Abstract
Background
There are emerging data indicating that sleep disturbance may be linked with an increase in opioid use. The majority of sickle cell disease (SCD) patients experience sleep disturbances, which can elevate pain severity and pain catastrophizing, both of which are important predictors of opioid consumption.
Purpose
We conducted a preliminary investigation on the association between previous night sleep disturbance and short-acting opioid use, as well as the potential mediating roles of pain severity and pain catastrophizing. Because sex is associated with sleep disturbance, pain-related experiences, and opioid use, we also explored the potential moderating role of sex.
Methods
Participants were 45 SCD patients who were prescribed opioids. For 3 months, sleep diaries were collected immediately upon participants’ awakening. Daily pain severity, pain catastrophizing, and prescription opioid use measures were collected before bedtime.
Results
Multilevel structural equation modeling revealed that wake time after sleep onset (WASO) during the previous night (Time 1) predicted greater short-acting opioid use during the next day (Time 2). Pain severity and pain catastrophizing measured during the next day (Time 2) also mediated the association between the two. Sex moderation analysis showed that the positive association between WASO and pain severity was largely driven by women.
Conclusion
These findings provide some preliminary evidence as to the mechanism linking sleep continuity disturbance and opioid requirement in SCD patients. Future studies should replicate and extend these findings with clearer temporal information and employing more refined measures of sleep continuity and prescription opioid use in a larger sample.
Keywords: Sickle cell disease, Sleep disturbance, Opioid use, Pain severity, Pain catastrophizing
Previous night’s sleep continuity disturbance is associated with greater prescription opioid use during the next day, and is mediated by pain severity and pain catastrophizing.
Introduction
Sickle cell disease (SCD) is a common and disabling inherited blood disorder. There are more than 100,000 individuals with SCD in the USA, and the number of patients is expected to increase by about 30% in the next 30 years [1]. Pain management is central to SCD patient care, as patients with SCD often experience acute pain (e.g., vaso-occlusive crisis) and chronic pain [2, 3]. The mainstay for pain management among many patients with SCD is long-term opioid therapy [4, 5]. However, there are numerous safety concerns in long-term opioid therapy such as dependence, elevated risk for respiratory depression, and opioid-induced hyperalgesia [6–8]. Identifying modifiable factors that can help patients attenuate their experience of pain and lower their opioid requirement, therefore, is an important goal for successful SCD management.
Investigating the effects of sleep disturbance on prescription opioid use is particularly relevant to addressing this goal for a number of reasons. First, the majority of SCD patients report experiencing significant sleep disturbances, including chronic insomnia [9, 10]. Second, there is growing evidence that sleep plays an important role in modulating pain sensitivity and inhibition in both healthy adults and individuals with chronic pain [11, 12]. Third, sleep disturbance is recognized as an important risk factor for the development, maintenance, and exacerbation of problematic substance use [13, 14]. Lastly, many sleep problems can be effectively treated through evidence-based interventions, such as cognitive–behavioral therapy for insomnia [15] and positive airway pressure (PAP) treatments [16]. Hence, examining the role of sleep disturbance in prescription opioid use may shed further light on helping patients with SCD better manage their pain with a lower need for opioids.
One previous study found that greater sleep continuity disturbance during the previous night was associated with higher doses of analgesic medication during the next day among adults hospitalized for burn injuries [17]. However, the underlying mechanisms of this association are unclear. The present study not only seeks to replicate this previous finding in a different clinical sample, but also extends the literature by examining factors that may help better understand the underlying mechanisms associating sleep disturbance and prescription opioid use.
Pain severity is a plausible mediator of this association. Sleep disturbance elevates both laboratory assessed pain sensitivity and clinical pain [11, 18, 19]. Greater pain, in turn, can elevate individuals’ daily use of prescription opioids [20]. In fact, in our previous study using daily diary data from SCD patients, we found that sleep continuity disturbance (i.e., the duration of wakefulness after sleep onset) predicts next-day increases in clinical pain severity [21]. We also found in this same study that higher daily pain severity is associated with greater short-acting opioid use [22]. However, whether daily pain severity significantly mediates the association between the two, and whether sleep disturbance directly predicts daily prescription opioid use over and above the effect of pain severity has not been examined.
Pain catastrophizing, a maladaptive cognitive-affective reaction to actual or anticipated pain [23, 24], is another promising candidate mediator of the association between sleep disturbance and opioid use. Previous studies showed that individuals with lower subjective sleep quality tend to report higher pain catastrophizing [25–27]. More recently, a daily diary study of fibromyalgia patients demonstrated that greater previous night nonrestorative sleep was associated with higher next morning pain catastrophizing, which in turn, predicted higher levels of pain and activity interference during the day [28]. Furthermore, pain catastrophizing also predicts postoperative opioid use and is an important risk factor for prescription opioid misuse [29–31]. Combined, these previous findings suggest that the effect of sleep disturbance on prescription opioid use may also be mediated by pain catastrophizing.
Emerging evidence supports the importance of investigating sex differences in associations among sleep disturbance, pain-related experiences, and opioid use. Women in general tend to have a greater predisposition to developing insomnia [32], are more susceptible to pain-related outcomes [33, 34], and report higher pain catastrophizing [35, 36]. On the other hand, men with chronic pain are at greater risk than women in progressing to high-dose opioid therapy and death due to opioid overdose [37]. In the present study, we explored the potential moderating role of sex in our proposed mediation model.
In sum, in the present study, we examined whether previous night sleep disturbance is associated with next day prescription opioid use through elevation of pain severity and pain catastrophizing among individuals with SCD. As an exploratory aim, we also tested the sex moderation effects of this mediation model.
