Abstract
Ecological stress during adolescent development may increase the sensitivity to negative emotional processes that can contribute to the onset and progression of internalizing behaviors during preadolescence. Although a small number of studies have considered the link among the relations between ecological stress, amygdala reactivity and internalizing symptoms in childhood and adolescence, these studies have largely been small, cross-sectional, and often do not consider unique roles of parenting or sex. In the current study, we evaluated the interrelations between ecological stress, amygdala functioning, subsequent internalizing symptoms, and the moderating roles of parenting and sex among 9- and 10-year-old preadolescents from the Adolescent Brain Cognitive Development (ABCD) Study ®. A subset of participants who met a priori quality control criteria for bilateral amygdala activation during the EN-back faces versus places contrast (N = 7,385; Mean Age = 120 months, SD = 7.52; 49.5% Female) were included in the study. A confirmatory factor analysis was performed to create a latent variable of ecological stress, and multiple structural equation models were tested to evaluate the association among baseline ecological stress and internalizing symptoms one year later, the mediating role of amygdala activity, and moderating effects of parental acceptance and sex. The results revealed a significant association between ecological stress and subsequent internalizing symptoms, which was greater in males than females. There was no association between amygdala activity during the Faces versus Places contrast and ecological stress or subsequent internalizing symptoms, and no mediating role of amygdala or moderating effect of parental acceptance on the association between ecological stress and internalizing symptoms. An alternative mediation model was tested which revealed that there was a small mediating effect of parental acceptance on the association between ecological stress and internalizing symptoms, demonstrating lower internalizing symptoms among preadolescents one year later. Given the lack of association in brain function, ecological stress and internalizing symptoms in preadolescents in this registered report, effects from comparable small studies should be reconsidered in larger samples.
Keywords: Preadolescence, Internalizing, Environment, Amygdala, Sex, Parenting
1. Introduction
The prevalence of internalizing problems, such as anxiety and depression, increases in preadolescence and becomes more pronounced during adolescence (Costello et al., 2003; Cyranowski et al., 2000; Kessler et al., 2012; Merikangas et al., 2010; Spear, 2000). The increased sensitivity of negative emotional processes during adolescence may have long-term maladaptive health outcomes (Tottenham & Galván, 2016), which can contribute to the onset and progression of chronic disease (Duffy et al., 2018). Ecological stress, defined here as elements of an individual’s environment (e.g. family, neighborhood, community) may have adverse effects on preadolescent emotional development (Caspi et al., 1987). Identifying the effects of ecological stress and neural mechanisms during preadolescence which may contribute to individual differences in emotional sensitivity, and how they both link to the emergence of internalizing problems could provide an important step towards early prognosis and preventive intervention.
Different efforts have demonstrated the adverse impacts of early ecological stressors on internalizing problems (Duffy et al., 2018; McEwen, 2012; McEwen & Gianaros, 2011) and amygdala functioning (McEwen & Gianaros, 2011; Swartz et al., 2015). Yet, despite recent evidence demonstrating that early adolescence is a behavioral and neurodevelopmental period by which individuals are highly sensitive to the influence of ecological stress (Gee & Casey, 2015), there is a paucity of studies linking the complex relations among ecological stress, amygdala reactivity and internalizing problems in preadolescence. Swartz and colleagues (2015) investigated how ecological stress, the brain, and internalizing symptoms may operate together, whereby amygdala reactivity was a mechanism through which stressful life events were associated with prospective internalizing problems among young adults. This work offered evidence of how amygdala reactivity was a mechanism that modified the impacts of ecological stress on internalizing problems. However, this study was conducted in a college sample and so it is unclear how these effects operate during the critical transitional period of preadolescence when internalizing symptoms become more prevalent.
Based on the framework of an ecological-transactional model (Lynch & Cicchetti, 1998), the development of internalizing problems may be a dynamic process influenced by ecological factors and neural mechanisms. Specifically, intermediate experiences may be necessary to sustain the progression of vulnerability (Hostinar & Miller, 2019) and maladaptive brain mechanisms (e.g., amygdala reactivity) may in turn sustain or amplify the initial vulnerability diathesis (Guyer, 2020). The overarching goal of this study is to examine how ecological stress relates to internalizing symptoms and amygdala function among preadolescents, and whether amygdala function may be a neural mechanism mediating the adverse impact of ecological stress on preadolescents’ internalizing symptoms.
According to the allostatic load perspective, cumulative stress factors, as opposed to specific ecological risks, are more predictive of a child’s developmental outcomes (Evans et al., 2013; Gach et al., 2018). Cumulative stress, however, is a heterogeneous concept (Evans et al., 2013) and various types of stress, such as, parental psychopathology (Boecker et al., 2014), family function (Farber et al., 2019; Vidal-Ribas et al., 2019), socioeconomic status (Noble et al., 2015), childhood neglect (McLaughlin et al., 2017; Zeanah et al., 2009) and/or abuse (J. A. Cohen et al., 2012; Jaffee, 2017) may have differential effects on symptom development and amygdala functioning (Farah, 2018; McLaughlin et al., 2017). Thus, it is important to pinpoint how specific types of stressors (i.e., ecological stress) may influence neural-behavioral development within a comprehensive model (McLaughlin et al., 2017). Specifically, our study focuses on understanding the adverse impact of ecological stress on brain-behavioral development for two reasons: 1) ecological stress tends to be a chronic stressor that affects the course of development and poses a high risk for intergenerational transmission, therefore understanding the adverse impact of ecological stress on brain-behavioral functioning may shed light on intervention strategies; and 2) among different types of stressors, ecological stress is one of the most common stressors across diverse populations and geographic locations, thus having strong implications for social policy.
While understanding how amygdala reactivity may mediate the effect of ecological stress and preadolescents’ internalizing problems is a fruitful start, work suggests that the role of parenting and sex may be important to consider. Prior studies have shown that parenting may moderate the association between stress and amygdala functioning (Farber et al., 2019) and buffer against the development of internalizing symptoms (Gorostiaga et al., 2019). Moreover, internalizing problems affect twice as many girls as boys beginning in late childhood, foreshadowing higher rates of anxiety and depression in females compared to males in adolescence (Pine et al., 1998) and into adulthood (Beesdo et al., 2009; Wittchen et al., 1998). Greater propensity for internalizing problems in females than in males may stem from differences in neural circuitry (Bangasser & Valentino, 2014). Given these findings, using an ecological-transactional model, as well as examining the impact of ecological stress on amygdala-internalizing outcomes, we examine how parenting and sex may moderate the complex associations among ecological stress, amygdala and internalizing problems. We use a large representative sample from the Adolescent Brain Cognitive Development (ABCD) Study to best capture variability in ecological stress and its impact on brain and behavior. How ecological stress is related to children’s internalizing symptoms and amygdala reactivity, and a rationale for how positive parenting and sex may moderate these links, is reviewed next.
1.1. The Impact of Multiple Ecological Stressors on Internalizing Problems and Amygdala Reactivity
Increasing evidence has demonstrated that exposure to multiple ecological risk factors (such as socioeconomic status [SES] and/or disadvantageous communities) has detrimental effects on children’s neuro-behavioral development beyond the effect of any singular risk factor (Evans et al., 2016; Gach et al., 2018). Specifically, childhood cumulative stress has been found to predict higher levels of internalizing problems among preadolescents (Evans et al., 2013). The impact of multiple risk factor exposure is often measured using a cumulative stress approach by dichotomizing each risk factor exposure and then summing the dichotomous scores. While this approach is good for parsimony and for preserving statistical power, it is sample- and context-specific. For example, the “cut-off’ threshold for high versus low income would be different if the sample was recruited from high versus low SES neighborhoods. This dichotomized approach thus may not be the most informative approach when using the ABCD sample that was recruited across multiple sites and diverse socio-demographic regions. An alternative approach that takes advantage of the characteristics of the ABCD sample is to use latent variable modeling to understand how multiple ecological factors and the underlying latent construct of ecological stress is associated with preadolescent internalizing symptoms and amygdala activity. Using a latent construct consisting of household financial strain, neighborhood problems and maternal psychological distress, Loukas and colleagues (2008) found that exposure to this multiple risk latent construct among 10- to 14- year old youths predicted subsequent internalizing symptoms 16 months later. Our study therefore first uses confirmatory factor analysis (CFA) to examine whether multiple ecological stress indicators available in the ABCD study consisting of household-income, neighborhood-SES-disadvantage, and neighborhood-safety comprise a coherent latent construct, to consider whether brain and behavior correlates can then be identified.
The brain plays a pivotal role in efferent and afferent processing of ecological factors, making its architecture a focal point of the effects of stress (McEwen et al., 2015). Recent reviews demonstrate the increasing role that ecological factors play on neural development (Farah, 2018; McEwen et al., 2015; McEwen & Akil, 2020), including its role in emotional processes, implicating the amygdala as one region whose function is often related to stress (Tottenham & Galván, 2016; Tottenham & Sheridan, 2010). Decreased family income in preadolescence has been shown to be associated with heightened activation in the amygdala to facial stimuli later in life (Kim et al., 2013). Moreover, Evans and colleagues (2016) demonstrated that preadolescent ecological stress (averaged across 9 and 13 years of age) was associated with elevated amygdala reactivity to emotional faces in adulthood. Likewise, in an epidemiologic cohort examining long-term outcomes of risk factors, Holz and colleagues (2017) reported that the accumulated psychosocial risks from 3-months until 11 years of age were inversely associated with amygdala reactivity in young adulthood. Although earlier studies evaluated ecological stress in childhood on the presentation of altered emotional reactivity in later development, there is limited evidence about neural reactivity during critical developmental stages. The ABCD sample offers a unique opportunity to evaluate the transitional window from childhood to adolescence which may have long-term implications for neural functioning in emotional processing, as well as potentially presenting high-value targets for intervention and prevention.
