Abstract
Background
With the emergence of coronary computed tomography (CT) angiography, anomalous aortic origin of a coronary artery (ANOCOR) is more frequently diagnosed. Fractional flow reserve derived from CT (FFRCT) is a noninvasive functional test providing anatomical and functional evaluation of the overall coronary tree. These unique features of anatomical and functional evaluation derived from CT could help for the management of patients with ANOCOR. We aimed to retrospectively evaluate the physiological and clinical impact of FFRCT analysis in the ANOCOR registry population.
Methods and Results
The ANOCOR registry included patients with ANOCOR detected during invasive coronary angiography or coronary CT angiography between January 2010 and January 2013, with a planned 5‐year follow‐up. We retrospectively performed FFRCT analysis in patients with coronary CT angiography of adequate quality. Follow‐up was performed with a clinical composite end point (cardiac death, myocardial infarction, and unplanned revascularization). We obtained successful FFRCT analyses and 5‐year clinical follow‐up in 54 patients (average age, 60±13 years). Thirty‐eight (70%) patients had conservative treatment, and 16 (30%) patients had coronary revascularization after coronary CT angiography. The presence of an ANOCOR course was associated with a moderate reduction of FFRCT value from 1.0 at the ostium to 0.90±0.10 downstream the ectopic course and 0.82±0.11 distally. No significant difference in FFRCT values was identified between at‐risk and not at‐risk ANOCOR. After a 5‐year follow‐up, only one unplanned percutaneous revascularization was reported.
Conclusions
The presence of ANOCOR was associated with a moderate hemodynamic decrease of FFRCT values and associated with a low risk of cardiovascular events after a 5‐year follow‐up in this middle‐aged population.
Keywords: anomalous aortic origin of coronary arteries, coronary computed tomography angiography, fractional flow reserve, fractional flow reserve computed tomography
Subject Categories: Echocardiography
Nonstandard Abbreviations and Acronyms
- ANOCOR
anomalous aortic origin of a coronary artery
- FFR
fractional flow reserve
- FFRCT
fractional flow reserve derived from computed tomography
- ICA
invasive coronary angiography
Clinical Perspective
What Is New?
Clinical decision making of patients with anomalous aortic origin of coronary arteries remains challenging.
Evaluation of abnormal coronary origin with fractional flow reserve derived from computed tomography and long‐term follow‐up were performed.
What Are the Clinical Implications?
Fractional flow reserve derived from computed tomography evaluation appears feasible in patients with anomalous aortic origin of coronary arteries. We observed favorable fractional flow reserve derived from computed tomography value and clinical outcome at 5 years.
Fractional flow reserve derived from computed tomography could help clinical decision making in patients with this abnormal coronary anatomical feature.
A broad spectrum of anatomical variations and anomalies of coronary arteries exists. 1 , 2 , 3 Anomalous aortic origin of a coronary artery (ANOCOR) is defined as a coronary connection from the contralateral artery, nonappropriate aortic sinus, or ascending aorta. Anomalous connections from the pulmonary artery are beyond the scope of this study. The estimated prevalence of ANOCOR is 0.5% among patients undergoing invasive coronary angiography (ICA) and 1.0% among patients undergoing coronary computed tomography angiography (CCTA). 4 True prevalence of ANOCOR in the general population is probably lower, but its detection will increase with the rising use of noninvasive cardiac imaging. The clinical impact of ANOCOR is challenging to predict and varies from none to the risk of sudden cardiac death. The general assumption is that some ANOCOR could induce myocardial ischemia, because of morphological modifications that appear as a slit‐like ostium shape, acute take‐off angle, ectopic course narrowing between the great vessels, and sometimes intramural aortic course. Myocardial ischemia is infrequently documented by noninvasive stress tests. Exercise test and stress myocardial perfusion imaging are often normal in patients with ANOCOR. 5 , 6 , 7 Invasive evaluation of ANOCOR with intravascular ultrasound and fractional flow reserve (FFR) has shown that, within the ectopic course, the lumen area is mildly to moderately reduced but changes from a round to an ovoid or elliptical shape, in most cases without significant decrease in FFR values. 8 , 9 However, this strategy is invasive, sometimes challenging to perform, and cost/time consuming. FFR derived from computed tomography (FFRCT) is an emerging noninvasive technology that provides anatomical and physiological evaluation of the overall coronary tree. 10 On the basis of several case reports, FFRCT could be of interest for the evaluation of ANOCOR vessels. 11 , 12 , 13 , 14 We retrospectively analyzed FFRCT in adult patients included in the ANOCOR registry between 2010 and 2013, and evaluated clinical outcomes at 5 years (Figure 1).
Figure 1. Flowchart.
