Summary
NAD(H) and NADP(H) have traditionally been viewed as co-factors (or co-enzymes) involved in a myriad of oxidation-reduction reactions including the electron transport in the mitochondria. However, NAD pathway metabolites have many other important functions, including roles in signaling pathways, post-translational modifications, epigenetic changes, and regulation of RNA stability and function via NAD-capping of RNA. Non-oxidative reactions ultimately lead to the net catabolism of these nucleotides, indicating that NAD metabolism is an extremely dynamic process. In fact, recent studies have clearly demonstrated that NAD has a half-life in the order of minutes in some tissues. Several evolving concepts on the metabolism, transport, and roles of these NAD pathway metabolites in disease states such as cancer, neurodegeneration, and aging have emerged in just the last few years. In this perspective we discuss key recent discoveries and changing concepts in NAD metabolism and biology that are re-shaping the field. In addition, we will pose some open questions in NAD biology, including: Why is NAD metabolism so fast and dynamic in some tissues? How are NAD and its precursors transported to cells and organelles? and How is NAD metabolism integrated with inflammation and senescence? Resolving these questions will lead to significant advancements in the field.
Keywords: NAD pathway metabolites, vitamin B3, NAD+, aging, humans, disease, transport, mitochondria
INTRODUCTION:
The current teaching on NAD pathway metabolites such as NAD and NADP is focused primarily on their roles in oxidation-reduction reactions, with little discussion about their non-oxidative roles or their metabolism. In fact, the most current biochemistry textbooks portray nicotinamide nucleotide metabolism as very static, placing importance mainly on the interconversion between oxidized and reduced forms of NAD and NADP. Publications over the past several decades, however, and particularly more recent studies, clearly demonstrate that NAD metabolism, transport, and function are very complex and dynamic (Figure 1) (Covarrubias et. al., 2020; Hogan et. al., 2018; Piedra-Quintero et. al., 2020). NAD can be converted into several molecules that play key roles in energy transduction and cell signaling such as NADP, NAADP, and cADPR. Products of NAD degradation such as nicotinamide and n-methyl-nicotinamide have also emerged as key regulators of energy metabolism, epigenetics, and disease states (Brachs et. al., 2019; Covarrubias et.al., 2020; Eckert et. al., 2019; Liu et. al., 2018; Piedra-Quintero et.al., 2020), making it clear that NAD pathway metabolites are involved in much more than the traditionally described oxidation-reduction reactions (Covarrubias et. al., 2020; Hogan et. al., 2018; Piedra-Quintero et. al., 2020).
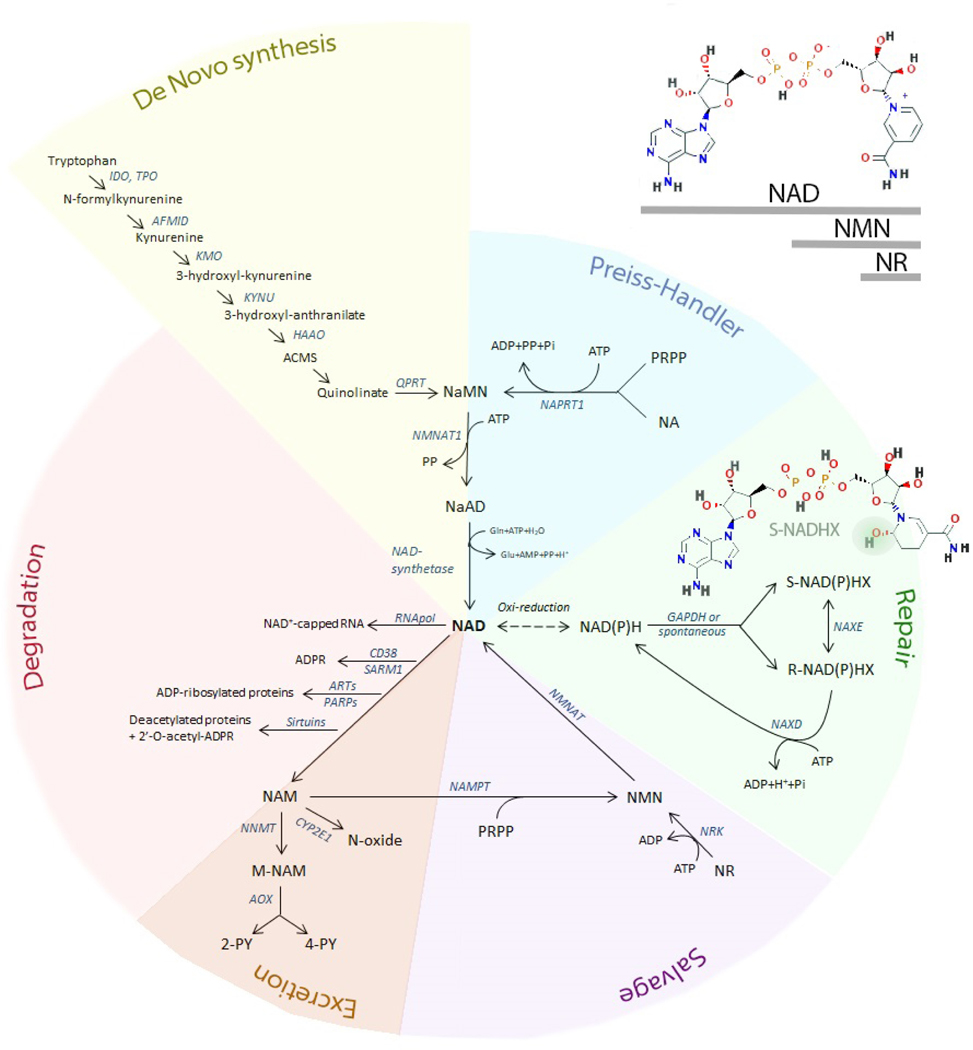
Figure 1. An overview of the NAD metabolome and metabolic pathways.
This figure is an integrative view of the networks related to NAD metabolism, including synthesis (de novo, Preiss-Handler, and salvage pathways), degradation, excretion, and repair pathways. The structure of NAD, NMN, and NR is shown (upper right). The structure of S-NADHX is shown (repair pathway - green) and the hydroxy group located at position 6 of the nicotinyl ring is highlighted. In the R-configuration the hydroxy group of the nicotinyl ring is located at position 2 (not shown). Cyclic forms of both S- and R-NAD(P)HX (not shown) are also toxic metabolites. 2-PY= N-methyl-2-pyridone-5-carboxamide; 4PY= N-methyl-4-pyridone-3-carboxamide; ACMS= 2-Amino-3-carboxymuconic acid semialdehyde; AFMID= Arylformamidase; AOX= aldehyde oxidase; ARTs= ADP-ribosyl-transferases; CYP2E1= Cytochrome P450 2E1; GAPDH= Glyceraldehyde-3-phosphate dehydrogenase; HAAO= 3-hydroxyanthranilate 3,4-dioxygenase; IDO= indoleamine 2,3-dioxygenase; KMO= kynurenine 3-monooxygenase; KYNU= kynureninase; M-NAM= methyl nicotinamide; NA= nicotinic acid; NaAD= nicotinic acid adenine dinucleotide; NAD= nicotinamide adenine dinucleotide; NAM= nicotinamide; NaMN= nicotinic acid mononucleotide; NAMPT= nicotinamide phosphoribosyltransferase; NaPRT1= nicotinic acid phosphoribosyltransferase 1; NAXD= NAD(P)HX Dehydratase; NAXE= NAD(P)H-hydrate epimerase; NMN= nicotinamide mononucleotide; NMNAT1= nicotinamide/nicotinic acid mononucleotide adenylyltransferase 1; NNMT= nicotinamide N-methyltranferase; NR= nicotinamide riboside; PARP= poly (ADP-ribose) polymerase; PRPP= phosphoribosyl pyrophosphate; QPRT= quinolinate phosphoribosyl transferase; SARM1= short for sterile alpha and Toll/interleukin receptor (TIR) motif-containing protein 1; TPO= tryptophan 2,3-dioxygenase;
NAD pathway metabolites can also serve as substrates for a group of diverse enzymes, including PARPs, sirtuins, CD38, ARTs, SARM1, and RNA polymerases, which are involved in several aspects of cellular homeostasis (Figure 1) (Coleman and Höke, 2020; Seman et. al., 2004). Different from oxidation-reduction reactions, these enzymes promote a net catabolism of nicotinamide nucleotides. Interestingly, cellular levels of these nucleotides appear to decrease during chronological aging, in progeroid states, and in several pathological conditions. In fact, dysregulation in NAD metabolism has emerged as a contributing factor in the pathogenesis of several disease states, and the so-called NAD-boosting or NAD-regenerative therapy has been proposed as an approach to treat human diseases (Chini, 2020; Lautrup et. al., 2019). This promising therapeutic concept has sparked a renewed interest in the biology of NAD pathway metabolites. Understanding the metabolism, transport, and biological roles of these nucleotides has been the focus of intensive investigation, leading to several key discoveries in the field. Many important questions, however, like the contribution of different topological forms of NADases such as CD38 and SARM1 to the NAD degradation process, and how NAD and its precursors are transported to cells, are only beginning to be addressed. In addition, emerging data indicate that an important, but largely neglected, aspect of NAD metabolism is the generation and repair of toxic NAD metabolites such as NAD(P)HX (Figure 1). Below we will discuss some of these new exciting discoveries in NAD biology and metabolism that are reshaping our understanding of the field. These sections will discuss many topics that are relevant, but still controversial, in the field of NAD biology and integrate some of these emerging concepts.
NAD-replacement therapy: a new approach to human diseases?
