Abstract
Early-life adversity (ELA), which includes maltreatment, neglect, or severe trauma in childhood, increases the life-long risk for negative health outcomes. Mitochondria play a key role in the stress response and may be an important mechanism by which stress is transduced into biological risk for disease. By responding to cues from stress-signaling pathways, mitochondria interact dynamically with physiological stress responses coordinated by the central nervous, endocrine, and immune systems. Preclinical evidence suggests that alterations in mitochondrial function and structure are linked to both early stress and systemic biological dysfunction. Early clinical studies support that increased mitochondrial DNA content and altered cellular energy demands may be present in individuals with a history of ELA. Further research should investigate mitochondria as a potential therapeutic target following ELA.
Keywords: Mitochondria, early-life adversity, stress, trauma, mechanisms
Introduction
Early experiences shape the development of emotional and physiological responses to stress and thereby exert long-term influences on health throughout the lifespan. Childhood is a developmental period in which individuals are especially vulnerable to the harmful consequences of stress as the brain is undergoing extensive experience-dependent growth and plasticity (Heim et al., 2019). Furthermore, because children rely on caregivers to meet their physical and emotional needs, the early environment exerts a profound influence on development and behavior (McLaughlin et al., 2017). This need for nurturing in childhood, combined with developmental changes to the nervous system, amplifies the neurobiological vulnerability to stressful environmental factors. Dynamic interactions between neural circuitry, genetics, and environmental stressors produce physiologic changes early in life, and result in downstream impact on health outcomes (Berens et al., 2017; Heim et al., 2019).
Early-life adversity (ELA) includes experiences of abuse, neglect, caregiver death, exposure to stressors associated with low socioeconomic status, and other traumatic events that threaten a child’s safety. Epidemiological studies provide robust evidence of associations between ELA and a variety of negative somatic and psychiatric health outcomes throughout the lifespan. A landmark study on adverse childhood experiences found a dose-dependent relationship between adverse childhood experiences and multiple health risk factors such as substance abuse disorders, depression, ischemic lung disease and cancer (Felitti et al., 1998). More recent meta-analyses continue to support that ELA increases risk for obesity, cardiovascular disease, other chronic diseases in adulthood (Danese and Tan, 2014; Gilbert et al., 2015; Li et al., 2019). Immune dysregulation, increased risk for developing cancer, and greater all-cause mortality risk have also been associated with adverse experiences in childhood (Baumeister et al., 2016; Boeck et al., 2016; Fagundes et al., 2013; Hughes et al., 2017; Rod et al., 2020). Finally, it has been well-documented that ELA is associated with many psychiatric disorders including major depressive disorder (MDD), post-traumatic stress disorder (PTSD), anxiety disorders, bipolar disorder, substance abuse, and behavioral problems (Gilbert et al., 2009; Syed and Nemeroff, 2017). Given the substantial evidence supporting the association between early adversity and later chronic health conditions, an understanding of the biological mechanisms transducing psychosocial stress into disease is needed to develop informed intervention and treatment strategies.
Mitochondria have gained attention in recent years for their potential role as targets of the stress response and mediators of stress-related dysregulation downstream (Picard et al., 2014). In response to acute stress exposure, mitochondria respond dynamically to cues from stress-signaling pathways enacted by the CNS, endocrine and immune systems, in order to adjust their activity to meet the current energetic demand (Manoli et al., 2007; Picard et al., 2018c). Evidence from rodent models suggests that mitochondria exhibit structural and functional changes with long-term exposure to stress, such as decreased respiratory enzymatic activity or mitochondrial membrane potential, resulting in an impaired capacity for energy production (Picard and McEwen, 2018a). Throughout this paper, we propose evidence to support the growing hypothesis that the mitochondrial response to stress is a key contributor to the broad range of pathological outcomes observed in individuals with ELA.
Foundations of Stress Physiology
CNS and Neuroendocrine Responses
The CNS and neuroendocrine systems are responsible for coordinating the physiological and behavioral responses to stress exposure. The cortico-limbic brain network, including regions such as the prefrontal cortex (PFC), the amygdala, and the hippocampus, perceives threat and coordinates responses to emotionally charged stimuli. The PFC regulates the amygdala, a region integral to fear and other emotional responses. Under acute stress, inhibition of the amygdala is released (Arnsten, 2015), which in turn activates the sympathetic-adrenal-medullary (SAM) axis and causes the release of catecholamines, such as adrenaline, from the adrenal gland. The hypothalamic-pituitary-adrenal (HPA) axis is also stimulated by the amygdala, driving the release of corticotropin-releasing factor (CRF) from the hypothalamus. CRF acts on the pituitary gland, precipitating the systemic release of adrenocorticotropic hormone (ACTH), which then stimulates the production of the glucocorticoid hormone, cortisol (Godoy et al., 2018).
Glucocorticoids serve a number of important functions in the stress response, including upregulating metabolic pathways, such as gluconeogenesis, lipolysis and proteolysis, to meet increased energy demands (Karatsoreos et al., 2010). Additionally, glucocorticoids facilitate the timely suppression of the physiological stress response and a return to basal conditions through negative feedback. Repetitive or excessive activation of the neuroendocrine system, especially during development, impairs negative feedback control and results in long-term dysregulation (Ceruso et al., 2020; van Bodegom et al., 2017). Both hypo- and hyper-reactive ACTH and cortisol levels have been observed in individuals with ELA at baseline and in response to acute stressors (Carpenter et al., 2007; Cicchetti et al., 2010; Cicchetti and Rogosch, 2001; Elzinga et al., 2008; Trickett et al., 2010; Tyrka et al., 2008; Young et al., 2020); the pattern of dysregulation may be determined by type and developmental timing of ELA (Carpenter et al., 2011; Miller et al., 2007; Tyrka et al., 2008), current age (Bunea et al., 2017), genetics (Trickett et al., 2010) and psychopathology (Berens et al., 2017). HPA axis dysregulation has clinical implications and has been linked with psychiatric disorders, including major depression and PTSD, and treatment response (Nikkheslat et al., 2020). Alterations in HPA axis functioning may provide the link between early stress, psychopathology, and metabolic outcomes such as obesity and metabolic syndrome (García-Eguren et al., 2019; Yu et al., 2014).
Impact of Stress on the Immune and Metabolic Systems
Glucocorticoids regulate immune and metabolic functioning in order to signal the possibility of injury or infection and increased systemic energy need when under threat. Individuals with chronic stress exposure in childhood demonstrate significant and graded elevations in inflammatory proteins and pro-inflammatory cytokines in adolescence and adulthood, an association not explained by mediators such as adult stressors or unhealthy behaviors (Baumeister et al., 2016; Danese and Lewis, 2017; do Prado et al., 2017). One study found that women with a history of childhood maltreatment exhibited greater pro-inflammatory cytokine release from peripheral blood mononuclear cells as compared to healthy controls in one small sample (Boeck et al., 2016). These pro-inflammatory responses were also associated with increased oxidative stress and altered mitochondrial function in immune cells, which will be discussed further below. Another study in women with childhood abuse-related post-traumatic stress disorder found that immune dysfunction following childhood maltreatment may be mediated by increased NF-κB pathway activity (Pace et al., 2012), which controls the expression of multiple pro-inflammatory cytokines (Liang et al., 2004). In rodent models, prolonged glucocorticoid activation has been shown to produce metabolic abnormalities which approximate metabolic syndrome (García-Eguren et al., 2019; Karatsoreos et al., 2010). Therefore, the association between ELA and cardiometabolic risk factors, such as obesity and unfavorable lipid profiles, may be driven by metabolic stress processes, which may be further exacerbated by additional exposures and health behaviors such as smoking, drug use, diet, and exercise (Duffy et al., 2018; Li et al., 2019). Discussed in more depth below, mitochondria may function as one avenue by which stress influences the immune and metabolic systems, as mitochondrial function is responsive to glucocorticoid signaling, inflammatory molecules, and availability of metabolic substrates (Boeck et al., 2016).
Stress throughout the lifespan also accelerates physiological aging of cells and the associated functional decline of organs within the body. Telomeres, the DNA-protein complexes that cap the ends of chromosomes, provide a buffer so that critical DNA segments are protected during replication (Epel and Prather, 2018). Telomeres are identified as an indicator of biological age, as telomeres shorten with aging and cellular stress (Han et al., 2019). Telomere shortening beyond a critical threshold leads to arrest of the cell cycle, cellular senescence, DNA damage and cell death. Over the past decade, a growing body of evidence has demonstrated a robust inverse relationship between telomere length and early adversity (Hanssen et al., 2017; Li et al., 2017; Ridout et al., 2018). Telomere attrition is also associated with chronic medical disorders, psychosocial stress and psychiatric disease; it may serve as one mechanism by which early-stress produces risk for multiple disease outcomes (Epel and Prather, 2018). While mitochondrial DNA does not contain telomeres, DNA damage and telomere shortening on nuclear chromosomes have been mechanistically linked with a decline in mitochondrial function, as both processes may be co-regulated within cells (Billard and Poncet, 2019; Sahin and DePinho, 2012). In human studies, telomere attrition and increased mitochondrial DNA copy number have been linked in the context of early stress (Cai et al., 2015; Tyrka et al., 2016).
