Abstract
In the past decades, numerous preclinical studies and several clinical trials have evidenced the feasibility of cell transplantation in treating heart diseases. Over the years, different delivery routes of cell therapy have emerged and broadened the width of the field. However, a common hurdle is shared by all current delivery routes: low cell retention. A myriad of studies confirm that cell retention plays a crucial role in the success of cell‐mediated cardiac repair. It is important for any delivery route to maintain donor cells in the recipient heart for enough time to not only proliferate by themselves, but also to send paracrine signals to surrounding damaged heart cells and repair them. In this review, we first undertake an in‐depth study of primary theories of cell loss, including low efficiency in cell injection, “washout” effects, and cell death, and then organize the literature from the past decade that focuses on cell transplantation to the heart using various cell delivery routes, including intracoronary injection, systemic intravenous injection, retrograde coronary venous injection, and intramyocardial injection. In addition to a recapitulation of these approaches, we also clearly evaluate their strengths and weaknesses. Furthermore, we conduct comparative research on the cell retention rate and functional outcomes of these delivery routes. Finally, we extend our discussion to state‐of‐the‐art bioengineering techniques that enhance cell retention, as well as alternative delivery routes, such as intrapericardial delivery. A combination of these novel strategies and more accurate assessment methods will help to address the hurdle of low cell retention and boost the efficacy of cell transplantation to the heart.
Keywords: cardiac repair, cell delivery, intracoronary, intramyocardial, intrapericardial, intravenous, retention rate
Subject Categories: Cell Therapy, Stem Cells, Translational Studies
Nonstandard Abbreviations and Acronyms
- CDC
cardiosphere‐derived cell
- CSC
cardiac stem/progenitor cell
- MSC
mesenchymal stromal/stem cell
- RCV
retrograde coronary venous
Although cell therapy has shown potential for treatment of heart diseases, 1 , 2 , 3 , 4 especially of myocardial infarction (MI), 3 , 5 one of the challenges still to overcome is poor engraftment of transplanted cells. 1 , 6 , 7 This problem may be explained by untargeted delivery routes and low cell retention at the injury sites after transplantation. 7 Cell retention and engraftment refer to the fraction of transplanted cells retained in the myocardium for a period of time (minutes to days). 6 , 7 While the therapeutic effect is premised on cell survival, 8 cell retention should be given more attention as a way to improve cell delivery fundamentally. Moreover, it has been confirmed by the literature that cell retention plays a crucial role in cardiac repair and regeneration. 8 It is important for any delivery route to maintain donor cells in the recipient heart for a long enough time for the cells to not only proliferate by themselves, but also to send paracrine signals to surrounding damaged heart cells 9 to repair them. Therefore, a higher retention rate is a prerequisite for the success of cell‐based heart regeneration.
Current Challenges to Cardiac Cell Therapy: Low Cell Retention and Engraftment
Notably, many preclinical studies and clinical trials have clearly demonstrated that the retention of transplanted cells in the heart by any current delivery method is low 7 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 (Table), especially in long‐term studies. Because the universal objective of all cell‐based therapies is for cells to act as a “drug” that treats the infarct area in the heart, it is preferable that they stay at the desired location for longer, rather than dying or being “washed out” before their death. In other words, above all other strategies to improve the efficacy of cell therapies, the first priority is to keep the cells at the desired location. However, regardless of delivery route, many have reported low cell retention in both animal studies and clinical trials. 7 , 10 , 11 , 12 Over the years, the reasons and factors influencing cell loss are explored by many hypotheses (Figure 1).
Table 1.
Literature Review of Reported Cell Retention and Related Functional Recovery
| Delivery Route | Species and Setting | Transplanted Cell Type | Cell Retention Rate, % | Time Point | Quantification Method | Functional Recovery | Reference |
|---|---|---|---|---|---|---|---|
| Intracoronary injection | Pig MI model | Bone marrow mononuclear cells | 1.0±0.8 | 1 h | Radiolabeling+PET‐CT | No data available | Mitsutake et al; 2017 13 |
| Pig MI model | Cardiac‐derived progenitor cells | 18 | 4 h | Radiolabeling+PET‐CT | ↑ LVEF | Crisostomo et al; 2019 14 | |
| Pig MI model | Mesenchymal stem cells | 13.74 | 4 h | Radiolabeling+nuclear imaging | No data available | Gathier et al; 2019 15 | |
| Pig MI model | Mesenchymal stem cells | 1.7±0.1 | 3 h | Luciferase/GFP labeling+bioluminescence/fluorescence microscopy | ↑ LVEF | Zlabinger et al; 2018 16 | |
| Pig MI model | Peripheral blood mononuclear cells | 2.6±0.3 | 1 h | Radiolabeling+γ‐emission counting | No data available | Hou et al; 2005 17 | |
| Pig I/R model | Cardiac stem/progenitor cells | 17.4±4.1 | 4 h | Radiolabeling+PET‐CT | No data available | Collantes et al; 2017 18 | |
| Patients with nonischemic dilated cardiomyopathy | CD34+ stem cell | 7.1±1.5 | 2 h | Radiolabeling+single‐photon emission CT | ↑ LVEF | Vrtovec et al; 2013 19 | |
| Patients with nonischemic dilated cardiomyopathy | CD34+ stem cell | 5.3±1.3 | 18 h | Radiolabeling+single‐photon emission CT | ↑ LVEF | Vrtovec et al; 2013 19 | |
| Patients with nonischemic dilated cardiomyopathy | CD34+ stem cell | 4.4±1.2 | 18 h | Radiolabeling+single‐photon emission CT | ↑ LVEF | Vrtovec et al; 2013 20 | |
| Patients with MI | Bone marrow mononuclear cells | 17.3±6.2 | 4 h | Radiolabeling+single‐photon emission CT | ↑ LVEF | Silva et al; 2009 21 | |
| Patients with MI | Bone marrow mononuclear cells | 10.6±6.1 | 24 h | Radiolabeling+single‐photon emission CT | ↑ LVEF | Silva et al; 2009 21 | |
| Patients with MI | CD34+ stem cell | 4.86 (1.7–7.6) | 1 h | Radiolabeling+single‐photon emission CT | No data available | Musialek et al; 2011 22 | |
| Systemic intravenous injection | Rat MI model | Mesenchymal stem cells | <1 | 1 d | Quantitative real‐time PCR | Not significant | Wang et al; 2011 23 |
| Rabbit MI model | Bone marrow multilineage‐differentiating stress enduring cells | 14.5±4.0 | 3 d | GFP Labeling+fluorescence microscopy | ↑ LVEF | Yamada et al; 2018 24 | |
| Pig I/R model | Embryonic endothelial progenitor cells | 0.5 | 1 h | Radiolabeling+single‐photon emission CT | Not significant | Kupatt et al; 2005 25 | |
| Retrograde coronary venous injection | Pig MI model | Mesenchymal stem cells | 2.89 | 4 h | Radiolabeling+nuclear imaging | No data available | Gathier et al; 2019 15 |
| Pig MI model | Peripheral blood mononuclear cells | 3.2±1 | 1 h | Radiolabeling+γ‐emission counting | No data available | Hou et al; 2005 17 | |
| Pig I/R model | Embryonic endothelial progenitor cells | 2.7 | 1 h | Radiolabeling+single‐photon emission CT | ↓ Infarct size | Kupatt et al; 2005 25 | |
| Patients with MI | Bone marrow mononuclear cells | 4.2±1.1 | 4 h | Radiolabeling+single‐photon emission CT | Not significant | Silva et al; 2009 21 | |
| Patients with MI | Bone marrow mononuclear cells | 3.2±0.3 | 24 h | Radiolabeling+single‐photon emission CT | Not significant | Silva et al; 2009 21 | |
| Intramyocardial injection (transendocardial) | Pig I/R model | Cardiac stem/progenitor cells | 13.4±3.4 | 4 h | Radiolabeling+PET‐CT | No data available | Collantes et al; 2017 18 |
| Pig MI model | Bone marrow mononuclear cells | 17.9±3.1 | 1 h | Radiolabeling+PET‐CT | No data available | Mitsutake et al; 2017 13 | |
| Pig MI model | Mesenchymal stem cells | 6.9±5.9 | 3 h | Luciferase/GFP labeling+bioluminescence/fluorescence microscopy | ↑ LVEF | Zlabinger et al; 2018 16 | |
| Patients with nonischemic dilated cardiomyopathy | CD34+ stem cell | 14±5 | 18 h | Radiolabeling+single‐photon emission CT | ↑ LVEF | Haddad et al; 2015 26 | |
| Patients with nonischemic dilated cardiomyopathy | CD34+ stem cell | 19.2±4.8 | 18 h | Radiolabeling+single‐photon emission CT | ↑ LVEF | Vrtovec et al; 2013 20 | |
| Intramyocardial injection (transepicardial) | Rat MI model | Cardiac‐derived stem cells | 17.6±11.5 | 1 h | Quantitative real‐time PCR | ↑ Fractional area change | Terrovitis et al; 2009 27 |
| Rat MI model | Cardiac‐derived stem cells | 17.8±7.3 | 1 h | Radiolabeling+PET | ↑ Fractional area change | Terrovitis et al; 2009 27 | |
| Pig MI model | Bone marrow mononuclear cells | 6.0±1.5 | 1 h | Radiolabeling+PET‐CT | No data available | Mitsutake et al; 2017 13 | |
| Pig MI model | Peripheral blood mononuclear cells | 11.3±3 | 1 h | Radiolabeling+γ‐emission counting | No data available | Hou et al; 2005 17 |
CT indicates computed tomography; GFP, green fluorescent protein; I/R, ischemia‐reperfusion; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCR, polymerase chain reaction; and PET, positron emission tomography.
Figure 1. Primary theories of low cell retention in the damaged heart.
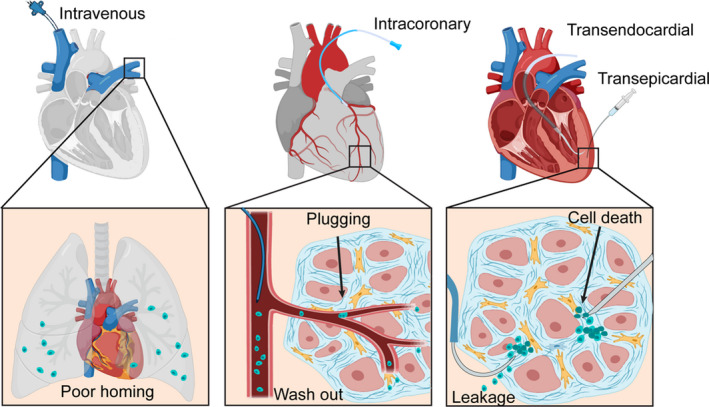
Factors Attributing to Cell Loss
Low Efficiency in Cell Injection
Early discussion of all cell delivery routes involves ensuring successful delivery of cells into the targeted site. Poor efficacy of transplantation can result not only in cell loss, but also in unpredictable cell distribution in vivo, which would be detrimental to the patient. This issue occurs with both systematic and local delivery of cells. Traditional systemic delivery of cells has been shown to be inefficient at delivering cells to the heart, and even causes adverse effects when the injected cells accumulate in other organs, such as the lungs. For local intracoronary injection, transplanted cells need to undergo transendothelial migration before they can reach the infarct zone. During their movement, plugging can happen as they pass through narrow capillaries, which have diameters similar to cells. Thus, those blocked cells become the barrier that prevents other cells from traveling from the coronary vessels to the parenchyma.
