Abstract
Background
There was little evidence about the role of objective sleep efficiency (SE) in the incidence of major cardiovascular disease (CVD) events. The purpose of this study was to investigate the correlation between objective SE and CVD based on polysomnography.
Methods and Results
A total of 3810 participants from the SHHS (Sleep Heart Health Study) were selected in the current study. CVD was assessed during an almost 11‐year follow‐up period. The primary composite cardiovascular outcome was major adverse cardiovascular events, defined as CVD mortality, congestive heart failure, myocardial infarction, and stroke. The secondary composite cardiovascular outcome was major adverse cardiovascular event plus revascularization. Objective measured SE, including SE and wake after sleep onset, was based on in‐home polysomnography records. Cox regression analysis was used to explore the association between SE and CVD. After multivariate Cox regression analysis, poor SE (<80%) was significantly associated with primary (hazard ratio [HR], 1.338; 95% CI, 1.025–1.745; P=0.032) and secondary composite cardiovascular outcomes (HR, 1.250; 95% CI, 1.027–1.521; P=0.026); it was also found to be a predictor of CVD mortality (HR, 1.887; 95% CI, 1.224–2.909; P=0.004). Moreover, wake after sleep onset of fourth quartile (>78.0 minutes) was closely correlated with primary (HR, 1.436; 95% CI, 1.066–1.934; P=0.017), secondary composite cardiovascular outcomes (HR, 1.374; 95% CI, 1.103–1.712; P=0.005), and CVD mortality (HR, 2.240; 95% CI, 1.377–3.642; P=0.001).
Conclusions
Poor SE and long wake after sleep onset, objectively measured by polysomnography, were associated with the increased risk of incident CVD.
Keywords: cardiovascular disease, major adverse cardiovascular event, sleep efficiency, wake after sleep onset
Subject Categories: Heart Failure, Myocardial Infarction, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- AHI
apnea‐hypopnea index
- ArI
arousal index
- SE
sleep efficiency
- SHHS
Sleep Heart Health Study
- SL
sleep latency
- SWS
slow wave sleep
- TST
total sleep time
- WASO
wake after sleep onset
Clinical Perspective
What Is New?
This community‐based cohort study included 3810 participants and found that sleep efficiency and wake after sleep onset were associated with major adverse cardiovascular events.
What Are the Clinical Implications?
Sleep efficiency based on polysomnography may be a useful indicator to predict cardiovascular disease.
Sleep is an essential physiological phenomenon throughout life. Obtaining sufficient quality sleep is vital to physical and mental health. 1 Questionnaires, such as the Pittsburgh Sleep Quality Index, are commonly used to evaluate sleep quality. 2 , 3 Previous studies usually used sleep quality assessed by Pittsburgh Sleep Quality Index, which was found to be associated with cardiovascular disease (CVD), diabetes mellitus, and metabolic syndrome. 4 , 5 , 6 Sleep efficiency (SE), defined as the ratio of the total time spent sleeping/the time spent in bed, is an objective indicator for the evaluation of sleep quality. SE of ≥85% is considered to be efficient sleep, whereas lower SE values usually lead to low energy, irritability, and depressed mood. 7 , 8
Several studies have showed that SE is closely related to cardiovascular risk factors. Massar et al revealed that poor habitual SE increased the reactivity of cardiovascular and cortisol stress in men. 9 In addition, low SE was reported to be correlated with high systolic blood pressure and high nighttime blood pressure. 10 Dorenbos et al also found that SE was negatively associated with insulin sensitivity in overweight and obese adolescents. 11 Besides, no significant association was found between SE and coronary artery calcification in Pittsburgh SleepSCORE Study. 12 , 13 Wake after sleep onset (WASO) is the time spent awake from sleep onset to final awakening, which are also used to assess objective SE. 14 Long WASO was positively associated with high levels of von Willebrand factor antigen and soluble tissue factor antigen, which could indicate endothelial damage. 14
However, there is little evidence to support an association between objective SE and major CVD based on polysomnography. Therefore, we conducted the present study using the database of the SHHS (Sleep Heart Health Study), a decade‐long community‐based study, to investigate the association between CVD and objectively measured SE.
Methods
Study Population
Anonymized data and materials have been made publicly available at the National Sleep Research Resource and can be accessed at https://doi.org/10.25822/ghy8‐ks59. The SHHS is a community‐based, prospective cohort study investigating cardiovascular consequences of sleep‐disordered breathing (ClinicalTrials.gov identifier: NCT00005275). Details of the study design have been previously reported. 15 The study population was selected from prospective cohort studies, including the ARIC (Atherosclerosis Risk in Communities) Study, the CHS (Cardiovascular Health Study), the Framingham Offspring and Omni Study, the SHS (Strong Heart Study), the Tucson Epidemiological Study of Obstructive Lung Disease, cohort studies of respiratory disease in Tucson, and cohort studies of hypertension in New York. Written consent was provided by all the participants, and the study protocol was approved by the institutional review board of each participating institution. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination of our research. The database was accessed on the basis of a signed agreement with the Brigham and Women’s Hospital. Eligible participants had: (1) complete polysomnography data and medical records; and (2) SE was not obviously affected during in‐home polysomnography (self‐administered morning survey questionnaires collected quality of sleep compared with usual determined on the basis of responses to questions such as “Compared to your usual night's sleep, how well did you sleep last night?”). Participants who had a history of heart failure, myocardial infarction (MI), stroke, and revascularization were excluded (Figure S1).
