Abstract
Background
A number of genetic markers linked to familial pulmonary fibrosis predict differential survival in interstitial lung disease (ILD) patients. Although genetic testing is not performed routinely for ILD, family history commonly is obtained and may inform outcome risk.
Research Question
Does survival vary between patients with and without self-reported familial pulmonary fibrosis?
Methods
Family history was acquired systematically for consecutive ILD patients who consented to clinical registry enrollment at the University of Texas Southwestern and the University of California at Davis. Patients were stratified by idiopathic pulmonary fibrosis (IPF) and non-IPF ILD diagnosis and were substratified by presence or absence of familial pulmonary fibrosis, defined as one or more additional affected family members. Transplant-free survival was compared using multilevel, mixed-effects Cox proportional hazards regression.
Results
Of the 1,262 patients included, 534 (42%) had IPF ILD and 728 (58%) had non-IPF ILD. Of those with non-IPF ILD, 18.5% had connective tissue disease, 15.6% had chronic hypersensitivity pneumonitis, and 23.5% had unclassifiable ILD. Familial pulmonary fibrosis was reported in 134 IPF ILD patients (25.1%) and 90 non-IPF ILD patients (12.4%). Those with familial IPF showed an 80% increased risk of death or transplantation compared with those with sporadic IPF (hazard ratio [HR], 1.8; 95% CI, 1.37-2.37; P < .001), whereas those with familial non-IPF ILD showed a twofold increased risk compared with their counterparts with sporadic disease (HR, 2.08; 95% CI, 1.46-2.96; P < .001). Outcome risk among those with familial non-IPF ILD was no different than for those with sporadic IPF ILD (HR, 1.27; 95% CI, 0.89-1.84; P = .19).
Interpretation
Patient-reported familial pulmonary fibrosis is predictive of reduced transplant-free survival in IPF and non-IPF ILD patients. Because survival among patients with familial non-IPF ILD approximates that of sporadic IPF ILD, early intervention should be considered for such patients. Until clinical genetic testing is widely available and provides actionable results, family history should be ascertained and considered in risk stratification.
Key Words: autoimmune, family history, hypersensitivity pneumonitis, ILD, pulmonary fibrosis, respiratory failure
Abbreviations: CHP, chronic hypersensitivity pneumonitis; CTD, connective tissue disease; FPF, familial pulmonary fibrosis; HR, hazard ratio; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; TFS, transplant-free survival; UCD, University of California at Davis; uILD, unclassifiable ILD; UTSW, University of Texas Southwestern
FOR EDITORIAL COMMENT, SEE PAGE 1709
Interstitial lung disease (ILD) comprises a heterogenous group of diffuse lung disorders with variable clinical, radiographic, and histologic features that commonly result in pulmonary fibrosis. Among the most common causes of ILD are connective tissue disease (CTD), including rheumatoid arthritis, systemic sclerosis, and idiopathic inflammatory myopathy, and chronic hypersensitivity pneumonitis (CHP) resulting from an inappropriate immunologic response to a chronically inhaled organic antigen.1,2 For those without an identifiable ILD cause, idiopathic pulmonary fibrosis (IPF) predominates, accounting for more than 50% of idiopathic cases and 20% of all ILD cases.3,4 An accurate ILD diagnosis is critical because IPF often follows a more progressive course and treatment varies between individual ILD patients.5,6
Familial pulmonary fibrosis (FPF) characterizes patients for whom ILD affects two or more blood relatives.7 Approximately 20% of IPF cases are familial,7, 8, 9 with most such patients demonstrating autosomal-dominant transmission with reduced penetrance. Rare pathogenic variants in genes involved in telomere maintenance10, 11, 12, 13, 14, 15, 16 and surfactant protein production have been implicated.17, 18, 19 Although IPF is the most common ILD subtype in patients with FPF, other ILDs of both known and unknown cause can be identified readily within FPF kindreds, with discordant diagnoses in up to 80% of kindreds with the same pathologic rare variants.20,21 The importance of ILD classification in such patients remains unclear, because individuals with pathogenic variants in telomere-related genes TERT, TERC, PARN, and RTEL1 have been shown to follow an “IPF-like” natural history regardless of ILD classification.21
Substantial progress has been made in characterizing the genetic landscape in patients with FPF; however, widespread use of clinical genetic testing has not been adopted by the ILD community. A pathogenic or likely pathogenic rare genetic variant may be identified in up to 30% of patients, but may go unrealized by clinicians and patients without a genetic workup. Although the natural history of FPF subsets harboring a causative telomere-related gene mutation has been characterized, results of studies assessing disease course among the broader groups of patients endorsing a family history have been mixed.22,23 In this investigation, we sought to determine whether patients with a self-reported family history of ILD display a unique phenotype regarding clinical characteristics and longitudinal outcomes. We hypothesized that self-reported family history of ILD would predict worse survival in patients with IPF, CTD-associated ILD, CHP, and unclassifiable ILD (uILD) when compared with their counterparts with sporadic disease.