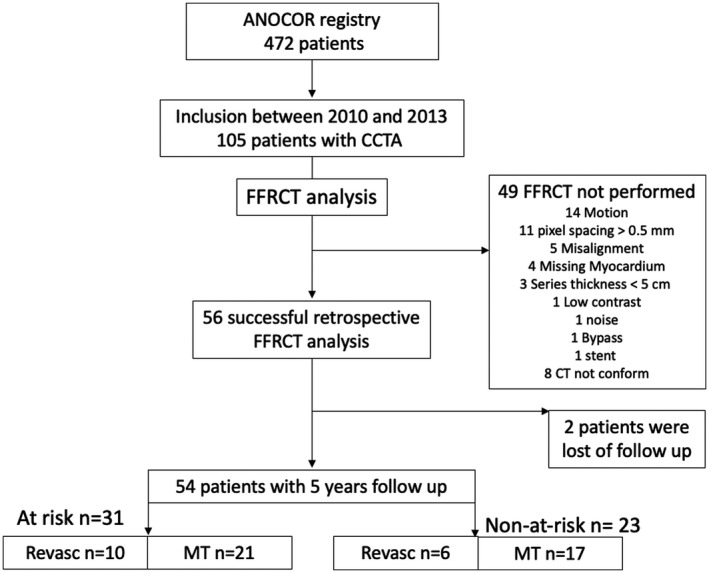
ANOCOR indicates anomalous aortic origin of a coronary artery; CCTA, coronary CT angiography; CT, computed tomography; FFRCT, fractional flow reserve derived from CT; MT, medical therapy; and Revasc, revascularization.
Methods
The data that support the findings of this study are available from the corresponding author and ANOCOR group on reasonable request. The ANOCOR registry is a multicentric observational study of young and adult patients with documented ANOCOR, validated by an independent committee of cardiologists and radiologists. This registry included 496 ANOCOR cases in 472 patients from 71 centers between January 2010 and January 2013 in France, Greece, and Switzerland. This cohort has been previously described. 15 The role of the committee was to classify each anomalous connection of coronary artery (type of artery, site of connection, ectopic course, and the presence of coronary artery disease). Therefore, we defined the type of artery involved: left main artery, left anterior descending artery, left circumflex artery, and right coronary artery. Site of connection was defined according to the connection site from the contralateral artery, nonappropriate aortic sinus, or ascending aorta (Figure 2). The ectopic course was defined as interarterial when proximal coronary vessel is placed between the aorta and pulmonary artery with or without intramural course. The retroaortic course was defined when the coronary artery travels posterior to the aorta. Prepulmonar course was defined when the coronary artery courses forward to the pulmonar trunk and pulmonar infundibulum. The retropulmonar course was defined when the coronary artery travels below the pulmonic annulus and right ventricular outflow tract and courses on the interventricular septum. Ectopic course is defined as the coronary path between the ostium and the point where the ANOCOR vessel meets up with an appropriate myocardial area. We defined ANOCOR involving interarterial course as ANOCOR at risk and other courses as not at risk (Figure 2).
Figure 2. Anomalous aortic origin of a coronary artery (ANOCOR) types: examples of fractional flow reserve derived from computed tomography (FFRCT) in the conservation group.
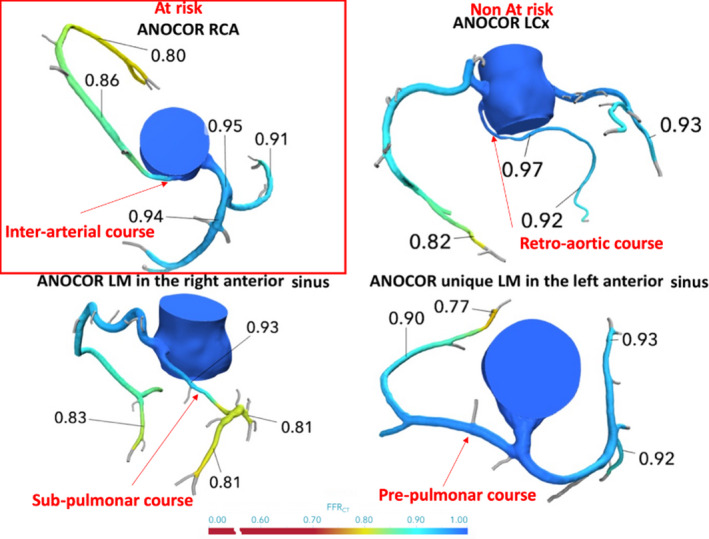
LCx indicates left circumflex; LM, left main; and RCA, right coronary artery.
ICA and CCTA, when performed, were reviewed by the committee to evaluate the degree of coronary stenosis in ANOCOR and non‐ANOCOR vessels, which was defined as angiographically significant stenosis when stenosis is ≥50%, assessed by visual estimation, and nonsignificant for stenosis <50% and normal coronary artery. 16 By definition, a stenosis observed in an interarterial course was defined as a non–coronary artery disease–related stenosis. All other stenoses were defined as coronary artery disease–related stenosis attributable to atheroma. Noninvasive stress tests were performed at discretion of the referring physician, and the positivity of these tests was defined according to the standard criteria. 17
Therapeutic decision making (conservative treatment, percutaneous revascularization, and surgical revascularization) for ANOCOR and non‐ANOCOR vessels was left at the discretion of the referring physician.
Follow‐up was performed by contacting the patient by telephone or letter or, in case of no answer, by calling the referring physician. Evaluation follow‐up criteria consisted of unplanned revascularization, myocardial infarction, or cardiac death. Clinical follow‐up was performed at 5 years.
