Abstract
Tissue-resident macrophages play an important role in maintaining tissue homeostasis and innate immune defense against invading microbial pathogens. Brain-resident macrophages can be classified into microglia in the brain parenchyma and non-parenchymal brain macrophages, also known as central nervous system-associated or border-associated macrophages, in the brain-circulation interface. Microglia and non-parenchymal brain macrophages, including meningeal, perivascular, and choroid plexus macrophages, are mostly produced during embryonic development, and maintained their population by self-renewal. Microglia have gained much attention for their dual roles in the maintenance of brain homeostasis and the induction of neuroinflammation. In particular, diverse phenotypes of microglia have been increasingly identified under pathological conditions. Single-cell phenotypic analysis revealed that microglia are highly heterogenous and plastic, thus it is difficult to define the status of microglia as M1/M2 or resting/activated state due to complex nature of microglia. Meanwhile, physiological function of non-parenchymal brain macrophages remain to be fully demonstrated. In this review, we have summarized the origin and signatures of brain-resident macrophages and discussed the unique features of microglia, particularly, their phenotypic polarization, diversity of subtypes, and inflammasome responses related to neurodegenerative diseases.
Keywords: brain-resident macrophages, central nervous system-associated macrophages, inflammasome, microglia, non-parenchymal brain macrophages
INTRODUCTION
The brain has been considered an immune-privileged organ because of the protection of the blood-brain barrier and the absence of lymphatic vessel drainage (Carson et al., 2006). This concept is now being re-evaluated by recent findings on immune cell trafficking and the presence of lymphatic vessels within surrounding barrier regions (Engelhardt et al., 2017). Brain parenchymal regions are sequestered from the external environment and circulating blood or cerebrospinal fluid (CSF) by the meningeal barrier, blood-brain barrier, and blood-CSF barrier (Mastorakos and McGavern, 2019). These barriers surrounding brain parenchyma prevent the efflux of parenchymal antigens and influx of circulating immune cells, thereby creating a site that is somewhat secure from peripheral immune surveillance (Engelhardt et al., 2017). In the brain parenchyma, major cell types are the neurons and glial cells, such as the astrocytes, oligodendrocytes, and microglia. Among these cells, microglia function as the main immune cell that monitor pathogen- or damage-associated molecular patterns in the brain (Li and Barres, 2018). In addition to microglia, other types of macrophages reside in the surrounding barrier or border regions, such as the meninges, perivascular space, and choroid plexus stroma. These non-parenchymal brain macrophages are also referred to as border-associated or central nervous system (CNS)-associated macrophages (CAMs) (Kierdorf et al., 2019).
In pathological conditions, circulating myeloid cells such as neutrophils or monocytes can infiltrate into brain parenchyma and some monocytes differentiate into macrophages or microglia-like cells. Contrary to these brain-infiltrated macrophages, parenchymal microglia and non-parenchymal CAMs reside in the brain under normal condition. In this context, we categorized microglia and non-parenchymal brain macrophages (termed as CAMs) into brain-resident macrophages in the current review. Microglia have diverse physiological non-immune functions, such as neuronal homeostasis regulation and synapse elimination (Li and Barres, 2018). Microglia exhibit unique homeostatic phenotypes depending on the CNS microenvironment (Colonna and Butovsky, 2017). Under pathological conditions, microglia undergo remarkable phenotypic changes into distinct subsets such as disease-associated or aged microglia, which are implicated in the neurodegenerative diseases, traumatic brain injury, and psychiatric diseases (Bar and Barak, 2019; Deczkowska et al., 2018; Safaiyan et al., 2016). Therefore, understanding of these microglial subsets during disease progression can provide significant insights to aid in the development of therapeutic strategies for neurologic disorders.
Here, we have summarized the development and specialized features of the brain-resident macrophages. Moreover, we have characterized distinct subtypes of microglia based on their regional heterogeneity and plasticity during a disease state. Finally, we have discussed the inflammasome responses of microglia related to neurological disorders.
DEVELOPMENT OF BRAIN-RESIDENT MACROPHAGES
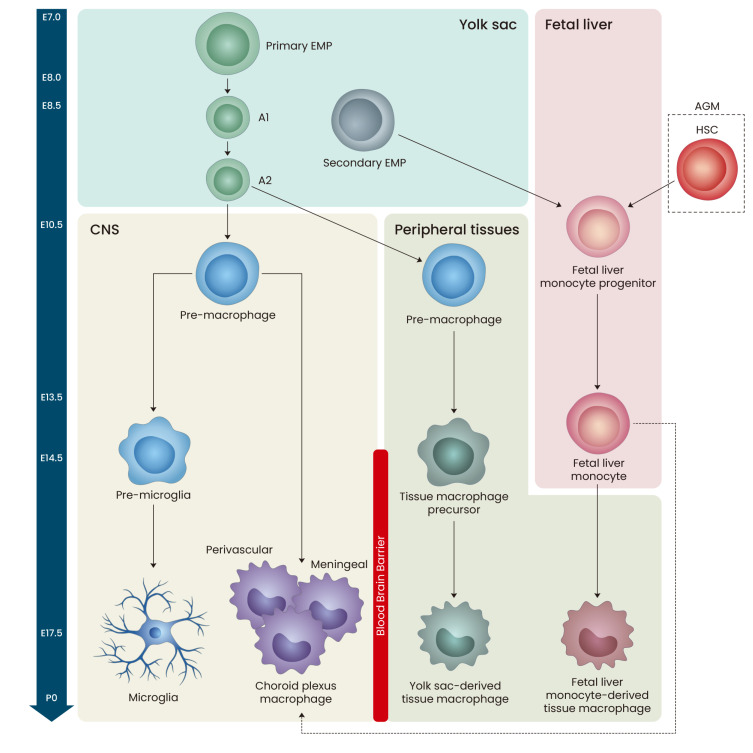
Brain-resident macrophages are classified into parenchymal microglia and non-parenchymal CAMs, such as meningeal, perivascular, and choroid plexus macrophages (Kierdorf et al., 2019; Li and Barres, 2018; Mrdjen et al., 2018). Microglia represent the largest population of immune cells in the brain parenchyma, whereas other cell types are localized at the interface between the brain parenchyma and circulation (Prinz et al., 2017). During the development of brain-resident macrophages, three waves of hematopoiesis occur in two major sites, yolk sac (YS) and fetal liver. In the first wave, “primitive” hematopoiesis generates primary erythro-myeloid progenitor (EMP) cells in the YS during embryonic day 7.0 (E7.0) and E.8.0 (Sevenich, 2018). Primary EMP cells predominantly generate YS macrophages (A1 and A2), which colonize the entire embryo to generate all types of tissue-resident macrophages, including those in the brain (Fig. 1) (Ginhoux and Guilliams, 2016; Hoeffel et al., 2015; Li and Barres, 2018). YS macrophages move to the CNS or peripheral region around E10.5 (Li and Barres, 2018; McGrath et al., 2003), then differentiate into microglia or non-parenchymal macrophages in the CNS and YS-derived tissue macrophages in the peripheral tissues.
