Abstract
Background:
Adiponectin, leptin, and pro- and anti-inflammatory cytokines are implicated in breast cancer risk and recurrence. Weight loss, via the dynamic interplay of energy balance through exercise and/or caloric restriction decreases risk of breast cancer recurrence.
Methods:
We investigated the effects of lifestyle modifications (exercise only, or combined caloric restriction and exercise) on adipokines, IL-2, IL-6, IL-8, IL-10, CRP, and TNF-α biomarkers in breast cancer survivors. Searches were completed in June and July of 2019 to identify randomized controlled trials that met inclusion criteria. Weighted mean difference was calculated using random- or fixed- effects models based on the heterogeneity of the studies.
Results:
2501 records were identified with 30 ultimately meeting inclusion criteria of the systematic review. 21 studies provided data suitable for meta-analysis. We observed leptin levels were significantly reduced in the exercise only group compared to sedentary control (WMD −5.66; 95% CI, −11.0 to −0.33, p = 0.04).
Conclusion:
Leptin may be a primary mediator of exercise–induced improvements in breast cancer recurrence.
Impact:
This is the first review and meta-analysis to examine combined exercise and caloric restriction programs in breast cancer survivors. Future studies should further examine combined programs and their efficacy for altering leptin.
Keywords: breast cancer, biomarkers, exercise, diet, cancer survivors
Introduction
In 2018, the five-year survival rate for breast cancer in the United States was 88.6%. (1) Recently reported rates of breast cancer recurrence in large cohorts of survivors followed over multiple decades are 59.7%. (2) Elucidating the mechanistic drivers of breast cancer recurrence and interventions such as diet and exercise to ameliorate these drivers of recurrence is necessary to improve public health recommendations for breast cancer survivors. Obesity results in the elevation of pro-inflammatory mediators that may contribute to breast cancer progression. (3) Mechanistically, excess adiposity may contribute to the risk of breast cancer recurrence by increasing the infiltration of immune cells into adipose tissue which contributes to the production of pro-inflammatory mediators. (4) Elevated inflammatory markers are associated with worse overall survival in survivors of breast cancer suggesting that inflammation may underlie, at least in part, the association between obesity and poor health outcomes in breast cancer survivors. (5–8) It is hypothesized that inflammation contributes to activation of dormant tumor cells which may enhance proliferation, cell survival, invasion/metastasis, and angiogenesis. (4,9)
Multiple cross-sectional studies and reviews suggest that diet and exercise habits play an important role in both risk and recurrence of breast cancer. (10,11) Exercise (caloric expenditure) and diet (caloric restriction), independently and concomitantly have the potential to induce weight loss which may be of particular importance for reducing breast cancer recurrence risk in survivors. From a dietary perspective, dietary restriction –induced weight loss is more important than a change in dietary composition. Two similar studies of breast cancer survivors, one employing a caloric restriction diet (WINS) and the other using an isocaloric diet with more healthful food options (WHEL) found opposing results. (12,13) Participants in the intervention arms of the WINS randomized controlled trial lost an average of 2.4 kg and had a decreased risk for recurrent breast cancer (hazard ratio 0.76; 95% CI, 0.60–0.98). (12) Conversely, the intervention group in the WHEL study noted an average weight gain of 1.1 kg and no decrease in recurrence risk. (13) A suggested mechanism for the weight loss-induced decrease in breast cancer risk is decreased levels of inflammatory cytokines and adipokines from adipocytes. (4) Chronic exercise has anti-inflammatory effects in addition to inducing weight loss, perhaps leading to similar advantages as a weight loss diet. (14–16) Collectively, the effects of exercise, caloric restriction, and weight loss have been consistently observed to reduce risk of recurrence in epidemiologic, observational studies of breast cancer survivors (17,18); however, the mechanistic pathways of this risk reduction are less well understood.
Recent studies have systematically reviewed the effects of diet and exercise on biomarkers of inflammation, metabolism, and sex hormones in breast cancer survivors. (14,19) Meneses-Eschavez and colleagues completed a recent systematic review and meta-analysis encompassing inflammatory markers in breast cancer survivors but excluded combined programs, focusing instead on exercise-only interventions (14). The present systematic review and meta-analysis examines the effects of exercise, as well as combined diet and exercise programs, on biomarkers of inflammation in the breast cancer survivor population. Combined diet and exercise programs have greater efficacy for weight loss as compared to exercise or diet alone. (16,20) Our systematic review and meta-analysis seeks to fill the gap in knowledge by a simultaneous review of exercise-only and combined interventions of exercise with caloric restriction for weight loss in breast cancer survivors. By assessing exercise and caloric restriction interventions broadly, we seek to better elucidate the interplay of energy balance (via any type of exercise and any type of caloric restriction) and the underlying mechanisms leading to decreased breast cancer recurrence risk. This approach reflects free-living conditions where individuals employ a variety of lifestyle changes to induce weight loss.
Materials and Methods
Protocol
We registered our protocol in the PROSPERO database and used the PRISMA statement to guide the drafting of our review. We developed PICO criteria and used these to develop our search strategy, inclusion, and exclusion criteria.
Search Strategy
The search strategy was developed with assistance from all authors and the reference librarian for systematic reviews using the following PICO format. The population was human breast cancer survivors over the age of 18. Survivor was defined as having completed curative therapy including surgery, radiation, or chemotherapy. Ongoing endocrine therapy is common in breast cancer survivors and as such this was not an exclusionary criterion. The interventions were defined as exercise or diet or a combination of the two. Exercise was defined as physical activity which is performed regularly, two to six times per week over months and done with the intention of improving physical fitness or maintaining physical function or health. Diet was defined as a change in eating habits with the goal of weight loss but excluded dietary interventions such as supplements that were not geared towards weight loss. All studies were required to have a control group defined as a group receiving standard of care therapy for the primary outcome of the study and could also include diet and exercise educational material but no specific diet or exercise intervention. Finally, the outcome of the study had to include a pro-inflammatory or anti-inflammatory biomarker including: IL-2, IL-6, IL-8, IL-10, CRP, TNF-α, adiponectin, leptin. Specific biomarkers were selected based on a review of literature including recent systematic reviews and published studies as well as consultation with experts in the field (14).
Searches were conducted in June and July of 2019 and included OVID Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), CINHAL and Scopus. No date limits were placed on the search. The search strategy was developed to identify the study type (randomized controlled trial), disease and population (breast cancer survivors), intervention (exercise, weight loss diet, or both), and outcome (IL-2, IL-6, IL-8, IL-10, CRP, TNF-α, adiponectin, leptin). A detailed search strategy is available in the Supplementary Methods and Materials. One reviewer (TJB) initially reviewed the titles and abstracts of all resulted manuscripts for inclusion or exclusion. Two reviewers (TJB, KMS) then reviewed the full texts resulted from the screening for eligibility and abstracted all relevant data from the included studies. A third reviewer (KHS) acted as a tie breaker in instances of disagreement on inclusion. Both reviewers also closely examined the references of each included manuscript for further texts to include in the review. Respected researchers in the field were also consulted regarding relevant trials either ongoing or published.
Criteria
Included studies met the following criteria: (a) randomized controlled trial including exclusively breast cancer survivors; (b) included a control group that received education only, usual care for the primary outcome of the study, or no intervention; (c) included one or more experimental group(s) that undertook an exercise (aerobic, resistance, yoga, tai chi, or some combination), dietary intervention aimed specifically at weight loss (education, meal replacement, or commercial weight-loss programs), or a program combining any of the accepted exercise and diet interventions; and (d) measured serum concentrations of one or more biomarkers of interest (IL-2, IL-6, IL-8, IL-10, CRP, TNF-α, adiponectin, leptin).
Studies were not excluded based on date published or sex or gender of the patient population. If raw biomarker values were not reported, the first and corresponding author made two attempts to contact the corresponding authors using the provided e-mail address to request raw data in order to include the study in the review. If data was unable to be obtained, the study was excluded from the meta-analysis but was still considered in the systematic review. The biomarker assay methods and coefficients of variation were abstracted and critically reviewed. Both abstractors (TJB, KMS) confirmed that each study to be included had appropriate ethics approval and informed consent.