Methods
Overview
The present study is a secondary data analysis of 3-month daily diary data previously collected from patients with SCD. Two papers were published using this data set from our research group. Moscou-Jackson et al. [21] investigated how sleep continuity variables are associated with daily experiences of pain in SCD. However, they did not examine the role of sleep in pain catastrophizing nor prescription opioid use, nor the moderating effects of sex. Finan et al. [22] examined how daily opioid use in SCD varies as a function of daily pain severity, pain catastrophizing, and affective states, but did not examine the role of sleep in daily prescription opioid use nor sex differences. The present study seeks to bridge these questions and differs from these two studies in that we are (i) testing the direct association between sleep continuity disturbance and short-acting opioid use during the next day; (ii) evaluating the potential mediating roles of pain severity and pain catastrophizing in the association between sleep disturbance and prescription opioid use; (iii) exploring sex moderation effects in the mediation model; and (iv) employing a more advanced analytic technique—multilevel structural equation modeling (MSEM)—that allows for testing multiple mediators simultaneously in a single model.
Participants
Participants were recruited from the community in Baltimore and Washington D.C. region using flyers and advertisements, as well as referrals from local SCD clinics. Inclusion criteria for the study were: (i) 18 years of age or older, (ii) diagnosis of a SCD hemoglobinopathy genotype (i.e., HbSS, HbSC, HbS/β-thalassemia), (iii) currently receiving a stable dose of pain medication including opioids, nonsteroidal anti-inflammatory drugs, or acetaminophen, and (iv) no experience of vaso-occlusive crisis within the past 3 weeks. Exclusion criteria were: (i) report of on-going substance abuse issues, (ii) a significant cognitive impairment or psychiatric disorder, (iii) current infection (including HIV with a neuropathy), (iv) diagnosis of an autoimmune disorder, or (v) currently pregnant or plan to become pregnant in the next 6 months of the study. Note that individuals on chronic transfusion were also allowed to participate in the current study.
As previously described [22], among a total of 84 SCD participants available from the parent study, the present study included only the 45 participants who were prescribed and using opioids. A flow chart that describes the selection process of this final sample used in our study is presented in Electronic Supplementary Fig. 1. A series of χ 2 and t-tests revealed that there were no significant socio-demographic differences (i.e., age, sex, race, education level, and marital status) between the included vs. excluded sample (p-values ranging from .21 to .65). Not surprisingly, the included sample reported significantly higher mean diary pain severity (0–100 numerical rating scale) compared to the excluded sample (Mincluded = 32.9 vs. Mexcluded = 9.7, p < .05).
Procedure
An initial telephone screening was conducted to ensure that interested participants met the eligibility criteria presented above. Those who passed the phone screening were invited for an in-person visit. Each participant provided written informed consent prior to the start of the baseline study session which included self-report questionnaires, a medical and psychiatric history, and a series of laboratory assessments of pain sensitivity (see [38] for more details). At the end of the baseline session, a research coordinator trained each participant to use an electronic PDA (Palm personal electronic organizer, Palm, Inc., U.S. Robotics, Sunnyvale, CA) for a 90-day diary assessment. Diaries were completed two times per day; one in the morning and the other in the evening. The morning assessment took place immediately upon participants’ awakening, and the evening assessment was measured just before the participants’ bedtime. Although participants did not receive any daily reminders to complete the diary, they were tracked by the research coordinator and helped with any technical issues that arose. All participants were provided with financial incentives based upon completion of their diary. All study procedures were approved by an institutional review board at Johns Hopkins School of Medicine.
Measures
Predictor variables
Daily sleep parameters.
Immediately upon awakening, participants were instructed to record their last night’s bedtime, how long it took to fall asleep, the total amount of time they were awake during the night, final wake up time, and time they got out of bed. These sleep diary items were used to calculate total sleep time (TST; duration from bedtime to final wake time), sleep onset latency (SOL; the amount of time it takes one to go from being awake to sleep), and wake after sleep onset (WASO; duration of wakefulness after sleep onset).
Mediators
Daily pain severity.
Participants rated their average pain level for the day before going to bed each night using an electronic slider on the PDA screen between 0 (“no pain”) and 100 (“pain as bad as you can imagine”).
Daily pain catastrophizing.
Using the evening diary, participants reported the average intensity of pain catastrophizing throughout the day. Three items were adapted from the Pain Catastrophizing Scale (PCS; [39]): (i) “I could not seem to keep the pain out of my mind,” (ii) “I thought the pain was never going to get better,” and (iii) “I kept thinking about how much it was going to hurt.” Each item represented one of the three main constructs (i.e., rumination, helplessness, and magnification) of PCS. Items were rated on a 0 (“not at all”) to 100 (“very much”) scale using the PDA electronic slider. By averaging these three items, we created a composite of daily pain catastrophizing. A number of previous studies have used this composite of daily pain catastrophizing scale [22, 40, 41]. The composite showed excellent internal consistency (Cronbach α = .99).
Outcome variable
Daily short-acting opioid use.
As previously described by Finan et al. [22], at the baseline session prior to diary assessment, participants who reported using opioids provided detailed information about their opioid prescriptions (e.g., generic name, dose, and frequency). If their prescription and/or dose changed during the study, participants were instructed to inform the research coordinator immediately so that the change in prescription opioid information is logged into our database. For the daily diary, participants were asked to indicate how many pills of their prescribed opioid/s they had consumed that day. At baseline, participants provided the name and dosage of each prescribed opioid medication. This information was used to compute morphine equivalent daily dosage (MEDD) separately for short- and long-acting opioids in order to standardize opioid intake across different prescription opioids. The total MEDD was defined as the product of the number of pills reported by daily diary and the morphine equivalent dose of each pill. The oral morphine equivalents (ME) conversion was based upon standard equianalgesic conversion calculation methods [42–44]. Details on ME conversion are available as Supplementary material. In the present study, we only used the short-acting opioid use variable based upon our previous study, which revealed extremely low within-person variability in daily long-acting opioid use [22].
Data Analytic Strategy
All main analyses were conducted using SPSS Version 26 and Mplus version 8.0 [45]. First, using SPSS, preliminary analyses examined diary completion rates, and descriptive statistics and intraclass correlations (ICCs) of study variables. Second, a multilevel structural equation model (MSEM) was conducted using Mplus in order to effectively test a model with multiple mediators [46] that included both pain severity and pain catastrophizing ratings. When conducting MSEM, Mplus in default automatically partitions the within- and between-person level variances using person-mean centering that is the conventional centering method in multilevel modeling [47]. Hence, regression (path) coefficients are directly interpreted at the corresponding levels of analysis [46]. The person-centered scores indicate day-to-day deviations from a participant’s own mean score over the entire diary period (e.g., 90 days) for that variable.
