Abstract
Background
Sub-Saharan Africa (SSA) carries the highest burden of maternal mortality, yet, the accurate maternal mortality ratios (MMR) are uncertain in most SSA countries. Measuring maternal mortality is challenging in this region, where civil registration and vital statistics (CRVS) systems are weak or non-existent. We describe a protocol designed to explore the use of CRVS to monitor maternal mortality in Zimbabwe—an SSA country.
Methods
In this study, we will collect deliveries and maternal death data from CRVS (government death registration records) and health facilities for 2007–2008 and 2018–2019 to compare MMRs and causes of death. We will code the causes of death using classifications in the maternal mortality version of the 10th revision to the international classification of diseases. We will compare the proportions of maternal deaths attributed to different causes between the two study periods. We will also analyse missingness and misclassification of maternal deaths in CRVS to assess the validity of their use to measure maternal mortality in Zimbabwe.
Discussion
This study will determine changes in MMR and causes of maternal mortality in Zimbabwe over a decade. It will show whether HIV, which was at its peak in 2007–2008, remains a significant cause of maternal deaths in Zimbabwe. The study will recommend measures to improve the quality of CRVS data for future use to monitor maternal mortality in Zimbabwe and other SSA countries of similar characteristics.
Introduction
WHO defines maternal death as the death of a woman while pregnant, giving birth or within 42 days of termination of pregnancy, irrespective of the duration and site of the pregnancy, from any cause related to or aggravated by the pregnancy or its management but not from accidental or incidental causes [1]. WHO also defines the cause of death as all those diseases, morbid conditions or injuries which either resulted in or contributed to death and the circumstances of the accident or violence which produced any such injuries [2]. Maternal deaths remain a major global health problem [3–5]. In 2017, there were an estimated 295,000 maternal deaths globally, 196,000 (66%) of which occurred in Sub-Saharan Africa (SSA), primarily due to avoidable causes [3].
Sustainable Development Goal (SDG) 3.1 aims to reduce the average global maternal mortality ratio (MMR) to less than 70 maternal deaths per 100,000 live births by 2030. Countries with a 2010 baseline above 420 should not have an MMR greater than 140 maternal deaths per 100,000 live births [6, 7]. Attaining the SDG 3.1.1 global target by 2030 is a mammoth task for SSA countries such as Zimbabwe, where the MMR is estimated above 400 maternal deaths per 100,000 live births [8–10]. More importantly, data will be required from all countries to measure progress towards SDG 3.1 [11], yet many countries lack comprehensive data systems to measure maternal mortality [12].
Maternal mortality data come from civil registration and vital statistics (CRVS), routine health information systems (HIS), population censuses, surveys and statistical modelling [13, 14]. SSA countries rely on modelling and population-based surveys that use the sisterhood method for their MMR estimates, all of which are unreliable [14, 15]. Estimates that models produce require countries to provide input data. These data are often unavailable in most SSA countries [3, 16, 17]. Sisterhood studies collect data for deaths from the past seven or more years; hence, they are prone to recall bias. Study respondents may not provide precise dates and causes of death. Hence sisterhood studies only identify pregnancy-related deaths and not maternal deaths. This makes sisterhood studies under-or over-estimate maternal mortality levels [14, 16].
As with most SSA countries, Zimbabwe estimates MMR using periodic population-based surveys that use the sisterhood method. These include Demographic and Health Surveys (DHS), Multiple Indicator Cluster Surveys (MICS) and population census. The country has set up a maternal death surveillance and response system (MDSR) following WHO recommendations, which is not fully functional [18].
CRVS data are widely used to estimate MMR in developed countries, but the first study to use CRVS in Zimbabwe was done in 2007–2008. The study combined CRVS (data from government death registration records) with data from health records (patient charts and antenatal care, delivery and mortuary registers) and the community (using verbal autopsy questionnaires) [19]. In Zimbabwe, CRVS is backed by legislation that mandates the notification, registration by the government and issuance of a certificate for births and deaths of citizens and residents.
In this study, we will analyse trends in MMR and causes of maternal deaths in Zimbabwe using data for 2007–2008 and 2018–2019 to see if the country’s MMR and causes of maternal mortality have changed in the past decade. We will assess changes in the contribution of HIV to maternal mortality since 2007–2008, when HIV mortality was at its peak in the country. We will also evaluate the validity of using CRVS to monitor maternal mortality in Zimbabwe and recommend measures to ensure its future use in SSA countries of similar characteristics.
Materials and methods
Study aim
To analyse the epidemiology of maternal mortality in Zimbabwe by assessing changes in the MMR and causes of maternal death over a decade and the validity of using CRVS data in future to monitor maternal mortality in Zimbabwe and similar countries.
Study objectives
To estimate the MMR for Zimbabwe in 2007–2008 and 2018–2019 and quantify the difference in MMR from these two time periods.
