Abstract
Natural killer (NK) cells are attractive effector cells of the innate immune system against human immunodeficiency virus (HIV) and cancer. However, NK cell therapies are limited by the fact that target cells evade NK cells, for example, in latent reservoirs (in HIV) or through upregulation of inhibitory signals (in cancer). To address this limitation, we describe a biodegradable nanoparticle-based “priming” approach to enhance the cytotoxic efficacy of peripheral blood mononuclear cell-derived NK cells. We present poly(lactic-co-glycolic acid) (PLGA) nanodepots (NDs) that co-encapsulate prostratin, a latency-reversing agent, and anti-CD25 (aCD25), a cell surface binding antibody, to enhance primary NK cell function against HIV and cancer. We utilize a nanoemulsion synthesis scheme to encapsulate both prostratin and aCD25 within the PLGA NDs (termed Pro-aCD25-NDs). Physicochemical characterization studies of the NDs demonstrated that our synthesis scheme resulted in stable and monodisperse Pro-aCD25-NDs. The NDs successfully released both active prostratin and anti-CD25, and with controllable release kinetics. When Pro-aCD25-NDs were administered in an in vitro model of latent HIV and acute T cell leukemia using J-Lat 10.6 cells, the NDs were observed to prime J-Lat cells resulting in significantly increased NK cell-mediated cytotoxicity compared to free prostratin plus anti-CD25, and other controls. These findings demonstrate the feasibility of using our Pro-aCD25-NDs to prime target cells for enhancing the cytotoxicity of NK cells as antiviral or antitumor agents.
Keywords: poly(lactic-co-glycolic acid) (PLGA) nanodepots, natural killer (NK) cells, human immunodeficiency virus (HIV), cancer, latency-reversing agent, antibody
1. Introduction
Natural killer (NK) cells are attractive immune effector cells for treating human immunodeficiency virus (HIV) and cancer, as they can serially lyse targets without the need for explicit donor-recipient matching [1, 2]. Further, unlike T cells which contain the required surface receptors for virion binding, NK cells cannot be infected with HIV, allowing them to exert cytotoxic pressure without the risk of viral spread [3]. However, the efficacy of an NK cell-based therapy can be limited if the cellular targets of NK cells evade detection, as is commonly observed with latent HIV reservoirs or immunosuppressive tumor microenvironments [4–8]. In these disease contexts, a promising strategy to maximize the benefits of using NK cells would be to “prime” disease sites to present cell-specific targets for enhanced NK cell-mediated cytotoxicity. Biodegradable polymeric nanodepots (NDs) represent a promising avenue for activating and potentiating the cytotoxicity of NK cells in these disease settings [9–12]. By packaging and delivering therapeutic agents using a single ND platform, one can prime a disease site for maximal NK cell-mediated antiviral or antitumor cytotoxicity.
Here we present a nano-immunoengineering approach to improve the cytotoxicity of NK cells as antiviral and antitumor agents. Specifically, we have generated a poly(lactic-co-glycolic acid) (PLGA) ND platform that co-encapsulates prostratin, a latency-reversing agent (LRA) that activates target cells such as those located in latently infected HIV reservoirs [13–15], and anti-CD25 (aCD25), an antibody that binds to CD25 expressed on the surface of the target cells (Fig. 1). PLGA is a suitable candidate for formulating the NDs because it has excellent biocompatibility and biodegradability and is US FDA-approved for oral and parenteral administration [16, 17]. PLGA allows the co-localization of multiple therapeutic agents [17, 18], which is important in applications that require their simultaneous presence. PLGA NDs have contributed to the field of immunoengineering, wherein they have been synthesized to target or modulate immune cells, thus improving the efficacy of existing immunotherapies [19].
Figure 1.
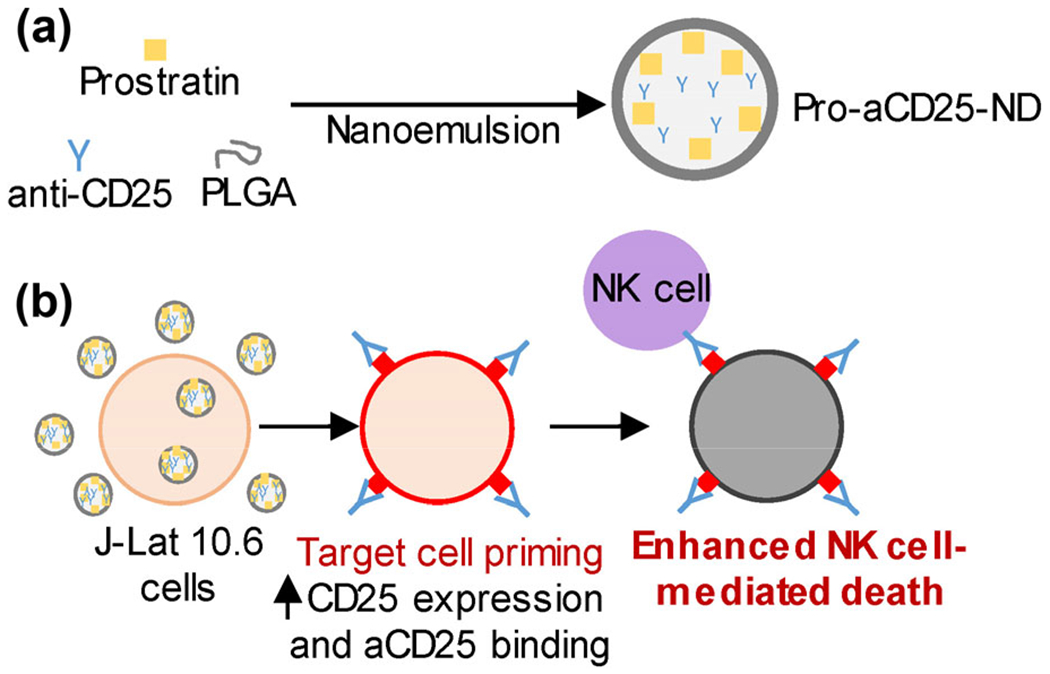
Schematic overview of our PLGA ND-based approach for enhancing NK cell cytotoxic function, (a) PLGA NDs encapsulating prostratin, a latency-reversing agent, and aCD25 were synthesized using a nanoemulsion scheme, (b) J-Lat 10.6 cells, a model of latent HIV and leukemia, treated with Pro-aCD25-ND increased CD25 expression in response to released prostratin, which enables increased aCD25 binding (also released from the NDs). These effects function in concern to prime the targeted cells for enhanced NK cell-mediated killing.
