Abstract
Background
Although Black adults are more likely to die from coronary heart disease (CHD) compared with White adults, few studies have examined the relationship between cigarette smoking and CHD risk among Black adults. We evaluated the relationship between cigarette smoking, incident CHD, and coronary artery calcification in the JHS (Jackson Heart Study).
Methods and Results
We classified JHS participants without a history of CHD (n=4432) by self‐reported baseline smoking status into current, former (smoked at least 400 cigarettes/life) or never smokers at baseline (2000–2004). We further classified current smokers by smoking intensity (number of cigarettes smoked per day [1–19 or ≥20]) and followed for incident CHD (through 2016). Hazard ratios (HR) for incident CHD for each smoking group compared with never smokers were estimated with adjusted Cox proportional hazard regression models. At baseline, there were 548 (12.4%) current, 782 (17.6%) former, and 3102 (70%) never smokers. During follow‐up (median, 13.8 years), 254 participants developed CHD. After risk factor adjustment, CHD risk was significantly higher in current smokers compared with never smokers (HR, 2.11; 95% CI, 1.39–3.18); the difference between former smokers and never smokers (HR, 1.37; 95% CI, 1.0–1.90) did not achieve statistical significance. Among current smokers, we did not observe a dose‐response effect for CHD risk. Additionally, in multivariable logistic regression models with a subset of our analytic cohort, current smokers had greater odds of coronary artery calcification score >0 compared with never smokers (odds ratio, 2.63; 95% CI, 1.88–3.68).
Conclusions
In a large prospective cohort of Black adults, current smoking was associated with a >2‐fold increased risk of CHD over a median follow‐up of greater than a decade.
Keywords: Black adults, cigarette smoking, coronary artery calcification, coronary heart disease, Jackson Heart Study
Subject Categories: Cardiovascular Disease, Epidemiology, Race and Ethnicity
Nonstandard Abbreviations and Acronyms
- JHS
Jackson Heart Study
Clinical Perspective
What Is New?
In a large community‐based longitudinal study assessing cardiovascular risk in Black adults, current cigarette smoking was associated with >2 times increased risk for coronary heart disease.
By coronary artery calcification score measurements (used to evaluate the burden of coronary atherosclerosis and predict the risk for future coronary heart disease events), we found a dose‐dependent increase in the odds of having coronary artery calcification scores >2 clinically important clinical thresholds (coronary artery calcification score >0 and coronary artery calcification score >400), among current Black smokers, after adjusting for other cardiovascular risk factors.
What Are the Clinical Implications?
In view of the high incidence of coronary heart disease among Black smokers, our findings highlight the need for more smoking cessation strategies directed toward this population.
Coronary heart disease (CHD) accounts for ≈1 in 7 deaths in the United States, 1 , 2 where an American has a heart attack every 40 seconds. 1 Black adults are more likely to die from CHD compared with White adults. 1 Higher rates of traditional risk factors such as diabetes mellitus, hypertension, and obesity among Black adults may account for some of this disparity. 3 , 4 , 5 Cigarette smoking is also an independent risk factor for the development of CHD. 6 , 7 , 8 Smoking causes inflammation, impaired endothelial function, platelet dysfunction, increased oxidative stress, and atherosclerosis, all of which are associated with the development of CHD. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 While previous studies have shown a significant association between cigarette smoking and CHD, this relationship has been understudied in Black adults. Although the prevalence of cigarette smoking has declined among Black adults in recent years, 14.9% of Black adults are current smokers, 18 and prior reports have shown longer smoking durations among Black adults compared with White adults and lower smoking cessation rates in Black adults compared with both White and Hispanic adults. 19 , 20 Thus, combined with higher rates of traditional risk factors for CHD, Black adults who smoke cigarettes may represent a particularly vulnerable population at risk of CHD.
Therefore, we assessed the relationships between cigarette smoking status, intensity, and dose with incident CHD in participants of the JHS (Jackson Heart Study), a large, well‐characterized community‐based Black cohort. We also evaluated the relationship between cigarette smoking status and intensity and coronary artery calcification (CAC) score measurements.
METHODS
Study Population
The JHS is a large, community‐based prospective cohort study designed to investigate the predictors and outcomes of cardiovascular diseases in Black adults. The study included 5306 participants aged 21 to 84 years who were recruited from the tri‐county area surrounding Jackson, Mississippi. Participants were evaluated at baseline from 2000 to 2004 (visit 1) and completed 2 subsequent study follow‐up visits (visit 2, 2005–2008; visit 3, 2009–2013) in addition to annual follow‐up telephone calls. The data, analytic methods, and study materials are available to other researchers for purposes of reproducing the results or replicating the analyses by following the JHS publications, procedures, and data use agreements. 21
The study was approved by the Institutional Review Boards of Jackson State University, Tougaloo College, and the University of Mississippi Medical Center in Jackson, Mississippi. All participants provided written informed consent.
Inclusion/Exclusion Criteria
We excluded all participants with a history of CHD (n=418); missing CHD data (n=190); history of cardiovascular disease (n=154); missing information on smoking status, intensity, and dose (n=46); or missing information on relevant study variables (ie, those included in multivariable analyses, n=66) at visit 1 (Figure 1).
Figure 1. Flow diagram for determination of final study population.