Methods
This retrospective investigation was conducted at the University of Texas Southwestern (UTSW) and the University of California at Davis (UCD) and was approved by the institutional review board at each institution (UTSW Identifiers: 082010-127 and 092017-007; UCD Identifier: 875917). Consecutive patients with a diagnosis of ILD consenting to clinical registry enrollment at each institution were screened (UTSW, 2003 through 2019; and UCD, 2016 through 2019). All included patients had longitudinal follow-up data and a multidisciplinary diagnosis of IPF, CTD-associated ILD, CHP, or uILD. CTD-associated ILD subtypes included rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, idiopathic inflammatory myopathy, mixed connective tissue disease, and Sjögren’s syndrome.
Patients were substratified by presence or absence of FPF, defined as patient-reported history of one or more additional family members affected with ILD. FPF presence was ascertained systematically for all patients by previsit questionnaire (UTSW and UCD), detailed family history at the time of initial evaluation at the ILD center (UTSW and UCD), at time of registry enrollment (UTSW), or a combination thereof. A positive family history was confirmed by provider interview during clinical evaluation for all patients who reported a family history of ILD on the questionnaire with number of affected family members ascertained. Only the proband of families with multiple affected members enrolled in the registries were included in this study.
Statistical Analysis
Continuous variables were reported as their means with SDs and were compared using a two-tailed Student t test. Categorical variables were reported as counts and percentages and were compared using the χ2 test or Fisher exact test, as appropriate. We assessed the association between FPF and transplant-free survival (TFS) using univariate and multivariate multilevel, mixed-effects Cox proportional hazards regression, which incorporated center as a random effect to control for center-level heterogeneity in TFS and patient-level covariates strongly collinear with center.24,25 Race and Gender, Age, Physiology stage (composite of sex, age, FVC and diffusion capacity for carbon monoxide percent predicted) were modeled as fixed effects.26 Survival was plotted using the Kaplan-Meier survival estimator and was compared using a log-rank test. Because two groups were being evaluated (IPF ILD and non-IPF ILD), statistical significance was set at .025 to correct for multiple testing. All statistical analyses were performed using Stata release 16 software (Stata Corp).
Results
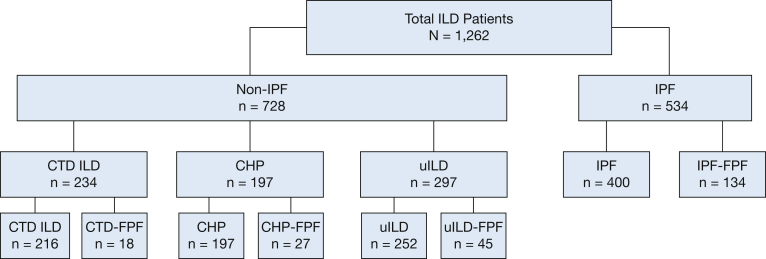
Of the 1,262 patients included in the analysis, 534 (42%) carried a diagnosis of IPF ILD and 728 (58%) carried a diagnosis of non-IPF ILD, including 234 (32%) with CTD-associated ILD, 197 (27%) with CHP, and 297 (41%) with uILD (Fig 1). The mean age ranged between 57 and 68 years. IPF patients were predominantly men and CTD-associated ILD patients were predominantly women, whereas CHP and uILD patients showed nearly equal sex ratios. Race for all subtypes predominantly was White, and roughly half of participants across all subtypes had a prior smoking history. Baseline lung function was similar across ILD subtypes (Table 1).
Figure 1.
Strengthening the Reporting of Observational Studies in Epidemiology diagram. CHP = chronic hypersensitivity pneumonitis; CTD = connective tissue disease; FPF = familial pulmonary fibrosis; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; uILD = unclassifiable ILD.
Table 1.
