Abstract
We sought to characterize the relationship between alcohol consumption and sexual risk-taking in an aging population in rural South Africa. A cross-sectional analysis was conducted using baseline data from Health and Ageing in Africa: a Longitudinal Study of an INDEPTH Community (HAALSI) cohort. We elicited information on sexual risk behavior and self-reported frequency of alcohol consumption among 5,059 adults ≥ 40 years old.
Multivariable models showed that more frequent alcohol consumption is associated with a higher number of sexual partners (β: 1.38, p < .001) and greater odds of having sex for money (OR: 42.58, p < .001) in older adults in South Africa. Additionally, daily drinkers were more likely to have sex without a condom (OR: 2.67, p = .01).
Older adults who drank more alcohol were more likely to engage in sexual risk-taking. Behavioral interventions to reduce alcohol intake should be considered to reduce STI and HIV transmission.
Keywords: Sexual risk behavior, Alcohol, HIV, older adults
INTRODUCTION
The United Nations has declared a goal to end the worldwide HIV epidemic by 2030 [1]. One of the targets to achieve this goal is primary prevention of HIV infection. An important component of HIV prevention efforts is reducing high-risk sexual behavior that increases risk of to HIV infection [2].
Sexual risk-taking is widely recognized as an important risk factor for HIV transmission, and includes inconsistent condom use, having multiple partners and not discussing sexual risk before intercourse [3,4]. Evidence has shown that alcohol use is associated with these types of risk behaviors and increased HIV vulnerability, due to its disinhibiting effects and impaired judgement in those who overuse or abuse it [5,6]. Consequently, alcohol consumption decreases the ability to engage in sexual risk reduction activities [7]. For instance, people who drink alcohol are more likely to have sex without a condom compared to those who do not [8,9]. Moreover, alcohol intoxication can decrease a person’s ability to negotiate condom use prior to intercourse [4].
In addition to this, evidence has shown an indirect association between alcohol use and sexual risk-taking [10–12]. For instance, social venues that serve alcohol allow individuals to not only buy alcohol but also meet potential sexual partners [10,12]. These settings especially play a role in HIV transmission in South Africa. One example of such social venues is shebeens, which are informal community-based social gathering places in South Africa. Prior research has shown that almost over 90% of the people living in Cape Town reported having met new sexual partners in these shebeens, suggesting they may play an important part in HIV transmission [10].
As a result of the association between alcohol and sexual risk-taking, interventions to reduce alcohol use have been explored as a way to decrease transmission of all sexually transmitted infections (STI), including HIV [13,14]. Given these relationships, it is particularly important to understand the role of alcohol use in driving sexual behavior in regions of high HIV prevalence such as South Africa, which happens to also rank among the top countries in the world with respect to alcohol consumption [15,16]. Earlier literature showed that 33.1% of the South African population drinks alcohol [17]. In addition, approximately one third of the population report heavy, episodic drinking behavior [18]. Major drivers of reported alcohol misuse are the ubiquitous availability of inexpensive alcoholic beverages and their promotion through advertisements and sponsorships of the alcohol industry [19].
Earlier studies have shown consistent associations between alcohol consumption and high-risk sexual behavior in southern Africa [15,20]. While much of this research has focused on alcohol and sexual risk-taking in adolescents and young people, there is minimal evidence to date regarding the relationship between alcohol consumption and HIV vulnerability due to sexual risk-taking among aging populations. However, this older population is an important one given that existing literature has revealed high rates of alcohol consumption among men and women over 50 years old in rural settings [21]. Moreover, there is growing evidence that this population is engaging in sexual risk behaviors, and may underestimate their risk of STIs including HIV. This has been demonstrated by increasing rates of STIs among older adults in this region [22].
As such, this study sought to evaluate the relationship between alcohol consumption and high-risk sexual behavior in a large cohort of older adults (40 years and above) in rural South Africa. We hypothesized that alcohol consumption may be linked to an increase in high-risk sexual behavior in this population.
METHODS
Study population
This study is a cross-sectional analysis using baseline data from the survey “Health and Aging in Africa: A Longitudinal Study of INDEPTH community in South Africa” (HAALSI). HAALSI is a cohort of adults aged ≥ 40 that was conducted in the Agincourt sub-district of Mpumalanga province, South Africa [23]. The study is nested within a health and socio- demographic surveillance site (HDSS) that includes 116,000 people from 31 villages [24]. Based on predetermined inclusion criteria, this study ultimately surveyed 5,059 randomly selected men and women (n = 2,345, 46.3% men; n = 2,714, 53.7% women). Household interviews were completed between November 2014 and November 2015 and queried the demographics, health- and economic conditions of all individual participants.
Data collection
The questionnaires were developed in English and then translated into the local language, Shangaan. To ensure reliability, the translated answers were back translated [25]. The data was collected via a computer-assisted personal interview (CAPI) with trained, local fieldworkers. The interviews were conducted in Shangaan. The in-person interview included questions about sociodemographic factors, health status, health risk behaviors, and living conditions.
Measures
Alcohol consumption
Alcohol consumption was assessed by asking participants about the frequency with which they consumed at least one alcoholic beverage in the past 30 days. Alcohol consumption was subcategorized into daily drinking, 5–6 days per week, 1–4 days per week, 1–3 days per month, less than once per month and does not currently drink. “Does not currently drink” included participants who did not consume an alcoholic drink in the last 30 days. Quantity of alcohol consumption was not assessed during the interviews.