Intersectional Role of Mitochondria
A great deal of research on stress physiology has focused on the effect of primary neuroendocrine mediators such as glucocorticoids, and secondary effects on metabolism, immune function, and telomere shortening. However, recent evidence suggests that mitochondria occupy a central and overlapping role in these processes. Mitochondria respond to signals from established stress-pathways, and alterations in mitochondrial function further modify the activity of these physiological systems (Figure 1). The intersectional role of mitochondria in multiple stress-related systems has important clinical implications. Individuals with psychiatric illness often have medical comorbidities, suggesting that stress may produce psychiatric and somatic disease via parallel mechanisms in different organ systems (Epel and Prather, 2018). As a highly energy-dependent organ, the brain is densely populated with mitochondria (Magistretti and Allaman, 2015). Genetic and functional abnormalities in mitochondria have been linked with anxiety behavior in rodents, as well as numerous psychiatric, neurodegenerative and somatic medical disorders in humans (Filiou and Sandi, 2019; Pei and Wallace, 2018; Picard et al., 2016). The mechanisms by which extreme or prolonged exposure to adversity and stress mediators alter mitochondrial DNA and function is described in depth in the following section.
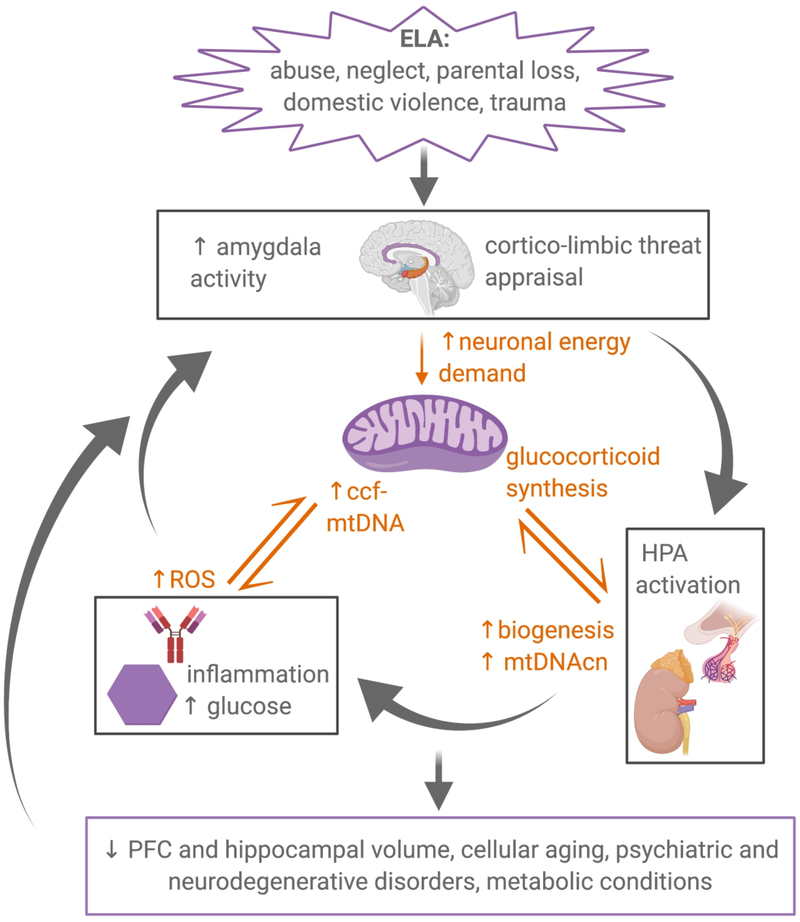
Figure 1.
Mitochondria occupy an intersecting role in stress responses. Early-life adversity (ELA) chronically activates physiological stress responses, coordinated by the cortico-limbic brain network, the HPA axis, the immune system and the metabolic system. Mitochondrial function and structure are altered by stress responses, which in turn exacerbates initial alterations in stress-related physiological systems. This feedforward cycle of dysfunction produces pathology across a variety of biological outcomes. ELA, early-life adversity; HPA, hypothalamic-pituitary-adrenal; mtDNA, mitochondrial DNA; mtDNAcn, mtDNA copy number; ccf-mtDNA, circulating cell-free mtDNA; PFC, prefrontal cortex.
Mitochondria and Mitochondrial Function
Mitochondria play a critical role in a broad range of functions within the cell and throughout the body, from energetics, epigenetics and inflammation, to hormone synthesis and metabolism (Juster et al., 2016; Picard et al., 2018c; Shaughnessy et al., 2014). Originating 1.5 billion years ago through the engulfment of a bacterial cell, mitochondria are the only non-nuclear organelle to contain their own genome-- the maternally-inherited, circular, double-stranded mitochondrial DNA (mtDNA; Hoffmann and Spengler, 2018; Sagan, 1967). The amount of mitochondrial DNA per cell, or the mitochondrial DNA copy number (mtDNAcn), varies between tissue types and individuals, and is subject to complex regulation (Lee and Wei, 2005; Moraes, 2001)). Although mtDNAcn is often used as a proxy measure of mitochondrial biogenesis (Picard et al., 2014; Wu et al., 1999), mtDNAcn is also influenced by the number of mtDNA copies per mitochondrion and the rate of mitophagy, or normal control process removing damaged mitochondria (Clay Montier et al., 2009; Frank et al., 2012). Importantly, mitochondria sense, integrate and signal information about cellular environments by responding to metabolites and neuroendocrine factors, driving inflammatory processes, and regulating the cell cycle (Picard and McEwen, 2018b).
Mitochondrial dysfunction is associated with many neuropsychiatric disorders as well as normal aging (Sun et al., 2016; Wang et al., 2019). Individuals with genetically inherited defects in mtDNA or mitochondrial proteins present with a variety of clinical symptoms indicative of CNS deficits, including neurological manifestations, atrophy of brain matter, or affective changes (Gorman et al., 2016; Kasahara and Kato, 2018). Additionally, mitochondrial abnormalities have been documented with psychiatric, neurodevelopmental, and neurodegenerative diseases such as schizophrenia (Bertolin et al., 2011; Gonçalves et al., 2018; Ichikawa et al., 2012; Martorell et al., 2006; Suárez-Méndez et al., 2020), mood disorders (Kato et al., 2018; Lindqvist et al., 2018; Scifo et al., 2018), Autism Spectrum Disorder (Chauhan et al., 2011; Chen et al., 2015; Singh et al., 2020), Alzheimer’s disease (AD; Huang et al., 2020; Lin et al., 2002; Smith et al., 1996; Trushina et al., 2012), and Parkinson’s disease (Gautier et al., 2016; Narendra et al., 2008), even in patients without inherited mitochondrial mutations. Work in animal models supports a causal association between mitochondrial dysfunction and changes representative of psychopathology, such as anxiety or depressive-like behaviors (Hollis et al., 2015; Kasahara et al., 2016), or neurodegeneration (Di Maio et al., 2016; Pickrell et al., 2015). For example, a pivotal recent study utilizing Caenorhabditis elegans and mouse models of AD found that stimulation of mitophagy could reverse memory impairment (Fang et al., 2019). New research showing that lifelong psychosocial factors influence cognitive-reserve and risk for dementia highlights the importance of further exploring the impact of early-life adversity on mitochondrial function and downstream disease-burden (H.-X. Wang et al., 2017).
Mitochondria in the Stress Response
Accumulating evidence supports a key role for mitochondria in stress-associated physiological systems. Energy demands increase with stress because the “fight or flight” behavioral response and allostatic biological systems are fueled by energy in the form of ATP. As a result, mitochondria respond to glucocorticoid signaling by increasing production cellular energy, generating signals to promote cellular adaptation, and undergoing biogenesis (Manoli et al., 2007; Picard et al., 2014). Furthermore, steroid hormones, including glucocorticoids, are both synthesized and metabolized by mitochondria (Bose et al., 2002; Lin et al., 1995). Indeed, preclinical evidence supports that mitochondrial dysfunction, induced by mutations in key mitochondrial genes, produces alterations in glucocorticoid production and the HPA response to stress (Meimaridou et al., 2012; Picard et al., 2015).