Baseline Characteristics Among ILD Subtypes
| Demographics | IPF (n = 534) | CTD-Associated ILD (n = 234) | CHP (n = 197) | uILD (n = 297) |
|---|---|---|---|---|
| Center | ||||
| UTSW | 377 (70.6) | 128 (54.7) | 112 (56.9) | 167 (56.2) |
| UCD | 157 (29.4) | 106 (45.3) | 85 (43.2) | 130 (43.8) |
| Age, y | 68.2 ± 10.1 | 57.0 ± 14.6 | 66.3 ± 10.7 | 65.8 ± 11.9 |
| Male sex | 397 (74.3) | 75 (32.1) | 106 (53.8) | 153 (51.5) |
| White race | 460 (86.1) | 143 (61.1) | 167 (84.8) | 232 (78.1) |
| Ever smoker | 342 (64.7) | 102 (43.6) | 89 (45.2) | 161 (54.2) |
| FVC, % predicted | 69.3 ± 18.5 | 68.9 ± 20.3 | 63.9 ± 20.2 | 68.7 ± 20.9 |
| Diffusion capacity, % predicted | 46.6 ± 18.3 | 45.9 ± 18.0 | 46.4 ± 18.8 | 47.3 ± 18.5 |
Data are presented as No. (%) or mean ± SD. CHP = chronic hypersensitivity pneumonitis; CTD = connective tissue disease; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; uILD = unclassifiable ILD; UCD = University of California at Davis; UTSW = University of Texas Southwestern.
A family history of ILD was reported in 134 probands with IPF (25.1%) and 90 probands with non-IPF ILD (12.4%) (Table 2), including 18 (7.7%) with CTD-associated ILD, 27 (13.7%) with CHP, and 45 (15.2%) with uILD. Among those with familial CTD-associated ILD, 11 of 18 (61%) had rheumatoid arthritis ILD. Most patients with FPF had only one other affected first-degree relative (n = 150 [67%]), whereas eight patients (3.6%) had only second-degree relatives who were affected (Table 2). When assessing baseline clinical characteristics between FPF cohorts, age, race, smoking history, and diffusing capacity of the lungs for carbon monoxide were similar (Table 3). A higher proportion of men characterized the familial IPF cohort and a higher proportion of women characterized the familial CTD-associated ILD cohort. Those in the familial CTD-associated ILD cohort showed a higher percent predicted FVC (Table 3) compared with other ILD subtypes. Compared with patients with sporadic disease, familial IPF patients were significantly younger with a lower proportion of men and smokers, whereas familial CTD-associated ILD patients were significantly older with a higher proportion of White people and smokers and higher percent predicted FVC (Table 3). Baseline characteristics were similar between patients with familial CHP and familial uILD and their sporadic counterparts.
Table 2.
Prevalence of ILD Family History Stratified by ILD Subtype
| Demographics | All Patients (N = 1,262) | IPF ILD (n = 534) | Non-IPF ILD (n = 728) |
|||
|---|---|---|---|---|---|---|
| Combined (n = 728) | CTD-Associated ILD (n = 234) | CHP (n = 197) | uILD (n = 297) | |||
| Family history of ILD | 224 (17.7) | 134 (25.1) | 90 (12.4) | 18 (7.7) | 27 (13.7) | 45 (15.2) |
| Affected first-degree relatives in FPF cohorts | ||||||
| 1 | 150 (67) | 81 (60.4) | 69 (76.7) | 16 (88.8) | 22 (81.5) | 31 (68.9) |
| 2 | 48 (21.4) | 34 (25.4) | 14 (15.6) | 0 | 4 (14.8) | 10 (22.2) |
| ≥ 3 | 18 (8) | 13 (9.7) | 5 (5.6) | 1 (5.6) | 1 (3.7) | 3 (6.7) |
| Affected second-degree relatives only | 8 (3.6) | 6 (4.5) | 2 (2.2) | 1 (5.6) | 0 | 1 (2.2) |
Data are presented as No. (%). CHP = chronic hypersensitivity pneumonitis; CTD = connective tissue disease; FPF = familial pulmonary fibrosis; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; uILD = unclassifiable ILD.
Table 3.
Comparison of Baseline Characteristics Between Familial and Sporadic ILD Subtypes
| Demographics | Familial IPF (n = 134) | Sporadic IPF (n =400) | Familial CTD-Associated ILD (n = 18) | Sporadic CTD-Associated ILD (n = 216) | Familial CHP (n = 27) | Sporadic CHP (n = 170) | Familial uILD (n = 45) | Sporadic uILD (n = 252) |
|---|---|---|---|---|---|---|---|---|
| Age, y | 64.6 ± 10.2a | 69.4 ± 9.7 | 67.5 ± 8.6a | 56.1 ± 14.7 | 64.9 ± 10.1 | 66.5 ± 10.8 | 63.2 ± 10.3 | 66.3 ± 12.1 |
| Male sex | 91 (67.9)a | 306 (76.5) | 5 (27.8) | 70 (32.4) | 11 (40.7) | 95 (55.9) | 22 (48.9) | 131 (52) |
| White race | 112 (83.6) | 348 (87) | 15 (83.3)a | 128 (59.3) | 21 (77.8) | 146 (85.9) | 36 (80.0) | 196 (77.8) |
| Ever smoker | 76 (56.7)a | 266 (67.3) | 12 (66.7)a | 90 (41.7) | 12 (44.4) | 77 (45.3) | 24 (53.3) | 137 (54.4) |
| FVC, % predicted | 68.9 ± 17.7 | 69.5 ± 18.7 | 80.7 ± 17.9a | 67.9 ± 20.2 | 61.9 ± 19.2 | 64.2 ± 20.4 | 71.3 ± 18.9 | 68.2 ± 21.2 |
| Diffusion capacity, % predicted | 48.5 ± 17.4 | 46 ± 18.6 | 52.7 ± 18.2 | 45.3 ± 17.9 | 46.5 ± 17.1 | 46.3 ± 19.1 | 50.9 ± 15.1 | 46.7 ± 19 |
Data are presented as No. (%) or mean ± SD. CHP = chronic hypersensitivity pneumonitis; CTD = connective tissue disease; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; uILD = unclassifiable ILD.