Sexual risk behavior
We explored several questions that assessed sexual risk behavior, including: (1) Do/did you use a condom with your most recent partner?; (2) Have you ever had sex in exchange for money?; (3) When having sex for money, did you us a condom?; (4) Have you ever had sex without a condom with someone HIV positive?; (5) How many different sex partners have you had in your lifetime?; (6) During the last 24 months, how many sexual partners have you had?.
With respect to the question “Do/did you use a condom with recent partner?”, participants only answered this question if they previously reported that they had a relationship in the last 24 months. All other sexual risk behavior questions were asked to all participants.
The responses to each of the sexual risk behavior questions were coded into binary outcomes that indicated harmful high-risk sexual behavior, except for the question regarding the number of sex partners in the last 24 months and lifetime, which was given in the form of a number. Participants who answered “I do not remember” were excluded from the analysis for that particular question. For the question about condom use (Question 1), the responses ‘always’ and ‘most of the time’ were categorized as high-risk sexual behavior, and “sometimes” and “never” were treated as low risk behavior.
HIV infection
HIV infection in the participants was determined by dried blood spot (DBS) HIV antibody testing [26]. Two categories were defined in the analysis; people without HIV (HIV-) and people with HIV (HIV+).
Demographic variables
Covariates included in the analyses included categorical age (40–49; 50–59; 60–69; 70–79; ≥ 80 years old), sex of the participant (male or female), country of birth (South Africa or Mozambique/other), education (no formal education; some primary (1–7 years); some secondary (8–11 years); secondary or more (≥ 12 years)), wealth quintiles derived from an asset index and marital status (never married; separated/divorced; widowed; currently married) [23].
Missing data
To account for missing data, we performed a complete case regression analysis in which participants were only included if they had complete data regarding the sexual risk behavior of interest and relevant covariates. Missing data are shown in Supplementary Appendix A.
Statistical analysis
The prevalence of each sexual risk behavior was calculated overall and by the frequency of alcohol use. Proportions were compared using a chi-squared test. In addition, multivariable logistic regression models were performed to investigate the adjusted association between alcohol use and high-risk sexual behaviors. A multivariable linear model was used to fit the number of lifetime sexual partners and sexual partners in the last 24 months. All models were adjusted for age, sex, education, marital status and household wealth.
The analyses were conducted using IBM SPSS Statistics 25.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Ethics Statement
Ethical approval for this study was granted by the University of Witwatersrand (#M141159), the Harvard T.H. Chan School of Public Health (#13–1608), and the Mpumalanga Provincial Research and Ethics Committee.
RESULTS
Baseline characteristics
Table I shows the characteristics of all participants. Almost half of the participants were men (46.3%), most (69.8%) were born in South Africa, 45.7% did not have a formal education, and 23.0% were living with HIV. In addition, 23.2% of the participants consumed alcohol in the last 30 days, of which 26.0% reported drinking alcohol at least five days per week. There were significant bivariate differences in alcohol consumption by sex (χ2df=5 = 638.5, p < .001), age (χ2df=20 = 45.8, p < .001), country of birth (χ2df=5 = 54.7, p < .001), education (χ2df=15 = 46.4, p < .001), marital status (χ2df=15 = 93.9, p < .001) and wealth categories (χ2df=20 = 176.5, p < .001) (see Table I). However, there was no difference in the frequency of alcohol consumption by HIV status (χ2df=5 = 0.7, p = 0.98).
Table I.
Participant Characteristics per Alcohol Consumption Category
| Daily (n=167) n (%) | 5–6 days per week (n=137) n (%) | 1–4 days per week (n=315) n (%) | 1–3 days per month (n=317) n (%) | Less than once a month (n=234) n (%) | Does not currently drink (n=3885) n (%) | Total (n=5055) n (%) | χ2 (df) | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| Male | 140 (83.8) | 111 (81.0) | 268 (85.1) | 244 (77.0) | 148 (6.2) | 1431 (36.8) | 2342 (46.3) | 638.5 (5) | <0.001** |
