Abstract
Background
Immunotherapy with chimeric antigen receptor (CAR) T cells is actively being explored for pediatric brain tumors in preclinical models and early phase clinical studies. At present, it is unclear which CAR target antigens are consistently expressed across different pediatric brain tumor types. In addition, the extent of HLA class I expression is unknown, which is critical for tumor recognition by conventional αβTCR T cells.
Methods
We profiled 49 low- and high-grade pediatric brain tumor patient-derived orthotopic xenografts (PDOX) by flow analysis for the expression of 5 CAR targets (B7-H3, GD2, IL-13Rα2, EphA2, and HER2), and HLA class I. In addition, we generated B7-H3-CAR T cells and evaluated their antitumor activity in vitro and in vivo.
Results
We established an expression hierarchy for the analyzed antigens (B7-H3 = GD2 >> IL-13Rα2 > HER2 = EphA2) and demonstrated that antigen expression is heterogenous. All high-grade gliomas expressed HLA class I, but only 57.1% of other tumor subtypes had detectable expression. We then selected B7-H3 as a target for CAR T-cell therapy. B7-H3-CAR T cells recognized tumor cells in an antigen-dependent fashion. Local or systemic administration of B7-H3-CAR T cells induced tumor regression in PDOX and immunocompetent murine glioma models resulting in a significant survival advantage.
Conclusions
Our study highlights the importance of studying target antigen and HLA class I expression in PDOX samples for the future design of immunotherapies. In addition, our results support active preclinical and clinical exploration of B7-H3-targeted CAR T-cell therapies for a broad spectrum of pediatric brain tumors.
Keywords: B7-H3, CAR T-cell immunotherapy, GD2, HLA, immunocompetent and PDOX models, pediatric brain tumor
Key Points.
1. B7-H3 and GD2 are consistently expressed in pediatric brain tumor PDOXs.
2. Downregulated HLA expression is a prominent feature of pediatric brain tumors except for gliomas.
3. B7-H3-CAR T cells have potent antitumor activity in PDOX and immunocompetent models.
Importance of the Study.
Using a large collection of pediatric brain tumor patient-derived orthotopic xenografts (PDOXs), we established an expression hierarchy of 5 CAR targets and demonstrate heterogenous antigen expression and tumor type-dependent downregulation of HLA class I expression. Lastly, we demonstrate the safety and efficacy of CAR T cells targeting B7-H3, in PDOXs and immune-competent brain tumor models. Thus, our study has implications for a broad range of immunotherapies that are being developed for pediatric brain tumors.
Pediatric brain tumors are the leading cause of cancer-related death in children and adolescents.1 Current treatment modalities are associated with suboptimal outcomes and significant acute and long-term side effects. Immunotherapy with T cells expressing chimeric antigen receptors (CARs) has the potential to improve outcomes and reduce treatment-related complications.2 Clinical CAR T-cell therapy studies for brain tumors have so far targeted human epidermal growth factor receptor 2 (HER2), interleukin 13 receptor subunit alpha 2 (IL-13Rα2), and epidermal growth factor receptor variant III (EGFRvIII), and have focused on adult patients except for one study.3–5 While CAR T-cell infusions were safe, antitumor activity was limited.3–5 Lack of efficacy is most likely multifactorial and includes, in particular for pediatric brain tumors, a limited knowledge of CAR target antigen expression.
CAR T cells recognize cell surface proteins/antigens and not peptides in the context of HLA class I molecules like conventional αβ T-cell receptor (TCR) T cells.6 Past studies have reported on the cell surface expression of CAR targets in pediatric brain tumors, identifying HER2, IL-13Rα2, ephrin type-A receptor 2 (EphA2), B7 homolog 3 (B7-H3), and disialoganglioside GD2 (GD2) as promising targets.7–13 However, a comprehensive comparison of the expression of these targets across different pediatric brain tumor types is much needed. In addition, previous studies relied on immunohistochemistry (IHC) and/or gene expression analyses, which does not allow for the reliable assessment of heterogenous antigen expression, which has emerged as major roadblock for CAR T-cell therapy.10,12–14
In addition, there is an existing controversy in the literature whether CAR T cells can exert their anti-brain tumor activity after intravenous (i.v.) delivery. While studies in xenograft models have demonstrated that systemically administered CAR T cells have limited antitumor activity in orthotopic brain tumor models,14,15 EGFRvIII-CAR T cells were readily detectable in glioma patients after i.v. administration.5
To address these gaps in our knowledge, we profiled the expression of HER2, IL-13Rα2, EphA2, B7-H3, and GD2 by flow cytometry in 49 patient-derived orthotopic xenograft (PDOX) samples. In addition, we analyzed HLA class I expression to assess the potential of standard αβTCR T cells to recognize pediatric brain tumors. We demonstrate that CAR target antigen expression is heterogenous, with B7-H3 and GD2 being most consistently expressed, and that downregulation of HLA expression is a prominent feature of pediatric brain tumors except for gliomas. Finally, we demonstrate that B7-H3-CAR T cells have potent antitumor activity after local and/or systemic administration in PDOX and immunocompetent brain tumor models.
Material and Methods
The following material and methods sections can be found in the Supplementary Material and Methods section: (1) Cell lines, (2) Generation of B7-h3 knockout GL261 cells, (3) Flow Cytometry, (4) PDOX Models, (5) B7-H3 IHC, (6) Generation of human B7-H3-CAR T cells, (7) Generation of retroviral particles for murine T-cell transduction, (8) Functional analysis of murine CAR T cells in vitro, (9) Functional analysis of CAR T cells in vivo, and (10) Bioluminescence imaging.
Ethics
Patient samples for tumor associated antigen (TAA) profiling were obtained under a St. Jude Children’s Research Hospital (St. Jude) IRB approved protocol, after informed consent was obtained in accordance with the Declaration of Helsinki. All animal experiments followed a protocol approved by the St. Jude Institutional Animal Care and Use Committee. Mice were euthanized when they met physical euthanasia criteria (significant weight loss, signs of distress), or when recommended by St. Jude veterinary staff.
Tumor Antigen Profiling
PDOX and patient samples were analyzed for the surface expression of the following antigens: EphA2, IL-13Rα2, GD2, B7-H3, HER2, HLA class I, CD47 (identification of human cells), and CD19 (negative control). A list of antibodies and matching controls are provided in the Supplementary Material and Methods section.
Animal Models
PDOXs were established using primary patient samples obtained from patients treated at St. Jude Children’s Research Hospital by biopsy, surgical resection, or autopsy procedures. Primary tumor samples were dissociated into single-cell suspensions using the Papain dissociation system (LK003150, Worthington Biochemical Corporation, Lakewood, NJ). Detailed description of PDOX establishment has been described previously.16 Briefly, dissociated cells were implanted orthotopically into the right hemisphere of the cerebral cortex of NOD/SCID gamma null (NSG) mice for medulloblastoma (MB), atypical teratoid rhabdoid tumor (ATRT), ependymoma (EPN), and embryonal tumor with multilayered rosette (ETMR), or in CD1 nude/nude for gliomas. If the cells engrafted and grew, tumors were harvested, mechanically dissociated, and reimplanted orthotopically into CD1 nude/nude mice to expand. Expanded tumors were harvested, mechanically dissociated, and cryopreserved. All animals used were 8- to 10-week-old male. CD1 nude/nude purchased from Charles River Laboratories (Wilmington, MA). The functional analysis of CAR T cells in animal models is described in the Supplementary Material and Methods section.
