Abstract
Background:
Critical care populations experience demographic shifts in response to trends in population and healthcare, with increasing severity and/or complexity of illness a common observation worldwide. Inflammation in critical illness impacts glucose–insulin metabolism, and hyperglycaemia is associated with mortality and morbidity. This study examines longitudinal trends in insulin sensitivity across almost a decade of glycaemic control in a single unit.
Methods:
A clinically validated model of glucose–insulin dynamics is used to assess hour–hour insulin sensitivity over the first 72 h of insulin therapy. Insulin sensitivity and its hour–hour percent variability are examined over 8 calendar years alongside severity scores and diagnostics.
Results:
Insulin sensitivity was found to decrease by 50–55% from 2011 to 2015, and remain low from 2015 to 2018, with no concomitant trends in age, severity scores or risk of death, or diagnostic category. Insulin sensitivity variability was found to remain largely unchanged year to year and was clinically equivalent (95% confidence interval) at the median and interquartile range. Insulin resistance was associated with greater incidence of high insulin doses in the effect saturation range (6–8 U/h), with the 75th percentile of hourly insulin doses rising from 4–4.5 U/h in 2011–2014 to 6 U/h in 2015–2018.
Conclusions:
Increasing insulin resistance was observed alongside no change in insulin sensitivity variability, implying greater insulin needs but equivalent (variability) challenge to glycaemic control. Increasing insulin resistance may imply greater inflammation and severity of illness not captured by existing severity scores. Insulin resistance reduces glucose tolerance, and can cause greater incidence of insulin saturation and resultant hyperglycaemia. Overall, these results have significant clinical implications for glycaemic control and nutrition management.
Keywords: glucose tolerance, glycaemic contro1, insulin resistance, insulin sensitivity, intensive care
Introduction
Critical illness is associated with increases in counter-regulatory hormones, such as glucagon, growth hormones, catecholamines, glucocorticoids, as well as cytokines, which are in turn associated with increased endogenous glucose production and hepatic and peripheral insulin resistance.1,2 Hyperglycaemia is a commonly observed manifestation of this stress response in critical care. While thought to be an adaptive response to redirect resources towards the immune response, 3 it has been associated with severity of illness and significant morbidities in intensive care.1,3–6 Additionally, insulin resistance and the resultant hyperglycaemia can be exacerbated in intensive care by glucocortoids, 7 inotropes,8,9 and excess nutrition.1,10
Hyperglycaemia is associated with increased mortality and morbidity, but causality and the role of tight glycaemic control in mitigating outcomes is still debated.11–15 Evidence in favour of the benefits of tight glycaemic control using insulin includes mechanistic arguments such as reducing inflammation and infection, reducing glucose-overload-related toxicity or avoidance of oxidative stress,16–23 as well as indications of the detrimental effects of hypoglycaemia24–26 and glycaemic variability27–29 associated with poor control. One study 30 examining survivors and nonsurvivors treated with glycaemic control concluded there is similar underlying metabolic variability in these two cohorts, and thus outcomes in studies showing benefit/neutrality/harm31–34 from glycaemic control are likely a function of the quality and safety of control delivered. In particular, an increase in hypoglycaemia with tight control is seen in most outcomes studies31–34 is due to inappropriate insulin dosing resulting in poor control rather than inherent patient characteristics. Hence, as intensive care unit (ICU) cohorts evolve it could be critical to understand how the ability or difficulty to control them, a function of their insulin sensitivity and its hour–hour variability, 35 evolves at the same time.
This evolution of insulin sensitivity evolution is affected by underlying cohort demographics, diagnoses, and disease progression/treatment. Shifts in ICU cohort demographics and diagnoses are evident worldwide. Much of the data describe trends from 1990 to the mid-2010s, observing increases in average age and (concomitant 36 ) prevalence of comorbidities 36 and/or complications 37 across the United States,37–39 Europe,40–42 the United Kingdom, 43 and Australia and New Zealand.44,45 Despite this increase in severity and complexity, mortality rates are dropping,39,42,44 reflecting medical and technological advances in care. Thus, it is hypothesised that there will be concomitant changes in underlying cohort insulin sensitivity over time due to changes in demographics and diagnoses, and overall illness severity.
This study analyses longitudinal changes in insulin sensitivity in a New Zealand critical care cohort receiving glycaemic control from 2011 to 2018. Given global trends in aging and the resulting increase in more severely ill or complex intensive care patients, it is hypothesised insulin sensitivity in glycaemic control cohorts will decrease over time, as insulin resistance is closely associated with stress response inflammation and immune response to critical illness and its severity.3,46 Changes in insulin sensitivity and its variability over time may have implications for the ability to achieve safe and effective glycaemic control both now and in the future. Achieving glycaemic control in turn has implications for clinical care and patient outcomes.
Methods
Clinical data collection
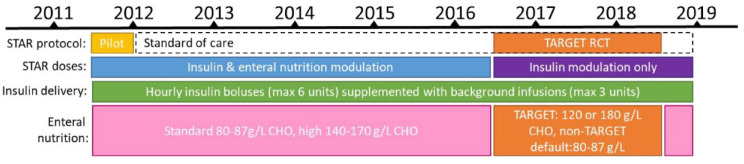
Glycaemic control data while on the STAR47–50 (Stochastic TARgeted) protocol at Christchurch Hospital ICU were analysed for the 2011–2018 calendar years. STAR was piloted during the second half of 2011, and implemented as standard of care from late 2011 onwards. Clinical context for each calendar year is shown in Figure 1.
Figure 1.
STAR implementation and clinical context for calendar years 2011–2019.
CHO is total nonfibre carbohydrate concentration. The TARGET randomised control trial (RCT) ran from mid-2016 to mid-2018.
STAR is a model-based glycaemic control protocol dosing insulin and nutrition based on current insulin sensitivity and statistical forward prediction of likely blood glucose (BG) outcomes.48,51 Clinical data inputs into the nurse-driven computerised protocol included all nutrition and insulin rates, and BG measurements, and these were automatically recorded and saved with double encryption to secure cloud storage by the STAR tablet computer for data-auditing purposes. Demographic data, including severity scores, risk of death (ROD) and hospital mortality were collected retrospectively from ICU admission records. Ethics approval for retrospective auditing of de-identified STAR data was approved by the New Zealand Health and Disabilities Ethics Committee (URA-12-EXP-004).
BG was typically measured using blood from an arterial line and an Arkray Super-Glucocard™ II glucometer (Arkray, Minneapolis, MN, USA) (2011–2012) or a Roche Accu-Chek Inform II (F. Hoffmann-La Roche Ltd., Basel, Switzerland) (2012–2018). Nutrition was predominantly enteral, unless otherwise clinically indicated, and delivered via a gastric tube. Typically, enteral nutrition was 1 kCal/ml and had a glucose content of 80–87 g/l, with less common use of higher carbohydrate options of 140–170 g/l. Participants recruited to a 2016–2018 TARGET randomised control trial (RCT) of nutrition 52 received nutrition at 120 g/l and 180 g/l, respectively across two arms; participants not recruited to this trial received standard nutrition under the same STAR glycaemic control conditions as the RCT and are included in this analysis. During the TARGET RCT, STAR only modulated insulin, nutrition rate was left to clinical discretion but accounted for in STAR insulin dose calculations.
Insulin was delivered as hourly intravenous boluses up to 6 U/h, with an additional 1–3 U/h insulin intravenous background infusion if required, all via an infusion pump. Insulin doses fall in increments of 0.5 U/h. The target glucose range was typically 4.4–8.0 mmol/l (4.4–9.0 mmol/l for patients with diabetes), but was raised to 4.4–10.0 mmol/l for all patients during the 2016–2018 RCT to standardise the trial across units. Patients were started on STAR if they had two BG measurements greater than 8.0 mmol/l within 4 h, or two BG greater than 10.0 mmol/l during the 2016–2018 RCT.