P < .05 compared with the sporadic form of the disease.
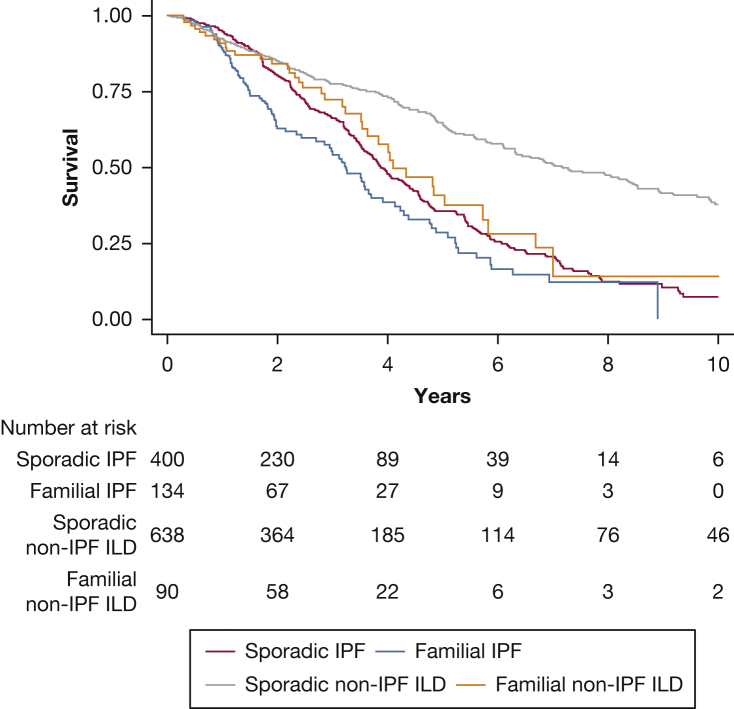
In outcome analysis, TFS was best among those with sporadic non-IPF ILD and worst among those with familial IPF ILD, with similar TFS in patients with sporadic IPF ILD and familial non-IPF ILD (Fig 2). When estimating outcome risk, those with familial IPF showed a 40% increased risk of death or lung transplantation compared with those with sporadic IPF, which increased to 80% after multivariate adjustment (hazard ratio [HR], 1.8; 95% CI, 1.37-2.37; P < .001) (Table 4). Among those with familial non-IPF ILD, outcome risk was nearly 80% higher when compared with those with sporadic non-IPF ILD, which increased to more than twofold after multivariate adjustment (HR, 2.08; 95% CI, 1.46-2.96; P < .001) (Table 4). Outcome risk among those with familial non-IPF ILD was no different than among those with sporadic IPF ILD (HR, 1.27; 95% CI, 0.89-1.84; P = .19).
Figure 2.
Line graph showing TFS for patients with IPF ILD and non-IPF ILD stratified by the presence of FPF. TFS was significantly worse for patients with familial IPF compared with those with sporadic IPF (P = .01, log-rank test) and familial non-IPF ILD compared with their counterparts with sporadic disease (P = .002, log-rank test). No difference was found in survival between patients with familial non-IPF ILD and sporadic IPF ILD (P = .42, log-rank test). FPF = familial pulmonary fibrosis; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; TFS = transplant-free survival.
Table 4.
Risk of Death or Lung Transplantation for Patients With Familial ILD When Compared With Their Sporadic Counterparts
| ILD Subtype | Unadjusted |
Adjusteda |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Familial IPF | 1.41 | 1.09-1.82 | .01 | 1.80 | 1.37-2.37 | < .001 |
| Familial non-IPF | 1.79 | 1.26-2.54 | .001 | 2.08 | 1.46-2.96 | < .001 |
| Familial CTDb | 2.63 | 1.11-6.23 | ... | ... | ... | ... |
| Familial CHPb | 1.84 | 1.00-3.40 | ... | ... | ... | ... |
| Familial uILDb | 1.19 | 0.73-1.96 | ... | ... | ... | ... |
CHP = chronic hypersensitivity pneumonitis; CTD = connective tissue disease; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; uILD = unclassifiable ILD.
