Abstract
Background
Coronary revascularization provides important long‐term clinical benefits to patients with high‐risk presentations of coronary artery disease, including those with chronic kidney disease. The cost‐effectiveness of coronary interventions in this setting is not known.
Methods and Results
We developed a Markov cohort simulation model to assess the cost‐effectiveness of percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) in patients with chronic kidney disease who were hospitalized with acute myocardial infarction or unstable angina. Model inputs were primarily drawn from a sample of 14 300 patients identified using the Medicare 20% sample. Survival, quality‐adjusted life‐years, costs, and cost‐effectiveness were projected over a 20‐year time horizon. Multivariable models indicated higher 30‐day mortality and end‐stage renal disease with both PCI and CABG, and higher stroke with CABG, relative to medical therapy. However, the model projected long‐term gains of 0.72 quality‐adjusted life‐years (0.97 life‐years) for PCI compared with medical therapy, and 0.93 quality‐adjusted life‐years (1.32 life‐years) for CABG compared with PCI. Incorporation of long‐term costs resulted in incremental cost‐effectiveness ratios of $65 326 per quality‐adjusted life‐year gained for PCI versus medical therapy, and $101 565 for CABG versus PCI. Results were robust to changes in input parameters but strongly influenced by the background costs of the population, and the time horizon.
Conclusions
For patients with chronic kidney disease and high‐risk coronary artery disease presentations, PCI and CABG were both associated with markedly increased costs as well as gains in quality‐adjusted life expectancy, with incremental cost‐effectiveness ratios indicating intermediate value in health economic terms.
Keywords: acute coronary syndrome, chronic kidney disease, coronary artery bypass grafting, cost‐effectiveness, percutaneous coronary intervention
Subject Categories: Cardiovascular Surgery, Percutaneous Coronary Intervention, Revascularization, Cost-Effectiveness
Nonstandard Abbreviations and Acronyms
- ICER
incremental cost‐effectiveness ratio
Clinical Perspective
What Is New?
In a large sample of Medicare patients with chronic kidney disease hospitalized with high‐risk presentations of coronary artery disease, coronary revascularization was associated with increased adverse events at 30 days, including end‐stage kidney disease and death.
Within a year, revascularization, both percutaneous coronary intervention and coronary artery bypass grafting, was associated with improved outcomes relative to medical therapy, leading to improved survival and quality‐adjusted survival in long‐term cohort projections.
Economic analysis of these cohorts predicts coronary revascularization to be of intermediate value, with incremental cost‐effectiveness ratios of $65 000 to $85 000 per quality‐adjusted life‐year gained for percutaneous coronary intervention and coronary artery bypass grafting relative to medical therapy.
What Are the Clinical Implications?
Time horizon and patient preferences for achievement or avoidance of specific outcomes (dialysis, stroke, or death) should influence the choice of treatment strategy for patients in these scenarios.
Given improved long‐term outcomes and acceptable cost‐effectiveness, the presence of chronic kidney disease in older patients should not preclude use of coronary revascularization on either clinical or economic grounds.
Chronic kidney disease (CKD) is known to increase the risk of death and other poor outcomes for patients with cardiovascular disease. 1 , 2 , 3 Although this could imply that patients with CKD have more to gain from coronary revascularization and other interventions than patients without CKD, strong evidence of this is lacking. At the same time, aggressive management of coronary artery disease (CAD) in patients with CKD definitely increases the risk of acute kidney injury and other complications, including stroke. Fear of acute kidney injury and the precipitation of end‐stage renal disease (ESRD) have led to possibly overcautious management of patients with CKD, an approach referred to as “renalism.” 4
Several major clinical trials have found that coronary revascularization does not improve survival or reduce myocardial infarction (MI) relative to medical therapy in patients with stable coronary disease. 5 , 6 This also holds true in patients with CKD and stable coronary disease with demonstrable ischemia, a group in whom an early invasive strategy was also associated with increased risk of stroke and dialysis. 7 Nonetheless, the role of coronary revascularization in patients with unstable coronary syndromes remains relatively unquestioned. 8 , 9
In a recent analysis of >34 000 Medicare patients, neither coronary artery bypass grafting (CABG) nor percutaneous coronary intervention (PCI) was found to improve survival in patients with CKD and low or medium clinical risk CAD relative to medical therapy, 10 a finding consistent with the clinical trial literature. However, in high‐risk patients, defined by admission for acute MI (AMI) or unstable angina, both CABG and PCI significantly improved survival compared with medical therapy. The current analysis sought to extend those findings by evaluating the cost‐effectiveness of PCI and CABG versus medical therapy in patients with CKD and unstable CAD.
Methods
Portions of the data, analytic methods, and study materials that support the findings of this study are available to other researchers from the corresponding author on reasonable request, but may be subject to data use agreements with the Centers for Medicare and Medicaid Services.
Overview
We conducted a Markov cohort analysis to compare the costs, life expectancy, quality‐adjusted life expectancy, and cost‐effectiveness of medical therapy, PCI, and CABG for patients with CKD and CAD. The principal source for inputs into this model was an analysis of Medicare claims data used for a recent clinical report. 10 As previously described, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM), diagnosis and procedure codes or Current Procedural Terminology codes were used to define the study cohort from the 20% Medicare sample from years 2007 to 2012. The Medicare sample was acquired and analyzed by the Chronic Disease Research Group at the Hennepin Healthcare Research Institute. The Medicare claims analysis was approved by the Human Subjects Research Committee at Hennepin County Medical Center/Hennepin Healthcare System, with a waiver of informed consent. CKD was defined using ICD‐9‐CM code 585.X. Patients with a history of dialysis treatment before the index hospital admission were excluded.
Within this cohort, qualifying CAD events were categorized as low, medium, and high risk. Low‐ and medium‐risk events were defined as the performance of a stress test or coronary angiogram in patients without (low risk) or with (medium risk) a preceding history of cardiovascular disease. Patients with hospital admission for unstable angina or AMI were designated as high risk. As the initial clinical outcomes assessment showed no evidence of improved survival with revascularization in the low‐ or medium‐risk patients, we elected to confine the cost‐effectiveness analysis to the high‐risk group (N=14 300).
