Multiple myeloma (MM) is an incurable malignancy of mature plasma cells. Treatment of MM focuses on obtaining a deep and durable remission to improve overall and progression-free survival (PFS). Patients with good functional status ≤70 years of age are generally considered eligible for treatment with bortezomib-based induction chemotherapy followed by autologous stem cell transplant (ASCT) which has demonstrated a PFS and overall survival (OS) benefit in large, randomized controlled trials.1-6 The use of lenalidomide maintenance (LM) following ASCT is based on four large randomized control trials and a meta-analysis demonstrating improvement in both PFS and OS.2,5,7-9
Currently, data validating the use of LM in the realworld, Canadian landscape, in which LM is publicly funded, is limited.10-13 An analysis of the survival impact and adverse effects of LM in large, real-world cohorts is of considerable importance. In order to address this knowledge gap, we conducted a retrospective, observational study of patients meeting International Myeloma Working Group (IMWG) criteria for MM who were treated with upfront bortezomib-based induction chemotherapy followed by ASCT.14 Data was collected from the Canadian Myeloma Research Group Database (CMRGDB), a comprehensive collaborative data-sharing platform that pools data from academic cancer centers across Canada and includes legacy data dating back to 2007. The project was approved by Health Research Ethics Board of Alberta. We included patients receiving either lenalidomide monotherapy as maintenance or no maintenance (non-LM). Charts were reviewed with regard to demographics, response and adverse effects. The primary outcomes of this analysis were PFS, OS and progressionfree survival 2 (PFS2) defined as time from initiation of second line chemotherapy to death, relapse or last follow- up. Progression was defined as per the IMWG criteria with an additional endpoint of near complete response (nCR) in whom complete response (CR) status was not confirmed by bone marrow biopsy.15 Data was analyzed using version 9.4 of the SAS system for Windows with Kaplan-Meier curves to evaluate PFS and OS. χ2 analysis was used for dichotomous variables. A P-value of <0.05 was considered statistically significant.
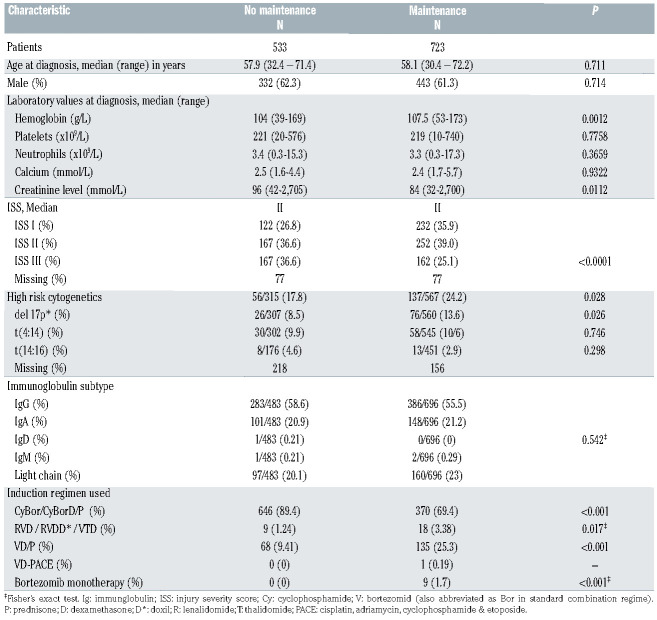
Table 1.
Baseline characteristics of lenalidomide maintenance and non-lenalidomide maintenance groups.
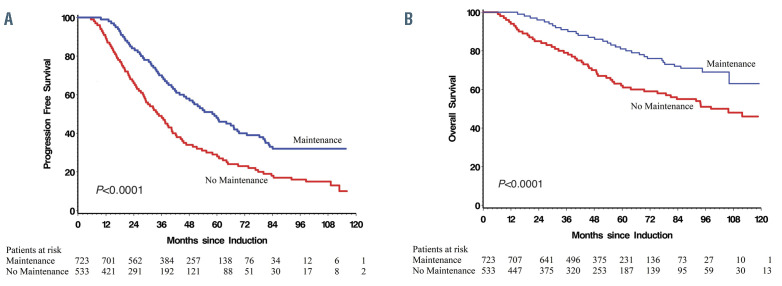
Figure 1.
