Abstract
Food allergy is a significant issue worldwide, particularly in Westernised countries. There is no clear explanation why food allergy appears to have increased so rapidly in recent years, particularly in young children, hence ongoing research to identify effective primary prevention strategies. Food allergy prevention guidelines for health professionals have been developed based on existing clinical trial evidence for effective translation and implementation. As these guidelines underpin clinical practice, it is important to ensure robust processes of development. We conducted a systematic review to identify food allergy prevention guidelines for health professional use; to compare the recommendations made by the identified guideline documents; and to assess the quality of the identified guideline documents.
We searched Medline, EMBASE, CINAHL, Scopus, Global Health and Guidelines International Network for the period 1990 to 13 August 2019, to identify articles referring to English-language food allergy prevention guidelines or the guidelines themselves. A grey literature search of Google Scholar and reference checking was also undertaken. The guidelines were compared for recommendation similarities and differences. An Appraisal Guidelines for Research and Evaluation (AGREE II) appraisal was undertaken to assess guideline quality.
The electronic database search yielded 1121 publications and reference checking identified an additional 16 publications. After title, abstract and full text screening, data extraction was undertaken on 156 publications and with additional reference checking, 28 food allergy prevention guidelines and advice documents were identified. Comparison of the recommendations within the guidelines and advice documents indicated the greatest variation in recommendations related to exclusive breastfeeding and timing of solid food introduction. Eight of the 10 guidelines and none of the 18 advice documents met the quality threshold set by the reviewers. Overall, documents specifically termed "guidelines" scored better than advice documents when assessed using the AGREE II tool.
Variation in recommendations may create confusion for health professionals and result in inconsistent advice being provided to parents, and less translation of the evidence into actual food allergy reduction in the population. Appraisal using the AGREE II tool identified that there is considerable room for improvement in the development of guidelines and advice documents for food allergy prevention. The AGREE II appraisal identified common areas of poorer quality development and/or documentation of processes to inform future guideline development. Based on this study, we recommend the use of validated guideline development tools, to direct food allergy prevention guideline review or development. Use of the AGREE II tool, to direct the review and development of guidelines, is very likely to improve guideline quality.
Keywords: Allergy prevention, Guidelines, Food allergy, Infant feeding
Introduction
Food allergy is a significant issue worldwide, particularly in Westernised countries.1, 2, 3, 4 While good epidemiological data are lacking, it is estimated that worldwide, more than 220 million people have a food allergy.5, 6, 7, 8 In developed countries, food allergy is more common in children,9 with verified food allergy prevalence ranging from 6% to 10% in infants and 2% and 5% in adults.10, 11, 12, 13 There is also evidence of a high prevalence of food allergy in developing countries, with a 2.5% incidence of challenge-proven food allergy observed in South Africa in 201514 and reported prevalence in China increasing from 3.5% to 7.7% between 1999-2009.14
Food allergy impacts greatly on the quality of life of children and their caregivers,15,16 and contributes significant direct health costs for the healthcare system and even larger costs for families with a food allergic child.17 Preventing food allergy is, therefore, a logical step in minimising the mortality, significant morbidity, and related costs associated with this condition.
Food allergy prevention strategies based on delaying introduction of common food allergens in high risk individuals have been largely ineffective,18,19 and, consequently, the search for effective primary prevention strategies has shifted to interventions including: timeframe for exclusive breastfeeding, breastmilk substitutes, early introduction of foods including common food allergen introduction, vitamin D and omega-3 fatty acid supplementation, and modification of the maternal and infant microbiome.20, 21, 22, 23, 24
In 2015, several randomised controlled trials (RCTs) and a meta-analysis of these trials examining the effect of early introduction of food allergens on the development of food allergy were published.24, 25, 26 Results from these trials have led to changes in infant feeding advice for food allergy prevention.24, 25, 26 Several large RCTs have examined the effect of early introduction of egg into the diet compared with delayed introduction and have shown some evidence that, depending on the baseline risk status of the treatment group, prevention of IgE-egg sensitisation or egg allergy may be associated with earlier introduction of egg.25,27,28 However, the Enquiring about Tolerance (EAT) study which examined the effect of introduction of 6 foods (cow's milk, egg, peanut, wheat, fish, sesame) to the diet of exclusively breastfed infants from 3 months of age,26 and the Prevention of Egg allergy with Tiny amount of InTake (PETIT) study in infants with well controlled eczema (high risk)28 showed the greatest benefits with cooked egg.
The Learning Early About Peanut (LEAP) study, which randomised 640 high risk infants aged 4–11 months to consume or avoid peanut until 60 months of age, was a pivotal peanut allergy prevention study.24 This study demonstrated an 86.1% relative reduction in peanut allergy prevalence in the consumption group compared to the control group.24 These studies provide a foundation for evidence-based food allergy prevention guidelines and advice documents.
Clinical practice guidelines are designed for health professionals and are important to ensure the best health outcomes for patients.29,30 Food allergy prevention guidelines for health professionals should be developed based on existing clinical trial evidence for effective translation and implementation. As guidelines are intended to underpin clinical practice, it is important that high quality evidence is integrated, and the development and reported process is robust.
The Appraisal Guidelines for Research and Evaluation (AGREE II) tool31,32 is a validated instrument used to assess guideline quality.33,34 The AGREE II tool assesses guidelines across 6 domains: scope/purpose; stakeholder involvement; rigor of development; presentation; applicability; and editorial independence. Assessing the quality of guidelines (including advice documents) is important to determine (1) if adequate guideline development processes were used; and (2) where the guideline documents differ in quality based on their nominated title (eg, guideline vs. consensus statement) and processes used in development.
This systematic review aimed to identify food allergy prevention guidelines for health professional use; to compare the recommendations made by the identified guideline documents; and to assess the quality of the identified guideline documents.
Methodology
In this review, the word "guideline" is defined as any document termed an evidence-based guideline, expert recommendation, consensus statement, joint statement, position paper, or clinical report/guidance document, in accordance with the World Health Organisation (WHO) definition of “any document that contains recommendation for clinical health practice or public health policy”.35 The AGREE II consortium acknowledges that documents specifically titled as "guidelines" generally score higher when appraised using the AGREE II tool than non-official guideline documents.36 Hence, for this AGREE II assessment, a comparison has been made between documents specifically named "guidelines" by their authors in their title and all other documents which have been grouped together as "advice documents".
Search strategy
The literature was systematically searched to identify guidelines and advice documents developed for health professional use for the primary prevention of food allergy. A two-phase search was employed with the initial phase identifying both guideline documents and publications that referred to a guideline document. The second phase involved sourcing all the guideline documents identified in phase 1.
The following databases were searched for the period 1990 to 13 August 2019: Medline, EMBASE, CINAHL, Scopus, Global Health and Guidelines International Network. A grey literature search of Google Scholar was also undertaken. In addition, a search of the reference lists from publications included in the full-text screen and references from identified guideline documents was undertaken. English language restrictions were applied. Guideline documents and studies reporting guideline documents between January 1990 and the date of the search (13 August 2019) were sought.
The following search terms were used: (“health professionals” OR “general practitioners” OR “nurses” OR “dietitians” OR “dieticians”) AND (“food allergy” OR “food hypersensitivity” OR “allergy”) AND (“guidelines” OR “guideline” OR “policies” OR “policy” OR “strategy” OR “recommendation” OR “statement” OR “protocol” OR “consensus” OR “clinical practice”) AND (“prevention” OR “primary prevention”).
Article selection
Publications identified from the search were exported to Endnote reference management software, version 8 (Clarivate Analytics, Philadelphia, PA), duplicates removed, then uploaded to Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) for screening. Two reviewers independently screened the titles and abstracts of all publications identified in the search. All publications meeting the inclusion criteria were retained. Where the titles and abstracts provided insufficient details, full-text publications were retrieved and screened again by both reviewers against the inclusion criteria. All disagreements were resolved by discussion between the reviewers without the need for moderation.
Two reviewers independently extracted data of interest using standardised data extraction forms developed for this review. The following information, where available, was extracted for each publication: authors; article title; name of guideline document; year of guideline document; name of organisation; country. Once identified through data extraction, the guideline documents were retrieved.
Selection criteria
For phase 1 of the search, the following were included: guideline documents whose stated purpose was the primary prevention of food allergy (including the original guideline document and articles referring to such guideline documents); guideline documents and articles in English; and guideline documents intended specifically for health professional use. Guideline documents for stated purposes other than primary prevention of food allergy were included only if they provided detailed, specific recommendations regarding food allergy prevention within their scope.
For phase 2 of the search, English language guideline documents whose stated purpose was the primary prevention of food allergy for health professionals were included; and guideline documents for stated purposes other than primary prevention of food allergy were included only if they provided detailed, specific recommendations regarding food allergy prevention within their scope.
If one professional organisation or government published more than one guideline document, all versions meeting the selection criteria were included in the review.
Guideline comparison
The recommendations contained within the guideline documents relating to maternal diet during pregnancy and lactation; breastfeeding substitutes; solid food timing; advice regarding introduction of common food allergens; specific advice regarding egg and peanut introduction; and spacing of introduction of new foods, were retrieved as these are key factors in relation to food allergy prevention. The guidelines were compared for their recommendations relating to these factors. Other interventions such as Vitamin D, omega-3 fatty acid supplementation, and modification of the maternal diet and the infant microbiome, were not included in this review.
