Abstract
Background
Influenza infection causes considerable morbidity and mortality in patients with cardiovascular disease. We assessed the effects of the influenza vaccine on mortality and cardiovascular outcomes in patients with cardiovascular disease.
Methods and Results
We searched PubMed, Embase, and the Cochrane Library through January 2020 for randomized controlled trials and observational studies assessing the effects of influenza vaccine on mortality and cardiovascular outcomes in patients with cardiovascular disease. Estimates were reported as random effects risk ratios (RRs) with 95% CIs. Analyses were stratified by study design into randomized controlled trials and observational studies. A total of 16 studies (n=237 058), including 4 randomized controlled trials (n=1667) and 12 observational studies (n=235 391), were identified. Participants' mean age was 69.2±7.01 years, 36.6% were women, 65.1% had hypertension, 31.1% had diabetes mellitus, and 23.4% were smokers. At a median follow‐up duration of 19.5 months, influenza vaccine was associated with a lower risk of all‐cause mortality (RR, 0.75; 95% CI, 0.60–0.93 [P=0.01]), cardiovascular mortality (RR, 0.82; 95% CI, 0.80–0.84 [P<0.001]), and major adverse cardiovascular events (RR, 0.87; 95% CI, 0.80–0.94 [P<0.001]) compared with control. The use of the influenza vaccine was not associated with a statistically significant reduction of myocardial infarction (RR, 0.73; 95% CI, 0.49–1.09 [P=0.12]) compared with control.
Conclusions
Data from both randomized controlled trials and observational studies support the use of the influenza vaccine in adults with cardiovascular disease to reduce mortality and cardiovascular events, as currently supported by clinical guidelines. Clinicians and health systems should continue to promote the influenza vaccine as part of comprehensive secondary prevention.
Keywords: cardiovascular disease, influenza vaccine, meta‐analysis, mortality
Subject Categories: Mortality/Survival
Nonstandard Abbreviations and Acronyms
- CDC
Centers for Disease Control and Prevention
- FLUCAD
Influenza Vaccination in Secondary Prevention From Coronary Ischemic Events in Coronary Artery Disease
- FLUVACS
Flu Vaccination in Acute Coronary Syndromes and Planned Percutaneous Coronary Interventions
- IAMI
Influenza Vaccination After Myocardial Infarction
- INVESTED
Influenza Vaccine to Effectively Stop Cardio Thoracic Events and Decompensated Heart Failure
- IVCAD
Efficacy of Influenza Vaccination in Reducing Cardiovascular Events in Patients With Coronary Artery Diseases
- IVVE
Influenza Vaccine in Patients With Heart Failure to Reduce Adverse Vascular Events
- MACE
major adverse cardiovascular events
Clinical Perspective
What Is New?
In a meta‐analysis of 4 randomized controlled trials and 12 observational studies, influenza vaccination was associated with 25% and 18% relative risk reduction in all‐cause and cardiovascular mortality, respectively, in patients with cardiovascular disease.
The mortality reduction was most likely driven in part by a 13% relative risk reduction in major adverse cardiovascular events.
What Are the Clinical Implications?
In the context of nearly half of individuals lacking routine influenza vaccination in the United States, this study reiterates the survival benefit and cardiovascular risk reduction achieved with the influenza vaccine in patients with cardiovascular disease.
These findings may help healthcare professionals and policymakers strongly advocate the influenza vaccination for secondary prevention of cardiovascular outcomes.
The US Centers for Disease Control and Prevention (CDC) estimated ≈39 to 56 million influenza illnesses and 24 000 to 62 000 influenza‐associated deaths during the year 2019 to 2020. 1 Adults with cardiovascular disease (CVD) are at notably higher risk of complications from influenza. Initial signals of the possible relationship between influenza and major adverse cardiovascular events (MACE) were noticed in the early 1900s after the influenza pandemic in Europe and the United States when the incidence of myocardial infarction (MI) and stroke peaked during the winter following respiratory infections. 2 This interrelation was later shown again in observational studies demonstrating a higher risk of cardiac events in patients with influenza infection. In an observational study published in the New England Journal of Medicine in 2018, the authors demonstrated a 6‐fold increased risk of MI within 7 days of confirmed influenza infection. 3 Similarly, in a population‐based study of adults hospitalized with influenza, almost 12% of patients had an acute cardiovascular event. 4
The American Heart Association/American College of Cardiology Guideline for Secondary Prevention for Patients With Coronary and Other Atherosclerotic Vascular Disease: 2006 Update recommends influenza vaccination in all patients with established coronary artery disease (class I, Level of Evidence B), 5 consistent with the CDC guidelines. 6 Even with the available evidence, influenza immunization rates remain low among individuals with CVD who reside in North America. 7 , 8 , 9 , 10 In a recent nationally representative sample of 19 793 patients with atherosclerotic CVD, 32.7% lacked influenza vaccination. 11 Several reasons were identified, including lack of systemic offering or awareness about the vaccine, lack of interest in vaccination, fears regarding the potential side effects of vaccination, and socioeconomic disparities among young or elderly individuals or ethnic/racial minorities limiting access to usual care. 9 , 11 Another survey of board‐certified cardiologists, endocrinologists, and pulmonologists found that cardiologists were least likely to stock influenza vaccine. 12 Among practitioners who did not stock the vaccine, the most common reason cited was the assumption that patients would receive the vaccine elsewhere.
Multiple studies have shown the potential for reduction in MACE with the influenza vaccine. 13 , 14 , 15 However, the degree of risk reduction has varied among trials and observational studies. 10 , 16 , 17 , 18 , 19 , 20 , 21 Therefore, we performed a systematic review and meta‐analysis aimed to investigate the effects of influenza vaccine on mortality and MACE in patients with CVD, stratified by study design.
Methods
Data Availability Statement
The authors declare that all supporting data are available within the article (and its online supplementary files).