As originally constructed, the high‐risk study cohort was defined by a qualifying event (admission for AMI or unstable angina) and a qualifying therapy (PCI, CABG, or intensified medical therapy, as evidenced by prescription of a new class of cardiovascular drug in part D claims) within 60 days of that qualifying event. 10 The qualifying events and qualifying therapies usually occurred during the same index hospitalization, but not always. For clinical outcomes, we followed the same convention as in the prior analysis, which was to make the start time of the analysis the date of the qualifying therapy. However, to ensure consistency between groups, the costs of the index hospitalization encompassing the qualifying event were included for all patients.
Markov Details
Our base case analysis was a Markov cohort simulation 11 with a cycle length of 1 month and a time horizon of 20 years. The starting age of the cohort was set at 75 years based on the mean cohort age of 78 years (Table 1). A 20‐year time horizon is therefore tantamount to lifetime. As cost inputs were derived from Medicare payment data, the analysis took the perspective of the Medicare program. An annual discount rate of 3% was applied to all future costs and benefits. 12 The model was programmed using TreeAge Pro 2019 software (Williamstown, MA).
Table 1.
Baseline Characteristics of the Study Population
| PCI | CABG | MedRx | Total | P Value | |
|---|---|---|---|---|---|
| (N=4608; 32.2%) | (N=1330; 9.3%) | (N=8362; 58.5%) | (N=14 300; 100%) | ||
| Age, mean±SD, y | 75.1±10.5 | 72.2±8.9 | 80.3±11.4 | 77.9±11.3 | <0.0001 |
| Age | <0.0001 | ||||
| <65 y | 591 (12.8) | 170 (12.8) | 689 (8.2) | 1450 (10.1) | |
| 65–74 y | 1441 (31.3) | 616 (46.3) | 1561 (18.7) | 3618 (25.3) | |
| 75–84 y | 1703 (37.0) | 465 (35.0) | 2585 (30.9) | 4753 (33.2) | |
| ≥85 y | 873 (18.9) | 79 (5.9) | 3527 (42.2) | 4479 (31.3) | |
| Sex | <0.0001 | ||||
| Men | 2310 (50.1) | 854 (64.2) | 3195 (38.2) | 6359 (44.5) | |
| Women | 2298 (49.9) | 476 (35.8) | 5167 (61.8) | 7941 (55.5) | |
| Race/ethnicity | <0.0001 | ||||
| White | 3747 (81.3) | 1076 (80.9) | 6409 (76.6) | 11 232 (78.5) | |
| Black | 526 (11.4) | 138 (10.4) | 1354 (16.2) | 2018 (14.1) | |
| Asian | 102 (2.2) | 40 (3.0) | 202 (2.4) | 344 (2.4) | |
| Hispanic | 122 (2.6) | 35 (2.6) | 248 (3.0) | 405 (2.8) | |
| Other/unknown | 111 (2.4) | 41 (3.1) | 149 (1.8) | 301 (2.1) | |
| Heart failure | 735 (16.0) | 125 (9.4) | 2131 (25.5) | 2991 (20.9) | <0.0001 |
| Diabetes mellitus | 2072 (45.0) | 677 (50.9) | 3476 (41.6) | 6225 (43.5) | <0.0001 |
| Cerebrovascular accident/TIA | 354 (7.7) | 77 (5.8) | 1039 (12.4) | 1470 (10.3) | <0.0001 |
| Peripheral artery disease | 554 (12.0) | 155 (11.7) | 1569 (18.8) | 2278 (15.9) | <0.0001 |
| Other cardiac disease | 251 (5.4) | 44 (3.3) | 691 (8.3) | 986 (6.9) | <0.0001 |
| Chronic lung disease | 775 (16.8) | 134 (10.1) | 1878 (22.5) | 2787 (19.5) | <0.0001 |
| Gastrointestinal bleeding, PUD, and reflux | 82 (1.8) | 14 (1.1) | 308 (3.7) | 404 (2.8) | <0.0001 |
| Liver disease | 51 (1.1) | 11 (0.8) | 83 (1.0) | 145 (1.0) | 0.639 |
| Dysrhythmia | 575 (12.5) | 112 (8.4) | 1625 (19.4) | 2312 (16.2) | <0.0001 |
| Anemia | 925 (20.1) | 211 (15.9) | 2667 (31.9) | 3803 (26.6) | <0.0001 |
| Cancer | 294 (6.4) | 64 (4.8) | 574 (6.9) | 932 (6.5) | 0.017 |
| Presentation | <0.0001 | ||||
| STEMI | 1671 (36.3) | 251 (18.9) | 1198 (14.3) | 3120 (21.8) | |
| NSTEMI | 2294 (49.8) | 812 (61.1) | 5967 (71.4) | 9073 (63.4) | |
| Unspecified | 643 (14.0) | 267 (20.1) | 1197 (14.3) | 2107 (14.7) | |
| CKD stage | <0.0001 | ||||
| Stage 1–3 | 1927 (41.8) | 579 (43.5) | 3038 (36.3) | 5544 (38.8) | |
| Stage 4–5 | 881 (19.1) | 263 (19.8) | 1979 (23.7) | 3123 (21.8) | |
| Unspecified | 1800 (39.1) | 488 (36.7) | 3345 (40.0) | 5633 (39.4) |
Values are number (percentage) unless otherwise noted. CABG indicates coronary artery bypass grafting; CKD, chronic kidney disease; MedRx, medical therapy; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; PUD, peptic ulcer disease; STEMI, ST‐segment–elevation myocardial infarction; and TIA, transient ischemic attack.
The model consisted of 5 health states representing the outcomes most likely to be affected by the management decision under study, on the basis of clinical judgment. Those health states and the permitted transitions between them are depicted in Figure 1. Although theoretically more transitions between states could have been considered (eg, between ESRD and stroke), some were not included to reduce model complexity. These states were characterized by high mortality and markedly reduced quality of life; thus, the impact of omitting transitions between them was believed to be negligible.
