Abstract
The study aimed to investigate the acute toxicity, antidiabetic potential (in-vitro and in-vivo) of the Operculina turpethum (L.) Silva Manso at fraction level. The plant was fractionated into different fractions, i.e., flavonoid fraction (OTFF), tannin fraction (OTTF), saponin fraction (OTSF). In-vitro alpha-amylase inhibition assay revealed that OTFF was found to be more potent than standard Acarbose. The plant fractions were evaluated by MTT assay at different concentrations ranging from 100 to 1000 µg/ml. All the fractions were further evaluated for their safety profile, and the biochemical, hematology and histopathology result exhibits that the OTFF fraction produces mild toxicity at organ level at a concentration of 2000 mg/kg in albino mice. The in-vivo antidiabetic study was carried out on Sprague-Dawley rats using high-fat diet (HFD) feeding streptozotocin (STZ) diabetic model, and the biochemical, histopathology research findings represent that OTFF at a concentration of 500 mg/kg, p.o. was found to be highly significant among all the fractions and found to be more potent than the standard Acarbose. LC–MS characterization of the bioactive fraction OTFF showed the presence of rutin with m/z 610.52 in 50.50% and Apigenin 7-O-6'' acetyl-glucoside with m/z 475.42 in 24.10%; from molecular docking study, it is predicted that the fraction primarily acts as an alpha-amylase inhibitor and PPAR gamma agonist. In conclusion, the plant’s OTFF fraction acts as a potential therapeutic agent for Type II diabetes mellitus.
Keywords: Operculina turpethum (L.) Silva Manso, Alpha-amylase, LC–MS, Molecular docking, Acute toxicity, Antidiabetic activity
Introduction
Diabetes mellitus (DM) occurs due to the impaired insulin release/resistance to insulin action or both in the biological system. It is allied with multiple organ dysfunctions due to hyperglycemia and associated with the risk of cardiovascular disease and obesity (Dharmani et al. 2019; Bindu and Narendhirakannan 2019; Latifi et al. 2019). DM is one of the foremost causes of death across the globe (Choudhary et al. 2020; Li et al. 2019a, b). In 2017, around 425 million adults in the age group of (20–79 years) reported to affect by diabetes and estimated to grow to 629 million by 2045 (Saeedi et al. 2019). Nowadays, combined therapy with several oral hypoglycemic agents is used to manage diabetes, but therapies possess numerous side effects (Choudhary et al. 2020; El Deeb et al. 2019; Ullah Jan et al. 2018). However, effective and safe treatment in curing diabetes is yet to be achieved. Therefore, there is a need to explore the phytochemical as they are considered less toxic than synthetic medicines and help manage type 2 DM (Shoaib et al. 2020; Choudhary et al. 2020; Eitah et al. 2019; Simon et al. 2018). The plant Operculina turpethum (L.) Silva Manso (Convolvulaceae) methanolic and aqueous extracts were earlier reported to have antidiabetic activity (Pulipaka et al. 2012; Gupta and Ved 2017). However, earlier studies were conducted at the pilot scale only as the methanol and aqueous extract was earlier reported to have antidiabetic activity. In addition, the plant’s extraction was not conducted systematically, responsible fraction, active phytoconstituents, and acute toxicity profile of the plant were not reported till now. Therefore, the current study focused on investigating the acute toxicity, in vitro, in vivo antidiabetic potential of the plant fractions and identifying the responsible phytoconstituents for its activity, and predicts the mechanism of action.
Materials and methods
Chemicals
STZ was purchased from CDH, New Delhi, India; ingredients of feed such as cholesterol and casein from CDH, New Delhi, formalin, sodium carboxymethylcellulose, and l-cysteine from Loba Chemicals, Mumbai, yeast powder from Molychem, Mumbai, mineral and vitamin mix from Sarabhai chemicals, Baroda, India, Ghee from Patanjali, Haridwar, Acarbose from Sisco Research Laboratories Pvt. Ltd.
Collection and authentication of plant material
Air-dried roots/rhizomes of Operculina turpethum (L.) Silva Manso were procured from Ludhiana (Punjab) and authenticated by Dr. Sunita Garg, Emeritus Scientist, Department of Raw Material Herbarium and Museum, National Institute of Sciences Communication and Information Resources, New Delhi under the voucher specimen number Ref. No. NISCAIR/RHMD/Consult/2018/3227/28-2.
Fractionation of total tannins and flavonoids
The plant’s roots/rhizomes were defatted using petroleum ether (40–60 °C), followed by extraction with chloroform and ethyl acetate using microwave-assisted extraction at 200 W by means of the magnetic shaker and at 50 °C temperature. The dried ethyl acetate extract, which was then dissolved in the aqueous phase and 10% NaCl solution, was added to the aqueous solution, centrifuge the solution to precipitate tannins (OTTF), the supernatant liquid was again partitioned with ethyl acetate to obtain the total flavonoids (OTFF) (Choudhary et al. 2011).
Fractionation of total saponin
The plant’s roots/rhizomes were defatted using petroleum ether (40–60 °C), followed by successive extraction using chloroform and methanol as a solvent by microwave-assisted extraction at 200 W by means of the magnetic shaker and at 50 °C temperature. The dried methanolic extract was suspended in water, and partitioning was done using diethyl ether, saturated n-butanol to isolate the total saponin (OTSF) (Majinda 2012).
In-vitro alpha-amylase inhibitory activity assay
The alpha-amylase inhibitory assay was carried out using the iodine-starch method. Acarbose was used as a standard. The assay depends on developing an iodine and starch complex, i.e., blue, and exhibits maximum absorbance at 580 nm. The positive control solution was prepared using alpha-amylase enzyme and starch in the absence of an inhibitor to achieving 100% enzymatic activity with minimum absorbance value. Simultaneously, the negative control solution contains the starch that converts into the dark green colored complex after the addition of iodine solution having maximum absorbance, due to the absence of inhibitor and alpha-amylase and possess no enzymatic activity. However, the test solution absorbance and color intensity should lie in the middle of positive and negative control absorbance (Soni et al. 2018).
Preparation of stock sample solution
The stock solution 1000 ppm concentration was prepared for each fraction by dissolving 10 mg compound in 10 ml methanol.
Preparation of sample solution
The various sample concentrations were prepared by withdrawing 0.5, 1, 1.5, 2, and 2.5 ml from stock solution into 10 ml of different volumetric flasks. Volume was prepared using methanol and labeled the sample solutions 50, 100, 150, 200, and 250 µg/ml.
MTT assay for cell viability and toxicity
Various fractions of the plant i.e. OTFF, OTTF and OTSF were analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay to identify the effect of these fractions on the cell viability and toxicity. In this context, normal mice fibroblast cell line (3T3 cell) was used, cell medium (3 × 103 cells per plate) was seeded into 96 well plates and incubated at 37 °C. Then various plant fractions were added at different concentrations (100–1000 µg/ml) into 96 well plates and incubated for 24 h at 37 °C. Furthermore, 20 µl of 5 mg/ml MTT was added to each well, incubate for 4 h. at 37 °C. The media was carefully removed and dimethyl sulfoxide (DMSO) 200 µl was added. The absorbance was recorded at 595 nm (Stefanowicz-Hajduk and Ochocka 2020; Fonseca et al. 2018).
Experimental animals
Female albino mice were procured from the central animal facility from the Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar for acute toxicity study. Sprague-Dawley (SD) rats of either sex were procured from the National Institutes of Pharmaceutical Education and Research, Mohali, for in-vivo antidiabetic study. The animals were kept in standard polypropylene cages and maintained under controlled room temperature (22 ± 2 °C) and humidity (55 ± 5%) with a 12:12 h light and dark cycle. All the rats and mice were supplied commercially free rat regular pellet diet (NPD) and water ad libitum before the dietary management. The animal experiments were conducted after approval from the Institutional Animal Ethics Committee of Lovely Professional University (Approval No. LPU/IAEC/2019/53), and experiments were conducted as per the guidelines of CPSCEA (Govt. of India).
Acute toxicity assay
The acute toxicity assay was carried out as per the OECD Test Guidelines 425 (Up and Down Procedure). In the study, non-pregnant female albino mice were used, having an age group 8–10 weeks, 28 ± 4 g weight was arbitrarily selected, and documented Table 1. The mice fasted for 3–4 h prior to dosing but had access to water ad libitum, and a single dose of 2000 mg/kg; p.o. was administered according to body weight to single mice from each test group. The animals were closely monitored initially for 30 min, then for 4 h for any sign of toxicity. The food was restored after 1–2 h of dosing. After the drug-treated mouse’s survival, all the remaining four mice were administered with the same dose. A similar protocol was carried out for all the vehicle control group mice by administering 1% carboxy methylcellulose (CMC) in the same volume as that of the treated group. All the groups were closely monitored for any toxic effect, and behavioral parameters were also recorded for the first 30 min, 4 h, and 24 h and after that at regular intervals for 14 days. The body weight of mice was measured at regular intervals. At the end of the protocol, the mice excised by cervical dislocation under diethyl-ether anesthesia and organ weight of the heart, liver, and kidney were measured (Xiong et al. 2019; Saleem et al. 2017). Blood samples were withdrawn by cardiac puncture and sent to the pathology laboratory to estimate biochemical and hematological parameters. The isolated organs, i.e., heart, liver, and kidney, were preserved in a 10% formalin solution for histopathological evaluation.
Table 1.
