Abstract
The retrospective cohort study aimed to evaluate the clinical outcomes of Ayurveda treatment exposure as an add-on to conventional care in early stage COVID-19 patients admitted at Samaras COVID care center, Ahmedabad, India. Conventional care included Vitamin-c, Azithromycin, and Paracetamol. Ayurveda formulations used as add-on were Dashamula and Pathyadi decoctions along with Trikatu powder, Sanshamani tablet, AYUSH-64 tablet AND Yastimadhu Ghana tablet for oral administration. Considering Add-on Ayurveda medicines as exposure of interest, patients who received Add-on Ayurveda medicines at least for 7 days were included in the exposed group while those who received only conventional care in unexposed group. Data was collected through record review and telephonic interviews. The outcomes of interest were the development of symptoms, duration of symptomatic phase in those progressing to symptomatic stage and mortality. Total 762 participants were included-[541 (71%) in the exposed group and 221 (29%) in the unexposed. Progression to symptomatic phase did not differ significantly between groups [27.6% in exposed, 24.6% in unexposed, adjusted RR 0.85; 95% CI 0.6-1.2]. The total duration of symptomatic phase among those progressing to the symptomatic stage was significantly decreased in the exposed group (x¯ = 3.66 ± 1.55 days in exposed (n = 133); x¯ = 5.34 ± 3.35 days in unexposed (n = 61), p < 0.001). No mortality was observed in either of the groups. Ayurveda Treatment as adjunctive to conventional care reduced the duration of symptomatic phase in early stage COVID-19 as compared to standalone conventional care. Add-on Ayurveda treatment has promising potential for management of early stage COVID-19.
Keywords: Ayurveda, COVID-19, cohort study, complementary medicine, SARS-CoV-2
Introduction
A worldwide outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the resulting COVID-19 cases and fatalities has challenged health systems globally. Globally, a total of 125 million cases including 2.7 million deaths have been reported by March 25, 2021. 1 Most of the patients with SARS-Cov-2 infection develop a mild illness, approximately 14% develop a severe disease that requires hospitalization and oxygen support, and 5% require admission to an intensive care unit. 2 As of now, mainly symptomatic supportive treatment is being provided to the patients whereas seriously ill individuals are treated with organ support. 3 The drug discovery process is accelerated at the moment with much focus on repurposing existing drugs. 4 The majority of the drugs used for treatment worldwide fall primarily under antiviral, antimalarial, anti-inflammatory, monoclonal antibodies categories 5 and are used solely on an empirical basis. 6 China has adopted integrative approach involving modern medicine and TCM (Traditional Chinese Medicine) in this pandemic situation and has reported encouraging outcomes. 7
In India, the traditional medicine Ayurveda is being explored for the prevention and treatment of COVID-19. Rasayana, unique concept of Ayurveda linked with the immunomodulation may play an important role in COVID-19 management. 8,9 The Ministry of AYUSH, Government of India released an advisory for public use detailing Ayurveda based preventive measures for COVID-19, 10 it was widely promoted and accessed. Pragmatic use of Ayurveda as per the COVID-19 disease severity was proposed as early as in March-April 2020. 11 Some states in India formally initiated the use of Ayurveda treatment along with conventional modern medicine treatment for COVID-19 earlier than the release of the National Protocol for Management of COVID-19 including Ayurveda interventions. 12 These include states of Gujarat, Haryana, Goa and Kerala where government orders supported such use of Ayurveda treatment. 13,14
COVID-19 being a new disease, sufficient reports of the role of Ayurveda treatment in its management are not available alike from other systems of medicine. Therefore, this study was conducted to evaluate the clinical outcomes of Ayurveda treatment exposure as an add-on to conventional care in the early stage of COVID-19 patients from a single center in Gujarat, India.