Data Sources and Searches
This meta‐analysis was conducted in accordance with the Cochrane Collaboration guidelines and was reported following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses). 22 , 23 A comprehensive literature search was performed without language restriction using the electronic databases PubMed, Embase, Cochrane Library, and ClinicalTrials.gov through January 2020. Additional online sources included websites of major cardiovascular and medicine journals (https://www.nejm.org, https://www.thelancet.com, https://www.thelancet.com/, https://jamanetwork.com, https://academic.oup.com/eurheartj, www.onlinejacc.org, http://annals.org/aim, and https://www.ahajournals.org/journal/circ); bibliographies of relevant studies; and meta‐analyses. The search algorithm was: ((((((((("influenza vaccines"[MeSH Terms] OR ("influenza"[All Fields] AND "vaccines"[All Fields])) OR "influenza vaccines"[All Fields]) OR ("influenza"[All Fields] AND "vaccine"[All Fields])) OR "influenza vaccine"[All Fields]) AND (((("cardiovascular diseases"[MeSH Terms] OR ("cardiovascular"[All Fields] AND "diseases"[All Fields])) OR "cardiovascular diseases"[All Fields]) OR ("cardiovascular"[All Fields] AND "disease"[All Fields])) OR "cardiovascular disease"[All Fields])) OR ((("mortality"[MeSH Terms] OR "mortality"[All Fields]) OR "mortalities"[All Fields]) OR "mortality"[MeSH Subheading])) OR (("heart failure"[MeSH Terms] OR ("heart"[All Fields] AND "failure"[All Fields])) OR "heart failure"[All Fields])) OR (("heart failure"[MeSH Terms] OR ("heart"[All Fields] AND "failure"[All Fields])) OR "heart failure"[All Fields])) OR ((("stroke"[MeSH Terms] OR "stroke"[All Fields]) OR "strokes"[All Fields]) OR "stroke s"[All Fields])) OR (("myocardial infarction"[MeSH Terms] OR ("myocardial"[All Fields] AND "infarction"[All Fields])) OR "myocardial infarction"[All Fields]).
Study Selection
The prespecified inclusion criteria were as follows: (1) RCTs or observational studies comparing the efficacy of influenza vaccine; (2) studies must have at least 50% of patients with established CVD (atherosclerotic CVD or heart failure [HF]) 24 , 25 ; (3) studies must report mortality and cardiovascular outcomes of interest; and (4) a follow‐up duration of at least 12 months assessing vaccine effectiveness for each influenza season. There were no restrictions on sample size or language.
After removing the duplicates and following the selection criteria, we screened the remaining articles at the title and abstract level and then at the full‐text level. The process of study search and selection was performed independently by 2 investigators (S.T. and A.N.L.). Any conflicts were resolved by discussion, mutual consensus, referring to the original study, and the third investigator's opinion (S.U.K.).
Data Extraction, Outcomes, and Quality Assessment
Two investigators (S.T. and A.N.L.) independently abstracted the data using prespecified data collection forms, appraised the abstractions' accuracy, and resolved any discrepancies by consensus after discussion with a third investigator (S.U.K.). The data were abstracted on the studies' characteristics, crude point estimates, number of events, sample sizes, and follow‐up duration. Two unblinded investigators (S.T. and A.N.L.) independently appraised the potential risks of bias of the trials using the Cochrane risk‐of‐bias tool and observational studies using the Newcastle‐Ottawa scale at the study level (Figure S1 and Table S1, respectively). 26 , 27 The end points of interest were all‐cause mortality, cardiovascular mortality, MACE, MI, and HF.
Data Synthesis and Analysis
Outcomes were pooled using a generic invariance random‐effects model. The DerSimonian and Laird method was used for estimation of τ2. 28 Analyses were stratified according to study design: RCTs and observational studies. 26 For the meta‐analysis, the threshold of at least 2 studies per study design was deemed compulsory. We reported effect sizes as risk ratios (RRs) with 95% CIs. We used I 2 statistics to measure the extent of unexplained statistical heterogeneity: I 2>50% was considered a high degree of between‐study statistical heterogeneity. For subgroup analyses, we assumed a common among‐study variance component among subgroups (pool within‐group estimates of τ2). 29 Publication bias was assessed using Egger regression test. Sensitivity analyses were performed by the exclusion of data reported exclusively in abstracts because of the lack of confirmation in subsequent publication 18 (Table S2). For all analyses, statistical significance was set at <5%. Comprehensive Meta‐Analysis Version 3.0 (Biostat Inc) was used.
Results
Of 858 827 articles, 666 were assessed for eligibility after removing duplicates and screening at the title and abstract level; 650 articles were removed based on a priori study selection criteria. Ultimately, a total of 16 studies (n=237 058) encompassing 4 RCTs (n=1667) and 12 observational studies (n=235 391) were included (Figure 1). Participants' mean age was 69.2±7.01 years, 36.6% were women, 65.1% had hypertension, 31.1% had diabetes mellitus, and 23.4% were smokers. The median follow‐up duration was 19.5 months (interquartile range, 12–43.3 months). Baseline characteristics of studies are reported in the Table. Data were available on all end points; however, we refrained from reporting HF end points since only 1 trial reported this outcome (Figure 2). Figure S2 demonstrates the summary of the effect of the influenza vaccine on mortality and cardiovascular end points.
Figure 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines reporting study selection process.

Table 1.