Acute toxicity studies (Acc. to OECD 425 guidelines)
| Groups | Treatment | Diet + dose and route of drug treatment | No. of animals in each group (mice) |
|---|---|---|---|
| I | Vehicle control | NPD + 1% w/v CMC (p.o.) (Vehicle) | 5 |
| II | Drug treated | NPD + OTFF (2000 mg/kg) (p.o.) | 5 |
| III | Drug treated | NPD + OTTF (2000 mg/kg) (p.o.) | 5 |
| IV | Drug treated | NPD + OTSF (2000 mg/kg) (p.o.) | 5 |
NPD: normal pellet diet; OTFF: Operculina turpethum flavonoid fraction; OTTF: Operculina turpethum tannin fraction; OTSF: Operculina turpethum saponin fraction
Biochemical analysis
All the samples were sent to the pathology lab (National Laboratories, Phagwara, Punjab) to analyze blood glucose, lipid profile, renal function tests, liver function tests, AST, ALT, alkaline phosphate, total protein, globulins, and albumin.
Hematological analysis
The blood samples were analyzed by pathology lab (National Laboratories, Phagwara, Punjab) in tubes containing ethylenediaminetetraacetic acid (EDTA) for hematological study. Hematology parameters, total Red blood cells (RBC), hemoglobin, mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), White blood cells (WBC) count, platelet (PLT) count, lymphocytes (LYMP), neutrophils (NEUT), eosinophils (EO), basophils (BASO), and monocytes (MONO).
Histopathological study
The isolated vital organs (heart, liver and kidney) of mice were fixed in 10% formalin after sacrificing. The organs during processing fixed in paraffin wax. 5 mm paraffin sections stained with eosin and hematoxylin. The slides were kept beneath the light microscope, and magnified tissue structure images were captured for analysis.
Statistical analysis
The results were expressed as Mean ± SD, and the statistical significance among the groups was analyzed by one-way ANOVA, followed by Tukey’s multiple comparison tests. P ≤ 0.05 is considered statistically significant.
In-vivo antidiabetic activity
Diabetes induction and in vivo experimental design
To evaluate and identify the potential novel antidiabetic agents from medicinal plants to cure type 2 diabetes mellitus. For this, Sprague-Dawley rats of either sex were taken for the development of high-fat diet (HFD) feeding and administering a low dose (35 mg/kg) of streptozotocin (STZ) (Nambirajan et al. 2018; Gaikwad et al. 2010; Srinivasan et al. 2005). The rats were categorized into two different dietary regimens, i.e., Normal pellet diet (NPD) and high-fat diet (HFD) composition (58% fat, 25% protein, and 17% carbohydrate, as a percentage of total kcal) ad libitum, respectively, for the initial period of 2 weeks (Priscilla et al. 2014). The NPD and HFD rats further divided into NPD, HFD + STZ, HFD + STZ + test compound OTFF, OTTF and OTSF (Table 2). The ingredients of HFD (Table 3) (Gaikwad et al. 2010). After 2 weeks of dietary manipulation, all the rats from the HFD-fed group were injected with STZ low dose (35 mg/kg; i.p.). After that, body weight and biochemical estimations were conducted on the 7th day. The test compound OTFF, OTTF, and OTSF were fed orally at two different concentrations, i.e., 250 mg/kg and 500 mg/kg continuous for 7 days. After that, the blood samples were collected to analyze the plasma glucose level, total cholesterol level, and triglyceride level. Histopathology of the pancreas was conducted after sacrificing 50% of the animals at the end of the protocol. The non-fasting rats with a plasma glucose level of ≥ 300 mg/dl were considered diabetic and chosen for further experimental studies. Animal water and feed water intake were also measured. The rats were allowed to continue with the feed as per the protocol.
Table 2.
Represents the different experimental groups for antidiabetic study
| Groups | Group name | Diet + dose and route of drug treatment | No. of animals in each group (SD rats) |
|---|---|---|---|
| I | Vehicle control | NPD + 0.5% w/v CMC (p.o.) (Vehicle) | 6 |
| II | Experimental control | HFD + 35 mg/kg of STZ (i.p.) | 6 |
| III | Standard drug | HFD + 35 mg/kg of STZ (i.p.) + Acarbose 10 mg/kg, p.o | 6 |
| IV | OTFF 250 mg/kg | HFD + 35 mg/kg of STZ (i.p.) + OTFF (250 mg/kg) (p.o.) | 6 |
| V | OTFF 500 mg/kg | HFD + 35 mg/kg of STZ (i.p.) + OTFF (500 mg/kg) (p.o.) | 6 |
| VI | OTTF 250 mg/kg | HFD + 35 mg/kg of STZ (i.p.) + OTTF (250 mg/kg) (p.o.) | 6 |
| VII | OTTF 500 mg/kg | HFD + 35 mg/kg of STZ (i.p.) + OTTF (500 mg/kg) (p.o.) | 6 |
| VIII | OTSF 250 mg/kg | HFD + 35 mg/kg of STZ (i.p.) + OTSF (250 mg/kg) (p.o.) | 6 |
| IX | OTSF 500 mg/kg | HFD + 35 mg/kg of STZ (i.p.) + OTSF (500 mg/kg) (p.o.) | 6 |
NPD: normal pellet diet; CMC: carboxymethyl cellulose; HFD: high-fat diet; STZ: streptozotocin; OTFF: Operculina turpethum flavonoid fraction; OTTF: Operculina turpethum tannin fraction; OTSF: Operculina turpethum saponin fraction
Table 3.
Represents the HFD composition
| S. no | Ingredients | Diet (g/kg) |
|---|---|---|
| 1 | Powdered NPD | 365 |
| 2 | Ghee | 310 |
| 3 | Casein | 250 |
| 4 | Cholesterol | 10 |
| 5 | Vitamin and mineral mix | 60 |
| 6 | l-Cysteine | 03 |
| 7 | Yeast powder | 01 |
| 8 | Sodium chloride | 01 |
Collection of blood and analytical methods
Blood was collected from rats’ retro-orbital plexus under light ether anesthesia using capillary tubes. The blood samples were analyzed for plasma glucose, total cholesterol, and triglycerides level.
Histopathological study
The rats’ isolated pancreas was fixed in 10% formalin after sacrificing, after processing fixed in paraffin wax. 5 mm paraffin sections were stained with eosin and hematoxylin. The slides were kept beneath the light microscope, and magnified tissue structure images were captured for analysis.
Statistical analysis
The results were expressed as Mean ± SD, and the statistical significance among the groups was analyzed by one way ANOVA followed by Turkey’s multiple comparison tests. P ≤ 0.05 was considered as statistically significant.
LC–MS of OTFF
The bioactive OTFF fraction obtained from the plant’s roots/rhizomes was characterized through LC–MS Spectra for bioactive molecules responsible for the activity. The LC–MS data components present in the OTFF fraction correlated with the mass fragments reported in the literature data (Jang et al. 2018; Brito et al. 2014).
Molecular docking
For this molecular modeling software, Autodock-Vina (Sharma et al. 2020; Kiametis et al. 2017; Trott and Olson 2010) was employed by the PDB or protein selected 3WY2, 4GQR, and 3SZ1 alpha-glucosidase, alpha-amylase, and PPAR-gamma proteins, respectively were extracted out from protein data bank. The structures of these molecules were drawn by ChemDraw and changed to 3D. Further minimizations of energy were carried out using the MM2 Interface program on ChemBio3D Ultra 12.0, and molecules were saved in pdb format (Cambridge Soft). For identifying the most active molecule, initially, the removal of the internal ligand was done, and docking was carried out in a similar pattern to an actual ligand.
Results
Fractionation
The various fractions were fractionated from the plant Operculina turpethum (L.) Silva Manso. The further yield of OTFF, OTTF, and OTSF was found to be 2.32, 1.57, and 3.37 (w/w), respectively.
In-vitro alpha-amylase inhibition assay
All the fractions of the plant were evaluated by alpha-amylases assay. The OTFF showed potent alpha-amylase inhibitor activity compared with standard Acarbose having inhibition concentration (IC50) values 100.69 and 812.83 µg/ml, respectively, whereas the OTTF and OTSF found to be less active with that of standard in terms of % inhibition (Table 4).
Table 4.
Represents the % inhibition and IC50 of various fractions of Operculina turpethum
| S. no | Conc. (µg/ml) | Log conc | % Inhibition acarbose | % Inhibition OTFF | % Inhibition OTTF | % Inhibition OTSF |
|---|---|---|---|---|---|---|
| 1 | 50 | 1.70 | 8.75 ± 0.67 | 31.58 ± 0.87 | 7.24 ± 0.62 | 3.29 ± 0.75 |
| 2 | 100 | 2.00 | 17.86 ± 0.87 | 45.39 ± 0.73 | 12.50 ± 0.74 | 5.92 ± 0.63 |
| 3 | 150 | 2.18 | 21.32 ± 0.53 | 55.92 ± 0.98 | 17.76 ± 0.95 | 7.89 ± 0.86 |
| 4 | 200 | 2.30 | 28.12 ± 0.78 | 71.05 ± 1.09 | 23.03 ± 1.09 | 10.53 ± 0.89 |
| 5 | 250 | 2.40 | 34.67 ± 0.97 | 88.82 ± 0.81 | 28.95 ± 0.93 | 12.50 ± 0.96 |
| IC50 | 812.83 | 100.69 | 1538.15 | – | ||
Values are expressed as Mean ± SD
OTFF: Operculina turpethum flavonoid fraction; OTTF: Operculina turpethum tannin fraction; OTSF: Operculina turpethum saponin fraction
MTPP assay
To identify the cell viability 3T3 cell line was used and MTT assay was performed for the various plant fractions i.e. OTFF, OTTF and OTSF, evaluated at different concentrations i.e. 100, 200, 300, 400, 500 and 1000 µg/ml. The assay results indicate that all the fractions of the plants were found to be safe and non toxic. at a higher concentration of 1000 µg/ml (Fig. 1).
Fig. 1.
