Abstract
In spite of the considerable achievements in the field of regenerative medicine in the past several decades, osteochondral defect regeneration remains a challenging issue among diseases in the musculoskeletal system because of the spatial complexity of osteochondral units in composition, structure and functions. In order to repair the hierarchical tissue involving different layers of articular cartilage, cartilage-bone interface and subchondral bone, traditional clinical treatments including palliative and reparative methods have showed certain improvement in pain relief and defect filling. It is the development of tissue engineering that has provided more promising results in regenerating neo-tissues with comparable compositional, structural and functional characteristics to the native osteochondral tissues. Here in this review, some basic knowledge of the osteochondral units including the anatomical structure and composition, the defect classification and clinical treatments will be first introduced. Then we will highlight the recent progress in osteochondral tissue engineering from perspectives of scaffold design, cell encapsulation and signaling factor incorporation including bioreactor application. Clinical products for osteochondral defect repair will be analyzed and summarized later. Moreover, we will discuss the current obstacles and future directions to regenerate the damaged osteochondral tissues.
Keywords: Osteochondral tissue engineering, Cartilage tissue engineering, Gradient scaffold, Bioreactors
Graphical abstract
Highlights
-
•
Building materials are summarized: polymers, bioceramics, metals and ECM-based ones.
-
•
The advancing gradient scaffolds include multiphasic and continuous gradient ones.
-
•
Signaling factors are sorted into biochemical, and physiochemical and physical ones.
-
•
The commercially available products are introduced and categorized into three types.
-
•
Four biggest challenges are proposed followed by some possible future directions.
1. Introduction
Osteoarthritis, a long-term chronic disease, is the most common degenerative musculoskeletal disorder affecting diarthrodial joints worldwide [1]. It can affect an estimated 9.6% of men and 18% of women aged more than 60 years [2] and cause a great physical and psychological burden on individuals as well as a considerable socioeconomic burden on the whole world [3,4]. Growing life expectancy and aging populations are expected to witness a consequent rise in the prevalence of such a chronic disease [5,6]. The clinical manifestations of osteoarthritis include pain, transient morning stiffness and impaired joint movement. Apart from age, osteoarthritis is associated with numerous risk factors such as obesity, occupational injury, and trauma [1]. Although it is now known to be a complex condition affecting the whole joint involving the cartilage, subchondral bone, synovium and ligaments, the early recognized mechanical degradation of articular cartilage and the alternation of subchondral bone play a pivotal role in its pathogenesis [7].
As an avascular and aneural tissue, the degenerated articular cartilage lacks the capability to self-heal after damaged. Without appropriate and timely intervention, the chondral defect may extend deep into the subchondral bone and require a joint replacement at the end stage [5,8]. The defects associated with articular cartilage can be classified into three different types based on the lesion depth, partial thickness chondral defect, full thickness chondral defect and osteochondral defect. To prevent and treat the early osteoarthritis indicated by the loss of articular cartilage, a wide range of clinical treatment methods have been developed and applied including palliative, reparative and regenerative treatments [9]. Palliative treatments intended to relieve the knee pain and improve the functional status are incapable to prevent the progression of further chondral defects [10]. In terms of reparative approaches such as bone marrow stimulating techniques, the chondral defect can be thoroughly filled, but the filled tissue made up of fibrocartilage is deficient in biomechanical and viscoelastic features comparable to the natural hyaline cartilage [11]. The later appeared osteochondral transplantation using autografts or allografts provides appealing opportunities for the treatment of even the most severe osteochondral defects. Nonetheless, autografts are faced with disadvantages like a limited origin of the donor, unmatched mechanical properties, and the morbidity of the donor site. Shortcomings of allografts include difficulties in graft preservation and management, risks of disease transmission, and immunogenicity [12]. Autologous chondrocyte implantation (ACI) is the first explored regenerative treatment which potentiates the production of mechanically and functionally stable hyaline cartilage and it has evolved with several generations to achieve optimization of the surgical process and cartilaginous restoration [13,14]. Whereas, its potential to regenerate osteochondral defect involving both articular cartilage and subchondral bone remains challenging [12].
In early 1990s, the emergence of tissue engineering field broadened new horizons for the improvement of the traditional regenerative treatments [15]. To repair the cartilage defects, cartilage and osteochondral tissue engineering have been proposed with three key components, scaffolds, cells and signaling factors [16,17]. Given that the study of both cartilage and bone has all come a long way over the past decades, we will focus on the recent development in osteochondral defect repair in this review. The regeneration of osteochondral defect which involves cartilage, cartilage-bone interface and subchondral bone is largely based on the knowledge advances of the two separate tissues and more importantly, the understanding of osteochondral interface. In designing the osteochondral scaffold, a variety of materials are available in tissue engineering and regenerative medicine fields, including polymers, inorganic materials, extracellular matrix (ECM)-based materials, and metals. Also, the architectures of the grafts have experienced a tremendous improvement from the simplest monophasic scaffolds to biphasic, triphasic, multiphasic, and most recently, gradient ones to better mimic the complex hierarchy of native osteochondral units. The incorporation of tissue forming cells and/or signaling factors is acknowledged to improve the ECM deposition and restoration efficacy by influencing the interaction between the adjacent native tissue and the artificial scaffold [18,19]. Among the molecular factors, there are biochemical, physiochemical and physical ones, the first of which are represented by growth factors, while the last physical ones are always shown in various bioreactors [20]. Although the options of the cells and signaling factors are relatively limited compared to those of the scaffolds, the importance cannot be ignored. Despite the considerable progress in the osteochondral tissue engineering, only a few have been translated into clinical products.
