Abstract
Heavy alcohol consumption in mid-adulthood is an established risk factor of colorectal cancer (CRC). Alcohol use in early adulthood is common, but its association with subsequent CRC risk remains largely unknown. We prospectively investigated the association of average alcohol intake in early adulthood (age 18-22) with CRC risk later in life among 191,543 participants of the Nurses’ Health Study ([NHS], 1988-2014), NHSII (1989-2015) and Health Professionals Follow-Up Study (1988-2014). Cox proportional hazards models were used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs), which were pooled using random effects models. We documented 2,624 CRC cases. High alcohol consumption in early adulthood (≥15 g/day) was associated with a higher CRC risk (multivariable HR 1.28, 95% CI 0.99 to 1.66, Ptrend=0.02; Pheterogeneity=0.44), after adjusting for potential confounding factors in early adulthood. Among never/light smokers in early adulthood, the risk associated with high alcohol consumption in early adulthood was elevated (HR 1.53, 95% CI 1.04 to 2.24), compared with those who had <1 g/day of alcohol intake. The suggestive higher CRC risk associated with high alcohol consumption in early adulthood was similar in those who had <15 g/day (HR 1.35, 95% CI 0.98 to 1.86) versus ≥15 g/day of midlife alcohol intake (HR 1.35, 95% CI 0.89 to 2.05), compared with nondrinkers in both life stages. The findings from these large prospective cohort studies suggest that higher alcohol intake in early adulthood may be associated with a higher risk of developing CRC later in life.
Keywords: alcohol, colorectal cancer, early adulthood, college drinking
INTRODUCTION
Colorectal cancer (CRC) is the 3rd leading cause of cancer incidence and mortality in the US [1]. The majority of the identified CRC risk factors have centered around exposures assessed in mid to later life stages [2]. Growing evidence from a range of cancers has suggested the importance of early life exposures in subsequent cancer risk later in life [3–6]. Exposure to cancer risk factors early in life can increase accumulation of the lifetime exposure, and by acting in a critical period, can induce structural or functional changes in tissues, organs and metabolism that lead to carcinogenesis [7, 8]. Recently identified marked increases in CRC among younger adults in multiple developed countries [9, 10] also point to the importance of early life exposures in CRC development.
Alcohol intake in early adulthood is an important public health agenda due to its high prevalence worldwide [11]. Specifically in the US, even with downward secular trends in alcohol consumption from 1980 to 2019, 62% of male and female college students aged 19-22 still consumed alcohol in the past month in 2019 [12]. Given daily consumption of ≥2 alcoholic drinks in mid-adulthood is an established risk factor of CRC [2], and alcohol use peaks during the transition from late adolescence to early adulthood [13, 14], it is important to investigate the link between alcohol intake in early adulthood and risk of developing CRC later in life. Studies have documented the link between early adulthood alcohol consumption and subsequent breast cancer risk [3, 15]; however, to our knowledge, no studies have yet examined its association with CRC, independent of mid-adulthood alcohol intake.
To address these knowledge gaps, we examined the association between alcohol intake in early adulthood (i.e. age 18-22) and risk of developing CRC later in life, leveraging three well-established, large, ongoing prospective cohort studies with detailed assessment of alcohol intake and other CRC risk factors covering early- to mid-adulthood.
METHODS
Study population
The Nurses’ Health Study (NHS), NHSII and Health Professionals Follow-Up Study (HPFS) are ongoing prospective cohort studies of female registered nurses and male health professionals residing in the US. NHS was established in 1976 including 121,700 nurses aged 30-55, NHSII in 1989 enlisting 116,429 nurses aged 25-42 and HPFS in 1986 recruiting 51,529 health professionals aged 40-75, respectively. In all three cohorts, detailed information on demographics, lifestyle factors and medical history was collected biennially via self-administered questionnaires. Dietary intake was assessed using validated semiquantitative food frequency questionnaires (FFQs) nearly every four years. Return of the completed questionnaire implied informed consent to participate in the study. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health and those of participating state cancer registries as required.
Ascertainment of colorectal cancer
The primary endpoint was the diagnosis of incident invasive CRC. For CRC diagnoses reported on a biennial questionnaire and lethal CRC cases identified from the National Death Index, tumor registries or death certificates, permission to review medical records or pathology reports was sought. Study physicians who were masked to information on self-reported risk factors reviewed the records and confirmed diagnosis, date, anatomic subsite, histology and stage at presentation for incident CRC cases. Cases were coded using the International Classification of Diseases, 8th revision (153 for colon cancer; 154 for rectal cancer).
Assessment of early and mid-adulthood alcohol intake
Information on alcohol intake in early adulthood was assessed in 1988 for NHS and HPFS and 1989 for NHSII. Participants were asked about the total number of alcoholic drinks usually consumed per week at age 18-22 defined as bottles/cans of beer, 4 oz (118 ml) glasses of wine and shots of liquor, with 5 categories ranging from “none” to “≥14/week” for NHS, 9 categories from “none or <1/month” to “≥40/week” for NHSII and 8 categories from “none” to “≥16/week” for HPFS. Alcohol consumption in grams per day at age 18-22 was estimated as the daily number of alcoholic beverages consumed during that time interval multiplied by the alcohol content of 12.8 g, equivalent to that of a 12 oz (355 ml) standard serving of beer. A prior study conducted among a subset of our study population showed that alcohol intake at age 18-22, regardless of participants’ current age, had been recalled with reasonable reproducibility (Spearman’s rank correlation coefficient: 0.66), which was higher than that of most foods [16].
