Abstract
The objective of this study was to evaluate effects of whole body radiation exposure early in life on influenza vaccination immune responses much later in life. A total of 292 volunteers recruited from the cohort members of ongoing Adult Health Study (AHS) of Japanese atomic bomb (A-bomb) survivors completed this observational study spanning two influenza seasons (2011–2012 and 2012–2013). Peripheral blood samples were collected prior to and three weeks after vaccination. Serum hemagglutination inhibition (HAI) antibody titers were measured as well as concentrations of 25 cytokines and chemokines in culture supernatant from peripheral blood mononuclear cells, with and without in vitro stimulation with influenza vaccine. We found that influenza vaccination modestly enhanced serum HAI titers in this unique cohort of elderly subjects, with seroprotection ranging from 18 to 48% for specific antigen/season combinations. Twelve percent of subjects were seroprotected against all three vaccine antigens post-vaccination. Males were generally more likely to be seroprotected for one or more antigens post-vaccination, with no differences in vaccine responses based on age at vaccination or radiation exposure in early life. These results show that early life exposure to ionizing radiation does not prevent responses of elderly A-bomb survivors to seasonal influenza vaccine.
Keywords: Influenza vaccine, Antibodies, Radiation, Atomic-bomb radiation, Cytokine, Chemokine
1. Introduction
Influenza is a major cause of illness and death among the elderly. Patient data pooled from 18 cohorts comprised of a total of 713,872 community-dwelling US adults older than 65 years studied across 10 seasons showed that receipt of seasonal influenza vaccine was associated with a 27% reduction in risk of hospitalization for pneumonia or influenza and a 48% reduction in death [1]. Based on this and previous studies, older individuals have been highly encouraged to obtain seasonal vaccination against influenza. However, the magnitude of benefit from such immunization has been widely debated, based primarily on concerns regarding potential selection bias that could inflate estimates of risk reduction for hospitalization and mortality (e.g. if those at highest risk do not receive vaccine) and on decreased immune responses of the elderly to vaccination [2–4].
Age has been shown to have a significant and consistent negative impact on immune response across both sexes and in genetically diverse populations. For example, while 70–90% of healthy young adults are protected from influenza after vaccination, such vaccination is at best only 50–60% effective in the elderly, and may be as low as 17% depending on circulating viruses [5–10]. Environmental factors such as diet, exposure to pollutants, and chronic infection can also alter immune responses, although the magnitude and consistency of the changes they induce are not as robust as those of age [11].
High doses of radiation have also been associated with significant dose-dependent decreases in overall immune responses, mediated by depletion of the cellular components of the immune response and potentially by damage to the stromal components of both primary and secondary lymphoid organs [12]. While many studies have examined the differential effects of acute exposure to ionizing radiation on immune function in both animals and humans, the effects of radiation exposure early in life (prior to age 25) on immune function in elderly humans have not been explored. Examination of the immune response of survivors of the atomic bombing of Hiroshima, Japan, which occurred more than 70 years ago, allows exploration of this question. In many individuals exposed to the large doses of ionizing radiation generated by the atomic bomb (A-bomb), circulating lymphocytes were destroyed and stem cells lost their capacity to produce new lymphocytes [13], leading to early death from infection [13,14]. However, in most A-bomb survivors, levels of hematopoietic cells returned to normal within months post-exposure [13], presumably followed by restored hematopoiesis. How prior radiation exposure affected immune responses years after recovery from its acute effects is of considerable interest, as this can affect quality of life as well as survival. The mechanisms by which radiation exposure can lead to long-term changes in immune response are many and have only recently begun to be elucidated. For example, normal tissue stem cells have been identified to undergo accelerated aging/senescence as a consequence of exposure to therapeutic radiation. Senescent stem cells are unable to self-replenish, leading to failure to maintain specific organs or cell populations. Radiation-injured cells may also develop a senescence-associated secretory phenotype, characterized by secretion of pro-inflammatory cytokines that can themselves contribute to disease processes [15]. We recently showed that prior exposure to A-bomb radiation enhanced age-related atrophy of the thymus, an important source of naïve T cells [16]. We also showed that A-bomb radiation had long term effects on dendritic cell generation and function, resulting in impaired antigen presentation in elderly A-bomb survivors [17].
This study was designed to test the hypothesis that radiation exposure early in life exacerbates age-associated decreases in immune function and thus further reduces the ability of elderly individuals to mount a protective adaptive immune response to prophylactic seasonal influenza vaccination. The response of elderly Japanese A-bomb survivors to influenza vaccination was assessed by measuring anti-influenza virus antibody titers and influenza antigen-induced cytokine production three weeks after vaccination. We present data that support the null hypothesis and show that early exposure to ionizing radiation, if survived, bears little impact upon functional immunity in the old age.
2. Methods
2.1. Study population
This study evaluated response to clinically indicated influenza vaccination in A-bomb survivors who were part of the Radiation Effects Research Foundation (RERF) Adult Health Study (AHS). This previously described AHS cohort [18–20] is part of a clinical research program established in July 1957 to follow approximately 20,000 A-bomb survivors in both Hiroshima and Nagasaki, Japan with health examinations every two years. Radiation exposure dose for each individual was estimated using the bone marrow doses calculated by dosimetry system DS02 [21–23]. Individuals with calculated radiation exposures less than 5 mGy were designated as controls.
Just over 3000 RERF-AHS subjects in Hiroshima, Japan were alive at the beginning of this study. Six hundred thirty two (632) potential radiation-exposed and non-exposed (control) subjects were randomly selected from the pool of AHS subjects and surveyed for study eligibility, using stratified sampling to enhance representation across the major subgroups of sex, age at vaccination, and radiation exposure dose groups. Eligibility for inclusion required that subjects had received influenza vaccinations within the past 5 years, were already planning to receive another influenza vaccination for the flu season being studied, and had an attending physician who agreed to cooperate with blood collection and vaccine administration. Subjects were excluded if they had a history of selected diseases or treatments that might affect responsiveness to vaccination, including a history of leukemia, lymphoma, myelodysplastic syndrome (MDS), rheumatoid arthritis, systemic lupus erythematosus (SLE), radiotherapy, current chemotherapy, steroid therapy, or interferon therapy. Of the 632 potential subjects surveyed, 88 subjects (14%) were unwilling to participate, 145 (23%) had no vaccination history or did not plan to be vaccinated in 2011 or 2012, 28 (4%) were currently in the hospital, and 69 (11%) did not respond to the survey. Thus, a total of 302 subjects (48%) consented to participate, including 118 non-exposed and 184 exposed subjects, Two hundred ninety two (292) of these subjects completed the study, with 157 in the 2011 flu season cohort and 135 in the 2012 flu season cohort (Supplementary Fig. S1). The demographics of the study population are shown in Table 1.
Table 1.