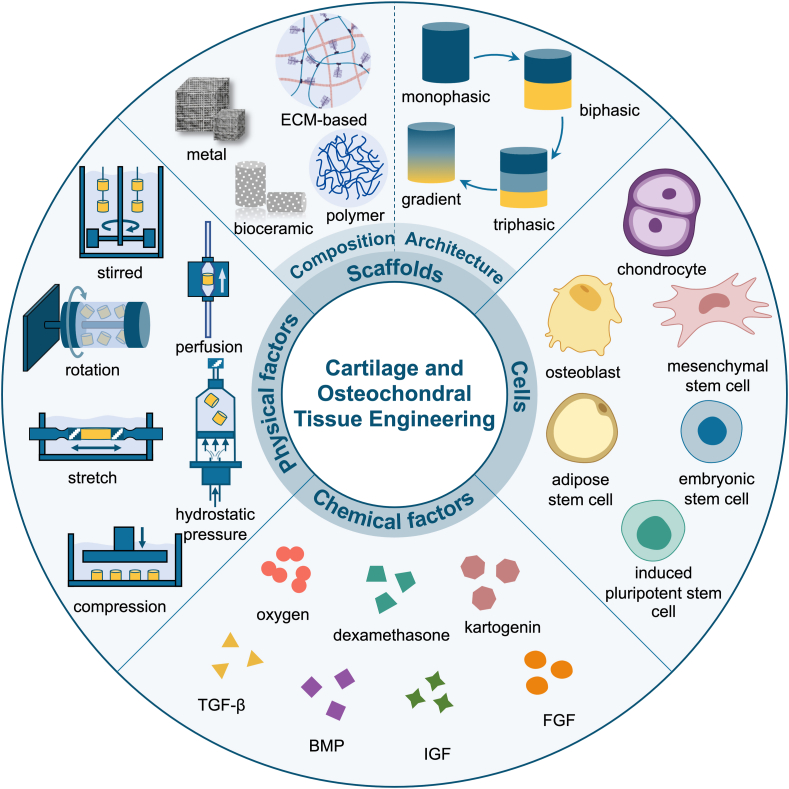
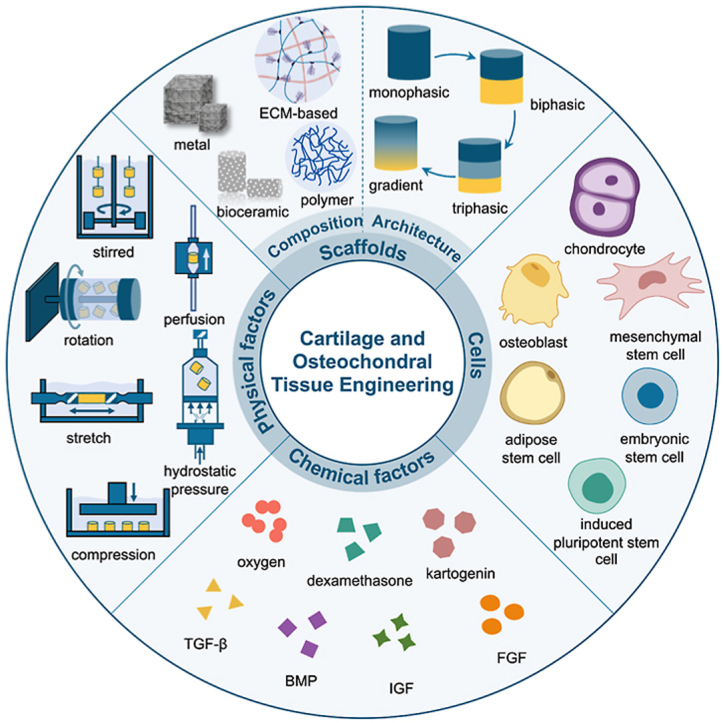
In this review, we will first present the basic anatomical stratified structure of osteochondral tissues, including articular cartilage, calcified cartilage and subchondral bone. Later, the defect classification and several clinical treatments as well as their corresponding advantages, disadvantages and development will be introduced. Furthermore, recent advances of cartilage and osteochondral tissue engineering will be overviewed from aspects of the three critical components, highlighting the compositions and architectures of the scaffolds. The architectures of the scaffolds are classified into four categories and four separate focuses are included. Summary is also conducted for the bioreactor related physiochemical and physical signaling factors. We then summarize the current clinical products for articular cartilage and osteochondral defect repair by sorting them into three types and finally, the four biggest challenges are proposed and corresponding future development directions in regenerating the damaged osteochondral tissues are envisioned (Fig. 1).
Fig. 1.
The schematic illustration of the key elements in cartilage and osteochondral tissue engineering including scaffold design from perspectives of composition and architecture, cell encapsulation and signaling factors which consist of chemical factors and physical factors (bioreactors).
2. Anatomical structure of the osteochondral unit
2.1. Articular cartilage
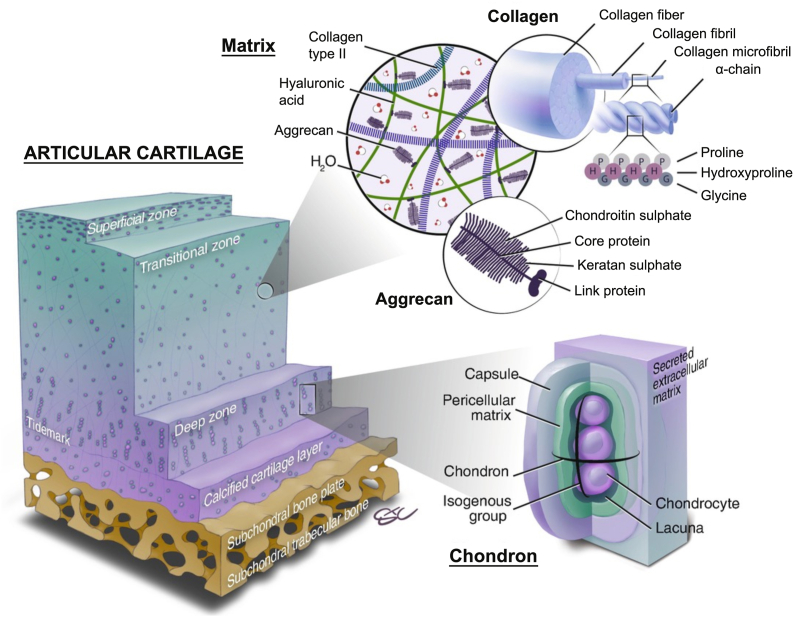
As a tough, durable and flexible form of the supporting connective tissue, cartilage plays the supportive and protective roles in the musculoskeletal system. Devoid of any blood and lymphatic vessels and nerves, it consists of a dense extracellular matrix and the embedded chondrocytes, the primary cell type within. According to the composition of the matrix especially the fibers, cartilage can be classified into three main types: hyaline cartilage, elastic cartilage and fibrocartilage, among which the first is the most common one found in the human body with a semitransparent appearance at fresh state and constitutes the articular cartilage [21,22]. Located on the surface of movable joints, articular cartilage is superficially lubricated and serves as the cushion to lower the friction between adjacent bones, transmit the mechanical loads into the deep subchondral bone plate and facilitate the bone movement. As the primary cell type in the articular cartilage, chondrocytes (<10% of total cartilage volume) are responsible for the synthesis, organization and maintenance of all ECM components. The articular cartilage ECM principally consists of water (68–85% of total wet weight, the main fluid phase), collagen (60–86% of dry weight), proteoglycan (15–40% of dry weight) and some other minor noncollagenous proteins and glycoproteins [23]. The water flow through the cartilage can not only transport the nutrients to chondrocytes but also provide lubrication on the articular surface [24]. The most abundant collagen in articular cartilage is type II collagen (90–95%), forming microfibrils, fibrils and later collagen fibers intertwined with proteoglycan aggregates [[25], [26], [27]]. Other collagens including type I, IV, V, VI, IX and XI are also present in minor proportions and can help increase the structural and elastic strength of cartilage along with type II collagen fiber network [24,28]. Proteoglycans are glycosylated protein monomers composed of a protein core and the covalently bonded negative glycosaminoglycans (GAGs) chains. They can then interact with hyaluronan chains via link proteins to form larger proteoglycan aggregates possessing different GAG compositions and functions [29]. The main proteoglycan type in articular cartilage is aggrecan, while the key GAGs are hyaluronic acid, chondroitin sulfate, keratan sulfate and dermatan sulfate. The GAGs with a high density of sulfate anions in the aggrecan can attract cations in water and offer the articular cartilage with osmotic properties [30]. The collagen fiber network and the attached proteoglycan aggregates collaboratively give rise to the compression resistance of the cartilage, as was biomechanically studied and systematically analyzed by Prof. Mow, who is the first bioengineer proposed the biphasic theory (a solid matrix phase and an interstitial fluid phase) of articular cartilage [31,32]. (Fig. 2).
Fig. 2.
The schematic presentation of the extracellular structure of osteochondral unit and its main individual components including the collagen and aggrecan in matrix, and chondron. Reproduced with permission [8]. 2019, Springer eBook.
