Abstract
Background
Presence of clonal hematopoiesis of indeterminate potential (CHIP) is associated with a higher risk of atherosclerotic cardiovascular disease, cancer, and mortality. The relationship between a healthy lifestyle and CHIP is unknown.
Methods and Results
This analysis included 8709 postmenopausal women (mean age, 66.5 years) enrolled in the WHI (Women's Health Initiative), free of cancer or cardiovascular disease, with deep‐coverage whole genome sequencing data available. Information on lifestyle factors (body mass index, smoking, physical activity, and diet quality) was obtained, and a healthy lifestyle score was created on the basis of healthy criteria met (0 point [least healthy] to 4 points [most healthy]). CHIP was derived on the basis of a prespecified list of leukemogenic driver mutations. The prevalence of CHIP was 8.6%. A higher healthy lifestyle score was not associated with CHIP (multivariable‐adjusted odds ratio [OR] [95% CI], 0.99 [0.80–1.23] and 1.13 [0.93–1.37]) for the upper (3 or 4 points) and middle category (2 points), respectively, versus referent (0 or 1 point). Across score components, a normal and overweight body mass index compared with obese was significantly associated with a lower odds for CHIP (OR, 0.71 [95% CI, 0.57–0.88] and 0.83 [95% CI, 0.68–1.01], respectively; P‐trend 0.0015). Having never smoked compared with being a current smoker tended to be associated with lower odds for CHIP.
Conclusions
A healthy lifestyle, based on a composite score, was not related to CHIP among postmenopausal women. However, across individual lifestyle factors, having a normal body mass index was strongly associated with a lower prevalence of CHIP. These findings support the idea that certain healthy lifestyle factors are associated with a lower frequency of CHIP.
Keywords: body mass index, clonal hematopoiesis of indeterminate potential, diet, lifestyle, physical activity, smoking
Subject Categories: Lifestyle, Biomarkers, Primary Prevention, Women
Nonstandard Abbreviations and Acronyms
- AHEI
Alternate Healthy Eating Index‐2010
- CHIP
clonal hematopoiesis of indeterminate potential
- WHI
Women's Health Initiative
Clinical Perspective
What Is New?
Is a healthy lifestyle associated with a lower prevalence of clonal hematopoiesis of indeterminate potential (CHIP)?
Adherence to a healthy lifestyle, on the basis of a composite score of various lifestyle factors (body mass index, smoking, physical activity, and diet quality), was not related to CHIP in postmenopausal women.
In detailed analyses across individual lifestyle factors, having a normal body mass index compared with being obese was strongly associated with a lower prevalence of CHIP. Having never smoked compared with current smoking tended to be related to a lower odds for CHIP.
What Are the Clinical Implications?
Certain healthy lifestyle factors are associated with a lower prevalence of CHIP in an older population.
These findings emphasize the importance of maintaining a normal body weight and nonsmoking status. The results may clarify ongoing disputes as how to handle “metabolically healthy” obesity.
Screening for CHIP and its major driver mutations can help to better identify and characterize (early or subclinical) health changes.
Human aging is associated with an increased frequency of somatic mutations in hematopoietic cells. The acquisition of specific somatic mutations in the absence of other criteria for hematologic neoplasia, dysplasia, or cytopenia is termed clonal hematopoiesis of indeterminate potential (CHIP). 1 The prevalence of CHIP in peripheral blood is low (<0.5%) from birth until 50 years of age, after which it may begin to increase, affecting 10% of people aged 70 to 80 years. 2 , 3 , 4 The most frequently mutated driver genes are DNMT3A and TET2, which play an important role in epigenetic regulation and inflammatory response. 1 , 2 , 3 , 4 , 5 , 6 More important, mutations in these driver genes may occur while blood counts remain normal and in people who are otherwise apparently healthy. 1 , 7 Although such somatic mutations greatly increase the risk of acquiring additional driver mutations and eventually developing a hematologic cancer, the main cause of death in individuals with CHIP is notably atherosclerotic cardiovascular disease (CVD). 2 , 3 , 8 , 9 , 10
Somatic mutations in hematopoietic cells, such as TET2, have been shown to contribute to the development of atherosclerosis mainly by interactions between clonal monocytes‐macrophages and the endothelium and the increased expression of proinflammatory genes. 7 , 11 , 12 As many of the CVD risk factors are influenced by lifestyle practices, we hypothesized that modifiable lifestyle factors may be associated with a lower presence of CHIP. Adherence to a healthy lifestyle is a major approach to controlling CVD at the population level, and is associated with more favorable CVD risk factor profiles and with lower CVD incidence and mortality. 13 , 14 , 15 , 16 However, at this time, it remains largely unknown whether a healthy lifestyle or individual lifestyle factors are related to CHIP, as detailed information on lifestyle has not been available in most studies on CHIP. 11 , 17 , 18 , 19 Moreover, most previous studies of CHIP have involved study populations who already experienced a high burden of atherosclerotic disease. 8 , 11 , 17 , 20 , 21 , 22 , 23
The aim of this study was first to examine the associations of adherence to a healthy lifestyle and presence of CHIP in postmenopausal women without history of CVD or cancer. Second, we aimed to relate the presence of CHIP in the most frequently affected driver mutations (DNMT3A and TET2) to a healthy lifestyle. We hypothesized that a healthy lifestyle would be associated with a lower frequency of CHIP.
Methods
The data, analytic methods, and study materials are made available to other researchers for purposes of reproducing the results or replicating the procedure. The data underlying our work can be obtained through 2 mechanisms. First, interested investigators can contact the WHI (Women's Health Initiative) Coordinating Center. Details about the procedures for data request can be found online (www.whi.org). Second, most data from the WHI can also be obtained from BioLINCC, a repository maintained by the National Heart, Lung, and Blood Institute. The BioLINCC website includes detailed information about the available data and the process to obtain such data (https://biolincc.nhlbi.nih.gov/home/).
Study Population
The study population consisted of women enrolled in the WHI. Participating women were recruited between 1993 and 1998 (baseline) at 40 clinical centers in the United States and were eligible if they were 50 to 79 years old, were in overall good health, and were postmenopausal at the time of enrollment. 24 Further details of the WHI have been described elsewhere. 25 , 26 , 27 , 28 Through the National Heart, Lung, and Blood Institute Trans‐Omics for Precision Medicine program, WHI had 11 085 participants undergo whole genome sequencing. The WHI Trans‐Omics for Precision Medicine sample was selected from a WHI case‐control ancillary substudy of venous thromboembolism and stroke cases (a virtual census) and controls (a stratified‐random sample). Whole genome sequencing was performed at the Broad Institute Sequencing Center at 38× coverage for >400 million variants using a centralized, rigorous approach to variant calling for germ‐line variants. 29 After strict quality check procedure, CHIP information was available for 11 029 WHI participants.