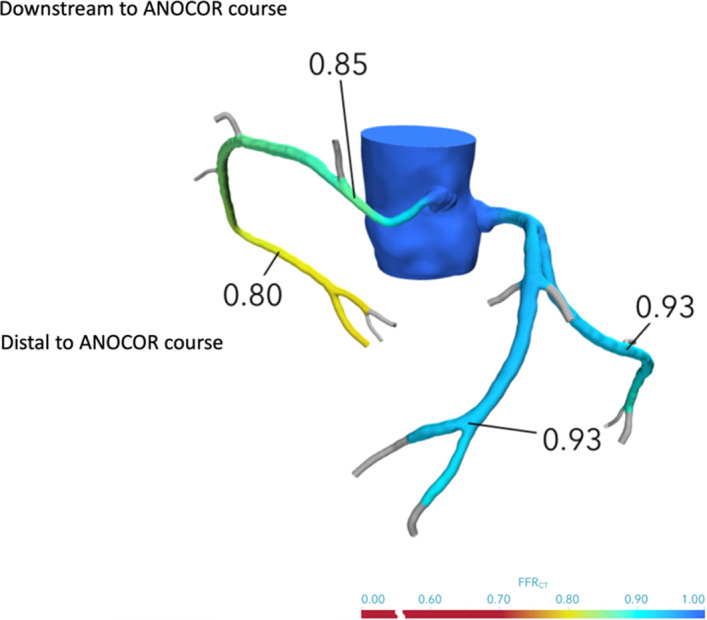
We evaluated the clinical presentation, the noninvasive stress tests performed, the coronary angiograms when performed, and the FFRCT analysis from CCTA. From the ANOCOR registry population of 472 patients, 105 had a CCTA. All CCTAs were sent for FFRCT analysis. All computed tomography (CT)–coded Digital imaging and communications in medicine files were submitted by protected internet transfer link to Heartflow (Redwood City, CA) for calculation. Results were transmitted via a protected website for evaluation by a blinded independent operator, unaware of the medical file of the patients. The computation of FFRCT is based on the following physiological assumptions: the baseline coronary blood flow is proportional to the volume of myocardial mass at resting condition, the coronary microvascular resistance at rest is inversely associated with the vessel size, and a predicable reduction of microcirculatory resistance is seen as response to infusion of vasodilators, such as adenosine. Three‐dimensional geometric models of coronary tree are obtained from CCTA images acquired from at least 64‐detector row CCTA. Lumen boundary surfaces of the aorta and coronary arteries are delineated and reconstructed in 3 dimensions. Baseline coronary blood flow is estimated and applied at the inlet as boundary condition. Hyperemic conditions are simulated, and the Navier‐Stokes equations are solved using computational fluid dynamic principles, assuming that the blood flow is a newtonian fluid and the vessel wall is rigid. As there is no preestablished method for the use of FFRCT to model ANOCOR, FFRCT evaluation consisted of collecting FFRCT values within the ANOCOR vessel, just downstream to its abnormal course location, by visual evaluation at the level of the first lateral branch and in the distal part of ANOCOR and non‐ANOCOR vessel locations, which were defined as the most distal analyzable part of main vessels (left anterior descending artery, left circumflex artery, and right coronary artery) obtained with FFRCT (Figure 3). The ANOCOR registry obtained ethical committee approval in France. All patients gave informed consent for the use of their imaging data for research purposes. Clinical decision making was based on the medical file without FFRCT analysis. We aimed to evaluate the hemodynamic impact of ANOCOR using FFRCT and the clinical outcome at 5 years of follow‐up.
Figure 3. Anomalous aortic origin of a coronary artery (ANOCOR), fractional flow reserve derived from computed tomography (FFRCT) analysis example.
Statistical Analysis
The summary of descriptive statistics is reported as mean (SD) or count (percentage), as appropriate. Normal distribution was tested with the Shapiro‐Wilk test. Comparisons between groups were performed by Mann‐Whitney or unpaired t test, as appropriate. A Cox regression model was used to adjust to the cofounding factors, defined as age, sex, diabetes mellitus, body mass index, and the presence of anatomical features at risk. Kaplan‐Meier curves were generated to evaluate the cumulative rate of the clinical end point (composite of cardiac death, myocardial infarction, and unplanned revascularization) in at‐risk and not at‐risk groups. Kaplan‐Meier curves were compared by the log‐rank test, according to at‐risk and not at‐risk anatomical features. P values were considered statistically significant if <0.05. All analyses were performed with Prism GraphPad 7.0 (GraphPad Software Inc, CA) and SPSS 21.0 (IBM Inc, NY).