Despite the same basic constituents throughout the entire articular cartilage, there are obvious regional variations in the structure and composition concentrations of the matrix as well as the morphology of the chondrocytes along the perpendicular direction: the superficial/tangential zone, the middle/transitional zone and the deep/radial zone (Fig. 2). These three zones make up approximately 10–20%, 40–60% and 30–40% of the articular cartilage thickness separately [33]. From the surface to the depth of the articular cartilage, the content of the proteoglycan aggregates undergoes an increasing trend, the content of the water and the chondrocytes decreases while the collagen fibrils' concentration remains nearly constant [34,35]. In the superficial zone, the type II collagen fibrils are tenuous, tightly packed and parallel to the articular surface, and the chondrocytes exhibit a flattened shape in the interstitial of the fibrils. In contact with the synovial fluid, this zone is vital in maintaining the lubrication and tensile properties of the tissue. Immediately beneath the superficial zone is the largest zone, the middle zone, where the collagen fibrils are thicker and randomly oriented, and the cells are rounder. As an anatomic and functional transition between the other two zones, the middle zone is the first line of defense in resisting the compressive forces imposed on the articulating surfaces. In the deep zone, the collagen fibrils are bunched into tough fibers with the largest diameter perpendicular to the cartilage's surface. The interstitial chondrocytes are oriented parallel to the collagen fibers in a columnar direction and may appear in groups of several cells. This radial zone contributes most of the compressive resistance during body movements [24].
2.2. Calcified cartilage
At the bottom of the deep zone in the articular cartilage, there is a thin wavy basophilic border distinguishing the non-calcified zones from the calcified cartilage below, which is known as the tidemark with a complex three-dimensional structure. The collagen fibers across this junction are continuous with those in the non-calcified and calcified zones. From a biomechanical perspective, the undulating shape of the tidemark provides a geometric pattern to resist the articular shearing. This junction in mature cartilage can also protect the relatively flexible fibers from being sheared at their anchoring point on the subchondral bone beneath the calcified cartilage [36,37]. As a result of endochondral ossification, the calcified cartilage combining both the characteristics of cartilage and bone provides an excellent structural integration to transfer and distribute mechanical loadings between the flexible uncalcified cartilage and the rigid subchondral bone during joint motion [38,39]. Chondrocytes in this zone express a hypertrophic phenotype and can produce type X collagen to calcify the ECM [22]. This chondro-osseous interface including the tidemark and the calcified cartilage is permeable to small nutritional solutes of low molecular weight and plays an important role in maintaining the microenvironment of the two distinct tissues [40].
2.3. Subchondral bone
The subchondral bone, the last region of the osteochondral tissue, is separated from the calcified cartilage by the cement line. Anatomically, it consists of the subchondral cortical bone and subchondral cancellous (or trabecular) bone. The former, also called the subchondral bone plate, is a thin lamella lying immediately beneath the calcified cartilage with low porosity and limited blood vessels. The latter containing randomly aligned supporting trabeculae is more porous, highly vascularized and metabolically active [41,42]. Detailed information on subchondral bone's morphology, function, pathology and its relationship with osteochondral diseases has been comprehensively reviewed by Prof. Madry [41]. The bone tissue is mainly composed of the extracellular bone matrix and three major cells including osteoblasts, osteocytes and osteoclasts. The organic contents in the calcified bone matrix are primarily the collagen fibers, 90% of which is type I collagen, and the amorphous interfibrillar matrix, mostly proteoglycans and their composites such as osteonectin, osteopontin, and osteocalcin. For the inorganic components, nearly 50% of the dry weight of the matrix, calcium hydroxyapatite (HAp, Ca10(PO4)6(OH)2) in the form of needle-like crystal is the most abundant, others including biocarbonate, citrate, magnesium, potassium and sodium ions are also present [21]. Associated with the inorganic minerals that are arranged in a complex hierarchical structure, the collagen fibers are responsible for the ultimate hardness and resistance of the bone tissue [43,44]. As a crucial part of the osteochondral junction, the stiffened and less pliable subchondral bone is shock-absorbing with supportive functions of transmitting the mechanical loads and providing the nutrient supply for the overlaying cartilage [45]. Although it has been long recognized that the degradation of articular cartilage is the primary hallmark of osteoarthritis, microstructural and histopathological changes of the subchondral bone are attracting increasing attention in the progression and pathogenesis of these osteochondral diseases like osteoarthritis. Besides, the highly innervated subchondral bone is probably the main contribution of the pain in disease [7,41].