As presence of CHIP increases strongly with age and has been shown to be associated with atherosclerotic CVD and cancer, we excluded women with history of any cancer (except nonmelanoma skin cancer) (n=722) or a history of coronary heart disease or stroke (n=368). Moreover, women with implausible total energy intake (<600 or >5000 kcal/d) (n=308), who were underweight (body mass index [BMI] ≤18.5 kg/m2) (n=80), who had missing information on any of the components composing the healthy lifestyle score (n=817), or who had insufficient CHIP data quality (n=25) were also excluded. The final study population consisted of 8709 women (Figure). Institutional review boards at participating institutions approved all study protocols, and all participants provided written informed consent.
Figure 1. Study inclusion criteria.
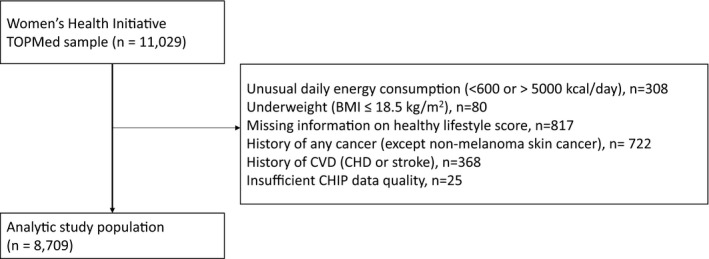
BMI indicates body mass index; CHD, coronary heart disease; CHIP, clonal hematopoiesis of indeterminate potential; CVD, cardiovascular disease; and TOPMed, Trans‐Omics for Precision Medicine.
Lifestyle Measures
We considered 4 lifestyle factors assessed at baseline: BMI, smoking, physical activity, and diet quality. 24 We categorized women as normal weight (18.5 kg/m2≤BMI<25 kg/m2), overweight (25 kg/m2≤BMI≤30 kg/m2), and obese (BMI >30 kg/m2). Smoking status was categorized as current, former, or never smoker on the basis of self‐report. To assess continued exposure to smoking and smoking intensity, pack‐years of smoking were further derived. Recreational physical activity was assessed using a questionnaire on frequency and duration of several activity types, which were summarized as metabolic equivalent task hours per week. 30 , 31 We categorized physical activity as inactive (eg, no report of moderate or vigorous physical activity), insufficiently active (ie, less active than recommendations: <150 min/wk of moderate physical activity, <75 min/wk of vigorous physical activity, or equivalent combination), or active (eg, meeting physical activity recommendations: ≥150 min/wk of moderate physical activity, ≥75 min/wk of vigorous physical activity, or an equivalent combination). 32 , 33 Finally, dietary intake was captured from a self‐administered WHI food frequency questionnaire. 24 , 34 , 35 Diet quality was derived using the Alternative Healthy Eating Index‐2010 (AHEI), which is typically used to broadly assess adherence to national dietary guidelines. 36 , 37 The AHEI includes 11 items, and each component score ranges from 0 (worst) to 10 (best). It emphasizes vegetables, fruits, whole grains, nuts, legumes, vegetable proteins, long‐chain omega‐3 polyunsaturated fatty acids, polyunsaturated fatty acids (excluding long‐chain omega‐3 polyunsaturated fatty acids), moderate alcohol consumption, avoidance of trans fat, and lower intakes of sugar‐sweetened beverages (including fruit juice), red and processed meats, and sodium. Total AHEI scoring can range from 0 (nonadherence) to 110 (perfect adherence).
Healthy Lifestyle Score
For this analysis, a healthy lifestyle score was created following prior studies. 16 , 33 , 38 , 39 Each lifestyle factor at baseline was dichotomized as healthy versus unhealthy, as follows: low‐scoring AHEI (quintiles 1–3) versus high‐scoring AHEI (quintiles 4 and 5), physically inactive or insufficiently active versus physically active, overweight or obese BMI (BMI ≥25 kg/m2) versus normal BMI (18.5 kg/m2≤BMI<25 kg/m2), and current smoker versus noncurrent smoker. Women received 1 point for every healthy criterion met, and points were summed to obtain the healthy lifestyle score (minimum 0 points/least healthy to maximum 4 points/most healthy).
Clonal Hematopoiesis of Indeterminate Potential
CHIP was determined by calling somatic variants from whole genome DNA sequencing from banked peripheral blood drawn at WHI enrollment between 1993 and 1998. When baseline DNA sample was not available, DNA sample from subsequent annual visits (AVs) were used (24.2% baseline, 23.0% AV1, 10.9% AV2, 20.4% AV3, 13.2% AV4, 1.3% AV5, 3.5% AV6, 2.5% AV7, 0.4% AV8, 0.5% AV9, and 0.2% AV10). 24 Specifically, the presence of CHIP was defined using the GATK Mutect2 somatic variant caller on the basis of 74 prespecified driver genes known to promote clonal expansion of hematopoietic stem cells using a variant allele frequency of >0.06 to minimize the false discovery rate. 11 , 29
Covariates
Information on covariates was obtained by self‐report questionnaires. 24 Hypertension was defined as current antihypertensive medication use or value of systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg. 40 Women were categorized as having high cholesterol on the basis of cholesterol‐lowering medication use. Diabetes mellitus was defined as self‐report of physician diagnosis or self‐report of taking diabetes mellitus medication.
Statistical Analysis
Descriptive statistics by categories of the healthy lifestyle score were generated to describe demographics and other characteristics. Baseline characteristics across categories of healthy lifestyle scoring were evaluated by Pearson χ2 test for categorical variables and by ANOVA test for continuous variables.
To assess the relationship between presence of CHIP and healthy lifestyle, we used multiple logistic regression models with covariate adjustment in 2 nested models: the first model adjusted for age at blood draw/CHIP assessment (continuous) and race/ethnicity; the second model additionally adjusted for education, family income, marital status, use of cholesterol‐lowering medication, history of treated diabetes mellitus, family history of cancer, hypertension, hormone therapy use, and WHI trial participation. To account for the sampling scheme of the WHI Trans‐Omics for Precision Medicine sample, both primary and secondary models used 3 strata (conditional logistic regression): case (stroke and venous thromboembolism) and control. The strata were not a covariate (ie, we assumed a different stratum‐specific baseline risk profile [intercept] but the same confounding/modification effect of the covariates). The main variable of interest for this analysis was the composite healthy lifestyle score. However, we also looked at each component of the healthy lifestyle score separately. For each explanatory variable, we computed the odds ratios (ORs) for the presence of CHIP for different categories compared with the reference category; P values for the difference between highest and lowest category and P values from trend test to look at a possible dose‐response relationship were calculated. In addition, we looked at these explanatory variables in their continuous forms. Participants with missing covariates were excluded from the analysis. Percentage of missing covariates ranged from 0% (age at blood draw/CHIP, race/ethnicity, and WHI trial participation) to 6% (annual income). In sensitivity analyses, we repeated the analysis for presence of CHIP in the DNMT3A and TET2 genes. We also undertook sensitivity analyses, restricting to participants aged ≥65 years, and by additionally adjusting our analyses between AHEI, physical activity, smoking, and CHIP for BMI.