Results
Study Population
From ANOCOR registry, including 472 patients with ANOCOR, we performed a retrospective study of CCTA performed in 105 patients; FFRCT could not be performed in 49 cases. In 6 cases, CCTA did not conform (not gated CT in 4 cases and cardiac phases missing in 2 cases), and therefore they were excluded from FFRCT analysis. Other reasons for unperformed FFRCT analysis are listed in Figure 1. Fifty‐six patients had a successful FFRCT analysis; 2 patients were lost to follow‐up just after inclusion and therefore excluded from the study: one patient was treated with conservative strategy, and the other one was treated with surgical revascularization (both patients had FFRCT values >0.80 in all distal main vessels). Finally, we included in this study 54 patients with 56 ANOCOR (2 patients had 2 ANOCOR) and 5 years of follow‐up (median of 7.5 years). Among the 54 patients included, 42 (78%) were men, and mean age was 60±13 years. Thirty‐eight (70%) patients had conservative treatment, and 16 (30%) patients had coronary revascularization with percutaneous angioplasty or coronary artery bypass grafting on at least one coronary artery (ANOCOR or non‐ANOCOR vessel) within the 6 months after the inclusion. In ANOCOR vessel, revascularization was performed in 5 cases, including 2 patients in association with valvular replacement and 2 percutaneous coronary interventions downstream the ANOCOR. Baseline characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics, Clinical Presentation, and Stress Testing of Patients With ANOCOR at Risk (ANOCOR Involving Interarterial Course) and Not at Risk
| Variable | Overall (N=54) | At Risk (N=31) | Not at Risk (N=23) | P Value for at Risk vs Not at Risk |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, y | 60±13 | 57±14 | 58±14 | 0.069 |
| Sex (men) | 42 (78) | 22 (71) | 20 (87) | 0.200 |
| BMI, kg/m2 | 26.5±3.9 | 26.7±3.8 | 26.2±3.9 | 0.823 |
| Hypertension | 23 (43) | 14 (45) | 9 (39) | 0.783 |
| Dyslipidemia | 16 (30) | 11 (35) | 5 (22) | 0.368 |
| Diabetes mellitus | 6 (11) | 3 (10) | 3 (13) | 0.999 |
| Current smoker | 6 (11) | 6 (19) | 0 | 0.030 |
| Physical activity* | 18 (33) | 9 (29) | 9 (39) | 0.370 |
| Previous MI | 4 (8) | 3 (10) | 1 (4) | 0.296 |
| Cardiomyopathy | 1 (2) | 0 | 1 (4) | 0.999 |
| Valvular heart disease | 3 (6) | 2 (6) | 1 (4) | 0.563 |
| Clinical presentation | ||||
| Asymptomatic | 11 (20) | 7 (17) | 4 (17) | 0.999 |
| Atypical angina | 10 (19) | 7 (21) | 3 (13) | 0.791 |
| Angina | 11 (20) | 6 (21) | 5 (22) | 0.913 |
| Dyspnea | 11 (20) | 7 (21) | 4 (17) | 0.741 |
| Palpitations | 11 (20) | 5 (29) | 6 (26) | 0.304 |
| Acute coronary syndrome | 5 (9) | 3 (8) | 2 (9) | 0.999 |
| Syncope | 3 (6) | 3 (8) | 0 | 0.267 |
| Resuscitated cardiac arrest | 1 (2) | 1 | 0 | 0.999 |
| Documented ventricular tachycardia | 2 (4) | 0 | 2 (9) | 0.158 |
| Noninvasive stress testing | ||||
| Noninvasive stress test | 23 (43) | 15 (48) | 8 (35) | 0.283 |
| Myocardial scintigraphy | 11 (39) | 8 (26) | 3 (13) | 0.319 |
| Positive exercise test | 16 (30) | 10 (32) | 6 (26) | 0.999 |
| Silent ischemia | 12 (22) | 6 (19) | 6 (26) | 0.999 |
Data are given as number (percentage), unless otherwise indicated. ANOCOR indicates anomalous aortic origin of a coronary artery; BMI, body mass index; and MI, myocardial infarction.
Physical activity: expressed as >20 minutes of exercise 3 times a week.
Clinical Presentation
Patients were asymptomatic in 20% of cases. Symptoms reported at inclusion were typical or atypical angina in 39% of cases, dyspnea in 20%, and palpitations in 20%. Acute coronary syndrome was noticed in 5 patients (9%), syncope in 3 patients (6%), and resuscitated cardiac arrest in 1 patient (2%). A functional test to assess ischemia was performed in 23 (43%) patients. Among them, 16 (70%) of patients had a positive test, 8 (35%) of exercise tests and 8 (35%) of single‐photon emission CT were positive. Silent ischemia was documented in 12 (52%) of patients with noninvasive stress test. No significant difference was observed between the 2 groups according to ANOCOR at risk and not at risk, except a significantly higher rate of current smoker in at‐risk group compared with not at‐risk group (19% versus 0%, respectively; P=0.030) (Table 1).
Angiographic Characteristics
ICA was performed in 50 (93%) patients. The degree of stenosis of ANOCOR and non‐ANOCOR vessels was detected and analyzed by visual estimation. In non‐ANOCOR vessels, no stenosis was observed in 17 (34%) patients, coronary stenosis <50% was observed in 14 (28%) patients, and coronary stenosis ≥50% was observed in 19 (38%) patients. In ANOCOR vessels, no stenosis was observed in 26 (52%) patients, coronary stenosis <50% was observed in 16 (32%) patients and coronary stenosis ≥50% was observed in 8 (16%) patients. No statistical difference was observed between ANOCOR at risk and ANOCOR not at risk in terms of visual assessment.
Morphological and FFRCT Evaluation
Among 56 ANOCOR vessels: left main artery was involved in 8 (14%) of cases, left circumflex artery was involved in 16 (29%) cases, and the right coronary artery was involved in 32 (57%) cases. The ANOCOR course involved the following reparation: preaortic arterial course was observed in 15 (27%) of cases, interarterial course was observed in 31 (55%) of cases, prepulmonar course was observed in 7 (13%) of cases, and subpulmonar course was observed in 3 (5%) of cases (Table 2 and Figure 2). Among the 31 interarterial courses, 30 involved right coronary artery and 1 involved left main artery.
Table 2.