Sleep Characteristics
All participants underwent in‐home overnight polysomnography (P‐Series; Compumedics, Abbotsville, Australia), as previously described. 16 , 17 SE was calculated as the ratio of the total time spent sleeping/the time spent in bed and divided into 4 groups (≥90%, 85%–89.9%, 80%–84.9%, and <80%). WASO was defined as the total amount of time spent awake after going to sleep and categorized in 4 groups (quartile IV: >78.0 minutes; quartile III: 47.5–78.0 minutes; quartile II: 29.5–47.0 minutes; and quartile I: <29.5 minutes). Sleep latency (SL) was defined as the time from lights out to the beginning of sleep. Total arousal index (ArI) was computed as the ratio of the total number of arousals/the total sleep time (TST). Slow wave sleep (SWS) was defined as stage 3 of non–rapid eye movement sleep. TST in the present study refers to the entire sleep time captured by polysomnography. Sleep duration was divided into short (<6 hours), long (>8 hours), and normal (6–8 hours). The apnea‐hypopnea index (AHI) was calculated as all apnea and hypopnea episodes per hour of sleep accompanied by at least a 4% drop in oxygen saturation. According to conventional clinical categories, AHI was classified as none (AHI <5 events/h), mild (AHI ≥5–<15 events/h), moderate (AHI ≥15–<30 events/h), and severe (AHI ≥30 events/h). 18 The Epworth Sleepiness Scale score was assessed using an 8‐item self‐report questionnaire. 19
Cardiovascular Disease
Incident cardiovascular events, including CVD mortality, MI, congestive heart failure (CHF), and stroke, were evaluated according to the parent cohorts based on exhaustive protocols. 20 , 21 , 22 , 23 , 24 , 25 , 26 Survival time was defined as the time from baseline polysomnography to the first episode of incident cardiovascular event. The primary composite cardiovascular outcome was the first occurrence of a major adverse cardiovascular event, defined as cardiovascular death, CHF, MI, and stroke. The secondary composite cardiovascular outcome was defined as major adverse cardiovascular event plus revascularization.
Participants’ age, sex, race, smoking status, history of diabetes mellitus and hypertension, body mass index, and polysomnography data were obtained from the baseline examination of SHHS.
Statistical Analysis
The comparisons of continuous variables and categorical variables were based on ANOVA and the χ2 test, respectively. Descriptive statistics are presented as percentages for categorical variables and mean±SD for continuous variables. Unadjusted Kaplan‐Meier plots were used to calculate cumulative incidence of composite cardiovascular outcomes in different SE categories. We also performed collinearity diagnostics between sleep parameters and clinically relevant factors when exploring the role of objective SE in the incidence of CVD. Univariate and multivariate Cox regression analyses were used to analyze the relationship between composite cardiovascular outcomes and objectively measured sleep parameters. All the covariates in the univariate analysis were included in the final multivariate Cox analysis (enter model). Multivariate Cox regression analysis, following the Harrell guideline, was performed to identify independent risk factors after adjusting for age, sex, race, smoking status, body mass index, hypertension, diabetes mellitus, AHI, sleep duration, and sleep parameters. 27 , 28 Furthermore, we identified the interaction between SE/WASO and AHI in the multivariate Cox regression analysis. P<0.05 was considered statistically significant. All statistical analyses were conducted using the SPSS version 24.0 (SPSS Inc, Chicago, IL).
Results
Participants’ Characteristics
This study involved 3810 participants (1713 men and 2097 women; aged 63.2±11.0 years). The 4 categories of SE (≥90%, 85%–89.9%, 80%–84.9%, and <80%) were present in 1125 (29.5%), 932 (24.5%), 672 (17.6%), and 1081 (28.4%) individuals, respectively. Participants with poor SE (<80%) were older and more likely to be men; they were also more likely to have been diagnosed with sleep apnea, diabetes mellitus, and hypertension when compared with participants with SE ≥90%. Moreover, individuals with SE ≥90% had the lowest body mass index than those with SE with 85% to 89.9%, 80% to 84.9%, and <80%. Table 1 reports the study populations’ characteristics, according to the 4 categories of SE.
Table 1.