Methods
Study Settings
Gujarat state is situated in Western India. It covers 196,024 km2 area with 62.7 million population approximately. Prevalence of major lifestyle disorders that impact COVID-19 is relatively lower in Gujarat than in India—overweight 6.5%-9.1% vs 14.6%, Obesity 1.1%-2.1% vs 3.4%, hypertension 2.1%-3% vs 5.2%, and diabetes 2.2%-3.9% vs 7.1% respectively. 15 On 29th April 2020, Gujarat became the second major hotspot for the COVID-19 outbreak in the country after Maharashtra. Gujarat had 3,744 cases of COVID-19 (11.94%) of the total 31,332 of all over India though Gujarat state has only 4.3% of the national population. Moreover, at the time of this study death rate in Gujarat was comparatively higher (4.8) as compared to the 3.21 death rate across India. 16 As per testing guidelines then, all symptomatic individuals who had undertaken international travel in the last 14 days, or had contacts of laboratory-confirmed cases, health care workers; all patients with Severe Acute Respiratory Illness (fever AND cough and/or shortness of breath); asymptomatic direct and high-risk contacts of a confirmed case were to be tested. 17 In hotspots/cluster and large migration gatherings/ evacuees centers, all symptomatic ILI (Influenza-like illness) (fever, cough, sore throat, runny nose) were to be tested within 7 days of illness (–RT-PCR and after 7 days of illness—Antibody test, if negative, confirmed by RT-PCR). COVID-19 patients were being managed as per then available Clinical Management guideline published by the Govt of India. 18 AYUSH health department of Gujarat adopted an integrative approach in the prevention and control of COVID-19. State AYUSH directorate issued standard Ayurveda treatment guideline for COVID-19 treatment for asymptomatic and mild conditions. This recommended Dashamula and Pathyadi Kwatha 20 ml each along with Trikatu Churna 2 g b.i.d., Sanshamani Vati 500 mg b.i.d., AYUSH-64 tablets 500 mg b.i.d. for oral administration and Yastimadhu Ghanvati 500 mg for chewing, 5 to 6 times a day. The group of drugs was selected based on Ayurveda principles to manage epidemics as these drugs are used primarily in respiratory and influenza like illness (Supplementary file S1). These Ayurvedic drugs were offered as add on treatment to asymptomatic and mild cases in addition to the conventional medicines comprising Vitamin-C 500 mg once a day and antibiotic Azithromycin 500 mg per day for the first 5 days to all patients and paracetamol (500 mg) s.o.s. in pyrexia.
Study Site
The study was conducted at Samras COVID care center in Ahmedabad city. Samaras COVID care center (CCC), with a capacity of 1400 beds was developed by Municipal Corporation that offered care only for those cases that have been clinically assigned as asymptomatic or mild cases and having a lower risk of severe COVID-19. COVID-19 patients diagnosed at various hospitals or quarantine zone in the city were referred to Samaras center after primary screening for severity risk by doctors.
Ayurveda physicians deputed to the CCC offered Ayurvedic medicines (as recommended in the state guideline-Supplementary file S1) to all patients without any influence. Patients were briefed about the possible role of Ayurveda medicines in disease prevention and management. Written consent was taken from each patient before medicines were given. Drug dose was adjusted in case of child patients. Ayurveda medicines were continued until the patient was discharged. Initially, the patient was discharged after 2 specimens tested negative for Sars-cov2 within a period of 24 hours, but later as guideline changed patient was discharged without testing, after 10 days of symptom onset if fever free for 3 consecutive days. At any point in time, before discharge from CCC, if the oxygen saturation dipped below 95%, the patient was moved to Dedicated COVID Health Centre (DCHC). Basic information of patients was documented manually in registers and data on clinical progress, treatment received, and transfer or discharge were documented electronically. Electronic records were maintained under AYUSH directorate office, Health Department. Ayurveda medicines provided were manufactured by Rajapipla Ayurved Pharmacy and supplied by state AYUSH directorate office. All facilities and treatments were provided free of cost. Center was functioning with 6 doctors, 6 paramedical staff from the conventional system along 5 medical officers from the Ayurveda system.