Figure 1. State‐transition diagram.
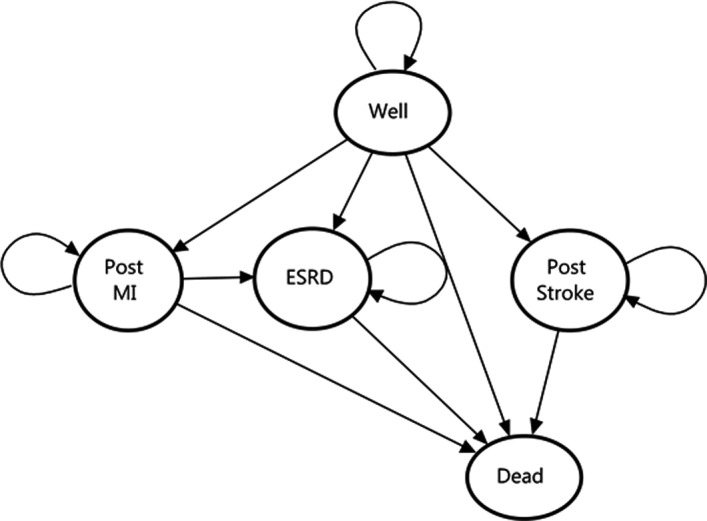
Circles denote discrete health states within the Markov model. Arrows indicate possible transitions between health states. ESRD indicates end‐stage renal disease; and MI, myocardial infarction.
The first 1‐month cycle of the model began the date of the qualifying therapy (as defined above), extending through 30 days, and was represented analytically as a decision tree. The proportion of each treatment cohort that experienced death, recurrent MI, stroke, or ESRD during the first 30 days then entered those respective health states in the Markov model. The remaining proportion of the cohort entered the “well” state. Each health state was associated with specific costs and utility (reflecting quality of life) values, as shown in Table 2, which were the same for each of the 3 treatment cohorts. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Over subsequent monthly Markov cycles, portions of each cohort could move between the health states, according to group‐specific transition probabilities, as described below.
Table 2.
Model Inputs
| Transition Probabilities | Base Case | Plausible Range | Source |
|---|---|---|---|
| Medical therapy, % | |||
| 30‐d Mortality | 6.8 | 6.2 to 7.3 | Claims analysis |
| 30‐d ESRD | 1.6 | 1.3 to 1.9 | Claims analysis |
| 30‐d Stroke | 0.7 | 0.5 to 0.9 | Claims analysis |
| 30‐d MI | 2.8 | 2.4 to 3.2 | Claims analysis |
| 31‐d to 12‐mo Mortality* | 27.7 | 26.7 to 28.7 | Claims analysis |
| 31‐d to 12‐mo ESRD | 2.2 | 1.9 to 2.6 | Claims analysis |
| 31‐d to 12‐mo Stroke | 2.7 | 2.4 to 3.1 | Claims analysis |
| 31‐d to 12‐mo MI | 10.6 | 9.9 to 11.3 | Claims analysis |
| 31‐d to 12‐mo PCI | 1.7 | 1.4 to 2.0 | Claims analysis |
| 31‐d to 12‐mo CABG | 1.3 | 1.0 to 1.5 | Claims analysis |
| All groups, % | |||
| CABG mortality (30 d) | 4.8 | 3.3 to 6.8 | 3 |
| PCI mortality (30 d) | 1.5 | 1.38 to 1.52 | 13 |
| MI mortality (30 d) | 12.5 | 10.1 to 14.9 | 14 |
| Stroke mortality (30 d) | 8.2 | 8.2 to 10.1 | 15 |
| Costs, $ | |||
| Initial hospitalization, MedRx | 20 769 | 18 589 to 22 948 | Claims analysis |
| Initial hospitalization, CABG | 64 666 | 61 978 to 67 354 | Claims analysis |
| Initial hospitalization, PCI | 30 198 | 27 901 to 32 494 | Claims analysis |
| Post–acute care, MedRx † | 2070 | 1826 to 2313 | Claims analysis |
| Post–acute care, CABG † | 2635 | 2332 to 2938 | Claims analysis |
| Post–acute care, PCI † | 1377 | 1118 to 1635 | Claims analysis |
| Event costs, $ | |||
| Stroke (initial event) | 15 180 | 15 000 to 15 400 | 16 |
| CABG (follow‐up event) | 56 694 | 39 262 to 65 441 | Claims analysis |
| PCI (follow‐up event) | 22 955 | 13 885 to 26 237 | Claims analysis |
| Acute MI | 19 621 | 18 510 to 20 732 | Claims analysis |
| Follow‐up costs, $ | |||
| Background monthly | 2101 | 2025 to 2176 | Claims analysis |
| Post‐stroke monthly | 12 584 | 5470 to 39 087 | Claims analysis |
| ESRD monthly | 8054 | 5326 to 14 364 | Claims analysis |
| Utilities | |||
| Well (CKD) | 0.79 | 0.7 to 0.89 | 17 |
| ESRD | 0.51 | 0.2 to 0.82 | 18 |
| Disutility post‐MI | −0.134 | −0.174 to −0.094 | 19 |
| Post‐stroke | 0.64 | 0.31 to 0.99 | 20 |
| Disutility CABG | −0.02 | 0 to 0.04 | 27 |
| Disutility PCI | −0.005 | 0 to 0.02 | 21 |
Binomial calculation used to generate 95% CI for proportions. Transition probabilities for the MedRx arm, as well as costs and utilities for all groups, are shown. Transition probabilities for the CABG and PCI arms were calculated using relative risks (as shown in Table 3) referent to the values for the MedRx group shown here. CABG indicates coronary artery bypass grafting; CKD, chronic kidney disease; ESRD, end‐stage renal disease; MedRx, medical therapy; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
Observed. See text for mortality calculations.
Per patient‐month, applied to first 30 days following index event.