All statistical tests were 2 sided. Analyses were performed by SAS statistical software version 9.4 (SAS Inc, Cary, NC).
Results
Baseline characteristics according to categories of healthy lifestyle scoring are presented in Table 1. Women with a higher score were older, were more likely to be non‐Hispanic White individuals, were more likely to be married, were more likely to have a college degree or higher, and had an annual income ≥35 000 US$ and were less likely to report a history of treated diabetes mellitus or hypertension. There was no difference in cholesterol medication use or family history of cancer. Most women participated in the WHI trials (≈59%).
Table 1.
Baseline Characteristics, According to the Healthy Lifestyle Score (n=8709)
| Characteristic | All (n=8709) | Healthy Lifestyle Score by Categories (Range) | P Value* | ||
|---|---|---|---|---|---|
|
Category 1 (0–1) (n=3531) |
Category 2 (2) (n=2922) |
Category 3 (3–4) (n=2256) |
|||
| Age at baseline, mean (SD), y | 66.5 (6.7) | 65.3 (6.8) | 67.0 (6.7) | 67.5 (6.4) | <0.0001 |
| Age, n (%) | <0.0001 | ||||
| 50–54 y | 480 (5.5) | 247 (7.0) | 142 (4.9) | 91 (4.0) | |
| 55–59 y | 914 (10.5) | 482 (13.7) | 263 (9.0) | 169 (7.5) | |
| 60–64 y | 1827 (21.0) | 819 (23.2) | 600 (20.5) | 408 (18.1) | |
| 65–69 y | 2343 (26.9) | 919 (26.0) | 771 (26.4) | 653 (28.9) | |
| 70–74 y | 2111 (24.2) | 735 (20.8) | 763 (26.1) | 613 (27.2) | |
| 75–79 y | 1034 (11.9) | 329 (9.3) | 383 (13.1) | 322 (14.3) | |
| Age at blood draw/CHIP assessment, mean (SD), y | 68.6 (6.9) | 67.4 (7.0) | 69.1 (6.7) | 69.8 (6.5) | <0.0001 |
| Race/ethnicity, n (%) | <0.0001 | ||||
| Non‐Hispanic White | 7064 (81.1) | 2669 (75.6) | 2401 (82.2) | 1994 (88.4) | |
| Non‐Hispanic African American | 1127 (12.9) | 657 (18.6) | 347 (11.9) | 123 (5.5) | |
| Hispanic/Latina | 252 (2.9) | 124 (3.5) | 82 (2.8) | 46 (2.0) | |
| Other | 266 (3.1) | 81 (2.3) | 92 (3.1) | 93 (4.1) | |
| Marital status, n (%) | 0.0001 | ||||
| Never married | 350 (4.0) | 143 (4.1) | 120 (4.1) | 87 (3.9) | |
| Divorced/separated | 1232 (14.2) | 537 (15.3) | 431 (14.8) | 264 (11.7) | |
| Widowed | 2059 (23.7) | 871 (24.7) | 690 (23.7) | 498 (22.1) | |
| Presently married | 5043 (58.1) | 1969 (55.9) | 1674 (57.4) | 1400 (62.2) | |
| Education, n (%) | <0.0001 | ||||
| Less than high school | 480 (5.6) | 273 (7.8) | 156 (5.4) | 51 (2.3) | |
| High school diploma/GED | 1614 (18.7) | 836 (23.8) | 495 (17.1) | 283 (12.6) | |
| School after high school | 3380 (39.1) | 1423 (40.6) | 1158 (39.9) | 799 (35.7) | |
| College or higher | 3174 (36.7) | 975 (27.8) | 1094 (37.7) | 1105 (49.4) | |
| Annual income, $ | <0.0001 | ||||
| <35 000 | 4054 (49.5) | 1888 (56.9) | 1400 (51.2) | 766 (36.0) | |
| ≥35 000 | 4128 (50.5) | 1433 (43.1) | 1336 (48.8) | 1359 (64.0) | |
| In WHI observational study, n (%) | 3543 (40.7) | 1098 (31.1) | 1220 (41.8) | 1225 (54.3) | <0.0001 |
| In WHI clinical trials, n (%) | 5166 (59.3) | 2433 (68.9) | 1702 (58.2) | 1031 (45.7) | <0.0001 |
| Clinical risk factors, n (%) | |||||
| High cholesterol, requiring pill | 1232 (14.4) | 504 (14.5) | 419 (14.6) | 309 (13.9) | 0.72 |
| History of treated diabetes mellitus | 546 (6.3) | 274 (7.8) | 203 (7.0) | 69 (3.1) | <0.0001 |
| History of hypertension | 4422 (51.2) | 1979 (56.6) | 1512 (52.1) | 931 (41.5) | <0.0001 |
| Family history of cancer | 5502 (66.1) | 2202 (65.5) | 1874 (67.2) | 1426 (65.7) | 0.35 |
| Hormone replacement therapy use | <0.0001 | ||||
| Never used | 4584 (52.7) | 2020 (57.3) | 1519 (52.1) | 1045 (46.4) | |
| Past user | 1683 (19.4) | 685 (19.4) | 565 (19.4) | 433 (19.2) | |
| Current user | 2429 (27.9) | 823 (23.3) | 832 (28.5) | 774 (34.4) | |
| Lifestyle score components | |||||
| BMI, mean (SD), kg/m2 | 28.8 (6.1) | 31.6 (5.7) | 28.7 (5.9) | 24.8 (4.3) | <0.0001 |
| AHEI score, mean (SD) | 51.1 (10.3) | 44.1 (6.8) | 52.9 (9.5) | 59.7 (8.0) | <0.0001 |
| Current smoking, n (%) | 642 (7.4) | 516 (14.6) | 104 (3.6) | 22 (1.0) | <0.0001 |
| Physical activity, mean (SD), MET h/wk | 11.4 (13.1) | 4.8 (6.2) | 11.0 (11.4) | 22.3 (15.6) | <0.0001 |
| Physically “inactive”, n (%) | 3779 (43.4) | 2256 (63.9) | 1232 (42.2) | 291 (12.9) | <0.0001 |
AHEI indicates Alternate Healthy Eating Index‐2010; BMI, body mass index; CHIP, clonal hematopoiesis of indeterminate potential; GED, general equivalency diploma; MET, metabolic equivalent task; and WHI, Women's Health Initiative.
P value was calculated by χ2 test for categorical variables and linear regression model comparing difference across 3 categories for continuous variables.