Generation of Murine CAR T Cells
CD3+ T cells were enriched from murine splenocytes by using Pan T-Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, spleens were collected from 6- to 8-week-old C57BL/6 mice (C57BL/6J, 000664, The Jackson Laboratory, Bar Harbor, ME) or from luciferase transgenic mice (B6; FVB-Ptprca Tg(CAG-luc-GFP)L2G85Chco Thy1a/J, 025854, The Jackson Laboratory) and passed through a 70-μm cell strainer using the back of a syringe plunger. Red blood cells were then lysed using ACK (ammonium-chloride-potassium) lysis buffer (A10492-01, Life Technologies, Carlsbad, CA), washed, and pelleted. CD3+ murine T cells were then collected using the Pan T-Cell Isolation Kit II (130-095-130, Miltenyi Biotec) as per the manufacturer’s protocol. Isolated T cells were washed and activated using plate-bound CD3/CD28 antibodies (553057 and 553294 respectively, BD Biosciences) in complete RPMI media supplemented with 50 U/mL of human IL-2 (Peprotech, Rocky Hill, NJ).
On Day 2 post-activation, T cells were transduced with retroviral particles on RetroNectin-coated plates (Clontech, Mountain View, CA) in complete RPMI media supplemented with 50 U/mL of IL-2. non-transduced (NT) T cells were prepared by adding activated T cells on RetroNectin-coated wells without retroviral particles. After 2 days, CAR T cells were harvested and expanded in the presence of IL-2 until Day 7 post-transduction. CAR detection was performed on Days 3-5 post-transduction using flow cytometry. Experiments were performed at or before Day 7 post-transduction.
Statistical Analyses
All experiments were performed at least in duplicates. For comparisons between 2 groups, a 2-tailed t test was used. For comparisons of 3 or more groups with a single independent variable, statistical significance was determined by 1-way ANOVA with Tukey’s multiple comparisons test. For comparison of 3 or more groups with 2 or more independent variables, statistical significance was determined by multiple t tests or 2-way ANOVA with Sidak’s or Tukey’s multiple comparisons test. Survival curves were plotted using the Kaplan-Meier method. Statistical significance between survival curves was determined using the log-rank (Mantel-Cox) test. Bioluminescence imaging data were analyzed using either ANOVA or t test. P-values were calculated using Prism (GraphPad Software, San Diego, CA). *P < .05; **P < .01; ***P < .001; ****P < .0001; ns, nonsignificant.
Results
B7-H3 and GD2 Are Consistently Expressed in Pediatric Brain Tumor PDOX Models
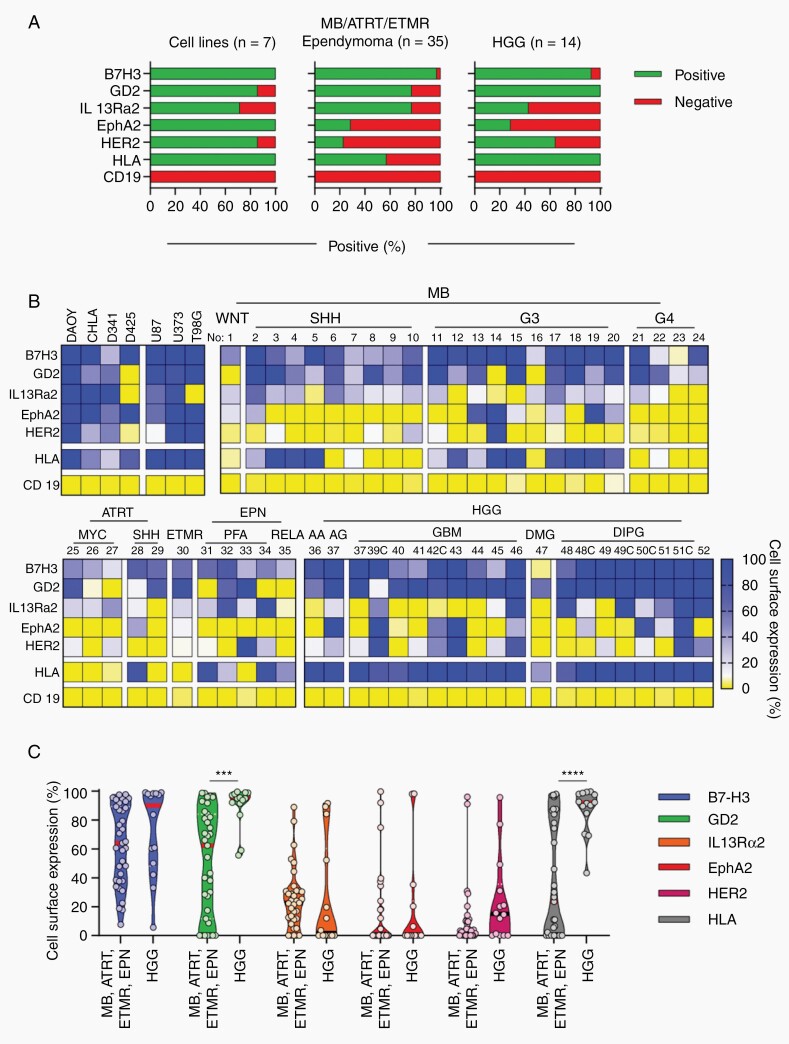
To identify potential CAR targets for pediatric brain tumors, we evaluated cell surface expression of 5 brain tumor antigens (B7-H3, GD2, IL-13Rα2, EphA2, HER2) in PDOX samples by flow cytometry analysis using CD19 as a negative control. In addition, we evaluated HLA class I expression. We profiled 49 PDOX samples, including 24 MBs, 5 ATRTs, 5 EPNs, 1 ETMRs, 14 high-grade gliomas (HGGs). In addition, we analyzed established human brain tumor cell lines (U373, U87, T98G, DAOY, D341, D425, CHLA-01-MED) as controls. We considered PDOX samples and cell lines positive when ≥10% of analyzed tumor cells were positive. Almost all established cell lines consistently expressed all target antigens except for couple of cases (Figure 1A and B). B7-H3 was expressed in 47 (95.9%), GD2 in 41 (83.7%), IL-13Rα2 in 33 (67.4%), EphA2 in 14 (28.6%), and HER2 in 18 (36.7%) PDOX samples (Figure 1A).
Fig. 1.
Heterogeneous CAR target and HLA expression in pediatric brain tumors. PDOX samples were stained for B7-H3, EphA2, GD2, HER2, IL-13Rα2, HLA class I, and CD19. CHLA, CHLA-01-MED; MB, medulloblastoma; ATRT, atypical teratoid rhabdoid tumor; EPN, ependymoma; ETMR, embryonal tumor with multilayered rosettes; HGG, high-grade glioma; DIPG, diffuse intrinsic pontine glioma. A, Percentage of positive/negative cell lines and PDOXs. B, Double color gradient heatmap: 0%: yellow, 10%: white, 100%: blue gradient. WNT, MB wingless subgroup; SHH, MB sonic hedgehog subgroup; G3, MB subgroup 3; G4, MB subgroup 4; MYC, MYC ATRT subgroup; III, HGG WHO grade III; IV, HGG WHO grade IV; No, PDOX model number. Details related to each model are provided in Supplementary Tables 1 and 2. C, Violin plots showing percentage of cell surface expression of TAAs in different pediatric brain tumor subtypes and subgroups (2-way ANOVA with Sidak’s test for multiple comparisons; ***P < .001; ****P < .0001).