Subject Characteristics by SE Categories
| Characteristics | SE, % | |||||
|---|---|---|---|---|---|---|
| Total (n=3810) | <80.0 (n=1081) | 80.0–84.9 (n=672) | 85.0–89.9 (n=932) | ≥90.0 (n=1125) | P Value | |
| Age, y | 63.2±11.0 | 67.4±10.5 | 64.5±10.8 | 62.2±10.3 | 59.0±10.4 | <0.001 |
| Sex, n (%) | <0.001 | |||||
| Men | 1713 (45.0) | 548 (50.7) | 334 (49.7) | 414 (44.4) | 417 (37.1) | … |
| Women | 2097 (55.0) | 533 (49.3) | 338 (50.3) | 518 (55.6) | 708 (62.9) | … |
| Body mass index, kg/m2 | 28.3±5.0 | 28.5±5.0 | 28.4±4.9 | 28.4±5.1 | 27.8±4.8 | 0.004 |
| Race, n (%) | 0.715 | |||||
| White | 3308 (86.8) | 932 (86.2) | 578 (86.0) | 814 (87.3) | 984 (87.5) | … |
| Other* | 502 (13.2) | 149 (13.8) | 94 (14.0) | 118 (12.7) | 141 (12.5) | … |
| Smoking status, n (%) | 0.019 | |||||
| Current smoker | 387 (10.2) | 103 (9.5) | 67 (10.0) | 94 (10.1) | 123 (10.9) | … |
| Former smoker | 1631 (42.8) | 512 (47.4) | 282 (42.0) | 392 (42.0) | 445 (39.6) | … |
| Never smoker | 1786 (46.9) | 462 (42.7) | 322 (47.9) | 446 (47.9) | 556 (49.4) | … |
| Diabetes mellitus, n (%) | 231 (6.1) | 94 (8.7) | 37 (5.5) | 61 (6.5) | 39 (3.5) | <0.001 |
| Hypertension, n (%) | 1376 (36.1) | 515 (47.6) | 251 (37.4) | 315 (33.8) | 295 (26.2) | <0.001 |
| AHI, n (%) | <0.001 | |||||
| <5.0 Events/h | 1919 (50.4) | 448 (41.4) | 326 (48.5) | 459 (49.2) | 686 (61.0) | … |
| 5.0–14.9 Events/h | 1147 (30.1) | 367 (34.0) | 190 (28.3) | 295 (31.7) | 295 (26.2) | … |
| 15.0–29.9 Events/h | 494 (12.9) | 177 (16.4) | 105 (15.6) | 120 (12.9) | 92 (8.2) | … |
| ≥30.0 Events/h | 250 (6.6) | 89 (8.2) | 51 (7.6) | 58 (6.2) | 52 (4.6) | … |
| WASO, min | 59.3±41.8 | 106.0±45.5 | 62.5±19.1 | 43.9±13.5 | 25.3±10.6 | <0.001 |
| TST, h | 6.1±1.0 | 5.3±0.9 | 6.1±0.8 | 6.4±0.8 | 6.7±0.9 | <0.001 |
| SWS, % | 17.7±11.8 | 16.5±12.2 | 16.6±12.1 | 17.5±11.3 | 19.8±11.5 | <0.001 |
| SL, min | 12.5±19.3 | 21.6±28.6 | 13.9±15.7 | 10.5±11.9 | 4.6±7.7 | <0.001 |
| Total ArI, events/h | 18.9±10.2 | 21.9±11.6 | 19.9±9.9 | 18.3±9.1 | 15.8±8.7 | <0.001 |
| Sleep duration, h | 7.3±0.9 | 7.4±0.9 | 7.4±0.9 | 7.3±0.9 | 7.2±0.9 | <0.001 |
| Follow‐up time, y | 10.9±2.8 | 10.3±3.2 | 10.9±2.8 | 11.3±2.5 | 11.3±2.6 | <0.001 |
Results are presented as mean±SD or number (percentage). The P values represent the difference among 4 groups. AHI indicates apnea‐hypopnea index; ArI, arousal index; SE, sleep efficiency; SL, sleep latency; SWS, slow wave sleep; TST, total sleep time; and WASO, wake after sleep onset.
*Other race included Black, Native American or Alaskan Native, Asian or Pacific Islanders, and Hispnic.
Cardiovascular Composite Outcomes and SE
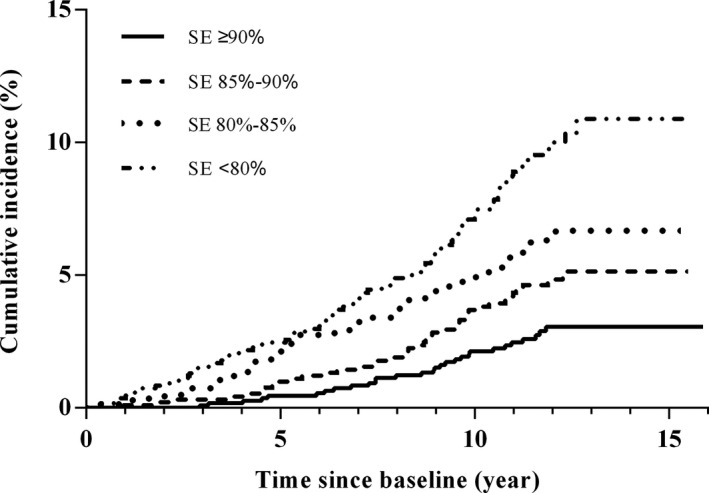
After a mean follow‐up period of 10.9±2.8 years from the baseline polysomnography, 474 (12.4%) cases of primary (major adverse cardiovascular event) and 839 (22.0%) cases of secondary cardiovascular composite outcomes occurred. Participants with SE <80% had the highest incidence of primary (17.5% versus 13.2% versus 10.5% versus 7.3%, respectively; P<0.001) and secondary composite cardiovascular outcomes (32.3% versus 23.3% versus 19.8% versus 14.6%, respectively; P<0.001) compared with those with 85% to 89.9%, 80% to 84.9%, and <80%. The distribution of CVD mortality, CHF, stroke, and MI was significantly higher in those with low SE than in those with high SE (Figure S2). In addition, participants with poor SE had an elevated cumulative incidence of primary and secondary composite cardiovascular outcomes and CVD mortality (Figures 1 and 2).
Figure 1. Kaplan‐Meier plots of cumulative risk for cardiovascular disease, stratified by sleep efficiency (SE) categories (≥90.0%, 85.0%–89.9%, 80.0%–84.9%, and <80.0%).