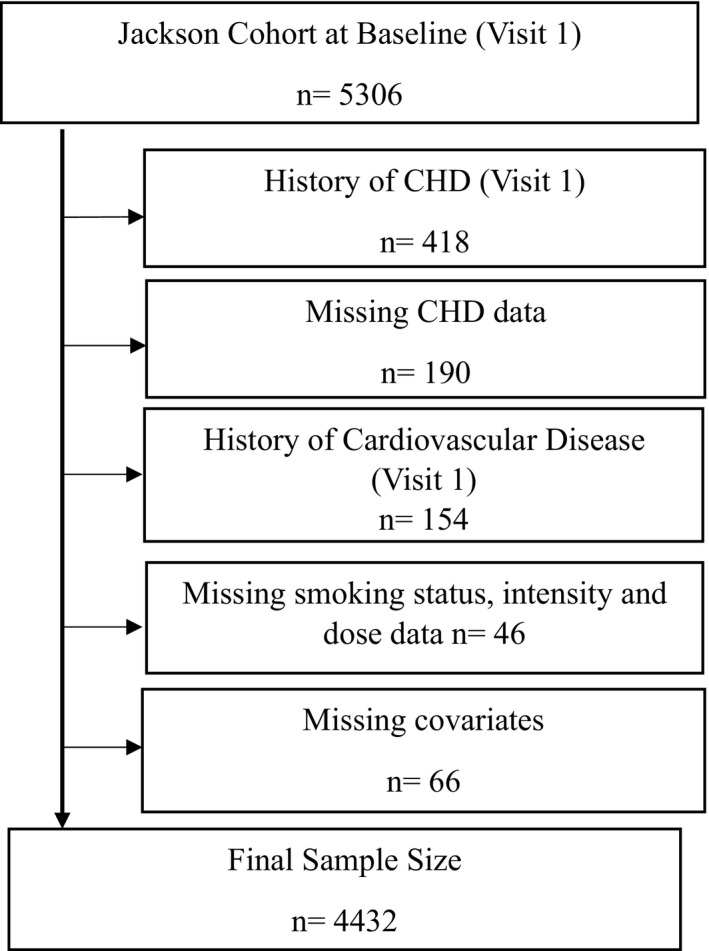
**171 participants were administratively censored, and 657 died during the follow‐up period in JHS. CHD indicates coronary heart disease; and JHS, Jackson Heart Study.
Smoking Information
Smoking information was obtained by a self‐reported questionnaire at baseline (visit 1). Participants who smoked >400 cigarettes in their lifetime were defined as ever smokers. Participants who gave a positive response to the question, “Do you now smoke cigarettes?” were classified as current smokers. Those who responded negatively to these questions were classified as never smokers. Participants who were classified as ever smokers who no longer smoked at the time of the examination were classified as former smokers. 22 Information concerning the number of cigarettes smoked daily was collected to determine smoking intensity, and smoking burden (pack‐years) was calculated by multiplying the number of cigarettes smoked daily by the number of years smoking.
Ascertainment of CHD Events
The JHS defined CHD as definite or probable myocardial infarction (MI) (chest pain, ECG changes, and changes in cardiac biomarker levels), definite fatal MI, definite fatal CHD (based on cause of death from death certificate) or cardiac procedures (percutaneous transluminal coronary angioplasty, stent placement, coronary revascularization, or coronary artery bypass grafting). 23 , 24 CHD events were ascertained via directed patient queries during annual telephone follow‐up and ongoing surveillance of hospitalizations, with subsequent transmission of hospital records and death certificates to a medical record abstraction unit for review. A computer‐generated diagnosis with physician adjudication was used to classify hospitalized and fatal CHD events. Details of these procedures have been described previously in this population. 25
Coronary Artery Calcification Score Measurement
Coronary artery calcification (CAC) measurements can be used to evaluate the burden of atherosclerosis. 26 , 27 An increased CAC score has been associated with incident CHD. 28 , 29 Therefore, we examined the relationship between cigarette smoking and the development of CHD in Black adults of the JHS, through CAC scores (measured at visit 2). CAC was measured by a 16‐channel multidetector computed tomography system equipped with cardiac gating (GE Healthcare Lightspeed 16 Pro, Waukesha, WI) during visit 2 (2005–2008) at the JHS Computed Tomography Data Acquisition Center located at the Jackson Medical Mall. The computed tomography research protocol of the JHS included imaging the heart, liver, and abdomen. 30 Computed tomography scan images were read at the Wake Forest University School of Medicine. CAC scores were reported using Agatston scoring at a threshold of 130 Hounsfield units and a minimum lesion size of 1 mm2. An Agatston score >0 was defined as the presence of CAC. Details of CAC scoring performed in the JHS have been described in previous studies. 31
Clinical Covariates
At the baseline exam (2000–2004), home interviews, clinic visits, physical examinations, and laboratory tests were conducted. Age and sex were recorded from questionnaires. Weight was measured using a balance scale in kilograms. Standing height was measured using a vertical ruler in centimeters. Body mass index was calculated as body weight (kilograms) divided the square of height (meters).
Physical activity survey instruments (adapted from the Baeke physical activity and ARIC [Atherosclerosis Risk in Communities] surveys) were administered by trained interviewers at baseline to calculate the physical activity score for each participant. 32 , 33 Participants were further categorized on the basis of their scores into poor, intermediate, ideal/recommended from the American Heart Association's recommended levels of physical activity 34 (ideal health indicator from physical activity). Each participant's self‐reported highest level of education was recorded and categorized: less than high school; high school graduate/General Educational Development; and attended vocational school, trade school, or college.
Blood pressure was calculated from the average of 2 sitting blood pressure measurements, at 5‐minute intervals. Hypertension was defined as BP ≥140/90 mm Hg or use of self‐reported blood pressure–lowering medication. Diabetes mellitus was defined as fasting glucose ≥126 mg/dL or hemoglobin A1c ≥6.5%, or use of diabetes mellitus medications within 2 weeks before the clinic visit. 22 , 35 Total cholesterol was measured from plasma with the use of a cholesterol oxidase method (Roche Diagnostics, Rotkreuz, Switzerland) on a Roche COBAS FARA centrifugal analyzer. Serum C‐reactive protein was measured by the latex particle immunoturbidimetric assay (Roche Diagnostics). 22 , 36 Information about self‐reported use of aspirin, statins, and antihypertensive medications was also collected at each visit.
