Abstract
The failure of T cells to eradicate tumour cells in the tumour microenvironment is mainly due to the dysfunction of T cells. Senescent T cells, with defects in proliferation and effector functions, accumulate in ageing, chronic viral infections, and autoimmune disorders where antigen stimulation persists. Increasing evidence suggests that inducing T cell senescence is a key strategy used by malignant tumours to evade immune surveillance. In this review, we summarize the general features, functional regulation, and signalling network of senescent T cells in tumour development and highlight their potential as prognostic biomarkers in multiple cancer treatments, including chemotherapy, radiotherapy, and immunotherapy. Moreover, we discuss possible therapeutic strategies for preventing or rejuvenating senescence in tumour-specific T cells. Understanding these critical issues may provide novel strategies to enhance cancer immunotherapy.
Keywords: Senescent T cell, tumour microenvironment, cancer immunotherapy, prognostic biomarkers, therapeutic targets
1. Introduction
Tumorigenesis is a process that escapes the immune system manifested by the dysfunction of immune surveillance and clearance. Novel tumour immune escape mechanisms are still under investigation. As the most powerful immune cells in clearing tumour cells, dysfunction of T cells has attracted the most interest.
The exhaustion and senescence of T cells are two dominant dysfunctional states in chronic infections and cancers [1]. The principle features of exhausted T cells is the elevated inhibitory receptors, including programmed cell death protein 1 (PD-1), T cell immunoglobulin and mucin domain containing-3 (Tim-3), and lymphocyte activation gene-3 (LAG-3) with impaired cytotoxicity and effector cytokine production [1]. Senescent T cells have a distinct phenotypes including downregulated expression of the costimulatory molecules CD27 and CD28, and high expression of CD57, killer cell lectin-like receptor subfamily G member 1 (KLRG-1), and CD45RA [2], [3], [4]. They share common features with senescent somatic cells such as DNA damage, declines in proliferation and activation, but are able to produce high amounts of proinflammatory cytokines [5]. The dysfunction of exhausted T cells can be reversed by immune checkpoint blockades whereas senescent seems to be irreversible [1]. The exhausted and senescent T cells share overlapping characteristics but they are two distinct dysfunctional states.
The accumulation of senescent T cells was first found in the peripheral blood of elderly people [6]. Therefore, T cell senescence is thought to be attributed to the failing efficacy of vaccination and the increased morbidity and mortality from infections and cancer in ageing [7,8]. Soon thereafter, an increase in senescent T cells was also detected in young patients with chronic viral infections or autoimmune disorders [5,9]. This phenomenon indicates that in addition to ageing, repeated antigenic stimulation and a chronic inflammatory environment can also lead to T cell senescence. Considering that T cells may be constitutively activated by antigens and influenced by numerous inflammatory cytokines, the tumour microenvironment may be the origin of senescent T cells. Recent studies have shed light on the potential roles of T cell senescence in cancer development. The interplay between senescent T cells and the tumour microenvironment has been reviewed recently [10]. However, its potential prognostic value upon various cancer treatments needs to be further clarified. In this review, we summarized the general features, functional regulation, and signalling network of senescent T cells in tumour development and their potential as prognostic biomarkers in cancer therapy. Moreover, possible therapeutic strategies for modulating T cell senescence to alter the initiation and progression of cancer are discussed.
2. Characteristics and potential roles of senescent T cells in tumours
2.1. General markers of senescent T cells
Senescent T cells share several common features with senescent somatic cells. Senescent T cells become larger and flatter and show positive senescence-associated β-galactosidase (SA-β-gal) staining [4,[11], [12], [13], [14]]. The telomeres become shortened and the DNA damage increases, which then leads to the upregulation of p53, p21, and p16, and the downregulation of Cdk2, Cdk6, and cyclin D3 to cause cell cycle arrest [[11], [12], [13],15]. Therefore, the proliferation of senescent T cells is greatly dampened. The senescence-associated secretory phenotype (SASP) [11,13,[15], [16], [17], [18], [19]] and metabolic changes (discussed further in the following sections) [13,17,20,21] are also present in senescent T cells.
In addition to the common features mentioned above, senescent T cells show distinct surface markers. Senescent T cells lose the expression of costimulatory receptors CD27/CD28 but express CD57 and KLRG-1, which indicates replicative senscent [2], [3], [4]. Senescent T cells also show a terminally differentiated phenotype with the downregulation of the chemokine receptors CCR7 and CD45RO but the upregulation of CD45RA [17,22,23]. Therefore, the T cell phenotype of CD27−CD28−CD57+KLRG-1+, or CCR7−CD45RA+ is a commonly used indicator of T cell senescence. T cells that express Tim-3 [24], T cell immunoreceptor with Ig and ITIM domains (TIGIT) [19], immunoglobulin-like transcript 2 (ILT2/CD85j) [25], or other natural killer like receptors (NKRs) [26] also showed senescent features, indicating that these may be newly defined senescent markers. Whether exhausted markers such as PD-1, and LAG-3 are expressed on senescent T cells is still under debate [19,27]. Since senescent T cells may show diversified phenotypes in different scenarios, we should better to combine functional markers and surface markers to track aged T cells accurately (Table 1).
Table 1.
Markers of senescent T cells
| Category | Makers | References | |
|---|---|---|---|
| Functional markers | SA-β-gal activity | SA-β-gal+ | [11], [12], [13], [14] |
| Cell cycle arrest | Proliferation ↓ | [11], [12], [13],15 | |
| p53, p21, p16 ↑ | [11], [12], [13] | ||
| DNA damage | ATM, and γH2AX ↑ | 13,28 | |
| SASP | IL-6, IL-8, TNF-α, IFN-γ ↑ | 11,13,[15], [16], [17], [18] | |
| IL-2, and Granzyme B ↓ | 15,19 | ||
| Metabolic changes | ROS ↑ | 17,20,21 | |
| Mitochondrial fitness ↓ | 17,20 | ||
| Glycolysis ↑ | 17,20 | ||
| Surface markers | Frequently used | CD28, CD27 ↓ | [11], [12], [13],51,81 |
| CCR7, CD45RO↓; CD45RA ↑ | 17,22,23 | ||
| KLRG-1, CD57 ↑ | [2], [3], [4],22,68 | ||
| Newly defined | TIGIT, Tim-3, ILT2/CD85j, NKRs ↑ | 19,[24], [25], [26] | |
| Under debate | PD-1, LAG-3 ? | 19,27 | |
ATM, ataxia-telangiectasia mutated; IFN-γ: interferon gamma; ILT2: immunoglobulin-like transcript 2; KLRG-1: killer cell lectin-like receptor subfamily G member 1; LAG-3: lymphocyte activation gene-3; NKRs: natural killer like receptors; PD-1: programmed cell death protein 1; SA-β-gal: senescence-associated β-galactosidase; SASP: senescence-associated secretory phenotype; TIGIT: T cell immunoreceptor with Ig and ITIM domains; Tim-3: T cell immunoglobulin and mucin domain containing-3; TNF-α: tumour necrosis factor alpha; γH2AX: phosphorylated H2AX.