Data Abstraction
After final selection of included studies, data was abstracted. The following data (with standard error where appropriate) was recorded: country, number of participants, age of participants, eligibility criteria, cancer stage, study design, details on the experimental and control interventions (including duration, session length, number of sessions, and session frequency), number of groups, number of participants per group, intensity of physical activity (for interventions with an exercise component), body composition markers (weight, BMI, waist circumference), biomarker collection timepoints, reported biomarker data (mean, standard error, and measurement tool), and key conclusions. The PEDro scale was used to assess the risk of bias and study quality. (21) This scale is based on the Delphi List and allows for the assessment of internal validity, external validity, and statistical soundness. (22)
Statistical Analysis
When at least two studies were included for an outcome, a meta-analysis was performed. An estimate for each study was calculated and weighted using standard deviation (SD). When change data was not presented, change from baseline to follow-up was calculated with the associated SD using two different correlations (r=0.25 and r=0.5) in order to perform a sensitivity analysis. No difference was noted between the two; therefore, the data using a correlation of r=0.5 was reported. All dependent variables were continuous and weighted mean difference (WMD) was calculated by summing the weighted study estimates (weight*mean difference) and dividing by the sum of all study weights. In fixed analyses, the weight was the inverse of the squared standard error whereas the Paule-Mandel method was used to calculate the weights in random analyses. Analysis models were selected based on the result of heterogeneity testing using Higgin I2 statistic. I2 < 50% indicated absence of heterogeneity and a fixed effects model was used. I2 > 50% indicated the presence of heterogeneity and the Paule-Mandel random effects model was used.
Results
Study Selection and Analysis
A flow diagram detailing the study selection is present in Figure 1. Briefly, 2501 records were identified after duplicates were removed, 2376 were excluded following title and abstract review. Of the 125 remaining studies, 30 met inclusion criteria and were included in the systematic review. Four studies did not report raw quantitative data and therefore were excluded from the meta-analysis. An additional five studies were excluded from the meta-analysis as they did not report mean with standard deviation or error. One study had two exercise intervention arms, one home-based and one supervised. (23) We combined the results from both of these intervention groups into one for analysis as the two were statistically similar. Insufficient studies implementing only caloric restriction were available for analysis and therefore the scope of this meta-analysis and systematic review was narrowed to compare exercise training programs to control and combined (exercise training + caloric restriction) programs to control. No studies with both exercise only and combined intervention arms were available meaning these interventions were not directly compared in our analysis. All included studies limited participants to only female breast cancer survivors.
Figure 1:
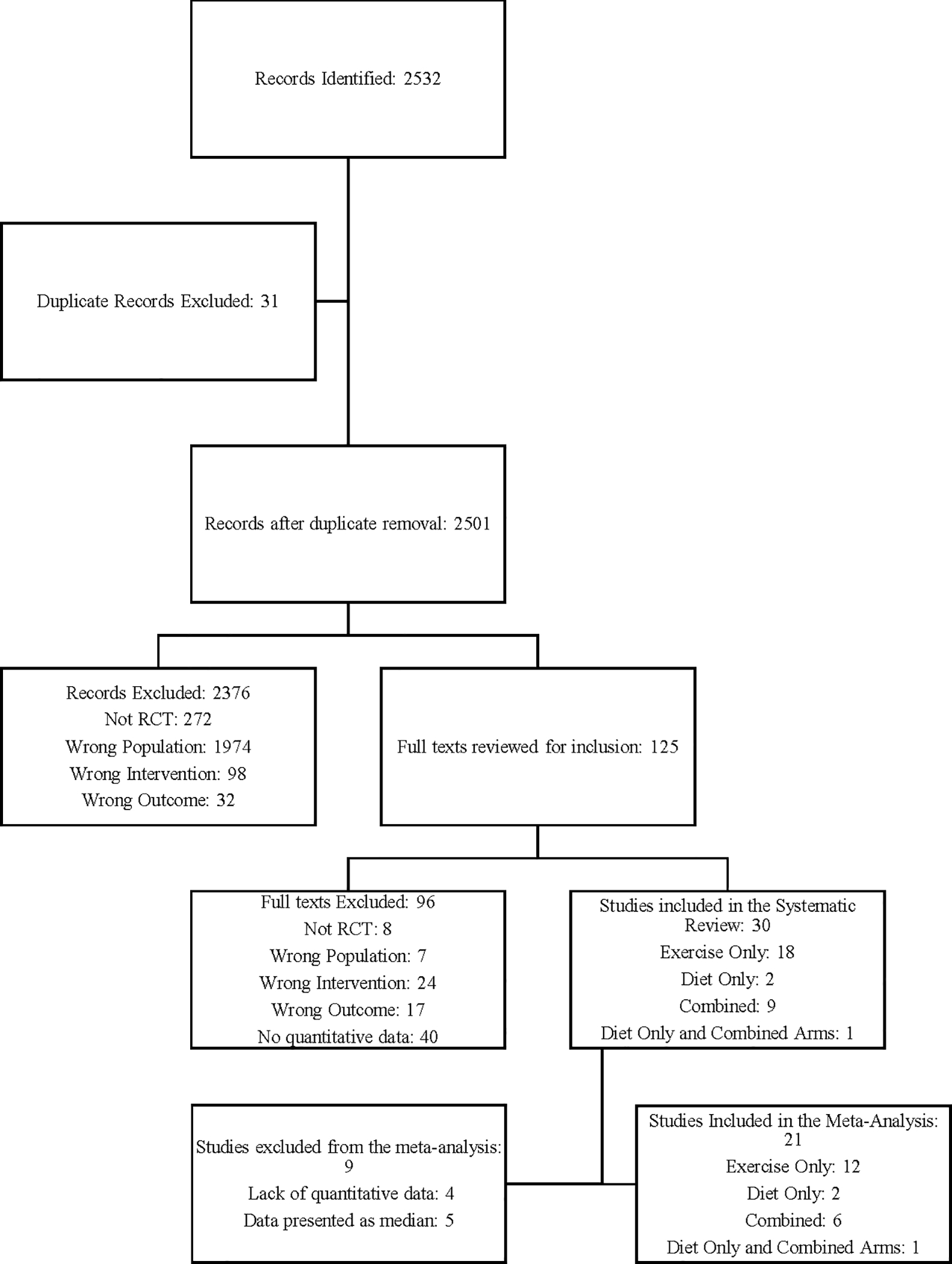
Flow chart of search strategy. The approach to identify eligible studies of inflammatory biomarkers in breast cancer survivors before and following an exercise only intervention, or, combined caloric restriction and exercise intervention, is presented.
A bias analysis was completed and all included studies met a threshold of at least 5 out of 10 on this scale in order to be included (Table 1). The most common unmet criteria was blinding of participants which was met by none of the studies due to the nature of the assessed interventions. A total of twenty studies (67%) explicitly reported blinding measurement assessors making a majority of studies at least partially blinded. A total of 1338 subjects participated in the 21 studies considered in the meta-analysis, an additional 544 participated in the additional 9 studies that were included only in the systematic review. All comparisons in the exercise only group were completed using the Paule-Mandel random effects model as all demonstrated high heterogeneity. In the combined group, Only CRP was compared using the random effects model as all other comparisons demonstrated low heterogeneity so a fixed effects model was used. Detailed characteristics of included studies are included in Table 1.
Table 1:
Assessment of methodologic quality and risk of bias using the PEDro scale. 1 indicates the criteria was met, 0 indicates the criteria was not met.
| Authors | Random Allocation | Concealed Allocation | Similar baseline | Participant blinding | Therapist Blinding | Assessor Blinding | < 15% drop out | Intention to treat analysis | between group difference reported | Point estimate and variability reported | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arikawa (2018) | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Bower (2014) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Dieli-Conwright (2018) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Ergun (2013) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Fairey (2005) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Giallauria (2014) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Gomez (2011) | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 7 |
| Greenlee (2016) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Guinan (2013) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Hagstrom (2016) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Harrigan (2016) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Irwin (2014) | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Janelsins (2011) | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| Jen (2004) | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 5 |
| Jones (2013) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Karimi (2013) | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Kim (2017) | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 |
| Ligibel (2009) | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 4 |
| Murillo-Ortiz (2017) | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
| Pakiz (2011) | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 |
| Patterson (2018) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Payne (2008) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Rogers (2013) | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 7 |
| Rogers (2014) | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Saxton (2014) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Scott (2013) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Sprod (2012) | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| Sturgeon (2018) | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
| Swisher (2015) | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 7 |
| Winters-Stone (2018) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
Insufficient studies reporting common anthropometric variables such as body weight, BMI, waist circumference, lean body mass, or fat mass were available to complete a meta-analysis of any one anthropometric measure and physical activity or the combination of physical activity and caloric restriction. Instead, we systematically reviewed the thirty included studies and found only eleven (37%) reported statistically significant improvements in any anthropometric measure with physical activity or the combination of physical activity and caloric restriction. Of these studies, only three (10%) reported weight loss of greater than five percent (24–26). Studies reviewed are presented in Table 2 with descriptions related to author and publication year, study participants, trial intervention(s), and biomarker outcomes.