Demographics of subjects studied.
| Characteristic | 2011 Flu season |
2012 Flu season |
||||
|---|---|---|---|---|---|---|
| Non-exposed (n = 59) | Low-mod. dose (n = 70) | High dose (n = 28) | Non-exposed (n = 59) | Low-mod. dose (n = 59) | High dose (n = 17) | |
| Sex | ||||||
| Female | 38 | 47 | 19 | 38 | 44 | 9 |
| Male | 21 | 23 | 9 | 21 | 15 | 8 |
| Age at vaccination | ||||||
| Median (range) | 80 (67–89) | 79 (66–87) | 76 (67–89) | 80 (67–88) | 81 (67–89) | 75* (69–84) |
| <75 y.o. | 17 | 17 | 13 | 20 | 10 | 7 |
| ≥75 y.o. | 42 | 53 | 15 | 39 | 49 | 10 |
| Age at time of bomb | ||||||
| Median (range) | 15 (1–23) | 14 (0.5–21) | 10 (2–24) | 13 (0.1–22) | 14 (0.5–22) | 8 (2 to18) |
| <12 y.o. | 22 | 22 | 16 | 26 | 14 | 11 |
| ≥12 y.o. | 37 | 48 | 12 | 33 | 45 | 6 |
| BM dose (mGy) | ||||||
| Median (range) | 0 (0–2) | 248 (10–982) | 1427 (1010–3496) | 0 (0–4) | 300 (5–974) | 1694 (1006–3135) |
| <5 mGy | 59 | 0 | 0 | 59 | 0 | 0 |
| 5–500 mGy | 0 | 48 | 0 | 0 | 37 | 0 |
| 500–1000 mGy | 0 | 22 | 0 | 0 | 22 | 0 |
| 1000 + mGy | 0 | 0 | 28 | 0 | 0 | 17 |
Wilcoxon rank sum tests were used to compare continuous variables and Fisher exact tests were used to compare categorical data. The distributions of sex and age at time of bomb were similar for all dose groups. However, the subjects in the high dose group vaccinated in the 2012 season were significantly younger at vaccination than those in the non-exposed and low-moderate dose groups for that season (p = 0.005), due to absence of subjects older than 84 years in the 2012 high dose cohort.
Written informed consent was obtained from all subjects prior to conducting any study-related procedures. This study was conducted in accordance with the Ethical Guidelines for Epidemiological Research established by the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labor, and Welfare of Japan, and in accordance with the Declaration of Helsinki. All study-related documents and procedures were approved by the RERF Ethical Committee.
2.2. Influenza vaccine
Non-overlapping cohorts of subjects were recruited during the 2011 and 2012 flu seasons, spanning October 2011-January 2012 and October 2012-January 2013 respectively, to receive a single subcutaneous dose of either the 2011 or 2012 trivalent inactivated influenza vaccine. The commercial influenza vaccines used were obtained from four suppliers: Kaketsuken, Kumamoto, Japan (119 subjects; 41%); Denka-Seiken, Tokyo, Japan (74 subjects; 25%); Biken, Osaka, Japan (49 subjects; 17%); and Kitasato Institute Research Center for Biologicals, Saitama, Japan (50 subjects; 17%). Vaccines were given at a dose of 1.0 mL that contained >30 μg/mL of hemagglutinin for each of the recommended components, following guidelines from the Japanese Ministry of Health, Labor, and Welfare. The antigenic strain compositions of the trivalent seasonal vaccines administered in 2010 (shown for reference), 2011, and 2012 are shown in Supplementary Table S1. All 3 antigens (H1N1, H3N2, and B) were classified as recall antigens for subjects in the 2011 flu season cohort. Similarly, the H1N1 antigen used in the 2012 flu season was classified as a recall antigen for the 2012 cohort, with the 2012 H3N2 and B antigens serving as potential neo-antigens. Given the different antigen compositions of the vaccines, responses from subjects from each flu season were analyzed separately by antigen, resulting in six different antigen-season groups for analysis: responses to H1N1, H3N2, and B antigens for subjects vaccinated in the 2011 or 2012 flu seasons.
2.3. Peripheral blood collection
Peripheral blood samples were collected and processed at baseline prior to immunization and at three weeks after influenza vaccination. Serum was separated from whole blood by centrifugation, then was immediately frozen and stored at −80 °C until used. Peripheral blood mononuclear cells (PBMCs) were purified from heparinized venous blood by banding in Ficoll-Paque (Organon Teknika Corporation, Durham, NC). Due to human subject restrictions indicated by the RERF Ethical Committee, total blood collection volume was restricted to 6 mL per subject at each time point and only one follow-up time point was approved.
2.4. Determination of influenza hemagglutination inhibition (HAI) titers
Serum antibody titers against specific influenza strain components of the trivalent vaccines were measured by HAI assay, following standard procedures [24]. Briefly, serum samples were treated with a receptor-destroying enzyme (RDE: Denka Seiken, Niigata, Japan) overnight at 37 °C, heat-inactivated for 60 min at 56 °C, centrifuged, then diluted 1:10, followed by serial two-fold dilutions. Each serum dilution was incubated for one hour at room temperature with four hemagglutination (HA) units of whole inactivated virus and a 0.5% suspension of chicken red blood cells for H1N1 and B, or turkey red blood cells for H3N2. The HAI titer was defined as the reciprocal of the highest dilution that prevented red blood cell agglutination. Samples were evaluated in duplicate. HAI geometric mean titer (GMT) levels in pre- and post-vaccination (day 21) samples and the vaccine response measures of seroprotection rate (SPR), seroconversion rate (SCR), and seroconversion factor (SCF) were calculated. Seroprotection was defined as having a post-vaccination HAI titer of at least 40, Seroprotection rate (SPR) was defined as the proportion of subjects with a post-vaccination HAI titer of at least 40, regardless of pre-vaccination titer level. Seroconversion Rate (SCR) was defined as the proportion of subjects with at least 4-fold increase in HAI titer following vaccination resulting in a post-vaccination HAI titer of at least 40. Seroconversion factor (SCF) was defined as the ratio of the post-vaccination GMT levels to pre-vaccination GMT levels.
2.5. In vitro PBMC restimulation with vaccine antigens
PBMCs obtained pre- and three weeks post-vaccination were suspended in AIM V medium (Life Technologies, Gibco, Grand Island, NY) at 1 × 106 cells/mL and cultured at 2 × 105 cells/well in a 96-well culture plate (Costar, Toronto, Canada) under stimulation with the appropriate flu season-specific influenza vaccine (Supplementary Table S1) at 0.1 μg HA/mL (Denka Seiken, Tokyo, Japan). Plates were incubated at 37 °C in 5% carbon dioxide. Supernatants harvested at 24 and 96 h were frozen at −80 °C for subsequent multiplexed measurement of cytokines/chemokines.
2.6. Multiplexed cytokine/chemokine analysis
Cytokine/chemokine profiling of PBMC culture supernatant was performed using a Luminex bead-based human 25-plex cytokine assay (Life Technologies-Novex, Grand Island, NY, USA) according to the manufacturer’s protocol, using overnight primary incubation periods. The specific analytes measured were: GM-CSF, TNF, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12(p40/p70), IL-13, IL-15, IL-17, IFN-α, IFN-γ, IL-2R, RANTES, MIG, MIP-1α, MCP-1, IP-10, MIP-1β, eotaxin, and IL-1RA. All samples from each flu season were run on lot-matched assay kits. The final observed concentration (pg/mL) of each analyte was quantified using a five-parameter logic curve fit of assay kit standards and Bioplex Manager software. The low limit of quantification (LLOQ) was determined for each analyte per assay plate and sample values at or below the LLOQ were replaced with a value equal to one half (i.e. midpoint) of the LLOQ. Multiplex Luminex assays were run by the Duke Human Vaccine Institute Regional Biocontainment Laboratory Immunology Unit (Durham, NC).
2.7. Assessment of thymus function
Thymus function was estimated based on age and radiation dose, as previously described [16].
2.8. Statistical analysis
The primary endpoint of this study was the absolute change in HAI titer from baseline to three weeks post-vaccination. Antigen-specific GMTs were calculated across subjects for pre- and post-vaccination samples. Additionally, subject-specific titers were also calculated across the antigen-specific antibodies. When vaccine response variables were analyzed as dichotomous variables, their incidence was assumed to be binomially distributed. Univariate and multivariable logistic regression models were used to assess the influence of demographic factors such as age at time of bomb, age at vaccination, sex, and exposure dose on HAI titers and PBMC cytokine/chemokine biomarkers (see below). Odds ratios were calculated along with 95% confidence intervals. When analyses were performed across all subjects, adjustment for flu season was included in those models. Age at vaccination, age at time of bomb, and bone marrow radiation dose variables were evaluated as continuous measures and as categorical and dichotomized measures. Dose exposure groups were defined as <5 mGy (non-exposed; reflecting no meaningful exposure), 5–500 mGy (low), 500–1000 mGy (moderate), and ≥1000 mGy (high). In addition, the dichotomized outcomes of high dose (≥1000 mGy) versus lesser/no dose and the combination of low and moderate dose groups were also evaluated. Differences in continuous measures between groups were analyzed using two-sided, two-sample Wilcoxon rank sum tests. Differences in categorical variables between groups were assessed using Fisher exact or chi-square tests, depending on group sizes.