3. Osteochondral defect classification and clinical treatments
Conducting an epidemiological survey in the general population is faced with a great challenge since that part of the patients with cartilage or osteochondral defects won't consult the doctors because of the lack of obvious clinical symptoms. Although difficult to acquire a thorough knowledge about the overall prevalence of the lesions in articular cartilage, it can be estimated that about 60–70% of all the patients submitted to an arthroscopic procedure are diagnosed with chondral or osteochondral lesions [[46], [47], [48]].
3.1. Defect classification
According to the depth of the lesion, articular osteochondral defects can simply be divided into three types: partial thickness chondral defect, full thickness chondral defect and osteochondral defect. For the first partial thickness chondral defect, the damage is confined only to the articular hyaline cartilage zone without affecting the calcified cartilage. The full thickness chondral defect will cause damage to the calcified cartilage layer, while the defect can be then defined as an osteochondral defect when the subchondral bone is totally exposed and the structure of the whole osteochondral tissue is destroyed [49].
To evaluate the severity of the articular cartilage lesions correctly for appropriate clinical treatment and scientific study, several classification systems have been developed, including the most accepted Outerbridge classification score and the evaluation score system recommended by the International Cartilage Regeneration & Joint Preservation Society (ICRS). The location, depth, size, area of the defects as well as the status of the surrounding cartilage are the primary defect parameters in classification [47]. Outerbridge first described a four grades’ classification system in 1961 according to the macroscopic aspects, depth and extension of the cartilage injury. In Grade I, the softening and swelling of the articular cartilage are present. Grade II denotes the fissuring or fragmentation of the cartilage surface in an area up to half an inch in diameter. Lesions with the same fissuring and cracks as Grade II, but an area more than half an inch are classified as Grade III. Finally, the cartilage lesions eroded down to the subchondral bone would be considered as Grade IV [50,51]. Another five grade classification system recommended by ICRS mainly takes the depth and extent of the lesions into consideration [47,52]. Different classification systems can be proposed with the development of newer imaging technologies, as Prof. Kennedy et al. has summarized for the osteochondral lesions of the talus [53].
3.2. Clinical treatment methods
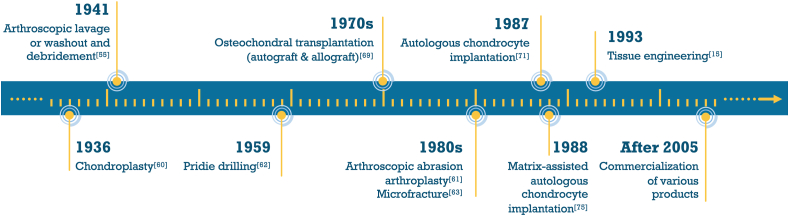
Focusing on the level of the repair for the chondral or osteochondral lesions, clinical treatment methods can be categorized to palliative treatments, reparative treatments and regenerative/restorative treatments. Palliative treatments strive to alleviate the symptoms of the patients like articular pain and usually the replacement of the injured tissue does not happen. Reparative treatments aim at repairing the defect area by stimulating the blood in the subchondral bone to recruit the cells or replacing with some biological materials. However, regenerative methods are the ideal ones that can help restore the defective osteochondral tissue [9]. (Fig. 3).
Fig. 3.
Major milestones in articular cartilage and osteochondral defect treatments.
3.2.1. Palliative treatments
Arthroscopic lavage or washout and debridement are the typical palliative treatments for chondral and osteochondral defects to relieve the knee pain and improve the functional status. As the least invasive surgical technique, arthroscopic debridement needs to wash and suck out all the cartilage or bone debris in the damaged area first and then remove the degenerated meniscus, ligament loose bodies, chondral fragments and the redundant synovia [[54], [55], [56]]. Although arthroscopic washout and debridement were considered to provide a short-term symptomatic relief by reducing the inflammatory response and resecting the pathological structures [57,58], the clinical usage of those procedures is in decline over the last decade since that weak support could be found for the benefit of arthroscopic lavage and debridement [54,59]. With the intent to produce a macroscopically smooth edge without damaging the surrounding cartilage by utilizing mechanical tools or radiofrequency energy, chondroplasty is thought to be palliative as well. Side effects include risks of osteonecrosis, damage to the surrounding cartilage and progression of the partial-thickness lesions [10,60].
3.2.2. Reparative treatments
Reparative procedures including arthroscopic abrasion arthroplasty, Pridie drilling and microfracture seek to realize a spontaneous and natural cartilage repair by promoting the hemorrhage from the subchondral bone, blood clot formation and consequent recruitment of bone marrow cells and fibrocartilage filling. The primary difference between the abrasion arthroplasty and subchondral bone drilling is the extent of the bone and vascularity exposure. As an extension of the debridement, the arthroplasty emphasizes the superficial abrasion and exposure of the viable bone and vascularity [61]. Pridie drilling, first introduced by Dr. Pridie K. H. in 1959, is a modification of debridement to create multiple deep holes into the subchondral bone marrow by a quarter-inch drill [62]. Inspired by the self-healing potential after drilling, microfracture was initially developed by Dr. Steadman in 1980s and then quickly gained ubiquitous clinical applications for patients with full-thickness chondral defects [63,64]. In the procedure, multiple perforations, approximately 3–4 mm apart and 2–4 mm in depth, are made on the exposed subchondral bone plate by a specially designed arthroscopic awl after thorough removal of the overlaying calcified cartilage. Despite the popularity of microfracture as the gold standard for knee cartilage repair and the fact that it is advocated by the FDA and many clinicians for the effective short-term functional improvement, less supportive data are found in the long-term treatment when possible deterioration happened [[65], [66], [67]]. Besides, the common shortcoming of these bone marrow stimulating techniques is the lack of the biomechanical and viscoelastic features of the filled fibrocartilage compared to the natural hyaline cartilage [11].