Quality appraisal of guideline documents
Quality assessment of all identified guideline documents was undertaken independently by 2 reviewers who reviewed and scored each guideline document using the AGREE II tool.31,32 The AGREE II tool assesses guidelines using the following domains: scope/purpose (objectives, question, population); stakeholder involvement (group membership, target population, target users); rigor of development (search methods, evidence criteria, evidence strengths and limitations, recommendations, benefits and harms considerations, recommendations and evidence link, external review, and updating procedures); presentation clarity (specific, unambiguous recommendations, management options, and identifiable key recommendations); applicability (application facilitators and barriers, implementation of advice/tools, resource implications, and monitor/audit criteria); and editorial independence (funding body, competing interests).
The reviewers referred to the AGREE II tool with the user's manual34 when assessing the guideline documents and were masked to scores assigned by the other reviewer. Each domain has a different number of quality assessment questions, each requiring a score between 1 and 7 (7 being the highest score). The quality scores were synthesised and domain scores for each guideline document calculated according to the AGREE II manual protocol.34 Domain scores are calculated by subtracting the minimum possible score for the domain from the obtained score for the domain; this is then divided by the maximum possible score for the domain minus the minimum possible score for the domain; this score is multiplied by 100 to achieve a percentage.34
Interpretation of domain scores
The AGREE II tool does not specify cut-off scores equating to guideline quality.34 For this review, consistent with other reviews,37,38 the quality threshold for guideline acceptability was defined as guideline documents achieving at least 50% for Domain 3 (rigor of development) and at least 50% for at least 2 other domains.
Statistical analysis
Data were analysed in SPSS 26.0 (SPSS Inc, Chicago, IL). Descriptive statistics for the AGREE II assessment were obtained, and comparison of the means of the guidelines compared to the advice documents was undertaken using an independent t-test.
Results
The electronic database search yielded 1121 publications and reference checking identified an additional 18 publications. After removal of duplicates (n = 477); title and abstract screening was undertaken on 660 publications; 239 publications underwent full-text screening. Data extraction was undertaken on 156 publications to identify guideline documents including: the name of the guideline, creator/owner, country, and date. Two additional guideline documents were identified through reference searching. This yielded 28 guideline documents (from 17 organisations) over a period of 21 years (Supplemental Figure 1). The AGREE II appraisal was undertaken on all 28 guideline documents identified.
Identified guideline documents
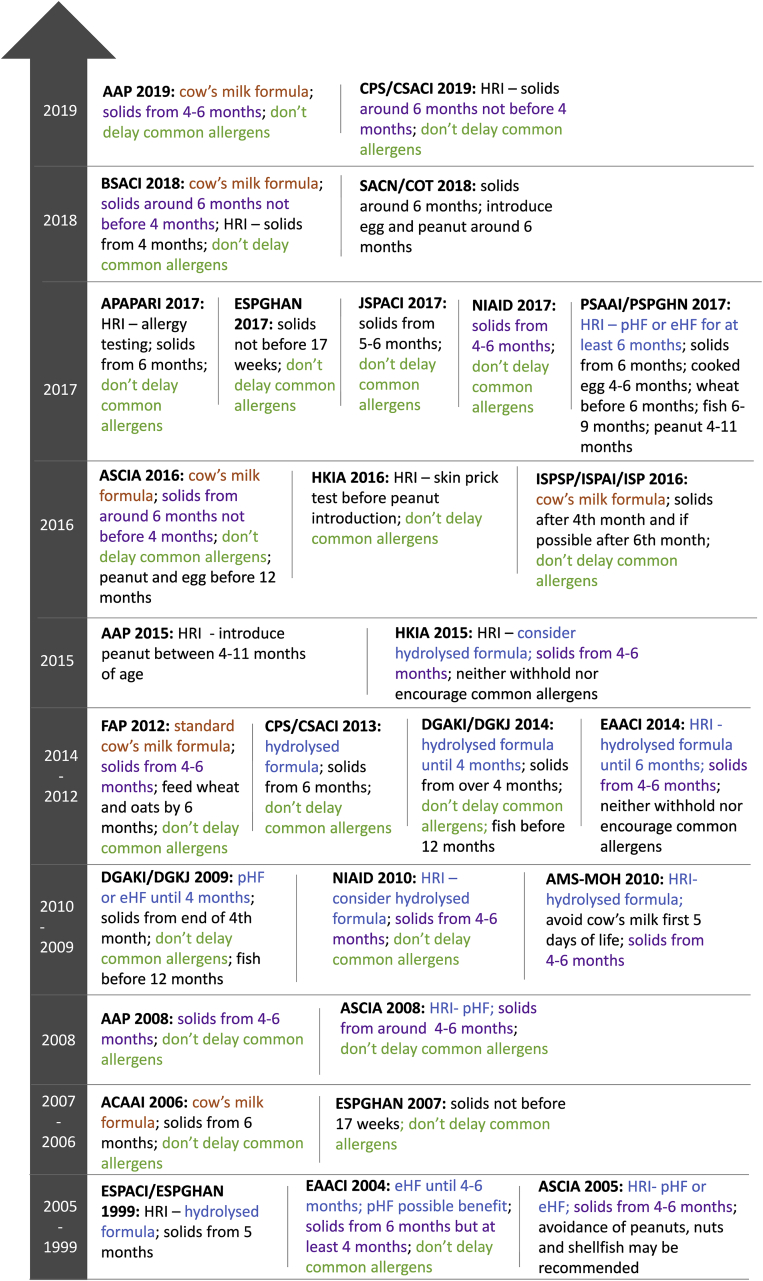
A summary of included guideline documents is provided in Table 1.39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 Of the 28 food allergy prevention guideline documents, 10 were specifically titled as "guidelines",46,51,55, 56, 57,60,62, 63, 64,66 and 18 were titled as consensus statements, position statements, joint statements or recommendations and were grouped together as "advice documents".39, 40, 41, 42, 43, 44, 45,48,50, 51, 52,54, 55, 56, 57,62,63,68 Where an organisation had more than 1 version of their guideline document, all versions were included. A timeline of the 28 food allergy prevention guidelines is provided in Fig. 1.
Table 1.
Summary of included guideline documents
| Organisation | Name of document | Author specified type of document | Region | Year |
|---|---|---|---|---|
| American Academy of Paediatrics | Effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods and hydrolysed formulas39 | Clinical report/Guidance | United States | 2008 |
| American Academy of Paediatrics | Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants40 | Consensus communication | United States | 2015 |
| American Academy of Paediatrics | The effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, hydrolysed formulas and timing of introduction of allergenic complementary foods41 | Clinical report/Guidance | United States | 2019 |
| American College of Allergy, Asthma and Immunology (ACAAI) | Food allergy and introduction of solid foods to infants: a consensus document42 | Consensus document | United States | 2006 |
| Asia Pacific Association of Paediatric Allergy, Respirology & Immunology (APAPARI) | Early introduction of allergenic foods for the prevention of food allergy from an Asian perspective - An APAPARI consensus statement43 | Consensus statement | Asia | 2017 |
| Australasian Society of Clinical Immunology and Allergy (ASCIA) | Australasian Society of Clinical Immunology and Allergy position statement: summary of allergy prevention in children44 | Position statement | Australia & New Zealand | 2005 |
| Australasian Society of Clinical Immunology and Allergy (ASCIA) | Infant feeding advice45 | Advice | Australia & New Zealand | 2008 |
| Australasian Society of Clinical Immunology and Allergy (ASCIA) | ASCIA Guidelines for infant feeding and allergy prevention46 | Guideline | Australia & New Zealand | 2016 |
| ASCIA Guidelines: Infant feeding and allergy prevention47 | ||||
| British Society for Allergy & Clinical Immunology (BSACI) | Preventing food allergy in higher risk infants: guidance for healthcare professionals48 | Guidance | United Kingdom | 2018 |
| Implementing primary prevention of food allergy in infants: New BSACI guidance published49 | ||||
| Canadian Paediatric Society (CPS) and Canadian Society of Allergy and Clinical Immunology (CSACI) | Dietary exposures and allergy prevention in high-risk infants50 | Joint statement | Canada | 2013 |
| Canadian Paediatric Society (CPS) and Canadian Society of Allergy and Clinical Immunology (CSACI) | Timing of introduction of allergenic solids for infants at high risk51 | Practice point | Canada | 2019 |
| European Academy of Allergy and Clinical Immunology (EAACI) | Dietary prevention of allergic diseases in infants and small children52 | Recommendations | Europe | 2004 |
| European Academy of Allergy and Clinical Immunology (EAACI) | EAACI Food Allergy and Anaphylaxis Guidelines. Primary prevention of food allergy53 | Guideline | Europe | 2014 |
| European Society for Paediatric Allergology and Clinical Immunology (ESPACI) and European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) | Dietary products used in infants for treatment and prevention of food allergy54 | Joint statement | Europe | 1999 |
| European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) | Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition55 | Position paper | Europe | 2007 |
| European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) | Complementary feeding: A position paper by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition56 | Position paper | Europe | 2017 |
| Finnish Allergy Programme | Allergy in children: practical recommendations of the Finish Allergy Programme 2008–2018 for prevention, diagnosis and treatment57 | Recommendations | Finland | 2012 |
| German Society for Allergology and Clinical Immunology (DGAKI) and the German Society for Paediatric and Adolescent Medicine (DGKJ) | Allergy Prevention58 | Clinical practice guideline | Germany | 2009 |
| German Society for Allergology and Clinical Immunology (DGAKI) and the German Society for Paediatric and Adolescent Medicine (DGKJ) | S3-Guideline on allergy prevention: 2014 update59 | Guideline | Germany | 2014 |
| Hong Kong Institute of Allergy (HKIA) | Guidelines for allergy prevention in Hong Kong60 | Guideline | Hong Kong | 2015 |
| Guidelines for Allergy Prevention in Hong Kong61 | ||||
| Hong Kong Institute of Allergy (HKIA) | HKIA position paper on prevention of peanut allergy in high risk infants62 | Position paper | Hong Kong | 2016 |
| Italian Society of Preventative and Social Paediatrics (ISPSP), the Italian Society of Paediatric Allergy and Immunology (ISPAI) and the Italian Society of Pediatrics (ISP) | Prevention of food and airway allergy: consensus of the Italian Society of Preventative and Social Paediatrics, the Italian Society of Paediatric Allergy and Immunology, and Italian Society of Pediatrics63 | Consensus statement | Italy | 2016 |
| Japanese Society of Paediatric Allergy and Clinical Immunology (JSPACI) | Japanese guidelines for food allergy 201764 | Guideline | Japan | 2017 |
| National Institute of Allergy and Infectious Diseases (NIAID) | Guidelines for the Diagnosis and Management of Food Allergy in the United States: Report of the NIAID-Sponsored Expert Panel65 | Guideline | United States | 2010 |
| National Institute of Allergy and Infectious Diseases (NIAID) | NIAID Addendum guidelines for prevention of peanut allergy in the United States66 | Guideline | United States | 2017 |
| Philippine Society of Allergy, Asthma and Immunology (PSAAI) and the Philippine Society for Paediatric Gastroenterology, Hepatology and Nutrition (PSPGHN) | Dietary prevention of allergic diseases in children: the Philippine guidelines67 | Guideline | Philippines | 2017 |
| Scientific Advisory Committee on Nutrition (SACN) and Committee on Toxicity of Chemicals in food Consumer products and the Environment (COT) | Assessing health benefits and risks of the introduction of peanut and hen's egg into the infant diet before six months of age in the UK68 | Joint statement | United Kingdom | 2018 |
| Academy of Medicine, Singapore Ministry of Health (AMS-MOH) | Management of food allergy69 | Clinical practice guideline | Singapore | 2010 |
| Academy of medicine, Singapore-Ministry of Health clinical practice guidelines: management of food allergy70 |
Fig. 1.