To assess changes in causes of maternal mortality in Zimbabwe between 2007–2008 and 2018–2019.
To assess changes in HIV-associated maternal mortality in Zimbabwe between 2007–2008 and 2018–2019.
To assess the validity of using CRVS to estimate national MMR and recommend measures to ensure their future use to monitor maternal mortality in Zimbabwe and similar countries.
Study design and setting
We will conduct an observational, population-based, trend analysis study comparing maternal mortality estimates and cause for two studies that are ten years apart.
Study methods
The study will use the reproductive age mortality survey (RAMOS) method, collecting data for all deaths of women of reproductive age (WRA) to identify maternal deaths [20, 21]. It will collect deaths for the one year from 1 May 2007 to 15 June 2008 and 1 May 2018 to 15 June 2019. The data will be collected from CRVS and health facilities (central, provincial, district, mission and rural hospitals and primary care clinics).
Study population
Women of reproductive age, 12–49 years in Zimbabwe.
Study outcomes
The primary study outcomes are change in MMR and changes in proportions of deaths due to the leading causes of maternal mortality in Zimbabwe from 2007–2008 to 2018–2019. Secondary study outcomes are changes in proportions of deaths of WRA and pregnancy-related deaths.
Sampling methods and sample size calculations
The sampling for 2007–2008 and 2018–2019 data is the same. The sample size was estimated using deliveries—the denominator for the MMR. The population was stratified into the ten provinces of the country, and one district was selected from each province using simple random sampling [22–24]. An extra district was sampled from Harare by proportional-to-size sampling, considering the city’s large population and two referral hospitals. In total, the study will be done in 11 districts and 291 health facilities.
The sample size of deliveries was estimated using the last verifiable MMR of Zimbabwe and the country’s total fertility rate (TFR), treating MMR as a single proportion [25]. It was calculated using the Wald test, which estimates a one-sample proportion against an expected proportion (the last verifiable MMR as a proportion). Power of 80%, z-value for two-sided 95% CI, normal-approximation continuity correction for the expected proportion, 2.5% error margin (for the alternative hypothesis of MMR outside the 95% CI of the last verified MMR) was applied. A design effect of 2 was also applied, based on the two-step sampling procedure of stratifying the study population into provinces and districts and randomly selecting the districts [23, 24, 26]. Deliveries and deaths occurring in the study districts are consecutively enrolled until the sample size of deliveries is reached.
Data collection procedures
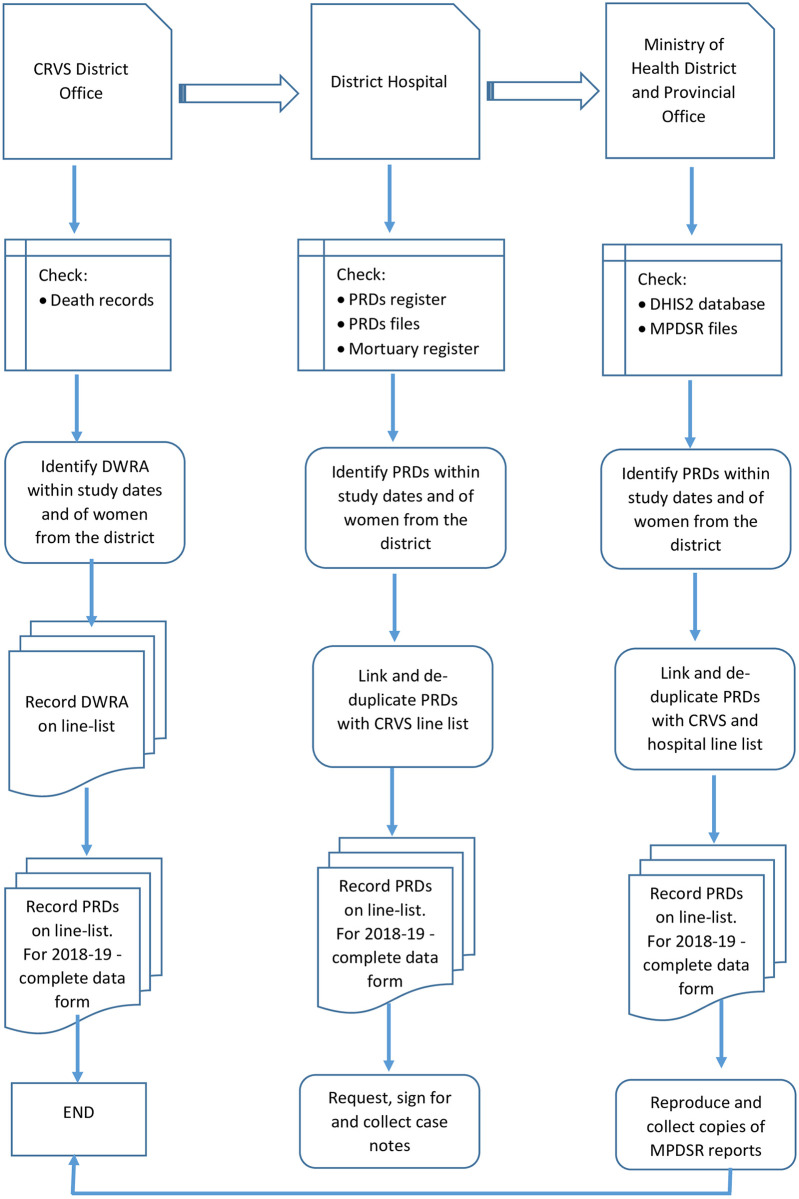
The data collection procedures are summarised in Fig 1.