Fig. 1. Embryonic development of brain-resident macrophages.
Between embryonic day 7.0 (E7.0) and E8.0 of mouse development, primary erythromyeloid progenitor (EMP) cells in the yolk sac (YS) generate YS macrophages (A1 and A2), which are able to produce all types of tissue-resident macrophages including the brain. Around E10.5, YS macrophages move to central nervous system (CNS) or peripheral regions, and can differentiate into microglia or non-parenchymal macrophages (perivascular, meningeal, or choroid plexus macrophages) in the CNS or YS-derived tissue macrophages in the peripheral tissues. Secondary EMP cells in the YS and hematopoietic stem cells (HSCs) in the aorta-gonad-mesonephros (AGM) of embryo migrate to fetal liver during E8.5-10. Then, this fetal liver progenitor cells differentiate into fetal liver monocytes, which then invade all peripheral tissues except CNS at E14.5 of embryonic development.
In the overlapping second and third waves, “definitive” hematopoiesis is initiated by hematopoietic progenitors, secondary EMPs in the YS and hematopoietic stem cells (HSCs) in the aorta-gonad-mesonephros of the embryo, during E8.5-E10 (Epelman et al., 2014b). Both progenitor cells migrate into the fetal liver during E9.5-E10.5 and differentiate into fetal liver monocytes. At approximately E14.5, the fetal liver monocytes invade all surrounding tissues except the brain parenchyma, where the blood-brain barrier, formed at approximately E13.5, presumably blocks their entry (Frade and Barde, 1998; Li and Barres, 2018). Fetal liver monocytes then develop into fetal liver-derived tissue macrophages or non-parenchymal macrophages in the brain. Notably, the two tissue macrophage populations, fetal liver- and YS-derived, cannot be distinguished in the peripheral tissues in adults (Li and Barres, 2018). Primary EMPs, secondary EMP-derived fetal liver monocytes, and HSC-derived fetal liver monocytes disproportionately contribute to all tissue macrophages. Fetal liver serves as the major hematopoietic organ during definitive hematopoiesis, and around birth, hematopoiesis starts being restricted to the bone marrow (Perdiguero and Geissmann, 2016).
Microglial population of the fetal brain is almost established before the onset of monocyte production in the fetal liver and blood-brain barrier closure (Ginhoux et al., 2013; Sevenich, 2018). Thus, microglia originate exclusively from YS-derived progenitors, whereas CAMs are replenished by fetal liver-derived progenitor cells during embryonic development (Sevenich, 2018). In this way, all brain-resident macrophages are predominantly generated by embryonic precursor cells and maintain their population by self-renewal under normal conditions except for the choroid plexus macrophages (Li and Barres, 2018). In adults, choroid plexus macrophages are further replenished by circulating HSC-derived progenitor cells (Fig. 2) (Goldmann et al., 2016; Li and Barres, 2018; Prinz et al., 2017).
Fig. 2. Location of brain-resident macrophages.
Microglia are the major innate immune cells in the brain parenchyma that are exclusively derived from yolk sac (YS)-derived embryonic precursor cells. All other brain-resident macrophages, including meningeal, perivascular, and choroid plexus macrophages, which originate from both YS- and fetal liver (FL)-derived progenitor cells, are located at the brain-circulation interface. Brain-resident macrophages maintain their population by self-renewal, whereas only the choroid plexus macrophages receive input from the circulating hematopoietic stem cell (HSC)-derived progenitors. Cardiac and intestinal macrophages also originate from the HSC-derived progenitor cells. Peripheral tissue macrophages in the epidermis and heart are derived from embryonic precursors. FL-derived precursor cells are committed to forming Kupffer cells in the liver and alveolar macrophages in the lung. CSF, cerebrospinal fluid; CNS, central nervous system.
Normally, microglia sustain the microglia pool via local clonal expansion throughout life (Butovsky and Weiner, 2018); however, fate-mapping studies have proposed that monocyte-derived macrophages, which are recruited into the brain parenchyma, can differentiate into the microglial population under certain physiological conditions, while maintaining their own unique identity (Cronk et al., 2018; Lund et al., 2018). In contrast, Huang et al. (2018) demonstrated that microglial depletion resulted in repopulation of microglia by remaining residual microglia but not by peripheral macrophages. Therefore, at present, it remains unclear whether peripheral macrophages can contribute to microglial pool.
Ontogeny of peripheral tissue macrophages
For a long time, it was believed that tissue-resident macrophage homeostasis relied on constant recruitment of bone marrow-derived blood monocytes (Sawyer et al., 1982; van Furth and Cohn, 1968; Volkman et al., 1983). However, many ontogenic studies revealed that a majority of tissue macrophages originated from embryonic precursors that were derived from the YS and fetal liver (Fig. 2) (Ginhoux and Guilliams, 2016; Li and Barres, 2018; Sevenich, 2018). Tissue-resident macrophages maintain themselves in adults by self-renewal except in the gut, dermis, and heart (Epelman et al., 2014a; Tamoutounour et al., 2013). The gut and dermis are considered open tissues with fast recruitment kinetics and differentiation of bone marrow-derived monocytes into macrophages (Ginhoux and Guilliams, 2016). For example, although at birth, embryonically derived macrophages are present in the gut, they are replaced by cells derived from an influx of CCR2-dependent Ly6Chi monocytes (Bain et al., 2014). Likewise, cardiac macrophages originate from the embryonic YS and fetal monocyte progenitors and give rise to embryonic resident macrophages; however, they can be replenished by bone marrow-derived monocytes, especially after a heart injury such as cardiac ischemia (Fig. 2) (Epelman et al., 2014a).