2.2. Loss of antigen-specific killing but gain of innate-like functions
In the tumour microenvironment, cytotoxic T cells are activated by tumour antigens through T-cell receptor (TCR) signalling to produce a durable and efficient antitumour immune response. However, the efficiency of TCR signalling is compromised in senescent T cells. The expression of key components of TCR signalling (CD3, Lck, Zap70, SLP-76, LAT, and PLCγ1) [26,28], as well as the costimulatory molecules CD27 and CD2811, 29 is decreased in senescent T cells. In addition, the phosphorylation of Zap70 is impaired following CD3 activation [26,28]. When stimulated with anti-CD3 plus anti-CD28, senescent T cells, especially those isolated from old individuals, produce significantly lower amounts of IL-2, interferon gamma (IFN-γ), tumour necrosis factor alpha (TNF-α), and granzyme B than other types of T cells [15,19]. Moreover, TCRβ diversity roughly declines linearly with age, especially in senescent CD8+ T cells [30,31]. These findings indicate that senescent T cells probably dampen TCR-dependent antigen-specific killing.
Senescent T cells also upregulate the expression of NKRs, including NKG2A/C and KLRG-1 [2,26]. Senescent T cells display higher levels of CD107a, granzyme B, and perforin than other subsets with no stimulation[19]. and under NKG2D stimulation [26]. In vitro experiments have shown that senescent T cells kill tumour cells independent of TCR, with the same efficiency as natural killer (NK) cells [26]. This evidence indicates that although antigen specific killing is lost, senescent T cells with strong nonspecific killing potential fight antitumour immunity to some extent.
2.3. Special SASPs
Senescent cells produce a complex mixture of factors termed SASPs. Senescent T cells are also able to produce pro- and anti-inflammatory cytokines to modulate the tumour microenvironment. Similar to fibroblasts, IL-6, IL-8, CXCR1, and CXCR2, classic SASP factors, are also increased in the senescent subset compared with the naïve subset [16]. In addition, the inflammatory and immune-modulatory cytokines and chemokines, such as IFN-γ, TNF-α, IL-18, IL-29, CCL5, CCL16, and CCL23, are significantly upregulated in senescent T cells [15,17,18]. SASP factors such as IL-6 [32] and TNF-α [33], act in an autocrine or paracrine manner to accelerate T cell senescence. SASP factors may also promote tumour development and suppress antitumour immunity. For example, the CCL5/CCR5 axis promotes tumour growth and migration, facilitates neovessel formation, and induces the immunosuppressive polarization of monocytes and myeloid cells, leading to M2 type tumour-associated macrophages and myeloid-derived suppressor cells (MDSCs) that induce the exhaustion of effector T cells [34]. IL-18, a proinflammatory cytokine in the IL-1 family that induces IFN-γ production, drives the generation of MDSCs, and augments the immunosuppressive activity of MDSCs in multiple myeloma [35]. High levels of IFN-γ in the absence of granzyme B also inhibit T cell cytotoxicity by inducing the expression of immune suppressive factors such as indoleamine 2, 3-dioxygenase (IDO), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) [36]. Therefore, SASP factors produced by senescent T cells may create and maintain an immunosuppressive environment.
2.4. Immunosuppression
In addition to modulating the tumour microenvironment by the SASPs, senescent T cells are also reported to be a unique subpopulation of regulatory T cells (Tregs) that directly suppress T cell functions. Senescent T cells inhibit proliferation and activation, decrease the secretion of proinflammatory cytokines, and induce the apoptosis of activated T cells in vitro [37,38]. Tumour-induced senescent T cells may also enhance the production of proinflammatory cytokines (TNF, IL-1β, and IL-6) and angiogenic factors [matrix metallopeptidase 9 (MMP-9), vascular endothelial growth factor A (VEGF-A) and IL-8] by monocytes/macrophages, which may promote tubulogenesis and tumour cell survival [39]. These data indicate that senescent T cells with suppressive functions could contribute to tumour immune evasion. However, whether senescent T cells play a beneficial role or a negative role in different types of tumours and the different stages of tumour progression still need further investigation.
2.5. Metabolic features
In T cells, more energy allows more proliferation and effector functions. As the most important energy-producing organelle, mitochondria are defective in senescent T cells, with a low mitochondrial mass, decreased mitochondrial membrane potential and elevated levels of reactive oxygen species (ROS) [17,20,21]. CD8+ T cells acquire a senescent phenotype faster than CD4+ T cells, because they have less fit and healthy mitochondria [20]. Senescent T cells express low levels of the glucose transporter type 1 (Glut1) [17,20], and fatty acid transporters FATP2 and FATP3 [20], resulting in impaired nutrient uptake. Therefore, as the principle mechanism, senescent T cells rely heavily on glycolysis, which is not the most productive way to generate energy, whereas effector T cells can use glycolysis and oxidative phosphorylation for energy generation [17,20]. Although p38 inhibition partially restores mitochondrial function, senescent T cells still engage glycolysis to provide the energy required for the increase in proliferation after activation and the increased energy demand is met by increased autophagy [17]. Moreover, glucose consumption caused by immunosuppressive cells such as natural regulatory T cells (nTregs) or tumour-derived γδ Treg cells also triggers T cell senescence [13]. Tumour cells and tumour-infiltrating T cells also compete for glucose within the tumour suppressive microenvironment, indicating that a lack of enough glucose highly consumed by tumour cells may result in responder T cell senescence as a new mechanism of tumour immune escape.
2.6. Key signalling molecules regulating T cell senescence
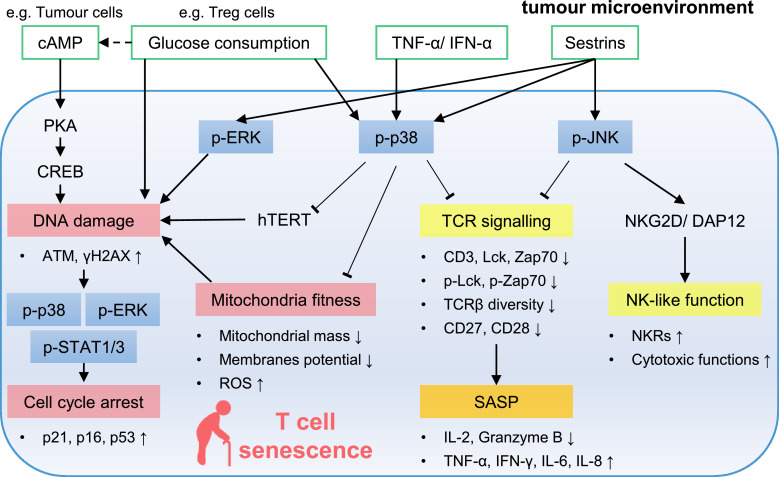
Mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated protein kinase (ERK), c-Jun N-terminal kinase (JNK) and p38, play an important role in regulating T cell senescence (Figure 1). In senescent T cells, MAPKs can be activated by DNA damage [28], metabolic disorders, such as glucose consumption [13,28] or the accumulation of cyclic adenosine monophosphate (cAMP) [14] proinflammatory cytokines, TNF-α [40] and interferon alpha (IFN-α) [29], and stress sensor, sestrins [26,41]. The activation of p38, JNK and ERK suppresses T cell proliferation [15,28,41] and causes senescence in different ways. On the one hand, after the phosphorylation of p38, the expression and activity of telomerase decrease [28,29,40,41], and mitochondria become dysfunctional [17], which further leads to DNA damage. In the tumour microenvironment, DNA damage in T cells is also triggered by glucose consumption [13,28] and the accumulation of cAMP, which can be reduced by the activation of TLR8 signalling [14]. Then, activated p38 and ERK cooperate with signal transducer and activator of transcription 1/3 (STAT1/3) to significantly increase the expression of cyclin-dependent kinase inhibitors (CKIs), such as p21, p16, and p53 to prevent T cell proliferation [13]. Two p53 isoforms also regulate ageing- and tumour-associated T cell senescence [16]. On the other hand, activated p3811, 28, 29 and JNK [26,41] inhibit the expression and activity of key components of TCR signalling as well as costimulatory receptors, CD27 and CD28, to form T cell-specific SASPs [18]. Moreover, JNK helps senescent T cells gain innate-like killing capacity by increasing NKG2D-DAP12 expression [26].