Fig 1. Data collection procedures for 2007–2008 and 2018–2019.
Procedures for 2007–2008 data collection
The 2007–2008 dataset will be built from data collected in the first study conducted in 2007–2008. We will validate the 2007–2008 data by re-visiting the vital registration (VR) and hospital records in the 11 districts and collect data for all deaths of WRAs and their causes of death for 2007–2008. We will collect case notes for the deaths from hospitals to code the causes of death. We will use the new data to clean up the data collected in 2007–2008.
Procedures for 2018–2019 data collection
We will collect the 2018–2019 data from the same 11 districts using the same method recording all deaths of WRA at the VR offices and hospitals. Depending on the level of the institution, sources of maternal deaths data in health institutions in Zimbabwe include maternal death line-list in reproductive health offices, delivery unit, casualty unit, theatre, intensive care unit, high dependency unit, female ward, and mortuary registers [27]. We will triangulate and collect additional data from the maternal death surveillance, and response (MDSR) reports. MDSR reports and VR records include community deaths. Deaths of WRA will be identified at the VR offices from birth and death registration forms filed by year in locked records rooms. We will record the data on line-listing forms, including demographic and cause of death information for each woman. A second line-listing form will record more detailed data for each PRD, including personal identifiers such as name, date of birth, national identification number, home address, place of residence, date of death, place of death and cause of death. We will use personal identifiers to track and de-duplicate the women across CRVS and health facility records.
PRDs will be identified at health facilities from maternity, delivery, maternal death and mortuary registers, and MDSR reports. The data will be recorded on the same line-listing forms. Case notes for all PRDs will be collected from hospitals. MDSR reports will be collected from the district and provincial health offices. PRDs identified in the RAMOS will be verified with those reported in the district health information system (DHIS2) by reporting month and institution to avoid missing some deaths. The 2007–2008 data were collected on a form adapted from WHO. We will use updated versions of the 2007–2008 questionnaire to collect the 2018–2019 data and use the newly collected 2007–2008 data to update the previous data forms. Variables collected in the form include demographic, antenatal, antepartum and postpartum information and causes of death. Four (4) trained research midwives will collect the data from all districts.
Coding of the causes of death in 2007–2008 and 2018–2019 pregnancy-related deaths (PRDs)
A panel of obstetricians will review all PRDs for 2007–2008 and 2018–2019 to confirm maternal deaths, the type of death (direct, indirect or incidental), category and the actual cause of death. They will use the WHO ICD-10 MM guide for the classification and coding. ICD-10 MM groups the causes of maternal death into nine groups, namely: 1: Pregnancies with the abortive outcome, 2: Hypertensive disorders in pregnancy, childbirth and the puerperium, 3: Obstetric haemorrhage, 4: Pregnancy-related infection, 5: Other obstetric complications, 6: Unanticipated complications of management, 7: Non-obstetric complications, 8: Unknown/undetermined causes and 9: Coincidental causes [1]. Groups 1–8 are maternal deaths, while group 9 are pregnancy-related deaths. Obstetrician reviews will be documented on an additional data form page attached to each PRD’s data form. The primary data form and line-listing forms were adapted from the WHO maternal mortality and morbidity systematic review tool [28]. The death registration forms used in Zimbabwe are aligned to ICD-10 MM.