A growing number of pre-clinical studies demonstrate that NAD levels decline in physiological states such as aging, progeroid states, and pathological conditions involving multiple organ systems including skeletal muscle, heart, kidney, central nervous system, hearing, and vision (Abdellatif al., 2021; Elhassan et. al., 2019; Katsyuba et. al., 2020; Ralto et.al., 2020). However, as with any other approach that relies on data in rodents and in vitro cell culture, significant skepticism exists regarding the translational potential of NAD-replacement therapy to humans. In fact, several studies with the NAD precursor nicotinamide riboside (NR) failed to demonstrate clinical, structural, or functional effects in the obese and the elderly (Dollerup et al. 2018; Dollerup et al., 2019; Dollerup et al., 2020; Martens et al., 2018). On the other hand, key studies using simpler forms of vitamin B3 such as nicotinic acid or nicotinamide have shown interesting positive results. However, even these positive studies still need to be validated by large, double-blind randomized studies before NAD-boosting therapy can be translated to human conditions. Here we will discuss three human studies which have indicated that NAD-boosting therapy may indeed have a salutary effect in specific pathological conditions. We will also discuss the pitfalls of these studies and highlight what we believe is needed in the field to eventually be able to translate NAD-boosting therapy to humans.
The first study, by Pirinen published in Cell Metabolism in 2020, evaluated 5 adult patients with progressive external ophthalmoplegia (PEO), a mitochondrial myopathy caused by mitochondrial DNA (mtDNA) deletions (Pirinen et al., 2020). The authors described that levels of NAD in skeletal muscle and blood were lower in patients with PEO when compared to age- and gender-matched healthy controls (Pirinen et. al., 2020). Although the specific mechanism of NAD decline observed in the PEO patients was not well characterized, it is possible that it is at least in part mediated by increased catabolism of NAD via PARPs, as described before in other progeroid or mitochondrial myopathy animal models (Bolderson et. al., 2019; Khan et. al., 2014). This is supported by the fact that the products of NAD hydrolysis, namely nicotinamide and ADPR, were higher in these patients (Pirinen et. al., 2020). Treatment of these patients with the vitamin B3 niacin (nicotinic acid) for 12 months increased both skeletal muscle and blood NAD levels in PEO patients (Pirinen et. al., 2020). Importantly, in their secondary outcomes, the authors observed several striking effects of niacin treatment in the PEO patients, including significant increases in muscle strength and mitochondrial biogenesis.
In the study by Poyan Mehr et. al. published in Nature Medicine (Poyan et. al., 2018), the authors demonstrated that impairment of de novo NAD biosynthesis from tryptophan appears to be involved in the development of acute kidney injury (AKI). The authors elegantly showed that the enzyme quinolinate phosphoribosyltransferase (QPRT) is crucial for protection against NAD decline and resistance to AKI in mice. Furthermore, they proposed that an elevated urinary quinolinate/tryptophan (uQ/T) ratio, as an index of reduced activity of QPRT, predicted AKI and other adverse outcomes in critically ill patients. Furthermore, the authors performed a small (31 patients) phase 1 placebo-controlled study of oral vitamin B3 (nicotinamide) administration preemptively before cardiac surgery and observed a dose-related increase in circulating NAD metabolites and less AKI in these patients. Thus, the authors suggested that impaired NAD biosynthesis may be a feature of high-risk hospitalizations for which NAD augmentation could be beneficial.
Next, we would like to highlight the phase III trial conducted by Chen and colleagues on the role of nicotinamide as a chemoprevention against non-melanoma skin cancer in high risk patients (Chen et. al., 2015). In this study the authors performed a double-blind, randomized, controlled trial with over 380 patients who had at least two non-melanoma skin cancers in the previous 5 years. Patients received 500 mg of nicotinamide or placebo twice daily for 12 months. Patients who received nicotinamide had a decrease in the incidence of non-melanoma skin cancer, including both basal-cell carcinomas and squamous-cell carcinomas, of about 20% and 30%, respectively (Chen et. al., 2015). In this study the authors did not perform mechanistic studies to evaluate the potential role of nicotinamide-induced NAD-boosting as the driver of the chemoprevention. However, it is possible that the increased NAD levels promoted protection against UV-radiation-induced DNA damage via activation of PARP1 and sirtuins (Malesu et. al., 2020). The authors further published a follow-up phase II study on the potential role of nicotinamide chemoprevention of skin cancer in recipients of kidney transplant. This study demonstrated a trend for a decrease in the incidence of skin cancer in the patients receiving nicotinamide but did not observe statistical differences between the groups. (Chen et. al., 2016). Follow-up studies on the role of vitamin B3 and other NAD-boosting approaches as chemoprevention for skin cancer are necessary to establish its potential clinical use (Bagcchi et. al., 2015).
Although the studies mentioned above provide evidence on the role of NAD decline and NAD replacement therapy in human subjects, they have obvious limitations that prevent their clinical implementation at this moment. In particular, larger, double-blinded, multicenter, and randomized studies are necessary to validate these clinical uses. Nevertheless, these studies provide important frameworks, including the use of blood and urinary NAD metabolome as biomarkers for certain diseases.
It is known that vitamin B3 can exist in multiple forms, including niacin, nicotinamide, NR, and nicotinamide mononucleotide (NMN), all of which are capable of supporting NAD synthesis in vivo (Elhassan et. al., 2019; Lautrup et. al., 2019; Remie et. al., 2020). However, it is not known if any of the NAD-boosting strategies is superior to the others, or if there are specific diseases that would benefit from different NAD precursors. In fact, as discussed above, some studies using the NAD precursor NR failed to demonstrate clinical benefits and had only modest effects in structure and function studies (Dollerup et al. 2018; Dollerup et al., 2019; Dollerup et al., 2020; Martens et al., 2018). For example, a randomized placebo-controlled clinical trial of NR in humans showed that NR is safe, however, it did not show benefits in the population studied. This clinical trial showed that NR did not improve insulin sensitivity and whole-body glucose metabolism in obese, insulin-resistant men (Dollerup et al., 2018). Also, glucose tolerance, β-cell secretory capacity, α-cell function, and incretin hormone secretion in nondiabetic males with obesity were also not changed (Dollerup et al., 2019). Finally, NR did not alter mitochondrial respiration, content, or morphology in skeletal muscle from obese and insulin-resistant men (Dollerup et al., 2020). Another study on NR administration in humans (Martens et al., 2018) found that chronic NR supplementation was well tolerated and elevated NAD levels in healthy middle-aged and older adults. However, the clinical outcomes in this study were not disease-specific and were mostly not affected by NR. The authors suggested that larger clinical trials should be performed to test the effect of NR in cardiovascular function and health in older adults (Martens et al., 2018), although the specific disease indication was not clearly defined.
Because translation of NAD-boosting therapy to humans has been limited, it requires standardization and rigorous clinical trials before it can be implemented in clinical practice. In order to define the optimal approach to promote NAD-boosting via administration of vitamin B3 derivatives, rigorous head-to-head comparisons between the different NAD precursors should be performed. These head-to-head comparisons are of great importance since different NAD-precursors are expected to have distinct biological effects. For example, nicotinic acid, in addition to being a NAD precursor, can also activate its own specific receptor, which appears to be responsible for its effects in cutaneous flushing and decrease in free fatty acids in the plasma (Lauring et al., 2012; Offermanns, 2006). On the other hand, precursors such as nicotinamide, NR, and NMN are not expected to activate the nicotinic acid receptor, unless they are converted to nicotinic acid.
Interestingly, pharmacokinetic studies indicate that both NR and NMN are significantly metabolized by the gut microbiome (Shats et. al., 2020) and by the first passage in the liver (Liu et. al., 2018). Thus, it appears that when NR and NMN are given orally, the vitamin B3 species that circulate and reach the peripheral tissues are likely nicotinamide and nicotinic acid (Liu et. al., 2018; Shats et. al., 2020). Niacin and nicotinamide have been extensively studied in humans, and its pharmacokinetics and safety profile are well known (Ralto et. al., 2020).
Understanding whether other approaches to NAD-boosting are superior or can improve niacin’s biological effects is of major importance to advance the field. New studies on NAD-boosting could use niacin and nicotinamide as gold standards by which other NAD-boosting compounds could be compared or added on to.
Other important issues to be addressed before NAD-replacement therapy can be translated to patients are determining if there is a threshold of NAD that needs to be achieved, and if very high levels of NAD could be detrimental in some situations. This is of particular importance considering the development of extremely potent NAD+-boosting compounds such as the reduced form of NR, namely NRH (Dihydronicotinamide riboside)(Giroud-Gerbetant et al., 2019; Sonavane et al., 2020; Yang et al., 2019). Interestingly, a recent study indicates that NRH could cause a cell specific cytotoxicity, likely via an oxidative stress pathway (Sonavane et al., 2020 ). On the other hand, an in vivo study demonstrates that its administration can prevent cisplatin-induced acute kidney injury (Giroud-Gerbetant et al., 2019 ). We believe that these studies in NAD-replacement therapies are indeed promoting a renewed interest in NAD metabolism and fueling basic scientific discoveries that are tremendously increasing our understanding of the biology of NAD.
Nicotinamide nucleotide metabolism is complex, fast and dynamic, but why?