Clinical Outcomes
Clinical outcomes depicted in the model were derived from the Medicare claims analysis wherever possible and based on ICD‐9‐CM codes, as previously described, 10 or on death dates obtained from the Medicare Master Beneficiary Summary File. A limited number of event probabilities were not easily calculable with the claims data and were derived from external literature, as cited in Table 2. Given the differential and shifting nature of risks with the 3 different treatment options, the assessment of outcomes was partitioned into 3 time periods: 0 to 30 days, 31 days to 1 year, and beyond 1 year. Surviving patients (N=7828) had a median follow‐up observation period of 22 months (interquartile range, 9–40 months).
For the medical therapy arm of the model, observed and unadjusted 30‐day event rates for death, stroke, MI, and ESRD were used (Table 2). Corresponding rates for these events in the PCI and CABG groups were calculated as relative risks, referent to medical therapy, using logistic regression models, adjusted for age, sex, race, CKD stage, ST‐segment–elevation MI versus non–ST‐segment–elevation MI presentation, and comorbidities (as shown in Table 1). The resulting relative risks used in the model are shown in Table 3.
Table 3.
Relative Risks for Clinical Event Probabilities
| Variable | 0–30 d, | 30 d–1 y, | >1 y, |
|---|---|---|---|
| OR (95% CI)* | HR (95% CI)* | HR (95% CI)* | |
| CABG vs MedRx | |||
| Death | 1.41 (1.09–1.83) | 0.55 (0.46–0.66) | 0.43 (0.36–0.52) |
| Stroke | 2.02 (1.04–3.90) | 0.77 (0.49–1.20) | 0.52 (0.33–0.81) |
| ESRD | 1.95 (1.37–2.78) | 1.81 (1.33–2.46) | 1.05 (0.74–1.48) |
| AMI | 0.97 (0.65–1.46) | 0.45 (0.34–0.59) | 0.40 (0.30–0.53) |
| PCI | 2.66 (1.10–6.46) | 1.27 (0.86–1.87) | 1.32 (0.94–1.84) |
| CABG | … | … | … |
| PCI vs MedRx | |||
| Death | 1.69 (1.46–1.96) | 0.58 (0.53–0.65) | 0.55 (0.50–0.61) |
| Stroke | 1.13 (0.70–1.84) | 0.73 (0.55–0.96) | 0.83 (0.65–1.06) |
| ESRD | 1.85 (1.42–2.41) | 0.91 (0.70–1.18) | 0.80 (0.61–1.05) |
| AMI | 1.30 (1.04–1.64) | 0.80 (0.69–0.91) | 0.70 (0.60–0.81) |
| Repeated PCI | 19.52 (11.16–34.15) | 4.85 (3.92–5.99) | 2.32 (1.84–2.91) |
| CABG | 5.05 (1.79–14.21) | 0.77 (0.55–1.08) | 0.85 (0.52–1.40) |
AMI indicates acute myocardial infarction; CABG, coronary artery bypass grafting; ESRD, end‐stage renal disease; HR, hazard ratio; MedRx, medical therapy; OR, odds ratio; and PCI, percutaneous coronary intervention.
ORs and HRs shown were derived from logistic regression and Cox proportional hazard models, respectively, adjusting for the following variables: age, sex, race, chronic kidney disease stage, ST‐segment–elevation myocardial infarction vs non–ST‐segment–elevation myocardial infarction presentation, and comorbidities, as listed in Table 1.
Long‐term survival in the medical therapy group was projected using a combination of both observed mortality rates following key clinical events in conjunction with a life‐table approach. First, 1‐, 2‐, and 3‐year mortality rates in patients experiencing ESRD, stroke, and MI were calculated from the observed data and programmed as the mortality risks for the associated health states in the model. The mortality rates from year 2 to year 3 were then carried forward over the 20‐year model time horizon.
Mortality rates from US life tables 22 were then applied to the “well” health state to calibrate the overall mortality rate predicted by the model for the medical therapy group. As patients in the medical therapy group were significantly older (mean age, 80.3 years) than those in the PCI and CABG groups (Table 1), we age adjusted observed mortality from 31 days to 4 years using the hazard ratio for age derived from a multivariable Cox proportional hazards model that included all 30‐day survivors. Plots of observed and age‐adjusted mortality for the medical therapy group are shown in Figure S1. Life table mortality rates were then adjusted with multiplicative factors until the annual total mortality rates from the model closely matched the age‐adjusted rates (as also shown in Figure S1). Long‐term risks for other key clinical events in the medical therapy group were taken directly from the claims analysis (see Table 2 for 31‐day to 12‐month risks, among patients surviving the first 30 days without events).
For the CABG and PCI groups, relative risks for all clinical events, including death, were calculated relative to medical therapy using Cox proportional hazards models, one for the 31‐day to 12‐month interval, and a second for beyond 12 months. Each of the Cox models was run on patients who had survived the preceding interval without experiencing the event of interest. The relative risks derived from these Cox models (Table 3) were multiplied by the baseline event rates in the medical therapy group to project outcomes for the PCI and CABG groups in the Markov model.
Costs
Most of the cost inputs for the model were also derived from the Medicare claims analysis (Table 2). Medicare reimbursement rates from parts A, B, and D were included and accepted as proxy for actual costs. We inflated costs from all years to 2015 US dollars using the Centers for Medicare and Medicaid Services market basket index.
For the index hospital admissions, costs were adjusted for baseline differences among the 3 treatment groups using a linear regression model. Covariates in that model included the same demographic and clinical variables used in the assessment of 30‐day clinical event rates. The adjusted mean costs for each treatment group were used for the index admission costs in the Markov model. The Markov model also included post–acute care costs following the index admission, which were more likely to be observed following CABG. Post–acute care costs included all reimbursements for skilled nursing, inpatient rehabilitation, home health, or hospice within 30 days of discharge from the index hospital admission, and were also adjusted with linear regression.