Mean antigen expression of positive tumors was 68% for B7-H3 (range: 17.7-99.0%), 74.1% for GD2 (range: 11.7%-99.6%), 37.5% for IL-13Rα2 (range: 11.7%-99.6%), 50.1% for EphA2 (range: 12.0%-99.8%), and 36.1% for HER2 (range: 10.0%-96.0%) (Figure 1B and C and Supplementary Tables 1 and 2). Using a cutoff of 50%, 33 (67.3%) PDOX samples were positive for B7-H3, 33 (67.3%) for GD2, 9 (18.4%) for IL-13Rα2, 5 (10.2%) for EphA2, and 4 (8.2%) for HER2. Comparing HGGs to other pediatric brain tumors (MB, ATRT, EPN, and ETMR), HGG expressed significantly higher levels of GD2 (Figure 1C). HGGs also expressed significantly higher levels (P < .0001) of HLA class I in comparison to all other pediatric brain tumor subtypes evaluated (Figure 1C). Thus, B7-H3, GD2, IL-13Rα2, EphA2, and HER2 expression is heterogenous in pediatric brain tumor PDOX models and there is a clear hierarchy: B7-H3 = GD2 >> IL-13Rα2 > HER2 = EphA2. In addition, HLA class I expression is downregulated in MB, ATRT, EPN, and ETMR, a finding that supports the development of CAR T-cell therapies since tumor cell recognition by CAR T cells is independent of HLA expression.2 Importantly, we observed similar findings when we analyzed PDOX samples from different passages (Supplementary Figure 2A) and patient samples (Supplementary Figure 2B and C). Lastly, cell surface expression did not correlate with existing gene expression (RNAseq) data of matching PDOX samples and samples from the Pediatric Cancer Genome Project (PCGP) (Supplementary Figures 3 and 4).
IHC Confirms B7-H3 Expression in Pediatric Brain Tumors
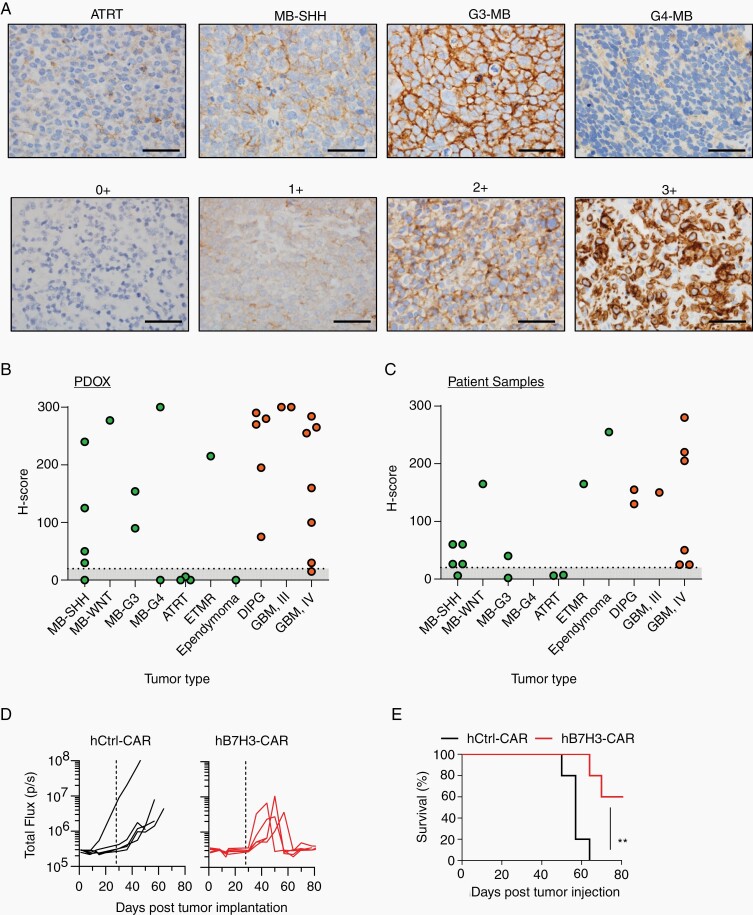
Based on our findings, we decided to focus on B7-H3 since it is not expressed in normal brain tissue in contrast to GD2.17 To validate B7-H3 expression, we performed IHC on paraffin-embedded tissue blocks of 29 pediatric brain tumor PDOX models (Figure 2A). Twenty-four were positive for B7-H3, with most of them (92%) demonstrating high-intensity staining (2+ or 3+). Eight out of 10 MBs were positive for B7-H3, and 13 out of 14 pediatric gliomas were positive for B7-H3 with mostly high-intensity staining (Figure 2B, Supplementary Figure 5). One ETMR showed positive staining for B7-H3. In contrast, all ATRTs and one evaluated EPN were negative for B7-H3. For most of the PDOX samples evaluated, we also had matching patient tumor samples that were collected for diagnostic purposes or at autopsy and had not been passaged in mice. We observed a similar pattern of B7-H3 expression in these samples, although the staining intensities were lower (Figure 2C, Supplementary Figure 5A).
Fig. 2.
B7-H3 is expressed in pediatric brain tumors. PDOXs were evaluated for B7-H3 expression by IHC. A, Top panel: representative images for different tumor subtypes, including ATRT, MB-SHH, G3-MB, and G4-MB stained for B7-H3. Bottom panel: B7-H3 staining-intensity scale: 0+: no staining, 1+: weak positive, 2+: moderate positive, 3+: strong positive. Scare bar, 50 µm. B and C, H-scores of PDOXs and pediatric brain tumor sections (evaluated by pathologist blinded to tumor type and expression status). D, MB-SHH PDOX tumor cells (Model No. 5, SJMBSHH-13-5634) expressing YFP.ffLuc were implanted into the cortices of NSG mice followed by i.v. injection of 5 × 106 hB7-H3− or hCtrl-CAR T cells on Day 28. Radiance (total flux) is shown. E, Kaplan-Meier survival analysis. Log-rank (Mantel-Cox) test; hCtrl− vs hB7-H3-CAR T cells; P = .0052).