Information on mid-adulthood alcohol consumption was collected using semiquantitative FFQs assessing dietary intake of ~130 items over the past 12 months [17–19]. The total alcohol intake in grams per day was calculated as the sum of the daily number of each alcoholic drink consumed multiplied by the corresponding alcohol content: 12.8 g of alcohol per 12 oz (355 ml) of regular beer; 11.3 g per 12 oz of light beer; 11.0 g per 4 oz (118 ml) of wine; and 14.0 g per 1.5 oz (44 ml) of liquor. In 2003 and thereafter, an increase in the portion size of wine to 5 oz (148 ml) was taken into account in estimation. Alcohol intake data were obtained in 1986 for NHS and HPFS and in 1989 and 1991 for NHSII, with updates every four years thereafter. A previous study using NHS and HPFS showed the validity of current alcohol intake assessed from the FFQ, given its high correlation with that measured from multiple, 1-week diet records (Spearman’s rank correlation coefficient: 0.90 in women and 0.86 in men) and serum high density lipoprotein levels (0.40 in women and 0.35 in men) [20].
Assessment of covariates
We asked participants to recall their health status and lifestyle in early adulthood. Using recalled weight data at age 18 for NHS and NHSII and at age 21 for HPFS and height, body mass index (BMI) in early adulthood was calculated. At baseline, participants reported average number of cigarettes smoked per day and duration of smoking at younger ages. Pack-years of smoking before age 30 were obtained as a proxy for early adulthood smoking. Physical activity at age 18-22, for NHS and HPFS, was assessed as the number of months per year that participants had engaged in strenuous activity at least twice weekly. For NHSII, using the questionnaire that showed reasonable reliability [21], participants reported average hours per week spent on a variety of physical activities. Metabolic equivalent of task (MET)-hours was calculated by multiplying the MET score by the time spent for each activity.
Characteristics in mid-adulthood assessed at baseline and then updated during follow-up included weight, menopausal status and menopausal hormone use (in NHS and NHSII only), family history of CRC, personal history of diabetes, smoking habits, physical activity, regular use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) and current use of multivitamins. Using FFQs, intake of total calories, red and processed meat, dietary fiber, total folate and total calcium was updated every four years. Diet quality was assessed with the Alternative Healthy Eating Index (AHEI)-2010, as described previously [22]. Biennially, participants were asked if they had received a colonoscopy/sigmoidoscopy over the past two years and if so, the reasons for the procedure.
Statistical analysis
We set the analytic baseline to 1988 for NHS and HPFS and 1989 for NHSII, when alcohol intake at age 18-22 was assessed. A total of 62,292 and 92,858 women and 36,393 men were included in the final analysis of NHS, NHSII and HPFS, respectively, after we excluded those who died or developed cancer or inflammatory bowel disease prior to baseline; those with implausible energy intake (<600 or >3500 kcal/day for women and <800 or >4200 kcal/day for men); and those who reported no information on early adulthood and baseline alcohol intake.
As our primary analysis, we investigated the associations between early adulthood alcohol intake (categorized as 0, 0.1-4.9, 5-14.9, ≥15 g/day) and risk of CRC later in life. Person-years of follow-up accrued from the date when participants had returned the baseline questionnaire (1988 for NHS and HPFS and 1989 for NHSII) to the diagnosis of CRC, death or the end of follow-up (June 2014 for NHS, June 2015 for NHSII and January 2014 for HPFS), whichever came first. Cox proportional hazards models were used to compute hazard ratios (HRs) with 95% confidence intervals (CIs). Models were stratified by age in months and biennial questionnaire cycle to provide finer control for confounding by these two time scales.
In multivariable models, first, potential confounding factors reflecting early adulthood lifestyle were included as covariates: race (white, nonwhite), height (continuous), BMI (at age 18 for NHS and NHSII; at age 21 for HPFS [continuous]), pack-years of smoking before age 30 (continuous) and physical activity at age 18-22 (in MET-hours/week for NHSII [continuous]; strenuous physical activity ≥2 times/week, 10-12 months/year for NHS and HPFS [yes, no]). Missing data were set to the median of each covariate. Additionally, we further adjusted for putative CRC risk factors assessed in mid-adulthood on a time-varying basis: cumulative alcohol intake (continuous), BMI (continuous), menopausal status and menopausal hormone use (premenopausal, postmenopausal never user, postmenopausal ever user, unknown menopausal status or hormone use) for females only, family history of CRC (yes, no), personal history of diabetes (yes, no), pack-years of smoking (continuous), physical activity (continuous), regular use of aspirin (yes, no) or NSAIDs (yes, no), current use of multivitamins (yes, no), intake of total calories, red and processed meat, dietary fiber, total folate (from foods and supplements) and total calcium (all continuous), AHEI-2010 score without alcohol (continuous) and lower endoscopy due to screening (yes, no) or for other indications within the past 10 years (yes, no). For missing data in these time-varying covariates, non-missing values from the prior questionnaire cycle were carried forward to the subsequent cycles. For alcohol intake, BMI, physical activity and dietary intake in mid-adulthood, we calculated the cumulative average of each covariate collected across all available questionnaires from the study baseline up to each questionnaire cycle to better represent long-term status reflecting true changes and minimizing the extent of measurement error and within-person variation.