Figure 1.
Signalling pathways involved in T cell senescence in the tumour microenvironment. In senescent T cells, MAPKs can be activated by DNA damage, metabolic disorders, proinflammatory cytokines, and sestrins. The activation of p38 inhibits telomerase activity and destroys mitochondria fitness which leads to DNA damage. DNA damage further activates p38, ERK, and STAT1/3 to pronounce the expression of CKIs to prevent T cell proliferation. P-p38 and p-JNK inhibit TCR signaling to form T cell specific SASPs. P-JNK also help senescent T cells gain innate-like killing capacity. ATM, ataxia-telangiectasia mutated; cAMP, cyclic adenosine monophosphate; CKIs, cyclin-dependent kinase inhibitors; CREB, cAMP response element-binding protein; ERK, extracellular signal-regulated protein kinase; hTERT, human telomerase reverse transcriptase; IFN-γ, interferon gamma; JNK, c-Jun N-terminal kinase; MAPKs, mitogen-activated protein kinases; NK, natural killer; NKRs, natural killer like receptors; PKA, phosphorylase kinase A; ROS, reactive oxygen species; SASP, senescence-associated secretory phenotype; STAT, signal transducer and activator of transcription; TCR, T-cell receptor; TNF-α, tumour necrosis factor alpha; Tregs, regulatory T cells; γH2AX, phosphorylated H2AX
3. Potential prognostic biomarkers in cancer treatment
3.1. General progression
Senescent T cells accumulate in the peripheral blood of patients with solid tumour, such as lung cancer [22], breast cancer [42,43], head and neck cancer [44], gastric cancer [45], glioblastoma [46], and haematologic malignancies, such as acute myeloid leukaemia (AML) [47,48], and chronic lymphocytic leukaemia (CLL) [49,50], and increase with clinical stage [51]. Senescent T cells show a decreasing tendency after tumour resection [44] or in long-term complete remission (CR) [47]. T cells also display features of senescence at the tumour site of non-small cell lung cancer (NSCLC) [22], multiple myeloma [52], ovarian cancer [2], breast cancer [2], and follicular lymphoma [53]. In NSCLC, although the percentage of CD57+ T cells in CD3+ tumour-infiltrating lymphocytes (TILs) is lower than that observed in the peripheral blood [22], the cytokine production and proliferation ability of senescent T cells is much more dampened at the tumour site [22,52]. In vitro experiments further confirmed that tumour cells [14,16,38,48] or Tregs in the tumour microenvironment [11,13] are able to induce T cell senescence.
Senescent T cells may also serve as biomarkers to predict clinical outcomes. In lung cancer [54], gastric cancer [45], renal cell carcinoma [55], glioblastoma [46], non-Hodgkin lymphoma [53], CLL [49], and AML [56], overall survival (OS) is significantly shorter in patients with higher levels of senescent T cells in the blood. CD8+CD57+ T cells are also able to predict the development of cutaneous squamous cell carcinoma in patients post kidney transplantation [57].
3.2. Chemo(radio)therapy
In addition to correlations with the progression and prognosis of multiple cancers, most studies have indicated that pretreatment levels of senescent T cells in the peripheral blood are also associated with outcomes in patients treated with chemo(radio)therapy. A lower level of non-senescent T cells independently predicts a worse early response to stereotactic ablative radiotherapy in patients with lung metastases from NSCLC [58]. Higher levels of senescent T cells also independently predict unfavourable OS and progression free survival (PFS) from diagnosis in advanced gastric cancer [45], NSCLC [51], breast cancer [59], and AML [56] patients. The change in senescent T cells after chemo(radio)therapy can also act as a prognostic biomarker for refractory disease or relapse. In AML, the frequency of senescent CD8+ T cells decreases in patients after achieving CR, but concomitantly increases in patients who develop refractory disease and/or relapse (RR) [48,56]. Patients with more senescent T cells in the peripheral blood have decreased levels of IFN-γ and increased levels of IL-6 [59], which may partially explain why senescent T cells predict poor chemo(radio)therapy efficacy.
Chemo(radio)therapy is reported to induce T cell senescence in lung cancer [60], breast cancer [54], and metastatic colorectal cancer [61]. One explanation is that chemo(radio)therapy, as a DNA-damaging agent, can induce T cell senescence by increasing the expression of phosphorylated H2AX (γH2AX) and p16, which are canonical biomarkers in senescent somatic cells [62,63], preventing proliferation[63] and modulating metabolism [64]. Another explanation is that senescent T cells accumulate after several cycles of chemo(radio)therapies because non-proliferative cells are resistant to chemo(radio)therapy [61,63]. Notably, senescent T cells induced by chemo(radio)therapy are impaired in cytotoxicity and the production of TNF-α and IFN-γ [61], which may further lead to a poor response.
3.3. Immune checkpoint inhibitors
As one of the most important developments in cancer therapy in the past decade, immune checkpoint inhibitors (ICIs) have been designed to restore a patient's own antitumour immune response that is attenuated during the process of tumour progression [65]. It is the exhausted T cells (PD-1+CD8+ T cells), but not senescent T cells can be re-activated by PD-1/PD-L1 blockades. However, preclinical research has demonstrated that the reinvigoration of exhausted CD8+ T cells by PD-1/PD-L1 blockade depends on CD28 signalling [66,67]. Therefore, the frequency of senescent T cells may act as a potential biomarker to predict the response to ICIs. In advanced NSCLC and melanoma patients treated with PD-1/PD-L1 inhibitors, a high level of circulating senescent CD8+ T cells, with limited proliferative capacity and lower IL-2 and higher TNF-α and IFN-γ production [68], was significantly correlated with resistance to ICIs [69] and a poor overall response rate (ORR), median PFS and OS [68,70].
