Key Points
Question
Do insertable cardiac monitors (ICMs) detect more atrial fibrillation than usual care in patients with a recent ischemic stroke attributed to large-vessel or small-vessel disease?
Findings
This randomized clinical trial included 492 patients with stroke attributed to large- or small-vessel disease evaluated with an ICM or usual care. Over 12 months, atrial fibrillation was detected in 12.1% of patients in the ICM group vs 1.8% in the usual care group, a difference that was statistically significant.
Meaning
Among patients with stroke attributed to large- or small-vessel disease, AF was more commonly detected via ICM than usual care, but whether this is of clinical importance is not addressed by the study findings.
Abstract
Importance
Patients with ischemic stroke attributed to large- or small-vessel disease are not considered at high risk for atrial fibrillation (AF), and the AF incidence rate in this population is unknown.
Objectives
To determine whether long-term cardiac monitoring is more effective than usual care for AF detection in patients with stroke attributed to large- or small-vessel disease through 12 months of follow-up.
Design, Setting, and Participants
The STROKE-AF trial was a randomized (1:1), multicenter (33 sites in the US) clinical trial that enrolled 496 patients between April 2016 and July 2019, with primary end point follow-up through August 2020. Eligible patients were aged 60 years or older or aged 50 to 59 years with at least 1 additional stroke risk factor and had an index stroke attributed to large- or small-vessel disease within 10 days prior to insertable cardiac monitor (ICM) insertion.
Interventions
Patients randomized to the intervention group (n = 242) received ICM insertion within 10 days of the index stroke; patients in the control group (n = 250) received site-specific usual care consisting of external cardiac monitoring, such as 12-lead electrocardiograms, Holter monitoring, telemetry, or event recorders.
Main Outcomes and Measures
Incident AF lasting more than 30 seconds through 12 months.
Results
Among 492 patients who were randomized (mean [SD] age, 67.1 [9.4] years; 185 [37.6%] women), 417 (84.8%) completed 12 months of follow-up. The median (interquartile range) CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, age 65 to 74 years, sex category) score was 5 (4-6). AF detection at 12 months was significantly higher in the ICM group vs the control group (27 patients [12.1%] vs 4 patients [1.8%]; hazard ratio, 7.4 [95% CI, 2.6-21.3]; P < .001). Among the 221 patients in the ICM group who received an ICM, 4 (1.8%) had ICM procedure–related adverse events (1 site infection, 2 incision site hemorrhages, and 1 implant site pain).
Conclusions and Relevance
Among patients with stroke attributed to large- or small-vessel disease, monitoring with an ICM compared with usual care detected significantly more AF over 12 months. However, further research is needed to understand whether identifying AF in these patients is of clinical importance.
Trial Registration
ClinicalTrials.gov Identifier: NCT02700945
This randomized clinical trial examines whether long-term cardiac monitoring with an insertable cardiac monitor was more effective than usual care for detection of atrial fibrillation in patients with stroke attributed to cervical or intracranial large artery atherosclerosis or small-vessel occlusion (large- or small-vessel disease).
Introduction
Data from 2020 indicate that the annual incidence of new or recurrent stroke in the US was approximately 795 000, of which 23% were recurrent strokes.1 Most strokes were ischemic (87%),1 and 2014 data suggests a confirmed diagnosis of atrial fibrillation (AF) was the presumed cause in 12% of cases each year,2 although this may be an underestimate because it did not account for undiagnosed AF.3 The risk of stroke was highest among patients with AF who had a stroke or a high CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischemic attack [TIA], vascular disease, age 65 to 74 years, sex category) score.4 The identification of AF in patients with stroke, whether it is the cause of the index stroke, unmasked by it, or coexistent, is therefore crucial for secondary stroke prevention with guideline-recommended oral anticoagulation (OAC) therapy, although the minimum burden of AF that requires anticoagulation is unknown.5
The use of prolonged cardiac monitoring has been proven to identify AF in a substantial proportion of patients with ischemic stroke and TIA.6 Observational studies of patients with other ischemic stroke subtypes have reported on newly diagnosed subclinical AF.7,8,9,10 However, there are scarce data on the yield of heart rhythm monitoring relative to routine follow-up across ischemic stroke subtypes. In particular, the optimum duration of cardiac monitoring and the rate of AF detection remain unknown in patients with a recent ischemic stroke classified as being due to a noncardioembolic etiology.
The Stroke of Known Cause and Underlying Atrial Fibrillation (STROKE-AF) trial assessed whether long-term cardiac monitoring with an insertable cardiac monitor (ICM) was more effective than usual poststroke care for AF detection in patients with stroke attributed to cervical or intracranial large-artery atherosclerosis or small-vessel occlusion (ie, large- or small-vessel disease). The hypothesis was that ICMs would detect more AF than usual care in this subset of patients with stroke who do not normally undergo prolonged cardiac monitoring.
Methods
Study Design
This study was a multicenter, randomized (1:1), parallel-group clinical trial comparing the rate of AF detection with an ICM vs a control group receiving usual care in patients with an index ischemic stroke classified by the enrolling investigator as being due to large-artery atherosclerosis (large-vessel disease) or small-vessel occlusion (small-vessel disease). The study was conducted in compliance with international ethical and scientific quality standards and the principles of the Declaration of Helsinki. The study design has been previously reported,11 and the trial protocol and statistical analysis plan are available in Supplement 1 and Supplement 2. The protocol was approved by all relevant institutional review boards and all patients provided written informed consent prior to initiation of any study-specific procedures. All protocol modifications made after study initiation are listed in eTable 1 in Supplement 3.
