Abstract
Peripheral artery disease (PAD) is a progressive atherosclerotic disease associated with high rates of morbidity and mortality. Symptomatic PAD typically presents with claudication, and symptom severity strongly associates with reduced health-related quality of life (HRQoL). Existing treatment strategies for PAD are aimed at reducing symptom severity and improving functional outcomes. However, there is a need to incorporate patient-reported outcome measures (PROMs) into PAD treatment and research in order to provide more patient-centered care. This review will discuss the impact of PAD on HRQoL, existing PROMs available to assess PAD-related HRQoL, utilization of PROMs in research studies and registries, and challenges and solutions related to the integration of PROMs into research and clinical settings.
Keywords: claudication, critical limb ischemia (CLI), outcomes, peripheral artery disease (PAD), quality of life
Introduction
The majority of patients with symptomatic peripheral artery disease (PAD) manifest with claudication, with symptom severity strongly associating with reduced health-related quality of life (HRQoL). As the focus of treating symptomatic PAD is to improve HRQoL, there is a need for patient-centered care and incorporation of patient-reported outcome measures (PROMs) to guide treatment decisions and evaluate the effectiveness of therapies. This review focuses on the impact of PAD on HRQoL, PROMs available to assess PAD-related HRQoL, implementation of PROMs in research studies and registries, and challenges and solutions related to the integration of PROMs into research and clinical settings.
Overview of PAD
PAD is an atherosclerotic disease process that most commonly involves the abdominal aorta and lower extremity arteries.1 PAD affects 8.5 million individuals in the United States and becomes increasingly prevalent with age.2,3 The disease is associated with significant morbidity and mortality and a three to sixfold higher risk of myocardial infarction, stroke, and death.4 The majority of patients with symptomatic PAD present with claudication, which includes recurrent fatigue, cramping, and pain in the lower extremities, that usually resolves within 10 minutes of rest.1 The most severe subtype of PAD is critical limb ischemia (CLI), defined as ischemic rest pain, non-healing wounds or ulcers, or gangrene in one or both legs.3 CLI affects up to 10% of patients with PAD and has a 5-year mortality rate of 50% to 60%.5
Current treatment options for PAD
Current therapies aim to reduce symptoms, improve function, and optimize the HRQoL of patients with PAD. The initial treatment strategy is modification of cardiovascular risk factors, including smoking cessation, blood pressure and lipid control, optimizing blood sugar control, and exercise.6 For symptomatic PAD, societal guidelines recommend a trial of medical treatment and supervised exercise therapy (SET) as first-line therapy,7,8 with revascularization reserved for residual symptoms.9 The Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study, which enrolled patients with claudication, found that SET with optimal medical therapy provided comparable durable improvement in functional status and HRQoL as compared with a combination of optimal medical therapy and revascularization.10 If these initial strategies are ineffective, patients may consider endovascular or surgical revascularization.6
Importance of assessing HRQoL in PAD
Treatment effectiveness studies in PAD primarily utilize clinical markers of disease severity (i.e. patency on duplex ultrasound), changes in functional classification systems (i.e. improvement in Fontaine or Rutherford classes), and changes in functional status (i.e. patients’ capacities to perform physical activities) as primary outcome measures. However, these endpoints do not comprehensively capture patients’ health status as they are derived from clinicians’ assessment of symptom severity, rather than elicited from patients directly. Studies comparing treatment strategies also inconsistently use one or more outcome measures, making the comparison of treatments across studies challenging.11,12
From the patient’s perspective, it is important to capture not only physical capacity, but also symptom burden, social limitations, and HRQoL, as impacted by their disease.13 Recently, there has been a growing emphasis on better understanding and measuring HRQoL in symptomatic PAD. Studies have shown that individuals with PAD living with claudication often avoid ambulating, further contributing to a decline in their functional capacity.13 Clinical metrics like a lower ankle–brachial index (ABI) score, indicating a higher disease severity, have been associated with worse HRQoL; however, two patients with identical ABIs may have very different symptoms, function, and HRQoL.14 Moreover, perceived stress and subjective pain adversely influence the health status and HRQoL of patients with PAD well beyond the influence of clinical indicators.15 Patients with PAD are at increased risk for significant social and emotional deficits, and are less likely to participate in social activities, engage in leisurely activity, and maintain work.13
Consequently, PAD-specific management should account not only for objective clinical outcomes, but also patient-centered outcomes. The 2016 AHA/ACC guidelines stress the importance of improved HRQoL beyond symptom relief when treating PAD.9 Although multiple patient-centered assessment tools are available, they have yet to be incorporated into clinical care or used consistently into clinical trials. Traditional measurements like ABI have been shown to poorly correlate with improvements in health status scores and are not sufficient substitutes for validated PROMs.16
Definition of patient-reported outcome measures (PROMs)
In the early 2000s, the World Health Organization defined health as a state of complete physical, mental, and social well-being, and not merely the absence of disease or infirmity.16 They also stated that QoL was determined by patients’ perceptions and HRQoL, referring to aspects of QoL related to health.17 PROMs are instruments used to systematically measure HRQoL, health status, and functional status. They include self-administered questionnaires, interviews, and other forms of data collection, and can be either disease-specific or generic. PROMs provide reports on patients’ perceptions of their disease and disability, satisfaction with their treatment and care, and/or adherence to prescribed treatments.18,19 Although widely applied in research for decades, the use of PROMs in clinical practice is especially limited for PAD.