Test for trend was performed using the median of each category of early adulthood alcohol intake as a continuous variable. We conducted analyses for each cohort first and pooled the study-specific estimates using a random effects model [23]. The extent of heterogeneity was assessed using Cochran’s Q test [24]. As secondary analysis, a joint association of alcohol and cigarette use in early adulthood as well as that of alcohol intake in early and mid-adulthood were examined in relation to incident CRC later in life. For early adulthood smoking, 5 pack-years, corresponding to the median of smokers in NHS, was used as a cutoff. A cutoff of 15 g/day was chosen to define high versus low alcohol use, discerning individuals who consumed ≥1 alcoholic drinks/day in early adulthood. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc.).
RESULTS
Over 26 years of follow-up in the three cohorts (4,531,613 person-years), 2,624 incident CRC cases were documented (1,257 in NHS, 345 in NHSII and 1,022 in HPFS). Overall, the average alcohol intake in early adulthood among our study participants was ≤5 g/day (Supplementary Table 1). Compared with nondrinkers, individuals with alcohol intakes ≥15 g/day in early adulthood were more likely to smoke before age 30, be physically active at age 18-22 and onward and use NSAIDs in mid-adulthood (Table 1). For the same comparison, they tended to consume more total calories and substantially more alcohol in mid-adulthood (Spearman’s rank correlation coefficients for early and mid-adulthood alcohol intake: 0.24 in NHS, 0.39 in NHSII and 0.37 in HPFS).
Table 1.
Age-standardized characteristics of person-years according to average daily alcohol intake in early adulthood in the NHS, NHSII and HPFSa
| Alcohol intake in early adulthood, g/day |
||||||
|---|---|---|---|---|---|---|
| NHS, 1988-2014 |
NHSII, 1989-2015 |
HPFS, 1988-2014 |
||||
| 0 | ≥15 | 0 | ≥15 | 0 | ≥15 | |
| Participants, No. | 26,894 | 358 | 24,113 | 3,207 | 9,205 | 1,695 |
| Age, years | 67.1 (9.9) | 62.4 (9.4) | 47.7 (8.8) | 44.7 (8.7) | 68.0 (11.2) | 62.7 (10.3) |
| Race, white, % | 97 | 99 | 89 | 96 | 89 | 93 |
| Height, cm | 164 (6.2) | 166 (6.3) | 164 (6.6) | 166 (6.6) | 178 (6.7) | 179 (6.7) |
| Characteristics in early adulthood | ||||||
| BMI in early adulthood, kg/m2b | 21.2 (2.9) | 22.0 (4.1) | 21.2 (3.4) | 21.8 (3.7) | 22.8 (2.8) | 23.6 (2.9) |
| Ever smokers before age 30, % | 35 | 82 | 15 | 63 | 24 | 66 |
| Pack-years among ever smokers before age 30 | 6.2 (5.2) | 9.9 (7.0) | 8.0 (5.7) | 10.9 (6.4) | 9.5 (6.2) | 13.9 (7.3) |
| Physical activity at age 18–22c | 6.2 | 13.6 | 38.0 (27.7) | 47.5 (34.1) | 19.2 | 22.9 |
| Characteristics in mid-adulthood | ||||||
| BMI, kg/m2 | 25.9 (4.8) | 27.7 (5.7) | 25.7 (5.7) | 26.3 (5.9) | 25.6 (3.3) | 26.5 (3.4) |
| Postmenopausal, % | 90 | 87 | 33 | 32 | NA | NA |
| Current menopausal hormone use among postmenopausal women, % | 30 | 34 | 33 | 30 | NA | NA |
| Family history of colorectal cancer, % | 16 | 14 | 7 | 9 | 13 | 13 |
| Screening lower endoscopy within the past 10 years, % | 28 | 25 | 16 | 15 | 47 | 46 |
| Lower endoscopy due to other indications within the past 10 years, % | 24 | 21 | 14 | 16 | 23 | 24 |
| Personal history of diabetes, % | 9 | 12 | 4 | 4 | 9 | 9 |
| Ever smokers, % | 38 | 84 | 16 | 64 | 31 | 72 |
| Pack-years among ever smokers | 23.3 (21.1) | 28.2 (21.8) | 14.5 (12.0) | 16.0 (12.3) | 20.4 (18.6) | 27.4 (21.4) |
| Physical activity, MET-hours/week | 17.4 (17.6) | 19.4 (21.0) | 19.7 (23.0) | 25.0 (27.6) | 30.8 (25.8) | 34.5 (26.2) |
| Regular use of aspirin, % | 36 | 40 | 11 | 12 | 49 | 52 |
| Regular use of non-aspirin NSAIDs, % | 22 | 30 | 28 | 36 | 14 | 24 |
| Current use of multivitamins, % | 54 | 57 | 47 | 48 | 49 | 54 |
| Dietary intake | ||||||
| Energy, kcal/day | 1722 (452) | 1862 (478) | 1769 (504) | 1898 (500) | 1946 (542) | 2141 (570) |