Model-based insulin sensitivity
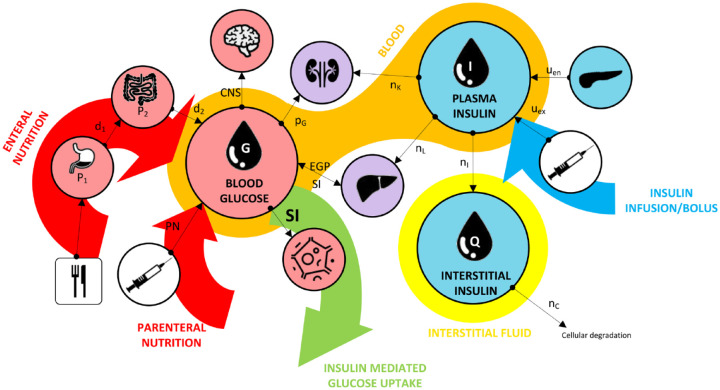
The Intensive Care Insulin-Nutrition-Glucose (ICING) model was used to compute insulin sensitivity based on measured glycaemic response to insulin and nutrition administration. The model has been clinically utilised in 30 the STAR glycaemic control protocol and a wide range of related clinical analyses for over a decade, and is clinically and mathematically well validated.7,30,47,53–55 Details can be found elsewhere,47,48,51 but core model dynamics are illustrated in Figure 2 30 and Equations 1–3.
Figure 2.
Illustration of key dynamics of the glucose–insulin model, where key compartments include blood glucose, plasma insulin, and interstitial insulin.
Arrows show the direction of glucose flux.
Figure originally published by Uyttendaele et al. 30
CNS, central nervous system; EGP, endogenous glucose production; PN, parenteral nutrition; SI, insulin sensitivity.
| (1) |
| (2) |
| (3) |
Where G is BG concentration, I is plasma insulin concentration, and Q is peripheral insulin concentration. Other parameter definitions and their units can be found in the supplementary appendix. BG dynamics include noninsulin-mediated and insulin-mediated glucose uptake. Insulin sensitivity (SI) is a saturable process, and SI is fitted on an hourly basis from clinical BG, nutrition, and insulin data using integral-based least-squares fitting. 56
Analyses and statistics
SI was identified hourly from clinical data and Equations 1–3. This study focused on (up to) the first 72 h of insulin therapy, where most patients in this analysis received insulin under the STAR protocol for 46–84 h. The following questions were examined:
Was insulin sensitivity level consistent or different across calendar years?
Was the insulin sensitivity variability consistent or different across calendar years?
Do trends in insulin sensitivity and/or its variability match trends in severity score or patient cohort composition (including age and diagnoses)?
Key outcomes are assessed for the 2012–2018 calendar years, while data from 2011 provides additional context. Patients who received very high glucose intake were excluded, where the linearity of differential equation coefficients in the model of Figure 2 and Equations 1–3 may not be valid, 57 due to saturation or other dynamics. Patients were excluded from this analysis if they received mean nutrition rates greater than 7 g/h (85 ml/h delivery at 81 g/l; 40–60 ml/h at 120–180 g/l) while on the STAR protocol, where most were part of the (very) high nutrition arm of the 2016–2018 TARGET RCT. 52
Insulin sensitivity variability was calculated as the percentage change in SI/h, %∆SI. Distributions were plotted for SI and %∆SI per calendar year. Hypothesis testing was used to examine statistical differences between cohorts. Equivalence testing, a method independent of hypothesis testing which assesses whether differences are impactful or measurable (e.g. within measurement noise), was used to assess whether differences are clinically relevant. 30
The Kolmogorov–Smirnov (KS) test was used to compare cumulative distributions, and bootstrapped statistics are used to compare median SI due to the relative large data densities from hourly SI. 30 During the bootstrapping process, data from each of two cohorts (A, B) was bootstrapped with replacement 10,000 times to generate 10,000 paired samples for A and B, of length n = minimum[length(A),length(B)]. To compare medians, the median difference of each bootstrapped sample pair (Ai - Bi) was computed, and the median confidence interval (CI) of this median difference is presented. Where the CI does not cross zero, samples A and B are statistically different to . 58 For example, for p ≤ 0.05, the 95% CI must not cross zero. To account for multiple comparisons, a Bonferroni correction is used. For 8 years of data (2011–2018), 28 comparisons can be made year–year, thus the corrected p-value for statistical significance was taken as (CI 99.8).
Equivalence testing in this analysis used a threshold of 15% absolute change in SI, based on previous work showing equivalence at a ~15–18%∆SI, based on a 7% BG measurement error. 30 Equivalence in median SI level between cohorts was accepted if the median (95% CI) of bootstrapped percentage difference between the two cohorts falls within ±15%. 30 Equivalence in median percentage SI variability was accepted if the raw difference in hour–hour %∆SI for each cohort is within ±15%.
Median [interquartile range (IQR)] in demographic data was compared using the rank-sum method. ROD across a cohort is represented by the mean (standard deviation) as the ROD statistic itself assumes normal distribution of likelihood for a given severity level.59–61 Mean ROD between cohorts was thus compared using a student’s t-test.
Results
Clinical data
Christchurch Hospital ICU averaged 1232 admissions per year from 2011 to 2018. Over the period analysed (mid-2011 to end-2018), 1147 glycaemic control ‘episodes’ of 8 h or more in length were recorded. A total of 143 (~12%) glycaemic control records were then excluded from this analysis as they could not be retrospectively matched against demographic data records due to missing, incomplete, or inaccurate identifiers in recorded STAR data, resulting in 1004 episodes from 757 (~110/year) individual patients. As many patients have more than one glycaemic control record/episode because they are later restarted on insulin, excluded patients represented ⩽12% of total patients who received glycaemic control. A further 58 (7.7%) patients were excluded from this analysis as they received mean nutrition rates greater than 7 g/h. Patients excluded on the basis of high nutrition were predominantly from the 2016 to 2018 TARGET RCT period (44 of 58, 75.9%), and were spread across different diagnoses, as would be expected. Cohort characteristics are given in Table 1. During the second half of 2011, STAR was first piloted, so cohort size is smaller.
Table 1.
Year to year baseline cohort characteristics and glycaemic control outcomes for all patients meeting the criteria for analysis.
| 2011 a | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|---|---|
| n, ICU admissions | 1243 | 1242 | 1267 | 980 | 1195 | 1258 | 1303 | 1366 |
| n, GC | 16+ | 100 | 172 | 99 | 96 | 73 | 80 | 61 |
| Age (years) | 63 (48–70) | 65 (55–72) | 65 (55–72) | ’62 (51–70) | 66 (58–74) | 67 (59–73) | 66 (58–72) | 63 (50–70) |
| LOS (days) | 20.9 (10.8–28.0) | 4.6 (2.6–11.3) | 4.8 (2.7–11.5) | 7.6 (3.6–10.8) | 4.6 (2.7–10.3) | 6.6 (3.5–14.5) | 5.6 (2.6–10.0) | 5.0 (3.0–11.3) |
| n, hours on GC | 147 (60–199) | 47 (23–98) | 47 (25–95) | 62 (34–120) | 42 (23–91) | 48 (26–115) | 42 (20–106) | 47 (28–80) |
| n, hours of SI b | 72 (53–72) | 46 (23–68) | 44 (24–69) | 54 (33–72) | 41 (23–72) | 44 (26–72) | 41 (20–72) | 47 (28–72) |
| STAR target range parameters (mmol/l) | 4.4–8.0 | 4.4–8.0 or 4.4–9.0 (DM) |
4.4–8.0 or4.4–9.0 (DM) | 4.4–8.0 or4.4–9.0 (DM) | 4.4–8.0 or4.4–9.0 (DM) | 4.4–8.0 or4.4–10.0 (June→) | 4.4–10.0 | 4.4–10.0 |
| BG b (mmol/l) | 6.5 (5.7–7.5) | 6.8 (6.1–8.0) | 7.5 (6.4–8.9) | 7.0 (6.3–8.0) | 7.3 (6.5–8.4) | 7.3 (6.5–8.5) | 7.2 (6.4–8.6) | 7.3 (6.5–8.8) |
| ΔBG (mmol/l/h) b | −0.0 (−0.5 to 0.4) | −0.0 (−0.4 to 0.3) | −0.0 (−0.5 to 0.4) | −0.0 (−0.4 to 0.3) | −0.1 (−0.5 to 0.3) | −0.1 (−0.4 to 0.3) | −0.1 (−0.5 to 0.3) | −0.1 (−0.4 to 0.3) |
| Starting BG (mmol/l) | 7.5 (5.8–8.8) | 10.3 (8.7–12.1) | 10.9 (9.2–13.5) | 9.9 (8.7–12.0) | 12.5 (10.4–14.9) | 12.4 (10.7–15.1) | 12.9 (11.3–15.9) | 12.3 (11.1–14.2) |
| % BG in 4.4–8.0 mmol/l b (adjusted c ) | 81.0 (82.4) | 71.6 (74.6) | 58.1 (61.2) | 72.0 (74.7) | 62.0 (66.6) | 59.5 (63.9) | 55.8 (60.9) | 61.5 (66.2) |
| % BG > 10.0 mmol/l
b
[per-patient med (IQR)] |
5.5 [2.1 (0.0– 7.5)] | 9.3 [3.7 (0.0– 15.4)] | 15.7 [6.8 (1.4–24.1)] | 9.2 [3.4 (0.0–14.6)] | 17.0 [11.8 (3.3–28.7)] | 14.1 [9.6 (4.5–21.0)] | 21.0 [16.1 (6.1–28.6)] | 15.1 [10.0 (4.1–25.9)] |
| % BG < 4.0 mmol/l b | 0.8 | 1.2 | 0.4 | 0.2 | 0.1 | 0.2 | 0.3 | 0.2 |
| % BG < 2.6 mmol/l b | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Hourly Insulin (U/hr) | 3.1 (1.0–6.0) | 2.0 (1.0–4.0) | 2.0 (0.1–4.0) | 2.9 (1.0–4.5) | 3.5 (2.0–6.0) | 4.0 (2.0–6.0) | 4.0 (2.0–6.0) | 3.5 (2.0–6.0) |
| Hourly Nutrition (g/hr) | 4.4 (1.7–5.5) | 3.2 (0.0–5.4) | 2.0 (0.0–4.9) | 3.7 (0.0–5.6) | 2.0 (0.0–4.9) | 2.5 (0.0–5.3) | 4.1 (0.0–5.8) | 3.2 (0.0–5.0) |
Averages are presented as median (IQR) where relevant. BG data are hourly resampled.