Data management
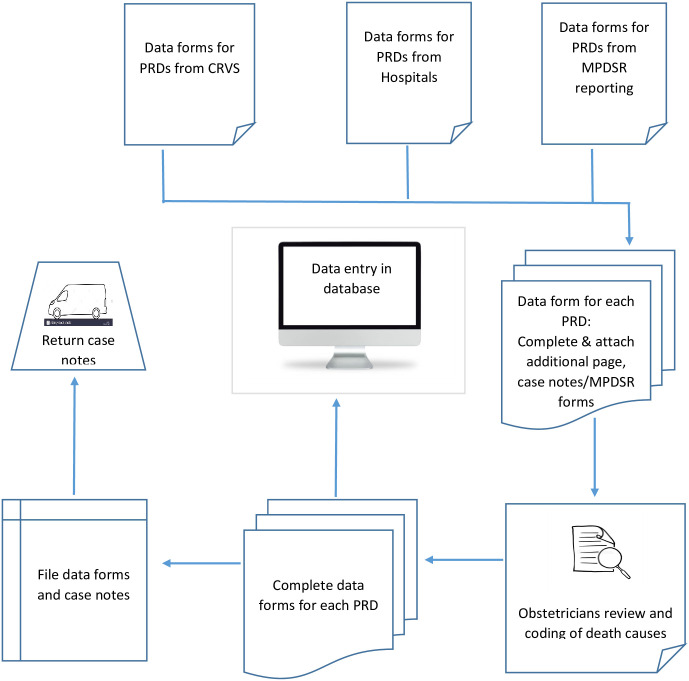
For 2007–2008 data, the additional data form page will be used to facilitate changes in the database. For 2018–2019, the line-listings of PRDs will be used to complete the main data form. The data form is completed in the field while data collectors still have access to other patient data from field records. After coding the causes of death, the data will be entered into a password protected MS Access database. The data processing procedures are summarised in Fig 2.
Fig 2. Data processing procedures.
Inclusion and exclusion criteria
The study will include deaths of WRA (12–49 years) from the study districts, including those who died during pregnancy or within 100 days of termination of pregnancy. We will include PRDs due to illnesses, injuries and accidents that were not pregnancy-induced. We will exclude these from MMR estimates. We will analyse these other deaths of WRA as secondary outcomes. Maternal deaths of unknown or non-specific causes coded in ICD-10 MM group 8 will be included in MMR estimates and cause of death analyses. Women who died within but originated from other districts will be excluded from MMR estimates and cause of death analyses but reported in secondary outcomes.
Quality control measures
The principal investigator (PI) will collect data with the research midwives at the first two districts and at two other districts midway through data collection. During this time, the PI will ensure that data collectors are following the protocol. PRDs identified in the study will be linked across all data sources to minimise missing data and avoid duplicating some deaths. The PI will review the completeness and quality of data on all forms. Obstetricians’ review of every PRD will be another quality control measure on the data.
Data analysis
Descriptive analysis
We will compare demographic and clinical characteristics of 2007–2008 and 2018–2019 deaths, including age, parity, gravidity and pregnancy complications, using proportions and mean/medians (with standard deviations) with tests of significance of difference.
Multivariable logistic regression
We will assess risk factors for maternal death using multivariable logistic regression models on 2007–2008 data, which has a comparison group of deliveries where the women did not die. We will include predictor variables with univariate p-values less than 0.25 in the initial model and test model fitness using Hosmer and Lemeshow Chi-square test [29]. Predictor variables with p-values less than 0.2 will be included in the final model [30]. This analysis will be design-adjusted and use survey weights [31, 32].
Changes in MMR
We will calculate and compare MMRs for 2007–2008 and 2018–2019 using their 95% CIs. We will calculate the MMRs by dividing the total number of maternal deaths with the total live births for that year, multiplied by 100,000 [33, 34]. MMR estimates with overlapping CIs will suggest that the MMR has not changed over the decade. However, we will also consider the clinical significance of differences in MMRs when CIs show no difference. Differences in MMR point estimates greater than 100 will be considered clinically significant [35, 36].
Changes in causes of death
We will calculate proportions (with 95% CIs) of deaths due to each underlying cause of death by dividing the number of deaths for each cause with the total number of deaths in the survey to identify the leading causes of maternal mortality in 2007–2008 and 2018–2019. We will use the 95% CIs to evaluate changes in the contribution of each cause to maternal mortality over the decade [37]. Subject to the suitability of the study design and availability of requisite data, competing risk analysis will be considered in cause-of-death analysis [38].
Changes in HIV-associated mortality
We will calculate and compare the proportion of HIV-associated maternal deaths in 2007–2008 and 2018–2019. HIV-associated maternal deaths are deaths where an AIDS-defining condition is present [28, 39, 40]. We will calculate the proportions by dividing the number of HIV-associated maternal deaths by the total number of maternal deaths in that year.
Validity of using CRVS to monitor maternal mortality and recommendations for future use
We will triangulate the number of maternal deaths identified from CRVS, health facilities and MDSR to quantify missingness in the study. We will classify the maternal deaths according to false positives, false negatives, true positives, true negatives, and missing (both true and false maternal deaths) to assess the validity of using CRVS to monitor maternal mortality in Zimbabwe. We will review the quality of records in CRVS records to evaluate its use to monitor maternal mortality. We will discuss the challenges identified in the data systems and recommend measures to strengthen CRVS for future use to monitor maternal mortality in Zimbabwe and similar countries.