To systematically investigate the role of sleep disturbance in prescription opioid use, we tested three different MSEM models. The first model only included previous night’s sleep continuity parameters (i.e., TST, WASO, and SOL) predicting next day short-acting opioid use. Then, we conducted a second model which tested the potential mediation effects of pain severity and pain catastrophizing. The last model examined sex moderation effects employing multiple group analysis. Multiple group analysis compares the model fits of two models using a chi-square difference test: (i) a configurable model that allows regression paths to be freely estimated across sex, and (ii) a constrained model that constrains regression paths to be equal across sex. If the result of the χ 2 difference test is significant (i.e., constrained model has a significantly worse model fit than the configurable model), it is assumed that there is an overall sex difference in the model. Then, each regression path is examined to see if there are any significant sex differences using the MODEL CONSTRAINT command from Mplus. To balance the chances of Type I and Type II errors, we set the alpha level at .01 for exploring sex moderation effects.
We only interpret and report the results of the within-person level (day level) model because (i) our research questions and hypotheses focus on the within-person level, and (ii) the associations among study variables at the between-person level are all cross-sectional because each variable in this level represents a person mean (i.e., average of daily diary assessments). Thus, the findings of the between-person level analyses cannot provide any directionality of regression results.
MSEM in Mplus uses the restricted maximum likelihood estimator, which can effectively handle non-normally distributed outcomes (e.g., short-acting opioid use). Missing data were handled by full information maximum likelihood (FIML) under the commonly used missing at random assumption [48]. The Rmediation software [49] was used to test the significance of the mediated effects of pain severity and pain catastrophizing in the association between sleep parameters and short-acting opioid use. Rmediation computes asymmetric confidence limits for the distribution of the product of a path and b path. This method for testing mediation has substantially better control of Type I error rates and has higher statistical power than traditional mediation analyses, such as the Sobel test. The statistical significance of mediated effects from Rmediation was determined by a 95% confidence interval, which is the preferable method for determining the statistical significance of mediated effects. It is assumed that there is a significant mediating effect if the confidence interval does not include zero.
Results
Preliminary findings
We investigated compliance with the daily diary protocol by calculating the diary completion ratio (i.e., the number of diaries completed out of the total number of days that the participant possessed the PDA). The average diary completion ratio was 72.3% (i.e., 2,386 days of 3,300 days) for the morning diary and 75.5% (i.e., 2,493 days out of 3,300 days) for the evening diary. Table 1 summarizes the demographic and clinical characteristics of our sample. Participants’ average age was 37.5 years and the majority self-identified themselves as Black. Approximately 71% (n = 32) of the participants were females, and most of them had the HbSS hemoglobinopathy genotype, were currently not living with a romantic partner, and had at least some college or technical school experience. A series of t-tests and χ 2 tests revealed that there were no significant sex differences in demographic and clinical characteristics, including the proportion of hemoglobinopathy genotype, hemoglobin levels, the number of white blood cells, use of hydroxyurea, morphine equivalents of short- and long-acting opioid use that were averaged across diary study periods, and body mass index level.
Table 1.
Sample characteristics (N = 45)
| Mean (SD) or % | ||||
|---|---|---|---|---|
| Variables | Female (n =32) | Male (n = 13) | Total | p |
| Age (years) | 35.88 (10.97) | 41.46 (11.33) | 37.49 (11.24) | .13 |
| Race | ||||
| African American | 93.8% | 100% | 95.5% | .38 |
| More than one race | 6.3% | 0% | 4.5% | |
| Hemoglobinopathy genotype | .68 | |||
| SS | 67.7% | 69.2% | 68.2% | |
| S-Beta0 thalassemia | 6.5% | 7.7% | 6.8% | |
| S-Beta+ thalassemia | 9.7% | 0% | 6.8% | |
| SC | 16.1% | 23.1% | 18.2% | |
| Hemoglobin level | 9.27 (1.98) | 8.78 (1.86) | 9.12 (1.93) | .45 |
| White blood cell count | 10273.55 (3572.41) | 10723.85 (6862.02) | 10406.59 (4699.75) | .78 |
| Taking hydroxyurea | .98 | |||
| Yes | 31.3% | 31.3% | 31.1% | |
| Average short-acting opioid use (ME) | 23.88 (46.20) | 23.56 (33.20) | 23.79 (42.48) | .98 |
| Average long-acting opioid use (ME) | 74.81 (142.51) | 38.81 (77.55) | 64.43 (127.37) | .40 |
| Body mass index (BMI) | 27.23 (6.10) | 24.64 (6.34) | 26.50 (6.20) | .22 |
| Education | .33 | |||
| Some high school | 0% | 7.7% | 2.2% | |
| High school graduate/GED | 15.6% | 23.1% | 17.8% | |
| Technical school graduate | 12.5% | 7.7% | 11.1% | |
| Some college | 43.8% | 23.1% | 37.8% | |
| College graduate | 25.0% | 23.1% | 24.4% | |
| Master’s/doctoral degree | 3.1% | 15.4% | 6.6% | |
| Marital status | .20 | |||
| Married/partnered | 25.0% | 25.0% | 25.0% | |
| Single | 62.5% | 50.0% | 59.1% | |
| Divorced/separated | 12.5% | 25.0% | 15.9% | |
Note. ME, morphine equivalents.
Table 2 displays descriptive statistics and ICCs of all study variables. On average participants reported approximately 7 hr of TST. Participants’ reported average WASO and SOL were all slightly longer than 30 min which are comparable to those with clinical insomnia disorder [50]. Overall, participants reported a moderate level of daily pain severity and pain catastrophizing. ICCs ranged from .30 (WASO) to .69 (short-acting opioid use), indicating the use of multilevel modeling was appropriate given that there was substantial within-person variation across days. For instance, only 30% of the variation was explained by between-person differences in WASO and the rest of the variation was explained by within-person changes. In terms of bivariate correlations, most of the study variables were correlated in expected directions. For instance, WASO was positively and significantly associated with daily pain severity (r = .15, p < .001) and pain catastrophizing (r = .07, p < .001).