Baseline Characteristics of the Included Studies
| Study/Author | Year | Group | No. | Age, y | Women, % | Hypertension, % | Diabetes Mellitus, % | Hyperlipidemia, % | Obesity, % | Smoker, % | Follow Up, mo |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RCTs | |||||||||||
| FLUVACS 17 | 2004 | Vaccine | 145 | 64.0 | 29.7 | 59.3 | 19.3 | … | … | 45.5 | 12 |
| No vaccine | 147 | 65.0 | 26.5 | 45.6 | 17.0 | … | … | 42.9 | |||
| FLUCAD 16 | 2008 | Vaccine | 325 | 58.8 | 28.9 | 69.7 | 19.8 | … | … | 20.7 | 12 |
| Placebo | 333 | 58.1 | 26.1 | 63.4 | 20.7 | … | … | 16.3 | |||
| IVCAD 18 | 2009 | Vaccine | 141 | 54.9 | 34.0 | 82.0 | … | 83.0 | … | … | 12 |
| Placebo | 137 | 54.5 | 33.0 | 84.0 | … | 90.0 | … | … | |||
| Phrommintikul et al 19 | 2011 | Vaccine | 221 | 65.0 | 39.0 | 63.1 | 29.0 | 44.3 | … | 13.7 | 12 |
| No Vaccine | 218 | 67.0 | 48.0 | 61.6 | 32.1 | 49.5 | … | 10.3 | |||
| Observational studies | |||||||||||
| de Diego et al 7 | 2009 | Vaccine | 860 | 76.7 | 52.0 | 66.7 | 32.1 | … | 24.0 | 6.5 | 40 |
| No vaccine | 480 | 75.5 | 53.8 | 60.6 | 32.7 | … | 17.7 | 8.1 | |||
| Liu et al 30 | 2012 | Vaccine | 2760 | 74.8 | 41.7 | 80.5 | 54.4 | 42.9 | … | … | 48 |
| No vaccine | 2288 | 75.7 | 48.2 | 76.2 | 53.2 | 38.8 | … | … | |||
| Wu et al 31 | 2014 | Vaccine | 2087 | 71.9 | 1.5 | … | … | … | … | … | 12 |
| No vaccine | 429 | 68.3 | 1.6 | … | … | … | … | … | |||
| Kopel et al 32 | 2014 | Vaccine | 501 | 75.8 | 44.0 | 73.0 | 45.0 | … | 23.0 | 32.0 | 48 |
| No vaccine | 1463 | 74.1 | 45.0 | 72.0 | 45.0 | … | 24.0 | 29.0 | |||
| Blaya‐Nováková et al 33 | 2016 | Vaccine | 1016 | 76.0 | 60.3 | 70.0 | 25.9 | 42.6 | 22.7 | … | 48 |
| No/partial vaccine | 1016 | 76.5 | 61.9 | 69.7 | 24.7 | 42.3 | 22.1 | … | |||
| Vardeny et al 10 | 2016 | Vaccine | 1769 | 67.9 | 19.8 | 68.9 | 40.9 | … | … | … | 27 |
| No vaccine | 6630 | 62.7 | 22.3 | 71.2 | 32.9 | … | … | … | |||
| Modin et al 34 | 2019 | Vaccine | 78 379 | 73.7 | 43.6 | 36.7 | 16.7 | … | … | … | 44.4 |
| No vaccine | 55 669 | 72.8 | 44.8 | 40.3 | 14.9 | … | … | … | |||
| Wu et al 20 | 2019 | Vaccine | 4350 | 76.3 | 35.1 | 90.1 | 53.7 | 57.6 | … | … | 12 |
| No vaccine | 4350 | 76.2 | 34.6 | 89.9 | 53.5 | 56.6 | … | … | |||
| Kaya et al 35 | 2016 | Vaccine | 265 | 60.0 | 28.3 | 35.1 | 24.0 | … | … | … | 15 |
| No vaccine | 391 | 63.0 | 27.6 | 34.3 | 21.0 | … | … | … | |||
| Jackson et al 36 | 2002 | Vaccine | 1016 | … | 34.9 | 49.3 | 24.1 | … | … | 23.3 | 27.6 |
| No vaccine | 362 | … | 27.3 | 42.8 | 17.1 | … | … | 41.4 | |||
| Lavallee et al 21 | 2014 | Vaccine | 5054 | 70.0 | 39.7 | 82.7 | 29.5 | 51.4 | … | 19.3 | 24 |
| No vaccine | 5054 | 69.9 | 40.0 | 82.9 | 29.9 | 51.6 | … | 19.5 | |||
| Mohseni et al 37 | 2017 | Vaccine | 59 202 | 74.7 | 49.9 | … | … | … | … | … | 12 |
| No vaccine | 74.7 | 49.9 | … | … | … | … | … | ||||
FLUCAD indicates Influenza Vaccination in Secondary Prevention From Coronary Ischemic Events in Coronary Artery Disease; FLUVACS, Flu Vaccination in Acute Coronary Syndromes and Planned Percutaneous Coronary Interventions; IVCAD, Efficacy of Influenza Vaccination in Reducing Cardiovascular Events in Patients With Coronary Artery Diseases; and RCTs, randomized control trials.
Figure 2. Effect of influenza vaccine on mortality and cardiovascular end points.
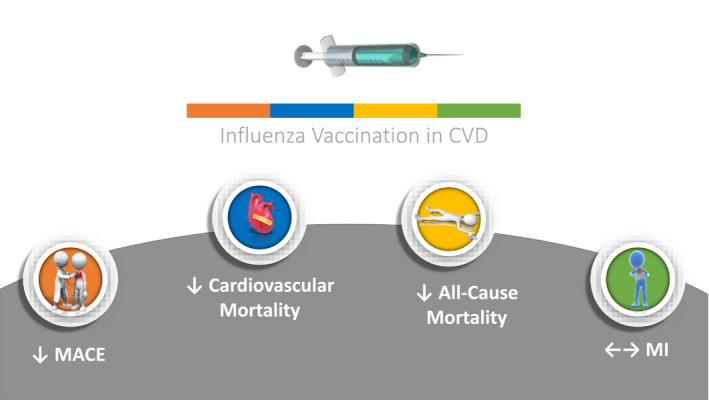
CVD indicates cardiovascular disease; MACE, major adverse cardiovascular events; and MI, myocardial infarction.