Senescent T cell markers are also enriched in the peripheral blood of NSCLC patients who respond to ICIs [23,65,71]; therefore, they can be used to determine candidates and predict therapeutic efficacy. Neoantigen-specific T cells towards terminally differentiated phenotype with enhanced CD57 and KLRG-1 are more frequently detected in responders than in nonresponders [23,71]. The terminally differentiated phenotype is indicative of recent antigen experience, suggesting that an effective antitumour T cell response may be ongoing in responders [71]. Moreover, a high percentage of highly differentiated CD4+ T cells at baseline was significantly correlated with objective responses [65]. Although lack CD27 and CD28 expression, highly differentiated CD4+ T cells do not highly express CD57 and γH2AX, indicating that they are not senescent T cells [65]. Therefore, whether senescent-like T cells with predictive ability for ICIs are bona fide senescent T cells still needs further clarification.
3.4. Adoptive cell transfer therapy
Adoptive cell transfer therapy, especially chimeric antigen receptor (CAR) T cell therapy, can evoke antitumour immune responses to prevent the progression of a variety of malignancies. However, continuous TCR or CAR stimulation would probably accelerate T cell differentiation to senescent subsets with limited in vivo persistence [72,73]. Compared with pre-infusion, post-infusion CAR-T cells express the senescent markers KLRG-1 and CD57, and lose the expression of the costimulatory molecule CD28 towards senescent-like phenotypes [74], [75], [76] The upregulation of CD57 on CAR T cells depends on the contact with tumour cells [75]. However, the mechanism by which these CAR T cells turn senescent still needs investigation.
It has been consistently reported that adoptive cell transfer of few senescent T cells demonstrate superior in vivo expansion, persistence, and antitumour capacities relative to the more senescent T cell subsets [77,78]. In B lymphoid haematologic malignancies, T cell senescence influences the response to CAR T cell therapy [79]. Responders who achieved CR and PR, had both a lower frequency of senescent populations and a higher frequency of less differentiated populations on CD8+ CAR T cells than nonresponders [80]. Another small patient cohort study also showed that responders had a lower percentage of senescent T cells in the apheresis product prior to the CAR T cell manufacturing process [81]. Moreover, according to preclinical experiments, enhancing proliferative and cytotoxic capacities and preventing the terminal differentiation of T cells by CD27 or CD28 transduction [82,83] or gamma chain cytokine treatment [84,85] may help restore antitumour activity. These data indicate that fewer senescent T cells may be more therapeutically effective.
3.5. Cancer vaccines
Cancer vaccines activate immune systems to treat existing cancers or prevent the development of cancer. However, cancer vaccines do not achieve good efficacy in limiting existing tumour cells or hurdling metastases in old individuals [7]. This is probably because the cancer vaccine fails to restore the proliferation of CD4+ and CD8+ T cells in elderly individuals [7,8]. CD8+ cytotoxic T cell responses to dominant tumour-associated antigens are profoundly weakened by ageing [86]. Loss of CD28 expression, which may indicate the accumulation of senescent T cells, was also found after vaccine challenge in the old group but not in the young group [87].
A clinical trial indicated that the proportion of senescent T cells at the beginning of the CIMAvax-EGF vaccine can be used as a predictive biomarker of efficacy in NSCLC patients [60]. Vaccinated patients with <24% of CD8+CD28− T cells pretreatment achieved a 20-month increase in median survival compared to control patients [60]. A reduction in senescent T cells by the temporary blockade of sestrins [41] or with a p38 MAPK inhibitor (losmapimod) [41,87] significantly enhances the number of antigen-specific T cells and increases the antiviral response after vaccination in older subjects. These findings suggest that delayed or reversed senescence in antigen-specific CD8+ T cells may be a new strategy to enhance antitumour immunity after vaccination during ageing.
4. Senescent T cells as therapeutic targets
4.1. Gamma chain cytokine treatment
The gamma chain cytokines, IL-2, IL-7, IL-15, and IL-21 serve as critical regulators of the survival and homeostasis of T cells [84,85,88,89]. Increasing evidence indicates that gamma chain cytokine-fuel cancer immunotherapy is associated with delayed or reversed senescence in antigen-specific CD8+ T or CAR T cells [84,85,88,89]. For example, IL-7 but not IL-2, or IL-15 inhibits tumour-induced T cell senescence by maintaining CD27/CD28 expression and proliferative capacity and reducing their suppressive function [88]. IL-15 and IL-21 prevent the terminal differentiation of tumour antigen-specific T cells and promote their expansion and effector functions [84,85,89]. The IL-15 super-agonist complex ALT-803 can also upregulate the expression of NKG2D and exhibit nonspecific cytotoxicity against tumour cells [90]. In clinical trials, ALT-803 efficiently reinduced responses in NSCLC or Merkel cell carcinoma patients who failed anti-PD-1 treatments [91,92] and in haematologic malignancy patients who relapsed after allogeneic haematopoietic cell transplantation [93]. Enhanced donor antitumour immune responses were probably due to an increase in the proliferation of CD8+ T cells [91,93].
The delayed or reversed senescence of antigen-specific CD8+ T or CAR T cells by gamma chain cytokine treatment relies on the activation of the JAK-STAT signalling pathway [89,94]. Therefore, a novel generation of CAR T cells that include domains to activate STAT signalling can prevent CAR T cell senescence by maintaining a less differentiated phenotype (CD8+CD45RA+CD62L+CCR7+ T cells) and proliferation ability to gain superior antitumour effects than other CAR T cells [94].
4.2. MAPK-related regulation
MAPKs play an important role in regulating T cell senescence. Therefore, inhibitors targeting the p38, ERK, JNK, and STAT signalling pathways may prevent the senescence of T cells according to preclinical experiments [13,17,18,28,29,40,41,95]. Notably, MAPK signalling is also critical for T cell activation and effector functions. Therefore, the temporal specificity of MAPK inhibitors for preventing senescence in tumour-reactive T cells is urgently needed. In mouse models, T cells were pretreated with p38 inhibitors before transfusion to test their antitumour function in vivo [95]. One possible solution is the CRISPR-Cas9 system, which may help to modulate MAPK signalling directly and stably. Another is to identify specific surface markers on senescent T cells to perform selective depletion [96].
The regulation of MAPK inhibition can also reduce T cell senescence. Knockdown of sestrins restored antigen-specific proliferation and cytokine production in T cells from old mice [41]. However, the restoration of sestrins may also increase senescent T cell NK-like clearance ability at the cost of TCR-dependent cytotoxic function [26]. Future clinical experiments are needed to illustrate the role of sestrins in modulating senescent T cell functions in different kinds of tumours.
4.3. Metabolic regulation
Senescence is the ultimate state of the coordinating effect from both inner and outer environments. Mitochondrial dysfunction, glucose deprivation and a hypoxic environment may cause T cell senescence. Therefore, metabolic regulation might be an important strategy to reverse or prevent T cell senescence in the tumour microenvironment. Rapamycin [84], a mammalian target of rapamycin complex 1 (mTORC1) inhibitor, metformin [97] and BIRB 796 [17], p38 inhibitors, can increase mitochondrial biogenesis and fitness to prevent T cell senescence. The addition of glucose can prevent Treg-induced senescence in responder T cells during their interactions [13]. A hypoxic environment created by the accumulation of adenosine and cAMP was reported to induce T cell senescence [14]. A prospective trial showed that hyperbaric oxygen therapy increases telomere length in peripheral blood cells and decreases the number of senescent T cells [98]. Similarly, physical exercise, which can increase the amount of oxygen in the blood, prevents cellular senescence in circulating leukocytes [99]. In the tumour microenvironment, the downregulation of cAMP by the specific cAMP pharmacological inhibitors, 7-ddA and H89, or by synthetic poly-G3 and natural TLR8 ligand (ssRNA40) to activate TLR8 signalling suppresses tumour-, Treg-, and γδ-Treg-induced T cell senescence [11,12,14]. Although many clinical trials on TLR agonists in solid tumours are currently ongoing [100], whether the enhanced antitumour immunity is partially due to the prevention of T cell senescence still needs further investigation.