Statistical Analysis
We compared baseline characteristics using the 1‐way ANOVA, chi‐square tests, Mann–Whitney U test, and Kruskal–Wallis tests for differences among never, past, and current smokers, as appropriate, on the basis of the underlying distribution. Cox proportional hazard regression models were used to estimate the association between smoking status at visit 1 and incident CHD yielding hazard ratios for incident CHD for each smoking group compared with never smokers and by smoking intensity and burden among current smokers. Model 1 was adjusted for age and education; model 2 was adjusted for age, sex, education level, body mass index, diabetes mellitus, systolic blood pressure, hypertension, total cholesterol, fasting triglycerides, physical activity, and alcohol intake in the past 12 months. Kaplan–Meier curves were constructed to assess the cumulative survival of participants free from incident CHD by smoking status and compared by log‐rank tests. Participants who died and were lost to follow‐up were censored at the time of death or the last follow‐up contact. Schoenfeld residuals were used to test the assumption of proportionality.
In secondary analyses, multivariable logistic and linear regression models were used to assess the associations among smoking status, smoking intensity, and CAC at visit 2. Model 1 was adjusted for age and education; model 2 was adjusted for age, sex, education level, body mass index, diabetes mellitus, systolic blood pressure, hypertension, total cholesterol, fasting triglycerides, physical activity, and alcohol intake in the past 12 months. CAC was transformed to [In (CAC+1)] and used in our models to have an all‐inclusive analysis (1 was added to the CAC scores to also include participants with CAC score of 0) 37 ; this analytical approach has been used in prior studies. 30 , 36 All statistical analyses were performed with STATA version 15 (StataCorp, College Station, TX) and SAS version 9.4 (SAS Institute, Cary, NC). A 2‐sided P<0.05 was considered statistically significant.
RESULTS
Among 4432 eligible participants (83.5% of overall cohort, mean age 54±13 years, 64% women), 3102 (70%) of participants were identified as never smokers, 782 (17.6%) were identified as former smokers, and 548 (12.4%) were identified as current smokers. Baseline characteristics of the participants grouped by smoking status are presented in Table 1. Compared with never smokers, current smokers and former smokers were more likely to be men (P<0.001). Current smokers were younger and had lower body mass index compared with never smokers and former smokers (all P<0.001). Former smokers had a higher prevalence of hypertension and diabetes mellitus compared with never smokers and current smokers (all P<0.001).
Table 1.
Baseline Characteristics at Visit 1 by Smoking Status
| Variables | Overall (n=4432) | Smoking Status | P Value | ||
|---|---|---|---|---|---|
| Never Smoker (n=3102) | Former Smoker (n=782) | Current Smoker (n=548) | |||
| Age, y | 53.9 (12.8) | 53.0 (13.0) | 59.1 (11.2) | 51.5 (11.1) | <0.001 |
| Sex, men, n (%) | 1593 (36) | 948 (31) | 368 (47) | 277 (51) | <0.001 |
| Body mass index, kg/m2 | 31.8 (7.2) | 32.2 (7.3) | 31.5 (6.6) | 29.9 (7.4) | <0.001 |
| Ideal health indicator via physical activity, n (%) | |||||
| Poor health | 2117 (48) | 1450 (47) | 343 (44) | 324 (59) | <0.001 |
| Intermediate health | 1431 (32) | 1023 (33) | 270 (35) | 138 (25) | |
| Ideal health | 884 (20) | 629 (20) | 169 (22) | 86 (16) | |
| Alcohol consumption in the past 12 mo, n (%) | 2087 (47) | 1302 (42) | 386 (50) | 399 (73) | <0.001 |
| Age of initiation of smoking, y | NA | NA | 18.8 (5.0) | 19.8 (6.1) | |
| Education, n (%) | |||||
| Less than high school | 724 (16) | 423 (14) | 183 (23) | 118 (22) | <0.001 |
| High school graduate/General Educational Development | 891 (20) | 591 (19) | 170 (22) | 130 (24) | |
| Attended vocational school, trade school, or college | 2817 (64) | 2088 (67) | 429 (55) | 300 (55) | |
| Hypertension, n (%) | 2366 (53) | 1610 (52) | 492 (63) | 264 (48) | <0.001 |
| Diabetes mellitus, n (%) | 955 (22) | 646 (21) | 210 (27) | 99 (18) | <0.001 |
| Total cholesterol, mg/dL | 199.7 (39.5) | 199.5 (38.6) | 201.8 (40.9) | 198.0 (42.3) | 0.212 |
| Fasting triglyceride, mg/dL | 105.5 (80.2) | 100.5 (71.7) | 113.1 (75.5) | 123.6 (120.9) | <0.001 |
| Antiplatelet medication use, n (%) | 916 (45) | 591 (43) | 228 (55) | 97 (37) | <0.001 |
| Statin use, n (%) | 499 (11) | 334 (11) | 124 (16) | 41 (8) | <0.001 |
| Prevalent atrial fibrillation, n (%) | 11 (0) | 5 (0) | 4 (1) | 2 (0) | 0.179 |
| C‐reactive protein level, mg/dL | 0.5 (0.9) | 0.5 (0.7) | 0.5 (0.8) | 0.6 (1.7) | 0.002 |
| Homocysteine level, μmol/L | 9.2 (4.5) | 9.03 (4.7) | 9.52 (3.3) | 9.91 (5.1) | <0.001 |
Continuous values are presented as mean (SD), and all other values are numbers (%). χ2 tests, 1‐way ANOVA, Mann–Whitney U test, and Kruskal–Wallis test were used to compare baseline characteristics of participants by smoking status. NA indicates not applicable.