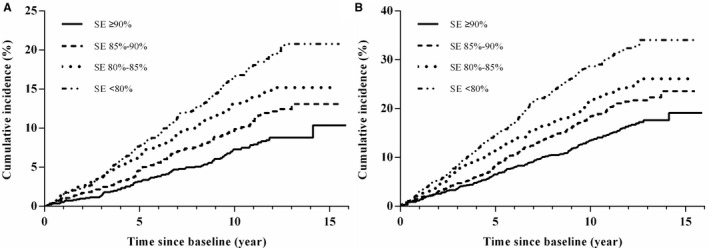
A, Primary composite cardiovascular end point. B, Secondary composite cardiovascular end point.
Figure 2. Kaplan‐Meier survival curves for the risk of cardiovascular disease mortality, stratified by sleep efficiency (SE) categories (≥90.0%, 85.0%–89.9%, 80.0%–84.9%, and <80.0%).
Variables associated with primary and secondary composite cardiovascular outcomes by unadjusted and adjusted Cox regression are shown in Table 2. In the final multivariate model, SE <80% was found to be an independent predictor of primary (hazard ratio [HR], 1.338; 95% CI, 1.025–1.745; P=0.032) and secondary (HR, 1.250; 95% CI, 1.027–1.521; P=0.026) composite cardiovascular outcomes after adjustment for age, sex, smoking status, body mass index, diabetes mellitus, hypertension, and AHI. Furthermore, participants with poor SE (<80%) had a high incidence of CVD mortality (HR, 1.887; 95% CI, 1.224–2.909; P=0.004) (Table 3). SE (per 1%) was also associated with the incidence of primary (HR, 0.988; 95% CI, 0.979–0.996; P=0.005) and secondary (HR, 0.989; 95% CI, 0.982–0.995; P=0.001) composite cardiovascular outcomes and CVD mortality (HR, 0.979; 95% CI, 0.967–0.991; P=0.001) (Table 2 and Table 3).
Table 2.
HRs and 95% CIs for SE (<80.0%, 80.0%–84.9%, 85.0%–89.9%, and ≥90.0%) Associated With Primary (MACE) and Secondary Composite Cardiovascular End Points
| Variable | Individuals, N | Person‐Years | Events, n (%) | Morbidity Rate* | Univariate Models | Multivariable Adjusted † | ||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||||
| Primary composite cardiovascular end point | 3810 | 40101.7 | 474 (12.4) | 11.8 | ||||
| SE, % | ||||||||
| <80.0 | 1081 (28.4) | 10581.0 | 185 (17.1) | 17.5 | 2.412 (1.874–3.103) | <0.001 | 1.338 (1.025–1.745) | 0.032 |
| 80.0–84.9 | 672 (17.6) | 7032.8 | 93 (13.8) | 13.2 | 1.815 (1.359–2.426) | <0.001 | 1.271 (0.943–1.713) | 0.115 |
| 85.0–89.9 | 932 (24.5) | 10113.3 | 106 (11.4) | 10.5 | 1.438 (1.086–1.904) | 0.011 | 1.112 (0.836–1.479) | 0.467 |
| ≥90 | 1125 (29.5) | 12374.7 | 90 (8.0) | 7.3 | 1 (Reference) | 1 (Reference) | ||
| Continuous (per 1%) | 0.969 (0.962–0.977) | <0.001 | 0.988 (0.979–0.996) | 0.005 | ||||
| Secondary composite cardiovascular end point | 3810 | 38130.4 | 839 (22.0) | 22.0 | ||||
| SE, % | ||||||||
| <80.0 | 1081 (28.4) | 9825.3 | 317 (29.3) | 32.3 | 2.218 (1.843–2.668) | <0.001 | 1.250 (1.027–1.521) | 0.026 |
| 80.0–84.9 | 672 (17.6) | 6682.3 | 156 (23.2) | 23.3 | 1.601 (1.290–1.987) | <0.001 | 1.126 (0.901–1.407) | 0.296 |
| 85.0–89.9 | 932 (24.5) | 9682.1 | 192 (20.6) | 19.8 | 1.361 (1.108–1.671) | 0.003 | 1.069 (0.868–1.317) | 0.530 |
| ≥90 | 1125 (29.5) | 11940.7 | 174 (15.5) | 14.6 | 1 (Reference) | 1 (Reference) | ||
| Continuous (per 1%) | 0.970 (0.965–0.976) | <0.001 | 0.989 (0.982–0.995) | 0.001 | ||||
HR indicates hazard ratio; MACE, major adverse cardiovascular event; and SE, sleep efficiency.
Crude event rate per 1000 person‐years.
Adjusted by age, sex, race, smoking status, body mass index, prevalent hypertension and diabetes mellitus, apnea‐hypopnea index, and sleep duration.
Table 3.