Participants
Patients were admitted to high-performing stroke centers where the treating physicians routinely categorize stroke types using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria.12 Investigators were instructed to assign stroke mechanism using the TOAST criteria as applied in clinical practice. A prespecified research instrument for TOAST classification was not required. Patients were eligible if they had an index stroke attributed by the investigators to large- or small-vessel disease within 10 days prior to ICM insertion. Patients were aged 60 years or older or aged 50 to 59 years with a documented medical history of at least 1 of the following additional stroke risk factors: congestive heart failure, hypertension, diabetes, ischemic stroke more than 90 days before the index stroke, or other ischemic vascular disease. Patients were excluded if they had cryptogenic stroke or embolic stroke of undetermined source, cardioembolic stroke, a history of documented AF or atrial flutter, or a known indication or contraindication for long-term OAC. To avoid confounding factors, patients were excluded if they had a medical history of untreated hyperthyroidism, myocardial infarction or cardiac surgery less than 1 month prior to index stroke, or mechanical heart valve or valvular disease requiring immediate surgery.
Randomization
Patients were randomized in a 1:1 ratio to the ICM or control group. SAS, version 9.4 (SAS Institute Inc), code was developed to generate an output of site-specific randomization schedules. The schedules consisted of randomly permuted blocks of 2 and 4. The block permutation kept the balance of participants receiving each group assignment and helped prevent the next group assignment from being guessed by site personnel. Sites were limited to 50 enrollments to reduce site-specific bias. The patients, investigative site personnel, and Medtronic personnel were blinded to the overall randomization sequence. However, due to the nature of the intervention, blinding on an individual patient level was not feasible. Aggregate efficacy results and adverse events, analyzed by randomized group, remained blinded to study personnel until completion of the primary end point analysis. The clinical events committee (CEC) had visibility to group assignment.
Interventions
Patients in the ICM group received an insertable cardiac monitor (REVEAL LINQ, Medtronic) within 10 days of the index stroke and after randomization. The follow-up period began on the day of randomization. Device insertion was performed in accordance with each site’s standard procedure practice and the instructions in the device’s clinician manual. Patients in the control group received usual care specific to each participating site.
Outcomes
The primary outcome was AF detection through 12 months between study groups, which included a subgroup analysis comparing patients with index stroke attributed to large-vessel disease vs small-vessel disease. The secondary outcome was AF detection between study groups through the duration of the study (up to 36 months). This analysis will therefore be performed at study end. Post hoc analyses included the following prespecified ancillary end points planned at 36 months that were explored at 12 months: comparison of incidence rates of AF detection at 6 months between study groups; the proportion of asymptomatic AF episodes in the ICM group (no event on the patient activation recorder); identification of the longest AF episode duration per patient in the ICM group; comparison of OAC use; and the incidence rate of recurrent stroke between study groups.11
Clinical and monitoring data were collected at baseline and 1, 6, and 12 months after randomization, and will continue at 6-month intervals up to 36 months or the end of ICM battery life. Additionally, patients randomized to the ICM group had data collected at device insertion and at 3 and 9 months. ICMs were programmed according to standardized requirements (eTable 2 in Supplement 3).
AF was defined as an episode of irregular heart rhythm, without detectable P waves, lasting more than 30 seconds and adjudicated by the CEC.13 In the control group, electrocardiogram or other cardiac rhythm monitoring was performed at the discretion of the treating physician. If an AF episode longer than 30 seconds was detected, source documentation was reviewed by the CEC. However, due to the ICM’s automatic detection algorithms, all detected episodes in the ICM group were at least 2 minutes in duration. Investigators were instructed to ascertain whether patients experienced a recurrent stroke or TIA at each follow-up visit and provide supporting details (eTable 3 in Supplement 3). All recurrent TIAs and strokes were determined by the stroke centers and confirmed by the CEC. Recurrent stroke was defined as any hemorrhagic or ischemic event with rapid onset of a focal or global neurological deficit or other neurological signs or symptoms consistent with stroke. TIA was defined as any new focal neurological deficit with rapid symptom resolution (usually 1-2 hours; always within 24 hours) and without tissue injury (based on neuroimaging). Stroke severity was assessed using the National Institutes of Health Stroke Scale.
Sample Size Calculation
The primary objective was to compare the incidence rates of AF detection through 12 months of follow-up between the ICM and control groups. A total of 23 AF events within 12 months of follow-up were required to demonstrate a statistically significant difference (P < .05) between the 2 groups with 85% power. This calculation was based on the assumptions that the AF incidence rate would be 8% in the ICM group and 2% in the control group (hazard ratio [HR] of 4.13), annual attrition rate of no greater than 10%, and annual crossover rate of 5%. The assumption for HR of of 4.13 was based on the results of previous studies, such as the CRYSTAL-AF trial.3 Due to population differences, we expected a lower rate of AF in the current trial than in the CRYSTAL-AF study. The sample size was estimated to require 486 randomized patients. To account for an assumed 2% attrition rate from enrollment to randomization, it was estimated that approximately 496 patients would be enrolled in the study.