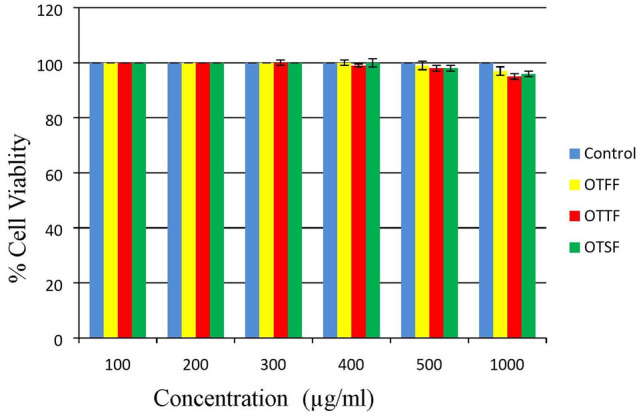
Represents the % Cell viability of the various fractions of the plant at different concentrations i.e. 100, 200, 300, 400, 500 and 1000 µg/ml in normal mice fibroblast cell line (3T3 cell). Values are presented as Mean ± SD, Statistical analysis was performed using one way ANOVA followed by Turkey’s multiple comparison test, OTFF: Operculina turpethum (L.) Silva Manso flavonoid fraction; OTTF: Operculina turpethum (L.) Silva Manso tannin fraction; OTSF: Operculina turpethum (L.) Silva Manso saponin fraction
Acute toxicity study
The acute toxicity was conducted as per OECD guidelines for the OTFF, OTTF, and OTSF at a concentration of 2000 mg/kg; mortality was not observed in any of the treated and vehicle control groups. All the animals were observed at regular intervals, and observations were noted during the study phase, i.e., 14 days.
Behavioral pattern and body weight
The sleepy and drowsing effects were observed in OTTF, OTSF fraction during the first 4 h. During the acute toxicity study, it was observed that there is a slight increase in body weight in the treated and vehicle control group (Table 5).
Table 5.
Effects of the various fractions on the body weight of mice
| Group | Day 1 | Day 7 | Day 14 |
|---|---|---|---|
| I | 28.39 ± 0.46 | 28.93 ± 0.63 | 29.17 ± 0.61 |
| II | 27.06 ± 0.34 | 27.54 ± 0.39 | 28.49 ± 0.41 |
| III | 25.84 ± 0.45 | 26.32 ± 0.41 | 26.72 ± 0.55 |
| IV | 29.89 ± 0.34 | 30.21 ± 0.30 | 30.68 ± 0.47 |
Values are expressed as Mean ± SD N = 5
Organ to body weight index
There was no significant difference observed in organ to body weight index for both treated and vehicle control groups. There were no lesions observed at the organ level (heart, liver, and kidney) in any of the groups at a 2000 mg/kg concentration, p.o. (Table 6).
Table 6.
Represents the mice organs weight index in treated and vehicle control group
| Group | Heart weight | Liver weight | Kidney weight |
|---|---|---|---|
| I | 0.736 ± 0.106 | 6.594 ± 0.091 | 1.532 ± 0.112 |
| II | 0.716 ± 0.171 | 6.588 ± 0.104 | 1.524 ± 0.132 |
| III | 0.727 ± 0.136 | 6.600 ± 0.114 | 1.533 ± 0.125 |
| IV | 0.744 ± 0.154 | 6.593 ± 0.133 | 1.533 ± 0.106 |
Values are presented as Mean ± SD, N = 5; Organ-to-body weight index = (organ weight × 100)/body weight
Biochemical analysis
In all the fractions (OTFF, OTTF, and OTSF), there is an increase in the total cholesterol, LDL, urea, creatinine, albumin, SGOT, SGPT, ALP levels when compared with vehicle control. Furthermore, there is a significant increase in globulin levels in OTFF, OTTF, and HDL in OTTF, OTSF only. The biochemical results indicate that all the fractions at a concentration of 2000 mg/kg produces only mild toxicity symptoms without producing any severe toxicity at the organ level in albino mice (Table 7).
Table 7.
Represents the biochemical analysis of various fractions of Operculina turpethum
| S. no | Parameters | Units | VC | OTFF | OTTF | OTSF |
|---|---|---|---|---|---|---|
| 1 | Glucose | mg/dl | 94.67 ± 1.96 | 96.59 ± 2.85 | 93.52 ± 4.01 | 95.81 ± 2.30 |
| Lipid profile | ||||||
| 2 | Total cholesterol | mg/dl | 98.67 ± 3.06 | 162.67 ± 2.52* | 117.33 ± 4.16* | 156.50 ± 1.50* |
| 3 | HDL cholesterol | mg/dl | 30.33 ± 1.53 | 42.67 ± 2.08* | 30.67 ± 1.53 | 39.20 ± 1.06* |
| 4 | LDL cholesterol | mg/dl | 45.93 ± 1.51 | 95.20 ± 1.31* | 64.07 ± 2.39* | 92.77 ± 0.50* |
| 5 | VLDL cholesterol | mg/dl | 22.40 ± 0.60 | 24.80 ± 1.06 | 22.60 ± 3.22 | 24.53 ± 1.11 |
| 6 | Triglycerides | mg/dl | 112 ± 3.00 | 124.00 ± 5.29 | 122.33 ± 4.04 | 122.63 ± 5.54 |
| 7 | CHOL/HDL ratio | 3.26 ± 0.11 | 3.82 ± 0.13* | 3.83 ± 0.10* | 3.99 ± 0.07* | |
| 8 | LDL/HDL ratio | 1.52 ± 0.08 | 2.24 ± 0.13* | 2.09 ± 0.16* | 2.37 ± 0.07* | |
| Kidney function tests | ||||||
| 9 | Urea | mg/dl | 37.33 ± 2.08 | 49.33 ± 1.53* | 47.00 ± 2.65* | 53.33 ± 1.53* |
| 10 | Creatinine | mg/dl | 0.53 ± 0.02 | 0.61 ± 0.02* | 0.59 ± 0.02* | 0.57 ± 0.02* |
| Liver function tests | ||||||
| 11 | BIT | mg/dl | 0.67 ± 0.06 | 0.75 ± 0.05 | 0.65 ± 0.06 | 0.79 ± 0.04* |
| 12 | BID | mg/dl | 0.25 ± 0.05 | 0.28 ± 0.02 | 0.19 ± 0.09 | 0.35 ± 0.05 |
| 13 | BII | mg/dl | 0.42 ± 0.02 | 0.46 ± 0.07 | 0.46 ± 0.03 | 0.44 ± 0.05 |
| 14 | Protein | mg/dl | 6.27 ± 0.21 | 6.40 ± 0.40 | 6.27 ± 0.25 | 6.60 ± 0.10 |
| 15 | Albumin | mg/dl | 2.27 ± 0.06 | 2.82 ± 0.20* | 2.83 ± 0.11* | 2.80 ± 0.09* |
| 16 | Globulin | mg/dl | 4.00 ± 0.17 | 3.58 ± 0.22* | 3.44 ± 0.16* | 3.79 ± 0.01 |
| 17 | A:G/ratio | 0.57 ± 0.02 | 0.79 ± 0.03* | 0.82 ± 0.02* | 0.74 ± 0.02* | |
| 18 | SGOT/AST | IU/l | 95.93 ± 1.79 | 131.33 ± 6.40* | 163.10 ± 7.07* | 140.33 ± 5.03* |
| 19 | SGPT/ALT | IU/l | 65.97 ± 5.31 | 95.43 ± 4.27* | 86.00 ± 9.64* | 78.72 ± 4.15* |
| 20 | ALP | IU/l | 94.97 ± 6.05 | 109.67 ± 5.51* | 130.67 ± 5.13* | 123.67 ± 5.13* |
Values are presented as Mean ± SD, N = 5, Statistical analysis was performed using one way ANOVA followed by Turkey’s multiple comparison test
VC: vehicle control; OTFF: Operculina turpethum flavonoid fraction; OTTF: Operculina turpethum tannin fraction; OTSF: Operculina turpethum saponin fraction
*Represents P < 0.05 vs. vehicle control
Hematological analysis
Both the plant’s fractions, i.e., OTFF and OTTF, causes a significant increase in the levels of HGB, RBC, HCT MCV, MCH, MCHC, PLT, MPV, P-LCR. Besides, there are also significant increases in WBC levels, PCT in OTTF and OTSF fractions. A hematology evaluation's research finding represents that all the fractions produce only mild toxicity symptoms in albino mice (Table 8).
Table 8.