Table 2:
Characteristics of included studies.
| Study | Type | Participants | Intervention | Supervision | Outcome |
|---|---|---|---|---|---|
| Arikawa (2018) | Combined | 20 overweight/obese (BMI>27), postmenopausal BC Survivors, curative treatment completed > 3 months prior EXP: n=10; 54.7 +/− 8.4 yrs CON: n=10; 58.4 +/− 7.6 yrs |
12 weeks with 6-week follow-up EXP: 1000 kcal/day deficit diet, mixed aerobic and resistance exercise with weekly increase (20 min to 35 min X 3 to 6/wk) CON: Education (60 min X 1/wk) |
Exercise: combined Diet: All meals provided |
Leptin, Adiponectin, CRP, IL-6, F2-Isoprostanes |
| Bower (2014) | Exercise | 31 BC survivors, curative treatment completed > 6 months prior EXP: n=13; 54.4 +/− 5.7 yrs CON: n=15; 53.3 +/− 4.9 yrs |
12 weeks with 3-month follow-up EXP: Iyengar Yoga (90 min X 2/wk) CON: Education (120 min X 1/wk) |
All supervised | TNF, CRP, IL-6 |
| Dieli-Conwright (2018) | Exercise | 100 sedentary, overweight or obese (BMI > 25, Waist circ. > 88) cm BC survivors (Stage 0-III), curative treatment completed < 6 months prior EXP: n=50; 52.8 +/− 10.6 yrs CON: n=50; 53.6 +/− 10.1 yrs |
16 weeks with 3-month follow-up (only EXP, so excluded third time point) EXP: 80 min (RE and AE) X 2 days/wk X + 50 min (AE only) X 1 day/wk CON: Usual care |
All supervised sessions | Leptin, Adiponectin, CRP, IL-6, IL-8, TNF |
| Ergun (2013) | Exercise | 58 BC survivors, curative treatment completed EXP 1: n=20; 49.65 +/− 8.25 yrs EXP 2: n=18; 55.05 +/− 6.85 yrs CON: n=20; 50.30 +/− 10.37 yrs |
12 weeks EXP 1: 45 mins (AE and RE) X 3 days/wk + 30 min (AE only) X 3 days/wk EXP 2: 30 mins (AE only) X 3 days/wk CON: education session |
EXP 1: Supervised EXP 2: Home |
IL-6, IL-8, TNF |
| Fairey (2005)* | Exercise | 52 BC Survivors, curative treatment completed EXP: n=24; 59 +/− 5 yrs CON: n=28; 58 +/− 6 yrs |
15 weeks EXP: Cycling at 75% O2Peak, 15 min to 35 min X 3 days/wk CON: Usual care |
All Supervised | |
| Giallauria (2014) | Combined | 94 BC Survivors at a high risk for recurrence EXP: n=61; 53.5 +/− 8.6 yrs CON: n=33; 52.3 +/− 7.0 yrs |
52 weeks EXP: Cycle ergometer or treadmill at 75% O2Peak, 30 min X 3 days/wk for 3 months, then 1 day/wk for 9 months; Cooking classes- Mediterranean diet CON: Education only |
Supervised | IL-6, CRP |
| Gomez (2011) | Exercise | 16 BC Survivors, 2–5 years after curative treatment completed that included surgery, chemotherapy, and radiation EXP: n=8; 50 +/− 5.6 yrs CON: n=8; 44.1 +/− 15.8 yrs |
8 weeks EXP: Cycling at 70% to 80% HRmax, 20 min to 30 min X 3 days/wk + RE, 60 min X 3 days/wk CON: Usual Care |
Supervised | IL-2, IL-6, IL-8, IL-10, TNF |
| Greenlee (2016) | Diet | 58 Spanish-speaking, Hispanic BC survivors > 3 months post curative treatment EXP: n=29; 55.1 +/− 9.1 yrs CON: n=29; 58 +/− 10.1 yrs |
12 weeks EXP: Nutrition education: 4 roundtables, 2 shopping trips, 3 cooking classes, 9 classes, 24 hours total CON: Written diet recommendations |
Supervised | IL-6, IL-8, IL-10, TNF, CRP |
| Guinan (2013) | Exercise | 24 BC survivors 2–6 months post curative treatment EXP: n=14; 50.05 +/− 8.27 yrs CON: n=10; 45.05 +/− 9.04 yrs |
8 weeks, with 3-month follow-up EXP: aerobic exercise (cycling, treadmill, and rowing), supervised sessions 21 to 42 min X 2 days/wk, Home sessions 21 to 42 min X 1 to 5 days/wk CON: Usual care |
Combination | CRP |
| Hagstrom (2016)* | Exercise | 32 sedentary BC survivors, completed curative treatment EXP: n=14; 51.2 +/− 8.5 yrs CON: n=18; 52.7 +/− 9.4 yrs |
16 weeks EXP: RE at 80% 1RM, 60 min X 3 days/wk CON: Usual care |
Supervised | IL-6, IL-10 CRP, TNF |
| Harrigan (2016) | Combined | 85 overweight or obese (BMI > 25) BC survivors 3 months to 5 years post curative treatment EXP1: n=30; 58.9 +/− 7.3 yrs EXP2: n=24; 60 +/− 7.7 yrs CON: n=31; 58 +/− 7.5 yrs |
6 months EXP1: nutrition counseling (500 kcal daily deficit) plus moderate intensity exercise counseling (150 min/week moderate intensity), 11 sessions, 4 weekly, then 4 bi-monthly, then 3 monthly. In person EXP2: same as 1 but on the phone CON: Usual care, education available |
Unsupervised | CRP, Leptin, Adiponectin, IL-6, TNF |
| Irwin (2014)* | Exercise | 90 BC survivors, >6 months post curative treatment suffering from insomnia EXP: n=38; 59.6 +/− 7.9 yrs CON: n=43; 60.0 +/− 9.3 yrs |
12 weeks EXP: Tai Chi, 120 mins/week over 3 weekly sessions CON: Cognitive behavioral therapy (standard of care), same time as EXP |
Supervised | CRP |
| Janelsins (2011) Sprod (2011) |
Exercise | 19 BC Survivors 1–30 months post curative treatment EXP: n=9; 54.33 (10.64) yrs CON: n=10; 52.7 (6.67) yrs |
12 weeks, 60 mins X 3 sessions/week EXP: Tai Chi CON: psychosocial support group |
Supervised | |
| Jen (2004) | Diet and Combined Arms | 48 obese (BMI > 30) BC survivors, 3 to 48 months post curative treatment EXP1: n=8 EXP2: n=9 EXP3: n=10 CON: n=12 No ages reported, inclusion criteria of 18 to 70 years old |
12 months EXP1: Weekly Weight Watchers EXP2: Individualized diet and exercise counseling weekly for 3 months, bi weekly for 3 months, monthly for 6 months EXP3: combined EXP½ CON: education material provided |
Supervised | Leptin |
| Jones (2013) | Exercise | 68 Sedentary BC survivors, at least 6 months post curative treatment EXP: n=36; 56.4 (9.6) yrs CON: n=32; 55.4 (7.6) yrs |
6 months EXP: moderate intensity aerobic exercise (60–80% HR Max) 15 to 30 mins X 5 sessions/week, 3 supervised, 2 at home CON: Usual care |
Combination | IL-6, CRP, TNF |
| Karimi (2013) | Exercise | 40 BC survivors, we excluded two supplement groups in analysis (n=20) EXP: n=10; 48 (6) yrs CON: n=10; 48 (6) yrs |
6 weeks EXP: water-based exercise (50–75% HRR) 40 to 80 min X 4 sessions/week CON: Placebo, usual care |
Supervised | Adiponectin |
| Kim (2017) | Exercise | 24 sedentary (<60 mins exercise/week) BC survivors at least 6 months post curative treatment EXP: n=11; 56 (6.5) CON: n=13; 49.3 (4.8) |
12 weeks EXP: combined AE/RE for 40 min X 3 sessions/week CON: Usual care |