“Washout” Effects
The washout theory is highlighted in many local cell delivery routes, especially intramyocardial injection (either epicardial or endocardial). 28 , 29 During invasive surgery, needles or catheters can cause mechanical damage to cardiac tissue, opening blood vessels and leaving a needle track in the myocardium. As soon as cells are injected into the myocardium in fluid medium, it is likely that they will drain through those gaps on the damaged blood vessels, whose diameter is much larger than that of cell's. What is more, given the fact that the heart is a contractile pump and has automaticity, the beating of surrounding cardiomyocytes squeezes the narrow space in the myocardium and adds extra pressure to transplanted cells, increasing their drainage throughout the circulation system. 29 Besides blood flow, the cells can also be washed out of the heart by lymphatic drainage. Together with blood vessels, the heart also has lymphatics that consist of capillary plexuses that continuously drain subendocardial, myocardial, and subepicardial areas, followed by lymphatic collecting vessels that lead the lymph out of the heart. Similarly, lymphatics become the other “exit” that helps transplanted cells escape from the harsh microenvironment of the heart.
Cell Death
In addition to physical washout from the heart, transplanted cells are likely to die before making an influence on the injured cardiac tissue. 30 First, as a foreign biologic, they will be recognized and cleared by the immune response. Inflammatory cells, such as macrophages and neutrophils, have the ability to secrete several cytokines that are cytotoxic to the transplanted cells, leading to their apoptosis. When many cells die, a necrotic core is likely to form, secreting proinflammatory cytokines that can kill more healthy cells as a consequence. Second, the damaged cardiomyocytes in the infarct zone can release reactive oxygen species, which also results in transplanted cell apoptosis. What is more, the infarct area's microenvironment is deficient in extracellular matrix infrastructure and oxygen and nutrient levels. As a result, many transplanted cells die in these unfavorable living conditions.
Current Cell Delivery Routes to the Heart
Intracoronary Injection
Intracoronary injection, also called intracoronary infusion, is one of the most practical routes for cell delivery. 10 , 11 , 31 , 32 , 33 , 34 , 35 , 36 , 37 As a regional injection method, it can send cells directly to the culprit coronary artery. This method is well accepted clinically because of its minimally invasive nature, in which the occluded blood vessels are dilated by an inflated balloon. Cells are then delivered by a catheter that has been placed into the distal coronary bed near the ischemic area. However, the transendothelial migration of cells from the coronary artery to the myocardium cannot be controlled by the operators and, unfortunately, is often unsatisfying, which leads to low cell retention and poor engraftment of cells in the infarct zone. One of the crucial factors affecting cells' movement is the formation of clogs in narrow capillaries through which they try to pass. These capillaries have diameters that are smaller or similar to that of cells. Furthermore, because of high pressure and perfusion in the artery, transplanted cells that are in a liquid environment become unstable and easily washed out of the heart. On the basis of these challenges, the benefits of intracoronary injection are not concrete, despite some literature demonstrating a positive outcome.
The intracoronary injection method has been applied in both animal studies and clinical trials during the past decades. 6 , 13 , 15 , 16 , 17 , 18 , 19 , 38 , 39 Campbell et al developed an ex vivo Langendorff heart perfusion model in rats to assess donor cell retention after intracoronary injection. 40 The bone marrow mononuclear cells that they injected by intracoronary route had a 20.1% retention rate at 5 minutes postinjection, whereas bone marrow–derived mesenchymal stromal/stem cells (MSCs), a larger cell type with median diameter of 11.5 μm (versus 7.0 μm for bone marrow mononuclear cells), were retained with a >3‐fold enhanced rate of 77.5%. The relationship between cell size and retention performance was reemphasized by the finding that retention rate decreased to <10% when the size of the injected cells was 5 to 6 μm. These retention data agree with the theory of cell clogging in coronary capillaries, whose diameter is 5 to 10 μm in humans and smaller in rodents. In a porcine MI model, Zlabinger et al found that intracoronary injection of MSCs led to cell retention of 1.7±0.1% (P=0.041) 3 hours postinjection. 16 At 24‐hour and 7‐day follow‐up, the number of labeled MSCs decreased in the heart continuously and had unfavorable biodistribution in bone marrow and other organs, even at 1 week postinjection. This explained the low retention in the heart and demonstrated the escape of donor cells into the circulation system. Vrtovec et al investigated long‐term effects of intracoronary CD34+ cell transplantation in dilated cardiomyopathy in a 5‐year clinical trial. 19 At 2 hours posttransplantation, the average cell engraftment was 7.1±1.5%. At 18 hours posttransplantation, the retention of cells in the myocardium decreased to 5.3±1.3% (P<0.001), which agreed with previous studies. In their trial, they confirmed the importance of cell retention for the success of stem cell therapy and found that patients with poor cell homing did not significantly improve left ventricular function at any time point. In addition, they showed that better homing is associated with better host response to stem cells.
The futility in intracoronary injections was mentioned in other clinical trials as well. 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 de Silva et al applied intracoronary infusion of mononuclear cells from either bone marrow or granulocyte colony‐stimulating factor mobilized apheresis product to a swine model of MI. 41 After 6 weeks, they found that intracoronary‐delivered cells, compared with the placebo group, did not improve cardiac repair according to systolic function, adverse ventricular remodeling, infarct size, and perfusion parameters. Similarly, Fernández‐Avilés et al reported the results of the CAREMI (Safety and Efficacy Evaluation of Intracoronary Infusion of Allogeneic Human Cardiac Stem Cells in Patients With AMI) trial, in which they administered allogeneic cardiosphere‐derived cells (CDCs) in patients with ST‐segment–elevation MI. 42 Among 49 patients, their data showed no significant immunologic reactions and no differences in cardiac magnetic resonance imaging–based efficacy parameters within 12 months, which demonstrated the safety of intracoronary delivery route of CDCs but also raised doubts as to its efficacy. Similar modest outcomes were reported in the ALLSTAR (Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration) trial 43 and some others. 44 , 46 Jeyaraman et al conducted a meta‐analysis to explore the clinical data supporting the effectiveness of intracoronary‐administered autologous bone marrow‐derived mesenchymal stem/stromal cells in patients with ST‐segment–elevation MI. 45 Their analysis, which included 3356 patients in 42 randomized controlled trials, concluded that there was no detectable reduction in mortality, arrhythmia, or infarct size and no improvement in myocardial function attributable to BMSC treatment. These clinical lines of evidence questioned intracoronary injection to a large extent.
Intravenous Injection
The systemic delivery route is traditionally used for drug delivery and gene therapy for heart diseases. 49 , 50 Because of its advantage of being minimally invasive, it has also been introduced in the area of nanomedicine 51 , 52 , 53 and cell therapy. 10 , 11 , 31 , 32 , 33 , 34 , 35 , 36 , 37 Increasing evidence shows that the homing of stem cells to the injured site is a natural function of the body regulated by signals from the heart under MI. 54 , 55 Systemic delivery of cells is conducted by injection through a needle or catheter into the peripheral vein. The stem cells entering circulation will be recruited from the blood vessels where they are delivered to the infarcted zone in the heart where ischemia occurs. However, the efficacy of homing and engraftment into the heart of transplanted cells is limited. 7 , 55 , 56 One of the most important reasons cells do not reach the heart is that they are entrapped or occluded in other organs, such as the lungs or spleen, because of these organs' complex microvasculature and capillary system. Another factor is that the recruitment of donor cells to the heart is highly dependent on the signaling pathways started in the ischemic cardiac tissue. 54 Therefore, if signals decrease with time or are weakened before fulfilling their roles, the homing effect will be even more disappointing. To conclude, despite the simple and minimally invasive procedure, systemic intravenous injection is an indirect delivery route that needs more mechanism research to optimize.
It was reported by Wang et al that when MSCs were injected in a rat MI model, most of the cells were entrapped in the lung after 10 to 14 days and <1% were found in the heart after 4 hours. 23 They found that around 52% of the intravenous‐injected MSCs were entrapped in the lungs and, similarly, <1% were retained in the heart at 1 day postinjection. 23 On the other hand, intravenous injection can also successfully target the heart. In a recent study, Yamada et al used bone marrow multilineage‐differentiating stress enduring cells for heart repair by intravenous infusion. 24 After 3 days, they found that 14.5±4.0% of the injected cells were engrafted into the heart, mainly at the border area, which is unexpectedly higher than other studies delivering cells to the heart by intravenous injection. And more surprisingly, those retained cells were proliferating in the heart and showed higher amount in the left ventricle at 2 weeks and as long as 2 months. This study implies that the type of transplanted cell and the homing mechanism play important roles in the success of intravenous injection, as homing is crucial to functional repair. Notably, because homing signals can decrease rapidly, systemic delivery is only effective during a narrow window. Thus, the benefits of this delivery route on treating cases of chronic myocardial ischemia or cardiomyopathies remain unclear because of the lack of homing mechanisms. 57 On the other hand, thanks to its noninvasive nature, repeated administration is possible. It has been reported that repeated intraventricular infusion has cumulative beneficial effects on cardiac function, although it does not increase cell engraftment significantly. 57 , 58 , 59 If repeated systemic intravenous injections can be optimized in future studies and proven to be safe and effective, this delivery route will be more promising for cardiac cell therapy.
Retrograde Coronary Venous Injection
Unlike the regular antegrade intracoronary injection, there is another retrograde intracoronary injection that delivers cells into circulation. 10 , 11 , 31 , 32 , 33 , 34 , 35 , 36 , 37 In this method, a catheter is passed percutaneously (eg, by the femoral vein), and will cannulate to the coronary sinus through the right atrium, reaching the target coronary vein. Cells are then injected during balloon occlusion of the distal coronary sinus to avoid rapid washout by the blood flow. Unfortunately, cell retention is still moderate in this injection route, although it shows higher clinical safety. 15 , 60 Because of the venous system's larger diameter and slower blood flow compared with the artery system, the retrograde coronary venous (RCV) injection provides a more feasible platform for cell delivery with minimal risk of occlusion and obstruction. In the past 20 years, there have been a few studies providing cell retention rates from RCV injection. 15 , 17 , 60 Most of the studies reported a retention rate of <10%, whereas Suzuki et al reported a remarkable result of >30%. 15 , 17 , 60 , 61 As a relatively safe delivery route, RCV injection has also been applied to clinical trials. Silva et al conducted a 30‐patient trial in which autologous bone marrow mononuclear cells were injected by RCV injection. 21 After 4 hours, cell retention was reported at 4.2±1.1% and decreased to 3.2±0.3% after 24 hours. However, a higher percentage of cell retention was found in patients with anterograde intracoronary artery injection compared with retrograde injection, which indicates that more studies are needed.
Intramyocardial Injection
As one of the most straightforward routes to deliver cells to the heart, intramyocardial injection has been popularly applied in preclinical animal study and clinical trials. 17 , 20 , 27 , 28 , 62 , 63 , 64 , 65 Depending on different techniques, intramyocardial injection can be performed on both sides of the myocardium, through either the epicardium or the endocardium. 10 , 11 , 31 , 32 , 33 , 34 , 35 , 36 , 37
Epicardial intramyocardial injection consists of puncturing the myocardium from the epicardium with a needle and injecting cells. This method requires open‐chest surgery and the exposure of the heart, which limits its clinical application. Despite this, this approach is still preferred in preclinical research using small or large animal models because of the simple equipment needed. 16 , 17 , 18 , 27 , 31 , 66 , 67 Also, it has been applied to deliver other cell‐derived therapeutic agents to the myocardium, such as exosomes, 68 and cell‐based therapeutics, such as cardiospheres. 69 What is more, compared with intracoronary or intravenous injection, the intramyocardial delivery route does not inject cells into the circulatory system but into the tissue directly, limiting the washout of cells by blood flow and thus enhancing cell retention.