Excessive stress is associated with changes to mitochondrial structure and function. In conditions of severe or prolonged exposure to stress, circulating levels of glucocorticoids and associated metabolic substrates are chronically elevated. Research in cell culture and animal models shows that this promotes mitochondrial fragmentation, or the breakdown of networks of communicating mitochondria within cells, which is associated with mtDNA damage, oxidative stress, and increased risk of cell death (Hoffmann and Spengler, 2018; Lee et al., 2004; Picard et al., 2014; Shutt and McBride, 2013). Similarly, preclinical evidence demonstrates that excessive or chronic elevations in glucocorticoids decrease mitochondrial function by dampening the mitochondrial membrane potential, decreasing respiratory chain enzymatic activity, lowering the threshold for apoptosis, and increasing the production of ROS (Du et al., 2009; Manoli et al., 2007; Picard et al., 2014; Yu et al., 2014). Overall, severe stress and associated alterations in glucocorticoid signaling promote mitochondrial dysfunction through altering mitochondrial dynamics.
Mitochondrial and immune function are closely intertwined, a relationship that may further link the physiological response to early adversity with systemic biological changes and health outcomes. Due to the evolutionary origin of mitochondria from bacteria, circulating cell-free mtDNA (ccf-mtDNA) and other mitochondrial components act as damage associated molecular patterns (DAMPs) when outside the cell, and activate the innate immune system via Toll-Like Receptors (West et al., 2011; Zhang et al., 2010). Inflammation is also associated with increased production of mitochondrial ROS, which has a detrimental impact on genetic stability and cellular functions (Boeck et al., 2016). Additionally, mitochondrial dysfunction promotes inflammasome activation, or the maturation of pro-inflammatory cytokines in response to innate immune danger signals (Zhou et al., 2011). In this feedforward cycle of dysfunction, inflammation promotes ROS generation and mitochondrial dysfunction, which in turn stimulates further inflammation by engaging a pro-inflammatory cytokine response. One recent study in human leukocytes has identified an association between mitochondrial respiratory chain activity with lipopolysaccharide-induced IL-6 production, finding that mitochondrial respiratory capacity may contribute to the inter-individual variability in pro-inflammatory responses (Karan et al., 2020). Neuroendocrine, metabolic and immune responses to stress are highly integrated, with mitochondria playing a critical role as shared actor in these converging pathways.
Animal Studies on Early Stress and Mitochondrial Alterations
Several studies using rodent models have confirmed that both chronic and acute environmental stressors, such as physical restraint or unpredictable habitat changes, impair mitochondrial function. Chronic unpredictable stress procedures in adult rodents have been shown to decrease oxygen consumption, respiratory chain enzymatic activity, and membrane potential, as well increase ROS production in both brain and peripheral tissues (Cai et al., 2015; Liu and Zhou, 2012; Picard and McEwen, 2018a). A study of 8-week-old male mice subjected to 6 weeks of mild unpredictable stressors exhibited depressive-like behaviors, which were associated with decreased rates of mitochondrial respiration in three brain regions as well as swollen, damaged mitochondrial structure (Gong et al., 2011). This study and others provide strong evidence that chronic stress produces mitochondrial abnormalities, with phenotypic consequences (Picard and McEwen 2018).
Most work on mitochondrial changes in response to stress has been done in adult animals, and the relationship between environmental stress exposure during the post-natal period and long-term mitochondrial function has been less extensively studied. While early stress likely activates the same adaptive processes as stressors occurring later in life, the particular alterations may be unique. For example, the severity and duration of dysregulation from early stress may be more severe. The plasticity of the brain in early-life, as well as the potential for intervention before adulthood pathology presents, necessitates research focused specifically on the effects of early-stress on mitochondrial function.
Post-natal maternal separation (MS), during which pups are separated from their mothers for several hours per day beginning shortly after birth, is a frequently tested model of ELA. Several studies have observed mitochondrial dysfunction in the rodent CNS following MS. For example, adult rodents who underwent MS had increased ROS, decreased ATP production, decreased and antioxidant levels in the hippocampus as compared to control animals (AminiKhoei et al., 2017; Fattahi Masrour et al., 2018). In these two studies, treatment with oxytocin, a neuromodulator with potential anti-inflammatory and anti-depressant properties (Matsushita et al., 2019; Matsuura et al., 2016), the antidepressant fluoxetine, or exercise in adulthood improved indicators of mitochondrial dysfunction after MS (Amini-Khoei et al., 2017; Fattahi Masrour et al., 2018). Similar alternations in mitochondrial function have been observed following MS in peripheral tissues, such as cardiac tissue and bowel fibroblasts, which were also mitigated with adult exercise (Khorjahani et al., 2020; Sahafi et al., 2018). Together, these studies provide early evidence that post-natal stress promotes later mitochondrial dysfunction, and that interventions which reduce inflammation and enhance antioxidant properties may attenuate the negative effects of early stress on mitochondrial function in rodents.
Importantly, most studies on the effects of stress on mitochondria occur in male animals, and information on sex differences in stress-responses and mitochondrial function is lacking (Picard and McEwen, 2018a; Ventura-Clapier et al., 2017). Both mitochondria and neuroendocrine pathways are sensitive to sex hormones, suggesting that ELA may have differential effects on mitochondrial function based on sex (Bekhbat and Neigh, 2018; Torrens-Mas et al., 2020). One recent study investigated the sex-specific effects of MS in rodent CNS (González-Pardo et al., 2020). While both sexes exhibited a decrease in mitochondrial oxidative capacity in cortico-limbic regions of the brain including the PFC, nucleus accumbens, and hippocampus, females exhibited a greater reduction in metabolic capacity after MS than males (González-Pardo et al., 2020). These findings highlight the importance of using both sexes for future animal research in the field, particularly when designing studies to test interventions that may mitigate the harmful effects of MS on mitochondrial function.
Although not a direct measure of mitochondrial function, mtDNAcn is a mitochondrial measure that can be readily studied in both animal models and humans. Chronic unpredictable stress (CUS) in adult mice increased mtDNAcn in blood and saliva (Cai et al., 2015). Increased mtDNAcn was associated with decreased oxidative phosphorylation capacity in stressed mice, suggesting that the elevation in mtDNAcn is indicative of either inefficient mitochondrial function or increased reliance on glycolysis for energy production (Cai et al., 2015). Furthermore, 8 weeks of glucocorticoid administration to adult female mice replicated the increase in mtDNAcn observed with CUS, suggesting that glucocorticoid signaling mediates stress-induced changes in mtDNAcn. After four weeks in a favorable environment, the mtDNAcn of stressed mice returned to normal levels. However, because this study utilized adult mice, it is unclear if changes to mtDNAcn induced by early stressors are also reversible. In contrast, a study utilizing a MS protocol found that early stress decreased mtDNAcn in muscle tissue of stressed adult mice (Ghosh et al., 2016). These discrepancies highlight that both timing of stressors and tissue location may influence stress-related changes to mtDNAcn, and additional research is needed to further elucidate these relationships.
Clinical Research on Early-life Adversity and Mitochondria
In the last decade, interest in mitochondrial dysfunction as a pathogenic mechanism in neuropsychiatric disease, as well as in medicine more broadly, has steadily increased (Picard et al., 2016). As such, there have been several clinical studies on the impact of ELA on mitochondrial function or mtDNAcn. One small study of 30 post-partum women found that a history of maltreatment was associated with an increase in mitochondrial respiratory activity in cryogenically-preserved peripheral blood mononuclear cells (PMBCs), which were both associated with increased spontaneous release of inflammatory cytokines (Boeck et al., 2016). Maltreatment was also associated with increased ROS production and decreased antioxidant levels in this sample. These findings suggest that PBMCs from individuals with a history of maltreatment have greater energy requirements than cells from controls and provide a possible link between abnormal mitochondrial function and increased inflammation in those with early stress. The sample size was small, and changes to mitochondrial function are likely tissue specific (Cai et al., 2015) and may be altered with cryogenic preservation, so further research that addresses these issues is needed.
Recently, this group also investigated whether observed changes in mitochondrial bioenergetics are transmitted intergenerationally from mothers to neonates (Gumpp et al., 2020). Gumpp et al. found that mitochondrial respiratory activity in cryopreserved PMBCs was increased in postpartum women with a history of childhood maltreatment, as compared to controls, which supports previous findings (Boeck et al., 2016; Gumpp et al., 2020). Additionally, the maltreated group had increased mitochondrial density in PMBCs, as measured by citrate synthase activity, suggesting that increased respiratory activity may be due to a higher number of mitochondria per cell. However, maternal childhood maltreatment was not significantly associated with neonatal mitochondrial bioenergetics measured from umbilical cord blood mononuclear cells (Gumpp et al., 2020).
Increased mtDNAcn has been reported in both adults and children with ELA. Our group examined mtDNAcn from whole blood leukocytes of 290 healthy unmedicated adults with and without a history of ELA, including childhood maltreatment and parental loss, as well as with and without a lifetime history of depressive, anxiety, and substance-use disorders. We observed that both ELA and lifetime psychopathology were independently associated with elevations of leukocyte mtDNAcn (Tyrka et al., 2016). The effect of ELA on mtDNAcn was not accounted for by measures of subclinical psychiatric symptoms, recent stressors, or resilience. Adults with both ELA and lifetime psychopathology had the highest mtDNAcn on average, though the burden of early adversity was also greatest in this group. We also found that the association between ELA and mtDNAcn was statistically accounted for by decreased promoter methylation of the glucocorticoid receptor gene NR3C1 (Ridout et al., 2020). This relationship between glucocorticoid signaling and ELA-associated mtDNAcn alterations is consistent with the Cai et al. findings in rodents that suggest a mechanistic role of glucocorticoids in the effects of early stress on mtDNAcn (Cai et al., 2015).