5. Outstanding questions
Increasing evidence suggests a link between T cell senescence and tumour progression. Studies have indicated that the tumour microenvironment promotes the senescence of T cells through multiple pathways. The accumulation of senescent T cells may be responsible for advanced cancer and the low response rate to chemo(radio)therapy as well as immunotherapy (Table 2). Thus, preventing and restoring T cell senescence could be novel therapeutic strategies for cancer treatment.
Table 2.
Potential prognostic role of senescent T cells in cancer treatment
| Treatment | Tumour type | Change of cell subsets | Clinical outcome | References |
|---|---|---|---|---|
| radiotherapy | NSCLC | high levels of pretreatment CD8+CD28− T cells | early treatment response ↓ | 58 |
| chemo(radio)therapy | NSCLC | high levels of pretreatment CD8+CD28− T cells | OS and PFS ↓ | 51 |
| chemotherapy | gastric cancer | high levels of pretreatment CD57+ T | cumulative 3-year survival ↓ | 45 |
| chemotherapy | metastatic breast cancer | high levels of pretreatment CD8+CD28− T cells | PFS ↓ | 59 |
| chemotherapy | AML | high levels of pretreatment CD28−CD57+CD8+ T cells | OS and EFS ↓ | 56 |
| chemotherapy | AML | posttreatment CD8+CD28−CD57+ T cells and CD8+CD57+ T cells ↑ | responders | 48,56 |
| ICIs | melanoma | high levels of pretreatment CD45RA+ CCR7−CD27−CD28−CD8+ T cells | OS ↓ | 70 |
| ICIs | melanoma | high level of pretreatment CD27−CD28−Tim-3+CD57+ T cells | resistance | 69 |
| ICIs | NSCLC | high level of pretreatment CD28−CD57+KLRG1+CD8+ T cells | ORR, median PFS and OS ↓ | 68 |
| ICIs | NSCLC | present of CD57+KLRG-1+ T cells after treatment | responders | 71 |
| ICIs | NSCLC | present of CD45RA+CCR7−CD28−CD95+CD8+ T cells after treatment | responders | 23 |
| CAR T cell therapy | B lymphoid haematologic malignancies | lower frequency of pretreatment CD27−CD28− T cells | responders | 81 |
| CAR T cell therapy | B lymphoid haematologic malignancies | lower frequency of CD57+CD39+CD28−CD8+ CAR T cells | responders | 80 |
| cancer vaccine | NSCLC | high levels of pretreatment CD28−CD8+ T cells | survival ↓ | 60 |
AML, acute myeloid leukaemia; CAR, chimeric antigen receptor; EFS, event-free survival; ICIs: immune checkpoint inhibitors; NSCLC, non-small cell lung cancer; ORR, overall response rate; OS, overall survival; PFS, progression free survival.
Although great progress has been made in this specific area of cancer research, gaps in our understanding of senescent T cells in cancer patients remain. First, the heterogeneity should be fully considered. The heterogeneity here refers to both the spatial and time dimensions. The role of senescent T cells in different types of tumours, even in different sites within the same patient varies. Likewise, senescent T cells in primary versus metastatic tumours, and in situ versus the peripheral blood also need to be further investigated. Senescent T cells lose TCR-dependent killing ability but gain TCR-independent cytotoxic functions [26,41], suggesting that rather than being dysfunctional, these cells acquire an alternative functional profile as they differentiate towards senescence. Therefore, the role of senescent T cells in the tumour microenvironment needs further study. T cells incubated with tumours at a low tumour to T cell ratio undergo transformation into a senescent phenotype [38], indicating that the number of TILs may also influence T cell senescence.
Second, the study techniques for T cell senescence should be improved. Since several features of senescence in aged mice do not necessarily represent the phenomenon in humans [19], it is worth finding suitable animal models to study the mechanisms involved. In addition, due to their limited proliferative abilities, more efficient and precise high-resolution techniques are needed because the quantity of senescent T cells is usually too low for in-depth phenotype or functional assays [71]. This effort may be aided by techniques such as single cell sequencing. Moreover, since senescence is the dynamic process upon antigen stimulation, it will be very helpful to trace overall changes in both the phenotype and corresponding function.
Third, targeting senescent T cells as a novel therapeutic strategy requires further analysis of the phenotype and causative mechanism under different scenarios. It is worth clarifying whether the existing therapies/strategies are engaged in regulating T cell senescence. If involved in T cell senescence upon a given therapy, parameters such as the dose, interval time, and treatment duration should be delineated precisely. Only with a comprehensive and objective understanding can disabled T cells regain their powerful lethality in tumour therapy.
6. Search strategy and selection criteria
Data for this Review were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles using the search terms “senescent T cell”, “CD8+CD57+ T cell”, “CD8+CD28− T cell”, “terminally differentiated T cells”, “cancer”, “immunotherapy”, and “chemotherapy”. Abstracts and reports from meetings were included only when they related directly to previously published work. Only articles published in English between 1998 and 2021 were included.
Author contributions
JZ and THH wrote the manuscript and designed the figures. LXX and HYG revised the draft and final approved of the version to be submitted. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by Beijing-Tianjin-Hebei Basic Research Cooperation Special Program of Natural Science Foundation of Beijing Municipality (J200015), National Natural Science Foundation of China (91749107), Youth Program of National Natural Science Foundation of China (81901570). All the funders did not play any role in paper design, interpretation, or writing of the paper.
Contributor Information
Lixiang Xue, Email: lixiangxue@bjmu.edu.cn.
Hongyan Guo, Email: bysyghy@163.com.