Nevertheless, the mechanical damage that intramyocardial injection inflicts on the myocardium is inevitable and nonnegligible. Zhao et al reported that the injection sites of an aging mouse heart showed obvious fibrotic staining, likely caused by needle injury, indicating intramyocardial injection may be dangerous for aging mice. 70 Their results also showed that intramyocardial‐injected CDCs did not improve heart function and systemic performance in aging mice. In another study, van den Akker et al used real‐time dynamics to visualize intramyocardial cell injections. 28 The results show a massive, immediate washout of injected cells via venous drainage after 5 minutes. Therefore, better assessment and optimization of cell retention using intramyocardial injection is needed. One such study is by Terrovitis et al, where positron emission tomography (PET) was applied to accurately quantify CDC retention in the heart after intramyocardial injection. 27 At 1 hour postdelivery, the retention rate was reported at 17.6±11.5% by polymerase chain reaction (PCR) and 17.8±7.3% by PET. What is more, to increase the retention performance, they developed strategies, such as sealing injection sites with fibrin glue and lowering ventricular rate by using adenosine, and found that all these optimization methods enhanced cell engraftment to different extents and have the potential for clinical translation. Although there are many clinical studies reporting safety and therapeutic benefits of intramyocardial injection of different cell types, 71 , 72 , 73 few have evaluated the retention of donor cells, which is likely a key factor in cardiac repair.
Endocardial intramyocardial injection also consists of penetration of the myocardium, but from the inside of the ventricular wall, or the endocardium, instead. Because of this, a catheter is used instead of a needle. Similar to RCV injection, a long catheter is threaded through the peripheral blood vessels into the left ventricle, where the cells are injected through the endocardium. Generally, this approach is not as invasive as epicardial injection because of the use of an external guidance system and precise instruments. For this reason, it is more widely used in clinics, as there is no need for open‐chest surgery. Consequently, it requires more advanced medical systems and higher expenses.
The transendocardial injection method has also been studied in both animal models and clinical trials. 13 , 20 , 65 , 74 Mitsutake et al evaluated cell retention after 1 hour using the Helix transendocardial delivery system (BioCardia Inc). 13 Bone marrow mononuclear cells were delivered with a catheter via the percutaneous transendocardial route using the Helix system and were found to have a higher retention rate (17.9±3.1%) compared with transepicardial injection (6.0±1.5%). Similarly, Vrtovec et al compared transendocardial CD34+ cell transplantation with intracoronary injection in a clinical trial of patients with nonischemic dilated cardiomyopathy. 20 At 18 hours postprocedure, they found the cell retention was nearly 5‐fold higher in the transendocardial group (19.2±4.8%) than in the intracoronary group (4.4±1.2%; P<0.01), which, in turn, provided improvement of cardiac function. There are also many other trials that did not discuss retention but verified the feasibility and practicality of this relatively safe delivery route. 64 , 75 , 76
Cell Sheet Transplantation
In the past decade, cell sheet transplantation has been reported in clinical trials led by Okano and Sawa. First applied in 1993, this method has been continuously applied to deliver different cell types to treat heart diseases, and has been implemented in many preclinical studies. 34 , 77 , 78 , 79 , 80 , 81 , 82 Okano and colleagues developed a novel bioengineering technology to generate cell sheets using thermoresponsive culture dishes. 81 This unique dish is coated with a temperature‐responsive polymer that is hydrophilic at 37°C, but hydrophobic at <25°C, so that cells can attach to the bottom and grow normally at 37°C. When the temperature goes down to ≤25°C, cells will be detached spontaneously, forming a scaffold‐free cell sheet. The cell sheets can be placed onto the epicardial surface to target the infarct zone of the heart. Thus, this approach is praised for its minimally invasive transplantation to the heart as well as its higher cell retention when compared with intramyocardial, intracoronary, and intravenous injection, which is confirmed by many animal studies. 34 , 77 , 78 , 79 , 80 , 81 , 82
Intrapericardial Injection
Similarly, many researchers have focused on the development of another innovative cell delivery route: intrapericardial injection. The pericardium is a double‐wall sac that surrounds the heart and protects it from the intrathoracic environment. There is a space between the parietal and the visceral pericardium called the pericardial cavity, which contains pericardial fluid. The pericardial fluid, an ultrafiltrate of plasma (similar composition but with fewer proteins, triglycerides, and cholesterol), has been shown to be beneficial to cell growth and migration. 83 The original function of pericardial fluid, which is secreted by the serous membrane, is to decrease friction between the heart and adjacent tissues. What is more, the relatively larger volume in the pericardial cavity is believed to accommodate more donor cells compared with the dense tissue of myocardium. 84 Therefore, more cells can be injected, and the chance to have more cells retained after hours or days is higher. The pericardium acts as a “film” to cover the transplanted cells, supporting retention. Unlike intramyocardial injection, which needs to penetrate the myocardium and damage the blood vessels inside, intrapericardial injection reaches the pericardial cavity without damage to the epicardium layer, lowering risk to the patient and increasing feasibility for clinical applications. 83 , 84 , 85
Intrapericardial injection has attracted scientists' attention since the end of the last century and has been studied continuously over the past decades. 86 , 87 , 88 , 89 , 90 , 91 In fact, many preclinical studies have shown the feasibility of intrapericardial administration for gene delivery and other pharmacological agents, including fibroblast growth factor, L‐arginine, and omega‐3 fatty acids. 86 , 87 , 88 , 89 , 90 , 91 When it comes to cell therapy, however, few studies have been done. Blázquez et al used a swine model to evaluate the in vivo biodistribution of MSCs injected into the pericardial cavity. 83 They tracked the cells by magnetic resonance imaging, histological examination, and Y‐chromosome amplification. All of these methods showed a clear, large presence of MSCs in the pericardium, both ventricles (left and right), and both atria 7 days postinjection. Unfortunately, they did not provide the quantification data for the cell retention rate, so we are unable to compare with intrapericardial injection and other current delivery routes. What is more, Blázquez et al also transferred this method to CDC delivery and confirmed its safety and feasibility. 85
Comparative Studies on Different Cell Delivery Routes
Since the beginning of cell therapy for heart diseases, generations of scientists have been comparatively discussing delivery methods in their preclinical animal studies or clinical trials. 13 , 15 , 16 , 17 , 18 , 20 , 21 , 74 , 92 , 93 Even if the strengths and weaknesses of these methods are indisputable, attitudes toward them vary from one scientist to the next, and these attitudes continue to evolve as technology develops/advances.
RCV Route Versus Intracoronary Route
Because of RCV injection's similarities with intracoronary injection of delivering cells through blood vessels via a catheter, the 2 have often been compared (Figure 2A). Gathier et al compared the 2 in a porcine model by injecting MSCs into swine hearts via both methods and measuring cell retention after 4 hours. 15 Their results show that retention was significantly lower after RCV injection (median RCV injection versus intracoronary: 2.89% versus 13.74%; P=0.002). What is more, they also found that RCV injection can lead to development of pericardial fluid and hematomas on the front wall of the heart, which suggested low safety and efficacy.
Figure 2. A summary of cell retention study.
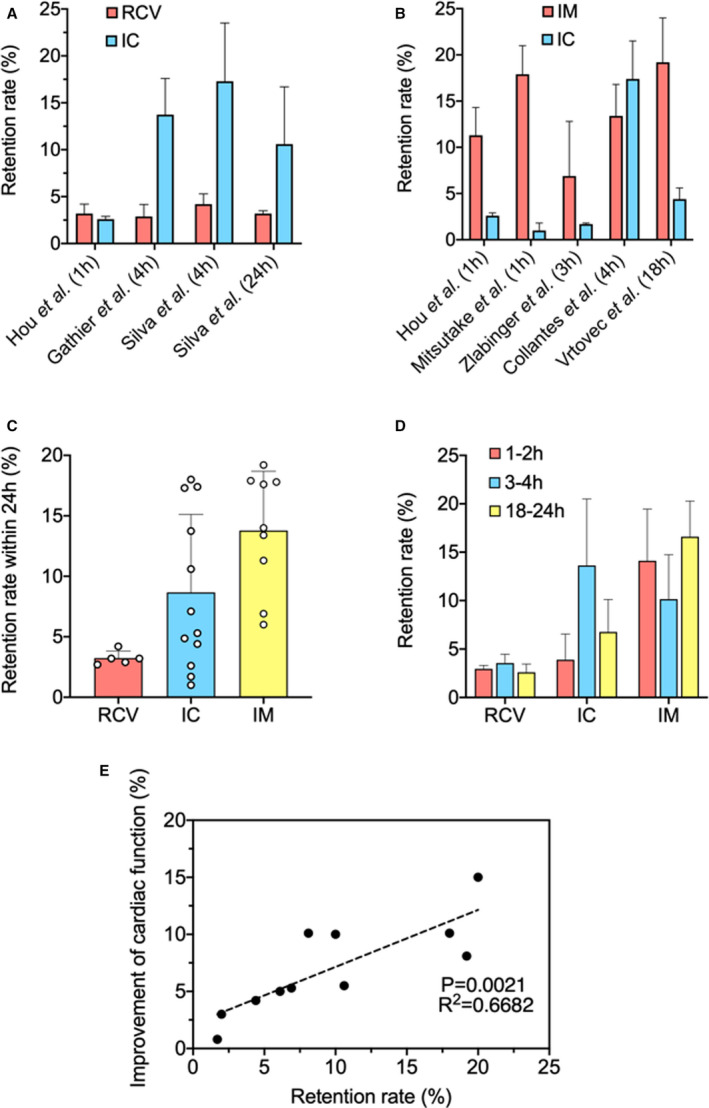
A, Representative comparative studies on retrograde coronary venous (RCV) injection and intracoronary (IC) injection. 15 , 17 , 21 B, Representative comparative studies on intramyocardial (IM) injection and IC injection. 13 , 16 , 17 , 18 , 20 C, Summarizing representative studies on RCV injection, IC injection, and IM injection based on short‐term retention. (All reported retention rate results herein were measured within 24 hours postinjection.) 13 , 14 , 15 , 17 , 18 , 19 , 21 , 22 , 23 , 24 , 25 , 26 , 27 D, Summarizing representative studies on RCV injection, IC injection, and IM injection based on specific time points. 13 , 14 , 15 , 17 , 18 , 19 , 21 , 22 , 23 , 24 , 25 , 26 , 27 E, Relationship between short‐term cell retention rate and long‐term improvement of cardiac function (represented by left ventricular ejection fraction) in representative studies. 14 , 16 , 20 , 21 , 94 , 95 , 96 , 97 All data are means±SD. Comparisons among groups were performed using 1‐way ANOVA, followed by post hoc Bonferroni test. The comparisons between samples are indicated by lines, and the statistical significance is indicated by asterisks above the lines.