SIGNATURES OF BRAIN-RESIDENT MACROPHAGES
Microglia express common macrophage markers such as F4/80, CD11b, CD45, Iba1, and CX3C chemokine receptor 1 (CX3CR1) (Li and Barres, 2018). Although many of these proteins are expressed by macrophages, their expression levels can be used to distinguish microglia from other related cell types (Butovsky and Weiner, 2018). For instance, CD45 and CD11b are downregulated in microglia than in monocytes, which makes it possible to distinguish resident microglia from infiltrated monocyte-derived cells in the brain (Bennett et al., 2016; Butovsky and Weiner, 2018; Li and Barres, 2018). CX3CR1 is expressed in other tissue macrophages; however, its expression is higher in microglia (Jung et al., 2000). Notably, CX3CR1 deficiency leads to transient reduction in microglia number during the early postnatal period and a consequent defect in synaptic pruning, synaptic transmission, and functional brain connectivity (Zhan et al., 2014). It is thus widely used to study the role of microglia in the CNS by using CX3CR1-deficient or CX3CR1-Cre mouse lines (Reshef et al., 2017; Wolf et al., 2013; Zhao et al., 2019). Besides, microglia express other highly restricted, specific molecules such as transmembrane protein 119 (TMEM119), P2Y purinoceptor 12 (P2RY12), and Sal-like protein 1 (SALL1) (Table 1) (Butovsky and Weiner, 2018; Buttgereit et al., 2016; Li and Barres, 2018).
Table 1.
Molecular signatures of brain macrophages
| Type | Name | Expression markers | |
|---|---|---|---|
| Brain-resident (parenchymal) | Microglia | CD45int, CD11bint, CX3CR1, IBA1, TMEM119, P2RY12, TREM2, SALL1, Siglec-H | |
| Brain-resident (non-parenchymal) | Meningeal macrophages | CD45hi , CD11b, CX3CR1, IBA1, LYVE1, MHCIIhi |
CD206, Siglec-1 (CD169), CD36 |
| Perivascular macrophages | |||
| Choroid plexus macrophages | SALL1, Siglec-H, CCR2, Ly6Chi | ||
| Brain-infiltrated (parenchymal) | Monocyte-derived macrophages | CD45hi, CD11bhi, IBA1, Siglec-1 (CD169), CCR2, Ly6Chi, CD44 | |
CX3CR1, CX3C chemokine receptor 1; IBA1, ionized calcium-binding adaptor molecule 1; TREM2, triggering receptor expressed on myeloid cells 2; SALL1, Sal-like protein 1; LYVE1, lymphatic vessel endothelial hyaluronan receptor 1; CCR2, C-C chemokine receptor 2.
Some microglial markers exhibit a distinct expression pattern depending on the surrounding environment. Triggering receptor expressed on myeloid cells 2 (TREM2) is a crucial transmembrane receptor in microglia to scavenge extracellular toxic molecules such as amyloid-β and its expression is restricted to some CNS regions (Poliani et al., 2015; Schmid et al., 2002). Although TREM2 mutation is considered a risk factor for non-familial Alzheimer’s disease (AD), its expression does not change in AD patients (Colonna and Wang, 2016; Del-Aguila et al., 2019). Additionally, the expression of CD33 (Siglec3), another transmembrane receptor of microglia, is elevated in AD patients, and the increased CD33 expression is associated with the inhibition of amyloid-β clearance and phagocytosis (Griciuc et al., 2013). CD68, a lysosomal protein, is highly expressed in activated microglia but not in resting microglia (Griciuc et al., 2013; Hopperton et al., 2018; Walker and Lue, 2015).
Non-parenchymal CAMs express pan-macrophage markers similar to those expressed by microglia, such as CX3CR1, CD45, CD11b, and Iba1 (Table 1) (Brioschi et al., 2020). Of note, higher expression of CD45 and MHCII in CAMs generally distinguishes them from microglia (Li and Barres, 2018). However, the presence of CD45low non-parenchymal brain macrophages makes it difficult to discriminate from microglia (Mrdjen et al., 2018). Additionally, lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1) was also expressed in perivascular and meningeal macrophages, but not in microglia (Ayata et al., 2018). During embryonic development, YS-derived progenitor cells (CD206-positive) can differentiate into non-parenchymal macrophages with a specific CD206 expression at E10.5 (Utz et al., 2020). Choroid plexus macrophages that originate from embryonic precursor cells express SALL1, whereas monocyte-derived choroid plexus macrophages do not (Buttgereit et al., 2016). Moreover, SALL1 is not expressed in meningeal or perivascular macrophages (Buttgereit et al., 2016). Recent studies using single-cell sequencing technologies revealed that meningeal and perivascular macrophages are considered as homogenous populations, while more heterogeneity was observed in choroid plexus macrophages (Jordao et al., 2019; Kierdorf et al., 2019).
PHENOTYPES AND FUNCTIONS OF MICROGLIA
M1/M2 polarization of macrophages
Tissue-specific or context-dependent microenvironments result in diverse macrophage phenotypes that show distinct gene expression profiles and specific time-dependent functions (Ivashkiv, 2013; Lawrence and Natoli, 2011). Depending on the extracellular conditions, such as cytokines, lipid mediators, or pattern-recognition receptor agonists, macrophages can be activated into two groups, M1 and M2 macrophages, which have distinct phenotypic and functional characteristics (Ginhoux et al., 2016; Ivashkiv, 2013). Given the intensive efforts to highlight previous works regarding M1/M2 polarization, macrophage polarization is not going to be rigorously discussed in this review. However, macrophage activation status cannot be simply classified into two groups. Macrophages do not show a clear M1 or M2 phenotype in physiological conditions and instead present with phenotypic plasticity in many homeostatic or pathological situations (Martinez and Gordon, 2014).