References
- 1.Zhao Y, Shao Q, Peng G. Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment. Cellular & molecular immunology. 2020;17(1):27–35. doi: 10.1038/s41423-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbarin A, Cayssials E, Jacomet F. Phenotype of NK-Like CD8(+) T Cells with Innate Features in Humans and Their Relevance in Cancer Diseases. Frontiers in immunology. 2017;8:316. doi: 10.3389/fimmu.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covre LP, De Maeyer RPH, Gomes DCO, Akbar AN. The role of senescent T cells in immunopathology. Aging cell. 2020;19(12):e13272. doi: 10.1111/acel.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu W, Stachura P, Xu HC. Senescent Tumor CD8(+) T Cells: Mechanisms of Induction and Challenges to Immunotherapy. Cancers. 2020;12(10) doi: 10.3390/cancers12102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akbar AN, Henson SM, Lanna A. Senescence of T Lymphocytes: Implications for Enhancing Human Immunity. Trends in immunology. 2016;37(12):866–876. doi: 10.1016/j.it.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Effros RB. Replicative senescence in the immune system: impact of the Hayflick limit on T-cell function in the elderly. American journal of human genetics. 1998;62(5):1003–1007. doi: 10.1086/301845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Provinciali M. Immunosenescence and cancer vaccines. Cancer immunology, immunotherapy : CII. 2009;58(12):1959–1967. doi: 10.1007/s00262-009-0665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira B, Xu XN, Akbar AN. Targeting Inflammation and Immunosenescence to Improve Vaccine Responses in the Elderly. Frontiers in immunology. 2020;11 doi: 10.3389/fimmu.2020.583019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbe-Tuana F, Funchal G, Schmitz CRR, Maurmann RM, Bauer ME. The interplay between immunosenescence and age-related diseases. Seminars in immunopathology. 2020;42(5):545–557. doi: 10.1007/s00281-020-00806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Hoft DF, Peng G. Senescent T cells within suppressive tumor microenvironments: emerging target for tumor immunotherapy. The Journal of clinical investigation. 2020;130(3):1073–1083. doi: 10.1172/JCI133679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye J, Huang X, Hsueh EC. Human regulatory T cells induce T-lymphocyte senescence. Blood. 2012;120(10):2021–2031. doi: 10.1182/blood-2012-03-416040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye J, Ma C, Hsueh EC. Tumor-derived gammadelta regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. Journal of immunology (Baltimore, Md : 1950) 2013;190(5):2403–2414. doi: 10.4049/jimmunol.1202369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Mo W, Ye J. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nature communications. 2018;9(1):249. doi: 10.1038/s41467-017-02689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye J, Ma C, Hsueh EC. TLR8 signaling enhances tumor immunity by preventing tumor-induced T-cell senescence. EMBO molecular medicine. 2014;6(10):1294–1311. doi: 10.15252/emmm.201403918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henson SM, Macaulay R, Riddell NE, Nunn CJ, Akbar AN. Blockade of PD-1 or p38 MAP kinase signaling enhances senescent human CD8(+) T-cell proliferation by distinct pathways. European journal of immunology. 2015;45(5):1441–1451. doi: 10.1002/eji.201445312. [DOI] [PubMed] [Google Scholar]
- 16.Mondal AM, Horikawa I, Pine SR. p53 isoforms regulate aging- and tumor-associated replicative senescence in T lymphocytes. The Journal of clinical investigation. 2013;123(12):5247–5257. doi: 10.1172/JCI70355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henson SM, Lanna A, Riddell NE. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8(+) T cells. The Journal of clinical investigation. 2014;124(9):4004–4016. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callender LA, Carroll EC, Beal RWJ. Human CD8(+) EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging cell. 2018;17(1) doi: 10.1111/acel.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song Y, Wang B, Song R. T-cell Immunoglobulin and ITIM Domain Contributes to CD8(+) T-cell Immunosenescence. Aging cell. 2018;17(2) doi: 10.1111/acel.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callender LA, Carroll EC, Bober EA, Akbar AN, Solito E, Henson SM. Mitochondrial mass governs the extent of human T cell senescence. Aging cell. 2020;19(2):e13067. doi: 10.1111/acel.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desdin-Mico G, Soto-Heredero G, Aranda JF. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science (New York, NY) 2020;368(6497):1371–1376. doi: 10.1126/science.aax0860. [DOI] [PubMed] [Google Scholar]
- 22.Huang B, Liu R, Wang P. CD8(+)CD57(+) T cells exhibit distinct features in human non-small cell lung cancer. Journal for immunotherapy of cancer. 2020;8(1) doi: 10.1136/jitc-2020-000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunert A, Basak EA, Hurkmans DP. CD45RA(+)CCR7(-) CD8 T cells lacking co-stimulatory receptors demonstrate enhanced frequency in peripheral blood of NSCLC patients responding to nivolumab. Journal for immunotherapy of cancer. 2019;7(1):149. doi: 10.1186/s40425-019-0608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu W, Larbi A. Markers of T Cell Senescence in Humans. International journal of molecular sciences. 2017;18(8) doi: 10.3390/ijms18081742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gustafson CE, Qi Q, Hutter-Saunders J. Immune Checkpoint Function of CD85j in CD8 T Cell Differentiation and Aging. Frontiers in immunology. 2017;8:692. doi: 10.3389/fimmu.2017.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira BI, De Maeyer RPH, Covre LP. Sestrins induce natural killer function in senescent-like CD8(+) T cells. Nature immunology. 2020;21(6):684–694. doi: 10.1038/s41590-020-0643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suen H, Brown R, Yang S. Multiple myeloma causes clonal T-cell immunosenescence: identification of potential novel targets for promoting tumour immunity and implications for checkpoint blockade. Leukemia. 2016;30(8):1716–1724. doi: 10.1038/leu.2016.84. [DOI] [PubMed] [Google Scholar]
- 28.Lanna A, Henson SM, Escors D, Akbar AN. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nature immunology. 2014;15(10):965–972. doi: 10.1038/ni.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanna A, Coutavas E, Levati L. IFN-alpha inhibits telomerase in human CD8(+) T cells by both hTERT downregulation and induction of p38 MAPK signaling. Journal of immunology (Baltimore, Md : 1950) 2013;191(7):3744–3752. doi: 10.4049/jimmunol.1301409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Britanova OV, Putintseva EV, Shugay M. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. Journal of immunology (Baltimore, Md : 1950) 2014;192(6):2689–2698. doi: 10.4049/jimmunol.1302064. [DOI] [PubMed] [Google Scholar]
- 31.Bjorkstrom NK, Beziat V, Cichocki F. CD8 T cells express randomly selected KIRs with distinct specificities compared with NK cells. Blood. 2012;120(17):3455–3465. doi: 10.1182/blood-2012-03-416867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herranz N, Gil J. Mechanisms and functions of cellular senescence. The Journal of clinical investigation. 2018;128(4):1238–1246. doi: 10.1172/JCI95148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parish ST, Wu JE, Effros RB. Modulation of T lymphocyte replicative senescence via TNF-{alpha} inhibition: role of caspase-3. Journal of immunology (Baltimore, Md : 1950) 2009;182(7):4237–4243. doi: 10.4049/jimmunol.0803449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aldinucci D, Borghese C, Casagrande N. The CCL5/CCR5 Axis in Cancer Progression. Cancers. 2020;12(7) doi: 10.3390/cancers12071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura K, Kassem S, Cleynen A. Dysregulated IL-18 Is a Key Driver of Immunosuppression and a Possible Therapeutic Target in the Multiple Myeloma Microenvironment. Cancer cell. 2018;33(4):634–648. doi: 10.1016/j.ccell.2018.02.007. e5. [DOI] [PubMed] [Google Scholar]