Guideline document timeline
Guideline comparison
The recommendations within the 28 guidelines document in relation to maternal diet, exclusive breastfeeding, breastmilk substitutes, timing of solid food introduction, and any recommendations for specific food introduction are summarised in Table 2.
Table 2.
Summary of comparison of recommendations.
| Guideline document | Maternal diet (pregnancy and breastfeeding) | Breastfeeding | Breastmilk substitutes | Solid food timing | Peanut and egg |
|---|---|---|---|---|---|
| AAP 2019 | No restrictions
|
Exclusive BF for at least 4 months
|
Hydrolysed formula not recommended
|
4–6 months of age: Do not delay common allergens
|
HRI - earliest age of peanut introduction is 4–6 months and consider evaluation of HRI by allergist before peanut introduction; Infants with mild-moderate eczema - earliest age of peanut introduction is around 6 months; Infants with no eczema or any food allergy - earliest age of peanut introduction is age appropriate and based on family meals and culture 2015 document ‘For HRI - introduce peanut between 4 and 11 months of age; Consider evaluation of HRI by allergist before peanut introduction’ |
| ACAAI 2006 | Not applicable | Exclusive BF for 6 months | Standard cow's milk formula | 6 months of age; Delay introduction of common allergens; Cooked, homogenised foods should be preferred over fresh counterparts if reduced allergenicity (e.g. beef and kiwifruit); egg, peanut, tree nuts fish and seafood introduction requires caution | Peanut and egg introduction requires caution |
| APAPARI 2017 | Not applicable | Continue BF alongside solid food introduction up to 2 years if possible, according to cultural practice | Not specified | HRI - recommend allergy testing to egg and peanut; At risk infants - no delay in introduction of allergenic foods; Healthy infants - 6 months of age | Allergy testing for HRI prior to introduction of egg and peanut |
| ASCIA 2016 | No restrictions; Healthy balanced diet; Up to 3 serves oily fish/week during pregnancy
|
At least 6 months (where possible) and for as long as mother and infant wish to continue
|
All infants - standard cow's milk formula
|
When infant is ready, around 6 months, not before 4 months; Introduce all common allergens; egg should be cooked; Continue to regularly include in infant diet once introduced
|
Introduce cooked egg and peanut before 12 months of age; Procedure for high risk infants
|
| BSACI 2018 | Not applicable | Exclusive BF for around 6 months; Continue to breastfeed while introducing solids if possible | Standard cow's milk formula | From around 6 months, but not before 4 months, when infant is ready; HRI - parents may wish to introduce solids from 4 months, cooked egg then peanut should be given, then other allergenic foods; egg should be cooked; Introduce before 12 months of age; Continue to regularly include in infant diet once introduced | HRI - may benefit from introduction of peanut and egg from 4 months alongside other foods |
| CPS and CSACI 2019 | Not applicable
|
Breastfeed for up to 2 years and beyond - 2013 document ‘Exclusive BF for first 6 months’ | Not applicable
|
HRI - around 6 months of age but not before 4 months; All other infants - around 6 months; Introduce all common allergens; Continue to regularly include in infant diet once introduced
|
Do not delay
|
| DGAKI and DGKJ 2014 | Balanced and varied diet; No restrictions; Fish should form part of the maternal diet
|
Predominantly breastfed up to 4 months of age
|
Hydrolysed infant formula until 4 months of age; Soy based formula is not recommended for allergy prevention
|
From over the age of 4 months; Common allergens should not be delayed; Fish should be introduced by 12 months of age
|
Not specified
|
| EAACI 2014 | No restrictions; No supplements while breastfeeding
|
Exclusive BF for 4–6 months
|
HRI - hydrolysed formula until 4 months of age then standard cow's milk formula; All other infants - standard cow's milk formula; Soy and hydrolysed formulas not recommended
|
From 4 to 6 months of age, when infant is ready; Neither withhold nor encourage exposure of common food allergens
|
Not specified
|
| ESPGHAN 2017 | Not applicable
|
Continue BF while introducing solid foods
|
Not specified
|
Not before 17 weeks; Do not delay common food allergens
|
High risk infants - introduce peanut between 4 and 11 months
|
| Finish Allergy Program 2012 | No restrictions | Exclusive BF for 4–6 months | Standard cow's milk formula | From 4 to 6 months while continuing BF; Introduce wheat and oats by 6 months of age | Do not delay |
| HKIA 2016 | Not applicable
|
Not applicable
|
Not applicable
|
HRI - SPT before introduction encouraged; Low risk infants - introduce peanut upon introduction of foods; Do not delay common food allergens
|
HRI - SPT; negative and mild positive SPT - 6g peanut protein/wk 3 times/wk until 5 years of age; Positive SPT - oral peanut challenge, include peanut if negative challenge an avoid peanut if positive challenge
|
| ISPSP and ISPAI and ISP 2016 | Fish oil supplementation not recommended | Exclusive BF for at least 4 months (possibly 6 months) | Standard cow's milk formula | After the 4th month and if possible after the 6th month; Introduce common food allergens in the same way as for children without allergic risk | Not specified |
| JSPACI 2017 | No restrictions | Insufficient evidence to indicate superiority of BF in the prevention of allergic disease | Insufficient evidence to support the use of hydrolysed formula | From 5 to 6 months of age when developmentally ready; Do not delay common food allergens | Introduce peanuts sooner rather than later after weaning |
| NIAID 2017 | Not applicable
|
Not applicable
|
Not applicable
|
4–6 months of age; Introduce common food allergens from 4 to 6 months of age
|
HRI - earliest age of peanut introduction is 4–6 months and consider evaluation of HRI by allergist before peanut introduction; Infants with mild-moderate eczema - earliest age of peanut introduction is around 6 months; Infants with no eczema or any food allergy - earliest age of peanut introduction is age appropriate and based on family meals and culture
|
| PSAAI and PSPGHN 2017 | No increased intake of certain foods recommended; No restrictions | Exclusive BF for at least 3–6 months | HRI – pHF or eHF recommended for at least 6 months; Soy milk not recommended | From 6 months of age; Cooked egg at 4–6 months; wheat before 6 months; fish at 6–9 months; peanut at 4–11 months | Cooked egg at 4–6 months; peanut at 4–11 months |
| SACN and COT 2018 | Not applicable | Exclusive BF for around 6 months | Not specified | Around 6 months of age; No information regarding common food allergens | Introduce peanut and egg around 6 months of age; If history of eczema or suspected food allergy, medical advice before peanut introduction may be sought; once introduced, peanut and egg should continue to be consumed as part of the usual infant diet |
| AMS-MOH 2010 | No restrictions | Exclusive BF for at least 4–6 months | HRI - hydrolysed formula recommended; Avoid cow's milk formula in the first 5 days of life | 4–6 months of age for all infants; No information regarding common food allergens | Not specified |