HRs and 95% CIs for SE Associated With CVD Mortality
| Variable | Total | SE <80.0% | SE 80.0%–84.9% | SE 85.0%–89.9% | SE ≥90.0% | Continuous (per 1%) |
|---|---|---|---|---|---|---|
| CVD mortality | ||||||
| No. of subjects | 3810 | 1081 | 672 | 932 | 1125 | |
| Person‐years | 41640.3 | 11115.7 | 7342.2 | 10488.1 | 12694.3 | |
| Events, n (%) | 199 (5.2) | 90 (8.3) | 39 (5.8) | 41 (4.4) | 29 (2.6) | |
| Morbidity rate* | 4.8 | 8.1 | 5.3 | 3.9 | 2.3 | |
| Hazard ratio for SE | ||||||
| Univariate models | 3.647 (2.399–5.543) † | 2.336 (1.445–3.778) ‡ | 1.709 (1.062–2.750) § | 1 (Reference) | 0.961 (0.951–0.971) † | |
| Age and sex adjusted | 2.356 (1.543–3.598) † | 1.747 (1.077–2.833) § | 1.351 (0.839–2.176) | 1 (Reference) | 0.973 (0.962–0.984) † | |
| Multivariable adjusted ‖ | 1.887 (1.224–2.909) ‡ | 1.587 (0.972–2.590) | 1.233 (0.764–1.991) | 1 (Reference) | 0.979 (0.967–0.991) ‡ | |
CVD indicates cardiovascular disease; HR, hazard ratio; and SE, sleep efficiency.
Crude event rate per 1000 person‐years.
P<0.001.
P<0.01.
P<0.05.
Adjusted by age, sex, race, smoking status, body mass index, prevalent hypertension and diabetes mellitus, apnea‐hypopnea index, and sleep duration.
We further explored the role of SE in the incidence of primary and secondary composite cardiovascular outcomes, and CVD mortality stratified by AHI (<5 events/h versus ≥5 events/h). Our results showed that SE was significantly associated with the incidence of both primary and secondary composite cardiovascular outcomes, and CVD mortality in participants with or without sleep‐disordered breathing. WASO was also associated with secondary composite cardiovascular outcomes and CVD mortality in participants with or without sleep‐disordered breathing. No significant interactions were found in these stratified analyses (Table S1).
Cardiovascular Composite Outcomes and WASO
The distribution of primary (18.3% versus 11.7% versus 10.8% versus 7.1%, respectively; P<0.001) and secondary composite cardiovascular outcomes (33.7% versus 22.8% versus 18.9% versus 14.2%, respectively; P<0.001) was significantly different in 4 categories of WASO (quartile IV: >78.0 minutes; quartile III: 47.5–78.0 minutes; quartile II: 29.5–47.0 minutes; and quartile I: <29.5 minutes). After multivariate Cox regression was adjusted, our findings revealed that WASO of the fourth quartile had a higher incidence of primary (HR, 1.436; 95% CI, 1.066–1.934; P=0.017) and secondary composite cardiovascular outcomes (HR, 1.374; 95% CI, 1.103–1.712; P=0.005) and CVD mortality (HR, 2.240; 95% CI, 1.377–3.642; P=0.001) compared with first quartile. WASO (per 1 minute) was also found to be a predictor for the incidence of primary (HR, 1.003; 95% CI, 1.001–1.005; P=0.012) and secondary composite cardiovascular outcomes (HR, 1.003; 95% CI, 1.001–1.004; P=0.001) and CVD mortality (HR, 1.005; 95% CI, 1.002–1.008; P=0.002) (Table 4).
Table 4.
HRs and 95% CIs for WASO Quartiles (Quartile I: <29.5 Minutes; Quartile II: 29.5–47.0 Minutes; Quartile III: 47.1–78.0 Minutes; and Quartile IV: >78.0 Minutes) Associated With Primary (MACE) and Secondary Composite Cardiovascular End Points
| Variable | Individuals, N | Person‐Years | Events, n (%) | Morbidity Rate* | Univariate Models | Multivariable Adjusted † | ||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||||
| Primary composite cardiovascular end point | ||||||||
| WASO, min | ||||||||
| Fourth quartile | 958 | 9379.5 | 172 (18.0) | 18.3 | 2.574 (1.960–3.381) | <0.001 | 1.436 (1.066–1.934) | 0.017 |
| Third quartile | 957 | 10094.8 | 118 (12.3) | 11.7 | 1.634 (1.222–2.185) | 0.001 | 1.169 (0.863–1.584) | 0.313 |
| Second quartile | 951 | 10224.0 | 110 (11.6) | 10.8 | 1.509 (1.124–2.026) | 0.006 | 1.334 (0.987–1.803) | 0.061 |
| First quartile | 944 | 10403.4 | 74 (7.8) | 7.1 | 1 (Reference) | 1 (Reference) | ||
| Continuous (per 1 min) | 1.007 (1.006–1.009) | <0.001 | 1.003 (1.001–1.005) | 0.012 | ||||
| Secondary composite cardiovascular end point | ||||||||
| WASO, min | ||||||||
| Fourth quartile | 958 | 8688.6 | 293 (30.6) | 33.7 | 2.378 (1.947–2.905) | <0.001 | 1.374 (1.103–1.712) | 0.005 |
| Third quartile | 957 | 9514.6 | 217 (22.7) | 22.8 | 1.606 (1.300–1.984) | <0.001 | 1.166 (0.935–1.455) | 0.173 |
| Second quartile | 951 | 9839.5 | 186 (19.6) | 18.9 | 1.334 (1.073–1.659) | 0.010 | 1.178 (0.943–1.473) | 0.149 |
| First quartile | 944 | 10087.6 | 143 (15.1) | 14.2 | 1 (Reference) | 1 (Reference) | ||
| Continuous (per 1 min) | 1.007 (1.006–1.009) | <0.001 | 1.003 (1.001–1.004) | 0.001 | ||||
| CVD mortality | ||||||||
| WASO, min | ||||||||
| Fourth quartile | 958 | 9852.3 | 88 (9.2) | 8.9 | 4.227 (2.671–6.691) | <0.001 | 2.240 (1.377–3.642) | 0.001 |
| Third quartile | 957 | 10479.5 | 49 (5.1) | 4.7 | 2.167 (1.320–3.556) | 0.002 | 1.447 (0.871–2.404) | 0.153 |
| Second quartile | 951 | 10645.0 | 39 (4.1) | 3.7 | 1.698 (1.014–2.842) | 0.044 | 1.431 (0.851–2.406) | 0.176 |
| First quartile | 944 | 10663.6 | 23 (2.4) | 2.2 | 1 (Reference) | 1 (Reference) | ||
| Continuous (per 1 min) | 1.009 (1.007–1.012) | <0.001 | 1.005 (1.002–1.008) | 0.002 | ||||
HR indicates hazard ratio; MACE, major adverse cardiovascular event; and WASO, wake after sleep onset.