As described above, with the growing interest in NAD-replacement therapy it becomes imperative to investigate the metabolism of NAD precursors and metabolites, and to understand the dynamics of their fluxes in normal and disease states. Furthermore, it is essential to determine the relative contributions of anabolic and catabolic pathways to tissue- and cell-specific NAD(P)(H) biology. Several pathways for NAD synthesis have been very well described in mammalian cells and tissues (Figure 1) (Yoshino et. al., 2017). These include de novo NAD synthesis from tryptophan that appears to be present predominantly in the liver, the salvage pathway via re-cycling or incorporation of nicotinamide, and the Preiss-Handler pathway (Figure 1). The precise and relative roles of these different pathways in mammalian synthetic fluxes of NAD pathway metabolites in health and disease states have not been fully characterized. However, new data on these topics are emerging. For example, a manuscript published by Liu et al. in Cell Metabolism (Liu et. al., 2018), described the NAD synthetic fluxes in cell lines and mouse tissues, and arrived at some expected and new conclusions. The expected conclusion was that the liver makes NAD from tryptophan, excreting nicotinamide to be used by other tissues. The exciting and unexpected discoveries in this study were about the NAD half-life in vivo. In particular, the authors described that NAD fluxes vary widely across tissues, with t1/2 life that varies from 15 min to 15 hours, high fluxes in the small intestine and spleen, and low fluxes in the skeletal muscle. Therefore, flux analysis can reveal distinct tissue-specific features of NAD metabolism. As with any important study, the study by Liu et. al. also opens new questions in the field. First, which are the specific catabolic pathways that are responsible for the differences in tissue turnover of NAD pathway metabolites? Second, why do some tissues recycle nicotinamide at such a high speed? The latter question is of particular interest since this “futile cycle” of NAD synthesis and degradation is predicted to be a very energy-demanding process.
To date, the impact of specific catabolic pathways on NAD fluxes and NAD half-life have not been explored. Some years ago, we showed that one of the main NAD consuming enzymes in mammalian tissues during aging is the enzyme CD38 (Camacho-Pereira et. al., 2016; Hogan et. al., 2019). CD38 is highly expressed in tissues that are rich in immune cells, such as spleen, bone marrow, and gut (Camacho-Pereira et. al., 2016; Chini et. al, 2020). Furthermore, two independent groups have shown that CD38 levels in tissues can be increased by induction of an inflammatory response that promotes invasion of tissue by CD38+ immune cells such as T cells, B cells, and macrophages (Chini et.al, 2020; Covarrubias et. al., 2020). These studies demonstrate that the accumulation of CD38 observed during aging is, at least in part, mediated by the accumulation of CD38+ immune cells which can be induced by senescent cells and their secretory phenotypes (Chini et.al., 2020; Covarrubias et. al., 2020). Thus, it is possible that the high turnover of NAD and the “futile cycle” of NAD synthesis and degradation in tissues such as spleen and gut are mediated by the presence of CD38-expressing immune cells. This observation raises yet another important question related to the specific cellular and tissue compartments where NAD degradation occurs. CD38 is oriented in great majority as an ecto-enzyme in cells (Chini et. al, 2020). This aspect of NAD biology and metabolism presents an important topological paradox where the CD38 NADase faces the extracellular compartment and the majority of NAD is intracellular (Chini et. al., 2020). We have recently approached this paradox experimentally using a CD38 blocking antibody in vivo and in vitro. Our results demonstrate that CD38 ecto-enzymatic activity plays a major role in NAD homeostasis by regulating the availability of NAD precursors such as NMN to cells and by degrading NAD that may “leak” out of cells (Chini et. al., 2020). Thus, examining the contribution of specific anabolic and catabolic pathways to nicotinamide nucleotide metabolism in health and disease is an important and complex undertaking that will further shape our understanding of NAD biology. Certainly, investigating the role of multiple synthetic and catabolic pathways such as the SARM1, ARTs, PARPs and sirtuins in nicotinamide fluxes in vivo will be crucial to provide a full understanding of the metabolism of NAD pathway metabolites.
Regarding the question of why some tissues turnover NAD so quickly, we can only speculate at this moment. It is possible that, as reported before, some immune cells are sensitive to an extracellular NAD-mediated cell death via an ART-mediated ADP-ribosylation of the P2RX7 purinergic receptor (Künzli et. al., 2020; Nolz, 2020; Seman et. al., 2003). Furthermore, it is possible that immune cells may hydrolyze extracellular precursors such as NMN, NAD, and NR to prevent their use by bacteria which are unable to synthetize their own NAD(P) pool (Hogan et. al., 2019). Finally, as will be discussed next, it is possible that the high turnover, low steady state levels and short half-life of NAD pathway metabolites in some tissues may decrease the likelihood that a static NAD(P) pool could be converted into side toxic metabolites, such as hydrated forms of NADH (Figure 1). Future experimentation will be necessary to provide responses to these provocative ideas.
Metabolite repair mechanisms in NAD(P) biology, and their potential implications for NAD-replacement therapy.
An important aspect of cellular metabolism is the recognition that several enzymes through side reactions or even spontaneous non-enzymatic reactions can generate potentially toxic products. In fact, mechanisms to restore these metabolites via metabolite repair enzymes exist (Bommer et. al., 2020). Interestingly, some of the oldest known metabolic repair mechanisms involve NAD pathway metabolites (Figure 1) (Bommer et. al., 2020). In fact, since the 1950s it has been known that a toxic metabolite of NADH, known as NADHX, can be generated through its hydration. This side reaction is catalyzed by the glycolytic enzyme glyceldehyde-3-phosphate dehydrogenase (GAPDH) (Figure 1). In addition, another metabolite NADPHX can be generated by a non-enzymatic conversion at physiological temperatures at a rate of 10% per hour (Bommer et. al., 2020). These metabolites exist as different isomers (R and S), as well as cyclic and non-cyclic forms. They are known to inhibit several dehydrogenases, and as a result, their accumulation should be prevented (Figure 1) (Bommer et. al., 2020). Extremely conserved repair enzymes named NAXE (NAD(P)H-hydrate epimerase) and NAXD (ATP-dependent (S)-NAD(P)H-hydrate dehydratase) play a key role in promoting the recycling of these metabolites back to their NAD(P)H forms, protecting several different metabolic pathways (Fig. 1) (Bommer et al., 2020). In eukaryotes, these enzymes are present in different cellular compartments including the cytosol, mitochondria, chloroplasts in plants, and endoplasmic reticulum in mammals (Bommer et. al., 2020), indicating that these enzymes are present in virtually all locations where pyridine nucleotides are present. Another extremely important aspect of these metabolites is that although there are repair mechanisms to detoxify cells from isomers of NADPHX, no mechanisms have been described for the detoxification of their cyclic forms.
Mutations in NADPHX repair enzymes have been described recently. These mutations promote accumulation of toxic metabolites, causing severe encephalopathy and death in kids after a febrile episode (Kremer et al., 2016; Van Bergen, et al., 2019). The predominant clinical features found in subjects with mutations in these repair pathways are sub-acute onset of ataxia, cerebellar edema, spinal myelopathy, and skin lesions (Kremer et al. 2016; Van Bergen, et al., 2019). Importantly, infection and fever appear to be triggers of these processes, indicating that a temperature-dependent conversion of NADPH to these toxic metabolites plays a role in the pathogenesis of these diseases.
Little is known about the dynamic changes in these toxic metabolites and repair enzymes under normal physiological and pathological conditions such as exercise, inflammation, and aging. Furthermore, it is not known if there is any differential accumulation of these toxic metabolites in specific tissues, or how the levels of these metabolites correlate with NAD fluxes. Interestingly, a new study by Sabatasso’s group highlights the need to understand the dynamics of the accumulation of toxic NADPH metabolites during pathological conditions (Aljakna Khan, et al., 2021). In this study, the authors describe that R-NADPHX is one of the top metabolites that accumulate in an ex-vivo rat heart Langendorff model of ischemia (Aljakna Khan, et al., 2021).
One possible reason NAD(P) metabolism is so dynamic could be to promote continue recycling of the NAD(P) pool to prevent accumulation of these toxic metabolites. A fast and dynamic flux could influence the accumulation of these toxic metabolites through different mechanisms. Since at least part of the generation of these metabolites is mediated by non-enzymatic processes, one would expect that the high turnover of the NAD and NADP pool in tissues would promote a kinetic disadvantage for the generation of the toxic metabolites. In addition, a high turnover of NAD in the spleen and gut are also accompanied by lower steady state levels that would be expected to decrease the mass effect that could drive generation of the toxic metabolites. Finally, it is also possible that these metabolites are directly degraded by the same NADases that are important for the high turnover of the NAD pool. For example, could inhibition of the NAD(P) degrading enzymes such as CD38, SARM1, and PARP1 lead to the accumulation of these toxic metabolites over time by increasing the NAD(P) pool? Thus, it is critical to understand the potential consequences of manipulations of NAD(P) metabolic fluxes in the accumulation and metabolism of NADHX and NADPHX toxic metabolites. In particular, the compartmentalization of the flux and metabolism of the NAD(P) pool and these toxic metabolites are open questions in the field.
Another important question on this topic is whether NAD(P) metabolizing enzymes can generate other side products that can be toxic to cells. For example, both CD38 and SARM1 hydrolyze NAD to ADPR and nicotinamide, but they also generate a small amount of the cyclic nucleotide cADPR. Traditionally, cADPR has been considered a second messenger. However, we can speculate that in mammalian cells the “vestigial” cyclase activity of CD38 and SARM1 may lead to an accumulation of cADPR that could be toxic to some cells.
Finally, as we have been approaching NAD-replacement therapy as a novel therapeutic modality, it is imperative to understand if different types of NAD-boosting therapies like vitamin B3 derivatives, NAD anabolism activators such as NAMPT activators, or NAD(P) catabolism inhibitors would lead to the accumulation or clearance of these toxic NAD(P)HX metabolites. In particular, investigating the long-term effect of these therapies in metabolite repair mechanisms will be extremely important.