Over a median follow‐up of 13.8 years, 254 participants developed CHD (incidence rate, 4.5/1000 person‐years). Incident CHD occurred in 148 never smokers, 68 former smokers, and 38 current smokers (Table 2). Both former and current smokers had a higher incidence of CHD compared with never smokers (log‐rank P <0.001) (Figure 2). Among current smokers using 1 to 19 cigarettes/day there were 28 CHD events, while those using ≥20 cigarettes/day had 10 CHD events. The results of our unadjusted analysis for incident CHD stratified by sex for each smoking category are presented in Table S1.
Table 2.
CHD Incidence by Smoking Status and Intensity From Visit 1 to 2016
| Never Smokers | Former Smokers | Current Smokers | Current (1–19 Cigarettes/d) | Current (≥20 Cigarettes/d) | |
|---|---|---|---|---|---|
| Incident CHD, n/total (%) | 148/3102 | 68/782 | 38/548 | 28/367 | 10/181 |
| 4.8 | 8.7 | 6.9 | 7.6 | 5.5 | |
| Crude CHD incidence rate (per 1000 person‐years) | 3.7 | 7.0 | 5.6 | 6.2 | 4.6 |
Figure 2. Kaplan‐Meier survival curves of the study participants by smoking status.
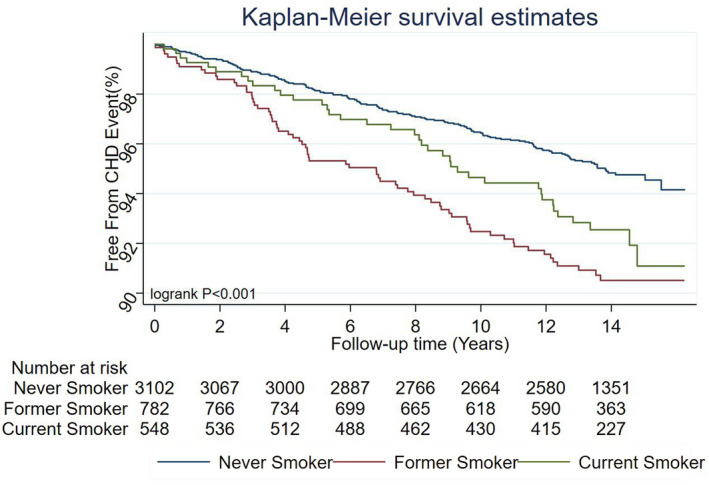
CHD indicates coronary heart disease.
After adjusting for age and sex (model 1), former smokers had a higher risk for CHD compared with never smokers, and current smokers also had a higher risk for CHD compared with never smokers (Table 3). After further adjustment for risk factors (model 2), current smoking was associated with about 2 times increased risk of incident CHD compared with never smoking. There was an increased risk of CHD in current smokers smoking 1 to 19 cigarettes/day; however, smoking ≥20 cigarettes/day was not significantly associated with CHD. Our results were similar with propensity‐adjusted analysis (Table S2) and modified Poisson regression with robust variance approach (Table S3).
Table 3.
Association Between Smoking Status and Incident CHD From Visit 1 to 2016
| Model | Smoking Status | Smoking Intensity | Smoking Dose/Burden | ||
|---|---|---|---|---|---|
| Former vs Never Smokers | Current vs Never Smokers | Current (1–19 Cigarettes/d) vs Never Smokers | Current (≥20 Cigarettes/d) vs Never Smokers | Exposure in Pack‐Years | |
| Model 1 |
1.37, P=0.036* (1.02–1.84) |
1.72, P=0.003* (1.20–2.48) |
1.93, P=0.002* (1.28–2.91) |
1.24, P=0.524 (0.64–2.37) |
0.99, P=0.069 (0.99–1.00) |
| Model 2 |
1.37, P=0.051 (1.0–1.90) |
2.11, P<0.001* (1.39–3.18) |
2.16, P=0.001* (1.36–3.42) |
1.36, P=0.413 (0.65–2.87) |
0.99, P=0.064 (0.98–1.00) |
Values are given as hazard ratio, P value (95% CI).
P‐values are statistically significant; Model 1: adjusted for age and sex; model 2: model 1 plus education level, diabetes mellitus, systolic blood pressure, body mass index, hypertension, total cholesterol, fasting triglycerides, physical activity, and alcohol intake in the past 12 months.
The type 3 P values for each smoking variable in our models were statistically significant and the values for our Akaike information criterion diminished from our age‐ and sex‐adjusted model (model 1) to our primary model (model 2), indicating better fit models as we adjusted for more covariates in our stepwise analysis, except in our models with smoking burden as the predictor variable.
We examined the relationship between cigarette smoking and the development of CHD, through CAC scores by smoking status and intensity (Tables 4 and 5; Table S5) in our multivariable logistic regression models at 2 clinically relevant cut points, 29 , 38 , 39 (CAC score >0 and >400).
Table 4.
Association Between Smoking Status and CAC Score >0 (Log Transformed) at Visit 2 Among JHS Participants
| Former vs Never Smokers | Current vs Never Smokers | Current (1–19 Cigarettes/D) vs Never Smokers | Current (≥20 Cigarettes/D) vs Never Smokers | |
|---|---|---|---|---|
| Model 1 |
1.33, P=0.017* (1.05–1.68) |
2.62, P<0.0001* (1.95–3.54) |
2.15, P<0.0001* (1.51–3.07) |
3.83, P<0.0001* (2.32–6.48) |
| Model 2 |
1.18, P=0.196 (0.92–1.51) |
2.63, P<0.0001* (1.88–3.68) |
1.95, P<0.001* (1.32–2.88) |
4.84, P<0.0001* (2.70–8.89) |
Multivariable logistic regression model for participants by smoking status and intensity with CAC score >0. n=2493; Values are given as odds ratio, P value (95% CI). CAC indicates coronary artery calcification; and JHS, Jackson Heart Study.