All‐Cause Mortality
Four RCTs (n=1667) and 8 observational studies (n=164 047) reported all‐cause mortality. 7 , 34 Overall, influenza vaccine was associated with reduction in all‐cause mortality compared with control (RR, 0.75; 95% CI, 0.60–0.93 [P=0.01]) (I 2=97%) (Figure 3). This benefit was consistent in RCTs (RR, 0.53; 95% CI, 0.28–0.99 [P=0.05]) and observational studies (RR, 0.79; 95% CI, 0.62–0.99 [P=0.04]) (P for interaction=0.24).
Figure 3. Effect of influenza vaccine on all‐cause mortality.
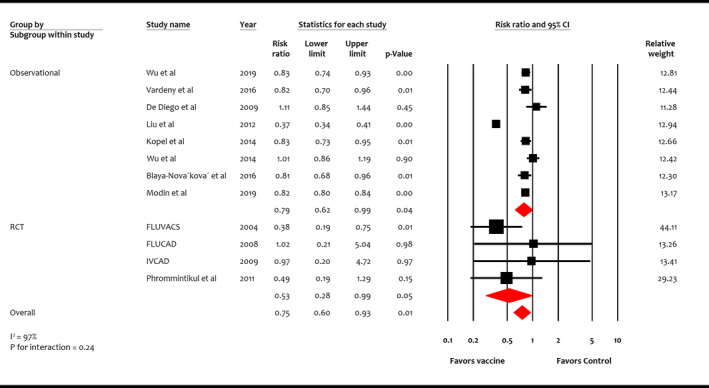
Studies included Wu et al, 20 Vardeny et al, 10 de Diego et al, 7 Liu et al, 30 Kopel et al, 32 Wu et al, 31 Blaya‐Nováková et al, 33 Modin et al, 34 FLUVACS (Flu Vaccination in Acute Coronary Syndromes and Planned Percutaneous Coronary Interventions), 17 FLUCAD (Influenza Vaccination in Secondary Prevention From Coronary Ischemic Events in Coronary Artery Disease), 16 IVCAD (Efficacy of Influenza Vaccination in Reducing Cardiovascular Events in Patients With Coronary Artery Diseases), 18 and Phrommintikul et al. 19 RCT indicates randomized control trial.
Cardiovascular Mortality
Four RCTs (n=1667) and 3 observational studies (n=136 082) reported cardiovascular mortality. 16 , 17 , 18 , 19 , 34 , 35 , 36 Overall, influenza vaccine was associated with reduction in cardiovascular mortality compared with control (RR, 0.82; 95% CI, 0.80–0.84 [P<0.001]) (I 2=31%) (Figure 4). This benefit was consistent among study designs but more pronounced in RCTs (RR, 0.44; 95% CI, 0.26–0.76 [P<0.001]) than observational studies (RR, 0.82; 95% CI, 0.80–0.84 [P<0.001]) (P for interaction=0.02).
Figure 4. Effect of influenza vaccine on cardiovascular mortality.
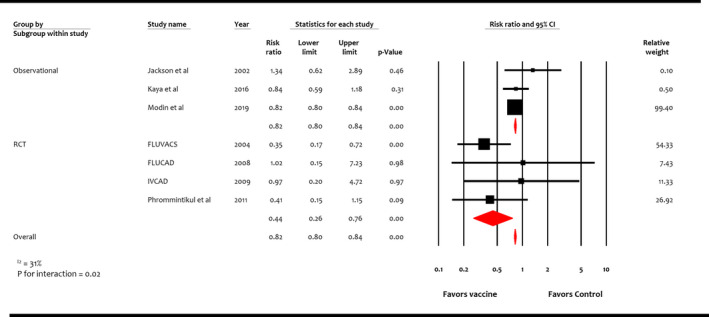
Studies included Jackson et al, 36 Kaya et al, 35 Modin et al, 34 FLUVACS (Flu Vaccination in Acute Coronary Syndromes and Planned Percutaneous Coronary Interventions), 17 FLUCAD (Influenza Vaccination in Secondary Prevention From Coronary Ischemic Events in Coronary Artery Disease), 16 IVCAD (Efficacy of Influenza Vaccination in Reducing Cardiovascular Events in Patients With Coronary Artery Diseases), 18 and Phrommintikul et al. 19 RCT indicates randomized control trial.
Major Adverse Cardiovascular Events
Four RCTs (n=1667) and 3 observational studies (n=27 207) reported MACE. 10 , 16 , 17 , 18 , 19 , 20 , 21 Overall, influenza vaccine was associated with reduction in MACE compared with control (RR, 0.87; 95% CI, 0.80–0.94 [P<0.001]) (I 2=51%) (Figure 5). While this benefit was consistent among study designs, reduction in RR was more pronounced in RCTs (RR, 0.57; 95% CI, 0.43–0.74 [P<0.001]) than observational studies (RR, 0.90; 95% CI, 0.83–0.98 [P=0.02]) (P for interaction <0.01).
Figure 5. Effect of influenza vaccine on major adverse cardiovascular events.
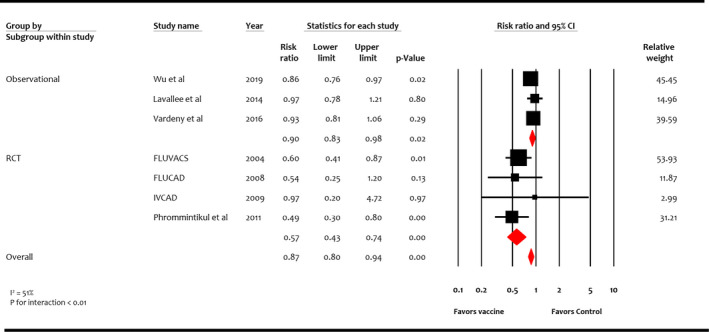
Studies included Wu et al, 20 Lavallee et al, 21 Vardeny et al, 10 FLUVACS (Flu Vaccination in Acute Coronary Syndromes and Planned Percutaneous Coronary Interventions), 17 FLUCAD (Influenza Vaccination in Secondary Prevention From Coronary Ischemic Events in Coronary Artery Disease), 16 IVCAD (Efficacy of Influenza Vaccination in Reducing Cardiovascular Events in Patients With Coronary Artery Diseases), 18 and Phrommintikul et al. 19 RCT indicates randomized control trial.