First introduced in 1970s, the osteochondral transplantation, consisting of autograft and allograft transplantation with various origins of the donor grafts, gained widespread attention in 1990s [68,69]. The donor of the autograft is the lesser-weight-bearing on the same joint or another joint of the patient himself/herself, while that of the allograft is from another person [12]. Both types can be further applied as mosaicplasty utilizing more than one cylindrical graft. Disadvantages of the autografts include the limited origin of the donor, the unmatched mechanical properties and the morbidity of the donor site. Allografts also present serious shortcomings like difficulties in graft preservation and management, risks of disease transmission and immunogenicity [12]. Problems in these technically demanding methods have accelerated the development of new procedures to restore the damaged articular cartilage.
3.2.3. Regenerative treatments
As the clinically recognized regenerative treatment method for cartilage defect, autologous chondrocyte implantation has evolved with several generations. The earliest animal trials for the homotransplantations of isolated chondrocytes in the orthopedic use can date back to 1960s [70]. More than 30 years ago in Sweden, Dr. Peterson and Dr. Brittberg introduced the first cell-based biological approach for full-thickness chondral defect treatment and opened a new era in cartilage repair [14,71]. In this procedure, healthy autologous chondrocytes from the cartilage biopsy samples are isolated from the injured knee during arthroscopy and then cultured in the laboratory, followed by injection into the lesion under a periosteal cover. The long-term results 10–20 years after the surgery of ACI have shown positive outcomes that the mechanically and functionally stable hyaline-like cartilage can be produced and shows integration into the adjacent cartilage and subchondral bone [13,14].
Complications associated with the first-generation ACI like periosteal hypertrophy, complexity of the operation, large-area joint exposure and long recovery time prompted the emergence of the second-generation ACI, when the periosteal patch was substituted by the synthetic collagen membrane to cover the treated defect [72,73]. However, the maintenance of the chondrocyte phenotype in the two-dimensional culture is still critical since that the chondrocyte may dedifferentiate into fibroblasts without the capacity for hyaline cartilage regeneration [74]. For the third-generation ACI, a matrix made of porcine type I/III collagen is used for the in vitro growth of the isolated autologous chondrocytes before the cell seeded matrix is transplanted into the lesion. The so-called matrix-assisted autologous chondrocyte transplantation (MACI) allows for not only the reduced operating time and surgical exposure, but also the enhanced proliferation, phenotype and the even distribution of the chondrocytes [74,75]. Nevertheless, a secondary operation is always necessary for ACI and the high expense warrants further investigation [17]. Moreover, the regenerative efficacy for osteochondral defect where the damage of the subchondral bone is involved is still challenging [12].
Later in the early 1990s, ACI are further improved with the emergence of tissue engineering. Combining the principles of engineering and biology for the functional regeneration, maintenance and improvement of the damaged tissue, tissue engineering consists of three principal components: scaffolds, cells and signaling factors [15]. In 2011, Brian J. Cole et al. reported the first clinical experience treating focal chondral defects in using cartilage autograft implantation system (CAIS), which combined an absorbable scaffold with autologous cartilage fragments based on ACI, and demonstrated its safety, feasibility, and possible effectivity in improving the long-term clinical outcomes [76]. The past few decades have witnessed the booming development in the study of both bone and cartilage tissue engineering [17,77]. In the following parts, a comprehensive summary of the tissue engineering advances in articular cartilage and osteochondral defects will be discussed from different aspects of the three components.
4. Strategies of the scaffolds for cartilage and osteochondral tissue engineering
As a temporary three-dimensional construct to fill the osteochondral defect, the scaffold can structurally and functionally imitate the native osteochondral tissue and provide an appropriate microenvironment for the restoration of the complex tissue. Generally, several requirements from different perspectives need to be considered in designing and fabricating the osteochondral scaffold: (1) Compositionally, the scaffold should be biocompatible with no or little immune rejection and stable physicochemical properties when implanted into the body, and at the same time, be biodegradable with harmless degradation products and a comparable degradation rate to the growth rate of the osteochondral tissue. (2) In regard to the structure, a stratified orientation, a continuous gradient interface as well as a porous structure with an appropriate pore size and porosity are required for the transit of nutrients and wastes, providing an optimal reticular skeleton to maintain a suitable environment for the cell attachment, proliferation and in-growth, and eventual establishment of the damaged tissue. (3) From a functional viewpoint, the ability to maintain the morphology and phenotype of chondrocytes, the biomechanical properties (such as tensile strength, compressive strength and superficial lubrication) to withstand the local stresses and forces, and the integration with the surrounding cartilage and bone should all be taken into consideration. (4) In terms of the preparation, the manufacturing techniques, which are convenient and versatile enough to support the individualized design of the scaffold with a patient-customized geometry, would render the widest acceptance [[78], [79], [80], [81], [82]].
The scaffold is the key factor in osteochondral tissue engineering and at the same time the paramount research focus for material scientists. Since the application of tissue engineering in osteochondral repair, the scaffolds have experienced deeper and more comprehensive research in aspects like compositions, structures, functions and preparation methods. In this review, we will mainly summarize the recent advances of the scaffolds from their compositions and structures.
4.1. Choices for the fundamental material composition
Types of scaffolds’ compositions applied in osteochondral tissue engineering include polymers, inorganic materials, ECM-based materials, metals and the composites of several kinds of the aforementioned materials, among which polymers are the most widely studied ones for biomedical applications because of the largest diversity and unique characteristic similarity to the natural ECMs [83].
4.1.1. Polymers
Polymers, usually presented in the form of hydrogels composed of ECM-mimicking networks, can be further divided into natural polymers, synthetic ones and their corresponding derivatives. The chemical structures, origins, advantages and limitations for cartilage and osteochondral tissue engineering of the commonly used polymers are summarized in Table 1.
Table 1.