The 2011 cohort consists of data from a 6-month pilot trial.
Constrained to a max 72 h.
Adjusted for starting BG: time in range after first BG < 9.0 mmol/L, or 6 h, whichever is first.
BG, blood glucose; DM, diagnosed diabetes mellitus; GC, glycaemic control; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; SI, insulin sensitivity.
Insulin sensitivity across calendar years
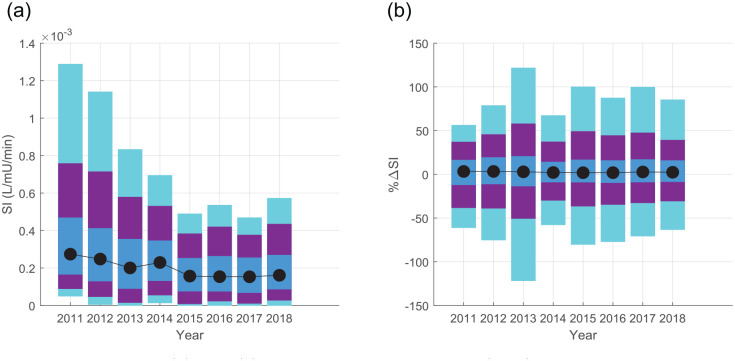
Figure 3 shows decreasing SI median and range over time, with a consistent trend in the reduction of both median SI and the 95th and 90th percentile extremes. The differences in median SI in Table 2 showed statistical difference for all but the 2015–2018 years (p < 0.0018), where Figure 3(a) shows the evolution of the distribution for each year, including a continual drop in 95th and 90th percentile SI to 2015. These differences in median SI between the 2015–2018 cohorts fall within the 15% equivalence range, suggesting the cohorts are clinically similar. The only other cohorts equivalent in median SI were 2012 and 2014. Overall, SI decreased in a statistically significant and nonequivalent manner from 2012 to 2015, after which median SI is equivalent, which is clear in Figure 3(a).
Figure 3.
Distributions of (a) SI and (b) its percentage change hour–hour (%∆SI). Shaded regions show the 5–95 (cyan), 10–90 (purple), and 25–75 (IQR, blue) ranges respectively. Model-based SI is drawn from the first 72 h of insulin therapy in an ICU admission, and Tables 2 and 3 detail differences between medians and statistical difference/equivalences.
ICU, intensive care unit; IQR, interquartile range; SI, insulin sensitivity.
Table 2.
Year to year comparison of SI. As comparisons are symmetric, the upper triangle presents the median (95% CI) percentage difference in SI, while the lower triangle gives statistical interpretation.
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|---|---|
| 2011 | 10.3 (2.8–19.6) | 31.1 (22.1–40.5) | 17.8 (11.5–26.0) | 54.6 (47.9–63.2) | 56.6 (48.9–65.2) | 56.6 (49.2–65.0) | 52.0 (45.3–60.1) | |
| 2012 | p < 0.0018 | 20.9 (16.4–25.1) | 7.5 (3.9–11.2) ⇔ | 44.9 (41.1–48.7) | 46.9 (42.5–51.3) | 47.0 (42.3–51.3) | 42.2 (37.5–46.9) | |
| 2013 | p < 0.0018 | p < 0.0018 | −13.5 (−16.9 to −9.5) | 24.5 (20.6–29.1) | 26.6 (21.9–31.8) | 26.7 (21.9–31.8) | 21.8 (16.7–27.4) | |
| 2014 | p < 0.0018 | p < 0.0018, ⇔ | p < 0.0018 | 37.7 (34.4–41.3) | 39.7 (35.6–43.6) | 39.8 (35.6–43.6) | 34.9 (30.8–39.2) | |
| 2015 | p < 0.0018 | p < 0.0018 | p < 0.0018 | p < 0.0018 | 2.1 (−2.5 to 6.6) ⇔ | 2.2 (−2.7 to 6.5) ⇔ | −2.9 (−7.6 to 1.9) ⇔ | |
| 2016 | p < 0.0018 | p < 0.0018 | p < 0.0018 | p < 0.0018 | p > 0.0018, ⇔ | 0.2 (−4.9 to 4.7) ⇔ | −4.9 (−10.0 to 0.2) ⇔ | |
| 2017 | p < 0.0018 | p < 0.0018 | p < 0.0018 | p < 0.0018 | p > 0.0018, ⇔ | p > 0.0018, ⇔ | −5.08 (−9.8 to 0.3) ⇔ | |
| 2018 | p < 0.0018 | p < 0.0018 | p < 0.0018 | p < 0.0018 | p > 0.0018, ⇔ | p > 0.0018, ⇔ | p > 0.0018, ⇔ |
Lower triangle bootstrapped p-values are relative to a corrected threshold of 0.0018, and ⇔ highlights equivalency to %ΔSI < +/−15%. Note, it is mathematically possible to be statistically different but clinically equivalent, as in the 2012–2014 comparison.
CI, confidence interval; SI, insulin sensitivity.
The lower insulin sensitivity in 2015–2018 was associated with slightly lower (5–10%) time in the 4.4–8.0 mmol/l range, and higher incidence of BG > 10 mmol/l, shown in Table 1. Table 1 also shows larger insulin doses under similar glucose intake conditions, matching the lower SI. The majority of the increase in hyperglycaemia was attributable to a small cohort of patients who experienced persistent hyperglycaemia (high individual % BG > 10 in Table 1) despite large insulin doses, and the ~25% or more of hourly insulin doses of ⩾6 U/h from 2015 to 2018 match expectations around saturation of insulin effect at 6–8 U/h.62–64 Thus nutrition reductions would be required to further reduce BG in these patient hours. Of note, BG time in range from 2011 to 2014 was lower than previously reported, as it includes only the first 72 h of data, which tends to be less glycaemically stable, and does not exclude patients with diabetes or who were not fed. 47
Insulin sensitivity variability across calendar years
In Table 3, percentage insulin sensitivity variability was equivalent at the median and upper quartile of the distributions in Figure 3(b). Table 3 also shows all cohorts are still equivalent at the 75th and 80th percentiles, showing equivalence across large portions of the distribution range and not just at the median, but only six comparisons (21%) are equivalent at the 90th percentile. Overall, insulin sensitivity variability was equivalent on average, and extremely similar across the middle of the range. Divergence in some cohorts at the extremes is not unexpected and is often influenced by small values.
Table 3.
Year to year comparison of hour–hour insulin sensitivity variability (%∆SI). As comparisons are symmetric, the upper triangle in presents the median (95% CI) difference in medians (50th percentile) between years, while the lower triangle gives the median (95% CI) difference at the upper quartile (75th percentile).
