Abstract
INTRODUCTION:
Supervised exercise interventions are expensive and time intensive. However, there are financial costs to consider in addition to the intervention itself, namely: advertising and recruitment, outcome assessments, and other trial-related costs.
OBJECTIVES:
In this analysis, we examine the financial costs associated with the administration of Investigating Gains in Neurocognition in an Intervention Trial of Exercise (IGNITE) to quantify the costs associated with large exercise intervention trials and to provide future investigators with financial estimates if they wish to pursue studies of a similar design.
METHODS:
Cost per randomized participant were calculated in four areas: (1) advertising and recruitment, (2) outcome assessments, (3) delivery of the intervention, and (4) other trial-related expenses. Overall trial costs associated with data analysis, faculty salaries, and indirect costs were estimated as well.
RESULTS:
The total cost per randomized participant was estimated to be $16,494. Outcome assessments accounted for the highest proportion of per-participant (75%) and total trial (38%) costs. Neuroimaging assessments (MRI & PET) cost $8,247 per randomized participant, accounting for two-thirds (67%) of outcome assessment costs and half (50%) of per-participant costs.
CONCLUSION:
Large clinical trials of exercise are expensive (~$21 million), particularly when administering several visits to assess study aims. Outcome assessments, specifically those involving neuroimaging, accounted for a significant proportion of total costs in this analysis. Future investigators must budget accordingly if they wish to conduct a comprehensive, multi-site exercise intervention trial that examines numerous physiological and psychological outcomes.
Keywords: Randomized clinical trial, Aging, Cognition, Physical activity, Neuroimaging, Aerobic exercise
Introduction
The administration of clinical trials often requires significant financial costs. Specifically, Phase III pharmaceutical clinical trials have been estimated to have average costs ranging from $11.5 million to $52.9 million [1]. Exercise interventions are a viable non-pharmaceutical approach for improving many health outcomes [2–5]. However, the costs associated with Phase III clinical trials of exercise are not well-documented. There is a great utility in reporting such costs because estimates like this can be used for similar types of exercise trials, including early-stage trials in which funding and feasibility of the study may be more precarious or questionable. Further, it is critical that sufficient resources are devoted to behavioral interventions to ensure rigor and transparency with the aim of influencing public health outcomes. Investigating Gains in Neurocognition in an Intervention Trial of Exercise (IGNITE) is a 12-month, multi-site, randomized dose-response Phase III exercise trial examining whether moderate intensity aerobic exercise improves brain health in cognitively normal adults aged 65–80. IGNITE represents an ideal opportunity to quantify the typical costs of conducting a rigorous exercise intervention in a large and well-characterized sample, analogous to those used in pharmaceutical trials. A more detailed description of the aims and protocol of IGNITE has been outlined previously by Erickson et al [6].
The targeted sample size of IGNITE is 639 participants across three intervention sites: University of Pittsburgh (Pittsburgh, PA), University of Kansas Medical Center (Kansas City, KS), and Northeastern University (Boston, MA). Given the coordination and organization needed for a trial of this magnitude, there are significant financial costs associated with (1) advertising and recruitment, (2) outcome assessments, (3) delivery of the intervention, and (4) other trial-related expenses. Reporting on these costs is the focus of the present paper.
To our knowledge, few studies have examined the financial costs associated with administering a large, multi-site exercise intervention trial, such as IGNITE. However, these studies have primarily focused on costs and cost-effectiveness of the delivery of the exercise intervention [7,8]. Here, in addition to the cost of intervention delivery, we present a detailed summary of the financial costs associated with advertising and recruitment, outcome assessments, and other trial-related expenses for conducting an exercise intervention. We also estimate overall trial costs associated with data analysis, faculty salaries, and indirect costs.
Methods
Details of the IGNITE protocol have already been published [6]. Briefly, there are several outcome assessments that are administered at baseline, during, and at follow-up of the 12-month intervention: (1) a battery of neuropsychological assessments of cognition, (2) a maximal cardiorespiratory fitness test on a treadmill (VO2 max), (3) a battery of psychosocial questionnaires, (4) a diet history questionnaire, (5) objective physical activity (PA) monitoring, (6) a dual-energy x-ray absorptiometry (DXA), pulse wave velocity (PWV), and physical function (PF) assessment, (7) a fasting blood draw and hair sample, (8) a magnetic resonance imaging (MRI) brain scan, and (9) a positron emission tomography (PET) brain amyloid scan. After successful completion of the baseline outcome assessments, participants are randomized into one of three exercise intervention arms: 150 min/week of moderate intensity aerobic exercise, 225 min/week of moderate intensity aerobic exercise, or 150 min/week of light intensity stretching and toning.
During the intervention, the cognitive assessments, psychosocial questionnaires, and blood draw are completed at a midpoint assessment (6 months). Objective PA monitoring is performed every two months (months 2, 4, 6, 8, 10) for a total of five PA monitoring timepoints during the intervention. After the completion of the 12-month trial, all baseline assessments (1–9 above) are repeated in order to assess the study aims [6].