1.2. Parenting as a Moderator of Risk
Parenting is an important factor that may modify the development of emotional reactivity to stress in preadolescence (Fox et al., 2010). Specifically, parental warmth (or parental acceptance) may be one of the protective factors that may reduce the adverse effect of stress on emotional reactivity (as indexed by amygdala structures and functions; Farber et al., 2019; Whittle et al., 2014), and the development of internalizing symptoms (Gorostiaga et al., 2019). For example, Whittle and colleagues (2014) examined the associations among disadvantaged communities, maternal behavior and amygdala volumes across adolescent development. They found that positive maternal interactions moderated the association between community disadvantages and amygdala volume, whereby youth in a community with higher disadvantages had similar amygdala volumes relative to those in lower SES communities when they experienced positive parenting. With respect to functional activation, Farber and colleagues (2019) further provided evidence that positive parenting serves an important role in stress and amygdala functioning by reducing amygdala reactivity to interpersonal emotional faces beyond ecological stress factors. These results offer preliminary evidence that parenting may moderate how the amygdala may react to emotional stimuli in an ecologically stressful environment.
Nevertheless, it is still unclear how the moderating effect of parenting on stress-amygdala reactivity may contribute to internalizing symptoms. According to a recent review by Gorostiaga and colleagues (2019), parental warmth may be one important factor that attenuates the rate of internalizing problems. As evidence for this, Hipwell and colleague’s (2008) meta-analysis of 141 studies found that higher parental warmth was associated with fewer internalizing symptoms. It is therefore important to examine whether parental warmth (or parental acceptance) may moderate the link between ecological stress and amygdala reactivity, as well as between amygdala reactivity and internalizing symptoms, in order to better understand the mechanisms that explain the buffering effect of parental warmth. This is especially critical to explore during preadolescence given that it is a time of high neural plasticity (Lillard & Erisir, 2011) when ecological factors may have direct effects on their neural and behavioral development (Hackman et al., 2010).
1.3. Moderating Role of Sex on Mechanisms of Risk
Understanding the relationship between ecological stress and internalizing symptoms requires understanding differences between sexes. It is well-documented that internalizing problems are more prevalent in females than males (Beesdo et al., 2009; Costello et al., 2003), a difference first emerges during adolescence (Essau et al., 2010), and is differentially affected by economic stressors in late childhood/preadolescence (Leventhal & Brooks-Gunn, 2011; Schneider et al., 2015). While it remains unclear whether sex differences in internalizing symptoms stem from differences in neural circuitry (e.g., amygdala reactivity), two particularly salient factors could be the effects of ecological stress and parenting. For instance, using a daily diary method, girls’ negative family interactions partially explained differences in internalizing symptoms, and positive family interactions reduced sex differences in internalizing symptoms (Telzer & Fuligni, 2013). In the case of income-to-needs ratio and cortical development, Whittle and collegues (2017) found that parenting served as a significant buffer in males but not in females. Collectively, when attempting to model why parenting and stress could affect internalizing symptoms, it is therefore important to consider sex differences in how parenting may moderate stress-amygdala-internalizing associations.
1.4. Current Study
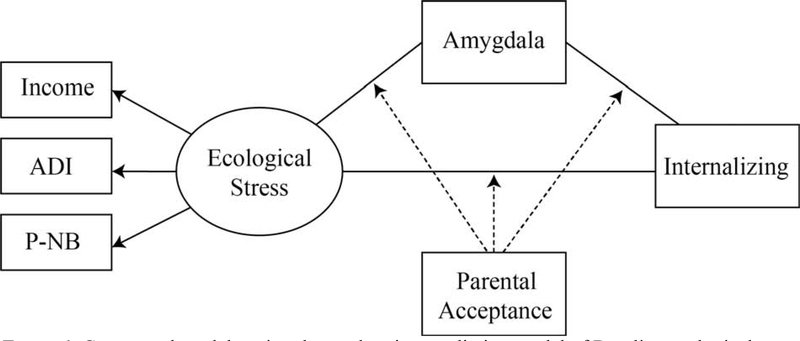
The current study evaluated the effects of an aggregated ecological stress factor on behavioral outcomes, neural mechanisms, and buffers in a large, representative sample of youth (Figure 1). Due to previous studies relying on variable definitions of ecological stress (Boecker et al., 2014; J. A. Cohen et al., 2012; Farber et al., 2019; Jaffee, 2017; Marshall et al., 2018; McLaughlin et al., 2017; Noble et al., 2015; Odgers et al., 2012; Vidal-Ribas et al., 2019; Zeanah et al., 2009), we use a multi-source latent factor that assesses ecological stress at the individual and neighborhood levels. When creating a comprehensive metric of individual ecological stress, it is critical to account for neighborhood-level resources and characteristics, such as safety, because they provide relevant and distinct information in addition to an individual’s report of income (Braveman et al., 2005; Kachmar et al., 2019). We hypothesized that higher ecological stress (as indexed by our latent construct) would be associated with higher parent-reported child internalizing symptoms (Hypothesis 1). Subsequently, we evaluated the mediating role of the amygdala on the association between ecological stress and internalizing symptoms. We hypothesized that increased ecological stress will be associated with increased amygdala activity, which in turn will be related to increased internalizing symptoms at 1-year follow-up (Hypothesis 2–3), and that consequently the direct relationship between ecological stress and internalizing will be significantly reduced (Hypothesis 4). Based on the evidence of parental warmth/acceptance functioning as a moderator of internalizing problems (Gorostiaga et al., 2019), we examined the moderating role of parenting, with the hypothesis that higher parental acceptance reported by the child will result in decreased associations between ecological stress and a) internalizing problems and b) amygdala reactivity, as well as c) the association between amygdala reactivity and internalizing problems (Hypothesis 5). Although prior studies suggest differences in internalizing problems and positive parenting in males and females (Cyranowski et al, 2000), there is little evidence to predict sex differences in the strength of relations between ecological stressors, internalizing problems, the mediating role of the amygdala and the moderating role of parenting, therefore, we performed exploratory analyses to identify the presence of sex differences for all hypotheses.
Figure 1:
Conceptual model testing the moderation-mediation model of Baseline ecological stress, amygdala reactivity (mediator), parental acceptance (moderator) and Year 1 internalizing problems.
Income = baseline parental self-reported income; ADI = baseline area deprivation index; P-NB = baseline parental self-report of neighborhood safety. Amygdala = baseline bilateral activation of amygdala on EN-back task contrasting faces versus places.
2. Method
2.1. Participants
Data were drawn from the release 3.0 (including Baseline and Year 1 data) of the ABCD study, a longitudinal national study of 11,878 9- and 10-year old adolescents across 22 sites (https://nda.nih.gov/study.html?id=901; Casey et al., 2018). A subset of participants, based on a priori criteria (described below), were identified and used in subsequent analyses, N = 7,385 (see inclusion flowchart, supplementary Figure S1); participants with missing information on the variable of interest (Figure 1) are not included in the final Structural Equation Model (SEM).
2.2. Procedures
For detailed description of study recruitment and procedures, see Garavan and colleagues (2018) and Casey and colleagues (2018). Access to the data was established via an unfunded agreement (19-UFA02466). Approval for data access was also granted by the University of Michigan Institutional Review Board (HUM# HUM00159925). Data were accessed by downloading release 3.0 (https://nda.nih.gov/study.html?id=901) via the National Institute of Mental health Data Archive (NDA); the pre-packaged data associated with these analyses are NDA ABCD Study #1182451 (complete R-markdown & Mplus files are available at https://osf.io/bq49h/). This manuscript is part of a Registered Report submission which was preregistered on the Open-Science Framework Registry (https://osf.io/46yru).
2.3. Measures
Income.
Parents reported combined household income in the past 12 months by selecting an income category ranging from (1) “less than $5,000” to (10) “$200,000 and greater”. Parents were also given the option to select “refuse to answer” or “don’t know”. For our main model (Figure 1) this item is reverse coded for the analyses, such that higher scores indicated lower income levels, to maintain the same direction on our ecological stress factor. The variable used in this analysis is assessed at baseline.
Area Deprivation Index.
The Area Community Service (ACS) Area Deprivation Index (a single item) uses census block group-level data to approximate neighborhood level of social-economic neighborhood disadvantage (Kind & Buckingham, 2018). Index scores are derived from 17 education, employment, housing-equality, and income measures within a 5-year period at the census block level. Index scores are percentiles converted to decile groups from 1 (groups 1 −10) to 10 (groups 91–100), where high ranked groups indicate more disadvantage. The variables used in this analysis are assessed at baseline.
Neighborhood safety.
A PhenX Toolkit measure (3 items) derived from the Neighborhood and Crime Safety scale (Echeverria et al., 2004; Mujahid et al., 2007) assessed parents self-reports of neighborhood safety. Parents rated statements that asked whether their neighborhoods were safe from crime, violence and if they could safely walk in their neighborhoods on a 5-point Likert scale ranging from (1) “strongly disagree” to (5) “strongly agree” (Zucker et al., 2018). Items were averaged such that higher scores indicated higher perception of neighborhood safety. For our final model (Figure 1) this item is reversed coded for the analyses, to maintain the same direction on our ecological stress factor such that higher scores reflect lower perception of neighborhood safety. The variables used in this analysis are assessed at baseline.
Internalizing Symptoms.
Parents’ reports on the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001), was used to assess their child’s internalizing symptoms using the broadband internalizing subscale (33 items). Parents rated their child’s withdrawn, somatic, anxious, and depressive symptoms on a 3-point Likert type scale from (0) “never” to (2) “often”. Parents’ ratings were used to create an internalizing problem summary score. Raw scores were normalized into t-scores (mean of 50, standard deviation of 10) to provide a common metric, with higher scores indicating more internalizing symptomatology. The variable used in this analysis is assessed at 1- year follow-up.
Parental Acceptance.
The acceptance subscale (5 items) from the Children’s Report of Parental Behavior Inventory (Schaefer, 1965) was used to assess parental warmth and acceptance. Preadolescents responded to items regarding the parent or caregiver who completed the ABCD parent protocol. This was typically the child’s primary caregiver, but it did not have to be. Statements such as “Makes me feel better after talking about my worries with him/her,” were rated on a 3-point Likert type scale ranging from (1) “Not like him/her” to (3) “A lot like him/her” (Zucker et al. 2018). Items were averaged such that higher scores indicated higher perceptions of parental acceptance. The variables used in this analysis are assessed at baseline.