Weighting the survey data
Some of our analysis will be weighted since this study has multistage sampling [41–43]. The weights will be calculated using non-proportional sample allocation to clusters and possible inter-cluster differences in response rates [22, 44]. The weights are calculated using the product of two probabilities, considering the two clustering levels of province and district in the sampling design. The first probability is for selecting a district in a province, which is one out of the number of districts in the province. The second probability is for identifying a maternal death in the district, estimated at 70% (0.7) by a verification exercise conducted in the 2007–2008 study [19]. The final sample weight is the inverse of the product of the two probabilities [44, 45]. Sample weights will be calculated per district since the probabilities vary by district.
Ethics approvals
The protocol was approved by the UNDP-UNFPA-UNICEF-WHO-World Bank Human Reproduction Program (HRP) (Date: 2019-03-27), World Health Organization Ethics Review Committee (Ref: ERC 0003348; Date: 2020-04-24), Medical Research Council of Zimbabwe (Ref: MRCZ/A/2613; Date: 2020-07-17) and University of Pretoria Faculty Health Research Ethics Committee (Ref: 339/2019; First submission approved– 2020-07-15; Amendment approved: 2021-01-21). The Ministry of Health and Child Care (MoHCC) and the Registrar General’s Department of Zimbabwe granted permissions for access to patient and civil registration records, respectively. Additional clearances for data collection will be received from provincial, district and station heads of the MoHCC and RG’s offices upon presentation of permission letters from their head offices. All approvals waived consenting of study participants since the study will collect data from existing records and not engage living human subjects.
Discussion
This study will assess the changes in MMR and causes of maternal deaths over a decade in a developing country. The study will assess changes in the contribution of HIV to maternal mortality since 2007–2008 when HIV mortality was at the peak in the country. The study will assess the completeness and accuracy of maternal death and cause-of-death data collected from different sources to explore the validity of using CRVS to monitor maternal mortality in Zimbabwe.
Our literature review indicated that few countries had estimated MMR using RAMOS methods and CRVS data in SSA [46]. Adomako in Ghana and Mgawadere in Malawi used the RAMOS method to estimate MMR for a district [47, 48]. Mohammed in Sudan, Walraven in Gambia and Zakariah and Geynisman in Ghana used the method to estimate MMR in urban and rural communities [49–52]. These studies did not use CRVS data as the systems did not exist in the countries. This will be one of the few studies to use the RAMOS method with CRVS data to produce a national MMR estimate.
Evaluation of decadal changes in MMR and causes of maternal mortality has not been done in many SSA countries. Other studies have shown that haemorrhage, hypertensive disease in pregnancy, pregnancies with abortive outcomes and pregnancy-related infections are the common causes of maternal mortality in SSA [53]. The contribution of caesarean sections to maternal mortality has been reported in Zimbabwe and other SSA countries like Sierra Leone [54, 55]. The contribution of epidemics like Ebola to maternal mortality has been observed in West African countries. Zimbabwe had a similar epidemic of Cholera in 2009–2009, which may have contributed to maternal mortality [56]. Preliminary results from the 2007–2008 survey suggest that HIV was the major cause of maternal mortality at that time [19]. HIV prevalence was high, and HIV treatment was still in the early rollout phase during these years [57]. However, Zimbabwe has since rolled out antiretroviral therapy (ART) to most of its health facilities, and ART coverage increased to more than 80% of people living with HIV [58]. Prevention of mother-to-child transmission (PMTCT) regimens was rolled out to nearly all hospitals and primary care clinics offering maternal and child health services in 2011–2012 and 2013, respectively [59]. The impact of ART on mortality has been observed in Zimbabwe [60], and elsewhere [61]. Thus, we expect ART to have reduced maternal mortality due to HIV, as seen in other SSA studies [62]. Other interventions implemented during this period, such as massive donor funding for maternal and child health programs through the health transition and health development funds, will be examined [63].
Our study will assess the feasibility of using CRVS to generate national MMR estimates. The study will recommend measures to strengthen CRVS for future use to produce national MMR estimates. The use of CRVS to monitor maternal mortality will strengthen the systems in the long run. It will make MMR estimates available annually, enabling the country to monitor trends. Our study will provide lessons for other SSA countries with similar characteristics as Zimbabwe.
Acknowledgments
The authors acknowledge the Zimbabwe Maternal and Perinatal Mortality Study (ZMPMS) group members for 2007–2008 and 2018–2019 (Thulani Magwali, Margaret Nyandoro†, Lennarth Nystrom, Bernard Madzima, Davidzoyashe Makosa, Chipo Chimamise, Michael Nyakura, Sunhurai Mukwambo). The support received from Jennifer Cresswell of WHO-Geneva and Jolivet Rima of the Human Reproduction Program in the study project and reviewing this manuscript is acknowledged. Assistance received from Cheryl Tosh of the University of Pretoria to copy-edit the article is also appreciated.