Statistical Analysis
Statistical analyses were performed using SAS, version 9.4, and R, version 3.6.0 (R Foundation for Statistical Computing). The analysis set consisted of all randomized patients (Figure 1). Patients were analyzed according to their randomization group. Survival estimates are reported for each group as well as an HR estimate for the effect with corresponding 2-sided 95% CIs. The Cox models analyzed time to first AF episode through 12 months. Prespecified subgroup analyses of patients by stroke subtype (large-vessel disease and small-vessel disease) were performed. A Cox proportional hazards model was used to test the interaction term between group and subgroup of stroke subtype. The proportional hazards assumption of the Cox models was assessed by examining the group by log(time) interaction term; the interaction term was not significant in each model (P > .28) and, consequently, the proportional hazard assumption was met. Because time-to-event methods were used, there were missing data only for participants with no follow-up time from randomization. Otherwise, each participant had data to contribute to the analyses. To evaluate whether the effect of ICM monitoring varied among the enrolling sites, a post hoc mixed-effects model analysis of the primary outcome that treated study site as a random effect was performed. Because of the potential for type I error due to multiple comparisons, findings for analyses of post hoc end points should be interpreted as exploratory. Statistical significance was set at a 2-sided P value of .05 for all analyses.
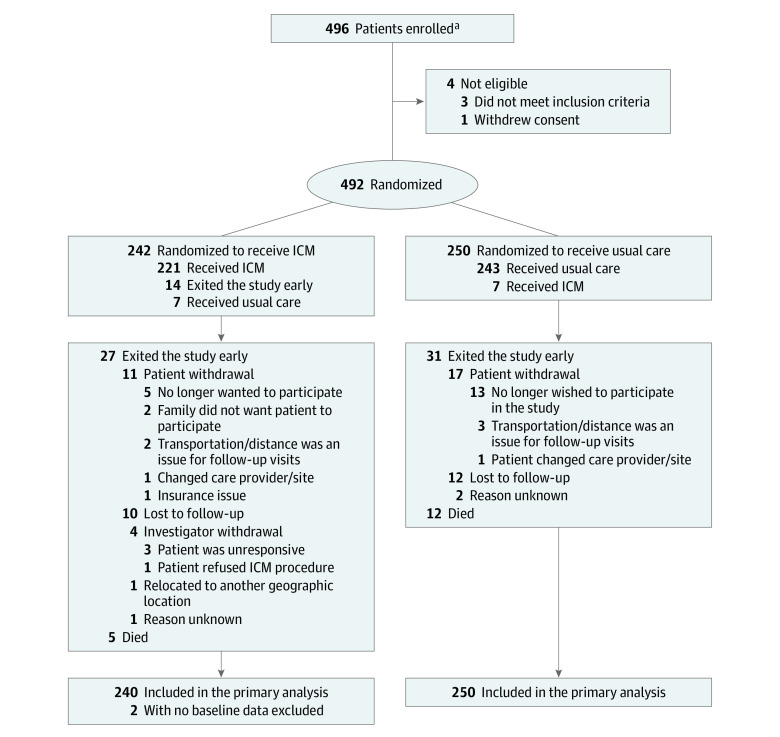
Figure 1. Patient Flow in a Study of Long-term Continuous Cardiac Monitoring vs Usual Care on Detection of Atrial Fibrillation in Patients With Stroke Attributed to Large- or Small-Vessel Disease.
ICM indicates insertable cardiac monitor.
aSites were not required to provide screening logs during the recruitment phase; therefore, the number of patients initially screened and reasons preventing their enrollment are not available.
Results
Study Population
Between April 2016 and July 2019, a total of 496 patients were enrolled at 33 sites in the US, of whom 492 were randomly assigned to the ICM (n = 242) or the control group (n = 250). All randomized patients were analyzed according to their randomization group (Figure 1). The median (interquartile range [IQR]) time from index stroke to randomization was 4 (2-6) days. Crossover occurred in 14 of 492 randomized patients: 6 assigned to receive an ICM did not and 8 assigned to receive usual care received an ICM. Device insertion was successful in all 223 patients who received an ICM; 219 (98.2%) underwent the procedure 10 days or less after randomization, with median (IQR) time from index stroke to ICM insertion of 5 (3-8) days. All patients were assessed for symptoms at each 6-month follow-up visit. Follow-up continued through August 2020 (from randomization to 12 months) for a mean (SD) duration of 331.4 (90.9) days.
In total, 417 patients (84.8%) completed 12 months of follow-up: 210 in the ICM group and 207 in the control group. Of the 17 patients who died, 2 met the primary end point prior to death (both in the ICM group). Of the 58 patients who left the study early, 1 in the ICM group met the primary end point prior to exiting. Reasons for incomplete follow-up are shown in Figure 1.
Compared with patients who completed the 12-month follow-up visit, baseline demographics of patients with incomplete follow-up (n = 75) were similar except for more frequent congestive heart failure (P = .04) (eTable 4 in Supplement 3). The Table shows baseline demographics and clinical characteristics for each group. The mean (SD) age of the population was 67.1 (9.4) years, 185 (37.6%) were women, and the median (IQR) CHA2DS2-VASc score was 5 (4-6). eTable 5 in Supplement 3 shows baseline characteristics according to TOAST subtype. Major echocardiographic features were similar between study groups (eTable 6 in Supplement 3).