Pubertal Development:
Parental reports on child’s pubertal stage were assessed using the Pubertal Development Scale (PDS; Petersen et al., 1988). The PDS is a text-based non-invasive measure to assess current pubertal status in females and males. For females, the items assess changes in 1) body hair, 2) breast development and 3) menstruation. For males, the items assess changes in 1) body hair, 2) hair on the face and 3) deepening voice. The pubertal category score provides a 1–5 reference for whether a participant is: (1) = “pre puberty”; (2) = “early puberty”; (3) = “mid puberty”; (4) = “late puberty”; or (5) = “post puberty”. The items are averaged to create an average pubertal developmental score. The variables used in this analysis are assessed at baseline.
2.4. MRI Acquisition
EmotionalN-Back (EN-back) Task.
The EN-back task (Casey et al., 2018; Cohen et al., 2016) taps into both working memory and emotion regulation processes, though in the present study, we focus on the emotion regulation application. Casey et al. (2018) described the EN-back task in the ABCD study. The task contains two runs with eight blocks in each run. Stimuli were either emotional faces (happy, fearful, neutral) or places. Emotional faces were taken from the NimStim (Tottenham et al., 2009) and the Racially Diverse Affective Expressions (Conley et al., 2018) stimuli sets. The EN-back task data used in these analyses was collected at baseline.
At the beginning of each block, instructions were given to specify to the participant whether it was a 0-back or a 2-back block. Each block consisted of 10 trials. Each trial began with a stimulus being presented for 2 seconds, during which time the participant would respond with whether the stimulus matched or did not match the target stimulus. Following the stimulus presentation, a fixation cross was presented for 500 milliseconds. There were 80 trials for each of the two memory load conditions (i.e., 0-back, 2-back) and 40 trials of each stimulus type (i.e., matching vs. not matching) for each of the two memory load conditions.
Behavioral performance on this task was used as an exclusion criterion. Participants with less than 60% accuracy were considered to have poor performance by the ABCD data analytic core and are thus excluded from the present analyses (Hagler et al., 2019).
MRI acquisition.
For additional information regarding MRI acquisition in the ABCD Study, see Casey et al. (2018). Scans were all performed on different 3T scanners from Siemens (Prisma VE11B-C, Siemens Medical Systems, Erlangen, Germany), Philips (Achieva dStream, Ingenia, Philips Medical Systems, Best, the Netherlands), or General Electric (GE) (MR750, DV25–26, General Electric, Milwaukee, WI, USA). At each site, children were desensitized to the scanner environment through either a mock scanner or a play tunnel that was the size of the scanner bore. To encourage motion compliance in the scanner, behavioral shaping was used to monitor head motion (Epstein et al., 2007) and provide feedback to the child. Additionally, to minimize head motion in the scanner, the head was stabilized with foam padding.
A Tl-weighted (T1w) anatomical scan was acquired using the following parameters: Siemens: TR = 2500ms, TE = 2.88ms, TI = 1060 ms, flip angle = 8°, 176 transverse slices; Philips: TR = 6.31ms, TE = 2.9ms, TI = 1060ms, flip angle = 8°, 225 transverse slices; GE: TR = 2500ms, TE = 2ms, TI = 1060ms, flip angle = 8°, 208 transverse slices. Voxel size was 1 × 1 × 1 mm for all three scanner types.
Multi-slice/multiband EPI functional MRI scans for the EN-back task were acquired using the following parameters on all scanner types: TR = 800ms, TE = 30ms, flip angle = 52°, 60 transverse slices, multiband acceleration = 6, voxel size = 2.4 × 2.4 × 2.4 mm.
2.5. Preprocessing and MRI data analysis.
The data analytic core in the ABCD consortium MRI preprocessing and analyses were performed by the ABCD consortium’s data analytic core using their in-house Multi-Modal Preprocessing Stream (v. 248 https://www.nitrc.org/proiects/abcd_study) This pipeline uses a combination of MATLAB functions and publicly available neuroimaging packages including Freesurfer v.5.3.0 (Fischl, 2012), AFNI v.2008_02_01_1144 (Cox, 1996) and FSL v.5.0.2.2-centos5_64 (Jenkinson et al., 2012; S. M. Smith et al., 2004). For more details, see Hagler and colleagues (2019).
Structural images.
T1w structural images were used during the preprocessing of functional MRI data and the parcellations used for the region of interest (ROI) time course extractions. T1w images were corrected for gradient non-linearity distortions (Jovicich et al., 2006) and for intensity inhomogeneity using B1-bias fields which are estimated using sparse spatial smoothing and white matter segmentation. Images were then resampled with 1mm isotropic voxels and registered to standard reference brain for subsequent subcortical segmentation using an automated, atlas-based volumetric segmentation procedure in Freesurfer (Fischl et al., 2002).
Functional images.
Functional images were corrected for head motion by registering each frame to the first using AFNI’s 3dvolreg (Cox, 1996) and B0 field inhomogeneity, which causes spatial and intensity distortions, using a reverse gradient method (Holland et al., 2010). Images were then corrected for gradient non-linearity distortions (Jovicich et al., 2006) and between-scan motion was corrected using alignment to a reference scan. Following this realignment, a registration matrix is calculated to allow for rigid body transformation between fMRI and T1W anatomical images. The fMRI images remain in “native-space” and have a 2.4mm isotropic resolution.
Following image preprocessing, the first 16 frames of the scan were removed to ensure equilibration of the T1W signal and then voxel time series were normalized by dividing by the mean across time of each voxel. Baseline and quadratic trends in the time-series data were removed using linear regression, as were motion estimates and their derivatives (Power et al., 2014). Volumes with greater than 0.9mm framewise displacement were censored (Siegel et al., 2014). Time course data were then bandpass filtered to remove signals between 0.31–0.43 Hz using an infinite impulse response notch filter.
The EN-back task was modeled in the data at the individual subject level using a general linear model implemented in AFNI’s 3dDeconolve (Cox, 1996). The HRF was modelled using a gamma basis function variate and its temporal derivative. The duration of cues in the EN-back task (2.5 seconds) were modeled as square waves that were convolved with the HRF. Incorrect trials were modeled as separate conditions.
ROI data.
The present study made use of tabulated data sheets resulting from the ABCD consortium’s data analytic core (Hagler et al., 2019). The average signal for the left and right amygdala were calculated based on atlas-based subcortical parcellations in Freesurfer (Fischl et al., 2002) for each run of the EN-back task separately across both the 0-back and 2-back conditions for the emotional faces vs places stimuli. The average signal across both runs was weighted by the number of frames remaining after motion censoring minus the number of model parameters (not accounting for temporal autocorrelation). Conditions with no valid trials were marked as undefined and had empty cells in the ABCD tabulated data. Conditions with few trials and extremely high standard error of the mean for the beta estimates were also censored following the ABCD protocol for exclusion. We did not have hypotheses regarding laterality, so we averaged the extracted values for the left and right amygdalae; however, lateral (left & right amygdala models) exploratory results are provided in supplementary materials. Although we cannot use a traditional method to get at mean activation in the amygdala for the aforementioned condition, beta values are used to provide a distribution of mean-level signal and overall effect of the task condition for the amygdala in supplementary materials. Further, participants were excluded if their fMRI data quality is labeled as ‘Reject’ based on the FreeSurfer Quality Control variable (fsqc_qc) and exceed 0.9 for mean framewise displacement, specific to our analyses.
2.6. Statistical Analysis
For exclusions relating to fMRI data Pearson correlation, descriptive statistics and missing data pattern analysis are conducted first. Variables (e.g., CBCL internalizing T-score) that are deemed to be significantly skewed are transformed (e.g., log). Mplus 7.2 (Muthén & Muthén, 2012) is used to test the hypothesized model (see Figure 1). First, Confirmatory Factor Analysis (CFA) of household income, Area Deprivation Index, and neighborhood safety is performed to examine whether various ecological risk factors load as one or multiple latent constructs. The CFA is run on the individual variables (e.g., reverse coded income, Area Deprivation Index, and the reverse coded neighborhood safety). Second, a SEM model is conducted to examine the associations between the construct from the CFA and amygdala reactivity and internalizing symptoms. Then, the mediation/indirect effects are tested using MODEL INDIRECT in Mplus. Third, to examine the moderating role of parental acceptance in the hypothesized stress-amygdala-internalizing mediation model, we examine the interaction effect of ecological stress by parental acceptance on amygdala and internalizing problems. Significant interaction terms are probed using simple slope analysis and partial residuals are plotted using the Visreg package in R to visualize the simple slope analysis. To examine the moderating effect of sex, we conduct a multi-group model within Mplus to examine whether the hypothesized associations vary between males and females. To account for the sampling effect of siblings within a family cluster, all CFA and path models are estimated using Taylor-series linearization using Type = Complex in Mplus. To account for the multiple site design of the ABCD study, we specified Stratification = ABCD site (to adjust for standard errors and that the chi-square test of model takes into account non-independent observations due to cluster sampling of study variables) for all models in Mplus. Covariates including puberty, race and scanner type (due to the variability of scanner type within some recruitment sites) are included in all models.
Multiple-fit statistics are reported and interpreted as outlined (Kline, 2015): (a) Pearson X2 for which nonsignificant values (p < .05) signify good fit and a X2/df ratio <3 is acceptable; (b) Comparative Fit Index (CFI) for which a value .90 is considered a good fit; and (3) root mean square error of approximation (RMSEA), for which a value <.08 is considered acceptable and < .05 is considered good. While missing data are handled using the Mplus Full Information Maximum Likelihood feature (Muthén & Muthén, 2012), additional analysis are conducted with and without participants with missing data to ensure that the results are not altered by missingness.
3. Results
3.1. Descriptive Statistics
Relating to participant amygdala ROI beta, to be included in these analyses, a participant had to have a) a beta estimate present, b) the FreeSurfer Quality Control variable (fsqc_qc) had been marked “Accept”, c) a mean framewise displacement that did not exceed .9, and d) behavioral performance during the EN-back task that had greater than 60% accuracy. Based on these four exclusion criteria, a subset of participants (N = 7,385) from the full sample were retained for subsequent analyses (flowchart, supplementary Figure S1).