SARM1: the killer within.
In our opinion one of the most remarkable discoveries in NAD biology in the last years came from Jeffrey Milbrandt’s laboratory at Washington University in St. Louis. Working with Wallerian degeneration models his laboratory found that the protein SARM1 (Sterile alpha and Toll/interleukin receptor (TIR) motif-containing protein 1) is an evolutionarily conserved executioner of this degenerative cascade (Essuman et. al., 2018; Gerdts et. al., 2015). They further described that the TIR domain of SARM1 is necessary for SARM1 activity, although in other proteins dimerized TIR domains serve as scaffolds for innate immune signaling. In SARM1, dimerization of TIR domains promotes consumption of NAD+ and induces neuronal destruction (Essuman et. al., 2018). Another interesting finding in SARM1 biology and Wallerian degeneration is that NMN may be an endogenous activator of SARM1, and NMN deamidase, an enzyme with NMN-consuming activity, delays axon degeneration in neuronal cultures (Di Stefano et. al., 2017).
Remarkably, the catalytic activity of dimerized SARM1 is nearly identical to the enzymatic activity of CD38, despite a complete lack of homology between these two proteins. Dimerized SARM1 has glycohydrolase activity that consumes NAD+, converting it to ADPR and nicotinamide, with a very small portion converted to cADPR (Essuman et.al., 2017; Essuman et. al., 2018). This mirrors the enzymatic activity of CD38 with one very important distinction, CD38 is mostly an ecto-enzyme and SARM1 is an intracellular enzyme. Thus, dimerization and activation of SARM1 enzymatic activity consumes NAD+ leading to metabolic collapse and cell death (Figure 2) (Essuman et. al., 2017). The specific role, if any, of SARM1-generated cADPR in neuronal cell death has not been clearly demonstrated at this point. Therefore, dissecting the specific mechanisms of regulation of cell death induced by SARM1 dimerization and activation is critical.
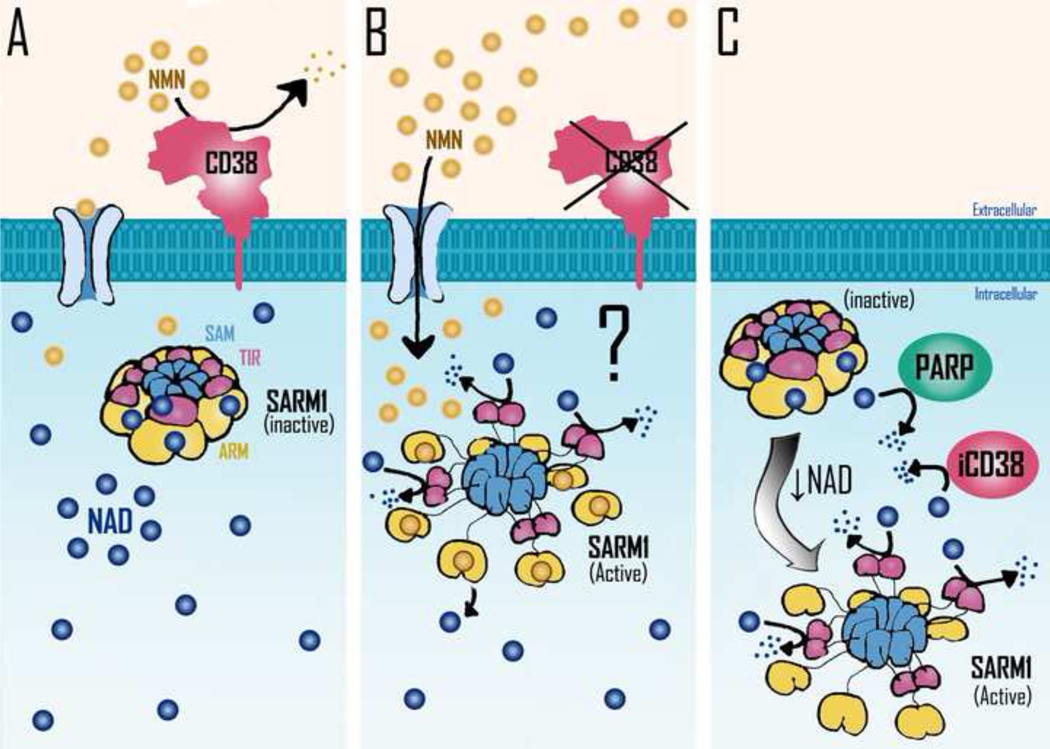
Figure 2. Potential mechanisms involved in Sarm1 activation.
SARM1 homo-octamer assumes a packed inactive conformation which is stabilized by NAD binding to allosteric sites located in the ARM domains. NAD decline leads to the disassembly of SARM1’s peripheral ARM ring, allowing the formation of TIR dimers, which are responsible for SARM1 NADase activity. It has been postulated that NMN may promote NAD displacement from SARM1 inhibitory allosteric sites, resulting in SARM1 NADase activation. A) In normal physiological context NAD is bound to allosteric sites in SARM1 oligomers, far from its catalytic sites. CD38 present in the cellular plasma membrane would lead to the degradation of extracellular NMN, preventing the increase of intracellular NMN levels. B) In a condition where extracellular CD38 activity is blocked, the consequent increase in intracellular NMN levels could lead to the displacement of NAD from SARM1’s allosteric inhibitory sites leading to SARM1 activation. C) Increased expression/activity of intracellular NADases such as PARPs or intracellular CD38 (iCD38) can lead to a decrease in intracellular NAD levels and consequent activation of SARM1 activity. Further decrease in NAD levels could lead to metabolic collapse and cell death. SAM= sterile alpha motif; TIR= toll/interleukin-1 receptor (TIR) homology domain; ARM= armadillo repeat domain.
Three recent studies explored the regulation of SARM1 using a structure-based approach ( Bratkowski et. al., 2020; Jiang et. al., 2020; Sporny et. al., 2020). These studies report the unique and unexpected finding that NAD+-binding to the armadillo/heat repeat motifs (ARM) domain of SARM1 facilitates the inhibition of the TIR-domain NADase through the domain interface, and that disruption of the NAD+ binding site or the ARM-TIR interaction causes constitutive activation of SARM1 and axonal degeneration (Figure 2). These findings suggest that NAD+ itself mediates self-inhibition of this central pro-neurodegenerative protein and controls its own fate (Figure 2). Another interesting finding was the demonstration that NAD+ binding to this inhibitory site can be displaced by the NAD+ precursor NMN. NMN displaces NAD+ from its binding site in the ARM, leading to activation of SARM1 (Figure 2). On the other hand, it has been proposed that an initial NAD+ decline induced by metabolic dysfunction or perhaps by DNA damage and PARP1 activation or activation of CD38 could release NAD+ from its ARM binding site. This NAD+ release could free the TIR domain to be dimerized and activated, leading to NAD consumption and metabolic collapse and neural cell death (Figure 2).
Further studies are necessary to clearly delineate the mechanisms involved in SARM1 activation in vivo. This is of particular importance when considering the potential use of NMN as an NAD precursor. It is essential to know if NMN supplementation can lead to displacement of NAD+ from the regulatory site on SARM1, inducing activation of SARM1 NADase activity, NAD+ metabolic collapse, and cell death. In fact, a recent study supports the notion that SARM1 is a sensor of the NMN/NAD ratio (Figley et al., 2021).
New findings and controversies in nicotinamide nucleotide transport.
Another important aspect of nicotinamide nucleotide biology being intensively investigated relates to the machinery used by cells and organelles to transport NAD pathway metabolites and precursors (Figure 3). This aspect of NAD biology is a source of heated debates (Grozio et. al., 2019a; Grozio et.al., 2019b; Schmidt and Brenner, 2019; Wu and Sinclair, 2019). Key questions in this field are how NAD(P) precursors are transported from the extracellular to the intracellular space and whether NAD is transported into the mitochondria.
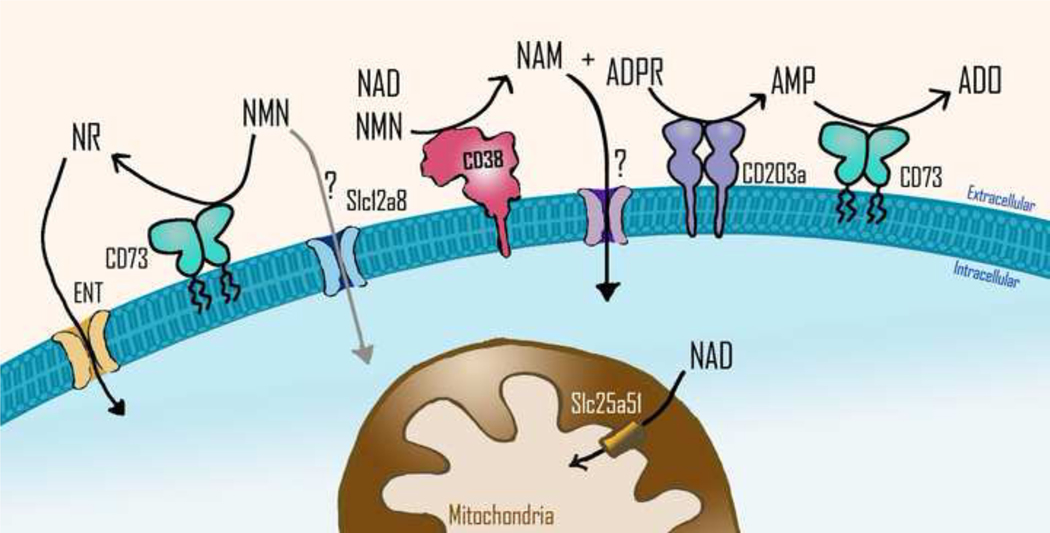
Figure 3. Nicotinamide nucleotides transport into cells and organelles.