In the largest study to date, Cai and colleagues studied 11,670 Chinese women with and without recurrent MDD, and found that higher salivary mtDNAcn was associated with a history of childhood sexual abuse (Cai et al., 2015). However, MDD moderated the relationship between ELA and mtDNAcn in this sample, as there was no association between sexual abuse and mtDNAcn in women without MDD. Conversely, MDD had associations with higher mtDNAcn that were independent of the early stress measures. Our group recently found that ELA-induced elevations in salivary mtDNAcn may begin in childhood (Ridout et al., 2019). In a sample of n=256 low-income preschool aged children, those with a confirmed case of maltreatment within the previous six months had significantly higher salivary mtDNAcn than non-maltreated children.
Together, these studies provide early evidence that forms of ELA such as maltreatment are associated with increased mtDNAcn, as measured from blood or saliva, presenting as early as in childhood. There is evidence that increases in mtDNAcn, as have been observed with ELA, can be a compensatory response to a decrease in mitochondrial function (Picard et al., 2014), that can serve to increase mitochondrial biogenesis and energy production (Clay Montier et al., 2009). However, because regulation of mtDNAcn is complex, it is also possible that alterations in mtDNAcn occur independently to changes in mitochondrial function.
Elevations in mtDNAcn have also been associated with lifetime mood and substance use disorders (Cai et al., 2015; Tsujii et al., 2019; Tyrka et al., 2016; X. Wang et al., 2017), Parkinson’s disease (Bury et al., 2017), and Autism Spectrum Disorder (Chen et al., 2015; Yoo et al., 2017). Nonetheless, there are important limitations with the use of mtDNAcn as a biomarker of mitochondrial function. First, while consistent elevations in mtDNAcn have been noted following ELA, the direction of change observed in mtDNAcn with psychiatric disorders, including MDD, PTSD, and bipolar disorder, has been less clear (Daniels et al., 2020). Variability in these findings could be due to differences in the nature of the samples (i.e., comorbidities, medications, history of trauma), or differing cell or tissue sources of mtDNAcn (Daniels et al., 2020). Furthermore, clinical studies measure mtDNAcn from tissue with mixed cell populations, such as blood and saliva, which may confound the measurement (Han et al., 2019; Picard et al., 2019). For example, one study has shown that mtDNAcn measured from whole blood overestimates the mtDNA contribution of leukocytes due to the presence of platelets, which possess mtDNA but not nDNA (Hurtado-Roca et al., 2016). Finally, clinical measurement of mtDNAcn does not distinguish intracellular mtDNA from extracellular ccf-mtDNA, or the cell type origin. While mtDNAcn can be reliably measured as it does not require the preservation of live cells, mtDNAcn is not a direct measure of mitochondrial function and thus findings should be interpreted with caution.
The Mitochondrial Health Index (MHI), developed by Picard and colleagues (Picard et al., 2018d), aims to overcome these challenges through integration of measures of both mitochondrial content (such as mtDNAcn) and mitochondrial enzymatic activity (reflecting mitochondrial function). In a study of 91 mothers, approximately half of whom were experiencing chronic stress in the form of caring for a child with autism, both stress exposure and mood were related to MHI, with lower MHI in individuals with more perceived stress, and higher MHI associated with positive mood. Interestingly, there was no significant relationship between the individual components of the MHI and caregiving status, indicating the MHI may be a valuable composite measure of mitochondrial functional capacity. These findings in a sample of adults with chronic stress warrant investigation of MHI as it relates to ELA.
Future research on ELA, mitochondrial dysfunction, inflammation, and negative health outcomes should include examinations of ccf-mtDNA concentrations in populations with ELA. Ccf-mtDNA is a mitokine, or a systemic signaling molecule originating from mitochondria, recently identified as a peripheral indicator of mitochondrial health and inflammation. It is present in low levels in healthy individuals, abundant in inflammatory disease, and significantly increased in critically ill hospitalized patients (Nakahira et al., 2013). Several recent small studies suggest a role for ccf-mtDNA in stress-related psychological states. Baseline concentrations of ccf-mtDNA were shown to be elevated in non-medicated suicide attempters and patients with MDD (Lindqvist et al., 2018, 2016). Furthermore, ccf-mtDNA was shown to increase in response to acute psychosocial stress in two samples of healthy adults (Hummel et al., 2018; Trumpff et al., 2019). Research on concentrations of ccf-mtDNA in adults who experienced ELA will further elucidate the role of this signaling molecule in psychiatric disease and stress responses.
Synthesis of Findings and Clinical Implications
Synthesizing findings from animal models and clinical research, we wish to highlight three important themes. First, ELA has been shown to impair mitochondrial function and increase salivary and leukocyte mtDNAcn in recent preclinical and clinical research (Amini-Khoei et al., 2017; Boeck et al., 2016; Cai et al., 2015; Fattahi Masrour et al., 2018; Ghosh et al., 2016; González-Pardo et al., 2020; Khorjahani et al., 2020; Ridout et al., 2019; Sahafi et al., 2018; Tyrka et al., 2016). While the existing findings in regards to ELA have been quite consistent, current research, especially in humans and even more so in children, is limited and thus conclusions should be interpreted cautiously. The second finding spanning research in both animals and humans is that glucocorticoid signaling may mediate the increase in mtDNAcn, as measured in human leukocytes and saliva, following ELA. This finding is in accordance with previous evidence that mitochondria are responsive to neuroendocrine signaling. Thus, changes in mtDNAcn may be a novel marker indicating that established stress signals impact cellular energetics through mitochondrial changes. Finally, limited research with animal models suggests that ELA-induced mitochondrial dysfunction is potentially reversible with timely intervention. Further investigation, both preclinical and clinical, is needed to determine if mitochondrial dysfunction is an appropriate target for intervention following ELA.
We hypothesize that mitochondrial alterations, such as increased oxidative stress and decreased respiratory capacity, may accelerate biological aging and thereby explain the increased prevalence of disease in adults with a history of ELA. For example, mitochondrial oxidative stress can propagate a wide range of disease outcomes, which are also associated with ELA, from metabolic syndrome to neurodegeneration and cancer (Franco-Iborra et al., 2018; Rani et al., 2016; Yang et al., 2016). Furthermore, recent studies have implicated mitochondrial dysfunction in the pathogenesis of mood and trauma-related disorders (Filiou and Sandi, 2019; Pei and Wallace, 2018; Preston et al., 2018; Srivastava et al., 2018). The density of mitochondria in the brain, as well as the organelle’s importance in other physiological systems, may explain why early adversity exerts such a profound influence on risk for both psychiatric and somatic disease.
There are multiple limitations to current research that limit our understanding of the effect of ELA on mitochondria and their role in health outcomes. First, most animal studies on chronic stress utilize fully matured animals. As adverse experiences in childhood are consistently associated with worse long-term health outcomes as compared to stressors occurring later in life, the developmental period of exposure is an important target for stress research. Furthermore, very limited research has considered the sex effects of stress on mitochondrial function, and future experimentation should utilize both male and female animals. It is also important to note that while rodent paradigms allow precise control of experimental conditions, they serve as imperfect models for the early adversities experienced by humans, which encompass a diverse range of socially complex experiences. There have been few studies in humans, and conclusions are limited due to variability in study design, such as definitions of ELA, type of mitochondrial measure and tissue of origin, and presence of comorbidities in the sample. Though several studies have examined mtDNAcn in clinical populations, there is a need for further work directly measuring mitochondrial function, especially utilizing prospective and longitudinal study designs.
While current evidence is still very limited, the potential to reverse ELA-induced mitochondrial dysfunction is promising and worthy of further investigation. Early stress is an especially deserving target for research due to the potential to intervene therapeutically prior to the conclusion of brain development and emergence of clinical pathology. Further work in this field, especially with an emphasis on clinical translations, has the potential to minimize the life-long health burden in a large population of individuals with ELA.
Acknowledgements
Figure 1 was created with BioRender.com.