Secondary study endpoints were in vitro stimulated PBMC cytokine and chemokine production profiles. Results were summarized individually as well as in relation to the vaccine response outcomes of interest (e.g. seroprotection, seroconversion, failure to seroprotect for any of the three vaccine antigens). These parameters were analyzed in relation to sex, age at vaccination, and radiation exposure dose. Univariate logistic regression models were evaluated, but overall significance in relation to vaccine outcome was calculated using multivariable models that adjusted for the key demographic factors described above. All subsets of regression approaches were used to evaluate optimal multivariable models that further incorporated multiple biomarkers in addition to the demographic factors. All analyses were conducted using the R statistical program (version 3.1.2. for Windows). No corrections were made for multiple comparisons. Statistical significance was defined as p < 0.05.
3. Results
3.1. Study cohort characteristics
A flow diagram describing how subjects were selected is present in Supplementary Fig. S1. Demographic characteristics of the study population are summarized in Table 1. 94% and 88% of subjects in the 2011 cohort had received influenza vaccine in the 2010 and 2009 flu seasons, respectively. For the 2012 cohort, 69% of subjects had received influenza vaccine in the 2011 flu season and 65% received vaccine in the 2010 flu season. The distributions of sex and age at time of bomb were similar for all dose groups. However, the high dose group vaccinated in the 2012 flu season contained no subjects older than 84 years at time of vaccination and thus the median age of this group was younger at the time of vaccination than in the non-exposed and low-moderate dose groups for that season (p = 0.005) (Table 1).
3.2. Humoral response to influenza vaccination
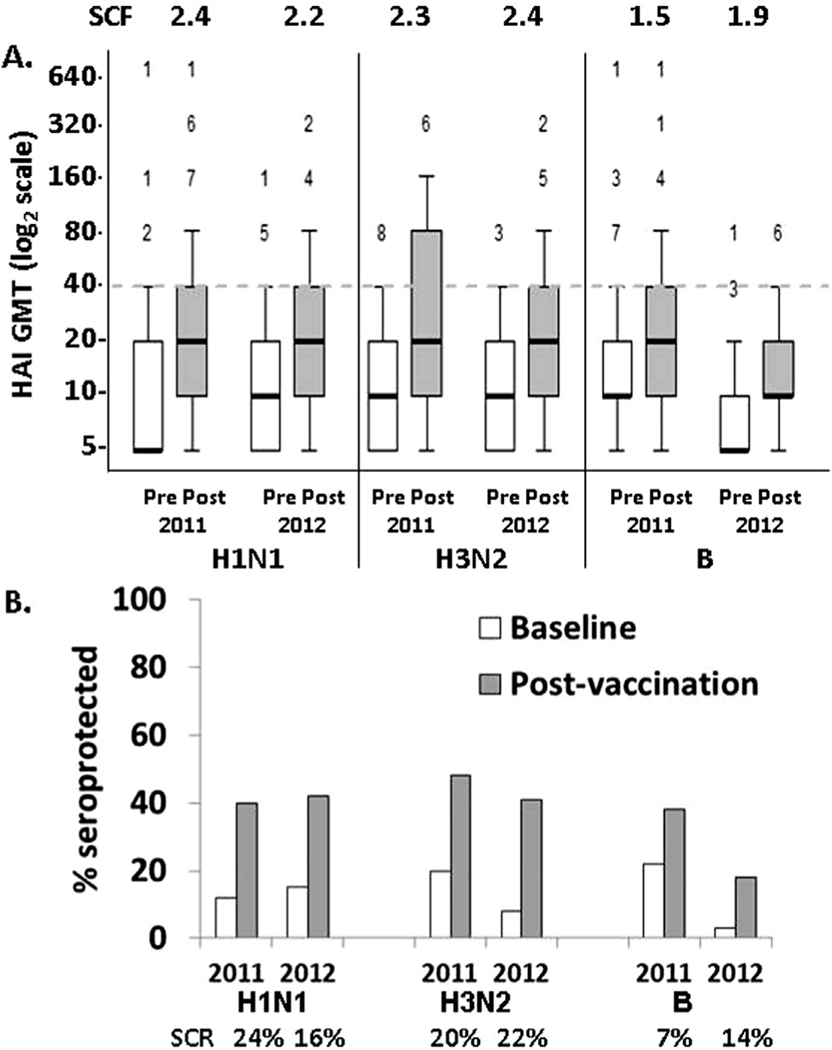
Antigen-specific response measures were evaluated to test the hypothesis that radiation exposure in early life negatively impacts immune function and the capacity for protective immune response to seasonal influenza vaccine in aged individuals. First, responses to each of the vaccine antigen/season combinations were evaluated for the overall study population, without stratification for factors such as age, dose, and sex. Influenza vaccination increased GMT against each antigen received (Fig. 1A; p < 0.0001). Accordingly, the % of subjects who were seroprotected for each antigen/season combination also increased, ranging from 19 to 48% post-vaccination (Fig. 1B; p = 0.0005). Antigen-specific responses against H1N1 and H3N2 were similar between the two season-based cohorts in terms of GMT and SPR post-vaccination. B antigen-specific pre-vaccination (baseline) and post-vaccination GMT and SPR were higher for the 2011 cohort when it was a recall antigen, compared to the 2012 cohort (Fig. 1A; p < 0.01). Consistent with its classification as a neo-antigen, only 2.5% of subjects demonstrated pre-existing (baseline) seroprotection to the new B antigenic strain in the 2012 vaccine formulation. However, the 2012 B antigen-specific SCF was lower than H1N1 and H3N2 in the 2011 cohort (p < 0.0002). Despite B potentially being a neo-antigen in the 2012 cohort, the B antigen-specific SCF trended notably lower than for H1N1 and H3N2 in the 2012 cohort (p = 0.06 and p = 0.044, respectively). Three subjects from the 2011 cohort (where the vaccine composition was identical to the previous flu season) were seroprotected against all three vaccine antigens at baseline. Including these subjects, only 12% of subjects were seroprotected against all three vaccine antigens post-vaccination. No differences in seroprotection were identified based on vaccine manufacturer.
Fig. 1.
Humoral HAI responses to vaccination by cohort flu season. (A) Box and whisker plot show HAI titers for each of the six antigen-season combinations studied for all participants in the study at baseline (white bars) and three weeks post-vaccination (gray bars). The “box” encloses the 25–75 percentile titers and the “whisker” (error bars) indicate 95% of responses. The number of outliers at higher titers is shown above each whisker. The seroconversion factor (SCF) for each antigen/season combination is also shown. (B) The percentages of subjects classified as seroprotected before and after vaccination and the seroconversion rate (SCR) are shown for these same antigen/season combinations. The 95% confidence intervals for SCF are presented in Supplementary Table S2.