It is believed that extracellular NAD and NMN are degraded into ADPR and NAM or NR by exonucleotidases such as CD38 or CD73, respectively, before being transported into the cells. The transport of NR is mediated by equilibrative nucleoside transporters (ENT). Whether NMN can be taken up into cells through the transporter Slc12a8 or any other transporter is still under debate. NAM is known to enter into the cells, but the protein that mediates its transport is still unknown. The CD38 product ADPR can be used as substrate by other nucleotidases such as the nucleotide pyrophosphatase CD203a, which generates AMP. AMP is the main substrate of CD73, which generates adenosine, an immunosuppressive metabolite. Slc25a51, a member of the solute carrier transporter family, has recently been identified as the NAD transporter in the mitochondrial inner membrane. NAM= nicotinamide; NMN= nicotinamide mononucleotide; NR= nicotinamide riboside; ADPR= adenosine diphosphate ribose; AMP= adenosine mononucleotide; ADO= adenosine.
Interestingly, pathogens such as H. aegyptius, H. influenzae, H. haemolyticus, H. parainfluenzae, and H. parahaemolyticus, that are responsible for a spectrum of infections, lack the ability to synthesize NAD+, and rely instead on uptake of extracellular NAD and NAD precursor molecules (e. g., NMN, NR) to support their metabolism and growth (Andersen et. al., 2003). In fact, NAD and its precursors are necessary for the growth of these bacteria and must be included in culture media as the V-factor. These bacteria express nucleotide transporters to facilitate intracellular incorporation of nicotinamide nucleotides, and it has been suggested that NR, NMN, and NAD can be transported through the membranes of H. influenzae through different putative channels (Andersen et. al., 2003). In contrast, in mammalian cells it has been proposed that only nicotinamide, nicotinic acid, NR, and a reduced form of NR (NRH) can be transported from outside to the inside of the cells through specific transporters. Conversion of extracellular NAD and NMN by CD38 and CD73, to either free nicotinamide or NR, would be the mechanism available to incorporate these extracellular precursors into cells (Figure 3). However, the debate on how extracellular NMN is incorporated into cells persists (Garavaglia et. al., 2012; Wilk et.al., 2020).
Regarding the transport of NMN, the study by Grozio and colleagues, and the subsequent criticism by Schmidt and Brenner deserve special attention (Grozio et. al., 2019a; Grozio et. al., 2019b; Schmidt and Brenner, 2019). NMN is a biosynthetic precursor of NAD+ known to promote cellular NAD+ production and to counteract age-associated pathologies in mice. However, it is still not clear how NMN is taken up into cells. Grozio et al proposed that the Slc12a8 gene, which has been previously named a salt transporter, encodes a specific NMN transporter that is regulated by NAD in the mouse small intestine (Grozio et. al., 2019a). They report that Slc12a8 knockdown abrogates the uptake of NMN in cultured cells and in a mouse knockdown model (Grozio et. al., 2019a). The authors further propose that Slc12a8 specifically transports NMN, but not NR, and that NMN transport depends on the presence of sodium ions (Grozio et. al., 2019a). However, Schmidt and Brenner claim that the analytical and transport data are not sound and do not support the transport of NMN by Slc12a8 (Schmidt and Brenner, 2019). In addition, they state that there is enough genetic, pharmacological, and kinetic evidence indicating that NMN is dephosphorylated to NR before entering the cell (Schmidt and Brenner, 2019). Therefore, two scenarios may exist, one where NMN is indeed transported into cells via a transporter such as Slc12a8, and another where NMN must be converted to NR by an enzyme such as CD73, to be incorporated by cells. To add to the controversy, studies on the role of CD73 in converting NMN to NR have come to very different conclusions (Mateuszuk et. al., 2020; Wilk et. al., 2020). In one study, it was shown that CD73 is necessary for the cellular effect of NMN both in vivo and in vitro in endothelial cells (Mateuszuk et. al., 2020). In contrast, in a different study, it has been proposed that CD73 does not efficiently convert NMN to NR, and that NMN uptake into some cancer cells occurs independently of CD73 (Wilk et. al., 2020). It is possible that these different mechanisms to incorporate NAD precursors are present, or more abundant, in specific cell types. Because studies in vitro usually focus in one or few cell types, a more complete picture is still missing. For example, it is possible that the mechanism to incorporate NAD and NMN in vivo could be cell, tissue, and context dependent. Thus, it appears that much is still to be learned about the mechanisms that cells use to uptake NAD precursors from the extracellular space (Figure 3).
Another key aspect of NMN metabolism and transport relates to the dramatically different levels of this metabolite reported in the circulation. It is worth noting that the range of NMN levels reported in the circulation varies from nearly undetectable to the μM range (Chini et al., 2020; Irie et al., 2020; Grozio et al., 2019; Liu et al., 2018; Mills et al., 2016; Ratajczak et al, 2016). The reason for these different results is not known, but it can likely be explained by the lack of standardization common to the field. In fact, collection of samples, extraction of nucleotides, storage, and the specific detection assays used could all be responsible for these differences. Therefore, we believe that standardization of experimental protocols on the analysis of NAD metabolites and precursors is imperative to be able to compare different studies and get to the bottom of the NMN levels and transport controversy. Interestingly, we have observed that after the administration of oral doses of NMN, NMN levels are nearly undetectable in plasma. However, NMN levels can be increased by inhibition of the enzyme CD38, indicating that this ecto-enzyme plays an important role in the pharmacokinetics of NMN in the plasma (Chini et al., 2020). One important factor to consider about NMN metabolism is the potential role of secreted exosomes containing the rate limiting enzyme for the synthesis of NMN (eNAMPT). These exosomes could potentially present a site for the synthesis of NMN in the circulation and, also a source of tissue crosstalk between adipocytes and the brain, as proposed by Imai’s group (Yoon et al., 2015). Although, these are intriguing possibilities, they still await experimental validation and confirmation in the literature. As we will discuss next, the uptake of NAD and its precursors by organelles such as the mitochondria is also the subject of very important new discoveries in the field, and may provide clues to discover how NAD and NMN may be incorporated by cells.
NAD+ can be transported into mammalian mitochondria after all.
One important topic that has been debated in the field of NAD precursor transport is whether NAD+ in mitochondria arises from nicotinamide, NMN, or NAD itself. Recently, a study by Davila et al. suggested the existence of an unrecognized NAD+ (or NADH) mitochondrial transporter (Davila et al, 2018), challenging the old view that mitochondria were impermeable to NAD+. To further expand on this important finding, three independent studies simultaneously identified the solute transporter MCART1/SLC25A51 as the first discovered mammalian mitochondrial NAD+ transporter (Figure 3) (Girardi et. al., 2020; Kory et. al., 2020; Luongo et. al., 2020).
The NAD+/NADH pair is particularly critical in mitochondria, as it connects substrate oxidation by the tricarboxylic acid (TCA) cycle to adenosine triphosphate (ATP) generation by the electron transport chain (ETC) and oxidative phosphorylation. Using different approaches, including gene essentiality data and gene interaction networks, MCART1 (SLC25A51) was identified as a mitochondrial NAD+ transporter. In support of that, all three studies showed that MCART1-null cells have significant decreases in mitochondrial NAD+ levels, TCA cycle flux, and mitochondrial respiration (for example, the NAD-dependent mitochondrial complex I activity). Furthermore, these three independent studies also demonstrated that isolated mitochondria from cells lacking MCART1 had impaired NAD+ uptake. Importantly, the functional consequences of the absence of MCART1 in cells could be prevented by the expression of NDT1, a yeast mitochondrial NAD+ transporter (Kory et. al., 2020; Luongo et al., 2020). Thus, these studies propose that MCART1 is the long sought mitochondrial transporter for NAD+ in human cells.
In light of these findings described above, one could predict that transporters for other forms of NAD pathway metabolites may be revealed in the near future, further enhancing our understanding of NAD metabolism and biology. Identification of these transporters may have important physiological and translational implications. For example, in addition to MCART1, humans have another homologous gene named MCART2 that appears to be expressed nearly exclusively in sperm cells (Girardi et. al., 2020; Kory et. al., 2020; Luongo et. al., 2020). It is possible that exploration of the specific role of the NAD mitochondrial transporter in sperm cells may lead to the development of a male-specific contraceptive. Also, the renewed interest in the transport of NAD and its precursors has now led to the identification of a transport mechanism for the most clinically used form of vitamin B3, namely nicotinic acid (Mathialagan et. al., 2020).
In conclusion, the renewed interest in NAD metabolism, transport, and its biology has promoted investigators to explore several extremely important open questions in the field that has led to an expansion of our understanding of NAD transport and metabolism in mammalian cells. The use of gene essentiality data and gene interaction networks will likely be instrumental to continue to investigate these questions.
NAD, inflammation-senescence, and aging: the good, the bad, and the ugly.
Aging is characterized by many changes at the cellular and molecular level, including epigenetic changes, genomic instability, mitochondrial dysfunction, and dysregulated nutrient sensing (López-Otín and Kroemer, 2020). Cells that do not properly function can either die or become senescent (López-Otín and Kroemer, 2020). In the senescent state, cells secrete many pro-inflammatory factors, including a variety of inflammatory cytokines and chemokines (SASP), resulting in a low-grade inflammation called “inflammaging” (Franceschi and Campisi, 2014). Another feature of aging and aging-associated pathologies is a decline in NAD+ levels in tissues, which may influence the activity of sirtuins and promote age-related metabolic disruption (McReynolds et. al., 2020). In order to better understand the role of NAD+ decline during aging, it is important to characterize NAD+ metabolism and its enzymes during aging. In particular, two critical topics need to be better understood: what the roles of NAD+ are in different cell types, including senescent cells, and whether senescent cells and “inflammaging” regulate NAD+ metabolism (Figure 4).