Intramyocardial Route Versus Intracoronary Route
As the 2 most popular methods, there is no lack of comparison and discussion between the intracoronary route and intramyocardial route (Figure 2B). In a swine preclinical model of chronic ischemia‐reperfusion, Collantes et al used PET–computed tomography imaging to quantify cell retention rate 4 hours after intracoronary and intramyocardial injection of cardiac stem/progenitor cells (CSCs) and made a head‐to‐head comparison. 18 Interestingly, their results showed similar levels of short‐term cell retention in both intramyocardial (13.4±3.4%) and intracoronary (17.4±4.1%) injected groups. In their study, however, cell engraftment performance and retention were not correlated, as engraftment was improved by intramyocardial injection. At 3 days postinjection, engrafted CSCs were only detected in intramyocardial injection by histological evaluation. In a similar study that also used the swine model of reperfused MI, Zlabinger et al found the highest MSC retention after intramyocardial injection 3 hours postdelivery (6.9±5.9%) versus intracoronary injection (1.7±0.1%; P=0.041). 16 Even after 24 hours and 7 days, the retention in the intramyocardial group was still significantly higher than in the intracoronary group. They also studied the mechanism behind stem cell homing and found there was a reduced C‐X‐C chemokine receptor type 4 (CXCR‐4) expression level in the intracoronary injection group, which was in line with the retention data. In addition to the animal models, a comparative study was also conducted in clinical trials. In a trial with 40 patients, Vrtovec et al reported that at 18 hours postprocedure, the retention of CD34+ cells was higher in the transendocardial group (19.2±4.8%) than in the intracoronary group (4.4±1.2%; P<0.01), which was associated with the improvement of cardiac function. 20
Multiple Comparisons
There are also cases of comparisons between >2 delivery methods. Hou et al evaluated the retention of peripheral blood mononuclear cells after intramyocardial, intracoronary, and interstitial RCV delivery in a swine model. 17 At 1 hour postinjection, they quantified cell retention in the heart by γ‐emission counting in terms of radioactivity and found significantly more retained cells after intramyocardial injection (11.3±3%) than after intracoronary injection (2.6±0.3%; P<0.05). Interstitial RCV infusion showed lower retention than intramyocardial (3.2±1%), even though there was no statistical significance. Among the intramyocardial group, they also noted that delivery efficiency was less consistent than in the intracoronary and interstitial RCV groups, suggesting the uncertain and uncontrollable factors of intramyocardial injection, such as an inaccurate injection site and needle‐piercing depth. This concern is reasonable because the intramyocardial method does not use blood vessels in its delivery mechanism. What is more, Hou et al also investigated the topographic distribution of delivered cells in the heart. 17 Cells delivered by intramyocardial injection were primarily detected in the anterolateral left ventricular wall, whereas cells delivered by intracoronary injection were distributed predominantly in the anterolateral and apical regions of the left ventricle, with some on the right ventricle. In the interstitial RCV route, cells were mainly distributed in the heart base, atria, and right ventricle. This finding could give some insight as to the therapeutic effect of cells transplanted by different routes. To summarize, many preclinical and clinical studies using intramyocardial delivery of cells result in higher cell retention when compared with intracoronary or intravenous delivery. In addition, among intramyocardial injection methods, transendocardial delivery demonstrated better cell retention performance compared with epicardial delivery, which could be explained by the transmural heterogeneity hemodynamic and physical surface tension in epicardial injection with greater mechanical extrusion to the heart.
Inconsistency of Cell Retention Results and Its Relationship to Cardiac Function
Generally, we have found a higher overall retention rate in intramyocardial injections than in intracoronary injections and RCV injections, based on representative studies in the past decade (Figure 2C and 2D). Notably, when we make a systematic, comparative study of the literature, differences in animal models, cell types/dose, timing of delivery, and cardiac injury (eg, arterial ligation versus ischemic reperfusion) can lead to varying degrees of inconsistencies.
Unsurprisingly, the inherent differences between small animal models and humans might attribute to the disappointments of cardiac cell therapy in some clinical trials. Although murine models could provide us with valuable preclinical information and expectations, they are still not faithful enough to completely represent heart diseases in humans. Interestingly, however, inconsistent results were also found between studies of similar animal models. For example, in the study by Gathier et al, the retention of injected MSCs in a swine model was reported to be 13.74% by intracoronary infusion 4 hours postinjection. 15 Meanwhile, in a similar study that was conducted by Zlabinger et al in a swine model of MI, MSCs were also delivered via intracoronary infusion and cell retention at 3 hours postinjection was considerably lower at 1.7±0.1%. 16 The differences in retention rates between these studies could be caused by the delayed treatment by cell delivery following the MI. In Gathier's study, cells were administrated 4 weeks post‐MI, whereas in Zlabinger's study, administration was at 1 week post‐MI (8±2 days). A similar phenomenon was found with intramyocardial injection. In Zlabinger's study, cell retention after intramyocardial injection of a swine model was 6.9±5.9% at 3 hours postinjection. However, in Collantes' study, CSCs were injected under the same setting but with a delayed treatment (30 days after intramyocardial versus 8±2 days in Zlabinger's). The retention rate at 4 hours postinjection was 13.4±3.4%, which was 2‐fold higher than that of Zlabinger's. The improved therapeutic effect of delayed cell delivery post‐MI on cell retention is not accidental but reasonable. It could be explained by the signaling mechanism, which is critical to the successful homing of transplanted cells to the heart. During the process of transendothelial migration to the parenchyma in the myocardium, adhesion molecules are crucial to the communication and migration of transplanted cells, and these molecules have a continuously increasing level of expression following MI. Thus, we can hypothesize that, within certain limits, a relatively longer period of delayed treatment post‐MI could result in higher donor cell retention, while notably, not necessarily leading to better functional recovery. The latter can be explained by transmurality of the infarct, which plays an important role in determining the recovery of cardiac function after most therapies. The recovery of segmental contractile function is inversely correlated with the transmural extent of MI. 98 A relatively longer period of delayed treatment can lead to a greater transmural extent, finally resulting in poorer functional recovery. But in general, within certain limits, a higher cell retention rate is positively correlated to better cardiac function, which is suggested by the left ventricular ejection fraction, infarct size, and other evaluations, according to our review. Assessable data from representative studies were plotted in a graph to indicate a positive correlation between short‐term cell retention rate and long‐term improvement of cardiac function (left ventricular ejection fraction) (Figure 2E). 94 , 95 , 96 , 97
Methods to Quantify Cell Retention in the Heart
Another possible explanation of the inconsistencies between retention data of different studies is in the method for cell detection and quantification. 7 In many studies, including Gathier's and Collantes', measuring radioactivity of radiolabeled cells using PET/computed tomography techniques was the evaluation standard for cell retention rate. 15 , 18 All the measurements were obtained in vivo without isolating the heart from the body. In some studies, including Zlabinger's, cells were labeled with bioluminescence and/or fluorescence, such as green fluorescent protein, before injection and quantified by ex vivo visualization of the luminescent/fluorescent cells. 16 Other quantification methods include quantitative real‐time PCR, 7 which focuses on cell‐specific sequences as targets instead of exogenous labels (eg, radioactivity or fluorescence).
For bioluminescence and fluorescence imaging, quantification is based on subjective visualization instead of quantitative analysis, which can be inconsistent as fluorescent background complicates objectively drawing a boundary between positive and negative fluorescence. In addition, most of the imaging methods were based on 2‐dimensional instead of 3‐dimensional images of the whole heart, leading to incomplete evaluation of all tissues. On the basis of these drawbacks, quantification based solely on histology is less reliable when compared with PET, computed tomography, and quantitative real‐time PCR, which are considered to be more accurate for cell tracking. The quantification method could therefore explain the higher retention rates achieved by certain studies using less accurate methods. However, notably, among those relatively rigorous methods, the application of PCR is limited to xenotransplantation models or sex‐mismatched transplantation because of its dependence on cell‐specific sequences. 7 In the latter, male cells with a Y chromosome‐specific SRY gene are injected into females as donor cells. Because female cell detection is concluded based solely on the absence of an amplicon, PCR based on Y chromosome SRY gene is not considered to be completely reliable. 7 What is more, the frequency of the deletion of the Y copy of the amelogenin gene has been reported to occur between 0.018% and 8%, meaning that a deleted‐amelogenin male would inaccurately be identified as a female. 99
Compared with PCR, PET does not require invasive procedures for accurate detection, which is favorable in clinics. However, in some studies using PET for the diagnosis of tumors, it was noted that radioactivity could cause false positives and be “overaccurate.” In these cases, dead cells in the heart were detected and internalized with other living cells, which could explain the higher retention rates in studies using PET. To overcome these limitations, reporter genes have been introduced in combination with PET. 7 Briefly, reporter genes encoding membrane receptor or enzymes are genetically integrated to the donor cells' genome by lentivirus or retrovirus vectors. Once injected, the transgenic cells can exclusively uptake and accumulate a systemically injected radiotracer. Only viable cells can generate reporter signals because the uptake process requires protein synthesis and metabolic activity, which limits false positives. In addition, similar to other genetic labeling methods, daughter cells will keep the reporter genes after proliferation. However, the accuracy and stability of this method remain unclear because the signals are generated indirectly. Despite the limitations it might have, PET/computed tomography is still the best choice among common methods at present. Generally, in future technique development, the methods of combining genetic labeling and in vivo tracking need to be further developed to have rigorous and noninvasive assessment of cell retention in both preclinical studies and clinics.
Strategies to Enhance Cell Retention
To improve the retention performance of donor cells in the heart, generations of scientists have been focusing on bioengineering strategies. 100 , 101 , 102 Many studies have proven that cell signaling and cytokines play an important role in cell homing and engraftment. 54 Thus, focusing on the signaling mechanisms is an ideal strategy to enhance cell retention. Chen et al exploited cysteine‐arginine‐glutamic acid‐lysine‐alanine to modify MSCs and enhance fibrin‐mediated homing ability in a rat model. 103 They found that cysteine‐arginine‐glutamic acid‐lysine‐alanine significantly enhanced MSCs' binding ability to fibrin clots. Remarkably, cysteine‐arginine‐glutamic acid‐lysine‐alanine MSCs showed 6.5‐fold higher accumulation than unmodified MSCs at 1 day postadministration, resulting in better functional recovery of the injured heart. Besides homing of donor cells, recruitment of preexisting stem cells is important to cardiac repair. Tilokee et al demonstrated that paracrine engineering of human cardiac stem cells to overexpress stromal cell–derived factor 1α enhances recruitment of endogenous stem cells, promotes myocyte/vessel formation, and salvages reversibly damaged myocardium to enhance cardiac repair in a mouse model of MI. 104 On the other hand, decreasing cell death and enhancing cell survival in the myocardium by paracrine engineering can also positively change cell engraftment. Jackson et al transplanted explant‐derived cells overexpressing insulin‐like growth factor‐1 into immunodeficient mice with MI and found that long‐term engraftment of transplanted cells was boosted, whereas apoptosis and long‐term myocardial scarring were reduced. 105
Another optimization strategy to improve injected cell retention is dedicated to the development of auxiliary equipment or devices to aid the process of cell delivery. Tabei et al demonstrated a newly developed device for direct intramyocardial injection of human‐induced pluripotent stem cell–derived cardiomyocyte spheroids. 106 Their data showed that direct epicardial injection using this device resulted in better distribution and retention of transplanted spheroids in a layer within the myocardium than conventional needle‐based intramyocardial injection procedures. Previously, our laboratory has used US Food and Drug Administration–approved ferumoxytol to decorate stem cells or fabricate nanoparticles to attempt magnetic targeting in the body. 94 , 107 , 108 , 109 As the technique continuously improves, an external magnetic field could be placed near the injury site. 94 , 107 , 108 , 109 During the injection of iron‐labeled (ferumoxytol) stem cells, a magnetic field attracts them to the injured cardiac tissue, enhancing both target delivery and cell retention in the heart. However, the use of a strong magnetic field during an operative procedure may have unexpected consequences on the patient and equipment. The development of a more biosafe targeting strategy is needed. Maxwell et al investigated the ability of electrical stimulation to enhance the retention and therapeutic function of pediatric cardiac‐derived c‐kit+ progenitor cells in a rat model of right ventricular heart failure. 110 Both cellular retention and cardiac function were higher in their electrically stimulated c‐kit+ progenitor cells. What is more, in their mechanism study, they found that upregulation of β1 and β5 integrins contributed to the increased retention of electrically stimulated c‐kit+ progenitor cells. Their findings showed the potential of electrical stimulation to increase the retention, survival, and therapeutic effect of human c‐kit+ progenitor cells without introduction of extra devices into the heart.