Adjusted for center as a random effect and race and Gender, Age, Physiology stage as fixed effects.
Proportional hazards assumption violated graphically so nominal, unadjusted effect estimates are reported.
When assessing center-specific outcomes, similar effect size and direction were observed for IPF and non-IPF ILD. FPF was associated with a 34% increased risk of death or lung transplantation in those with IPF followed up at UTSW (HR, 1.34; 95% CI, 1.10-1.77) and 90% increased risk in those with IPF followed up at UCD (HR, 1.9; 95% CI, 0.94-3.38). In those with non-IPF ILD, FPF was associated with a 95% increased risk of death or lung transplantation in those followed up at UTSW (HR, 1.95; 95% CI, 1.30-2.92) and a 54% increased risk in those followed up at UCD (HR, 1.54; 95% CI, 0.73-3.23). Interaction testing showed no heterogeneity between centers with regard to FPF-associated outcome risk for patients with IPF ILD (P = .22 for interaction) and non-IPF ILD (P = .54 for interaction).
Sensitivity analysis performed for patients (n = 46) from UTSW with known pathologic variants in telomere-related genes showed similar results. Self-reported family history remained significantly associated with increased outcome risk for those with IPF ILD (HR, 1.49; 95% CI, 1.08-2.05) and non-IPF ILD (HR, 2.26; 95% CI, 1.45-3.52). Results also were similar when excluding patients with a known pathogenic mutation (data not shown). The number of affected first-degree relatives also did not affect mortality risk for IPF or non-IPF ILD with family history when modeled as ordinal or categorical variables (data not shown). When assessing non-IPF ILD subgroups, FPF was associated with an increased nominal risk of death or transplantation in each group (Table 4). The proportional hazards assumption was not met for some non-IPF ILD subgroups depending on the model used, so nominal effect estimates are reported without formal inference testing.
Discussion
In this multicenter study, we showed that a self-reported family history of pulmonary fibrosis predicts reduced TFS for patients with IPF and non-IPF ILD. Importantly, although a positive family history is present less frequently in patients with non-IPF forms of ILD, when it is identified, the patients have a risk of TFS similar to that associated with sporadic IPF. Although accurately classifying ILD subtype has important implications for disease management, this finding argues that the presence of family history of ILD is more predictive of prognosis than ILD classification and that patients with FPF are uniquely at risk for more progressive disease. Taken together, these data suggest that the FPF designation may be a useful prognostic modifier to the current ILD diagnostic schema.
Similar to prior studies, we demonstrated that familial IPF patients account for approximately 25% of all IPF cases and exhibit earlier disease onset when compared with patients with sporadic disease.7,20 Although IPF is the most common phenotype of probands with FPF, a significant minority of other forms of ILD also may be identified as familial. Herein, we showed that the proportion of probands with family history of ILD is remarkably consistent across major subsets of non-IPF ILDs, including 15.2% of uILD, 13.7% of CHP, and 15.7% of rheumatoid arthritis-associated ILD, whereas only 2.9% of non-rheumatoid arthritis CTD-associated ILD patients have a family history of ILD. This is consistent with other studies that have demonstrated higher frequency the MUC5B rs3570590 single nucleotide polymorphism,27, 28, 29 telomere-related gene mutations,21,30,31 and short age-adjusted telomere length27,29,32 in uILD, CHP, and rheumatoid arthritis-associated ILD. Although prior studies assessing outcomes in FPF have been mixed,22,23 our data support prior work by Krauss and colleagues22 showing that the presence of family history alone predicts worse survival. This effect seemed to be strong in the CTD-associated ILD group, in which family history of ILD was associated with a more than twofold increased risk of death or transplantation, despite higher percent predicted FVC at baseline. This association could not be explored further, however, given the small number of patients and violation of the proportional hazards assumption. The familial CTD-associated ILD group is older and has higher percentage of smokers, which may have affected the nominal risk estimate for this group, because both variables previously were associated with increased mortality in some CTD-associated ILDs.33, 34, 35 Patients with familial CHP and familial uILD have similar demographics and lung function compared with their counterparts with sporadic disease, so these factors likely did not influence our nominal outcome risk estimates.