Funding: This work was supported by NIH grants R01HD086487 and R01MH101107 (ART). Dr. Daniels received support from R25 MH101076 (ART). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
All authors report nothing to disclose. All authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amini-Khoei H, Mohammadi-Asl A, Amiri S, Hosseini M-J, Momeny M, Hassanipour M, Rastegar M, Haj-Mirzaian A, Mirzaian AH-, Sanjarimoghaddam H, Mehr SE, Dehpour AR, 2017. Oxytocin mitigated the depressive-like behaviors of maternal separation stress through modulating mitochondrial function and neuroinflammation. Progress in Neuro-Psychopharmacology and Biological Psychiatry 76, 169–178. 10.1016/j.pnpbp.2017.02.022 [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, 2015. Stress weakens prefrontal networks: molecular insults to higher cognition. Nature Neuroscience 18, 1376–1385. 10.1038/nn.4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V, 2016. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry 21, 642–649. 10.1038/mp.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Neigh GN, 2018. Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain, Behavior, and Immunity 67, 1–12. 10.1016/j.bbi.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens AE, Jensen SKG, Nelson CA, 2017. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Medicine 15, 135. 10.1186/s12916-017-0895-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolin C, Magri C, Barlati S, Vettori A, Perini GI, Peruzzi P, Mostacciuolo ML, Vazza G, 2011. Analysis of complete mitochondrial genomes of patients with schizophrenia and bipolar disorder. J Hum Genet 56, 869–872. 10.1038/jhg.2011.111 [DOI] [PubMed] [Google Scholar]
- Billard P, Poncet DA, 2019. Replication Stress at Telomeric and Mitochondrial DNA: Common Origins and Consequences on Ageing. International Journal of Molecular Sciences 20, 4959. 10.3390/ijms20194959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeck C, Koenig AM, Schury K, Geiger ML, Karabatsiakis A, Wilker S, Waller C, Gündel H, Fegert JM, Calzia E, Kolassa I-T, 2016. Inflammation in adult women with a history of child maltreatment: The involvement of mitochondrial alterations and oxidative stress. Mitochondrion 30, 197–207. 10.1016/j.mito.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Bose HS, Lingappa VR, Miller WL, 2002. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature 417, 87–91. 10.1038/417087a [DOI] [PubMed] [Google Scholar]
- Bunea IM, Szentágotai-Tătar A, Miu AC, 2017. Early-life adversity and cortisol response to social stress: a meta-analysis. Translational Psychiatry 7, 1–8. 10.1038/s41398-017-0032-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury AG, Pyle A, Elson JL, Greaves L, Morris CM, Hudson G, Pienaar IS, 2017. Mitochondrial DNA changes in pedunculopontine cholinergic neurons in Parkinson disease. Annals of Neurology 82, 1016–1021. 10.1002/ana.25099 [DOI] [PubMed] [Google Scholar]
- Cai N, Chang S, Li Yihan, Li Q, Hu Jingchu, Liang J, Song L, Kretzschmar W, Gan X, Nicod J, Rivera M, Deng H, Du B, Li Keqing, Sang W, Gao J, Gao S, Ha B, Ho H-Y, Hu C, Hu Jian, Hu Z, Huang G, Jiang G, Jiang T, Jin W, Li G, Li Kan, Li Yi, Li Yingrui, Li Youhui, Lin Y-T, Liu L, Liu T, Liu Ying, Liu Yuan, Lu Y, Lv L, Meng H, Qian P, Sang H, Shen J, Shi J, Sun J, Tao M, Wang Gang, Wang Guangbiao, Wang Jian, Wang L, Wang Xueyi, Wang Xumei, Yang H, Yang L, Yin Y, Zhang J, Zhang K, Sun N, Zhang W, Zhang X, Zhang Z, Zhong H, Breen G, Wang Jun, Marchini J, Chen Y, Xu Q, Xu X, Mott R, Huang G-J, Kendler K, Flint J, 2015. Molecular Signatures of Major Depression. Curr Biol 25, 1146–1156. 10.1016/j.cub.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH, 2007. Decreased ACTH and Cortisol Responses to Stress in Healthy Adults Reporting Significant Childhood Maltreatment. Biol Psychiatry 62, 1080–1087. 10.1016/j.biopsych.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH, 2011. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl) 214, 367–375. 10.1007/s00213-010-2007-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceruso A, Martínez-Cengotitabengoa Mayte, Peters-Corbett A, Diaz-Gutierrez MJ, Martínez-Cengotitabengoa Monica, 2020. Alterations of the HPA Axis Observed in Patients with Major Depressive Disorder and Their Relation to Early Life Stress: A Systematic Review. NPS 1–11. 10.1159/000506484 [DOI] [PubMed] [Google Scholar]
- Chauhan A, Gu F, Essa MM, Wegiel J, Kaur K, Brown WT, Chauhan V, 2011. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J Neurochem 117, 209–220. 10.1111/j.1471-4159.2011.07189.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Li Z, He Y, Zhang F, Li H, Liao Y, Wei Z, Wan G, Xiang X, Hu M, Xia K, Chen X, Tang J, 2015. Elevated mitochondrial DNA copy number in peripheral blood cells is associated with childhood autism. BMC Psychiatry 15. 10.1186/s12888-015-0432-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, 2001. Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol 13, 677–693. 10.1017/s0954579401003145 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL, 2010. The Differential Impacts of Early Physical and Sexual Abuse and Internalizing Problems on Daytime Cortisol Rhythm in School-Aged Children. Child Dev 81, 252–269. 10.1111/j.1467-8624.2009.01393.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay Montier LL, Deng JJ, Bai Y, 2009. Number matters: control of mammalian mitochondrial DNA copy number. Journal of Genetics and Genomics 36, 125–131. 10.1016/S1673-8527(08)60099-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Lewis SJ, 2017. Psychoneuroimmunology of Early-Life Stress: The Hidden Wounds of Childhood Trauma? Neuropsychopharmacology 42, 99–114. 10.1038/npp.2016.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Tan M, 2014. Childhood maltreatment and obesity: systematic review and meta-analysis. Molecular Psychiatry 19, 544–554. 10.1038/mp.2013.54 [DOI] [PubMed] [Google Scholar]
- Daniels TE, Olsen EM, Tyrka AR, 2020. Stress and Psychiatric Disorders: The Role of Mitochondria. Annual Review of Clinical Psychology 16, 165–186. 10.1146/annurev-clinpsy-082719-104030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio R, Barrett PJ, Hoffman EK, Barrett CW, Zharikov A, Borah A, Hu X, Mccoy J, Chu CT, Burton EA, Hastings TG, Greenamyre JT, 2016. Alpha-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci. Transl. Med. 8, 342ra78. 10.1126/scitranslmed.aaf3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Prado CH, Grassi-Oliveira R, Daruy-Filho L, Wieck A, Bauer ME, 2017. Evidence for Immune Activation and Resistance to Glucocorticoids Following Childhood Maltreatment in Adolescents Without Psychopathology. Neuropsychopharmacology 42, 2272–2282. 10.1038/npp.2017.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, Machado-Vieira R, McEwen BS, Manji HK, 2009. Dynamic regulation of mitochondrial function by glucocorticoids. Proceedings of the National Academy of Sciences 106, 3543–3548. 10.1073/pnas.0812671106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy KA, McLaughlin KA, Green PA, 2018. Early life adversity and health-risk behaviors: proposed psychological and neural mechanisms. Ann N Y Acad Sci 1428, 151–169. 10.1111/nyas.13928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P, 2008. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: A study among healthy young subjects. Psychoneuroendocrinology 33, 227–237. 10.1016/j.psyneuen.2007.11.004 [DOI] [PubMed] [Google Scholar]
- Epel ES, Prather AA, 2018. Stress, Telomeres, and Psychopathology: Toward a Deeper Understanding of a Triad of Early Aging. Annu. Rev. Clin. Psychol. 14, 371–397. 10.1146/annurev-clinpsy-032816-045054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Kiecolt-Glaser JK, 2013. Stressful early life experiences and immune dysregulation across the lifespan. Brain, Behavior, and Immunity 27, 8–12. 10.1016/j.bbi.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM, Caponio D, Dan X, Rocktäschel P, Croteau DL, Akbari M, Greig NH, Fladby T, Nilsen H, Cader MZ, Mattson MP, Tavernarakis N, Bohr VA, 2019. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nature Neuroscience 22, 401–412. 10.1038/s41593-018-0332-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattahi Masrour F, Peeri M, Hosseini MJ, Azarbayjani MA, 2018. Exercise During Adolescence Attenuated Depressive-Like Behaviors and Hippocampal Mitochondrial Dysfunction Following Early Life Stress in Adult Male Rats. Iran J Pharm Res 17, 124–133. [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, 1998. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine 14, 245–258. 10.1016/S0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- Filiou MD, Sandi C, 2019. Anxiety and Brain Mitochondria: A Bidirectional Crosstalk. Trends in Neurosciences 42, 573–588. 10.1016/j.tins.2019.07.002 [DOI] [PubMed] [Google Scholar]