Supervised, additional unsupervised encouraged | CRP, Adiponectin, Leptin |
| Ligibel (2009) | Exercise | 83 sedentary, overweight or obese (BMI > 25), BC survivors at least 3 months post curative treatment EXP: n=40; 52 (9) yrs CON: n=43; 53 (9) yrs |
16 weeks EXP: RE for 50 min X 2 sessions/week supervised; AE X 90 min/week, self-determined |
RE supervised, AE unsupervised | Leptin, Adiponectin |
| Murillo-Ortiz (2017) | Diet | 100 post-menopausal BC survivors on tamoxifen at least 1 year after diagnosis and having completed curative treatment EXP: n=50; 50.45 (7.94) yrs CON: n=50; 52.26 (6.11) yrs |
6 months EXP: low-fat diet aimed at weight loss; 5 meals/day provided CON: typical diet, 5 meals/day provided |
Supervised (all meals provided) | Adiponectin |
| Patterson (2018)* | Combined | 154 post-menopausal, non-diabetic, BC Survivors, within 10 years of completing curative treatment EXP: n=78 CON: n=76 |
6 months EXP: 12 phone counseling sessions encouraging 500–1000 kcal deficits/day and 300 minutes of walking/week CON: educational material |
Unsupervised | CRP |
| Pakiz (2011) | Combined | 68 Overweight or obese (BMI > 25) BC survivors within 14 years of curative treatment | 16 weeks EXP: Weekly group education sessions on weight loss with individualized phone follow-up CON: Waitlist |
Unsupervised | TNF, IL-6, IL-8 |
| Payne (2008)* | Exercise | 18 postmenopausal BC survivors with fatigue symptoms on hormonal therapy EXP: n=9 CON: n=9 |
12 weeks EXP: walking, 20 min X 4 session/week CON: Usual care |
Unsupervised | IL-6 |
| Rogers (2013) | Exercise | 22 sedentary BC survivors having completed curative treatment at least 8 weeks prior EXP: n=12; 58.0 (6.1) CON: n=10; 53.8 (13.9) |
12 weeks EXP: Supervised exercise program transitioning to at home gradually over first 6 weeks. Goal 150 min AE per week and 2 RE sessions per week. Group and individual counseling CON: written educational material |
Combined | IL-6, IL-8, IL-10, TNF, Leptin, Adiponectin |
| Rogers (2014) | Exercise | 42 Postmenopausal BC survivors at least 4 weeks post curative treatment EXP: n=18; 57.2 (5.5) yrs CON: n=22; 55.2 (9.1) yrs |
12 weeks EXP: AE 40 min X 4 sessions/week, 50% supervised, RE X 2 sessions/week, all supervised, 6 support groups over duration CON: Usual care |
Combined | IL-6, IL-8, IL-10, TNF |
| Saxton (2014)* Scott (2013)* |
Combined | 85 Overweight or obese (BMI > 25) BC survivors 3–18 months post curative treatment EXP: n=44; 55.8 (10) yrs CON: n=41; 55.3 (8.8) yrs |
24 weeks EXP: AE 30 mins, RE 10–15 mins X 3 sessions/week, supervised at 65–85% of maximum heart rate, Individualized diet counseling and education for a daily 600 kcal deficit CON: usual care |
Supervised | IL-6, TNF, CRP, Leptin |
| Sturgeon (2018) | Combined | 35 Overweight (BMI > 23) BRCA ½ positive BC survivors having completed curative treatment >4 months prior and prophylactic oophorectomy >2 years prior EXP: n=19; 45.1 (4) yrs CON: n=16; 47.2 (3.8) yrs |
12 Months EXP: RE 3 days/week, AE 2 days/week, active recovery 1 day/week, total 160 minutes of exercise; Daily online education and new nutrition habits every 2 weeks CON: Usual Care |
Unsupervised | IL-6, IL-8, TNF |
| Swisher (2015)* | Combined | 23 Overweight (BMI > 25) BC survivors at least 3 months post curative treatment EXP: n=13; 53.8 (43–65) yrs (range) CON: n=10; 53.6 (36–71) yrs (range) |
12 weeks EXP: AE 30 min X 3 days/week supervised, 2 days/week at home; 2 diet counseling sessions CON: Educational material |
Combined | CRP, IL-6, TNF, Adiponectin, Leptin |
| Winters-Stone (2018) | Exercise | Postmenopausal BC survivors at least 1 year post curative treatment, do not regularly perform RE EXP: n=109; 59.8 (11.4) yrs CON: n=106; 59.3 (11.6) yrs |
12 months EXP: RE, 60 min X 2 days/week supervised, 45 mins X 1 day/week at home, 60–80% 1RM CON: stretching program with same time commitment |
Combined | CRP, IL-6, IL-8, TNF, Adiponectin, Leptin |
indicates studies not included in the meta-analysis. Abbreviations: EXP: experimental group. CON: control group. AE: aerobic exercise. RE: resistance exercise.
Leptin
A total of nine studies reported leptin levels. Five studies (56%, 5/9) in the exercise-only group involving 363 subjects included leptin as a biomarker (Figure 2A). (27–31) We observed leptin levels were significantly reduced in the exercise groups as compared to the control (WMD −5.66; 95% CI, −11.0 to −0.33, p = 0.04). A sensitivity analysis (Supplementary Figure S1) was completed which revealed that removal of Dieli-Conwright (2018) (27), Kim (2017) (28), or Rogers (2013) (30) caused the comparison to become nonsignificant (p > 0.05). There were no differences (p = 0.14) in the combined group compared to controls, although only two studies (22%, 2/9) reported leptin in this group (Figure 2B). (24,25) Two additional studies (22%, 2/9) were excluded from the combined analysis as only median and inter-quartile range were reported. Scott and colleagues reported a median decrease in leptin of 3,351 pg/ml in the intervention group (45 min aerobic exercise + resistance exercise and 600 kcal deficit daily diet counseling) which was significantly different than an increase of 4,553 pg/ml in the control group (p = 0.005). Furthermore, a change in body weight and waist circumference was associated with change in leptin in both the intervention and control groups (p < 0.01) (32). Similarly, Swisher and colleagues report a decrease in leptin in a combined intervention but their results did not reach significance (33).
Figure 2:
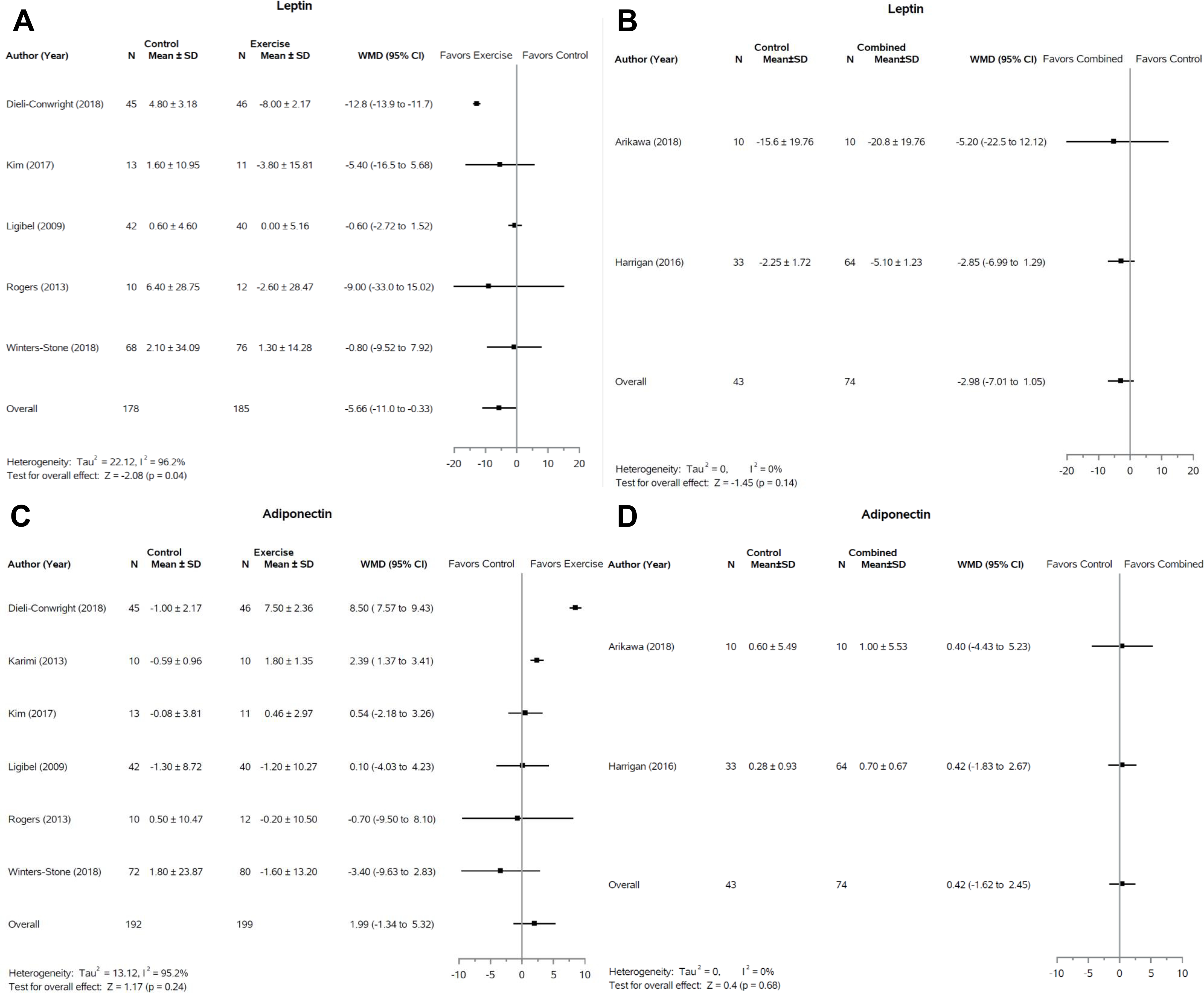
Forest plots of change in adipokine levels following exercise only or combination interventions. Response of leptin to exercise interventions (A) and combined (weight loss + exercise) interventions (B) are presented. Response of adipokine to exercise interventions (C) and combined interventions (D) are also displayed. SD, standard deviation; WMD, weighted mean difference; CI, confidence interval.