The prevalence of CHIP in our study sample was 8.57% (746/8709). A higher healthy lifestyle score was not associated with presence of CHIP (OR [95% CI], 0.99 [0.80–1.23] and 1.13 [0.93–1.37] after full adjustment for the upper and middle category versus referent [P‐trend 0.95], respectively) (Table 2). Across the individual lifestyle factors, neither higher AHEI nor higher levels of physical activity were related to the presence of CHIP. Compared with women with obesity, a normal or overweight BMI (overweight BMI: OR, 0.83 [95% CI, 0.68–1.01]; or normal BMI: OR, 0.71 [95% CI, 0.57–0.88]; P‐trend 0.0015) was significantly associated with presence of CHIP. Having never smoked or being a past smoker, compared with a current smoker, tended to be associated with a lower odds for the presence of CHIP (never smoker: OR, 0.81 [95% CI, 0.58–1.13]; or past smoker: OR, 1.01 [95% CI, 0.72–1.41]; P‐trend 0.0168).
Table 2.
Presence of CHIP in Relation to Individual Lifestyle Factors and Healthy Lifestyle Score (n=8709)
| Variable | CHIP (Yes/No) | Minimally Adjusted Model* | Fully Adjusted Model † | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Healthy lifestyle score (range) | |||||
| Category 1 (0–1) | 274/3257 | Referent | 0.48 | Referent | 0.35 |
| Category 2 (2) | 271/2651 | 1.11 (0.93–1.32) | 1.13 (0.93–1.37) | ||
| Category 3 (3–4) | 201/2055 | 1.01 (0.83–1.23) | 0.99 (0.80–1.23) | ||
| P trend ‡ | 0.82 | 0.95 | |||
| Healthy lifestyle score, continuous | 1.00 (0.92–1.08) | 0.96 | 0.98 (0.90–1.08) | 0.72 | |
| AHEI (range) | |||||
| Quintile 1 (18–42.3) | 133/1606 | Referent | 0.37 | Referent | 0.22 |
| Quintile 2 (42.3–48.1) | 138/1604 | 0.99 (0.77–1.27) | 1.05 (0.80–1.37) | ||
| Quintile 3 (48.1–53.4) | 137/1605 | 0.96 (0.74–1.23) | 0.93 (0.71–1.23) | ||
| Quintile 4 (53.4–59.9) | 170/1573 | 1.17 (0.92–1.49) | 1.24 (0.96–1.61) | ||
| Quintile 5 (60–91) | 168/1575 | 1.12 (0.88–1.43) | 1.13 (0.86–1.48) | ||
| P trend ‡ | 0.14 | 0.16 | |||
| AHEI, continuous | 1.00 (1.00–1.01) | 0.20 | 1.01 (1.00–1.01) | 0.22 | |
| Physical activity | |||||
| Inactive | 306/3473 | Referent | 0.51 | Referent | 0.71 |
| Insufficiently active | 233/2363 | 1.11 (0.93–1.33) | 1.08 (0.89–1.31) | ||
| Active | 207/2127 | 1.07 (0.89–1.29) | 1.03 (0.84–1.26) | ||
| P trend ‡ | 0.42 | 0.73 | |||
| MET h/wk, continuous | 1.00 (1.00–1.01) | 0.62 | 1.00 (0.99–1.01) | 0.87 | |
| BMI | |||||
| Obese (BMI >30 kg/m2) | 271/2794 | Referent | 0.0246 | Referent | 0.0062 |
| Overweight (25 kg/m2≤BMI≤30 kg/m2) | 271/2815 | 0.89 (0.74–1.06) | 0.83 (0.68–1.01) | ||
| Normal (18.5 kg/m2≤BMI≤25 kg/m2) | 204/2354 | 0.76 (0.63–0.93) | 0.71 (0.57–0.88) | ||
| P trend ‡ | 0.0065 | 0.0015 | |||
| BMI, continuous | 1.02 (1.00–1.03) | 0.0148 | 1.02 (1.01–1.04) | 0.0028 | |
| Smoking | |||||
| Current smoker | 48/594 | Referent | 0.0205 | Referent | 0.0336 |
| Past smoker | 339/3238 | 1.08 (0.79–1.49) | 1.01 (0.72–1.41) | ||
| Never smoker | 359/4131 | 0.87 (0.63–1.19) | 0.81 (0.58–1.13) | ||
| P trend ‡ | 0.0199 | 0.0168 | |||
| Pack‐year of smoking, continuous | 1.00 (1.00–1.01) | 0.0094 | 1.00 (1.00–1.01) | 0.0103 | |
AHEI indicates Alternate Healthy Eating Index‐2010; BMI, body mass index; CHIP, clonal hematopoiesis of indeterminate potential; MET, metabolic equivalent task; and OR, odds ratio.
Model adjusted for age at CHIP and race/ethnicity, stratified by case type (stroke, venous thromboembolism, and controls).
Model adjusted for age at CHIP, race/ethnicity, education, income, marital status, use of cholesterol‐lowering medication, history of treated diabetes mellitus, hypertension, family history of cancer and hormone therapy use, and WHI (Women's Health Initiative) trial participation, stratified by case type (stroke, venous thromboembolism, and controls).
P value for trend test was obtained from the regression model by fitting the categorical variable as a continuous variable to evaluate a dose‐response relationship.
In analyses stratified by presence of a CHIP‐defining DNMT3A or TET2 mutation, no relationship between healthy lifestyle score and CHIP was detected (Table 3). Having a normal or overweight BMI, compared with obese BMI, was associated with presence of CHIP for both driver mutations in a graded manner (DNMT3A: normal BMI, OR, 0.69 [95% CI, 0.52–0.91]; overweight BMI, OR, 0.78 [95% CI, 0.61–1.00] [P‐trend 0.0070]; and TET2: normal BMI, OR, 0.59 [95% CI, 0.38–0.92]; overweight BMI, OR, 0.72 [95% CI, 0.48–1.07] [P‐trend 0.0188]). Having never smoked, compared with being a current smoker, tended to be related to a lower odds for presence of a CHIP‐defining DNMT3A mutation (P‐trend 0.0057). No association between smoking and presence of a CHIP‐defining TET2 mutation was detected, but there was an insufficient number of cases available.
Table 3.