Crude event rate per 1000 person‐years.
Adjusted by age, sex, race, smoking status, body mass index, prevalent hypertension and diabetes mellitus, apnea‐hypopnea index, and sleep duration.
Cardiovascular Composite Outcomes and SL, SWS, Total ArI, or TST
Our study also investigated the associations between CVD and SL, SWS, total ArI, and TST. Short TST (<5 hours) was found to be associated with CVD mortality (HR, 2.049; 95% CI, 1.200–3.500; P=0.009). No significant association was found between CVD and SL, SWS, and total ArI (Table S2).
Discussion
Previous studies demonstrated a correlation between SE and CVD risk factors. 10 , 29 , 30 In this study, we used several objective indicators from polysomnography, including SE, WASO, SL, total ArI, SWS, and TST, to explore the role of objective sleep characteristics in CVD. Our results showed that low SE and long WASO were prone to have a high proportion of incident CVD. SE and WASO were significantly associated with primary/secondary composite cardiovascular outcomes and CVD mortality.
Monitoring of SE, a significant feature of poor health and pathological conditions, is commonly used in the objective evaluation of sleep quality. 7 Efficient sleep means a deeper sleep of higher quality with fewer interruptions. An efficient sleep usually had an SE with ≥85%. SE >90% is considered to be good, whereas <85% is considered poor. 7 SE has been found to be a determinant of insulin sensitivity in overweight or obese adolescents. 11 Massar et al also showed that poor habitual SE is associated with increased cardiovascular and cortisol stress reactivity in men. 9 In addition, objectively measured SE strongly predicts mortality in patients with CHF. 31 However, there is little evidence supporting an association between SE and CVD. SE in the current study was based on in‐home polysomnography, and participants whose sleep quality was affected by polysomnography were excluded. Our analyses showed that participants with SE <80% had significantly higher incident CVD and CVD mortality than other participants. In the final Cox regression model, poor SE (<80%) was associated with the incidence of primary and secondary composite cardiovascular outcomes. SE was also found to be a strong predictor of CVD mortality in our study. WASO, which calculates the total wake time after going to sleep, was also identified as an important indicator reflecting sleep quality. Our results showed that longer WASO leads to an elevated incidence of cardiovascular mortality.
AHI has been proven to be a strong risk factor for CVD mortality, CHF, stroke, MI, and diabetes mellitus. 32 , 33 An AHI of >5 events per hour is considered as sleep apnea and may accompanied with microarousal during the nighttime. We considered that AHI may be a confounding factor in the association between AHI and coronary artery disease. Therefore, we performed stratified analyses and used an interaction term to detect the association between SE and CVD in participants with and without sleep‐disordered breathing. Our results showed that SE was associated with CVD in individuals with or without sleep‐disordered breathing. Besides, no significant interaction was found in these stratified analyses.
On the basis of our findings, SE and WASO were associated with the risk of cardiovascular events. Improving SE may be a way to reduce the risk of CVD. As we know, chronic diseases, such as chronic bronchitis, angina pectoris, gastric ulcer, anxiety, pain, and kidney disease, reduced the SE during the night. In addition, sleep environment, sleeping habit, daytime nap habit, and the intake of tea, coffee, and alcohol were also important influence factors for SE. 7 Comfortable bedtime environment, good eating habit before going to bed, regular exercise, relaxing activity before sleep, and limiting the time of daytime nap may help people improve SE. 34 , 35 Cognitive behavioral therapy for insomnia is also a highly effective way to improve SE. 36
Although there might be a relationship between SE and CVD, the pathophysiological mechanism remains obscure. Poor sleep may cause an increase in stress response, further activating the hypothalamic‐pituitary‐adrenal axis and the sympathetic nervous system, which could lead to elevated blood pressures, increased heart rates, decreased variability of heart rates, increased urinary cortisol and catecholamines, and change of blood flow. 37 All these may contribute to endothelial dysfunction and atherosclerosis. Moreover, poor quality of sleep was also found to be associated with metabolic disturbances and quality of life. 1 In this study, participants with poor sleep were more likely to have hypertension, diabetes mellitus, and sleep apnea‐hypopnea syndrome, which are strong risk factors for CVD.