| Female | 27 (16.2) | 26 (19.0) | 47 (14.9) | 73 (23.0) | 86 (36.8) | 2454 (63.2) | 2713 (53.7) | ||
| Age groups | |||||||||
| 40–49 | 23 (13.8) | 24 (17.5) | 66 (21.0) | 52 (16.4) | 37 (15.8) | 715 (18.4) | 917 (18.1) | 45.8 (20) | <0.001** |
| 50–59 | 44 (26.3) | 34 (24.8) | 84 (26.7) | 77 (24.3) | 57 (24.4) | 1112 (28.6) | 1409 (27.9) | ||
| 60–69 | 62 (37.1) | 39 (28.5) | 83 (26.3) | 80 (25.2) | 52 (22.2) | 998 (25.4) | 1304 (25.8) | ||
| 70–79 | 27 (16.2) | 32 (23.4) | 54 (17.1) | 72 (22.7) | 59 (25.2) | 634 (16.3) | 878 (17.4) | ||
| ≥80 | 11 (6.6) | 8 (5.8) | 28 (8.9) | 36 (11.4) | 29 (12.4) | 436 (11.2) | 548 (10.8) | ||
| Country of birth | |||||||||
| South Africa | 95 (56.9) | 67 (48.9) | 212 (67.3) | 213 (67.2) | 154 (65.8) | 2786 (71.8) | 3527 (69.8) | 54.7 (5) | <0.001** |
| Mozambique or other | 72 (43.1) | 70 (51.1) | 103 (32.7) | 104 (32.8) | 80 (34.2) | 1094 (28.2) | 1523 (30.2) | ||
| Education | |||||||||
| No formal education | 94 (56.3) | 87 (63.5) | 148 (47.0) | 155 (49.2) | 118 (50.4) | 1702 (44.0) | 2304 (45.7) | 46.4 (15) | <0.001** |
| Some primary (1–7 years) | 53 (31.7) | 30 (21.9) | 112 (35.6) | 104 (33.0) | 78 (33.3) | 1339 (34.6) | 1716 (34.1) | ||
| Some secondary (8–11 years) | 13 (7.8) | 13 (9.5) | 34 (10.8) | 34 (10.8) | 28 (12.0) | 451 (11.7) | 573 (11.4) | ||
| Secondary or more (12+ years) | 7 (4.2) | 7 (5.1) | 21 (6.7) | 22 (7.0) | 10 (4.3) | 378 (9.8) | 445 (8.8) | ||
| Marital status | |||||||||
| Never married | 17 (10.2) | 14 (10.2) | 23 (7.3) | 27 (8.5) | 13 (5.6) | 196 (5.1) | 290 (5.7) | 93.9 (15) | <0.001** |
| Separated/ divorced | 29 (17.4) | 25 (18.2) | 52 (16.5) | 37 (11.7) | 32 (13.7) | 474 (12.2) | 649 (12.8) | ||
| Widowed | 33 (19.8) | 28 (20.4) | 53 (16.8) | 71 (22.4) | 54 (23.1) | 1299 (33.5) | 1538 (30.4) | ||
| Currently Married | 88 (52.7) | 70 (51.1) | 187 (59.4) | 182 (57.4) | 135 (57.7) | 1912 (49.3) | 2574 (51.0) | ||
| HIV status | |||||||||
| HIV- | 113 (75.8) | 98 (79.0) | 210 (75.8) | 223 (77.2) | 161 (78.2) | 2703 (77.0) | 3508 (77.0) | 0.7 (5) | 0.98 |
| HIV+ | 36 (24.2) | 26 (21.0) | 67 (24.2) | 66 (22.8) | 46 (22.2) | 807 (23.0) | 1048 (23.0) | ||
| Wealth index status | |||||||||
| Quantile 1 | 75 (44.9) | 50 (36.5) | 93 (29.5) | 76 (24.0) | 55 (23.5) | 694 (17.9) | 1043 (20.6) | 176.5 (20) | <0.001** |
| Quantile 2 | 39 (23.4) | 36 (26.3) | 58 (18.4) | 78 (24.6) | 48 (20.5) | 741 (19.1) | 1000 (19.8) | ||
| Quantile 3 | 27 (16.2) | 19 (13.9) | 65 (20.6) | 58 (18.3) | 48 (20.5) | 774 (19.9) | 991 (19.6) | ||
| Quantile 4 | 10 (6.0) | 19 (13.9) | 61 (19.4) | 51 (16.1) | 55 (23.5) | 811 (20.9) | 1007 (19.9) | ||
| Quantile 5 | 16 (9.6) | 13 (9.5) | 38 (12.1) | 54 (17.0) | 28 (12.0) | 865 (22.3) | 1014 (20.1) | ||
p < .05.
p < .01, χ2 tests were used to compare proportions
Alcohol consumption and sexual risk behavior
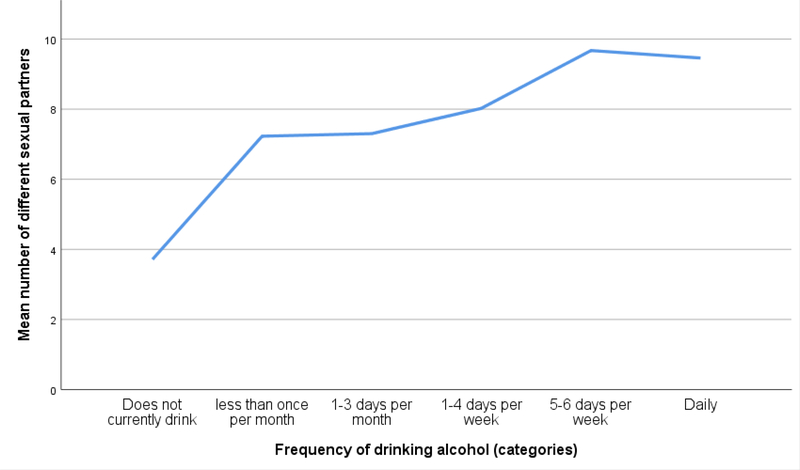
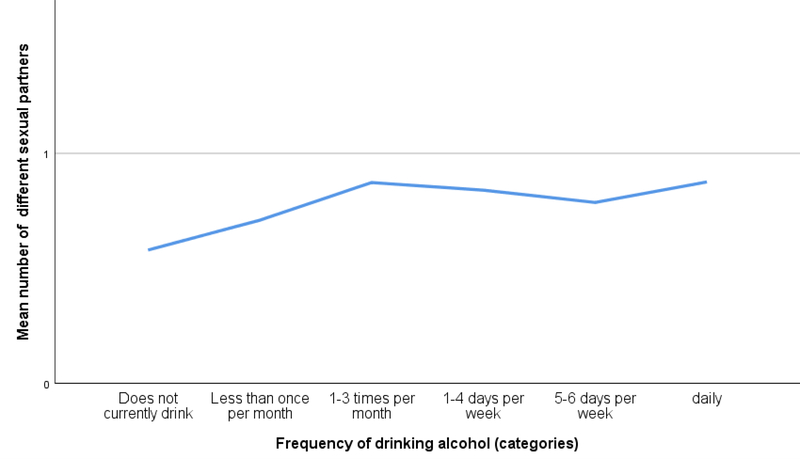
More frequent alcohol consumption was associated with a greater number of sexual partners over the life course and in the past 24 months, as shown in Figure 1 and 2. Controlling for background characteristics, there was a significant association between the number of sexual partners over the lifetime and frequency of alcohol consumption (β: 1.38 95% CI: 1.21–1.55, p < .001, see Table II). Moreover, this association was also seen between the number of sexual partners in the past 24 months and frequency of alcohol consumption (β: 0.07 95% CI: 0.06–0.09, p < .001, see Table II). In addition, linear regression models showed that women had a fewer number of sexual partners during their lifetime and in the past 24 months as compared to men (β: −6.06 95% CI: −6.52- −5.67, p < .001; β: −0.50 95% CI: −0.54- −0.47, p < .001). Greater educational attainment was also associated with a greater number of lifetime sexual partners and sexual partners in the last 24 months (β: 0.58 95% CI: 0.35– 0.82, p < .001; β: 0.12 95% CI: 0.10– 0.14, p < .001). Furthermore, younger age and a greater wealth were associated with a greater number of sexual partners in the last 24 months (β: −0.13 95% CI: −0.14- −0.11, p < .001; β: 0.04 95% CI: 0.02–0.05, p < .001).