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|---|---|
| 2011 | 0.0 (−3.5 to 3.6) ⇔ | 0.4 (−3.5 to 4.0) ⇔ | 1.2 (−2.2 to 4.4) ⇔ | 1.4 (−2.1 to 4.8) ⇔ | 1.4 (−1.9 to 4.5) ⇔ | 0.6 (−2.7 to 3.8) ⇔ | 0.9 (−2.3 to 4.1) ⇔ | |
| 2012 | −2.8 (−8.2 to 3.3) ⇔ a | 0.4 (−1.4 to 2.1) ⇔ | 1.2 (−0.6 to 2.7) ⇔ | 1.4 (−0.3 to 3.2) ⇔ | 1.4 (−0.6 to 3.0) ⇔ | 0.6 (−1.2 to 2.4) ⇔ | 0.9 (−1.1 to 2.9) ⇔ | |
| 2013 | −4.2 (−9.9 to 2.3) ⇔a,b | −1.3 (−4.3 to 1.6) ⇔ a | 0.8 (−0.9 to 2.3) ⇔ | 1.0 (−0.8 to 2.8) ⇔ | 1.0 (−1.1 to 2.8) ⇔ | 0.2 (−1.7 to 2.2) ⇔ | 0.5 (−1.6 to 2.9) ⇔ | |
| 2014 | 2.2 (−2.4 to 7.7) ⇔ a | 5.1 (2.2 to 7.6) ⇔ a | 6.4 (3.6 to 8.9) ⇔ a | 0.2 (−1.4 to 1.8) ⇔ | 0.1 (−1.6 to 1.8) ⇔ | −0.6 (−2.2 to 1.3) ⇔ | −0.3 (−2.2 to 1.8) ⇔ | |
| 2015 | −0.3 (−6.2 to 5.4) ⇔ a | 2.6 (−0.3 to 5.3) ⇔a,b | 3.9 (0.9 to 6.7) ⇔ a | −2.5 (−4.9 to −0.1) ⇔ a | −0.1 (−1.8 to 1.7) ⇔ | −0.8 (−2.6 to 1.0) ⇔ | −0.5 (−2.5 to 1.4) ⇔ | |
| 2016 | 0.5 (−5.6 to 6.7) ⇔ a | 3.4 (0.2 to 6.5) ⇔ a,b | 4.7 (1.5 to 7.8) ⇔ a | −1.7 (−4.4 to 1.1) ⇔ a | 0.8 (−2.2 to 3.8) ⇔ a | −0.8 (−2.4 to 1.16) ⇔ | −0.5 (−2.4 to 1.6) ⇔ | |
| 2017 | −0.6 (−7.1 to 5.1) ⇔ a | 2.3 (−1.1 to 5.6) ⇔a,b | 3.6 (−0.1 to 7.0) ⇔ a | −2.8 (−5.7 to −0.0) ⇔ a | −0.3 (−3.5 to 2.5) ⇔a,b | −1.1 (−4.4 to 2.1) ⇔ a | 0.3 (−1.6 to 2.2) ⇔ | |
| 2018 | 0.6 (−4.7 to −6.5) ⇔ a | 3.5 (0.2−6.8) ⇔ a | 4.8 (1.1–8.1) ⇔ a | −1.6 (−4.5 to 1.4) ⇔a,b | 0.9 (−2.3 to 4.5) ⇔ a | 0.1 (−3.0 to 3.3) ⇔ a | 1.2 (−2.1 to 4.8) ⇔ a |
⇔ shows equivalent to %∆SI < +/−15%, where the median and IQR in Figure 3 are equivalent across all years. Bolded values are independently statistically different to p < 0.0018.
Also equivalent at the 80th percentile.
Also equivalent at the 90th percentile.
CI, confidence interval; SI, insulin sensitivity.
Trends in severity score or patient cohort composition across calendar years
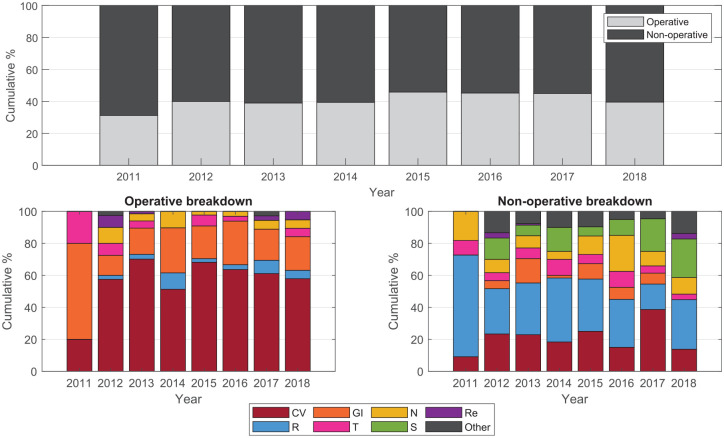
Table 4 shows no overall trend in severity score, mortality, or APACHE III diagnosis code grouping in patients who received insulin therapy from 2012 to 2017. During 2011, the data were collected as part of a half-year pilot trial, so patient numbers are both low and possibly not reflective of all patients with insulin requirements. The percentage of operative patients was consistent at ~40%, and 50–70% of these patients were cardiac surgery patients. Cardiac or respiratory diagnoses made up the majority of nonoperative patients across most years, while other diagnoses categories fluctuated year to year with no discernible trend (Figure 4).
Table 4.
Cohort characteristics and severity scores. Statistics are reported as median (IQR), and mean (standard deviation) for ROD or number (percentage).
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|---|---|
| n, ICU admissions | 1243 | 1242 | 1267 | 980 | 1195 | 1258 | 1303 | 1366 |
| n, GC analysis | 16 | 100 | 172 | 99 | 96 | 73 | 80 | 61 |
| n, DM | N/A | 21 (21%) a | 56 (32.5%) a | 37 (37.4%) a | 46 (47.9%) a | 19 (26%) | 34 (42.5%) | 16 (26.2%) |
| Age (years) | 63 (48–70) | 65 (55–72) | 65 (55–72) | ’62 (51–70) | 66 (58–74) | 67 (59–73) | 66 (58–72) | 64 (53–70) |
| n, Age ⩾80 | 2 (12.5%) | 3 (3.0%) | 18 (10.5%) | 4 (4.0%) | 7 (7.3%) | 7 (9.6%) | 4 (5.0%) | 0 (0) |
| n, Age ⩾70 | 5 (31.3%) | 33 (33.0%) | 56 (32.6%) | 27 (27.3%) | 35 (36.5%) | 30 (41.1%) | 29 (36.3%) | 17 (27.9%) |
| APACHE II | ||||||||
| Score | 22 (17–28) | 21 (15–26) | 20 (16–25) | 20 (14–24) | 21 (16–26) | 19 (15–26) | 20 (15–28) | 18 (13–26) |
| ROD | 0.387 (0.254) | 0.343 (0.240) | 0.365 (0.251) | 0.315 (0.219) | 0.376 (0.255) | 0.341 (0.272) | 0.464 (0.289) | 0.195 (0.232) |
| APACHE III | ||||||||
| Score | 89 (63–105) | 70 (54–93) | 74 (58–92) | 70 (52–88) | 79 (60–101) | 77 (65–90) | 80 (60–102) | 71 (55–92) |
| ROD | 0.412 (0.277) | 0.294 (0.255) | 0.301 (0.263) | 0.270 (0.238) | 0.355 (0.290) | 0.304 (0.256) | 0.373 (0.309) | 0.273 (0.268) |
| n, operative | 5 (31.3%) | 40 (40.0%) | 67 (39.0%) | 39 (39.4%) | 44 (45.8%) | 33 (45.2%) | 36 (45.0%) | 22 (36.1%) |
| Mortality | ||||||||
| ICU mort | 4 (25.0%) | 23 (23.0%) | 36 (20.9%) | 16 (16.2%) | 23 (24.0%) | 13 (17.8%) | 20 (25.0%) | 13 (21.3) |
| Hospital mort | 4 (25.0%) | 26 (26.0%) | 50 (29.1%) | 25 (25.3%) | 25 (26.0%) | 17 (23.3%) | 24 (30.0%) | 15 (24.6) |
| SMR (A-II) | 0.65 | 0.76 | 0.80 | 0.80 | 0.69 | 0.68 | 0.65 | 1.26 |
| SMR (A-III) | 0.61 | 0.88 | 0.97 | 0.94 | 0.73 | 0.77 | 0.80 | 0.90 |
APACHE II and APACHE III are calibrated by the ANZICS for use in New Zealand and Australian ICUs.
Estimated from clinical selection of higher target range, which may over-estimate occurrence.
A-II, APACHE II; A-III, APACHE III; DM, diagnosed diabetes mellitus; GC, glycaemic control; ICU, intensive care unit; IQR, interquartile range; mort, mortality; ROD, risk of death; SMR, standardised motility ratio.
Figure 4.