PROMs used for disease-specific and generic health conditions
Disease-specific PROMs have previously been applied to assess various health conditions, including angina (Seattle Angina Questionnaire [SAQ]),20 dyspnea (Rose Dyspnea Scale [RDS]),20 heart failure (Kansas City Cardiomyopathy Questionnaire [KCCQ]),21 depression (Patient Health Questionnaire-2 [PHQ-2] and Patient Health Questionnaire-9 [PHQ-9]),22 and anxiety (General Anxiety Disorder-7 [GAD-7]).23 These assessments have been tested in diverse settings and delivered through platforms like smartphones and electronic tablets, creating an opportunity to capture information on symptoms pre- and post-treatment in a standardized fashion. Generic, non-disease-specific HRQoL measurement tools include the Short Form-12 (SF-12),24 Short Form-36 (SF-36),24 EuroQoL-5 Dimensions (EQ-5D),25 Nottingham Health Profile,26 and Sickness Impact Profile.27 These instruments assess the impact of overall health (PAD as well as other comorbidities) on the functional status, health status, and HRQoL of individuals living with chronic illness. Although previously utilized in studies on patients with claudication,10,26,28 these generic instruments are not tailored to monitor claudication symptoms or PAD-related HRQoL.
PAD-specific PROMs questionnaires
To assess HRQoL and functional impairment in PAD, disease-specific PROMs have been developed and validated against gold standard metrics (Table 1). Unlike functional outcomes that measure what patients can do when pushed, these questionnaires measure what patients are doing daily. Disease-specific PROMs correlate with traditional endpoints, including mortality,10 repeat revascularization,28 and healthcare costs.29 The Walking Impairment Questionnaire (WIQ), which examines functionality, has been applied in multiple research studies on patients with claudication to evaluate therapies.10,28,30,31 The WIQ is the most specific questionnaire to evaluate walking ability in patients with PAD, is sensitive to changes over time, and correlates highly with ambulatory limitations as measured by treadmill tests, 6-minute walk tests, and ABI.32–34
Table 1.
PAD-specific PROMs questionnaires.
| Instrument | Domains (number of levels) | Response options (scale) | Scoring | Mode of administration and/or time needed to complete |
|---|---|---|---|---|
| Intermittent Claudication Questionnaire (ICQ)41 | Health-related quality of life (16) | Likert scale: 5 | Summing scores and transforming to a 0–100 scale | Self-completed (3.7 minutes) |
| Impact of PAD on Quality of Life Questionnaire (PAD-QoL)40 | Social relationships and interactions (9), self-concept and feelings (7), symptoms and limitations in physical functioning (8), fear and uncertainty (4), positive adaptation (7) | Likert scale: 5 | Summed and transformed score 0–100% | Self-completed (5–10 minutes) |
| Peripheral Artery Questionnaire (PAQ)37 | Physical limitation (7), symptoms (4), quality of life (3), social function (3), treatment satisfaction (3) | Likert scale: 5 | 0–100 (lower scores indicating worse performance) | Self-completed |
| Vascular Quality of Life Questionnaire (VascuQoL)36 | Pain (4), activity (8), emotional (7), symptoms (4), social (2) | Likert scale: 7 | 1 (the worst) to 7 (the best possible) | Self-completed |
| Walking Impairment Questionnaire (WIQ)31 | Symptom severity (8), walking distance (7), walking speed (4), stair-climbing (3) | Likert scale: 5 | 0 (unable to do) to 4 (no difficulty) | Self-completed (6 minutes) |
PAD, peripheral artery disease; PROMs, patient-reported outcome measures.
Unlike the WIQ that is aimed at examining patients’ functionality, the Vascular Quality of Life Questionnaire (VascuQoL-25) assesses HRQoL across the spectrum of symptom severity. It was first utilized in the Bypass vs Angioplasty in Severe Ischaemia of the Leg (BASIL) trial and shown to be a reliable and valid outcome measure.11,35,36 The Peripheral Artery Questionnaire (PAQ) is another tool used to assess HRQoL that is sensitive to PAD-specific risk-factors and clinical change; it also helps discriminate between patients with or without symptoms.37,38 Considered a departure from previous questionnaires that focus on walking impairment, symptomatology, and/or physical, social, and emotional functioning, the PAD Quality of Life Questionnaire (PAD-QoL) emphasizes the perceived burden of the disease and its effects on well-being and HRQoL. It has been found to have strong internal consistency and construct validity.39,40 Other questionnaires include the Intermittent Claudication Questionnaire (ICQ), which evaluates physical limitations, anxiety, and activity interference in patients with claudication, and the Claudication Symptom Instrument (CSI), which assesses claudication in the leg or foot.12,41
Guideline-recommended uses of PROMS
In 2000, the TransAtlantic Inter-Society Consensus Document on Management of Peripheral Artery Disease (TASC I) was developed with the goal of providing recommendations for the evaluation, diagnosis, treatment, and follow-up of patients with PAD.42 The updated TASC II guidelines primarily emphasize the effect of treatment on the anatomic and physiologic status of affected limbs, as well as on patients’ ambulatory status as determined by conventional functional outcome measures.42 Less emphasized is the use of PROMs in clinical practice. Although the guidelines briefly comment that HRQoL should be considered the ideal primary endpoint in treatment effectiveness studies on PAD, they note a relative lack of accurate assessment tools for this endpoint. They recommend the use of SF-36 or WIQ to measure HRQoL among patients with claudication.42 It is notable that even more contemporary PAD society guidelines, such as the 2016 AHA/ACC Guidelines on the Management of Patients with Lower Extremity Peripheral Artery Disease, fail to discuss or incorporate the utility of PROMs to evaluate and monitor improvement in HRQoL.9
Prevalence of PROMs in PAD research
The body of literature on the application of PROMs in PAD in both research trials and clinical practice is limited (Table 2). Most treatment effectiveness studies in PAD emphasize anatomic outcomes rather than patient HRQoL. For instance, interventional trials rely on arterial patency to evaluate for efficacy, which may not correlate with abatement in patient symptoms. Improvements in symptoms and HRQoL are the principal benefits of these procedures in patients with stable disease and can be assessed with PROMs.
Table 2.