| Alcohol, g/day | 4.1 (7.8) | 8.2 (11.8) | 1.5 (3.7) | 6.8 (8.6) | 5.7 (10.1) | 18.9 (19.2) |
| Red and processed meat, servings/week | 6.1 (3.7) | 7.2 (3.9) | 6.3 (4.2) | 6.5 (4.2) | 6.0 (4.2) | 7.6 (4.7) |
| Dietary fiber, g/day | 19.1 (4.9) | 17.0 (4.5) | 19.3 (5.6) | 18.5 (5.2) | 23.4 (6.8) | 20.8 (6.1) |
| Total folate, μg/day | 492 (204) | 473 (204) | 516 (252) | 525 (234) | 555 (248) | 550 (239) |
| Total calcium, mg/day | 1143 (449) | 1071 (413) | 1099 (453) | 1112 (409) | 984 (389) | 927 (358) |
| Alternative Healthy Eating Index-2010 scored | 48.7 (9.4) | 46.4 (9.6) | 45.1 (10.0) | 45.5 (10.0) | 48.9 (10.1) | 47.0 (9.8) |
Abbreviations: BMI, body mass index; HPFS, Health Professionals Follow-Up Study; MET, metabolic equivalent of task; NA, not applicable; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; NSAID, nonsteroidal anti-inflammatory drug
Data are presented as mean (standard deviation) of person-years unless otherwise indicated. All values other than age were directly standardized to age distribution (in 5-year intervals) of all participants.
BMI at age 18 for NHS and NHSII and at age 21 for HPFS, respectively.
Physical activity at age 18-22 as a frequency of strenuous activity ≥2 times/week, 10-12 months/year for NHS and HPFS and in MET-hours/week for NHSII, respectively.
Without alcohol intake.
Alcohol intake in early adulthood appeared to be associated with a higher risk of CRC later in life after adjusting for potential confounding factors in early adulthood, including race, height, BMI, pack-years of smoking and physical activity. Compared with nondrinkers, individuals who consumed ≥15 g/day of alcohol in early adulthood had a 28% higher risk of CRC later in life (pooled multivariable HR 1.28, 95% CI 0.99 to 1.66, Ptrend=0.02, n=70 CRC cases, Table 2). Additional adjustment for cumulative alcohol intake in mid-adulthood slightly attenuated the magnitude of the association (pooled HR 1.20, 95% CI 0.92 to 1.56, Ptrend=0.09, Supplementary Table 2). We observed a less than 10% of change in our findings when putative CRC risk factors in mid-adulthood were taken into account further.
Table 2.
Alcohol intake in early adulthood and risk of colorectal cancer
| Alcohol intake in early adulthood, g/day |
P for trenda | P for heterogeneityb | ||||
|---|---|---|---|---|---|---|
| 0 | 0.1-4.9 | 5-14.9 | ≥15 | |||
| Pooled | ||||||
| Age-adjusted HR (95% CI) | 1 | 1.11 (1.01 to 1.22) | 1.19 (1.06 to 1.34) | 1.42 (1.10 to 1.83) | 0.004 | 0.20 |
| Multivariable HR (95% CI)c | 1 | 1.07 (0.98 to 1.17) | 1.11 (0.98 to 1.26) | 1.28 (0.99 to 1.66) | 0.02 | 0.44 |
| NHS | ||||||
| Cases | 534 | 564 | 149 | 10 | ||
| Person-years | 614,205 | 642,212 | 166,524 | 7,903 | ||
| Age-adjusted HR (95% CI) | 1 | 1.16 (1.03 to 1.31) | 1.29 (1.07 to 1.55) | 2.01 (1.07 to 3.77) | <0.001 | |
| Multivariable HR (95% CI)c | 1 | 1.11 (0.98 to 1.25) | 1.18 (0.97 to 1.43) | 1.72 (0.91 to 3.24) | 0.02 | |
| NHSII | ||||||
| Cases | 86 | 120 | 125 | 14 | ||
| Person-years | 600,740 | 717,191 | 919,772 | 79,678 | ||
| Age-adjusted HR (95% CI) | 1 | 1.21 (0.92 to 1.60) | 1.09 (0.82 to 1.44) | 1.50 (0.85 to 2.66) | 0.31 | |
| Multivariable HR (95% CI)c | 1 | 1.18 (0.89 to 1.56) | 1.03 (0.77 to 1.37) | 1.33 (0.74 to 2.39) | 0.58 | |
| HPFS | ||||||
| Cases | 276 | 509 | 191 | 46 | ||
| Person-years | 192,044 | 395,627 | 157,839 | 37,879 | ||
| Age-adjusted HR (95% CI) | 1 | 1.02 (0.88 to 1.18) | 1.14 (0.94 to 1.38) | 1.28 (0.93 to 1.76) | 0.05 | |
| Multivariable HR (95% CI)c | 1 | 0.99 (0.85 to 1.15) | 1.08 (0.88 to 1.31) | 1.17 (0.85 to 1.63) | 0.20 | |
Abbreviations: CI, confidence interval; HPFS, Health Professionals Follow-Up Study; HR, hazard ratio; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II
Test for trend was performed using the median of each category of alcohol intake in early adulthood as a continuous variable.