Human B7-H3-CAR T Cells Have Antitumor Activity in a PDOX Models In Vivo
Having established that B7-H3 is a promising CAR target for pediatric brain tumors, we next evaluated if B7-H3-positive PDOX tumors can be recognized and killed by human (h) B7-H3-CAR T cells in vivo. We generated hB7-H3-CAR and nonfunctional control (hCtrl)-CAR T cells for these studies (Supplementary Figure 6A and B), and used a B7-H3-positive orthotopic SHH-MB PDOX model (model 5; Supplementary Figure 6C). On Day 0, SHH-MB PDOX expressing YFP.ffLuc were orthotopically implanted into NSG mice. At Day 28, mice received a single i.v. dose of 5 × 106 hB7-H3-CAR or hCtrl-CAR T cells. Tumor growth was monitored by weekly bioluminescence imaging. hB7-H3-CAR T cells induced tumor regression compared to hCtrl-CAR T cells, resulting in a significant overall survival advantage (Figure 2D and E, P = .0052). Two tumor-free animals in the B7-H3-CAR T-cell treatment group died due to a rapid weight loss, which could be explained by xenogeneic graft-vs-host disease (GVHD). However, we did not observe any other clinical signs of GVHD (eg, scruffy fur, loss of whiskers). Next, we performed similar studies using DIPG (diffuse intrinsic pontine glioma) PDOX model (model 48). hB7-H3-CAR T cells showed significant control of tumor burden and an overall survival advantage when compared to Ctrl-CAR T cells (Supplementary Figure 7). Thus, hB7-H3-CAR T cells have potent antitumor activity in pediatric PDOX models in vivo.
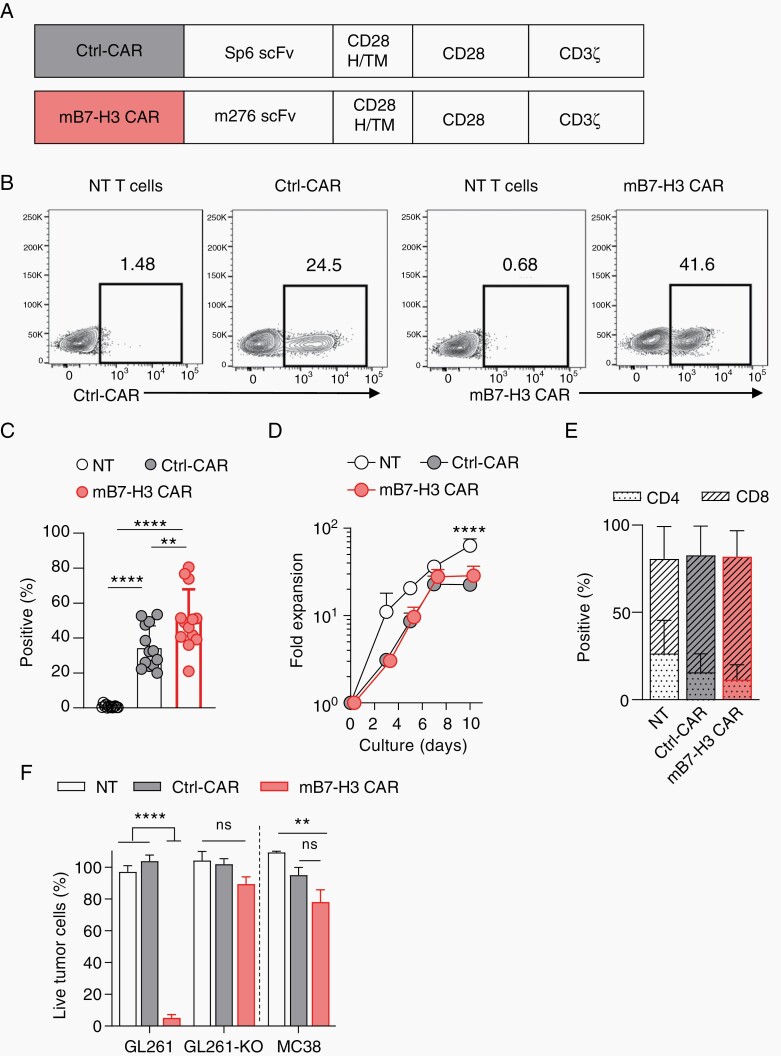
mB7-H3-CAR T Cells Recognize and Kill Murine Glioma Cells In Vitro
Having shown that hB7-H3-CAR T cells can recognize and eradicate tumors, we next sought to evaluate the safety and efficacy of B7-H3-CAR T-cell therapy in an immunocompetent glioma mouse model. To generate murine (m) B7-H3-CAR T cells, we designed a retroviral vector encoding a second-generation CD28.ζ CAR with an antigen recognition domain derived from the mB7-H3-specific MAb m27618 (Figure 3A, Supplementary Figure 8A). As a control CAR (Ctrl-CAR), we used a previously described Sp6-CAR vector with the same CD28.ζ endodomain.19 CAR T cells were generated by standard retroviral transduction and a mean CAR transduction efficiency of 50% (SD ±17.19%) for mB7-H3-CAR and 35% (SD ±12.23%) for Ctrl-CAR was achieved (Figure 3B and C). For subsequent experiments, cells were used within the first 7 days’ post-transduction since there was no difference in expansion and viability between NT, Ctrl-CAR, and mB7-H3-CAR T cells (Figure 3D, Supplementary Figure 8B). Phenotypic analysis revealed a mixture of CD4+ and CD8+ T cells with no significant differences between NT, Ctrl-CAR, or mB7-H3-CAR T cells (Figure 3E). In addition, transduction did not affect T-cell subset composition (memory: CD44+/CD62L−; central memory: CD44+/CD62L+; naïve CD44−/CD62L+; Supplementary Figure 8C). To evaluate if mB7-H3-CAR T cells recognize and kill target cells in an antigen-dependent manner, we used a 72 hours’ MTS assay with the following targets: mB7-H3-positive GL261 (murine glioma) cells, GL261 mB7-H3 knockout cells (GL261-KO), and mB7-H3-negative MC38 (murine colon adenocarcinoma) cells (Supplementary Figure 9). Only mB7-H3-CAR T cells had significant (P < .0001) cytolytic activity against GL261 in contrast to NT or Ctrl-CAR T cells (Figure 3F). This result was confirmed with sorted Ctrl-CAR and mB7-H3 CAR T cells (Supplementary Figure 10). No mB7-H3-specific cytolytic activity was observed against GL261-KO and MC38 cells (Figure 3F), confirming specificity.
Fig. 3.
Functional characterization of mB7-H3-CAR T cells. A, Scheme of retroviral vectors; H/TM: hinge/transmembrane domain. B, CAR expression evaluated using flow cytometry analysis at Days 3-5 post-transduction. Representative flow plots for NT, Ctrl-CAR, and mB7-H3-CAR expression in transduced murine T cells. C, Summary plot of %F(ab’)2-positive T cells (n = 13, mean ± SEM, 1-way ANOVA with Tukey’s test for multiple comparisons). D, T cells were expanded with IL-2 (50 U/mL) until Day 10 post-transduction. Fold expansion over time (n = 6, mean ± SEM, 2-way ANOVA with Tukey’s test for multiple comparisons). E, Summary plot depicts the proportion of CD4+ and CD8+ cells in NT, Ctrl-CAR, and mB7-H3-CAR T cells on Day 5 post-transduction (n = 10, mean ± SEM, multiple t tests for within-group analysis and 2-way ANOVA with Tukey’s test for multiple comparisons). F, MTS assay with B7-H3-positive (GL261) and B7-H3-negative (MC38, GL261-KO) targets at an effector to target (E:T) ratio of 0.25; n = 5 (GL261, GL261-KO), n = 3 (MC38), mean ± SEM, 2-way ANOVA with Tukey’s test for multiple comparisons).