Table 2.
Descriptive statistics and bi-variate within-person correlations of study variables
| Variables | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1.Total sleep time (TST) | – | –.32*** | –.23*** | –.07*** | –.09*** | –.05** |
| 2.Wake after sleep onset (WASO) | – | .15*** | .13*** | .15*** | .07*** | |
| 3.Sleep onset latency (SOL) | – | .03 | .03 | .02 | ||
| 4.Daily pain severity | – | .56*** | .22*** | |||
| 5.Daily pain catastrophizing | – | .22*** | ||||
| 6.Daily short-acting opioid use | – | |||||
| Mean | 7.16 | 35.13 | 38.38 | 30.26 | 18.73 | 36.25 |
| SD | 2.62 | 61.18 | 51.19 | 24.88 | 25.76 | 68.31 |
| ICC | .39 | .30 | .47 | .60 | .56 | .69 |
Note. *p < .05, **p < .01, *** p < .001. In order to examine pure level-1 (within-person) correlations, all variables were person-mean centered. As a result, some descriptive statistics are slightly different from Finan et al.’s [22] study which were based upon between-person means (i.e., level-1 variables averaged across days).
Associations between sleep parameters and daily short-acting opioid use
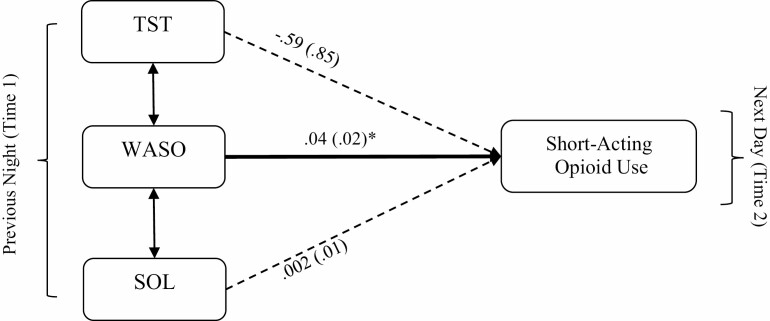
Models examined were fully saturated, and thus, the model fit indices were not generated. Fig. 1 presents a summary of the first model which included only the sleep parameters as predictors. On days following greater than usual WASO, participants reported using more short-acting opioids. Specifically, for every 10-min increase of WASO, daily short-acting opioid use increased by 0.4 ME. The rest of the sleep parameters (TST and SOL) did not significantly predict short-acting opioid use during the next day.
Fig. 1.
A multi-level path model that excludes mediators. Note. All path estimates are unstandardized regression coefficients. Values in brackets are standard error estimates. Single-headed arrows indicate regression paths. Double-headed arrows indicate correlations. Bold lines indicate statistically significant paths. *p < .05.
Mediating effects of daily pain severity and pain catastrophizing
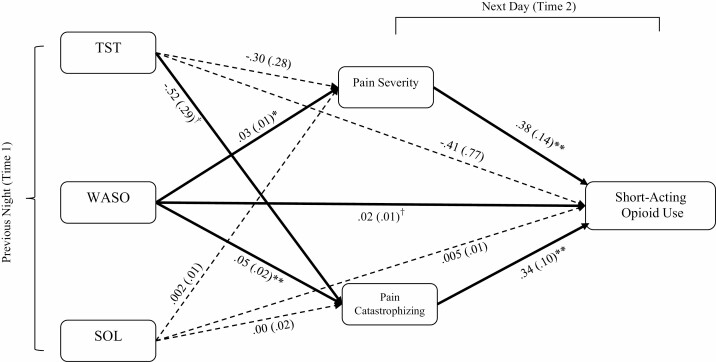
Fig. 2 summarizes findings of the second model which expanded the first model by including two mediators. Greater previous night WASO was associated with a higher level of daily pain severity (p = .011) and pain catastrophizing (p = .002). Daily pain severity (p = .008) and pain catastrophizing (p = .001), in turn, were significantly related to greater use of short-acting opioids. For instance, for every 10 unit increase of pain severity (from a 0–100 numerical rating scale), daily short-acting opioid use increased by 3.8 ME. Table 3 shows detailed point estimates and confidence intervals of each mediated effect that was tested in this model. Both pain severity (95% CI: .002, .025) and pain catastrophizing (95% CI: .003, .039) significantly mediated the relation between WASO and short-acting opioid use. The effects of TST and SOL on short-acting opioid use were not significantly mediated by pain severity or pain catastrophizing (see Table 3). However, a trend was observed in the association between TST and pain catastrophizing (p = .075), such that greater previous night TST was related to lower pain catastrophizing reported during the next day. It is also noteworthy that the direct effect of WASO on short-acting opioid use trended toward significance (p = .072), even when controlling for the effects of two mediators.
Fig. 2.
A multi-level path model that includes mediators. Note. All path estimates are unstandardized regression coefficients. Values in brackets are standard error estimates. Single-headed arrows indicate regression paths. Double-headed arrows indicate correlations. Bold lines indicate statistically significant paths. †p < .10, *p < .05, **p < .01.
Table 3.
Mediated effects of daily pain severity and pain catastrophizing and 95% asymmetric confidence interval (CI)
| Predictors | Mediators | a Path ß (SE) | b Path ß (SE) | ab correlation | Point estimate | 95% Asymmetric CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| TST | –.30 (.28) | .38 (.14) | –.003 | –.1141 | –.388 | .095 | |
| WASO | Pain severity | .03 (.01) | .38 (.14) | .029 | .0114* | .002 | .025 |
| SOL | .002 (.01) | .38 (.14) | .028 | .0008 | –.007 | .010 | |
| TST | –.52 (.29) | .34 (.10) | .089 | –.1742 | –.420 | .016 | |
| WASO | Pain catastrophizing | .05 (.02) | .34 (.10) | .186 | .0174* | .003 | .039 |
| SOL | .00 (.02) | .34 (.10) | .153 | .0003 | –.013 | .016 |
Note. TST, total sleep time; WASO, wake after sleep onset; SOL, sleep onset latency; *, statistically significant mediated effect.