Adiponectin
A total of nine studies reported adiponectin levels. There was no significant effect of exercise alone or in combination with dietary restriction on adiponectin (Figure 2C and 2D). All studies evaluating exercise interventions (66%, 6/9) that reported adiponectin were included in the meta-analysis (27–31,34). One study (11%, 1/9) (33), in the combined group was excluded from the meta-analysis but reported no change in adiponectin in either the control or intervention groups as did both studies (22%, 2/9) included in the meta-analysis (24,25).
C-Reactive Protein
There was no significant effect of exercise alone or in combination with dietary restriction on CRP (Figure 3A and 3B). Six (40%, 6/15) (28,35–38) and three (20%, 3/15) (24,25,39) studies, respectively, were included in the meta-analysis of exercise and combined interventions (Fifteen studies included CRP levels). All exercise studies not included in the meta-analysis reported no significant change in CRP in the intervention group (27%, 4/15) (40–43). Scott and colleagues reported that change in body weight and waist circumference was associated with change in CRP across control and combined intervention groups (p < 0.01) but change in CRP was not significantly different between groups (32). Swisher and colleagues also found no significant change in CRP between combined intervention and control groups (33).
Figure 3:
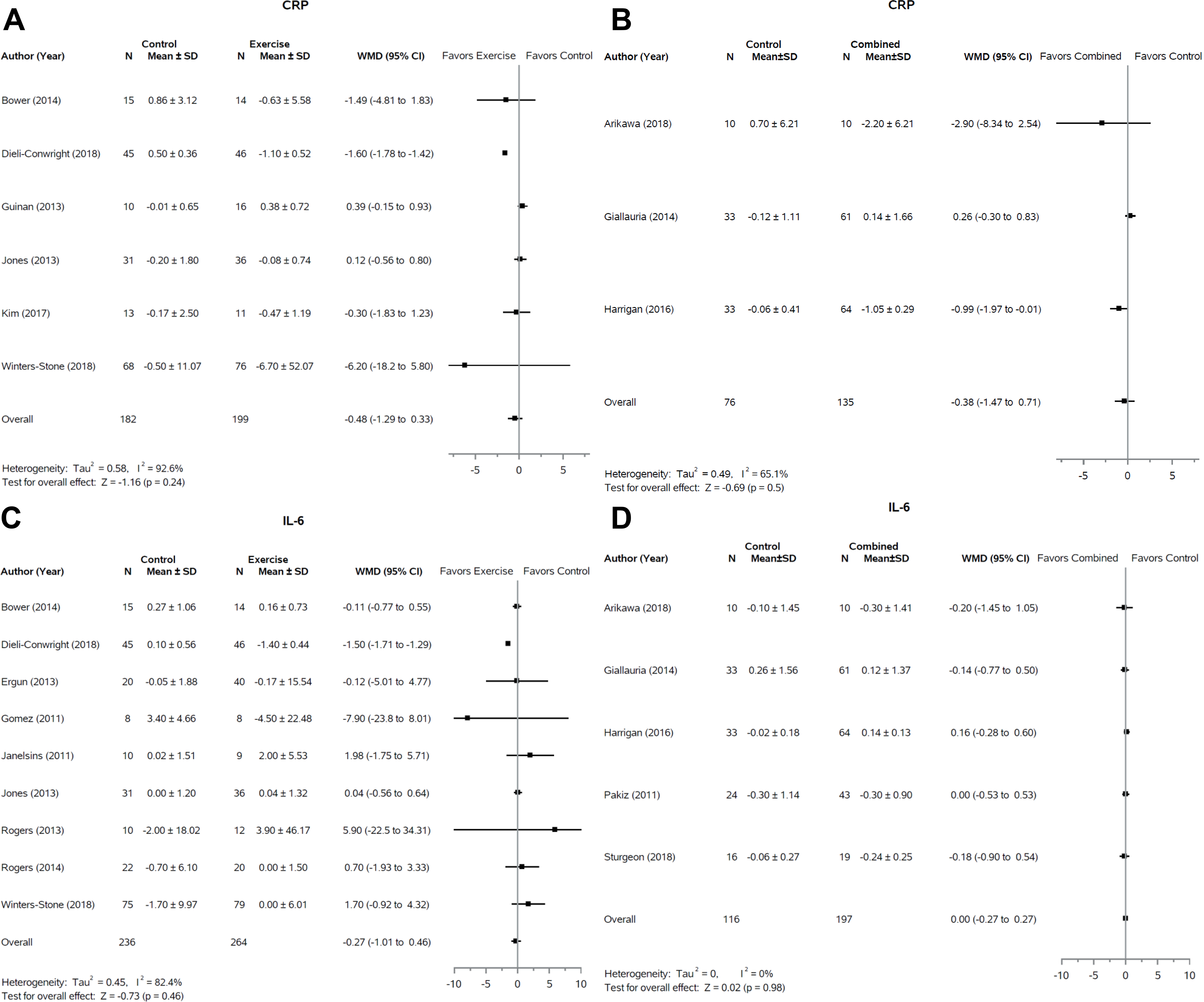
Forest plots of change in CRP and IL-6 levels following exercise only or combination interventions. Response of CRP to exercise interventions (A) and combined (weight loss + exercise) interventions (B) are presented. Response of IL-6 to exercise interventions (C) and combined interventions (D) are also displayed. SD, standard deviation; WMD, weighted mean difference; CI, confidence interval.
IL-6
The largest number of studies, both in the exercise only and combined groups reported data on IL-6 (nineteen total studies), nine (47%, 9/19) and five (26%, 5/19) studies respectively (Figure 3C and 3D). In both cases there was no effect of the intervention compared to the control (p = 0.46 and p = 0.98 respectively). For the exercise and combined groups, an additional two studies each (11%, 2/19) also reported no significant differences between control and intervention groups but were not included in the meta-analysis (33,41,44,45).
TNF-α
There was no significant effect of exercise or combined interventions on TNF-α across fourteen studies (Figure 4A and 4B). Eight studies (57%, 8/14) were included in the exercise only group, totaling 481 subjects, (23,30,35,36,38,46,47). One study (7%, 1/14) was excluded from the analysis which also showed no significant effect of exercise on TNF-α (41). Three studies (21%, 3/14) in the combined group were included in the meta-analysis (25,26,48) while two (14%, 2/14) were excluded (33,45). Swisher and colleagues reported an increase in TNF-α in the intervention group compared to no change in the control group that approached significance (p=0.11) (33).