Study Design
An observational study was planned using routinely collected data at the Samaras COVID care center. A retrospective cohort study design was used to study the effect of add on Ayurveda treatment on disease progression and severity. Eligible participants enrolled in the cohort were inpatients at study site admitted on or after 15th April, 2020 and discharged or referred by 31st May, 2020 who had tested positive for COVID-19 by a RT-PCR test and had no symptoms of COVID-19 at the time of admission. Participants included were asymptomatic patients of either sex and any age group, diagnosed with COVID-19 through RTPCR with and without comorbidities. The add-on Ayurveda treatment was the exposure of interest. Therefore, patient who received Add-on Ayurveda treatment for 7 days were included in the exposed group while those who received only conventional treatment were included in the un-exposed (no exposure of add on Ayurveda treatment) group. Patients who had comparatively lower exposure of add-on treatment (less than 7 days) were not considered for analysis presuming that a minimum 7 days of drug exposure is required for drug effect.
The outcomes of interest were the development of symptoms, duration of symptomatic phase in those progressing to symptomatic stage, and death. Incidence of the symptom of COVID-19 was considered positive if at least one of these symptoms was found: fever, cough, cold, sore throat, shortness of breath, body ache, and lethargy. The total symptomatic phase was counted from the initiation of the first symptoms to the disappearance of the last symptoms. Participants were followed up to 1 month from admission.
Data Collection
Basic information of participants such as date of being COVID positive, demography, and transfer or discharge details were retrieved from the center’s physical records maintained in registers at the site. Knowledge about the source of infection, medicines provided, and symptoms development were retrieved from electronic format maintained by the AYUSH directorate office. Site Co-investigator and trained research assistants (Ayurved graduates) reviewed data after permission was obtained. A total of 12 assistants were involved in data collection. Patients were telephonically approached for post discharge outcomes at 1 month after discharge and to complete missing data if any. Another call was made the next day if the first call was unsuccessful. Follow up call was made after 7 days if symptoms were reported at the time of the first call. Oral consent was taken at the time of the telephonic interview for additional data collection. Patients who did not respond to follow up call or did not provide consent for the additional data were also excluded.
Data collection format included basic information i.e. gender, age, socioeconomic condition, education, comorbidities; disease-related information i.e. occurrence and the total duration of COVID-19 symptoms, and drug-related information i.e. onset and duration of medicine consumed and adverse events if any. Diabetes mellitus, hypertension, chronic lung disease, cardiovascular disease, liver and kidney diseases, and cancer were listed as comorbid conditions enquired for. Information was also sought if the patient was a contact defined as a patient who had a close contact with an infected person for at least 15 minutes. The total duration of symptoms was retrieved by asking the dates when the first symptoms appeared and the last symptom subsided; if dates could not be recalled by participant total period of symptoms was asked.
Data collection was stopped as a minimum predefined sample size (200 participants eligible) was achieved in each group. Total 3 weeks period (26th May to 15th June 2020) was required for data collection.
Statistical Analysis
Data analysis was conducted by SPSS statistics (IBM, Armonk, New York, USA). Continuous variables are expressed as the mean ± standard deviation and categorical variables as numbers and percentages. p values of less than 0.05 were considered to be significant. Incidence rates and risk ratios were calculated. Logistic regression was performed to adjust risks for age and comorbidities. Independent samples t-test was used to analyze total days of symptomatic phase in COVID patients. The standardized effect size was evaluated by Cohen’s d. Data imputation for missing values was performed by linear interpolation method. Missing values were observed below 1% for most of the variables except economic status (hence not presented).