Abbreviations: BF = breastfeeding; HRI = High risk infants; SPT = Skin prick test; AAP = American Academy of Pediatrics; ACAAI = American College of Allergy, Asthma and Immunology; APAPARI = Asia Pacific Association of Paediatric Allergy, Respirology & Immunology; ASCIA = Australasian Society of Clinical Immunology and Allergy; BSACI = British Society for Allergy & Clinical Immunology; CPS = Canadian Paediatric Society; CSACI = Canadian Society of Allergy and Clinical Immunology; DGAKI = German Society for Allergology and Clinical Immunology; DGKJ = German Society for Paediatric and Adolescent Medicine; EAACI = European Academy for Allergy and Clinical Immunology; ESPACI = European Society for Paediatric Allergology and Clinical Immunology; ESPGHAN = European Society for Paediatric Gastroenterology, Hepatology and Nutrition; HKIA = Hong Kong Institute of Allergy; ISPSP = Italian Society of Preventative and Social Paediatrics; ISPAI = Italian Society of Paediatric Allergy and Immunology; ISP = Italian Society of Pediatrics; JSPACI = Japanese Society of Paediatric Allergy and Clinical Immunology; NIAID = National Institute of Allergy and Infectious Diseases; PSAAI = Philippine Society of Allergy, Asthma and Immunology; PSPGHN = Philippine Society for Paediatric Gastroenterology, Hepatology and Nutrition; SACN = Scientific Advisory Committee on Nutrition; COT = Committee on Toxicity of Chemicals in food, consumer products and the environment; AMS-MOH = Academy of Medicine, Singapore Ministry of Health
Maternal diet
Of the 28 guideline documents, 1739,41,44, 45, 46, 47,50,52,53,57, 58, 59, 60, 61,63, 64, 65,67,69,70 included recommendations regarding maternal diet during pregnancy and lactation. Eleven guideline documents did not include maternal dietary recommendations as they were guidelines specific to infant feeding. Of the 17 with maternal diet recommendations, all stipulated "no dietary restrictions".39,41,44, 45, 46, 47,50,52,53,57, 58, 59, 60, 61,63, 64, 65,67,69,70 In addition to the "no dietary restrictions" recommendation, 4 guideline documents also recommended a healthy balanced maternal diet;46,47,53,58,59 3 guideline documents recommended the inclusion of fish;46,47,58,59 and 1 document stipulated that fish oil supplements were not recommended.63
Exclusive breastfeeding
Twenty-five guideline documents made recommendations regarding exclusive breastfeeding.39,41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61,63, 64, 65,67, 68, 69 The remaining 3 made no exclusive feeding recommendations, as these guideline documents were specifically related to peanut introduction.40,62,66 Of those that did make breastfeeding recommendations, 6 recommended "exclusive/predominantly exclusive breastfeeding for at least 4 months".39,41,52,58,59,63 "Exclusive breastfeeding for 4–6 months" was recommended by 7 guideline documents;44,53,54,57,60,61,65,69,70 7 recommended "exclusive breastfeeding for 6 months or around 6 months or at least 6 months".42,45, 46, 47, 48, 49, 50,55,68 Two guideline documents provided no recommendation regarding exclusive breastfeeding but stipulated that breastfeeding "should continue up to 2 years or longer".43,52
Breastmilk substitutes
Twenty of the 28 guideline documents provided recommendations for breastmilk substitutes for primary prevention of allergy;39,41,42,44, 45, 46, 47, 48, 49, 50,52, 53, 54,57, 58, 59, 60, 61,63, 64, 65,67,69,70 4 of the remaining documents focussed on solid food introduction, and, therefore, were not intended to include breastfeeding information,40,51,62,66 and 4 made no recommendation.43,55,56,68 Of these, soy formula was not recommended by 10 documents.39,44,45,50,52,53,58,59,65,67 Specific recommendations for high risk infants were made in 8 documents,44,45,53,54,60,61,65,67,69,70 with partially hydrolysed formula (pHF) recommended in all 8 documents44,45,53,54,60,61,65,67,69,70 and extensively hydrolysed formula (eHF) recommended in 7 of the 8 documents.44,53,54,60,61,65,67,69,70 Where documented, the majority of documents that recommended the use of hydrolysed formulas were based on evidence suggesting potential reduction in allergic disease generally;44,45,50 atopic eczema;52, 53, 54,60,61,67,69,70 asthma and allergic rhinitis;60,61,67 food intolerance;44,50,52,53 or food allergy.52,53,65,69,70
Timing of solid food introduction into the infant diet
Twenty-seven documents made recommendations regarding the timing of solid food introduction.39,41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 The remaining guideline document was specific to peanut introduction, hence made no general solid food timing recommendations.40 There was variability in the wording for timing of the introduction of solid foods; however, all were within the range of 4 to 6 months. The most common wording (used in 10 documents) stipulated "from 4 to 6 months";39,41,44,45,53,57,60,61,65,66,69,70 4 documents stipulated "from 6 months";42,50,67,68 3 documents stipulated "around 6 months but not before 4 months".46, 47, 48, 49,51 When comparing timing of solid food introduction in guideline documents developed between 2015 and 2019 with such recommendations,40,41,43,46, 47, 48, 49,51,56,60,61,63,64,66, 67, 68 there was still similar variability in the wording of recommendations regarding timing of solid food introduction.
Specific recommendations for high risk infants were included in 4 documents.43,48,49,51,62 Of these, one document recommended introduction of solid foods "at 4 months" (compared to "6 months" for infants not at risk),48,49 and 1 document recommended introduction of solids "around 6 months but not before 4 months";51 while 2 documents recommended allergy testing before introduction of solid foods.43,62
Common food allergens
Twenty-four documents made recommendations regarding timing of common food allergen introduction.39,41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53,55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67 Sixteen documents recommended that the common food allergen introduction should not be delayed;39,41, 42, 43,45, 46, 47,50, 51, 52,55,56,58,59,62,64, 65, 66 1 document recommended delaying common food allergen introduction;42 and 1 document recommended delaying peanut, nuts and shellfish.44 Three documents recommended neither withholding nor encouraging common food allergen introduction.53,60,61,63 Five documents made recommendations about specific foods;57, 58, 59,62,67 1 recommended wheat and oat introduction by 6 months of age;57 2 recommending fish introduction by 12 months of age;58,59 and 1 recommended egg, wheat, fish, and peanut introduction at specified ages.67 Of those guideline documents produced between 2016 to 2019,41,43,46, 47, 48, 49,51,56,62, 63, 64,66, 67, 68 all 12 stipulated that common food allergen introduction should not be delayed.
Recommendations specific to peanut and egg introduction
Nineteen documents included recommendations regarding peanut and egg.40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51,55, 56, 57,63,64,66, 67, 68 Of these, 6 indicated peanut and egg should not be delayed,39,45,50,51,55,57 and 1 stipulated introduction by 12 months of age.46,47 One document recommended caution regarding introduction,42 and another recommended avoidance.44 Special advice for high risk infants was provided in 8 documents,40,41,43,48,49,56,62,66,68 with 6 of these documents recommending testing/evaluation by an allergist.40,41,43,62,66,68 Six documents were updated after publication of the LEAP study and include peanut allergy specific information.41,46,47,51,56,62,66
Spacing of introduction of new foods
Only 5 documents made recommendations regarding the spacing (time between introduction of each new food) of introducing new foods, with all 5 documents recommended introducing 1 new food at a time.42,44,46,47,51,55
Quality appraisal using AGREE II
An overview of the domain scores for each of the 28 guideline documents is presented in Table 3. Eight46,47,53,58,59,65, 66, 67,69,70 of the 10 guidelines46,47,53,58, 59, 60, 61,64, 65, 66, 67,69,70 examined achieved the quality threshold, and of these, 1 guideline53 scored equal to or greater than 50% across all domains. None of the advice documents39, 40, 41, 42, 43, 44, 45,48, 49, 50, 51, 52,54, 55, 56, 57,62,63,68 met the quality threshold. Domain 3 (rigor of development) was considered integral to developing a quality guideline: only the 8 guidelines46,47,53,58,59,65, 66, 67,69,70 that met the quality threshold achieved at least 50% for this domain.
Table 3.