We organized the trial into four primary areas when calculating the total per-participant expenses: advertising and recruitment, outcome assessments (areas 1–9 described above), delivery of the intervention, and other trial-related expenses. Within each of these four areas, we calculated costs associated with administration, participant compensation, and data management and quality control. Administration costs include items such as staff time involved with administering each assessment, time spent scheduling, organizing paperwork, setting up equipment, as well as any specific fees associated with the assessment (e.g., costs of MRI and PET scans per participant). Participant compensation includes the reimbursement that IGNITE provides each participant for completing the associated study visit. Finally, data management and quality control accounts for staff time spent entering, verifying, analyzing, and managing the data associated with each study visit. We calculated the costs of each of the nine outcome assessments in a similar format.
When calculating these costs, we estimated each item based on the cost needed for one participant to successfully complete the screening-to-randomization process. It is important to note that this is not the same as the cost associated with one participant independently going through screening and each baseline assessment. For example, not all participants who undergo a telephone screening are randomized to the intervention because they may not meet eligibility criteria. This means that staff involved in telephone screening must speak with several potential participants for each participant that is ultimately randomized. Outcome assessments are also affected in this manner, specifically baseline assessments, because they occur earlier in the study timeline. Staff time and participant compensation are inherently greater in these early baseline assessments because participants may be deemed ineligible for randomization at a subsequent session. We accounted for these discrepancies by calculating assessment-specific randomization ratios: the number of participants who were randomized divided by the total number of participants who completed the assessment (Table 1). By taking the inverse of these randomization ratios we calculated an “Increased Cost Factor” which was multiplied by the costs associated with each assessment per participant. This is due to the number of participants needed to complete each assessment, in order to achieve randomization targets, varies by assessment. For instance, any costs associated with phone screening for one participant must be multiplied by 5.95 to account for the fact that only 0.168 (16.8%) of participants who are screened are ultimately randomized. In other words, about 6 people need to complete the phone screen in order to have 1 person successfully randomized.
Table 1.
Increased costs associated with baseline outcome assessments per randomized participant.
| Assessment | Participants Assessed | Participants Randomized | Randomization Ratio | Increased Cost Factor |
|---|---|---|---|---|
| Phone screening | 2938 | 494 | 0.168 | 5.95 |
| Cognitive* | 673 | 494 | 0.734 | 1.36 |
| Questionnaires | 646 | 494 | 0.765 | 1.31 |
| VO2 max | 563 | 494 | 0.877 | 1.14 |
| DHQ | 404 | 494 | 1.223 | NA |
| PA monitoring | 517 | 494 | 0.956 | 1.05 |
| DXA/PWV/PF | 545 | 494 | 0.906 | 1.10 |
| Blood/Hair | 542 | 494 | 0.911 | 1.10 |
| MRI scan | 530 | 494 | 0.932 | 1.07 |
| PET scan** | 349 | 362 | 1.037 | NA |
Both visits
PET scan from Pitt and KU sites only
VO2max: Maximal Cardiorespiratory Fitness Test; DHQ: Diet History Questionnaire; PA: Physical Activity; DXA: Dual-energy X-ray Absorptiometry; PWV: Pulse Wave Velocity; PF: Physical Function; MRI: Magnetic Resonance Imaging; PET: Positron Emission Tomography.
Any assessments during the intervention or at follow-up were not affected by these calculations as all participants at these timepoints would have already been randomized (i.e., they would exhibit a 1:1 randomization ratio). There were, however, participants who were randomized without completing the Diet History Questionnaire (DHQ) and PET scans, which resulted in what would appear to be higher than 100% randomization ratios for these two outcomes. Therefore, we used a 1:1 randomization ratio for these calculations as, ideally, all participants would complete these assessments prior to randomization. It is also important to note that the PET outcome data from Northeastern University are not included because, at time of writing, PET amyloid data were only being collected at Kansas and Pittsburgh sites. As costs and other incidental expenses vary by site (Pittsburgh, Kansas City, Boston), we used salary rates and costs from the University of Pittsburgh site as a proxy for costs across all three sites.
As intent-to-treat is a standard practice for many clinical trials, including IGNITE, all randomized participants, regardless of adherence and compliance to the intervention, were invited to return for follow-up assessments. It is also worth noting that when this analysis was conducted, IGNITE had randomized approximately 77% of the target sample size (494 out of 639 participants). Therefore, these assessment-specific randomization ratios may be different at the conclusion of the study.
We also calculated additional costs that were accrued by the study coordinating center at the University of Pittsburgh. These costs are primarily associated with items related to data collection, storage, and management (e.g., physical activity monitoring equipment, data server, software, etc.). Total supplementary costs (e.g., data analysis, faculty salaries, and indirect costs) were estimated as well. Both the coordinating center and supplementary costs were assessed separately from the per-participant costs because items such as final data analysis will occur after the conclusion of the trial. Likewise, equipment costs and investigator salaries were budgeted prior to receiving grant funding. Hence, enrollment and randomization ratios would not affect these estimates.
Given the ongoing concerns surrounding SARS-CoV-2, many clinical trials are experiencing and projecting additional expenses, and this is also the case for IGNITE. There have been additional costs related to the purchasing of personal protective equipment (PPE) and other sanitization supplies, which are not reported in this paper. We expect that these costs will continue to accumulate and given the rapidly shifting environment and public health safety guidelines, we cannot accurately estimate costs related to SARS-CoV-2 at this time.