Over the last few decades, our understanding of the complex genetic architecture underlying ILD has expanded greatly. We now recognize that pathogenic rare variants in genes involved in telomere maintenance and surfactant production,10, 11, 12, 13, 14, 15, 16, 17, 18, 19 common single nucleotide polymorphisms,36, 37, 38, 39, 40, 41 and short age-adjusted leukocyte telomere length42, 43, 44 can be inherited traits that contribute to familial and sporadic ILD development risk. Of these genomic markers, rare variants in TERT, TERC, PARN, and RTEL1 genes, common single nucleotide polymorphisms in the MUC5B (rs35705950) and TOLLIP (rs5743890) genes, and short age-adjusted leukocyte telomere length27,29,32,45,46 are associated with differential survival risk. Although these previously identified genetic and genomic markers predict disease susceptibility and clinical course, studies performed in patients for whom these markers have been assessed suggest that they are present in the minority of FPF patients. Our findings support that the broader group of FPF patients, not just those harboring specific genomic markers, are at risk for reduced survival, thus offering a potentially clinically relevant prognostic modifier to current ILD diagnostic categorization.
Despite our growing appreciation of genetic variation in ILD, the implementation of such information in the clinical setting remains largely unrealized. Research efforts are ongoing to discover additional genetic variants that explain the missing heritability of FPF, but it remains unclear how such discoveries should be implemented in clinical practice. Many challenges exist to widespread clinical genetic testing, including lack of access to genetic counselors, uncertainty in interpretation of genetic testing results, lack of infrastructure to expand testing to other affected and unaffected kindred, and potentially burdensome costs deferred to the patient. Unfortunately, no published practice recommendations exist for ILD patients indicating who to test, when to test, or how to test for genetic variants in patients with FPF. This leaves a significant vacuum in the field and inhibits the advancement of clinical genetic testing for FPF. However, our study suggests that a family history of ILD is clinically informative with regard to differential outcome risk. As opposed to genetic testing, acquiring an in-depth family history can be performed easily with low attendant costs or infrastructure disruptions through self-directed questionnaires, thoughtful discussions with providers, or both.
The results of the current study should inform designs of future investigations. Large-scale cohort studies and clinical trials must capture family history of ILD systematically as a relevant clinical data point. Although we demonstrated differential mortality risk associated with FPF in these two large cohorts, this requires external validation. Studies assessing outcome associations with known or novel genomic markers also should account for risk attributed to FPF to measure additive effects of the genetic markers themselves. This will be a necessary step to transition research genetic testing into provider-driven clinical testing. Additionally, preferential study of FPF patients may inform potential pharmacogenomic associations, thus potentially paving the way for an individualized approach to ILD management.
Our study has a number of limitations. First, family history of ILD was assessed by patient questionnaire and clinical interview, which is prone to recall bias. Second, we did not perform systematic genetic sequencing in the cohorts. Our focus was to define the predictive ability of a positive family history of ILD in the entire cohort, as opposed to limiting our analyses to the minority of patients who would be expected to harbor specific genetic variants. Given the ubiquitous nature of family history ascertainment, we believe that this approach improves the generalizability to the larger ILD community. We excluded known related family members from our data set to reduce potential double-counting of individuals with similar genomic background; however, the lack of sequencing data means that we could not account for kindred with cryptic relatedness. Third, we were unable to obtain longitudinal clinical information for all affected relatives. Instead, our study focused on the probands of FPF kindred to inform risk stratification for those who were treated at our ILD centers. Future studies of well-phenotyped kindred are needed to determine if longitudinal outcomes are similar within and across FPF relatives. Fourth, our data did not provide for assessment of the impact of treatment received by enrolled patients. Prior post hoc analyses and retrospective cohort studies suggest that outcome risk may be mediated by pharmacogenomic interactions,47,48 but confirmatory clinical trials are lacking. The enrollment time frame for the two ILD centers differed and represent changes in practice patterns that may influence outcome associations. The UTSW cohort spanned the eras before and after antifibrotics, whereas the UCD cohort spanned only the era after antifibrotics. However, family history of ILD remained significantly associated with poor outcomes in both cohorts with similar effect sizes. Finally, our institutions are large ILD referral centers, which may confound representation of the larger ILD population.
Conclusions
A family history of ILD is more common in patients with IPF, but is present across a wide variety of ILDs and predicts worse survival in patients with IPF and non-IPF forms of ILD. Although widespread clinical genetic testing may identify specific prognostic genetic markers, it has not been implemented widely for ILD patients. Our study argues for the systematic ascertainment of detailed family histories for all patients with ILD to inform prognosis and expected disease behavior.
Take-home Points.
Study Question: Does survival vary between interstitial lung disease (ILD) patients with and without self-reported family history of pulmonary fibrosis?
Results: Compared with their sporadic disease counterparts, patients with familial IPF showed an 80% increased risk of death or transplantation, and patients with familial non-IPF ILD showed a twofold increased risk of death.
Interpretation: Family history should be ascertained in all patients with ILD and early intervention considered for those with familial non-IPF ILD, as survival in this group approximates that of sporadic IPF.