Myocardial Infarction
Four RCTs (n=1667) and 3 observational studies (n=70 688) reported MI. 16 , 17 , 18 , 19 , 21 , 36 , 37 Overall, influenza vaccine was not significantly associated with reduction in MI compared with control (RR, 0.73; 95% CI, 0.49–1.09 [P=0.12]) (I 2=64%) (Figure 6). This effect was consistent among study designs: RCTs (RR, 0.74; 95% CI, 0.38–1.44 [P=0.38]) and observational studies (RR, 0.73; 95% CI, 0.44–1.19 [P=0.20]) (P for interaction=0.95).
Figure 6. Effect of influenza vaccine on myocardial infarction.
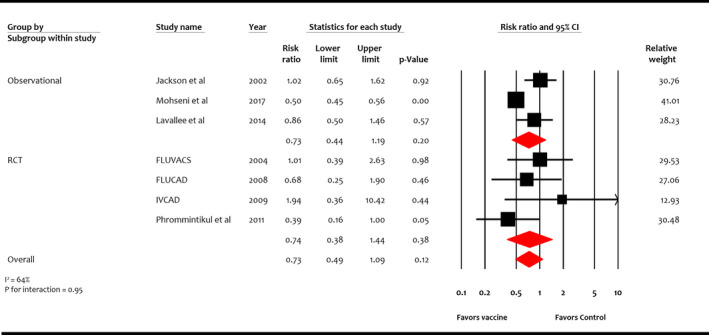
Studies included Jackson et al, 36 Mohseni et al, 37 Lavallee et al, 21 FLUVACS (Flu Vaccination in Acute Coronary Syndromes and Planned Percutaneous Coronary Interventions), 17 FLUCAD (Influenza Vaccination in Secondary Prevention From Coronary Ischemic Events in Coronary Artery Disease), 16 IVCAD (Efficacy of Influenza Vaccination in Reducing Cardiovascular Events in Patients With Coronary Artery Diseases), 18 and Phrommintikul et al. 19 RCT indicates randomized control trial.
Sensitivity analyses by excluding abstract data 18 did not influence mortality and MACE end points. Egger regression test did not detect small study bias (P=0.63).
Discussion
In this meta‐analysis of 237 058 patients with CVD, influenza vaccination was associated with a significant reduction in all‐cause mortality, cardiovascular mortality, and MACE. While a numerical reduction in MI was associated with the influenza vaccine, statistical significance was not achieved. The effects of the influenza vaccine were consistent among RCTs and observational studies. The influence of influenza vaccine on HF was not reported in the main results because of the paucity of randomized data. However, the summary estimate was consistent with a 29% RR reduction in HF, predominantly driven by the 27% reduction noted from observational data.
There have been multiple postulated mechanisms that could explain an increased cardiovascular risk after influenza infection, including atherosclerotic plaque destabilization and subsequent thrombosis, deposition of immune complexes in atherosclerotic plaques, and elevation of macrophage circulation into the arteries resulting in coronary vascular events. 38 , 39 , 40 Proinflammatory cytokine release, endothelial dysfunction, sympathetic activation, and exaggerated fluid shifts leading to volume overload are few mechanisms explaining acute HF development. 41 , 42 , 43 , 44 Our meta‐analysis provides further confirmation that preventing influenza infection through vaccination can reduce MACE and mortality risk. Despite lack of statistically significant reduction for MI, the directionality of the effect estimates with numerical 27% RR reduction appears to influence MACE, which consequently resulted in the observed survival benefit with influenza vaccine. 10 , 18 , 19 , 21 However, since these findings do not represent causative effect, these observations should be considered hypothesis‐generating.
We compared our results with other meta‐analyses. 13 , 14 , 15 Loomba et al 14 analyzed 3 RCTs and 2 observational studies including 292 383 patients and showed a reduction in all‐cause mortality (odds ratio [OR], 0.61 [95% CI, 0.57–0.64]), MI (OR, 0.73 [95% CI, 0.57–0.93]), and MACE (OR, 0.47 [95% CI, 0.29–0.74]) in patients who received influenza vaccination. This study was a mixed cohort of patients with and without CVD and did not comment on whether mortality benefit was persistent in secondary CVD prevention. Moreover, the influence of study design on summary estimates was not examined. Udell et al 15 analyzed 6 RCTs comprising only 36.2% of patients with CVD and showed a reduction in composite cardiovascular events with influenza vaccination versus control (2.9% versus 4.7%; RR, 0.64 [95% CI, 0.48–0.86]). However, the influenza vaccine was not shown to reduce cardiovascular mortality (RR, 0.81 [95% CI, 0.36–1.83]). The most likely explanation of the difference in mortality estimate between their study and ours was the limited mean follow‐up duration (7.9 months) of studies included in Udell et al. Whereas, we included studies with a follow‐up duration of at least 12 months to demonstrate significant differences between rare events, such as mortality. 45 This is important given that differences in rare events between interventions, such as mortality, take a longer follow‐up duration to emerge. Clar et al 13 performed a meta‐analysis of 8 RCTs encompassing patients with and without CVD. In 4 RCTs focused on the secondary prevention of cardiovascular outcomes, cardiovascular mortality was reduced by influenza vaccination (RR, 0.45; 95% CI, 0.26–0.76). However, this study differed from our meta‐analysis by excluding observational data. In our unique meta‐analysis, we attempted to generate consensus regarding persistent cardiovascular benefits between “real‐world data” and RCTs regarding the efficacy of the influenza vaccine in CVD.