mB7-H3-CAR T Cells Maintain Their Effector Function in Repeat Stimulation Assays
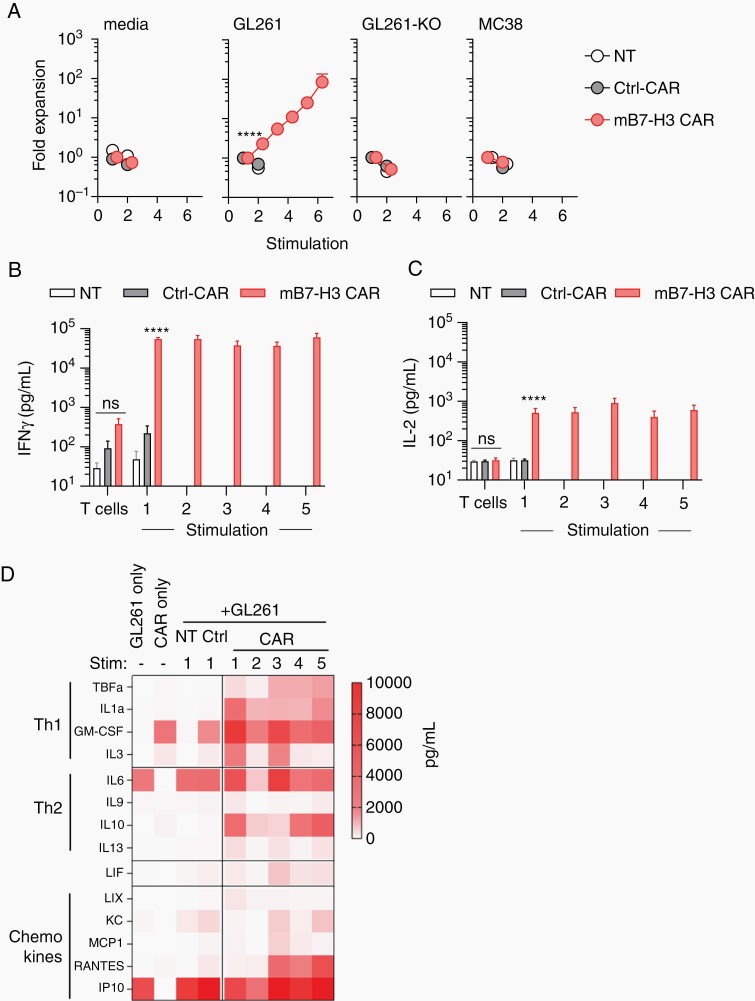
To test if CAR T cells retain their ability to recognize and kill tumor cells after multiple stimulations, we set up a repeated-stimulation assay. Effector T-cell populations were co-cultured with GL261, GL261-KO, or MC38 tumor cells at an effector to target (E:T) ratio of 2:1 without any exogenous cytokines. Every 3 days, T cells were counted and restimulated with fresh tumor cells at the same E:T ratio, until they stopped expanding. In addition, co-culture supernatants were collected at 24 hours after each stimulation, and the concentrations of IFN-γ and IL-2 were determined by ELISA. In addition, we also evaluated other Th1 (TNF-α, IL-1α, GM-CSF, IL-3) and Th2 (IL-6, IL-9, IL-10, IL-13) cytokines, and chemokines (LIX, KC, MCP1, RANTES, IP10) by Multiplex analysis.
Only mB7-H3-CAR T cells expanded for up to 6 stimulations with GL261. This expansion was antigen-specific, since we observed no expansion after stimulation with mB7-H3-negative tumor cells (GL261-KO, MC38; Figure 4A). Compared to NT or Ctrl-CAR T cells, mB7-H3-CAR T cells secreted significantly (P < .0001) higher levels of IFN-γ and IL-2 after the first stimulation with GL261 (Figure 4B and C; Supplementary Figure 11). With subsequent stimulations, IFN-γ and IL-2 secretion was only sustained by mB7-H3-CAR T cells. In addition to IFN-γ and IL-2, mB7-H3-CAR T cells also consistently produced IL-1α, GM-CSF, IL-10, and RANTES (Figure 4D). Consistent with published data,20,21 IL-6 and IP-10 were produced by GL261 tumor cells.
Fig. 4.
mB7-H3-CAR T cells expand and secrete cytokines in repeat stimulation assay. CAR T cells were co-cultured with tumor cells at a 2:1 ratio without exogenous cytokines followed by enumeration and re-stimulation with fresh tumor cells every 3 days until T cells stopped expanding. A, Average fold expansion of T cells upon successive stimulations (x-axis: each stimulation is a 3-day co-culture with fresh tumor cells, n = 11 for media and GL261, n = 6 for GL261-KO, n = 5 for MC38, mean ± SEM, 2-way ANOVA with Tukey’s test for multiple comparisons). B-C, CAR T-cell production of IFN-γ and IL-2 cytokines 24 hours’ post-stimulation at 2:1 ratio against GL261 tumor cells (n = 11, mean ± SEM, multiple t tests). D, Heatmap of production of Th1 and Th2 cytokines, and chemokines post-repeat stimulations (n = 4, mean).
mB7-H3-CAR T Cells Have Potent Anti-Glioma Activity Post-Local or Systemic Administration
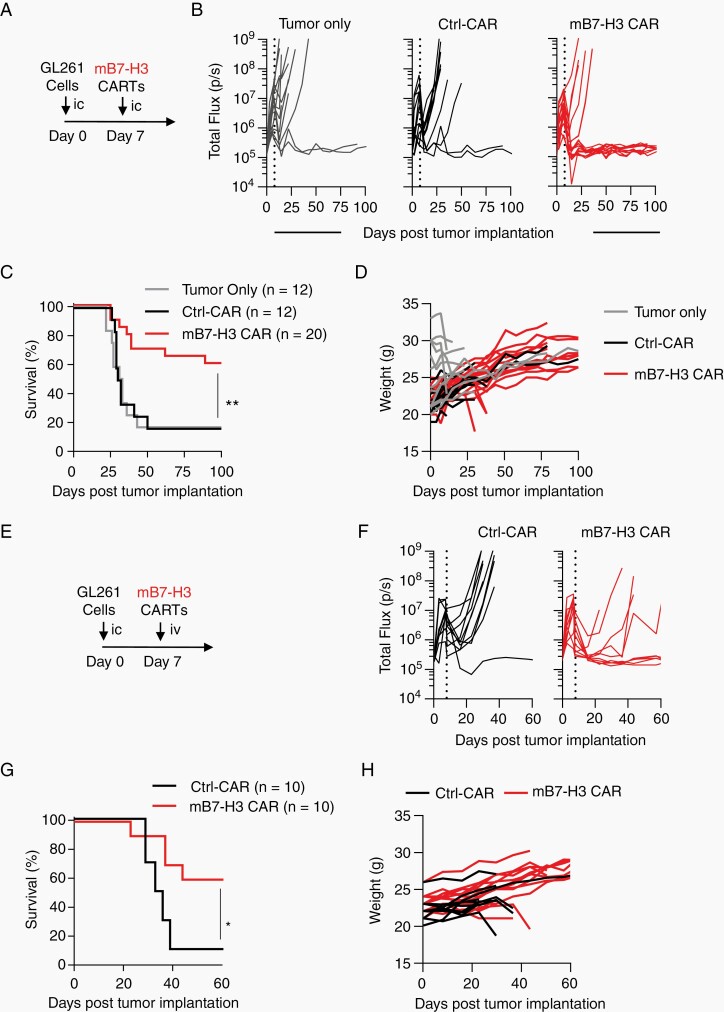
Finally, we evaluated the antitumor activity of mB7-H3-CAR T cells in an immune-competent model in vivo. We selected the orthotopic GL261 murine glioma model, since GL261 tumors faithfully recapitulate human disease22 and express murine B7-H3. On Day 0, GL261.eGFP.ffLuc cells were implanted into the brains of C57BL/6 mice, followed by intra-tumoral injection of 2 × 106 CAR T cells on Day 7 (Figure 5A). Tumor burden was monitored by weekly IVIS imaging. Intra-tumoral injection of mB7-H3-CAR T cells resulted in a complete tumor regression in 12 out of 20 (60%) treated mice (Figure 5B; Supplementary Figure 12). This resulted in a significant increase in overall survival without any signs of toxicity as judged by weight measurements, compared to untreated mice and mice injected with Ctrl-CAR T cells (Figure 5C and D, P = .006).