Cochran’s Q test was used to test heterogeneity of the trend across the studies.
Additionally adjusted for race (white, nonwhite), height (continuous), BMI in early adulthood (at age 18 for NHS and NHSII; at age 21 for HPFS [continuous]), pack-years of smoking before age 30 (continuous) and physical activity at age 18-22 (in metabolic equivalent of task-hours/week for NHSII [continuous]; strenuous physical activity ≥2 times/week, 10-12 months/year for NHS and HPFS [yes, no]).
In the joint analysis combining alcohol consumption and cigarette use in early adulthood in relation to future CRC development, compared with nondrinkers who smoked <5 pack-years, a 53% elevated risk was noted among those who had alcohol intake of ≥15 g/day with the same extent of pack-years of smoking (pooled HR 1.53, 95% CI 1.04 to 2.24, n=28 CRC cases, Fig. 1). For the same reference group, individuals who drank ≥15 g/day of alcohol and smoked ≥5 pack-years had a marginally significant 34% higher risk (pooled HR 1.34, 95% CI 0.97 to 1.84, n=42 CRC cases), suggesting that at least the results were not driven by cigarette smoking and early adulthood alcohol intake contributes to colorectal carcinogenesis, despite the limited number of cases in each of these groups. We observed a slightly attenuated association after further adjustment for cumulative alcohol intake and putative CRC risk factors in mid-adulthood (Supplementary Table 3).
Fig. 1. Joint association of alcohol and cigarette use in early adulthood with risk of colorectal cancer.
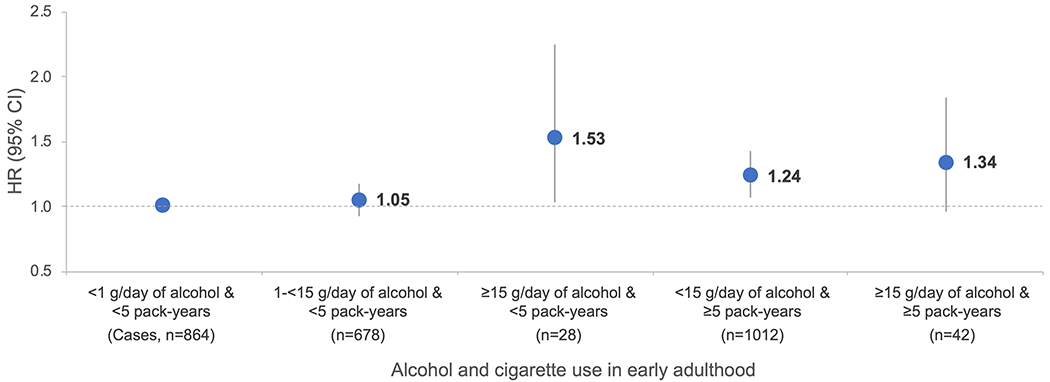
Abbreviations: CI, confidence interval; HR, hazard ratio. HR was adjusted for covariates in multivariable model of Table 2, except pack-years of smoking before age 30
In joint analyses of early and mid-adulthood alcohol intake, compared with those who consumed <1 g/day of alcohol in both life stages, positive associations of similar magnitude were observed for high alcohol consumption in early adulthood and/or midlife (pooled HRs ranged 27-35% for alcohol consumption of ≥15 g/day, Fig. 2). Further adjustment for CRC risk factors in mid-adulthood yielded slight increases in the effect sizes (Supplementary Table 4).
Fig. 2. Joint association of alcohol intake in early and mid-adulthood with risk of colorectal cancer.
