Abstract
Peripheral artery disease (PAD) is a common condition with increasing prevalence domestically and worldwide. Patients with PAD have a poor prognosis, as PAD is associated with high rates of myocardial infarction, ischemic stroke, and cardiovascular disease death. The primary symptom of PAD, claudication, significantly reduces quality of life and functional status and is associated with depression.
In addition to several advances in medications for PAD over the last decade, endovascular device therapy has seen a significant breakthrough in the form of paclitaxel-coated devices (PCDs), which significantly reduce rates of restenosis relative to non-PCDs, a finding which has been demonstrated in numerous randomized clinical trials. After their introduction to the market in 2012 (paclitaxel-eluting stents) and 2014 (paclitaxel-coated balloons) their use surged as they replaced non-PCDs and were designated the first-line endovascular therapy by society guidelines.
This trend was abruptly reversed, however, after a meta-analysis of summary-level data was published in December of 2018 that reported an elevated mortality associated with PCDs compared with non-PCDs 2–5 years after treatment. This meta-analysis has been criticized for considerable methodological flaws. The Food and Drug Administration conducted a review and concluded that insufficient data existed to make a definitive statement regarding the safety of PCDs. They called for restriction of the use of PCDs to the highest-risk patient populations. At the same time, the FDA deemed pursuing new RCTs to better evaluate PCDs unfeasible due to the high numbers of patients and long follow-up time that would be required.
In this setting, real-world data emerged as a powerful source of information for the evaluation of PCDs. Real-world data offers advantages over randomized-controlled trials including expeditious access to and analysis of data and the availability of large numbers of patients. Several retrospective observational studies demonstrate no difference in long-term all-cause mortality in patients treated with PCDs relative to those treated with non-PCDs. This paclitaxel controversy has illustrated the critical role that real-world data is assuming in long-term safety monitoring of medical devices.
Keywords: peripheral artery disease, endovascular revascularization, paclitaxel-coated devices, real-world data
Introduction
Peripheral artery disease (PAD) is a common disease in the United States and worldwide.1,2 It has significant negative consequences for patients including local effects on limbs, such as pain and tissue loss, and is an indicator of high mortality because it is associated with high rates of myocardial infarction (MI), ischemic stroke, and cardiovascular disease (CVD) death.3 Despite its high and growing prevalence, PAD remains an underdiagnosed and undertreated condition, particularly in the Black population.4,5
Claudication, the most common symptom of PAD, significantly reduces quality of life (QoL) and functional status.6,7 Treatment of claudication, as in all patients with PAD, requires a multi-pronged approach which includes medical therapies, exercise therapy, and consideration of endovascular or surgical revascularization when appropriate. Significant gains have been made in evidence-based medical therapies for PAD in recent years including the addition of PCSK9 inhibitors and the use of low-dose rivaroxaban.5 Supervised exercise therapy (SET) continues to be a critical intervention for all patients with PAD and its use has been designated a class 1A recommendation in the 2016 ACC/AHA Guidelines for the Management of Lower Extremity Peripheral Artery Disease.8
Revascularization is also central to the management of claudication in patients with symptoms that are refractory to a combination of maximal medical therapy and SET. Identifying patients for revascularization requires a nuanced assessment of symptom severity, patient preferences, risk tolerance, physical limitations, and QoL. Endovascular strategies are typically recommended prior to consideration of surgical revascularization for most lesions as endovascular therapies have high efficacy, but lower rates of morbidity and mortality relative to surgical approaches.9 Unfortunately, despite major advances in endovascular technology, rates of restenosis associated with percutaneous transluminal angioplasty (PTA) and bare-metal stenting (BMS) remain high, affecting 40–60% of patients within 1 year of treatment.10,11
Paclitaxel-coated devices (PCDs), including paclitaxel-coated balloons (PCBs) and paclitaxel-eluting stents (PES) represent an enormous advance in endovascular therapies for claudication and PAD overall. Lined with paclitaxel, which inhibits smooth muscle cell proliferation, they significantly reduce rates of restenosis and target-lesion revascularization (TLR) compared with non-PCDs.12 PCD use has increased significantly and has supplanted non-PCD use in contemporary practice.13
Despite their significant growth and widespread use for patient care, sparse long-term safety data have been available with respect to PCDs. In December of 2018, Katsanos et al. performed a summary-level meta-analysis of 28 randomized clinical trials comparing PCDs to non-PCDs and reported a 68% increase in risk of mortality at 2 years and a 93% increase in risk of mortality in 5 years for patients treated with PCDs compared with non-PCDs.14 This meta-analysis has been criticized for significant methodological flaws which may have biased their conclusions. In spite of the substantial concerns surrounding bias in the meta-analysis, PCD use and patient care were greatly affected. Two major clinical trials of PCDs were stopped.15 PCD use dropped precipitously.16 The FDA convened a large panel to review the available evidence concerning PCDs. They concluded that no ultimate decision could be made due to insufficient data. PCDs were allowed to remain on the market with the recommendation that their use be restricted to patients at high-risk of reintervention.17 This left the vascular community at a stalemate: PCDs were being withheld from patients in need, but RCTs with the potential to provide insight into the safety of PCDs were not being conducted.
This controversy over paclitaxel has underscored the importance of real-world data in providing insight into the safety and efficacy of medical devices. The FDA felt that an RCT powered to establish a mortality difference in PCDs versus non-PCDs would require a very high number of patients over a long period of time so as to make it unfeasible. There are several advantages to the use of real-world data for providing safety evaluations, including the ability to access data on high numbers of patients and the ability to do this in a timely fashion. In addition, no additional patients need be treated with a device of questionable safety, as these analyses are retrospective.
In this review, we discuss key features of claudication and the advantages of PCDs for its management. We review the meta-analyses that reported a signal of harm for use of PCDs and the application of real-world data that consistently demonstrate the safety of PCDs.