Integration of PROMs into PAD treatment effectiveness studies.
| Study | Population screened | Treatment arms | Functional outcome tools | PROMs tools | Follow-up period | Changes |
|---|---|---|---|---|---|---|
| Murphy (2015)10 (CLEVER study) | 111 patients with aortoiliac PAD, multicenter | Optimal medical care alone; both SET and medical care; or both revascularization and medical care | Treadmill-based walking, ABI | SF-12, WIQ, PAQ | 18 months | Combination arms had greater improvements in disease-specific PROMs; SET cohort had greater improvements in PAQ scores compared to revascularization cohort |
| Gray (2015)28 (STROLL study) | 250 subjects with de novo or restenotic lesions of superficial femoral artery, multicenter | One or more SMART® bare-metal stents | Rutherford/Becker classification, resting or exercise ABI, angiographic studies | PAQ, WIQ, EQ-5D, SF-12 | 3 years | PAQ summary scores showed significant health status gains at 1 month, with more than 85% of the gain maintained during a 3-year period |
| Mustapha (2019)43 (LIBERTY 360° study) | 1200 patients with claudication and CLI, multicenter | Treatment with an FDA-approved peripheral endovascular device intervention | Rutherford classification, angiography imaging, duplex ultrasonography | VascuQoL, EQ-5D | 12 months, up to 5 years | VascuQoL scores improved significantly from baseline to 30 days and persisted at 12 months across all domains in all groups, paralleling estimates of freedom from major amputation and maintenance of artery patency |
| Devine (2016)12 | 323 adults with moderate to severe claudication, multicenter | Medical management (including physician-recommended exercise therapy, smoking cessation, and/or medications) or surgical/endovascular revascularization | ABI | 3 domains of modified WIQ (distance, speed, stair-climbing); WIQ pain domain, VascuQoL, EQ-5D, CSI | 12 months | At 12 months, both the medical and revascularization groups had improved WIQ, VascuQoL, and EQ-5D scores, and fewer symptoms as measured by CSI, but the revascularization group had greater improvement as compared to medical management alone |
| McDermott (2018)30 (HONOR study) | 200 patients with PAD, multicenter | Usual care or a home-based exercise program with a wearable activity monitor and telephone coaching | 6-minute walk distance | Subscales of WIQ, SF-36, and PROMIS | 9 months | Exercise intervention was associated with a worse PROMIS pain interference score and a smaller degree of improvement in walking distance |
ABI, ankle–brachial index; CLI, critical limb ischemia; CSI, Claudication Symptom Instrument; EQ-5D, EuroQoL-5 Dimensions; EuroQoL, European Quality of Life; FDA, Food and Drug Administration; PAD, peripheral artery disease; PAQ, Peripheral Artery Questionnaire; PROMIS, Patient-Reported Outcomes Measurement Information System; PROMs, patient-reported outcome measures; SET, supervised exercise therapy; SF-12, Short Form-12; SF-36, Short Form-36; VascuQoL, Vascular Quality of Life Questionnaire; WIQ, Walking Impairment Questionnaire.
In the CLEVER trial, 111 patients with aortoiliac PAD were randomized to three treatment arms (optimal medical care alone, SET and optimal medical care, or stent revascularization and optimal medical care) across 29 centers in the United States and Canada with a follow-up period of 18 months.10 Outcome and clinical measurements included treadmill-based walking, ABI, and subjective HRQoL measured by both generic and PAD-specific questionnaires: the SF-12, WIQ, and PAQ. At 18 months, the authors found that the combination arms with SET or revascularization had greater improvements in disease-specific PROMs than the arm with medical therapy alone. Furthermore, the SET cohort had greater improvements in PAQ-symptoms, PAQ-treatment satisfaction, PAQ-QoL, and PAQ-summary scores as compared with the revascularization cohort.
In the prospective, observational STROLL study, 250 subjects with de novo or restenotic lesions of the superficial femoral artery across 39 centers were treated with one or more SMART® bare-metal stents (Cordis Corp, Miami Lakes, FL, USA) and evaluated 1 and 3 years post-treatment.28 Clinical outcomes were reported based on the Rutherford classification system, resting or exercise ABI measurements, and angiographic studies. Health status was evaluated with the PAQ, WIQ, EQ-5D, and SF-12. The study concluded that revascularization with the stent improved PAQ summary scores at 1 month, and more than 85% of the improvement was maintained over the 3-year follow-up period. Health benefits were similarly maintained as demonstrated by improvement in component scores on both PAD-specific and generic questionnaires. Notably, these gains mirrored the clinically determined improvements during the same period, such as lower rates of repeat revascularization and loss of target vessel patency.
In the prospective, post-market LIBERTY 360° trial, 1200 patients with claudication and CLI stratified by Rutherford classes were treated with a Food and Drug Administration-approved peripheral endovascular device intervention and followed over 12 months.43 Outcomes included freedom from major adverse events, defined as death, major amputation, and target vessel revascularization, as assessed with angiography imaging and duplex ultrasonography. HRQoL was measured with the VascuQoL and EQ-5D. Over the follow-up period, the VascuQoL scores improved significantly from baseline to 30 days and persisted at 12 months across all domains in all groups, paralleling estimates of freedom from major amputation and maintenance of arterial patency.
In a prospective, observational cohort study conducted at the University of Washington, 323 adults with moderate to severe claudication underwent either medical management (including physician-recommended exercise therapy, smoking cessation, and/or medications) or surgical/endovascular revascularization interventions.12 Primary outcomes assessed at baseline, 6 months, and 12 months were evaluated by three domains of the modified WIQ: distance, speed, and stair-climbing. Secondary outcomes were scores on the WIQ pain domain, VascuQoL, EQ-5D, and CSI. At 12 months, both the medical and revascularization groups had improved function as measured by the WIQ, improved HRQoL as measured by the VascuQoL and EQ-5D, and fewer symptoms as measured by the CSI. In addition, the revascularization group had greater improvement in these measures as compared with the medical management group alone.