Domain Scores – Guidelines and advice documents
| Type of document for comparison | Domain 1 (%) | Domain 2 (%) | Domain 3 (%) | Domain 4 (%) | Domain 5 (%) | Domain 6 (%) | Meets quality threshold | |
|---|---|---|---|---|---|---|---|---|
| ASCIA 2016 | Guideline | 94.4 | 52.7 | 60.4 | 83.3 | 29.2 | 79.2 | Yes |
| DGAKI/DGKJ 2009 | Guideline | 88.9 | 47.2 | 66.7 | 72.2 | 6.2 | 70.8 | Yes |
| DGAKI/DGKJ 2014 | Guideline | 97.2 | 4.4 | 78.1 | 75.0 | 47.9 | 83.8 | Yes |
| EAACI 2014 | Guideline | 94.4 | 91.7 | 89.6 | 91.7 | 56.2 | 54.2 | Yes |
| HKIA 2015 | Guideline | 50.0 | 2.7 | 19.8 | 61.6 | 4.2 | 45.8 | No |
| JSPACI 2017 | Guideline | 58.3 | 41.7 | 22.9 | 61.1 | 12.5 | 62.5 | No |
| NIAID 2010 | Guideline | 100.0 | 91.7 | 82.3 | 86.1 | 27.1 | 62.5 | Yes |
| NIAID 2017 | Guideline | 97.2 | 91.7 | 71.9 | 88.9 | 20.8 | 50.0 | Yes |
| PSAAI/PSPGHN 2017 | Guideline | 94.4 | 91.7 | 74.0 | 86.1 | 8.3 | 70.8 | Yes |
| AMS-MOH 2010 | Guideline | 88.9 | 88.9 | 52.1 | 91.7 | 29.2 | 12.5 | Yes |
| AAP 2008 | Advice document | 88.9 | 13.9 | 19.8 | 47.2 | 2.1 | 4.2 | No |
| AAP 2015 | Advice document | 75.0 | 22.2 | 21.9 | 41.7 | 6.2 | 4.2 | No |
| AAP 2019 | Advice document | 83.3 | 25.0 | 28.1 | 52.8 | 2.1 | 62.5 | No |
| ACAAI 2006 | Advice document | 88.9 | 36.1 | 33.3 | 58.3 | 12.5 | 91.7 | No |
| APAPARI 2017 | Advice document | 75.0 | 8.3 | 20.8 | 36.1 | 16.7 | 4.2 | No |
| ASCIA 2008 | Advice document | 88.9 | 36.1 | 20.1 | 50.0 | 14.6 | 62.5 | No |
| ASCIA 2005 | Advice document | 72.2 | 30.6 | 24.0 | 52.8 | 4.2 | 12.5 | No |
| BSACI guidance | Advice document | 66.7 | 58.3 | 13.5 | 66.7 | 16.7 | 45.8 | No |
| CPS/CSACI 2013 | Advice document | 91.7 | 50.0 | 25.0 | 66.7 | 12.5 | 4.2 | No |
| CPS/CSACI 2019 | Advice document | 97.2 | 16.7 | 12.5 | 50.0 | 4.2 | 4.2 | No |
| EAACI 2004 | Advice document | 61.1 | 25.0 | 46.9 | 44.4 | 8.3 | 8.3 | No |
| ESPACI/ESPGHAN 1999 | Advice document | 91.7 | 52.8 | 12.5 | 61.1 | 2.1 | 4.2 | No |
| ESPGHAN 2007 | Advice document | 94.4 | 36.1 | 17.7 | 58.3 | 8.3 | 12.5 | No |
| ESPGHAN 2017 | Advice document | 100.0 | 50.0 | 28.1 | 50.0 | 16.7 | 4.2 | No |
| Finnish AP 2012 | Advice document | 69.4 | 36.1 | 12.5 | 41.7 | 10.4 | 8.3 | No |
| HKIA 2016 | Advice document | 44.4 | 19.4 | 7.3 | 33.3 | 8.3 | 0.0 | No |
| ISPSP/ISPAI/ISP 2016 | Advice document | 83.3 | 33.3 | 44.8 | 61.1 | 6.2 | 33.3 | No |
| SACN/COT 2018 | Advice document | 94.4 | 38.9 | 47.9 | 63.9 | 6.2 | 4.2 | No |
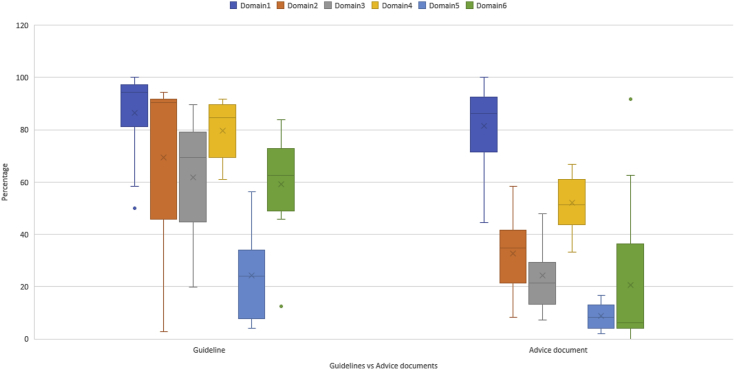
The highest scores were achieved for Domain 1 (scope and purpose) and Domain 4 (clarity of presentation) with mean scores of 83.2% and 61.9%, respectively. Domains 5 (applicability) and 6 (editorial independence) achieved the lowest mean scores of 14.3% and 34.3%, respectively.
Domains 2 (stakeholder engagement) and 6 (editorial independence) had the greatest range in scores, both having a range of 91.7% despite having different minimum scores (2.7% and 0.0% respectively).
A comparison of mean scores for the guidelines and advice documents identified a significant difference at the 5% level for all domains except Domain 1 (scope and purpose) for which both guidelines and advice documents achieved the highest mean scores of 86.4 and 81.5, respectively (Supplemental Table 1). Fig. 2 shows a graphical comparison of the domain scores for guidelines versus advice documents.
Fig. 2.
Domain scores comparison for guidelines versus advice documents
Discussion
To our knowledge, this systematic review provides the only review comparing food allergy prevention guidelines (created over a 21-year timeframe, 1999–2019) and the first comprehensive appraisal of the quality of these guidelines. In searching for primary prevention guidelines for food allergy, we identified 28 food allergy prevention documents that meet the WHO definition of a guideline,35 even though they varied in their titles (eg, guidelines, consensus statement, joint statement) and their processes of development. Since undertaking this study, European Academy of Allergy and Clinical Immunology (EAACI) updated their 2014 guidelines which were in press at the time of finalising this article for publication.71 The updated EAACI guidelines were not included in the AGREE II appraisal nor the comparison of guideline recommendations; however, some references have been made to EAACI guidelines recommendations in the discussion.
Comparison and consistency of recommendations
Children may be sensitised in utero or while breastfeeding, and as such, maternal diet during pregnancy and breastfeeding has become an increasing focus for food allergy prevention.72,73 As such, key factors which should be included in guidelines, in relation to food allergy prevention, include maternal diet during pregnancy and breastfeeding; timeframe for exclusive breastfeeding; breastmilk substitutes; timing of introduction of solid foods; and recommendations regarding common food allergen introduction.21,74 Other interventions such as vitamin D and omega 3- fatty acid supplementation and modification of the maternal and infant microbiome, remain more controversial20 and were considered beyond the scope of this review.
Some guideline documents were clearly designed to contain comprehensive recommendations relating to all aspects of infant feeding and allergy prevention;39,41,44, 45, 46, 47, 48, 49, 50,52,53,57, 58, 59, 60, 61,63, 64, 65,67,69,70 whereas others were clearly deliberately targeted at single recommendations, such as those specifically developed or updated in response to the LEAP study results24 to provide advice specifically related to the prevention of peanut allergy.40,51,62,66,68 As such, comparisons regarding the comprehensiveness of all guidelines are not necessarily overly meaningful.
Overall, all 28 guideline documents were consistent in recommending no maternal dietary restrictions during pregnancy and breastfeeding for allergy prevention. However, 4 documents provided additional recommendations regarding maternal diet, relating to a balanced diet46,47,58, 59, 60, 61 and include fish.58,59 In the case of the most recent Australasian Society of Cinical Immunology and Allergy (ASCIA) guideline46,47 the recommendation regarding a balanced maternal diet was included to be consistent with national dietary recommendations,75 and factors such as this may be the reason for inclusion in the other guideline documents.