Table. Baseline Characteristics of Participants in a Study of Long-term Continuous Cardiac Monitoring vs Usual Care on Detection of Atrial Fibrillation in Patients With Stroke Attributed to Large- or Small-Vessel Disease.
| Characteristic | No. (%) | |
|---|---|---|
| Insertable cardiac monitor (n = 242)a | Usual care (n = 250) | |
| Age, mean (SD), y | 66.6 (9.3) [n = 240] | 67.5 (9.5) |
| Age <65 y | 107/240 (44.2) | 108 (43.2) |
| Age 65-74 y | 81/240 (33.5) | 77 (30.8) |
| Age ≥75 y | 52/240 (21.5) | 65 (26.0) |
| Men | 144/240 (60.0) | 161 (64.4) |
| Women | 96/240 (40.0) | 89 (35.6) |
| CHA2DS2-VASc score, median (IQR)b | 5.0 (4.0-5.0) | 5.0 (4.0-6.0) |
| Comorbidities/risk factors | ||
| Stroke | 242 (100.0) | 250 (100.0) |
| Hypertension | 197 (81.4) | 200 (80.0) |
| Smoking tobacco | 130 (53.7) | 133 (53.2) |
| Diabetes | 87 (36.0) | 100 (40.0) |
| Vascular disease | 45 (18.6) | 47 (18.8) |
| Congestive heart failure | 28 (11.6) | 23 (9.2) |
| TOAST classificationc | ||
| Large-vessel disease | 140 (57.9) | 142 (56.8) |
| Small-vessel disease | 100 (41.3) | 108 (43.2) |
| CT only | 13 (5.4) | 11 (4.4) |
| MRI only | 11 (4.5) | 20 (8.0) |
| Both CT and MRI | 211 (87.2) | 219 (87.6) |
| Neither CT nor MRI | 7 (2.9) | 0 |
| NIHSS score, median (IQR)d | 2.0 (1.0-4.0) | 2.0 (1.0-5.0) |
Abbreviations: CT, computed tomography; IQR, interquartile range; MRI, magnetic resonance imaging; TIA, transient ischemic attack.
Unless otherwise noted. Two patients in the ICM group for whom age and sex are not known exited the study early. One patient exited the same day of randomization and the other patient exited a day after being randomized. Both patients met the inclusion criterion stating that the patient had an ischemic stroke believed to be due to small-vessel disease or large-vessel cervical or intracranial atherosclerosis within the past 10 days of enrollment. This information was used to calculate a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke or TIA, vascular disease, age 65 to 74 years, sex category) score of 2. Type of qualifying stroke event was provided at the enrollment case report form and was used as the TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification of subtypes of acute ischemic stroke.
Scores on the CHA2DS2-VASc risk assessment range from 0 to 9, with higher scores indicating a greater risk of stroke. A score of 5 corresponds to an estimated stroke risk of 7.2% per year.14
The TOAST classification categorizes subtypes of ischemic stroke based on etiology into (1) large-artery atherosclerosis (or large-vessel disease), (2) cardioembolism, (3) small-vessel occlusion (or small-vessel disease), (4) stroke of other determined etiology, and (5) stroke of undetermined etiology.
Scores on the National Institutes of Health Stroke Scale (NIHSS) range from 0 to 42, with higher scores indicating more severe neurologic deficits. An NIHSS score of 2 corresponds to a relatively mild stroke.
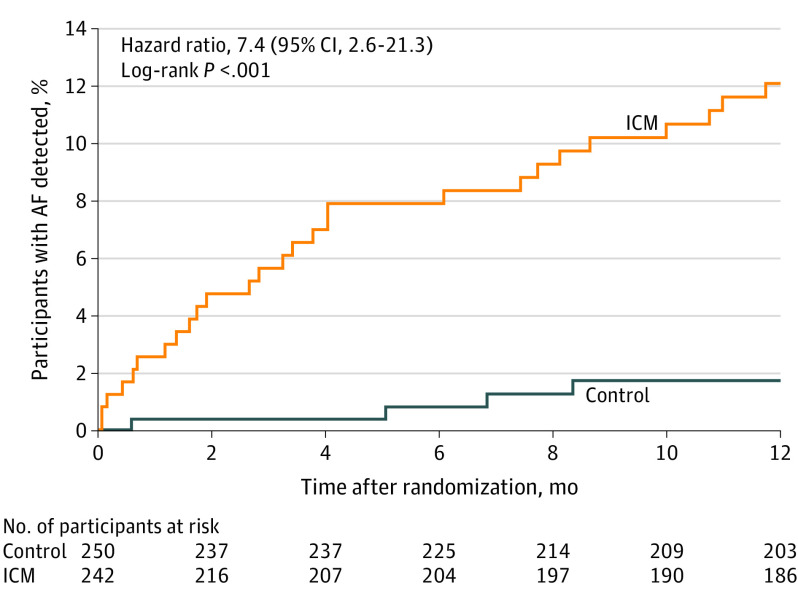
Primary End Point
The incidence of AF at 12 months was 12.1% (n = 27) in the ICM group vs 1.8% (n = 4) in the control group (HR, 7.4 [95% CI, 2.6-21.3]; P < .001) (Figure 2). The median (IQR) time from randomization to AF detection was 99 (36-235) days for the ICM group and 181 (86-231) days for the control group. AF episodes detected in the control group resulted from a total of 76 electrocardiograms in 57 patients, 26 Holter monitors/event recorders in 25 patients, and 2 mobile cardiac telemetry devices in 2 patients.