Table 1 shows the relevant descriptive statistics for the key variables in the study, both for the participants included in this study (N = 7,385) and those excluded from the study for reasons outlined above (N = 4,493). There were some notable differences between those included and excluded from these analyses. On average, the included sample were approximately 2 months older than the excluded sample and were in a higher family income decile compared to the excluded sample (decile #3, $75,000 through $99,999 and decile #4, $50,000 through $74,999, respectively). These effects can both be considered as small in magnitude (Cohen, 1988). The included sample was more likely to be White. In addition, compared to the excluded sample, the included sample had higher neighborhood safety (d = .19) and were associated with a lower area deprivation index (d = −.14) – however, the size of these differences were minimal-to-small (Cohen, 1988).
Table 1.
Table showing differences in key demographic and cognitive variables between the included and excluded samples at baseline.
| Included Sample (Baseline) | Excluded Sample (Baseline) | Effect size | |
|---|---|---|---|
| N=7385 | N=4493 | ||
| Sex | Φ = .04 | ||
| Female | 3656 (49.5%) | 2026 (45.1%) | |
| Male | 3729 (50.5%) | 2467 (54.9%) | |
| Race | Φ = .18 | ||
| White | 4259 (57.7%) | 1925 (42.8%) | |
| Black | 787 (10.7%) | 998 (22.2%) | |
| Hispanic | 1414 (19.1%) | 997 (22.2%) | |
| Asian | 165 (2.2%) | 89 (2.0%) | |
| Other | 760 (10.3%) | 487 (10.8%) | |
| M(SD) | M(SD) | ||
| Age (Months) | 120 (7.52) | 118 (7.33) | d = .24 |
| Family Income Deciles | 7.6 (2.2) | 6.6 (2.7) | d = .38 |
| 1. Less than $5,000 | 151 (2.0%) | 266 (5.9%) | |
| 2. $5,000 through $11,999 | 194 (2.6%) | 227 (5.1%) | |
| 3. $12,000 through $15,999 | 138 (1.9%) | 136 (3.0%) | |
| 4. $16,000 through $24,999 | 273 (3.7%) | 251 (5.6%) | |
| 5. $25,000 through $34,999 | 355 (4.8%) | 299 (6.7%) | |
| 6. $35,000 through $49,999 | 541 (7.3%) | 393 (8.7%) | |
| 7. $50,000 through $74,999 | 944 (12.8%) | 555 (12.3%) | |
| 8. $75,000 through $99,999 | 1061 (14.4%) | 511 (11.4%) | |
| 9. $100,000 through $199,999 | 2325 (31.5%) | 990 (22.0%) | |
| 10. $200,000 and greater | 885 (12.0%) | 365 (8.1%) | |
| Refuse to answer | 278 (3.8%) | 234 (5.2%) | |
| Don’t Know | 240 (3.2%) | 264 (5.9%) | |
| Pubertal Development | 1.73 (0.86) | 1.79 (0.88) | d = −.07 |
| ADI | 91.4 (24.4) | 94.9 (25.4) | d = −.14 |
| Internalizing Symptoms | 48.20 (10.4) | 48.90 (11.0) | d = −.06 |
| Neighborhood Safety | 3.96 (0.93) | 3.77 (1.04) | d = .19 |
| Parental Acceptance | 2.79 (0.29) | 2.77 (0.32) | d = .06 |
Note: Unless specified otherwise, each cell refers to the N of that group and the percentage that group composes of the given variable within either the included or excluded sample. Positive effects refer to higher mean scores for the included sample; negative effects refer to higher mean scores for the excluded sample.
Sex differences in pubertal development at baseline were expected given the known differences in the trajectories of pubertal development by age between males and females (Susman et al., 2010). As expected, on a scale between 1 (prepuberty) and 5 (post puberty), females (M = 2.2, SD = 0.9) showed significantly more pubertal development than males (M = 1.3, SD = 0.6), t(5965.4) = 45.9, p < .001, d = 1.1. There was also a significant sex difference for Year 1 internalizing between males (M = 49.1, SD = 10.5) and females (M = 47.7, SD = 10.3), t(7100)=18.9, p < .001; however, this effect was small (d = −0.13).
All distributions for variables used in these analyses are reported in supplementary materials, see figures in Supplemental Section 2.2 to Section 2.3. Given that our self-report items did not have issues of skew or kurtosis, no variables were transformed for subsequent analyses.
3.2. Correlations
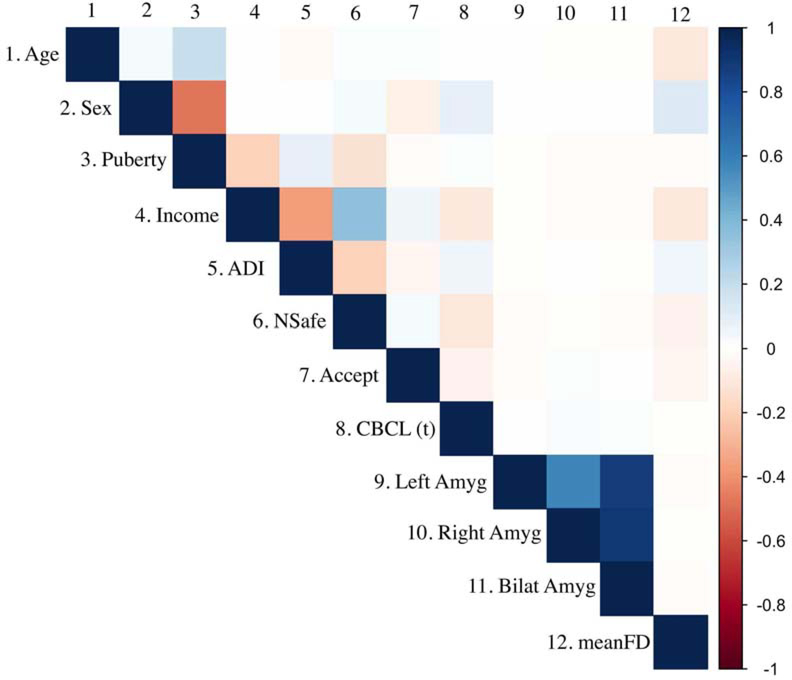
Correlations were conducted between key demographic variables (sex, age, income, pubertal development), three indicators of ecological stress (parental income, area deprivation index, parental neighborhood safety), internalizing symptoms, amygdala activations (left, right and bilateral) and EN-back mean framewise displacement. Figure 2 shows the relations between all aforementioned variables; select key correlations are reviewed below.
Figure 2.
Correlation table Showing the direction and magnitude of effects between key study variables. Positive correlations are shown in blue; negative correlations are shown in red. The associated magnitude is represented by the color in the colorbar.
ADI = Area Deprivation Index; NSafe = Parental Report Neighborhood Safety; Accept = Parental Acceptance; Bilat Amyg = Bilateral Amygdala; meanFD = Mean Framewise Displacement during EN-back. Sex: Male = 1, Female = 0. CBCL(t) = Internalizing Symptoms
As expected, pubertal development had a large negative correlation with sex (r = −.48), moderate positive correlation with age (r = .19) and negative correlation with income (r = −.19). Specifically, females, older preadolescents and preadolescents with lower household income were self-reported by parents to be further along in pubertal development. Lower income was correlated with higher (implying more disadvantage) scores for the Area Deprivation Index (r = .36) and lower neighborhood safety (r = .35), both with moderate effect sizes. Area Deprivation Index and neighborhood safety had a small negative correlation with each other (r = −.20). As expected, bilateral, left and right amygdala activations (see mean beta estimates in supplemental section 2.2, Figures S6 and S7) were highly correlated with each other, r’s = .88 - .90.
3.3. Confirmatory Factor Analysis: Ecological Stress
The results of the CFA revealed that household income (reverse coded; factor loading: β = .81, p < .001), Area Deprivation Index (factor loading: β = .45, p < .001) and neighborhood safety (reverse coded; factor loading: β = .44, p < .001) are loaded as a single latent construct.
3.4. Structural Equation Models (SEM)
Hypothesis 1: Ecological stress latent construct will positively associate with preadolescents ‘ internalizing symptoms.
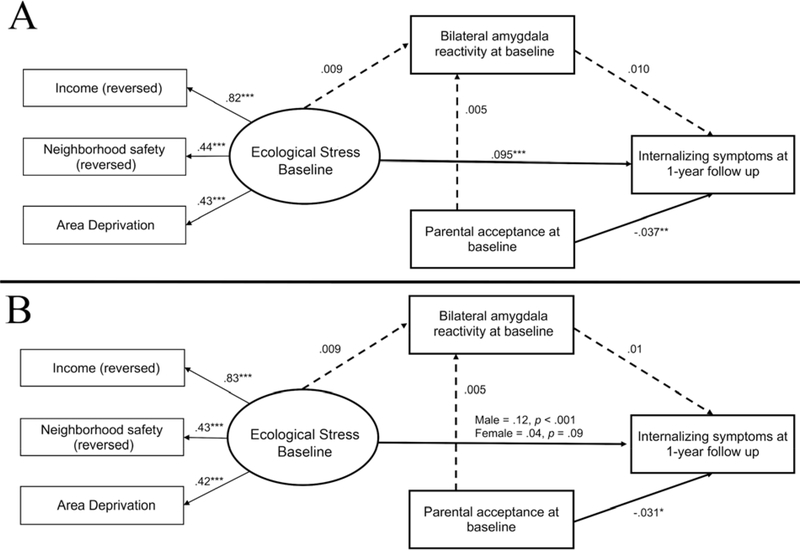
Supporting our hypothesis, the SEM results revealed that higher levels of ecological stress (latent construct at baseline) was significantly associated with higher levels of internalizing symptoms at the 1-year follow-up (Figure 3A). Model fit indices indicated adequate fit in the model: χ2(17) = 174, p < .001, RMSEA = .04, CFI = .93, SRMR = .02.
Figure 3:
Results from the models testing the main model proposed in Figure 1. A) Full model evaluating the role of ecological stress, bilateral amygdala activation, moderating effect of parental acceptance on follow-up internalizing symptoms; B) Model freely estimating ecological stress to follow-up internalizing symptoms, indicating a difference across sex. Both models controlled for age, pubertal development, race, scanner type, clustered by family, stratified by site.