Resting and activated microglia
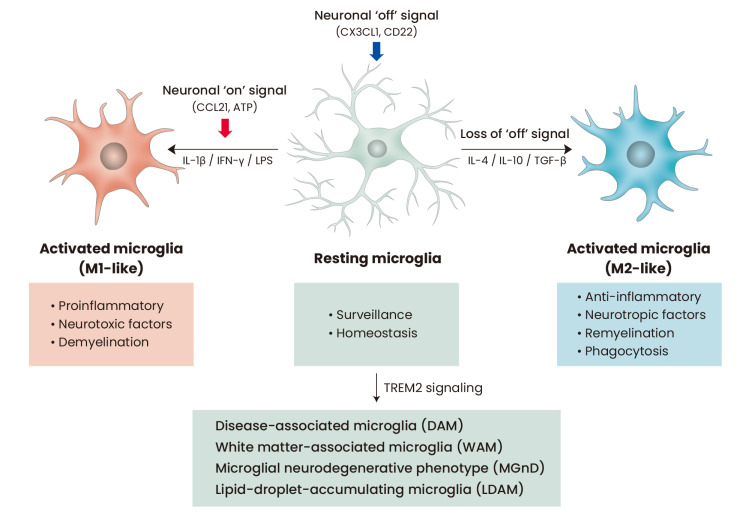
Under normal and physiological conditions, microglia exist in a so-called “resting state.” Resting microglia, characterized by a highly ramified morphology, continuously attempt to detect any pathological or homeostatic changes in the brain parenchyma (Nimmerjahn et al., 2005). On observing a disturbance or damage in the CNS homeostasis, the microglia shift toward an “activated state.” (Davalos et al., 2005; Kawabori and Yenari, 2015) Further, upon sensing foreign molecules associated with an infection and damage-associated factors from damaged neurons, microglia undergo transformation from their resting state to an activated state, which can in turn initiate protective or detrimental microglial functions (Fig. 3) (Butovsky et al., 2005). However, the nomenclature of “resting” and “activated” microglia has been recently challenged because of the highly dynamic surveillance in the resting status (Nimmerjahn et al., 2005; Sierra et al., 2014). Interestingly, microglial activation is also controlled by two types of signals from neurons, namely, the “on” and “off” signals (Biber et al., 2007; Szepesi et al., 2018). Neuronal “off” signals include constitutive production of CX3CL1, CD22, neurotransmitters, or neutrophins from healthy neurons to keep the microglia in a resting state (Biber et al., 2007). Conversely, damaged or stressed neurons rapidly trigger the activation of microglia by producing “on” signals, such as CCL21, CCL10, or ATP production. Although microglial activation can also be classified into M1 and M2 polarization just like macrophage activation (Hu et al., 2015; Orihuela et al., 2016), microglia show more heterogeneous phenotypes than peripheral macrophages due to a brain-specific regional difference and pathological conditions (Ginhoux et al., 2016). Thus, neither the terms “resting” and “activated,” nor M1 and M2 are sufficient for defining and explaining the complex plasticity of microglia.
Fig. 3. Diversity of microglial phenotypes.
Neuronal “off” signals, such as CX3CL1, constantly inhibit microglial activation in physiological conditions. Microglia in this environment show a ramified morphology with a low expression of CD68 and are referred to as “resting” microglia. Resting microglia can be activated by PAMPs (LPS) or DAMPs (ATP) inside the CNS into M1-like microglia and are characterized by ameboid morphology, high CD68 expression, and proinflammatory phenotypes. Neuronal “on” signals also contribute to M1 microglial polarization. In contrast, loss of “off” signals or anti-inflammatory cytokine-rich milieu can induce the activation of microglia into neuroprotective M2-like phenotype. Recently, diverse microglial phenotypes have been identified in pathological conditions, such as DAM, WAM, MGnD and LDAM. PAMP, pathogen-associated molecular pattern; DAMP, damage-associated molecular pattern; LPS, lipopolysaccharide.
Microenvironments in the brain may drive the differentiation of distinct microglial subtypes, resulting in microglial regional heterogeneity (Stratoulias et al., 2019). Microglial regional heterogeneity includes microglial density, morphology, molecular signatures, and functions across different brain regions (Tan et al., 2020). Notably, microglial subtypes in each brain region respond differently to identical stimuli or conditions (Furube et al., 2018; Hui et al., 2018; Tay et al., 2017). For example, cerebellar and hippocampal microglia exist in a more “immune-vigilant” state than the microglia in the forebrain regions (Grabert et al., 2016). Furthermore, cerebellar microglia express increased gene expression associated with the detection and phagocytosis of apoptotic cells than microglia in striatum or cortex (Ayata et al., 2018). Microglia within the subventricular zone and rostral migratory stream exhibit lower expression of purinoreceptors and less phagocytic ability than cortical microglia (Ribeiro Xavier et al., 2015). This distinct microglial phenotype contributes to the support of neurogenesis in the subventricular zone. On the contrary, microglia in the cortex shows more rapid chemotactic ability towards ATP than subventricular zone microglia (Ribeiro Xavier et al., 2015).
Disease-associated and aged microglia
Under pathological conditions, several microglial subtypes, such as disease-associated microglia (DAM) and aged microglia, are reportedly associated with neurodegenerative diseases. DAM have been recently identified as a new subset of microglia that are found at neurodegeneration sites and show unique transcriptional and functional signatures (Deczkowska et al., 2018; Keren-Shaul et al., 2017). DAM show downregulation of homeostatic genes such as TMEM19, P2RY12, and CX3CR1 and upregulation of TREM2, CST7, and Axl (Brioschi et al., 2020). TREM2 signaling plays a pivotal role in DAM activation (Keren-Shaul et al., 2017). DAM are frequently detected under conditions of accumulating degenerating neurons, myelin debris, or extracellular protein aggregates and reportedly alleviate the damage; however, it is not clear whether they have a protective or disease-inducing function (Butovsky and Weiner, 2018; Haruwaka et al., 2019; Liddelow et al., 2017; Simard et al., 2006).
Aging of microglia is also a potent risk factor for the development of neurodegenerative diseases. Aged microglia are characterized by functional impairment, including decreased phagocytic activity, lowered threshold of immune stimuli activation, and enhanced release of inflammatory cytokines (Niraula et al., 2017; Perry and Holmes, 2014; Safaiyan et al., 2016; Spittau, 2017). Thus, age-related changes in the microglia are likely to be related to the onset and progress of age-related neurodegenerative diseases (Spittau, 2017). Recently, white matter-associated microglia (WAMs) were identified in the white matter of aged mouse brain with a similar molecular signature to DAM (Safaiyan et al., 2021). Safaiyan et al. (2021) revealed that WAMs are formed in a TREM2-dependent, but ApoE-independent manner and required to remove degenerated myelin. Unlike WAM, TREM2-ApoE signaling is a major regulator of microglial phenotypic change into microglial neurodegenerative phenotype (MGnD) in neurodegenerative diseases (Krasemann et al., 2017). Lipid-droplet-accumulating microglia (LDAM) were also recently identified as dysfunctional and proinflammatory microglial phenotype in the aged brain (Marschallinger et al., 2020). Interestingly, LDAM showed impaired phagocytosis and might contribute to chronic neuroinflammation and neurodegenerative phenotypes (Marschallinger et al., 2020). Further research endeavors will likely clarify the correlation of these recently-identified disease- or age-associated microglial subtypes and the regulation by neuronal on/off signals.