Sex moderation effects
The result of the Satorra-Bentler scaled χ 2 difference test showed that there was a statistically significant model fit difference between the constrained and configurable (freely estimated) models [χ2(32) = 99.00, p < .001], indicating significant sex differences in the mediation model. We further explored which regression paths were significantly different between men and women. There was a significant sex moderation effect in the association between WASO and pain severity (p = .009, 95% CI: –.099, –.014). Specifically, while there was a positive and significant association between WASO and pain severity among women (B = .05, SE = .02, p = .003), there was no significant association between the two among men (B = –.01, SE = .02, p = .596). As a result of this sex moderation effect, the WASO → pain severity → short-acting opioid Use mediation was statistically significant among women (point estimate = .019, 95% CI: .003, .040), but not among men (point estimate = –0.004, 95% CI: –0.021, 0.012). The rest of the regression paths did not significantly differ between men and women.
Findings of Post-hoc Analyses
Many SCD patients experience vaso-occlusive crises (VOC), which is commonly characterized by extreme acute pain, and prescription opioids are frequently used to manage VOC. Thus, VOC can be an important factor to consider in examining the association between daily pain severity and prescription short-acting opioid use. We conducted a post-hoc analysis to examine whether the association of daily pain severity and short-acting opioid use remains statistically significant after controlling for the experience of VOC. As expected, we found that daily VOC significantly predicted short-acting opioid use (B = 12.92, SE = 5.45, p = .018). Additionally, with VOC as a covariate in the model, both daily pain severity (B = .27, SE = .12, p = .029) and pain catastrophizing (B = .27, SE = .09, p = .002) remained significant predictors of short-acting opioid use.
Previous studies have shown that daily levels of negative affect are closely associated with pain catastrophizing and pain severity [51]. In order to test the robustness of the mediated effects of daily pain severity and pain catastrophizing, we included negative affect as an additional covariate, which was measured by a composite of six items (i.e., feeling anxious, nervous, sad, alone, tired, and blue) derived from the Positive and Negative Affect Schedule and Profile of Mood State [52, 53]. Results showed that even after controlling for daily negative affect, both daily pain severity (B = .39, SE = .16, p = .012) and pain catastrophizing (B = .37, SE = .13, p = .004) significantly predicted short-acting opioid use.
As indicated by ICC, short-acting opioid use has substantial between-person level variability. We explored whether person-mean of sleep continuity variables, pain severity, and pain catastrophizing can significantly account for the between-person variability of short-acting opioid use. We controlled for some between-person factors (i.e., use of hydroxyurea, ethnicity, age, and BMI) that can potentially confound the associations among our study variables. Partial correlation analysis revealed that in controlling for these covariates, only average pain severity had a significant association with average short-acting opioid use (r = .34, p = .046).
Discussion
The present study conducted a preliminary investigation of whether pain severity and pain catastrophizing mediate the daily association between sleep disturbance and prescription opioid use among individuals with SCD. We also explored whether sex differences moderate the mediating effects of pain severity and pain catastrophizing in the relationship between sleep disturbance and short-acting opioid use. Although these findings require replication, our study suggests that WASO and short-acting opioid use are quite robustly associated, and both pain severity and pain catastrophizing also partially mediate the association between WASO and short-acting opioid use. In addition, we found that sex significantly moderated the association between WASO and pain severity, such that the association between the two was significant among women but not among men.
Prior to including mediators in the model, we found that longer WASO during the previous night was significantly associated with greater short-acting opioid use during the next day (see Fig. 1). This is consistent with findings of Raymond et al.’s [17] study which demonstrated that greater WASO is associated with more opioid intake during the same night and during the next day among hospitalized burn injury patients. Even after including daily pain severity and pain catastrophizing, which are potent predictors of prescription opioid use [20, 29, 54] as mediators in the model, the direct association between WASO and short-acting opioid use still trended toward significance, potentially indicating its robustness. We also found some preliminary evidence that the association of sleep disturbance and daily opioid use in SCD could also be explained through daily changes in pain severity and pain catastrophizing. Post-hoc analyses revealed that these mediated effects remained significant, even after controlling for the experience of vaso-occlusive crisis and daily negative affect. As demonstrated in previous longitudinal studies which displayed significant predictive effects of pain severity and pain catastrophizing on prescription opioid use among individuals with chronic pain [20, 29–31], targeting the reduction of pain severity and pain catastrophizing, in addition to sleep continuity disturbance, appears to be an important clinical target in helping individuals with SCD to lower their opioid requirement. It is important to note, however, that pain severity, pain catastrophizing, and short-acting opioid use were all measured at the same assessment time point. Future studies should measure these variables more frequently during the day to delineate temporal differences among them.
Interestingly, among various sleep parameters, we found that only WASO was significantly associated with daily pain severity and pain catastrophizing. Although speculative, one possibility is that compared to total sleep time and sleep onset latency, WASO (i.e., the duration of wakefulness after sleep onset) appears to more directly capture disruptions in slow wave and rapid eye moment (REM) sleep, which are associated with one’s pain perception and inhibition [11, 55]. In fact, a recent experimental sleep disruption study found that forced awakening after sleep onset, which targeted increasing WASO, produced a significantly larger reduction in slow wave sleep and increase in REM latency than the restricted sleep onset condition which targeted increasing sleep onset latency [56]. Furthermore, findings indicate that prolonged middle of the night awakenings are the most common sleep disturbance associated with a variety of chronic pain conditions [11]. Some recent findings from experimental and intervention studies further provide support for the unique effects of WASO on pain and pain catastrophizing. For instance, using a healthy female sample, Smith et al. [57] demonstrated that experimentally disrupting sleep continuity significantly decreased endogenous pain inhibition and increased spontaneous pain severity. However, these effects were not exhibited in the delayed sleep onset group or the control group that did not experience any sleep disturbances [57]. Another study by Lerman et al. [41] found that improvement of WASO in an early treatment phase of cognitive behavioral therapy for insomnia among individuals with knee osteoarthritis was associated with reduced trait and nocturnal pain catastrophizing in a later treatment phase. Future studies which employ experimental sleep disruption will be needed to determine the mechanisms through which sleep disruption during the sleep period, relative to other sleep continuity deficits such as delayed onset of sleep, leads to higher pain severity, and pain catastrophizing.