The PLGA NDs co-encapsulating both prostratin and aCD25 (termed Pro-aCD25-NDs) are synthesized using a customized nanoemulsion technique [20, 21]. Administration of the Pro-aCD25-NDs in combination with primary NK cells as a therapeutic ensemble co-localizes the actions of three sequential immunological effects important for NK cell-mediated cytotoxicity of targeted cells. First, the prostratin released from the NDs activates the targeted cells. This activation results in increased CD25 expression on their cell surface. Simultaneously, aCD25 released from the Pro-aCD25-NDs binds to the targeted cells with increased (prostratin-driven) CD25 expression. The NK cells that are present in the vicinity of the NDs are then stimulated to effect antibody-dependent cellular cytotoxicity (ADCC) via this specific binding interaction [22]. Encapsulation and release of these therapeutic agents using biodegradable NDs with controllable release kinetics offers the potential for improved priming of targeted cells, and consequently, improved NK cell-mediated cytotoxicity.
CD25 represents an effective target in both HIV and cancer. In HIV, eliminating CD25-expressing HIV+ T cells has been shown to block viral production and spread [23]. In cancer, aCD25 antibodies have been used to deplete regulatory T cells and block oncogenic signaling in leukemic cells [24, 25]. We hypothesize that the co-localization of an LRA, a target cell-specific antibody, and primary NK cells will trigger a coordinated and enhanced eradication of target cells. To test this hypothesis, we present the physicochemical characterization of the Pro-aCD25-NDs—size distributions, morphology, charge, stability, encapsulation efficiency, and drug release. We confirm feasibility of our therapeutic approach in a J-Lat 10.6 (J-Lat) cell model of latent HIV and leukemia [26]. If successful, our NK cell-ND-based strategy can be applied to both latent viral infections as well as tumor sites for improved antiviral and antitumor responses.
2. Results and discussion
Modular PLGA NDs were synthesized to encapsulate either vehicle (Blank NDs), aCD25 (aCD25-NDs), prostratin (Pro-NDs), or both prostratin and aCD25 (Pro-aCD25-NDs) using the nanoemulsion synthesis technique (see Experimental section) [21]. All NDs exhibited monodisperse size distributions (poly-dispersity index < 0.2 for each ND) and mean hydrodynamic diameters between 190.1–220.2 nm. The NDs remained stable when stored in water at room temperature (RT) over twenty-two days, as demonstrated by consistent size distributions measured using dynamic light scattering (DLS) (Fig. 2(a)). Pro-aCD25-NDs exhibited uniform spherical morphology as measured by scanning electron microscopy (SEM) (Fig. 2(b)). Neither the absorbance spectra (Fig. 2(c)) nor the surface charge (zeta potential, Fig. 2(d)) of the NDs were notably altered when prostratin and/or aCD25 were encapsulated in the NDs, which demonstrates that our nanoemulsion scheme yielded modular NDs with consistent size, stability, and surface charges regardless of their encapsulated contents, an important aspect for maintaining stable physiological interactions.
Figure 2.
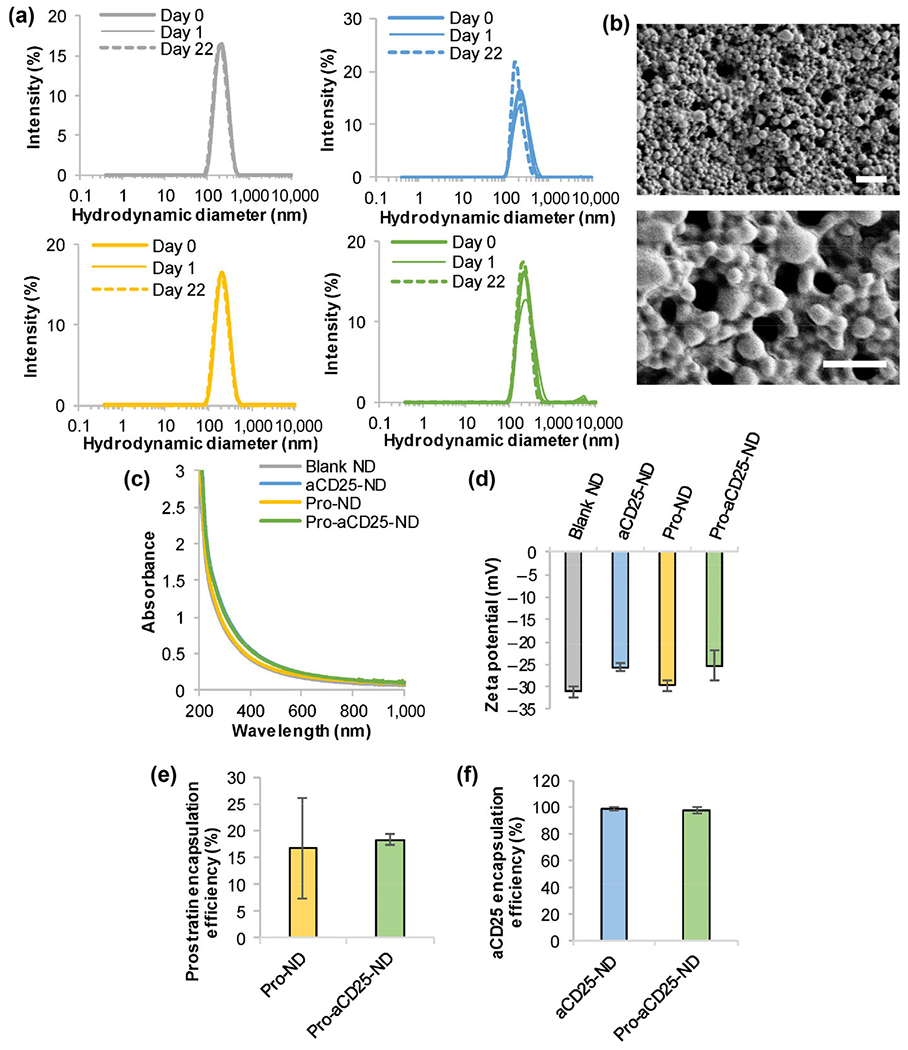
Stable and monodisperse PLGA NDs co-encapsulate prostratin and aCD25. (a) ND hydrodynamic diameters were measured by DLS; gray: Blank NDs, blue: aCD25-NDs, yellow: Pro-NDs, green: Pro-aCD25-NDs. (b) SEM images of Pro-aCD25-NDs at increasing magnifications (top to bottom). Scale bar = 2 μm (top) and 1 μm (bottom), (c) Absorbance spectra were analyzed using UV–Vis–NIR spectroscopy, (d) Zeta potential was measured by electrophoretic light scattering. The encapsulation efficiency of (e) prostratin was determined by absorbance spectroscopy and that of (f) aCD25 was determined by BCA protein assay.