Represents the hematological analysis of various fractions of Operculina turpethum
| S. no | Parameters | Unit | VC | OTFF | OTTF | OTSF |
|---|---|---|---|---|---|---|
| 1 | HGB | g/dl | 13.35 ± 0.05 | 13.82 ± 0.10* | 14.30 ± 0.10* | 14.43 ± 0.06* |
| 2 | RBCs | 106/ul | 8.48 ± 0.08 | 9.10 ± 0.03* | 9.32 ± 0.04* | 9.15 ± 0.03* |
| 3 | HCT | % | 44.68 ± 0.16 | 45.77 ± 0.32* | 49.82 ± 0.08* | 48.94 ± 0.06* |
| 4 | MCV | fL | 52.67 ± 0.52 | 50.31 ± 0.22* | 53.47 ± 0.12* | 53.49 ± 0.11* |
| 5 | MCH | Pg | 15.74 ± 0.19 | 15.19 ± 0.07* | 15.35 ± 0.05* | 15.77 ± 0.02 |
| 6 | MCHC | g/dl | 29.88 ± 0.07 | 30.19 ± 0.11* | 28.71 ± 0.16* | 29.49 ± 0.09* |
| 7 | RDW-SD | fL | 19.69 ± 0.11 | 21.78 ± 0.07* | 22.07 ± 0.06* | 20.56 ± 0.07* |
| 8 | RDW-CV | % | 19.06 ± 0.05* | 19.85 ± 0.05* | 19.94 ± 0.04* | 19.15 ± 0.06* |
| 9 | WBCs | 103/ul | 4.94 ± 0.03 | 4.79 ± 0.04* | 4.94 ± 0.04 | 4.86 ± 0.03* |
| 10 | NEUT% | % | 20.45 ± 0.41 | 20.79 ± 0.62 | 20.74 ± 0.49 | 20.90 ± 0.81 |
| 11 | LYMPH% | % | 76.37 ± 0.33 | 75.07 ± 0.57 | 75.27 ± 0.55 | 74.99 ± 0.70 |
| 12 | MONO% | % | 1.00 ± 0.12 | 1.50 ± 0.08* | 1.45 ± 0.10* | 1.39 ± 0.07* |
| 13 | EO% | % | 2.18 ± 0.12 | 2.65 ± 0.08* | 2.54 ± 0.08* | 2.71 ± 0.07* |
| 14 | BASO% | % | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 15 | IG% | % | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 16 | NEUT# | 103/ul | 1.01 ± 0.02 | 1.00 ± 0.04 | 1.02 ± 0.03 | 1.02 ± 0.04 |
| 17 | LYMPH# | 103/ul | 3.78 ± 0.03 | 3.60 ± 0.01* | 3.72 ± 0.01* | 3.64 ± 0.04* |
| 18 | MONO# | 103/ul | 0.05 ± 0.01 | 0.07 ± 0.00* | 0.07 ± 0.00* | 0.07 ± 0.00* |
| 19 | EO# | 103/ul | 0.11 ± 0.01 | 0.13 ± 0.00* | 0.13 ± 0.00* | 0.13 ± 0.00* |
| 20 | BASO# | 103/ul | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 21 | IG# | 103/ul | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 22 | PLT | 103/ul | 692.33 ± 2.52 | 764.67 ± 2.52* | 785.00 ± 4.00* | 795.00 ± 3.00* |
| 23 | PDW | fL | 6.75 ± 0.05 | 6.75 ± 0.05 | 7.14 ± 0.04* | 7.17 ± 0.04* |
| 24 | MPV | fL | 6.25 ± 0.05 | 6.65 ± 0.06* | 7.03 ± 0.03* | 6.73 ± 0.04* |
| 25 | P-LCR | % | 3.04 ± 0.01 | 4.18 ± 0.08* | 6.43 ± 0.03* | 4.74 ± 0.04* |
| 26 | PCT | % | 0.51 ± 0.02 | 0.51 ± 0.01 | 0.66 ± 0.03* | 0.83 ± 0.03* |
Values are presented as Mean ± SD, N = 5, Statistical analysis was performed using one way ANOVA followed by Turkey’s multiple comparison test
VC: vehicle control; OTFF: Operculina turpethum flavonoid fraction; OTTF: Operculina turpethum tannin fraction; OTSF: Operculina turpethum saponin fraction
*Represents P < 0.050 vs. vehicle control
Histopathology analysis
All the fractions OTTF, OTTF, and OTSF of the plant did not exhibit any severe sign of toxicity at the organ level, which indicates that all the fractions are safe at a concentration of 2000 mg/kg, p.o. The histopathological research findings are summarized for the heart (Fig. 2), Kidney (Fig. 3), and liver (Fig. 4). The results indicate that OTFF and OTSF fractions produce only mild toxicity at heart, liver, and kidney, but OTTF fraction produces mild to moderate toxicity at the organ level. The organ also supports these results to body weight index, biochemical and hematology findings.
Fig. 2.
Histopathological observations of heart for the various fractions of the plant when evaluated for acute oral toxicty study at 2000 mg/kg, a (VC): Nothing abnormal detected; b (OTFF): Operculina turpethum (L.) Silva Manso flavanoid fraction, mild fatty infiltration (MFI) in the myocardial fibers; c (OTTF): Operculina turpethum (L.) Silva Manso tannin fraction, mild to moderate vacuolar degeneration (MVD) in the myocardial fibers; d (OTSF): Operculina turpethum (L.) Silva Manso saponin fraction, mild degenerative changes (MDC) in the myocardial fibers
Fig. 3.
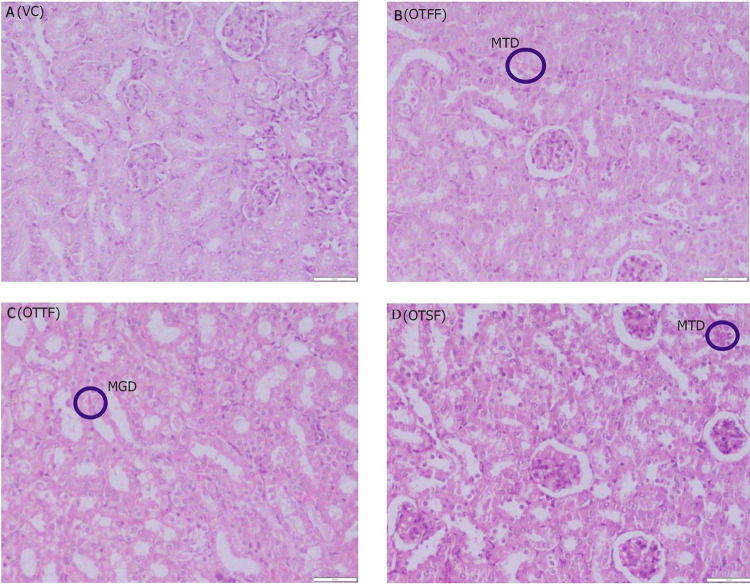
Histopathological observations of kidney for the various fractions of the plant when evaluated for acute oral toxicty study at 2000 mg/kg, a (VC): Nothing abnormal detected; b (OTFF): Operculina turpethum (L.) Silva Manso flavanoid fraction, mild degeneration in the tubular epithelial cells (MTD); c (OTTF): Operculina turpethum (L.) Silva Manso tannin fraction, moderate granular degeneration (MGD) in the tubular epithelial cells; d (OTSF): Operculina turpethum (L.) Silva Manso saponin fraction, mild degenerative changes (MTD) in the tubular epithelial cells
Fig. 4.
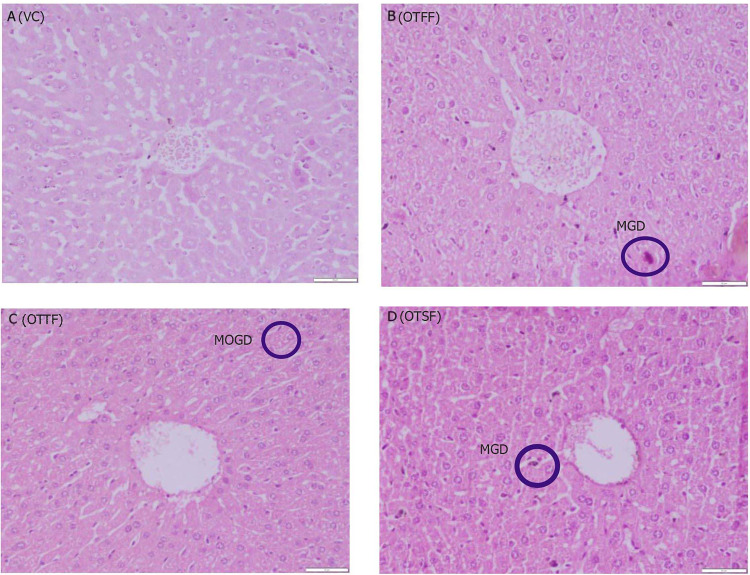
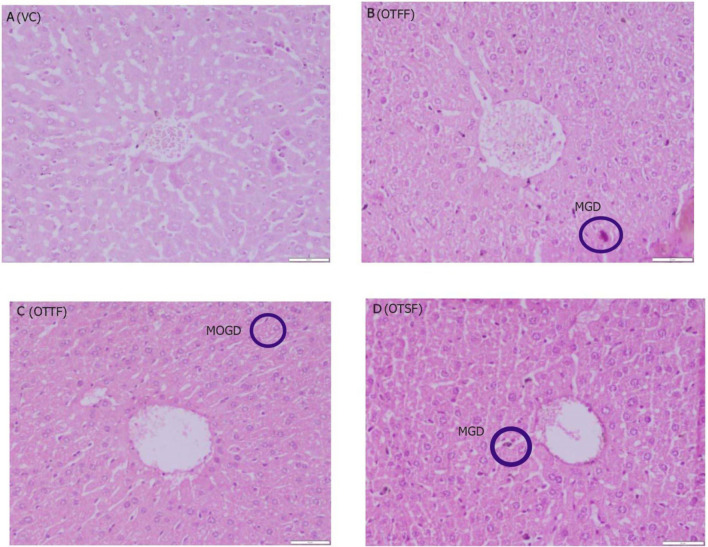
Histopathological observations of liver for the various fractions of the plant when evaluated for acute oral toxicty study at 2000 mg/kg, a (VC): Nothing abnormal detected; b (OTFF): Operculina turpethum (L.) Silva Manso flavanoid fraction, mild granular degeneration (MGD) in hepatocyctes of the liver; c (OTTF): Operculina turpethum (L.) Silva Manso tannin fraction, moderate granular degeneration (MOGD) in hepatocyctes of the liver; d (OTSF): Operculina turpethum (L.) Silva Manso saponin fraction, mild granular degeneration (MGD) in hepatocyctes of the liver
In-vivo antidiabetic activity
Biochemical analysis
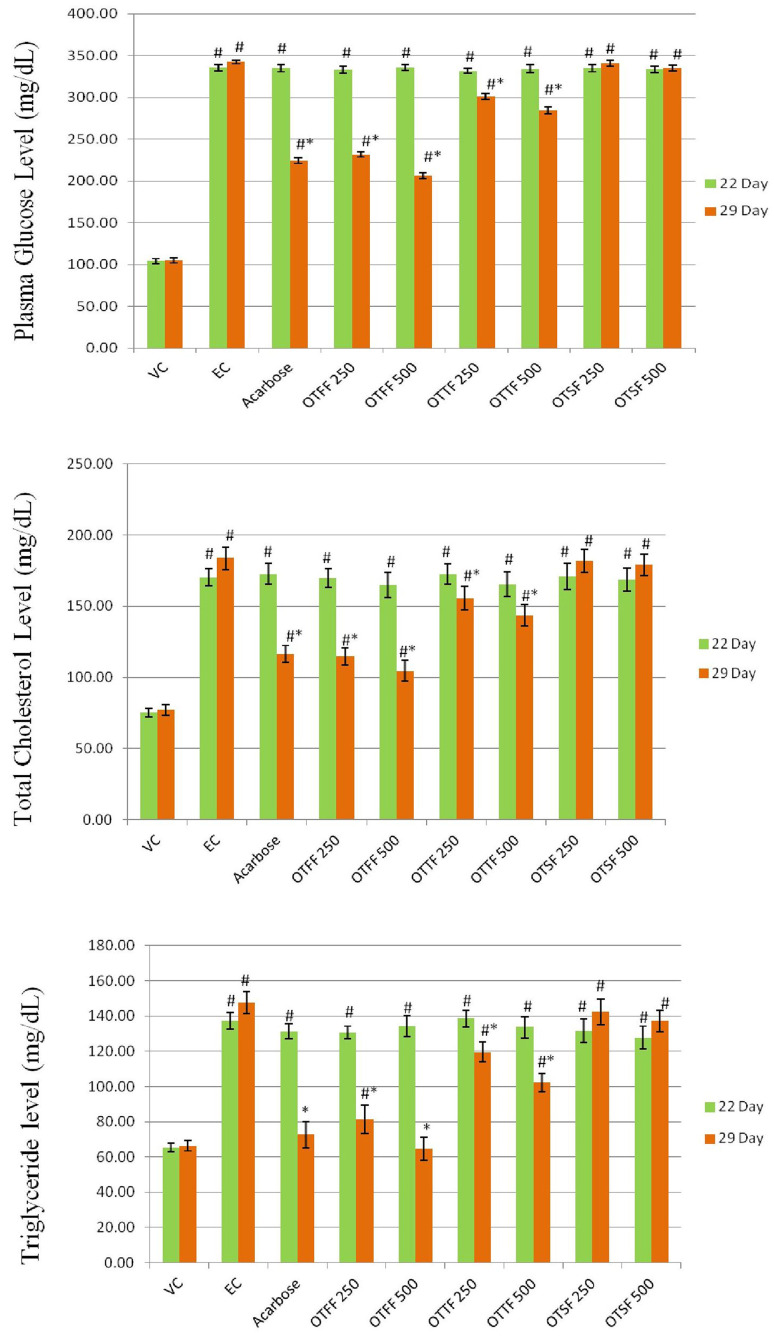