Monthly background medical care costs were estimated from 5023 patients who survived 18 months from the date of their qualifying event and experienced none of ESRD, AMI, PCI, CABG, or stroke. The first 90 days of follow‐up were excluded from the calculation of background costs to avoid the inclusion of costs related to the qualifying events and treatments. Unadjusted mean costs for all of these patients were applied as background costs for all patients in the “well” health state.
Costs for the ESRD health state were estimated from 736 patients who developed ESRD at the time of the qualifying therapy and survived to 12 months without additional clinical events. As the distribution of costs in this group was extremely skewed, we selected the median monthly cost from this group for the Markov model. The Medicare claims were also used to derive event costs for MI, PCI, and CABG that occurred during follow‐up.
Health State Utilities
Health state utilities are summary measures of quality of life, taking values from 0 to 1, that are applied to life expectancy to calculate quality‐adjusted life‐years (QALYs). Each health state other than death was assigned a utility obtained from previously published literature (Table 2). Transient utility decrements (or “disutilities)” for follow‐up CABG and PCI events were applied in the model as tolls.
Cost‐Effectiveness Analysis
Our base case analysis calculated incremental cost‐effectiveness ratios (ICERs), expressed as dollars per QALY gained. The 3 therapies were sorted in ascending order of total 20‐year costs, which corresponded with ascending QALYs. ICERs were then calculated comparing each therapy with its next‐best alternative. ICERs were also calculated as dollars per life‐year gained.
Model uncertainty was explored with both 1‐way sensitivity analysis and probabilistic sensitivity analysis. For 1‐way sensitivity analysis, individual model parameters were varied across the range of plausible values (as shown in Tables 2 and 3, in most cases 95% CIs). Results were compiled and presented as tornado diagrams. Probabilistic sensitivity analysis was performed by replacing each model input with a probability distribution. For clinical events, β distributions were used on the basis of observed event rates (Table S1). Log‐normal distributions were used for costs, and β distributions were used for literature‐based probabilities. Second‐order Monte Carlo simulation was then performed, in which each probability distribution was independently resampled over 1000 model iterations. The resulting ICERs from each model iteration were then plotted on the cost‐effectiveness plane. Cost‐effectiveness acceptability curves were then constructed, plotting the probability that each clinical strategy would be cost‐effective across a range of willingness‐to‐pay thresholds.
Results
The clinical characteristics of the patients with CKD with hospital admission for AMI or unstable angina are shown in Table 1. Of the 14 300 patients in this sample, 58% were treated medically. The patients treated with medical therapy were significantly older, more often women, less often White race, and more likely to have several comorbid conditions, including heart failure, chronic lung disease, prior stroke/transient ischemic attack, and peripheral artery disease, than the patients in the other groups. The medical therapy patients were also more likely to have non–ST‐segment–elevation MI and stage 4 to 5 (or unclassified) CKD than the patients who underwent revascularization.
The Markov model projected both highest long‐term survival (Figure S2) and costs (Figure S3) with CABG, and lowest with medical therapy. The results of our base case cost‐effectiveness analysis are shown in Table 4. PCI was projected to add 0.72 QALYs relative to medical therapy, with an ICER of $65 326 per QALY gained. CABG was projected to add an additional 0.93 QALYs relative to PCI, with an ICER of $101 565 per QALY gained. If CABG were compared directly with medical therapy (eg, for patients suitable for CABG but not PCI), then the gain in QALYs would be 1.65 and the ICER $85 678 per QALY gained.
Table 4.
Base Case Results With 20‐Year Time Horizon
| Strategy | Total Cost, $ | LYs | QALYs | Incremental Cost, $ | LYs Gained | QALYs Gained | ICER, $/QALYs Gained | ICER, $/LYs Gained |
|---|---|---|---|---|---|---|---|---|
| MedRx | 180 681 | 4.38 | 3.32 | |||||
| PCI | 227 834 | 5.36 | 4.04 | 47 153 | 0.97 | 0.72 | 65 326 | 48 498 |
| CABG | 321 750 | 6.67 | 4.96 | 93 916 | 1.32 | 0.93 | 101 565 | 71 289 |
By convention, treatments are ordered from the top row down in the table according to increasing cumulative cost. Incremental costs, benefits, and ICERs are then shown for each treatment compared with its next best alternative. CABG indicates coronary artery bypass grafting; ICER, incremental cost‐effectiveness ratio; LY, life‐year; MedRx, medical therapy; PCI, percutaneous coronary intervention; and QALY, quality‐adjusted LY.
One‐way sensitivity analyses indicated that the model results were not highly influenced by changes in any single model parameter (Figure S4A and B). The ICER of PCI versus medical therapy was most sensitive to changes in the discount rate, the assumed utility of the “well” health state (representing patients with CKD), and the longitudinal cost of the ESRD health state, but in no case exceeded $75 000 per QALY gained. The ICER for CABG versus medical therapy was most sensitive to the longitudinal costs of the ESRD and stroke health states, and the starting age of the cohort. The results were also influenced by the high (~$2100/month) background medical care costs for patients in the “well” health state. When such costs were omitted, the ICERs decreased to $32 345 and $79 459 per QALY gained for PCI versus medical therapy and for CABG versus PCI, respectively.
Figure 2A and B show ICER scatterplots from the probabilistic sensitivity analysis, reflecting overall model uncertainty. The results indicate that PCI was highly likely to be both more effective and more costly than medical therapy, and that CABG was highly likely to be both more effective and more costly than PCI. In both cases, most model replications produced an ICER between $50 000 and $150 000 per QALY gained. The ICERs for PCI (relative to medical therapy) and CABG (relative to PCI) were <$150 000 per QALY gained in 94% and 78% of model replications, respectively. The cost‐effectiveness acceptability curve (Figure 3) indicates that medical therapy, PCI, and CABG would be preferred at low (<$50 000 per QALY), medium (roughly $50 000 to $80 000 per QALY), and high (>$80 000 per QALY) willingness to pay thresholds, respectively.
Figure 2. Probabilistic sensitivity analysis scatterplot.