- 36.Castro F, Cardoso AP, Goncalves RM, Serre K, Oliveira MJ. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Frontiers in immunology. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Liu Q, Xiang AP. CD8+CD28- T cells: not only age-related cells but a subset of regulatory T cells. Cellular & molecular immunology. 2018;15(8):734–736. doi: 10.1038/cmi.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montes CL, Chapoval AI, Nelson J. Tumor-induced senescent T cells with suppressor function: a potential form of tumor immune evasion. Cancer research. 2008;68(3):870–879. doi: 10.1158/0008-5472.CAN-07-2282. [DOI] [PubMed] [Google Scholar]
- 39.Ramello MC, Tosello Boari J, Canale FP. Tumor-induced senescent T cells promote the secretion of pro-inflammatory cytokines and angiogenic factors by human monocytes/macrophages through a mechanism that involves Tim-3 and CD40L. Cell death & disease. 2014;5(11):e1507. doi: 10.1038/cddis.2014.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Mitri D, Azevedo RI, Henson SM. Reversible senescence in human CD4+CD45RA+CD27- memory T cells. Journal of immunology (Baltimore, Md : 1950) 2011;187(5):2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- 41.Lanna A, Gomes DC, Muller-Durovic B. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nature immunology. 2017;18(3):354–363. doi: 10.1038/ni.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trintinaglia L, Bandinelli LP, Grassi-Oliveira R. Features of Immunosenescence in Women Newly Diagnosed With Breast Cancer. Frontiers in immunology. 2018;9:1651. doi: 10.3389/fimmu.2018.01651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onyema OO, Decoster L, Njemini R. Chemotherapy-induced changes and immunosenescence of CD8+ T-cells in patients with breast cancer. Anticancer research. 2015;35(3):1481–1489. [PubMed] [Google Scholar]
- 44.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(-) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer immunology, immunotherapy : CII. 2003;52(10):599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akagi J, Baba H. Prognostic value of CD57(+) T lymphocytes in the peripheral blood of patients with advanced gastric cancer. International journal of clinical oncology. 2008;13(6):528–535. doi: 10.1007/s10147-008-0789-8. [DOI] [PubMed] [Google Scholar]
- 46.Fornara O, Odeberg J, Wolmer Solberg N. Poor survival in glioblastoma patients is associated with early signs of immunosenescence in the CD4 T-cell compartment after surgery. Oncoimmunology. 2015;4(9) doi: 10.1080/2162402X.2015.1036211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan J, Chen S, Lu Y. Higher PD-1 expression concurrent with exhausted CD8+ T cells in patients with de novo acute myeloid leukemia. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2017;29(5):463–470. doi: 10.21147/j.issn.1000-9604.2017.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knaus HA, Berglund S, Hackl H. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI insight. 2018;3(21) doi: 10.1172/jci.insight.120974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nunes C, Wong R, Mason M, Fegan C, Man S, Pepper C. Expansion of a CD8(+)PD-1(+) replicative senescence phenotype in early stage CLL patients is associated with inverted CD4:CD8 ratios and disease progression. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(3):678–687. doi: 10.1158/1078-0432.CCR-11-2630. [DOI] [PubMed] [Google Scholar]
- 50.Gothert JR, Eisele L, Klein-Hitpass L. Expanded CD8+ T cells of murine and human CLL are driven into a senescent KLRG1+ effector memory phenotype. Cancer immunology, immunotherapy : CII. 2013;62(11):1697–1709. doi: 10.1007/s00262-013-1473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu C, Jing W, An N. Prognostic significance of peripheral CD8+CD28+ and CD8+CD28- T cells in advanced non-small cell lung cancer patients treated with chemo(radio)therapy. Journal of translational medicine. 2019;17(1):344. doi: 10.1186/s12967-019-2097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zelle-Rieser C, Thangavadivel S, Biedermann R. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. Journal of hematology & oncology. 2016;9(1):116. doi: 10.1186/s13045-016-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magnano L, Martinez A, Carreras J. T-cell subsets in lymph nodes identify a subgroup of follicular lymphoma patients with favorable outcome. Leukemia & lymphoma. 2017;58(4):842–850. doi: 10.1080/10428194.2016.1217525. [DOI] [PubMed] [Google Scholar]
- 54.Onyema OO, Decoster L, Njemini R. Shifts in subsets of CD8+ T-cells as evidence of immunosenescence in patients with cancers affecting the lungs: an observational case-control study. BMC cancer. 2015;15:1016. doi: 10.1186/s12885-015-2013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Characiejus D, Pasukoniene V, Kazlauskaite N. Predictive value of CD8highCD57+ lymphocyte subset in interferon therapy of patients with renal cell carcinoma. Anticancer research. 2002;22(6B):3679–3683. [PubMed] [Google Scholar]
- 56.Tang L, Wu J, Li CG. Characterization of Immune Dysfunction and Identification of Prognostic Immune-Related Risk Factors in Acute Myeloid Leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020;26(7):1763–1772. doi: 10.1158/1078-0432.CCR-19-3003. [DOI] [PubMed] [Google Scholar]
- 57.Bottomley MJ, Harden PN, Wood KJ. CD8+ Immunosenescence Predicts Post-Transplant Cutaneous Squamous Cell Carcinoma in High-Risk Patients. Journal of the American Society of Nephrology : JASN. 2016;27(5):1505–1515. doi: 10.1681/ASN.2015030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu C, Hu Q, Hu K. Increased CD8+CD28+ T cells independently predict better early response to stereotactic ablative radiotherapy in patients with lung metastases from non-small cell lung cancer. Journal of translational medicine. 2019;17(1):120. doi: 10.1186/s12967-019-1872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song G, Wang X, Jia J. Elevated level of peripheral CD8(+)CD28(-) T lymphocytes are an independent predictor of progression-free survival in patients with metastatic breast cancer during the course of chemotherapy. Cancer immunology, immunotherapy : CII. 2013;62(6):1123–1130. doi: 10.1007/s00262-013-1424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saavedra D, Garcia B, Lorenzo-Luaces P. Biomarkers related to immunosenescence: relationships with therapy and survival in lung cancer patients. Cancer immunology, immunotherapy : CII. 2016;65(1):37–45. doi: 10.1007/s00262-015-1773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruni E, Cazzetta V, Donadon M. Chemotherapy accelerates immune-senescence and functional impairments of Vdelta2(pos) T cells in elderly patients affected by liver metastatic colorectal cancer. Journal for immunotherapy of cancer. 2019;7(1):347. doi: 10.1186/s40425-019-0825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanoff HK, Deal AM, Krishnamurthy J. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. Journal of the National Cancer Institute. 2014;106(4):057. doi: 10.1093/jnci/dju057. :dju. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arina A, Beckett M, Fernandez C. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nature communications. 2019;10(1):3959. doi: 10.1038/s41467-019-11906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li HH, Wang YW, Chen R, Zhou B, Ashwell JD, Fornace AJ., Jr. Ionizing Radiation Impairs T Cell Activation by Affecting Metabolic Reprogramming. International journal of biological sciences. 2015;11(7):726–736. doi: 10.7150/ijbs.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zuazo M, Arasanz H, Fernandez-Hinojal G. Functional systemic CD4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO molecular medicine. 2019;11(7):e10293. doi: 10.15252/emmm.201910293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hui E, Cheung J, Zhu J. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science (New York, NY) 2017;355(6332):1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamphorst AO, Wieland A, Nasti T. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science (New York, NY) 2017;355(6332):1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferrara R, Naigeon M, Auclin E. Circulating T-cell Immunosenescence in Patients with Advanced Non-small Cell Lung Cancer Treated with Single-agent PD-1/PD-L1 Inhibitors or Platinum-based Chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2021;27(2):492–503. doi: 10.1158/1078-0432.CCR-20-1420. [DOI] [PubMed] [Google Scholar]