Fig. 5.
mB7-H3-CAR T cells have potent anti-glioma activity in immunocompetent GL261 model. A-D, 1 × 105 GL261.eGFP.ffLuc cells were injected orthotopically into albino C57BL/6 mice, followed by intra-tumoral injection of media (tumor-only group), 2 × 106 Ctrl-CAR, or 2 × 106 mB7-H3-CAR T cells. A, Experimental scheme. B, Quantitative bioluminescent signal of tumor burden over time. C, Kaplan-Meier survival curve (log-rank Mantel-Cox test; n = 12 for tumor-only and Ctrl-CAR, n = 20 for mB7-H3-CAR, P = .006). D, Changes in mice body weight determined weekly. E-H, 1 × 105 GL261.eGFP.ffLuc cells were injected orthotopically into albino C57BL/6 mice, followed by i.v. injection of 3 × 106 Ctrl-CAR or 3 × 106 CAR T cells. E, Experimental scheme. F, Quantitative bioluminescent signal of tumor burden over time. G, Kaplan-Meier survival curve (log-rank Mantel-Cox test; n = 10 for Ctrl-CAR, n = 15 for CAR, P = .0112). H, Changes in mice body weight determined weekly.
Next, we examined the antitumor activity of mB7-H3-CAR T cells post-systemic administration. CAR T cells (3 × 106/mouse) were injected i.v. at Day 7 post-tumor implantation (Figure 5E). mB7-H3-CAR T cells induced complete eradication of tumors in 6 out of 10 (60%) treated mice, whereas Ctrl-CAR T cells had minimal antitumor activity (Figure 5F), resulting in a significant survival advantage for mice receiving the mB7-H3-CAR (Figure 5G, P = .0112). Systemic T-cell administration did not cause weight loss in any of the treated animals (Figure 5H).
Systemic Administration of mB7-H3-CAR T Cells Is Safe
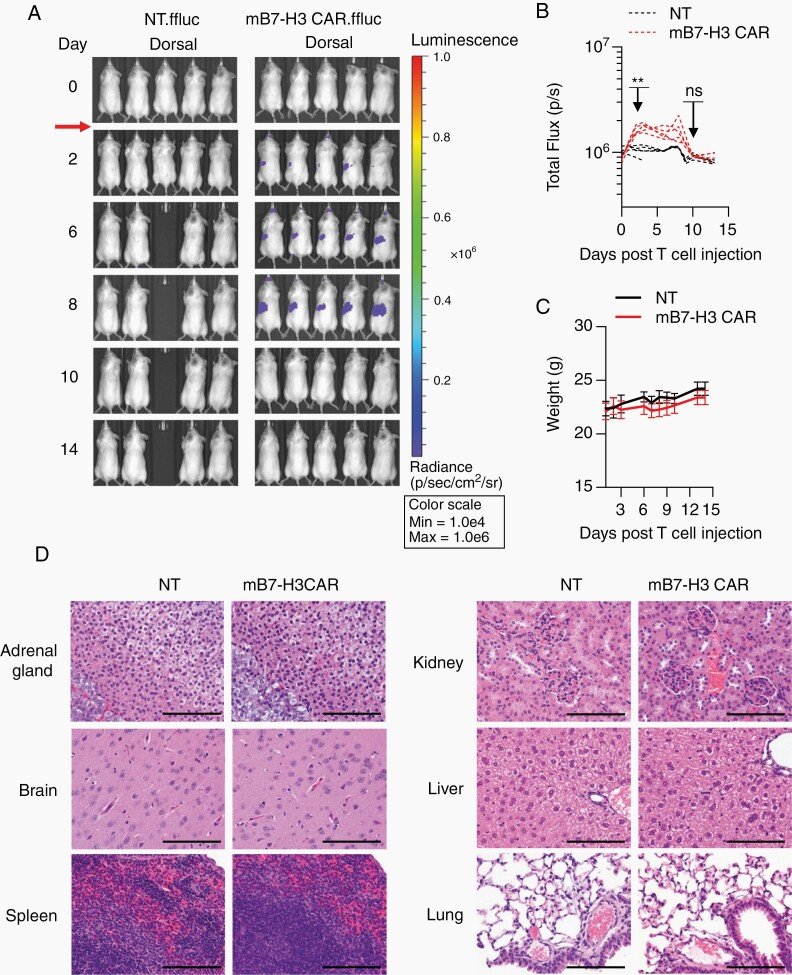
B7-H3 is expressed at low levels on some normal tissues like adrenal glands, liver, and reproductive organs18,23; thus, we sought to evaluate the safety of mB7-H3-CAR T cells in tumor-free C57BL/6 mice. To generate CAR T cells, we used CD3+ T cells from the splenocytes of luciferase transgenic mice to track CAR T-cell migration and expansion over time using bioluminescence imaging. Tumor-free mice received one i.v. dose of 1 × 107 NT or mB7-H3-CAR T cells. We observed only minor, transient mB7-H3-CAR T-cell expansion compared to that in the NT T-cell group (Figure 6A and B; Supplementary Figure 13A and B). Mice did not lose weight or develop any signs of morbidity or weakness upon daily follow-up (Figure 6C). One out of 5 mice in the NT group died during imaging at Day 4 posttreatment due to unknown reasons. Mice from both groups were euthanized on Day 14 post–T-cell injection and subjected to histopathological evaluation. No notable histologic differences were observed in the examined tissues between the 2 groups (Figure 6D, Supplementary Figure 13C). One out of 5 mice that received mB7-H3-CAR T cells had mild liver inflammation, as judged by numerous mixed-cell foci in the liver parenchyma. The observed inflammation was most likely the result of a bacterial shower from the gut rather than due to mB7-H3-CAR T cells since it was not located around the periportal/biliary tract area and did not consist of lymphocytes. Additionally, extensive analysis of major brain structures revealed no treatment-related side effects (Supplementary Figure 14). Similarly, safety studies in younger mice (4 weeks old) revealed no evidence of CAR T-cell–mediated toxicity (Supplementary Figure 15).
Fig. 6.