Less consistency was observed in the recommendations relating to duration of exclusive breastfeeding. The challenge with recommendations regarding exclusive breastfeeding is balancing the WHO guidelines,76 for exclusive breastfeeding until 6 months (primarily to ensure adequate nutrition in all infants in developing as well as developed regions) against primary prevention of allergic disease. This may also be the case for infant feeding guidelines for the general population within different countries. In Australia, for example, general population infant feeding guidelines77 aim to be consistent with the WHO guidelines, stipulating "at around 6 months" with no mention of 4 months; whereas the evidence for food allergy prevention supports introduction of common food allergens from around 6 months, but not before 4 months, indicating that if the infant is ready at 4 months, solids can be introduced.24, 25, 26, 27, 28 Therefore, food allergy prevention guidelines are likely to provide different advice to general infant feeding recommendations which may cause some confusion for healthcare providers and parents. Further to this, despite recent studies being unable to make conclusions regarding the role of breastfeeding in the prevention of food allergy development,78 guidelines continue to make recommendations regarding exclusive breastfeeding. However, interestingly, the updated EAACI guidelines make no recommendations regarding timing of exclusive breastfeeding, nor the timing of introduction of solids.71
While breastfeeding is promoted for its many benefits,79 recommendations regarding infant formulas are important for mothers who cannot breastfeed or who choose not to breastfeed. Overall, there was consistency in not recommending soy formula for allergy prevention39,44,45,50,52,53,58,65,67 and some consistency regarding the use of hydrolysed infant formulas in high risk infants.44,45,50,52, 53, 54,58, 59, 60, 61,65,67,69,70 However, in the last 5 years (2015–2019), in response to a recent systematic review and meta-analysis,80 a trend to recommended standard cow's milk formula for all infants including those at high risk of allergy was observed in the newer guidelines41,46, 47, 48, 49,63 compared to older guidelines which tended to recommend hydrolysed infant formula.60,61,67
While the introduction of solid foods is an important milestone,21 it is also important for the child's immediate nutritional status and long-term health, including immune programming.81 In our review, recommendations regarding the timing of solid food introduction varied in wording usually related to their recommendations regarding duration of exclusive breastfeeding. The most common recommendation for solid food introduction was "4–6 months".39,41,44,45,53,57,58,65,66,69,70 When considering only the 14 guideline documents published between 2015 and 2019, greater variation in recommendations regarding solid food introduction was observed than in the previous 5-year period. In the Australian context, this may be explained by the need to align with current infant feeding advice for the general population, as timing of solid food introduction is dependent on the recommendations relating to exclusive breastfeeding.21
There was more consistency with regard to recommendations regarding common allergenic food introduction, with recommendations that it should not be delayed. In the last 5 years, (2015–2019), 10 out of 14 documents recommended that common food allergen introduction should not be delayed, consistent with recent studies to support "early" introduction of common food allergens.41,43,46, 47, 48, 49,51,56,62, 63, 64,66
With the publishing of the LEAP study,24 we would expect to see guideline documents including recommendations specific to peanut and possibly other specific foods. From 2016 to 2019, recommendations regarding specific food allergens, most commonly egg and peanut, were included in four guideline documents.46,47,66, 67, 68
Overall quality of the guideline documents
Of the 28 food allergy prevention guideline documents identified, only 8 met the AGREE II quality threshold and of these all eight were titled "guidelines" by their authors. Further, only 1 guideline achieved a domain score of at least 50% across all domains. Therefore, most documents either did not have robust processes of development in place, or they did not fully report their processes of development. Rigour of development (Domain 3) was considered a critical component of guideline quality for this AGREE II assessment, as it relates to the evidence base used to underpin the recommendations as well as the process of formulating the recommendations. It is important to communicate development processes so that health professionals can easily identify whether guidelines are evidence-based. Ensuring that high quality evidence underpins recommendations for food allergy prevention is critical, particularly as the evidence has changed over time and the implications of poor advice from a low quality guideline can have a lifelong impact on the individual. For example, the evidence regarding food allergen introduction has changed from delaying common food allergen introduction to introducing common food allergens within the first year of life.24, 25, 26, 27, 28 If guidelines with recommendations about common food allergen introduction are not up to date with current evidence, the "window of opportunity" to introduce the common food allergens and hence potentially prevent food allergy, could be missed. The updated EAACI guidelines document has used the AGREE II framework as the basis for the guideline development.71
As food allergy is common, parents concerned about the risk of their baby developing food allergies seek guidance from health professionals, particularly in relation to introducing common food allergens into their baby's diet. Clinical immunology/allergy specialists, general practitioners, paediatricians, child health nurses, and dietitians all rely on food allergy prevention guidelines to provide evidence-based advice to parents. Health professionals need to consider the robustness of the evidence underpinning the recommendations contained within each guideline document, as clinical practice should be governed by best available evidence.29,30 In this review, we found that some guideline documents lacked adequate reporting of the relevant stakeholders involved in the development process (domain 2), particularly consumer representation. We also found a lack of detailed information regarding funding sources and clarification regarding author conflicts of interest (Domain 6). These two domains (2 and 6) had the greatest variation in scores compared to other domain score ranges. This indicates that documentation of the stakeholders involved in the development process (domain 2) and the editorial independence (domain 6), ranged from poorly described or lacking to well documented within each domain. The main reason for the lower scores pertaining to stakeholder involvement, was how clearly consumer engagement was documented, if at all. Given that improving patient outcomes is the focus of clinical practice guidelines,82 the consumer perspective is important. Timely and appropriate consumer engagement can provide insight into the relevance, practicality, and achievability of proposed recommendations, and can also contribute to development of appropriate implementation strategies after guidelines have been developed.82
Most documents reviewed clearly detailed the scope and purpose of the document (Domain 1) and recommendations were clearly presented (Domain 4), thereby meeting the 50% quality threshold. This allows health professionals to easily identify the aim of the document, the target audience, and the recommendations with regards to food allergy prevention. Having clearly documented food allergy prevention recommendations assists busy health professionals to easily identify the advice they should be providing to parents.
There are often barriers to implementing recommendations as evidenced by low scores in Domain 5 (applicability). Guidelines should consider potential outcomes and implementation factors such as how to successfully carry out the intervention, cost effectiveness, and the workforce required to implement the guideline recommendations.82 This is important for food allergy prevention, because if recommendations are not achievable, the potential to reduce food allergy is lost. For example, if screening for peanut allergy by skin prick testing is recommended prior to introducing peanut, access to skin prick testing must occur before the recommended timeframe for introduction to peanut (ie, before 1 year of age or earlier in some settings). These issues will be specific to the region for which the guidelines are being developed. In the United States where allergy specialists are accessible, screening prior to peanut introduction is recommended.66 However, in Australia, allergy testing prior to peanut introduction is not feasible, as access to allergy specialists to conduct skin prick testing to screen for peanut sensitisation is unlikely before the child is 12 months of age, and the opportunity to introduce peanut to prevent peanut allergy would be missed. As a result, the ASCIA guidelines do not recommend screening for peanut sensitisation prior to peanut introduction.46,47 Tools for both health professionals and parents to support implementation of food allergen prevention guidelines are also important to help allay fears and encourage recommendation adoption. As the updated EAACI guideline has used the AGREE II tool in developing the guideline, the barriers and facilitators to implementation as well as audit criteria and resource implications have been considered and clearly communicated.71
A question to consider is whether food allergy prevention guideline documents which score poorly using the AGREE II tool translate to having poor quality recommendations. The documents titled guidelines by their authors were more likely to meet the quality threshold than the advice documents, of which none met the quality threshold. Does this mean that the advice document recommendations are substandard? While the AGREE II tool appraises the methodology of guideline development and reporting, it does not appraise the clinical appropriateness of the recommendations themselves, or alignment with evidence.32 Domain 3 (rigor of development) evaluates the process of identifying and grading the evidence that underpins the recommendations, to the extent it relies on this information being communicated within the document or in supporting material that is publicly available. Clearly reporting the process of guideline development may increase health professional confidence to adhere to and implement the guidelines, an important factor with food allergy prevention. If health professionals fail to implement food allergy prevention guidelines, the "window of opportunity" to introduce common food allergens in the first year of life could be missed, resulting in an increased risk of food allergy. As some food allergies will be lifelong, this poses a significant impact on quality of life15,16 and, in some cases, poses a threat to life.
Conclusion
This review identified 28 food allergy prevention guideline documents published over 21 years. While we have compared the recommendations within the identified guideline documents for similarities, we must acknowledge that some of the variation in the recommendations may relate to country specific issues such as the general-population-based infant feeding recommendations. Overall, the greatest variation in recommendations was related to duration of exclusive breastfeeding and timing of solid food introduction. This may create confusion for health professionals and result in inconsistent advice being provided to parents, and less translation of the evidence into actual food allergy reduction in the population at large.
Documents specifically termed guidelines by the authors in their title scored higher using the AGREE II appraisal, principally because they included more information about their development process. Assessment using the AGREE II tool identified areas of improvement for future guideline development. The 2 key areas for improvement in food allergy prevention guidelines identified by this study include: documentation of stakeholder involvement, particularly consumer engagement, and clear documentation of editorial independence. Based on this study, we would recommend use of validated guideline development tools to direct development and review of food allergy prevention guidelines and ensure robust development and reporting processes.
Funding
Not applicable.
Consent for publication
All authors consent to this work being published.
Author contributions
All authors have contributed substantially to the writing of this publication.
Availability of data and materials
Data is available upon request.
Potential competing interests
Ms. Vale reports I am employed by the Australasian Society of Clinical Immunology and Allergy.
Ms Lobb has nothing to disclose.
Dr. Netting has nothing to disclose.
Dr. Murray has nothing to disclose.
Dr. Clifford has nothing to disclose.
Prof. Campbell reports other from DBV Technologies, other from DBV Technologies, other from Allergenis, other from Westmead Fertility Centre, and Research Grant (P.I., collaborator or consultant; pending and received grants) - National Health and Medical Research Council of Australia (NHMRC), Allergy and Immunology Foundation of Australasia, Nestle Health Sciences outside the submitted work.
Dr. Salter has nothing to disclose.
Ethics approval
Not applicable.