The encapsulation efficiency of prostratin was determined using absorbance spectroscopy by measuring the concentration of unencapsulated prostratin in the supernatant of the NDs collected after synthesis. We calculated the molar extinction coefficient for prostratin and utilized the linear relationship between prostratin concentration and optical absorbance (at 260 nm) in order to quantify unknown prostratin concentrations (Fig. S1(a) in the Electronic Supplementary Material (ESM)). Similarly, the encapsulation efficiency of aCD25 was determined using a bicinchoninic acid (BCA) protein assay by measuring the concentration of unencapsulated antibody in the supernatant of the NDs collected after synthesis. A standard curve was established to define the linear relationship between aCD25 concentration and optical absorbance at 562 nm (Fig. S1(b) in the ESM). Pro-NDs and Pro-aCD25-NDs encapsulated prostratin with encapsulation efficiencies of 16.8% and 18.3%, respectively (Fig. 2(e)), while aCD25-NDs and Pro-aCD25-NDs encapsulated aCD25 with encapsulation efficiencies of 98.4% and 96.9%, respectively (Fig. 2(f)). To test whether the water-oil-water emulsion scheme had an adverse effect on the functionality of the encapsulated antibody, we conducted a study wherein the synthesis components were mixed in the absence of PLGA, i.e. antibody in phosphate buffered saline (PBS) was added to acetonitrile, which was then added to the aqueous emulsifier (5% polyvinyl alcohol (PVA)). Importantly, aCD25 maintained 65.5% of the functionality as measured by an ELISA, despite direct exposure to acetonitrile and 5% PVA in the absence of PLGA for 3 h (Fig. S2 in the ESM). Additionally, our synthesis scheme was premised on extensive literature using nanoemulsion to encapsulate antibody, protein, or peptide therapeutics [11, 27–32]. Should we anticipate a significant drop in antibody efficacy, we will modify our synthesis scheme to investigate the effect of emulsifiers, cryoprotectants, and/or carrier proteins [33, 34]. These findings demonstrate that our synthesis scheme results in co-encapsulation of prostratin and aCD25 within the NDs, which is important for their co-administration.
To determine release of the agents from the NDs of interest, Pro-aCD25-NDs were incubated in PBS at 37 °C to mimic a physiological environment. At predetermined time intervals, the Pro-aCD25-ND solution was centrifuged, and supernatants were analyzed for prostratin content (by absorbance spectroscopy) and antibody content (by BCA protein assay). Pro-aCD25-NDs released 43.2% of the encapsulated prostratin and 34.5% of the encapsulated antibody by 24 h in PBS (Figs. 3(a) and 3(b)). We note a higher burst release of the water-soluble aCD25 after 1 h versus the organic-soluble prostratin, reflecting the individual agents’ intrinsic proclivity toward the PBS solvent. We predict the prostratin to incorporate homogenously within the PLGA ND shell; thus, as PLGA gradually degrades in PBS at 37 °C, the prostratin is increasingly released into the surrounding solvent. Conversely, we predict our synthesis scheme to result in aCD25 encapsulation just interior to the exterior PLGA surface; thus, when the Pro-aCD25-NDs are incubated in a solvent amenable to dissolving antibody, aCD25 quickly diffuses into the PBS. Our results are consistent with studies encapsulating small molecules (similar to prostratin) and macromolecules (similar to aCD25) in Refs. [35–38]. To further characterize the aCD25 release kinetics and demonstrate the NDs’ potential for sustained release and functionality over time, we conducted a release study in the presence of solvent replacement. Here, compared with the study in Fig. 3(b) that achieved equilibrium release, Pro-aCD25-NDs were incubated in PBS at 37 °C with replacement of fresh PBS at specified time intervals (0 h, 15 min, 16 h, 24 h). This yielded additional antibody release with every solvent replacement (addition of fresh PBS). In this scheme of PBS replacement, 42.6% more aCD25 was released after 24 h compared to no replacement (77.1% vs. 34.5%) (Fig. 3(c)). Our synthesis scheme was also amenable to synthesizing NDs that could be used for longer-term release. Accordingly, Pro-aCD25-NDs were allowed to incubate at 4 °C for 24 h after synthesis, thereby “hardening” the PLGA NDs. Under this synthesis scheme, both prostratin (Fig. 3(d)) and aCD25 (Fig. 3(e)) exhibited gradual and sustained release kinetics over six days. These findings demonstrate that PLGA NDs serve as a versatile platform for controllable encapsulation and longitudinal release of both prostratin and aCD25, which is important for co-localizing their immunological effects for priming targeted cells.
Figure 3.

Pro-aCD25-NDs exhibit tunable release kinetics of both prostratin and aCD25. (a) and (b) Pro-aCD25-NDs were incubated in PBS at 37 °C. Cumulative release of (a) prostratin and (b) aCD25 into the solution was analyzed at the listed time periods by UV–Vis spectroscopy and BCA protein assay, respectively, (c) Pro-aCD25-NDs were incubated in PBS at 37 °C for 24 h. At indicated timepoints, release of aCD25 from NDs into the solution was measured by BCA assay. Fresh PBS was used for replacement at each timepoint in the replacement group (shaded circles). No fresh PBS was added to the no replacement group (empty circles), (d) and (e) After synthesis, Pro-aCD25-NDs were incubated at 4 °C. After 24 h, Pro-aCD25-NDs were incubated in PBS at 37 °C for six days. At each listed timepoint, an aliquot was centrifuged, and released (d) prostratin and (e) aCD25 was measured by UV-Vis spectroscopy and BCA assay, respectively. Fresh PBS was replaced at every timepoint measured (i.e. 24, 48, and 72 h). Values represent means ± standard deviation (n = 3/group).
Since the nano-immunotherapeutic platform relies on the co-administration of both NDs and NK cells, we investigated the effect of the NDs on NK cell activation. Primary NK cells were isolated from peripheral blood mononuclear cells (PBMC) and cultured for 24 h with free agents (vehicle, aCD25, prostratin, or prostratin + aCD25) or each of the modular NDs. NK cells were then harvested, stained with fluorescent antibodies, and analyzed for representative activation markers NKG2D, NKp30, and NKp46 by flow cytometry. Specifically, the percentage of cells staining positive for each marker was measured for each treatment condition, and normalized to the percentage of positively stained cells generated by vehicle treatment. Neither the free agents nor any of the NDs had significant effect on NKG2D or NKp30 expression on NK cells (Figs. 4(a) and 4(b)) as measured by fold change over the vehicle. NKp46 appeared to be marginally (but not statistically significantly) increased with treatments, with maximal expression after incubation with prostratin + aCD25 and the correlative Pro-aCD25-NDs (Fig. 4(c)). These data suggest that co-administering the NDs with NK cells does not significantly alter the phenotype of NK cells; rather, Pro-aCD25-NDs may increase NK cell activation, although further studies would be needed to describe these potential effects.