Funding Statement
This work is supported, in part, by the Bill & Melinda Gates Foundation through a grant to the Improving Maternal Health Measurement (IMHM) Project at the Women & Health Initiative of the Harvard T.H. Chan School of Public Health [Grant Number OPP1169546]. It is also funded from the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a cosponsored programme executed by the World Health Organization (WHO). The Zimbabwe Ministry of Health and Child Care is also cosponsoring the study through funding from its partners.
References
- 1.WHO. The WHO Application of ICD-10 to deaths during pregnancy, childbirth and the puerperium: ICD-MM. Volume 1. World Health Organization. Geneva, 2011. [Google Scholar]
- 2.WHO. International Statistical Classification of Diseases and Related Health Problems. Instruction manual. Volume 2. Department of Health Statistics. World Health Organization. Geneva:2011. [Google Scholar]
- 3.WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. World Health Organization. Geneva: 2019. [Google Scholar]
- 4.Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet (London, England) 2016, 387(10017):462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassebaum NJ, Arora M, Barber RM, Bhutta Z, Brown J, Carter A, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet 2016, 388(10053):1603–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Sustainable Development Goal 3: Health. World Health Organization. Geneva: 2020. https://www.who.int/topics/sustainable-development-goals/targets/en/. Accessed 14 Nov 2020. [Google Scholar]
- 7.Maternal Health Task Force. The Sustainable Development Goals and Maternal Mortality. 2020. https://www.mhtf.org/topics/the-sustainable-development-goals-and-maternal-mortality/. Accessed 14 Nov 2020. [Google Scholar]
- 8.ZimStat and UNICEF. Zimbabwe Multiple Indicator Cluster Survey 2014: Final Report. Harare, Zimbabwe: 2015. [Google Scholar]
- 9.ZimStat and ICF International. Zimbabwe Demographic and Health Survey 2015: Final Report. Zimbabwe National Statistics Agency (ZIMSTAT), Harare and ICF International Rockville, Maryland, USA: 2016. [Google Scholar]
- 10.ZimStat and UNICEF. Zimbabwe Multiple Indicator Cluster Survey 2017: Final Report. Harare: 2019. [Google Scholar]
- 11.Marchant T, Boerma T, Diaz T, Huicho L, Kyobutungi C, Mershon C-H, et al. Measurement and accountability for maternal, newborn and child health: fit for 2030? BMJ Global Health 2020, 5(7):e002697. doi: 10.1136/bmjgh-2020-002697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grove J, Claeson M, Bryce J, Amouzou A, Boerma T, Waiswa P, et al. Maternal, newborn, and child health and the Sustainable Development Goals: a call for sustained and improved measurement. The Lancet 2015, 386(10003):1511–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham WJ, Ahmed S, Stanton C, Abou-Zahr C, Campbell OM. Measuring maternal mortality: an overview of opportunities and options for developing countries. BMC Med 2008, 6:12. doi: 10.1186/1741-7015-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mgawadere F, Kana T, van den Broek N. Measuring maternal mortality: a systematic review of methods used to obtain estimates of the maternal mortality ratio (MMR) in low- and middle-income countries. Br Med Bull 2017, 121(1):121–134. doi: 10.1093/bmb/ldw056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munjanja SP. Joining the dots: a plea for precise estimates of the maternal mortality ratio in sub-Saharan Africa. BJOG 2009, 116 Suppl 1(Suppl 1):7–10. doi: 10.1111/j.1471-0528.2009.02337.x [DOI] [PubMed] [Google Scholar]
- 16.Alkema L, Zhang S, Chou D, Gemmill A, Moller A-B, Fat DM, et al. A Bayesian approach to the global estimation of maternal mortality. Appl Stat 2017, 11(3):1245–1274. [Google Scholar]
- 17.Kassebaum NJ, Barber RM, Bhutta ZA, Dandona L, Gething PW, Hay SI, et al. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet 2016, 388(10053):1775–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimbabwe Ministry of Health and Child Care. Maternal and Perinatal Death Surveillance and Response: 2019 Annual Report. Family Health Depatment. Harare: 2020. [Google Scholar]
- 19.Munjanja SP, Nystrom L, Nyandoro M, Magwali T. Zimbabwe Maternal and Perinatal Mortality Study 2007–2008: Short Report. Ministry of Health and Child Welfare, Harare: 2008. [Google Scholar]