Coronary Angiography, CT, and FFRCT Evaluation, According to Patients With ANOCOR at Risk and Not at Risk
| Variable | Overall (N=54) | At Risk (N=31) | Not at Risk (N=23) | P Value for at Risk vs Not at Risk |
|---|---|---|---|---|
| Coronary angiography for non‐ANOCOR vessels | N=50 | N=31 | N=19 | |
| No atheroma | 17 (34) | 12 (38) | 5 (26) | 0.220 |
| Atheroma <50% | 14 (28) | 8 (26) | 6 (32) | 0.506 |
| Atheroma >50% | 19 (38) | 11 (35) | 8 (42) | 0.999 |
| Coronary angiography for ANOCOR vessel | N=50 | N=31 | N=19 | |
| No stenosis | 26 (52) | 16 (52) | 10 (53) | 0.999 |
| Stenosis <50% | 16 (32) | 12 (39) | 4 (21) | 0.999 |
| Stenosis >50% | 8 (16) | 3 (10) | 5 (26) | 0.236 |
| CCTA of ANOCOR vessel | N=56 | N=33 | N=23 | |
| Left main artery | 8 (14) | 2 (6) | 6 (26) | 0.053 |
| Left circumflex artery | 16 (29) | 0 | 16 (69) | 0.001 |
| Right coronary artery | 32 (57) | 31 (94) | 1 (4) | <0.001 |
| CCTA ANOCOR course | N=56 | N=33 | N=23 | |
| Retroaortic | 15 (27) | 2 (6) | 13 (57) | <0.001 |
| Interarterial | 31 (55) | 31 (94) | 0 | <0.001 |
| Prepulmonar | 7 (13) | 0 | 7 (30) | 0.003 |
| Subpulmonar | 3 (5) | 0 | 3 (13) | 0.058 |
| FFRCT values | ||||
| Downstream to ANOCOR course | 0.87±0.20 | 0.90±0.10 | 0.91±0.09 | 0.516 |
| Distal to ANOCOR | 0.82±0.11 | 0.83±0.11 | 0.81±0.11 | 0.499 |
| Left anterior descending artery | 0.85±0.09 | 0.85±0.10 | 0.86±0.06 | 0.803 |
| Left circumflex artery | 0.91±0.08 | 0.92±0.07 | 0.90±0.09 | 0.224 |
| Right coronary artery | 0.89±0.05 | 0.86±0.05 | 0.90±0.06 | 0.201 |
Data are given as number (percentage) or mean±SD. ANOCOR indicates anomalous aortic origin of a coronary artery; CCTA, coronary CT angiography; CT, computed tomography; and FFRCT, fractional flow reserve derived from CT.
FFRCT could be obtained in 54 patients. The overall ANOCOR course reduced the FFRCT mean values from 1 in the aorta to 0.87±0.20 just downstream to the ANOCOR course. The mean FFRCT value in the distal segment of the ANOCOR was significantly lower than immediately after the ectopic segment of ANOCOR (0.82±0.11 versus 0.87±0.20, respectively; P<0.0001; Table 2). Mean FFRCT values between at‐risk and not at‐risk ANOCOR were similar (P>0.05 for all comparisons) (Table 2). FFRCT values are summarized in Figure 4, Table 1, and Figures S1 and S2.
Figure 4. Frequency distribution of anomalous aortic origin of a coronary artery (ANOCOR) vessel and non‐ANOCOR vessel.
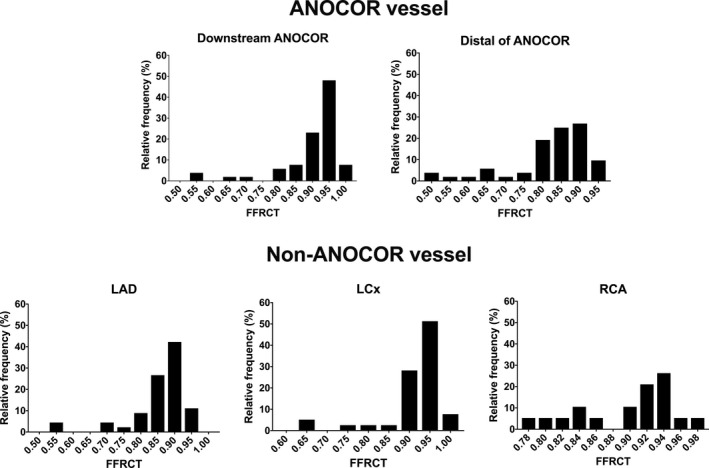
FFRCT indicates fractional flow reserve derived from computed tomography; LAD, left anterior descending; LCx, left circumflex; and RCA, right coronary artery.
Revascularization
In the group of patients at risk (n=31), 11 (35%) patients underwent revascularization. Five patients had coronary artery bypass grafting. Six patients had percutaneous coronary intervention revascularization. Among them, 1 patient had percutaneous coronary intervention of the left anterior descending artery downstream an ANOCOR course. In the group of patients not at risk (n=23), 6 (26%) patients underwent revascularization. Two patients had surgery with aortic valve replacement and coronary artery bypass grafting. Four patients had percutaneous coronary intervention revascularization.
Follow‐Up
Median follow‐up was 7.5 years. During the 5‐year follow‐up period, 1 patient (2%) presented an acute coronary syndrome associated with unplanned revascularization in the at‐risk group. Cox regression adjusted for the following confounding factors (age, sex, diabetes mellitus, body mass index, and anatomical features at risk), which were not statistically significant (P>0.05) (data not shown). The composite end point was not statistically different between the at‐risk and not at‐risk group (P=0.39) (Figure 5A and 5B).