Breakdown of APACHE III diagnosis by operative and nonoperative codes. Codes are grouped according to the ANZICS-modified APACHE III score. 65
CV, cardiovascular; GI, gastrointestinal (including hepatic/pancreatic); N, neurological; R, respiratory; Re, renal; S, sepsis; T, trauma; and other includes all other codes.
Cohort age was lowest in 2014 [62 (51–70) years] and highest in 2016 [67 (59–73) years], with a general overall trend to increasing median age by ~1 year from 2012 to 2017, with no particular increase in the percentage of patients who were aged ⩾70 or ⩾80 years. However, year to year comparisons show no statistical difference. For 18/21 cohort comparisons between years, p > 0.05 for age. No age comparisons were statistically significant to corrected p < 0.0018, with the three lowest p-values at 0.025 (2014 versus 2015, 62 versus 66), 0.030 (2014 versus 2017, 62 versus 66), and 0.007 (2014 versus 2016, 62 versus 67).
APACHE II scores were lowest in 2016 [19 (15–26)] and highest in 2011 [22 (17–28)], while APACHE III scores were lowest in 2014 [70 (52–88)] and highest in 2011 [89 (63–105)]. All cohort comparisons were not statistically different to p < 0.0018, where the minimum p-value for APACHE II scores was p = 0.08 (20/21 comparisons had p > 0.17), and for APACHE III the minimum was p = 0.0109 (3/21 comparisons had p < 0.05). Similarly, cohort comparisons for ROD scores for APACHE II and III were not significantly different to p < 0.0018 in all but one case (APACHE II ROD, 2014 versus 2017, p < 0.0001). For APACHE II RO 17/21 comparisons had p > 0.05, while for APACHE III ROD 18/21 had p > 0.05 and the minimum was p = 0.013 (2014 versus 2017). Overall, APACHE II and III scores and ROD did not increase or decrease overall over the 2012–2017 period, and were generally not significantly different year to year.
Discussion
Main results
Over the 2012–2017 calendar years insulin sensitivity decreased, despite no trends in severity scores, or other underlying demographic features such as age and illness/injury diagnosis. Median insulin sensitivity and distributions appear to steadily decrease then stabilise at some significantly lower (and tighter) range from 2015 to 2018. Insulin sensitivity variability was overall similar year to year. This decreasing insulin sensitivity, and thus increasing insulin resistance, implies a shift in patient metabolic characteristics for those requiring insulin in the ICU, and which is not already reflected in severity scores.
Insulin sensitivity in this cohort was lower compared to a 2005–2007 analysis of glycaemic control cohorts from the same unit, although hour–hour percent change in insulin sensitivity is similar. 66 While glycaemic control cohort and target range (4.0–6.1 mmol/l) were different, the analysis is based on the same underlying physiological model, nutrition composition was similar, 66 and thus overall results support the trend in insulin sensitivity seen in this study. Insulin sensitivity in Christchurch has been previously shown to be lower than in Belgium and Hungary, 67 though nutrition type and/or delivery in these two centres differed.
There was no overall change in the APACHE II and III severity scores over this time period, and no trends in cohort makeup either by age or diagnosis. Similarly, there was no overall change in ROD, though this number is perhaps less insightful as its calibration accuracy deteriorates over time as ICU survival improves. 68 Forecasts predict the number of very elderly patients (age >80 years) will double by 2050, 69 but no overall change in the number of patients greater than 70 or 80 years of age was seen in this study. Data from 2011 were limited and reflects a clinical pilot of a new glycaemic control protocol, thus patients are most different in this year for diagnoses and length of ICU stay. Thus, for patients who received insulin for glycaemic control in the period 2012–2017, there appears to be no underlying shift in demographic, or diagnosis as broadly categorised by the APACHE III system.
Apparent changes in insulin sensitivity can be caused by changes underlying patient metabolism, changes in the way insulin is stored/prepared and delivered, changes in glucose monitoring and the tightness of glycaemic control, or changes in other clinical therapies such as nutrition, inotrope, or steroid use. The ~45–48% relative drop in median insulin sensitivity between 2012 and 2015–2018 is effectively double the 20–25% difference in ICU patients receiving versus not receiving corticosteroids, 7 or the higher end value of ~30% error in SI when timing and (now outdated) glucometer errors are combined. 70 Given improvements in the last decade in bedside glucose measurement accuracy and the magnitude of the 2012–2015 insulin sensitivity decrease, the results observed are unlikely to be solely attributable to changes in steroid use or glucose measurement practice alone.
Data on steroid or inotrope use were not available for this study, but as previously discussed, the magnitude of the insulin sensitivity decrease is unlikely to be solely attributable to a change in dosing in this area from 2012 to 2015. Both of these therapies were standard care prior to 2012 and there have been no clinical practice change implementations related to these since 2011, though usage may have gradually increased with increased evidence for benefit. Figure 4 shows no trends in the number of operative or nonoperative cardiovascular patients receiving glycaemic control. Future work could examine trends in insulin sensitivity related to steroid or inotrope usage, though direct clinical outcomes of higher insulin requirements with increasing insulin resistance remain.
There is the possibility of insulin adsorption affecting apparent insulin sensitivity by requiring greater insulin doses. 71 Trends in consumables have tended towards a better understanding of adsorption as an issue, so it seems unlikely that apparent insulin sensitivity would drop due to adsorptive effects. Equally, adsorption is only an issue in the adult ICU at the flow rates and concentrations over the first 1–3 h, 71 where insulin concentrations may get as low as 80% of intended, and thus adsorption is not going to have a significant effect on 72-h SI. The authors also know of no other changes in insulin type, storage, preparation, or delivery over this period, mitigating the potential bias from these effects.
The most significant clinical change was a change in nutrition during the TARGET RCT trial from mid-2016 to mid-2018. Changes in nutrition glucose concentrations are automatically accounted for in the model-based insulin sensitivity. Patients on the high calorie arm (1.5 kcal/ml, 180 g/l carbohydrate) were mostly excluded in this analysis by the 7 g/l glucose intake threshold. Of patients on the 1.0 kcal/ml arm, the higher glucose content (120 g/l) is balanced by a decrease in fat, while protein is comparable to the standard care (and nonenrolled) nutrition. Given similarities in protein content and the fact that insulin sensitivity was low even before the mid-2016 start of the RCT, it is unlikely the decrease in insulin sensitivity is attributable to a change in nutrition type.
Superficially, lack of a shift in demographic or diagnostics implies cohort severity remains unchanged. However, the decreasing whole-body insulin sensitivity implies greater peripheral insulin resistance and/or higher hepatic endogenous glucose production. Both of these factors may point to a potential underlying increase in acute inflammation3,46 not captured by commonly used severity or diagnostic scores. Overall, while equivalent in broad demographics and diagnostics, patients may be more complex, particularly when ICU bed spaces are under increasing demand, 72 as in Christchurch over this period. New Zealand has a relatively low number of ICU beds per capita compared to other developed countries.73–76 Increasing patient complexity would match widely observed global trends in increasing comorbidities in intensive care patients.36–40,42,44,45
Shifts in intensive care cohort demographics towards older and more severely ill, or more complex, patients are evident worldwide,36–43 including Australia and New Zealand.44,45 Of studies reporting severity scores, these scores (SAPS, APACHE) were generally stable 41 or decreased slightly. However, long term data on these scores is scarce and complicated by changes in score use and/or calibration. One Austrian study published in 2007 looked directly at insulin resistance using the HOMA metric and saw insulin resistance increase proportionally to the APACHE III score, 77 a different result from this study. This discrepancy could relate to differences in cohorts both by decade and geography, or be a function of a single n = 25 cohort versus a longitudinal multi-year approach. Finally, while comparatively easy to calculate, the HOMA metric is also relatively insensitive compared to other means of estimating insulin sensitivity. 78
The glycaemic control cohort analysed here was on average 2–7 years older than a broader 2017 Australian and New Zealand intensive care published cohort analysis (62–67 vs. 60 years old). 79 APACHE scores and ROD were more similar to the subcohort of patients with a length of stay >21 days, 79 implying those who require insulin are generally more severely ill and more complex. Similar to the results in Table 4, a short 2000–2004 longitudinal analysis in this same study saw no statistically significant increase in average age in or chronic health conditions in long-stay patients, suggesting longer analysis intervals may be required in the current context to observe cohort changes based on APACHE scores or similar. Equally, APACHE scores were designed for mortality risk assessment across a cohort,59,80 and may not capture longitudinal changes well.