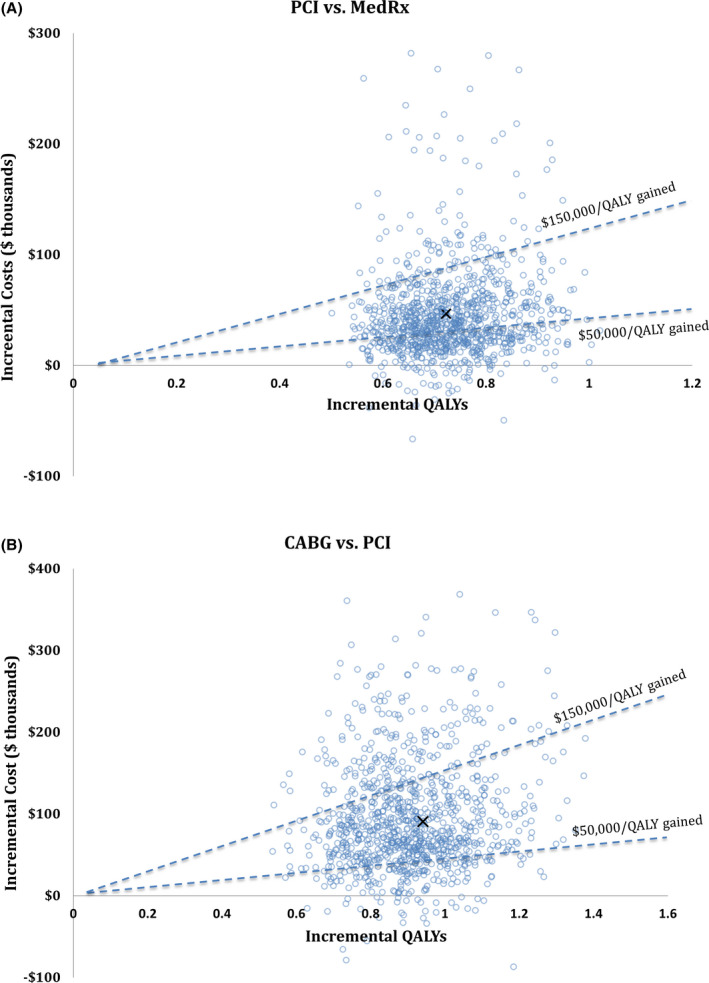
Incremental quality‐adjusted life‐years (QALYs) (x‐axis) and incremental costs (y‐axis) are shown for 1000 model replications (open circles). For each model replication, individual input parameters were drawn separately at random from probability distributions. The “x” represents the base case result. A, Comparison of percutaneous coronary intervention (PCI) vs medical therapy (MedRx). B, Comparison of coronary artery bypass grafting (CABG) vs PCI. The dashed lines represent willingness‐to‐pay thresholds of $50 000 and $150 000 per QALY gained. Points below and rightward to those lines indicate cost‐effective results at those thresholds.
Figure 3. Cost‐effectiveness acceptability curve.
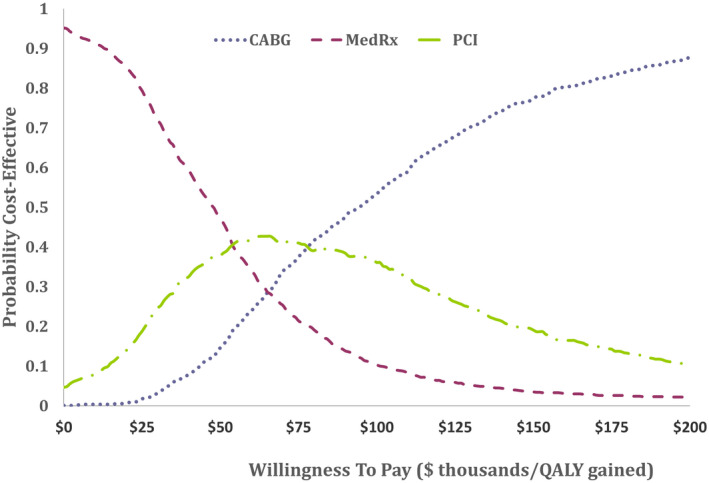
The probability (y‐axis) that each of the 3 assessed therapies was cost‐effective across a range of willingness‐to‐pay thresholds (x‐axis) is shown. These probabilities are derived from the probabilistic sensitivity analysis. On health economic grounds, medical therapy (MedRx) would be preferred at low willingness‐to‐pay thresholds (<$50 000 per quality‐adjusted life‐year [QALY] gained) and coronary artery bypass grafting (CABG) would be preferred at higher thresholds (>$75 000). PCI indicates percutaneous coronary intervention.
The base case results were found to be highly sensitive to the model time horizon. Through 2 years, a slight survival advantage emerged for CABG (Figure S2), but because CABG was associated with higher early rates of stroke and ESRD, cumulative QALYs at 2 years showed smaller differences (Figure S5), with just a 0.04 increase for CABG relative to both PCI and medical therapy. The ICERs fall below $150 000 per QALY gained at time horizons >5 years (Figure S6).
Discussion
Using Medicare claims data to inform a Markov cohort simulation, we assessed the long‐term cost‐effectiveness of coronary revascularization in 75‐year‐old patients with CKD and high‐risk presentations of CAD. Although healthcare costs and mortality were high in all groups, our model projected gains of ~1 to 2.3 life‐years and ~0.7 to 1.6 QALYs over time with PCI and CABG compared with medical therapy. High incremental costs with revascularization resulted in ICERs of roughly $65 000 per QALY gained for PCI compared with medical therapy and $101 000 per QALY gained for CABG compared with PCI, both within the range indicating intermediate health economic value, as defined by US cardiovascular professional societies. 23 These results were relatively insensitive to variation of the model inputs, but were clearly impacted by the high observed background healthcare costs of the study population, as well as time horizon.