Influenza vaccination was associated with a 25% reduced risk of all‐cause death comparable in size effect to guideline‐directed therapy with β‐blockers and angiotensin‐converting enzyme inhibitors with reductions in mortality of ≈20% to 25%, respectively. 34 , 46 , 47 , 48 This is a substantial reduction in mortality given the safety, feasibility, and cost‐efficiency of influenza vaccination, and thus should be considered alongside other cardiovascular prevention therapies. In the recent INVESTED (Influenza Vaccine to Effectively Stop Cardio Thoracic Events and Decompensated Heart Failure) trial, high‐dose vaccine showed comparable outcomes compared with a standard vaccine in high‐risk patients with CVD. 49 Two other ongoing RCTs, 50 , 51 the IAMI (Influenza Vaccination After Myocardial Infarction) trial that randomized patients with acute coronary syndrome undergoing coronary angiography to the influenza vaccine versus placebo, 50 and the IVVE (Influenza Vaccine in Patients With Heart Failure to Reduce Adverse Vascular Events) trial that compared a composite cardiovascular end point in patients with HF who received the influenza vaccine compared with placebo, 51 might further aid in strengthening the evidence in favor of efficacy profile of the influenza vaccine in specific patients with CVD.
Our analysis was limited by inherent shortcomings of study‐level meta‐analysis, such as study design, baseline variables of population, definition of end points, and heterogenous follow‐up durations. While we included studies of only patients with prevalent CVD, there were limited and inconsistent data reported in the studies regarding specific underlying CVD subtypes (coronary artery disease, peripheral artery disease, cerebrovascular disease, or HF); we were unable to further stratify by these subgroups. Event rates were low, compromising the power of specific end points such as MI. Similarly, scarcity of data on other hard end points, such as stroke, did not allow us to explore the influence of relevant cardiovascular end points on mortality and MACE. Some studies carried higher relative weight in pooled estimates than other studies. For instance, FLUVACS (Flu Vaccination in Acute Coronary Syndromes and Planned Percutaneous Coronary Interventions) 17 contributed >50% relative weight and can influence the end points. That said, the directionality of the majority of component studies of this meta‐analysis favored the influenza vaccine for survival improvement.
Conclusions
The influenza vaccine was associated with a lower risk of total and cardiovascular mortality in patients with CVD. Influenza is the most common respiratory infection. 52 Yet, only 45% of adults in the United States were vaccinated against influenza during the 2018 to 2019 season despite the available evidence favoring survival benefit in CVD. 53 Influenza vaccination in patients with or at risk for CVD is a standard of care, and all providers should assume the responsibility of inquiring about vaccination status, providing education, and ensuring that patients have the opportunity to be vaccinated. The current study may help care clinicians and health policymakers to strongly advocate the influenza vaccination for secondary prevention of cardiovascular outcomes.
Sources of Funding
Dr Michos is supported by the Blumenthal Scholars Award in Preventive Cardiology at Johns Hopkins University.
Disclosures
Dr Navar has received funding for research to her institution from Amarin, Amgen, Janssen, Regeneron, and Sanofi; and honoraria and consulting fees from Amarin, Amgen, AstraZeneca, BI, Cerner, 89Bio, Esperion, Janssen, Lilly, New Amsterdam, Novartis, NovoNordisk, Pfizer, Regeneron, Sanofi, and The Medicines Company. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figures S1–S2
(J Am Heart Assoc. 2021;10:e019636. DOI: 10.1161/JAHA.120.019636.)
*Dr Yedlapati and Dr Safi U. Khan contributed equally to this work.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Centers for Disease Control and Prevention . US flu season: preliminary burden estimates. 2019–2020. Available at: https://www.cdc.gov/flu/about/burden/2019‐2020.html. Accessed January 9, 2021.
- 2. Collins SD. Excess mortality from causes other than influenza and pneumonia during influenza epidemics. Public Health Rep. 1932;47:2159–2179. DOI: 10.2307/4580606.19315373 [DOI] [Google Scholar]
- 3. Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, Katz K, Ko DT, McGeer AJ, McNally D, et al. Acute myocardial infarction after laboratory‐confirmed influenza infection. N Engl J Med. 2018;378:345–353. DOI: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 4. Chow EJ, Rolfes MA, O'Halloran A, Anderson EJ, Bennett NM, Billing L, Chai S, Dufort E, Herlihy R, Kim S, et al. Acute cardiovascular events associated with influenza in hospitalized adults: a cross‐sectional study. Ann Intern Med. 2020;173:605–613. DOI: 10.7326/M20-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith SC, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113:2363–2372. DOI: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 6. Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices — United States, 2019–20 influenza season. MMWR Recomm Rep. 2019;68:1–21. DOI: 10.15585/mmwr.rr6803a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Diego C, Vila‐Corcoles A, Ochoa O, Rodriguez‐Blanco T, Salsench E, Hospital I, Bejarano F, del Puy Muniain M, Fortin M, Canals M, et al. Effects of annual influenza vaccination on winter mortality in elderly people with chronic heart disease. Eur Heart J. 2009;30:209–216. DOI: 10.1093/eurheartj/ehn498. [DOI] [PubMed] [Google Scholar]