Footnotes
Full list of author information is available at the end of the article.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2021.100550.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Jackson K.D., Howie L.D., Akinbami O.J. National Center for Health Statistics; Hyattsville, MD, USA: 2013. Trends in Allergic Conditions Among Children: United States, 1997–2011; NCHS Data Brief, No. 121. [PubMed] [Google Scholar]
- 2.Sicherer S.H., Muñoz-Furlong A., Godbold J.H., Sampson H.A. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 3.McKean M., Caughey A.B., Leong R.E., Wong A., Cabana M.D. The timing of infant food introduction in families with a history of atopy. Clin Pediatr. 2015;54:745–751. doi: 10.1177/0009922815584927. [DOI] [PubMed] [Google Scholar]
- 4.Osborne N.J., Koplin J.J., Martin P.E. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127(3):668–676. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer S.H., Sampson H.A. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141:41–58. doi: 10.1016/j.jaci.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Dunlop J.H., Keet C.A. Epidemiology of food allergy. Immunol Allergy Clin Immunol. 2018;38:13–25. doi: 10.1016/j.iac.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Sicherer S.H. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Jones S.M., Burks A.W. Food allergy. N Engl J Med. 2017;377:1168–1176. doi: 10.1056/NEJMcp1611971. [DOI] [PubMed] [Google Scholar]
- 9.Berin M.C., Sampson H.A. Food allergy: an enigmatic epidemic. Trends Immunol. 2013;34:390–397. doi: 10.1016/j.it.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schussler E., Sobel J., Hsu J. Workgroup report by the joint task force involving American Academy of allergy, asthma & immunology (AAAAI); food allergy, anaphylaxis, dermatology and drug allergy (FADDA)(Adverse reactions to foods committee and adverse reactions to drugs, biologicals, and latex committee); and the centers for disease control and prevention botulism clinical treatment guidelines work group - allergic reactions to botulinum antitoxin: a systematic review. Clin Infect Dis. 2017;66:S65–S72. doi: 10.1093/cid/cix827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki M., Koplin J.J., Dharmage S.C. Prevalence of clinic-defined food allergy in early adolescence: the School Nuts study. J Allergy Clin Immunol. 2017;141(1):391–398. doi: 10.1016/j.jaci.2017.05.041. [DOI] [PubMed] [Google Scholar]
- 12.Australian Institute of Health and Welfare . 2001. Chronic Diseases and Associated Risk Factors in Australia.https://www.aihw.gov.au/reports/chronic-disease/associated-risk-factors-australia-2001/contents/table-of-contents AIHW cat no. PHE 33. Available from: [Google Scholar]
- 13.De Martinis M., Sirufo M.M., Suppa M., Ginaldi L. New perspectives in food allergy. Int J Mol Sci. 2020;21:1474. doi: 10.3390/ijms21041474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agnes S.Y., Leung G.W., Wong K., Tang M.L.K. Food allergy in the developing world. J Allergy Clin Immunol. 2018;141(1):76–78 E1. doi: 10.1016/j.jaci.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Avery N.J., King R.M., Knight S., Hourihane J.O.B. Assessment of quality of life in children with peanut allergy. Pediatr Allergy Immunol. 2003;4:378–382. doi: 10.1034/j.1399-3038.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 16.Sicherer S.H., Noone S.A., Muñoz-Furlong A. The impact of childhood food allergy on quality of life. Ann Allergy Asthma Immunol. 2001;87(2):461–464. doi: 10.1016/S1081-1206(10)62258-2. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R., Holdford D., Bilaver L., Dyer A., Holl J.L., Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167(11):1026–1031. doi: 10.1001/jamapediatrics.2013.2376. [DOI] [PubMed] [Google Scholar]
- 18.Chin B., Chan E.S., Goldman R.D. Early exposure to food and food allergy in children. Can Fam Physician. 2014;60(4):338–339. [PMC free article] [PubMed] [Google Scholar]
- 19.Chan E.S., Abrams E.M., Hildebrand K.J., Watson W. Early introduction of foods to prevent food allergy. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):57. doi: 10.1186/s13223-018-0286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.du Toit George, Tsakok Teresa, Lack Simon, Lack Gideon. Prevention of food allergy. J Allergy Clin Immunol. 2016;137(4):998–1010. doi: 10.1016/j.jaci.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Netting M.J., Allen K.J. Advice about infant feeding for allergy prevention: a confusing picture for Australian consumers? JPCH. 2017;53:870–875. doi: 10.1111/jpc.13594. [DOI] [PubMed] [Google Scholar]
- 22.Grimshaw K.E., Allen K., Edwards C.A. Infant feeding and allergy prevention: a review of current knowledge and recommendations. A EuroPrevall state of the art paper. Allergy. 2009;64:1407–1416. doi: 10.1111/j.1398-9995.2009.02172.x. [DOI] [PubMed] [Google Scholar]
- 23.Koplin J.J., Allen K.J. Optimal timing for solids introduction – why are the guidelines always changing? Clin Exp Allergy. 2013;43:826–834. doi: 10.1111/cea.12090. [DOI] [PubMed] [Google Scholar]
- 24.Du Toit G., Roberts G., Sayre P.H. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803–813. doi: 10.1056/NEJMoa1414850. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkin M.R., Logan K., Tseng A. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374(18):1733–1743. doi: 10.1056/NEJMoa1514210. [DOI] [PubMed] [Google Scholar]
- 26.Perkin M.R., Logan K., Marrs T. Enquiring about Tolerance (EAT) study: feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol. 2016;137(5):1477–1486. doi: 10.1016/j.jaci.2015.12.1322. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavery W.J., Assa’ad A. How to prevent food allergy during infancy: what has changed since 2013? Curr Opin Allergy Clin Immunol. 2018;18:265–270. doi: 10.1097/ACI.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natsme O., Kabashima S., Nakazato J. Two-step egg introduction for prevention of egg allergy in high-riak infants with eczema (PETIT): a radnomized, double-blind, placebo-controlled trial. Lancet. 2017;389:276–286. doi: 10.1016/S0140-6736(16)31418-0. [DOI] [PubMed] [Google Scholar]
- 29.Shekelle Paul G., Woolf Steven H., Eccles Martin, Grimshaw Jeremy. Developing guidelines. BMJ. 1999;318:593. doi: 10.1136/bmj.318.7183.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Consensus report, Institute of Medicine . 2011. Clinical Practice Guidelines We Can Trust.http://www.iom.edu/Reports/2011/Clinical-Practice-Guidelines-We-Can-Trust.aspx March 23. [Google Scholar]
- 31.Brouwers M.C., Kerkvliet K., Spithoff K. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ. 2016;352:i1152. doi: 10.1136/bmj.i1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brouwers M.C., Kho M.E., Browman G.P., Burgers J.S., Cluzeau F., Feder G. AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ (Can Med Assoc J) 2010;182(18):E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ. 2016;352:i1152. doi: 10.1136/bmj.i1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AGREE Next Steps Consortium . 2017. The AGREE II Instrument.http://www.agreetrust.org Retrieved April 25th, 2020 from. [Google Scholar]
- 35.World Health Organisation . second ed. WHO Library Cataloguing-in-Publication Data; 2014. WHO Handbook for Guideline Development. ISBN 978 92 4 154896 0. [Google Scholar]
- 36.AGREE II FAQ. https://www.agreetrust.org/resource-centre/agree-ii/faq-agree-ii-2/Last
- 37.Zhang Z., Guo J., Su G., Li J., Wu H., Xie X. Evaluation of the quality of guidelines for myasthenia gravis with the AGREE II instrument. PloS One. 2014;9(11) doi: 10.1371/journal.pone.0111796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Y., Wang J., Luo X. Quality appraisal of clinical practice guidelines for diabetes mellitus published in China between 2007 and 2017 using the AGREE II instrument. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greer F.R., Sicherer S.H., Burks A.W. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121(1):183–191. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 40.Fleischer D.M., Sicherer S., Greenhawt M. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. Ann Allergy Asthma Immunol. 2015 Aug;115(2):87–90. doi: 10.1016/j.anai.2015.06.001. Epub 2015 Jun 27. PMID: 26122934. [DOI] [PubMed] [Google Scholar]
- 41.Greer F.R., Sicherer S.H., Burks A.W. Committee on nutrition; section on allergy and immunology. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics. 2019 Apr;143(4) doi: 10.1542/peds.2019-0281. Epub 2019 Mar 18. PMID: 30886111. [DOI] [PubMed] [Google Scholar]
- 42.Fiocchi A., Assa'ad A., Bahna S. Adverse reactions to foods committee; American College of allergy, asthma and immunology. Food allergy and the introduction of solid foods to infants: a consensus document. Adverse reactions to foods committee, American College of allergy, asthma and immunology. Ann Allergy Asthma Immunol. 2006 Jul;97(1):10–20. doi: 10.1016/s1081-1206(10)61364-6. quiz 21, 77, PMID: 16892776. [DOI] [PubMed] [Google Scholar]
- 43.Tham E.H., Shek L.P., Van Bever H.P. Asia pacific association of pediatric allergy, Respirology & immunology (APAPARI). Early introduction of allergenic foods for the prevention of food allergy from an asian perspective-an Asia pacific association of pediatric allergy, Respirology & immunology (APAPARI) consensus statement. Pediatr Allergy Immunol. 2018 Feb;29(1):18–27. doi: 10.1111/pai.12820. Epub 2017 Nov 22. PMID: 29068090. [DOI] [PubMed] [Google Scholar]
- 44.Prescott S.L., Tang M.L.K. The Australasian Society of Clinical Immunology and Allergy position statement: summary of allergy prevention in children. Med J Aust. 2005;182(9):464–467. doi: 10.5694/j.1326-5377.2005.tb06787.x. [DOI] [PubMed] [Google Scholar]
- 45.Australasian Society of Clinical Immunology and Allergy . 2010. ASCIA Infant Feeding Advice.https://www.allergy.org.au/images/stories/hp/info/ASCIA_Infant_Feeding_Advice_2010.pdf [Google Scholar]