Figure 1.
Number of sexual partners over the lifetime per category of alcohol use frequency
Figure 2.
Number of sexual partners in the last 24 months per category of alcohol use frequency.
Table II.
Multivariable regression to identify the association between alcohol usage and sexual risk behavior
| Multiple logistic regression analysis | Linear regression analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex without condom with recent partner (n=2740) | Sex without condom with someone HIV positive (n=4804) | Sex for Money (n=4872) | Number of sex partners in lifetime (n=4494) | Number of sex partners in the last 24 months (n=4882) | ||||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | Β (95% CI) | p-value | Β (95% CI) | p-value | |
| Alcohol usage | 1.38 (1.21–1.55) | <0.001** | 0.07 (0.06–0.09) | <0.001** | ||||||
| Does not currently drink | REF | REF | REF | - | - | - | - | |||
| Less than once a month. | 1.13 (0.68–1.87) | 0.64 | 1.15 (0.35–3.79) | 0.82 | 0.00 (0.00) | 1.00 | - | - | - | - |
| 1–3 days per month | 1.82 (1.13–2.94) | 0.01* | 2.52 (1.17–5.42) | 0.02* | 12.05 (2.93–49.62) | <0.001** | - | - | - | - |
| 1–4 days per week | 1.25 (0.82–1.91) | 0.30 | 3.21 (1.61–6.44) | <0.001** | 9.61 (1.97–46.97) | 0.04* | - | - | - | - |
| 5–6 days per week | 1.48 (0.75–2.92) | 0.25 | 1.16 (0.27–5.04) | 0.84 | 12.83 (2.08–79.10) | 0.01* | - | - | - | - |
| Daily drinking | 2.67 (1.27–5.58) | 0.01* | 1.72 (0.58–5.11) | 0.22 | 42.58 (10.48–173.02) | <0.001** | - | - | - | - |
| Sex | −6.06 (−6.52– −5.67) | <0.001** | −0.50 (−0.54– −0.47) | <0.001** | ||||||
| Male | REF | REF | REF | - | - | - | - | |||
| Female | 1.40 (1.08–1.80) | 0.01* | 0.80 (0.46–1.39) | 0.43 | 3.45 (1.15–10.30) | 0.03* | - | - | - | - |
| Age | −0.11 (−0.18–0.20) | 0.24 | −0.13 (−0.14– −0.11) | <0.001** | ||||||
| 40–49 | REF | REF | REF | - | - | - | - | |||
| 50–59 | 1.41 (1.07–1.86) | 0.01* | 1.14 (0.64–2.05) | 0.64 | 0.52 (0.15–1.77) | 0.29 | - | - | - | - |
| 60–69 | 1.98 (1.39– 2.83) | <0.001** | 0.52 (0.25–1.09) | 0.08 | 0.48 (0.12–1.87) | 0.29 | - | - | - | - |
| 70–79 | 3.37 (2.03–5.60) | <0.001** | 0.25 (0.10–0.73) | 0.01* | 1.33 (0.34–5.23) | 0.68 | - | - | - | - |
| ≥80 | 4.37 (1.91–9.99) | <0.001** | 0.18 (0.04–0.83) | 0.03* | 0.00 (0.00) | 0.99 | - | - | - | - |
| Education | 0.58 (0.35–0.82) | <0.001** | 0.12 (0.10–0.14) | <0.001** | ||||||
| No formal education | REF | REF | REF | - | - | - | - | |||
| Some primary | 1.00 (0.75–1.33) | 0.98 | 1.46 (0.83–2.56) | 0.19 | 1.30 (0.47–3.60) | 0.62 | - | - | - | - |
| Some secondary | 0.79 (0.56–1.14) | 0.21 | 1.42 (0.66–3.10) | 0.37 | 0.92 (0.18–4.79) | 0.92 | - | - | - | - |
| Secondary or more | 0.94 (0.62–1.43) | 0.76 | 1.31 (0.51–3.40) | 0.57 | 0.91 (0.09–0.17) | 0.94 | - | - | - | - |
| Marital status | 0.17 (−0.09–0.43) | 0.21 | 0.30 (0.28–0.32) | <0.001** | ||||||
| Never married | REF | REF | REF | - | - | - | - | |||
| Separated/ divorced | 0.73 (0.45–1.20) | 0.21 | 2.19 (0.72–6.69) | 0.17 | 0.50 (0.14–1.79) | 0.29 | - | - | - | - |
| Widowed | 0.81 (0.47–1.39) | 0.44 | 1.94 (0.61–6.15) | 0.26 | 0.08 (0.01–0.48) | 0.01* | - | - | - | - |
| Currently Married | 3.44 (2.22–5.32) | <0.001** | 1.43 (0.49–4.15) | 0.51 | 0.25 (0.07–0.85) | 0.03* | - | - | - | - |
| Wealth index status | 0.05 (−0.11–0.22) | 0.51 | 0.04 (0.02–0.05) | <0.001** | ||||||
| Quantile 1 | REF | REF | REF | - | - | - | - | |||
| Quantile 2 | 0.65 (0.45–0.93) | 0.02* | 0.89 (0.46–1.71) | 0.71 | 0.63 (0.21–1.92) | 0.42 | - | - | - | - |
| Quantile 3 | 0.73 (0.49–1.07) | 0.10 | 0.78 (0.39–1.55) | 0.47 | 0.32 (0.07–1.52) | 0.15 | - | - | - | - |
| Quantile 4 | 0.86 (0.58–1.26) | 0.42 | 0.56 (0.26–1.21) | 0.14 | 0.40 (0.08–1.95) | 0.26 | - | - | - | - |
| Quantile 5 | 0.88 (0.59–1.33) | 0.55 | 0.66 (0.30–1.46) | 0.31 | 0.23 (0.03–2.00) | 0.18 | - | - | - | - |
p < .05.
p < .01.
Multivariable logistic regression models of alcohol consumption and sexual risk behavior showed a greater odds of having sex for money for participants that consumed alcohol more frequently (see Table II and in Supplementary Appendix B). Though few people in the cohort reported having sex for money (0.4%), daily drinkers had 46 times greater odds of reporting this behavior than those who did not consume alcohol (OR: 42.58 95% CI: 10.48–173.02, p < .001). Moreover, women were more likely than men to have exchanged sex for money, controlling for other risk factors (OR: 3.45 95% CI: 1.15–10.30, p = .03).