Figure 4:
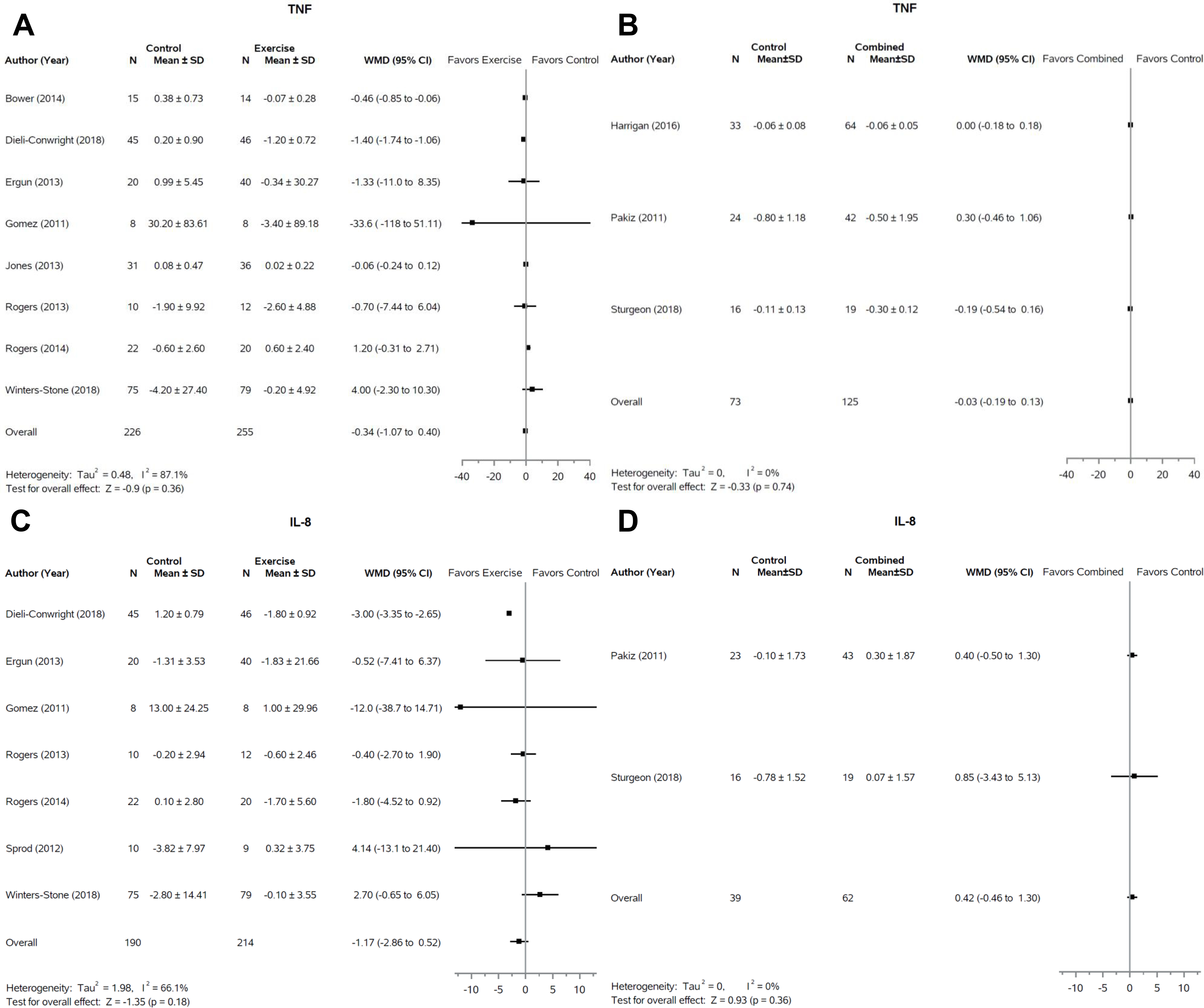
Forest plots of change in TNF-α and IL-8 levels following exercise only or combination interventions. Response of TNF-α to exercise interventions (A) and combined (weight loss + exercise) interventions (B) are presented. Response of IL-8 to exercise interventions (C) and combined interventions (D) are also displayed. SD, standard deviation; WMD, weighted mean difference; CI, confidence interval.
IL-8
All studies that evaluated IL-8 were included in the meta-analysis with seven (77%, 7/9) in the exercise group (23,27,30,31,46,47) and two (22%, 2/9) in the combined group (26,48). No significant effects of either intervention type on IL-8 were reported.
IL-10
No combined intervention studies reported data on IL-10. Three studies (75%, 3/4) in the exercise group were included in the meta-analysis (30,46,47). There was no difference between the exercise intervention or control groups (P=0.46) and one study (25%, 1/4) was excluded but also demonstrated no significant difference between control and intervention groups (41).
IL-2
Due to limited study availability, IL-2 was excluded from the meta-analysis. No studies with combined interventions were available for analysis. Two studies with exercise only interventions both noted no significant results (46,49).
Discussion
Our systematic review and meta-analysis assessing the effects of exercise-only interventions, as well as, combined exercise and caloric restriction interventions on biomarkers of inflammation in breast cancer survivors revealed a significant effect of exercise on leptin but otherwise demonstrated no significant effects of exercise or combined exercise and caloric restriction interventions on biomarkers of inflammation. The significant decrease in leptin levels via exercise interventions appears to be independent of change in any anthropometric measures. We also observed that despite a combined program of caloric restriction with exercise, there was no uniform decrease in anthropometric measures. A lack of change in inflammatory biomarkers in the combined group may be explained by the lack of significant weight loss.
Elevated leptin levels due to excess or dysfunctional adipose tissue are thought to increase pro-inflammatory signaling that subsequently increases the likelihood of breast cancer cell reactivation out of dormancy in survivors (50). Our meta-analysis is the first to report a significant effect of exercise on leptin in the breast cancer survivor population. Additional studies evaluating the combination of exercise and dietary restriction should assess leptin to determine if the beneficial effect observed with exercise is magnified by the combined dietary energy restriction and exercise. A recent review and meta-analysis of 72 trials found exercise interventions of greater than 2 weeks across numerous populations including breast, prostate, and colon cancer as well as chronic diseases such as hypertension and diabetes significantly improved leptin levels as compared to control (51). This improvement was associated with a decrease in percent body fat. While significant, the results of our leptin analysis should be interpreted critically as demonstrated by our sensitivity analysis. All five studies in our analysis employed combined aerobic and resistance exercise interventions (27–31). Weekly time spent exercising varied from 120 to 210 minutes. Winters-Stone and colleagues employed a twelve month intervention while the other included studies were 12–16 weeks. Dieli-Conwright and colleagues as well as Kim and colleagues used entirely supervised interventions with participants attending three exercise session per week. Rogers and colleagues also used a similar supervised model that gradually transitioned to an unsupervised at home exercise routine over the first six weeks of the twelve-week intervention. Conversely, the two studies that did not independently drive the leptin analysis both used supervised resistance training sessions but encouraged unsupervised aerobic exercise at home (29,31). Supervision of exercise sessions therefore may have increased adherence to the intervention leading to a greater improvement in leptin levels. Further studies with large populations are still necessary to validate our findings.
Our meta-analysis on leptin levels for the combined group only included two studies totaling 117 subjects. Two additional studies with a total of 106 subjects were not included in the meta-analysis but may have influenced the overall effect had they been included (32,33). Scott and colleagues implemented a 600 kcal/day caloric deficit diet combined with three weekly exercise sessions in 83 subjects and found a median decrease of 3,351 pg/ml in the intervention group compared to an increase of 4,553 pg/ml in the control group (p=0.005) (32). Change in leptin was also associated with change in body weight and waist circumference in this study. Winters-Stone and colleagues reported no significant effect of a resistance training intervention on leptin; however, following a sub-group analysis they found that women in the intervention group who lost weight noted a statistically significant decrease in leptin (31). The relationship between leptin and body adiposity suggests that weight loss may be the driver of the decrease in leptin levels. While we did not observe significant weight loss in our meta-analysis, all studies included in the exercise only leptin analysis employed exercise regimens with aerobic and resistance training components suggesting that changes in body composition may better explain the observed benefit. (27–31) Exercise programs with both aerobic and resistance training therefore may decrease fat mass while also increasing lean body mass leading to no change in overall body mass while still decreasing leptin levels. The total number of participants in the combined group was also less than that of the exercise group analysis suggesting our meta-analysis may not have had sufficient power to demonstrate significant differences in this group.
An important limitation of our simultaneous review and meta-analysis is the inability to directly compare exercise interventions to combined interventions as such a comparison would require both groups in the same study. Indeed, clinic trials such as WISER Survivor (NCT01515124) are designed specifically to make these direct comparisons by including three intervention arms and a control arm. Forthcoming biomarker analysis from this and similar studies will allow for direct comparison of these diverse interventions and the effects on biomarkers.
A previous meta-analysis of 13 cohort studies reported that elevated adiponectin levels were associated with a decreased breast cancer risk implicating the importance of increasing adiponectin to prevent recurrent neoplasm (52). Adiponectin is hypothesized to exert a cancer prevention effect by mediating apoptosis and reducing proliferation of neoplastic cells (53). Past studies in healthy populations and breast cancer survivors have demonstrated variable effects of exercise on adiponectin (14,54,55). Our meta-analysis is consistent with these findings demonstrating a variable effect in both the combined and exercise only groups (Figure 2C, D). Although no prior reports examine the effects of combined weight loss and exercise programs on adiponectin, a review of caloric restriction diets reported a highly variable effect of caloric restriction on adiponectin levels with a majority of reviewed trials reporting no effect (56). The aforementioned review also postulates that weight loss of at least 10% is necessary to induce increases in adiponectin as studies with weight loss < 10% universally did not induce increases in adiponectin (56). Only eleven of the thirty studies included in our review report statistically significant changes in body composition with only one (24) reporting a 10% decrease in weight suggesting the weight loss accomplished in these studies may be insufficient to increase adiponectin levels.
A significant number of studies have explored the relationship between inflammatory mediators and breast cancer recurrence, as chronic inflammation is a link to metastatic progression (57). Across all pro- and anti-inflammatory markers that we analyzed, including IL-2, IL-8, IL-10, CRP, and TNF-α, no significant effects of exercise-only interventions or combined interventions on any of the inflammatory mediators in breast cancer survivors were observed. Our findings are consistent with prior meta-analyses of exercise only interventions (14,55); however, Monteiro-Junior and colleagues report a significant effect of exercise on CRP (p=0.007) and a near significant effect on TNF-α (p=0.07) in a meta-analysis of exercise interventions in healthy older adults (58). Whether this reflects a variation in inflammatory markers and response in breast cancer survivors versus the general elderly population is unknown.