Incidence of PAD
PAD, defined as atherosclerotic disease of the aorta or arteries of the lower extremities, affects up to 12 million people in the United States and 200 million people worldwide.18,19,20 The prevalence of the disease rises with age, affecting over 10% of individuals in their seventh and eighth decades of life and over 20% of those over age eighty.19,20 There is a higher rate of PAD in men than women, particularly for more severe or symptomatic disease. Among races, Black individuals have the highest rates of PAD.21,22 PAD is associated with substantial morbidity and mortality and has a crude five-year death rate of approximately 33%.3 Those with PAD have a 3-to 6-fold increase in the risk of MI, stroke, and death.23 Patients with PAD have CVD risk profiles similar to those who have sustained a prior MI.23 The two conditions share risk factors including type 2 diabetes mellitus (T2DM), smoking, obesity, hypertension, dyslipidemia, family history of PAD, CVD, and stroke. The presence of three or more of these risk factors confers a 10-fold risk of developing PAD.22 The most significant risk factor for the development of PAD is active smoking, which also has the greatest impact on disease severity.20
Pathophysiology of Claudication
PAD is most commonly due to an atherosclerotic occlusive process affecting the aorta or lower extremity arteries. Factors that can contribute to the development of atherosclerosis are similar to those associated with PAD, including dyslipidemia, tobacco use, endothelial dysfunction, and increased platelet activity.24 Plaque buildup narrows the arterial lumen, typically in the proximal or middle segments, restricting blood flow and resulting in the characteristic disease symptomatology.25 The supply-demand mismatch is exacerbated by walking, which may cause the oxygen demand of muscles to exceed the supply of oxygen delivered by blood flow. This results in ischemic pain with activity that is relieved by rest. Disease progression is characterized by the increasing failure of collateral blood supply to meet the metabolic demands of muscles.
Clinical Signs and Symptoms of Claudication
Patients with PAD can present with a spectrum of symptoms, the most common of which are no symptoms (~50%), claudication (~10 to 30%), and critical limb ischemia (CLI) (~10%).18 The majority of patients with symptomatic PAD experience typical claudication, which consists of recurrent fatigue, cramping, and pain in the lower extremities associated with walking that usually resolves within 10 minutes of rest.18 Patients may also present with atypical claudication. Atypical symptoms include exertional leg pain that occasionally begins at rest and exertional leg pain that does not cause the patient to stop walking.26 Prior studies have found that the prevalence of patients with atypical symptoms is between 20% to 40%.18 CLI, which is defined as chronic ischemic rest pain, non-healing wounds or ulcers, or gangrene proven to be caused by occlusive atherosclerosis, is the most severe subtype.20 CLI affects up to 10% of patients in the US and is associated with a mortality rate of 50% to 60% over 5 years.27 Approximately 5% to 10% of patients with typical or asymptomatic subtypes of PAD will progress to CLI over a five-year period.27
Establishing the Diagnosis and Severity of PAD
To diagnose PAD, key points in the history should be elicited. This includes an accurate assessment of walking ability and cardiovascular risk factors. A physical exam may reveal reduced or absent pulses in the lower extremities, which can be confirmed with an ankle-brachial index (ABI), which is an objective measurement of lower extremity arterial perfusion obtained by comparing systolic blood pressures in the ankle and the arm. An ABI value less-than-or-equal-to 0.90 is suggestive of PAD.23 For patients with PAD and non-distensible arteries, a value of greater than 1.4 also suggests disease. A skin exam may reveal atrophy, reduced hair growth, cyanosis, and evidence of poor wound healing in areas of restricted blood flow.28
The Fontaine and Rutherford classification systems have been widely used to characterize the severity of PAD based on symptoms (Table 1). The Fontaine system assigns four stages based solely on the clinical presentation.29 The Rutherford system, which mimics Fontaine but is more widely used, classifies symptoms based on increasing severity from asymptomatic (Stage 0) to severe ischemia (Stage 6). This system also incorporates objective findings from Doppler ultrasound, toe or ankle pressures, ABI, and vascular treadmill exercise testing. Patients with PAD may also be classified by the anatomic distribution of their atherosclerotic lesions.29 The Bollinger classification system grades invasive angiograms based on the number of lesions and divides lower extremity arteries into segments that are subsequently characterized by the pattern of occlusion. Although not widely used clinically, the Bollinger classification has been used in research studies, such as the Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial, to follow disease progression over time.30,9
Table 1.
Classification of the Severity of Peripheral Artery Disease Based on Symptoms using the Fontaine and Rutherford Systems. Adapted from Dave et al. Int J Rest Med Sci. 2018;6(5):1474–1483.62
| Fontaine Clinical Classification | Rutherford Clinical Classification | ||||
|---|---|---|---|---|---|
| Stage | Clinical Aspects | Grade | Category | Clinical Aspects | Objective Findings |
| I | Asymptomatic | 0 | 0 | Asymptomatic | Normal treadmill test |
| IIa | Mild claudication | I | 1 | Mild claudication | Completes treadmill test; ankle pressure after test > 50 mmHg but at least 20 mmHg lower than resting value |
| IIb | Moderate-severe claudication | I | 2 | Moderate claudication | Between categories 1 and 3 |
| I | 3 | Severe claudication | Cannot complete treadmill test; ankle pressure after test < 50 mmHg | ||
| III | Rest pain | II | 4 | Rest pain | Resting ankle pressure < 40mmHg; flat or barely pulsatile ankle or metatarsal pulse volume recording; toe pressure < 30 mmHg |
| IV | Ulceration or gangrene | Ill | 5 | Ischemic ulcer of the digits of the foot (minor tissue loss) | Resting ankle pressure < 60 mmHg; ankle or metatarsal pulse volume recording flat or barely pulsatile; toe pressure < 40 mmHg |
| Ill | 6 | Severe ischemic ulcers or gangrene (major tissue loss) | Same as category 5 | ||
Assessing functional status – patients’ capacities to perform physical activities – is also an important part of evaluating the severity of PAD (Table 2). Peak performance or walking ability is commonly measured with metrics such as the maximal walking distance or absolute claudication distance and maximal walking time or peak walking time. Claudication onset is measured with pain-free walking distance or initial claudication distance and pain-free walking time or claudication onset time.31 Time and/or distance to onset of claudication and maximal time and/or distance walked until leg pain arises are commonly used as endpoints in randomized trials.32 Prior studies have demonstrated that vascular treadmill testing can be predictive of long-term morbidity and mortality outcomes in PAD.33 The 6-minute walk test (6MWT), which eliminates the need for equipment, is another measure of functional status that has been found to be highly reproducible, valid, and sensitive for patients with claudication.34,35
Table 2.