Systemic administration of mB7-H3-CAR T cells is safe. High dose (1 × 107) of NT and mB7-H3-CAR T cells expressing GFP.ffluc were injected i.v. into tumor-free albino C57BL/6 mice. A. Representative dorsal bioluminescence images. B. Radiance signal (total flux) over time (n = 5, 2-way ANOVA with Sidak’s test for multiple comparisons; **P < .01; ns, nonsignificant). C. Change in murine body weight after T-cell injection. D. Representative images of H&E-stained sections for each treatment group are shown at 40× magnification, scale bar: 100 µm.
Discussion
Here we established an expression hierarchy of 5 CAR targets: B7-H3 = GD2 >> IL-13Rα2 > HER2 = EphA2 in pediatric brain tumor PDOX samples and demonstrate significant inter- and intratumor heterogeneity in regard to antigen expression. We further demonstrate that MB, ATRT, EPN, and ETMR PDOXs express lower levels of HLA class I than HGG/DIPGs. Lastly, we generate B7-H3-CAR T cells and show that these CAR T cells have potent antitumor activity after local or systemic delivery in orthotopic brain tumor models.
Few studies have compared the expression of tumor antigens in pediatric brain tumors. One study analyzed the expression of cell surface and intracellular antigens by RT-qPCR in 5 HGGs, 4 low-grade gliomas, 10 juvenile pilocytic astrocytomas, and 7 EPNs.13 EphA2, IL-13Rα2, and HER2 were included in the analysis, and all analyzed tumors expressed EphA2, 15/26 expressed IL-13Rα2, and 14/26 expressed HER2. Another study analyzed the expression of EphA2, IL-13Rα2, and survivin in 15 pediatric brain stem gliomas (BSGs) and 12 non-brain stem gliomas (NBSGs), which included high-grade as well as low-grade histologies.12 EphA2 was expressed in 7/15 BSGs and 12/12 NBSGs, and IL-13Rα2 was expressed in 10/15 BSGs and 7/9 NBSGs. Finally, a recent study demonstrated expression of EphA2, HER2, and IL-13Rα2 in wide array of MBs by IHC, which is in contract to our findings.14
Numerous studies have reported the expression of single antigens in pediatric brain tumors. For example, evaluating HER2 expression in MBs, investigators found that 60/70 tumors were positive with 32.9% of tumors having greater than 50% positivity by IHC analysis.24 Modak et al. first reported high levels of B7-H3 expression in 33/47 pediatric brain tumors including HGG, mixed glioma, oligodendroglioma, astrocytoma, meningioma, schwannoma, and MB.25 High expression of B7-H3 in pediatric brain tumors was recently confirmed by others.10 Expression of IL-13Ra2 was also examined in 58 pediatric brain tumors using in situ hybridization and IHC26; 11/11 high-grade astrocytomas, 26/33 of low-grade astrocytomas, 4/6 MBs, and 2/3 EPNs were positive for IL-13Rα2. Lastly, Mount et al. recently reported on consistent and high levels of GD2 expression in H3 K27M-mutant diffuse midline gliomas including DIPGs.11
Our study now extends these findings and performs for the first time a comprehensive analysis of 49 pediatric brain tumor PDOXs. It is also the first study to use flow analysis for all 5 antigens, which significantly reduces the risk of false-positive antigen detection and enables reliable detection of heterogenous antigen expression in contrast to standard IHC. Based on our analysis, using a stringent cutoff of at least 10% positive cells, we unequivocally establish a rank order of expression for B7-H3, GD2, EphA2, IL-13Rα2, and HER2 (B7-H3 = GD2 >> IL-13Rα2 > HER2 = EphA2). In addition, we demonstrate that target antigen expression is heterogenous in PDOX tumors, in contrast to established cell lines, highlighting (i) the importance of using PDOX models to evaluate suitable CAR T-cell targets and (ii) the need to target multiple antigens to reduce the risk of immune escape when a single antigen is targeted. Importantly, our results indicate that gene expression analysis does not correlate with cell surface expression of a particular TAA, emphasizing the significance of analyzing protein expression.
We also analyzed HLA class I expression and demonstrate that MB, ATRT, EPN, and ETMR expressed significant lower levels of HLA class I than HGG/DIPG. Low levels of HLA class I expression have been reported in MB.27 One study has also demonstrated that H3 K27M-mutant induces HLA class I expression in DIPG via VCX3A.28 Our study now firmly established significant differences in HLA class I expression between non-glial brain tumors and HGGs. This has potential implications for immunotherapeutic approaches for pediatric brain tumors that are currently being developed since checkpoint blockade, vaccines, and αβTCR T-cell therapies rely on HLA class I expression to exert their antitumor activity in contrast to CAR T-cell or natural killer-cell therapies.6
We focused our therapeutic approach on targeting B7-H3 with CAR T cells since it was expressed consistently across all pediatric brain tumor types. We demonstrate that human B7-H3-CAR T cells have potent antitumor activity in a B7-H3-positive SHH-MB and DIPG PDOX models, extending findings by others that B7-H3-CAR T cells have significant antitumor activity against MB and ATRT in xenograft models.10,15 To establish the safety and efficacy of B7-H3-CAR T cells in an immunocompetent brain tumor model, we adapted a well-characterized GL261 murine glioma model.22 We demonstrate that mB7-H3-CAR T cells recognize and kill B7-H3-positive murine tumor cells in vitro and have potent anti-glioma activity in vivo after local or systemic T-cell delivery. We did not observe any evidence of on-target/off-tumor toxicity after local or systemic delivery. This finding is consistent with one other study that demonstrated that the i.v. injection of mB7-H3-CAR T cells does not induce systemic toxicities in immunocompetent models despite reports that B7-H3 is expressed at low levels in several healthy tissues.23
The observed antitumor activity in our immunocompetent animal model is noteworthy given its physiological relevance. While other immunocompetent animal models have relied on genetically modifying murine brain tumor cells to overexpress CAR targets, including human IL-13Rα2, human EGFRvIII, murine CD70 or murine GD2 and GD3 synthases,18,29–32 we used a glioma cell line that was not genetically modified to express the target antigen, mimicking physiological antigen expression.
Antitumor activity of systemic administration of CAR T cells in orthotopic brain tumor xenograft models has been model dependent; several studies have reported limited or no antitumor activity while others have reported responses, including complete tumor regressions.9,10,14,15,33 In addition, H3.3 K27M mutant-specific αβTCR T cells had potent anti-DIPG activity in xenograft models,34 and CD70−, GD2−, or EGFRvIII-CAR T cells in immunocompetent models after i.v. administration.30,31 In humans, T cells also had antitumor activity against brain tumors and/or were detected within the central nervous system (CNS) after i.v. administration. For example, Epstein-Barr virus (EBV)-specific T cells have induced complete remissions in patients with EBV-positive CNS lymphoma,35 and CD19-CAR or EGFRvIII-CAR T cells are readily detected in the cerebrospinal fluid or at glioma sites, respectively.5,36 In addition, 1 patient with a centrally located HGG had a remarkable response post-i.v. administration of HER2-CAR T cells.3 Lastly, infusion of tumor-infiltrating lymphocytes (TILs) in patients with melanoma brain metastases was associated with an intracranial objective response of 28% in one clinical study.37 Thus, at present, it is unclear what the optimal route of administration of CAR T cells for brain tumor is; most likely, it will depend on anatomic localization and the targeted antigen. For example, if a targeted antigen is not expressed in normal brain tissue, but at low levels outside the CNS, then locoregional delivery might be advantageous to prevent on-target/off-cancer toxicity.