The comparative effectiveness of medical therapy, PCI, and CABG for CAD has been studied in several randomized trials 6 , 24 , 25 , 26 and large‐scale observational studies. 27 Our current analysis is not directly comparable with any of those studies, which focused exclusively on stable patients with CAD 6 , 24 or enrolled a minority of patients with unstable angina or recent acute coronary syndrome. 25 , 26 In addition, patients with CKD were either excluded or generally underrepresented in those trials.
Despite those differences, a few of our findings were consistent with previous studies. In multiple studies comparing alternative revascularization strategies, long‐term results favored CABG over PCI, particularly for patients with diabetes mellitus and for patients with more advanced/complex CAD, although CABG was associated with a higher risk of stroke. 25 , 26 , 27 Health economic evaluation of each of those studies found CABG to be economically attractive long‐term, relative to PCI. 28 , 29 , 30
Our study provides unique perspective on the outcomes of patients with CKD and high‐risk presentations of CAD, including a large group of patients managed only with medical therapy, for whom clinical trial enrollment would likely be infeasible. Often in clinical practice, a desire to avoid short‐term harm leads to conservative management decisions. Indeed, we observed that at 30 days, both PCI and CABG nearly doubled the risk of ESRD, and CABG doubled the risk of stroke (Table 3). The absolute rates of these adverse events were relatively low in our directly observed data (<5% for ESRD and <1% for stroke at 30 days) such that with moderate to long time horizons, the clinical benefits of revascularization far outweighed the early risks at the group level. Nonetheless, treatment decisions for these high‐risk patients involve difficult trade‐offs. If patients strongly weight short‐term outcomes over long‐term outcomes, or value the avoidance of certain adverse events (eg, stroke or ESRD) more highly than longevity, then more conservative initial treatment approaches may be appropriate.
The incremental costs associated with PCI and CABG in our study were high, resulting in ICERs above the outdated 31 but often cited $50 000 per QALY threshold adopted to indicate high economic value. 23 This may in part have resulted from our inclusion of all Medicare part A, B, and D claims in our estimation of background medical costs, which were >$20 000 per year in the patients with no adverse clinical events. This reflects an economic reality of extending life in an older population of patients with chronic health conditions. As such, we believe it is appropriate to include these background costs, as we have done in the assessment of other health technologies primarily used in elderly patients. 32 Nonetheless, the ICERs we calculated fall well below the $150 000 per QALY threshold suggested as indicating poor economic value. 23
Our study has several noteworthy limitations. Most important, our analysis is anchored by observational data derived solely from Medicare claims. The treatment decisions studied in our analysis are obviously not made randomly, and the medical therapy patients in our sample, in particular, differed from the other patients in several important ways. Although we adjusted both the clinical outcomes and costs for measured confounders, the potential for residual unmeasured confounding is high, and would remain so even if alternative analytic techniques were used. For example, the claims data lack information on the results of laboratory and other clinical testing, such that the severity of CKD, the presence or absence of left ventricular systolic dysfunction, and the severity and complexity of the CAD were not known. It should not therefore be assumed that all patients were equally suitable for both PCI and CABG.
The reliance on diagnostic codes from claims data to define the cohorts and their outcomes can introduce further problems. In particular, definitions of MI in clinical practice as well as in the context of clinical trials vary. With the availability of highly sensitive biomarkers, there is the potential for myocardial injury attributable to various causes to be misclassified as MI, in addition to conflating type 2 MI with unstable coronary syndromes. 33
We believe the impact of these limitations would most likely be to overstate the poor prognosis of the medically treated patients, leading to an overestimation of the benefits of revascularization in this population. Unfortunately, patients with CKD have been adequately represented in few clinical trials of coronary revascularization. Furthermore, it would likely be infeasible to use randomized methods to study the high‐risk patients involved in our study, which would be the only way to estimate these treatment effects with true precision. As a result, the limitations inherent in observational research methods were to a large degree unavoidable.
Conclusions
Using clinical and economic outcomes data from a moderately large Medicare sample, we projected the long‐term cost‐effectiveness of coronary revascularization in patients with CKD and high‐risk CAD presentations. We found that PCI and CABG were both associated with up‐front risks and markedly increased costs but produced eventual gains in quality‐adjusted life expectancy, with ICERs indicating intermediate value in health economic terms. The magnitude of the modeled health benefits, although uncertain, suggests that revascularization should not be withheld on either clinical or economic grounds when safely feasible.
Sources of Funding
This work was supported by the National Institutes of Health (grant HL118314‐01).
Disclosures
None.