Figure 2. Time to First Detection of Atrial Fibrillation at 12 Months in a Study of Long-term Continuous Cardiac Monitoring vs Usual Care on Detection of Atrial Fibrillation (AF) in Patients With Stroke Attributed to Large- or Small-Vessel Disease.
At 6 months, the AF incidence was 7.9% in the ICM group vs 0.8% in the control group (hazard ratio, 9.9 [95% CI, 2.3-43.5]; P < .001). The median (interquartile range) time from randomization to AF detection was 99 (36.0-235.0) days for the insertable cardiac monitor (ICM) group and 181 (86.0-231.0) days for the control group. The median (interquartile range) observation time was 365 (365-365) days for all randomized patients for each group.
Two additional patients in the control group had mention of possible AF episodes with insufficient evidence for the CEC to adjudicate these as confirmed AF. Results of a sensitivity analysis that added these 2 AF episodes to the control group still showed a significantly increased detection incidence of AF in the ICM group compared with the control group (12.1% [n = 27] vs 2.6% [n = 6]; HR, 4.9 [95% CI, 2.0-11.9]; P < .001). In the ICM group, all AF episodes contributing to the primary end point were detected by the ICM.
A subgroup analysis was performed to compare the detection incidence of AF between patients with an index stroke classified as large-vessel disease (284 patients [57.3%]) and small-vessel disease (208 patients [42.3%]) (Table). A Cox model that included a term for the interaction between stroke subtype and group showed that the interaction term as well as stroke subtype were not significant (P = .42 and P = .46), and only the group effect was significant (P = .009). AF incidence at 12 months was significantly higher in the ICM group vs the control group in patients with large-vessel (15 [11.7%] vs 3 [2.3%]; HR, 5.3 95% CI, 1.5-18.2]; P < .001) and small-vessel (12 [12.6%] vs 1 [1.0%]; HR, 13.8 [95% CI, 1.8-111.1]; P < .001) disease (Figure 3). Among patients in the ICM group, there was no significant difference in AF detection in participants with large- vs small-vessel stroke (15 [11.7%] vs 12 [12.6%]; HR, 0.9 [95% CI, 0.4-1.9]; P = .74).
Figure 3. Time to First Detection of Atrial Fibrillation at 12 Months in a Study of Long-term Continuous Cardiac Monitoring vs Usual Care in Patients With Stroke Attributed to Large- or Small-Vessel Disease.
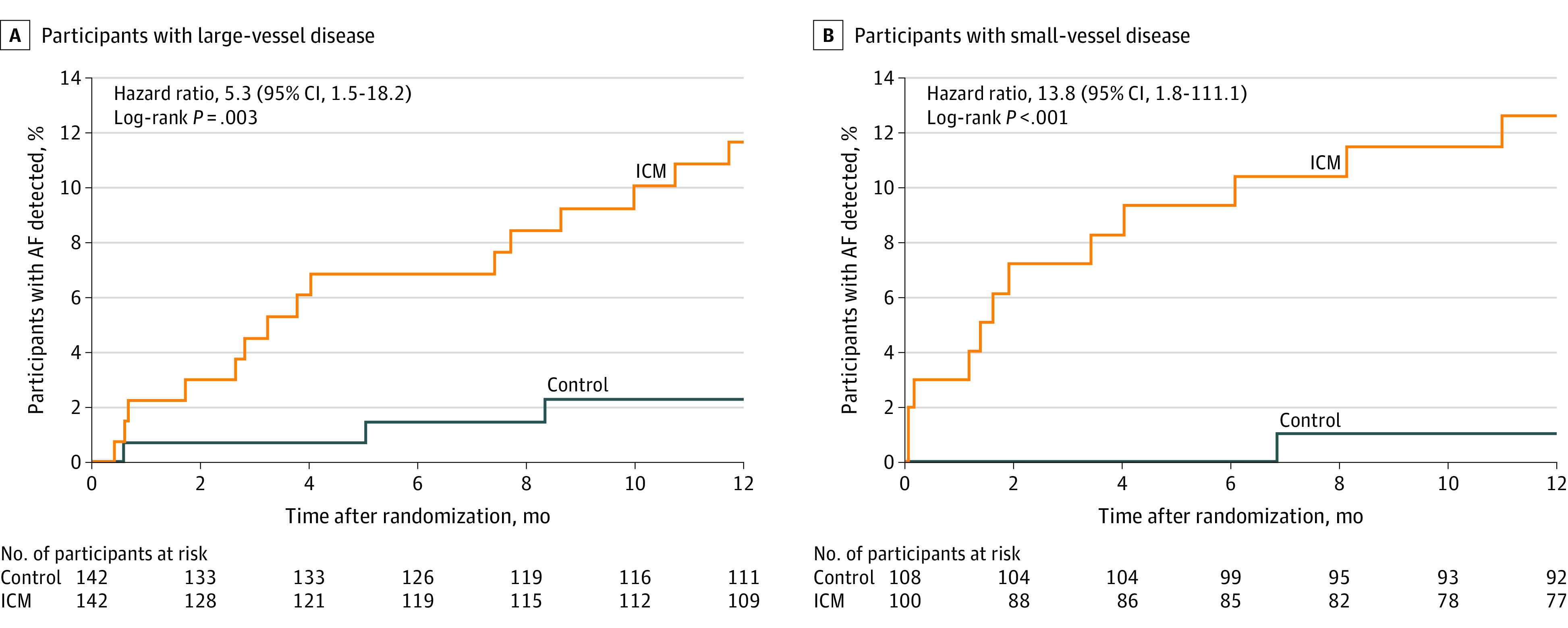
The median (interquartile range) observation time was 365 (365-365) days for all randomized patients for each group. ICM indicates insertable cardiac monitor.