Conventional Measures of Functional Capacity in PAD.
| Measure | Definition | How to Measure |
|---|---|---|
| Maximal walking distance (or absolute claudication distance) | The walking distance until claudication pain forces the patient to stop | Standardized progressive treadmill test with a constant speed of 3.2 km/h starting with 0% incline, increasing every 2 minutes by 2% |
| Maximal walking time (or peak walking time) | The walking time until claudication pain forces the patient to stop | Standardized progressive treadmill test with a constant speed of 3.2 km/h starting with 0% incline, increasing every 2 minutes by 2% |
| Pain-free walking distance (or initial claudication distance) | The walking distance until the onset of claudication pain | Standardized progressive treadmill test with a constant speed of 3.2 km/h starting with 0% incline, increasing every 2 minutes by 2% |
| Pain-free walking time (or claudication onset time) | The walking time until the onset of claudication pain | Standardized progressive treadmill test with a constant speed of 3.2 km/h starting with 0% incline, increasing every 2 minutes by 2% |
| 6-minute walk test (6MWT) | Measures the distance patient is able to walk over a total of six minutes on a hard, flat surface | Test performed in a 30 meter, pre-measured flat walking area with interval markings every 3 meters |
Given the significant impact of symptomatic PAD on health-related QoL (HRQoL), there has been growing interest in incorporating patient-reported outcome measures (PROMs) into research and clinical settings.36 The 2016 AHA/ACC guidelines underscore the importance of improving both HRQoL and symptom burden when treating PAD.8 Disease-specific PROMs, such as the Walking Impairment Questionnaire and the Peripheral Artery Questionnaire, were developed to evaluate symptom burden, functional impairment, social limitations, and HRQoL.37,38 Validated against gold standard metrics, these disease-specific PROMs correlate with traditional indicators like mortality39, repeat revascularization40, and healthcare costs.41
PROMs and HRQoL combined with objective testing and functional status assessments are required for selecting patients most likely to benefit from revascularization.
Importance of Endovascular Therapy for Managing Claudication
Revascularization may be necessary for limb salvage in PAD, but it is also used widely for patients with claudication that is refractory to maximal medical therapy, lifestyle interventions such as smoking cessation, and SET. Endovascular therapy has undergone significant advances in the last decade and is associated with patency rates similar to those of surgical revascularization, but with lower rates of morbidity and mortality.9 In 2017, the ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Disease designated endovascular therapy as first-line, prior to surgical approaches, for femoropopliteal lesions <25 cm.42
Despite this progress in endovascular technology, PTA and BMS are associated with very high rates of restenosis, affecting 40–60% of patients with 1 year of treatment.10,11 In 2012 and 2014, PES and PCBs, respectively, were introduced into the market. PCDs represent a significant innovation in endovascular therapy as paclitaxel inhibits vascular smooth muscle cells and fibroblasts, meaningfully reducing rates of restenosis.43 Several clinical trials have now been published establishing the efficacy of PCDs and their superiority to non-PCDs.
First, the THUNDER trial, a multicenter randomized controlled trial (RCT), randomly assigned 154 patients with femoropopliteal lesions to PCB, POBA with paclitaxel in the contrast medium, or POBA to evaluate late lumen loss. At 6 months, treatment with PCB resulted in significantly lower late lumen loss compared to treatment with POBA (0.4±1.2 mm vs. 1.7±1.8, p<0.001). In addition, patients treated with PCB had significantly lower rates of target lesion revascularization (TLR) compared with patients treated with POBA (4% vs. 37%, p<0.001). This was also demonstrated at 24 months (15% vs. 52%, p<0.001).44 After 5 years, treatment with PCB resulted in significantly lower rates of TLR than treatment with POBA (21% vs. 56%, p=0.0005).45 There was no difference in serious adverse events, including amputation and death, between patients treated with PCB and those treated with POBA.
In the IN.PACT SFA trial, 331 patients with femoropopliteal lesions were evaluated for TLR.46 Patients were randomly assigned to PCB or PTA. At 5 years, treatment with PCB resulted in lower rates of TLR compared with treatment with PTA (Kaplan-Meier estimate of 74.5% vs. 65.3%, log-rank p=0.02). There was no difference in all-cause mortality or major adverse events among those treated with PCB versus PTA.
The Zilver PTX was a randomized-controlled trial comparing PES to BMS over 5 years. 474 patients were assigned to PES or PTA.47 Clinical benefit, patency, and freedom from TLR were significantly higher for PES at 5 years. Specifically, freedom from reintervention was 83.1% for PES and 67.6% for PTA, p<0.01.
The efficacy of PCDs is well-established; however, a paucity of data on their long-term safety existed prior to their introduction into the market, use for patient care, or widespread incorporation of these devices into clinical practice.36
A Late Mortality Signal Associated with Paclitaxel
A major challenge for the treatment of patients with claudication arose in December of 2018 when Katsanos et al. published a meta-analysis of clinical trials that showed a higher mortality associated with long-term treatment with PCDs compared with non-PCDs.14 This meta-analysis of summary-level data from 28 randomized-clinical trials reported no difference in mortality at 1 year, a 68% increase in mortality at 2 years, and a 93% increase in mortality at 5 years associated with PCDs.
Importantly, this meta-analysis had several methodological flaws that must be considered. First, the trials in the meta-analysis were powered to assess efficacy at 6 months to 2 years with primary endpoints including primary patency, late lumen loss, diameter stenosis, target lesion revascularization, and in-stent binary restenosis. This resulted in significant loss-to-follow-up when primary endpoints were met. This can be seen in the numbers of patients analyzed: at 1 year, there were 28 trials and 4432 patients, at 2 years, there were 12 trials and 2316 patients, and at 5 years there were only 3 trials and 868 patients. Asymmetric loss to follow up between PCD and non-PCD arms could bias the results. Second, because the study did not analyze individual-level patient data, they could not adjust for the heterogeneity in patient populations including burden of patient comorbidities, lesion characteristics, and types of endovascular therapies used. Using summary-level data also precluded use of time-to-event analyses or the ability to censor patients who were lost to follow-up. Third, though the meta-analysis reported a dose-response relationship between paclitaxel and mortality, subsequent studies using more robust methods have not successfully replicated this signal. Fourth, no plausible mechanism has been reported to link paclitaxel to mortality.