Presence of CHIP in DNMT3A and TET2 Genes in Relation to Individual Lifestyle Factors and Healthy Lifestyle Score
| Variable |
DNMT3A CHIP (Yes/No) |
DNMT3A (n=8395) |
TET2 CHIP (Yes/No) |
TET2 (n=8126) |
||
|---|---|---|---|---|---|---|
| OR (95% CI)* | P Value | OR (95% CI)* | P Value | |||
| Healthy lifestyle score (range) | ||||||
| Category 1 (0–1) | 166/3257 | Referent | 0.96 | 60/3257 | Referent | 0.95 |
| Category 2 (2) | 144/2651 | 1.02 (0.79–1.30) | 57/2651 | 1.03 (0.69–1.54) | ||
| Category 3 (3–4) | 122/2055 | 1.04 (0.79–1.37) | 46/2055 | 0.96 (0.62–1.50) | ||
| P trend † | 0.78 | 0.89 | ||||
| Healthy lifestyle score, continuous | 0.99 (0.89–1.11) | 0.90 | 1.00 (0.83–1.20) | 0.98 | ||
| AHEI (range) | ||||||
| Quintile 1 (18–42.3) | 79/1606 | Referent | 0.0466 | 22/1606 | Referent | 0.16 |
| Quintile 2 (42.3–48.1) | 75/1604 | 0.92 (0.65–1.30) | 38/1604 | 1.86 (1.03–3.36) | ||
| Quintile 3 (48.1–53.4) | 75/1605 | 0.83 (0.59–1.19) | 28/1605 | 1.29 (0.69–2.42) | ||
| Quintile 4 (53.4–59.9) | 111/1573 | 1.33 (0.97–1.84) | 31/1573 | 1.42 (0.76–2.63) | ||
| Quintile 5 (60–91) | 92/1575 | 1.01 (0.71–1.42) | 44/1575 | 1.88 (1.03–3.41) | ||
| P trend † | 0.28 | 0.18 | ||||
| AHEI, continuous | 1.01 (0.99–1.02) | 0.32 | 1.01 (1.00–1.03) | 0.15 | ||
| Physical activity | ||||||
| Inactive | 179/3473 | Referent | 0.89 | 72/3473 | Referent | 0.88 |
| Insufficiently active | 132/2363 | 1.06 (0.83–1.36) | 47/2363 | 0.91 (0.61–1.36) | ||
| Active | 121/2127 | 1.02 (0.79–1.32) | 44/2127 | 0.93 (0.62–1.41) | ||
| P trend † | 0.85 | 0.72 | ||||
| MET h/wk, continuous | 1.00 (0.99–1.00) | 0.33 | 1.01 (1.00–1.02) | 0.14 | ||
| BMI | ||||||
| Obese (BMI >30 kg/m2) | 169/2794 | Referent | 0.0210 | 61/2794 | Referent | 0.06 |
| Overweight (25 kg/m2≤BMI≤30 kg/m2) | 151/2815 | 0.78 (0.61–1.00) | 58/2815 | 0.72 (0.48–1.07) | ||
| Normal (18.5 kg/m2≤BMI≤25 kg/m2) | 112/2354 | 0.69 (0.52–0.91) | 44/2354 | 0.59 (0.38–0.92) | ||
| P trend † | 0.0070 | 0.0188 | ||||
| BMI, continuous | 1.02 (1.01–1.04) | 0.0085 | 1.03 (1.00–1.06) | 0.0338 | ||
| Smoking | ||||||
| Current smoker | 33/594 | Referent | 0.0203 | 5/594 | Referent | 0.27 |
| Past smoker | 203/3238 | 0.87 (0.58–1.30) | 66/3238 | 2.20 (0.79–6.13) | ||
| Never smoker | 196/4131 | 0.67 (0.45–1.00) | 92/4131 | 2.31 (0.83–6.40) | ||
| P trend † | 0.0057 | 0.23 | ||||
| Pack‐year of smoking, continuous | 1.01 (1.00–1.01) | 0.0028 | 1.00 (0.99–1.01) | 0.78 | ||
AHEI indicates Alternate Healthy Eating Index‐2010; BMI, body mass index; CHIP, clonal hematopoiesis of indeterminate potential; MET, metabolic equivalent task; and OR, odds ratio.
Model adjusted for age at CHIP, race/ethnicity, education, income, marital status, use of cholesterol‐lowering medication, history of treated diabetes mellitus, hypertension, family history of cancer and hormone therapy use, and WHI (Women's Health Initiative) trial participation, stratified by case type (stroke, venous thromboembolism, and controls).
P value for trend test was obtained from the regression model by fitting the categorical variable as a continuous variable to evaluate a dose‐response relationship.
As presence of CHIP strongly increases with age, we undertook additional sensitivity analyses, where we restricted our study population to participants aged ≥65 years (Table 4). The main results did not change. A higher healthy lifestyle score was not related to the presence of CHIP, whereas a normal or an overweight BMI, compared with an obese BMI, remained associated with presence of CHIP in a graded manner (normal BMI: OR, 0.66 [95% CI, 0.52–0.85]; overweight BMI: OR, 0.74 [95% CI, 0.59–0.93] [P‐trend 0.001]). Smoking status was not associated with presence of CHIP. In stratified analysis by presence of CHIP‐defining DNMT3A and TET2 mutations in participants aged ≥65 years, a normal or overweight BMI remained strongly related to a CHIP‐defining DNMT3A mutation (normal BMI: OR, 0.65 [95% CI, 0.47–0.90]; or overweight BMI: OR, 0.76 [95% CI, 0.57–1.01] [P‐trend 0.0082]), but the association was slightly attenuated in case of TET2 (normal BMI: OR, 0.61 [95% CI, 0.38–1.00]; or overweight BMI: OR, 0.65 [95% CI, 0.41–1.02] [P‐trend 0.0499]), most likely because of lower sample sizes (Table S1). No relationship between healthy lifestyle scoring, AHEI, physical activity, or smoking status and presence of CHIP‐defining DNMT3A and TET2 mutations was detected in older women.
Table 4.