Result
Study Participants
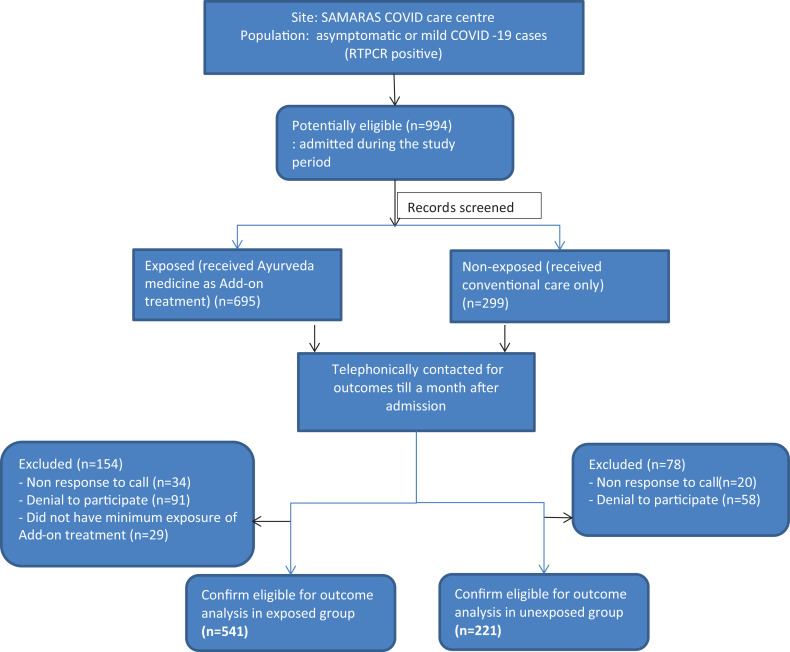
A total of 994 potential participants’ data admitted during the study period were screened. Out of them, 695 participants were exposed to Add-on Ayurveda medicines and 299 were unexposed. Including both groups, total 54 patients could not be contacted due to either incorrect contact number or no response to the phone call made. A total of 178 were excluded after screening including 149 who did not consent to participate (in exposed, n = 91; in unexposed, n = 58; possibly due to unknown fear or stigma related to disease) and 29 who did not have minimum exposure (7 days of add-on Ayurveda treatment). Hence, total 762 patients were included in analysis, 541 (71%) of these had been exposed to add-on Ayurveda medicines and 221 (29%) were unexposed (received only supportive conventional care) (Figure 1).
Figure 1.
Flow chart of the study procedure.
Characteristics of Study Participants
Background characteristics of patients are presented in Table 1. The mean age of patients in the exposed and unexposed group was 35.33 and 33.86 years respectively. Of the study participants, nearly three-fourths (546, 74%) were males and most (97.2%) did not have any comorbidity. The confounders (age, sex, and comorbidities) were distributed uniformly across both groups (p > 0.05). Of the total, 37% (n = 282) patients knew their source contact of infection. The mean duration of exposure of add-on Ayurveda treatment was 13.2 ± 4.9 days.
Table 1.
Background Characteristics of Participants (n = 762).
| Variables | Category | Exposed group (n = 541, 71%) | Unexposed group (n = 221, 29%) | Total | P-value |
|---|---|---|---|---|---|
| Age (Mean ± SD) | – | 35.33 ± 11.85 | 33.86 ± 11.82 | 34.9 ± 11.85 | 0.12 |
| Age | below 20 years | 42 (7.8%) | 26 (11.8%) | 68 (8.9%) | 0.231 |
| 21 to 40 years | 341 (63%) | 139 (62.9%) | 480 (63%) | ||
| 41 to 60 years | 144 (26.6%) | 53 (24%) | 197 (25.9%) | ||
| Above 60 years | 14 (2.6%) | 3 (1.4%) | 17 (2.2%) | ||
| Sex, n (%) | Male | 410 (75.8%) | 154 (69.7%) | 564 (74%) | 0.081 |
| Female | 131 (24.2%) | 67 (30.3%) | 198 (26%) | ||
| Education, n (%) | Primary | 152 (28.1%) | 75 (33.9%) | 227 (29.8%) | 0.011 |
| High school | 219 (40.5%) | 64 (29%) | 283 (37.1%) | ||
| Graduate | 170 (31.4%) | 82 (37.1%) | 252 (33.1%) | ||
| Co-morbidities | No | 526 (97.2%) | 215 (97.3%) | 741 (97.2) | 0.965 |
| YES | 15 (2.8%) | 6 (2.7%) | 21 (2.8) | ||
| Known to have close contact with a confirmed case of SARS CoV 2 infection, no. (%) | No | 375 (69.3%) | 105 (47.5%) | 480 (63%) | <0.001 |
| Yes | 166 (30.7%) | 116 (52.5%) | 282 (37%) |
SD-Standard Deviation, *analyzed by Chi square test.