Overview of some commonly used natural and synthetic polymers for cartilage and osteochondral tissue engineering.
| Polymer Type | Polymer Name | Chemical Structure | Existence in Osteochondral Tissue and/or Origin | Advantages | Limitations | References |
|---|---|---|---|---|---|---|
| Natural polymers: Polysaccharide | Hyaluronic acid (HA) | 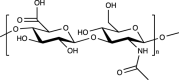 |
Yes. The most abundant GAG in native cartilage | ECM component (vital in the structural and functional maintenance of cartilage: the morphogenesis and proliferation of chondrocytes, formation of proteoglycans and collagen II, water adsorption and retention, lubrication and compression bearing, immune system modulation), easy to be functionalized | Poor mechanical properties, rapid degradation, week cell adhesion | [[84], [85], [86], [87]] |
| Chondroitin sulfate |
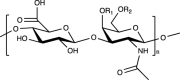 
|
Yes. A sulfated GAG ubiquitous in native cartilage ECM | ECM component (beneficial in reducing pain and functional limitation associated with knee osteoarthritis, anti-inflammatory activity, role in cell recognition and signaling), easy to be functionalized | Poor mechanical properties, rapid degradation | [[88], [89], [90], [91], [92]] | |
| Alginate | 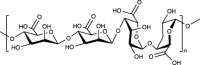 |
No. A natural unbranched negative polysaccharide obtained from brown algae and bacterial sources |
High functionality, fast cross-linking, low cost, injectable for bioprinting, structural similarity to GAGs | Poor mechanical strength, low cell-matrix interaction, varying levels of purity due to source variability, immunogenicity | [83,[93], [94], [95], [96], [97]] | |
| Agarose | 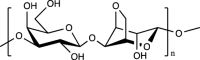 |
No. A natural neutral polysaccharide found in red algae |
High functionality, thermoreversible gelation, low cost, structural similarity to GAGs | Limited mechanical performance, low bioactivity, poor cell attachment | [[98], [99], [100], [101]] | |
| Chitosan | 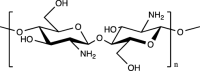 |
No. A chemically partial deacetylated derivative of chitin, mainly exploited from two marine crustaceans, shrimps and crabs |
Intrinsic antibacterial ability, pH and temperature responsiveness, cationic characteristic for the electrostatic interactions with the anionic GAGs in ECM, low cost, structural similarity to GAGs | Poor water solubility in physiological conditions, potential allergenic risks, inferior mechanical properties, low cell-matrix interaction | [[102], [103], [104], [105], [106]] | |
| Gellan gum | No. A linear negatively charged polysaccharide produced by the Sphingononas group bacteria |
pH and temperature responsiveness, structural similarity to GAGs | Weak mechanical strength, poor stability, low bioactivity, relatively high gelation temperature, small temperature window | [[107], [108], [109], [110]] | ||
| Natural polymers: Protein based materials | Collagen |
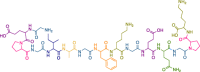 type II collagen type II collagen |
Yes. The most prevalent protein component constituting the ECM | ECM components, good cell-matrix interaction | Potential of immunogenicity, relatively low mechanical strength, high cost, religious issues, limited sterilizability | [25,[111], [112], [113]] |
| Gelatin | 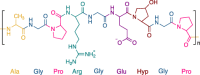 |
Yes. A derivative of collagen by partial hydrolysis with much lower antigenicity | Biologically active for cellular interaction, low immunogenicity in comparison to collagen, ease of processing and functionalization | Poor mechanical properties, rapid degradation, low thermal stability | [98,[114], [115], [116]] | |
| Silk fibroin | 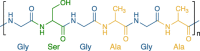 |
No. The major protein component of natural silk |
High mechanical strength, low immunogenicity, structural similarity to collagen, morphologic flexibility, good sterilizability | Source variability, low biodegradability of the β-sheet crystals | [[117], [118], [119], [120]] | |
| Synthetic polymers |
Poly(ethylene glycol) (PEG) Poly(ethylene oxide) (PEO) |
 |
No | Good biocompatibility, versatility in processing and functionalization, mechanical adjustability, low immunogenicity | Biologically inert for cellular interaction, non-biodegradability | [[121], [122], [123], [124]] |
|
Polylactic acid (PLA) Polyglycolic acid (PGA) Poly(lactic acid-co-glycolic acid) (PLGA) |
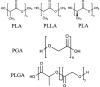 |
No | Good biocompatibility and biodegradability, ease of functionalization, low immunogenicity | Low bioactivity, acidic degradation products eliciting inflammatory response | [[125], [126], [127], [128]] | |
| Polycaprolactone (PCL) | 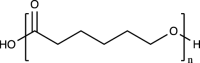 |
No | Relatively low melting temperature for 3D printing, long-term mechanical stability, ease to manufacture | Poor bioactivity, hydrophobicity | [[129], [130], [131], [132]] | |
| Poly(vinyl alcohol) (PVA) | 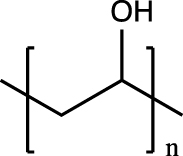 |
No | Good water adsorption and retention ability, chemical resistance, good mechanical properties, ease of aqueous processing | Biologically inert, non-degradability | [[133], [134], [135], [136]] | |
| Poly(l-glutamic acid) | 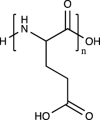 |
No. But its degradation product, l-glutamic acid, is the most abundant amino acid in articular cartilage |
No antigenicity or immunogenicity, good biological and physio-chemical properties, hydrophilicity. | Non-injectability | [[137], [138], [139], [140]] | |
| Poly(propylene fumarate (PPF) | 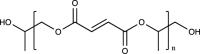 |
No | High mechanical strength, good degradability, biocompatible degradation products, injectability, thermal and photochemical crosslinkability | Deficient bioactivity | [[141], [142], [143], [144]] | |
| Poly(N-isopropyl acrylamide) (PNIPAAm) | 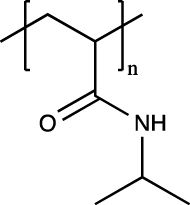 |
No | Thermoresponsiveness over a wide range of ionic strengths and pH, ease of modification | Poor cell affinity | [[145], [146], [147], [148]] |
Natural polymers including polysaccharide (typically hyaluronic acid (HA), chondroitin sulfate, alginate, agarose, chitosan and gellan gum) and protein-based materials (commonly collagen, gelatin and silk fibroin) have acquired widespread explorations due to the structural similarity to native GAGs and the consequent biocompatibility, biodegradability and little inflammatory response. Nevertheless, problems like the difficulty in purification, poor mechanical properties, uncontrollable degradation time and batch-dependent performances remain to be resolved. As the most abundant GAG in native cartilage, HA plays a pivotal role in the structural and functional maintenance of cartilage. To overcome its disadvantages for cartilage and osteochondral regeneration like inadequate mechanical properties and degradability, much effort has been taken through the incorporation of additional polymer networks [149] or by chemical modifications at the hydroxyl and carboxyl functional groups using methacrylate [86], thiol [150], enzyme [151] and amino acid [152]. Chondroitin sulfate is similar with hyaluronan in fundamental structural and biological processes. It has been reported to be beneficial in reducing pain and functional limitation associated with knee osteoarthritis [89] and improving the mechanical and signaling properties in composite materials for cartilage engineering [88,153,154]. Alginate, agarose, chitosan and gellan gum are all natural polysaccharides with similar structures to GAGs and thus have been widely explored in cartilage and osteochondral tissue engineering. Their inferior mechanical strength and low bioactivity are always addressed by combining with other polymers and encapsulating some bioactive molecules [93,100,104,108]. Collagen, as the main protein component constituting the ECM, and its derivative gelatin, are biologically active but are faced with the same drawback of poor mechanical properties. Another popular protein based natural polymer for cartilage and osteochondral restoration that is worth mentioning is silk fibroin. Apart from the structural similarity to collagen, it can be processed into reinforcing fibers and then incorporated into hydrogels to improve the mechanical strength [120,155].