The plasma glucose, total cholesterol, and total triglycerides were compared on the 22nd and 29th day (Table 9). After 1 week of STZ injection, i.e., on the 22nd day, there is a highly significant increase in glucose, cholesterol, and triglyceride level in all the HFD + STZ treated groups that confirm the type 2 diabetic condition. After 1 week of treatment, i.e., 29th day, there is a highly significant decrease in the glucose, cholesterol, and triglyceride level in OTTF fraction at a dose of 500 mg/kg, p.o. when compared with the experimental group and found to be more potent than the standard Acarbose. Furthermore, the OTTF fraction also significantly reduces all the biochemical levels when compared with the observational group. There is no significant difference observed with the OTSF fraction treated group compared to the experimental group. The results represent that the OTFF was effective at both the dose, but highly effective at a higher dose compared to the standard group (Fig. 5).
Table 9.
Represents the plasma glucose level, total cholesterol and triglyceride level in SD rats
| Group number | Plasma glucose level (mg/dl) | Total cholesterol level (mg/dl) | Triglyceride level (mg/dl) | |||
|---|---|---|---|---|---|---|
| 22 Day | 29 Day | 22 Day | 29 Day | 22 Day | 29 Day | |
| I | 104.33 ± 3.06 | 105.33 ± 3.06 | 75.33 ± 3.06 | 77.33 ± 3.79 | 65.33 ± 2.52 | 66.33 ± 3.06 |
| II | 335.33 ± 3.51# | 342.33 ± 2.52# | 170.33 ± 6.03# | 183.67 ± 8.08# | 137.33 ± 4.73# | 147.67 ± 6.11# |
| III | 334.67 ± 4.16# | 224.33 ± 3.51#* | 172.67 ± 7.51# | 116.33 ± 6.11#* | 131.33 ± 4.16# | 72.67 ± 7.37* |
| IV | 333.33 ± 4.16# | 231.67 ± 3.06#* | 169.67 ± 6.51# | 114.67 ± 6.03#* | 130.67 ± 3.79# | 81.33 ± 8.02#* |
| V | 335.67 ± 3.21# | 206.33 ± 3.79#* | 164.67 ± 8.62# | 104.67 ± 7.37#* | 134.33 ± 5.69# | 64.67 ± 6.51* |
| VI | 331.67 ± 3.06# | 301.33 ± 3.51#* | 172.67 ± 7.09# | 155.67 ± 8.33#* | 138.67 ± 4.73# | 119.67 ± 5.51#* |
| VII | 334.33 ± 5.03# | 284.33 ± 4.04#* | 165.33 ± 8.74# | 143.67 ± 7.64#* | 133.67 ± 6.11# | 102.33 ± 5.03#* |
| VIII | 335.00 ± 4.36# | 341.33 ± 3.51# | 170.67 ± 9.29# | 181.67 ± 8.08# | 131.67 ± 6.66# | 142.33 ± 7.09# |
| IX | 333.67 ± 4.04# | 334.67 ± 3.51# | 168.67 ± 8.02# | 179.00 ± 7.55# | 127.67 ± 6.51# | 137.33 ± 6.03# |
Values are presented as Mean ± SD, n = 6. Statistical analysis was performed using one way ANOVA followed by Turkey’s multiple comparison test
OTFF: Operculina turpethum flavonoid fraction; OTTF: Operculina turpethum tannin fraction; OTSF: Operculina turpethum saponin fraction
#Represents P < 0.05 vs. vehicle control
*Represents P < 0.05 vs. experimental control
Fig. 5.
Effect of different groups on plasma glucose level, total cholesterol and triglycerides for the various fractions of the plant when evaluated for in-vivo antidibaetic activity at two different concentrations i.e. 250 and 500 mg/kg using HFD and STZ induced diabetic model in SD rats. Values are presented as Mean ± SD, n = 6. Statistical analysis was performed using one way ANOVA followed by Turkey’s multiple comparison test, # represents P < 0.05 vs. vehicle control, * represents P < 0.05 vs. experimental control; OTFF: Operculina turpethum (L.) Silva Manso flavonoid fraction; OTTF: Operculina turpethum (L.) Silva Manso tannin fraction; OTSF: Operculina turpethum (L.) Silva Manso saponin fraction
Histopathology analysis
After a single dose of STZ administration, there is the development of type-2 diabetes and leads to necrosis to islets β-cell, which can be noted in the experimental group. After a week of administration of OTFF fraction, the islets of pancreatic cells retain their standard structure, and mild necrosis was observed that supports the potential antidiabetic effect of the OTFF fraction, whereas, in the OTTF fraction, there is less recovery of islet cell. However, no recovery is observed in the OTSF treated group compared to the experimental control group (Fig. 6).
Fig. 6.
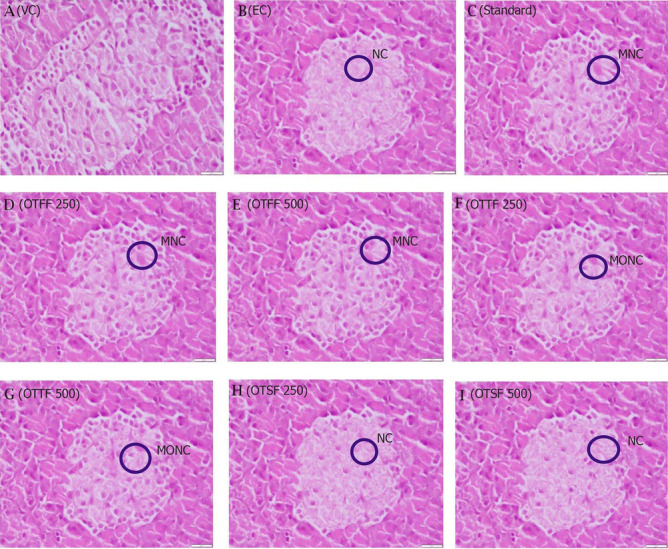
Histopathological observations of Pancreas for the various fractions of the plant when evulated for in-vivo antidibaetic activity at two different concentrations i.e. 250 and 500 mg/kg using HFD and STZ induced diabetic model in SD rats. a (VC): Nothing abnormal detected; b (EC): Necrosis (NC) of Islets of Langerhans; c (Standard): Mild necrosis (MNC) of Islets of Langerhans; d (OTFF 250 mg/kg): Mild necrosis (MNC) of Islets of Langerhans; e (OTFF 500 mg/kg): Mild necrosis (MNC) of Islets of Langerhans; f (OTTF 250 mg/kg): Moderate necrosis (MONC) of Islets of Langerhans ; g (OTTF 500 mg/kg): Moderate necrosis (MONC) of Islets of Langerhans; h (OTSF 250 mg/kg): Necrosis (NC) of Islets of Langerhans; i (OTSF 500 mg/kg): Necrosis (NC) of Islets of Langerhans
Characterization by LC–MS
The in-vitro result showed that the OTFF posses maximum activity among all the fractions. The OTFF fraction was analyzed by LC–MS Spectra and predicted two significant compounds, i.e., Rutin (RU) having retention time 7.00 min with a mass of m/z 610.52 in 50.50% and Apigenin 7-O-6'' acetyl-glucoside (AAG) at 7.62 retention time with a mass of m/z 475.42 in 24.10% (Table 10).