- Franco-Iborra S, Vila M, Perier C, 2018. Mitochondrial Quality Control in Neurodegenerative Diseases: Focus on Parkinson’s Disease and Huntington’s Disease. Front. Neurosci. 12. 10.3389/fnins.2018.00342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS, 2012. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1823, 2297–2310. 10.1016/j.bbamcr.2012.08.007 [DOI] [PubMed] [Google Scholar]
- García-Eguren G, Giró O, Romero M del M, Grasa M, Hanzu FA, 2019. Chronic hypercortisolism causes more persistent visceral adiposity than HFD-induced obesity. Journal of Endocrinology 242, 65–77. 10.1530/JOE-19-0168 [DOI] [PubMed] [Google Scholar]
- Gautier CA, Erpapazoglou Z, Mouton-Liger F, Muriel MP, Cormier F, Bigou S, Duffaure S, Girard M, Foret B, Iannielli A, Broccoli V, Dalle C, Bohl D, Michel PP, Corvol J-C, Brice A, Corti O, 2016. The endoplasmic reticulum-mitochondria interface is perturbed in PARK2 knockout mice and patients with PARK2 mutations. Hum Mol Genet 25, 2972–2984. 10.1093/hmg/ddw148 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Banerjee KK, Vaidya VA, Kolthur-Seetharam U, 2016. Early Stress History Alters Serum Insulin-Like Growth Factor-1 and Impairs Muscle Mitochondrial Function in Adult Male Rats. Journal of Neuroendocrinology 28. 10.1111/jne.12397 [DOI] [PubMed] [Google Scholar]
- Gilbert LK, Breiding MJ, Merrick MT, Thompson WW, Ford DC, Dhingra SS, Parks SE, 2015. Childhood Adversity and Adult Chronic Disease: An Update from Ten States and the District of Columbia, 2010. American Journal of Preventive Medicine 48, 345–349. 10.1016/j.amepre.2014.09.006 [DOI] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S, 2009. Burden and consequences of child maltreatment in high-income countries. The Lancet 373, 68–81. 10.1016/S0140-6736(08)61706-7 [DOI] [PubMed] [Google Scholar]
- Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH, 2018. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 12. 10.3389/fnbeh.2018.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves VF, Cappi C, Hagen CM, Sequeira A, Vawter MP, Derkach A, Zai CC, Hedley PL, Bybjerg-Grauholm J, Pouget JG, Cuperfain AB, Sullivan PF, Christiansen M, Kennedy JL, Sun L, 2018. A comprehensive analysis of nuclear-encoded mitochondrial genes in schizophrenia. Biol Psychiatry 83, 780–789. 10.1016/j.biopsych.2018.02.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Chai Y, Ding J-H, Sun X-L, Hu G, 2011. Chronic mild stress damages mitochondrial ultrastructure and function in mouse brain. Neuroscience Letters 488, 76–80. 10.1016/j.neulet.2010.11.006 [DOI] [PubMed] [Google Scholar]
- González-Pardo H, Arias JL, Gómez-Lázaro E, López Taboada I, Conejo NM, 2020. Sex-Specific Effects of Early Life Stress on Brain Mitochondrial Function, Monoamine Levels and Neuroinflammation. Brain Sciences 10, 447. 10.3390/brainsci10070447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, Suomalainen A, Thorburn DR, Zeviani M, Turnbull DM, 2016. Mitochondrial diseases. Nature Reviews Disease Primers 2, 1–22. 10.1038/nrdp.2016.80 [DOI] [PubMed] [Google Scholar]
- Gumpp AM, Boeck C, Behnke A, Bach AM, Ramo-Fernández L, Welz T, Gündel H, Kolassa I-T, Karabatsiakis A, 2020. Childhood maltreatment is associated with changes in mitochondrial bioenergetics in maternal, but not in neonatal immune cells. PNAS 117, 24778–24784. 10.1073/pnas.2005885117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han LKM, Verhoeven JE, Tyrka AR, Penninx BWJH, Wolkowitz OM, Månsson KNT, Lindqvist D, Boks MP, Révész D, Mellon SH, Picard M, 2019. Accelerating research on biological aging and mental health: Current challenges and future directions. Psychoneuroendocrinology 106, 293–311. 10.1016/j.psyneuen.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen LM, Schutte NS, Malouff JM, Epel ES, 2017. The Relationship Between Childhood Psychosocial Stressor Level and Telomere Length: A Meta-Analysis. Health Psychol Res 5. 10.4081/hpr.2017.6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim CM, Entringer S, Buss C, 2019. Translating basic research knowledge on the biological embedding of early-life stress into novel approaches for the developmental programming of lifelong health. Psychoneuroendocrinology, Festschrift for Dirk Hellhammer 105, 123–137. 10.1016/j.psyneuen.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Spengler D, 2018. The Mitochondrion as Potential Interface in Early-Life Stress Brain Programming. Front. Behav. Neurosci. 12. 10.3389/fnbeh.2018.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis F, Kooij MA van der, Zanoletti O, Lozano L, Cantó C, Sandi C, 2015. Mitochondrial function in the brain links anxiety with social subordination. PNAS 112, 15486–15491. 10.1073/pnas.1512653112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Yan Q, Wang Y, Zou Q, Li J, Liu Z, Cai Z, 2020. Role of Mitochondrial Dysfunction in the Pathology of Amyloid-β. J Alzheimers Dis. 10.3233/JAD-200519 [DOI] [PubMed] [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, Jones L, Dunne MP, 2017. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public Health 2, e356–e366. 10.1016/S2468-2667(17)30118-4 [DOI] [PubMed] [Google Scholar]
- Hummel EM, Hessas E, Müller S, Beiter T, Fisch M, Eibl A, Wolf OT, Giebel B, Platen P, Kumsta R, Moser DA, 2018. Cell-free DNA release under psychosocial and physical stress conditions. Transl Psychiatry 8. 10.1038/s41398-018-0264-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-Roca Y, Ledesma M, Gonzalez-Lazaro M, Moreno-Loshuertos R, Fernandez-Silva P, Enriquez JA, Laclaustra M, 2016. Adjusting MtDNA Quantification in Whole Blood for Peripheral Blood Platelet and Leukocyte Counts. PLOS ONE 11, e0163770. 10.1371/journal.pone.0163770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Arai Makoto, Miyashita M, Arai Mayumi, Obata N, Nohara I, Oshima K, Niizato K, Okazaki Y, Doi N, Itokawa M, 2012. Schizophrenia: Maternal inheritance and heteroplasmy of mtDNA mutations. Molecular Genetics and Metabolism 105, 103–109. 10.1016/j.ymgme.2011.09.034 [DOI] [PubMed] [Google Scholar]
- Juster R-P, Russell JJ, Almeida D, Picard M, 2016. Allostatic load and comorbidities: A mitochondrial, epigenetic, and evolutionary perspective. Development and Psychopathology 28, 1117–1146. 10.1017/S0954579416000730 [DOI] [PubMed] [Google Scholar]
- Karan KR, Trumpff C, McGill MA, Thomas JE, Sturm G, Lauriola V, Sloan RP, Rohleder N, Kaufman BA, Marsland AL, Picard M, 2020. Mitochondrial respiratory capacity modulates LPS-induced inflammatory signatures in human blood. Brain, Behavior, & Immunity - Health 5, 100080. 10.1016/j.bbih.2020.100080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS, 2010. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 151, 2117–2127. 10.1210/en.2009-1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara T, Kato T, 2018. What Can Mitochondrial DNA Analysis Tell Us About Mood Disorders? Biological Psychiatry, Mitochondrial Dynamics and Bioenergetics in Psychiatry 83, 731–738. 10.1016/j.biopsych.2017.09.010 [DOI] [PubMed] [Google Scholar]
- Kasahara T, Takata A, Kato TM, Kubota-Sakashita M, Sawada T, Kakita A, Mizukami H, Kaneda D, Ozawa K, Kato T, 2016. Depression-like episodes in mice harboring mtDNA deletions in paraventricular thalamus. Molecular Psychiatry 21, 39–48. 10.1038/mp.2015.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato TM, Kubota-Sakashita M, Fujimori-Tonou N, Saitow F, Fuke S, Masuda A, Itohara S, Suzuki H, Kato T, 2018. Ant1 mutant mice bridge the mitochondrial and serotonergic dysfunctions in bipolar disorder. Mol Psychiatry 23, 2039–2049. 10.1038/s41380-018-0074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorjahani A, Peeri M, Azarbayjani MA, 2020. The Therapeutic Effect of Exercise on Anxiety and Bowel Oxidative Stress in the Maternal Separation Animal Model. Basic Clin Neurosci 11, 69–78. 10.32598/bcn.9.10.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-C, Wei Y-H, 2005. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. The International Journal of Biochemistry & Cell Biology 37, 822–834. 10.1016/j.biocel.2004.09.010 [DOI] [PubMed] [Google Scholar]