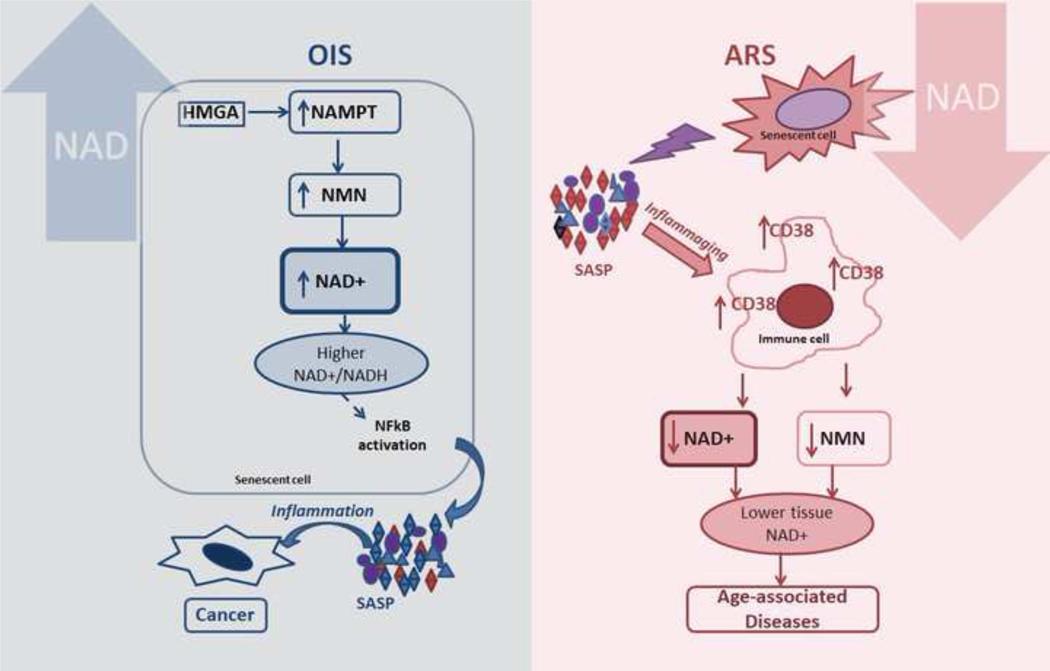
Figure 4. NAD, inflammation, and senescence: double edged sword?
In oncogene-induced senescence (OIS), high mobility group A protein (HMGA) upregulates the expression of nicotinamide phosphoribosyltransferase (NAMPT), the rate liming enzyme for the NAD salvage pathway. The HMGA-mediated NAMPT expression induces NAD synthesis, leading to higher NAD+/NADH ratios. This metabolic change can induce a higher pro-inflammatory SASP, which accelerates cancer progression in surrounding cells. During aging-related senescence (ARS), the chronic SASP causes a low-level pro-inflammatory environment, leading to inflammaging and upregulation of CD38 expression in M1 macrophages and other immune cells. Induction of CD38 expression in turn causes a reduction in NAD and NMN levels in surrounding tissues. Pro-inflammatory immune cells also have reduced NAD levels, but this reduction depends on other enzymes in addition to CD38, like PARP1. NAD boosting-therapies are attractive therapeutical approaches, but it is crucial to consider them carefully. NAD= nicotinamide adenine dinucleotide; NMN= nicotinamide mononucleotide; SASP= senescence associated secretory phenotype; OIS= oncogene-induced senescence
Regarding NAD+ metabolism in senescent cells, a recent study proposes that NAD+ metabolism plays a key role in senescent cells by determining the strength of the pro-inflammatory SASP (Figure 4). In a model of high pro-inflammatory SASP associated senescence, oncogene induced senescence (OIS), significant increases were observed in the NAD+/NADH ratio, NAD+ levels, and NAMPT expression, and knockdown of NAMPT decreased not only NAD+ levels, but also secretion of the pro-inflammatory SASP (Nacarelli et. al., 2019). Thus, this study proposes that in OIS the high pro-inflammatory SASP appears to be predominantly driven by the NAD+/NADH ratio (Nacarelli et. al., 2019). However, in a model of replicative senescence (RS), which is a low pro-inflammatory SASP-associated senescence, the authors found that NAMPT was downregulated and the NAD+/NADH ratio was decreased. It appears that in RS, senescence is primarily driven by DNA damage. In addition, this study reported that increasing the NAD+/NADH ratio in RS by ectopic oncogenic RAS or NMN supplementation significantly enhanced the SASP in vitro (Nacarelli et. al., 2019) Because pro-inflammatory SASP has been shown to be tumorigenic, this study suggests that supplementation with NAD precursors should be approached with caution, since it may be pro-inflammatory or tumorigenic in some conditions (Nacarelli et. al., 2019).
In contrast to the hypothesis that NAD+ increase in senescent cells may be pro-inflammatory, a new study published in Science (Desdín-Micó et. al., 2020) showed that mitochondrial dysfunction in T cells generated defective T cells that initiated an inflammaging process in distal tissues of these animals. The senescence observed in these mice was accompanied by low NAD+/NADH ratios in peripheral tissues (Desdín-Micó et. al., 2020). Notably, administration of NR restored the NAD+/NADH ratio, prevented transcriptional changes related to aging, and rescued the multimorbidity syndrome in these mice (Desdín-Micó et. al., 2020). In support of this study, a study by Elhassan and colleagues in 2019 showed that NR supplementation in aged individuals depressed levels of some circulating inflammatory cytokines, proposing that NR administration in aged humans may have anti-inflammatory effects (Elhassan et. al., 20219). In view of these findings, several issues need to be addressed to better understand the role of NAD+ metabolism in the development of senescence during aging. It is critical to fully characterize the role of NAD synthesizing and consuming enzymes in senescent cells and to investigate the role of NAD metabolism in other cellular models of senescence. More importantly, it is necessary to manipulate NAD+ levels through different mechanisms in vivo, using different NAD precursors or manipulating NAD metabolism enzymes, to have a clear picture of their role in the development of senescence and age-related inflammation in vivo.
Another interesting topic in NAD metabolism and aging is how senescent cells and their secretory phenotype regulate NAD metabolism in non-senescent cells. Three recent studies, including two by our group (Chini et. al., 2019, Chini et. al., 2020, Covarrubias et. al., 2020), explored this topic and provided a key connection between senescent cells and the NAD+-degrading ecto-enzyme CD38. These studies show that immune cells are the main cells that accumulate CD38 during aging (Chini et. al., 2019; Chini et. al., 2020; Covarrubias et. al., 2020). In addition, the SASP induces expression of CD38 in immune cells in vitro and in vivo, especially in macrophages, but also in non-immune cells (Figure 4) (Chini et. al., 2019, Chini et. al., 2020, Covarrubias et. al., 2020). Induction of senescence in vivo increased CD38 expression in white adipose tissue and liver (Chini et. al., 2020, Covarrubias et. al., 2020). Importantly, depletion of senescent cells or their secretory phenotype in vivo, decreased CD38 levels and partially rescued NAD+ levels in aging tissues, demonstrating that senescent cells contribute to the NAD+ decline observed during aging (Chini et. al., 2020). Therefore, increased CD38 expression in immune cells during aging is a key event that connects accumulation of senescent cells with inflammation and NAD+ metabolism. Because CD38 degrades not only intracellular NAD+, but also extracellular NMN, blocking the extracellular CD38 activity with a specific antibody increased both NAD and NMN levels in tissues of aging mice (Chini et. al., 2020). This finding raises the possibility that inhibition of CD38 ecto-enzymatic activity with specific antibodies could be used together with other NAD boosting therapies in studies of NAD-replacement therapies. It would also be interesting to investigate the effects of CD38 inhibition in combination with other aging therapies, such as senolytic or senomorphic agents, to improve aging-related diseases. More important, however, is the need to determine if an increase in CD38 expression during the aging process may have a potential negative feedback effect on the SASP in tissues enriched in senescent cells (Wu and Zhang, 2020). Thus, whether tissue NAD-decline during aging is a friend or foe has not been completely elucidated (Wu and Zhang, 2020).
Concluding Remarks
Research in nicotinamide nucleotide metabolism in physiological and disease states has rapidly evolved in the last few years and has transformed our understanding of the roles of these nucleotides in the metabolism. However, at this moment, it appears that we are still far from understanding the complete picture of how all these NAD metabolites are integrated. Many open questions remain to be explored for us to improve our understanding of the biology of these nucleotides and their potential therapeutic roles. First, how do NAD precursors get into cells and organelles? This is still a point of debate, especially due to the use of different cells and tissues in specific studies. Does it matter which precursors are used and which pathways are targeted? Why is nicotinamide nucleotide metabolism so fast and dynamic? Do NAD toxic metabolites such as R-S-NADPHX accumulate during different physiological and pathological states? How are multiple arms of nicotinamide nucleotide metabolism integrated with other metabolic pathways such as the methionine cycle? What are the emerging non-oxidative roles of NAD and its derivatives, such as the role of NAD-capping of RNA? Answering these questions would provide us with a clear picture on the roles and biology of these nucleotides.