As the most thriving strategy to alter transplanted cells' low retention, biomaterials have been innovated in the past decade to be protected from washout in the heart. 100 , 101 , 102 , 111 It has been confirmed in many in vitro/in vivo studies that polymeric materials could benefit cellular adhesion and proliferation, establishing a foundation for the use of biomaterials. 112 , 113 , 114 There are 2 main kinds of biomaterials for the application of heart repair: hydrogels and cardiac patches. Many injectable hydrogels have been designed with different combinations of base materials and cell types. 84 , 115 , 116 , 117 , 118 Many of the hydrogels are delivered to the heart through intramyocardial injection. 115 , 116 , 118 Previously, our laboratory demonstrated the safety and efficacy of encapsulating CSCs in thermosensitive poly(N‐isopropylacrylamine‐co‐acrylic acid) or P(NIPAM‐AA) nanogel in mouse and swine models of MI. 118 In a recent study, we created a hydrophilic and negatively charged microenvironment by poly(N‐isopropylacrylamide‐co‐itaconic acid), which is favorable for maintaining high viability of CSCs. 115 The results revealed that hydrogel‐encapsulated CSCs promote cardiac repair through angiogenesis and inhibition of apoptosis with an improved cell retention rate. Another revolutionary biomaterial is the cardiac patch. 62 , 119 , 120 , 121 , 122 , 123 Delivering cells in a cardiac patch increases cell retention and fulfills many other functionalization aims. 62 , 119 , 120 , 121 , 122 , 123 With the assistance of cardiac patches, scientists have successfully developed dual stem cell therapy to treat MI. 62 Park et al delivered both cardiomyocytes derived from human‐induced pluripotent stem cells by intramyocardial injection and a human MSC‐loaded patch simultaneously to amplify cardiac repair in a rat MI model. 62 Their results showed a synergistic effect of 2 different cell types post‐MI. Epicardially implanted human MSC‐loaded patch created a complimentary microenvironment that enhanced vascular regeneration through prolonged secretion of beneficial paracrine factors. More important, the favorable niche created could improve retention, distribution, engraftment, and maturation of cardiomyocytes derived from human‐induced pluripotent stem cells, which ultimately improved heart function and restored the injured myocardium. Another cardiac patch example is from our group, which has reported a novel strategy for creating a vascularized cardiac patch using biomimetic microvessels in a fibrin gel spiked with human CSCs. 119 Our results show that the endothelialized biomimetic microvessels can mimic the natural architecture and function of capillaries and that the vascularized cardiac patch (biomimetic microvessel–CSC patch) has great regenerative potential, which was also confirmed in a pig model of MI. 120 Meanwhile, to address the hurdle of slow integration with host myocardium, our group engineered an innovative microneedle patch integrated with cardiac stromal cells using polymeric microneedles to create communication channels between host myocardium and therapeutic CSCs. 122 In a recent study, we fabricated an “off‐the‐shelf” cardiac patch made of a porcine extracellular matrix scaffold and synthetic CSCs, which are encapsulated secreted human cardiac cellular factors to support cardiac recovery in a rat model of MI, showing the translational potential of the cardiac patch. 121 Unfortunately, cardiac patch–based strategies typically require open‐chest surgery, which brings additional complications, such as pain. What is more, in transmural infarcts, solely placing a patch on the epicardium may not be sufficient to be therapeutic, especially when the research models are transited from small to large animals, which needs attention in future designs of cardiac patches. In contrast, hydrogels have demonstrated their capability to be injected via microinvasive operation, which explains the popularity of this biomaterial in both preclinical studies and clinical trials.
Beside increasing cell engraftment in the heart, biomaterials can also shift the paracrine activity of transplanted cells. In an immunodeficient mouse model of ischemic cardiomyopathy, Kanda et al found that changing the physical properties of nanoporous gel cocoons could not only lead to higher cell retention in the myocardium, but also prompt explant‐derived cardiac stem cells to produce greater amounts of cytokines, nanovesicles, and microRNAs that boosted therapeutic repair after injury. 124 Remarkably, in this study, they also noticed that treatment with 2% nanoporous gel cocooned cells had equivalent effects on myocardial function as the suspended cells, even though nanoporous gel enhanced both short‐ and long‐term cell engraftment. The authors hypothesized from this observation that once a cell dose threshold has been reached, no higher engraftment of cells would trigger further functional improvements. When the paracrine repertoire is limited, it is important to generate endogenous cardiac repair to boost therapeutic effects, rather than solely relying on indirect cardiac repair. Kanda et al also used a microfluidic platform to dissect the impact of cocoon size and intracapsular cell number on the regenerative potential of transplanted heart explant–derived cells in the mouse model of ischemic cardiomyopathy. 125 They found that deterministic increases in cocoon size boosted the proportion of multicellular aggregates within cocoons, reduced vascular clearance of transplanted cells, and enhanced stimulation of endogenous repair.
Conclusions
To date, there have been important breakthroughs and advancements in cell delivery methods for heart disease therapeutics. However, none has been optimized to be universally accepted by researchers and clinicians. Despite continuous innovation, each method still has many advantages and disadvantages to be considered when designing a therapeutic approach. For example, intracoronary and intravenous injections are minimally invasive procedures but present the risk of blocking blood vessels, which can result in a second infarction, and the accelerated blood flow in which the cells are inserted results in higher cell washout. On the other hand, intramyocardial delivery routes have higher cell retention but require open‐chest surgery, which adds risk. This method also has relatively uncertain efficiency according to an overview of past preclinical and clinical studies. For the reasons highlighted in this review, innovative bioengineering techniques and alternative delivery routes need to be explored. Novel procedures, in combination with accurate assessment methods, should aim to overcome the existing limitations, especially that of low cell retention, which plays a crucial role in heart repair (Figure 3).
Figure 3. A new era of cell retention study combining novel delivery strategies and accurate assessment methods.
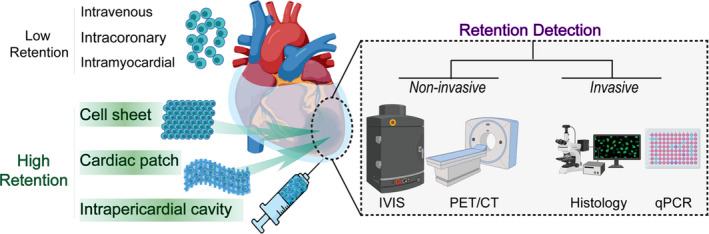
CT indicates computed tomography; IVIS, in vivo imaging system; PET, positron emission tomography; and qPCR, quantitative real‐time polymerase chain reaction.
Sources of Funding
This work was supported by grants from the National Institutes of Health (HL123920, HL137093, HL144002, HL146153, HL147357, and HL149940 to Dr Cheng) and the American Heart Association (18TPA34230092 and 19EIA34660286 to Dr Cheng).
Disclosures
None.
Acknowledgments
Author Contributions: Conception and design: all authors; administrative support: Dr Cheng; provision of study materials: Dr Cheng; collection and assembly of data: all authors; data analysis and interpretation: all authors; manuscript writing: all authors; final approval of manuscript: all authors. All authors have contributed to the writing of this article. All authors have approved the final version of this article. Final grammatical edits were completed by B. López de Juan Abad. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
(J Am Heart Assoc. 2021;10:e020402. DOI: 10.1161/JAHA.120.020402.)
For Sources of Funding and Disclosures, see pages 13 and 14.