- Lee Y, Jeong S-Y, Karbowski M, Smith CL, Youle RJ, 2004. Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis. MBoC 15, 5001–5011. 10.1091/mbc.e04-04-0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Pereira SMP, Power C, 2019. Childhood maltreatment and biomarkers for cardiometabolic disease in mid-adulthood in a prospective British birth cohort: associations and potential explanations. BMJ Open 9. 10.1136/bmjopen-2018-024079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, He Y, Wang D, Tang J, Chen X, 2017. Association between childhood trauma and accelerated telomere erosion in adulthood: A meta-analytic study. Journal of Psychiatric Research 93, 64–71. 10.1016/j.jpsychires.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Liang Y, Zhou Y, Shen P, 2004. NF-kappaB and its regulation on the immune system. Cell Mol Immunol 1, 343–350. [PubMed] [Google Scholar]
- Lin D, Sugawara T, Strauss JF, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL, 1995. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267, 1828–1831. 10.1126/science.7892608 [DOI] [PubMed] [Google Scholar]
- Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF, 2002. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum Mol Genet 11, 133–145. 10.1093/hmg/11.2.133 [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Fernström J, Grudet C, Ljunggren L, Träskman-Bendz L, Ohlsson L, Westrin Å, 2016. Increased plasma levels of circulating cell-free mitochondrial DNA in suicide attempters: associations with HPA-axis hyperactivity. Transl Psychiatry 6, e971. 10.1038/tp.2016.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Picard M, Ohlsson L, Bersani FS, Fernström J, Westrin Å, Hough CM, Lin J, Reus VI, Epel ES, Mellon SH, 2018. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology 43, 1557–1564. 10.1038/s41386-017-0001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Zhou C, 2012. Corticosterone reduces brain mitochondrial function and expression of mitofusin, BDNF in depression-like rodents regardless of exercise preconditioning. Psychoneuroendocrinology 37, 1057–1070. 10.1016/j.psyneuen.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Allaman I, 2015. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron 86, 883–901. 10.1016/j.neuron.2015.03.035 [DOI] [PubMed] [Google Scholar]
- Manoli I, Alesci S, Blackman MR, Su YA, Rennert OM, Chrousos GP, 2007. Mitochondria as key components of the stress response. Trends in Endocrinology & Metabolism 18, 190–198. 10.1016/j.tem.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Martorell L, Segués T, Folch G, Valero J, Joven J, Labad A, Vilella E, 2006. New variants in the mitochondrial genomes of schizophrenic patients. European Journal of Human Genetics 14, 520–528. 10.1038/sj.ejhg.5201606 [DOI] [PubMed] [Google Scholar]
- Matsushita H, Latt HM, Koga Y, Nishiki T, Matsui H, 2019. Oxytocin and Stress: Neural Mechanisms, Stress-Related Disorders, and Therapeutic Approaches. Neuroscience 417, 1–10. 10.1016/j.neuroscience.2019.07.046 [DOI] [PubMed] [Google Scholar]
- Matsuura T, Motojima Y, Kawasaki M, Ohnishi H, Sakai A, Ueta Y, 2016. Relationship Between Oxytocin and Pain Modulation and Inflammation. J. UOEH 38, 325–334. 10.7888/juoeh.38.325 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Nelson CA, 2017. Neglect as a Violation of Species-Expectant Experience: Neurodevelopmental Consequences. Biological Psychiatry, Stress: Mechanisms in Gut and Brain 82, 462–471. 10.1016/j.biopsych.2017.02.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meimaridou E, Kowalczyk J, Guasti L, Hughes CR, Wagner F, Frommolt P, Nürnberg P, Mann NP, Banerjee R, Saka HN, Chapple JP, King PJ, Clark AJL, Metherell LA, 2012. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nature Genetics 44, 740–742. 10.1038/ng.2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES, 2007. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 133, 25–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Moraes CT, 2001. What regulates mitochondrial DNA copy number in animal cells? Trends Genet. 17, 199–205. 10.1016/s0168-9525(01)02238-7 [DOI] [PubMed] [Google Scholar]
- Nakahira K, Kyung S-Y, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, Lawler LA, Christie JD, Meyer NJ, Causland F.R.Mc., Waikar SS, Waxman AB, Chung RT, Bueno R, Rosas IO, Fredenburgh LE, Baron RM, Christiani DC, Hunninghake GM, Choi AMK, 2013. Circulating Mitochondrial DNA in Patients in the ICU as a Marker of Mortality: Derivation and Validation. PLoS Med 10. 10.1371/journal.pmed.1001577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen D-F, Youle RJ, 2008. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183, 795–803. 10.1083/jcb.200809125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkheslat N, McLaughlin AP, Hastings C, Zajkowska Z, Nettis MA, Mariani N, Enache D, Lombardo G, Pointon L, Cowen PJ, Cavanagh J, Harrison NA, Bullmore ET, Pariante CM, Mondelli V, 2020. Childhood trauma, HPA axis activity and antidepressant response in patients with depression. Brain, Behavior, and Immunity 87, 229–237. 10.1016/j.bbi.2019.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM, 2012. Increased peripheral NF-κB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain, Behavior, and Immunity 26, 13–17. 10.1016/j.bbi.2011.07.232 [DOI] [PubMed] [Google Scholar]
- Pei L, Wallace DC, 2018. Mitochondrial Etiology of Neuropsychiatric Disorders. Biological Psychiatry, Mitochondrial Dynamics and Bioenergetics in Psychiatry 83, 722–730. 10.1016/j.biopsych.2017.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Juster R-P, McEwen BS, 2014. Mitochondrial allostatic load puts the “gluc” back in glucocorticoids. Nature Reviews Endocrinology 10, 303–310. 10.1038/nrendo.2014.22 [DOI] [PubMed] [Google Scholar]
- Picard M, McEwen BS, 2018a. Psychological Stress and Mitochondria: A Systematic Review. Psychosom Med 80, 141–153. 10.1097/PSY.0000000000000545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McEwen BS, 2018b. Psychological Stress and Mitochondria: A Conceptual Framework. Psychosom Med 80, 126–140. 10.1097/PSY.0000000000000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McEwen BS, Epel ES, Sandi C, 2018a. An energetic view of stress: Focus on mitochondria. Front Neuroendocrinol 49, 72–85. 10.1016/j.yfrne.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK, Seifert EL, McEwen BS, Wallace DC, 2015. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. PNAS 112, E6614–E6623. 10.1073/pnas.1515733112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Prather AA, Puterman E, Cuillerier A, Coccia M, Aschbacher K, Burelle Y, Epel ES, 2018b. A Mitochondrial Health Index Sensitive to Mood and Caregiving Stress. Biological Psychiatry, Mechanisms of Depression and Antidepressant Treatment 84, 9–17. 10.1016/j.biopsych.2018.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Trumpff C, Burelle Y, 2019. Mitochondrial psychobiology: foundations and applications. Current Opinion in Behavioral Sciences, SI: 28: Psychoneuroimmunology (2019) 28, 142–151. 10.1016/j.cobeha.2019.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Wallace DC, Burelle Y, 2016. The Rise of Mitochondria in Medicine. Mitochondrion 30, 105–116. 10.1016/j.mito.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Huang C-H, Kennedy SR, Ordureau A, Sideris DP, Hoekstra JG, Harper JW, Youle RJ, 2015. Endogenous Parkin Preserves Dopaminergic Substantia Nigral Neurons following Mitochondrial DNA Mutagenic Stress. Neuron 87, 371–381. 10.1016/j.neuron.2015.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G, Kirdar F, Kozicz T, 2018. The role of suboptimal mitochondrial function in vulnerability to post-traumatic stress disorder. Journal of Inherited Metabolic Disease 41, 585–596. 10.1007/s10545-018-0168-1 [DOI] [PubMed] [Google Scholar]
- Rani V, Deep G, Singh RK, Palle K, Yadav UCS, 2016. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sciences 148, 183–193. 10.1016/j.lfs.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Ridout KK, Coe JL, Parade SH, Marsit CJ, Kao H-T, Porton B, Carpenter LL, Price LH, Tyrka AR, 2020. Molecular markers of neuroendocrine function and mitochondrial biogenesis associated with early life stress. Psychoneuroendocrinology 116, 104632. 10.1016/j.psyneuen.2020.104632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, Price LH, Tyrka AR, 2018. Early life adversity and telomere length: a meta-analysis. Molecular Psychiatry 23, 858–871. 10.1038/mp.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout KK, Parade SH, Kao H-T, Magnan S, Seifer R, Porton B, Price LH, Tyrka AR, 2019. Childhood maltreatment, behavioral adjustment, and molecular markers of cellular aging in preschool-aged children: A cohort study. Psychoneuroendocrinology 107, 261–269. 10.1016/j.psyneuen.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rod NH, Bengtsson J, Budtz-Jørgensen E, Clipet-Jensen C, Taylor-Robinson D, Andersen A-MN, Dich N, Rieckmann A, 2020. Trajectories of childhood adversity and mortality in early adulthood: a population-based cohort study. The Lancet 396, 489–497. 10.1016/S0140-6736(20)30621-8 [DOI] [PubMed] [Google Scholar]