Acknowledgments
The work in Dr. Chini’s laboratory is supported in part by grants from the Helen Diller Family Foundation, Ted Nash Long Life Foundation, the Glenn Foundation for Medical Research via the Paul F. Glenn Laboratories for the Biology of Aging at the Mayo Clinic (E.N.C), sponsored research funding from Calico Life Sciences, the Mayo and Noaber Foundations, NIH National Institute of Aging (NIA) grants AG-26094 (to E.N.C.), AG58812 (to E.N.C.), CA233790 (to E.N.C.).
Footnotes
Declaration of Interests
E.N.C. holds a patent on the use of CD38 inhibitors for metabolic diseases that is licensed by Elysium health. E.N.C. is a consultant for TeneoBio, Calico, Mitobridge and Cytokinetics. E.N.C. is on the advisory board of Eolo Pharma. E.N.C. own stocks in Teneobio. Research in E.N.C laboratory has been conducted in compliance with Mayo Clinic Conflict of Interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Abdellatif M, Trummer-Herbst V, Koser F, Durand S, Adão R, Vasques-Nóvoa F, Freundt JK, Voglhuber J, Pricolo MR, Kasa M, et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med. 2021; 13:eabd7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljakna Khan A, Bararpour N, Gorka M, Joye T, Morel S, Montessuit CA, Grabherr S, Fracasso T, Augsburger M, Kwak BR, Thomas A, Sabatasso S. Detecting early myocardial ischemia in rat heart by MALDI imaging mass spectrometry. Sci Rep. 2021. March 4;11(1):5135. doi: 10.1038/s41598-021-84523-z. PMID: 33664384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen C, Maier E, Kemmer G, Blass J, Hilpert AK, Benz R, Reidl J. Porin OmpP2 of Haemophilus influenzae shows specificity for nicotinamide-derived nucleotide substrates. J Biol Chem. 2003; 278:24269–24276. [DOI] [PubMed] [Google Scholar]
- Bagcchi S. Nicotinamide yields impressive results in skin cancer. Lancet Oncol. 2015; 16: e591. [DOI] [PubMed] [Google Scholar]
- Bolderson E, Burgess JT, Li J, Gandhi NS, Boucher D, Croft LV, Beard S, Plowman JJ, Suraweera A, Adams MN, et al. Barrier-to-autointegration factor 1 (Banf1) regulates poly [ADP-ribose] polymerase 1 (PARP1) activity following oxidative DNA damage. Nat Commun. 2019; 10:5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommer GT, Van Schaftingen E, Veiga-da-Cunha M. Metabolite Repair Enzymes Control Metabolic Damage in Glycolysis. Trends Biochem Sci. 2020; 45:228–243. [DOI] [PubMed] [Google Scholar]
- Brachs S, Polack J, Brachs M, Jahn-Hofmann K, Elvert R, Pfenninger A, Bärenz F, Margerie D, Mai K, Spranger J, Kannt A. Genetic Nicotinamide N-Methyltransferase (Nnmt) Deficiency in Male Mice Improves Insulin Sensitivity in Diet-Induced Obesity but Does Not Affect Glucose Tolerance. Diabetes. 2019; 68:527–542. [DOI] [PubMed] [Google Scholar]
- Bratkowski M, Xie T, Thayer DA, Lad S, Mathur P, Yang YS, Danko G, Burdett TC, Danao J, Cantor A, et al. Structural and Mechanistic Regulation of the Pro-degenerative NAD Hydrolase SARM1. Cell Rep. 2020; 32:107999. [DOI] [PubMed] [Google Scholar]
- Camacho-Pereira J, Tarragó MG, Chini CCS, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, Chini EN CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016; 23:1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986; 8:1245–1255. [DOI] [PubMed] [Google Scholar]
- Chen AC, Martin AJ, Choy B, Fernández-Peñas P, Dalziell RA, McKenzie CA, Scolyer RA, Dhillon HM, Vardy JL, Kricker A, et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N Engl J Med. 2015; 373:1618–1626. [DOI] [PubMed] [Google Scholar]
- Chen AC, Martin AJ, Dalziell RA, McKenzie CA, Lowe PM, Eris JM, Scolyer RA, Dhillon HM, Vardy JL, Bielski VA, et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br J Dermatol. 2016; 175:1073–1075. [DOI] [PubMed] [Google Scholar]
- Chini C, Hogan KA, Warner GM, Tarragó MG, Peclat TR, Tchkonia T, Kirkland JL, Chini E. The NADase CD38 is induced by factors secreted from senescent cells providing a potential link between senescence and age-related cellular NAD+ decline. Biochem Biophys Res Commun. 2019; 513:486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini CCS, Peclat TR, Warner GM, Kashyap S, Espindola-Netto JM, de Oliveira GC, Gomez LS, Hogan KA, Tarragó MG, Puranik AS, et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nat Metab. 2020; 2:1284–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini EN Of Mice and Men: NAD+ Boosting with Niacin Provides Hope for Mitochondrial Myopathy Patients. Cell Metab. 2020; 31:1041–1043. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Höke A. Programmed axon degeneration: from mouse to mechanism to medicine. Nat Rev Neurosci. 2020; 4:183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias AJ, Kale A, Perrone R, Lopez-Dominguez JA, Pisco AO, Kasler HG, Schmidt MS, Heckenbach I, Kwok R, Wiley CD, et al. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat Metab. 2020; 2:1265–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2020; 22:119–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila A, Liu L, Chellappa K, Redpath P, Nakamaru-Ogiso E, Paolella LM, Zhang Z, Migaud ME, Rabinowitz JD, Baur JA Nicotinamide adenine dinucleotide is transported into mammalian mitochondria. Elife. 2018; 7:e33246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desdín-Micó G, Soto-Heredero G, Aranda JF, Oller J, Carrasco E, Gabandé-Rodríguez E, Blanco EM, Alfranca A, Cussó L, Desco M, et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. 2020; 368:1371–1376. [DOI] [PubMed] [Google Scholar]
- Di Stefano M, Loreto A, Orsomando G, Mori V, Zamporlini F, Hulse RP, Webster J, Donaldson LF, Gering M, Raffaelli N, et al. NMN Deamidase Delays Wallerian Degeneration and Rescues Axonal Defects Caused by NMNAT2 Deficiency In Vivo. Curr Biol. 2017; 27:784–794. [DOI] [PubMed] [Google Scholar]
- Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, Stødkilde-Jørgensen H, Møller N, Brenner C, Treebak JT, et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018; 108:343–353. [DOI] [PubMed] [Google Scholar]
- Dollerup OL, Chubanava S, Agerholm M, Søndergård SD, Altıntaş A, Møller AB, Høyer KF, Ringgaard S, Stødkilde-Jørgensen H, Lavery GG, et al. Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. J Physiol. 2020; 598:731–754. [DOI] [PubMed] [Google Scholar]
- Dollerup OL, Trammell SAJ, Hartmann B, Holst JJ, Christensen B, Møller N, Gillum MP, Treebak JT, Jessen N. Effects of Nicotinamide Riboside on Endocrine Pancreatic Function and Incretin Hormones in Nondiabetic Men with Obesity. J Clin Endocrinol Metab. 2019; 104:5703–5714. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Coscia F, Chryplewicz A, Chang JW, Hernandez KM, Pan S, Tienda SM, Nahotko DA, Li G, Blaženović I, et al. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature. 2019; 569:723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, Cartwright DM, Oakey L, Burley CV, Jenkinson N, et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Antiinflammatory Signatures. Cell Rep. 2019; 28:1717–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, Milbrandt J. The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD+ Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron. 2017; 93:1334–1343.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essuman K, Summers DW, Sasaki Y, Mao X, Yim AKY, DiAntonio A, Milbrandt J. TIR Domain Proteins Are an Ancient Family of NAD+-Consuming Enzymes. Curr Biol. 2018; 28:421–430.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figley MD, Gu W, Nanson JD, Shi Y, Sasaki Y, Cunnea K, Malde AK, Jia X, Luo Z, Saikot FK, et al. SARM1 is a metabolic sensor activated by an increased NMN/NAD+ ratio to trigger axon degeneration. Neuron. 2021; S0896–6273 00083–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014; 69 Suppl 1:S4–9. [DOI] [PubMed] [Google Scholar]
- Garavaglia S, Bruzzone S, Cassani C, Canella L, Allegrone G, Sturla L, Mannino E, Millo E, De Flora A, Rizzi M. The high-resolution crystal structure of periplasmic Haemophilus influenzae NAD nucleotidase reveals a novel enzymatic function of human CD73 related to NAD metabolism. Biochem J. 2012; 441:131–141. [DOI] [PubMed] [Google Scholar]
- Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD⁺ destruction. Science. 2015; 348:453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi E, Agrimi G, Goldmann U, Fiume G, Lindinger S, Sedlyarov V, Srndic I, Gürtl B, Agerer B, Kartnig F, et al. Epistasis-driven identification of SLC25A51 as a regulator of human mitochondrial NAD import. Nat Commun. 2020; 11:6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroud-Gerbetant J, Joffraud M, Giner MP, Cercillieux A, Bartova S, Makarov MV, Zapata-Pérez R, Sánchez-García JL, Houtkooper RH, Migaud ME, et al. A reduced form of nicotinamide riboside defines a new path for NAD+ biosynthesis and acts as an orally bioavailable NAD+ precursor. Mol Metab. 2019; 30:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozio A, Mills KF, Yoshino J, Bruzzone S, Sociali G, Tokizane K, Lei HC, Cunningham R, Sasaki Y, Migaud ME, et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat Metab. 2019; 1:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozio A, Mills K, Yoshino J, Bruzzone S, Sociali G, Tokizane K, Lei HC, Sasaki Y, Migaud M, Imai SI Reply to: Absence of evidence that Slc12a8 encodes a nicotinamide mononucleotide transporter. Nat Metab. 2019; 1:662–665. [DOI] [PubMed] [Google Scholar]