P‐values are statistically significant; Model 1: adjusted for age and sex; Model 2: Model 1 plus education level, diabetes mellitus, systolic blood pressure, body mass index, hypertension, total cholesterol, fasting triglycerides, physical activity, and alcohol intake in the past 12 months.
Table 5.
Association Between Smoking Status and CAC Score >400 (Log Transformed) at Visit 2 Among JHS Participants
| Former vs Never Smokers | Current vs Never Smokers | Current (1–19 Cigarettes/d) vs Never Smokers | Current (≥20 Cigarettes/d) vs Never Smokers | |
|---|---|---|---|---|
| Model 1 |
1.55, P=0.010* (1.10–2.17) |
2.08, P=0.002* (1.30–3.23) |
1.95, P=0.020* (1.08–3.35) |
2.41, P=0.012* (1.16–4.69) |
| Model 2 |
1.44, P=0.052 (0.99–2.07) |
2.02, P<0.001* (1.17–3.40) |
1.71, P=0.114 (0.85–3.27) |
2.82, P=0.013* (1.19–6.24) |
Multivariable logistic regression model for participants by smoking status and intensity with CAC score >400. n=2493; values are given as odds ratio, P value (95% CI). CAC indicates coronary artery calcification; and JHS, Jackson Heart Study.
P‐values are statisitically significant; model 1: adjusted for age and sex; model 2: model 1 plus education level, diabetes mellitus, systolic blood pressure, body mass index, hypertension, total cholesterol, fasting triglycerides, physical activity, and alcohol intake in the past 12 months.
In our age‐ and sex‐adjusted logistic regression model for the odds of CAC score >0 (Table 4), current and former smoking was associated with increased odds of CAC score >0 compared with never smokers (model 1). Current smokers smoking 1 to 19 and ≥20 cigarettes/day also had higher odds of CAC score >0 compared with never smokers. After further adjustment for conventional risk factors (model 2), former smoking was not significantly associated with increased odds of CAC score >0 compared with never smokers. However, current smokers had higher odds of CAC score >0 compared with never smokers (odds ratio [OR], 2.63; 95% CI, 1.88–3.68). Furthermore, the odds of having a CAC score >0 increased as daily cigarette use also increased among current smokers compared with never smokers (OR, 1.95; 95% CI, 1.32–2.88; and OR, 4.84; 95% CI, 2.70–8.89 for 1–19 and ≥20 cigarettes/day, respectively).
Furthermore, in our age‐ and sex‐adjusted logistic regression model for the odds of CAC score >400 (Table 5), current and past smoking were associated with increased odds of CAC score >400 compared with never smokers (model 1). Current smokers smoking 1 to 19 cigarettes/day had higher odds of CAC score >400 compared with never smokers and current smokers using ≥20 cigarettes/day also had higher odds of CAC score >400 compared with never smokers. In multivariable‐adjusted models (model 2), former smoking was not significantly associated with increased odds of CAC score >400 compared with never smokers. However, current smokers had higher odds of CAC score >400 compared with never smokers (OR, 2.02; 95% CI, 1.17–3.40). Current smokers using ≥20 cigarettes/day also had higher odds of CAC score >400 compared with never smokers (OR, 2.82; 95% CI, 1.19–6.24). The characteristics of JHS participants with CAC scores and the distribution of CAC scores among participants by smoking status for our analysis (at visit 2) are presented in Table 6, Figure 3, Table S4, and Figure S1. Our multivariable linear regression models by smoking status and intensity are also shown in Table S5.
Table 6.
Characteristics of Participants With CAC Scores at Visit 2 Among JHS Participants by Smoking Status
| Never Smoker | Former Smoker | Current Smoker | Total | |
|---|---|---|---|---|
| Number of participants by smoking status | 1784 | 462 | 247 | 2493 |
| CAC=0, n (%) | 1077 (60.37) | 203 (43.94) | 100 (40.49) | 1380 |
| 0 < CAC ≤100, n (%) | 402 (22.53) | 123 (26.62) | 79 (31.98) | 604 |
| 100 < CAC <400, n (%) | 177 (9.92) | 66 (14.29) | 38 (15.38) | 281 |
| CAC ≥400, n (%) | 128 (7.17) | 70 (15.15) | 30 (12.15) | 228 |
n=2493 for total number of participants with CAC scores after exclusion of participants with missing covariates. CAC indicates coronary artery calcification; and JHS, Jackson Heart Study.
Figure 3. Distribution of log‐transformed CAC scores at visit 2 among JHS participants by smoking status.
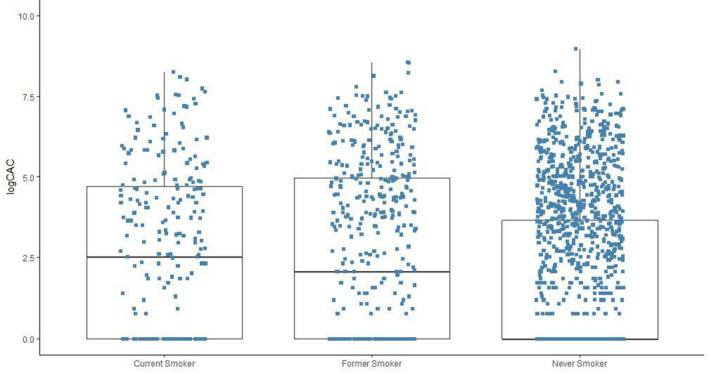
CAC indicates coronary artery calcification; CHD, coronary heart disease; and JHS, Jackson Heart Study.