FUNCTIONS OF NON-PARENCHYMAL BRAIN MACROPHAGES
Because of their unique anatomical location, non-parenchymal CAMs primarily support the barrier function against external antigens (Li and Barres, 2018). Although functional studies are limited, CAMs reportedly monitor or filter the CSF for any harmful antigens and metabolites (Kierdorf et al., 2019), and also contribute to the drainage of CNS-derived antigens (Mundt et al., 2019). CAM-specific markers CD206 is potentially responsible for their scavenging function (Kierdorf et al., 2019). Among brain-circulation barrier regions, perivascular spaces in the brain are surrounded by diverse cell types, including astrocytes, pericytes and endothelial cells. In this context, perivascular macrophages can reciprocally interact with these surrounding cells. Of interest, perivascular macrophages drive the activation of hypothalamo-pituitary-adrenal axis through prostanoid production and the anti-inflammatory action on endothelial cells upon systemic inflammation (Serrats et al., 2010). Moreover, perivascular macrophages can clear amyloid β in a CCR2-dependent manner in a mouse model of AD (Mildner et al., 2011). Nevertheless, the physiological function of CAMs is still largely unknown and remains to be further elucidated.
Non-parenchymal CAMs exhibit distinct morphologies. Perivascular and meningeal macrophages have more elongated shape than microglia (Kierdorf et al., 2019), whereas choroid plexus macrophages are characterized by stellate shape similar to Langerhans cells (Goldmann et al., 2016). In addition, CAMs in the dura mater, the most outer layer of the meninges, have bipolar structure with more dendrites. Meanwhile, the phenotypic heterogeneity of CAMs is recently being examined and thus requires further extensive investigation. The diversity of CAMs is supported by recent study demonstrating the expression of five core signature genes including mannose receptor 1 (Mrc1, encoding CD206). In particular, the expression of these CAM-specific genes was downregulated in the mouse model of neuroinflammation (Jordao et al., 2019). Thus, it will be intriguing to clarify the phenotypic diversity of CAMs under pathological condition.
INFLAMMASOME-MEDIATED RESPONSE OF MICROGLIA
In the brain, interleukin‐1β (IL-1β) and tumor necrosis factor α (TNF-α) are the key proinflammatory cytokines that contribute to CNS inflammation (Clausen et al., 2008). TNF-α is produced by the engagement of TLRs in glial or myeloid cells, with diverse ligands associated with microbial infection or neuronal damage (Rodgers et al., 2020). However, active IL-1β production requires further cytosolic inflammasome activation along with TLR-mediated transcriptional induction of pro-IL-1β (Yu and Lee, 2016). Inflammasome assembly results in caspase-1 activation, which then induce the maturation and gasdermin D-dependent secretion of IL-1β (Evavold et al., 2018; Schroder and Tschopp, 2010). Thus, unlike TNF-α, mature IL-1β production is restricted to inflammasome-active myeloid cells such as the microglia. It remains to be determined whether inflammasome activation occurs in non-parenchymal brain macrophages, but a previous study reported the expression of inflammasome components in the perivascular macrophages (Kawana et al., 2013).
Inflammasome is normally composed of sensor proteins, such as NOD-like receptor (NLR) family, pyrin domain-containing 3 (NLRP3) or CARD domain-containing 4 (NLRC4), adaptor protein, apoptosis-associated speck-like protein containing a CARD (ASC), and procaspase-1 (Rathinam and Fitzgerald, 2016). This inflammasome components are assembled only upon the detection of specific inflammasome-stimulating agonists by the sensor proteins in the cytoplasm (Yu and Lee, 2016). In particular, inflammasome activation in microglia has been implicated in neurodegenerative disease progression (Labzin et al., 2018). Indeed, NLRP3 sensor protein is activated by recognizing protein aggregates such as amyloid-β and α-synuclein or abnormal endogenous metabolites such as 25-hydroxycholesterol in the microglia (Codolo et al., 2013; Gordon et al., 2018; Halle et al., 2008; Jang et al., 2016; Venegas et al., 2017). This microglial inflammasome activation contributes to neuronal cell death, ultimately leading to neurodegeneration (Gordon et al., 2018; Heneka et al., 2013; Lee et al., 2019). In the brain parenchyma, microglial NLRP3 may function as a key sensor for cellular stress-associated molecules resulting from neuronal injury and protein inclusions leading to the progression of numerous neurological disorders, such as AD, Parkinson’s disease, multiple sclerosis, stroke, and traumatic brain injury (Voet et al., 2019; Walsh et al., 2014).
Microglia show robust NLRP3 expression particularly in the presence of lipopolysaccharide (LPS) stimulation (Gustin et al., 2015). Other inflammasome sensor proteins such as NLRC4 are also detected at lower levels (Walsh et al., 2014). However, it is not certain whether sensors other than NLRP3 are able to induce inflammasome activation in microglia under physiological conditions. Microglial NLRP3 inflammasome responses are more persistent than those by macrophages because of a lack of negative regulation of pro-IL-1β expression (Burm et al., 2015). Burm et al. (2015) raised the possibility that microglial NLRP3 inflammasome signaling may be more harmful to the microenvironment than that by macrophages due to a persistent inflammasome activation. Intriguingly, peripheral inflammation impairs the amyloid-β clearing ability of microglia through the NLRP3 inflammasome (Tejera et al., 2019). This finding suggests that microglial NLRP3 inflammasome response can alter the microglial phenotype contributing to neurodegeneration. Therefore, further understanding of microglial inflammasome response should shed light on the development of therapeutic strategies that target neuroinflammation-mediated neurological disorders.
CONCLUDING REMARKS
Brain-resident microglia continuously surveil the brain to detect homeostatic and pathological changes. Along with playing a central role in host defense against invading pathogens, microglia maintain tissue homeostasis and develop inflammation-mediated diseases. Non-parenchymal CAMs may strengthen the barrier function at the brain-circulation interface to maintain the CNS immune privilege. Although microglia and CAMs share many phenotypic features, they also have unique functional differences that result in different responses to homeostatic and pathological conditions. Additionally, microglia exhibit plasticity and regional heterogeneity according to a specific surrounding environment. In turn, diverse phenotypes of microglia participate differently in disease progression by driving different immunological responses in a disease-associated environment. Therefore, phenotypic approaches can provide important insight into elucidating the pathological mechanisms and developing novel therapeutic approaches in a variety of inflammatory diseases by targeting specific subsets of microglia. Furthermore, brain inflammasome activation may contribute to the development of neurodegenerative diseases as well as other neurological defects (Heneka et al., 2018). It will be thus intriguing to investigate molecular mechanisms by which inflammasome signaling is implicated in diseases such as sleep and neuropsychiatric disorders.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (2020R1A2B5B02001823, 2020R1A4A1019009).