Finally, in the Home-Based Monitored Exercise for PAD (HONOR) trial, 200 patients with PAD were randomized to undergo either usual care or a home-based exercise program with a wearable activity monitor and telephone coaching, and followed over a period of 9 months.30 The primary outcome was a change in 6-minute walk distance, and secondary outcomes were a change in the specific domains of the WIQ, SF-36, and Patient-Reported Outcomes Measurement Information System (PROMIS). PROMIS is a government-funded classification system that measures generic health status and aims to enhance and standardize the measurement of patient-reported outcomes, including pain interference, fatigue, physical functioning, emotional distress, and satisfaction with social participation, in patients with chronic diseases. Although limited by its use of generic measures that are generally less sensitive than disease-specific measures of PAD, the study found that exercise intervention was associated with a worse PROMIS pain interference score and a smaller degree of improvement in walking distance at 9 months.
Utilization of PROMs in registries
There are a number of large-scale PAD registries, originating from societies (American College of Cardiology’s National Cardiovascular Data Registry and Peripheral Vascular Intervention Registry and the Society for Vascular Surgery’s Vascular Quality Initiative), as well as investigator-initiated.44,45 However, few collect data on PROMs. To address the limitations of these registries, the Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories (PORTRAIT) study is prospectively collecting serial PAD-specific health status data from 1275 patients with abnormal ABIs and new, or worsened, claudication symptoms across 16 international centers.46 In addition to collecting PAD diagnostic information, the registry monitors health status over time using the PAQ and EQ-5D, as well as psychosocial status using the PHQ and GAD-7. Its aims are to examine variations in treatment by patient characteristics, quantify PAD-specific health status outcomes (symptoms, HRQoL, and function) in patients, and study the association between treatments and health status outcomes. The inclusion of PROMs provides a unique opportunity to better understand patient- and treatment-related factors that are associated with improved or worsened health status over time.
Challenges and potential solutions related to the implementation of PROMs in research settings
Previous studies administering PROMs to patients with PAD are limited by their design, methodology, and clinical interpretability. Owing to the range of questionnaires available to assess PAD-specific symptomology and functional limitations, the use of these tools in the research context has been inconsistent and non-standardized. PAD-specific questionnaires are often used in conjunction with more generic questionnaires. This increases the time needed to administer these instruments and potentially limits participation.
In a 2017 systematic review of randomized controlled trials that included HRQoL-measuring questionnaires, 14 different questionnaires were used in 31 studies to survey over 3000 patients.47 The most commonly utilized tool was the SF-36 (23 out of the 31 studies), followed by the WIQ (8 out of the 31). Three studies utilized two or more questionnaires. Notably, many of these studies did not fully report HRQoL data and ~24% of studies were missing at least one domain of these questionnaires. A 2016 systematic review analyzed 14 peer-reviewed studies that reported PROMs in patients with symptomatic PAD.48 Among 1594 patients, the most frequently utilized generic assessments were the SF-36 and EQ-5D, and the most common disease-specific questionnaires were the VascuQoL and WIQ. Instruments like the WIQ were limited by its lack of measures for PAD-related HRQoL.
Overall, the use of the PAQ or VascQoL is supported by prior literature. To measure other dimensions of health status, the inclusion of valid, brief, psychometrically sound instruments, such as the PHQ, may be added. Although the SF-36 has the most positive evidence favoring its use in a PAD population,48 generic questionnaires are much less sensitive and specific than disease-specific measures of HRQoL.38
Another key challenge is difficulty interpreting the significance of score changes in PROMs. The minimal clinically meaningful difference (MCID) represents the smallest threshold change in PROMs that patients consider beneficial. The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA)49 and Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients with HF with Reduced Ejection Fraction (DEFINE-HF)50 trials examined the absolute difference in proportions of patients achieving the MCID in disease-specific questionnaires (the SAQ and KCCQ, respectively), and defined the number of patients needed to treat. In the context of PAD, an eight-point change between the baseline and 1-year summary PAQ scores is considered the MCID. This benchmark is supported by prior work demonstrating that the medium effect size is represented by a change of 8% on the instrument’s 100-point scale.51 A recent study on a cohort of 120 patients with symptomatic PAD assigned to different exercise therapies examined the MCIDs for small, moderate, and large changes in the WIQ and SF-36.52 Using both distribution-and anchor-based statistical methods, they found that relatively higher sample sizes (~400–500 patients) were needed to detect meaningful changes with 80% power for specific domains of both questionnaires.
Additional studies with relatively large sample sizes are needed to better understand the clinical changes and prognostic significance of existing PAD-specific questionnaires. Furthermore, as MCIDs have been shown to vary with cohort characteristics like demographics, baseline PROMs scores, and disease severity, the context in which these values are derived must be adequately understood.53–55 This is especially important if PROMs are to be universally applied in research or clinical settings.56
Expanding upon the progress made with the VascuQoL,57 SAQ,58 and KCCQ,59 PROMs questionnaires should also be shortened, while preserving the psychometric properties of the full instrument. A 2011 meta-analysis of 20 studies analyzing response rates in relation to questionnaire length found that attrition rates were lower when shorter questionnaires were administered.60 This is especially important for patients with PAD who may find it difficult to complete long questionnaires due to multiple comorbidities or disabilities. Shortened tools are also useful within the time-limited nature of the clinic.
Finally, disease-specific PROMs have primarily been validated in patients with claudication, while there remains a need for validated questionnaires in patients with CLI. This subtype of patients has unique issues (e.g. wound healing, rest pain, etc.) and multiple chronic comorbid conditions, which are not adequately captured in the domains of available questionnaires. There is also a need for more treatment effectiveness studies utilizing PROMs among patients with CLI. Despite increased utilization of endovascular procedures in recent years, prior studies of these patients have typically examined open surgical revascularization. The BASIL trial is a notable exception in which outcomes of amputation-free survival after angioplasty versus surgery were studied in 452 patients with severe lower limb ischemia.11 Patients demonstrated sustained improvement in all VascuQoL domains, SF-36 health domains, and EQ-5D at the 3-year follow-up period, with the largest gains seen within 3 months.29 No statistically significant differences were noted between the two treatment groups. As the paradigm shifts toward the use of validated PROMs to guide optimal treatment strategies in PAD, additional studies are needed to advance understanding on how to better manage and treat this unique cohort.