DISCUSSION
In this well‐characterized community‐based cohort of Black adults, current cigarette smoking was associated with higher incidence of CHD. Furthermore, current cigarette smoking and high intensity smoking (at least 20 cigarettes/day) were associated with increased odds of CAC compared with never smokers after adjusting traditional risk factors.
Previous studies have investigated the relationship between cigarette smoking and CHD. In the Nurses' Health Study, 119 404 female nurses free of diagnosed CHD were followed up for 6 years. Investigators found that current but not former cigarette smoking was associated with an adjusted increased risk for CHD; the risk of fatal CHD and nonfatal MI was higher as the number of cigarettes smoked increased, compared with women who were nonsmokers, Those who smoked 1 to 14 cigarettes/day had adjusted relative risk of 2.3 for total nonfatal MI and fatal CHD, while women who smoked ≥25 cigarettes/day had adjusted relative risk of 6.1 for total nonfatal MI and fatal CHD. 40 The effect of smoking on incident CHD was similar in our study. We also found a similar association between current smoking and increasing risk of CHD in current smokers who smoked 1 to 19 cigarettes/day (low‐intensity smokers) after adjusting for traditional risk factors in our analysis (hazard ratio, 2.16; 95% CI, 1.36–3.42). We did not demonstrate a significant dose–response relationship between cigarette smoking and the risk of CHD in current smokers using ≥20 cigarettes/day. This finding could be partly attributable to the modest number of participants who smoked ≥20 cigarettes/day (about 4% of our study sample) in our subgroup comparisons between current smoking levels and never smokers, and probably attributable to smokers using ≥20 cigarettes/day developing other diseases or the competing risk of death (high‐intensity smokers may have died prematurely). Unfortunately, the JHS did not capture sufficient data to assess smoking cessation among those who smoked. This may partly explain the paradoxical finding that high‐intensity smokers had lower rates of CHD compared with current smokers smoking 1 to 19 cigarettes/day. It is possible that participants who developed CHD, other diseases, or disease risk factors subsequently reduced their smoking intensity. Furthermore, we did not distinguish between fatal and nonfatal CHD in our analysis, and the participants of Nurses' Health Study were primarily White women.
In a community‐based study investigating the relationship between cigarette smoking and the long‐term risk of atherosclerotic diseases, smoking was dose‐dependently associated with increased risk for CHD. 41 However, we did not demonstrate a significant dose–response relationship between cigarette smoking and the risk of CHD in current smokers using ≥20 cigarettes/day.
In a pooled cohort of 1838 men from the Albany Cardiovascular Health Center Study and 2282 men from the Framingham Heart study, followed up for an average of 6 and 8 years, respectively, to assess the impact of cigarette smoking on CHD, former smoking was not associated with an increased risk for CHD compared with never smokers, and the risk for MI was 3 times higher among current smokers who smoked >20 cigarettes/day compared with never smokers. 42 We also found no association between former smoking and incident CHD in our study. However, in our analysis, we grouped “all incident CHD” events together. Additionally, the participants of the pooled cohort of the Albany Cardiovascular Health Center Study and the Framingham Heart study were all men, and racial differences were not examined. Regardless, taken together, these data imply that smoking cessation may reduce the risk of CHD to levels at or near those of never smokers. Unfortunately, the JHS did not capture time since quitting, so we were unable to adequately assess the true effect of smoking cessation on this relationship.
Black adults are more likely to die from CHD compared with White adults 1 and currently, there are limited data directly evaluating the relationship between cigarette smoking and CHD in Black adults. To the best of our knowledge, our study is the first prospective study that has investigated the relationship between cigarette smoking and incident CHD exclusively among a large cohort of Black adults while assessing multiple traditional risk factors for cardiovascular diseases among groups subdivided by smoking status, intensity, and burden. Additionally, we investigated the relationship between cigarette smoking and the development of CHD by evaluating the burden of atherosclerosis among groups stratified by smoking status and intensity through CAC score measurements at clinically relevant thresholds.
CAC scores can predict the risk for future CHD events. 28 , 29 A CAC score of 0 is associated with low risk for CHD, 28 and a CAC score >400 is associated with high risk for CHD. 43 Prior reports have shown a 4‐fold increase in risk for CHD in individuals with CAC >400 compared with CAC score of 0. 44 In our analysis, we evaluated the relationship between smoking status and intensity with CAC scores at 2 important thresholds of CAC score >0 and >400. Smoking status and intensity were associated with increased risk of CAC score >0 and >400 after controlling for traditional risk factors. Our findings suggest that an increased burden of coronary atherosclerosis could be a mechanism for the development of CHD among Black smokers.
Our study has some limitations that warrant consideration: Our study was observational; we cannot infer causality, and we cannot rule out residual confounding. We adjusted for potential confounders at visit 1 but acknowledge that smoking status and covariates may have changed between the baseline and when CHD events occurred. Cigarette smoking information was obtained by self‐report and was not confirmed by urine cotinine levels, giving room for misclassification. The information about the type of cigarette smoked by participants in our study was not available for our analysis. Also, it is possible that participants used other types of tobacco products including pipes, cigars, and cigarillos, which were not captured and may have affected our results. The JHS was conducted within a single geographic area of Black people, so our findings may not be generalizable to other racial groups or regions. Despite the large sample size of our study, we had a modest number of events; subgroup comparisons between current smoking levels (1–19 versus 20+ cigarettes/day) and between current versus former smokers may be underpowered. Finally, CAC was measured only at visit 2, which could limit our ability to show causality, and the number of participants with available CAC data was limited (n=2614), which may also affect the interpretation of our results.
In conclusion, in a large prospective cohort of Black adults, current cigarette smoking was independently associated with incident CHD. Additionally, there was no significant difference in the risk of incident CHD between former and never smokers, suggesting that smoking cessation may have potential benefits in reducing the incidence of CHD in Black adults.