Figure 5. Kaplan‐Meier curve of event‐free rate of patients at 5 years of follow‐up in the overall population and according to the anatomical feature at risk.
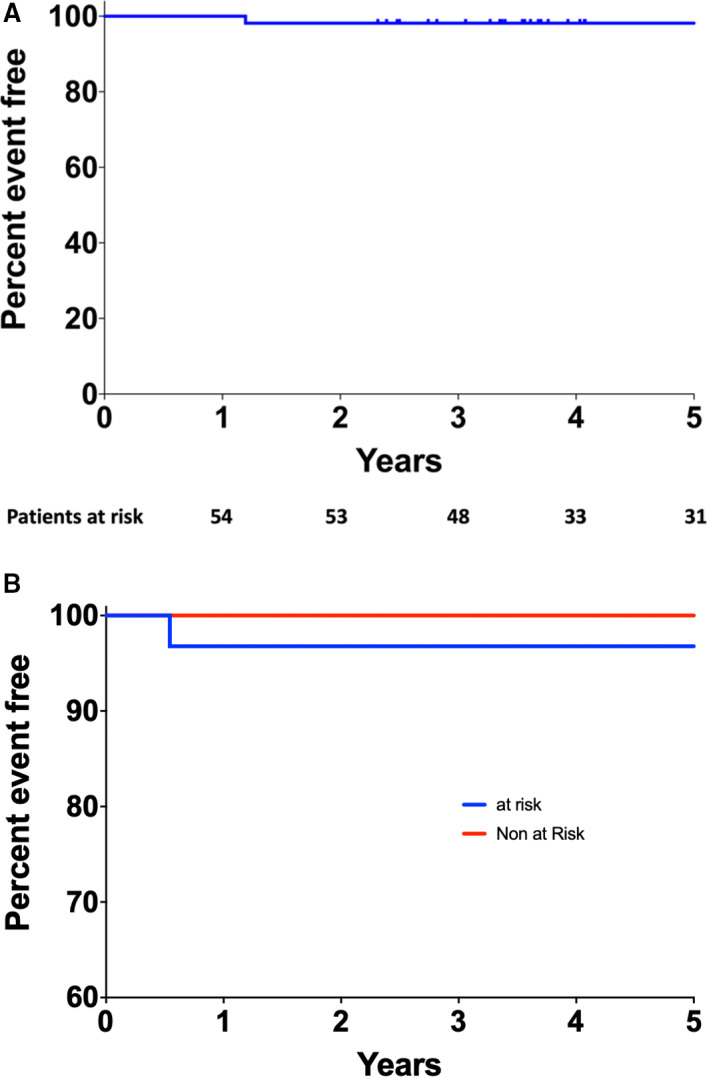
A, Kaplan‐Meier curve of event‐free rate of patients at 5 years of follow‐up. Number of patients at risk are presented below in the figure. B, Kaplan‐Meier curves of event‐free rate of patients at 5 years of follow‐up, according to anatomical feature at risk. No statistical differences were observed with log‐rank test (P=0.39).
Discussion
In our study, FFRCT showed a low hemodynamic impact on coronary flow of vessels, as evidenced by a low FFRCT gradient across different types of ANOCOR. This observation was associated with good outcomes in patients with ANOCOR at 5 years of clinical follow‐up. The growing use of cardiac CT will increase the number of patients recognized as having ANOCOR. However, clinical decision making for ANOCOR at risk remains challenging. Cheezum et al recommend a personalized approach based on clinical presentation, type of ANOCOR, and risk‐benefit balance of surgery. 4 This risk stratification can lead us to propose a surgical correction in patients with chest pain or syncope during exercise, documented myocardial ischemia, aborted sudden death, and ANOCOR with an interarterial or preaortic course. Coronary bypass graft surgery is not recommended in absence of high‐grade coronary stenosis. Accordingly, several surgical methods to treat ANOCOR, such as unroofing, coronary reimplantation, or neo‐ostia formation, are occasionally used. Noninvasive stress tests were performed in our study in 23 patients and were considered positive in 16 patients (70% of noninvasive stress tests). A clear mismatch was observed between ischemia evaluated with stress ECG and myocardial scintigraphy compared with FFRCT evaluation in our study population of ANOCOR. However, previous studies and meta‐analysis showed that myocardial scintigraphy has a low sensitivity compared with FFR. 18
FFRCT provides valuable data on the hemodynamic impact of ANOCOR all along its course based on cardiac CT calculation of myocardial mass and 3‐dimensional reconstruction of the coronary artery tree. On the other hand, ICA appears limited in its ability to obtain good images, and intravascular ultrasound and FFR evaluation is challenging. These evaluations were of value to understand that the 2‐dimensional coronary “stenosis” of ANOCOR proximal course has in fact a morphological elliptical shape with preserved lumen size assessed by intravascular ultrasound, and in most cases FFR values were >0.80. 8 Similarly, FFRCT showed, in 3‐dimensional reconstruction with color coding, both anatomical and hemodynamic aspects of ANOCOR, with an elliptical shape of the vessel and minor changes in FFRCT values within the ANOCOR course. In this population, our study showed favorable FFRCT values and excellent outcomes with a conservative strategy, with most of FFRCT values >0.80. Current guidelines support surgical revascularization (class IA) in patients with ANOCOR and symptoms or diagnostic evidence consistent with coronary ischemia attributable to the anomalous coronary artery. 19 , 20 Furthermore, guidelines accept that surgery or continued observation may be reasonable for asymptomatic patients with ANOCOR without ischemia or anatomic or physiological evaluation, suggesting potential for compromise of coronary perfusion, such as intramural course, fish mouth‐shaped orifice, or acute angle (class IIb). 20 However, our study showed the limited clinical impact of current positive noninvasive stress tests and anatomical evaluation of the coronary tree, whereas FFRCT could be an evaluation of interest for tackling difficult decisions on treatment of patients with ANOCOR.