Our review found no significant effect of exercise-only interventions or combined interventions on IL-6. This differs from one prior meta-analysis which found significant beneficial effects of exercise interventions on IL-6 in breast cancer survivors (14); however, a more recent meta-analysis was consistent with our results (55). The lack of a beneficial effect of exercise on IL-6 levels that we and others (55) have observed may be specific to breast cancer patients. A recent meta-analysis of exercise interventions in healthy older adults found a significant beneficial effect of exercise on IL-6 (58). This suggests that breast cancer survivors may have impaired response to exercise interventions relative to a non-cancer population. Such observations are compelling given that chronically elevated plasma levels of IL-6 are associated with reduced survival in breast cancer survivors (5).
Timing of cytokine sampling is an important methodological issue to consider. We were unable to ascertain the timing of blood sampling from individual studies reported in the current review. Additionally, it may be that the type of activity/dietary intervention and time since cancer treatment are important variables influencing the relationship between activity and circulating cytokines in breast cancer survivors. Lastly, inflammatory cytokines are produced and released from a variety of immune and other cell types. We have demonstrated that inflammatory markers are reliably detected in circulating immune cells or in response to in vitro stimulation, but are not consistently raised in plasma in healthy, older adults (59). Thus, exercise and the combination of dietary restriction and exercise interventions may be altering inflammatory cytokine production, but by assaying these mediators in plasma rather than from isolated cells, no detectable effect is observed. Indeed, several studies we reviewed examined functional immune outcomes such as natural killer cell cytotoxic activity and cytokine production (40,41). Both reported significant beneficial changes in intervention groups (both exercise) suggesting that evaluation of additional immunological endpoints in future studies may provide greater insight into the effects of exercise-only interventions and combined weight loss programs on the biological pathways that may be important in mediating a weight loss-induced reduction in breast cancer recurrence. Due to the low number of studies with these markers that met other inclusion criteria in our review, we did not analyze these markers in our meta-analysis.
As individuals advance in age, maintaining healthy weight and fitness levels are increasingly difficult due to the natural aging process. Indeed, two studies report stable levels of TNF-α and IL-8 respectively in intervention groups (both combined interventions) compared to worsening levels in control groups (32,33). Future analysis should consider these observations as maintaining health in this population may be a more realistic goal than outright improvement at least at the biomarker level. Long-term follow-up will also be necessary to determine if exercise and/or weight loss diet interventions lead to lasting health maintenance and diminished recurrent risk of breast cancer.
Overall, many studies report variable adherence to study protocols and variable definitions of adherence, indicating the importance of compliance for data integrity and efficacy of exercise-only and combined interventions. Winters-Stone and colleagues had the largest population in our analysis and found that increased strength was associated with decreased CRP (31). Furthermore, subjects who had no change in strength also had increases in IL-6 suggesting that marked changes in body composition drive biomarker response (31). The use of clinical markers of adherence such as measurable improvements in fitness, weight, or other markers of body composition allows for greater stratification of study populations that may clarify the effects of exercise, diet-induced weight loss, and combined programs on biomarkers of inflammation in breast cancer survivors. We were unable to perform a stratified meta-analysis based on compliance, intervention specifics such as exercise type or intervention duration, or degree of weight loss as doing so limited many groups to single studies. Further, the goal of this meta-analysis was to assess the effects of exercise and weight loss on inflammatory markers regardless of intervention specifics. Future analyses as more studies become available would benefit from such stratification.
There are several limitations to our study. First, our sensitivity analysis revealed that our significant results of exercise only interventions on leptin levels were significantly altered by the removal of any one of three manuscripts. Our results therefore must be cautiously interpreted and continually reassessed as studies with a greater number of participants continue to become available. Second, across our meta-analysis the number of studies with combined exercise and diet weight loss groups was limited. Furthermore, two prominent studies with combined intervention groups were excluded from the meta-analysis due to the data formatting issues (data was presented as median with interquartile ratio) (32,33). Collectively, the ability to only include two studies for many outcomes limited the power of this analysis and may have contributed to our largely null results. Despite this limitation, combined interventions often had similar results to exercise only interventions suggesting a need for more studies including both types of lifestyle interventions to allow for direct comparison of the interventions. Additionally, our definition of exercise-only interventions was broad. We included exercise-only interventions that encompassed aerobic exercise, resistance exercise, yoga, tai chi, or some combination. Thus, frequency, intensity, time, and type (F.I.T.T. exercise physiology principles) may be different between exercise-only interventions, and modulation of F.I.T.T can yield different physiological responses. Despite this limitation, the goal of our review is broadened by it in that we sought to assess changes in energy balance via decreasing energy in or increasing energy out, without regard for method to accomplish the change in energy balance.
Our review and meta-analysis reveals that exercise interventions and combined exercise and diet interventions had little effect on circulating inflammatory mediators. However, exercise had a significant effect on leptin levels in breast cancer survivors suggesting that leptin may be a possible mediator of exercise–induced changes in breast cancer recurrence. Future studies exploring the combination of exercise and dietary restriction should evaluate leptin to determine if the reduction in leptin observed following exercise alone is enhanced by the combination of exercise and dietary restriction. Additional mechanistic studies are needed to explore if leptin is contributing to the exercise-induced reduction in breast cancer recurrence. (10)
Supplementary Material
Acknowledgments
We would like to thank Esther Dell and Amy Knehans for their guidance regarding systematic review methodology and protocol.
Financial Support: This publication was supported, in part, by Grant 5UL1TR002014 and 5KL2TR002015 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
References
- 1.U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2017 submission data (1999–2015). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; <https://gis.cdc.gov/Cancer/USCS/DataViz.html.> Accessed 2019. [Google Scholar]
- 2.Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thurlimann B, et al. Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results From the International Breast Cancer Study Group Trials I to V. J Clin Oncol 2016;34:927–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argolo DF, Hudis CA, Iyengar NM. The Impact of Obesity on Breast Cancer. Curr Oncol Rep 2018;20:47. [DOI] [PubMed] [Google Scholar]
- 4.Crespi E, Bottai G, Santarpia L. Role of inflammation in obesity-related breast cancer. Curr Opin Pharmacol 2016;31:114–22 [DOI] [PubMed] [Google Scholar]
- 5.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol 2009;27:3437–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J Nutr 2020;150:663–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agnoli C, Berrino F, Abagnato CA, Muti P, Panico S, Crosignani P, et al. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: a nested case-control study. Nutr Metab Cardiovasc Dis 2010;20:41–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 2010;123:627–35 [DOI] [PubMed] [Google Scholar]
- 9.Modzelewska P, Chludzinska S, Lewko J, Reszec J. The influence of leptin on the process of carcinogenesis. Contemp Oncol (Pozn) 2019;23:63–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiseman M The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 2008;67:253–6 [DOI] [PubMed] [Google Scholar]
- 11.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014;25:1901–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst 2006;98:1767–76 [DOI] [PubMed] [Google Scholar]
- 13.Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 2007;298:289–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meneses-Echavez JF, Correa-Bautista JE, Gonzalez-Jimenez E, Schmidt Rio-Valle J, Elkins MR, Lobelo F, et al. The Effect of Exercise Training on Mediators of Inflammation in Breast Cancer Survivors: A Systematic Review with Meta-analysis. Cancer Epidemiol Biomarkers Prev 2016;25:1009–17 [DOI] [PubMed] [Google Scholar]