Results
Per-participant Costs
The overall total cost for one randomized participant to successfully complete the entire IGNITE trial is estimated to be $16,494. The IGNITE study has a target sample of 639 participants; therefore, the total participant-related costs for conducting the trial would approximate $10.5 million (this figure does not include investigator effort and salaries, institutional indirect costs, or equipment to perform the intervention and outcome assessments). Below, the costs are broken down into the four aforementioned subsections: (1) advertising and recruitment, (2) outcome assessments, (3) delivery of the intervention, and (4) other trial-related expenses.
Per-participant Costs: Advertising & Recruitment
Advertising and recruitment costs were estimated from combining all outlets of study advertising (e.g., bus ads, mailed postcards, newspaper ads, magazine articles, etc.) and staff hours associated with conducting phone screens, scheduling assessments, acquiring necessary clearance documents, and organizing participant files. The cost related to advertising and recruitment per randomized participant is estimated to be $590. Note that these are purely administrative costs (Table 2) as participants are not compensated until attending outcome assessments and there are no data management and quality control necessary during this step because participants have not yet enrolled in the trial.
Table 2.
IGNITE Financial Costs for conducting the trial (excluding faculty salaries and analysis).
| Cost/Randomized Participant (USD) | ||||
|---|---|---|---|---|
| Item | Administration | Participant Compensation | Data Management & Quality Control | Total |
| Advertising & Recruitment | 590 | NA | NA | 590 |
| Outcome Assessments | 9,257 | 805 | 2,281 | 12,343 |
| Intervention | 3,059 | NA | 342 | 3,401 |
| Other | 161 | NA | NA | 161 |
| Total | 13,067 | 805 | 2,622 | 16,494 |
USD: United States Dollars
Per-participant Costs: Outcome Assessments
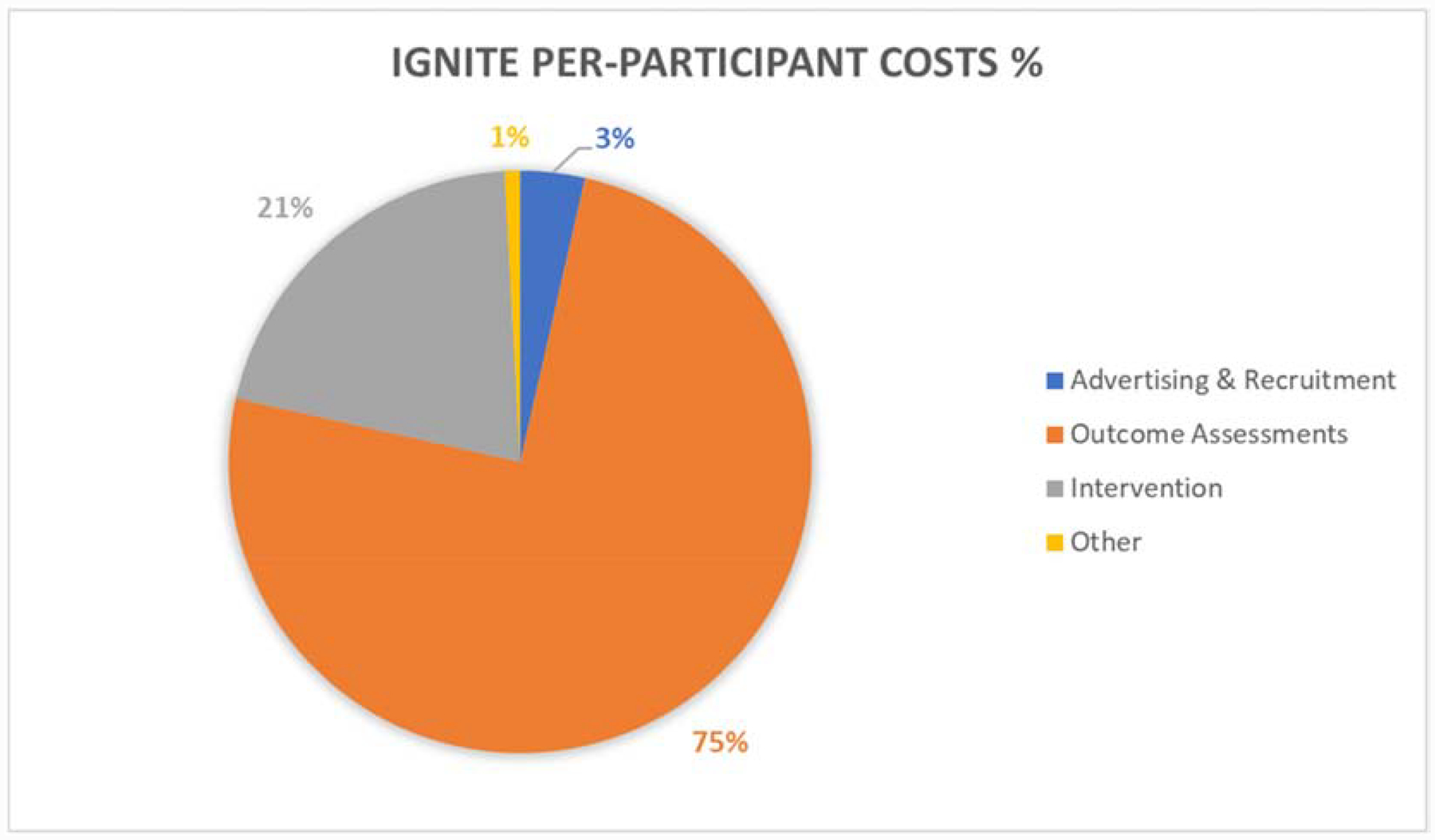
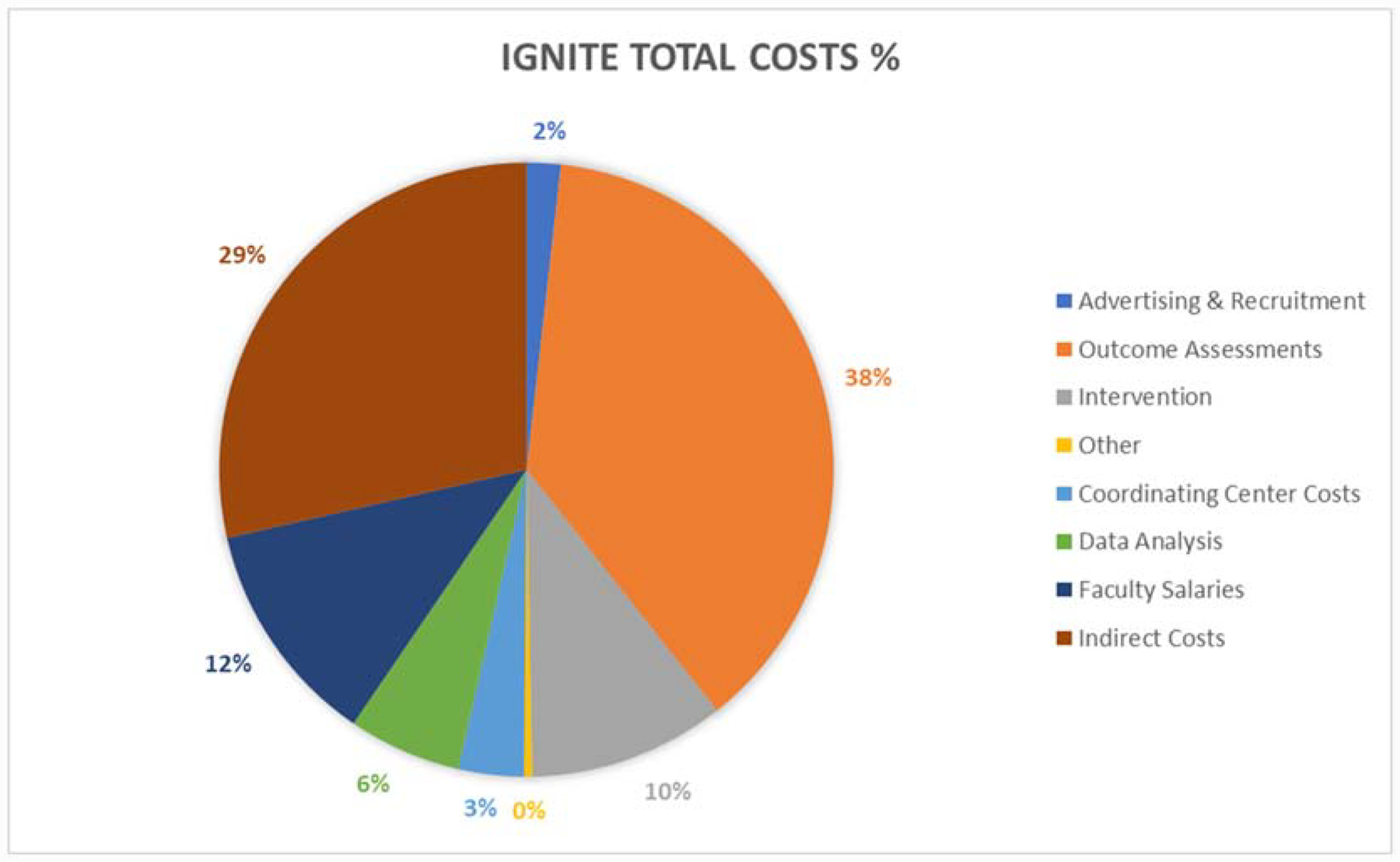
Outcome assessments are the most expensive aspect of IGNITE, accounting for 75% of per-participant costs (Figure 1) and 38% of total trial costs (Figure 2) with an estimated cost of $12,343 per randomized participant (Table 2). This is largely due to the fact that IGNITE has nine separate outcome assessment sessions that are conducted pre- and post-intervention, as well as several assessments conducted at the midpoint and throughout the 12-month intervention period.
Figure 1.
Percentages associated with cost per individual IGNITE participant (excluding faculty salaries and analysis).
Figure 2.
Percentages associated with IGNITE total study costs.