Figure 4.
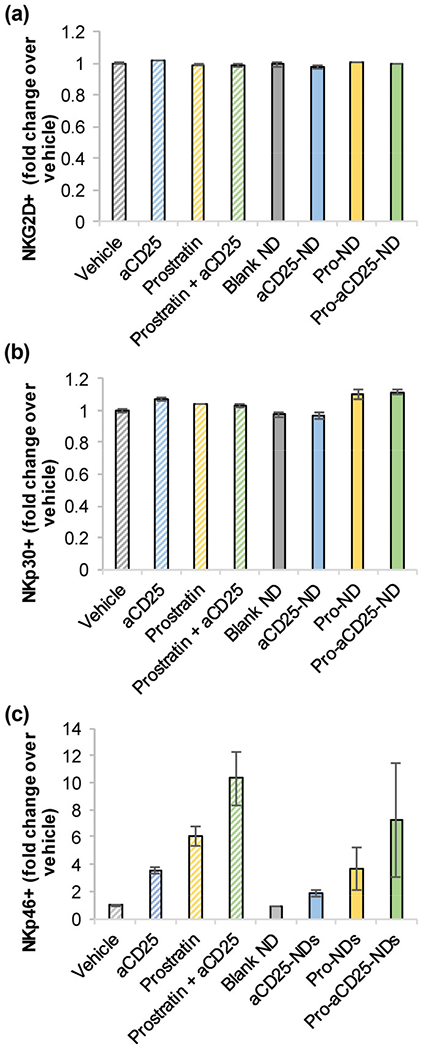
PLGA NDs and their encapsulated contents do not significantly alter NK cell phenotype. Primary NK cells were cultured with NDs, their constituent components, or controls. After 24 h, (a) NKG2D, (b) NKp30, and (c) NKp46 expressions were measured by flow cytometry. Values represent means ± standard deviation (n = 2/group).
Next, we investigated the effect of the NDs on the targeted J-Lat cells. J-Lat is an immortalized T cell line derived from the parental Jurkat acute T cell leukemia cell line. J-Lat cells have been virally infected with a full HIV-1 retroviral construct containing the full-length HIV-1 genome with a non-functional Env gene; further, GFP replaces Nef in the genome, such that when latent HIV transcription is reactivated, the cells generate GFP [26]. Therefore, these cells model blood cancer, and represent a functional model for latent HIV infection where they will generate GFP when they have been reactivated out of latency. Here, we used GFP expression as a functional read-out for viral reactivation by prostratin. As seen in Refs. [39, 40], our studies demonstrated that GFP expression of J-Lat cells increases as a function of increasing prostratin concentration and in a time-dependent manner (Figs. S3(a)–S3(e) in the ESM) with maximal prostratin-dependent GFP expression at 24 h with 1,000 ng/mL prostratin (60.6%). When J-Lat cells are activated by prostratin, they also express CD25 on their cell surface in a prostratin concentration- and time-dependent manner (Figs. S3(f)–S3(j) in the ESM), once again with maximal CD25 expression at 24 h at 1,000 ng/mL prostratin (68.7%). CD25 is marginally expressed under basal conditions (6.03% cells express CD25 after 24 h treatment with dimethyl sulfoxide (DMSO)). Importantly, prostratin released from Pro-NDs maintains its functionality to induce CD25 expression on J-Lat cells similarly to free prostratin, in a dose-dependent manner (Fig. S4 in the ESM). These studies illustrate the ability of prostratin to prime a disease site (i.e. targeted J-Lat cells), providing an activation-specific cell target for potentially improved antiviral and antitumor NK cell cytotoxic effect.
To further confirm functionality of prostratin once it has been released from the NDs, we treated J-Lat cells with NDs and measured GFP expression after 24 h. Prostratin from both Pro-NDs and Pro-aCD25-NDs significantly increased GFP expression in the J-Lat cells as measured by flow cytometry, suggesting HIV-specific activation out of latency (Figs. 5(a)–5(c)). The level of GFP expression was 3-fold higher for both Pro-NDs and Pro-aCD25-NDs (14.2% and 13.8%, respectively) than untreated and controls (~ 4% GFP expression), indicative of effective latency reversal. The lower levels of GFP expression induced by the NDs than that observed for free prostratin (Fig. S3 in the ESM) can be potentially attributed to the presence of the NDs interfering with the measurement of GFP+ cells, or the kinetics of release from the NDs. Complementary to the reactivation studies, we sought to determine the binding of released aCD25 from the NDs to the surface of the prostratin-activated J-Lat cells. To visualize this, we stained J-Lat cells with a fluorescent secondary antibody to quantify primary aCD25 binding. We found that 76.0% of J-Lat cells bound aCD25 released from Pro-aCD25-NDs (Figs. 5(d)–5(f)). These results illustrate the ability of the NDs to induce prostratin-driven CD25 expression on J-Lat cells, which in turn increased binding of the aCD25 to the J-Lat cells. Importantly, NDs alone did not dramatically impact J-Lat cell viability (Fig. S5 in the ESM). Similar viability levels were observed for Blank ND-treated and vehicle-treated cells (95.5% and 97.7%, respectively, with 1 μL treatment for 24 h). These data confirm the functionality of our Pro-aCD25-NDs wherein the co-encapsulated and co-released agents function in concern to reactivate target cells via prostratin (GFP expression) and provide a ligand for the cell-specific antibody (aCD25) to bind, through which the NK cells can exert their cytotoxic function.
Figure 5.