Incidence and Relative Risk of Developing Symptoms
The incidence of developing COVID-19 symptoms was 24.6% (133 out of 541) in the exposed group compared to 27.6% (61 out of 221) in the unexposed group. The Crude Risk Ratio was 0.85 (CI: 0.60-1.22) and adjusted RR was 0.85 (CI, 0.6-1.21). Thus, risk of progressing from non-symptomatic to symptomatic COVID-19 disease was not significantly different between groups (p = 0.37). (Table 2)
Table 2.
Incidence and Risk of Developing Symptoms.
| Variable | Category | Incidence of symptoms (n) | Crude RR (CI) | P-value | Adjusted RRa (CI) | P-value | |
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| Add-on Ayurveda Exposure status | Unexposed | 160 (72.4%) | 61 (27.6%) | 0.85 (0.60-1.22) | 0.39 | 0.85 (0.60-1.21) | 0.37 |
| Exposed | 408 (75.42%) | 133 (24.58%) | |||||
RR, Risk Ratio; CI, confidence interval; aAdjusted for exposure status, age category (above and below 60 years) and comorbidities status through binary logistic regression.
Duration of Symptomatic Phase
The total duration of the symptomatic period in exposed group was 3.66 days compared to 5.34 days in unexposed group. However, Levene’s test of equality of error variance performed for the total duration of symptoms present in patients was found significant (p < 0.001), indicating variance of the dependent variable was not equally distributed across groups. Therefore, independent samples t-test (2-tailed) was performed with equal variances not assumed. T-test for total duration of symptoms was found significant (p < 0.001). The magnitude of the clinical effect of the drugs measured using Cohen’s d was found to be 0.739 (Table 3). Add-on Ayurveda treatment significantly reduced the duration of symptomatic phase.
Table 3.
Total Duration of Symptomatic Phase.
| Groups | Mean | SD | F value | P-value | Cohen’s d |
|---|---|---|---|---|---|
| Unexposed (n = 61) | 5.34 | 3.35 | 26.181 | 0.001* | 0.739 (0.425-1.050) |
| Exposed (n = 132) | 3.66 | 1.55 |
SD-Standard Deviation, *analyzed by t-test.
Discussion
This retrospective observational study to evaluate the impact of Ayurveda treatment exposure on COVID-19 may be the first of such a pharmaco-epidemiological study. Results indicate the potential of add-on treatment by providing symptomatic relief in early stage COVID-19 patients.
In this study, exposure to Ayurveda treatment as add on showed a significant decrease in the total duration of the symptomatic phase-patients on add on treatment had 2 days shorter symptomatic phase. Cohen’s d value is calculated to show clinically meaningful outcomes by the standardized effect size. 19 0.739 value of Cohen’s d indicates medium effect size (Cohen’s d value range 0.5 to 0.8 for medium effect size). Reduction in the total duration of the symptomatic phase indicates that Ayurveda treatment improves clinical recovery. However, no remarkable difference in progression to the symptomatic case was observed in add-on Ayurveda treatment compared to standard care.
In this study, the study site (Samaras center) provided care to early-stage and low-risk COVID patients therefore the proportions of old age group and patients with comorbidities were low. Our results of no mortality in any of the groups should be interpreted in this background.
The Ayurveda intervention in this study comprised a mix of selected formulations with several actions. Dashamula, a group of ten herbs, alone and its combination with aspirin showed anti-inflammatory, analgesic, and anti-platelet effects comparable to aspirin. 20 Pathyadi Kwatha is a classical Ayurveda polyherbal formulation prescribed for the treatment of upper respiratory tract infections and different types of headaches. 21 Some of these ingredients have been evaluated in experimental models of inflammation and pain and have shown to possess anti-inflammatory and analgesic activities. 22,23 Trikatu powder augments the metabolic process by quick absorption of nutrients. 24,25 Piperine, an alkaloid, active component of Pippali increases the bioavailability of drugs and nutrients. 26 Guduchi Ghana (also known as Samshamani Vati) is important “Rasayana” 27 medicine found to have anti-inflammatory, 28 immunomodulatory, 29 anti-allergic, 30 and anti-diabetic 31 properties. AYUSH-64 is proprietary a polyherbal formulation recognized for malarial fever. 32 A recent pilot study claimed AYUSH-64 as add-on to standard care is safe and helped recover from Influenza-like illness (ILI) symptoms 33 however, generalizability of its outcomes to COVID-19 is cautioned. 34 Yastimadhu (Glycyrrhiza glabra Linn.) possesses many pharmacological activities like antiviral, antimicrobial, anti-inflammatory, and antitumor activities. 35 Pharmacological actions, target and mechanism of action of these multi herbal formulations with numerous bioactive compounds in COVID-19 are remain unexplored. The network pharmacology approach may serve as a valuable tool for evidence-based Ayurveda to understand the medicines’ putative actions, indications, and mechanisms. 36 Further, multimodal Ayurveda treatment may be better explained by whole system approach in its own theoretical framework.