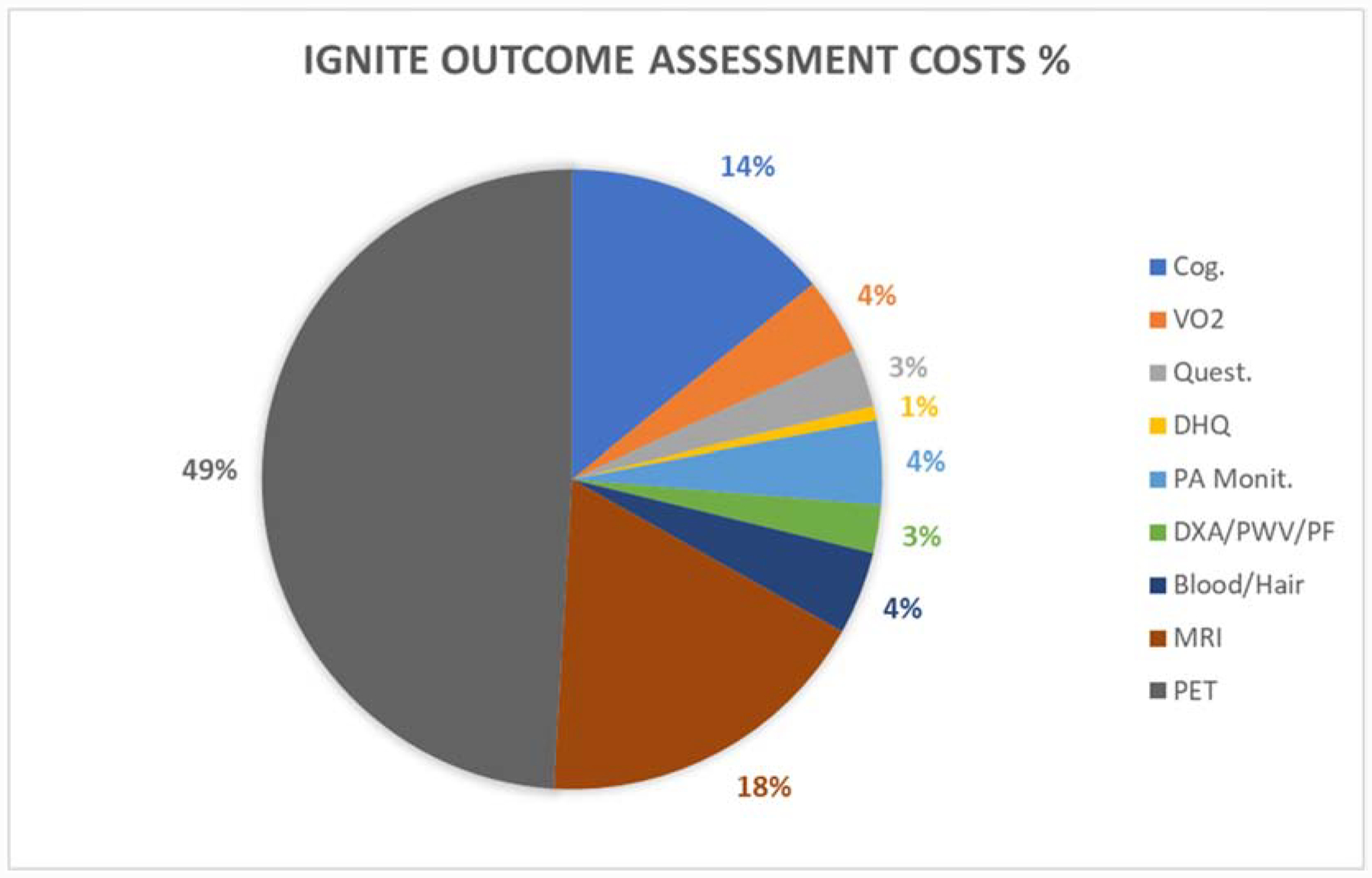
Table 3 shows the cost of each individual outcome assessment across the duration of the trial. The two most expensive assessments are related to neuroimaging – the MRI and PET brain scans – totaling to $2,192 and $6,055 per randomized participant, respectively. The overwhelming majority of these neuroimaging assessment costs is attributed to the administration of the scans (75% of total cost for MRI and 96% of total cost for PET), which includes staff time and the necessary equipment and technology. This pattern also continues when considering neuroimaging costs relative to the total cost of all outcome assessments; although only two of the nine outcome assessments involve neuroimaging (22%), these visits account for 67% (18% for MRI, 49% for PET) of total outcome assessment costs (Figure 3). Furthermore, the two neuroimaging assessments alone total to $8,247, which comprises half of the total per-participant costs (50%).
Table 3.
IGNITE Outcome Assessment Financial Costs.
| Cost/Randomized Participant (USD) | ||||
|---|---|---|---|---|
| Outcome Assessment | Administration | Participant Compensation | Data Management & Quality Control | Total |
| Cognitive* | 874 | 168 | 707 | 1,748 |
| VO2 max | 322 | 75 | 100 | 497 |
| Questionnaires | 57 | 26 | 293 | 376 |
| DHQ | NA | 20 | 70 | 90 |
| PA monitoring | 47 | 35 | 456 | 538 |
| DXA/PWV/PF | 169 | 42 | 99 | 310 |
| Blood/Hair | 360 | 31 | 145 | 536 |
| MRI scan | 1,643 | 207 | 342 | 2,192 |
| PET scan | 5,786 | 200 | 69 | 6,055 |
| Total | 9,257 | 805 | 2,281 | 12,343 |
Two separate visits
USD: United States Dollars; VO2 max: Maximal Cardiorespiratory Fitness Test; DXA: Dual-energy X-ray Absorptiometry; PWV: Pulse Wave Velocity; PF: Physical Function; MRI: Magnetic Resonance Imaging; PET: Positron Emission Tomography; PA: Physical Activity; DHQ: Diet History Questionnaire.