- 8. Kadoglou NPE, Bracke F, Simmers T, Tsiodras S, Parissis J. Influenza infection and heart failure‐vaccination may change heart failure prognosis? Heart Fail Rev. 2017;22:329–336. DOI: 10.1007/s10741-017-9614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singleton JA, Wortley P, Lu PJ. Influenza vaccination of persons with cardiovascular disease in the United States. Tex Heart Inst J. 2004;31:22–27. [PMC free article] [PubMed] [Google Scholar]
- 10. Vardeny O, Claggett B, Udell JA, Packer M, Zile M, Rouleau J, Swedberg K, Desai AS, Lefkowitz M, Shi V, et al. Influenza vaccination in patients with chronic heart failure: the PARADIGM‐HF trial. JACC Heart Fail. 2016;4:152–158. DOI: 10.1016/j.jchf.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 11. Grandhi GR, Mszar R, Vahidy F, Valero‐Elizondo J, Blankstein R, Blaha MJ, Virani SS, Andrieni JD, Omer SB, Nasir K. Sociodemographic disparities in influenza vaccination among adults with atherosclerotic cardiovascular disease in the United States. JAMA Cardiol. 2021;6:87–91. DOI: 10.1001/jamacardio.2020.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis MM, Wortley PM, Ndiaye SM, Cowan AE, Osta AD, Clark SJ. Influenza vaccine for high‐risk non‐elderly adults: a national survey of subspecialists. Hum Vaccin. 2008;4:229–233. DOI: 10.4161/hv.4.3.5516. [DOI] [PubMed] [Google Scholar]
- 13. Clar C, Oseni Z, Flowers N, Keshtkar‐Jahromi M, Rees K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst Rev. 2015;5:CD005050. DOI: 10.1002/14651858.CD005050.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loomba RS, Aggarwal S, Shah PH, Arora RR. Influenza vaccination and cardiovascular morbidity and mortality: analysis of 292,383 patients. J Cardiovasc Pharmacol Ther. 2012;17:277–283. DOI: 10.1177/1074248411429965. [DOI] [PubMed] [Google Scholar]
- 15. Udell JA, Zawi R, Bhatt DL, Keshtkar‐Jahromi M, Gaughran F, Phrommintikul A, Ciszewski A, Vakili H, Hoffman EB, Farkouh ME, et al. Association between influenza vaccination and cardiovascular outcomes in high‐risk patients: a meta‐analysis. JAMA. 2013;310:1711–1720. DOI: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 16. Ciszewski A, Bilinska ZT, Brydak LB, Kepka C, Kruk M, Romanowska M, Ksiezycka E, Przyluski J, Piotrowski W, Maczynska R, et al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J. 2008;29:1350–1358. DOI: 10.1093/eurheartj/ehm581. [DOI] [PubMed] [Google Scholar]
- 17. Gurfinkel EP, Leon de la Fuente R, Mendiz O, Mautner B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) Study. Eur Heart J. 2004;25:25–31. DOI: 10.1016/j.ehj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 18. Keshtkar‐Jahromi M, Vakili H, Rahnavardi MT. The efficacy of influenza vaccination in reducing cardiovascular events in patients with coronary artery diseases: IVCAD study. Clin Microbiol Infect. 2009;15:S395–S396. [Google Scholar]
- 19. Phrommintikul A, Kuanprasert S, Wongcharoen W, Kanjanavanit R, Chaiwarith R, Sukonthasarn A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J. 2011;32:1730–1735. DOI: 10.1093/eurheartj/ehr004. [DOI] [PubMed] [Google Scholar]
- 20. Wu HH, Chang YY, Kuo SC, Chen YT. Influenza vaccination and secondary prevention of cardiovascular disease among Taiwanese elders‐a propensity score‐matched follow‐up study. PLoS One. 2019;14:e0219172. DOI: 10.1371/journal.pone.0219172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lavallee PC, Labreuche J, Fox KM, Lavados P, Mattle H, Steg PG, Amarenco P; PERFORM, OPTIC, and AMISTAD Investigators A . Influenza vaccination and cardiovascular risk in patients with recent TIA and stroke. Neurology. 2014;82:1905–1913. DOI: 10.1212/WNL.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 22. van Tulder M, Furlan A, Bombardier C, Bouter L; Editorial Board of the Cochrane Collaboration Back Review Group . Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine. 2003;28:1290–1299. DOI: 10.1097/01.BRS.0000065484.95996.AF. [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. DOI: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 24. Khan SU, Khan MU, Valavoor S, Khan MS, Okunrintemi V, Mamas MA, Leucker TM, Blaha MJ, Michos ED. Association of lowering apolipoprotein B with cardiovascular outcomes across various lipid‐lowering therapies: systematic review and meta‐analysis of trials. Eur J Prev Cardiol. 2020;27:1255–1268. DOI: 10.1177/2047487319871733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koskinas KC, Siontis GCM, Piccolo R, Mavridis D, Räber L, Mach F, Windecker S. Effect of statins and non‐statin LDL‐lowering medications on cardiovascular outcomes in secondary prevention: a meta‐analysis of randomized trials. Eur Heart J. 2018;39:1172–1180. DOI: 10.1093/eurheartj/ehx566. [DOI] [PubMed] [Google Scholar]
- 26. Khan SU, Khan MU, Ghani AR, Lone AN, Arshad A, Kaluski E. Meta‐analysis of antithrombotic therapy in atrial fibrillation after percutaneous coronary intervention. Am J Cardiol. 2018;121:1200–1206. DOI: 10.1016/j.amjcard.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. DOI: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. DOI: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29. Borenstein M, Higgins JP. Meta‐analysis and subgroups. Prev Sci. 2013;14:134–143. DOI: 10.1007/s11121-013-0377-7. [DOI] [PubMed] [Google Scholar]
- 30. Liu IF, Huang CC, Chan WL, Huang PH, Chung CM, Lin SJ, Chen JW, Leu HB. Effects of annual influenza vaccination on mortality and hospitalization in elderly patients with ischemic heart disease: a nationwide population‐based study. Prev Med. 2012;54:431–433. DOI: 10.1016/j.ypmed.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 31. Wu WC, Jiang L, Friedmann PD, Trivedi A. Association between process quality measures for heart failure and mortality among US veterans. Am Heart J. 2014;168:713–720. DOI: 10.1016/j.ahj.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kopel E, Klempfner R, Goldenberg I. Influenza vaccine and survival in acute heart failure. Eur J Heart Fail. 2014;16:264–270. DOI: 10.1002/ejhf.14. [DOI] [PubMed] [Google Scholar]