This analysis did not examine glycaemic control outcomes, focusing instead on underlying metabolic insulin sensitivity. Insulin sensitivity captures the net efficacy of insulin at mediating glucose uptake, and was calculated based on net glucose and insulin inputs, and BG measurements. Thus, two patients can have the same underlying insulin sensitivity, but different insulin/nutrition ratios and BG outcomes. Given the well-validated model and methods, year–year glycaemic control protocol differences were not expected to significantly affect underlying insulin sensitivity identified,67,81 particularly since the lower target of the target range, which primarily determines insulin dose size, remained unchanged over the whole period.
Limitations
This study presents clinical results from a single centre, based on data observationally collected from an electronic glycaemic control protocol. Patients meeting starting criteria for insulin therapy (2 BG > 8 or 10 mmol/l within 4 h), and who spent more than 8 h on insulin therapy, make up about 7–15% of total ICU admissions. Thus changes in insulin sensitivity are thus only directly observed in patients requiring insulin therapy due to hyperglycaemia. No data on comorbidities were available for this study, where comorbidities contribute to patient complexity. No data on inflammatory markers were available in this retrospective study, and future work should assess the link between trends in apparent insulin resistance and inflammation.
Incidence of people with pre-existing diabetes was not recorded clinically pre-2016, although clinical selection of a higher target range provides a surrogate. Equally, undiagnosed diabetes or metabolic disorders is common in this cohort. It is possible increasing insulin resistance reflects greater prevalence of undiagnosed diabetes, where general trends point towards increasing incidence of diabetes within New Zealand and worldwide.82–84
The physiological model presented here has been widely and successfully used as a standard of care in a range of ICUs over a number of years.47,49,50,55 The whole-body time-varying model-based insulin sensitivity used in this analysis has been well used clinically,7,30,47–50,55,66,67,84,86 but relies on population parameters for endogenous glucose production and insulin production/clearance. It has been a useful marker of overall insulin efficacy, and is responsive to changes in drugs and therapies.7,66,84,86 As inflammatory and stress responses are primary drivers of insulin resistance in the ICU, changes in SI capture both increased endogenous glucose production and greater peripheral insulin resistance, where the overall effect is to require greater insulin dosing to manage nutrition and glycaemia.
The STAR protocol was used throughout 2011–2018, but was modified for use during the TARGET RCT during mid 2016–2018, including a higher upper target on the target range (10 mmol/l versus 8 mmol/l), and leaving nutrition rates solely to clinical discretion. Insulin doses under STAR are a function of the lower target of the range, 48 where the upper target allows longer intervals between measurements when BG is stable, and thus a change in the upper target is unlikely to significantly affect overall time in range, and, indirectly, SI. The effect of modulating insulin only, and not nutrition as well, was an increase in hyperglycaemia (Table 1) from 2016 to 2018, the majority of which comes from persistent hyperglycaemia in a handful of patients. This is attributable to the fact STAR was not allowed to recommend nutrition reductions during the TARGET RCT trial, and thus had reduced capacity to treat persistent hyperglycaemia in the face of high insulin doses.
Only data from patients who received less than 7 g/h mean glucose intake in enteral nutrition was used in this analysis, excluding 73 (9.6%) patients, where most were part of the (very) high nutrition arm of the 2016–2018 TARGET RCT. 52 The physiological model used here was designed for normal clinical conditions, where dynamics are more nonlinear in the presence of very high glucose and/or insulin intake 57 and the exact nature and threshold of insulin effect saturation is hard to precisely quantify in a patient-specific manner, but is somewhere in the vicinity of 6–8 U/h,.62–64 If the analysis is repeated with data from all nutrition rates (not shown here) the trends in insulin sensitivity, and its hour–hour variability overall, remain. However, for the integrity of this analysis, patient data within normal clinical and proven physiological ranges are used.
Clinical implications
Increasing insulin resistance in intensive care means hyperglycaemia and glycaemic dysregulation are likely more common, irrespective of the underlying cause of insulin resistance. Further, greater insulin resistance requires larger insulin doses to achieve comparable nutrition intake and BG levels. This aspect is directly observed in Tables 1 and 2, where decreasing SI is seen alongside rising insulin doses and comparable nutrition. Where insulin ‘effect,’ or insulin-mediated glucose uptake, saturates (6–8 U/h,62–64), decreasing insulin sensitivity in tandem with maintenance of relatively high nutrition intake will result in hyperglycaemia,1,10,87 and subsequently reduced outcomes.21,88–90 It should be noted that STAR typically modulated both insulin and nutrition, so persistent hyperglycaemia is rare, resulting in good control to target range, as nutrition rate may be temporarily lowered to bring insulin-saturated high BG into range.
The ability to achieve tight and safe glycaemic control is foremost a function of underlying metabolic variability.30,35 Across the 2011–2018 calendar years metabolic variability, as reflected by percent change in insulin sensitivity, was largely unchanged. This lack of change suggests equal ability to control these cohorts, 30 as hour–hour variability and change is equivalent, within a range of clinical parameters. Where SI is low, glycaemic control can suffer when glucose intake is too high and insulin dosing has reached saturation.1,10,87 In these cases, greater incidence of transient and persistent hyperglycaemia is expected, particularly under rigid nutrition targets and dosing. Hence, as SI declines the ability to safely and effectively control all patients may diminish due to insulin effect saturation. Equally, as SI declines, greater reductions in nutrition may be required in a small number of patients to achieve glycaemic targets.
Irrespective of underlying causes, increasing insulin resistance has implications for care, both in understanding and managing the relationship between metabolism and the immune response, and, more directly, for the modulation of nutrition and implementation of insulin therapy for tight glycaemic control. As hyperglycaemia is the manifestation of insulin resistance, future studies of glycaemic control may be easily confounded by insulin resistance and resulting lowered glucose tolerance. Resolution of the debate around the benefits or harms of glycaemic control, and optimum targets and thresholds for intervention or care, is likely to become more difficult if the increasing insulin resistance observed here, whether caused by underlying population changes (such as aging and/or comorbidities, or metabolic changes including trends in diabetes or obesity) or prevalence of other clinical therapies, generalises more broadly to other centres and countries.
Conclusions
Over the 2012–2018 calendar years, SI steadily decreased from 2012 to 2014 (p < 0.0018, statistically nonequivalent) then stabilised at a significantly lower (and tighter) range from 2015 to 2018 (statistically equivalent), despite no trends in severity scores, or other underlying demographic features such as age and illness/injury diagnosis. Changes in SI may reflect increasing inflammation, patient complexity, or other clinical therapies, and are associated with an increase in high insulin doses. Future work should assess whether other intensive care units are also seeing an increase in insulin resistance. The clinical implications of decreasing SI (increasing resistance) are that hyperglycaemia and glycaemic dysregulation are likely more common because of insulin saturation at high insulin doses, thus having implications for glycaemic control and nutrition management, irrespective of the underlying cause of insulin resistance.
Supplemental Material
Supplemental material, sj-pdf-1-tae-10.1177_20420188211012144 for Increased insulin resistance in intensive care: longitudinal retrospective analysis of glycaemic control patients in a New Zealand ICU by Jennifer L. Knopp, J. Geoffrey Chase and Geoffrey M. Shaw in Therapeutic Advances in Endocrinology and Metabolism
Footnotes
Author contribution(s): Jennifer L. Knopp: Conceptualization; Data curation; Formal analysis; Methodology; Validation; Visualization; Writing-original draft; Writing-review & editing.
J. Geoffrey Chase: Formal analysis; Supervision; Writing-review & editing.
Geoffrey M. Shaw: Data curation; Investigation; Supervision; Writing-review & editing.
Conflicts of interest: There has been a provisional filing of a patent regarding insulin sensitivity forecasting (JGC and JLK) for licensee Tiro Medical. Insulin sensitivity and its forecasting are well published in the literature, and forecasting is not used in this analysis. No other conflicts of interest are declared.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the NZ National Science Challenge 7, Science for Technology and Innovation (2019-S3-CRS).
Ethics approval: Glycaemic control using STAR is provided as a standard of care at Christchurch Hospital ICU. Ethics approval for retrospective auditing of de-identified STAR glycaemic control data was approved by the New Zealand Health and Disabilities Ethics Committee (URA-12-EXP-004).
ORCID iD: Jennifer L. Knopp  https://orcid.org/0000-0001-9343-3961
https://orcid.org/0000-0001-9343-3961
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jennifer L. Knopp, Department of Mechanical Engineering, University of Canterbury, Private Bag 4800, Christchurch, 8140, New Zealand.