Acknowledgments
Author contributions: J. M. O. is the guarantor of the content of the manuscript, including the data and analysis. J. M. O. and C. A. N. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. C. C. C., C. K. G., J. M. O., and C. A. N. were responsible for study design, interpretation of results, and the writing of the manuscript. W. S. B., N. D., and J. V. P. contributed to data collection and interpretation of results.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. M. O. reports consulting fees from Genentech and Boehringer Ingelheim unrelated to the submitted work. None declared (C. C. C., W. S. B., N. D., C. K. G., J. V. P.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: C. K. G. is supported by the National Institutes of Health [Grant R01HL093096]. W. S. B. is supported by the National Institutes of Health [Grant T32HL007013]. J. M. O. is supported by the National Heart, Lung, and Blood Institute [Grant K23HL138190]. C. A. N. is supported by the National Heart, Lung, and Blood Institute [Grant K23HL148498] and by the National Center for Advancing Translational Sciences [Grant UL1TR001105].
J. M. Oldham and C. A. Newton contributed equally to this manuscript.
References
- 1.Fischer A., du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380(9842):689–698. doi: 10.1016/S0140-6736(12)61079-4. [DOI] [PubMed] [Google Scholar]
- 2.Selman M., Pardo A., King T.E., Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186(4):314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 3.Lederer D.J., Martinez F.J. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 4.Neurohr C., Behr J. Changes in the current classification of IIP: a critical review. Respirology. 2015;20(5):699–704. doi: 10.1111/resp.12553. [DOI] [PubMed] [Google Scholar]
- 5.Bjoraker J.A., Ryu J.H., Edwin M.K. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157(1):199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 6.Oldham J.M., Adegunsoye A., Valenzi E. Characterisation of patients with interstitial pneumonia with autoimmune features. Eur Respir J. 2016;47(6):1767–1775. doi: 10.1183/13993003.01565-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Sancho C., Buendia-Roldan I., Fernandez-Plata M.R. Familial pulmonary fibrosis is the strongest risk factor for idiopathic pulmonary fibrosis. Respir Med. 2011;105(12):1902–1907. doi: 10.1016/j.rmed.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Hodgson U., Laitinen T., Tukiainen P. Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: evidence of founder effect among multiplex families in Finland. Thorax. 2002;57(4):338–342. doi: 10.1136/thorax.57.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loyd J.E. Pulmonary fibrosis in families. Am J Respir Cell Mol Biol. 2003;29(3 suppl):S47–S50. [PubMed] [Google Scholar]
- 10.Tsakiri K.D., Cronkhite J.T., Kuan P.J. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104(18):7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armanios M.Y., Chen J.J., Cogan J.D. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 12.Stuart B.D., Choi J., Zaidi S. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat Genet. 2015;47(5):512–517. doi: 10.1038/ng.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kropski J.A., Mitchell D.B., Markin C. A novel dyskerin (DKC1) mutation is associated with familial interstitial pneumonia. Chest. 2014;146(1):e1–e7. doi: 10.1378/chest.13-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cogan J.D., Kropski J.A., Zhao M. Rare variants in RTEL1 are associated with familial interstitial pneumonia. Am J Respir Crit Care Med. 2015;191(6):646–655. doi: 10.1164/rccm.201408-1510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannengiesser C., Borie R., Revy P. Pulmonary fibrosis associated with TINF2 gene mutation: is somatic reversion required? Eur Respir J. 2014;44(1):269–270. doi: 10.1183/09031936.00038714. [DOI] [PubMed] [Google Scholar]
- 16.Alder J.K., Stanley S.E., Wagner C.L., Hamilton M., Hanumanthu V.S., Armanios M. Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest. 2015;147(5):1361–1368. doi: 10.1378/chest.14-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Kuan P.J., Xing C. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84(1):52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogee L.M., Dunbar A.E., III, Wert S.E., Askin F., Hamvas A., Whitsett J.A. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344(8):573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 19.Campo I., Zorzetto M., Mariani F. A large kindred of pulmonary fibrosis associated with a novel ABCA3 gene variant. Respir Res. 2014;15:43. doi: 10.1186/1465-9921-15-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steele M.P., Speer M.C., Loyd J.E. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172(9):1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newton C.A., Batra K., Torrealba J. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur Respir J. 2016;48(6):1710–1720. doi: 10.1183/13993003.00308-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krauss E., Gehrken G., Drakopanagiotakis F. Clinical characteristics of patients with familial idiopathic pulmonary fibrosis (f-IPF) BMC Pulm Med. 2019;19(1):130. doi: 10.1186/s12890-019-0895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H.L., Ryu J.H., Wittmer M.H. Familial idiopathic pulmonary fibrosis: clinical features and outcome. Chest. 2005;127(6):2034–2041. doi: 10.1378/chest.127.6.2034. [DOI] [PubMed] [Google Scholar]