The proportional hazard assumption was checked by examining the group by log(time) interaction term. The interaction term was not significant in each model (P > .28). Consequently, the proportional hazard assumption was met. The effect of ICM monitoring was evaluated among the 33 enrolling sites with a post hoc mixed-effects model analysis of the primary end point. Sites were random effects and their effect was not significant (P = .10).
Post Hoc End Points
At 6 months, the incidence of AF was 7.9% (n = 18) in the ICM group vs 0.8% (n = 2) in the control group (HR, 9.9 [95% CI, 2.3-43.5]; P = .002). Few AF episodes were detected in the ICM group during the first 30 days of follow-up (6 [2.6%]), suggesting that 30 days of continuous monitoring (eg, mobile telemetry) would have missed 78% (21 of 27) of the patients detected by the ICM at 12 months. The first AF episode detected in the ICM group was asymptomatic in 26 of 27 patients (96.3%).
Among patients with AF in the ICM group, the median (IQR) duration for the longest single episode of AF detected was 88 (10-526) minutes, and was longer in those with small-vessel vs large-vessel stroke (267 [49-438] vs 44 [8-526] minutes). Furthermore, 15 patients (55.5%) with AF in the ICM group had an episode longer than 1 hour. A histogram showing the duration of the longest AF episode per patient is provided in eFigure 1 in Supplement 3.
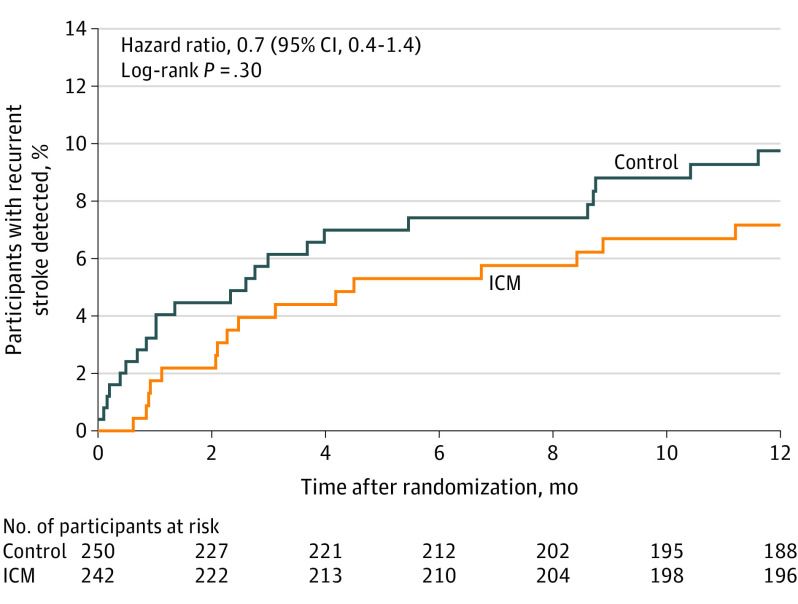
At 12 months, 38 patients (15.7%) in the ICM group and 14 (5.6%) in the control group were prescribed OAC (unadjusted odds ratio, 3.1 [95% CI 1.7-6.0]; P < .001). Among patients who were prescribed OAC, 20 patients in the ICM group and 11 patients in the control group did not have AF. In patients in whom AF was detected, OAC was prescribed more often in the ICM vs the control group (18 [7.4%] vs 3 [1.2%]; unadjusted odds ratio, 6.6 [95% CI, 1.9-22.8]; P < .001). Direct oral anticoagulants were the predominant type of OAC prescribed (43 of 52 [83%]). In addition, 1 patient in the ICM group underwent left atrial appendage occlusion and no patients underwent ablation. The incidence of recurrent ischemic and hemorrhagic stroke at 12 months was 7.2% (n = 16) in the ICM group and 9.8% (n = 23) in the control group (HR, 0.7 [95% CI, 0.4-1.4]; P = .30) (Figure 4). Classification of first recurrent ischemic stroke according to TOAST subtype is shown in eTable 7 in Supplement 3. Of the 16 recurrent strokes in the ICM group, only 1 occurred in a patient who had AF detected prior to the stroke, and this patient was not prescribed anticoagulants until after the recurrent stroke. Additionally, 1 patient without AF detected prior to their recurrent stroke was prescribed OAC prior to the recurrent event. Of the 23 recurrent strokes in the control group, there were no patients with AF detected and no patients who were prescribed OAC. The rate of recurrent TIA at 12 months was 1.8% (n = 4) in the ICM group and 0.4% (n = 1) in the control group (HR, 4.1 [95% CI, 0.5-36.8]; P = .21). Hemorrhagic stroke was reported in 1 patient in each group, and frequency of recurrent strokes were similar in the large- vs small-vessel subtypes (27 [10.3%] vs 12 [6.1%]; HR 1.7 [95% CI 0.9-3.4]; P = .12).