Limitations
Our study has several limitations. Inherent to its retrospective design, it is not possible to compare revascularization strategies and outcome. Our population was selected by interventional cardiologists who mostly discover ANOCOR in patients with suspected coronary artery disease during ICA. Therefore, our population was men in 78% of cases, with a mean age of 60 years, and younger patients were not diagnosed. FFRCT analysis was limited to retrospective cardiac CT scans, performed between 2010 and 2013. The main reason is that radiologists performed cardiac CT to evaluate the ANOCOR position and course. Therefore, most of the quality criteria for FFRCT calculations were not respected. A second reason for not being able to obtain an FFRCT analysis could be the difficulty in avoiding coronary spacing in ANOCOR along the cardiac cycle with CT because of extra motion compared with standard anatomical coronary positions. We acknowledge the small number of patients included. However, this study is the largest reported with ANOCOR functional evaluation and 5 years of follow‐up.
Conclusions
In this cohort of adult patients with ANOCOR, we did not identify a hemodynamic impact of ANOCOR using FFRCT. Clinical outcome after a 5‐year follow‐up was favorable in this population, mostly treated medically. Further studies are needed to confirm the clinical value of FFRCT measurements in patients with ANOCOR, especially in the young adult population.
Appendix
Abnormal Coronary Origin Investigators
W. Abi Khalil, L. Aguirre, A. Akesbi, P. Aubry, Y. Banus, L. Belle, H. Benamer, Y. Biron, E. Boiffard, R. Bouallal, O. Boudvillain, R. Bourkaïb, C. Brasselet, E. Bressollette, P. Brunel, D. Champagnac, M. Coco, P. Commeau, S. Cook, P. Couppie, F. de Poli, L. Delorme, F. Descoutures, R. Didier, G. Ducrocq, P. Dupouy, C. Durier, R. El Mahmoud, J.‐B. Estève, B. Faurie, E. Garbarz, J.‐L. Georges, B. Gérardin, G. Gibault‐Genty, M. Gilard, M. Godin, J.‐J. Goy, C. Haffner‐Debus, X. Halna du Fretay, M. Hanssen, S. Hascoët, L. Jacquemin, J. Jeanneteau, T. Joseph, J.‐M. Juliard, B. Karsenty, R. Koning, E. La Scala, P. Leddet, G. Lemesle, G. Leurent, R. Levy, B. Livarek, C. Loubeyre, L. Maillard, L. Mangin, S. Marlière, M. Nejjari, P. Ohlmann, N. Poulos, A. Py, S. Ranc, A. Rialan, R. Roriz, P. Rougier, P. Staat, C. Thuaire, M. Togni, J. van Rothem, O. Varenne, V. Voudris.
Sources of Funding
None.
Disclosures
None.
Supporting information
Figures S1–S2
Acknowledgments
Heartflow (Redwood City, CA) provided the fractional flow reserve derived from computed tomography calculation for research purposes.
(J Am Heart Assoc. 2021;10:e018593. DOI: 10.1161/JAHA.120.018593.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018593
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Julien Adjedj, Email: julienadjedj@hotmail.com.
the Abnormal Coronary Origin investigators:
W. Abi Khalil, L. Aguirre, A. Akesbi, P. Aubry, Y. Banus, L. Belle, H. Benamer, Y. Biron, E. Boiffard, R. Bouallal, O. Boudvillain, R. Bourkaïb, C. Brasselet, E. Bressollette, P. Brunel, D. Champagnac, M. Coco, P. Commeau, S. Cook, P. Couppie, F. de Poli, L. Delorme, F. Descoutures, R. Didier, G. Ducrocq, P. Dupouy, C. Durier, R. El Mahmoud, J.‐B. Estève, B. Faurie, E. Garbarz, J.‐L. Georges, B. Gérardin, G. Gibault‐Genty, M. Gilard, M. Godin, J.‐J. Goy, C. Haffner‐Debus, X. Halna du Fretay, M. Hanssen, S. Hascoët, L. Jacquemin, J. Jeanneteau, T. Joseph, J.‐M. Juliard, B. Karsenty, R. Koning, E. La Scala, P. Leddet, G. Lemesle, G. Leurent, R. Levy, B. Livarek, C. Loubeyre, L. Maillard, L. Mangin, S. Marlière, M. Nejjari, P. Ohlmann, N. Poulos, A. Py, S. Ranc, A. Rialan, R. Roriz, P. Rougier, P. Staat, C. Thuaire, M. Togni, J. van Rothem, O. Varenne, and V. Voudris
References
- 1. Lluri G, Aboulhosn J. Coronary arterial development: a review of normal and congenitally anomalous patterns. Clin Cardiol. 2014;37:126–130.DOI: 10.1002/clc.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation. 2007;115:1296–1305.DOI: 10.1161/CIRCULATIONAHA.106.618082. [DOI] [PubMed] [Google Scholar]
- 3. Jacobs ML, Mavroudis C. Anomalies of the coronary arteries: nomenclature and classification. Cardiol Young. 2010;20(suppl 3):15–19.DOI: 10.1017/S1047951110001046. [DOI] [PubMed] [Google Scholar]