Supporting information
Table S1
Figures S1–S6
(J Am Heart Assoc. 2021;10:e019391. DOI: 10.1161/JAHA.120.019391.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Anavekar NS, McMurray JJV, Velazquez EJ, Solomon SD, Kober L, Rouleau J‐L, White HD, Nordlander R, Maggioni A, Dickstein K, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. DOI: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. DOI: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3. Charytan DM, Yang SS, McGurk S, Rawn J. Long and short‐term outcomes following coronary artery bypass grafting in patients with and without chronic kidney disease. Nephrol Dial Transplant. 2010;25:3654–3663. DOI: 10.1093/ndt/gfq328. [DOI] [PubMed] [Google Scholar]
- 4. Chertow GM, Normand SL, McNeil BJ. "Renalism": inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol. 2004;15:2462–2468. DOI: 10.1097/01.ASN.0000135969.33773.0B. [DOI] [PubMed] [Google Scholar]
- 5. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. DOI: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 6. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, López‐Sendón J, Alexander KP, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–1407. DOI: 10.1056/NEJMoa1915922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bangalore S, Maron DJ, O’Brien SM, Fleg JL, Kretov EI, Briguori C, Kaul U, Reynolds HR, Mazurek T, Sidhu MS, et al. Management of coronary disease in patients with advanced kidney disease. N Engl J Med. 2020;382:1608–1618. DOI: 10.1056/NEJMoa1915925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann F‐J, Robertson DH, DeLucca PT, DiBattiste PM, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879–1887. DOI: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 9. FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators . Invasive compared with non‐invasive treatment in unstable coronary‐artery disease: FRISC II prospective randomised multicentre study. Lancet. 1999;354:708–715. [PubMed] [Google Scholar]
- 10. Charytan DM, Natwick T, Solid CA, Li S, Gong T, Herzog CA. Comparative effectiveness of medical therapy, percutaneous revascularization, and surgical coronary revascularization in cardiovascular risk subgroups of patients with CKD: a retrospective cohort study of Medicare beneficiaries. Am J Kidney Dis. 2019;74:463–473. DOI: 10.1053/j.ajkd.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 11. Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–328. DOI: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 12. Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, et al. Recommendations for conduct, methodological practices, and reporting of cost‐effectiveness analyses: second panel on cost‐effectiveness in health and medicine. JAMA. 2016;316:1093–1103. DOI: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 13. Bricker RS, Valle JA, Plomondon ME, Armstrong EJ, Waldo SW. Causes of mortality after percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes. 2019;12:e005355. DOI: 10.1161/CIRCOUTCOMES.118.005355. [DOI] [PubMed] [Google Scholar]
- 14. Krumholz HM, Normand ST, Wang Y. Twenty‐year trends in outcomes for older adults with acute myocardial infarction in the United States. JAMA Netw Open. 2019;2:e191938. DOI: 10.1001/jamanetworkopen.2019.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freeman JV, Hutton DW, Barnes GD, Zhu RP, Owens DK, Garber AM, Go AS, Hlatky MA, Heidenreich PA, Wang PJ, et al. Cost‐effectiveness of percutaneous closure of the left atrial appendage in atrial fibrillation based on results from PROTECT AF versus PREVAIL. Circ Arrhythm Electrophysiol. 2016;9:e003407. DOI: 10.1161/CIRCEP.115.003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang G, Zhang Z, Ayala C, Dunet DO, Fang J, George MG. Costs of hospitalization for stroke patients aged 18–64 years in the United States. J Stroke Cerebrovasc Dis. 2014;23:861–868. DOI: 10.1016/j.jstrokecerebrovasdis.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta‐analysis of utility‐based quality of life in chronic kidney disease treatments. PLoS Med. 2012;9:e1001307. DOI: 10.1371/journal.pmed.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khattak A, Mandel EI, Reynolds MR, Charytan DM. Percutaneous coronary intervention versus optimal medical therapy for stable angina in advanced CKD: a decision analysis. Am J Kidney Dis. 2017;69:350–357. DOI: 10.1053/j.ajkd.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sehested TSG, Bjerre J, Ku S, Chang A, Jahansouz A, Owens DK, Hlatky MA, Goldhaber‐Fiebert JD. Cost‐effectiveness of canakinumab for prevention of recurrent cardiovascular events. JAMA Cardiol. 2019;4:128–135. DOI: 10.1001/jamacardio.2018.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luengo‐Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM, Oxford VS. Quality of life after TIA and stroke: ten‐year results of the Oxford Vascular Study. Neurology. 2013;81:1588–1595. DOI: 10.1212/WNL.0b013e3182a9f45f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen DJ, Taira DA, Berezin R, Cox DA, Morice MC, Stone GW, Grines CL. Cost‐effectiveness of coronary stenting in acute myocardial infarction: results from the stent primary angioplasty in myocardial infarction (stent‐PAMI) trial. Circulation. 2001;104:3039–3045. DOI: 10.1161/hc5001.100794. [DOI] [PubMed] [Google Scholar]
- 22. Arias E, Xu JQ. Unites States life tables, 2017. National Vital Statistics Reports 2019; 68(7). Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- 23. Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129:2329–2345. DOI: 10.1161/CIR.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 24. BARI 2D Study Group , Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serruys PW, Morice M‐C, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, et al. Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. DOI: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 26. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. DOI: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 27. Weintraub WS, Grau‐Sepulveda MV, Weiss JM, O'Brien SM, Peterson ED, Kolm P, Zhang Z, Klein LW, Shaw RE, McKay C, et al. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–1476. DOI: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Magnuson EA, Farkouh ME, Fuster V, Wang K, Vilain K, Li H, Appelwick J, Muratov V, Sleeper LA, Boineau R, et al. Cost‐effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes mellitus and multivessel coronary artery disease: results from the FREEDOM trial. Circulation. 2013;127:820–831. DOI: 10.1161/CIRCULATIONAHA.112.147488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen DJ, Osnabrugge RL, Magnuson EA, Wang K, Li H, Chinnakondepalli K, Pinto D, Abdallah MS, Vilain KA, Morice M‐C, et al. Cost‐effectiveness of percutaneous coronary intervention with drug‐eluting stents versus bypass surgery for patients with 3‐vessel or left main coronary artery disease: final results from the Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery (SYNTAX) trial. Circulation. 2014;130:1146–1157. DOI: 10.1161/CIRCULATIONAHA.114.009985. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Z, Kolm P, Grau‐Sepulveda MV, Ponirakis A, O'Brien SM, Klein LW, Shaw RE, McKay C, Shahian DM, Grover FL, et al. Cost‐effectiveness of revascularization strategies: the ASCERT study. J Am Coll Cardiol. 2015;65:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neumann PJ, Cohen JT, Weinstein MC. Updating cost‐effectiveness–the curious resilience of the $50,000‐per‐QALY threshold. N Engl J Med. 2014;371:796–797. DOI: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 32. Reynolds MR, Magnuson EA, Wang K, Lei Y, Vilain K, Walczak J, Kodali SK, Lasala JM, O'Neill WW, Davidson CJ, et al. Cost‐effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (cohort B). Circulation. 2012;125:1102–1109. DOI: 10.1161/CIRCULATIONAHA.111.054072. [DOI] [PubMed] [Google Scholar]
- 33. McCarthy C, Murphy S, Cohen JA, Rehman S, Jones‐O'Connor M, Olshan DS, Singh A, Vaduganathan M, Januzzi JL Jr, Wasfy JH. Misclassification of myocardial injury as myocardial infarction: implications for assessing outcomes in value‐based programs. JAMA Cardiol. 2019;4:460–464. DOI: 10.1001/jamacardio.2019.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S6