Figure 4. Rate of Recurrent Stroke at 12 Months in a Study of Long-term Continuous Cardiac Monitoring vs Usual Care on Detection of Atrial Fibrillation in Patients With Stroke Attributed to Large- or Small-Vessel Disease.
The median (interquartile range) observation time was 365 (365-365) days for all randomized patients for each group. ICM indicates insertable cardiac monitor.
Adverse Events
Among the 221 patients in the ICM group who received an ICM, 4 (1.8%) had insertion procedure–related adverse events: 1 had site infection (0.5%), 2 had incision site hemorrhages (0.9%), and 1 had implant site pain (0.5%).
Discussion
In this randomized clinical trial, among patients with a recent ischemic stroke attributed to large- or small-vessel disease, monitoring with an ICM resulted in a significantly higher rate of AF detection compared with usual care at 12 months.
The results presented are comparable to a small observational study of a noncryptogenic ischemic stroke cohort that found AF detected in 6 of 51 patients (12%) after monitoring with an ICM for 13 months.7 Other studies using prolonged continuous cardiac monitoring (64 hours to 21 days)8,9,10 in patients with acute and nonacute ischemic stroke have shown higher rates of AF detection compared with short-term monitoring. However, those cohorts included unselected patients with ischemic stroke in their reported AF detection rates, making comparisons of incidence rates between studies unreliable. The recently completed but unpublished PERDIEM randomized trial defined clinically actionable AF as lasting at least 2 minutes and compared the rates of AF at 1 year with an ICM vs 4 weeks of external loop recording in 300 adults.15 However, patients were enrolled up to 6 months after ischemic stroke and, although all ischemic subtypes were eligible, patients with cryptogenic stroke made up approximately 70% of the sample. The present study, on the other hand, provided evidence of the higher yield of AF detection by ICM in patients with noncryptogenic stroke starting within 10 days of the index stroke.
However, it is important to note that in the absence of a comparator group of patients with similar characteristics but without stroke, it is not possible from this study design to determine whether the rate of AF detection was related to the stroke or represented background asymptomatic AF that would have been detected at a similar rate in the absence of stroke. This study also did not address whether identifying AF in this setting affects clinical outcomes.
The CRYSTAL-AF study showed a 12.4% AF detection rate using ICMs in the first year after cryptogenic stroke,3 a stroke subtype in which paroxysmal AF was strongly suspected. Results of that study led to guideline recommendations for long-term cardiac monitoring in cryptogenic stroke.5,16 Monitoring is now frequently performed in patients with embolic stroke of undetermined source,17 because empirical anticoagulation in these patients in the absence of ICM-detected AF has not been proven effective.18,19
In contrast to those trials, the present study recruited only patients with stroke attributed to large- or small-vessel disease, a population of patients with ischemic stroke for whom long-term cardiac monitoring data are sparse. Because these strokes are generally thought to be due to atherosclerosis or lipohyalinosis,20 long-term cardiac monitoring is neither currently recommended nor routinely performed. However, many recurrent strokes do not have the same mechanism as the initial stroke,21 so patients with an index stroke due to large- or small-vessel disease can still be at risk for future AF-related stroke, especially if they are only treated with antiplatelet therapy.
Although some of the index strokes in this study may have been due to undetected paroxysmal AF and mistakenly attributed to large- or small-vessel disease, this could not be determined. Prior studies have demonstrated that up to 1 in 6 patients presenting with classic lacunar syndromes have multiple infarctions demonstrated on diffusion-weighted imaging, consistent with a proximal embolic source.22 The importance of detecting AF is not to ascribe an alternative etiology to the index stroke, but rather that it represents a risk for future cardioembolic stroke, which may be more effectively prevented by OAC than antiplatelet therapy.
The minimum duration of AF needed to benefit from long-term OAC remains unknown, but, in this study, a post hoc analysis identifying the longest AF episode at 12 months showed that AF events frequently lasted more than 1 hour and all patients had a minimum CHA2DS2-VASc score of 2 (prior stroke). For patients with a clinical diagnosis of AF, the overall median CHA2DS2-VASc score of 5 observed in this population corresponds to an estimated stroke risk of 7.2% per year.14 Ongoing studies may shed light on the clinical significance of ICM-detected moderate-burden AF for primary stroke prevention.23,24
Limitations
This study has several limitations. First, the attribution of stroke mechanism is subjective and may have led to the enrollment of a population at higher risk of underlying embolism; however, this attribution reflects real-world practice and the sample in this trial is representative of patients for whom ICM decisions need to be made. Second, due to study design, neither patients, physicians, nor the committee members who adjudicated AF events or recurrent stroke events were blinded to randomization assignment. However, the primary end point of AF detection was adjudicated using objective criteria. Third, there was a higher frequency of congestive heart failure among patients who did not complete the 12-month follow-up. Given that this is a risk factor for the development of AF, the results may have underestimated the true incidence of AF. Fourth, the study was not powered to detect a significant difference in rates of recurrent stroke. Fifth, although the duration definition for AF in the study was 30 seconds, patients in the ICM group would not have their AF detected unless the episode persisted for at least 2 minutes due to the requirements of the automatic detection algorithm. However, this limitation makes estimates of the AF detection rate in the ICM group more conservative. Sixth, electrocardiogram monitoring follow-up in the control group was variable and limited, further reducing the ability to detect AF in these patients. This may reflect standard of care practice for patients with strokes of “known” origin attributed to large- and small-vessel disease in whom a cardioembolic etiology is deemed unlikely. Seventh, because TOAST classifications were applied as customary in clinical practice, these classifications may have greater interrater variation than if a validated formal TOAST classification algorithm was used. Eighth, because of the absence of a comparator group with similar characteristics but without a stroke, and the absence of clinical outcomes, this study can make no conclusions about the clinical value of such testing.