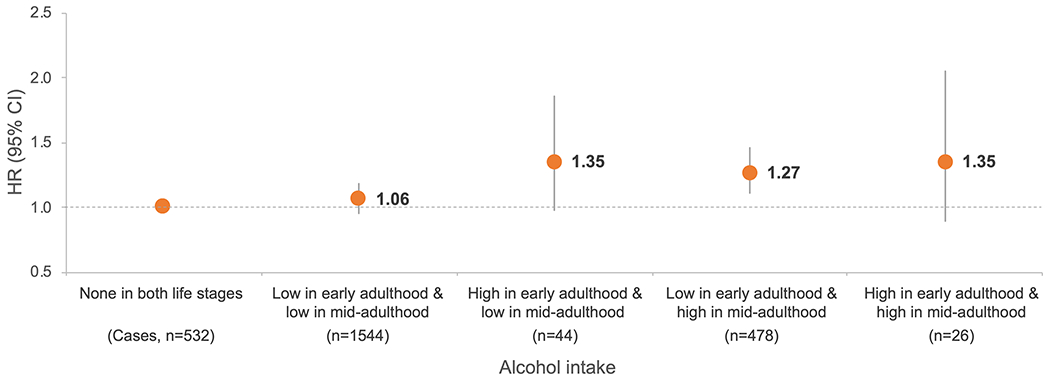
Abbreviations: CI, confidence interval; HR, hazard ratio. Alcohol intake of none was defined as <1 g/day, low as <15 g/day and high as ≥15 g/day. HR was adjusted for covariates in multivariable model of Table 2
DISCUSSION
In three large prospective cohort studies of US men and women, we found that alcohol consumption of ≥15 g/day in early adulthood was associated with a higher risk of CRC later in life. Although this association was slightly attenuated when midlife alcohol intake was taken into account, it was largely irrespective of the level of smoking in early adulthood and midlife alcohol intake, pointing to early adulthood as a potential relevant period of increased susceptibility to colorectal carcinogenesis. This study adds unique evidence evaluating the association of early adulthood alcohol intake with CRC risk, either alone or in combination with other risk factors such as smoking or midlife alcohol intake for which the relevant literature is sparse.
Alcohol is an established CRC risk factor [2]. Based on a meta-analysis of 16 prospective studies, each 10 g of daily alcohol intake in midlife was associated with a 7% higher CRC risk, with a stronger association being observed in men (8%) compared with women (4%) [25]. Nevertheless, the existing evidence on the link between alcohol and cancer risk is predominantly based on midlife alcohol consumption [25–30], except breast cancer [3, 15, 31] and prostate cancer [32] for which data on early life intake is available. Interestingly, we observed a 28% higher CRC risk among those who had alcohol intake of ≥15 g/day in early adulthood, with a stronger association in women than in men. Increased susceptibility to alcohol in women could be due to lower levels of alcohol dehydrogenase and differences in body composition resulting in higher blood alcohol concentrations [33, 34]. Considering that more measurement error is expected for assessment of remote than current alcohol intake and that early adulthood drinking comprised consumption only within a 5-year window, the comparable effect estimates we observed for high alcohol intake in early adulthood only versus that continued through mid-adulthood may indicate that alcohol intake of ≥15 g/day in early adulthood likely contributes to CRC development to some extent. In line with our current and previous observation of the joint association between alcohol and cigarette use [35], several prospective studies have documented similar findings in relation to CRC risk [36, 37]; however, these were mostly limited to exposure assessments in midlife. Although we had limited power to examine the synergistic effects of early adulthood alcohol and smoking, our findings suggest at least the association between early adulthood alcohol intake and CRC risk was not driven by smoking.
Alcohol-induced colorectal carcinogenesis could be through acetaldehyde – a primary, carcinogenic metabolite of ethanol [38] – that accumulates in the colon due to the low activity of acetaldehyde dehydrogenase in the colonic mucosa [39]. Acetaldehyde causes DNA damage [40–42], inhibits folate absorption and interferes with one-carbon metabolism, which in turn alters DNA methylation and expression of oncogenes and tumor suppressor genes [42, 43]. Accruing evidence supports the role of gut microbiota in colorectal carcinogenesis [44, 45], including alcohol-associated mechanisms [46, 47]. Causing dysbiosis and endotoxemia [48], high alcohol could result in systemic inflammation, insulin resistance, obesity and type 2 diabetes [49], all of which are metabolic conditions associated with CRC risk [50]. Thus, we speculate that initiation of alcohol consumption at young ages can contribute to colorectal carcinogenesis via accrual of time at risk [7, 8] and high-risk drinking behaviors (i.e. binge drinking) during this relevant period [51] and may induce genetic or epigenetic changes that are not entirely reversible and increase likelihood of CRC development later in life.
Strengths of our study comprise the large number of study participants, long-term follow-up and ability to account for a wide variety of potential confounding factors across different life stages, which have been considered methodological challenges to study the potential link between early life exposures and cancer development [52]. Specifically, our analyses included 191,543 US men and women followed over 26 years of time span. Using alcohol intake at age 18-22 assessed across all three cohorts, we were able to prospectively investigate the associations with future CRC risk in both men and women. Information on additional risk profiles around this life stage as well as that on dietary intake, lifestyle factors, family history of CRC, history and indications of lower endoscopy in mid-adulthood, all of which were assessed repeatedly in a prospective manner, helped account for numerous potential confounding factors in testing our hypothesis.