This study also found that alcohol consumption is associated with having sex without condom for people with HIV(PWH) (Table II and Supplementary Appendix B). The participants who consumed alcohol 1–3 days per month (OR: 2.52 95% CI: 1.17–5.42, p = .02) and 1–4 days per week (OR: 3.21 95% CI: 1.61–6.44, p < .001) had a greater odds of having sex without a condom with PWH than those who drank no alcohol. Moreover, older participants were less likely to engage in this risk behavior (≥ 80 years old; OR: 0.18 95% CI: 0.04–0.83, p = .03). Among those who reported sex without a condom, only the participants who reported drinking 1–3 days per month (OR: 1.82 95% CI: 1.13–2.94, p = .01) or daily drinkers (OR: 2.67 95% CI: 1.27–5.58, p = .01) had a greater odds of ever having sex without condom compared to those who did not currently drink (Table II). In addition, older participants were more likely to have ever had sex without a condom with their most recent partner compared to younger participants (>80 years old; OR: 4.37 95% CI: 1.91–9.99, p < .001) as did those who were currently married (OR: 3.44 95% CI: 2.22–5.32, p < .001).
DISCUSSION
In this study, we found that more frequent consumption of alcohol was associated with several key high-risk sexual behaviors in a population of older adults living in rural South Africa. Frequent alcohol use was most strongly associated with the number of sex partners, having sex without a condom and having sex for money. These findings are important and novel because this study was conducted among older adults in a region of high HIV prevalence, an often overlooked population in terms of HIV vulnerability. These findings suggest that the reduction of alcohol use may be a potential target for behavioral interventions that aim to reduce sexual risk-taking in older age groups.
According to prior research, South Africa ranks among the countries with the highest consumption of alcohol in the world [27,28]. In this study, 23% of the participants reported current alcohol use. This prevalence is lower than what has been found in previous studies, which have reported that as many as 33.1% of people in South Africa are current alcohol users [17]. This difference might be explained by the fact that this study was conducted in an older population or may have to do with other local differences in alcohol use patterns.
Furthermore, the association between alcohol consumption and sexual risk-taking is consistent with findings from previous studies, most of which have been conducted in younger populations [15,29,30]. In a prior study that explored the relationship between alcohol use and sexual behavior in South Africa, there was a consistent association observed between quantity of alcohol consumption and increased sexual risk-taking [15,30]. Our study adds to these findings and confirms that these relationships are also present in people aged 40 and older. In addition, earlier findings showed that people in South Africa had a higher risk of acquiring HIV when they consume more alcohol [29]. There are several explanations that have been proposed to explain this association, such as the impact of alcohol on decision making that can lead to sexual risk-taking and the biological effects of alcohol consumption on HIV transmission [31]. However, literature has shown that this association is strictly correlated to quantity of alcohol and not frequency of alcohol intake [15,29]. Our study found no differences in the frequency of alcohol use by HIV status, though we did not measure quantity consumed, as in prior studies.
This study showed that sexual risk behavior is also prevalent in this older population in rural South Africa and is linked to drinking behavior. Similar to other studies, older participants were less likely to have sex with a condom compared to younger participants [32–34]. This could be explained by the fact that older adults may lack awareness of the risk factors for acquiring HIV, especially given the relatively little HIV prevention education that targets older populations [35,36]. A study in sub-Saharan Africa found that people older than 50 had lower levels of knowledge regarding HIV prevention compared to younger adults [37]. This indicates that older adults are less well-equipped to make informed decisions about their own sexual risk behavior. However, age seemed to be protective for having sex with someone known to be HIV positive.