Challenges and potential solutions related to the implementation of PROMs in clinical settings
In recent years, there has been growing interest in the implementation of PROMs to supplement routine clinical assessments of chronic diseases, such as PAD. At Beth Israel Deaconess Medical Center’s Vascular Medicine Section, there is an ongoing effort to establish a user-friendly, electronically based system to administer the PAQ in the routine course of care for symptomatic patients with PAD recommended to undergo SET or revascularization.61 Patients are asked to fill out a baseline REDCAP survey on their phone or computer, and are subsequently emailed follow-up surveys at pre-determined intervals. This early feasibility study aims to assess the yield of integrating PROMs into the clinical setting, as well as determine the degree of benefit and predictors of symptom improvement after treatment.
In two pilot studies administering the SAQ, RDS, and PHQ-2 via tablet or phone to patients undergoing elective coronary angiography, Blumenthal et al. discussed the challenges of implementing programs in existing clinic workflows from an administrative viewpoint.62 Barriers included the perception of increased workload by staff, hospital technological requirements (specifically the daily reconfiguration of tablets to ensure data privacy), and patients’ difficulty self-administering the survey due to disability or language barriers. They also noted difficulty engaging patients in follow-up, yielding absolute response rates between 34% and 42%. A literature review of 14 studies that implemented PROMs in routine oncology care described similar barriers.63 At the system-wide level, the most common issues were lack of integration of PROMs into clinical workflow and inadequate technological infrastructure to efficiently collect information. Major challenges faced by patients included the time required and inability to complete the surveys on electronic devices. Finally, healthcare providers reported insufficient time and knowledge to interpret and explain patient-reported data to their patients. A summary of these barriers is displayed in Figure 1.
Figure 1.
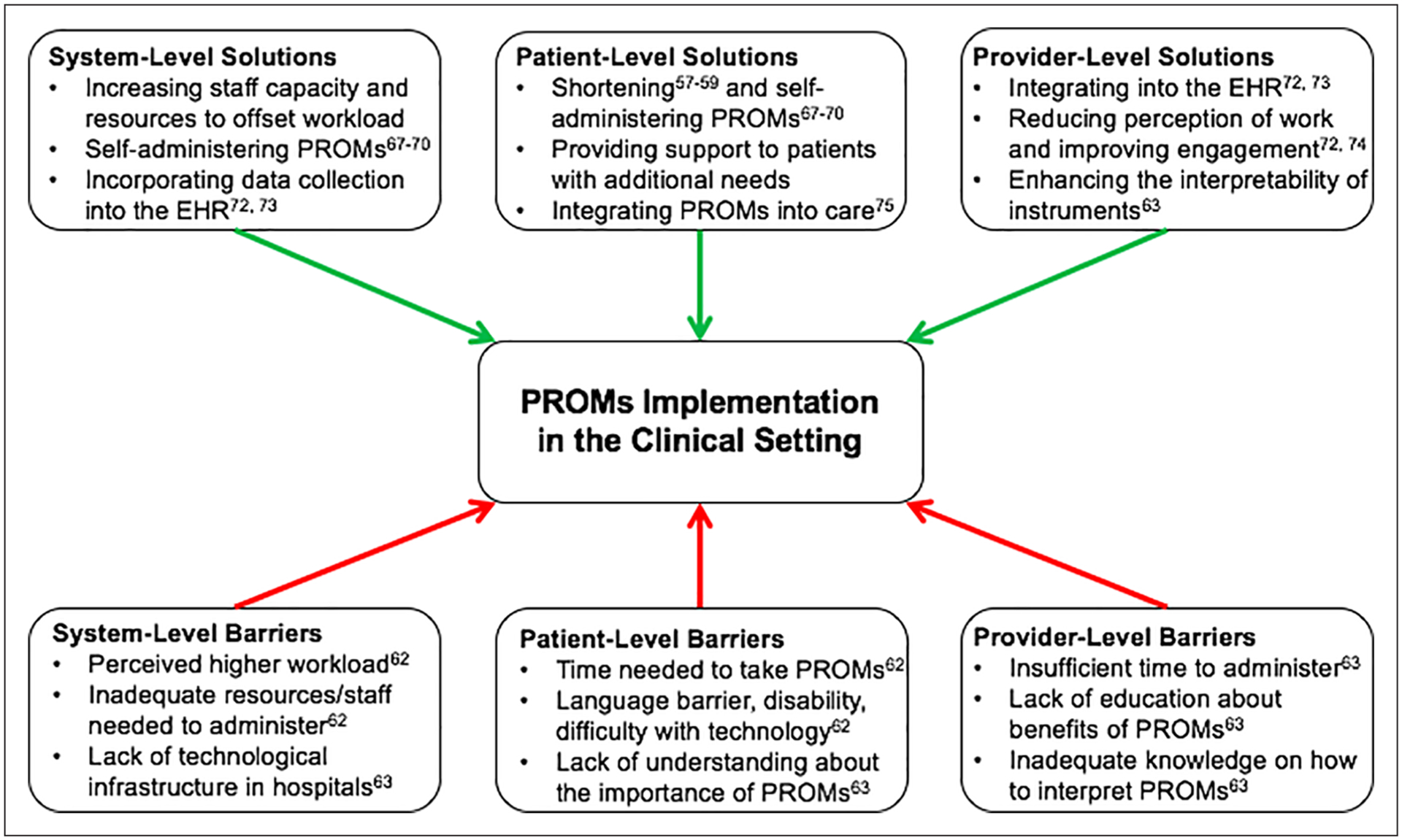
Barriers and solutions related to the implementation of PROMs in the clinical setting. EHR, electronic health records; PROMs, patient-reported outcome measures.