Scaffolds based on synthetic polymers such as poly(ethylene glycol) (PEG) [121], polylactic acid (PLA) [128], polyglycolic acid (PGA) [126], poly(lactic acid-co-glycolic acid) (PLGA) [126], polycaprolactone (PCL) [130], poly(vinyl alcohol) (PVA), poly(l-glutamic acid) [139], poly(propylene fumarate) (PPF) [141] and poly(N-isopropyl acrylamide) (PNIPAAm) [145] have also been extensively reported in osteochondral tissue engineering for their better controllability and reproducibility over the molecular weight, degradation rate as well as industrial scale production. However, the synthetic polymers are faced with challenges like limited bioactivity and complicated structural design and preparation processes. Based on star-shaped PLA, nanofibrous hollow microspheres were fabricated by the group of Prof. Peter Ma as the injectable chondrocyte carriers for knee cartilage repair. The microspheres were designed to mimic the structure of collagen fibers in ECM for cell-material interactions and showed channels/pores at multiple scales with a high porosity for cell migration, proliferation and tissue regeneration [156]. Zhao et al. fabricated an ultra-tough PVA-based hydrogel under physiological conditions through a self-reinforcing mechanism, which exhibited effective cartilage regeneration inducement results through the in vivo evaluation on a rabbit model [136]. PPF-based materials with appropriate mechanical strength, degradation rate and bioactivity are capable of promoting the cell proliferation and differentiation, the mineralization for bone healing and remodeling [143]. Moreover, composites of two or more natural and synthetic polymers have gained an increasing interest in cartilage and osteochondral regeneration because of the advantages of the different polymers can be combined in the composites to achieve comprehensive superiorities that no single biopolymer is comparable to. Gao et al. introduced a hydrogen bond-strengthened supramolecular polymer into the methacrylated gelatin hydrogel and realized a significant improvement in the mechanical properties of the hydrogel. This biohybrid hydrogel scaffold showed positive results in concurrent regeneration of cartilage and subchondral bone in a rat model [114].
4.1.2. Bioceramics
The above-mentioned polymers are mostly designed to mimic the natural hyaline cartilage tissue environment to facilitate cartilage tissue regeneration. Nevertheless, in an attempt to optimize the full chondro-osseous tissue restoration in osteochondral defects where the lesion of subchondral bone is involved as well, the successful engineering of the underlying bone is another key that cannot be neglected. As the most ubiquitous and widely acknowledged type of materials for the replacement and reconstruction of damaged bone tissues, bioceramics have been extensively reported to be incorporated with natural or synthetic polymers and serve as the components in promoting subchondral bone regeneration for their biocompatibility, bioactivity and mechanical strength. They include both crystalline and amorphous inorganic biomaterials from a general perspective such as hydroxyapatite (Ca5(PO4)3(OH)), tricalcium phosphate (TCP, Ca3(PO4)2), calcium silicate and amorphous bioactive glass (BG) [[157], [158], [159]]. Studies on the bioceramics with the simultaneous promotion for restoring cartilage and subchondral bone are rare but still available [160,161].
HAp, the major component of the natural bone tissue, is particularly attractive and beneficial as an additive material for engineering of bone and bone-related tissues including the articular osteochondral unit for its intrinsic bioactivity [108,129,[162], [163], [164]]. Zhou et al. generated a stratified graft composed of collagen upper layer and collagen/HAp lower layer. The different promotion superiority on mesenchymal stem cells (MSCs) of the two layers was demonstrated that the lower collagen/HAp layer was more efficient in promoting the osteogenic induction than either the collagen layer or pure HAp [165].
TCP is another common member of calcium phosphate, among which β-TCP is the most popular in bone tissue engineering because of its lower formation temperature and better structural, mechanical and biological performances [158]. With the similar chemical composition, structure and bioactivity to the natural bone mineral component, TCP is also popular as the osseous compartment in the osteochondral scaffold [[166], [167], [168], [169], [170]]. Deng et al. incorporated the manganese (Mn) into β-TCP for regenerating osteochondral defects, and found that the Mn-doped TCP scaffolds promoted not only the proliferation and differentiation of the embedded chondrocytes and MSCs, but also the regeneration of both cartilage and subchondral bone tissues from in vivo results [171].
Calcium silicate is a simple silicate bioceramic with osteogenic and angiogenic abilities and the released silicon ions were recognized to play a vital role [172,173]. Most other silicate ceramics which have been explored in bone and osteochondral tissue engineering are complex in chemical compositions containing various ions besides Ca2+ and Si4+ [174,175], for instance, Li+ [160], Sr2+ [161,176,177], Mg2+ [[178], [179], [180]]. Chen et al. 3D printed an osteochondral scaffold using pure-phase lithium calcium silicate bioceramic. In addition to the good mechanical strength and biodegradability, dual bioactivities for the regeneration of both cartilage and subchondral bone simultaneously in rabbit osteochondral defects were demonstrated [160].
BG has experienced an expanded development in the compositions, hierarchical structures, synthesis and performance for orthopedic applications since the first discovery by Hench in 1971, and the well-known 45S5 Bioglass® is composed of SiO2, CaO, Na2O and P2O5 [[181], [182], [183]]. Able to bound rapidly with the living bone and stimulate the osteoprogenitor cells at a genetic level, BG is attracting growing interest in regenerating bone and subchondral bone tissues [[184], [185], [186], [187]]. Zhu et al. reported a continuous stratified sodium alginate construct in which the BG was blended as the subchondral bone layer. The osteoblast differentiation of bone marrow mesenchymal stem cells (BMSCs) in this layer was proved from the upregulated gene expression results of the alkaline phosphatase and collagen I [187].