References
- 1. Tompkins BA, Balkan W, Winkler J, Gyöngyösi M, Goliasch G, Fernández‐Avilés F, Hare JM. Preclinical studies of stem cell therapy for heart disease. Circ Res. 2018;122:1006–1020. DOI: 10.1161/CIRCRESAHA.117.312486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. DOI: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gyöngyösi M, Haller PM, Blake DJ, Martin RE. Meta‐analysis of cell therapy studies in heart failure and acute myocardial infarction. Circ Res. 2018;123:301–308. DOI: 10.1161/CIRCRESAHA.117.311302. [DOI] [PubMed] [Google Scholar]
- 4. Murry CE, MacLellan WR. Stem cells and the heart—the road ahead. Science. 2020;367:854–855. DOI: 10.1126/science.aaz3650. [DOI] [PubMed] [Google Scholar]
- 5. Tang J, Cores J, Huang K, Cui X, Luo L, Zhang J, Li T, Qian L, Cheng K. Concise review: is cardiac cell therapy dead? Embarrassing trial outcomes and new directions for the future. Stem Cells Transl Med. 2018;7:354–359. DOI: 10.1002/sctm.17-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnston PV, Sasano T, Mills K, Evers R, Lee S‐T, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere‐derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. DOI: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Terrovitis JV, Smith RR, Marbán E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–494. DOI: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu J, Narsinh KH, Lan F, Wang LI, Nguyen PK, Hu S, Lee A, Han L, Gong Y, Huang M, et al. Early stem cell engraftment predicts late cardiac functional recovery: preclinical insights from molecular imaging. Circ Cardiovasc Imaging. 2012;5:481–490. DOI: 10.1161/CIRCIMAGING.111.969329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. DOI: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Z, Mikrani R, Zubair HM, Taleb A, Naveed M, Baig MMFA, Zhang Q, Li C, Habib M, Cui X, et al. Systemic and local delivery of mesenchymal stem cells for heart renovation: challenges and innovations. Eur J Pharmacol. 2020;876:173049. DOI: 10.1016/j.ejphar.2020.173049. [DOI] [PubMed] [Google Scholar]
- 11. Al Kindi A, Ge Y, Shum‐Tim D, Chiu RC. Cellular cardiomyoplasty: routes of cell delivery and retention. Front Biosci. 2008;13:2421–2434. DOI: 10.2741/2855. [DOI] [PubMed] [Google Scholar]
- 12. Menasché P. Cell therapy trials for heart regeneration—lessons learned and future directions. Nat Rev Cardiol. 2018;15:659–671. DOI: 10.1038/s41569-018-0013-0. [DOI] [PubMed] [Google Scholar]
- 13. Mitsutake Y, Pyun WB, Rouy D, Foo CWP, Stertzer SH, Altman P, Ikeno F. Improvement of local cell delivery using helix transendocardial delivery catheter in a porcine heart. Int Heart J. 2017;58:435–440. DOI: 10.1536/ihj.16-179. [DOI] [PubMed] [Google Scholar]
- 14. Crisostomo V, Baez C, Abad JL, Sanchez B, Alvarez V, Rosado R, Gómez‐Mauricio G, Gheysens O, Blanco‐Blazquez V, Blazquez R. Dose‐dependent improvement of cardiac function in a swine model of acute myocardial infarction after intracoronary administration of allogeneic heart‐derived cells. Stem Cell Res Ther. 2019;10:1–17. DOI: 10.1186/s13287-019-1237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gathier WA, van der Naald M, van Klarenbosch BR, Tuinenburg AE, Bemelmans JLM, Neef K, Sluijter JPG, van Slochteren FJ, Doevendans PA, Chamuleau SAJ. Lower retention after retrograde coronary venous infusion compared with intracoronary infusion of mesenchymal stromal cells in the infarcted porcine myocardium. BMJ Open Sci. 2019;3:e000006. DOI: 10.1136/bmjos-2018-000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zlabinger K, Lukovic D, Hemetsberger R, Gugerell A, Winkler J, Mandic L, Traxler D, Spannbauer A, Wolbank S, Zanoni G. Matrix metalloproteinase‐2 impairs homing of intracoronary delivered mesenchymal stem cells in a porcine reperfused myocardial infarction: comparison with intramyocardial cell delivery. Front Bioeng Biotechnol. 2018;6:35. DOI: 10.3389/fbioe.2018.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hou D, Youssef EA‐S, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150–I156. DOI: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 18. Collantes M, Pelacho B, García‐Velloso MJ, Gavira JJ, Abizanda G, Palacios I, Rodriguez‐Borlado L, Álvarez V, Prieto E, Ecay M. Non‐invasive in vivo imaging of cardiac stem/progenitor cell biodistribution and retention after intracoronary and intramyocardial delivery in a swine model of chronic ischemia reperfusion injury. J Transl Med. 2017;15:1–11. DOI: 10.1186/s12967-017-1157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre‐Amione G, Haddad F. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5‐year follow‐up. Circ Res. 2013;112:165–173. DOI: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- 20. Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, Cernelc P, Torre‐Amione G, Haddad F, Wu JC. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128:S42–S49. DOI: 10.1161/CIRCULATIONAHA.112.000230. [DOI] [PubMed] [Google Scholar]
- 21. Silva SA, Sousa ALS, Haddad AF, Azevedo JC, Soares VE, Peixoto CM, Soares AJS, Issa AFC, Felipe LRV, Branco RVC, et al. Autologous bone‐marrow mononuclear cell transplantation after acute myocardial infarction: comparison of two delivery techniques. Cell Transplant. 2009;18:343–352. DOI: 10.3727/096368909788534951. [DOI] [PubMed] [Google Scholar]
- 22. Musialek P, Tekieli L, Kostkiewicz M, Majka M, Szot W, Walter Z, Zebzda A, Pieniazek P, Kadzielski A, Banys RP, et al. Randomized transcoronary delivery of CD34+ cells with perfusion versus stop‐flow method in patients with recent myocardial infarction: early cardiac retention of 99m Tc‐labeled cells activity. J Nucl Cardiol. 2011;18:104–116. DOI: 10.1007/s12350-010-9326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang W, Jiang Q, Zhang H, Jin P, Yuan X, Wei Y, Hu S. Intravenous administration of bone marrow mesenchymal stromal cells is safe for the lung in a chronic myocardial infarction model. Regen Med. 2011;6:179–190. DOI: 10.2217/rme.10.104. [DOI] [PubMed] [Google Scholar]
- 24. Yamada Y, Wakao S, Kushida Y, Minatoguchi S, Mikami A, Higashi K, Baba S, Shigemoto T, Kuroda Y, Kanamori H, et al. S1P–S1PR2 axis mediates homing of muse cells into damaged heart for long‐lasting tissue repair and functional recovery after acute myocardial infarction. Circ Res. 2018;122:1069–1083. DOI: 10.1161/CIRCRESAHA.117.311648. [DOI] [PubMed] [Google Scholar]
- 25. Kupatt C, Hinkel R, Lamparter M, von Brühl M‐L, Pohl T, Horstkotte J, Beck H, Müller S, Delker S, Gildehaus F‐J. Retroinfusion of embryonic endothelial progenitor cells attenuates ischemia‐reperfusion injury in pigs: role of phosphatidylinositol 3‐kinase/AKT kinase. Circulation. 2005;112:I117–I122. DOI: 10.1161/CIRCULATIONAHA.104.524801. [DOI] [PubMed] [Google Scholar]
- 26. Haddad F, Sever M, Poglajen G, Lezaic L, Yang P, Maecker H, Davis M, Kuznetsova T, Wu JC, Vrtovec B. Immunologic network and response to intramyocardial CD34+ stem cell therapy in patients with dilated cardiomyopathy. J Card Fail. 2015;21:572–582. DOI: 10.1016/j.cardfail.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 27. Terrovitis J, Lautamäki R, Bonios M, Fox J, Engles JM, Yu J, Leppo MK, Pomper MG, Wahl RL, Seidel J, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac‐derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619–1626. DOI: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van den Akker F, Feyen DAM, van den Hoogen P, van Laake LW, van Eeuwijk ECM, Hoefer I, Pasterkamp G, Chamuleau SAJ, Grundeman PF, Doevendans PA. Intramyocardial stem cell injection: go (ne) with the flow. Eur Heart J. 2017;38:184–186. DOI: 10.1093/eurheartj/ehw056. [DOI] [PubMed] [Google Scholar]
- 29. Teng CJ, Luo J, Chiu RCJ, Shum‐Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg. 2006;132:628–632. DOI: 10.1016/j.jtcvs.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 30. Abdelwahid E, Kalvelyte A, Stulpinas A, De Carvalho KAT, Guarita‐Souza LC, Foldes G. Stem cell death and survival in heart regeneration and repair. Apoptosis. 2016;21:252–268. DOI: 10.1007/s10495-015-1203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanelidis AJ, Premer C, Lopez J, Balkan W, Hare JM. Route of delivery modulates the efficacy of mesenchymal stem cell therapy for myocardial infarction: a meta‐analysis of preclinical studies and clinical trials. Circ Res. 2017;120:1139–1150. DOI: 10.1161/CIRCRESAHA.116.309819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caplan H, Olson SD, Kumar A, George M, Prabhakara KS, Wenzel P, Bedi S, Toledano‐Furman NE, Triolo F, Kamhieh‐Milz J, et al. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front Immunol. 2019;10:1645. DOI: 10.3389/fimmu.2019.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cismaru A, Cismaru G. Optimal delivery strategy for stem cell therapy in patients with ischemic heart disease. In: Sharma R, ed. Stem Cells in Clinical Practice and Tissue Engineering. London, England: IntechOpen Ltd; 2018:225–243. [Google Scholar]
- 34. Fukushima S, Sawa Y, Suzuki K. Choice of cell‐delivery route for successful cell transplantation therapy for the heart. Future Cardiol. 2013;9:215–227. DOI: 10.2217/fca.12.85. [DOI] [PubMed] [Google Scholar]
- 35. Suzuki K. Cell delivery routes for cardiac stem cell therapy. In: Cardiac Regeneration and Repair. Cambridge: Woodhead Publishing; 2014:99–117. DOI: 10.1533/9780857096708.2.99. [DOI] [Google Scholar]
- 36. Campbell NG, Suzuki K. Cell delivery routes for stem cell therapy to the heart: current and future approaches. J Cardiovasc Transl Res. 2012;5:713–726. DOI: 10.1007/s12265-012-9378-3. [DOI] [PubMed] [Google Scholar]
- 37. Golpanian S, Schulman IH, Ebert RF, Heldman AW, DiFede DL, Yang PC, Wu JC, Bolli R, Perin EC, Moyé L, et al. Concise review: review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl Med. 2016;5:186–191. DOI: 10.5966/sctm.2015-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ishigami S, Ohtsuki S, Eitoku T, Ousaka D, Kondo M, Kurita Y, Hirai K, Fukushima Y, Baba K, Goto T, et al. Intracoronary cardiac progenitor cells in single ventricle physiology: the PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) randomized phase 2 trial. Circ Res. 2017;120:1162–1173. DOI: 10.1161/CIRCRESAHA.116.310253. [DOI] [PubMed] [Google Scholar]
- 39. Bolli R, Tang X‐L, Sanganalmath SK, Rimoldi O, Mosna F, Abdel‐Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–131. DOI: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Campbell NG, Kaneko M, Shintani Y, Narita T, Sawhney V, Coppen SR, Yashiro K, Mathur A, Suzuki K. Cell size critically determines initial retention of bone marrow mononuclear cells in the heart after intracoronary injection: evidence from a rat model. PLoS One. 2016;11:e0158232. DOI: 10.1371/journal.pone.0158232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Silva R, Raval AN, Hadi M, Gildea KM, Bonifacino AC, Yu Z‐X, Yau YY, Leitman SF, Bacharach SL, Donahue RE. Intracoronary infusion of autologous mononuclear cells from bone marrow or granulocyte colony‐stimulating factor‐mobilized apheresis product may not improve remodelling, contractile function, perfusion, or infarct size in a swine model of large myocardial. Eur Heart J. 2008;29:1772–1782. DOI: 10.1093/eurheartj/ehn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fernández‐Avilés F, Sanz‐Ruiz R, Bogaert J, Casado Plasencia A, Gilaberte I, Belmans A, Fernández‐Santos ME, Charron D, Mulet M, Yotti R, et al. Safety and efficacy of intracoronary infusion of allogeneic human cardiac stem cells in patients with ST‐segment elevation myocardial infarction and left ventricular dysfunction: a multicenter randomized, double‐blind, and placebo‐controlled clinical trial. Circ Res. 2018;123:579–589. DOI: 10.1161/CIRCRESAHA.118.312823. [DOI] [PubMed] [Google Scholar]