Potential solutions to improve integration of PROMs at the system- and patient-level include increasing staff capacity and resources to offset the workload, incorporating data collection into existing electronic health records systems, providing patients with the flexibility to complete questionnaires in their own time and on their personal devices, and employing shortened questionnaires. Although prior studies found that patients who were administered PROMs by interviewers reported lower health impairment than those who self-administered the surveys,64–66 many studies found no difference.67–70 In a 2011 prospective cohort study of 2261 participants with AIDS who completed multiple PROMs instruments, including the EQ-5D-5L, the authors reported no meaningful differences in scores between administration formats.71 Although similar studies on patients with PAD are needed, the current evidence supports the continued use of self-administered PROMs in the clinical setting, thus minimizing the burden on both research staff and participants. However, support should be provided to patients with language barriers, disabilities, or difficulty using technology, as these are all potential barriers to self-administration.
To incentivize participation by healthcare providers, educational sessions centered on the benefits of PROMs are needed. Prior studies have demonstrated that the regular implementation of PROMs in the clinical setting can improve workflow efficiency and save time. This is due to the timely identification of patients’ needs through positive responses to PROMs questions. Integration of PROMs into electronic health records with user-friendly interfaces also allows providers to input and receive results in real time.72,73 Discussing PROMs with patients and incorporating results into counseling sessions have also been shown to improve the patient–physician relationship and professional satisfaction.72,74 In a case report outlining the 5-year effort at Partners HealthCare to implement PROMs, Wagle described how PROMs results can be converted into numerical and visual graphics aimed at clarifying the risks and benefits of treatments. He noted that response rates approached 100% among providers who regularly utilized these data to aid in shared decision-making.75
In addition to the perceived time intensiveness of utilizing PROMs, healthcare providers commonly cite a lack of education about the appropriate thresholds for score changes to guide their clinical decision-making.63 Available MCIDs from prior studies should be disseminated widely, with the added caveat that they cannot be applied to every patient. As providers see patients with varying disease severity and baseline HRQoL in the clinical setting, it should also be acknowledged that additional studies on diverse cohorts of patients with PAD are needed before these thresholds can be broadly applied. Overall, reducing healthcare providers’ perception of additional workload, improving their engagement, and enhancing interpretability of instruments are important factors in facilitating the integration of PROMs into the clinical setting. A summary of potential solutions can be found in Figure 1.
Conclusion
PROMs can play a critical role in the evaluation, treatment selection, and monitoring of patients with symptomatic PAD. There is a need to design standardized approaches to develop and validate PROMs for use in PAD. Given the significant impact of this disease on HRQoL, it is essential that PROMs be more readily integrated into the research and clinical landscape as a means to assess the clinical effectiveness and durability of treatments. The implementation of PROMs in routine clinical care can pave the way forward for patient-centered care and shared decision-making in PAD.
Funding
Eric Secemsky is funded by the NIH/NHLBI K23HL150290.
Footnotes
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Eric A. Secemsky: Consulting/Speakers Bureau/Advisory Board: Bard, Cook, CSI, Janssen, Medtronic, Philips; Grants to Institution: AstraZeneca, Bard, Boston Scientific, Cook, CSI, Medtronic, Philips. Robert W. Yeh: Consulting/Scientific Advisory Board/Research Grants: Abbott Vascular, AstraZeneca, Boston Scientific, Medtronic; Research Grants: Bard, Cook, Philips. The other authors have nothing to disclose.
References
- 1.Olin JW, White CJ, Armstrong EJ, et al. Peripheral artery disease: Evolving role of exercise, medical therapy, and endovascular options. J Am Coll Cardiol 2016; 67: 1338–1357. [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Conte MS, Cutlip DE, et al. Evaluation and treatment of patients with lower extremity peripheral artery disease: Consensus definitions from Peripheral Academic Research Consortium (PARC). J Am Coll Cardiol 2015; 65: 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shu J, Santulli G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 2018; 275: 379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olin JW, Sealove BA. Peripheral artery disease: Current insight into the disease and its diagnosis and management. Mayo Clin Proc 2010; 85: 678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duff S, Mafilios MS, Bhounsule P, et al. The burden of critical limb ischemia: A review of recent literature. Vasc Health Risk Manag 2019; 15: 187–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zemaitis MR, Boll JM, Dreyer MA. Peripheral arterial disease. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 7.McDermott MM. Exercise rehabilitation for peripheral artery disease: A review. J Cardiopulm Rehabil Prev 2018; 38: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treat-Jacobson D, McDermott MM, Beckman JA, et al. Implementation of Supervised Exercise Therapy for Patients With Symptomatic Peripheral Artery Disease: A Science Advisory From the American Heart Association. Circulation 2019; 140: e700–e710. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017; 135: e686–e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: The CLEVER study. J Am Coll Cardiol 2015; 65: 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): Multicentre, randomised controlled trial. Lancet 2005; 366: 1925–1934. [DOI] [PubMed] [Google Scholar]
- 12.Devine EB, Alfonso-Cristancho R, Yanez ND, et al. Effectiveness of a medical vs revascularization intervention for intermittent leg claudication based on patient-reported outcomes. JAMA Surg 2016; 151: e162024. [DOI] [PubMed] [Google Scholar]