Table 10.
Identification of major compounds in the OTFF fraction of Operculina turpethum
| S. no. | Retention time (min.) | % AUC | Mass (m/z) | Mass fragments (m/z) | Identified compound |
|---|---|---|---|---|---|
| 1 | 7.00 | 50.50 | 610.52 | 633, 611, 465, 449, 303 |
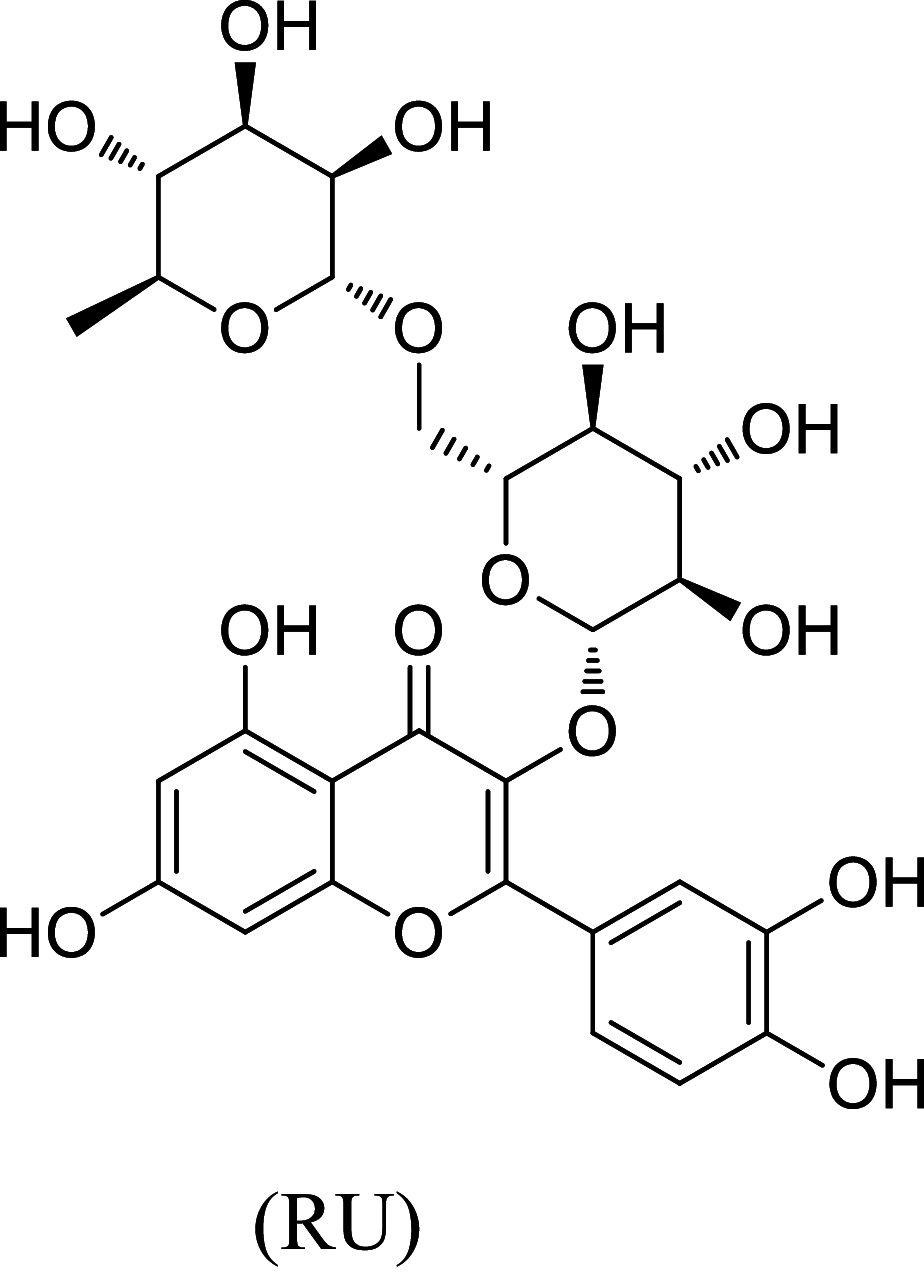
|
| 2 | 7.62 | 24.10 | 475.42 | 429, 323, 161, 221 |
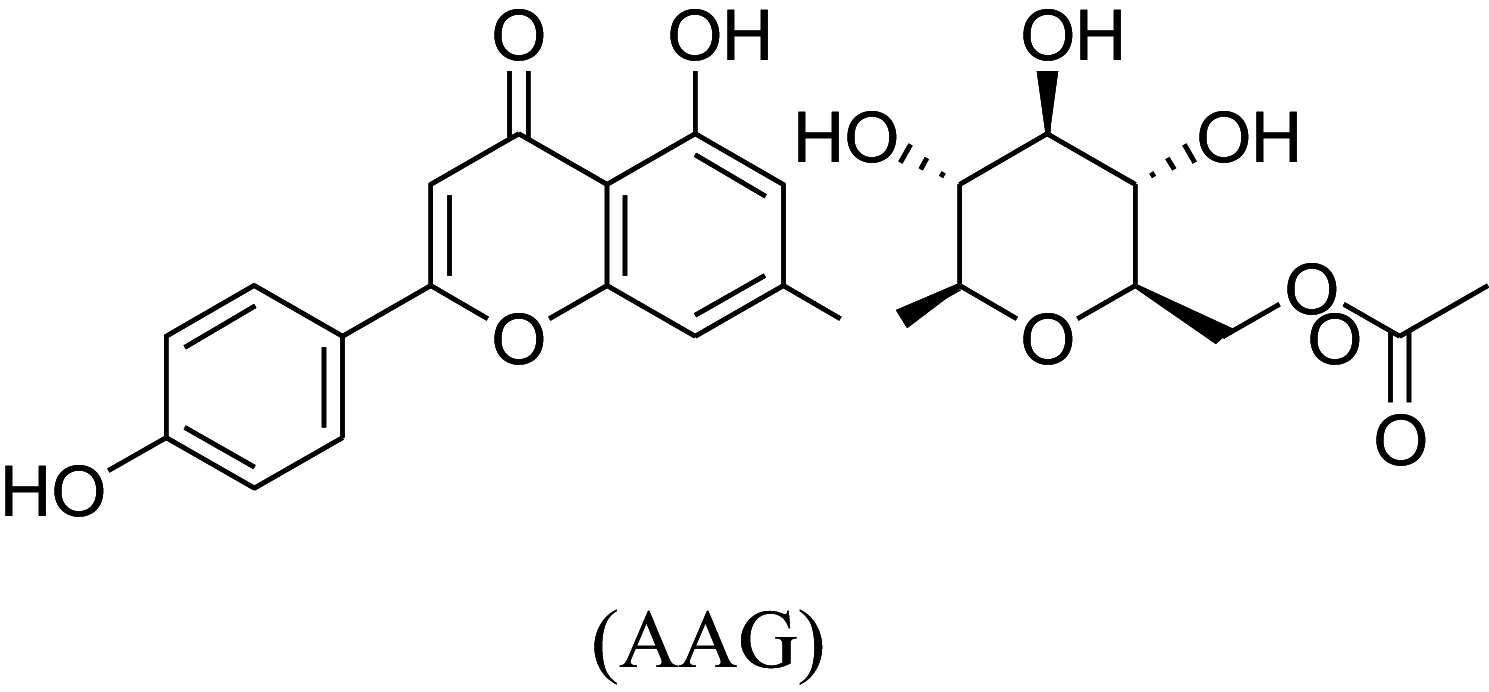
|
Molecular docking studies
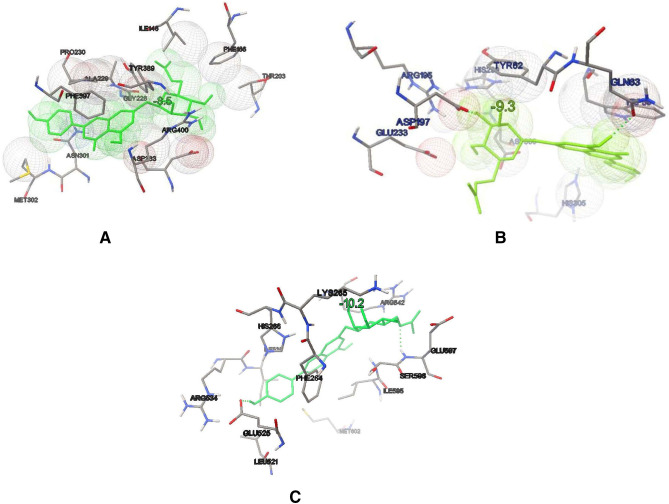
Based on LC–MS prediction, major constituents were studied in-silico for their antidiabetic activity choosing alpha-glucosidase, alpha-amylase, and PPAR gamma receptors. Among the predicted major phytochemicals, AAG has shown better binding affinities to alpha-glucosidase, alpha-amylase, and PPAR-gamma receptors with − 8.5 kcal/mol, − 9.3 kcal/mol, and − 7.0 kcal/mol on alpha-glucosidase, alpha-amylase, and PPAR gamma, respectively, in comparison to standard Acarbose (binding affinities − 4.4 kcal/mol, − 6.9 kcal/mol) and pioglitazone (binding affinities − 10.3 kcal/mol) (Table 11). A detailed interaction for AAG was studied for alpha-glucosidase, alpha-amylase, and PPAR gamma. In the alpha-glucosidase receptor, the THR203, GLY228, ALA 229, ASP333, ARG400, ASN301, MET302, TYR389, and PHE166 amino acid residues were identified as having interaction with AAG. The phytoconstituents AAG showed hydrophobic interaction with PHE397 with the aromatic ring, while aliphatic with ILE146 and THR203. Hydrophilic interaction of phenolic hydroxyl to ASP333 at the binding site of alpha-glucosidase (Fig. 7a). Different amino acid residues were involved in binding on amylase receptor, i.e., ARG195, ASP197, GLU233, TYR62, HIS305, GLN63, ASP300, HIS299, and TRP59. AAG showed two hydrogen bonds include ASP197 and GLN63, while the aromatic ring was also found to have the hydrophobic interaction of TRP59 has hydrophobic interaction with alpha-amylase (Fig7b). A study on PPAR gamma with AAG two hydrogen bonds with GLU525 and SER596, while PHE264 having hydrophobic interaction (Fig. 7c).