Footnotes
AUTHOR CONTRIBUTIONS
E.L., J.C.E., C.L., and J.W.Y. wrote and reviewed the manuscript.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Ayata P., Badimon A., Strasburger H.J., Duff M.K., Montgomery S.E., Loh Y.E., Ebert A., Pimenova A.A., Ramirez B.R., Chan A.T., et al. Epigenetic regulation of brain region-specific microglia clearance activity. Nat. Neurosci. 2018;21:1049–1060. doi: 10.1038/s41593-018-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain C.C., Bravo-Blas A., Scott C.L., Perdiguero E.G., Geissmann F., Henri S., Malissen B., Osborne L.C., Artis D., Mowat A.M. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar E., Barak B. Microglia roles in synaptic plasticity and myelination in homeostatic conditions and neurodevelopmental disorders. Glia. 2019;67:2125–2141. doi: 10.1002/glia.23637. [DOI] [PubMed] [Google Scholar]
- Bennett M.L., Bennett F.C., Liddelow S.A., Ajami B., Zamanian J.L., Fernhoff N.B., Mulinyawe S.B., Bohlen C.J., Adil A., Tucker A., et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K., Neumann H., Inoue K., Boddeke H.W. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Brioschi S., Zhou Y., Colonna M. Brain parenchymal and extraparenchymal macrophages in development, homeostasis, and disease. J. Immunol. 2020;204:294–305. doi: 10.4049/jimmunol.1900821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burm S.M., Zuiderwijk-Sick E.A., ‘t Jong A.E., van der Putten C., Veth J., Kondova I., Bajramovic J.J. Inflammasome-induced IL-1β secretion in microglia is characterized by delayed kinetics and is only partially dependent on inflammatory caspases. J. Neurosci. 2015;35:678–687. doi: 10.1523/JNEUROSCI.2510-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O., Talpalar A.E., Ben-Yaakov K., Schwartz M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Mol. Cell. Neurosci. 2005;29:381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Butovsky O., Weiner H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018;19:622–635. doi: 10.1038/s41583-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit A., Lelios I., Yu X., Vrohlings M., Krakoski N.R., Gautier E.L., Nishinakamura R., Becher B., Greter M. Sall1 is a transcriptional regulator defining microglia identity and function. Nat. Immunol. 2016;17:1397–1406. doi: 10.1038/ni.3585. [DOI] [PubMed] [Google Scholar]
- Carson M.J., Doose J.M., Melchior B., Schmid C.D., Ploix C.C. CNS immune privilege: hiding in plain sight. Immunol. Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen B.H., Lambertsen K.L., Babcock A.A., Holm T.H., Dagnaes-Hansen F., Finsen B. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J. Neuroinflammation. 2008;5:46. doi: 10.1186/1742-2094-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codolo G., Plotegher N., Pozzobon T., Brucale M., Tessari I., Bubacco L., de Bernard M. Triggering of inflammasome by aggregated α-synuclein, an inflammatory response in synucleinopathies. PLoS One. 2013;8:e55375. doi: 10.1371/journal.pone.0055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M., Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M., Wang Y. TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 2016;17:201–207. doi: 10.1038/nrn.2016.7. [DOI] [PubMed] [Google Scholar]
- Cronk J.C., Filiano A.J., Louveau A., Marin I., Marsh R., Ji E., Goldman D.H., Smirnov I., Geraci N., Acton S., et al. Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J. Exp. Med. 2018;215:1627–1647. doi: 10.1084/jem.20180247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D., Grutzendler J., Yang G., Kim J.V., Zuo Y., Jung S., Littman D.R., Dustin M.L., Gan W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Deczkowska A., Keren-Shaul H., Weiner A., Colonna M., Schwartz M., Amit I. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell. 2018;173:1073–1081. doi: 10.1016/j.cell.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Del-Aguila J.L., Benitez B.A., Li Z., Dube U., Mihindukulasuriya K.A., Budde J.P., Farias F.H.G., Fernandez M.V., Ibanez L., Jiang S., et al. TREM2 brain transcript-specific studies in AD and TREM2 mutation carriers. Mol. Neurodegener. 2019;14:18. doi: 10.1186/s13024-019-0319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B., Vajkoczy P., Weller R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- Epelman S., Lavine K.J., Beaudin A.E., Sojka D.K., Carrero J.A., Calderon B., Brija T., Gautier E.L., Ivanov S., Satpathy A.T., et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014a;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S., Lavine K.J., Randolph G.J. Origin and functions of tissue macrophages. Immunity. 2014b;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold C.L., Ruan J., Tan Y., Xia S., Wu H., Kagan J.C. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 2018;48:35–44.e6. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade J.M., Barde Y.A. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/s0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- Furube E., Kawai S., Inagaki H., Takagi S., Miyata S. Brain region-dependent heterogeneity and dose-dependent difference in transient microglia population increase during lipopolysaccharide-induced inflammation. Sci. Rep. 2018;8:2203. doi: 10.1038/s41598-018-20643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Lim S., Hoeffel G., Low D., Huber T. Origin and differentiation of microglia. Front. Cell. Neurosci. 2013;7:45. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Schultze J.L., Murray P.J., Ochando J., Biswas S.K. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat. Immunol. 2016;17:34–40. doi: 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- Goldmann T., Wieghofer P., Jordão M.J., Prutek F., Hagemeyer N., Frenzel K., Amann L., Staszewski O., Kierdorf K., Krueger M., et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016;17:797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R., Albornoz E.A., Christie D.C., Langley M.R., Kumar V., Mantovani S., Robertson A.A.B., Butler M.S., Rowe D.B., O'Neill L.A., et al. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018;10:eaah4066. doi: 10.1126/scitranslmed.aah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabert K., Michoel T., Karavolos M.H., Clohisey S., Baillie J.K., Stevens M.P., Freeman T.C., Summers K.M., McColl B.W. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 2016;19:504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griciuc A., Serrano-Pozo A., Parrado A.R., Lesinski A.N., Asselin C.N., Mullin K., Hooli B., Choi S.H., Hyman B.T., Tanzi R.E. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin A., Kirchmeyer M., Koncina E., Felten P., Losciuto S., Heurtaux T., Tardivel A., Heuschling P., Dostert C. NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS One. 2015;10:e0130624. doi: 10.1371/journal.pone.0130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A., Hornung V., Petzold G.C., Stewart C.R., Monks B.G., Reinheckel T., Fitzgerald K.A., Latz E., Moore K.J., Golenbock D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruwaka K., Ikegami A., Tachibana Y., Ohno N., Konishi H., Hashimoto A., Matsumoto M., Kato D., Ono R., Kiyama H., et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019;10:5816. doi: 10.1038/s41467-019-13812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M.T., Kummer M.P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T.C., et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M.T., McManus R.M., Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018;19:610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- Hoeffel G., Chen J., Lavin Y., Low D., Almeida F.F., See P., Beaudin A.E., Lum J., Low I., Forsberg E.C., et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopperton K.E., Mohammad D., Trépanier M.O., Giuliano V., Bazinet R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer's disease: a systematic review. Mol. Psychiatry. 2018;23:177–198. doi: 10.1038/mp.2017.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Leak R.K., Shi Y., Suenaga J., Gao Y., Zheng P., Chen J. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Xu Z., Xiong S., Sun F., Qin G., Hu G., Wang J., Zhao L., Liang Y.X., Wu T., et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 2018;21:530–540. doi: 10.1038/s41593-018-0090-8. [DOI] [PubMed] [Google Scholar]
- Hui C.W., St-Pierre A., El Hajj H., Remy Y., Hébert S.S., Luheshi G.N., Srivastava L.K., Tremblay M. Prenatal immune challenge in mice leads to partly sex-dependent behavioral, microglial, and molecular abnormalities associated with schizophrenia. Front. Mol. Neurosci. 2018;11:13. doi: 10.3389/fnmol.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkiv L.B. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34:216–223. doi: 10.1016/j.it.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J., Park S., Jin Hur H., Cho H.J., Hwang I., Pyo Kang Y., Im I., Lee H., Lee E., Yang W., et al. 25-hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome. Nat. Commun. 2016;7:13129. doi: 10.1038/ncomms13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordao M.J.C., Sankowski R., Brendecke S.M., Sagar , Locatelli G., Tai Y.H., Tay T.L., Schramm E., Armbruster S., Hagemeyer N., et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019;363:eaat7554. doi: 10.1126/science.aat7554. [DOI] [PubMed] [Google Scholar]
- Jung S., Aliberti J., Graemmel P., Sunshine M.J., Kreutzberg G.W., Sher A., Littman D.R. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 2000;20:4106–4114. doi: 10.1128/MCB.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabori M., Yenari M.A. The role of the microglia in acute CNS injury. Metab. Brain Dis. 2015;30:381–392. doi: 10.1007/s11011-014-9531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawana N., Yamamoto Y., Ishida T., Saito Y., Konno H., Arima K., Satoh J. Reactive astrocytes and perivascular macrophages express NLRP3 inflammasome in active demyelinating lesions of multiple sclerosis and necrotic lesions of neuromyelitis optica and cerebral infarction. Clin. Exp. Neuroimmunol. 2013;4:296–304. [Google Scholar]
- Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., David E., Baruch K., Lara-Astaiso D., Toth B., et al. A unique microglia type associated with restricting development of Alzheimer's disease. Cell. 2017;169:1276–1290.e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Kierdorf K., Masuda T., Jordao M.J.C., Prinz M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat. Rev. Neurosci. 2019;20:547–562. doi: 10.1038/s41583-019-0201-x. [DOI] [PubMed] [Google Scholar]
- Krasemann S., Madore C., Cialic R., Baufeld C., Calcagno N., El Fatimy R., Beckers L., O'Loughlin E., Xu Y., Fanek Z., et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47:566–581.e9. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labzin L.I., Heneka M.T., Latz E. Innate immunity and neurodegeneration. Annu. Rev. Med. 2018;69:437–449. doi: 10.1146/annurev-med-050715-104343. [DOI] [PubMed] [Google Scholar]
- Lawrence T., Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- Lee E., Hwang I., Park S., Hong S., Hwang B., Cho Y., Son J., Yu J.W. MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ. 2019;26:213–228. doi: 10.1038/s41418-018-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Barres B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018;18:225–242. doi: 10.1038/nri.2017.125. [DOI] [PubMed] [Google Scholar]
- Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W.S., Peterson T.C., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund H., Pieber M., Parsa R., Grommisch D., Ewing E., Kular L., Han J., Zhu K., Nijssen J., Hedlund E., et al. Fatal demyelinating disease is induced by monocyte-derived macrophages in the absence of TGF-β signaling. Nat. Immunol. 2018;19:1–7. doi: 10.1038/s41590-018-0091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschallinger J., Iram T., Zardeneta M., Lee S.E., Lehallier B., Haney M.S., Pluvinage J.V., Mathur V., Hahn O., Morgens D.W., et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020;23:194–208. doi: 10.1038/s41593-019-0566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos P., McGavern D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 2019;4:eaav0492. doi: 10.1126/sciimmunol.aav0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath K.E., Koniski A.D., Malik J., Palis J. Circulation is established in a stepwise pattern in the mammalian embryo. Blood. 2003;101:1669–1676. doi: 10.1182/blood-2002-08-2531. [DOI] [PubMed] [Google Scholar]
- Mildner A., Schlevogt B., Kierdorf K., Bottcher C., Erny D., Kummer M.P., Quinn M., Bruck W., Bechmann I., Heneka M.T., et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer's disease. J. Neurosci. 2011;31:11159–11171. doi: 10.1523/JNEUROSCI.6209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrdjen D., Pavlovic A., Hartmann F.J., Schreiner B., Utz S.G., Leung B.P., Lelios I., Heppner F.L., Kipnis J., Merkler D., et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48:380–395.e6. doi: 10.1016/j.immuni.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Mundt S., Greter M., Flugel A., Becher B. The CNS immune landscape from the viewpoint of a T cell. Trends Neurosci. 2019;42:667–679. doi: 10.1016/j.tins.2019.07.008. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Niraula A., Sheridan J.F., Godbout J.P. Microglia priming with aging and stress. Neuropsychopharmacology. 2017;42:318–333. doi: 10.1038/npp.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela R., McPherson C.A., Harry G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016;173:649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero E.G., Geissmann F. The development and maintenance of resident macrophages. Nat. Immunol. 2016;17:2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V.H., Holmes C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014;10:217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- Poliani P.L., Wang Y., Fontana E., Robinette M.L., Yamanishi Y., Gilfillan S., Colonna M. TREM2 sustains microglial expansion during aging and response to demyelination. J. Clin. Invest. 2015;125:2161–2170. doi: 10.1172/JCI77983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M., Erny D., Hagemeyer N. Ontogeny and homeostasis of CNS myeloid cells. Nat. Immunol. 2017;18:385–392. doi: 10.1038/ni.3703. [DOI] [PubMed] [Google Scholar]