Several limitations need to be considered while interpreting our findings. First, alcohol intake at young ages was recalled in mid-adulthood. However, prior studies in our study population showed the adolescent and early adulthood dietary data recalled over an extended time span had reasonable validity and reproducibility, independent of participants’ age at recall and current intake [16, 53, 54]. More importantly, as alcohol intake in early adulthood was assessed before CRC diagnosis, misclassification of alcohol intake would typically be nondifferential with respect to outcome. Second, as with every observational study, residual confounding cannot be ruled out completely. However, we have adjusted for a wide variety of potential confounding factors including midlife alcohol intake and putative CRC risk factors. Third, statistical power might have been limited due to the relatively small number of CRC cases (n=70) who had ≥15 g/day of alcohol intake in early adulthood. Although we tried to improve statistical power by pooling the cohort-specific estimates, a cautious interpretation is recommended especially for the joint analyses. Fourth, we could not further investigate the observed associations according to different types of alcoholic beverages or drinking patterns such as binge drinking that may be particularly relevant to this life stage. Future research on these aspects would be important to address evidence gaps and expand the current understanding of alcohol and CRC risk. Fifth, the vast majority of our study population is comprised of white nurses and health professionals. The generalizability of our findings to other populations remains to be investigated, though adult alcohol consumption has been consistently associated with CRC across diverse racial groups.
In conclusion, higher alcohol intake in early adulthood was associated with a higher risk of CRC later in life that was not driven by early adulthood smoking and midlife alcohol intake, pointing to early adulthood as a plausibly susceptible window for CRC development. Various lines of data in our study, together with future studies corroborating our findings, will help identify a critical window of susceptibility for colorectal neoplasia that could, in turn, contribute to elucidating a more effective and modifiable target to intervene at young ages and thus accomplishing the maximal benefit of CRC prevention.
Supplementary Material
Acknowledgments:
We would like to thank the participants and staff of the Nurses’ Health Study (NHS), NHSII and Health Professionals Follow-Up Study for their valuable contributions as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington and Wyoming. The authors assume full responsibility for analyses and interpretation of these data.
Funding:
This work was supported by the National Institutes of Health (NIH) grants (UM1 CA186107, P01 CA87969, U01 CA176726, U01 CA167552 as cohort infrastructure grants; R03 CA197879, R21 CA222940 and R21 CA230873 to KW; K07 CA218377 to YC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. KW is supported by an Investigator Initiated Grant from the American Institute for Cancer Research.
Abbreviations:
- AHEI
alternative healthy eating index
- BMI
body mass index
- CI
confidence interval
- CRC
colorectal cancer
- FFQ
food frequency questionnaire
- HPFS
Health Professionals Follow-Up Study
- HR
hazard ratio
- MET
metabolic equivalent of task
- NHS
Nurses’ Health Study
- NHSII
Nurses’ Health Study II
- NSAID
nonsteroidal anti-inflammatory drug
Footnotes
Conflicts of interest: None declared.
Ethics approval: The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health and those of participating state cancer registries as required.
Consent to participate: Return of the completed questionnaire implied informed consent to participate in the study.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and colorectal cancer. Continuous Update Project Expert Report. 2018.
- 3.Colditz GA, Frazier AL. Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev. 1995;4(5):567–71. [PubMed] [Google Scholar]
- 4.Sutcliffe S, Colditz GA. Prostate cancer: is it time to expand the research focus to early-life exposures? Nat Rev Cancer. 2013;13(3):208–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke MA, Joshu CE. Early life exposures and adult cancer risk. Epidemiol Rev. 2017;39(1):11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nimptsch K, Wu K. Is timing important? The role of diet and lifestyle during early life on colorectal neoplasia. Curr Colorectal Cancer Rep. 2018;14(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31(2):285–93. [PubMed] [Google Scholar]
- 8.Biro FM, Deardorff J. Identifying opportunities for cancer prevention during preadolescence and adolescence: puberty as a window of susceptibility. J Adolesc Health. 2013;52(5 Suppl):S15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–64. [DOI] [PubMed] [Google Scholar]
- 10.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179–85. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Global status report on alcohol and health 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 12.Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the Future national survey results on drug use, 1975–2019: volume II, college students and adults ages 19–60. Ann Arbor: Institute for Social Research, The University of Michigan; 2020. [Google Scholar]
- 13.Schulenberg JE, Maggs JL. A developmental perspective on alcohol use and heavy drinking during adolescence and the transition to young adulthood. J Stud Alcohol Suppl. 2002(14):54–70. [DOI] [PubMed] [Google Scholar]
- 14.Britton A, Ben-Shlomo Y, Benzeval M, Kuh D, Bell S. Life course trajectories of alcohol consumption in the United Kingdom using longitudinal data from nine cohort studies. BMC Med. 2015;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Colditz GA, Rosner B, et al. Alcohol intake between menarche and first pregnancy: a prospective study of breast cancer risk. J Natl Cancer Inst. 2013;105(20):1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazier AL, Willett WC, Colditz GA. Reproducibility of recall of adolescent diet: Nurses’ Health Study (United States). Cancer Causes Control. 1995;6(6):499–506. [DOI] [PubMed] [Google Scholar]
- 17.Willett W Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 2012. [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26. [DOI] [PubMed] [Google Scholar]
- 19.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–6. [DOI] [PubMed] [Google Scholar]
- 20.Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133(8):810–7. [DOI] [PubMed] [Google Scholar]
- 21.Baer HJ, Schnitt SJ, Connolly JL, et al. Early life factors and incidence of proliferative benign breast disease. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2889–97. [DOI] [PubMed] [Google Scholar]
- 22.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith-Warner SA, Spiegelman D, Ritz J, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163(11):1053–64. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 25.World Cancer Research Fund/American Institute for Cancer Research. The associations between food, nutrition and physical activity and the risk of colorectal cancer. World Cancer Research Fund International Systematic Literature Review. 2017.