- 33. Blaya‐Nováková V, Prado‐Galbarro FJ, Sarria‐Santamera A. Effects of annual influenza vaccination on mortality in patients with heart failure. Eur J Public Health. 2016;26:890–892. DOI: 10.1093/eurpub/ckw141. [DOI] [PubMed] [Google Scholar]
- 34. Modin D, Jorgensen ME, Gislason G, Jensen JS, Kober L, Claggett B, Hegde SM, Solomon SD, Torp‐Pedersen C, Biering‐Sorensen T. Influenza vaccine in heart failure. Circulation. 2019;139:575–586. DOI: 10.1161/CIRCULATIONAHA.118.036788. [DOI] [PubMed] [Google Scholar]
- 35. Kaya H, Beton O, Acar G, Temizhan A, Cavusoğlu Y, Guray U, Zoghi M, Ural D, Ekmekci A, Gungor H, et al. Influence of influenza vaccination on recurrent hospitalization in patients with heart failure. Herz. 2017;42:307–315. DOI: 10.1007/s00059-016-4460-2. [DOI] [PubMed] [Google Scholar]
- 36. Jackson LA, Yu O, Heckbert SR, Psaty BM, Malais D, Barlow WE, Thompson WW; Vaccine Safety Datalink Study Group . Influenza vaccination is not associated with a reduction in the risk of recurrent coronary events. Am J Epidemiol. 2002;156:634–640. DOI: 10.1093/aje/kwf073. [DOI] [PubMed] [Google Scholar]
- 37. Mohseni H, Kiran A, Khorshidi R, Rahimi K. Influenza vaccination and risk of hospitalization in patients with heart failure: a self‐controlled case series study. Eur Heart J. 2017;38:326–333. DOI: 10.1093/eurheartj/ehw411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Conti CR. Vascular events responsible for thrombotic occlusion of a blood vessel. Clin Cardiol. 1993;16:761–762. DOI: 10.1002/clc.4960161103. [DOI] [PubMed] [Google Scholar]
- 39. Hebsur S, Vakil E, Oetgen WJ, Kumar PN, Lazarous DF. Influenza and coronary artery disease: exploring a clinical association with myocardial infarction and analyzing the utility of vaccination in prevention of myocardial infarction. Rev Cardiovasc Med. 2014;15:168–175. [DOI] [PubMed] [Google Scholar]
- 40. Kinlay S, Ganz P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am J Cardiol. 1997;80:11I–16I. DOI: 10.1016/S0002-9149(97)00793-5. [DOI] [PubMed] [Google Scholar]
- 41. Tomiyama H, Yamashina A. Vascular dysfunction: a key player in chronic cardio‐renal syndrome. Intern Med. 2015;54:1465–1472. DOI: 10.2169/internalmedicine.54.4502. [DOI] [PubMed] [Google Scholar]
- 42. Marti CN, Georgiopoulou VV, Kalogeropoulos AP. Acute heart failure: patient characteristics and pathophysiology. Curr Heart Fail Rep. 2013;10:427–433. DOI: 10.1007/s11897-013-0151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davies MJ. The composition of coronary‐artery plaques. N Engl J Med. 1997;336:1312–1314. DOI: 10.1056/NEJM199705013361809. [DOI] [PubMed] [Google Scholar]
- 44. Chan NN, Colhoun HM, Vallance P. Cardiovascular risk factors as determinants of endothelium‐dependent and endothelium‐independent vascular reactivity in the general population. J Am Coll Cardiol. 2001;38:1814–1820. DOI: 10.1016/S0735-1097(01)01669-2. [DOI] [PubMed] [Google Scholar]
- 45. Khan SU, Rahman H, Talluri S, Kaluski E. The clinical benefits and mortality reduction associated with catheter ablation in subjects with atrial fibrillation: a systematic review and meta‐analysis. JACC Clin Electrophysiol. 2018;4:626–635. DOI: 10.1016/j.jacep.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 46. Flather MD, Yusuf S, Kober L, Pfeffer M, Hall A, Murray G, Torp‐Pedersen C, Ball S, Pogue J, Moye L, et al. Long‐term ACE‐inhibitor therapy in patients with heart failure or left‐ventricular dysfunction: a systematic overview of data from individual patients. ACE‐Inhibitor Myocardial Infarction Collaborative Group. Lancet. 2000;355:1575–1581. DOI: 10.1016/S0140-6736(00)02212-1. [DOI] [PubMed] [Google Scholar]
- 47. McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta‐analysis: beta‐blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–794. DOI: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 48. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. DOI: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 49. Vardeny O, Kim K, Udell JA, Joseph J, Desai AS, Farkouh ME, Hegde SM, Hernandez AF, McGeer A, Talbot HK, et al. Effect of high‐dose trivalent vs standard‐dose quadrivalent influenza vaccine on mortality or cardiopulmonary hospitalization in patients with high‐risk cardiovascular disease: a randomized clinical trial. JAMA. 2021;325:39–49. DOI: 10.1001/jama.2020.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Frobert O, Gotberg M, Angeras O, Jonasson L, Erlinge D, Engstrom T, Persson J, Jensen SE, Omerovic E, James SK, et al. Design and rationale for the Influenza vaccination After Myocardial Infarction (IAMI) trial. A registry‐based randomized clinical trial. Am Heart J. 2017;189:94–102. DOI: 10.1016/j.ahj.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 51. Loeb M, Dokainish H, Dans A, Palileo‐Villanueva LM, Roy A, Karaye K, Zhu J, Liang Y, Goma F, Damasceno A, et al. Randomized controlled trial of influenza vaccine in patients with heart failure to reduce adverse vascular events (IVVE): rationale and design. Am Heart J. 2019;212:36–44. DOI: 10.1016/j.ahj.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Warren‐Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9:601–610. DOI: 10.1016/S1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 53. Chung JR, Rolfes MA, Flannery B, Prasad P, O’Halloran A, Garg S, Fry AM, Singleton JA, Patel M, Reed C, et al. Effects of influenza vaccination in the United States during the 2018–2019 influenza season. Clin Infect Dis. 2020;71:e368–e376. DOI: 10.1093/cid/ciz1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S2
Data Availability Statement
The authors declare that all supporting data are available within the article (and its online supplementary files).