Notably, the path from WASO to pain severity was moderated by sex such that there was a positive and statistically significant association between the two among women, but not among men. As a result, while only pain catastrophizing mediated the association between WASO and short-acting opioid use among men, both pain severity and pain catastrophizing emerged as significant mediators in this association among women. A recent laboratory study [58] supports this divergent sex effect in the association between WASO and pain severity. The authors found that temporal summation, a common feature observed in many chronic pain disorders, is significantly elevated by 2 days of laboratory-induced sleep continuity disturbance only in healthy women [58]. Our findings suggest that decreasing sleep continuity disturbance may be particularly important for female SCD patients in improving pain severity, which in turn, may also lower their requirements for short-acting opioids.
Observing small effect associations at the within-person level using daily diary or ecological momentary assessment methods is quite common (e.g., [59–61]), and the effect sizes in our study were also quite small overall. However, these small effects may still have some clinically meaningful implications. For instance, we found that for every 10-min increase of WASO, daily short-acting opioid use increased by 0.4 ME. However, it should be noted that sleep continuity disturbance is quite common among individuals with SCD. If an SCD patient had a bad night and had 2 hr of WASO, which is approximately a 1.5 SD above our sample mean of WASO (see Table 2), daily short-acting opioid use can be increased by nearly 5 ME, which may no longer be trivial. To further ascertain this possibility, we suggest that future studies examine growth trajectories over longer periods of time to determine whether the small mediated and direct effects of WASO on daily short-acting opioid use accumulate over time and exert a clinically meaningful risk of dose escalation of opioids over longer periods of time, or even increase risk of opioid misuse or abuse, as has been observed in other groups [13, 14].
We are also mindful that our findings are specifically based upon patients with SCD prescribed opioids. Although a large proportion of patients with SCD experience chronic pain, they exhibit disease characteristics that are substantially different from other chronic pain conditions (e.g., chronic low back pain, fibromyalgia, arthritis, temporomandibular disorders, etc.). For instance, the vast majority of patients with SCD are of African or Hispanic heritage and they often experience vaso-occlusive crises that cause severe pain. In addition, SCD is a congenital blood disorder, whereas most chronic pain conditions are acquired over long periods of time. Thus, the extent to which findings from our study are generalizable to patients with other chronic pain conditions is an open question. Given that sleep disturbance, pain severity, pain catastrophizing, and opioid use are regarded as important factors for the management of chronic pain, evaluating the present model in other non-SCD chronic pain populations would be important in future studies.
Although our findings are preliminary, we believe that it is important to help patients with SCD to adequately manage their sleep and pain in order to help them lower their opioid requirement and improve their quality of life. Indeed, there are a number of evidence-based psychosocial interventions, such as cognitive-behavioral therapy (CBT) and mindfulness-based intervention (MBI), which can effectively improve sleep disturbance, as well as pain severity and catastrophic thinking patterns [62–65]. However, the accessibility of these interventions among patients with chronic pain is a major issue [66], especially in light of the current COVID-19 pandemic [67]. Utilizing an online version of these interventions may be particularly helpful in reducing this important clinical gap in helping patients with SCD to improve their sleep, pain severity, pain catastrophizing, and opioid use. Online CBT and MBIs are not only more accessible, but also have shown effect sizes comparable to those found in interventions delivered in person [68, 69].
Limitations
There are a number of limitations in the present study. First, although participants provided quite extensive daily diary data (i.e., assessed up to 90 days), the sample size (N=45) was fairly small, particularly for the moderation analysis. In addition, we had a much smaller number of male participants than female participants. Thus, some other important sex differences may have not been detected due to suboptimal statistical power. Despite this limitation, exploring sex effects is still an important endeavor in research and clinical care. Second, our sleep measures were based upon subjective sleep diary reports. Although sleep diary is the gold standard for measuring various sleep parameters in behavioral sleep treatments, more objective sleep continuity data through actigraphy or an ambulatory sleep monitoring device should be collected in future studies. Third, our diary data was measured only twice per day (i.e., morning and at the end of the day). Multiple daily assessments of our study variables could further expand our nascent understanding of the dynamic associations among pain severity, pain catastrophizing, and prescription opioid use, as these were measured at a single point in time at the end of the day. Fourth, our measure of daily prescription opioid use did not provide information as to whether participants engaged in problematic opioid use (e.g., misuse). Future studies should expand upon the present analyses by assessing opioid use intention and deviation from specific prescription guidelines in order to capture participants’ potential problematic opioid use. Lastly, daily prescription opioid use was also dependent upon participants’ self-report of daily use. To increase the precision of this measure, future studies should consider using an electronic medication packaging device or monitor that can more objectively record dosing events and store these records electronically [70].
Conclusion
We found preliminary evidence that an increase in time awake during the previous night’s sleep period was associated with greater pain severity and pain catastrophizing the next day, which in turn, related to greater short-acting opioid use. Women, compared to men, were more likely to report greater pain severity when they had higher WASO during the previous night. Replication and extension of the current findings through future studies that employ enhanced methods in a large sample are needed. Such methods should include more frequent diary or ecological momentary assessment to improve temporal resolution and more objective measures of sleep continuity and prescription opioid use. If consistently replicated, we may be able to further improve our existing nonpharmacological pain management strategies for SCD, reduce sex disparities, and also lower requirements of prescription opioids.