Despite the major shortcomings of this study, its publication had a major impact on the use of PCD technology. The U.S. Food and Drug Administration (FDA) recommended that only high-risk patients be treated with PCDs until the mortality signal could be better investigated.48 As a result, use of PCDs in clinical practice sharply declined. Major RCTs, BASIL III and SWEDEPAD I and II, that could provide information regarding PCDs were stopped.15
To better evaluate the late mortality signal and determine a course of action regarding use of PCDs, the FDA assembled a Medical Device Advisory Panel in June of 2019.17 The FDA replicated the late mortality signal identified by Katsanos et al., using the data provided in their publication. The FDA also performed an internal analysis of patient-level data from 4 pivotal RCTs of US-approved PCDs which included ZILVER PTX, LEVANT 2, IN.PACT SFA I & II, and ILLUMENATE, all of which had been included in the Katsanos meta-analysis (Figure 1), as well as a larger analysis that included any registry with at least 200 patients and at least 2-years of follow-up and any RCT. These analyses showed a late mortality signal.49 The FDA concluded, however, that there was no definitive evidence of a dose-response relationship between paclitaxel and mortality and that there was substantial missing data and small sample sizes. They reviewed observational data from the Medicare, Optum Claims, and Vascular Quality Initiative databases, all of which did not demonstrate a higher mortality in association with paclitaxel.
Figure 1. Randomized-clinical trials included in the Katsanos meta-ananlysis, the FDA internal meta-analysis, and the VIVA physicians meta-analysis.
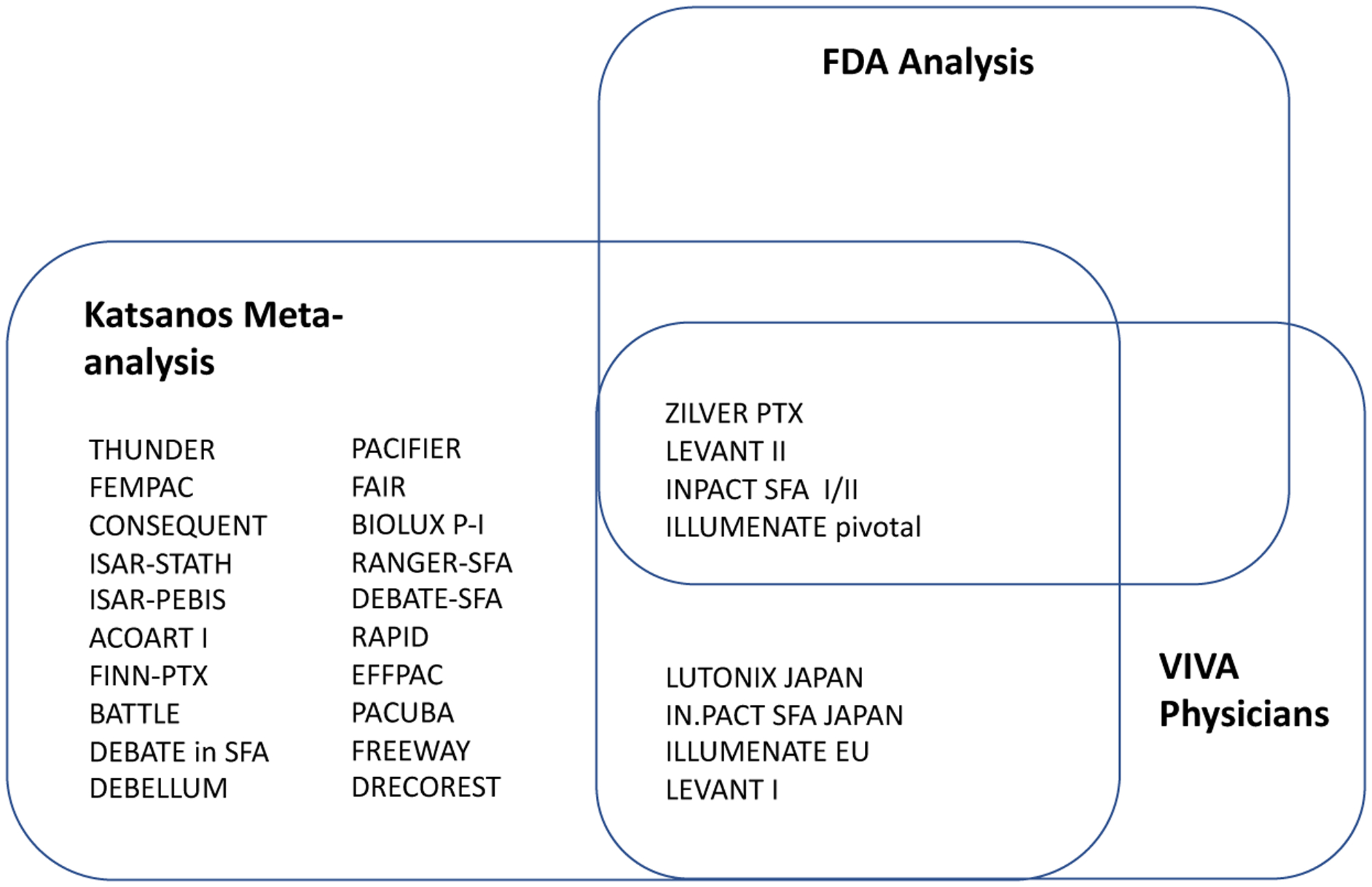
The Katsanos meta-analysis14, the FDA internal analysis49, and the VIVA physicians meta-analysis51 examined an overlapping group of randomized-clinical trials.