3.3. Impact of sex on seroprotection and seroconversion rates
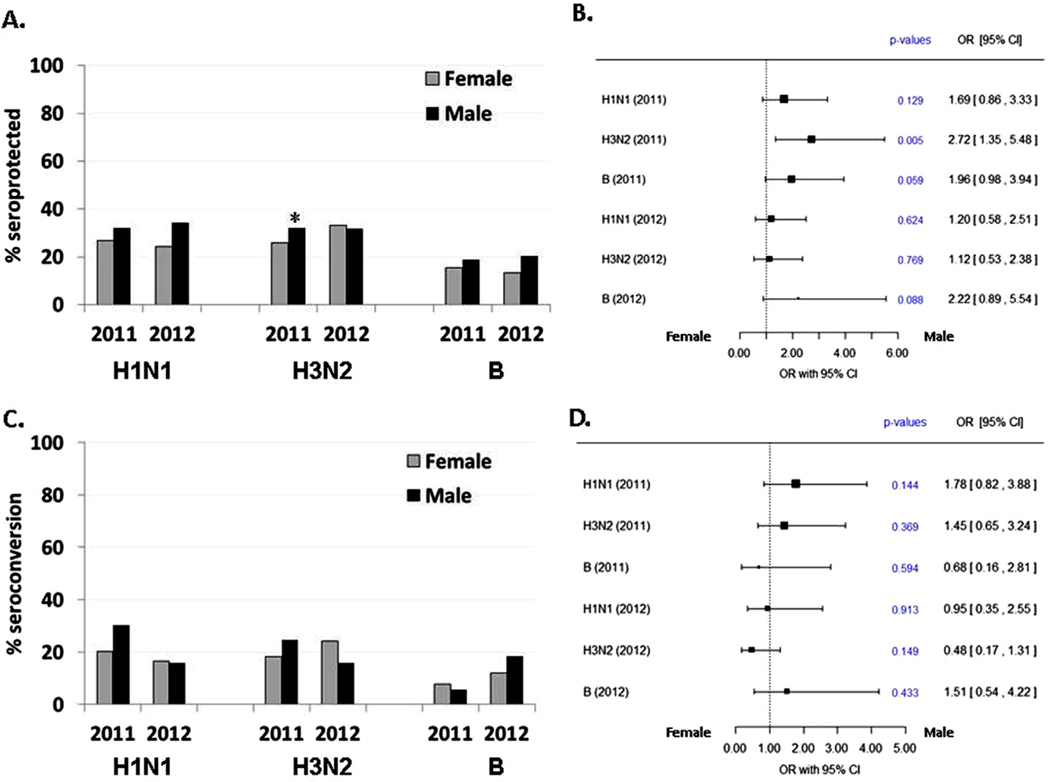
The influence of male versus female sex on SPR and SCR was next examined for each of the six different antigen-season groups, with multivariable analyses that adjusted for possible confounding effects of age and radiation exposure dose. For SPR (Fig. 2A and B), an overall trend is apparent in that males were generally more likely (odds ratios greater than 1) to mount a seroprotective response to each of the antigens than females. However, this trend is only significant for response to the 2011 H3N2 antigen, where men were more likely to achieve seroprotection (OR = 2.7, p = 0.005). Males and females had similar SCRs (Fig. 2C and D).
Fig. 2.
Multivariable analysis of sex effect on humoral response to influenza vaccine antigens. Vaccination response odds ratios (OR) and 95% confidence interval for sex effect on seroprotection rate (A and B) and seroconversion rate (C and D), adjusted for vaccination the prior season and for dichotomized age at vaccination (≥75 vs. <75 yrs), and radiation dose (<1 Gy vs. ≥1 Gy).
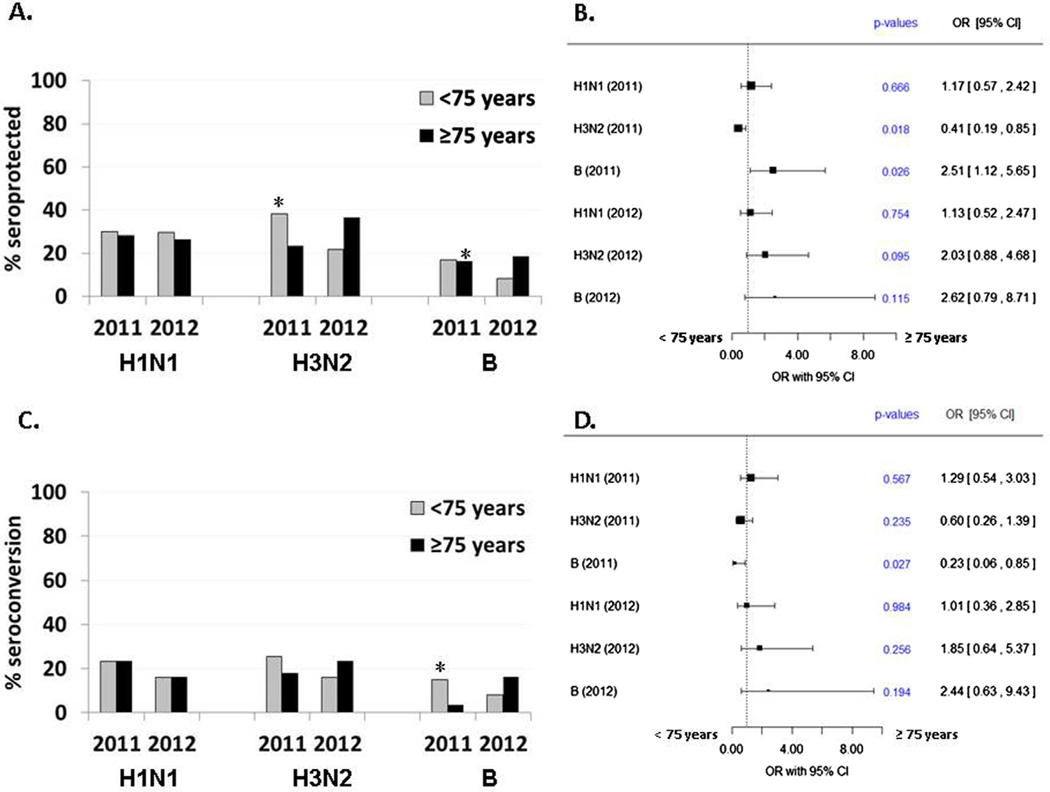
3.4. Impact of age at vaccination on seroprotection and seroconversion rates
A similar approach was taken to investigate the impact of age on influenza vaccine response measures (Fig. 3). Since the bombing occurred in 1945, the entire study population was aged 66 years or older at the time of vaccination. The multivariable analyses thus evaluated age at vaccination as a continuous variable and also compared subjects aged ≥75 years versus those <75 years at the time of vaccination. Individuals ≥75 years old were less likely than those <75 years (OR = 0.4; 95%CI: 0.2–0.9) to be seroprotected against the H3N2 antigen in the 2011 flu season and more likely to have seroprotection to B antigen after vaccination in the 2011 season (OR = 2.5; 95% CI: 1.1–5.7) (Fig. 3A and B). Similar findings were also observed when looking at age at vaccination as a continuous variable (2011 H3N2: OR = 0.9, p = 0.02; 2011 B: OR = 1.1, p = 0.06). However, the likelihood of SCR only increased for the 2011 B antigen in younger subjects (OR for age = 0.9, p = 0.07; OR for <75 years old = 0.2, p = 0.03; Fig. 3C and D). Overall, these analyses suggest that pre-existing immunity (e.g. number of subjects who entered the study seroprotected pre-vaccination) drove the observed age-related differences in seroprotection rates for the 2011 B antigen, since over half of subjects (34/60 = 57%) with seroprotection after vaccination were already seroprotected prior to vaccination. Further, none of those with baseline seroprotection for the 2011 B antigen achieved the additional requirement of a 4-fold change in titer levels used to define seroconversion.
Fig. 3.
Multivariable analysis of age at vaccination effect on humoral response to influenza vaccine antigens. Vaccination response odds ratios (OR) and 95% confidence interval for age effect on seroprotection rate (A and B) and seroconversion rate (C and D), adjusted for vaccination the prior season, sex, and radiation dose (<1 Gy vs. ≥1 Gy).