Supplementary Material
Acknowledgements
Funding for this research was supported by the National Heart, Lung, and Blood Institute R01HL098110 (J.A.H.), National Institute on Drug Abuse F32DA049393 (C.J.M.), and National institute of neurological disorders and stroke T32NS070201 (postdoctoral training for C.J.M.).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors declare that they have no conflict of interest.
Authors’ Contributions C.M.C., and J.A.H. designed and conducted the study with contribution from P.H.F., M.T.S., C.P.C., J.M.S., and S.M.L. C.J.M. analyzed data. C.J.M., and P.H.F. wrote the manuscript. All authors critically reviewed the manuscript.
Ethnical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study. These procedures were approved by an institutional review board at Johns Hopkins School of Medicine.
References
- 1. Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010–2050: Modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10:e1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. [DOI] [PubMed] [Google Scholar]
- 3. Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: Frequency, etiology, and prognostic significance. Am J Hematol. 2005;79:17–25. [DOI] [PubMed] [Google Scholar]
- 4. Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762–769. [DOI] [PubMed] [Google Scholar]
- 5. Ruta NS, Ballas SK. The opioid drug epidemic and sickle cell disease: Guilt by association. Pain Med. 2016;17:1793–1798. [DOI] [PubMed] [Google Scholar]
- 6. Barry DT, Marshall BDL, Becker WC, et al. Duration of opioid prescriptions predicts incident nonmedical use of prescription opioids among US veterans receiving medical care. Drug Alcohol Depend. 2018;191:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colvin LA, Fallon MT. Opioid-induced hyperalgesia: A clinical challenge. BJA Br J Anaesth. 2010;104:125–127. [DOI] [PubMed] [Google Scholar]
- 8. Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: A meta-analysis of effectiveness and side effects. Can Med Assoc J. 2006;174:1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mann-Jiles V, Thompson K, Lester J. Sleep impairment and insomnia in sickle cell disease: A retrospective chart review of clinical and psychological indicators. J Am Assoc Nurse Pract. 2015;27:441–449. [DOI] [PubMed] [Google Scholar]
- 10. Sharma S, Efird JT, Knupp C, et al. Sleep disorders in adult sickle cell patients. J Clin Sleep Med. 2015;11:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. J Pain. 2013;14:1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schrimpf M, Liegl G, Boeckle M, Leitner A, Geisler P, Pieh C. The effect of sleep deprivation on pain perception in healthy subjects: A meta-analysis. Sleep Med. 2015;16:1313–1320. [DOI] [PubMed] [Google Scholar]
- 13. Brower KJ, Perron BE. Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Med Hypotheses. 2010;74:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Med Rev. 2012;16:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trauer JM, Qian MY, Doyle JS, Rajaratnam SMW, Cunnington D. Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Ann Intern Med. 2015;163:191–204. [DOI] [PubMed] [Google Scholar]
- 16. Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: Results of a meta-analysis. Sleep. 2004;27:249–253. [DOI] [PubMed] [Google Scholar]
- 17. Raymond I, Ancoli-Israel S, Choinière M. Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries. Sleep Med. 2004;5:551–559. [DOI] [PubMed] [Google Scholar]
- 18. Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. [DOI] [PubMed] [Google Scholar]
- 19. Sivertsen B, Lallukka T, Petrie KJ, Steingrímsdóttir ÓA, Stubhaug A, Nielsen CS. Sleep and pain sensitivity in adults. Pain. 2015;156:1433–1439. [DOI] [PubMed] [Google Scholar]
- 20. Carpenter RW, Lane SP, Bruehl S, Trull TJ. Concurrent and lagged associations of prescription opioid use with pain and negative affect in the daily lives of chronic pain patients. J Consult Clin Psychol. 2019;87:872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moscou-Jackson G, Finan PH, Campbell CM, Smyth JM, Haythornthwaite JA. The effect of sleep continuity on pain in adults with sickle cell disease. J Pain. 2015;16:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Finan PH, Carroll CP, Moscou-Jackson G, et al. Daily opioid use fluctuates as a function of pain, catastrophizing, and affect in patients with sickle cell disease: An electronic daily diary analysis. J Pain. 2018;19:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gatchel RJ, Neblett R. Pain catastrophizing: What clinicians need to know. Pract Pain Manag. 2015;15:70–75. [Google Scholar]
- 24. Sullivan MJL, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. [DOI] [PubMed] [Google Scholar]
- 25. Liedberg GM, Björk M, Börsbo B. Self-reported nonrestorative sleep in fibromyalgia–relationship to impairments of body functions, personal function factors, and quality of life. J Pain Res. 2015;8:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fabian LA, McGuire L, Goodin BR, Edwards RR. Ethnicity, catastrophizing, and qualities of the pain experience. Pain Med. 2011;12:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilt JA, Davin S, Scheman J. A multilevel path model analysis of the relations between sleep, pain, and pain catastrophizing in chronic pain rehabilitation patients. Scand J Pain. 2016;10:122–129. [DOI] [PubMed] [Google Scholar]
- 28. Mun CJ, Davis MC, Campbell CM, Finan PH, Tennen H. Linking nonrestorative sleep and activity interference through pain catastrophizing and pain severity: An intraday process model among individuals with fibromyalgia. J Pain. 2020;21:546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martel MO, Wasan AD, Jamison RN, Edwards RR. Catastrophic thinking and increased risk for prescription opioid misuse in patients with chronic pain. Drug Alcohol Depend. 2013;132:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dunn LK, Durieux ME, Fernández LG, et al. Influence of catastrophizing, anxiety, and depression on in-hospital opioid consumption, pain, and quality of recovery after adult spine surgery. J Neurosurg Spine. 2018;28:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wright D, Hoang M, Sofine A, Silva JP, Schwarzkopf R. Pain catastrophizing as a predictor for postoperative pain and opiate consumption in total joint arthroplasty patients. Arch Orthop Trauma Surg. 2017;137:1623–1629. [DOI] [PubMed] [Google Scholar]