- 43. Makkar RR, Kereiakes DJ, Aguirre F, Kowalchuk G, Chakravarty T, Malliaras K, Francis GS, Povsic TJ, Schatz R, Traverse JH, et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): a randomized, placebo‐controlled, double‐blinded trial. Eur Heart J. 2020;41:3451–3458. DOI: 10.1093/eurheartj/ehaa541. [DOI] [PubMed] [Google Scholar]
- 44. Traverse JH, Henry TD, Pepine CJ, Willerson JT, Chugh A, Yang PC, Zhao DXM, Ellis SG, Forder JR, Perin EC, et al. TIME trial: effect of timing of stem cell delivery following ST‐elevation myocardial infarction on the recovery of global and regional left ventricular function: final 2‐year analysis. Circ Res. 2018;122:479–488. DOI: 10.1161/CIRCRESAHA.117.311466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jeyaraman MM, Rabbani R, Copstein L, Sulaiman W, Farshidfar F, Kashani HH, Qadar SMZ, Guan Q, Skidmore B, Kardami E, et al. Autologous bone marrow stem cell therapy in patients with ST‐elevation myocardial infarction: a systematic review and meta‐analysis. Can J Cardiol. 2017;33:1611–1623. DOI: 10.1016/j.cjca.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 46. Sürder D, Manka R, Lo Cicero V, Moccetti T, Rufibach K, Soncin S, Turchetto L, Radrizzani M, Astori G, Schwitter J, et al. Intracoronary injection of bone marrow–derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation. 2013;127:1968–1979. DOI: 10.1161/CIRCULATIONAHA.112.001035. [DOI] [PubMed] [Google Scholar]
- 47. Rafatian G, Davis DR. The frustration and futility of intracoronary stem cell therapy. Can J Cardiol. 2017;33:1510–1512. DOI: 10.1016/j.cjca.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 48. Tyler JM, Kereiakes DJ, Henry TD. No Risk, No Reward. Circ Res. 2018;123:521–523. DOI: 10.1161/circresaha.118.313593. [DOI] [PubMed] [Google Scholar]
- 49. Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out‐of‐hospital cardiac arrest: a randomized trial. JAMA. 2009;302:2222–2229. DOI: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 50. Zhu T, Zhou L, Mori S, Wang Z, McTiernan CF, Qiao C, Chen C, Wang DW, Li J, Xiao X. Sustained whole‐body functional rescue in congestive heart failure and muscular dystrophy hamsters by systemic gene transfer. Circulation. 2005;112:2650–2659. DOI: 10.1161/CIRCULATIONAHA.105.565598. [DOI] [PubMed] [Google Scholar]
- 51. Liang H, Huang KE, Su T, Li Z, Hu S, Dinh P‐U, Wrona EA, Shao C, Qiao LI, Vandergriff AC, et al. Mesenchymal stem cell/red blood cell‐inspired nanoparticle therapy in mice with carbon tetrachloride‐induced acute liver failure. ACS Nano. 2018;12:6536–6544. DOI: 10.1021/acsnano.8b00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Su T, Huang KE, Ma H, Liang H, Dinh P‐U, Chen J, Shen D, Allen TA, Qiao LI, Li Z, et al. Platelet‐inspired nanocells for targeted heart repair after ischemia/reperfusion injury. Adv Funct Mater. 2019;29:1803567. DOI: 10.1002/adfm.201803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJAM, Geertsma RE. Particle size‐dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. DOI: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 54. Taghavi S, George JC. Homing of stem cells to ischemic myocardium. Am J Transl Res. 2013;5:404. [PMC free article] [PubMed] [Google Scholar]
- 55. Sheikh AY, Lin S, Cao F, Cao Y, van der Bogt KEA, Chu P, Chang C, Contag CH, Robbins RC, Wu JC. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677–2684. DOI: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Luger D, Lipinski MJ, Westman PC, Glover DK, Dimastromatteo J, Frias JC, Albelda MT, Sikora S, Kharazi A, Vertelov G, et al. Intravenously delivered mesenchymal stem cells: systemic anti‐inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ Res. 2017;120:1598–1613. DOI: 10.1161/CIRCRESAHA.117.310599. [DOI] [PubMed] [Google Scholar]
- 57. Wysoczynki M, Khan A, Bolli R. New paradigms in cell therapy: repeated dosing, intravenous delivery, immunomodulatory actions, and new cell types. Circ Res. 2018;123:138–158. DOI: 10.1161/CIRCRESAHA.118.313251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tokita Y, Tang X‐L, Li Q, Wysoczynski M, Hong KU, Nakamura S, Wu W‐J, Xie W, Li D, Hunt G, et al. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ Res. 2016;119:635–651. DOI: 10.1161/CIRCRESAHA.116.308937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tang X, Nakamura S, Li Q, Wysoczynski M, Gumpert AM, Wu W, Hunt G, Stowers H, Ou Q, Bolli R. Repeated administrations of cardiac progenitor cells are superior to a single administration of an equivalent cumulative dose. J Am Heart Assoc. 2018;7:e007400. DOI: 10.1161/JAHA.117.007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gathier WA, van Ginkel DJ, van der Naald M, van Slochteren FJ, Doevendans PA, Chamuleau SAJ. Retrograde coronary venous infusion as a delivery strategy in regenerative cardiac therapy: an overview of preclinical and clinical data. J Cardiovasc Transl Res. 2018;11:173–181. DOI: 10.1007/s12265-018-9785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Suzuki K, Murtuza B, Fukushima S, Smolenski RT, Varela‐Carver A, Coppen SR, Yacoub MH. Targeted cell delivery into infarcted rat hearts by retrograde intracoronary infusion: distribution, dynamics, and influence on cardiac function. Circulation. 2004;110:II‐225–II‐230. DOI: 10.1161/01.CIR.0000138191.11580.e3. [DOI] [PubMed] [Google Scholar]
- 62. Park S‐J, Kim RY, Park B‐W, Lee S, Choi SW, Park J‐H, Choi JJ, Kim S‐W, Jang J, Cho D‐W. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat Commun. 2019;10:1–12. DOI: 10.1038/s41467-019-11091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ascheim DD, Gelijns AC, Goldstein D, Moye LA, Smedira N, Lee S, Klodell CT, Szady A, Parides MK, Jeffries NO, et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation. 2014;129:2287–2296. DOI: 10.1161/CIRCULATIONAHA.113.007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC‐HFT randomized trial. JAMA. 2014;311:62–73. DOI: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vrtovec B, Poglajen G, Sever M, Zemljic G, Frljak S, Cerar A, Cukjati M, Jaklic M, Cernelc P, Haddad F. Effects of repetitive transendocardial CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circ Res. 2018;123:389–396. DOI: 10.1161/CIRCRESAHA.117.312170. [DOI] [PubMed] [Google Scholar]
- 66. Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, et al. Allogeneic transplantation of iPS cell‐derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–391. DOI: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- 67. Huang P, Wang L, Li Q, Tian X, Xu J, Xu J, Xiong Y, Chen G, Qian H, Jin C. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells‐derived exosomes in acute myocardial infarction via up‐regulating long non‐coding RNA H19. Cardiovasc Res. 2020;116:353–367. DOI: 10.1093/cvr/cvz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Qiao LI, Hu S, Liu S, Zhang H, Ma H, Huang KE, Li Z, Su T, Vandergriff A, Tang J, et al. microRNA‐21‐5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J Clin Invest. 2019;129:2237–2250. DOI: 10.1172/JCI123135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shen D, Cheng K, Marbán E. Dose‐dependent functional benefit of human cardiosphere transplantation in mice with acute myocardial infarction. J Cell Mol Med. 2012;16:2112–2116. DOI: 10.1111/j.1582-4934.2011.01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhao Z‐A, Han X, Lei W, Li J, Yang Z, Wu J, Yao M, Lu X‐A, He L, Chen Y. Lack of cardiac improvement after cardiosphere‐derived cell transplantation in aging mouse hearts. Circ Res. 2018;123:e21–e31. DOI: 10.1161/CIRCRESAHA.118.313005. [DOI] [PubMed] [Google Scholar]
- 71. Yau TM, Pagani FD, Mancini DM, Chang HL, Lala A, Woo YJ, Acker MA, Selzman CH, Soltesz EG, Kern JA, et al. Intramyocardial injection of mesenchymal precursor cells and successful temporary weaning from left ventricular assist device support in patients with advanced heart failure: a randomized clinical trial. JAMA. 2019;321:1176–1186. DOI: 10.1001/jama.2019.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hendrikx M, Hensen K, Clijsters C, Jongen H, Koninckx R, Bijnens E, Ingels M, Jacobs A, Geukens R, Dendale P. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114:I‐101–I‐107. DOI: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 73. Yagyu T, Yasuda S, Nagaya N, Doi K, Nakatani T, Satomi K, Shimizu W, Kusano K, Anzai T, Noguchi T, et al. Long‐term results of intracardiac mesenchymal stem cell transplantation in patients with cardiomyopathy. Circ J. 2019;83:1590–1599. DOI: 10.1253/circj.CJ-18-1179. [DOI] [PubMed] [Google Scholar]
- 74. Suncion VY, Ghersin E, Fishman JE, Zambrano JP, Karantalis V, Mandel N, Nelson KH, Gerstenblith G, DiFede Velazquez DL, Breton E, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally? An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res. 2014;114:1292–1301. DOI: 10.1161/CIRCRESAHA.114.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DXM, Silva GV, Lai D, Thomas JD, Kronenberg MW, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS‐CCTRN trial. JAMA. 2012;307:1717–1726. DOI: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. DOI: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tano N, Narita T, Kaneko M, Ikebe C, Coppen SR, Campbell NG, Shiraishi M, Shintani Y, Suzuki K. Epicardial placement of mesenchymal stromal cell‐sheets for the treatment of ischemic cardiomyopathy; in vivo proof‐of‐concept study. Mol Ther. 2014;22:1864–1871. DOI: 10.1038/mt.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, Sakakida SK, Kondoh H, Aleshin AN, Shimizu T, et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–1341. DOI: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 79. Sekine H, Shimizu T, Dobashi I, Matsuura K, Hagiwara N, Takahashi M, Kobayashi E, Yamato M, Okano T. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng Part A. 2011;17:2973–2980. DOI: 10.1089/ten.tea.2010.0659. [DOI] [PubMed] [Google Scholar]
- 80. Kondoh H, Sawa Y, Miyagawa S, Sakakida‐Kitagawa S, Memon IA, Kawaguchi N, Matsuura N, Shimizu T, Okano T, Matsuda H. Longer preservation of cardiac performance by sheet‐shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovasc Res. 2006;69:466–475. DOI: 10.1016/j.cardiores.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 81. Imanishi Y, Miyagawa S, Maeda N, Fukushima S, Kitagawa‐Sakakida S, Daimon T, Hirata A, Shimizu T, Okano T, Shimomura I. Induced adipocyte cell‐sheet ameliorates cardiac dysfunction in a mouse myocardial infarction model: a novel drug delivery system for heart failure. Circulation. 2011;124:S10–S17. DOI: 10.1161/CIRCULATIONAHA.110.009993. [DOI] [PubMed] [Google Scholar]
- 82. Miyagawa S, Saito A, Sakaguchi T, Yoshikawa Y, Yamauchi T, Imanishi Y, Kawaguchi N, Teramoto N, Matsuura N, Iida H. Impaired myocardium regeneration with skeletal cell sheets—a preclinical trial for tissue‐engineered regeneration therapy. Transplantation. 2010;90:364–372. DOI: 10.1097/TP.0b013e3181e6f201. [DOI] [PubMed] [Google Scholar]