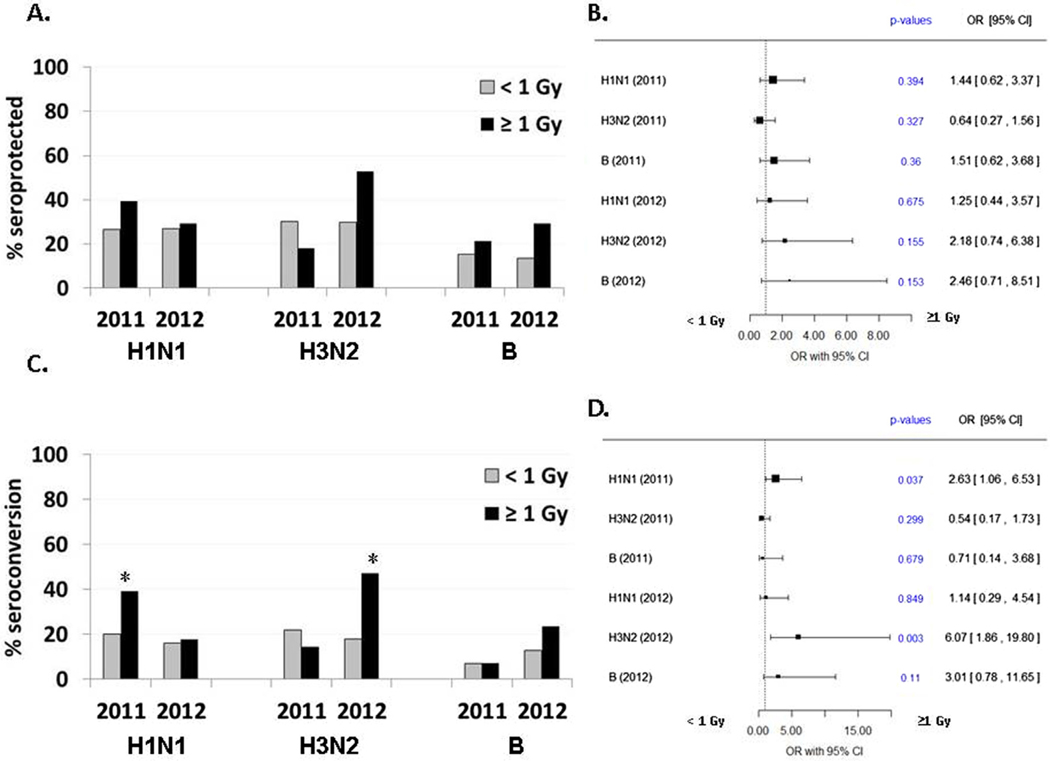
3.5. Impact of radiation dose on seroprotection and seroconversion rates
To examine the impact of early life exposure to A-bomb radiation on vaccine responses later in life, individuals exposed to ≥1 Gy radiation were compared to those who received lower doses or were not exposed to radiation, in addition to evaluating bone marrow dose exposure as a continuous measure. Multivariable analysis showed no differences in SPRs when comparing those with high dose radiation exposure (≥1 Gy) versus those exposed to lesser doses (Fig. 4A and B). There were also no observed influences of continuous dose exposure on seroprotection. However, radiation exposure did appear to influence seroconversion, although only for the 2011 H1N1 and 2012 H3N2 antigens. Specifically, individuals exposed to ≥1 Gy were more likely to seroconvert to H1N1 in the 2011 flu season (OR = 2.6; 95% CI = 1.1–6.5, p = 0.04) and to H3N2 in the 2012 season (OR = 6.1; 95% CI = 1.9–19.8, p = 0.003) (Fig. 4C and D). These overall findings were consistent when evaluating radiation exposure as a continuous measure (p = 0.10 and p = 0.004, respectively). It is worth noting that radiation exposure generated similar trends toward seroprotection for the 2012 H3N2 antigens (≥1 Gy OR = 2.2, p = 0.16; continuous dose exposure, p = 0.11). These differences may not have been as pronounced in relation to seroprotection, given that 27% of subjects who were seroprotected after vaccination were also seroprotected at baseline for the 2012 H3N2 antigen.
Fig. 4.
Multivariate analysis of radiation dose effect on humoral response to influenza vaccine antigens. Vaccination response odds ratios (OR) and 95% confidence interval for radiation dose effect on seroprotection rate (A and B) and seroconversion rate (C and D), adjusted for vaccination the prior season, sex, and for dichotomized age at vaccination (≥75 vs. <75 yrs).
3.6. Incidence of failure to develop seroprotection
A negative impact on immune function by factors such as sex, age, and radiation exposure may ultimately lead to the inability to mount a seroprotective response to any vaccine antigens. In a univariate analysis, women were more likely to fail to develop seroprotective responses to any of the three vaccine antigens tested in a given flu season, compared to men (38% vs. 23%; p = 0.01, Table 2). This difference remained (p = 0.009) after accounting for other factors (age at vaccination, radiation dose, prior season vaccination) in a multivariable logistic regression model. Failure to develop any seroprotective responses did not correlate with age at vaccination, age at time of bomb, or radiation dose (Table 2).
Table 2.
Vaccine efficacy as a function of sex, age at vaccination and at time of bomb, and radiation dose.
| Characteristic | Vaccine success (seroprotection for ≥1 antigen) | Vaccine failure (seroprotection for NO antigens) | p-value |
|---|---|---|---|
| Sex | |||
| Female | 120 (62%) | 75 (38%) | 0.01 |
| Male | 75 (77%) | 22 (23%) | |
| Age at vaccination | |||
| Median (range) | 79 (66–89) | 81 (67–89) | 0.44 |
| <75 y.o. | 55 (65%) | 29 (35%) | 0.87 |
| 75+ y.o. | 140 (67%) | 68 (33%) | |
| Age at time of bomb | |||
| Median (range) | 13.4 (0.5–23.2) | 14.3 (0.1–23.6) | 0.54 |
| <12 y.o. | 78 (70%) | 33 (39%) | 0.39 |
| 12+ y.o. | 117 (65%) | 64 (35%) | |
| Dose | |||
| Median (range) | 110 (0–3496) | 81 (0–2553) | 0.94 |
| <5 mGy | 80 (68%) | 38 (32%) | 0.2 |
| 5 to <500 mGy | 57 (67%) | 28 (33%) | |
| 500 to <1000 mGy | 24 (55%) | 20 (45%) | |
| 1000+ mGy | 34 (76%) | 11 (24%) | |
| <1000 mGy | 161 (65%) | 86 (35%) | 0.24 |
| 1000+ mGy | 34 (76%) | 11 (24%) |
3.7. PBMC cytokine response to in vitro restimulation with influenza vaccine
PBMC responses to in vitro stimulation with influenza vaccine were examined at baseline and three weeks after vaccination to identify possible cytokine/chemokine signatures that might correlate with observed vaccine responses. In vitro production of 25 cytokines and chemokines was similar between vaccine responders and non-responders (subjects who failed to develop seroprotective responses, as defined above), both at baseline (unstimulated) and 24 and 96 h following stimulation with vaccine antigens (Supplementary Fig. S2). Multivariable analysis showed no consistent effects of age at vaccination, sex, or radiation exposure, or in vitro cytokine/chemokine production on the likelihood of mounting a seroprotective response across the entire cohort. However, multivariable analysis that also adjusted for immunization season showed that higher in vitro production of IL-1β, IL-2, IL-6, IL-8, RANTES, or IFN-γ and lower production of MIP-1α, eotaxin, or IL-15 were associated with mounting seroprotective responses against at least one antigen, either for the entire cohort or when analyses were restricted to those subjects who were not seroprotected prior to vaccination (p = 0.008–0.05).