- 13.Mays RJ, Casserly IP, Kohrt WM, et al. Assessment of functional status and quality of life in claudication. J Vasc Surg 2011; 53: 1410–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu A, Coresh J, Selvin E, et al. Lower extremity peripheral artery disease and quality of life among older individuals in the community. J Am Heart Assoc 2017; 6: e004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aquarius AE, De Vries J, Henegouwen DP, et al. Clinical indicators and psychosocial aspects in peripheral arterial disease. Arch Surg 2006; 141: 161–166; discussion 166. [DOI] [PubMed] [Google Scholar]
- 16.Mehta T, Venkata Subramaniam A, Chetter I, et al. Assessing the validity and responsiveness of disease-specific quality of life instruments in intermittent claudication. Eur J Vasc Endovasc Surg 2006; 31: 46–52. [DOI] [PubMed] [Google Scholar]
- 17.Karimi M, Brazier J. Health, health-related quality of life, and quality of life: What is the difference? Pharmacoeconomics 2016; 34: 645–649. [DOI] [PubMed] [Google Scholar]
- 18.Brédart A, Marrel A, Abetz-Webb L, et al. Interviewing to develop Patient-Reported Outcome (PRO) measures for clinical research: Eliciting patients’ experience. Health Qual Life Outcomes 2014; 12: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black N Patient reported outcome measures could help transform healthcare. BMJ 2013; 346: f167. [DOI] [PubMed] [Google Scholar]
- 20.Qintar M, Grantham JA, Sapontis J, et al. Dyspnea among patients with chronic total occlusions undergoing percutaneous coronary intervention: Prevalence and predictors of improvement. Circ Cardiovasc Qual Outcomes 2017; 10: e003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green CP, Porter CB, Bresnahan DR, et al. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: A new health status measure for heart failure. J Am Coll Cardiol 2000; 35: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 22.Arroll B, Goodyear-Smith F, Crengle S, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med 2010; 8: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutter LA, Brown TA. Psychometric properties of the Generalized Anxiety Disorder Scale-7 (GAD-7) in outpatients with anxiety and mood disorders. J Psychopathol Behav Assess 2017; 39: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huo T, Guo Y, Shenkman E, et al. Assessing the reliability of the Short Form 12 (SF-12) health survey in adults with mental health conditions: A report from the wellness incentive and navigation (WIN) study. Health Qual Life Outcomes 2018; 16: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagy KV, Szeplaki G, Perge P, et al. Quality of life measured with EuroQol-five dimensions questionnaire predicts long-term mortality, response, and reverse remodelling in cardiac resynchronization therapy patients. Europace 2018; 20: 1506–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wann-Hansson C, Hallberg IR, Risberg B, et al. A comparison of the Nottingham Health Profile and Short Form 36 Health Survey in patients with chronic lower limb ischaemia in a longitudinal perspective. Health Qual Life Outcomes 2004; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prcic A, Aganovic D, Hadziosmanovic O. Sickness Impact Profile (SIP) score, a good alternative instrument for measuring quality of life in patients with ileal urinary diversions. Acta Inform Med 2013; 21: 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray WA, Feiring A, Cioppi M, et al. and STROLL Study Investigators. S.M.A.R.T. self-expanding nitinol stent for the treatment of atherosclerotic lesions in the superficial femoral artery (STROLL): 1-year outcomes. J Vasc Interv Radiol 2015; 26: 21–28. [DOI] [PubMed] [Google Scholar]
- 29.Forbes JF, Adam DJ, Bell J, et al. and BASIL trial Participants. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: Health-related quality of life outcomes, resource utilization, and cost-effectiveness analysis. J Vasc Surg 2010; 51: 43S–51S. [DOI] [PubMed] [Google Scholar]
- 30.McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: The HONOR randomized clinical trial. JAMA 2018; 319: 1665–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regensteiner JG, Steiner JF, Panzer RJ, et al. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol 1990; 2: 142–152. [Google Scholar]
- 32.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg 1996; 23: 104–115. [DOI] [PubMed] [Google Scholar]
- 33.Criqui MH, Denenberg JO, Bird CE, et al. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med 1996; 1: 65–71. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001; 286: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 35.Larsen ASF, Reiersen AT, Jacobsen MB, et al. Validation of the Vascular Quality of Life Questionnaire – 6 for clinical use in patients with lower limb peripheral arterial disease. Health Qual Life Outcomes 2017; 15: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan MB, Crayford T, Murrin B, et al. Developing the Vascular Quality of Life Questionnaire: A new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg 2001; 33: 679–687. [DOI] [PubMed] [Google Scholar]
- 37.Spertus J, Jones P, Poler S, et al. The peripheral artery questionnaire: A new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J 2004; 147: 301–308. [DOI] [PubMed] [Google Scholar]
- 38.Hoeks SE, Smolderen KG, Scholte Op Reimer WJ, et al. Clinical validity of a disease-specific health status questionnaire: The peripheral artery questionnaire. J Vasc Surg 2009; 49: 371–377. [DOI] [PubMed] [Google Scholar]
- 39.Nehler MR, McDermott MM, Treat-Jacobson D, et al. Functional outcomes and quality of life in peripheral arterial disease: Current status. Vasc Med 2003; 8: 115–126. [DOI] [PubMed] [Google Scholar]
- 40.Treat-Jacobson D, Lindquist RA, Witt DR, et al. The PADQOL: Development and validation of a PAD-specific quality of life questionnaire. Vasc Med 2012; 17: 405–415. [DOI] [PubMed] [Google Scholar]
- 41.Chong PF, Garratt AM, Golledge J, et al. The intermittent claudication questionnaire: A patient-assessed condition-specific health outcome measure. J Vasc Surg 2002; 36: 764–771; discussion 863–864. [PubMed] [Google Scholar]
- 42.Norgren L, Hiatt WR, Dormandy JA, et al. and TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45(Suppl S): S5–67. [DOI] [PubMed] [Google Scholar]
- 43.Mustapha J, Gray W, Martinsen BJ, et al. One-year results of the LIBERTY 360 study: Evaluation of acute and midterm clinical outcomes of peripheral endovascular device interventions. J Endovasc Ther 2019; 26: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desai SS, Kaji AH, Upchurch G Jr. Practical guide to surgical data sets: Society for Vascular Surgery Vascular Quality Initiative (SVS VQI). JAMA Surg 2018; 153: 957–958. [DOI] [PubMed] [Google Scholar]