Conclusions
Among patients with stroke attributed to large- or small-vessel disease, monitoring with an ICM compared with usual care detected significantly more AF over 12 months. However, further research is needed to understand whether identifying AF in these patients is of clinical importance.
Trial protocol
Statistical analysis plan
eMethods
STROKE AF-Investigators
Data sharing statement
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2.Kernan WN, Ovbiagele B, Black HR, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 3.Sanna T, Diener HC, Passman RS, et al. ; CRYSTAL-AF Investigators . Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478-2486. doi: 10.1056/NEJMoa1313600 [DOI] [PubMed] [Google Scholar]
- 4.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22(8):983-988. doi: 10.1161/01.STR.22.8.983 [DOI] [PubMed] [Google Scholar]
- 5.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104-132. doi: 10.1016/j.jacc.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 6.Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14(4):377-387. doi: 10.1016/S1474-4422(15)70027-X [DOI] [PubMed] [Google Scholar]
- 7.Katz JM, Eng MS, Carrazco C, et al. Occult paroxysmal atrial fibrillation in non-cryptogenic ischemic stroke. J Neurol. 2018;265(10):2237-2242. doi: 10.1007/s00415-018-8982-9 [DOI] [PubMed] [Google Scholar]
- 8.Rabinstein AA, Fugate JE, Mandrekar J, et al. Paroxysmal atrial fibrillation in cryptogenic stroke: a case-control study. J Stroke Cerebrovasc Dis. 2013;22(8):1405-1411. doi: 10.1016/j.jstrokecerebrovasdis.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 9.Rizos T, Güntner J, Jenetzky E, et al. Continuous stroke unit electrocardiographic monitoring versus 24-hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke. 2012;43(10):2689-2694. doi: 10.1161/STROKEAHA.112.654954 [DOI] [PubMed] [Google Scholar]
- 10.Wachter R, Gröschel K, Gelbrich G, et al. ; Find-AFrandomised Investigators and Coordinators . Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AFRANDOMISED): an open-label randomised controlled trial. Lancet Neurol. 2017;16(4):282-290. doi: 10.1016/S1474-4422(17)30002-9 [DOI] [PubMed] [Google Scholar]
- 11.Bernstein RA, Kamel H, Granger CB, Kowal RC, Ziegler PD, Schwamm LH. Stroke of known cause and underlying atrial fibrillation (STROKE-AF) randomized trial: design and rationale. Am Heart J. 2017;190:19-24. doi: 10.1016/j.ahj.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 12.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35-41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 13.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Heart Rhythm. 2017;14(10):e445-e494. doi: 10.1016/j.hrthm.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 14.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33(12):1500-1510. doi: 10.1093/eurheartj/ehr488 [DOI] [PubMed] [Google Scholar]
- 15.Buck BH, Hill MD, Quinn FR, et al. PERDIEM post-embolic rhythm detection with implantable versus external monitoring. Oral presentation at: 5th European Stroke Organisation Conference; May 23, 2019; Italy, Milan. [Google Scholar]
- 16.Kirchhof P, Benussi S, Kotecha D, et al. ; ESC Scientific Document Group . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 17.Implantable cardiac monitors to detect atrial fibrillation after cryptogenic stroke. National Institute for Health and Care Excellence . Published September 2, 2020. Accessed November 11, 2020. https://www.nice.org.uk/guidance/dg41
- 18.Diener HC, Sacco RL, Easton JD, et al. ; RE-SPECT ESUS Steering Committee and Investigators . Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380(20):1906-1917. doi: 10.1056/NEJMoa1813959 [DOI] [PubMed] [Google Scholar]
- 19.Hart RG, Connolly SJ, Mundl H. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;379(10):987. doi: 10.1056/NEJMoa1802686 [DOI] [PubMed] [Google Scholar]
- 20.Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32(8):871-876. doi: 10.1212/WNL.32.8.871 [DOI] [PubMed] [Google Scholar]
- 21.Benavente OR, Coffey CS, Conwit R, et al. ; SPS3 Study Group . Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507-515. doi: 10.1016/S0140-6736(13)60852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ay H, Oliveira-Filho J, Buonanno FS, et al. Diffusion-weighted imaging identifies a subset of lacunar infarction associated with embolic source. Stroke. 1999;30(12):2644-2650. doi: 10.1161/01.STR.30.12.2644 [DOI] [PubMed] [Google Scholar]
- 23.Kirchhof P, Blank BF, Calvert M, et al. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the non-vitamin K antagonist oral anticoagulants in patients with atrial high rate episodes (NOAH-AFNET 6) trial. Am Heart J. 2017;190:12-18. doi: 10.1016/j.ahj.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopes RD, Alings M, Connolly SJ, et al. Rationale and design of the apixaban for the reduction of thrombo-embolism in patients with device-detected sub-clinical atrial fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137-145. doi: 10.1016/j.ahj.2017.04.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods
STROKE AF-Investigators
Data sharing statement