Presence of CHIP in Relation to Individual Lifestyle Factors and Healthy Lifestyle Score Among Participants Aged ≥65 Years at Baseline (n=5488)
| Variable | CHIP (Yes/No) | Minimally Adjusted Model* | Fully Adjusted Model † | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Healthy lifestyle score (range) | |||||
| Category 1 (0–1) | 187/1796 | Referent | 0.40 | Referent | 0.28 |
| Category 2 (2) | 212/1705 | 1.15 (0.93–1.42) | 1.17 (0.94–1.47) | ||
| Category 3 (3–4) | 163/1425 | 1.04 (0.83–1.30) | 1.01 (0.79–1.29) | ||
| P trend ‡ | 0.72 | 0.89 | |||
| Healthy lifestyle score, continuous | 1.01 (0.92–1.11) | 0.78 | 1.00 (0.90–1.10) | 0.96 | |
| AHEI (range) | |||||
| Quintile 1 (18–42.3) | 86/861 | Referent | 0.50 | Referent | 0.28 |
| Quintile 2 (42.3–48.1) | 102/948 | 1.06 (0.78–1.44) | 1.16 (0.83–1.60) | ||
| Quintile 3 (48.1–53.4) | 106/990 | 1.05 (0.78–1.42) | 1.10 (0.80–1.53) | ||
| Quintile 4 (53.4–59.9) | 131/1027 | 1.24 (0.93–1.66) | 1.39 (1.01–1.90) | ||
| Quintile 5 (60–91) | 137/1100 | 1.19 (0.90–1.59) | 1.24 (0.90–1.71) | ||
| P trend ‡ | 0.11 | 0.10 | |||
| AHEI, continuous | 1.01 (1.00–1.01) | 0.23 | 1.01 (1.00–1.02) | 0.24 | |
| Physical activity | |||||
| Inactive | 236/2100 | Referent | 0.79 | Referent | 0.99 |
| Insufficiently active | 162/1457 | 0.98 (0.80–1.22) | 0.99 (0.79–1.24) | ||
| Active | 164/1369 | 1.06 (0.86–1.31) | 1.01 (0.80–1.27) | ||
| P trend ‡ | 0.62 | 0.95 | |||
| MET h/wk, continuous | 1.00 (1.00–1.01) | 0.57 | 1.00 (0.99–1.01) | 0.94 | |
| BMI | |||||
| Obese (BMI >30 kg/m2) | 197/1543 | Referent | 0.0131 | Referent | 0.0027 |
| Overweight (25 kg/m2≤BMI≤30 kg/m2) | 203/1801 | 0.81 (0.66–1.00) | 0.74 (0.59–0.93) | ||
| Normal (18.5 kg/m2≤BMI≤25 kg/m2) | 162/1582 | 0.72 (0.57–0.90) | 0.66 (0.52–0.85) | ||
| P trend ‡ | 0.0036 | 0.0010 | |||
| BMI, continuous | 1.02 (1.01–1.04) | 0.0018 | 1.03 (1.01–1.05) | 0.0005 | |
| Smoking | |||||
| Current smoker | 24/260 | Referent | 0.05 | Referent | 0.09 |
| Past smoker | 254/1981 | 1.28 (0.82–1.99) | 1.12 (0.71–1.75) | ||
| Never smoker | 284/2685 | 1.03 (0.66–1.60) | 0.90 (0.58–1.41) | ||
| P trend ‡ | 0.11 | 0.07 | |||
| Pack‐year of smoking, continuous | 1.00 (1.00–1.01) | 0.06 | 1.00 (1.00–1.01) | 0.10 | |
AHEI indicates Alternate Healthy Eating Index‐2010; BMI, body mass index; CHIP, clonal hematopoiesis of indeterminate potential; MET, metabolic equivalent task; and OR, odds ratio.
Model adjusted for age at CHIP and race/ethnicity, stratified by case type (stroke, venous thromboembolism, and controls).
Model adjusted for age at CHIP, race/ethnicity, education, income, marital status, use of cholesterol‐lowering medication, history of treated diabetes mellitus, hypertension, family history of cancer and hormone therapy use, and WHI (Women's Health Initiative) trial participation, stratified by case type (stroke, venous thromboembolism, and controls).
P value for trend test was obtained from the regression model by fitting the categorical variable as a continuous variable to evaluate a dose‐response relationship.
Finally, we undertook sensitivity analyses to investigate the relationship between AHEI, physical activity, and smoking with CHIP by additionally adjusting for BMI (Table S2). The association between AHEI, physical activity, and presence of CHIP remained nonsignificant, whereas never smoking or past smoking, compared with being a current smoker, again showed a stronger relationship (never smoker: OR, 0.78 [95% CI, 0.55–1.09]; past smoker: OR, 0.96 [95% CI, 0.68–1.35] [P‐trend=0.0120]).
Discussion
A healthy lifestyle at midlife has been shown to be associated with higher life expectancy free of major chronic diseases, including CVD and cancer. 16 , 41 At the same time, available evidence suggests that 95% of individuals in midlife already possess low levels of hematopoietic somatic mutations, predominantly in the DNMT3A and TET2 regions. 21 These findings have led to the compelling hypothesis that age‐associated chronic diseases, both CVD and non‐CVD, may be influenced by somatic mutations that lead to CHIP. 12 Therefore, the question emerges if a healthy lifestyle, which is a mainstay to CVD and cancer primary prevention, is associated with a lower prevalence of CHIP.
In a large cohort of community‐dwelling ambulatory postmenopausal women free of CVD or cancer at baseline, we found that a higher healthy lifestyle score, a composite measure of 4 prespecified lifestyle factors (BMI, smoking, diet quality, and physical activity), was not associated with presence of CHIP. However, there was a suggestion that individual healthy lifestyle components (namely, normal BMI and never smoking) are associated with a lower prevalence of CHIP.
Compared with obese women, normal weight women had a significantly lower presence of CHIP, defined by either DNMT3A or TET2 mutations. Recently, CRISPR technology has been used to evaluate the consequences of inactivating mutations in TET2 and DNMT3A. 42 The inactivation of either DNMT3A or TET2 led to increased cytokine expression, but the effects differed qualitatively and quantitatively, suggesting that they confer gene‐specific effects. Although inactivation of DNMT3A resulted in greater cardiac hypertrophy and increased fibrosis after angiotensin‐II administration, TET2‐mediated CHIP lead to the upregulation of interleukin‐6/interleukin‐1β because TET2 acts as a negative regulator of proinflammatory macrophage activation and induces atherosclerosis. 43 , 44 On the other hand, genetically reduced interleukin‐6 signaling can decrease CVD risk in CHIP carriers. 45 Adipose tissue can synthesize cytokines, such as tumor necrosis factor‐α and interleukin‐6, and has been shown to promote inflammation and atherogenesis, independent of effects on insulin resistance or lipoproteins. 46 , 47 Most recently, high‐sensitivity CRP (C‐reactive protein) has been found to be associated with CHIP. 48 Thus, obesity and CHIP may be related to one another through increased activation of proinflammatory pathways. 46 Nonetheless, data on the relationship between obesity and CHIP are largely missing, and evidence is conflicting. 11 , 17 , 18 , 49 However, such information is of importance. 12 Our data can help to better characterize early weight‐associated health changes. Specifically, screening for CHIP and its major driver mutations may help to clarify ongoing disputes on how to handle “metabolically healthy” obesity. The presence of CHIP in obese individuals may prompt clinicians to consider more intensified interventions, such as individualized weight loss strategies or even interleukin‐6/interleukin‐1β blockade, with the aim of preventing atherosclerotic lesion development and accumulation of further somatic mutations at an early subclinical disease stage. 7 , 12 , 45 Further outcome research, aimed at examining body weight and adiposity trajectories and associated metabolic factors in relation to presence of CHIP, is needed.