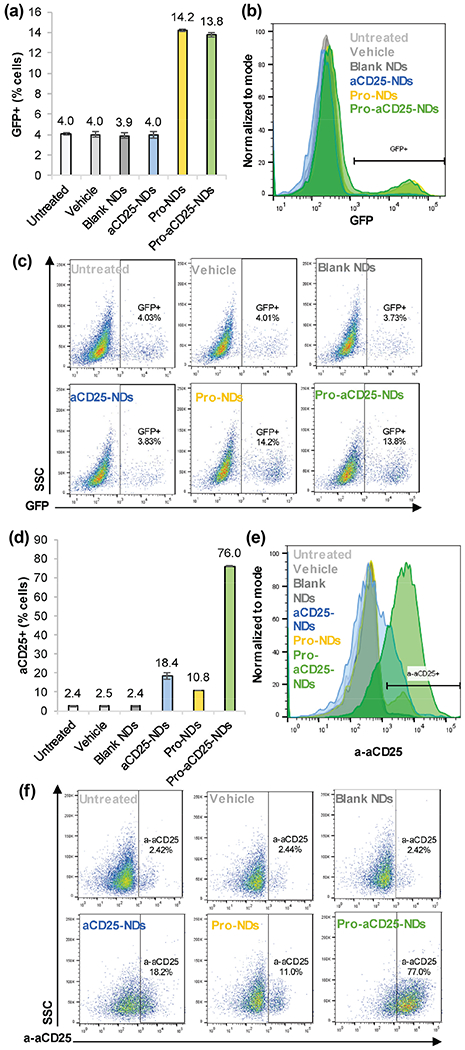
NDs function to generate activation of antibody binding to J-Lat cells, (a) J-Lat cells were cultured with NDs and controls. After 24 h> GFP expression was analyzed as a measure of J-Lat cell activation by flow cytometry, (b) Representative histograms of J-Lat cell GFP expression after 24 h culture with NDs and controls, (c) Representative scatter plots of J-Lat cell GFP expression after 24 h culture with NDs and controls, (d) J-Lat cells were cultured with NDs and controls. After 24 h, aCD25 binding to J-Lat cells was analyzed by detecting secondary antibody binding via flow cytometry, (e) Representative histograms of aCD25 binding to J-Lat cells after 24 h culture with NDs and controls, (f) Representative scatter plots of aCD25 binding to J-Lat cells after 24 h culture with NDs and controls. Values represent means ± standard deviation (n = 3/group).
The final goal of this proof-of-concept study is to test whether NK cells exhibited enhanced cytotoxic function against targeted cells. To test our platform, we first incubated J-Lat cells with NK cells at various effector:target (E:T; NK:J-Lat) cell ratios to understand basal intrinsic NK cell cytotoxicity on J-Lat cells. We found that J-Lat cells were killed in a dose-dependent manner; as fewer NK cells were added to J-Lat cell co-culture, a higher percentage of J-Lat cells remained viable (Figs. 6(a)–6(c)). J-Lat viability was minimal for 1:2 E:T ratio (10.5%) and highest for 1:100 E:T ratio (94.6%, similar to untreated controls 97.4%). We then combined NKs with the Pro-aCD25-NDs to test their effects on NK cell cytotoxic function. Pro-aCD25-NDs significantly enhanced NK cell-based cytotoxicity; that is, NK cells alone killed 77.7% of J-Lat cells, while NK cells in combination with Pro-aCD25-NDs were able to kill 90.8% of J-Lat cells (increasing target cell death by 13.1%; Figs. 6(d)–6(f)). The co-encapsulation of prostratin and aCD25 within the NDs yielded a 2.1% increase in cytotoxicity for the target J-Lat cells relative to the free prostratin and aCD25 administered at an equivalent concentration, which suggests that the encapsulated and released agents maintain functionality similar to free agents in vitro. Together, these data illustrate the ability of NDs to work in concern with NK cells, wherein the released prostratin activates the J-Lat cells, increasing CD25 expression for improved binding of the co-encapsulated and released aCD25, which in turn facilitates enhanced NK cell-mediated cytotoxicity. When a similar study was performed using a different E:T ratio (1:1) and the killing was quantified on a per NK cell basis, we found that NK cells + Pro-aCD25-NDs killed significantly more J-Lat cells relative to NK cells alone (9.1 and 4.3 cells, respectively) generating a 2.1-fold increase in cytotoxicity in the presence of the NDs (Fig. S6 in the ESM). These findings demonstrate that the Pro-aCD25-NDs prime J-Lat cells for significantly increased NK cell-mediated cytotoxicity.
Figure 6.
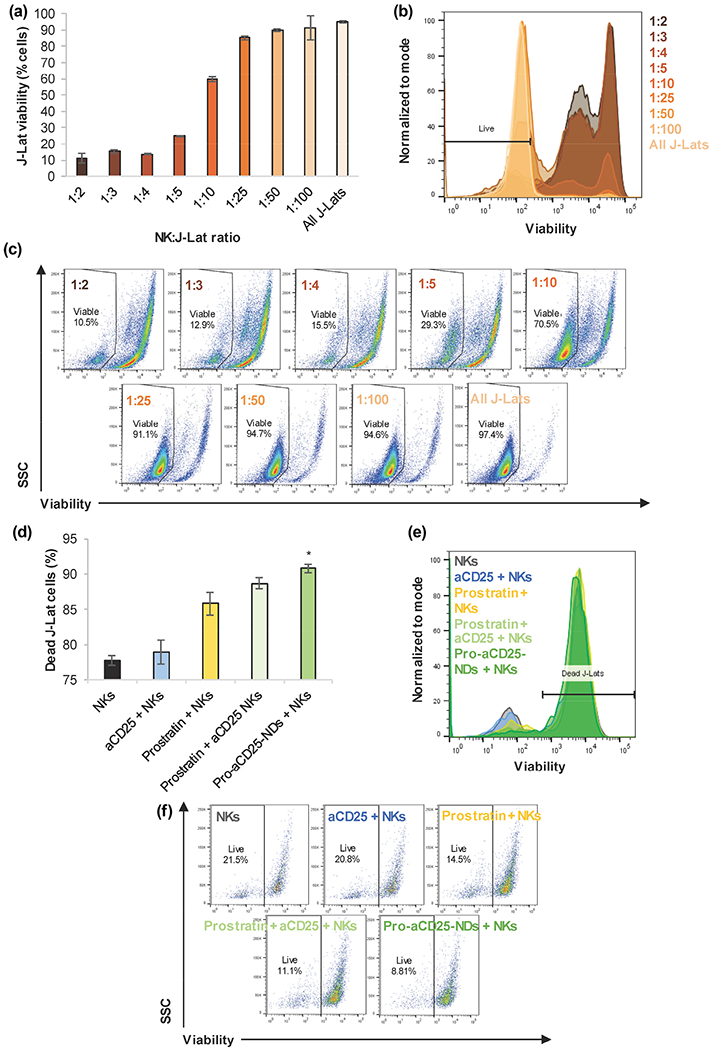
NDs enhance NK cell cytotoxic function on J-Lat cells, (a) NK cells were co-cultured with J-Lat cells at listed E:T ratios for 24 h. J-Lat cell viability was measured by flow cytometry, (b) Representative histograms of J-Lat cell viability after 24 h co-culture with NK cells at varied E:T ratios, (c) Representative scatter plots of J-Lat cell viability after 24 h co-culture with NK cells at varied E:T ratios, (d) NK cells, in combination with the listed additional agents, were co-cultured with J-Lat cells for 24 h at a 1:1 E:T ratio; J-Lat cell death was measured by flow cytometry, (e) Representative histograms of J-Lat cell death after 24 h co-culture with NK cells + additional agents, (f) Representative scatter plots of J-Lat cell death after 24 h co-culture with NK cells + additional agents. Values represent means ± standard deviation (n = 3/group), *p < 0.05 (versus all other groups).