This study also found gender differences in sexual risk behavior. Women were more likely to have sex without a condom and have sex for money, whereas men were more likely to have sex without a condom when drunk. This is an interesting finding, since women engage in different kinds of sexual risk behaviors than men and because we found that women drink less than men, which is also consistent with prior research [20,38,39]. The finding that women are more likely to have sex without a condom may also be explained by gender power imbalances that can significantly affect women’s ability to decide to use a condom. Previously literature from South Africa has shown that these gender dynamics around condom use are relevant to STI and HIV vulnerability [40,41].
As mentioned above, our findings suggest that interventions to reduce alcohol consumption may be effective to reduce sexual risk-taking [42]. Previous research has begun to explore the effectiveness of various alcohol reduction interventions in older adults (≥ 55 years old). These studies have shown that intensive interventions may be more effective in this population, including physician advice, personalized feedback, educational materials, and follow-up with a physician or other healthcare provider [43–45]. Furthermore, the use of alcohol reduction interventions has been evaluated in sub-Saharan Africa but these types of interventions have not been widely implemented [22]. These studies have also shown that individual models, which have been explored more commonly in high-income settings, do not seem to be fully applicable to collectivist cultures. As such, multi-level interventions may be more effective than individual-level interventions to reduce alcohol use in some communities such as the one in which this study is located [46]. In addition, there is evidence that multi-faceted policy approaches, such as increasing alcohol prices and reducing marketing and availability of alcoholic beverages, may decrease alcohol consumption and in turn reduce high-risk sexual behavior [47–49]. For instance, a study showed that alcohol tax increase in Illinois led to a decrease of 21% of state-wide rates of gonorrhea [48]. A second study showed that the increase in the drinking age in the United States reduced STI rates in youth [49]. Therefore, the evaluation of long-term, multilevel interventions to reduce alcohol consumption in older populations in South Africa is of great importance to determine their effectiveness in reducing sexual risk-taking and other alcohol related harms.
There are several limitations in this study. First of all, alcohol consumption and sexual risk behavior were both self-reported. Consequently, this could lead to recall or reporting bias which could affect the results. In particular, the themes of both alcohol use and sexual risk behavior could be perceived as shameful or stigmatized, which may further exacerbate under-reporting of these behaviors. Secondly, the data used in this study described frequency of alcohol consumption but did not address quantity or patterns of binge drinking. We were thus unable to explore associations between more infrequent but still harmful drinking behavior, namely that episodic binge drinking is linked to sexual risk-taking and HIV vulnerability [7]. Future studies should include quantity of alcohol consumed in addition to frequency. Lastly, the study was cross sectional and thus we were unable to establish causality or examine these relationships over time.
In conclusion, we show that there is a strong association between alcohol consumption and high-risk sexual behavior in an aging population in rural South Africa with high HIV prevalence. Interventions to reduce alcohol intake have the potential to decrease risk of both STIs and HIV acquisition in South Africa.
Supplementary Material
Acknowledgments
Funding
This work was funded by a Grant from the National Institute on Aging of the National Institutes of Health (P01 AG041710), HAALSI – Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in South Africa). The funder took no part in the study design, in the data-collection, analysis, interpretation of the results and writing the research article. The HAALSI study is located within the Agincourt Health and socio-Demographic Surveillance System site, funded by the University of the Witwatersrand and Medical Research Council, South Africa, and the Wellcome Trust, UK (058893/Z/99/A; 069683/Z/02/Z; 085477/Z/08/Z; 085477/B/08/Z). TB was supported by the Alexander von Humboldt Foundation through the Alexander von Humboldt Professor award, funded by the German Federal Ministry of Education and Research; the Wellcome Trust; and from NICHD of NIH (R01-HD084233), NIA of NIH (P01-AG041710), NIAID of NIH (R01-AI124389 and R01-AI112339) as well as FIC of NIH (D43-TW009775). JMG was supported by Grant Number T32 AI007433 from the National Institute of Allergy and Infectious Diseases. The contents of this research are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Competing interests
The authors have no conflicts of interests to declare.