- 32. Zhang B, Wing YK. Sex differences in insomnia: A meta-analysis. Sleep. 2006;29:85–93. [DOI] [PubMed] [Google Scholar]
- 33. Mogil JS. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–866. [DOI] [PubMed] [Google Scholar]
- 34. Fillingim RB, King CD, Ribeiro-Dasilva MC, Riley JL III. Sex, gender, and pain: A review of recent clinical and experimental findings. J Pain. 2009;10:447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: A path analytic description. Pain. 2004;112:188–196. [DOI] [PubMed] [Google Scholar]
- 36. Forsythe LP, Thorn B, Day M, Shelby G. Race and sex differences in primary appraisals, catastrophizing, and experimental pain outcomes. J Pain. 2011;12:563–572. [DOI] [PubMed] [Google Scholar]
- 37. Kaplovitch E, Gomes T, Camacho X, Dhalla IA, Mamdani MM, Juurlink DN. Sex differences in dose escalation and overdose death during chronic opioid therapy: A population-based cohort study. PLoS One. 2015;10:e0134550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Campbell CM, Moscou-Jackson G, Carroll CP, et al. An evaluation of central sensitization in patients with sickle cell disease. J Pain. 2016;17:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 40. Carriere JS, Lazaridou A, Martel MO, et al. The moderating role of pain catastrophizing on the relationship between partner support and pain intensity: A daily diary study in patients with knee osteoarthritis. J Behav Med. 2020;43:1–10. [DOI] [PubMed] [Google Scholar]
- 41. Lerman SF, Finan PH, Smith MT, Haythornthwaite JA. Psychological interventions that target sleep reduce pain catastrophizing in knee osteoarthritis. Pain. 2017;158:2189–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fine PG, Portenoy RK. A Clinical Guide to Opioid Analgesia. Minneapolis: McGraw Hill Healthcare Information; 2004. [Google Scholar]
- 43. Vieweg WVR, Lipps WFC, Fernandez A. Opioids and methadone equivalents for clinicians. Prim Care Companion J Clin Psychiatry. 2005;7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Von Korff M, Saunders K, Ray GT, et al. Defacto long-term opioid therapy for non-cancer pain. Clin J Pain. 2008;24:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Muthén LK, Muthén BO. Mplus User’s Guide. Eighth Edition (1998-2017). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- 46. Preacher KJ, Zyphur MJ, Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods. 2010;15:209–233. [DOI] [PubMed] [Google Scholar]
- 47. Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychol Methods. 2007;12:121–138. [DOI] [PubMed] [Google Scholar]
- 48. Enders CK. Applied Missing Data Analysis. New York: Guilford Press; 2010. [Google Scholar]
- 49. Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43:692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 51. Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: A critical review. Expert Rev Neurother. 2009;9:745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded Form. Ames: The University of Iowa; 1994. [Google Scholar]
- 53. Heuchert JP, McNair DM. Profile of Mood States, 2nd Edition: POMS-2. North Tonawanda, NY: Multi-Health Systems, Inc.; 2012. [Google Scholar]
- 54. Papaioannou M, Skapinakis P, Damigos D, Mavreas V, Broumas G, Palgimesi A. The role of catastrophizing in the prediction of postoperative pain. Pain Med. 2009;10:1452–1459. [DOI] [PubMed] [Google Scholar]
- 55. Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. [DOI] [PubMed] [Google Scholar]
- 56. Finan PH, Quartana PJ, Smith MT. The effects of sleep continuity disruption on positive mood and sleep architecture in healthy adults. Sleep. 2015;38:1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith PA, Selley DE, Sim-Selley LJ, Welch SP. Low dose combination of morphine and Δ9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol. 2007;571:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Smith MT Jr, Remeniuk B, Finan PH, et al. Sex differences in measures of central sensitization and pain sensitivity to experimental sleep disruption: Implications for sex differences in chronic pain. Sleep. 2018;42:zsy209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Biddle SJH, Gorely T, Marshall SJ, Cameron N. The prevalence of sedentary behavior and physical activity in leisure time: A study of Scottish adolescents using ecological momentary assessment. Prev Med (Baltim). 2009;48:151–155. [DOI] [PubMed] [Google Scholar]
- 60. Mun CJ, Thummala K, Davis MC, Karoly P, Tennen H, Zautra AJ. Predictors and social consequences of daily pain expectancy among adults with chronic pain. Pain. 2017;158:1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Finan PH, Zautra AJ, Davis MC. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosom Med. 2009;71:474–482. [DOI] [PubMed] [Google Scholar]
- 62. Finan PH, Buenaver LF, Runko VT, Smith MT. Cognitive-behavioral therapy for comorbid insomnia and chronic pain. Sleep Med Clin. 2014;9:261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: Systematic review and meta-analysis. Ann Behav Med. 2017;51:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gong H, Ni CX, Liu YZ, et al. Mindfulness meditation for insomnia: A meta-analysis of randomized controlled trials. J Psychosom Res. 2016;89:1–6. [DOI] [PubMed] [Google Scholar]
- 65. van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Med Rev. 2018;38:3–16. [DOI] [PubMed] [Google Scholar]
- 66. Becker WC, Dorflinger L, Edmond SN, Islam L, Heapy AA, Fraenkel L. Barriers and facilitators to use of non-pharmacological treatments in chronic pain. BMC Fam Pract. 2017;18:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Eccleston C, Blyth FM, Dear BF, et al. Managing patients with chronic pain during the COVID-19 outbreak: Considerations for the rapid introduction of remotely supported (eHealth) pain management services. Pain. 2020;161:889–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zachariae R, Lyby MS, Ritterband LM, O’Toole MS. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia—A systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1–10. [DOI] [PubMed] [Google Scholar]
- 69. Spijkerman MPJ, Pots WTM, Bohlmeijer ET. Effectiveness of online mindfulness-based interventions in improving mental health: A review and meta-analysis of randomised controlled trials. Clin Psychol Rev. 2016;45:102–114. [DOI] [PubMed] [Google Scholar]
- 70. Lam WY, Fresco P. Medication adherence measures: An overview. Biomed Res Int. 2015;2015:217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.