- Rathinam V.A., Fitzgerald K.A. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165:792–800. doi: 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef R., Kudryavitskaya E., Shani-Narkiss H., Isaacson B., Rimmerman N., Mizrahi A., Yirmiya R. The role of microglia and their CX3CR1 signaling in adult neurogenesis in the olfactory bulb. Elife. 2017;6:e30809. doi: 10.7554/eLife.30809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Xavier A.L., Kress B.T., Goldman S.A., Lacerda, de Menezes J.R., Nedergaard M. A distinct population of microglia supports adult neurogenesis in the subventricular zone. J. Neurosci. 2015;35:11848–11861. doi: 10.1523/JNEUROSCI.1217-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers K.R., Lin Y., Langan T.J., Iwakura Y., Chou R.C. Innate immune functions of astrocytes are dependent upon tumor necrosis factor-alpha. Sci. Rep. 2020;10:7047. doi: 10.1038/s41598-020-63766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan S., Besson-Girard S., Kaya T., Cantuti-Castelvetri L., Liu L., Ji H., Schifferer M., Gouna G., Usifo F., Kannaiyan N., et al. White matter aging drives microglial diversity. Neuron. 2021;109:1100–1117.e10. doi: 10.1016/j.neuron.2021.01.027. [DOI] [PubMed] [Google Scholar]
- Safaiyan S., Kannaiyan N., Snaidero N., Brioschi S., Biber K., Yona S., Edinger A.L., Jung S., Rossner M.J., Simons M. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 2016;19:995–998. doi: 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R.T., Strausbauch P.H., Volkman A. Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab. Invest. 1982;46:165–170. [PubMed] [Google Scholar]
- Schmid C.D., Sautkulis L.N., Danielson P.E., Cooper J., Hasel K.W., Hilbush B.S., Sutcliffe J.G., Carson M.J. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J. Neurochem. 2002;83:1309–1320. doi: 10.1046/j.1471-4159.2002.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1038/nm.3893,. [DOI] [PubMed] [Google Scholar]
- Serrats J., Schiltz J.C., Garcia-Bueno B., van Rooijen N., Reyes T.M., Sawchenko P.E. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenich L. Brain-resident microglia and blood-borne macrophages orchestrate central nervous system inflammation in neurodegenerative disorders and brain cancer. Front. Immunol. 2018;9:697. doi: 10.3389/fimmu.2018.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A., Tremblay M.E., Wake H. Never-resting microglia: physiological roles in the healthy brain and pathological implications. Front. Cell. Neurosci. 2014;8:240. doi: 10.3389/fncel.2014.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard A.R., Soulet D., Gowing G., Julien J.P., Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Spittau B. Aging microglia-phenotypes, functions and implications for age-related neurodegenerative diseases. Front. Aging Neurosci. 2017;9:194. doi: 10.3389/fnagi.2017.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratoulias V., Venero J.L., Tremblay M., Joseph B. Microglial subtypes: diversity within the microglial community. EMBO J. 2019;38:e101997. doi: 10.15252/embj.2019101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szepesi Z., Manouchehrian O., Bachiller S., Deierborg T. Bidirectional microglia-neuron communication in health and disease. Front. Cell. Neurosci. 2018;12:323. doi: 10.3389/fncel.2018.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamoutounour S., Guilliams M., Montanana Sanchis F., Liu H., Terhorst D., Malosse C., Pollet E., Ardouin L., Luche H., Sanchez C., et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Tan Y.L., Yuan Y., Tian L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry. 2020;25:351–367. doi: 10.1038/s41380-019-0609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay T.L., Mai D., Dautzenberg J., Fernández-Klett F., Lin G., Sagar , Datta M., Drougard A., Stempfl T., Ardura-Fabregat A., et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 2017;20:793–803. doi: 10.1038/nn.4547. [DOI] [PubMed] [Google Scholar]
- Tejera D., Mercan D., Sanchez-Caro J.M., Hanan M., Greenberg D., Soreq H., Latz E., Golenbock D., Heneka M.T. Systemic inflammation impairs microglial Abeta clearance through NLRP3 inflammasome. EMBO J. 2019;38:e101064. doi: 10.15252/embj.2018101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz S.G., See P., Mildenberger W., Thion M.S., Silvin A., Lutz M., Ingelfinger F., Rayan N.A., Lelios I., Buttgereit A., et al. Early fate defines microglia and non-parenchymal brain macrophage development. Cell. 2020;181:557–573.e18. doi: 10.1016/j.cell.2020.03.021. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z.A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas C., Kumar S., Franklin B.S., Dierkes T., Brinkschulte R., Tejera D., Vieira-Saecker A., Schwartz S., Santarelli F., Kummer M.P., et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer's disease. Nature. 2017;552:355–361. doi: 10.1038/nature25158. [DOI] [PubMed] [Google Scholar]
- Voet S., Srinivasan S., Lamkanfi M., van Loo G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019;11:e10248. doi: 10.15252/emmm.201810248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman A., Chang N.C., Strausbauch P.H., Morahan P.S. Differential effects of chronic monocyte depletion on macrophage populations. Lab. Invest. 1983;49:291–298. [PubMed] [Google Scholar]
- Walker D.G., Lue L.F. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res. Ther. 2015;7:56. doi: 10.1186/s13195-015-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J.G., Muruve D.A., Power C. Inflammasomes in the CNS. Nat. Rev. Neurosci. 2014;15:84–97. doi: 10.1038/nrn3638. [DOI] [PubMed] [Google Scholar]
- Wolf Y., Yona S., Kim K.W., Jung S. Microglia, seen from the CX3CR1 angle. Front. Cell. Neurosci. 2013;7:26. doi: 10.3389/fncel.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.W., Lee M.S. Mitochondria and the NLRP3 inflammasome: physiological and pathological relevance. Arch. Pharm. Res. 2016;39:1503–1518. doi: 10.1007/s12272-016-0827-4. [DOI] [PubMed] [Google Scholar]
- Zhan Y., Paolicelli R.C., Sforazzini F., Weinhard L., Bolasco G., Pagani F., Vyssotski A.L., Bifone A., Gozzi A., Ragozzino D., et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Zhao X.F., Alam M.M., Liao Y., Huang T., Mathur R., Zhu X., Huang Y. Targeting microglia using Cx3cr1-Cre lines: revisiting the specificity. Neuro. 2019;6:ENEURO.0114-19.2019. doi: 10.1523/ENEURO.0114-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]