Figure 3.
Percentages associated with costs of conducting the IGNITE outcome assessments.
Per-participant Costs: Delivery of Intervention
The delivery of the 12-month exercise intervention for one participant is estimated to be $3,401 (Table 2). As participants are not compensated for their adherence to exercise sessions, there are not additional costs associated with monetary incentives. Most of the costs (90%) are associated with the administration of the intervention, i.e., salaries for exercise trainers who work with each participant approximately three days per week. It is important to note that one exercise trainer supervises several participants (~3–4) at the same time (i.e., the intervention is typically conducted in a small group format), which is factored into this calculation. Other intervention-related costs included retention items, such as water bottles, towels, and exercise bands. Importantly, costs related to exercise machines (e.g., treadmills, recumbent bikes, etc.) were not included in this analysis because each intervention location already had this equipment on site prior to the start of IGNITE.
Per-participant Costs: Other trial-related expenses
Other trial-related expenses that were not directly related to advertising and recruitment, outcome assessments, or delivery of the intervention were calculated and totaled to $161 per randomized participant. These costs include replacing or repairing lost or damaged equipment, administrative tasks such as Institutional Review Board (IRB) modifications and reporting adverse events, and participant retention events.
Overall Costs: Coordinating Center Equipment Costs
At the University of Pittsburgh coordinating center, equipment costs totaled to $72,376 (Table 4). Supplies and storage of samples from the blood draw assessment contributed the most to this area of expenses, totaling approximately $28,000. The server that was purchased to store study data was also a significant contributor at a cost of $21,000.
Table 4.
Coordinating Center Equipment Costs (USD)
| Desktop PCs | 4,545 |
| Software and Subscriptions | 4,946 |
| Heart rate monitors and PA monitoring equipment* | 13,885 |
| Blood supplies and storage | 28,000 |
| Data server | 21,000 |
| Total | 72,376 |
Includes ActiLife software needed for analysis of PA monitoring data.
USD: United States Dollars.
Overall Costs: Estimated Supplementary Costs
Additionally, we estimated the costs associated with analysis of the blood assays, genotyping, and neuroimaging (MRI and PET) data, and testing of the primary aims (all of which are not included in the per-participant estimates). We expect these analyses to cost approximately $350,000 for blood analytes (e.g., staff time, kits, supplies), $100,000 for genotyping (including staff and supplies), $500,000 to cover staff time for all planned neuroimaging analyses, and $300,000 to organize, clean, and analyze cognitive outcome data. These supplementary estimates are provided in Table 5. Notably, the machinery and service contracts needed for outcome assessments (e.g., DXA, MRI, and PET scanners) are not included in these calculations.
Table 5.
Estimated Supplementary Costs (USD)
| Data analysis | |
| Blood analytes | 350,000 |
| Genotyping | 100,000 |
| Neuroimaging | 500,000 |
| Testing primary aims (cognition) | 300,000 |
| Faculty salaries | 2,500,000 |
| Indirect costs | 6,000,000 |
| Total | 9,750,000 |
USD: United States Dollars.
Estimates for faculty salaries and indirect costs related to IGNITE are provided in Table 5. Nearly 20 faculty members across several universities are involved with the trial, including individuals with expertise in various disciplines: neuroscience, psychology, exercise science, engineering, Alzheimer’s disease and amyloid pathology, neuropsychology, biostatistics, genetics, and blood assays. Overall, the total estimate of funds for salaries and fringe benefits was $2.5 million over the course of the trial. Indirect costs were approximated to be $6.0 million. Finally, when combining all per-participant costs and overall study costs, it is estimated that the total cost of the entire trial would be $21,013,543 (Table 6).
Table 6.
IGNITE Total Costs (USD)
| Item | Per-participant costs | Total |
|---|---|---|
| Advertising & Recruitment | 590 | 377,285 |
| Outcome Assessments | 12,343 | 7,886,870 |
| Intervention | 3,401 | 2,173,053 |
| Other | 161 | 102,560 |
| Coordinating Center Costs | NA | 723,776 |
| Data Analysis | NA | 1,250,000 |
| Faculty Salaries | NA | 2,500,000 |
| Indirect Costs | NA | 6,000,000 |
| Total | 16,494 | 21,013,543 |
USD: United States Dollars.
Discussion
The total cost per randomized participant in IGNITE was estimated to be $16,494. Outcome assessment visits accounted for 75% of these costs, followed by the exercise intervention (21%), advertising and recruitment (3%), and other trial-related costs (1%). Both neuroimaging assessments (MRI & PET) cost $8,247 combined per randomized participant, accounting for about two-thirds (67%) of the outcome assessment costs and half (50%) of total per-participant costs.