Note: *p < 0.05, **p < 0.01, ***p < 0.001
A multi-group SEM (and Wald Test of Parameter Constraints for path coefficient differences) was conducted to test whether the effect of ecological stress on internalizing symptoms differed by sex. Results indicated that the effect of ecological stress on preadolescents’ internalizing symptoms was stronger in males than females (Wald χ2 (1) = 5.43, p = .02; see Figure 3B).
Hypothesis 2: Increased ecological stress will be associated with increased amygdala activity
No association was found between ecological stress and bilateral amygdala reactivity (Figure 3A), nor left or right amygdala activity (see Supplementary Figure S10). No sex difference was found (Figure 3B).
Hypothesis 3: Increased amygdala activity at baseline will be related to increased internalizing problems at 1-year follow.
No association was found between any amygdala activity and internalizing symptoms. (Figure 3A; Supplementary Figure S10). No sex difference was found (Figure 3B).
Hypothesis 4: The direct relationship between ecological stress and internalizing will be significantly reduced when including amygdala reactivity as a mediator
Bilateral (or left or right) amygdala activity at baseline did not mediate the link between ecological stress and internalizing symptoms at the 1-year follow-up (Figure 3A). No sex difference was found (Figure 3B). The lack of mediation is expected given that hypothesis 2 and 3, mentioned above, were not supported.
Hypothesis 5: The moderating role of parental acceptance
High parental acceptance at baseline was negatively associated with preadolescents’ internalizing symptoms at 1-year follow-up. However, parental acceptance did not moderate any links (ecological stress-internalizing problems, ecological stress-amygdala reactivity, and amygdala-internalizing symptoms) in our model (Figure 3A). No sex difference was found (Figure 3B).
3.5. Post Hoc Analyses
Given that parental acceptance was significantly associated with preadolescents’ internalizing symptoms and we did not find a moderating effect of parental acceptance, we also tested for potential mediation effects. Specifically, we conducted a mediation analysis using MODEL INDIRECT in Mplus to test whether parental acceptance mediated the associations between ecological stress at baseline and preadolescents’ internalizing symptoms at the 1-year follow-up, with the same covariates (pubertal development, race and scanner type), cluster (family) and stratification (ABCD site) in the model. Results indicated that there was a significant indirect effect (est = .002, p = .025), such that lower parental acceptance mediated the associations between ecological stress and internalizing symptoms, indicating that the effect of ecological stress on internalizing symptomatology was higher for preadolescents reporting lower 2 parental acceptance (Supplementary Figure S11). Model fit indices indicated an adequate fit, χ2 (43) = 161, p < .001, RMSEA = .04, CFI = .94, SRMR = .02.
Multi-group analyses of the effect of sex were also conducted. The indirect effect of parental acceptance did not reveal a significant mediation effect when examining the model separately by sex.
3.6. Sensitivity Analyses
The original registration of this project proposed four exclusion criteria for the amygdala ROI beta estimates that were central to the model: a) a participant had to have a beta estimate present, b) the FreeSurfer Quality Control variable (fsqc_qc) had to have been labeled as “Accept”, c) mean framewise displacement did not exceed .9, and d) behavioral performance during the EN-back task had to be greater than 60% accuracy. Upon downloading the Release 3.0 (NDA ABCD Study #1182451), we examined the ABCD Consortium recommendations for inclusion/exclusions criteria related to the EN-back task data. Their recommendations were more stringent than those we had originally proposed. As such, it was prudent to check the results of our models with these stricter exclusion criteria, as artifacts in the neural data may contribute additional noise into our models and impact the results related to amygdala activation.
In addition to our original criteria (a-d), there were eight additional criteria used for inclusion/exclusion to perform our sensitivity analysis. We ensured that both the Raw 1) Functional EN-back (iqc_nback_ok_ser) and 2) T1 images (iqc_t1_ok_ser) passed quality control; 3) > 200 degrees of freedom were available for the EN-back task (tfmri_nback_all_beta_dof); 4) each participant had >100 trials of EN-back data (tfmri_nb_all_beh_total_nt) ; 5) there was a match with the E-Prime data (iqc_nback_ep_t_series_match); 6) the B0 fieldmaps were available (apqc_fmri_bounwarp_flag); 7) there were no discrepancies between manual raters in fMRI QC (fmri_postqc_qc); and 8) the ventral FOV cutoff score was < 60 (apqc_fmri_fov_cutoff_ventral). This resulted in a sensitivity sample of N = 5,807 (see flowchart in Supplementary Figure S12). The reduction in eligible participants was largely driven by the exclusion due to rater discrepancy (FMRI Manual QC), whereby N = 1,476 were excluded due to discrepancy between raters.
Our results with respect to the links between amygdala, ecological stress, internalizing and parental acceptance did not change when applying this sensitivity check. Furthermore, the Pearson correlations among the variables between the full (N = 7,385) and constrained sample (N = 5,807) remained relatively stable. Specifically, with the exception of two bivariate correlations that had a change of r = |0.02|, a small fraction of bivariate associations reflect a change of r < |.015|. The SEM and Pearson correlations for the constrained sample are reported in Supplementary Section 4: Sensitivity Analyses.
4. Discussion
In light of the paucity of the extant literature on the mediating role of brain function in ecological stress and behavioral outcomes, the overarching goal of this study was to evaluate the neurodevelopmental link between ecological stress, amygdala reactivity and internalizing problems, and the buffering effect of parental acceptance, in a large, geographically and socio-economically diverse sample of preadolescents. In this well-powered registered report, we created a latent construct of ecological stress based on household income, self-reported neighborhood safety, and Area Deprivation Index. Using this construct, we confirmed our hypothesis that there would be a significant association between ecological stress at baseline and subsequent internalizing symptoms at the 1-year follow-up; however, this association varied by sex. We did not find evidence to support our hypothesis that there would be a link between ecological stress and amygdala activation during the EN-back task. Hence, we found no support for the hypothesis that amygdala activation mediated the relationship between ecological stress and internalizing symptoms at the 1-year follow-up. Finally, although we did not find a moderating effect of parental acceptance on either of the relationships in the model, post hoc analyses did suggest a mediating role of parental acceptance on the association between ecological stress and internalizing symptoms.
It is well documented that ecological stress has adverse effects on psychological development (Duffy et al., 2018; McEwen, 2012), such as internalizing problems (Evans et al., 2013). Consistent with this broad literature, in this large longitudinal sample of preadolescents, we found a significant association between baseline ecological stress, measured at 9–10 years of age, and internalizing symptoms 1-year later. This finding is consistent with work reporting that multiple risk factors during early adolescence predicted higher rates of internalizing symptoms at a 16-month follow-up (Loukas et al., 2008). The present study extends the adolescent literature to show that ecological stress also impacts internalizing symptoms during preadolescent development in a large sample.
Although previous work on sex differences in internalizing symptoms during adolescence suggests that rates of internalizing problems are higher in females than males (Beesdo et al., 2009; Costello et al., 2003), in the present study, the association between ecological stress and internalizing symptoms was stronger in males than females. This finding might be partially explained by the suggestion that sex differences in internalizing symptoms become more pronounced in adolescence with pubertal development (Angold et al., 1998; Salk et al., 2017). The preadolescents in our sample were in relatively early stages of puberty, based on parent self-reported Pubertal Development Scale (M = 1.73 on a scale of 1–5), and thus it may be too early to observe the post-pubertal onset increase in internalizing symptoms (Gutman & Codiroli McMaster, 2020). Additionally, the effect size of the mean difference in internalizing symptoms was very small, explaining < 1% of variance (issues of effect sizes in ABCD are discussed below). As the ABCD sample gets older, it will be essential to consider how sex differences in internalizing symptoms change and become more pronounced over time.
A central hypothesis of the present study considered the role of amygdala activation in relation to ecological stress and internalizing symptoms. Previous work has found evidence for an association between childhood poverty and amygdala reactivity to emotional faces in early adulthood (Evans et al., 2016; Kim et al., 2013) and evidence that amygdala activation mediates the association between ecological stress and adult internalizing symptoms (Swartz et al., 2015). In the present study, however, we did not find support for either the association between ecological stress and amygdala reactivity or the mediating role of amygdala activation between ecological stress and internalizing symptoms in the transitional window of preadolescence. Estimates were not significant even when the analyses were constrained to a smaller sample that met rigorous quality control standards for the fMRI data or when we modeled the left and right hemispheres separately. We only examined the role of the amygdala using the faces versus places contrast during the EN-back task. Therefore, it is possible that amygdala reactivity to other contrasts or other tasks may be more related to ecological stress and internalizing symptoms. It is also tenable that the influence of ecological stress on amygdala reactivity is stronger when risk is assessed in early childhood when the amygdala is more sensitive to affective stimuli (Silvers et al., 2017), or when the association is examined in adolescence or early adulthood when rates of internalizing symptoms increase (Salk et al., 2017). These possibilities highlight the need to understand how developmental timing may influence the effect of ecological stress on the amygdala and subsequent internalizing symptoms (Cohodes et al., 2020). Future work in the ABCD sample could examine whether changes in or trajectories of amygdala reactivity across development may mediate the associations between ecological risk and internalizing symptoms as the sample ages using the ABCD longitudinal fMRI design.
Moreover, our study found no association between parental acceptance and amygdala activation (during emotional faces versus places contrasts). This differs from work that has reported the important role of positive parenting on amygdala activity in response to angry faces versus shapes in a sample of young adults (Farber et al., 2019). These results may differ as a function of age and units of analyses. While it is beyond the scope of our study, future work in the ABCD study may consider how parental acceptance is associated with fronto-amygdala connectivity, and whether or not the association between parenting and amygdala reactivity changes with development.