3.8. Predicted impact of thymus function on vaccine response
The age-associated decline in immune responses to vaccination has been hypothesized to be due, at least in part, to age-related loss of T cells with appropriate specificity, combined with inadequate replacement due to thymic atrophy. We have previously found increased thymic involution in individuals exposed to as little as 5 mGy radiation, when adjusted for age, using a unique archive of tissues obtained from 165 A-bomb survivors, 47% of whom were >75 yrs at the time of their natural death [16]. We applied a model developed in that work to the current population using the non-invasive demographic factors of age, radiation dose (as a continuous variable), sex, and their interaction. This estimated the percentage of residual thymus area involved in thymopoiesis (functional cortical area) for study subjects as between 0 and 7.5% (median 0, mean 0.8%). This estimated area did not differ between subjects who became seroprotected for at least one antigen versus subjects who did not (mean 0.75% vs. 0.79%; p = 0.80).
4. Discussion
The ability to respond to influenza vaccine is an important public health issue. We investigated effects of prior early life radiation exposure on the immunological capacity of elderly Japanese A-bomb survivors to respond to influenza vaccination as indicated by anti-influenza virus antibody titers and cytokine production three weeks after vaccination. Administration of seasonal influenza vaccine was potentially beneficial in this cohort of elderly Japanese subjects, based on vaccine-induced increase in serum GMTs and the increased percentage of subjects who were seroprotected for at least one antigen following vaccination. However, overall response to vaccination was modest, with post-vaccination seroprotection ranging from 18 to 48% for the various antigen/season combinations and only 12% of subjects were seroprotected against all three vaccine antigens post-vaccination.
A quantitative review of 31 influenza vaccination studies from 1986 to 2002 showed an adjusted odds-ratio (OR) for seroconversion and seroprotection to all three antigens in elderly versus young adults that ranged from 0.24 to 0.59 [10]. Vaccination responses in our elderly cohort were similar to those in other elderly cohorts [5–10] and the data we present here show that early life exposure to A-bomb radiation did not further affect responses. Of interest, similar conclusions were recently reached by our group in a controlled mouse irradiation and aging experiment, where irradiation in youth did not affect lifelong lymphocyte homeostasis beyond the initial recovery period, and also did not affect late-life responses to vaccination followed by potentially lethal virus challenge [25].
No difference in seroprotection for at least one antigen, was seen based on age at vaccination, age at time of bomb, or radiation dose when considered either as a continuous or a categorical variable (Table 2). However, although only 33% of study subjects were male, males were more likely than females (77% vs. 62%; p = 0.01) to develop seroprotection to at least one vaccine antigen. This is also reflected in the increased odds ratios for seroconversion by males to each of the 6 antigen/season combinations (Fig. 2), although statistical significance was reached only for the 2011 H3N2 antigen. Our studies were not able to determine whether this reflects true sex-based differences in immune response, history of previous exposure to influenza, or underlying differences in health status that affected participation in the study. However, total numbers of dendritic cells were recently shown to decrease with age, with absolute numbers of plasmacytoid dendritic cells decreased specifically in female A-bomb survivors [17], providing a possible mechanism for the decreased vaccine efficacy observed in females in the current study. Individuals exposed to ≥1 Gy radiation showed increased seroconversion to the H1N1 antigen in 2011 and to the H3N2 in 2012. Why these responses were limited to certain antigen/season combinations is currently unclear, but likely reflect past exposure (or lack thereof) to these antigens or other characteristics of individuals with higher levels of radiation exposure that were not assessed in this study.
To potentially provide a mechanistic basis for response or lack of serologic response to vaccination, cytokine production by peripheral blood mononuclear cells was assessed in response to stimulation with the season-specific vaccine in vitro. Although no differences were seen in univariate analysis of the entire cohort, changes in production of a limited number of cytokines/chemokines by vaccine-stimulated peripheral blood mononuclear cells were associated with seroprotection against at least one vaccine antigen in multivariable analyses that included age at vaccination, sex, radiation exposure, and immunization season. Limitations on blood draw volume prevented assessment of specific responses to purified H1N1, H3N2, and B proteins that would be required to determine with certainty that vaccine-stimulated cytokine responses in any given subject were related to mounting seroprotection rather than to quantitative variations in vaccine components, including impurities such as mRNAs or residual non-HA proteins. In addition, cytokine and chemokine responses induced by vaccine in vitro may not always reflect cellular immune responses that result in seroprotection in vivo. Nevertheless, the information reported here remains potentially useful for generating hypotheses regarding biomarkers that may potentially be relevant to vaccine responses that can be tested in future studies.
The immune system is highly dynamic and ability to respond to any specific antigen is in part dependent on environmental factors including history of antigen exposure. Changes documented with increasing age include a decrease in thymic T cell production, frequent clonal expansion of memory T cell populations, increases in autoimmune diseases/syndromes, and diminished response to prophylactic vaccinations [26–29]. A-bomb survivors have provided a wealth of information on the recovery from acute radiation damage, as well as the immediate and intermediate consequences of radiation [13,30–32]. Since survivors recovered their ability to produce blood cells following their radiation exposure, it is not surprising that the proportion of survivors who were affected by or died of infectious diseases, such as tuberculosis, has not exhibited a significant increase with radiation dose. Similarly, the incidence of autoimmune diseases, such as rheumatoid arthritis, has shown no relation to dose of radiation [32–36]. On the other hand, there are reports of slight dose-dependent decreases in immunity against viral infection [37,38], including a subtle decline of immunity against Epstein-Barr virus (EBV). Moreover, the proportion of carriers of the hepatitis B virus has appeared to increase with radiation dose [39,40] suggesting an increased risk for hepatocellular carcinoma. Finally, it has been suggested that long-term impairments of T cell immunity to infections by acute, and in particular persistent, microorganisms such as the cytomegalovirus (CMV) may cause chronic inflammation that may lead to increased risk of atherosclerotic diseases [19]. In that regard, we have reported that the percentages of helper T cells are decreased in A-bomb survivors who have a history of myocardial infarction [35,41,42].
Although individuals exposed to greater than 1 Gy radiation have demonstrated approximately a 2% decrease in CD4+ T cells [35,42–46], this shift has not resulted in increased frequency nor severity of infections or autoimmune diseases [47–49]. The results of biennial examinations of the A-bomb survivors indicated that there were dose-related changes in white blood cell counts and erythrocyte sedimentation rates as early as 1958 [50]. Since the levels of both markers are known to rise in response to a variety of inflammatory stimuli [51], it seemed reasonable to assume that low levels of inflammation may well have been present in the survivors for very long periods. Whether this inflammation was due to injured cells that developed the senescence-associated secretory phenotype [15] is unknown. A large-scale study revealed radiation-dose dependent increases in serum IgM (in both males and females) and IgA (in females) [52]. Another study in a subset of Hiroshima survivors has demonstrated that IgM, IgG and IgA levels tend to increase with radiation dose [53]. The reason for this enhanced B cell immune response in the survivors is unclear. Recently a positive association was demonstrated between the inflammation marker C-reactive protein and anti-Chlamydia pneumoniae antibody levels, especially in more heavily exposed (≥1 Gy) A-bomb survivors, although the antibody levels appeared to decrease with radiation dose among a total survivor population examined [19]. This suggested that the diminished immune response to Chlamydia pneumoniae might be related to chronic inflammatory reactions. Alternatively, elevated levels of cytokines such as IL-6 [54] may be associated with enhanced antibody production in A-bomb survivors, but it is unlikely that A-bomb irradiation has shifted the balance of regulatory mechanisms in favor of Th2 immunity. Whether exposure to A-bomb radiation results in subtle decreases in T regulatory cell numbers or function is currently unknown.