Prior evidence mainly based on study populations experiencing a high burden of atherosclerotic disease suggests that smoking is positively associated with the presence of CHIP, although findings have been inconsistent. 8 , 11 , 17 , 50 In our analysis consisting of relatively healthy postmenopausal women, having never smoked compared with being a current smoker tended to be associated with a lower frequency of CHIP, which is in support of the underlying pathophysiological hypothesis. Unfortunately, however, our sample sizes were low and our results were not consistent across all subgroups, as in case of TET2 driver carriers. In fact, the prevalence of current smoking was ≈7% in women included in this analysis. In contrast, in the United States, the prevalence was 17% and 14% among women aged 60 to 74 years in the National Health and Nutrition Examination Survey 1988 to 1994 and the National Health and Nutrition Examination Survey 1999 to 2002, respectively. 51
In contrast to our hypothesis, we did not observe a relationship between diet quality, assessed via AHEI, and presence of CHIP. These results did not change after additional adjustment for BMI. There may be several explanations for the lack of an association. The AHEI is based on the US Dietary Guidelines for Americans recommendations for optimal overall health and prevention of major chronic diseases, such as CVD and type 2 diabetes mellitus. 37 There may be other dietary factors, not accurately assessed using the AHEI, that are potentially associated with CHIP, and further research is warranted to understand whether other dietary patterns or specific foods are relevant to CHIP. Studies in mice show that a life‐long, high‐fat diet does not affect hematopoietic stem cell function, but caloric restriction can prevent age‐dependent increases in bone marrow cellularity. 52 Although dietary intake was assessed on the basis of a validated food‐frequency questionnaire in our cohort, measurement error may be present and data reflecting dietary changes over time are missing. Physical activity, or lack thereof, plays a role in maintaining genomic stability and greatly influences the immune and inflammatory systems, and atherosclerosis risk factors and clinical end points. 53 To this point, to our knowledge, specific evidence on a relationship between physical activity and presence of CHIP is missing. Again, in contrast to our hypothesis, we did not observe a significant association between physical activity and presence of CHIP. Considering potential methodological issues, physical activity was ascertained using a self‐report questionnaire in WHI, and the potential for misclassification is present. 54 Prior investigations in mice demonstrate that exercise can increase hematopoietic stem cell quantity and is associated with more activated, differentiated hematopoietic cells. However, this expansion may not improve or impair hematopoietic stem cell function. 55 , 56 Thus, our results on diet quality, physical activity, and CHIP are hypothesis generating and need further replication in both epidemiological and basic science investigations.
Our investigation is the first to report on the association of a healthy lifestyle, as a composite score and individual factors, with CHIP among a diverse group of older women. Strengths of this study include a large ethnically diverse sample size without a history of CVD or cancer and a well‐structured cohort with availability of a wide range of confounding factors. Among the limitations, most important, our results stem from a cross‐sectional assessment of lifestyle factors and presence of CHIP, and causation cannot be inferred. Herein, we studied older postmenopausal women in whom CHIP frequency already is relatively high because of aging‐related influences. Additional data from human studies are needed to evaluate the prospective association between lifestyle habits and their change over time with development of CHIP. Moreover, our study population was not adequately powered to investigate other driver mutations, such as TP53, SF3B1, and JAK2, with only 7, 13, and 32 women, respectively, with CHIP attributable to these driver mutations in our study sample.
Conclusions
A healthy lifestyle, based on a composite score, was not related to presence of CHIP among postmenopausal women free of CVD and cancer. Across individual lifestyle factors, compared with obese women, normal weight women had a significantly lower presence of CHIP, defined by either DNMT3A or TET2 mutations. Moreover, having never smoked, compared with being a current smoker, tended to be associated with lower frequency of CHIP. Our data suggest that certain healthy lifestyle factors are associated with a lower prevalence of CHIP in an older population.
Sources of Funding
The WHI (Women's Health Initiative) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), US Department of Health and Human Services, through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. Drs Reiner and Whitsel were supported by NIH R01 HL148565. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the US Department of Health and Human Services/NIH. This publication was supported by the Open Access Publication Fund of the University of Würzburg.
Disclosures
Dr Natarajan reports investigator‐initiated grants from Amgen, Apple, and Boston Scientific, and is a scientific advisor to Apple and Blackstone Life Sciences, all unrelated to the present work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Acknowledgments
The authors thank the WHI (Women's Health Initiative) participants, clinical sites, investigators, and staff for their dedicated efforts. A list of WHI investigators is available online at: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf.
Open access funding enabled and organized by ProjektDEAL.
(J Am Heart Assoc. 2021;10:e018789. DOI: 10.1161/JAHA.120.018789.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018789
For Sources of Funding and Disclosures, see page 10.
References
- 1. Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019;366:eaan4673. 10.1126/science.aan4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fuster JJ, Walsh K. Somatic mutations and clonal hematopoiesis: unexpected potential new drivers of age‐related cardiovascular disease. Circ Res. 2018;122:523–532. 10.1161/CIRCRESAHA.117.312115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ebert BL, Libby P. Clonal hematopoiesis confers predisposition to both cardiovascular disease and cancer: a newly recognized link between two major killers. Ann Intern Med. 2018;169:116–117. 10.7326/M18-0737 [DOI] [PubMed] [Google Scholar]
- 4. Laurie CC, Laurie CA, Rice K, Doheny KF, Zelnick LR, McHugh CP, Ling H, Hetrick KN, Pugh EW, Amos C, et al. Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat Genet. 2012;44:642–650. 10.1038/ng.2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buscarlet M, Provost S, Zada YF, Barhdadi A, Bourgoin V, Lepine G, Mollica L, Szuber N, Dube MP, Busque L. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. 2017;130:753–762. 10.1182/blood-2017-04-777029 [DOI] [PubMed] [Google Scholar]
- 6. Busque L, Patel JP, Figueroa ME, Vasanthakumar A, Provost S, Hamilou Z, Mollica L, Li J, Viale A, Heguy A, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44:1179–1181. 10.1038/ng.2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood Adv. 2018;2:3404–3410. 10.1182/bloodadvances.2018020222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, et al. Age‐related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. 10.1056/NEJMoa1408617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, Kosmider O, Le Couedic JP, Robert F, Alberdi A, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360:2289–2301. [DOI] [PubMed] [Google Scholar]