- 4. Cheezum MK, Liberthson RR, Shah NR, Villines TC, O'Gara PT, Landzberg MJ, Blankstein R. Anomalous aortic origin of a coronary artery from the inappropriate sinus of Valsalva. J Am Coll Cardiol. 2017;69:1592–1608. DOI: 10.1016/j.jacc.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 5. Brothers J, Carter C, McBride M, Spray T, Paridon S. Anomalous left coronary artery origin from the opposite sinus of Valsalva: evidence of intermittent ischemia. J Thorac Cardiovasc Surg. 2010;140:e27–e29.DOI: 10.1016/j.jtcvs.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 6. Logstrup BB, Buhl J, Nielsen AD, Smerup MH, Norgaard BL, Kristensen LD. Which exercise test to use for chest pain from an anomalous coronary artery. Congenit Heart Dis. 2014;9:E6–E10.DOI: 10.1111/chd.12046. [DOI] [PubMed] [Google Scholar]
- 7. Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501.DOI: 10.1016/S0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 8. Driesen BW, Warmerdam EG, Sieswerda G‐J, Schoof PH, Meijboom FJ, Haas F, Stella PR, Kraaijeveld AO, Evens FCM, Doevendans PAFM, et al. Anomalous coronary artery originating from the opposite sinus of Valsalva (ACAOS), fractional flow reserve‐ and intravascular ultrasound‐guided management in adult patients. Catheter Cardiovasc Interv. 2018;92:68–75.DOI: 10.1002/ccd.27578. [DOI] [PubMed] [Google Scholar]
- 9. Lee SE, Yu CW, Park K, Park KW, Suh J‐W, Cho Y‐S, Youn T‐J, Chae I‐H, Choi D‐J, Jang H‐J, et al. Physiological and clinical relevance of anomalous right coronary artery originating from left sinus of Valsalva in adults. Heart. 2016;102:114–119.DOI: 10.1136/heartjnl-2015-308488. [DOI] [PubMed] [Google Scholar]
- 10. Douglas PS, De Bruyne B, Pontone G, Patel MR, Norgaard BL, Byrne RA, Curzen N, Purcell I, Gutberlet M, Rioufol G, et al. 1‐Year outcomes of FFRCT‐guided care in patients with suspected coronary disease: the platform study. J Am Coll Cardiol. 2016;68:435–445.DOI: 10.1016/j.jacc.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 11. Adjedj J, Ferrara A, Penicka M, Van Mighem C, Wijns W. Coronary artery anomaly and evaluation by FFR computed tomography. Eur Heart J Cardiovasc Imaging. 2016;17:468.DOI: 10.1093/ehjci/jev356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zimmermann FM, Kobayashi Y, Mullen WL, Fearon WF. Non‐invasive FFRCT revealing severe inducible ischaemia in an anomalous right coronary artery. Eur Heart J. 2017;38:2569. [DOI] [PubMed] [Google Scholar]
- 13. Miki T, Miyoshi T, Watanabe A, Osawa K, Amioka N, Ito H. Anomalous aortic origin of the right coronary artery with functional ischemia determined with fractional flow reserve derived from computed tomography. Clin Case Rep. 2018;6:1371–1372.DOI: 10.1002/ccr3.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pascoe HM, Lui E, Joshi SB, Bui J, Hawson J, Iyer R, McCusker MW, Langenberg F, Better N. On‐site CT‐FFR of an anomalous right coronary artery to influence revascularization strategy. J Nucl Cardiol. 2019;26:1020–1022.DOI: 10.1007/s12350-018-01591-x. [DOI] [PubMed] [Google Scholar]
- 15. Koutsoukis A, Halna du Fretay X, Dupouy P, Ou P, Laissy JP, Juliard JM, Hyafil F, Aubry P. Interobserver variability in the classification of congenital coronary abnormalities: a substudy of the anomalous connections of the coronary arteries registry. Congenit Heart Dis. 2017;12:726–732. [DOI] [PubMed] [Google Scholar]
- 16. Genders TSS, Steyerberg EW, Alkadhi H, Leschka S, Desbiolles L, Nieman K, Galema TW, Meijboom WB, Mollet NR, de Feyter PJ, et al. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J. 2011;32:1316–1330.DOI: 10.1093/eurheartj/ehr014. [DOI] [PubMed] [Google Scholar]
- 17. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2019;41:407–477.DOI: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 18. Pontone G, Guaricci AI, Palmer SC, Andreini D, Verdecchia M, Fusini L, Lorenzoni V, Guglielmo M, Muscogiuri G, Baggiano A, et al. Diagnostic performance of non‐invasive imaging for stable coronary artery disease: a meta‐analysis. Int J Cardiol. 2020;300:276–281.DOI: 10.1016/j.ijcard.2019.10.046. [DOI] [PubMed] [Google Scholar]
- 19. Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines: developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123–e210. DOI: 10.1016/j.jacc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 20. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;73:1494–1563. DOI: 10.1016/j.jacc.2018.08.1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S2