Our studies found that the predicted percentage of residual thymus area involved in thymopoiesis did not differ between subjects who became seroprotected to at least one antigen versus subjects who did not, despite exposure to radiation. This is perhaps not unexpected, since although radiation exposure significantly accelerates loss of thymic area with active thymopoiesis [16], minimal thymic function is typically present even in non-exposed individuals at the ages present in this cohort. Poor vaccine responses in the elderly have generally been hypothesized to be due to a decline in T cell function. The role of age-related changes in B cells has been less well studied. A recent study using genetically engineered mice bearing limited numbers of potentially responsive B cells and a vaccine against an HIV-related antigen suggested that generation of priming responses could occur when at least one appropriate B cell precursor was present [55]. Additional studies will be required to determine the relative contributions of age- or radiation-induced changes in numbers and/or function of antigen-presenting cells, T cells, and B cells to vaccine responses in humans. Likewise, the mechanisms that resulted in a trend toward increased seroconversion and decreased vaccine failure in males will require further study. It is tempting to speculate that similar or enhanced immune responsiveness to vaccination may be due to possible overall constitutional and immunological “robustness” of the individuals who survived more than 65 years after being exposed to high early-life doses of radiation.
Our finding that elderly A-bomb survivors who had received over 1 Gy radiation showed similar seroprotection and similar or higher seroconversion after influenza vaccination compared with those who received less than 1 Gy or were non-exposed is consistent with other studies that showed general immune recovery and detectable but generally limited effects of radiation in this population [56]. They speak to a remarkable resiliency of the immune system to withstand a single global and widespread radiation-induced damage event.
In conclusion, this study found that influenza vaccination of a unique cohort of elderly subjects who were exposed to A-bomb radiation early in life modestly increased their serum HAI titers, similar to matched non-exposed subjects, indicating that early life exposure to ionizing radiation does not prevent vaccine responses later in life. Further study will be necessary to identify methods to increase immune responses to influenza vaccination in the elderly, with and without a history of radiation exposure.
Supplementary Material
Acknowledgements
We greatly appreciate the contribution of attending doctors, Hiroshima Medical Association, Asa Medical Association, Aki Medical Association, and Saiki Medical Association to this vaccination study. We thank P. Morrow and K. Riebe (Duke University) for technical support.
Funding
The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan, is a private, non-profit foundation funded by the Japanese Ministry of Health, Labor and Welfare and the United States of America Department of Energy (US-DOE), the latter in part through DOE Award DE-HS0000031 to the US National Academy of Sciences. This study was based on RERF Research Protocol RP#3-09 and was supported by the US National Institutes of Health (NIAID Contract HHSN272200900059C). Research at Duke University was performed in the Duke Regional Biocontainment Laboratory which received partial support for construction from the US National Institutes of Health (NIAID; UC6-AI058607). The views of the authors do not necessarily reflect those of the two governments.
Abbreviations:
- A-bomb
atomic bomb
- CI
confidence interval
- HA
hemagglutinin
- HAI
hemagglutination inhibition
- GMT
geometric mean titer
- LLOQ
lower limit of quantitation
- OR
odds ratio
- PBMC
peripheral blood mononuclear cells
- SPR
seroprotection rate
- SCR
seroconversion rate
- SCF
seroconversion factor
Footnotes
Conflict of interest
All authors report no potential conflicts of interest.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2018.09.054.
References
- [1].Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med 2007;357:1373–81. [DOI] [PubMed] [Google Scholar]
- [2].Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 2007;7:658–66. [DOI] [PubMed] [Google Scholar]
- [3].Fireman B, Lee J, Lewis N, Bembom O, van der Laan M, Baxter R. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol 2009;170:650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Trucchi C, Paganino C, Orsi A, De Florentiis D, Ansaldi F. Influenza vaccination in the elderly: why are the overall benefits still hotly debated? J Prev Med Hyg 2015;56:E37–43. [PMC free article] [PubMed] [Google Scholar]
- [5].Candore G, Balistreri CR, Listi F, Grimaldi MP, Vasto S, Colonna-Romano G, et al. Immunogenetics, gender, and longevity. Ann N Y Acad Sci 2006;1089:516–37. [DOI] [PubMed] [Google Scholar]
- [6].Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. Jama 1994;272:1661–5. [PubMed] [Google Scholar]
- [7].Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med 1994;331:778–84. [DOI] [PubMed] [Google Scholar]
- [8].Ahmed AE, Nicholson KG, Nguyen-Van-Tam JS. Reduction in mortality associated with influenza vaccine during 1989–90 epidemic. Lancet 1995;346:591–5. [DOI] [PubMed] [Google Scholar]
- [9].Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med 1995;123:518–27. [DOI] [PubMed] [Google Scholar]
- [10].Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006;24:1159–69. [DOI] [PubMed] [Google Scholar]
- [11].Campbell A. Inflammation, neurodegenerative diseases, and environmental exposures. Ann N Y Acad Sci 2004;1035:117–32. [DOI] [PubMed] [Google Scholar]
- [12].Shah DJ, Sachs RK, Wilson DJ. Radiation-induced cancer: a modern view. Br J Radiol 2012;85:e1166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Oughtersen AW, Warren S. Hematology of atomic bomb injuries, pathology of atomic bomb injuries. In: Medical effects of the atomic bomb in Japan National Nuclear Energy Series Division III. New York: McGraw-Hill; 1956. p. 191–430. [Google Scholar]
- [14].Ohkita T. Biological effects. A. Acute effects. J Radiat Res (Tokyo) 1975;16 (Suppl. 1):49–66 [In A review of Thirty Years Study of Hiroshima and Nagasaki Atomic Bomb Survivors]. [DOI] [PubMed] [Google Scholar]
- [15].Citrin DE. Recent developments in radiotherapy. N Engl J Med 2017;377:1065–75. [DOI] [PubMed] [Google Scholar]
- [16].Ito R, Hale LP, Geyer SM, Li J, Sornborger A, Kajimura J, et al. Late effects of exposure to ionizing radiation and age on human thymus morphology and function. Radiat Res 2017;187:589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kajimura J, Lynch H, Geyer S, Yamaoka M, Shterev I, Sempowski G, et al. Radiation and age-associated changes in peripheral blood dendritic cell populations among aging atomic bomb survivors in Japan. Rad Res 2018;189:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Beebe GW, Fujiwara H, Yamasaki M. Adult health study reference papers, A: selection of the sample, and B: characteristics of the sample. ABCC Technical Report 1960. [Google Scholar]
- [19].Hakoda M, Kasagi F, Kusunoki Y, Matsuura S, Hayashi T, Kyoizumi S, et al. Levels of antibodies to microorganisms implicated in atherosclerosis and of C-reactive protein among atomic bomb survivors. Radiat Res 2006;166:360–6. [DOI] [PubMed] [Google Scholar]
- [20].Hayashi T, Ito R, Cologne J, Maki M, Morishita Y, Nagamura H, et al. Effects of IL-10 haplotype and atomic bomb radiation exposure on gastric cancer risk. Radiat Res 2013;180:60–9. [DOI] [PubMed] [Google Scholar]