- 20.Geubbels E. Epidemiology of Maternal Mortality in Malawi. Malawi Med J 2006, 18(4):206–225. doi: 10.4314/mmj.v18i4.10923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adegoke A, Unkels R, Mgawadere F, van den Broek N. Measuring maternal mortality using a Reproductive Age Mortality Study (RAMOS). In: BMC Pregnancy and Childbirth. vol. 16; 2016: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fowler FJ. Survey research methods, 4th ed. edn. Los Angeles (i.e. Thousand Oaks, Calif.): SAGE Publications; 2009. [Google Scholar]
- 23.Rajeev K. Sample size calculation. In: indian journal of ophthalmology. vol. 60; 2012: 582. doi: 10.4103/0301-4738.103809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez Del Águila M, GonzÁlez-Ramírez A. Sample size calculation. Allergol Immunopathol (Madr) 2014, 42(5):485–492. doi: 10.1016/j.aller.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 25.Munjanja S, Nyandoro M, Magwali T, Mataya R, Magure T, Mushangwe V, et al. The maternal mortality ratio of Zimbabwe in 2007. International Journal of Gynecology and Obstetrics 2009, 107(Supplement 2):S278. [Google Scholar]
- 26.Killip S, Mahfoud Z, Pearce K. What is an intracluster correlation coefficient? Crucial concepts for primary care researchers. Annals of family medicine 2004, 2(3):204–208. doi: 10.1370/afm.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marotta C, Pisani L, Di Gennaro F, Cavallin F, Bah S, Pisani V, et al. Epidemiology, Outcomes, and Risk Factors for Mortality in Critically Ill Women Admitted to an Obstetric High-Dependency Unit in Sierra Leone. Am J Trop Med Hyg 2020, 103(5):2142–2148. doi: 10.4269/ajtmh.20-0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villar J, Piaggio G, BetrÁn A, lmezoglu A, Say L. WHO systematic review of maternal mortality and morbidity: methodological issues and challenges. BMC Medical Research Methodology. vol. 4; 2004: 1–8. doi: 10.1186/1471-2288-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilbe JM H. Logistic Regression Models. First Edition. Florida: Chapman & Hall/CRC; 2009. [Google Scholar]
- 30.Vittinghoff E GD, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. New York: Second edition; 2005. [Google Scholar]
- 31.Longest KC. Using Stata for quantitative analysis, Second edition. Los Angeles: SAGE; 2015. [Google Scholar]
- 32.Rabe-Hesketh S, Everitt B. A handbook of statistical analyses using Stata, 4th ed. Boca Raton FL: Chapman & Hall/CRC; 2007. [Google Scholar]
- 33.Bradshaw D, Dorrington RE. Maternal mortality ratio—trends in the vital registration data: original article. South African Journal of Obstetrics and Gynaecology 2012, 18(2):38–42. [Google Scholar]
- 34.Dako-Gyeke P, Moyer CA, Adanu RM. Facility-based delivery and maternal and early neonatal mortality in sub-Saharan Africa: a regional review of the literature: review article. African Journal of Reproductive Health 2013, 17(3):30–43. [PubMed] [Google Scholar]
- 35.Dupont WD. Statistical Modeling for Biomedical Researchers: a Simple Introduction to the Analysis of Complex Data. In. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 36.Spencer NH. Essentials of multivariate data analysis. Boca Raton: CRC Press/Taylor & Francis Group; 2014. [Google Scholar]
- 37.Hanley JA, Hagen CA, Shiferaw T. Confidence Intervals and Sample-size Calculations for the Sisterhood Method of Estimating Maternal Mortality. Stud Fam Plann 1996, 27(4):220. [PubMed] [Google Scholar]
- 38.Prentice RL, Kalbfleisch JD, Peterson AV, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics 1978, 34(4):541–554. [PubMed] [Google Scholar]
- 39.Posner S, Wojdyla D, BetrÁn A, lmezoglu A. National estimates for maternal mortality: an analysis based on the WHO systematic review of maternal mortality and morbidity. BMC Public Health. vol. 5; 2005: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosen JE, de Zoysa I, Dehne K, Mangiaterra V, Abdool-Karim Q. Understanding methods for estimating HIV-associated maternal mortality. J Pregnancy 2012, 2012:958262. doi: 10.1155/2012/958262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gnanadesikan R. Methods for statistical data analysis of multivariate observations. New York: Wiley; 1977. [Google Scholar]
- 42.Henkel RE. Tests of significance. Beverly Hills, Calif.: Sage Publications; 1976. [Google Scholar]
- 43.Ott L, Longnecker M. An introduction to statistical methods and data analysis, 5th ed. / edn. Australia;: Duxbury; 2001. [Google Scholar]
- 44.Levy PS, Lemeshow S. Sampling of populations: methods and applications, 4th ed. edn. Hoboken, NJJ: Wiley; 2008. [Google Scholar]