- 10. Desai P, Mencia‐Trinchant N, Savenkov O, Simon MS, Cheang G, Lee S, Samuel M, Ritchie EK, Guzman ML, Ballman KV, et al. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat Med. 2018;24:1015–1023. 10.1038/s41591-018-0081-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. 10.1056/NEJMoa1701719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Libby P, Sidlow R, Lin AE, Gupta D, Jones LW, Moslehi J, Zeiher A, Jaiswal S, Schulz C, Blankstein R, et al. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC review topic of the week. J Am Coll Cardiol. 2019;74:567–577. 10.1016/j.jacc.2019.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corlin L, Short MI, Vasan RS, Xanthakis V. Association of the duration of ideal cardiovascular health through adulthood with cardiometabolic outcomes and mortality in the Framingham Offspring Study. JAMA Cardiol. 2020;5:549–556. 10.1001/jamacardio.2020.0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ford ES, Bergmann MM, Boeing H, Li C, Capewell S. Healthy lifestyle behaviors and all‐cause mortality among adults in the United States. Prev Med. 2012;55:23–27. 10.1016/j.ypmed.2012.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mozaffarian D, Afshin A, Benowitz NL, Bittner V, Daniels SR, Franch HA, Jacobs DR, Kraus WE, Kris‐Etherton PM, Krummel DA, et al. Population approaches to improve diet, physical activity, and smoking habits: a scientific statement from the American Heart Association. Circulation. 2012;126:1514–1563. 10.1161/CIR.0b013e318260a20b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Schoufour J, Wang DD, Dhana K, Pan AN, Liu X, Song M, Liu G, Shin HJ, Sun QI, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. 2020;368:l6669. 10.1136/bmj.l6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou‐El‐Ardat K, Schmid T, Brüne B, Wagner S, Serve H, et al. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. 2019;4:25–33. 10.1001/jamacardio.2018.3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mas‐Peiro S, Hoffmann J, Fichtlscherer S, Dorsheimer L, Rieger MA, Dimmeler S, Vasa‐Nicotera M, Zeiher AM. Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur Heart J. 2020;41:933–939. 10.1093/eurheartj/ehz591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. King KY, Huang Y, Nakada D, Goodell MA. Environmental influences on clonal hematopoiesis. Exp Hematol. 2020;83:66–73. 10.1016/j.exphem.2019.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, et al. Clonal hematopoiesis and blood‐cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. 10.1056/NEJMoa1409405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML‐associated mutations is ubiquitous in healthy adults. Nat Commun. 2016;7:12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McKerrell T, Park N, Moreno T, Grove CS, Ponstingl H, Stephens J; Understanding Society Scientific Group , Crawley C, Craig J, Scott MA, Hodkinson C, et al. Leukemia‐associated somatic mutations drive distinct patterns of age‐related clonal hemopoiesis. Cell Rep. 2015;10:1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, Hyman DM, Solit DB, Robson ME, Baselga J, et al. Therapy‐related clonal hematopoiesis in patients with non‐hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21:374–382.e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. The Women's Health Initiative Study Group . Design of the women's health initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 25. Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. [DOI] [PubMed] [Google Scholar]
- 26. Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative observational study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. [DOI] [PubMed] [Google Scholar]
- 27. Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, et al. Low‐fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–642. [DOI] [PubMed] [Google Scholar]
- 28. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 29. Bick AG, Weinstock JS, Nandakumar SK, Fulco CP, Leventhal MJ, Bao EL, Nasser J, Zekavat SM, Szeto MD, Laurie C, et al. Inherited causes of clonal hematopoiesis of indeterminate potential in TOPMed whole genomes. bioRxiv, preprint. 2019:782748. [Google Scholar]
- 30. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36), I: conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 31. Hays RD, Sherbourne CD, Mazel RM. The rand 36‐item health survey 1.0. Health Econ. 1993;2:217–227. 10.1002/hec.4730020305 [DOI] [PubMed] [Google Scholar]
- 32. US Department of Health & Human Services . Physical activity guidelines for Americans. 2008. Available at:https://health.gov/sites/default/files/2019‐09/paguide.pdf, Accessed June 12, 2020.
- 33. Agha G, Loucks EB, Tinker LF, Waring ME, Michaud DS, Foraker RE, Li W, Martin LW, Greenland P, Manson JE, et al. Healthy lifestyle and decreasing risk of heart failure in women: the Women's Health Initiative observational study. J Am Coll Cardiol. 2014;64:1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–S17. 10.1016/S1047-2797(03)00043-7 [DOI] [PubMed] [Google Scholar]
- 35. Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs‐Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. 10.1016/S1047-2797(98)00055-6 [DOI] [PubMed] [Google Scholar]
- 36. McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr. 2006;9:152–157. 10.1079/PHN2005938 [DOI] [PubMed] [Google Scholar]
- 37. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Y, Pan AN, Wang DD, Liu X, Dhana K, Franco OH, Kaptoge S, Di Angelantonio E, Stampfer M, Willett WC, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. 2018;138:345–355. 10.1161/CIRCULATIONAHA.117.032047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reeves MJ, Rafferty AP. Healthy lifestyle characteristics among adults in the United States, 2000. Arch Intern Med. 2005;165:854–857. 10.1001/archinte.165.8.854 [DOI] [PubMed] [Google Scholar]
- 40. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, et al. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 41. Liu G, Li Y, Hu Y, Zong G, Li S, Rimm EB, Hu FB, Manson JE, Rexrode KM, Shin HJ, et al. Influence of lifestyle on incident cardiovascular disease and mortality in patients with diabetes mellitus. J Am Coll Cardiol. 2018;71:2867–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K. CRISPR‐mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res. 2018;123:335–341. 10.1161/CIRCRESAHA.118.313225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu C‐L, Sano S, Muralidharan S, Rius C, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. 10.1126/science.aag1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, et al. Tet2‐mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL‐1beta/NLRP3 inflammasome. J Am Coll Cardiol. 2018;71:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bick AG, Pirruccello JP, Griffin GK, Gupta N, Gabriel S, Saleheen D, Libby P, Kathiresan S, Natarajan P. Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation. 2020;141:124–131. 10.1161/CIRCULATIONAHA.119.044362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- 47. Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C‐reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. 10.1161/01.ATV.19.4.972 [DOI] [PubMed] [Google Scholar]
- 48. Busque L, Sun M, Buscarlet M, Ayachi S, Feroz Zada Y, Provost S, Bourgoin V, Mollica L, Meisel M, Hinterleitner R, et al. High‐sensitivity C‐reactive protein is associated with clonal hematopoiesis of indeterminate potential. Blood Adv. 2020;4:2430–2438. 10.1182/bloodadvances.2019000770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee MKS, Dragoljevic D, Bertuzzo Veiga C, Wang N, Yvan‐Charvet L, Murphy AJ. Interplay between clonal hematopoiesis of indeterminate potential and metabolism. Trends Endocrinol Metab. 2020;31:525–535. 10.1016/j.tem.2020.02.005 [DOI] [PubMed] [Google Scholar]
- 50. Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I, Thorgeirsson TE, Sigurdsson A, Gudjonsson SA, Gudmundsson J, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130:742–752. 10.1182/blood-2017-02-769869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Flegal KM. The effects of changes in smoking prevalence on obesity prevalence in the United States. Am J Public Health. 2007;97:1510–1514. 10.2105/AJPH.2005.084343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lazare S, Ausema A, Reijne AC, van Dijk G, van Os R, de Haan G. Lifelong dietary intervention does not affect hematopoietic stem cell function. Exp Hematol. 2017;53:26–30. 10.1016/j.exphem.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 53. Nahrendorf M, Swirski FK. Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res. 2015;116:884–894. 10.1161/CIRCRESAHA.116.303550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test‐retest reliability of the Women's Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41:530–538. 10.1249/MSS.0b013e31818ace55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Lisio M, Parise G. Characterization of the effects of exercise training on hematopoietic stem cell quantity and function. J Appl Physiol (1985). 2012;113:1576–1584. 10.1152/japplphysiol.00717.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Patel VS, Ete Chan M, Rubin J, Rubin CT. Marrow adiposity and hematopoiesis in aging and obesity: exercise as an intervention. Curr Osteoporos Rep. 2018;16:105–115. 10.1007/s11914-018-0424-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