- 83. Blázquez R, Sánchez‐Margallo FM, Crisóstomo V, Báez C, Maestre J, García‐Lindo M, Usón A, Álvarez V, Casado JG. Intrapericardial administration of mesenchymal stem cells in a large animal model: a bio‐distribution analysis. PLoS One. 2015;10:e0122377. DOI: 10.1371/journal.pone.0122377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Garcia JR, Campbell PF, Kumar G, Langberg JJ, Cesar L, Wang L, García AJ, Levit RD. A minimally invasive, translational method to deliver hydrogels to the heart through the pericardial space. JACC Basic Transl Sci. 2017;2:601–609. DOI: 10.1016/j.jacbts.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Blázquez R, Sánchez‐Margallo FM, Crisóstomo V, Báez C, Maestre J, Álvarez V, Casado JG. Intrapericardial delivery of cardiosphere‐derived cells: an immunological study in a clinically relevant large animal model. PLoS One. 2016;11:e0149001. DOI: 10.1371/journal.pone.0149001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ladage D, Turnbull IC, Ishikawa K, Takewa Y, Rapti K, Morel C, Karakikes I, Hadri L, Müller‐Ehmsen J, Costa KD, et al. Delivery of gelfoam‐enabled cells and vectors into the pericardial space using a percutaneous approach in a porcine model. Gene Ther. 2011;18:979–985. DOI: 10.1038/gt.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Polizzotti BD, Arab S, Kühn B. Intrapericardial delivery of gelfoam enables the targeted delivery of periostin peptide after myocardial infarction by inducing fibrin clot formation. PLoS One. 2012;7:e36788. DOI: 10.1371/journal.pone.0036788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Laham RJ, Rezaee M, Post M, Novicki D, Sellke FW, Pearlman JD, Simons M, Hung D. Intrapericardial delivery of fibroblast growth factor‐2 induces neovascularization in a porcine model of chronic myocardial ischemia. J Pharmacol Exp Ther. 2000;292:795–802. [PubMed] [Google Scholar]
- 89. Kolettis TM, Kazakos N, Katsouras CS, Niokou D, Pappa L, Koulouras V, Stefanou P, Seferiadis C, Malamou‐Mitsi V, Michalis LK, et al. Intrapericardial drug delivery: pharmacologic properties and long‐term safety in swine. Int J Cardiol. 2005;99:415–421. DOI: 10.1016/j.ijcard.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 90. Li Z, Zhu D, Hui Q, Bi J, Yu B, Huang Z, Hu S, Wang Z, Caranasos T, Rossi J. Injection of ROS‐responsive hydrogel loaded with basic fibroblast growth factor into the pericardial cavity for heart repair. Adv Funct Mater. 2021:2004377. [Google Scholar]
- 91. Zhu D, Li Z, Huang K, Caranasos TG, Rossi JS, Cheng K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat Commun. 2021;12. DOI: 10.1038/s41467-021-21682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dinh P‐UC, Paudel D, Brochu H, Popowski KD, Gracieux MC, Cores J, Huang K, Hensley MT, Harrell E, Vandergriff AC. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat Commun. 2020;11:1–14. DOI: 10.1038/s41467-020-14344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu M, Lutz H, Zhu D, Huang K, Li Z, Dinh PC, Gao J, Zhang Y, Cheng K. Bispecific antibody inhalation therapy for redirecting stem cells from the lungs to repair heart injury. Adv Sci. 2021;8:2002127. DOI: 10.1002/advs.202002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cheng K, Malliaras K, Li T‐S, Sun B, Houde C, Galang G, Smith J, Matsushita N, Marbán E. Magnetic enhancement of cell retention, engraftment, and functional benefit after intracoronary delivery of cardiac‐derived stem cells in a rat model of ischemia/reperfusion. Cell Transplant. 2012;21:1121–1135. DOI: 10.3727/096368911X627381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Huang Z, Shen Y, Sun A, Huang G, Zhu H, Huang B, Xu J, Song Y, Pei N, Ma J, et al. Magnetic targeting enhances retrograde cell retention in a rat model of myocardial infarction. Stem Cell Res Ther. 2013;4:1–13. DOI: 10.1186/scrt360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mayfield AE, Tilokee EL, Latham N, McNeill B, Lam B‐K, Ruel M, Suuronen EJ, Courtman DW, Stewart DJ, Davis DR. The effect of encapsulation of cardiac stem cells within matrix‐enriched hydrogel capsules on cell survival, post‐ischemic cell retention and cardiac function. Biomaterials. 2014;35:133–142. DOI: 10.1016/j.biomaterials.2013.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shen Y, Liu X, Huang Z, Pei N, Xu J, Li Z, Wang Y, Qian J, Ge J. Comparison of magnetic intensities for mesenchymal stem cell targeting therapy on ischemic myocardial repair: high magnetic intensity improves cell retention but has no additional functional benefit. Cell Transplant. 2015;24:1981–1997. DOI: 10.3727/096368914X685302. [DOI] [PubMed] [Google Scholar]
- 98. Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long‐term improvement in contractile function. Circulation. 2001;104:1101–1107. DOI: 10.1161/hc3501.096798. [DOI] [PubMed] [Google Scholar]
- 99. Drobnič K. A new primer set in a SRY gene for sex identification. Int Congr Ser. 2006;1288:268–270. DOI: 10.1016/j.ics.2005.08.020. [DOI] [Google Scholar]
- 100. Li Z, Hu S, Cheng K. Chemical engineering of cell therapy for heart diseases. Acc Chem Res. 2019;52:1687–1696. DOI: 10.1021/acs.accounts.9b00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Huang K, Hu S, Cheng K. A new era of cardiac cell therapy: opportunities and challenges. Adv Healthc Mater. 2019;8:1801011. DOI: 10.1002/adhm.201801011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li J, Hu S, Cheng K. Engineering better stem cell therapies for treating heart diseases. Ann Transl Med. 2020;8:569. DOI: 10.21037/atm.2020.03.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chen J, Song Y, Huang Z, Zhang N, Xie X, Liu X, Yang H, Wang Q, Li M, Li Q, et al. Modification with CREKA improves cell retention in a rat model of myocardial ischemia reperfusion. Stem Cells. 2019;37:663–676. DOI: 10.1002/stem.2983. [DOI] [PubMed] [Google Scholar]
- 104. Tilokee EL, Latham N, Jackson R, Mayfield AE, Ye B, Mount S, Lam B‐K, Suuronen EJ, Ruel M, Stewart DJ, et al. Paracrine engineering of human explant‐derived cardiac stem cells to over‐express stromal‐cell derived factor 1α enhances myocardial repair. Stem Cells. 2016;34:1826–1835. DOI: 10.1002/stem.2373. [DOI] [PubMed] [Google Scholar]
- 105. Jackson R, Tilokee EL, Latham N, Mount S, Rafatian G, Strydhorst J, Ye B, Boodhwani M, Chan V, Ruel M, et al. Paracrine engineering of human cardiac stem cells with insulin‐like growth factor 1 enhances myocardial repair. J Am Heart Assoc. 2015;4:e002104. DOI: 10.1161/JAHA.115.002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tabei R, Kawaguchi S, Kanazawa H, Tohyama S, Hirano A, Handa N, Hishikawa S, Teratani T, Kunita S, Fukuda J, et al. Development of a transplant injection device for optimal distribution and retention of human induced pluripotent stem cell‐derived cardiomyocytes. J Heart Lung Transplant. 2019;38:203–214. DOI: 10.1016/j.healun.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 107. Cheng K, Li T‐S, Malliaras K, Davis D, Zhang Y, Marbán E. Magnetic targeting enhances engraftment and functional benefit of iron‐labeled cardiosphere‐derived cells in myocardial infarction. Circ Res. 2010;106:1570. DOI: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cheng K, Shen D, Hensley MT, Middleton R, Sun B, Liu W, De Couto G, Marbán E. Magnetic antibody‐linked nanomatchmakers for therapeutic cell targeting. Nat Commun. 2014;5:1–9. DOI: 10.1038/ncomms5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Vandergriff AC, Hensley TM, Henry ET, Shen D, Anthony S, Zhang J, Cheng K. Magnetic targeting of cardiosphere‐derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials. 2014;35:8528–8539. DOI: 10.1016/j.biomaterials.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 110. Maxwell JT, Trac D, Shen M, Brown ME, Davis ME, Chao MS, Supapannachart KJ, Zaladonis CA, Baker E, Li ML. Electrical stimulation of pediatric cardiac‐derived c‐kit+ progenitor cells improves retention and cardiac function in right ventricular heart failure. Stem Cells. 2019;37:1528–1541. DOI: 10.1002/stem.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Roche ET, Hastings CL, Lewin SA, Shvartsman DE, Brudno Y, Vasilyev NV, O'Brien FJ, Walsh CJ, Duffy GP, Mooney DJ. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials. 2014;35:6850–6858. DOI: 10.1016/j.biomaterials.2014.04.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci. 2011;2011:1–19. DOI: 10.1155/2011/290602. [DOI] [Google Scholar]
- 113. Cheng K, Kisaalita WS. Exploring cellular adhesion and differentiation in a micro‐/nano‐hybrid polymer scaffold. Biotechnol Prog. 2010;26:838–846. DOI: 10.1002/btpr.391. [DOI] [PubMed] [Google Scholar]
- 114. Fisher OZ, Khademhosseini A, Langer R, Peppas NA. Bioinspired materials for controlling stem cell fate. Acc Chem Res. 2010;43:419–428. DOI: 10.1021/ar900226q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cui X, Tang J, Hartanto Y, Zhang J, Bi J, Dai S, Qiao SZ, Cheng K, Zhang H. NIPAM‐based microgel microenvironment regulates the therapeutic function of cardiac stromal cells. ACS Appl Mater Interfaces. 2018;10:37783–37796. DOI: 10.1021/acsami.8b09757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cheng KE, Blusztajn A, Shen D, Li T‐S, Sun B, Galang G, Zarembinski TI, Prestwich GD, Marbán E, Smith RR, et al. Functional performance of human cardiosphere‐derived cells delivered in an in situ polymerizable hyaluronan‐gelatin hydrogel. Biomaterials. 2012;33:5317–5324. DOI: 10.1016/j.biomaterials.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ichihara Y, Kaneko M, Yamahara K, Koulouroudias M, Sato N, Uppal R, Yamazaki K, Saito S, Suzuki K. Self‐assembling peptide hydrogel enables instant epicardial coating of the heart with mesenchymal stromal cells for the treatment of heart failure. Biomaterials. 2018;154:12–23. DOI: 10.1016/j.biomaterials.2017.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tang J, Cui X, Caranasos TG, Hensley MT, Vandergriff AC, Hartanto Y, Shen D, Zhang H, Zhang J, Cheng K. Heart repair using nanogel‐encapsulated human cardiac stem cells in mice and pigs with myocardial infarction. ACS Nano. 2017;11:9738–9749. DOI: 10.1021/acsnano.7b01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Su T, Huang KE, Daniele MA, Hensley MT, Young AT, Tang J, Allen TA, Vandergriff AC, Erb PD, Ligler FS, et al. Cardiac stem cell patch integrated with microengineered blood vessels promotes cardiomyocyte proliferation and neovascularization after acute myocardial infarction. ACS Appl Mater Interfaces. 2018;10:33088–33096. DOI: 10.1021/acsami.8b13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Su T, Huang KE, Mathews KG, Scharf VF, Hu S, Li Z, Frame BN, Cores J, Dinh P‐U, Daniele MA, et al. Cardiac stromal cell patch integrated with engineered microvessels improves recovery from myocardial infarction in rats and pigs. ACS Biomater Sci Eng. 2020;6:6309–6320. DOI: 10.1021/acsbiomaterials.0c00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Huang KE, Ozpinar EW, Su T, Tang J, Shen D, Qiao LI, Hu S, Li Z, Liang H, Mathews K, et al. An off‐the‐shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci Transl Med. 2020;12:eaat9683. DOI: 10.1126/scitranslmed.aat9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tang J, Wang J, Huang KE, Ye Y, Su T, Qiao LI, Hensley MT, Caranasos TG, Zhang J, Gu Z, et al. Cardiac cell–integrated microneedle patch for treating myocardial infarction. Sci Adv. 2018;4:eaat9365. DOI: 10.1126/sciadv.aat9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tang J, Vandergriff A, Wang Z, Hensley MT, Cores J, Allen TA, Dinh P‐U, Zhang J, Caranasos TG, Cheng K. A regenerative cardiac patch formed by spray painting of biomaterials onto the heart. Tissue Eng Part C Methods. 2017;23:146–155. DOI: 10.1089/ten.tec.2016.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kanda P, Alarcon EI, Yeuchyk T, Parent S, de Kemp RA, Variola F, Courtman D, Stewart DJ, Davis DR. Deterministic encapsulation of human cardiac stem cells in variable composition nanoporous gel cocoons to enhance therapeutic repair of injured myocardium. ACS Nano. 2018;12:4338–4350. DOI: 10.1021/acsnano.7b08881. [DOI] [PubMed] [Google Scholar]
- 125. Kanda P, Benavente‐Babace A, Parent S, Connor M, Soucy N, Steeves A, Lu A, Cober ND, Courtman D, Variola F, et al. Deterministic paracrine repair of injured myocardium using microfluidic‐based cocooning of heart explant‐derived cells. Biomaterials. 2020;247:120010. DOI: 10.1016/j.biomaterials.2020.120010. [DOI] [PubMed] [Google Scholar]