Sources of Funding
The Jackson Heart Study is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute and the National Institute for Minority Health and Health Disparities. Dr Hall has also received support from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 1K08DK099415‐01A1, National Institutes of Health/National Institute of General Medical Sciences P20GM104357 and National Institutes of Health/National Institute of General Medical Sciences 5U54GM115428. Dr Benjamin was funded by the American Heart Association, 18SFRN34110082 and 2U54HL120163. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
Disclosures
Dr Benjamin serves as an uncompensated member for the MyHeartLab Steering Committee. The MyHeartLab Study is a principal investigator–initiated study from the University of California San Francisco: principal investigator, Jeffrey Olgin, MD, through a research grant to University of California San Francisco from Samsung. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S5
Figure S1
Acknowledgments
The authors would like to thank the participants of the Jackson Heart Study for their time and participation.
(J Am Heart Assoc. 2021;10:e017320. DOI: 10.1161/JAHA.120.017320.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017320
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. DOI: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2. Leigh JA, Alvarez M, Rodriguez CJ. Ethnic minorities and coronary heart disease: an update and future directions. Curr Atheroscler Rep. 2016;18:9. DOI: 10.1007/s11883-016-0559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferdinand KC. Coronary artery disease in minority racial and ethnic groups in the United States. Am J Cardiol. 2006;97:12A–19A. DOI: 10.1016/j.amjcard.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 4. Saab KR, Kendrick J, Yracheta JM, Lanaspa MA, Pollard M, Johnson RJ. New insights on the risk for cardiovascular disease in African Americans: the role of added sugars. J Am Soc Nephrol. 2015;26:247–257. DOI: 10.1681/ASN.2014040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev. 2015;11:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inoue T. Cigarette smoking as a risk factor of coronary artery disease and its effects on platelet function. Tob Induc Dis. 2004;2:27–33. DOI: 10.1186/1617-9625-2-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation. 1997;96:3243–3247. DOI: 10.1161/01.CIR.96.9.3243. [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US) . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking‐Attributable Disease: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention (US); 2010. 6, Cardiovascular Diseases. Available at: https://www.ncbi.nlm.nih.gov/books/NBK53012/. Accessed January 4, 2020. [PubMed] [Google Scholar]
- 9. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. DOI: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 10. McEvoy JW, Nasir K, DeFilippis AP, Lima JAC, Bluemke DA, Hundley WG, Barr RG, Budoff MJ, Szklo M, Navas‐Acien A, et al. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi‐Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1002–1010. DOI: 10.1161/ATVBAHA.114.304960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis JW, Davis RF. Acute effect of tobacco cigarette smoking on the platelet aggregate ratio. Am J Med Sci. 1979;278:139–143. DOI: 10.1097/00000441-197909000-00004. [DOI] [PubMed] [Google Scholar]
- 12. Rival J, Riddle JM, Stein PD. Effects of chronic smoking on platelet function. Thromb Res. 1987;45:75–85. DOI: 10.1016/0049-3848(87)90258-1. [DOI] [PubMed] [Google Scholar]
- 13. Fusegawa Y, Goto S, Handa S, Kawada T, Ando Y. Platelet spontaneous aggregation in platelet‐rich plasma is increased in habitual smokers. Thromb Res. 1999;93:271–278. DOI: 10.1016/S0049-3848(98)00184-4. [DOI] [PubMed] [Google Scholar]
- 14. Renaud S, Blache D, Dumont E, Thevenon C, Wissendanger T. Platelet function after cigarette smoking in relation to nicotine and carbon monoxide. Clin Pharmacol Ther. 1984;36:389–395. DOI: 10.1038/clpt.1984.193. [DOI] [PubMed] [Google Scholar]
- 15. Tibuakuu M, Kamimura D, Kianoush S, DeFilippis AP, Al Rifai M, Reynolds LM, White WB, Butler KR, Mosley TH, Turner ST, et al. (2017) The association between cigarette smoking and inflammation: the Genetic Epidemiology Network of Arteriopathy (GENOA) study. PLoS One. 2017;12:e0184914. DOI: 10.1371/journal.pone.0184914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herbert WH. Cigarette smoking and arteriographically demonstrable coronary artery disease. Chest. 1975;67:49–52. DOI: 10.1378/chest.67.1.49. [DOI] [PubMed] [Google Scholar]
- 17. Ramsdale DR, Faragher EB, Bray CL, Bennett DH, Ward C, Beton DC. Smoking and coronary artery disease assessed by routine coronary arteriography. BMJ. 1985;290:197–200. DOI: 10.1136/bmj.290.6463.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention . Current cigarette smoking among adults—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1225–1232.30408019 [Google Scholar]
- 19. Jones MR, Joshu CE, Navas‐Acien A, Platz EA. Racial/ethnic differences in duration of smoking among former smokers in the National Health and Nutrition Examination Surveys. Nicotine Tob Res. 2018;20:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White WB, Cain LR, Benjamin EJ, DeFilippis AP, Blaha MJ, Wang W, Okhomina V, Keith RJ, Al Rifai M, Kianoush S, et al. High‐intensity cigarette smoking is associated with incident diabetes mellitus in black adults: the Jackson Heart Study. J Am Heart Assoc. 2018;7:e007413. DOI: 10.1161/JAHA.117.007413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackson Heart Study . “JHS data Access”. Available at: https://www.jacksonheartstudy.org/Research/Study‐Data/Data‐Access. Accessed November 11, 2019.