Ultimately, the FDA decided that no definitive statement could be made regarding the safety of PCDs and they recommended more rigorous long-term safety monitoring of patients treated with PCDs and requested the observational analyses from Medicare, Optum, and VQI to be extended over time. For patient care, they recommended that use of PCDs be limited to those at high-risk of restenosis or repeat intervention if benefits outweigh risks. They recommended that providers engage patients in risk-benefit discussions to inform them of a possible risk of increased mortality. Last, they recommended that device labeling be changed to reflect the possibility of an increased mortality signal associated with paclitaxel.50
In 2020, Rocha-Singh et al.51, performed a meta-analysis of patient-level data from 8 RCTs of PCDs in which they gathered additional data to reduce the loss-to-follow-up. All 8 of these trials had been included in the Katsanos meta-analysis and 4 had been analyzed in the internal meta-analysis by the FDA. When loss-to-follow-up was 27% and 25% in the treatment and control arms, the hazard ratio for all-cause mortality associated with PCDs was 1.38 (95% CI 1.06–1.80). At loss-to-follow-up rates of 10% and 9%, the HR was reduced to 1.27 (95% CI 1.03–1.58). There was no evidence of a dose-response relationship between paclitaxel and mortality. Overall, the reduction in the HR of mortality and paclitaxel with reductions in loss-to-follow-up indicates that the loss-to-follow-up may have been non-random and had the potential to bias results.
It is critical to note that the three meta-analyses described above used overlapping RCTs as their data source (Figure 1), RCTs shown to have significant missing data and that were designed to study short-term efficacy endpoints. The FDA, having recognized this limitation of the RCTs in their internal meta-analysis as well as in the Katsanos meta-analysis, called for more data comparing PCDs to non-PCDs in order that their safety could be rigorously evaluated. The FDA deemed additional RCTs to be unfeasible, as RCTs would require significant numbers of patients as well as several years of follow up time to draw sound conclusions.
Real-World Data Evaluating the Safety of Paclitaxel-Coated Devices
The controversy caused by the possibility of an increase in mortality associated with PCDs has highlighted the growing role of real-world evidence in medical product development, safety monitoring, and FDA regulation. The 21st Century Cures Act is a law passed in December of 2017 designed to expedite medical product development and implementation.52 A key feature of this law is using real-world data, data derived from sources other than randomized clinical trials, to support approval of a drug or to satisfy post-approval requirements. Real-world data have distinct advantages over clinical trials including rapid access to data, large numbers of patients, low cost, and inclusion of broad patient populations.
Given the challenges of obtaining clinical trial data to assess the late mortality signal in paclitaxel, real-world data took a front seat in the long-term safety analysis of paclitaxel. Numerous studies using real-world data have now been published (Table 3). The primary studies included observational data from the Medicare claims database, the Optum claims database, and the German BARMER health system.
Table 3.
Studies of the Safety of Paclitaxel-Coated Devices using Real-World Data.
| Studies of PCDs using Real-World Data | Time to Follow Up | Outcome |
|---|---|---|
| Secemsky et al. JAMA. 2019.53 (Medicare) | Median 389 days, up to 600 days |
No Increase in Mortality for PCDs - unadjusted cumulative incidence through 600 days: 32.5% PCD vs 34.3% non-PCD (P=0.007) - aHR 0.97, 95% CI 0.91–1.04 (p=0.43) |
| Secemsky et al. JACC. 2019.54 (Medicare) | Median 2 years, up to 4.1 years |
No Increase in Mortality for PCDs - unadjusted cumulative incidence through 4.1 years: 51.7% PES vs. 50.1% BMS (P=0.16) - aHR 0.98; 95%CI 0.93–1.03 (p=0.53) |
| Secemsky et al. EuroIntervention. 2020.56 (Optum Claims) | Median 2.66 years, up to 4.75 years |
No Increase in Mortality for PCDs - aHR for mortality of PCDs relative to non-PCDs: 1.03, 95% CI 0.96–1.10 (p=0.39) |
| Freisinger et al. ESC. 2019.57 (BARMER) | Median 92 months, up to 11 years |
No Increase in Mortality for PCDs - HR at 5 years for PES vs non-PCD: 1.01, 95% CI 0.83–12.3 (p=0.91) - HR at 5 years for PCB vs non-PCD: 0.97, 95% CI 0.89–1.06 (p=0.492) |
| Behrendt et al. Eur. J. Vasc. Surg. 2020.58 (BARMER) | 5 years |
No Increase in Mortality for PCDs - aHR for survival in PCD vs non-PCD in patients with IC: 0.87, 95% CI 0.76–0.99 - aHR for survival in PCD vs non-PCD in patients with CLTI: 0.83, 95% CI 0.77–0.90 |
| Bohme et al. JACC CI.2020.63 | Median 51 months |
No Increase in Mortality for PCDs - Mortality rate 27.5% after PTA vs 16.9% after PCB (p<0.001) |
| Bohme et al. Catheter Cardiovasc. Interv. 2020.64 | 3–7 years, mean of 52 months |
No Increase in Mortality for PCDs - Unadjusted mortality incidence for PES vs BMS: 22.6 vs 32.3% (p<0.033) - Adjusted mortality incidence for PES vs BMS: 24.1% vs 32.5% (p=0.264) |
| Hess et al. TCT Connect. 2020.61 | Median of 31 months |
No Increase in Mortality for PCDs - adjusted HR 0.95, 95% CI 0.83–1.09 (p=0.49) |
| Weissler et al. 2021.65 (ACC PVI Registry) | 2 years |
No Increase in Mortality for PCDs - aRR for all-cause mortality in PCD vs non-PCD: 0.98, 95% CI 0.77–1.24 (p=0.844) |
| Bertges et al. 2020.66 (VQI) | 1 year |
No Increase in Mortality for PCDs - aHR for mortality in PCDs compred with non-PCDs: 0.82, 95% CI 0.68–0.98 (p=0.03) |
Secemsky et al. performed two large analyses in the Medicare database evaluating all-cause mortality in patients who were treated with PCDs relative to non-PCDs. First, a study of 16,560 patients compared 5989 (36.2%) patients who were treated with PCDs to 10,571 patients treated with non-PCDs over a median follow-up time of 389 days (IQR 277–508 days). Treatment with PCDs was associated with a lower cumulative incidence of all-cause mortality compared with treatment with non-PCDs (32.% vs. 34.3%, respectively; log-rank p= 0.007).53 After adjustment, there was no difference in all-cause mortality among patients treated with PCDs relative to non-PCDs (aHR 0.97; 95% CI 0.91–1.04; p=0.43). The same outcome was observed in patients with claudication when stratified by severity of disease. In a second study, 51,456 patients who underwent PES or BMS were compared over a median follow up of 2.0 years (IQR 1.2–3.0 years).54 There was no difference in mortality among patients treated with PES compared with BMS through 4.1 years (51.7% vs. 50.1%, respectively; log-rank p=0.16). There was no association between stent type and mortality after adjustment (aHR: 0.98; 95% CI 0.93–1.03; p=0.53). There is now an ongoing long-term safety analysis, the Safety Assessment of Femoropopliteal Endovascular treatment with Paclitaxel-coated Devices (SAFE-PAD), to further evaluate the safety of PCDs in the Medicare population through a median follow-up time of 5 years (clinicaltrials.gov NCT04496544).55
Secemsky et al. also performed an evaluation of the late-mortality signal associated with paclitaxel in 16,796 patients insured by Medicare Advantage over a median follow-up time of 2.66 years (IQR 2.02–3.52).56 Of the cohort, 4,427 (26.4%) patients were treated with PCDs. After adjustment, there was no difference in mortality between patients treated with PCDs compared with those treated with non-PCDs (adjusted HR 1.03; 95% CI 0.96–1.10; p=0.39). This finding was true for patients treated with balloons alone or with stents.