- 15.Nieman DC, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci 2019;8:201–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev 2006:CD003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiPietro L, Buchner DM, Marquez DX, Pate RR, Pescatello LS, Whitt-Glover MC. New scientific basis for the 2018 U.S. Physical Activity Guidelines. J Sport Health Sci 2019;8:197–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McTiernan A, Friedenreich CM, Katzmarzyk PT, Powell KE, Macko R, Buchner D, et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med Sci Sports Exerc 2019;51:1252–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meneses-Echavez JF, Jimenez EG, Rio-Valle JS, Correa-Bautista JE, Izquierdo M, Ramirez-Velez R. The insulin-like growth factor system is modulated by exercise in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer 2016;16:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz KH, Troxel AB, Dean LT, DeMichele A, Brown JC, Sturgeon K, et al. Effect of Home-Based Exercise and Weight Loss Programs on Breast Cancer-Related Lymphedema Outcomes Among Overweight Breast Cancer Survivors: The WISER Survivor Randomized Clinical Trial. JAMA Oncol 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cashin AG, McAuley JH. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J Physiother 2019 [DOI] [PubMed] [Google Scholar]
- 22.Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235–41 [DOI] [PubMed] [Google Scholar]
- 23.Ergun M, Eyigor S, Karaca B, Kisim A, Uslu R. Effects of exercise on angiogenesis and apoptosis-related molecules, quality of life, fatigue and depression in breast cancer patients. European Journal of Cancer Care 2013;22:626–37 [DOI] [PubMed] [Google Scholar]
- 24.Arikawa AY, Kaufman BC, Raatz SK, Kurzer MS. Effects of a parallel-arm randomized controlled weight loss pilot study on biological and psychosocial parameters of overweight and obese breast cancer survivors. Pilot and Feasibility Studies 2018;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, et al. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: The lifestyle, exercise, and nutrition (LEAN) study. Journal of Clinical Oncology 2016;34:669–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pakiz BF SW:Bardwell WA:Rock CL:Mills PJ Effects of a weight loss intervention on body mass, fitness, and inflammatory biomarkers in overweight or obese breast cancer survivors. International Journal of Behavioral Medicine 2011;18:333–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Buchanan TA, et al. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: A randomized controlled trial. Journal of Clinical Oncology 2018;36:875–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim TH, Chang JS, Park KS, Park J, Kim N, Lee JI, et al. Effects of exercise training on circulating levels of Dickkpof-1 and secreted frizzled-related protein-1 in breast cancer survivors: A pilot single-blind randomized controlled trial. PLoS One 2017;12:e0171771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ligibel JAG-H A:Olenczuk D:Campbell N:Salinardi T:Winer EP:Mantzoros CS Impact of a mixed strength and endurance exercise intervention on levels of adiponectin, high molecular weight adiponectin and leptin in breast cancer survivors. Cancer Causes & Control 2009;20:1523–8 [DOI] [PubMed] [Google Scholar]
- 30.Rogers LQF A:Trammell R:Hopkins-Price P:Vicari S:Rao K:Edson B:Verhulst S:Courneya KS:Hoelzer K Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integrative Cancer Therapies 2013;12:323–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winters-Stone KM, Wood LJ, Stoyles S, Dieckmann NF. The effects of resistance exercise on biomarkers of breast cancer prognosis: A pooled analysis of three randomized trials. Cancer Epidemiology Biomarkers and Prevention 2018;27:146–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott E, Daley AJ, Doll H, Woodroofe N, Coleman RE, Mutrie N, et al. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long-term prognosis after early-stage breast cancer: A randomized controlled trial. Cancer Causes and Control 2013;24:181–91 [DOI] [PubMed] [Google Scholar]
- 33.Swisher AK, Abraham J, Bonner D, Gilleland D, Hobbs G, Kurian S, et al. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Supportive Care in Cancer 2015;23:2995–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karimi NR VD Change in adiponectin and oxidative stress after modifiable lifestyle interventions in breast cancer cases. Asian Pacific Journal of Cancer Prevention: Apjcp 2013;14:2845–50 [DOI] [PubMed] [Google Scholar]
- 35.Bower JE, Greendale G, Crosswell AD, Garet D, Sternlieb B, Ganz PA, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Psychoneuroendocrinology 2014;43:20–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dieli-Conwright CM, Parmentier JH, Sami N, Lee K, Spicer D, Mack WJ, et al. Adipose tissue inflammation in breast cancer survivors: effects of a 16-week combined aerobic and resistance exercise training intervention. Breast Cancer Research and Treatment 2018;168:147–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guinan EH J:Broderick JM:Lithander FE:O’Donnell D:Kennedy MJ:Connolly EM The effect of aerobic exercise on metabolic and inflammatory markers in breast cancer survivors--a pilot study. Supportive Care in Cancer 2013;21:1983–92 [DOI] [PubMed] [Google Scholar]
- 38.Jones SBT GA:Hesselsweet SD:Alvarez-Reeves M:Yu H:Irwin ML Effect of exercise on markers of inflammation in breast cancer survivors: the Yale exercise and survivorship study. Cancer Prevention Research 2013;6:109–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giallauria F, Gentile M, Chiodini P, Berrino F, Mattiello A, Maresca L, et al. Exercise training reduces high mobility group box-1 protein levels in women with breast cancer: Findings from the DIANA-5 study. Monaldi Archives for Chest Disease - Cardiac Series 2014;82:61–7 [DOI] [PubMed] [Google Scholar]
- 40.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. Journal of Applied Physiology 2005;98:1534–40 [DOI] [PubMed] [Google Scholar]
- 41.Hagstrom AD, Marshall PWM, Lonsdale C, Papalia S, Cheema BS, Toben C, et al. The effect of resistance training on markers of immune function and inflammation in previously sedentary women recovering from breast cancer: a randomized controlled trial. Breast Cancer Research and Treatment 2016;155:471–82 [DOI] [PubMed] [Google Scholar]
- 42.Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, et al. Tai Chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: A randomized controlled trial. Volume 20142014. p 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patterson RE, Marinac CR, Sears DD, Kerr J, Hartman SJ, Cadmus-Bertram L, et al. The effects of metformin and weight loss on biomarkers associated with breast cancer outcomes. Journal of the National Cancer Institute 2018;110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncology nursing forum 2008;35:635–42 [DOI] [PubMed] [Google Scholar]
- 45.Saxton JM, Scott EJ, Daley AJ, Woodroofe MN, Mutrie N, Crank H, et al. Effects of an exercise and hypocaloric healthy eating intervention on indices of psychological health status, hypothalamic-pituitary-adrenal axis regulation and immune function after early-stage breast cancer: A randomised controlled trial. Breast Cancer Research 2014;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gómez AM, Martínez C, Fiuza-Luces C, Herrero F, Pérez M, Madero L, et al. Exercise training and cytokines in breast cancer survivors. International journal of sports medicine 2011;32:461–7 [DOI] [PubMed] [Google Scholar]
- 47.Rogers LQ, Fogleman A, Trammell R, Hopkins-Price P, Spenner A, Vicari S, et al. Inflammation and psychosocial factors mediate exercise effects on sleep quality in breast cancer survivors: Pilot randomized controlled trial. Psycho-Oncology 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sturgeon KM, Foo W, Heroux M, Schmitz K. Change in inflammatory biomarkers and adipose tissue in BRCA1/2þ breast cancer survivors following a yearlong lifestyle modification program. Cancer Prevention Research 2018;11:545–50 [DOI] [PubMed] [Google Scholar]
- 49.Janelsins MC, Davis PG, Wideman L, Katula JA, Sprod LK, Peppone LJ, et al. Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clinical Breast Cancer 2011;11:161–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blucher C, Stadler SC. Obesity and Breast Cancer: Current Insights on the Role of Fatty Acids and Lipid Metabolism in Promoting Breast Cancer Growth and Progression. Front Endocrinol (Lausanne) 2017;8:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fedewa MV, Hathaway ED, Ward-Ritacco CL, Williams TD, Dobbs WC. The Effect of Chronic Exercise Training on Leptin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med 2018;48:1437–50 [DOI] [PubMed] [Google Scholar]
- 52.Liu LY, Wang M, Ma ZB, Yu LX, Zhang Q, Gao DZ, et al. The role of adiponectin in breast cancer: a meta-analysis. PLoS One 2013;8:e73183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarde T, Perrier S, Vasson MP, Caldefie-Chezet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer 2011;47:33–43 [DOI] [PubMed] [Google Scholar]
- 54.Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring) 2008;16:241–56 [DOI] [PubMed] [Google Scholar]
- 55.Kang DW, Lee J, Suh SH, Ligibel J, Courneya KS, Jeon JY. Effects of exercise on insulin, IGF axis, adipocytokines, and inflammatory markers in breast cancer survivors: A systematic review and meta-analysis. Cancer Epidemiology Biomarkers and Prevention 2017;26:355–65 [DOI] [PubMed] [Google Scholar]
- 56.Klempel MC, Varady KA. Reliability of leptin, but not adiponectin, as a biomarker for diet-induced weight loss in humans. Nutr Rev 2011;69:145–54 [DOI] [PubMed] [Google Scholar]
- 57.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 2014;10:455–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monteiro-Junior RS, de Tarso Maciel-Pinheiro P, da Matta Mello Portugal E, da Silva Figueiredo LF, Terra R, Carneiro LSF, et al. Effect of Exercise on Inflammatory Profile of Older Persons: Systematic Review and Meta-Analyses. J Phys Act Health 2018;15:64–71 [DOI] [PubMed] [Google Scholar]
- 59.Oh ES, Petersen KS, Kris-Etherton PM, Rogers CJ. Spices in a High-Saturated-Fat, High-Carbohydrate Meal Reduce Postprandial Proinflammatory Cytokine Secretion in Men with Overweight or Obesity: A 3-Period, Crossover, Randomized Controlled Trial. J Nutr 2020;150:1600–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