Given the benefits of ND-based co-localization of the effects of latency reversal, activated target cell-specific antibodies, and NK cell-mediated cytotoxicity in vitro, we anticipate similar advantages of using the NK-ND approach in vivo, where the pharmacokinetics are expected to be different than co-administration of the individual agents. Evidence in the literature suggests that nanoparticle encapsulation, especially in the context of co-administering two independent drugs or compounds, is more effective than administering free drugs separately [41–43]. Accordingly, we hypothesize that the ND approach described here will provide higher bioavailability and slower clearance of the encapsulated agents than the individual agents alone, thereby improving their functionality. Ongoing studies are testing this hypothesis.
3. Conclusions
Here, we have presented the feasibility of co-localizing a tripartite nano-immunotherapy using PLGA NDs and primary NK cells to eradicate latent HIV and cancer in vitro. The NDs co-encapsulated and co-released an LRA (prostratin) and an activated target cell-specific antibody (aCD25) with controllable release kinetics. These Pro-aCD25-NDs enhanced NK cell-mediated killing of J-Lat cells, which served as an in vitro model of virally infected cells and tumor cells. Building on this proof-of-concept study, we envision that our NDs can be customized to encapsulate other therapeutic agents for specific diseases of interest and administered in combination with NK cells for maximal therapeutic benefit.
4. Experimental section
4.1. Synthesis of PLGA ND
PLGA, DMSO, PVA, acetonitrile, PBS, and prostratin were purchased from Millipore Sigma. Recombinant human anti-CD25 antibody was purchased from Creative Biolabs. PLGA NDs were synthesized using the nanoemulsion technique [20, 21]. Briefly, prostratin (0.4 mg in 20 μL) or equivalent amount of DMSO (stock solvent for prostratin) was added to 5 mg/mL PLGA in 1 mL acetonitrile and vortexed for 20 s. Subsequently, recombinant human aCD25 (75 μg in 25 μL PBS) or equivalent amount of PBS (stock solvent for aCD25) was added to the solution, vortexed for 20 s, and sonicated on ice at 40% amplitude for 30 s. The resultant emulsion was then added to 10 mL of 5% PVA under stirring on a magnetic stirrer at 400 RPM, followed by increasing the speed immediately to 600 RPM. After stirring for 3 h, resultant NDs were collected by centrifugation at 10,000g for 30 min. Supernatant was then centrifuged at 10,000g for 30 min. Resultant pellets were combined and sonicated on ice at 30% amplitude for 30 s. Supernatant was saved for encapsulation assays.
4.2. Physical characterization of NDs
ND size (hydrodynamic diameter) and surface charge (zeta potential) distributions were measured using DLS and electrophoretic light scattering, respectively, on a Zetasizer Nano ZS (Malvern Instruments). SEM was performed on a FEI Teneo LV Field Emission Scanning Electron Microscope. ND absorbance spectra were measured on the Genesys 10S spectrophotometer and analyzed using the VISIONlite software (ThermoFisher Scientific).
4.3. Quantification of encapsulation of and release from NDs
ND encapsulation of prostratin was determined by measuring prostratin concentration in supernatants after collection of NDs by centrifugation. Prostratin concentrations were calculated by measuring absorbance at 260 nm on the Genesys 10S spectrophotometer and analyzed using the VISIONlite software (ThermoFisher Scientific). These absorbance values were converted to molar concentration via a prostratin standard curve and its intrinsic molar extinction coefficient (Fig. S1(a) in the ESM). ND encapsulation of anti-CD25 was determined by measuring protein content of supernatants after collection of NDs by centrifugation via Pierce BCA Protein Assay Kit (ThermoFisher Scientific, 23225) and standard curve generation (Fig. S1(b) in the ESM). To determine release of prostratin and anti-CD25 from NDs, NDs were incubated in PBS at 37 °C. At predetermined time intervals, NDs were centrifuged at 15,000g for 5 min, and supernatants were analyzed for prostratin content (by absorbance spectroscopy) and antibody content (by BCA protein assay kit).
4.4. NK cell generation, culture, and phenotyping
Peripheral blood mononuclear cells (PBMC) were isolated from healthy donor peripheral blood obtained from Dr. John Barrett at NIH (IRB: Pro00004033) by Ficoll-Paque PLUS density gradient separation (GE Life Sciences, 17144002). NK cells were isolated from cryopreserved PBMC using NK cell isolation kit (Stemcell Technologies, 17955) and rested overnight in 15 ng/mL IL-15 (R&D, 247-ILB-025). NK cells were stimulated twice with irradiated K562 cells, 200 U/mL IL-2 (R&D, 202-IL-500), and 15 ng/mL IL-15 (R&D, 247-ILB-025) at a 2:1 K562:NK cell ratio. K562 cells were genetically modified to express membrane-bound IL-15 and 41BBL [44, 45] (gifted from Baylor College of Medicine), and were irradiated at 200 Gy prior to stimulation. NK cells were expanded in Stem Cell Growth Media (Cellgenix, 20802-0500) supplemented with 10% fetal bovine serum (FBS) (Hyclone, SH30910.03) and 1% GlutaMax (ThermoFisher Scientific, 35050061). Seven days following the second stimulation, NK cells were cryopreserved in freeze media made of 50% Roswell Park Memorial Institute (RPMI) medium (Hyclone, SH30096.01), 40% FBS (Hyclone, SH30910.03), and 10% DMSO (VWR, WN182-10ML). Cryopreserved NK cells were thawed in a 37 °C water bath and rested overnight in 200 U/mL IL-2 (R&D, 202-IL-500). NK cells were treated with vehicle (water), aCD25, prostratin, prostratin + aCD25, Blank NDs, aCD25-NDs, Pro-NDs, or Pro-aCD25-NDs for 24 h and then stained with Fc Block (BD Pharmingen, 564220), CD56 FITC (BioLegend, 318304), NKG2D PE (BioLegend, 320805), NKp30 PE (BioLegend, 325207), CD3 PECy7 (BioLegend, 344816), Annexin APC (BioLegend, 550474), and NKp46 APC Fire750 (BioLegend, 137631). NK cells were identified as CD56+/CD3−. Flow cytometry was run on a CytoFLEX S (Beckman Coulter, C01158) and flow data was analyzed using FlowJo 10.5.3 software.