Table 11.
Molecular docking results of possible phytoconstituents in OTFF fraction
| S. no. | Molecule | Binding affinity (kcal/mol) | ||
|---|---|---|---|---|
| Alpha-glucosidase (3WY2) | Alpha-amylase (4GQR) | PPAR gamma (3SZ1) | ||
| 1 | RU | − 6.6 | − 8.9 | − 7.6 |
| 2 | AAG | − 8.5 | − 9.3 | − 10.2 |
| 3 | Acarbose | − 4.4 | − 6.9 | NA |
| 4 | Pioglitazone | NA | NA | − 10.3 |
Fig. 7.
Binding interaction of the bioactive fraction Operculina turpethum (L.) Silva Manso flavanoid fraction (OTFF) with the alpha-amylase, alpha-glucosidase and PPAR gamma a alpha-glucosidase (atom color) and AAG (in green color) b alpha-amylase (atom color) and AAG (in green color) c PPAR gamma (atom color) and AAG (in green color)
Discussion
DM is a metabolic disorder, i.e., characterized by hyperglycemia and inadequate production of pancreas insulin. Diabetes mellitus is associated with the risk of cardiovascular disease and obesity (Das et al. 2019; Kulkarni et al. 2019). Phytoconstituents play a vital part in the management of diabetes and are found to possess fewer side effects and less toxic when compared to the available synthetic medicines (Widjajakusuma et al. 2019). Many phytochemicals are reported to have a potential therapeutic effect on metabolic diseases (Feng et al. 2019; Li et al. 2019a, b). Many plant extracts and their isolated compounds were found to be safe and effective in various in-vitro enzyme inhibitory assays (Tewari et al. 2020; Sharma and Singh 2014). The plant’s available literature shows that the methanol and aqueous extract were effective in treating diabetes (Pulipaka et al. 2012; Gupta and Ved 2017). However, the study was conducted on a pilot scale only. No reports were available for the responsible secondary metabolites or compounds responsible for its therapeutic activity, and toxicity studies were also not conducted on the fractions to establish the plant’s safety profile. Therefore, our research mainly focuses on exploring the safety profile, in-vitro and in-vivo antidiabetic potential of the plant at the fraction level, identifying the responsible phytoconstituents, and predicting its mechanism action. The plant’s roots/rhizomes were fractionated into different fractions and screened for in vitro alpha-amylase assay in this context.
The OTFF fraction showed potent alpha-amylase inhibitor activity compared to standard Acarbose having IC50 values 100.69 and 812.83 µg/ml, respectively, whereas the other fractions showed less potent activity. The research finding suggests the possible role of OTFF in reducing the conversion rate of starch to monosaccharides. The MTT assay revealed that all the 3T3 cells were found viable for all the plant fractions i.e. OTFF, OTTF and OTSF at a concentration of 1000 µg/ml. This is due to the release of the NADPH dependent oxidoreductase enzyme in the cell which was further reacted with the MTT and leads to the formation of purple color formazan crystals. This represents that all the fractions of the plants were found to be safe and non toxic.
Furthermore, the OTFF, OTTF, and OTSF were evaluated for their acute toxicity at a concentration of 2000 mg/kg, p.o. in albino mice. The behavioral, biochemical, hematology, and histopathology study represent that all OTFF and OTSF fractions produce only mild toxicity at heart, liver, and kidney. In contrast, OTTF fraction produces mild to moderate toxicity in the liver, and all the fractions have an LD50 more than 2000 mg/kg.
The fractions (OTFF, OTTF, and OTSF) were evaluated for their in-vivo antidiabetic potential in HFD + STZ induced Type 2 DM. The biochemical results represent that after 1 week of OTFF administration at a 500 mg/kg dose, p.o. There is a highly significant decrease in the glucose, cholesterol, and triglyceride level compared with the experimental group, and OTFF was found to be more potent than the standard Acarbose. However, the OTTF fraction also significantly reduces all the biochemical levels when compared with the observational group. There is no significant difference observed with the OTSF fraction treated group compared to the experimental group. The histopathology findings of OTFF fraction indicate that the islets of pancreatic cells retain their standard structure, and mild necrosis was observed that supports the potential antidiabetic effect of the OTFF fraction. In contrast, in the OTTF fraction, moderate necrosis was observed. However, in that respect, no recovery is observed in the OTSF treated group than the experimental control group.
The in-vitro and in-vivo antidiabetic activity represents that the OTFF was more potent than standard Acarbose. Therefore, the bioactive OTFF fraction was further characterized by LC–MS, and two major components were identified, i.e. RU and AAG, which are primarily responsible for the fraction’s therapeutic activity. These flavonoids RU and AAG are reported for antidiabetic and antioxidant activity (Ghorbani 2017; Malik et al. 2017). Thus, we studied a molecular docking study using three major targets like alpha-glucosidase, alpha-amylase, and PPAR gamma, to predict their action toward these targets. Among the predicted major phytochemicals AAG showed better binding affinities to alpha-glucosidase, alpha-amylase, and PPAR-gamma receptors with − 8.5 kcal/mol, − 9.3 kcal/mol, and − 7.0 kcal/mol on alpha-glucosidase, alpha-amylase, and PPAR gamma, respectively, in comparison to standard Acarbose (binding affinities − 4.4 kcal/mol, − 6.9 kcal/mol) and pioglitazone (binding affinities − 10.3 kcal/mol). It is predicted that the OTFF fraction primarily acts as an alpha-amylase inhibitor and agonist for PPAR gamma that supports the potential role of the OTFF fraction in type 2 DM.
Conclusion
The research findings of the current study represent that OTFF fraction was found to posse’s dose-dependently potent antidiabetic activity in both in vitro and in vivo diabetic models. In contrast, the OTTF was found to be less active, and the OTSF fraction did not produce any therapeutic activity. The MTP assay represents that the entire plant fractions were safe when evaluated in normal mice fibroblast cell line (3T3 cell). Furthermore, all the fractions did not produce any sign of severe toxicity. Only mild toxicity was noted at a dose of 2000 mg/kg, p.o. In conclusion, the OTFF fraction enhanced the overall diabetic situation by acting as an alpha-amylase inhibitor and PPAR gamma agonist.
Acknowledgements
We thank the management of Lovely Professional University, Phagwara, for providing the necessary support and research facility for conducting the research work. We express our gratitude towards Prof Dr. Kuldeep Gupta, Department of Veterinary Pathology, Guru Angad Dev University, and Ludhiana for interpretation of histopathological slides and Dr. M.P Singh National Laboratories, Phagwara for providing support in biochemical and hematology analysis.