Two retrospective observational studies were published using data from the German BARMER health insurance claims records. Neither study showed an increase in mortality in patients treated with PCDs relative to non-PCDs. First, Freisinger et al. extracted data from 64,771 patients who underwent endovascular revascularization from 2007 to 2015 and followed them until December of 2017.57 At 5 years of follow up, PCDs were not associated with a higher mortality than non-PCDs (PES: HR 1.01, 95% CI 0.83–1.23, p=0.91; PCB: HR 0.97, 95% CI 0.89–1.06, p=0.49). A minority of patients were followed to 8–11 years and, similarly, PCDs were not associated with greater mortality over this time period (PES: HR 0.64, 95% CI 0.40–1.01, p=0.06; PCB: HR 1.02, 95% CI 0.74–1.40, p=0.92). Second, Behrendt et al. evaluated 37,914 patients who underwent endovascular revascularization. In the intermittent claudication cohort, mortality was significantly lower after using PCB (HR 0.87, 95% CI 0.76–0.99) or combined PCB and PES (HR 0.88, 95% CI 0.80–0.98) relative to non-PCDs.58
VOYAGER PAD and SWEDEPAD
Despite the decision not to initiate de novo clinical trials, new trial data has recently become available. The SWEDEPAD trial, the Swedish Drug Elution Trial in Peripheral Arterial Disease, is a multicenter, open-label, registry-based clinical trial designed to evaluate amputation rates among patients with CLI as well as health-related quality of life among patients with intermittent claudication.59 Enrollment in the trial was stopped in December of 2018 due to the concern over an increase in mortality associated with paclitaxel raised by the Katsanos meta-analysis. An unplanned interim analysis was conducted on data from the SWEDEPAD trial with the single endpoint of all-cause mortality.59 Of 2289 patients included in the trial, 1149 were randomly assigned to treatment with drug-coated devices and 1140 to uncoated devices. Patients were followed for 1 to 4 years with a mean of 2.49 years. There was no difference in all-cause mortality between patients treated with PCDs (25.5%) compared with those treated with uncoated devices (24.6%) (HR 1.06; 95% CI 0.92 to 1.22). When patients were stratified by CLI and intermittent claudication, there was no difference in incidence of death between patients with intermittent claudication treated with PCDs (10.9%) relative to those treated with uncoated devices (9.4%) (HR 1.18; 95% CI 0.72 to 1.93).
In addition, Hess, et al., performed a subgroup analysis of data from the VOYAGER PAD trial60 examining mortality rates of patients treated with PCDs relative to non-PCDs. In VOYAGER PAD, patients who underwent endovascular revascularization of the lower extremities were randomized to treatment with rivaroxaban 2.5 mg bid or placebo following revascularization. All patients were treated with aspirin 100 mg daily. Of the trial population, 4,379 patients underwent endovascular revascularization, 31% (1,358) of whom received a PCD. After adjustment for confounders, there was no difference in mortality between patients treated with PCDs and those treated with non-PCDs over a median follow-up period of 31 months.61
Conclusion
In 2021, claudication and underlying PAD remain common and undertreated conditions. Both medical and endovascular therapies have undergone significant advances over the last decade. Specifically, PCDs represent a critical breakthrough in endovascular technology as they have demonstrated superiority to non-PCDs as well as a similar efficacy to surgical revascularization with lower morbidity and mortality. Despite the methodological flaws in the Katsanos meta-analysis, the FDA restricted use of PCDs to the most high-risk patients following its publication. The vascular community found itself unable to widely offer PCDs, but with no RCTs on the horizon to definitively establish the safety of PCDs. In this setting, real-world data has emerged as powerful source of data with which to evaluate these devices. Now, numerous studies from sources including Medicare, Optum, and the BARMER German claims databases as well as registries including the Vascular Quality Initiative have demonstrated no signal of harm associated with PCDs compared with non-PCDs. Advances in real-world data have set the stage for expedient and inexpensive methods of monitoring the safety of FDA-approved drugs and devices and PCDs have been a proof of this concept.
Funding:
Dr. Secemsky is supported by NIH/NHLBI K23HL150290 and Harvard Medical School’s Shore Faculty Development Award
Relationships with Industry:
ES: Consulting/Scientific Advisory Board: Abbott, Bayer, BD, Boston Scientific, Cook, CSI, Inari, Janssen, Medtronic, Philips, and Venture Medical.; Research Grants: AstraZeneca, BD, Boston Scientific, Cook, CSI, Laminate Medical, Medtronic, and Philips.
Abbreviations:
- ABI
Ankle-brachial index
- BMS
Bare metal stent
- CLI
Critical limb ischemia
- CVD
Cardiovascular disease
- HRQoL
Health-related quality of life
- IQR
Interquartile range
- MI
Myocardial Infarction
- PCB
Paclitaxel-coated balloon
- PCDs
Paclitaxel-coated devices
- PES
Paclitaxel-eluting stent
- PTA
Percutaneous transluminal angioplasty
- PAD
Peripheral artery disease
- QoL
Quality of life
- SET
Supervised exercise therapy
- FDA
United States Food and Drug Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors have nothing to disclose.