- 26.Park SY, Wilkens LR, Setiawan VW, Monroe KR, Haiman CA, Le Marchand L. Alcohol intake and colorectal cancer risk in the Multiethnic Cohort Study. Am J Epidemiol. 2019;188(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishihara R, Wang M, Qian ZR, et al. Alcohol, one-carbon nutrient intake, and risk of colorectal cancer according to tumor methylation level of IGF2 differentially methylated region. Am J Clin Nutr. 2014;100(6):1479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moskal A, Norat T, Ferrari P, Riboli E. Alcohol intake and colorectal cancer risk: a dose-response meta-analysis of published cohort studies. Int J Cancer. 2007;120(3):664–71. [DOI] [PubMed] [Google Scholar]
- 29.Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22(9):1958–72. [DOI] [PubMed] [Google Scholar]
- 30.McNabb S, Harrison TA, Albanes D, et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int J Cancer. 2020;146(3):861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Tamimi RM, Colditz GA, Bertrand KA. Alcohol consumption across the life course and mammographic density in premenopausal women. Breast Cancer Res Treat. 2018;167(2):529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michael J, Howard LE, Markt SC, et al. Early-life alcohol intake and high-grade prostate cancer: results from an equal-access, racially diverse biopsy cohort. Cancer Prev Res. 2018;11(10):621–8. [DOI] [PubMed] [Google Scholar]
- 33.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322(2):95–9. [DOI] [PubMed] [Google Scholar]
- 34.Saunders JB, Davis M, Williams R. Do women develop alcoholic liver disease more readily than men? Br Med J. 1981;282(6270):1140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Y, Willett WC, Rimm EB, Stampfer MJ, Giovannucci EL. Light to moderate intake of alcohol, drinking patterns, and risk of cancer: results from two prospective US cohort studies. BMJ. 2015;351:h4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otani T, Iwasaki M, Yamamoto S, et al. Alcohol consumption, smoking, and subsequent risk of colorectal cancer in middle-aged and elderly Japanese men and women: Japan Public Health Center-based prospective study. Cancer Epidemiol Biomarkers Prev. 2003;12(12):1492–500. [PubMed] [Google Scholar]
- 37.Tsong WH, Koh WP, Yuan JM, Wang R, Sun CL, Yu MC. Cigarettes and alcohol in relation to colorectal cancer: the Singapore Chinese Health Study. Br J Cancer. 2007;96(5):821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans, volumes 1-122. Lyon, France: International Agency for Research on Cancer; 2019. [PMC free article] [PubMed] [Google Scholar]
- 39.Salaspuro M. Microbial metabolism of ethanol and acetaldehyde and clinical consequences. Addict Biol. 1997;2(1):35–46. [DOI] [PubMed] [Google Scholar]
- 40.Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc. 2006;65(3):278–90. [DOI] [PubMed] [Google Scholar]
- 41.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7(2):149–56. [DOI] [PubMed] [Google Scholar]
- 42.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7(8):599–612. [DOI] [PubMed] [Google Scholar]
- 43.Giovannucci E. Alcohol, one-carbon metabolism, and colorectal cancer: recent insights from molecular studies. J Nutr. 2004;134(9):2475S–81S. [DOI] [PubMed] [Google Scholar]
- 44.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–72. [DOI] [PubMed] [Google Scholar]
- 45.O’Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engen PA, Green SJ, Voigt RM, Forsyth CB, Keshavarzian A. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res. 2015;37(2):223–36. [PMC free article] [PubMed] [Google Scholar]
- 47.Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin Gastroenterol Hepatol. 2019;17(2):275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mutlu EA, Gillevet PM, Rangwala H, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G966–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. [DOI] [PubMed] [Google Scholar]
- 50.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138(6):2029–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanny D, Naimi TS, Liu Y, Lu H, Brewer RD. Annual total binge drinks consumed by U.S. adults, 2015. Am J Prev Med. 2018;54(4):486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahabir S, Aagaard K, Anderson LM, et al. Challenges and opportunities in research on early-life events/exposures and cancer development later in life. Cancer Causes Control. 2012;23(6):983–90. [DOI] [PubMed] [Google Scholar]
- 53.Maruti SS, Feskanich D, Colditz GA, et al. Adult recall of adolescent diet: reproducibility and comparison with maternal reporting. Am J Epidemiol. 2005;161(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maruti SS, Feskanich D, Rockett HR, Colditz GA, Sampson LA, Willett WC. Validation of adolescent diet recalled by adults. Epidemiology. 2006;17(2):226–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