Although parental acceptance was not found to be an important moderator, it was associated with lower internalizing symptoms at the 1-year follow-up and, in post hoc analyses, was found to mediate the relationship between ecological stress and internalizing symptoms. Parenting behavior is thought to impact the effect of stress exposure on youth (Gorostiaga et al., 2019). Indeed, research building on the Family Stress Model (Conger et al., 2002) suggests that parenting behavior may be an important proximal mechanism (mediator) through which ecological stress may increase risk for negative child mental health outcomes (Hyde et al., 2020). This is often discussed in the context of harsh parenting (Gard et al., 2020); however, previous work has also pointed to positive parenting or parental warmth as a proximal factor that relates to child emotion regulation skills (Morris et al., 2017) and reduces this risk for internalizing symptoms (Garthe et al., 2015). Further, research suggests that the absence of positive parenting practices may be an additional risk factor for youth exposed to ecological stress (Yamaoka & Bard, 2019). Given that the size of the indirect (mediating) effect in the present study was small, future research should test whether an index of harsh parenting is a stronger mediator in the ABCD sample and should also consider the possible mechanistic role that parental acceptance plays in the link between ecological stress and other outcomes (Gorostiaga et al., 2019).
While these analyses are the first to examine the mediating role of amygdala function in the association between baseline ecological stress and 1-year follow-up internalizing symptoms in the ABCD data, several existing cross-sectional analyses have considered associations between socioeconomic variables and brain structure and cognitive function. In a cross-sectional analysis of the baseline ABCD study data, Assari (2020) reported that parental SES, as measured by neighborhood income (ADI), and family income mediated the relationship between ethnoracial differences and amygdala volume. Meanwhile, in their cross-sectional analysis, Taylor and colleagues (2020) probed the relationship between neighborhood poverty as measured by 9 of 17 ADI variables, hippocampal and prefrontal cortex (PFC) volumes, and cognitive assessments across seven cognitive domains from the National Institutes of Health (NIH) toolbox. In their results, Taylor and colleagues (2020) found that higher household income was positively associated with hippocampal and PFC volumes, and that neighborhood poverty was negatively related to the volume of some PFC regions and the right hippocampus. They also found that higher neighborhood poverty was negatively associated with scores across all cognitive domains. Likewise, Vargas and colleagues (2020) reported that neighborhood deprivation, as measured by ADI quantiles (1–5; five being the highest deprivation), was negatively related to cognitive function and that this association was partially mediated by PFC surface area. Although all of these analyses considered cross-sectional associations, it will be important to consider how SES and brain associations change across time - a strength of the ABCD study design. Furthermore, although the brain was identified as mediator in cross-sectional analyses of the ABCD sample (Assari, 2020; Assari et al., 2020; Vargas et al., 2020), it will be important to reexamine the mechanistic role of specific brain regions using the longitudinal ABCD data to distinguish between cross-sectional and longitudinal effects. Finally, to describe how SES manifests in the brain (Farah, 2017) and relates to cognitive and behavioral outcomes, future work should examine differences in the analytic pipeline that contribute to convergence and differences in findings.
4.1. Study Considerations
This study’s findings are novel and provide insight into the relations between ecological stress, internalizing symptoms and amygdala function. The major strengths of this study include its large sample size that is far greater than the median sample size, N < 20, in experimental and clinical fMRI studies (Szucs & Ioannidis, 2020), and our attempt to reduce concerns relating to questionable research practices (i.e., p-hacking, publication bias, hypothesizing after results are known, etc.; Chambers et al., 2014) and researcher degrees of freedom (Simmons et al., 2011) by using a registered report format. Nevertheless, this study must be understood within the context of several important factors that affect its generalizability.
First, there continue to be important differences in how ecological stress and early adversity is defined and measured. Competing theoretical frameworks exist which support examining neural and behavior correlates of early life stress by modeling adversity as a multidimensional (McLaughlin et al., 2014) or unidimensional construct (Smith & Pollak, 2020). Additionally, there is variability in the items included in early life stress constructs that may be conceptually different (e.g., home, community). These approaches all have merit, though the heterogeneity in constructs can make it difficult to compare results across studies. Therefore, it is worth noting that our a priori ecological stress construct may differ from others and thus may influence the generalizability of our results. Specifically, the latent factor derived from the set of variables in our confirmatory factor analysis was weighted towards self-reported household income, which is a family level stress and may be different from nuanced community level factors that employ cost-of-living adjustments.
Importantly, a substantial portion (approximately 38%) of the overall participants who had at least partially complete data available needed to be excluded from the present analyses based on MRI exclusion criteria. Analyses of the differences in demographic variables between included and excluded participants suggest that the included sample 1) is less ethnically diverse and 2) has a higher average SES than the excluded sample, although the differences are small in magnitude. While the decision to exclude participants with particularly low accuracy on the working memory measure (Hagler et al., 2019) and with comparatively high amounts of motion in the scanner in order to reduce noise are justified (Caballero-Gaudes & Reynolds, 2017), it could be argued that such exclusions risk the models in these analyses not reflecting children with lower working memory skills or with less ability to remain still during imaging. This issue is not new in the field and requires continued efforts to create developmentally appropriate cognitive measures and correct for in-scanner motion while excluding as little data as possible.
The effect sizes in this study are comparably smaller than what might be expected based on the extant literature; however, there is reason to conclude that they may be closer to the “true effects” for this population. Given that it has been established that smaller samples tend to artificially inflate effects due to their comparatively increased sample variability (Button et al., 2013; Szucs & Ioannidis, 2017), and that the estimated median sample in fMRI studies is less than 20 (Szucs & Ioannidis, 2020), former studies may be underpowered to find small effects. Furthermore, Owens and colleagues (2020) argues that given that the median correlation between questionnaire and task variables in the ABCD study is only r = .03, which by the standards set in Cohen (1988) would be considered very small, it is likely that the size of effects in behavioral psychology has been overestimated due to inflation from underpowered studies. Therefore, although the effects are small compared to the previous literature, there should be relative confidence in the magnitude of the effects found in these analyses.
Finally, to probe amygdala activation in these analyses, we focused on the contrast of faces versus places during the EN-back task. Although there is a basis for behavioral and neural effects of emotional faces in development (Somerville et al., 2011), there are likely differences in explicit, implicit and passive-viewing designs that elicit different activation (García-García et al., 2016). Thus, this analytic choice may contribute to differences in the results, as this would change the mean beta estimates of amygdala activity. While keeping in mind the limitations in the subtraction process of task-based fMRI (Cacioppo et al., 2003; Price & Friston, 2005), future work should consider how brain function in alternative contrasts relates to ecological stress.
4.2. Conclusion
The ABCD study presents a large-scale opportunity to learn about adolescent brain development and the role of ecological stress. The present study investigated how ecological stress, defined as household and community economic resources and neighborhood safety, could increase risk for preadolescents’ development of internalizing symptoms. Ecological stress was indeed associated with developing more internalizing symptoms one year later and to a slightly larger extent for males compared to females. In contrast to what has been reported among adolescent and young adult samples, amygdala reactivity to emotional stimuli (in the faces versus places contrast) did not mediate the relation between ecological stress and preadolescent’s internalizing symptoms. In addition, parental acceptance did not lessen the association between ecological stress, amygdala reactivity, and internalizing symptoms. However, parental acceptance was meaningfully related to ecological stress and internalizing symptoms with higher rates of acceptance relating to lower internalizing symptoms one year later. This finding has strong implications for the design of parenting intervention that aims to reduce internalizing problems among preadolescents. These results also suggest that the amygdala activity alone does not play a meaningful role in the association between ecological stress and internalizing symptoms in this age group and in our sample. Additionally, the conclusions shed light on alternative mechanisms for the role of parenting in stressful environments in a preadolescent sample.
Supplementary Material
Acknowledgments
MD & LG are funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Developmental Training grant (T32HD007109-34, McLoyd & Monk). KI is funded by the Susan Nolen-Hoeksema Postdoctoral Fellowship.