In conclusion, our study establishes for pediatric brain tumors an expression hierarchy of 5 CAR target antigens that are currently being explored in preclinical as well as clinical studies. In addition, it highlights that downregulation of HLA class I expression is a prominent feature of non-glial pediatric brain tumors. Lastly, we demonstrate that CAR T cells targeting B7-H3, one of the two antigens that is expressed in the majority of pediatric brain tumors, have potent antitumor activity in PDOX and immunocompetent animal models, warranting further active exploration of B7-H3-targeted CAR T-cell therapies for pediatric brain tumors.
Supplementary Material
Acknowledgments
We would like to thank Dr. Brooke Prinzing for constructive discussions and reviewing our manuscript and Dr. Deanna Langfitt for assistance with flow cytometry.
Funding
This work was supported by the National Institute of Health (NIH) grant R01NS106379-02 (S.G., G.K.), the Assisi Foundation of Memphis (G.K.), and the American Lebanese Syrian Associated Charities (ALSAC; J.C., C.D., S.J.B., M.F.R., S.G., G.K.). The Center for In Vivo Imaging and Therapeutics (CIVIT) is supported in part by NIH grants P01CA096832 (M.F.R., S.J.B.) and R50CA211481. The Center for Advanced Genome Engineering is supported in part by the National Cancer Institute (NCI)/NIH grant P30CA021765, and the Sequencing (Hartwell) Center by NCI/NIH grant P30CA021765.
Conflict of interest statement. G.K. and S.G. have patent applications in the field of immunotherapy.
Authors’ contribution. D.H. and G.K. designed the study. D.H., H.H., J.C., Z.Y., and G.K. developed methodologies. D.H., H.H., J.C., Z.Y., Z.O., K.C., X.Z., K.S.M., and J.L.S. performed experiments. S.J.B. and M.F.R. provided PDOX models, and C.D. provided the human B7-H3-CAR. All authors contributed to data analysis and manuscript preparation. G.K. supervised the study.
References
- 1. Ostrom QT, Gittleman H, Liao P, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahmed N, Brawley V, Hegde M, et al. . HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3(8):1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown CE, Alizadeh D, Starr R, et al. . Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Rourke DM, Nasrallah MP, Desai A, et al. . A single dose of peripherally infused EGFRvIII-directed CAR T-cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399):eaaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris DT, Kranz DM. Adoptive T cell therapies: a comparison of T cell receptors and chimeric antigen receptors. Trends Pharmacol Sci. 2016;37(3):220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmed N, Ratnayake M, Savoldo B, et al. . Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67(12):5957–5964. [DOI] [PubMed] [Google Scholar]
- 8. Krenciute G, Krebs S, Torres D, et al. . Characterization and functional analysis of scFv-based chimeric antigen receptors to redirect T-cells to IL13Rα2-positive glioma. Mol Ther. 2016;24(2):354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chow KK, Naik S, Kakarla S, et al. . T-cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther. 2013;21(3):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Majzner RG, Theruvath JL, Nellan A, et al. . CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25(8):2560–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mount CW, Majzner RG, Sundaresh S, et al. . Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat Med. 2018;24(5):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okada H, Low KL, Kohanbash G, McDonald HA, Hamilton RL, Pollack IF. Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J Neurooncol. 2008;88(3):245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang JG, Kruse CA, Driggers L, et al. . Tumor antigen precursor protein profiles of adult and pediatric brain tumors identify potential targets for immunotherapy. J Neurooncol. 2008;88(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donovan LK, Delaidelli A, Joseph SK, et al. . Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat Med. 2020;26(5):720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Theruvath J, Sotillo E, Mount CW, et al. . Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat Med. 2020;26(5):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith KS, Xu K, Mercer KS, et al. . Patient-derived orthotopic xenografts of pediatric brain tumors: a St. Jude resource. Acta Neuropathol. 2020;140(2):209–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richman SA, Nunez-Cruz S, Moghimi B, et al. . High-affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol Res. 2018;6(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seaman S, Zhu Z, Saha S, et al. . Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell. 2017;31(4):501–515.e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116(19):3875–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kober C, Weibel S, Rohn S, Kirscher L, Szalay AA. Intratumoral INF-γ triggers an antiviral state in GL261 tumor cells: a major hurdle to overcome for oncolytic vaccinia virus therapy of cancer. Mol Ther Oncolytics. 2015;2:15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dzaye OD, Hu F, Derkow K, et al. . Glioma stem cells but not bulk glioma cells upregulate IL-6 secretion in microglia/brain macrophages via toll-like receptor 4 signaling. J Neuropathol Exp Neurol. 2016;75(5):429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oh T, Fakurnejad S, Sayegh ET, et al. . Immunocompetent murine models for the study of glioblastoma immunotherapy. J Transl Med. 2014;12:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Du H, Hirabayashi K, Ahn S, et al. . Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T-cells. Cancer Cell. 2019;35(2):221–237.e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilbertson RJ, Perry RH, Kelly PJ, Pearson AD, Lunec J. Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res. 1997;57(15):3272–3280. [PubMed] [Google Scholar]
- 25. Modak S, Kramer K, Gultekin SH, Guo HF, Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 2001;61(10):4048–4054. [PubMed] [Google Scholar]
- 26. Kawakami M, Kawakami K, Takahashi S, Abe M, Puri RK. Analysis of interleukin-13 receptor alpha2 expression in human pediatric brain tumors. Cancer. 2004;101(5):1036–1042. [DOI] [PubMed] [Google Scholar]
- 27. Smith C, Santi M, Rajan B, et al. . A novel role of HLA class I in the pathology of medulloblastoma. J Transl Med. 2009;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deng H, Zeng J, Zhang T, et al. . Histone H3.3K27M mobilizes multiple cancer/testis (CT) antigens in pediatric glioma. Mol Cancer Res. 2018;16(4):623–633. [DOI] [PubMed] [Google Scholar]
- 29. Pituch KC, Miska J, Krenciute G, et al. . Adoptive transfer of IL13Ralpha2-specific chimeric antigen receptor T-cells creates a pro-inflammatory environment in glioblastoma. Mol Ther. 2018;26(4):986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sampson JH, Choi BD, Sanchez-Perez L, et al. . EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res. 2014;20(4):972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jin L, Ge H, Long Y, et al. . CD70, a novel target of CAR-T-cell therapy for gliomas. Neuro Oncol. 2018;20(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murty S, Haile ST, Beinat C, et al. . Intravital imaging reveals synergistic effect of CAR T-cells and radiation therapy in a preclinical immunocompetent glioblastoma model. Oncoimmunology. 2020;9(1):1757360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nellan A, Rota C, Majzner R, et al. . Durable regression of medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J Immunother Cancer. 2018;6(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chheda ZS, Kohanbash G, Okada K, et al. . Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med. 2018;215(1):141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. . Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119(11):2644–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. . T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mehta GU, Malekzadeh P, Shelton T, et al. . Outcomes of adoptive cell transfer with tumor-infiltrating lymphocytes for metastatic melanoma patients with and without brain metastases. J Immunother. 2018;41(5):241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.