meta-analysis. Sports Med. 2020;50:1107-1128. [DOI] [PubMed] [Google Scholar]
- 66. Snyder-Mackler L, De Luca PF, Williams PR, Eastlack ME, Bartolozzi AR, 3rd. Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1994;76:555-560. [DOI] [PubMed] [Google Scholar]
- 67. Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res. 2008;186:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Utter AC, Robertson RJ, Green JM, Suminski RR, McAnulty SR, Nieman DC. Validation of the Adult OMNI Scale of perceived exertion for walking/running exercise. Med Sci Sports Exerc. 2004;36:1776-1780. [DOI] [PubMed] [Google Scholar]
- 69. Washabaugh E, Guo J, Chang CK, Remy D, Krishnan C. A portable passive rehabilitation robot for upper-extremity functional resistance training. IEEE Trans Biomed Eng. 2019;66:496-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Washabaugh EP, Claflin ES, Gillespie RB, Krishnan C. A novel application of eddy current braking for functional strength training during gait. Ann Biomed Eng. 2016;44:2760-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Washabaugh EP, Krishnan C. A wearable resistive robot facilitates locomotor adaptations during gait. Restor Neurol Neurosci. 2018;36:215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Webster KE, Wittwer JE, O’Brien J, Feller JA. Gait patterns after anterior cruciate ligament reconstruction are related to graft type. Am J Sports Med. 2005;33:247-254. [DOI] [PubMed] [Google Scholar]
- 73. Weier AT, Pearce AJ, Kidgell DJ. Strength training reduces intracortical inhibition. Acta Physiol (Oxf). 2012;206:109-119. [DOI] [PubMed] [Google Scholar]
- 74. Williams G, Kahn M, Randall A. Strength training for walking in neurologic rehabilitation is not task specific: a focused review. Am J Phys Med Rehabil. 2014;93:511-522. [DOI] [PubMed] [Google Scholar]
- 75. Yang YR, Wang RY, Lin KH, Chu MY, Chan RC. Task-oriented progressive resistance strength training improves muscle strength and functional performance in individuals with stroke. Clin Rehabil. 2006;20:860-870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sph-10.1177_1941738120955184 for Functional Resistance Training to Improve Knee Strength and Function After Acute Anterior Cruciate Ligament Reconstruction: A Case Study by Scott R. Brown, Edward P. Washabaugh, Aviroop Dutt-Mazumder, Edward M. Wojtys, Riann M. Palmieri-Smith and Chandramouli Krishnan in Sports Health: A Multidisciplinary Approach