REFERENCES
- 1.Jones J, Sullivan PS, Curran JW. Progress in the HIV epidemic: Identifying goals and measuring success. PLOS Med. Public Library of Science; 2019;16:e1002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGowan JP, Shah SS, Ganea CE, Blum S, Ernst JA, Irwin KL, et al. Risk Behavior for Transmission of Human Immunodeficiency Virus (HIV) among HIV‐Seropositive Individuals in an Urban Setting. Clin Infect Dis. Oxford University Press; 2004;38:122–7. [DOI] [PubMed] [Google Scholar]
- 3.Thato R, Daengsaard E, Sukrak N. The Effect of a Brief HIV Prevention Program on Risk Reduction Behaviors Among Thai Men Diagnosed With Sexually Transmitted Infections. Asian Nurs Res (Korean Soc Nurs Sci). Elsevier; 2018;12:265–72. [DOI] [PubMed] [Google Scholar]
- 4.Cho YH, Span SA. The effect of alcohol on sexual risk-taking among young men and women. Addict Behav. Pergamon; 2010;35:779–85. [DOI] [PubMed] [Google Scholar]
- 5.Fisher JC, Bang H, Kapiga SH. The association between HIV infection and alcohol use: a systematic review and meta-analysis of African studies. Sex Transm Dis. 2007;34:856–63. [DOI] [PubMed] [Google Scholar]
- 6.Palepu A, Raj A, Horton NJ, Tibbetts N, Meli S, Samet JH. Substance abuse treatment and risk behaviors among HIV-infected persons with alcohol problems. J Subst Abuse Treat. Pergamon; 2005;28:3–9. [DOI] [PubMed] [Google Scholar]
- 7.Brown JL, Gause NK, Northern N. The Association between Alcohol and Sexual Risk Behaviors among College Students: A Review. Curr Addict reports. NIH Public Access; 2016;3:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbey A, Saenz C, Buck PO. The cumulative effects of acute alcohol consumption, individual differences and situational perceptions on sexual decision making. J. Stud. Alcohol Alcohol Research Documentation Inc.; 2005. p. 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis KC, Hendershot CS, George WH, Norris J, Heiman JR. Alcohol’s effects on sexual decision making: An integration of alcohol myopia and individual differences. J Stud Alcohol Drugs. Alcohol Research Documentation Inc.; 2007;68:843–51. [DOI] [PubMed] [Google Scholar]
- 10.Scott-Sheldon LAJ, Carey KB, Carey MP, Cain D, Simbayi LC, Kalichman SC. Alcohol use disorder, contexts of alcohol use, and the risk of HIV transmission among South African male patrons of shebeens. Drug Alcohol Depend. Elsevier Ireland Ltd; 2014;140:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn JA, Woolf-King SE, Muyindike W. Adding fuel to the fire: Alcohol’s effect on the HIV epidemic in sub-saharan africa. Curr HIV/AIDS Rep. 2011;8:172–80. [DOI] [PubMed] [Google Scholar]
- 12.Watt MH, Aunon FM, Skinner D, Sikkema KJ, Kalichman SC, Pieterse D. “Because he has bought for her, he wants to sleep with her”: Alcohol as a currency for sexual exchange in South African drinking venues. Soc Sci Med. Soc Sci Med; 2012;74:1005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bountress KE, Metzger IW, Maples-Keller JL, Gilmore AK. Reducing sexual risk behaviors: secondary analyses from a randomized controlled trial of a brief web-based alcohol intervention for underage, heavy episodic drinking college women. Addict Res Theory. NIH Public Access; 2017;25:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan C-W, Scott-Sheldon LAJ, Carey KB, Johnson BT, Carey MP. Alcohol and sexual risk reduction interventions among people living in Russia: a systematic review and meta-analysis. AIDS Behav. NIH Public Access; 2014;18:1835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol Use and Sexual Risks for HIV/AIDS in Sub-Saharan Africa: Systematic Review of Empirical Findings. Prev Sci. Kluwer Academic Publishers-Plenum Publishers; 2007;8:141–51. [DOI] [PubMed] [Google Scholar]
- 16.Organization WH, Department WHOSA, of Mental Health WHOD, Abuse S. Global status report on alcohol 2004. World Health Organization; 2004. [Google Scholar]
- 17.Vellios NG, Van Walbeek CP. Self-reported alcohol use and binge drinking in South Africa: Evidence from the National Income Dynamics Study, 2014 – 2015. S Afr Med J. 2017;108:33–9. [DOI] [PubMed] [Google Scholar]
- 18.Bello B, Moultrie H, Somji A, Chersich MF, Watts C, Delany-Moretlwe S. Alcohol use and sexual risk behaviour among men and women in inner-city Johannesburg, South Africa. BMC Public Health. BioMed Central Ltd.; 2017;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letsela L, Weiner R, Gafos M, Fritz K. Alcohol Availability, Marketing, and Sexual Health Risk Amongst Urban and Rural Youth in South Africa. AIDS Behav. Springer New York LLC; 2019;23:175–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bello B, Moultrie H, Somji A, Chersich MF, Watts C, Delany-Moretlwe S. Alcohol use and sexual risk behaviour among men and women in inner-city Johannesburg, South Africa. BMC Public Health. BioMed Central; 2017;17:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Negin J, Cumming R, de Ramirez SS, Abimbola S, Sachs SE. Risk factors for non-communicable diseases among older adults in rural Africa. Trop Med Int Heal. John Wiley & Sons, Ltd; 2011;16:640–6. [DOI] [PubMed] [Google Scholar]
- 22.Syme ML, Cohn TJ, Barnack-Tavlaris J. A Comparison of Actual and Perceived Sexual Risk Among Older Adults. J Sex Res. Routledge; 2017;54:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gómez-Olivé FX, Montana L, Wagner RG, Kabudula CW, Rohr JK, Kahn K, et al. Cohort Profile: Health and Ageing in Africa: A Longitudinal Study of an INDEPTH Community in South Africa (HAALSI). Int J Epidemiol. Oxford University Press; 2018;47:689–690j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn K, Collinson MA, Gomez-Olive FX, Mokoena O, Twine R, Mee P, et al. Profile: Agincourt Health and Socio-demographic Surveillance System. Int J Epidemiol. 2012;41:988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.HAALSI Data | HAALSI [Internet]. [cited 2019 Mar 18]. Available from: https://haalsi.org/data