- Hogan KA, Chini CCS, Chini EN The Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases. Front Immunol. 2019; 10:1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie J, Inagaki E, Fujita M, Nakaya H, Mitsuishi M, Yamaguchi S, Yamashita K, Shigaki S, Ono T, Yukioka H, et al. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr J. 2020; 67:153–160. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liu T, Lee CH, Chang Q, Yang J, Zhang Z. The NAD+-mediated self-inhibition mechanism of pro-neurodegenerative SARM1. Nature. 2020; 588:658–663. [DOI] [PubMed] [Google Scholar]
- Katsyuba E, Romani M, Hofer D, Auwerx J. NAD+ homeostasis in health and disease. Nat Metab. 2020; 2:9–31. [DOI] [PubMed] [Google Scholar]
- Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsström S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J, Suomalainen A. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med. 2014; 6:721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kory N, Uit de Bos J, van der Rijt S, Jankovic N, Güra M, Arp N, Pena IA, Prakash G, Chan SH, Kunchok T, et al. MCART1/SLC25A51 is required for mitochondrial NAD transport. Sci Adv. 2020; 6:eabe 5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer LS, Danhauser K, Herebian D, Petkovic Ramadža D, Piekutowska-Abramczuk D, Seibt A, Müller-Felber W, Haack TB, Płoski R, Lohmeier K, et al. NAXE Mutations Disrupt the Cellular NAD(P)HX Repair System and Cause a Lethal Neurometabolic Disorder of Early Childhood. Am J Hum Genet. 2016; 99:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzli M, Schreiner D, Pereboom TC, Swarnalekha N, Litzler LC, Lötscher J, Ertuna YI, Roux J, Geier F, Jakob RP, et al. Long-lived T follicular helper cells retain plasticity and help sustain humoral immunity. Sci Immunol. 2020; 5:eaay5552. [DOI] [PubMed] [Google Scholar]
- Lauring B, Taggart AK, Tata JR, Dunbar R, Caro L, Cheng K, Chin J, Colletti SL, Cote J, Khalilieh S, et al. Niacin lipid efficacy is independent of both the niacin receptor GPR109A and free fatty acid suppression. Sci Transl Med. 2012; 4:148ra115. [DOI] [PubMed] [Google Scholar]
- Lautrup S, Sinclair DA, Mattson MP, Fang EF NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019; 30:630–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, He A, Chu J, Chen C, Zhang S, He Y, Tao W, Lu M, Hua M, Ju W, Fang Z. Serum N1-methylnicotinamide is Associated with Left Ventricular Systolic Dysfunction in Chinese. Sci Rep. 2018; 8:8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Su X, Quinn WJ 3rd, Hui S, Krukenberg K, Frederick DW, Redpath P, Zhan L, Chellappa K, White E, et al. Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. Cell Metab. 2018; 27:1067–1080.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Kroemer G. Hallmarks of Health. Cell. 2021; 184:33–63. [DOI] [PubMed] [Google Scholar]
- Luongo TS, Eller JM, Lu MJ, Niere M, Raith F, Perry C, Bornstein MR, Oliphint P, Wang L, McReynolds MR, et al. SLC25A51 is a mammalian mitochondrial NAD+ transporter. Nature. 2020; 588:174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malesu R, Martin AJ, Lyons JG, Scolyer RA, Chen AC, McKenzie CA, Madore J, Halliday GM, Damian DL Nicotinamide for skin cancer chemoprevention: effects of nicotinamide on melanoma in vitro and in vivo. Photochem Photobiol Sci. 2020; 19:171–179. [DOI] [PubMed] [Google Scholar]
- Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018; 9:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateuszuk Ł, Campagna R, Kutryb-Zając B, Kuś K, Słominska EM, Smolenski RT, Chlopicki S. Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochem Pharmacol. 2020; 178:114019. [DOI] [PubMed] [Google Scholar]
- Mathialagan S, Bi YA, Costales C, Kalgutkar AS, Rodrigues AD, Varma MVS Nicotinic acid transport into human liver involves organic anion transporter 2 (SLC22A7). Biochem Pharmacol. 2020; 174:113829. [DOI] [PubMed] [Google Scholar]
- McReynolds MR, Chellappa K, Baur JA Age-related NAD+ decline. Exp Gerontol. 2020; 134:110888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016; 24:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S. The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharmacol Sci. 2006; 27:384–390. [DOI] [PubMed] [Google Scholar]
- Nacarelli T, Lau L, Fukumoto T, Zundell J, Fatkhutdinov N, Wu S, Aird KM, Iwasaki O, Kossenkov AV, Schultz D, et al. NAD+ metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol. 2019; 21:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz JC P2X7R: The Achilles heel of follicular helper memory T cells. Sci Immunol. 2020; 5:eaba8097. [DOI] [PubMed] [Google Scholar]
- Piedra-Quintero ZL, Wilson Z, Nava P, Guerau-de-Arellano M. CD38: An Immunomodulatory Molecule in Inflammation and Autoimmunity. Front Immunol. 2020; 11:597959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirinen E, Auranen M, Khan NA, Brilhante V, Urho N, Pessia A, Hakkarainen A, Kuula J, Heinonen U, Schmidt MS, et al. Niacin Cures Systemic NAD+ Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy. Cell Metab. 2020; 31:1078–1090.e5. [DOI] [PubMed] [Google Scholar]
- Poyan Mehr A, Tran M T, Ralto KM, Leaf DE, Washco V, Messmer J, Lerner A, Kher A, Kim SH, Khoury CC, et al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat Med. 2018; 24:1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralto KM, Rhee EP, Parikh SM NAD+ homeostasis in renal health and disease. Nat Rev Nephrol. 2020; 16:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Joffraud M, Trammell SA, Ras R, Canela N, Boutant M, Kulkarni SS, Rodrigues M, Redpath P, Migaud ME, et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat Commun. 2016; 7:13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remie CME, Roumans KHM, Moonen MPB, Connell NJ, Havekes B, Mevenkamp J, Lindeboom L, de Wit VHW, van de Weijer T, Aarts SABM, et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am J Clin Nutr. 2020; 112:413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MS, Brenner C. Absence of evidence that Slc12a8 encodes a nicotinamide mononucleotide transporter. Nat Metab. 2019; 1:660–661. [DOI] [PubMed] [Google Scholar]
- Seman M, Adriouch S, Haag F, Koch-Nolte F. Ecto-ADP-ribosyltransferases (ARTs): emerging actors in cell communication and signaling. Curr Med Chem. 2004; 11:857–872. [DOI] [PubMed] [Google Scholar]
- Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, Deterre P, Haag F, Koch-Nolte F. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity. 2003; 19:571–582. [DOI] [PubMed] [Google Scholar]
- Shats I, Williams JG, Liu J, Makarov MV, Wu X, Lih FB, Deterding LJ, Lim C, Xu X, Randall TA, et al. Bacteria Boost Mammalian Host NAD Metabolism by Engaging the Deamidated Biosynthesis Pathway. Cell Metab. 2020; 31:564–579.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Enriquez A, Rapadas M, Martin EMMA, Wang R, Moreau J, Lim CK, Szot JO, Ip E, Hughes JN, et al. NAD Deficiency, Congenital Malformations, and Niacin Supplementation. N+ Engl J Med.; 377:544–552. [DOI] [PubMed] [Google Scholar]
- Sporny M, Guez-Haddad J, Khazma T, Yaron A, Dessau M, Shkolnisky Y, Mim C, Isupov MN, Zalk R, Hons M, Opatowsky Y. Structural basis for SARM1 inhibition and activation under energetic stress. Elife. 2020; 9:e6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonavane M, Hayat F, Makarov M, Migaud ME, Gassman NR Dihydronicotinamide riboside promotes cell-specific cytotoxicity by tipping the balance between metabolic regulation and oxidative stress. PLoS One. 2020; 15:e0242174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bergen NJ, Guo Y, Rankin J, Paczia N, Becker-Kettern J, Kremer LS, Pyle A, Conrotte JF, Ellaway C, Procopis P, et al. NAD(P)HX dehydratase (NAXD) deficiency: a novel neurodegenerative disorder exacerbated by febrile illnesses. Brain. 2019; 142:50–58. [DOI] [PubMed] [Google Scholar]
- Wilk A, Hayat F, Cunningham R, Li J, Garavaglia S, Zamani L, Ferraris DM, Sykora P, Andrews J, Clark J, et al. Extracellular NAD+ enhances PARP-dependent DNA repair capacity independently of CD73 activity. Sci Rep. 2020; 10:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LE, Sinclair DA The elusive NMN transporter is found. Nat Metab. 2019; 1:8–9. [DOI] [PubMed] [Google Scholar]
- Wu S, Zhang R. CD38-expressing macrophages drive age-related NAD+ decline. Nat Metab. 2020; 2:1186–1187. [DOI] [PubMed] [Google Scholar]
- Yang Y, Mohammed FS, Zhang N, Sauve AA Dihydronicotinamide riboside is a potent NAD+ concentration enhancer in vitro and in vivo. J Biol Chem. 2019; 294:9295–9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MJ, Yoshida M, Johnson S, Takikawa A, Usui I, Tobe K, Nakagawa T, Yoshino J, Imai S. SIRT1-Mediated eNAMPT Secretion from Adipose Tissue Regulates Hypothalamic NAD+ and Function in Mice. Cell Metab. 2015; 21:706–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Baur JA, Imai SI NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018; 27:513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]