Some potential limitations of this study should be noted. The average age of the study population was >60 years, and most of the participants were White people. Therefore, the results obtained should not be extended to all ethnic groups or younger populations. Depression, exercise, drinking tea and coffee, and drug use may be important covariates in the association between SE/WASO and incident coronary artery disease. However, we did not adjust these variables because of lack of data. Although the participants whose sleep qualities were affected by in‐home polysomnography were excluded in this study, 1‐night polysomnography may not fully represent the real SE. Multiple in‐home polysomnography recordings over a long period of time may provide additional details and decrease measurement error. Besides, wrist actigraphy is particularly useful in the long‐period documentation of sleep patterns. We will further explore the fluctuation in SE and the relationship between repeated‐measure SE and CVD based on wrist actigraphy.
Conclusions
In this community‐based cohort study, we investigated the role of SE in CVD based on polysomnography records. SE and WASO were demonstrated to be associated with the incidence of primary (major adverse cardiovascular event) and secondary composite cardiovascular outcomes. Moreover, SE was also a predictor for CVD mortality. This indicates that SE and WASO may predict the incidence of CVD.
Sources of Funding
This study was supported by National Heart, Lung, and Blood Institute cooperative agreements U01HL53916 (University of California, Davis), U01HL53931 (New York University), U01HL53934 (University of Minnesota), U01HL53937 and U01HL64360 (Johns Hopkins University), U01HL53938 (University of Arizona), U01HL53940 (University of Washington), and U01HL53941 (Boston University).
Disclosures
None.
Supporting information
Tables S1–S2
Figures S1–S2
Acknowledgments
We appreciate the Brigham and Women’s Hospital for sharing the data sets of SHHS (Sleep Heart Health Study). Besides, SHHS acknowledges the ARIC (Atherosclerosis Risk in Communities) Study, the CHS (Cardiovascular Health Study), the Framingham Offspring and Omni Study, the SHS (Strong Heart Study), Tucson Epidemiological Study of Obstructive Lung Disease, the cohort studies of respiratory disease in Tucson, and cohort studies of hypertension in New York. SHHS is particularly grateful to the members of these cohorts who agreed to participate in SHHS as well. SHHS further recognizes all of the investigators and staff who have contributed to its success. A list of SHHS investigators, staff, and their participating institutions is available on the SHHS website (www.jhucct.com/shhs). We also thank Jie Zheng and Fan Gao for their support on statistical approach.
Author contributions: Drs Yan, Yang, Zhao, Wang, and Ma raised the idea for the study. Drs Yan, Yang, and Fan contributed to the study design and writing and review of the report. Drs Yan and Ma acquired the data in SHHS, and Drs Yan and Ma participated in further data analysis. Dr Ma handled supervision in our study. All authors approved the final version of the report.
(J Am Heart Assoc. 2021;10:e06056. DOI: 10.1161/JAHA.120.016201.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016201
For Sources of Funding and Disclosures, see page 9.
References
- 1. St‐Onge MP, Grandner MA, Brown D, Conroy MB, Jean‐Louis G, Coons M, Bhatt DL, American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council of Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council . Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–e386. DOI: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. DOI: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 3. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. DOI: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 4. Cai S, Tan S, Gluckman PD, Godfrey KM, Saw SM, Teoh OH, Chong YS, Meaney MJ, Kramer MS, Gooley JJ, et al. Sleep quality and nocturnal sleep duration in pregnancy and risk of gestational diabetes mellitus. Sleep. 2017;40. DOI: 10.1093/sleep/zsw058. [DOI] [PubMed] [Google Scholar]
- 5. Okubo N, Matsuzaka M, Takahashi I, Sawada K, Sato S, Akimoto N, Umeda T, Nakaji S, Hirosaki University Graduate School of Medicine . Relationship between self‐reported sleep quality and metabolic syndrome in general population. BMC Public Health. 2014;14:562. DOI: 10.1186/1471-2458-14-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoevenaar‐Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12‐year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487–1492. DOI: 10.5665/sleep.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jung DW, Lee YJ, Jeong DU, Park KS. New predictors of sleep efficiency. Chronobiol Int. 2017;34:93–104. DOI: 10.1080/07420528.2016.1241802. [DOI] [PubMed] [Google Scholar]
- 8. Wong ML, Lau EY, Wan JH, Cheung SF, Hui CH, Mok DS. The interplay between sleep and mood in predicting academic functioning, physical health and psychological health: a longitudinal study. J Psychosom Res. 2013;74:271–277. DOI: 10.1016/j.jpsychores.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 9. Massar SAA, Liu JCJ, Mohammad NB, Chee MWL. Poor habitual sleep efficiency is associated with increased cardiovascular and cortisol stress reactivity in men. Psychoneuroendocrinology. 2017;81:151–156. DOI: 10.1016/j.psyneuen.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 10. Doyle CY, Ruiz JM, Taylor DJ, Smyth JW, Flores M, Dietch JR, Ahn C, Allison M, Smith TW, Uchino BN. Associations between objective sleep and ambulatory blood pressure in a community sample. Psychosom Med. 2019;81:545–556. DOI: 10.1097/PSY.0000000000000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dorenbos E, Rijks JM, Adam TC, Westerterp‐Plantenga MS, Vreugdenhil AC. Sleep efficiency as a determinant of insulin sensitivity in overweight and obese adolescents. Diabetes Obes Metab. 2015;17:90–98. DOI: 10.1111/dom.12515. [DOI] [PubMed] [Google Scholar]