- 26.Manne-Goehler J, Montana L, Gómez-Olivé FX, Rohr J, Harling G, Wagner RG, et al. The ART Advantage: Health Care Utilization for Diabetes and Hypertension in Rural South Africa. J Acquir Immune Defic Syndr. NIH Public Access; 2017;75:561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawana N, Booysen F. Decomposing socioeconomic inequalities in alcohol use by men living in South African urban informal settlements. BMC Public Health. BioMed Central; 2018;18:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vellios NG, Van Walbeek CP. Self-reported alcohol use and binge drinking in South Africa: Evidence from the National Income Dynamics Study, 2014 – 2015. S Afr Med J. S Afr Med J; 2017;108:33–9. [DOI] [PubMed] [Google Scholar]
- 29.Campbell C, Williams B, Gilgen D. Is social capital a useful conceptual tool for exploring community level influences on HIV infection? An exploratory case study from South Africa. AIDS Care. Taylor & Francis Group; 2002;14:41–54. [DOI] [PubMed] [Google Scholar]
- 30.Morojele NK, Kachieng’a MA, Nkoko MA, Moshia KM, Mokoko E, Parry CDH, et al. Perceived effects of alcohol use on sexual encounters among adults in South Africa. Afr J Drug Alcohol Stud. 2004;3:1–20. [Google Scholar]
- 31.Rehm J, Probst C, Shield KD, Shuper PA. Does alcohol use have a causal effect on HIV incidence and disease progression? A review of the literature and a modeling strategy for quantifying the effect. Popul. Health Metr BioMed Central Ltd.; 2017. p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman E, Anglewicz P. HIV prevalence and sexual behaviour at older ages in rural Malawi. Int J STD AIDS. NIH Public Access; 2012;23:490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peltzer K, Setswe G, Setswe G, Ramlagan S, Weiss SM, Rodriguez VJ, et al. Sexual risk behaviour among hiv-infected women in the first twelve months after delivery in south africa. J Psychol Africa. Taylor and Francis Ltd.; 2018;28:330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gott CM. Sexual activity and risk-taking in later life. Heal Soc Care Community. John Wiley & Sons, Ltd; 2001;9:72–8. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg MS, Gómez-Olivé FX, Rohr JK, Houle BC, Kabudula CW, Wagner RG, et al. Sexual Behaviors and HIV Status: A Population-Based Study among Older Adults in Rural South Africa. J Acquir Immune Defic Syndr. Lippincott Williams and Wilkins; 2017;74:e9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Negin J, Nemser B, Cumming R, Lelerai E, Amor Y Ben, Pronyk P. HIV attitudes, awareness and testing among older adults in Africa. AIDS Behav. AIDS Behav; 2012;16:63–8. [DOI] [PubMed] [Google Scholar]
- 37.Odimegwu CO, Mutanda N. Covariates of high-risk sexual behaviour of men aged 50 years and above in sub-Saharan Africa. Sahara J. Routledge; 2017;14:162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parry CDH, Plüddemann A, Steyn K, Bradshaw D, Norman R, Laubscher R. Alcohol use in South Africa: Findings from the first demographic and health survey (1998). J. Stud. Alcohol Alcohol Research Documentation Inc.; 2005. p. 91–7. [DOI] [PubMed] [Google Scholar]
- 39.Bello B, Moultrie H, Somji A, Chersich MF, Watts C, Delany-Moretlwe S. Alcohol use and sexual risk behaviour among men and women in inner-city Johannesburg, South Africa. BMC Public Health. BioMed Central Ltd.; 2017;17:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langen TT. Gender power imbalance on women’s capacity to negotiate self-protection against HIV/AIDS in Botswana and South Africa. Afr Health Sci. Makerere University Medical School; 2005;5:188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teitelman AM, Jemmott JB, Bellamy SL, Icard LD, O’Leary A, Anita Heeren G, et al. Partner violence, power, and gender differences in south african adolescents’ HIV/sexually transmitted infections risk Behaviors. Heal Psychol. American Psychological Association Inc.; 2016;35:751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaner EFS, Dickinson HO, Beyer FR, Campbell F, Schlesinger C, Heather N, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. John Wiley & Sons, Ltd; 2007; [DOI] [PubMed] [Google Scholar]
- 43.Kelly S, Olanrewaju O, Cowan A, Brayne C, Lafortune L. Interventions to prevent and reduce excessive alcohol consumption in older people: a systematic review and meta-analysis. Age Ageing. Oxford University Press; 2018;47:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schonfeld L, Klne-Kallimani BL, Duchene DM, Etheridge RL, Herrera JR, Barry KL, et al. Screening and brief intervention for substance misuse among older adults: The Florida BRITE project. Am J Public Health. Am J Public Health; 2010;100:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleming MF, Manwell LB, Barry KL, Adams W, Stauffacher EA. Brief Physician Advice for Alcohol Problems in Older Adults: A Randomized Community-Based Trial. J Fam Pract. J Fam Pract; 1999;48:378–84. [PubMed] [Google Scholar]
- 46.Carrasco MA, Esser MB, Sparks A, Kaufman MR. HIV-Alcohol Risk Reduction Interventions in Sub-Saharan Africa: A Systematic Review of the Literature and Recommendations for a Way Forward. AIDS Behav. Springer US; 2016;20:484–503. [DOI] [PubMed] [Google Scholar]
- 47.WHO | Global strategy to reduce harmful use of alcohol. WHO. World Health Organization; 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staras SAS, Livingston MD, Christou AM, Jernigan DH, Wagenaar AC. Heterogeneous population effects of an alcohol excise tax increase on sexually transmitted infections morbidity. Addiction. 2014;109:904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chesson H, Harrison P, Kassler WJ. Sex under the influence: the effect of alcohol policy on sexually transmitted disease rates in the United States. J Law Econ. 2000;43:215–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.