Limitations
This study has some limitations such as participants were included only from single-center which has limitations to the representativeness of participants. The sample size was small for analysis on mortality and randomization was not possible this being a retrospective study. Considerable proportion of potentially eligible patients could not be accessed due to their nonresponse or denial to participate. Some parts of data collection through telephonic interview may induce recall bias and observer bias. The possibility of erroneous records cannot be denied, although it was small and will not change the results. Participants were treated with multiple formulations, so the individual effect of these drugs on disease is out of the scope of the study. “Time taken for RT-PCR test negative” could not be analyzed because testing policy varied throughout the study period. The results can be constricted to the early stage of COVID-19 patients with a lower risk of disease severity.
Conclusion
Exposure of Ayurveda treatment as adjunctive might be more effective in mild/ early stage COVID-19 patients as compared to standalone conventional care. This may provide leads to generate hypotheses for designing future studies such as by helping identify the potential drug candidates from Ayurveda treatment and provide preliminary evidence for further clinical use in COVID-19 patients.
Supplemental Material
Supplemental Material, sj-pdf-1-chp-10.1177_2515690X211020685 for Add-on Ayurveda Treatment for Early Stage COVID-19: A Single Center Retrospective Cohort Study From Gujarat, India by Anup Thakar, Kalpesh Panara, Falgun Patel, Shital Bhagiya, Mandip Goyal, Sagar Bhinde, Swapnil Chaudhari and Sarika Chaturvedi in Journal of Evidence-Based Integrative Medicine
Acknowledgments
We acknowledge Dr. Bhushan Patwardhan (Vice-Chairman, University Grants Commission, New Delhi) and Dr. N. Srikanth (Deputy Director-General, CCRAS, New Delhi) for their overall guidance and technical support.
Authors’ Note: Anup Thakar and Kalpesh Panara: Preparation of study design, supervising and coordinating the study, analyzing data, drafting and submitting the manuscript. Falgun Patel and Shital Bhagiya: Collecting data, performing the study and coordinating the study. Mandip Goyal, Sagar Bhinde, Swapnil Chaudhari: Supporting in preparation of study design, data management, and manuscript revision. Sarika Chaturvedi: Technical inputs to study design, writing and revising the manuscript. The study protocol was approved by the Institutional Ethics Committee, Institute for Post Graduate Teaching and Research in Ayurveda (Approval no. PGT/7/-A/Ethics/2020-21/239).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Institute for Post Graduate Teaching and Research in Ayurveda, Gujarat Ayurved University, Jamnagar, Gujarat, India, 361008 (Grant order no. PGT/COVID19/Research/15).
ORCID iD: Anup Thakar, PhD  https://orcid.org/0000-0003-2863-6927
https://orcid.org/0000-0003-2863-6927
Supplemental Material: Supplemental material for this article is available online.