In terms of total trial costs, we estimated IGNITE to cost $21,013,543. The majority of these totals can be attributed to outcome assessments (38%). Faculty salaries (12%), intervention administration (10%), and data analysis costs (6%) were also notable contributors. Costs associated with the coordinating center, advertising and recruitment, and other participant-related costs were small (just under 6%) relative to the rest of the estimated total (Figure 2).
Although it is evident that a clinical trial of IGNITE’s magnitude is expensive, there are ways in which costs have been mitigated. For instance, the University of Pittsburgh site has accomplished much of its recruitment through a free online website run by the university. On the other hand, advertising methods such as direct mailing have been relatively successful at attracting participants [9] but are costly – approximately 69% of advertisement costs were from direct mailing (data not shown). Outcome assessments are the predominant cost associated with IGNITE, with the neuroimaging assessments (MRI and PET scans) contributing substantially to these expenses. Unfortunately, due to the nature of the technology and equipment needed for these visits (e.g., scanner maintenance, PET radioactive tracer, etc.), costs could not be cut from this area of the trial. Other trials that wish to incorporate these types of advanced neuroimaging techniques must be prepared to cover these expenses and should ensure that the appropriate funding and facilities are available to support them. Moreover, these findings demonstrate that a behavioral modification intervention, such as IGNITE, can be as costly as pharmaceutical interventions [1]. Modifiable lifestyle behaviors have a notable impact on dementia risk [10]; therefore, behavioral interventions are important investments for government funding agencies because cognitive decline is a significant public health issue [11]. In addition, if other exercise trials (including those at earlier stages of development) do not receive adequate funding to ensure rigor and transparency, there could be significant loss of time and resources.
SARS-CoV-2 health concerns have added an additional layer of complication to intervention delivery in that some participants may be hesitant to engage in in-person exercise sessions, especially in a group setting. One possible strategy could be to shift exercise sessions to a virtual format (e.g., Skype, FaceTime, or Zoom) as other studies have shown that virtual delivery methods may prove to be a promising method for future large-scale exercise interventions [12]. However, this delivery method is not without its limitations as it is significantly more challenging to monitor compliance and safety remotely, particularly in vulnerable samples such as older adults [13]. Thus, potential financial cost-savings might come with other costs related to scientific rigor, transparency, and supervision for monitoring adherence of intensity, frequency, and duration of the exercise sessions. These factors should be weighed carefully in determining whether the design of an exercise intervention will use remote exercise sessions, rather than in-person, supervised exercise.
When considering the coordinating center equipment costs, it is important to note that some of these costs were experienced by the other intervention sites in Kansas City and Boston as well. For instance, items such as personal computers and heart rate monitors needed to be purchased at each intervention site. On the other hand, equipment such as the data server were only purchased by the Pittsburgh site because all electronic data forms and biological samples are housed at the study coordinating center. Other multi-site trials must keep these logistical items in mind when budgeting for initial equipment costs.
This analysis is informative for assessing the financial costs of a Phase III clinical trial of exercise, but it is not without limitations. An important limitation to consider is that all costs are estimates from a partially completed ongoing trial; thus, the enrollment and recruitment ratios may change before the trial is completed. Further, while we have information on staff salaries and hours, advertisement costs, participant compensation, and current recruitment and enrollment numbers, many of these calculations have been estimated per randomized participant. For example, it is impossible to know how many people have viewed a study advertisement while riding the bus; therefore, we cannot calculate exactly how many people viewed each ad for each person that was randomized. On the other hand, the cost estimates for items such as participant compensation associated with the outcome assessments are much more precise. We know exactly how many participants have completed each assessment and which of these participants were ultimately randomized. Another potential limitation is that this analysis used staff salaries from only the Pittsburgh site. Staff salaries clearly vary in Kansas City and Boston due to differences in cost of living associated with these geographic regions. However, according to a 2018 cost of living report [14], Pittsburgh had a higher cost of living than Kansas City and a lower cost of living than Boston; consequently, we used the Pittsburgh site’s salaries in this analysis to provide a rough “average” between all three intervention sites.
There are also several costs which were not accounted for in the per-participant costs that should be considered. As part of the IGNITE protocol, the blood for each participant will be processed to examine various analytes and for genotyping. However, this will not be performed until the conclusion of the trial, so the costs associated with these analyses were estimated a priori. There will also be additional costs of running analyses of neuroimaging data, statistical testing of the primary aims, reducing and cleaning data, and providing data access to other investigators. These costs were all estimated and described above, but a dataset of this size will also likely lead to secondary, tertiary, or exploratory analyses and the time, resources, and costs associated with these analyses cannot be estimated here. In addition, while some of these data are processed immediately after the outcome assessments, more specific procedures and additional staff time will be necessary to assess the study aims at the conclusion of the trial. A future analysis should explore these subsequent costs once all data is collected, analyzed, and prepared for publication.