Abbreviations
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BASO
Basophills
- BID
Bilirubin direct
- BII
Bilirubin indirect
- BIT
Bilirubin total
- CMC
Carboxy methyl cellulose
- DM
Diabetes mellitus
- EDTA
Ethylenediaminetetraacetic acid
- EO
Eosinophils
- HCT
Hematocrit
- HDL
High-density lipoprotein
- HFD
High-fat diet
- HGB
Hemoglobin
- IG
Immature granulocyte count
- LDL
Low-density lipoprotein
- LYMP
Lymphocytes
- MCH
Mean corpuscular hemoglobin
- MCHC
Mean corpuscular hemoglobin concentration
- MCV
Mean corpuscular volume
- MONO
Monocytes
- MPV
Mean platelet volume
- NEUT
Neutrophils
- P-LCR
Platelet large cell ratio
- PCT
Plateletcrit
- PDW
Platelet distribution width
- PLT
Platelet
- RBC
Red blood cells
- RDW-CV
Red blood cell distribution width-coefficient of variance
- RDW-SD
Red blood cell distribution width-standard deviation
- SGOT
Serum glutamic oxaloacetic transaminase
- SGPT
Serum glutamic pyruvic transaminase
- STZ
Streptozotocin
- VLDL
Very low-density lipoprotein
- WBC
White blood cells
Declarations
Conflicts of interest
The authors have declared no conflict of interest.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s13205-024-04047-x
Change history
8/19/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s13205-024-04047-x
References
- Bindu J, Narendhirakannan RT (2019) Role of medicinal plants in the management of diabetes mellitus: a review. 3 Biotech 9:1–17. 10.1007/s13205-018-1528-0 10.1007/s13205-018-1528-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito A, Ramirez JE, Areche C et al (2014) HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 19:17400–17421. 10.3390/molecules191117400 10.3390/molecules191117400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary N, Bijjem KRV, Kalia AN (2011) Antiepileptic potential of flavonoids fraction from the leaves of Anisomeles malabarica. J Ethnopharmacol 135:238–242. 10.1016/j.jep.2011.02.019 10.1016/j.jep.2011.02.019 [DOI] [PubMed] [Google Scholar]
- Choudhary N, Khatik GL, Suttee A (2020) The possible role of Saponin in Type-II diabetes—a review. Curr Diabetes Rev 17:107–121. 10.2174/1573399816666200516173829 10.2174/1573399816666200516173829 [DOI] [PubMed] [Google Scholar]
- Das SK, Samantaray D, Sahoo SK et al (2019) Bioactivity guided isolation of antidiabetic and antioxidant compound from Xylocarpus granatum J. Koenig bark. 3 Biotech 9:1–9. 10.1007/s13205-019-1711-y 10.1007/s13205-019-1711-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmani M, Kamarulzaman K, Giribabu N et al (2019) Effect of Marantodes pumilum Blume (Kuntze) var. alata on β-cell function and insulin signaling in ovariectomised diabetic rats. Phytomedicine 65:153101. 10.1016/j.phymed.2019.153101 10.1016/j.phymed.2019.153101 [DOI] [PubMed] [Google Scholar]
- Eitah HE, Maklad YA, Abdelkader NF et al (2019) Modulating impacts of quercetin/sitagliptin combination on streptozotocin-induced diabetes mellitus in rats. Toxicol Appl Pharmacol 365:30–40. 10.1016/j.taap.2018.12.011 10.1016/j.taap.2018.12.011 [DOI] [PubMed] [Google Scholar]
- El Deeb KS, Eid HH, Ali ZY et al (2019) Bioassay-guided fractionation and identification of antidiabetic compounds from the rind of Punica Granatum Var. nana. Nat Prod Res. 10.1080/14786419.2019.1655411 10.1080/14786419.2019.1655411 [DOI] [PubMed] [Google Scholar]
- Feng X, Sureda A, Jafari S et al (2019) Berberine in cardiovascular and metabolic diseases: from mechanisms to therapeutics. Theranostics 9(7):1923–1951. 10.7150/thno.30787 10.7150/thno.30787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca AG, Ribeiro Dantas LLSF, Fernandes JM et al (2018) In vivo and in vitro toxicity evaluation of hydroethanolic extract of Kalanchoe brasiliensis (Crassulaceae) leaves. J Toxicol. 10.1155/2018/6849765 10.1155/2018/6849765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaikwad AB, Gupta J, Tikoo K (2010) Epigenetic changes and alteration of Fbn1and Col3A1gene expression under hyperglycaemic and hyperinsulinaemic conditions. Biochem J 432:333–341. 10.1042/BJ20100414 10.1042/BJ20100414 [DOI] [PubMed] [Google Scholar]
- Ghorbani A (2017) Mechanisms of antidiabetic effects of flavonoid rutin. Biomed Pharmacother 96:305–312. 10.1016/j.biopha.2017.10.001 10.1016/j.biopha.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Gupta S, Ved A (2017) Operculinaturpethum (Linn.) Silva Manso as a medicinal plant species: a review on bioactive components and pharmacological properties. Pharmacogn Rev 11:158. 10.4103/phrev.phrev_6_17 10.4103/phrev.phrev_6_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang GH, Kim HW, Lee MK et al (2018) Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC–DAD–QTOF/MS. Saudi J Biol Sci 25:1622–1631. 10.1016/j.sjbs.2016.08.001 10.1016/j.sjbs.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiametis AS, Silva MA, Romeiro LAS et al (2017) Potential acetylcholinesterase inhibitors: molecular docking, molecular dynamics, and in silico prediction. J Mol Model 23:67–76. 10.1007/s00894-017-3228-9 10.1007/s00894-017-3228-9 [DOI] [PubMed] [Google Scholar]
- Kulkarni P, Lohidasan S, Mahadik K (2019) Isolation, characterisation and investigation of in vitro antidiabetic and antioxidant activity of phytoconstituents from fruit of Momordica charantia Linn. Nat Prod Res. 10.1080/14786419.2019.1613400 10.1080/14786419.2019.1613400 [DOI] [PubMed] [Google Scholar]
- Latifi E, Mohammadpour AA, Fathi B, Nourani H (2019) Antidiabetic and antihyperlipidemic effects of ethanolic Ferula assa-foetida oleo-gum-resin extract in streptozotocin-induced diabetic wistar rats. Biomed Pharmacother 110:197–202. 10.1016/j.biopha.2018.10.152 10.1016/j.biopha.2018.10.152 [DOI] [PubMed] [Google Scholar]
- Li BY, Xu XY, Gan RY et al (2019a) Targeting gut microbiota for the prevention and management of diabetes mellitus by dietary natural products. Foods 8(10):440–458. 10.3390/foods8100440 10.3390/foods8100440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Wang J, Zhang B, Li X, Liu Y (2019b) Diabetes mellitus and cause-specific mortality: a population-based study. Diabetes Metab J 43:319–341. 10.4093/dmj.2018.0060 10.4093/dmj.2018.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majinda RRT (2012) Extraction and isolation of saponins. Methods Mol Biol 864:415–426. 10.1007/978-1-61779-624-1 10.1007/978-1-61779-624-1 [DOI] [PubMed] [Google Scholar]
- Malik S, Suchal K, Khan SI et al (2017) Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-ĸB-TNF-α and TGF-β1-MAPK-fibronectin pathways. Am J Physiol Renal Physiol 313:414–422. 10.1152/ajprenal.00393.2016 10.1152/ajprenal.00393.2016 [DOI] [PubMed] [Google Scholar]
- Nambirajan G, Karunanidhi K, Ganesan A et al (2018) Evaluation of antidiabetic activity of bud and flower of Avaram Senna (Cassia auriculata L.) in high fat diet and streptozotocin induced diabetic rats. Biomed Pharmacother 108:1495–1506. 10.1016/j.biopha.2018.10.007 10.1016/j.biopha.2018.10.007 [DOI] [PubMed] [Google Scholar]
- Priscilla DH, Roy D, Suresh A et al (2014) Naringenin inhibits α-glucosidase activity: a promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem Biol Interact 210:77–85. 10.1016/j.cbi.2013.12.014 10.1016/j.cbi.2013.12.014 [DOI] [PubMed] [Google Scholar]
- Pulipaka S, Challa SR, Pingili RB (2012) Comparative antidiabetic activity of methanolic extract of Operculina turpethum stem and root against healthy and streptozotocin induced diabetic rats. Int Curr Pharm J 1:272–278. 10.3329/icpj.v1i9.11618 10.3329/icpj.v1i9.11618 [DOI] [Google Scholar]
- Saeedi P, Petersohn I, Salpea P et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157:107843. 10.1016/j.diabres.2019.107843 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- Saleem U, Amin S, Ahmad B et al (2017) Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. Toxicol Rep 4:580–585. 10.1016/j.toxrep.2017.10.005 10.1016/j.toxrep.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Singh M (2014) Ameliorative effects of operculina turpethum and its isolated stigma-5,22dien-3-o-β-d-glucopyranoside on the hematological parameters of male mice exposed to n-nitrosodimethylamine, a potent carcinogen. Toxicol Int 21(1):29–36. 10.4103/0971-6580.128789 10.4103/0971-6580.128789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Joshi T, Joshi T et al (2020) In silico screening of potential antidiabetic phytochemicals from Phyllanthus emblica against therapeutic targets of type 2 diabetes. J Ethnopharmacol 248:112268. 10.1016/j.jep.2019.112268 10.1016/j.jep.2019.112268 [DOI] [PubMed] [Google Scholar]
- Shoaib A, Salem-Bekhit MM, Siddiqui HH et al (2020) Antidiabetic activity of standardized dried tubers extract of Aconitum napellus in streptozotocin-induced diabetic rats. 3 Biotech 10:1–8. 10.1007/s13205-019-2043-7 10.1007/s13205-019-2043-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JP, Baskaran UL, Shallauddin KB et al (2018) Evidence of antidiabetic activity of Spirulina fusiformis against streptozotocin-induced diabetic Wistar albino rats. 3 BIotech 8:1–12. 10.1007/s13205-018-1156-8 10.1007/s13205-018-1156-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni LK, Dobhal MP, Arya D et al (2018) In vitro and in vivo antidiabetic activity of isolated fraction of Prosopis cineraria against streptozotocin-induced experimental diabetes: a mechanistic study. Biomed Pharmacother 108:1015–1021. 10.1016/j.biopha.2018.09.099 10.1016/j.biopha.2018.09.099 [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Viswanad B, Asrat L et al (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 52:313–320. 10.1016/j.phrs.2005.05.004 10.1016/j.phrs.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Stefanowicz-Hajduk J, Ochocka JR (2020) Real-time cell analysis system in cytotoxicity applications: usefulness and comparison with tetrazolium salt assays. Toxicol Rep 7:335–344. 10.1016/j.toxrep.2020.02.002 10.1016/j.toxrep.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari D, Zengin G, Ak G et al (2020) Phenolic profiling, antioxidants, multivariate, and enzyme inhibitory properties of Wild Himalayan Fig (Ficus palmata Forssk.): a potential candidate for designing innovative nutraceuticals and related products. Anal Lett. 10.1080/00032719.2020.1804395 10.1080/00032719.2020.1804395 [DOI] [Google Scholar]
- Trott O, Olson AJ (2010) Software news and update AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. 10.1002/jcc.21334 10.1002/jcc.21334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah Jan N, Ali A, Ahmad B et al (2018) Evaluation of antidiabetic potential of steroidal alkaloid of Sarcococca saligna. Biomed Pharmacother 100:461–466. 10.1016/j.biopha.2018.01.008 10.1016/j.biopha.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Widjajakusuma EC, Jonosewojo A, Hendriati L et al (2019) Phytochemical screening and preliminary clinical trials of the aqueous extract mixture of Andrographis paniculata (Burm. f.) Wall. ex Nees and Syzygium polyanthum (Wight.) Walp leaves in metformin treated patients with type 2 diabetes. Phytomedicine 55:137–147. 10.1016/j.phymed.2018.07.002 10.1016/j.phymed.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Xiong A, Shao Y, Fang L et al (2019) Comparative analysis of toxic components in different medicinal parts of Gynura japonica and its toxicity assessment on mice. Phytomedicine 54:77–88. 10.1016/j.phymed.2018.06.015 10.1016/j.phymed.2018.06.015 [DOI] [PubMed] [Google Scholar]