- 45.Wall FJ: Statistical data analysis handbook. New York: McGraw-Hill; 1986. [Google Scholar]
- 46.Musarandega R, Machekano R, Munjanja SP, Pattinson R. Methods used to measure maternal mortality in Sub-Saharan Africa from 1980 to 2020: A systematic literature review. Int J Gynaecol Obstet 2021. doi: 10.1002/ijgo.13695 [DOI] [PubMed] [Google Scholar]
- 47.Mgawadere Florence RU, Adetoro Adegoke & Nynke van den Broek. Measuring maternal mortality using a Reproductive Age Mortality Study (RAMOS). BMC Pregnancy and Childbirth 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adomako J, Asare GQ, Ofosu A, Iott BE, Anthony T, Momoh AS, et al. Community-based surveillance of maternal deaths in rural Ghana. Bull World Health Organ 2016, 94(2):86–91. doi: 10.2471/BLT.15.154849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohammed AA, Elnour MH, Mohammed EE, Ahmed SA, Abdelfattah AI. Maternal mortality in Kassala State—Eastern Sudan: community-based study using reproductive age mortality survey (RAMOS). BMC pregnancy and childbirth 2011, 11:102. doi: 10.1186/1471-2393-11-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geynisman J, Latimer A, Ofosu A, Anderson FWJ. Improving maternal mortality reporting at the community level with a 4-question modified reproductive age mortality survey (RAMOS). International Journal of Gynecology & Obstetrics 2011, 114(1):29–32. [DOI] [PubMed] [Google Scholar]
- 51.Zakariah AY, Alexander S, van Roosmalen J, Buekens P, Kwawukume EY, Frimpong P. Reproductive age mortality survey (RAMOS) in Accra, Ghana. Reproductive health 2009, 6:7. doi: 10.1186/1742-4755-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walraven G, Telfer M, Rowley J, Ronsmans C. Maternal mortality in rural Gambia: levels, causes and contributing factors. Bull World Health Organ 2000, 78(5):603–613. [PMC free article] [PubMed] [Google Scholar]
- 53.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. The Lancet Global Health 2014, 2(6):e323–e333. doi: 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 54.Di Gennaro F, Marotta C, Pisani L, Veronese N, Pisani V, Lippolis V, et al. Maternal caesarean section infection (MACSI) in Sierra Leone: a case-control study. Epidemiol Infect 2020, 148:e40. doi: 10.1017/S0950268820000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ngwenya S. Factors associated with maternal mortality from sepsis in a low-resource setting: a five-year review at Mpilo Central Hospital, Bulawayo, Zimbabwe. Trop Doct 2020, 50(1):12–15. doi: 10.1177/0049475519884436 [DOI] [PubMed] [Google Scholar]
- 56.Cuneo CN, Sollom R, Beyrer C. The Cholera Epidemic in Zimbabwe, 2008–2009: A Review and Critique of the Evidence. Health and human rights 2017, 19(2):249–264. [PMC free article] [PubMed] [Google Scholar]
- 57.NAC. Zimbabwe National and Sub-national HIV Estimates Report 2017. National AIDS Council, Harare: 2018. [Google Scholar]
- 58.ICAP. Zimbabwe Population-based HIV Impact Assessment (ZIMPHIA) 2015–2016. ICAP Global Health Action. ICAP at Columbia University, New York: 2018. [Google Scholar]
- 59.Musarandega R, Robinson J, Sen PD, Hakobyan A, Mushavi A, Mahomva A, et al. Using the critical path method to rollout and optimise new PMTCT guidelines to eliminate mother-to-child transmission of HIV in Zimbabwe: a descriptive analysis. BMC Health Services Research 2020, 20(1). doi: 10.1186/s12913-020-05900-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gregson S, Mugurungi O, Eaton J, Takaruza A, Rhead R, Maswera R, et al. Documenting and explaining the HIV decline in east Zimbabwe: the Manicaland General Population Cohort. BMJ Open 2017, 7(10):e015898. doi: 10.1136/bmjopen-2017-015898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384(9947):1005–1070. doi: 10.1016/S0140-6736(14)60844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calvert C, Marston M, Slaymaker E, Crampin AC, Price AJ, Klein N, et al. Direct maternal deaths attributable to HIV in the era of antiretroviral therapy: evidence from three population-based HIV cohorts with verbal autopsy. AIDS 2020, 34(9):1397–1405. doi: 10.1097/QAD.0000000000002552 [DOI] [PubMed] [Google Scholar]
- 63.D’Aquino L, Pyone T, Nigussie A, Salama P, Gwinji G, van den Broek N. Introducing a sector-wide pooled fund in a fragile context: mixed-methods evaluation of the health transition fund in Zimbabwe. BMJ Open 2019, 9(6):e024516. doi: 10.1136/bmjopen-2018-024516 [DOI] [PMC free article] [PubMed] [Google Scholar]