J. Geoffrey Chase, Department of Mechanical Engineering, University of Canterbury, Christchurch, New Zealand.
Geoffrey M. Shaw, Department of Intensive Care, Christchurch Hospital, Christchurch, New Zealand
References
- 1. McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin 2001; 17: 107–124. [DOI] [PubMed] [Google Scholar]
- 2. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet 2009; 373: 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dhar A, Castillo L. Insulin resistance in critical illness. Curr Opin Pediatr 2011; 23: 269–274. [DOI] [PubMed] [Google Scholar]
- 4. Weiss SL, Alexander J, Agus MSD. Extreme stress hyperglycemia during acute illness in a pediatric emergency department. Pediatr Emerg Care 2010; 26: 626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ognibene KL, Vawdrey DK, Biagas KV. The association of age, illness severity, and glycemic status in a pediatric intensive care unit. Pediatr Crit Care Med 2011; 12: e386–e390. [DOI] [PubMed] [Google Scholar]
- 6. Bjerke HS, Shabot MM. Glucose intolerance in critically ill surgical patients: relationship to total parenteral nutrition and severity of illness. Am Surg 1992; 58: 728–731. [PubMed] [Google Scholar]
- 7. Pretty C, Chase JG, Lin J, et al. Impact of glucocorticoids on insulin resistance in the critically ill. Comput Methods Programs Biomed 2011; 102: 172–180. [DOI] [PubMed] [Google Scholar]
- 8. Bratusch-Marrain PR. Insulin-counteracting hormones: their impact on glucose metabolism. Diabetologia 1983; 24: 74–79. [DOI] [PubMed] [Google Scholar]
- 9. Khoury N, McGill JB. Changes in insulin sensitivity following administration of the clinically-used low-dose pressor, norepinephrine. Diabetes Metab Res Rev 2011; 27: 604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nasraway SA. Hyperglycemia during critical illness. JPEN J Parenter Enteral Nutr 2006; 30: 254–258. [DOI] [PubMed] [Google Scholar]
- 11. Marik PE. Tight glycemic control in acutely ill patients: low evidence of benefit, high evidence of harm! Intensive Care Med 2016; 42: 1475–1477. [DOI] [PubMed] [Google Scholar]
- 12. Schultz MJ, Harmsen RE, Spronk PE. Clinical review: strict or loose glycemic control in critically ill patients - implementing best available evidence from randomized controlled trials. Crit Care 2010; 14: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gunst J, Van den Berghe G. Blood glucose control in the ICU: don’t throw out the baby with the bathwater! Intensive Care Med 2016; 42: 1478–1481. [DOI] [PubMed] [Google Scholar]
- 14. Preiser JC, Straaten HMOV. Glycemic control: please agree to disagree. Intensive Care Med 2016; 42: 1482–1484. [DOI] [PubMed] [Google Scholar]
- 15. Chase JG, Dickson JL. Traversing the valley of glycemic control despair. Crit Care 2017; 21: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jafar N, Edriss H, Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am J Med Sci 2016; 351: 201–211. [DOI] [PubMed] [Google Scholar]
- 17. Hansen TK, Thiel S, Wouters PJ, et al. Intensive insulin therapy exerts anti inflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab 2003; 88: 1082–1088. [DOI] [PubMed] [Google Scholar]
- 18. Das UN. Insulin in sepsis and septic shock. J Assoc Physicians India 2003; 51: 695–700. [PubMed] [Google Scholar]
- 19. Jeschke MG, Klein D, Bolder U, et al. Insulin attenuates the systemic inflammatory response in endotoxemic rats. Endocrinology 2004; 145: 4084–4093. [DOI] [PubMed] [Google Scholar]
- 20. Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann Surg 2004; 239: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vanhorebeek I, Langouche L. Molecular mechanisms behind clinical benefits of intensive insulin therapy during critical illness: glucose versus insulin. Best Pract Res Clin Anaesthesiol 2009; 23: 449–459. [DOI] [PubMed] [Google Scholar]
- 22. Vanhorebeek I, De Vos R, Mesotten D, et al. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet 2005; 365: 53–59. [DOI] [PubMed] [Google Scholar]
- 23. Mesotten D, Van den Berghe G. Clinical benefits of tight glycaemic control: focus on the intensive care unit. Best Pract Res Clin Anaesthesiol 2009; 23: 421–429. [DOI] [PubMed] [Google Scholar]
- 24. Egi M, Bellomo R, Stachowski E, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc 2010; 85: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krinsley J, Schultz MJ, Spronk PE, et al. Mild hypoglycemia is strongly associated with increased intensive care unit length of stay. Ann Intensive Care 2011; 1: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 2007; 35: 2262–2267. [DOI] [PubMed] [Google Scholar]
- 27. Egi M, Bellomo R. Reducing glycemic variability in intensive care unit patients: a new therapeutic target? J Diabetes Sci Technol 2009; 3: 1302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Egi M, Bellomo R, Stachowski E, et al. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 2006; 105: 244–252. [DOI] [PubMed] [Google Scholar]
- 29. Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med 2008; 36: 3008–3013. [DOI] [PubMed] [Google Scholar]
- 30. Uyttendaele V, Dickson JL, Shaw GM, et al. Untangling glycaemia and mortality in critical care. Crit Care 2017; 21: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Griesdale DEG, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 2009; 180: 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA 2008; 300: 933–944. [DOI] [PubMed] [Google Scholar]
- 33. Marik PE, Preiser JC. Toward understanding tight glycemic control in the ICU: a systematic review and meta-analysis. Chest 2010; 137: 544–551. [DOI] [PubMed] [Google Scholar]
- 34. Yamada T, Shojima N, Noma H, et al. Glycemic control, mortality, and hypoglycemia in critically ill patients: a systematic review and network meta-analysis of randomized controlled trials. Intensive Care Med 2017; 43: 1–15. [DOI] [PubMed] [Google Scholar]
- 35. Chase JG, Le Compte AJ, Suhaimi F, et al. Tight glycemic control in critical care – The leading role of insulin sensitivity and patient variability: a review and model-based analysis. Comput Methods Programs Biomed 2011; 102: 156–171. [DOI] [PubMed] [Google Scholar]
- 36. Adhikari NK, Fowler RA, Bhagwanjee S, et al. Critical care and the global burden of critical illness in adults. Lancet 2010; 376: 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jentzer JC, van Diepen S, Barsness GW, et al. Changes in comorbidities, diagnoses, therapies and outcomes in a contemporary cardiac intensive care unit population. Am Heart J 2019; 215: 12–19. [DOI] [PubMed] [Google Scholar]
- 38. Sjoding MW, Prescott HC, Wunsch H, et al. Longitudinal changes in ICU admissions among elderly patients in the United States. Crit Care Med 2016; 44: 1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vincent JL, Lefrant JY, Kotfis K, et al. Comparison of European ICU patients in 2012 (ICON) versus 2002 (SOAP). Intensive Care Med 2018; 44: 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blot S, Cankurtaran M, Petrovic M, et al. Epidemiology and outcome of nosocomial bloodstream infection in elderly critically ill patients: a comparison between middle-aged, old, and very old patients. Crit Care Med 2009; 37: 1634–1641. [DOI] [PubMed] [Google Scholar]
- 41. Ihra GC, Lehberger J, Hochrieser H, et al. Development of demographics and outcome of very old critically ill patients admitted to intensive care units. Intensive Care Med 2012; 38: 620–626. [DOI] [PubMed] [Google Scholar]
- 42. Jakob SM, Rothen HU. Intensive care 1980–1995: change in patient characteristics, nursing workload and outcome. Intensive Care Med 1997; 23: 1165–1170. [DOI] [PubMed] [Google Scholar]
- 43. Parker A, Wyatt R, Ridley S. Intensive care services; a crisis of increasing expressed demand. Anaesthesia 1998; 53: 113–120. [DOI] [PubMed] [Google Scholar]
- 44. Bagshaw SM, Webb SA, Delaney A, et al. Very old patients admitted to intensive care in Australia and New Zealand: a multi-centre cohort analysis. Crit Care 2009; 13: R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Williams TA, Ho KM, Dobb GJ, et al. Changes in case-mix and outcomes of critically ill patients in an Australian tertiary intensive care unit. Anaesth Intensive Care 2010; 38: 703–709. [DOI] [PubMed] [Google Scholar]
- 46. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010; 72: 219–246. [DOI] [PubMed] [Google Scholar]
- 47. Stewart KW, Pretty CG, Tomlinson H, et al. Safety, efficacy and clinical generalization of the STAR protocol: a retrospective analysis. Ann Intensive Care 2016; 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fisk LM, Le Compte AJ, Shaw GM, et al. STAR Development and Protocol Comparison. IEEE Trans Biomed Eng 2012; 59: 3357–3364. [DOI] [PubMed] [Google Scholar]