- Sagan L, 1967. On the origin of mitosing cells. Journal of Theoretical Biology 14, 225–IN6. 10.1016/0022-5193(67)90079-3 [DOI] [PubMed] [Google Scholar]
- Sahafi E, Peeri M, Hosseini M-J, Azarbyjani MA, 2018. Cardiac oxidative stress following maternal separation stress was mitigated following adolescent voluntary exercise in adult male rat. Physiology & Behavior 183, 39–45. 10.1016/j.physbeh.2017.10.022 [DOI] [PubMed] [Google Scholar]
- Sahin E, DePinho RA, 2012. Axis of ageing: telomeres, p53 and mitochondria. Nature Reviews Molecular Cell Biology 13, 397–404. 10.1038/nrm3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scifo E, Pabba M, Kapadia F, Ma T, Lewis DA, Tseng GC, Sibille E, 2018. Sustained molecular pathology across episodes and remission in major depression. Biol Psychiatry 83, 81–89. 10.1016/j.biopsych.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy DT, McAllister Kimberly, Worth Leroy, Haugen Astrid C., Meyer Joel N, Domann Frederick E, Van Houten Bennett Mostoslavsky Raul, Bultman Scott J, Baccarelli Andrea A, Begley Thomas J, Sobol Robert W, Hirschey Matthew D, Ideker Trey, Santos Janine H., Copeland William C., Tice Raymond R., Balshaw David M., Tyson Frederick L., 2014. Mitochondria, Energetics, Epigenetics, and Cellular Responses to Stress. Environmental Health Perspectives 122, 1271–1278. 10.1289/ehp.1408418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutt TE, McBride HM, 2013. Staying cool in difficult times: Mitochondrial dynamics, quality control and the stress response. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, Protein Import and Quality Control in Mitochondria and Plastids 1833, 417–424. 10.1016/j.bbamcr.2012.05.024 [DOI] [PubMed] [Google Scholar]
- Singh K, Singh IN, Diggins E, Connors SL, Karim MA, Lee D, Zimmerman AW, Frye RE, 2020. Developmental regression and mitochondrial function in children with autism. Ann Clin Transl Neurol 7, 683–694. 10.1002/acn3.51034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Perry G, Richey PL, Sayrec LM, Anderson VE, Beal MF, Kowall N, 1996. Oxidative damage in Alzheimer’s. Nature 382, 120–121. 10.1038/382120b0 [DOI] [PubMed] [Google Scholar]
- Srivastava R, Faust T, Ramos A, Ishizuka K, Sawa A, 2018. Dynamic Changes of the Mitochondria in Psychiatric Illnesses: New Mechanistic Insights From Human Neuronal Models. Biological Psychiatry, Mitochondrial Dynamics and Bioenergetics in Psychiatry 83, 751–760. 10.1016/j.biopsych.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Méndez S, García-de la Cruz DD, Tovilla-Zárate CA, Genis-Mendoza AD, Ramón-Torres RA, González-Castro TB, Juárez-Rojop IE, 2020. Diverse roles of mtDNA in schizophrenia: Implications in its pathophysiology and as biomarker for cognitive impairment. Progress in Biophysics and Molecular Biology 155, 36–41. 10.1016/j.pbiomolbio.2020.04.004 [DOI] [PubMed] [Google Scholar]
- Sun N, Youle RJ, Finkel T, 2016. The Mitochondrial Basis of Aging. Mol Cell 61, 654–666. 10.1016/j.molcel.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed SA, Nemeroff CB, 2017. Early Life Stress, Mood, and Anxiety Disorders. Chronic Stress (Thousand Oaks) 1. 10.1177/2470547017694461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens-Mas M, Pons D-G, Sastre-Serra J, Oliver J, Roca P, 2020. Sexual hormones regulate the redox status and mitochondrial function in the brain. Pathological implications. Redox Biology, Sexual Dimorphisms in Redox Biology 31, 101505. 10.1016/j.redox.2020.101505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett PK, NOLL JG, SUSMAN EJ, SHENK CE, PUTNAM FW, 2010. Attenuation of cortisol across development for victims of sexual abuse. Dev Psychopathol 22, 165–175. 10.1017/S0954579409990332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpff C, Marsland AL, Basualto-Alarcón C, Martin JL, Carroll JE, Sturm G, Vincent AE, Mosharov EV, Gu Z, Kaufman BA, Picard M, 2019. Acute psychological stress increases serum circulating cell-free mitochondrial DNA. Psychoneuroendocrinology 106, 268–276. 10.1016/j.psyneuen.2019.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushina E, Nemutlu E, Zhang S, Christensen T, Camp J, Mesa J, Siddiqui A, Tamura Y, Sesaki H, Wengenack TM, Dzeja PP, Poduslo JF, 2012. Defects in Mitochondrial Dynamics and Metabolomic Signatures of Evolving Energetic Stress in Mouse Models of Familial Alzheimer’s Disease. PLoS One 7. 10.1371/journal.pone.0032737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii N, Otsuka I, Okazaki S, Yanagi M, Numata S, Yamaki N, Kawakubo Y, Shirakawa O, Hishimoto A, 2019. Mitochondrial DNA Copy Number Raises the Potential of Left Frontopolar Hemodynamic Response as a Diagnostic Marker for Distinguishing Bipolar Disorder From Major Depressive Disorder. Front. Psychiatry 10. 10.3389/fpsyt.2019.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Price LH, Kao H-T, Porton B, Philip NS, Welch ES, Carpenter LL, 2016. Alterations of Mitochondrial DNA Copy Number and Telomere Length With Early Adversity and Psychopathology. Biological Psychiatry, Borderline Personality Disorder: Mechanisms of Emotion Dysregulation 79, 78–86. 10.1016/j.biopsych.2014.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL, 2008. Childhood Parental Loss and Adult Hypothalamic-Pituitary-Adrenal Function. Biol Psychiatry 63, 1147–1154. 10.1016/j.biopsych.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bodegom M, Homberg JR, Henckens MJAG, 2017. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front Cell Neurosci 11. 10.3389/fncel.2017.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R, Moulin M, Piquereau J, Lemaire C, Mericskay M, Veksler V, Garnier A, 2017. Mitochondria: a central target for sex differences in pathologies. Clin Sci (Lond) 131, 803–822. 10.1042/CS20160485 [DOI] [PubMed] [Google Scholar]
- Wang H-X, MacDonald SWS, Dekhtyar S, Fratiglioni L, 2017. Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: A community-based cohort study. PLOS Medicine 14, e1002251. 10.1371/journal.pmed.1002251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Luo Z, Jin M, Sheng W, Wang H-T, Long X, Wu Y, Hu P, Xu H, Zhang X, 2019. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging (Albany NY) 11, 3117–3137. 10.18632/aging.101966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sundquist K, Rastkhani H, Palmér K, Memon AA, Sundquist J, 2017. Association of mitochondrial DNA in peripheral blood with depression, anxiety and stress- and adjustment disorders in primary health care patients. European Neuropsychopharmacology 27, 751–758. 10.1016/j.euroneuro.2017.06.001 [DOI] [PubMed] [Google Scholar]
- West AP, Shadel GS, Ghosh S, 2011. Mitochondria in innate immune responses. Nat Rev Immunol 11, 389–402. 10.1038/nri2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM, 1999. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell 98, 115–124. 10.1016/S0092-8674(00)80611-X [DOI] [PubMed] [Google Scholar]
- Yang Y, Karakhanova S, Hartwig W, D’Haese JG, Philippov PP, Werner J, Bazhin AV, 2016. Mitochondria and Mitochondrial ROS in Cancer: Novel Targets for Anticancer Therapy. Journal of Cellular Physiology 231, 2570–2581. 10.1002/jcp.25349 [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Park M, Kim SA, 2017. Difference in mitochondrial DNA copy number in peripheral blood cells between probands with autism spectrum disorders and their unaffected siblings. World J. Biol. Psychiatry 18, 151–156. 10.1080/15622975.2016.1234069 [DOI] [PubMed] [Google Scholar]
- Young ES, Doom JR, Farrell AK, Carlson EA, Englund MM, Miller GE, Gunnar MR, Roisman GI, Simpson JA, 2020. Life stress and cortisol reactivity: An exploratory analysis of the effects of stress exposure across life on HPA-axis functioning. Development and Psychopathology 1–12. 10.1017/S0954579419001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Yu B, He J, Zheng P, Mao X, Han G, Chen D, 2014. Chronic Glucocorticoid Exposure-Induced Epididymal Adiposity Is Associated with Mitochondrial Dysfunction in White Adipose Tissue of Male C57BL/6J Mice. PLOS ONE 9, e112628. 10.1371/journal.pone.0112628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ, 2010. Circulating Mitochondrial DAMPs Cause Inflammatory Responses to Injury. Nature 464, 104–107. 10.1038/nature08780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J, 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225. 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]