Survival outcomes in patients treated with and without lenalidomide maintenance post autologous stem cell transplant. (A) Progression free survival. (B) Overall survival.
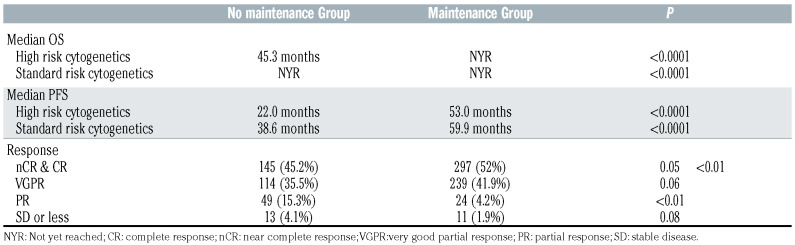
Table 2.
Survival and response outcomes in lenalidomide maintenance and non-lenalidomide maintenance groups.
We included 1,256 patients beginning induction treatment between January 2007 and January 2016. Seven hundred and twenty-three patients (57.6%) received LM and 533 (42.4%) did not. All relevant baseline characteristics of each group are illustrated in Table 1.
The median follow-up was 49.1 months in the LM group (range, 8.6–124.8 months) and 45.3 months in the non-LM group (range, 4.5–141.1 months). At the time of analysis, 397 (54.9%) of the LM group had not yet progressed compared to 198 (37.2%) of patients in the non- LM group.
The median PFS was 58.2 months (95% Confidence Interval [CI]: 52.0–64.0) in the LM group compared to 34.6 months in the non-LM group (95%CI: 30.7–37.7, P<0.0001), Figure 1A. The 5-year OS was 81% in the LM group compared to 61.5% in the non-LM cohort. The median OS was 98.3 months in the non-LM cohort (95%CI: 83.5) but not reached (>124 months) in the LM group (P<0.0001, Figure 1B). There was no difference in PFS (P=0.66) or OS (P=0.75) between 21/28 day and 28/28 lenalidomide dosing schedules. Median PFS2 was NYR in the LM cohort compared to 64.2 months (95%CI: 55.3–74.8, P<0.0001). Response rates were deeper in the LM cohort including nCR/CR (52.0% vs. 45.2%, P=0.05) and ≥VGPR (93.9% vs. 80.7%, P<0.01). The PFS benefit of lenalidomide persisted in those achieving a nCR/CR (P<0.0001), VGPR (P=0.0006) and PR (P=0.03). The presence of high-risk cytogenetics was associated with reduced PFS and OS in all patients (Table 2). While the worse outcome could not be overcome entirely with the use of maintenance both median PFS and OS were improved regardless of cytogenetic risk (Table 2).
In further data available for 226 patient in Edmonton, Alberta common indication for dose reduction or medication discontinuation were cytopenias (27.7%), rash (10.8%), infection (9.5%) and fatigue (8.1%). 19.6% discontinued therapy prior to relapse. Venous and arterial thrombosis during frontline treatment was not significantly different between the groups at 2.6% in the non- LM group compared to 5.4% in the LM group (P=0.5). Rates of secondary primary malignancy (SPM) were observed in 6.4% of the non-LM group and 3.4% of the LM patients (P=0.32). Primary sites included skin, lung, bladder and prostate in the non-LM group and breast, brain, lung, kidney and one case of CLL in the LM group.
This analysis from the CMRG-DB is the first dataset analyzing the use of LM following ASCT in the realworld Canadian landscape. Our data validates findings of large, phase 3 randomized controlled trials illustrating a positive impact of LM on PFS and OS in a real-world setting. 2,4,7-9 The median PFS data demonstrated a clear advantage for LM. The median OS data also strongly favored the use of LM. The disease control and survival advantage of LM persisted in patients with standard- or high-risk cytogenetics. Interestingly, patients with highrisk cytogenetics treated with LM had superior PFS and OS compared with standard-risk patients in the non-LM cohort, a finding that supports using LM even in patients anticipated to have the poorer survival outcomes associated with these cytogenetic abnormalities. Lastly, the improved PFS2 outcomes in LM patients demonstrates that non-LM patients do not “catch up” to their maintenance counterparts with second-line therapy.