- [21].Cullings HM, Fujita S, Funamoto S, Grant EJ, Kerr GD, Preston DL. Dose estimation for atomic bomb survivor studies: its evolution and present status. Radiat Res 2006;166:219–54. [DOI] [PubMed] [Google Scholar]
- [22].Okubo T. Results of review of basic information used in individual dose estimates and recalculation of adiation risks. RERF Update. 2013;24:25–7. [Google Scholar]
- [23].Cullings HM, Pierce DA, Kellerer AM. Accounting for neutron exposure in the Japanese atomic bomb survivors. Radiat Res 2014;182:587–98. [DOI] [PubMed] [Google Scholar]
- [24].Kendal AP, Pereira MS, Skehel J. Concepts and procedures for laboratory based influenza surveillance. Geneva: World Health Organization; 1982. [Google Scholar]
- [25].Pugh JL, Foster SA, Sukhina AS, Petravic J, Uhrlaub JL, Padilla-Torres J, et al. Acute systemic DNA damage in youth does not impair immune defense with aging. Aging Cell 2016;15:686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fulop T, Larbi A, Pawelec G. Human T cell aging and the impact of persistent viral infections. Front Immunol 2013;4:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Haynes L, Maue AC. Effects of aging on T cell function. Curr Opin Immunol 2009;21:414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol 2009;21:418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Murasko DM, Goonewardene IM. T-cell function in aging: mechanisms of decline. Ann Rev Gerontol Geriatrics 1990;10:71–96. [DOI] [PubMed] [Google Scholar]
- [30].Preston DL, Pierce DA, Shimizu Y, Cullings HM, Fujita S, Funamoto S, et al. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat Res 2004;162:377–89. [DOI] [PubMed] [Google Scholar]
- [31].Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 2007;168:1–64. [DOI] [PubMed] [Google Scholar]
- [32].Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950–1997. Radiat Res 2003;160:381–407. [DOI] [PubMed] [Google Scholar]
- [33].Shimizu Y, Pierce DA, Preston DL, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, Part II. Noncancer mortality: 1950–1990. Radiat Res 1999;152:374–89. [PubMed] [Google Scholar]
- [34].Preston DL, Pierce DA, Shimizu Y. Age-time patterns for cancer and noncancer excess risks in the atomic bomb survivors. Radiat Res 2000;154:733–4 [discussion 4–5]. [PubMed] [Google Scholar]
- [35].Hakoda M, Masunari N, Yamada M, Fujiwara S, Suzuki G, Kodama K, et al. Serum uric acid concentration as a risk factor for cardiovascular mortality: a longterm cohort study of atomic bomb survivors. J Rheumatol 2005;32:906–12. [PubMed] [Google Scholar]
- [36].Imaizumi M, Usa T, Tominaga T, Neriishi K, Akahoshi M, Nakashima E, et al. Radiation dose-response relationships for thyroid nodules and autoimmune thyroid diseases in Hiroshima and Nagasaki atomic bomb survivors 55–58 years after radiation exposure. JAMA 2006;295:1011–22. [DOI] [PubMed] [Google Scholar]
- [37].Kusunoki Y, Kyoizumi S, Ozaki K, Cologne JB, Akiyama M. Studies on immune response to Epstein-Barr virus among A-bomb survivors. In: Sugahara T, Sagan LA, Aoyama T, editors. Low dose irradiation and biological defense mechanisms. Amsterdam: Excerpta Medica; 1992. p. 63–6. [Google Scholar]
- [38].Kusunoki Y, Kyoizumi S, Fukuda Y, Huang H, Saito M, Ozaki K, et al. Immune responses to Epstein-Barr virus in atomic bomb survivors: study of precursor frequency of cytotoxic lymphocytes and titer levels of anti-Epstein-Barr virus-related antibodies. Radiat Res 1994;138:127–32. [PubMed] [Google Scholar]
- [39].Fujiwara S, Sharp GB, Cologne JB, Kusumi S, Akahoshi M, Kodama K, et al. Prevalence of hepatitis B virus infection among atomic bomb survivors. Radiat Res 2003;159:780–6. [DOI] [PubMed] [Google Scholar]
- [40].Sharp GB, Mizuno T, Fukuhara T, Tokuoka S. Lack of association between acute exposure to ionizing radiation and liver cirrhosis. Int J Radiat Biol 2006;82:231–40. [DOI] [PubMed] [Google Scholar]
- [41].Kusunoki Y, Kyoizumi S, Yamaoka M, Kasagi F, Kodama K, Seyama T. Decreased proportion of CD4 T cells in the blood of atomic bomb survivors with myocardial infarction. Radiat Res 1999;152:539–43 [published erratum appears in Radiat Res 2000 Jul;154(1):119]. [PubMed] [Google Scholar]
- [42].Kusunoki Y, Hayashi T. Long-lasting alterations of the immune system by ionizing radiation exposure: implications for disease development among atomic bomb survivors. Int J Radiat Biol 2008;84:1–14. [DOI] [PubMed] [Google Scholar]
- [43].Kusunoki Y, Hayashi T, Kyoizumi S. Immunity polarization in the atomic bomb survivors: from the view point of the Th1/Th2 paradigm. RERF Update 1998;9:10–1. [Google Scholar]
- [44].Kusunoki Y, Hayashi T, Morishita Y, Yamaoka M, Maki M, Bean MA, et al. T-cell responses to mitogens in atomic bomb survivors: a decreased capacity to produce interleukin 2 characterizes the T cells of heavily irradiated individuals. Radiat Res 2001;155:81–8. [DOI] [PubMed] [Google Scholar]
- [45].Kusunoki Y, Yamaoka M, Kasagi F, Hayashi T, Koyama K, Kodama K, et al. T cells of atomic bomb survivors respond poorly to stimulation by Staphylococcus aureus toxins in vitro: does this stem from their peripheral lymphocyte populations having a diminished naive CD4 T-cell content? Radiat Res 2002;158:715–24. [DOI] [PubMed] [Google Scholar]
- [46].Yamaoka M, Kusunoki Y, Kasagi F, Hayashi T, Nakachi K, Kyoizumi S. Decreases in percentages of naive CD4 and CD8 T cells and increases in percentages of memory CD8 T-cell subsets in the peripheral blood lymphocyte populations of A-bomb survivors. Radiat Res 2004;161:290–8. [DOI] [PubMed] [Google Scholar]
- [47].Shimizu Y, Mabuchi K, Preston DL, Shigematsu I. Mortality study of atomic-bomb survivors: implications for assessment of radiation accidents. World Health Stat Q Rapport trimestriel de statistiques sanitaires mondiales 1996;49:35–9. [PubMed] [Google Scholar]
- [48].Ozasa K, Shimizu Y, Sakata R, Sugiyama H, Grant EJ, Soda M, et al. Risk of cancer and non-cancer diseases in the atomic bomb survivors. Radiat Prot Dosim 2011;146:272–5. [DOI] [PubMed] [Google Scholar]
- [49].Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, et al. Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res 2012;177:229–43. [DOI] [PubMed] [Google Scholar]
- [50].Sawada H, Kodama K, Shimizu Y, Kato H. Adult health study report 6. Results of six examination cycles, 1968–1980, Hiroshima and Nagasaki. RERF TR 3–861986. [Google Scholar]
- [51].Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990;265:621–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fujiwara S, Carter RL, Akiyama M, Akahoshi M, Kodama K, Shimaoka K, et al. Autoantibodies and immunoglobulins among atomic bomb survivors. Radiat Res 1994;137:89–95. [PubMed] [Google Scholar]
- [53].Hayashi T, Morishita Y, Kubo Y, Kusunoki Y, Hayashi I, Kasagi F, et al. Long-term effects of radiation dose on inflammatory markers in atomic bomb survivors. Am J Med 2005;118:83–6. [DOI] [PubMed] [Google Scholar]
- [54].Hayashi T, Kusunoki Y, Hakoda M, Morishita Y, Kubo Y, Maki M, et al. Radiation dose-dependent increases in inflammatory response markers in A-bomb survivors. Int J Radiat Biol 2003;79:129–36. [PubMed] [Google Scholar]
- [55].Sok D, Briney B, Jardine JG, Kulp DW, Menis S, Pauthner M, et al. Priming HIV-1 broadly neutralizing antibody precursors in human Ig loci transgenic mice. Science 2016;353:1557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jordan BR. The Hiroshima/Nagasaki survivor studies: discrepancies between results and general perception. Genetics 2016;203:1505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.