- 12. Matthews KA, Strollo PJ Jr, Hall M, Mezick EJ, Kamarck TW, Owens JF, Buysse DJ, Reis SE. Associations of Framingham risk score profile and coronary artery calcification with sleep characteristics in middle‐aged men and women: Pittsburgh SleepSCORE study. Sleep. 2011;34:711–716. 10.5665/SLEEP.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lutsey PL, McClelland RL, Duprez D, Shea S, Shahar E, Nagayoshi M, Budoff M, Kaufman JD, Redline S. Objectively measured sleep characteristics and prevalence of coronary artery calcification: the Multi‐Ethnic Study of Atherosclerosis Sleep study. Thorax. 2015;70:880–887. DOI: 10.1136/thoraxjnl-2015-206871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Kanel R, Loredo JS, Ancoli‐Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest. 2007;131:733–739. DOI: 10.1378/chest.06-2006. [DOI] [PubMed] [Google Scholar]
- 15. Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O'Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 16. Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley JP; Sleep heart health research group. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 17. Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, Surovec S, Nieto FJ. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–757. DOI: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 18. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, et al. Sleep‐disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. DOI: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, Reis SE, Matthews KA. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4:563–571. DOI: 10.5664/jcsm.27351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener‐West M, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. DOI: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chambless LE, Folsom AR, Sharrett AR, Sorlie P, Couper D, Szklo M, Nieto FJ. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003;56:880–890. DOI: 10.1016/S0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 22. Toole JF, Lefkowitz DS, Chambless LE, Wijnberg L, Paton CC, Heiss G. Self‐reported transient ischemic attack and stroke symptoms: methods and baseline prevalence: the ARIC Study, 1987–1989. Am J Epidemiol. 1996;144:849–856. DOI: 10.1093/oxfordjournals.aje.a009019. [DOI] [PubMed] [Google Scholar]
- 23. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1:263–276. DOI: 10.1016/1047-2797(91)90005-W. [DOI] [PubMed] [Google Scholar]
- 24. Price TR, Psaty B, O'Leary D, Burke G, Gardin J. Assessment of cerebrovascular disease in the cardiovascular health study. Ann Epidemiol. 1993;3:504–507. DOI: 10.1016/1047-2797(93)90105-D. [DOI] [PubMed] [Google Scholar]
- 25. Lee ET, Cowan LD, Welty TK, Sievers M, Howard WJ, Oopik A, Wang W, Yeh J, Devereux RB, Rhoades ER, et al. All‐cause mortality and cardiovascular disease mortality in three American Indian populations, aged 45–74 years, 1984–1988: the Strong Heart Study. Am J Epidemiol. 1998;147:995–1008. [DOI] [PubMed] [Google Scholar]
- 26. Section 34: Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Study, 30‐year follow‐up. In: Kannel WB, Wolf PA, Garrison RJ, eds. The framingham heart study: An epidemiological investigation of cardiovascular disease. US Department of Health and Human Services: Bethesda, MD; 1987:1–26. [Google Scholar]
- 27. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173–e180. DOI: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harrell F. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer Verlag; 2001. [Google Scholar]
- 29. Koolhaas CM, Kocevska D, Te Lindert BHW, Erler NS, Franco OH, Luik AI, Tiemeier H. Objectively measured sleep and body mass index: a prospective bidirectional study in middle‐aged and older adults. Sleep Med. 2019;57:43–50. DOI: 10.1016/j.sleep.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 30. Ramos AR, Weng J, Wallace DM, Petrov MR, Wohlgemuth WK, Sotres‐Alvarez D, Loredo JS, Reid KJ, Zee PC, Mossavar‐Rahmani Y, et al. Sleep patterns and hypertension using actigraphy in the Hispanic Community Health Study/Study of Latinos. Chest. 2018;153:87–93. DOI: 10.1016/j.chest.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reinhard W, Plappert N, Zeman F, Hengstenberg C, Riegger G, Novack V, Maimon N, Pfeifer M, Arzt M. Prognostic impact of sleep duration and sleep efficiency on mortality in patients with chronic heart failure. Sleep Med. 2013;14:502–509. DOI: 10.1016/j.sleep.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 32. Chami HA, Resnick HE, Quan SF, Gottlieb DJ. Association of incident cardiovascular disease with progression of sleep‐disordered breathing. Circulation. 2011;123:1280–1286. DOI: 10.1161/CIRCULATIONAHA.110.974022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, Diener‐West M, Sanders MH, Wolf PA, Geraghty EM, et al. Obstructive sleep apnea‐hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. DOI: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23–36. DOI: 10.1016/j.smrv.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kline CE, Irish LA, Krafty RT, Sternfeld B, Kravitz HM, Buysse DJ, Bromberger JT, Dugan SA, Hall MH. Consistently high sports/exercise activity is associated with better sleep quality, continuity and depth in midlife women: the SWAN sleep study. Sleep. 2013;36:1279–1288. DOI: 10.5665/sleep.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik OE, Kvale G, Nielsen GH, Nordhus IH. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295:2851–2858. DOI: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 37. Lao XQ, Liu X, Deng H‐B, Chan T‐C, Ho KF, Wang F, Vermeulen R, Tam T, Wong MCS, Tse LA, et al. Sleep quality, sleep duration, and the risk of coronary heart disease: a prospective cohort study with 60,586 adults. J Clin Sleep Med. 2018;14:109–117. DOI: 10.5664/jcsm.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S2