References
- 1. WHO Coronavirus Disease (COVID-19) Dashboard. Updated March 25, 2021. Accessed March 25, 2021. https://covid19.who.int/
- 2. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China. China CDC Weekly. 2020;2(8):113–122. [PMC free article] [PubMed] [Google Scholar]
- 3. Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020;7(1):4. doi:10.1186/s40779-020-0233-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol. 2020;38(4):379–381. doi:10.1038/d41587-020-00003-1 [DOI] [PubMed] [Google Scholar]
- 5. Balachandar V, Kaavya J, Mahalaxmi I, et al. COVID-19: a promising cure for the global panic. Sci Total Environ. 2020;725:138277. doi.org/10.1016/j.scitotenv.2020.138277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaturvedi S, Kumar N, Tillu G, Deshpande S, Patwardhan B. AYUSH, modern medicine and the COVID-19 pandemic. Indian J Med Ethics. 2020;1-4. doi:10.20529/IJME.2020.058 [DOI] [PubMed] [Google Scholar]
- 7. Chan KW, Wong VT, Tang SCW. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese-Western medicine for the management of 2019 novel coronavirus disease. Am. J. Chin. Med. 2020;48(3):737–762. doi:10.1142/S0192415X20500378 [DOI] [PubMed] [Google Scholar]
- 8. Balasubramani SP, Venkatasubramanian P, Kukkupuni SK, Patwardhan B. Plant-based Rasayana drugs from Ayurveda. Chin J Integr Med. 2011;17(2):88–94. [DOI] [PubMed] [Google Scholar]
- 9. Girish T, Sarika C, Arvind C, Patwardhan B. Public health approach of Ayurveda and Yoga for COVID-19 prophylaxis. J Altern Complement Med. 2020;26(5):360–364. doi:10.1089/acm.2020.0129 [DOI] [PubMed] [Google Scholar]
- 10. Ministry of AYUSH. Ayurveda’s immunity boosting measures for self-care during COVID19 crisis. New Delhi: M/o AYUSH. Published 2020. Accessed May 19, 2021. https://www.mohfw.gov.in/pdf/ImmunityBoostingAYUSHAdvisory.pdf
- 11. Rastogi S, Pandey DN, Singh RH. COVID-19 pandemic: a pragmatic plan for Ayurveda intervention. J Ayurveda Integr Med. 2020;S0975-S9476(20)30019-X. doi:10.1016/j.jaim.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ministry of AYUSH. National Clinical Management Protocol based on Ayurveda and Yoga for the management of Covid-19. New Delhi: M/o AYUSH. Published 2020. Accessed May 19, 2021. https://main.ayush.gov.in/event/national-clinical-management-protocol-based-ayurveda-and-yoga-management-covid-19
- 13. Sohini D. Coronavirus outbreak: India plans ayurvedic trials on patients soon. Published 2020. Accessed May 19, 2021. https://www.business-standard.com/article/economy-policy/coronavirus-outbreak-india-plans-ayurvedic-trials-on-patients-soon-120041500073_1.html
- 14. Directorate of AYUSH. Ayurveda medicine to asymptomatic Patients of Corona positive. office order no. TLM/983745/2020. Published 2020. Accessed July 2, 2020.
- 15. Vennu V, Abdulrahman TA, Bindawas SM. The prevalence of overweight, obesity, hypertension, and diabetes in India: analysis of the 2015-2016 National Family Health Survey. Int J Environ Res Public Health. 2019;16(20):3987. doi:10.3390/ijerph16203987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Why Is Gujarat a COVID-19 Hotspot? Science the Wire. Published 2020. Accessed May 19, 2021. https://science.thewire.in/health/why-is-gujarat-a-covid-19-hotspot
- 17. Strategy of COVID19 testing in India (Version 4), Indian Council of Medical Research, Department of Health Research. Published 2020. Accessed May 19, 2021. https://www.icmr.gov.in/cteststrat.html
- 18. Revised Guidelines on Clinical Management of COVID-19, Ministry of Health & Family Welfare, Government of India. Published 2020. Accessed May 19, 2021. https://www.mohfw.gov.in/pdf/RevisedNationalClinicalManagementGuidelineforCOVID1931032020.pdf
- 19. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1):2–18. doi:10.1037/a0024338 [DOI] [PubMed] [Google Scholar]