References
- 1.Virani SS et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 141, (2020). [DOI] [PubMed] [Google Scholar]
- 2.Song P et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob. Health 7, e1020–e1030 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Caro J, Migliaccio-Walle K, Ishak KJ & Proskorovsky I The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc. Disord 5, 14 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger JS & Ladapo JA Underuse of Prevention and Lifestyle Counseling in Patients With Peripheral Artery Disease. J. Am. Coll. Cardiol 69, 2293–2300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevan GH & White Solaru KT Evidence-Based Medical Management of Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol 40, 541–553 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Dumville JC, Lee AJ, Smith FB & Fowkes FGR The health-related quality of life of people with peripheral arterial disease in the community: the Edinburgh Artery Study. Br. J. Gen. Pract 54, 826–831 (2004). [PMC free article] [PubMed] [Google Scholar]
- 7.Smolderen KG, Pelle AJ, Kupper N, Mols F & Denollet J Impact of peripheral arterial disease on health status: A comparison with chronic heart failure. J. Vasc. Surg 50, 1391–1398 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Gerhard-Herman MD et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol 69, e71–e126 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Bradbury AW et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: An intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J. Vasc. Surg 51, 5S–17S (2010). [DOI] [PubMed] [Google Scholar]
- 10.Johnston KW Femoral and popliteal arteries: reanalysis of results of balloon angioplasty. Radiology 183, 767–771 (1992). [DOI] [PubMed] [Google Scholar]
- 11.Rocha-Singh KJ et al. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv 69, 910–919 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Katsanos K et al. Economic analysis of endovascular drug-eluting treatments for femoropopliteal artery disease in the UK. BMJ Open 6, e011245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohapatra A et al. Nationwide trends in drug-coated balloon and drug-eluting stent utilization in the femoropopliteal arteries. J. Vasc. Surg 71, 560–566 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konstantinos Katsanos, Stavros Spiliopoulos, Panagiotis Kitrou, Miltiadis Krokidis, & Dimitrios Karnabatidis. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc 7, e011245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKeown LA (2018, December 17). Two Trials Halted in Wake of Study Linking Paclitaxel-Coated Devices to Deaths in PAD. Retrieved from https://www.tctmd.com/news/two-trials-halted-wake-study-linking-paclitaxel-coated-devices-deaths-pad. Accessed 12/19/18.
- 16.Monteleone PP et al. The Market Reacts Quickly: Changes in Paclitaxel Vascular Device Purchasing Within the Ascension Healthcare System. J. Invasive Cardiol 32, 18–24 (2020). [DOI] [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration. June 19–20, 2019: Circulatory System Devices Panel of the Medical Device Advisory Committee Meeting Announcement. Available at: https://www.fda.gov/advisory-committees/advisory-committeecalendar/june-19-20-2019-circulatory-systemdevices-panel-medical-devices-advisory-committee-meeting.
- 18.Peripheral Artery Disease: Evolving Role of Exercise, Medical Therapy, and Endovascular Options. - Google Search. https://www.google.com/search?q=Peripheral+Artery+Disease%3A+Evolving+Role+of+Exercise%2C+Medical+Therapy%2C+and+Endovascular+Options.&rlz=1C5CHFA_enUS794US794&oq=Peripheral+Artery+Disease%3A+Evolving+Role+of+Exercise%2C+Medical+Therapy%2C+and+Endovascular+Options.&aqs=chrome..69i57.892j0j7&sourceid=chrome&ie=UTF-8. [DOI] [PubMed]
- 19.Patel MR et al. Evaluation and Treatment of Patients With Lower Extremity Peripheral Artery Disease. J. Am. Coll. Cardiol 65, 931–941 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu J & Santulli G Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 275, 379–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Criqui Michael H & Aboyans Victor. Epidemiology of Peripheral Artery Disease. Circ. Res 116, 1509–1526 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Criqui Michael H et al. Ethnicity and Peripheral Arterial Disease. Circulation 112, 2703–2707 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Olin JW & Sealove BA Peripheral Artery Disease: Current Insight Into the Disease and Its Diagnosis and Management. Mayo Clin. Proc 85, 678–692 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novo S et al. Prevalence of risk factors in patients with peripheral arterial disease. A clinical and epidemiological evaluation. Int. Angiol. J. Int. Union Angiol 11, 218–229 (1992). [PubMed] [Google Scholar]
- 25.Zemaitis MR, Boll JM & Dreyer MA Peripheral Arterial Disease. in StatPearls (StatPearls Publishing, 2020). [PubMed] [Google Scholar]
- 26.Nehler MR, McDermott MM, Treat-Jacobson D, Chetter I & Regensteiner JG Functional outcomes and quality of life in peripheral arterial disease: current status. Vasc. Med. Lond. Engl 8, 115–126 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Duff S, Mafilios MS, Bhounsule P & Hasegawa JT The burden of critical limb ischemia: a review of recent literature. Vasc. Health Risk Manag 15, 187–208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cutaneous Manifestations of Chronic Vascular Disease - ScienceDirect. https://www.sciencedirect.com/science/article/abs/pii/S0033062018300549?via%3Dihub. [DOI] [PubMed]
- 29.Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) -Journal of Vascular Surgery. https://www.jvascsurg.org/article/S0741-5214(06)02296-8/abstract. [DOI] [PubMed]
- 30.Hardman RL, Jazaeri O, Yi J, Smith M & Gupta R Overview of Classification Systems in Peripheral Artery Disease. Semin. Interv. Radiol 31, 378–388 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vemulapalli S et al. Comparative Effectiveness of Medical Therapy, Supervised Exercise, and Revascularization for Patients With Intermittent Claudication: A Network Meta-analysis. Clin. Cardiol 38, 378–386 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruidenier LM et al. Functional claudication distance: a reliable and valid measurement to assess functional limitation in patients with intermittent claudication. BMC Cardiovasc. Disord 9, 9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leeper NJ et al. Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial disease. J. Vasc. Surg 57, 728–733 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDermott MM et al. Response to Letter Regarding Article, “Six-Minute Walk Is a Better Outcome Measure Than Treadmill Walking Tests in Therapeutic Trials of Patients With Peripheral Artery Disease”. Circulation 131, e407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montgomery PS & Gardner AW The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J. Am. Geriatr. Soc 46, 706–711 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Raja A, Spertus J, Yeh RW & Secemsky EA Assessing Health-Related Quality of Life among Patients with Peripheral Artery Disease: A Review of the Literature and Focus on Patient-Reported Outcome Measures. Vasc. Med. Lond. Engl 1358863X20977016 (2020) doi: 10.1177/1358863X20977016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coyne KS et al. Evaluating effects of method of administration on Walking Impairment Questionnaire. J. Vasc. Surg 38, 296–304 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Spertus J, Jones P, Poler S & Rocha-Singh K The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am. Heart J 147, 301–308 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Murphy TP et al. Supervised Exercise, Stent Revascularization, or Medical Therapy for Claudication Due to Aortoiliac Peripheral Artery Disease: A Randomized Clinical Trial. J. Am. Coll. Cardiol 65, 999 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray WA et al. S.M.A.R.T. Self-Expanding Nitinol Stent for the Treatment of Atherosclerotic Lesions in the Superficial Femoral Artery (STROLL): 1-Year Outcomes. J. Vasc. Interv. Radiol 26, 21–28 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Forbes JF et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: Health-related quality of life outcomes, resource utilization, and cost-effectiveness analysis. J. Vasc. Surg 51, 43S–51S (2010). [DOI] [PubMed] [Google Scholar]
- 42.Aboyans V et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J 39, 763–816 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Ng VG, Mena C, Pietras C & Lansky AJ Local delivery of paclitaxel in the treatment of peripheral arterial disease. Eur. J. Clin. Invest 45, 333–345 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Tepe G et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N. Engl. J. Med 358, 689–699 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Tepe G et al. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc. Interv 8, 102–108 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Laird John A et al. Long-Term Clinical Effectiveness of a Drug-Coated Balloon for the Treatment of Femoropopliteal Lesions. Circ. Cardiovasc. Interv 12, e007702 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dake MD et al. Durable Clinical Effectiveness With Paclitaxel-Eluting Stents in the Femoropopliteal Artery: 5-Year Results of the Zilver PTX Randomized Trial. Circulation 133, 1472–1483; discussion 1483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Food & Drug Administration (2019, January 17). Treatment of Peripheral Arterial Disease with Paclitaxel-Coated Balloons and Paclitaxel-Eluting Stents Potentially Associated with Increased Mortality - Letter to Health Care Providers. Retrieved from https://www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm629589.htm. Accessed 1/21/19.
- 49.FDA Executive Summary. https://www.fda.gov/media/127698/download. Accessed July 1, 2019.
- 50.Health, C. for D. and R. August 7, 2019 UPDATE: Treatment of Peripheral Arterial Disease with Paclitaxel-Coated Balloons and Paclitaxel-Eluting Stents Potentially Associated with Increased Mortality. FDA (2019). [Google Scholar]
- 51.Rocha-Singh Krishna J. et al. Mortality and Paclitaxel-Coated Devices. Circulation 141, 1859–1869 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.21st Century Cures Act. https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act. Accessed January 1, 2021.
- 53.Secemsky EA et al. Association of Survival With Femoropopliteal Artery Revascularization With Drug-Coated Devices. JAMA Cardiol. 4, 332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Secemsky EA et al. Drug-Eluting Stent Implantation and Long-Term Survival Following Peripheral Artery Revascularization. J. Am. Coll. Cardiol 73, 2636–2638 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Safety Assessment of Femoropopliteal Endovascular Treatment with PAclitaxel-coated Devices (SAFE-PAD Study) (SAFE-PAD)(Clinicaltrials website). 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04496544. Accessed 11/15/2020.
- 56.Secemsky EA et al. Long-Term Safety of Drug-Coated Devices for Peripheral Revascularisation. EuroIntervention https://eurointervention.pcronline.com/article/long-term-safety-of-drug-coated-devices-for-peripheral-revascularisation doi: 10.4244/EIJ-D-20-01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freisinger E et al. Mortality after use of paclitaxel-based devices in peripheral arteries: a real-world safety analysis. Eur. Heart J (2019) doi: 10.1093/eurheartj/ehz698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Behrendt C-A et al. Long Term Survival after Femoropopliteal Artery Revascularisation with Paclitaxel Coated Devices: A Propensity Score Matched Cohort Analysis. J. Vasc. Surg 71, 1815 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Nordanstig J et al. Mortality with Paclitaxel-Coated Devices in Peripheral Artery Disease. N. Engl. J. Med 383, 2538–2546 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Bonaca MP et al. Rivaroxaban in Peripheral Artery Disease after Revascularization. N. Engl. J. Med 382, 1994–2004 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Hess Connie. TCT Connect 2020: Late Breaking Clinical Trials and Science. Long-term Safety of Drug-Coated Devices for Peripheral Artery Revascularization: Insights from Voyager-PAD.
- 62.Dave B & Shah R Peripheral stent technology and current status for endovascular treatment of femoropopliteal artery disease: a clinical review. Int. J. Res. Med. Sci 6, 1474–1483 (2018). [Google Scholar]
- 63.Böhme T et al. Evaluation of Mortality Following Paclitaxel Drug-Coated Balloon Angioplasty of Femoropopliteal Lesions in the Real World. JACC Cardiovasc. Interv 13, 2052–2061 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Evaluation of mortality following paclitaxel drug-coated stent angioplasty of femoropopliteal lesions in real world - Böhme - 2020 - Catheterization and Cardiovascular Interventions -Wiley Online Library. https://onlinelibrary.wiley.com/doi/10.1002/ccd.29267. [DOI] [PubMed]
- 65.Weissler EH et al. Paclitaxel-coated devices in the treatment of femoropopliteal stenosis among patients ≥65 years old: An ACC PVI Registry Analysis. Am. Heart J 233, 59–67 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bertges Daniel J et al. Mortality After Paclitaxel Coated Balloon Angioplasty and Stenting of Superficial Femoral and Popliteal Artery in the Vascular Quality Initiative. Circ. Cardiovasc. Interv 13, e008528 (2020). [DOI] [PubMed] [Google Scholar]