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/scientists/workgroups/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, & Rescorla L (2001). Manual for the ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. Burlington, VT: : ASEBA. https://trove.nla.gov.au/version/1167240 [Google Scholar]
- Angold A, Costello EJ, & Worthman CM (1998). Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychological Medicine, 28(1), 51–61. 10.1017/S003329179700593X [DOI] [PubMed] [Google Scholar]
- Assari S (2020). Socioeconomic Status Inequalities Partially Mediate Racial and Ethnic Differences in Children’s Amygdala Volume. Studies in Social Science Research, 1(2), 62–79. 10.22158/sssr.v1n2p62 [DOI] [PubMed] [Google Scholar]
- Assari S, Boyce S, Bazargan M, & Caldwell CH (2020). Family Income Mediates the Effect of Parental Education on Adolescents’ Hippocampus Activation During an N-Back Memory Task. Brain Sciences, 10(8), 520. 10.3390/brainsci10080520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, & Valentino RJ (2014). Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Frontiers in Neuroendocrinology, 35(3), 303–319. 10.1016/j.yfrne.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, & Pine DS (2009). Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. The Psychiatric Clinics of North America, 32(3), 483–524. 10.1016/j.psc.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, Wolf I, Baumeister S, Meyer-Lindenberg A, Banaschewski T, Brandeis D, & Laucht M (2014). Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PloS One, 9(8), e104185. 10.1371/journal.pone.0104185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, & Posner S (2005). Socioeconomic Status in Health Research: One Size Does Not Fit All. JAMA, 294(22), 2879–2888. 10.1001/jama.294.22.2879 [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, & Munafò MR (2013). Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14(5), 365–376. 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Caballero-Gaudes C, & Reynolds RC (2017). Methods for cleaning the BOLD fMRI signal. NeuroImage, 154, 128–149. 10.1016/j.neuroimage.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Lorig TS, Norris CJ, Rickett E, & Nusbaum H (2003). Just because you’re imaging the brain doesn’t mean you can stop using your head: A primer and set of first principles. Journal of Personality and Social Psychology, 85(4), 650–661. 10.1037/0022-3514.85.4.650 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, Orr CA, Wager TD, Banich MT, Speer NK, Sutherland MT, Riedel MC, Dick AS, Bjork JM, Thomas KM, … ABCD Imaging Acquisition Workgroup. (2018). The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience, 32, 43–54. 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Bolger N, & Eckenrode J (1987). Linking person and context in the daily stress process. Journal of Personality and Social Psychology, 52(1), 184–195. 10.1037/0022-3514.52.1.184 [DOI] [PubMed] [Google Scholar]
- Chambers CD, Feredoes E, Muthukumaraswamy SD, & Etchells P (2014). Instead of “playing the game” it is time to change the rules: Registered Reports at AIMS Neuroscience and beyond. AIMS Neuroscience, 1(1), 4–17. 10.3934/Neuroscience.2014.1.4 [DOI] [Google Scholar]
- Cohen AO, Breiner K, Steinberg L, Bonnie RJ, Scott ES, Taylor-Thompson KA, Rudolph MD, Chein J, Richeson JA, Heller AS, Silverman MR, Dellarco DV, Fair DA, Galván A, & Casey BJ (2016). When Is an Adolescent an Adult? Assessing Cognitive Control in Emotional and Nonemotional Contexts. Psychological Science, 27(4), 549–562. 10.1177/0956797615627625 [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences. Academic Press [Google Scholar]
- Cohen JA, Mannarino AP, Kliethermes M, & Murray LA (2012). Trauma-focused CBT for youth with complex trauma. Child Abuse & Neglect, 36(6), 528–541. 10.1016/j.chiabu.2012.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohodes EM, Kitt ER, Baskin-Sommers A, & Gee DG (2020). Influences of early-life stress on frontolimbic circuitry: Harnessing a dimensional approach to elucidate the effects of heterogeneity in stress exposure. Developmental Psychobiology, n/a(n/a). 10.1002/dev.21969 [DOI] [PubMed] [Google Scholar]
- Conger RD, Wallace LE, Sun Y, Simons RL, McLoyd VC, & Brody GH (2002). Economic pressure in African American families: A replication and extension of the family stress model. Developmental Psychology, 38(2), 179–193. [PubMed] [Google Scholar]
- Conley MI, Dellarco DV, Rubien-Thomas E, Cohen AO, Cervera A, Tottenham N, & Casey B (2018). The racially diverse affective expression (RADIATE) face stimulus set. Psychiatry Research, 270, 1059–1067. 10.1016/j.psychres.2018.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, & Angold A (2003). Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry, 60(8), 837–844. 10.1001/archpsyc.60.8.837 [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal, 29(3), 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, & Shear MK (2000). Adolescent onset of the gender difference in lifetime rates of major depression: A theoretical model. Archives of General Psychiatry, 57(1), 21–27. 10.1001/archpsyc.57.1.21 [DOI] [PubMed] [Google Scholar]
- Duffy KA, McLaughlin KA, & Green PA (2018). Early life adversity and health-risk behaviors: Proposed psychological and neural mechanisms. Annals of the New York Academy of Sciences, 1428(1), 151–169. 10.1111/nyas.13928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria SE, Diez-Roux AV, & Link BG (2004). Reliability of self-reported neighborhood characteristics. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 81(4), 682–701. 10.1093/jurban/jth151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Casey BJ, Tonev ST, Davidson M, Reiss AL, Garrett A, Hinshaw SP, Greenhill LL, Vitolo A, Kotler LA, Jarrett MA, & Spicer J (2007). Assessment and Prevention of Head Motion During Imaging of Patients with Attention Deficit Hyperactivity Disorder. Psychiatry Research, 155(1), 75–82. 10.1016/j.pscychresns.2006.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essau CA, Lewinsohn PM, Seeley JR, & Sasagawa S (2010). Gender differences in the developmental course of depression. Journal of Affective Disorders, 127(1–3), 185–190. 10.1016/j.jad.2010.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Li D, & Whipple SS (2013). Cumulative risk and child development. Psychological Bulletin, 139(6), 1342–1396. 10.1037/a0031808 [DOI] [PubMed] [Google Scholar]
- Evans GW, Swain JE, King AP, Wang X, Javanbakht A, Ho SS, Angstadt M, Phan KL, Xie H, & Liberzon I (2016). Childhood Cumulative Risk Exposure and Adult Amygdala Volume and Function. Journal of Neuroscience Research, 94(6), 535–543. 10.1002/jnr.23681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ (2017). The Neuroscience of Socioeconomic Status: Correlates, Causes, and Consequences. Neuron, 96(1), 56–71. 10.1016/j.neuron.2017.08.034 [DOI] [PubMed] [Google Scholar]
- Farah MJ (2018). Socioeconomic status and the brain: Prospects for neuroscience-informed policy. Nature Reviews. Neuroscience, 19(7), 428–438. 10.1038/s41583-018-0023-2 [DOI] [PubMed] [Google Scholar]
- Farber MJ, Kim MJ, Knodt AR, & Hariri AR (2019). Maternal overprotection in childhood is associated with amygdala reactivity and structural connectivity in adulthood. Developmental Cognitive Neuroscience, 40, 100711. 10.1016/j.dcn.2019.100711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B (2012). FreeSurfer. NeuroImage, 62(2), 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, & Dale AM (2002). Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron, 33(3), 341–355. 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- Fox SE, Levitt P, & Nelson CA (2010). How the timing and quality of early experiences influence the development of brain architecture. Child Development, 81(1), 28–40. 10.1111/j.1467-8624.2009.01380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gach EJ, Ip KI, Sameroff AJ, & Olson SL (2018). Early cumulative risk predicts externalizing behavior at age 10: The mediating role of adverse parenting. Journal of Family Psychology: JFP: Journal of the Division of Family Psychology of the American Psychological Association (Division 43), 32(1), 92–102. 10.1037/fam0000360 [DOI] [PubMed] [Google Scholar]
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, Jernigan T, Potter A, Thompson W, & Zahs D (2018). Recruiting the ABCD sample: Design considerations and procedures. Developmental Cognitive Neuroscience, 32, 16–22. 10.1016/j.dcn.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-García I, Kube J, Gaebler M, Horstmann A, Villringer A, & Neumann J (2016). Neural processing of negative emotional stimuli and the influence of age, sex and task-related characteristics. Neuroscience & Biobehavioral Reviews, 68, 773–793. 10.1016/j.neubiorev.2016.04.020 [DOI] [PubMed] [Google Scholar]
- Gard AM, McLoyd VC, Mitchell C, & Hyde LW (2020). Evaluation of a longitudinal family stress model in a population-based cohort. Social Development, 29(4), 1155–1175. 10.1111/sode.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe RC, Sullivan T, & Kliewer W (2015). Longitudinal Relations Between Adolescent and Parental Behaviors, Parental Knowledge, and Internalizing Behaviors Among Urban Adolescents. Journal of Youth and Adolescence, 44(4), 819–832. 10.1007/s10964-014-0112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, & Casey BJ (2015). The Impact of Developmental Timing for Stress and Recovery. Neurobiology of Stress, 1, 184–194. 10.1016/j.ynstr.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostiaga A, Aliri J, Balluerka N, & Lameirinhas J (2019). Parenting Styles and Internalizing Symptoms in Adolescence: A Systematic Literature Review. International Journal of Environmental Research and Public Health, 16(17). 10.3390/ijerph16173192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman LM, & Codiroli McMaster N (2020). Gendered Pathways of Internalizing Problems from Early Childhood to Adolescence and Associated Adolescent Outcomes. Journal of Abnormal Child Psychology, 48(5), 703–718. 10.1007/s10802-020-00623-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE (2020). Adolescent Psychopathology: The Role of Brain-Based Diatheses, Sensitivities, and Susceptibilities. Child Development Perspectives, 14(2), 104–109. 10.1111/cdep.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, & Meaney MJ (2010). Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews. Neuroscience, 11(9), 651–659. 10.1038/nrn2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS, Sutherland MT, Casey BJ, Barch DM, Harms MP, Watts R, Bjork JM, Garavan HP, Hilmer L, Pung CJ, Sicat CS, Kuperman J, Bartsch H, Xue F, … Dale AM (2019). Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage, 202, 116091. 10.1016/j.neuroimage.2019.116091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipwell A, Keenan K, Kasza K, Loeber R, Stouthamer-Loeber M, & Bean T (2008). Reciprocal influences between girls’ conduct problems and depression, and parental punishment and warmth: A six year prospective analysis. Journal of Abnormal Child Psychology, 36(5), 663–677. 10.1007/s10802-007-9206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, Kuperman JM, & Dale AM (2010). Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. NeuroImage, 50(1), 175–183. 10.1016/j.neuroimage.2009.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz NE, Boecker-Schlier R, Buchmann AF, Blomeyer D, Jennen-Steinmetz C, Baumeister S, Plichta MM, Cattrell A, Schumann G, Esser G, Schmidt M, Buitelaar J, Meyer-Lindenberg A, Banaschewski T, Brandeis D, & Laucht M (2017). Ventral striatum and amygdala activity as convergence sites for early adversity and conduct disorder. Social Cognitive and Affective Neuroscience, 12(2), 261–272. 10.1093/scan/nsw120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, & Miller GE (2019). Protective factors for youth confronting economic hardship: Current challenges and future avenues in resilience research. American Psychologist, 74(6), 641–652. 10.1037/amp0000520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Gard AM, Tomlinson RC, Burt SA, Mitchell C, & Monk CS (2020). An ecological approach to understanding the developing brain: Examples linking poverty, parenting, neighborhoods, and the brain. The American Psychologist, 75(9), 1245–1259. 10.1037/amp0000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee SR (2017). Child Maltreatment and Risk for Psychopathology in Childhood and Adulthood. Annual Review of Clinical Psychology, 13, 525–551. 10.1146/annurev-clinpsy-032816-045005 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, & Smith SM (2012). FSL. NeuroImage, 62(2), 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, & Dale A (2006). Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. NeuroImage, 30(2), 436–443. 10.1016/j.neuroimage.2005.09.046 [DOI] [PubMed] [Google Scholar]