4.5. J-Lat cell culture and phenotyping
J-Lat 10.6 cells were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: J-Lat Full Length Cells (10.6) from Dr. Eric Verdin [26]. J-Lat cells were left untreated or treated with vehicle (water), Blank NDs, aCD25-NDs, Pro-NDs, or Pro-aCD25-NDs for 24 h; the cells were then washed, blocked with Fc Block, and stained with PE anti-human secondary antibody (Biolegend, HP6017). J-Lat cells were then visualized on a BD FACSCelesta flow cytometer and analyzed in FlowJo 10.5.3 software for viral reactivation (GFP expression) and aCD25 binding (by secondary antibody binding).
Viability of J-Lat cells after incubation with NK cells was performed as follows: NK cells at varying ratios were added to 100,000 J-Lat cells in U-bottom 96-well plates. Cells were co-cultured for 24 h in media in the presence of vehicle (water), aCD25, prostratin, prostratin + aCD25, or Pro-aCD25-NDs. Concentration of free drug was determined by calculating the equivalent amount released by the Pro-aCD25-NDs after 24 h (Figs. 3(a) and 3(b)). Pro-aCD25-NDs encapsulated 96.9% of added aCD25 (72.7 μg/mL), and released 34.5% of encapsulated antibody in 24 h (25.1 μg/mL). To achieve a biologically relevant concentration (as determined in Fig. S1(a) in the ESM), we diluted the Pro-aCD25-NDs 41-fold (5 μL Pro-aCD25-NDs in 200 μL media), such that 612 ng/mL would release after 24 h. As such, we added the equivalent concentration of 612 ng/mL free aCD25 to the cells. Similarly, Pro-aCD25-NDs encapsulated 18.3% of added prostratin (73.2 μg/mL), and released 43.2% of encapsulated drug in 24 h (31.6 μg/mL). Using the 41-fold dilution, Pro-aCD25-NDs would release 771 ng/mL after 24 h. As such, we added the equivalent concentration of 771 ng/mL free prostratin to the cells. Cells were then washed and stained with a PE-conjugated primary anti-CD56 antibody (Biolegend, 304605) and Zombie Violet Fixable Viability Kit (Biolegend, 423114). Cells were then visualized on a BD FACSCelesta flow cytometer and analyzed in FlowJo 10.5.3 software.
Supplementary Material
Acknowledgements
Research reported in this publication was supported in part by the George Washington Cancer Center and by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R21AI136102. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Electronic Supplementary Material: Supplementary material (detailing prostratin and antibody ND encapsulation quantification (Fig. S1), aCD25’s retained functionality after acetonitrile exposure during synthesis (Fig. S2), the ability of free prostratin (Fig. S3) and encapsulated/released prostratin (Fig. S4) to activate and induce CD25 expression on target J-Lat cells, the intrinsic toxicity of NDs on target J-Lat cells (Fig. S5), and the effect of Pro-aCD25-NDs to enhance NK cell cytotoxic function (normalized to viable NK cells) on J-Lat cells (Fig. S6)) is available in the online version of this article at https://doi.org/10.1007/s12274-020-2684-1.
References
- [1].Mikulak J; Oriolo F; Zaghi E; Di Vito C; Mavilio D Natural killer cells in HIV-1 infection and therapy. AIDS 2017, 31, 2317–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guillerey C; Huntington ND; Smyth MJ Targeting natural killer cells in cancer immunotherapy. Nat. Immunol 2016, 17, 1025–1036. [DOI] [PubMed] [Google Scholar]
- [3].Breunig M; Lungwitz U; Liebl R; Goepferich A Breaking up the correlation between efficacy and toxicity for nonviral gene delivery. Proc. Natl. Acad. Sci. USA 2007, 104, 14454–14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Daher M; Rezvani K Next generation natural killer cells for cancer immunotherapy: The promise of genetic engineering. Curr. Opin. Immunol 2018, 51, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Florea BI; Meaney C; Junginger HE; Borchard G Transfection efficiency and toxicity of polyethylenimine in differentiated Calu-3 and nondifferentiated COS-1 cell cultures. AAPS PharmSci 2002, 4, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kafil V; Omidi Y Cytotoxic impacts of linear and branched polyethylenimine nanostructures in a431 cells. Bioimpacts 2011, 1, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].De Maria A; Fogli M; Costa R; Murdaca G; Puppo F; Mavilio D; Moretta A; Moretta L The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44). Eur. J. Immunol 2003, 33, 2410–2418. [DOI] [PubMed] [Google Scholar]
- [8].Katz JD; Mitsuyasu R; Gottlieb MS; Lebow LT; Bonavida B Mechanism of defective NK cell activity in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. II. Normal antibody-dependent cellular cytotoxicity (ADCC) mediated by effector cells defective in natural killer (NK) cytotoxicity. J. Immunol 1987, 139, 55–60. [PubMed] [Google Scholar]
- [9].Cheng JJ; Teply BA; Sherifi I; Sung J; Luther G; Gu FX; Levy-Nissenbaum E; Radovic-Moreno AF; Langer R; Farokhzad OC Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 25, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li YP; Pei YY; Zhang XY; Gu ZH; Zhou ZH; Yuan WF; Zhou JJ; Zhu JH; Gao XJ PEGylated PLGA nanoparticles as protein carriers: Synthesis, preparation and biodistribution in rats. J. Control. Release 2001, 77, 203–211. [DOI] [PubMed] [Google Scholar]
- [11].Gdowski A; Ranjan A; Mukeijee A; Vishwanatha J Development of biodegradable nanocarriers loaded with a monoclonal antibody. Int. J. Mol. Sci 2015, 16, 3990–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Langer R; Folkman J Polymers for the sustained release of proteins and other macromolecules. Nature 1976, 263, 797–800. [DOI] [PubMed] [Google Scholar]
- [13].Spivak AM; Planelles V Novel latency reversal agents for HIV-1 cure. Annu. Rev. Med 2018, 69, 421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kulkosky J; Culnan DM; Roman J; Domadula G; Schnell M; Boyd MR; Pomerantz RJ Prostratin: Activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood 2001, 95, 3006–3015. [DOI] [PubMed] [Google Scholar]
- [15].Desimio MG; Giuliani E; Ferraro AS; Adorno G; Doria M In vitro exposure to prostratin but not bryostatin-1 improves natural killer cell functions including killing of CD4+ T cells harboring reactivated human immunodeficiency virus. Front. Immunol 2018, 9, 1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Makadia HK; Siegel SJ Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Danhier F; Ansorena E; Silva JM; Coco R; Le Breton A; Preat V PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [DOI] [PubMed] [Google Scholar]