LM further improved survival outcomes in each response category, including those in the nCR/CR group. This suggests that the impact of LM on PFS and OS goes beyond simply improving patients’ response criteria and offers additional survival advantages even in those who achieve a nCR/CR, perhaps through an immune as well as a cytotoxic effect.
Although most patients required a dose reduction or medication discontinuation at some point during their treatment, only 19.6% of patients discontinued therapy prior to relapse. This suggests that LM is well-tolerated.
Limitations of our study include its retrospective, observational nature. Patients were enrolled who started chemotherapy prior to 2016 and significant changes have emerged in the field of myeloma in recent years, particularly with regards to novel chemotherapeutic agents in the setting of relapsed disease. These may have influenced the OS data which depends in part on treatment received for disease recurrence. Given that the non-LM cohort largely comprises of those starting chemotherapy prior to 2012, these patients may not have had the same access to clinical trials or novel combination therapy as their LM counterparts. On the other hand, patients progressing on LM might be expected to experience poorer results with second-line therapy, which was not the case based on PFS2 data. However, the similarity of our data when compared to large scale, randomized, controlled trials suggests that the impact of this temporal relationship between the LM and non-LM groups may not significantly impact our results. Further, the availability of additional agents only affects the PFS2 and OS outcomes. The cohort presented here is still reflective of the impact of LM on disease control in the post-ASCT setting as measured by PFS.
Lastly, the recent adoption of LM limits our ability to see its full impact on survival outcomes as many of LM patients have not yet experienced their first relapse. Longer follow-up will allow further assessment of the impact of maintenance therapy, particularly in the era of improved therapy for relapsed disease.
Despite the limitations of retrospective data, large multicenter datasets have undeniable merits as they allow for evaluation of the generalizability of new treatments in patients who would not meet eligibility criteria for a clinical trial. Such datasets also provide lengthy and detailed follow-up of real-world data beyond the line of treatment in question, which can be challenging to collect in prospective randomized control trials.2,5,9-11 Furthermore, those with early relapse as well as long-term disease-free survivors are easily identified in these large, retrospective datasets. Examination of their data will be useful in the determination of contributing and prognosticating factors in these, and other, patient subsets.
In summary, our retrospective cohort validates the data seen in large phase 3 trials demonstrating the positive impact LM has had on PFS, OS and response in the realworld setting. This data supports the ongoing use of LM as a current standard of care.
Acknowledgments
The authors would like to thank the Canadian Myeloma Research Group for their support and our patients for generously donating their data for analysis.
References
- 1.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91-97. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35(29):3279-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan GJ, Davies FE, Hawkins K, et al. Follow up analysis for MRC Myeloma VII Trial. Blood. 2004;104(11):927. [Google Scholar]
- 4.Palumbo A, Bringhen S, Petrucci MT, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104(10):3052-3057. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895-905. [DOI] [PubMed] [Google Scholar]
- 6.Palumbo A, Gay F, Falco P, et al. Bortezomib as induction before autologous transplantation, followed by lenalidomide as consolidation- maintenance in untreated multiple myeloma patients. J Clin Oncol. 2010;28(5):800-807. [DOI] [PubMed] [Google Scholar]
- 7.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782-1791. [DOI] [PubMed] [Google Scholar]
- 8.Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20(1):57-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherniawsky H, Sandhu I, Chu MP, et al. The survival impact of lenalidomide maintenance chemotherapy in multiple myeloma patients treated with autologous stem cell transplant and bortezomib- based induction: an analysis of real world data. Blood. 2017; 130(Suppl 1):S3132. [Google Scholar]
- 11.Cote J, Phillips M, Atenafu EG, et al. Pattern of first relapse in multiple myeloma (MM) patients (Pts) after a cybord induction regimen and autologous stem cell transplantation (ASCT): impact of maintenance therapy in the real-world setting. Blood. 2016;128(22):2137. [Google Scholar]
- 12.Yang C, Jiang H, Masih-Khan E, et al. Tolerability and efficacy of post transplant lenalidomide maintenance therapy in multiple myeloma: a real world single centre experience. Blood. 2017;130(Suppl 1):S3462. [Google Scholar]
- 13.Lonial S, Anderson KC. Association of response endpoints with survival outcomes in multiple myeloma. Leukemia. 2014;28(2):258-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016; 17(8):e328-e346. [DOI] [PubMed] [Google Scholar]