- 69.Moreira A, Gross S, Kirchberger MC, Erdmann M, Schuler G, Heinzerling L. Senescence markers: Predictive for response to checkpoint inhibitors. International journal of cancer. 2019;144(5):1147–1150. doi: 10.1002/ijc.31763. [DOI] [PubMed] [Google Scholar]
- 70.Wistuba-Hamprecht K, Martens A, Heubach F. Peripheral CD8 effector-memory type 1 T-cells correlate with outcome in ipilimumab-treated stage IV melanoma patients. European journal of cancer (Oxford, England : 1990) 2017;73:61–70. doi: 10.1016/j.ejca.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fehlings M, Jhunjhunwala S, Kowanetz M. Late-differentiated effector neoantigen-specific CD8+ T cells are enriched in peripheral blood of non-small cell lung carcinoma patients responding to atezolizumab treatment. Journal for immunotherapy of cancer. 2019;7(1):249. doi: 10.1186/s40425-019-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kasakovski D, Xu L, Li Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. Journal of hematology & oncology. 2018;11(1):91. doi: 10.1186/s13045-018-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scherer LD, Brenner MK, Mamonkin M. Chimeric Antigen Receptors for T-Cell Malignancies. Frontiers in oncology. 2019;9:126. doi: 10.3389/fonc.2019.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brudno JN, Maric I, Hartman SD. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(22):2267–2280. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu X, Prasad S, Gaedicke S, Hettich M, Firat E, Niedermann G. Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget. 2015;6(1):171–184. doi: 10.18632/oncotarget.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang C, Palashati H, Rong Z. Pre-depletion of TRBC1+ T cells promotes the therapeutic efficacy of anti-TRBC1 CAR-T for T-cell malignancies. Molecular cancer. 2020;19(1):162. doi: 10.1186/s12943-020-01282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Powell DJ, Jr., Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105(1):241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krishna S, Lowery FJ, Copeland AR. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science (New York, NY) 2020;370(6522):1328–1334. doi: 10.1126/science.abb9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wudhikarn K, Park JH. Dissecting factors influencing response to CAR T cell therapy in B lymphoid hematologic malignancies: from basic to practice. Leukemia & lymphoma. 2020;61(10):2324–2334. doi: 10.1080/10428194.2020.1761967. [DOI] [PubMed] [Google Scholar]
- 80.Beider K, Besser MJ, Schachter J. Upregulation of Senescent/Exhausted Phenotype of CAR T Cells and Induction of Both Treg and Myeloid Suppressive Cells Correlate with Reduced Response to CAR T Cell Therapy in Relapsed/Refractory B Cell Malignancies. Blood. 2019;134(Supplement 1):3234. [Google Scholar]
- 81.Worel N, Pfistershammer K, Pickl W. Influence of CD27(-) CD28(-) T-Cells on the Therapeutic Outcome in Adult Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma after CART-Infusion. Blood. 2019:134. [Google Scholar]
- 82.Kowolik CM, Topp MS, Gonzalez S. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer research. 2006;66(22):10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 83.Song DG, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ., Jr. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119(3):696–706. doi: 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- 84.Alizadeh D, Wong RA, Yang X. IL15 Enhances CAR-T Cell Antitumor Activity by Reducing mTORC1 Activity and Preserving Their Stem Cell Memory Phenotype. Cancer immunology research. 2019;7(5):759–772. doi: 10.1158/2326-6066.CIR-18-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stach M, Ptackova P, Mucha M, Musil J, Klener P, Otahal P. Inducible secretion of IL-21 augments anti-tumor activity of piggyBac-manufactured chimeric antigen receptor T cells. Cytotherapy. 2020;22(12):744–754. doi: 10.1016/j.jcyt.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 86.Jackaman C, Gardner JK, Tomay F. CD8(+) cytotoxic T cell responses to dominant tumor-associated antigens are profoundly weakened by aging yet subdominant responses retain functionality and expand in response to chemotherapy. Oncoimmunology. 2019;8(4) doi: 10.1080/2162402X.2018.1564452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vukmanovic-Stejic M, Chambers ES, Suarez-Farinas M. Enhancement of cutaneous immunity during aging by blocking p38 mitogen-activated protein (MAP) kinase-induced inflammation. The Journal of allergy and clinical immunology. 2018;142(3):844–856. doi: 10.1016/j.jaci.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Pfannenstiel LW, Bolesta E. Interleukin-7 inhibits tumor-induced CD27-CD28- suppressor T cells: implications for cancer immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(15):4975–4986. doi: 10.1158/1078-0432.CCR-10-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weng J, Moriarty KE, Baio FE. IL-15 enhances the antitumor effect of human antigen-specific CD8(+) T cells by cellular senescence delay. Oncoimmunology. 2016;5(12) doi: 10.1080/2162402X.2016.1237327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu W, Jones M, Liu B. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer research. 2013;73(10):3075–3086. doi: 10.1158/0008-5472.CAN-12-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wrangle JM, Velcheti V, Patel MR. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. The Lancet Oncology. 2018;19(5):694–704. doi: 10.1016/S1470-2045(18)30148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drusbosky L, Nangia C, Nguyen A. Complete response to avelumab and IL-15 superagonist N-803 with Abraxane in Merkel cell carcinoma: a case study. Journal for immunotherapy of cancer. 2020;8(2) doi: 10.1136/jitc-2020-001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romee R, Cooley S, Berrien-Elliott MM. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131(23):2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kagoya Y, Tanaka S, Guo T. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nature medicine. 2018;24(3):352–359. doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gurusamy D, Henning AN, Yamamoto TN. Multi-phenotype CRISPR-Cas9 Screen Identifies p38 Kinase as a Target for Adoptive Immunotherapies. Cancer cell. 2020;37(6):818–833. doi: 10.1016/j.ccell.2020.05.004. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshida S, Nakagami H, Hayashi H. The CD153 vaccine is a senotherapeutic option for preventing the accumulation of senescent T cells in mice. Nature communications. 2020;11(1):2482. doi: 10.1038/s41467-020-16347-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bharath LP, Agrawal M, McCambridge G. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell metabolism. 2020;32(1):44–55. doi: 10.1016/j.cmet.2020.04.015. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hachmo Y, Hadanny A, Abu Hamed R. Hyperbaric oxygen therapy increases telomere length and decreases immunosenescence in isolated blood cells: a prospective trial. Aging. 2020;12(22):22445–22456. doi: 10.18632/aging.202188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Werner C, Furster T, Widmann T. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120(24):2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- 100.Anwar MA, Shah M, Kim J, Choi S. Recent clinical trends in Toll-like receptor targeting therapeutics. Medicinal research reviews. 2019;39(3):1053–1090. doi: 10.1002/med.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]