- [18].Español L; Larrea A; Andreu V; Mendoza G; Arruebo M; Sebastian V; Aurora-Prado MS; Kedor-Hackmann ERM; Santoro MIRM; Santamaria J Dual encapsulation of hydrophobic and hydrophilic drugs in PLGA nanoparticles by a single-step method: Drug delivery and cytotoxicity assays. RSC Adv. 2016, 6, 111060–111069. [Google Scholar]
- [19].Goldberg MS Immunoengineering: How nanotechnology can enhance cancer immunotherapy. Cell 2015, 161, 201–204. [DOI] [PubMed] [Google Scholar]
- [20].Martínez Rivas CJ; Tarhini M; Badri W; Miladi K; Greige-Gerges H; Nazari QA; Galindo Rodríguez SA; Román RÁ; Fessi H; Elaissari A Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm 2017, 532, 66–81. [DOI] [PubMed] [Google Scholar]
- [21].Astete CE; Sabliov CM Synthesis and characterization of PLGA nanoparticles. J. Biomat. Sci., Polym Ed 2006, 77, 247–289. [DOI] [PubMed] [Google Scholar]
- [22].Wang W; Erbe AK; Hank JA; Morris ZS; Sondel PM NK Cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front. Immunol 2015, 6, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ramilo O; Bell KD; Uhr JW; Vitetta ES Role of CD25+ and CD25− T cells in acute HIV infection in vitro. J. Immunol 1993, 750, 5202–5208. [PubMed] [Google Scholar]
- [24].Arce Vargas F; Furness AJS; Solomon I; Joshi K; Mekkaoui L; Lesko MH; Miranda Rota E; Dahan R; Georgiou A; Sledzinska A et al. Fc-optimized Anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity 2017, 46, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Flynn MJ; Hartley JA The emerging role of anti-CD25 directed therapies as both immune modulators and targeted agents in cancer. Brit. J. Haematol 2017, 7 79, 20–35. [DOI] [PubMed] [Google Scholar]
- [26].Jordan A; Bisgrove D; Verdin E HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J 2003, 22, 1868–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen MS; Ouyang HC; Zhou SY; Li JY; Ye YB PLGA-nanoparticle mediated delivery of anti-OX40 monoclonal antibody enhances anti-tumor cytotoxic T cell responses. Cell. Immunol 2014, 257, 91–99. [DOI] [PubMed] [Google Scholar]
- [28].Sousa F; Cruz A; Pinto IM; Sarmento B Nanoparticles provide long-term stability of bevacizumab preserving its antiangiogenic activity. Acta Biomater. 2018, 75, 285–295. [DOI] [PubMed] [Google Scholar]
- [29].Feczkó T; Tóth J; Dósa G; Gyenis J Optimization of protein encapsulation in PLGA nanoparticles. Chem. Eng. Process 2011, 50, 757–765. [Google Scholar]
- [30].Son S; Lee WR; Joung YK; Kwon MH; Kim YS; Park KD Optimized stability retention of a monoclonal antibody in the PLGA nanoparticles. Int. J. Pharm 2009, 368, 178–185. [DOI] [PubMed] [Google Scholar]
- [31].Lee YH; Lai YH Synthesis, characterization, and biological evaluation of anti-HER2 indocyanine green-encapsulated PEG-coated PLGA nanoparticles for targeted phototherapy of breast cancer cells. PLoS One 2016, 77, e0168192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee YH; Chang DS Fabrication, characterization, and biological evaluation of anti-HER2 indocyanine green-doxorubicin-encapsulated PEG-b-PLGA copolymeric nanoparticles for targeted photochemotherapy of breast cancer cells. Sci. Rep 2011, 7, 46688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sainz V; Peres C; Ciman T; Rodrigues C; Viana AS; Afonso CAM; Barata T; Brocchini S; Zloh M; Gaspar RS et al. Optimization of protein loaded PLGA nanoparticle manufacturing parameters following a quality-by-design approach. RSC Adv. 2016, 6, 104502–104512. [Google Scholar]
- [34].Fonte P; Soares S; Costa A; Andrade JC; Seabra V; Reis S; Sarmento B Effect of cryoprotectants on the porosity and stability of insulin-loaded PLGA nanoparticles after freeze-drying. Biomatter 2012, 2, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hines DJ; Kaplan DL Poly(lactic-co-glycolic) acid-controlled-release systems: Experimental and modeling insights. Crit. Rev. Ther. Drug. Carrier Syst 2013, 30, 257–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fredenberg S; Wahlgren M; Reslow M; Axelsson A The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems-a review. Int. J. Pharm 2011, 415, 34–52. [DOI] [PubMed] [Google Scholar]
- [37].Jeong B; Bae YH; Kim SW Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J. Control. Release 2000, 63, 155–163. [DOI] [PubMed] [Google Scholar]
- [38].Faisant N; Siepmann J; Richard J; Benoit JP Mathematical modeling of drug release from bioerodible microparticles: Effect of gamma-irradiation. Eur. J. Pharm. Biopharm 2003, 56, 271–279. [DOI] [PubMed] [Google Scholar]
- [39].Williams SA; Chen LF; Kwon H; Fenard D; Bisgrove D; Verdin E; Greene WC Prostratin antagonizes HIV latency by activating NF-κB. J. Biol. Chem 2004, 279, 42008–42017. [DOI] [PubMed] [Google Scholar]
- [40].Spina CA; .Anderson J; .Archin NM; Bosque A; Chan J; Famiglietti M; Greene WC; Kashuba A; Lewin SR; Margolis DM et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 2013, 9, e1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lv SX; Tang ZH; Li MQ; Lin J; Song WT; Liu HY; Huang YB; Zhang YY; Chen XS Co-delivery of doxorubicin and paclitaxel by PEG-polypeptide nanovehicle for the treatment of non-small cell lung cancer. Biomaterials 2014, 35, 6118–6129. [DOI] [PubMed] [Google Scholar]
- [42].Guo ST; Lin CM; Xu ZH; Miao L; Wang YH; Huang L Co-delivery of cisplatin and rapamycin for enhanced anticancer therapy through synergistic effects and microenvironment modulation. ACS Nano 2014, 5, 4996–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang Y; Gao SJ; Ye WH; Yoon HS; Yang YY Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat. Mater 2006, 5, 791–796. [DOI] [PubMed] [Google Scholar]
- [44].Fujisaki H; Kakuda H; Shimasaki N; Imai C; Ma J; Lockey T; Eldridge P; Leung WH; Campana D Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009, 69, 4010–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cho D; Campana D Expansion and activation of natural killer cells for cancer immunotherapy. Korean J. Lab. Med 2009, 29, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