- 45.Bhardwaj B, Spertus JA, Kennedy KF, et al. Bleeding complications in lower-extremity peripheral vascular interventions: Insights from the NCDR PVI Registry. JACC Cardiovasc Interv 2019; 12: 1140–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smolderen KG, Gosch K, Patel M, et al. PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories): Overview of design and rationale of an international prospective peripheral arterial disease study. Circ Cardiovasc Qual Outcomes 2018; 11: e003860. [DOI] [PubMed] [Google Scholar]
- 47.Harwood AE, Totty JP, Broadbent E, et al. Quality of life in patients with intermittent claudication. Gefasschirurgie 2017; 22: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poku E, Duncan R, Keetharuth A, et al. Patient-reported outcome measures in patients with peripheral arterial disease: A systematic review of psychometric properties. Health Qual Life Outcomes 2016; 14: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ISCHEMIA Trial Research Group, Maron DJ, Hochman JS, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J 2018; 201: 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: The DEFINE-HF Trial. Circulation 2019; 140: 1463–1476. [DOI] [PubMed] [Google Scholar]
- 51.Sloan J, Symonds T, Vargas-Chanes D, et al. Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Drug Inf J 2003; 37: 23–31. [Google Scholar]
- 52.Gardner AW, Montgomery PS, Wang M. Minimal clinically important differences in treadmill, 6-minute walk, and patient-based outcomes following supervised and home-based exercise in peripheral artery disease. Vasc Med 2018; 23: 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beaton DE, Boers M, Wells GA. Many faces of the minimal clinically important difference (MCID): A literature review and directions for future research. Curr Opin Rheumatol 2002; 14: 109–114. [DOI] [PubMed] [Google Scholar]
- 54.Niebauer K, Dewilde S, Fox-Rushby J, et al. Impact of omalizumab on quality-of-life outcomes in patients with moderate-to-severe allergic asthma. Ann Allergy Asthma Immunol 2006; 96: 316–326. [DOI] [PubMed] [Google Scholar]
- 55.Nordin A, Taft C, Lundgren-Nilsson A, et al. Minimal important differences for fatigue patient reported outcome measures – A systematic review. BMC Med Res Methodol 2016; 16: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Revicki D, Hays RD, Cella D, et al. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008; 61: 102–109. [DOI] [PubMed] [Google Scholar]
- 57.Nordanstig J, Wann-Hansson C, Karlsson J, et al. Vascular Quality of Life Questionnaire-6 facilitates health-related quality of life assessment in peripheral arterial disease. J Vasc Surg 2014; 59: 700–707. [DOI] [PubMed] [Google Scholar]
- 58.Chan PS, Jones PG, Arnold SA, et al. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes 2014; 7: 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spertus JA, Jones PG. Development and validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes 2015; 8: 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rolstad S, Adler J, Ryden A. Response burden and questionnaire length: Is shorter better? A review and meta-analysis. Value Health 2011; 14: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 61.Raja A, Chowdhury M, Carroll B, et al. Improvement in patient reported outcome measures following supervised exercise therapy or peripheral vascular intervention for symptomatic peripheral artery disease. Poster 102-B. Vasc Med 2020; 25: NP1–NP31. [Google Scholar]
- 62.Blumenthal DM, Strom JB, Valsdottir LR, et al. Patient-reported outcomes in cardiology. Circ Cardiovasc Qual Outcomes 2018; 11: e004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen H, Butow P, Dhillon H, et al. A review of the barriers to using Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs) in routine cancer care. J Med Radiat Sci. Epub ahead of print 19 August. DOI: 10.1002/jmrs.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheung YB, Goh C, Thumboo J, et al. Quality of life scores differed according to mode of administration in a review of three major oncology questionnaires. J Clin Epidemiol 2006; 59: 185–191. [DOI] [PubMed] [Google Scholar]
- 65.Feveile H, Olsen O, Hogh A. A randomized trial of mailed questionnaires versus telephone interviews: Response patterns in a survey. BMC Med Res Methodol 2007; 7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bowling A Mode of questionnaire administration can have serious effects on data quality. J Public Health (Oxf) 2005; 27: 281–291. [DOI] [PubMed] [Google Scholar]
- 67.Weinberger M, Oddone EZ, Samsa GP, et al. Are health-related quality-of-life measures affected by the mode of administration? J Clin Epidemiol 1996; 49: 135–140. [DOI] [PubMed] [Google Scholar]
- 68.Gundy CM, Aaronson NK. Effects of mode of administration (MOA) on the measurement properties of the EORTC QLQ-C30: A randomized study. Health Qual Life Outcomes 2010; 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Irvine EJ, Feagan BG, Wong CJ. Does self-administration of a quality of life index for inflammatory bowel disease change the results? J Clin Epidemiol 1996; 49: 1177–1185. [DOI] [PubMed] [Google Scholar]
- 70.Wu AW, Jacobson DL, Berzon RA, et al. The effect of mode of administration on medical outcomes study health ratings and EuroQol scores in AIDS. Qual Life Res 1997; 6: 3–10. [DOI] [PubMed] [Google Scholar]
- 71.Puhan MA, Ahuja A, Van Natta ML, et al. and Studies of Ocular Complications of AIDS Research Group. Interviewer versus self-administered health-related quality of life questionnaires – does it matter? Health Qual Life Outcomes 2011; 9: 30.21554737 [Google Scholar]
- 72.Rotenstein LS, Huckman RS, Wagle NW. Making patients and doctors happier – The potential of patient-reported outcomes. N Engl J Med 2017; 377: 1309–1312. [DOI] [PubMed] [Google Scholar]
- 73.Abernethy AP, Herndon JE 2nd, Wheeler JL, et al. Improving health care efficiency and quality using tablet personal computers to collect research-quality, patient-reported data. Health Serv Res 2008; 43: 1975–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Derksen FA, Olde Hartman TC, Bensing JM, et al. Managing barriers to empathy in the clinical encounter: A qualitative interview study with GPs. Br J Gen Pract 2016; 66: e887–e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagle NW. Implementing patient-reported outcome measures. NEJM Catal Innov Care Deliv 2017; October, https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0373 (accessed November 21, 2020).