- 22. Hall ME, Wang W, Okhomina V, Agarwal M, Hall JE, Dreisbach AW, Juncos LA, Winniford MD, Payne TJ, Robertson RM, et al. Cigarette smoking and chronic kidney disease in African Americans in the Jackson Heart Study. J Am Heart Assoc. 2016;5:e003280. DOI: 10.1161/JAHA.116.003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moran KE, Ommerborn MJ, Blackshear CT, Sims M, Clark CR. Financial stress and risk of coronary heart disease in the Jackson Heart Study. Am J Prev Med. 2019;56:224–231. DOI: 10.1016/j.amepre.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 24. Effoe VS, Wagenknecht LE, Echouffo Tcheugui JB, Chen H, Joseph JJ, Kalyani RR, Bell RA, Wu W‐C, Casanova R, Bertoni AG, et al. Sex differences in the association between insulin resistance and incident coronary heart disease and stroke among blacks without diabetes mellitus: the Jackson Heart Study. J Am Heart Assoc. 2017;6:e004229. DOI: 10.1161/JAHA.116.004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keku E, Rosamond W, Taylor HA Jr, Garrison R, Wyatt SB, Richard M, Jenkins B, Reeves L, Sarpong D. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethn Dis. 2005;15:S6‐62‐70. [PubMed] [Google Scholar]
- 26. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. DOI: 10.1016/0735-1097(90)90282-T. [DOI] [PubMed] [Google Scholar]
- 27. Dixon AK, Coulden RA. Coronary artery calcification on computed tomography. Lancet. 1997;350:1265. DOI: 10.1016/S0140-6736(05)62470-1. [DOI] [PubMed] [Google Scholar]
- 28. Church TS, Levine BD, McGuire DK, Lamonte MJ, Fitzgerald SJ, Cheng YJ, Kimball TE, Blair SN, Gibbons LW, Nichaman MZ, et al. Coronary artery calcium score, risk factors, and incident coronary heart disease events. Atherosclerosis. 2007;190:224–231. DOI: 10.1016/j.atherosclerosis.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 29. LaMonte MJ, FitzGerald SJ, Church TS, Barlow CE, Radford NB, Levine BD, Pippin JJ, Gibbons LW, Blair SN, Nichaman MZ, et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162:421–429. DOI: 10.1093/aje/kwi228. [DOI] [PubMed] [Google Scholar]
- 30. Sung J, Yeboah J, Lee J, Smith C, Terry J, Sims M, Samdarshi T, Musani S, Fox E, Ge Y, et al. Diagnostic value of coronary artery calcium score for cardiovascular disease in African Americans: the Jackson Heart Study. Br J Med Med Res. 2016;11:BJMMR/2016/21449. DOI: 10.9734/BJMMR/2016/21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carr JJ, Nelson JC, Wong ND, McNitt‐Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC, et al. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of Multi‐Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. DOI: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 32. Dubbert PM, Carithers T, Ainsworth BE, Taylor HA Jr, Wilson G, Wyatt SB. Physical activity assessment methods in the Jackson Heart Study. Ethn Dis. 2005;15:S6–S61. [PubMed] [Google Scholar]
- 33. Djoussé L, Petrone AB, Blackshear C, Griswold M, Harman JL, Clark CR, Talegawkar S, Hickson DA, Gaziano JM, Dubbert PM, et al. Prevalence and changes over time of ideal cardiovascular health metrics among African‐Americans: the Jackson Heart Study. Prev Med. 2015;74:111–116. DOI: 10.1016/j.ypmed.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al.; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. DOI: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 35. Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131–144. DOI: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 36. Oshunbade AA, Yimer WK, Valle KA, Clark D III, Kamimura D, White WB, DeFilippis AP, Blaha MJ, Benjamin EJ, O'Brien EC, et al. Cigarette smoking and incident stroke in Blacks of the Jackson Heart Study. J Am Heart Assoc. 2020;9:e014990. DOI: 10.1161/JAHA.119.014990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Divers J, Palmer ND, Langefeld CD, Brown WM, Lu L, Hicks PJ, Smith SC, Xu J, Terry JG, Register TC, et al. Genome‐wide association study of coronary artery calcified atherosclerotic plaque in African Americans with type 2 diabetes. BMC Genet. 2017;18:105. DOI: 10.1186/s12863-017-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. DOI: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 39. Neves PO, Andrade J, Monção H. Coronary artery calcium score: current status. Radiol Bras. 2017;50:182–189. DOI: 10.1590/0100-3984.2015.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Willett WC, Green A, Stampfer MJ, Speizer FE, Colditz GA, Rosner B, Monson RR, Stason W, Hennekens CH. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med. 1987;317:1303–1309. DOI: 10.1056/NEJM198711193172102. [DOI] [PubMed] [Google Scholar]
- 41. Ding N, Sang Y, Chen J, Ballew SH, Kalbaugh CA, Salameh MJ, Blaha MJ, Allison M, Heiss G, Selvin E, et al. Cigarette smoking, smoking cessation, and long‐term risk of 3 major atherosclerotic diseases. J Am Coll Cardiol. 2019;74:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doyle JT, Dawber TR, Kannek WB, Heslin AS, Kahn HA. Cigarette smoking and coronary heart disease. Combined experience of the Albany and Framingham studies. N Engl J Med. 1962;266:796–801. DOI: 10.1056/NEJM196204192661602. [DOI] [PubMed] [Google Scholar]
- 43. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74:243–252. DOI: 10.4065/74.3.243. [DOI] [PubMed] [Google Scholar]
- 44. Uddin SMI, Mirbolouk M, Kianoush S, Orimoloye OA, Dardari Z, Whelton SP, Miedema MD, Nasir K, Rumberger JA, Shaw LJ, et al. Role of coronary artery calcium for stratifying cardiovascular risk in adults with hypertension. Hypertension. 2019;73:983–989. DOI: 10.1161/HYPERTENSIONAHA.118.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figure S1