- 20. Parekar RR, Bolegave SS, Marathe PA, Rege NN. Experimental evaluation of analgesic, anti-inflammatory and anti-platelet potential of Dashamoola. J Ayurveda Integr Med. 2015;6(1):11–18. doi:10.4103/0975-9476.146565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abraham A, Samuel S, Mathew L. Phytochemical analysis of Pathyashadangam kwath and its standardization by HPLC and HPTLC. J Ayurveda Integr Med. 2020;11(2):153–158. doi:10.1016/j.jaim.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rasool M, Sabina EP. Anti-inflammatory effect of the Indian Ayurvedic herbal formulation Triphala on adjuvant-induced arthritis in mice. Phytother Res. 2007;21(9):889–894. doi:10.1002/ptr.2183 [DOI] [PubMed] [Google Scholar]
- 23. Hewlings SJ, Kalman DS. Curcumin: a review of its effects on human health. Foods 2017;6(10):92. doi:10.3390/foods6100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johri RK, Zutshi U. An Ayurvedic formulation “Trikatu” and its constituents. J Ethnopharmacol. 1992;37(2):85–91. doi:10.1016/0378-8741(92)90067-2 [DOI] [PubMed] [Google Scholar]
- 25. Pole S. Ayurvedic Medicine: The Principles of Traditional Practices. Churchill Livingstone, Elsevier; 2006:302–303. [Google Scholar]
- 26. Lee KB, Shin KH, Wan WS. Central nervous depressant and anti-inflammatory activity of piperine. Archives Pharma Res. 1984:1:127–132. [Google Scholar]
- 27. The Ayurvedic Pharmacopoeia of India, Part 1, Vol. 1 . Dept. of AYUSH, Ministry of Health and Family Welfare, Govt. of India. New Delhi. 2001:54-168. [Google Scholar]
- 28. Pendse VK, Dadhich AP, Mathur PN, Bal MS, Madam BR. Anti-inflammatory, immunosuppressive and some related pharmacological actions of the water extract of Neem Giloe (Tinospora cordifolia): a preliminary report. Indian J Pharm. 1977;9(3):221–224. [Google Scholar]
- 29. Thatte UM, Dahanukar SA. Immunotherapeutic modification of experimental infections by Indian medicinal plants. Phytother Res. 1989;3(2):43–49. [Google Scholar]
- 30. Nayampalli SS, Desai NK, Ainapure SS. Anti-allergic properties of Tinospora cardifolia in animal models. Indian J Pharm. 1986;18(4):250. [Google Scholar]
- 31. Wadood N, Wadood A, Shah SA. Effect of Tinospora cordifolia on blood glucose and total lipid levels of normal and alloxan-diabetic rabbits. Planta Med. 1992;58(2):131–136. [DOI] [PubMed] [Google Scholar]
- 32. Sharma KD, Kapoor ML, Vaidya SP, et al. A clinical trial of “AYUSH 64” (a coded antimalarial medicine) in cases of malaria. Jour Res Ay Sid. 1981;2(4):309–326. [Google Scholar]
- 33. Gundeti MS, Bhurke LW, Mundada PS, et al. AYUSH 64, a polyherbal Ayurvedic formulation in Influenza like illness: results of a pilot study. J Ayurveda Integr Med. 2020;S0975-S9476(20)30025-5. doi:10.1016/j.jaim.2020.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pandey DN, Rastogi S, Agarwal GG, Lakhotia SC. Influenza like illness related clinical trial on AYUSH-64 requires cautious interpretation. J Ayurveda Integr Med. 2020:S0975-S9476(20)30058-30059. doi:10.1016/j.jaim.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang L, Yang R, Yuan B, et al. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm Sin B. 2015;5(4):310–315. doi:10.1016/j.apsb.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chandran U, Mehendale N, Patil S, et al. Network Pharmacology . Innovative Approaches in Drug Discovery. 2017;127–164. doi:10.1016/B978-0-12-801814-9.00005-2 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-chp-10.1177_2515690X211020685 for Add-on Ayurveda Treatment for Early Stage COVID-19: A Single Center Retrospective Cohort Study From Gujarat, India by Anup Thakar, Kalpesh Panara, Falgun Patel, Shital Bhagiya, Mandip Goyal, Sagar Bhinde, Swapnil Chaudhari and Sarika Chaturvedi in Journal of Evidence-Based Integrative Medicine