- Kachmar AG, Connolly CA, Wolf S, & Curley MAQ (2019). Socioeconomic Status in Pediatric Health Research: A Scoping Review. The Journal of Pediatrics, 213, 163–170. 10.1016/j.jpeds.2019.06.005 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Costello EJ, Georgiades K, Green JG, Gruber MJ, He J, Koretz D, McLaughlin KA, Petukhova M, Sampson NA, Zaslavsky AM, & Merikangas KR (2012). Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Archives of General Psychiatry, 69(4), 372–380. 10.1001/archgenpsychiatry.2011.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, Liberzon I, & Phan KL (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18442–18447. 10.1073/pnas.1308240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind AJH, & Buckingham WR (2018). Making Neighborhood-Disadvantage Metrics Accessible—The Neighborhood Atlas. The New England Journal of Medicine, 378(26), 2456–2458. 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB (2015). Principles and Practice of Structural Equation Modeling, Fourth Edition. Guilford Publications [Google Scholar]
- Leventhal T, & Brooks-Gunn J (2011). Changes in neighborhood poverty from 1990 to 2000 and youth’s problem behaviors. Developmental Psychology, 47(6), 1680–1698. 10.1037/a0025314 [DOI] [PubMed] [Google Scholar]
- Lillard AS, & Erisir A (2011). Old Dogs Learning New Tricks: Neuroplasticity Beyond the Juvenile Period. Developmental Review: DR, 31(4), 207–239. 10.1016/j.dr.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas A, Prelow HM, Suizzo M-A, & Allua S (2008). Mothering and Peer Associations Mediate Cumulative Risk Effects for Latino Youth. Journal of Marriage and Family, 70(1), 76–85. 10.1111/j.1741-3737.2007.00462.x [DOI] [Google Scholar]
- Lynch M, & Cicchetti D (1998). An ecological-transactional analysis of children and contexts: The longitudinal interplay among child maltreatment, community violence, and children’s symptomatology. Development and Psychopathology, 10(2), 235–257. [DOI] [PubMed] [Google Scholar]
- Marshall NA, Marusak HA, Sala-Hamrick KJ, Crespo LM, Rabinak CA, & Thomason ME (2018). Socioeconomic disadvantage and altered corticostriatal circuitry in urban youth. Human Brain Mapping, 39(5), 1982–1994. 10.1002/hbm.23978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2012). Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences, 109(Supplement 2), 17180–17185. 10.1073/pnas.1121254109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Akil H (2020). Revisiting the Stress Concept: Implications for Affective Disorders. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 40(1), 12–21. 10.1523/JNEUROSCI.0733-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2011). Stress- and Allostasis-Induced Brain Plasticity. Annual Review of Medicine, 62(1), 431–445. 10.1146/annurev-med-052209-100430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gray J, & Nasca C (2015). Recognizing Resilience: Learning from the Effects of Stress on the Brain. Neurobiology of Stress, 1, 1–11. 10.1016/j.ynstr.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood Adversity and Neural Development: Deprivation and Threat as Distinct Dimensions of Early Experience. Neuroscience and Biobehavioral Reviews, 47, 578–591. 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Nelson CA (2017). Neglect as a Violation of Species-Expectant Experience: Neurodevelopmental Consequences. Biological Psychiatry, 82(7), 462–471. 10.1016/j.biopsych.2017.02.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, & Swendsen J (2010). Lifetime Prevalence of Mental Disorders in US Adolescents: Results from the National Comorbidity Study-Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry, 49(10), 980–989. 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AS, Criss MM, Silk JS, & Houltberg BJ (2017). The Impact of Parenting on Emotion Regulation During Childhood and Adolescence. Child Development Perspectives, 11(4), 233–238. 10.1111/cdep.12238 [DOI] [Google Scholar]
- Mujahid MS, Diez Roux AV, Morenoff JD, & Raghunathan T (2007). Assessing the measurement properties of neighborhood scales: From psychometrics to ecometrics. American Journal of Epidemiology, 165(8), 858–867. 10.1093/aje/kwm040 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2012). Mplus: Statistical Analysis with Latent Variables – User’s Guide
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ, Murray SS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Van Zijl P, … Sowell ER (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18(5), 773–778. 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odgers CL, Caspi A, Russell MA, Sampson RJ, Arseneault L, & Moffitt TE (2012). Supportive parenting mediates neighborhood socioeconomic disparities in children’s antisocial behavior from ages 5 to 12. Development and Psychopathology, 24(3), 705–721. 10.1017/S0954579412000326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MM, Potter A, Hyatt C, Albaugh M, Thompson WK, Jernigan T, Yuan D, Hahn S, Allgaier N, & Garavan H (2020). Recalibrating expectations about effect size: A multi-method survey of effect sizes in the ABCD study. PsyArXiv. 10.31234/osf.io/tn9u4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, & Ma Y (1998). The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry, 55(1), 56–64. 10.1001/archpsyc.55.1.56 [DOI] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, & Petersen SE (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341. 10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, & Friston KJ (2005). Functional ontologies for cognition: The systematic definition of structure and function. Cognitive Neuropsychology, 22(3), 262–275. 10.1080/02643290442000095 [DOI] [PubMed] [Google Scholar]
- Salk RH, Hyde JS, & Abramson LY (2017). Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychological Bulletin, 143(8), 783–822. 10.1037/bul0000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ES (1965). Children’s reports of parental behavior: An inventory. Child Development, 36(2), 413–424. 10.2307/1126465 [DOI] [PubMed] [Google Scholar]
- Schneider W, Waldfogel J, & Brooks-Gunn J (2015). The Great Recession and Behavior Problems in 9-Year Old Children. Developmental Psychology, 51(11), 1615–1629. 10.1037/dev0000038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, & Petersen SE (2014). Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Human Brain Mapping, 35(5), 1981–1996. 10.1002/hbm.22307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin R, Weber J, Mischel W, Casey BJ, & Ochsner KN (2017). The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Developmental Cognitive Neuroscience, 25, 128–137. 10.1016/j.dcn.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, & Simonsohn U (2011). False-positive psychology: Undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Science, 22(11), 1359–1366. 10.1177/0956797611417632 [DOI] [PubMed] [Google Scholar]
- Smith KE, & Pollak SD (2020). Rethinking Concepts and Categories for Understanding the Neurodevelopmental Effects of Childhood Adversity. Perspectives on Psychological Science, 1745691620920725. 10.1177/1745691620920725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, & Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23 Suppl 1, S208–219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Somerville LH, Fani N, & McClure-Tone EB (2011). Behavioral and neural representation of emotional facial expressions across the lifespan. Developmental Neuropsychology, 36(4), 408–428. 10.1080/87565641.2010.549865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews, 24(4), 417–463. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Houts RM, Steinberg L, Belsky J, Cauffman E, DeHart G, Friedman SL, Roisman GI, & Halpern-Felsher BL (2010). Longitudinal Development of Secondary Sexual Characteristics in Girls and Boys Between Ages 9½ and 15½ Years. Archives of Pediatrics & Adolescent Medicine, 164(2), 166–173. 10.1001/archpediatrics.2009.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, & Hariri AR (2015). A neural biomarker of psychological vulnerability to future life stress. Neuron, 85(3), 505–511. 10.1016/j.neuron.2014.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs D, & Ioannidis JPA (2017). Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLOS Biology, 15(3), e2000797. 10.1371/journal.pbio.2000797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs D, & Ioannidis JPA (2020). Sample size evolution in neuroimaging research: An evaluation of highly-cited studies (1990–2012) and of latest practices (2017–2018) in high-impact journals. Neuroimage, 221, 117164. 10.1016/j.neuroimage.2020.117164 [DOI] [PubMed] [Google Scholar]
- Taylor RL, Cooper SR, Jackson JJ, & Barch DM (2020). Assessment of Neighborhood Poverty, Cognitive Function, and Prefrontal and Hippocampal Volumes in Children. JAMA Network Open, 3(11). 10.1001/jamanetworkopen.2020.23774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, & Fuligni AJ (2013). Positive daily family interactions eliminate gender differences in internalizing symptoms among adolescents. Journal of Youth and Adolescence, 42(10), 1498–1511. 10.1007/s10964-013-9964-y [DOI] [PubMed] [Google Scholar]
- Tottenham N, & Galván A (2016). Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neuroscience and Biobehavioral Reviews, 70, 217–227. 10.1016/j.neubiorev.2016.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, & Sheridan MA (2010). A Review of Adversity, The Amygdala and the Hippocampus: A Consideration of Developmental Timing. Frontiers in Human Neuroscience, 3. 10.3389/neuro.09.068.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, & Nelson C (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas T, Damme KSF, & Mittal VA (2020). Neighborhood deprivation, prefrontal morphology and neurocognition in late childhood to early adolescence. NeuroImage, 220, 117086. 10.1016/j.neuroimage.2020.117086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Ribas P, Benson B, Vitale AD, Keren H, Harrewijn A, Fox NA, Pine DS, & Stringaris A (2019). Bidirectional Associations Between Stress and Reward Processing in Children and Adolescents: A Longitudinal Neuroimaging Study. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(10), 893–901. 10.1016/j.bpsc.2019.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Simmons JG, Dennison M, Vijayakumar N, Schwartz O, Yap MBH, Sheeber L, & Allen NB (2014). Positive parenting predicts the development of adolescent brain structure: A longitudinal study. Developmental Cognitive Neuroscience, 8, 7–17. 10.1016/j.dcn.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Vijayakumar N, Simmons JG, Dennison M, Schwartz O, Pantelis C, Sheeber L, Byrne ML, & Allen NB (2017). Role of Positive Parenting in the Association Between Neighborhood Social Disadvantage and Brain Development Across Adolescence. JAMA Psychiatry, 74(8), 824–832. 10.1001/jamapsychiatry.2017.1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Nelson CB, & Lachner G (1998). Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychological Medicine, 28(1), 109–126. 10.1017/s0033291797005928 [DOI] [PubMed] [Google Scholar]
- Yamaoka Y, & Bard DE (2019). Positive Parenting Matters in the Face of Early Adversity. American Journal of Preventive Medicine, 56(4), 530–539. 10.1016/j.amepre.2018.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, & Guthrie D (2009). Institutional rearing and psychiatric disorders in Romanian preschool children. The American Journal of Psychiatry, 166(7), 777–785. 10.1176/appi.ajp.2009.08091438 [DOI] [PubMed] [Google Scholar]
- Zucker RA, Gonzalez R, Feldstein Ewing SW, Paulus MP, Arroyo J, Fuligni A, Morris AS, Sanchez M, & Wills T (2018). Assessment of culture and environment in the Adolescent Brain and Cognitive Development Study: Rationale, description of measures, and early data. Developmental Cognitive Neuroscience, 32, 107–120. 10.1016/j.dcn.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.