- 49. Uyttendaele V, Knopp JL, Pirotte M, et al. Preliminary results from the STAR-Liège clinical trial: Virtual trials, safety, performance, and compliance analysis. IFAC-PapersOnLine 2018; 51: 355–360. [Google Scholar]
- 50. Knopp JL, Lynn AM, Shaw GM, et al. Safe and effective glycaemic control in premature infants: observational clinical results from the computerised STAR-GRYPHON protocol. Arch Dis Child 2019; 104: F205-F211. [DOI] [PubMed] [Google Scholar]
- 51. Evans A, Le Compte A, Tan CS, et al. Stochastic Targeted (STAR) glycemic control: design, safety, and performance. J Diabetes Sci Technol 2012; 6: 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. The TARGET Investigators, for the ANZICS Clinical Trials Group. Energy-Dense versus routine enteral nutrition in the critically ill. New Engl J Med 2018; 379: 1823–1834. [DOI] [PubMed] [Google Scholar]
- 53. Lin J, Razak NN, Pretty CG, et al. A physiological Intensive Control Insulin-Nutrition-Glucose (ICING) model validated in critically ill patients. Comput Methods Programs Biomed 2011; 102: 192–205. [DOI] [PubMed] [Google Scholar]
- 54. Chase JG, Pretty CG, Pfeifer L, et al. Organ failure and tight glycemic control in the SPRINT study. Crit Care 2010; 14: R154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lin J, Lee D, Chase JG, et al. Stochastic modelling of insulin sensitivity and adaptive glycemic control for critical care. Comput Methods Programs Biomed 2008; 89: 141–152. [DOI] [PubMed] [Google Scholar]
- 56. Docherty PD, Chase JG, David T. Characterisation of the iterative integral parameter identification method. Med Biol Eng Comput 2012; 50: 127–128. [DOI] [PubMed] [Google Scholar]
- 57. Chase JG, Andreassen S, Pielmeier U, et al. A glucose–insulin pharmacodynamic surface modeling validation and comparison of metabolic system models. Biomed Signal Process Control 2009; 4: 355–363. [Google Scholar]
- 58. Motulsky H. Intuitive biostatistics: a nonmathematical guide to statistical thinking, 3rd ed. New York: Oxford University Press, 2014. [Google Scholar]
- 59. Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 60. Del Bufalo C, Morelli A, Bassein L, et al. Severity scores in respiratory intensive care: APACHE II predicted mortality better than SAPS II. Respir Care 1995; 40: 1042–1047. [PubMed] [Google Scholar]
- 61. Ledoux D, Finfer S, Mckinley S. Impact of operator expertise on collection of the APACHE II score and on the derived risk of death and standardized mortality ratio. Anaesth Intensive Care 2005; 33: 585–590. [DOI] [PubMed] [Google Scholar]
- 62. Docherty PD, Chase JG, Hann CE, et al. The identification of insulin saturation effects during the dynamic insulin sensitivity test. Open Med Inform J 2010; 4: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Natali A, Gastaldelli A, Camastra S, et al. Dose-response characteristics of insulin action on glucose metabolism: a non-steady-state approach. Am J Physiol Endocrinol Metab 2000; 278: E794–E801. [DOI] [PubMed] [Google Scholar]
- 64. Prigeon RL, Jr, Røder ME, Porte D, et al. The effect of insulin dose on the measurement of insulin sensitivity by the minimal model technique. Evidence for saturable insulin transport in humans. J Clin Investig 1996; 97: 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Paul E, Bailey M, Pilcher D. Risk prediction of hospital mortality for adult patients admitted to Australian and New Zealand intensive care units: development and validation of the Australian and New Zealand risk of death model. J Crit Care 2013; 28: 935–941. [DOI] [PubMed] [Google Scholar]
- 66. Pretty CG, Le Compte AJ, Chase J, et al. Variability of insulin sensitivity during the first 4 days of critical illness: implications for tight glycemic control. Ann Intensive Care 2012; 2: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dickson JL, Stewart KW, Pretty CG, et al. Generalisability of a virtual trials method for glycaemic control in intensive care. IEEE Trans Biomed Eng 2017; 65: 1543–1553. [DOI] [PubMed] [Google Scholar]
- 68. Paul E, Bailey M, Van Lint A, et al. Performance of APACHE III over time in Australia and New Zealand: a retrospective cohort study. Anaesth Intensive Care 2012; 40: 980–994. [DOI] [PubMed] [Google Scholar]
- 69. Nguyen YL, Angus DC, Boumendil A, et al. The challenge of admitting the very elderly to intensive care. Ann Intensive Care 2011; 1: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pretty CG, Signal M, Fisk L, et al. Impact of sensor and measurement timing errors on model-based insulin sensitivity. Comput Methods Programs Biomed 2014; 114: e79–e86. [DOI] [PubMed] [Google Scholar]
- 71. Knopp JL, Chase JG. Clinical recommendations for managing the impact of insulin adsorptive loss in hospital and diabetes care. J Diabetes Sci Technol 2020; 1932296820915875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chase JG, Shaw GM. How standard is the “S” in SMR? Intensive Care Med 2012; 38: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Prin M, Wunsch H. International comparisons of intensive care: informing outcomes and improving standards. Curr Opin Crit Care 2012; 18: 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wallace DJ, Angus DC, Seymour CW, et al. Critical care bed growth in the United States. A comparison of regional and national trends. Am J Respir Crit Care Med 2015; 191: 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rhodes A, Ferdinande P, Flaatten H, et al. The variability of critical care bed numbers in Europe. Intensive Care Med 2012; 38: 1647–1653. [DOI] [PubMed] [Google Scholar]
- 76. Wong DJN, Popham S, Wilson AM, et al. Postoperative critical care and high-acuity care provision in the United Kingdom, Australia, and New Zealand. Br J Anaesth 2019; 122: 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zauner A, Nimmerrichter P, Anderwald C, et al. Severity of insulin resistance in critically ill medical patients. Metabolism 2007; 56: 1–5. [DOI] [PubMed] [Google Scholar]
- 78. Docherty PD, Chase JG, Lotz T, et al. DISTq: an iterative analysis of glucose data for low-cost, real-time and accurate estimation of insulin sensitivity. Open Med Inform J 2009; 3: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Crozier TM, Pilcher DV, Bailey MJ, et al. Long-stay patients in Australian and New Zealand intensive care units: demographics and outcomes. Crit Care Resusc 2007; 9: 327–333. [PubMed] [Google Scholar]
- 80. Vincent JL, Opal SM, Marshall JC. Ten reasons why we should NOT use severity scores as entry criteria for clinical trials or in our treatment decisions. Crit Care Med 2010; 38: 283–287. [DOI] [PubMed] [Google Scholar]
- 81. Chase JG, Suhaimi F, Penning S, et al. Validation of a model-based virtual trials method for tight glycemic control in intensive care. Biomed Eng Online 2010; 9: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zimmet P, Alberti KG, Magliano DJ, et al. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol 2016; 12: 616–622. [DOI] [PubMed] [Google Scholar]
- 83. Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011; 94: 311–321. [DOI] [PubMed] [Google Scholar]
- 84. American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018; 41: 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jamaludin UK, Docherty PD, Geoffrey Chase J, et al. Impact of haemodialysis on insulin kinetics of acute kidney injury patients in critical care. J Med Biol Eng 2015; 35: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pri AS, Chase JG, Pretty CG, et al. Insulin sensitivity variability during hypothermia. IFAC Proc Vol 2014; 47: 10162–10167. [Google Scholar]
- 87. Nguyen N, Ching K, Fraser R, et al. The relationship between blood glucose control and intolerance to enteral feeding during critical illness. Intensive Care Med 2007; 33: 2085–2092. [DOI] [PubMed] [Google Scholar]
- 88. Finney SJ, Zekveld C, Elia A, et al. Glucose control and mortality in critically ill patients. JAMA 2003; 290: 2041–2047. [DOI] [PubMed] [Google Scholar]
- 89. Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 2003; 78: 1471–1478. [DOI] [PubMed] [Google Scholar]
- 90. Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000; 355: 773–778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tae-10.1177_20420188211012144 for Increased insulin resistance in intensive care: longitudinal retrospective analysis of glycaemic control patients in a New Zealand ICU by Jennifer L. Knopp, J. Geoffrey Chase and Geoffrey M. Shaw in Therapeutic Advances in Endocrinology and Metabolism