- 24.Todd J.L., Neely M.L., Kopetskie H. Risk factors for acute rejection in the first year after lung transplant. A multicenter study. Am J Respir Crit Care Med. 2020;202(4):576–585. doi: 10.1164/rccm.201910-1915OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin P.C. A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev. 2017;85(2):185–203. doi: 10.1111/insr.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ley B., Ryerson C.J., Vittinghoff E. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ley B., Newton C.A., Arnould I. The MUC5B promoter polymorphism and telomere length in patients with chronic hypersensitivity pneumonitis: an observational cohort-control study. Lancet Respir Med. 2017;5(8):639–647. doi: 10.1016/S2213-2600(17)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juge P.A., Lee J.S., Ebstein E. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med. 2018;379(23):2209–2219. doi: 10.1056/NEJMoa1801562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newton C.A., Oldham J.M., Ley B. Telomere length and genetic variant associations with interstitial lung disease progression and survival. Eur Respir J. 2019;53(4) doi: 10.1183/13993003.01641-2018. 1801641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley B., Torgerson D.G., Oldham J.M. Rare protein-altering telomere-related gene variants in patients with chronic hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2019;200(9):1154–1163. doi: 10.1164/rccm.201902-0360OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juge P.A., Borie R., Kannengiesser C. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J. 2017;49(5) doi: 10.1183/13993003.02314-2016. 1602314. [DOI] [PubMed] [Google Scholar]
- 32.Ley B., Liu S., Elicker B.M. Telomere length in patients with unclassifiable interstitial lung disease: a cohort study. Eur Respir J. 2020;56(2) doi: 10.1183/13993003.00268-2020. 2000268. [DOI] [PubMed] [Google Scholar]
- 33.Winstone T.A., Assayag D., Wilcox P.G. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest. 2014;146(2):422–436. doi: 10.1378/chest.13-2626. [DOI] [PubMed] [Google Scholar]
- 34.Solomon J.J., Chung J.H., Cosgrove G.P. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2016;47(2):588–596. doi: 10.1183/13993003.00357-2015. [DOI] [PubMed] [Google Scholar]
- 35.Olson A.L., Swigris J.J., Sprunger D.B. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183(3):372–378. doi: 10.1164/rccm.201004-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mushiroda T., Wattanapokayakit S., Takahashi A. A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J Med Genet. 2008;45(10):654–656. doi: 10.1136/jmg.2008.057356. [DOI] [PubMed] [Google Scholar]
- 37.Fingerlin T.E., Murphy E., Zhang W. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45(6):613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fingerlin T.E., Zhang W., Yang I.V. Genome-wide imputation study identifies novel HLA locus for pulmonary fibrosis and potential role for auto-immunity in fibrotic idiopathic interstitial pneumonia. BMC Genet. 2016;17(1):74. doi: 10.1186/s12863-016-0377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noth I., Zhang Y., Ma S.F. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1(4):309–317. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen R.J., Porte J., Braybrooke R. Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study. Lancet Respir Med. 2017;5(11):869–880. doi: 10.1016/S2213-2600(17)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen R.J., Guillen-Guio B., Oldham J.M. Genome-wide association study of susceptibility to idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;201(5):564–574. doi: 10.1164/rccm.201905-1017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diaz de Leon A., Cronkhite J.T., Katzenstein A.L. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS One. 2010;5(5) doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alder J.K., Cogan J.D., Brown A.F. Ancestral mutation in telomerase causes defects in repeat addition processivity and manifests as familial pulmonary fibrosis. PLoS Genet. 2011;7(3) doi: 10.1371/journal.pgen.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldman F., Bouarich R., Kulkarni S. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc Natl Acad Sci U S A. 2005;102(47):17119–17124. doi: 10.1073/pnas.0505318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuart B.D., Lee J.S., Kozlitina J. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med. 2014;2(7):557–565. doi: 10.1016/S2213-2600(14)70124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai J., Cai H., Li H. Association between telomere length and survival in patients with idiopathic pulmonary fibrosis. Respirology. 2015;20(6):947–952. doi: 10.1111/resp.12566. [DOI] [PubMed] [Google Scholar]
- 47.Oldham J.M., Ma S.F., Martinez F.J. TOLLIP, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2015;192(12):1475–1482. doi: 10.1164/rccm.201505-1010OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newton C.A., Zhang D., Oldham J.M. Telomere length and use of immunosuppressive medications in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;200(3):336–347. doi: 10.1164/rccm.201809-1646OC. [DOI] [PMC free article] [PubMed] [Google Scholar]