In conclusion, this analysis shows that Phase III clinical trials of exercise are expensive given the large number of participants, staff, resources, and technology needed for these trials to run successfully. The majority of IGNITE’s costs stem from outcome assessments, particularly, those associated with neuroimaging. If an exercise intervention is not collecting these brain-related outcomes, these costs could be eliminated. Potentially, costs could also be reduced by altering the delivery of the intervention to a virtual format, but that may alter the nature of the intervention and is not without limitations related to study validity and participant safety. The costs for conducting clinical trials of exercise can be comparable to that of pharmaceutical trials and of equal importance when considering their impact on public health recommendations. We believe that the financial estimates reported in this paper will provide other investigators with a useful framework for assessing the costs associated with Phase III clinical trials of exercise and aid in developing cost-effective methods for conducting future trials of this nature.
Acknowledgements
We would like to thank the dedicated IGNITE participants and all research staff from the Pittsburgh, Kansas, and Northeastern sites who have contributed to the success of the trial thus far.
Conflicts/Funding
This study is funded by the National Institutes of Health (R01 AG053952) awarded to JB, CHH, AFK, EM, and KIE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Sertkaya A, Wong HH, Jessup A, & Beleche T (2016). Key cost drivers of pharmaceutical clinical trials in the United States. Clinical trials (London, England), 13(2), 117–126. doi: 10.1177/1740774515625964 [DOI] [PubMed] [Google Scholar]
- [2].Dipietro L, Campbell WW, Buchner DM, Erickson KI, Powell KE, Bloodgood B, Hughes T, Day KR, Piercy KL, Vaux-Bjerke A, Olson RD, & 2018 PHYSICAL ACTIVITY GUIDELINES ADVISORY COMMITTEE* (2019). Physical Activity, Injurious Falls, and Physical Function in Aging: An Umbrella Review. Medicine and science in sports and exercise, 51(6), 1303–1313. doi: 10.1249/MSS.0000000000001942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kandola A, Vancampfort D, Herring M, Rebar A, Hallgren M, Firth J, & Stubbs B (2018). Moving to Beat Anxiety: Epidemiology and Therapeutic Issues with Physical Activity for Anxiety. Current psychiatry reports, 20(8), 63. doi: 10.1007/s11920-018-0923-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, McMurdo M, & Mead GE (2013). Exercise for depression. The Cochrane database of systematic reviews, (9), CD004366. doi: 10.1002/14651858.CD004366.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stillman CM, Donahue PT, Williams MF, Callas M, Lwanga C, Brown C, Wollam ME, Jedrziewski MK, Kang C, & Erickson KI (2018). Weight-Loss Outcomes from a Pilot Study of African Dance in Older African Americans. Obesity (Silver Spring, Md.), 26(12), 1893–1897. doi: 10.1002/oby.22331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Erickson KI, Grove GA, Burns JM, Hillman CH, Kramer AF, McAuley E, Vidoni ED, Becker JT, Butters MA, Gray K, Huang H, Jakicic JM, Kamboh MI, Kang C, Klunk WE, Lee P, Marsland AL, Mettenburg J, Rogers RJ, Stillman CM, … Wollam ME (2019). Investigating Gains in Neurocognition in an Intervention Trial of Exercise (IGNITE): Protocol. Contemporary clinical trials, 85, 105832. doi: 10.1016/j.cct.2019.105832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Groessl EJ, Kaplan RM, Castro Sweet CM, Church T, Espeland MA, Gill TM, Glynn NW, King AC, Kritchevsky S, Manini T, McDermott MM, Reid KF, Rushing J, Pahor M, & LIFE Study Group (2016). Cost-effectiveness of the LIFE Physical Activity Intervention for Older Adults at Increased Risk for Mobility Disability. The journals of gerontology. Series A, Biological sciences and medical sciences, 71(5), 656–662. doi: 10.1093/gerona/glw001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Groessl EJ, Kaplan RM, Blair SN, Rejeski WJ, Katula JA, King AC, Fielding RA, Glynn NW, & Pahor M (2009). A cost analysis of a physical activity intervention for older adults. Journal of physical activity & health, 6(6), 767–774. doi: 10.1123/jpah.6.6.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vidoni ED, Szabo-Reed A, Kang C, Shaw AR, Perales-Puchalt J, Grove G, Hamill M, Henry D, Burns JM, Hillman C, Kramer AF, McAuley E, & Erickson KI (2020). The IGNITE trial: Participant recruitment lessons prior to SARS-CoV-2. Contemporary clinical trials communications, 20, 100666. doi: 10.1016/j.conctc.2020.100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, … Mukadam N (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England), 396(10248), 413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Subjective Cognitive Decline — A Public Health Issue. (2019, July 30). Centers for Disease Control and Prevention. https://www.cdc.gov/aging/aginginfo/subjective-cognitive-decline-brief.html
- [12].Argent R, Daly A, & Caulfield B (2018). Patient Involvement With Home-Based Exercise Programs: Can Connected Health Interventions Influence Adherence?. JMIR mHealth and uHealth, 6(3), e47. doi: 10.2196/mhealth.8518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stevens JA, Mahoney JE, & Ehrenreich H (2014). Circumstances and outcomes of falls among high risk community-dwelling older adults. Injury epidemiology, 1(1), 5. doi: 10.1186/2197-1714-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].CNN Money. (2018, January). Cost of living: How far will my salary go in another city? CNN. https://money.cnn.com/calculator/pf/cost-of-living/index.html [Google Scholar]