- 46.Joshi P.A., Smith J., Vale S., Campbell D.E. The Australasian Society of Clinical Immunology and Allergy infant feeding for allergy prevention guidelines. MJA. 2019;210(2):89–93. doi: 10.5694/mja2.12102. [DOI] [PubMed] [Google Scholar]
- 47.Australasian Society of Clinical Immunology and Allergy (ASCIA) 2016. ASCIA Guidelines: Infant Feeding and Allergy Prevention.https://allergy.org.au/hp/papers/infant-feeding-and-allergy-prevention [Google Scholar]
- 48.Stiefel G., Anagnostou K., Boyle R.J. BSACI guideline for the diagnosis and management of peanut and tree nut allergy. Clin Exp Allergy. 2017;47:719–739. doi: 10.1111/cea.12957. [DOI] [PubMed] [Google Scholar]
- 49.Turner P.J., Feeney M., Meyer R., Perkin M.R., Fox A.T. Implementing primary prevention of food allergy in infants: new BSACI guidance published. Clin Exp Allergy. 2018;48:912–915. doi: 10.1111/cea.13218. [DOI] [PubMed] [Google Scholar]
- 50.Chan E.S., Cummings C. Canadian paediatric society, community Paediatrics committee and allergy section. Dietary exposures and allergy prevention in high-risk infants: a joint statement with the Canadian society of allergy and clinical immunology. Paediatr Child Health. 2013;18(10):545–554. doi: 10.1093/pch/18.10.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abrams E.M., Hildebrand K., Blair B., Chan E.S. Timing of introduction of allergenic solids for infants at high risk. Paediatr Child Health. 2019 Feb;24(1):56–57. doi: 10.1093/pch/pxy195. Epub 2019 Feb 15. PMID: 30833823; PMCID: PMC6376285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muraro A., Dreborg S., Halken S. Dietary prevention of allergic diseases in infants and small children. Part III: critical review of published peer-reviewed observational and interventional studies and final recommendations. Pediatr Allergy Immunol. 2004 Aug;15(4):291–307. doi: 10.1111/j.1399-3038.2004.00127.x. PMID: 15305938. [DOI] [PubMed] [Google Scholar]
- 53.Muraro A.∗, Halken S.∗, Arshad S.H. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy. 2014;69:590–601. doi: 10.1111/all.12398. [DOI] [PubMed] [Google Scholar]
- 54.Høst A., Koletzko B., Dreborg S. Dietary products used in infants for treatment and prevention of food allergy. Joint statement of the European society for paediatric Allergology and clinical immunology (ESPACI) committee on hypoallergenic formulas and the European society for paediatric Gastroenterology, Hepatology and nutrition (ESPGHAN) committee on nutrition. Arch Dis Child. 1999 Jul;81(1):80–84. doi: 10.1136/adc.81.1.80. PMID: 10373144; PMCID: PMC1717972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agostoni C., Decsi T., Fewtrell M., ESPGHAN Committee on Nutrition Complementary feeding: a commentary by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2008 Jan;46(1):99–110. doi: 10.1097/01.mpg.0000304464.60788.bd. PMID: 18162844. [DOI] [PubMed] [Google Scholar]
- 56.Fewtrell M., Bronsky J., Campoy C. Complementary feeding: a position paper by the European society for paediatric Gastroenterology, Hepatology, and nutrition (ESPGHAN) committee on nutrition. J Pediatr Gastroenterol Nutr. 2017 Jan;64(1):119–132. doi: 10.1097/MPG.0000000000001454. PMID: 28027215. [DOI] [PubMed] [Google Scholar]
- 57.Pelkonen A.S., Kuitunen M., Dunder T. Allergy in children: practical recommendations of the Finnish Allergy Programme 2008-2018 for prevention, diagnosis, and treatment. Pediatr Allergy Immunol. 2012 Mar;23(2):103–116. doi: 10.1111/j.1399-3038.2012.01298.x. PMID: 22432881. [DOI] [PubMed] [Google Scholar]
- 58.Muche-Borowski C., Kopp M., Reese I., Sitter H., Werfel T., Schäfer T. Allergy prevention. Dtsch Arztebl Int. 2009;106(39):625–631. doi: 10.3238/arztebl.2009.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schäfer T., Bauer C.P., Beyer K. S3-Guideline on allergy prevention: 2014 update: guideline of the German society for Allergology and clinical immunology (DGAKI) and the German society for pediatric and adolescent medicine (DGKJ) Allergo J Int. 2014;23(6):186–199. doi: 10.1007/s40629-014-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan A.W.M., Chan J.K.C., Lee T.H., Leung T.F., Tam A.Y.C. 2015. Guidelines for Allergy Prevention in Hong Kong.http://www.allergy.org.hk/HKIA%20-%20Guidelines%20for%20Allergy%20Prevention%20in%20Hong%20Kong%20(Final).pdf [DOI] [PubMed] [Google Scholar]
- 61.Chan A.W.M., Chan J.K.C., Tam A.Y.C., Lee T.H. Guidelines for allergy prevention in Hong Kong. Medical Practice. 2016;22(3):279–285. doi: 10.12809/hkmj154763. [DOI] [PubMed] [Google Scholar]
- 62.Chan J.K.C., Chan A.W.M., Ho M.H.K., Lee T.H. 2016. HKIA Position Paper on Prevention of Peanut Allergy in High Risk Infants.http://www.allergy.org.hk/HKIA%20-%20Guildelines%20for%20Prevention%20of%20Peanut%20Allergy%20(Final).pdf [Google Scholar]
- 63.di Mauro G., Bernardini R., Barberi S. Prevention of food and airway allergy: consensus of the Italian society of preventive and social Paediatrics, the Italian society of paediatric allergy and immunology, and Italian society of Pediatrics. World Allergy Organ J. 2016;9:28. doi: 10.1186/s40413-016-0111-6. Published 2016 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ebisawa M., Ito K., Fujisawa T. Committee for Japanese pediatric guideline for food allergy, the Japanese society of pediatric allergy and clinical immunology, the Japanese society of Allergology. Japanese guidelines for food allergy 2017. Allergol Int. 2017 Apr;66(2):248–264. doi: 10.1016/j.alit.2017.02.001. Epub 2017 Mar 10. PMID: 28285847. [DOI] [PubMed] [Google Scholar]
- 65.Boyce J.A., Assa’ad A., Burks A.W., NIAID-Sponsored Expert Panel Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Togias A., Cooper S.F., Acebal M.L. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. World Allergy Organ J. 2017;10(1):1. doi: 10.1186/s40413-016-0137-9. Published 2017 Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Recto M.S.T., Genuino M.L.G., Castor M.A.R. Dietary primary prevention of allergic diseases in children: the Philippine guidelines. Asia Pac Allergy. 2017;7(2):102–114. doi: 10.5415/apallergy.2017.7.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joint Statement from the Scientific Advisory Committee on Nutrition and the Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. Assessing the Health Benefits and Risks of the Introduction of Peanut and Hen's Egg into the Infant Diet before Six Months of Age in the UKA. 2018. https://cot.food.gov.uk/sites/default/files/jointsacncotallergystatementfinal2.pdf [Google Scholar]
- 69.Ministry of Health Singapore . 2010. Management of Food Allergy: AMS-MOH Clinical Practice Guidelines 2.https://www.moh.gov.sg/docs/librariesprovider4/guidelines/cpg_management-of-food-allergy.pdf [Google Scholar]
- 70.Lee B.W., Aw M.M., Chiang W.C. Academy of medicine, Singapore-Ministry of Health clinical practice guidelines: management of food allergy. Singapore Med J. 2010 Jul;51(7):599–607. Erratum in: Singapore Med J. 2013 Aug;54(8):474. Aw, M [corrected to Aw, M M]; Shek, L [corrected to Shek, L P]. PMID: 20730402. [PubMed] [Google Scholar]
- 71.Halken Susanne. EAACI guideline: preventing the development of food allergy in infants and young children (2020 update) Pediatr Allergy Immunol. 2020 doi: 10.1111/pai.13496. [DOI] [PubMed] [Google Scholar]
- 72.Jeurink P.V., Knipping K, Wiens F. Importance of maternal diet in the training of the infant’s immune system during gestation and lactation. Crit Rev Food Sci Nutr. 2019;59(8):1311–1319. doi: 10.1080/10408398.2017.1405907. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y., Allen K.J., Koplin J.J. Dietary intervention for preventing food allergy in children. Curr Opin Pediatr. 2017;29(6):704–710. doi: 10.1097/MOP.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 74.Grimshaw K.E., Allen K., Edwards C.A. Infant feeding and allergy prevention: a review of current knowledge and recommendations. A EuroPrevall state of the art paper. Allergy. 2009;64:1407–1416. doi: 10.1111/j.1398-9995.2009.02172.x. [DOI] [PubMed] [Google Scholar]
- 75.National Health and Medical Research Council . 2013. The Australian Dietary Guidelines: Summary.https://www.eatforhealth.gov.au/sites/default/files/files/the_guidelines/n55a_australian_dietary_guidelines_summary_book.pdf [Google Scholar]
- 76.World Health Organization, UNICEF . World Health Organization; Geneva, Switzerland: 2003. Global Strategy for Infant and Young Child Feeding. [Google Scholar]
- 77.National Health and Medical Research Council . The Council; Canberra: 2012. Infant Feeding Guidelines. [DOI] [PubMed] [Google Scholar]
- 78.Greer F.R., Sicherer S.H., Burks A.W. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics. 2019;143 doi: 10.1542/peds.2019-0281. [DOI] [PubMed] [Google Scholar]
- 79.Faucher M.A. An updated scientific review of the benefits of breastfeeding with additional resources for use in everyday practice. J Midwifery Wom Health. 2012;57:422–423. doi: 10.1111/j.1542-2011.2012.00195_4.x. [DOI] [PubMed] [Google Scholar]
- 80.Boyle R.J., Ierodiakonou D., Khan T. Hydrolysed formula and risk of allergic or autoimmune disease: systematic review and meta-analysis. BMJ. 2016 Mar 8;352:i974. doi: 10.1136/bmj.i974. PMID: 26956579; PMCID: PMC4783517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prescott S.L., Smith P., Tang M. The importance of early complementary feeding in the development of oral tolerance: concerns and controversies. Pediatr Allergy Immunol. 2008;19:375–380. doi: 10.1111/j.1399-3038.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 82.Shekelle P., Aronson M.D., Givens J. Overview of clinical practice guidelines. UpToDate. Apr. 2020;8 https://www-uptodate-com.ezproxy.library.uwa.edu.au/contents/overview-of-clinical-practice-guidelines/print?search=Overview%20of%20clinical%20practice%20guidelines&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon request.