4.1.3. Metals
Metals and their alloys are another suitable candidate as bone implants with a long application history, the mostly used ones of which include titanium, titanium alloy, tantalum and cobalt based alloys on account of their good mechanical strength and biocompatibility [[188], [189], [190], [191]]. Porosity is often introduced, not only for a better ingrowth of the bone tissue, but also to alleviate the bone resorption and even implant failure caused by the stress shielding as a result of the much larger stiffness than native bones [192]. Bearing the recognition that the mechanical support from the subchondral bone plays an essential part in articular cartilage protection [193], researchers have investigated the effects of employing the metallic materials as the bony phase on osteochondral regeneration [[194], [195], [196], [197], [198]]. Bal et al. compared the in vivo chondral defect repairing outcomes of porous tantalum, bioglass and allograft bone cylinders and found that the tantalum was superior to the allograft in host bone integration, hyaline-like tissue regeneration and type II collagen expression [194]. Whereas, the lack of biodegradability of metals has been one of the main obstacles on their way to a wider acceptance and application [195]. Besides, metals can hardly mimic the articular cartilage tissue in osteochondral units.
4.1.4. ECM-based materials
Despite the tremendous progress that has been achieved to fabricate tissue engineering scaffolds with biomaterials simulating the native osteochondral tissue in the field of materials science, these materials can hardly mimic the tissue's microenvironment in full. Extracellular matrix, the natural template for the biomaterials with a complex 3D environment, offers another possibility [199,200]. Composed of various collagens, proteoglycans and the incorporated multiple biochemical and biophysical signal factors, ECM not only serves as a physical framework to support the structural integrity of the organism, but also delineates specific microenvironment for the survival, organization and differentiation of the resident cells [201]. Given the complexity and difficulty in totally mimicking the highly sophisticated native ECM with comparable composition and architecture, decellularization of whole tissues or organs by removing all cells and genetic material while maintaining the physical and biochemical characteristics has emerged as a possible alternative method for cartilage and osteochondral repair [[201], [202], [203]]. The resulting decellularized extracellular matrix (dECM) can be further processed to serve as a whole scaffold [[204], [205], [206], [207], [208]], a solubilized hydrogel component [123,[209], [210], [211]], a bioink for additive manufacturing [[212], [213], [214], [215], [216]] or pulverized particles for composite scaffold fabrication [205,[217], [218], [219]]. Lin et al. produced a biphasic decellularized osteochondral ECM scaffold by combining physical freezing-thawing methods and chemical treatments. The rabbit model results after 12 weeks surgery demonstrated that the scaffold with low immunogenicity and high bioactivity promoted the osteochondral regeneration dependent on the stratified constituents [208]. Limited standards and guidelines for the decellularization procedures and poor reproducibility are the primary challenging problems that remain to be solved in the future.
4.2. Architectures of the scaffolds
To successfully construct an ideal scaffold for the regeneration of cartilage or osteochondral defects, the architecture is another crucial factor in addition to the materials’ composition. The past decades have witnessed the development of osteochondral scaffolds in tissue engineering from the simplest monophasic scaffolds to biphasic, triphasic and multiphasic ones. In recent years, gradient scaffolds have attracted increasing attention in order to achieve a more sophisticated mimicry of the natural heterogeneity found in native osteochondral tissue.
4.2.1. Monophasic scaffolds
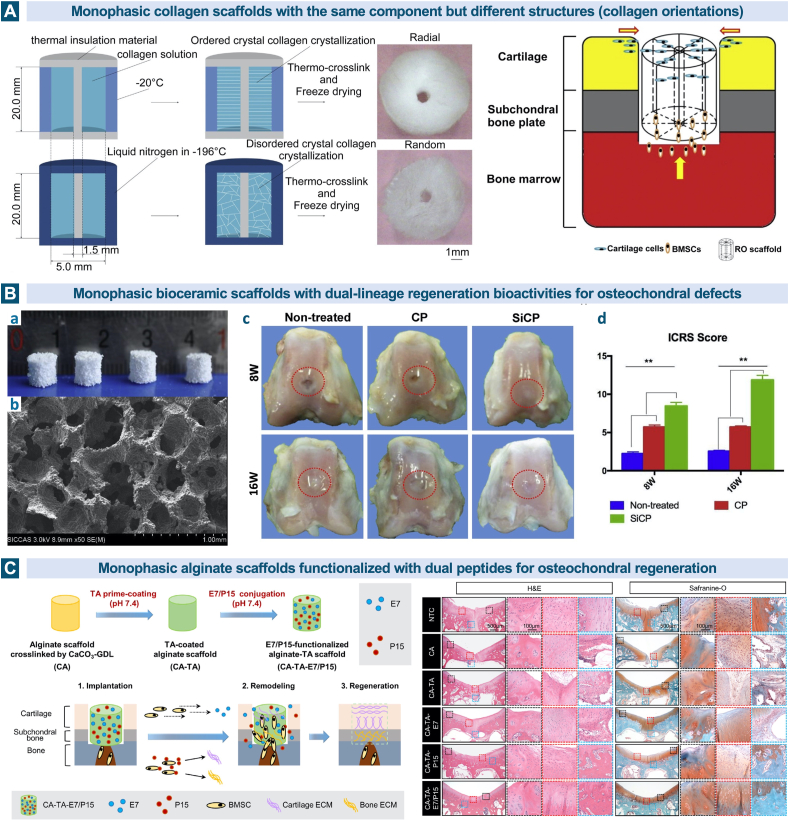
Containing a uniform material composition and structure, single phased scaffold is one of the first explored strategies for its simplicity in processing [78]. Although consistent throughout the whole scaffold, different component combinations of varying natural and synthetic materials mentioned in the sections before [179,220,221] as well as the structural designs including macroscopic shapes (hydrogels, fibers et al.), pore size and pore topology [[222], [223], [224], [225], [226]] can induce distinct efficacy for tissue restoration. Chen et al. designed two kinds of monophasic collagen scaffolds with radial and random orientation separately and measured the effects on in vitro MSCs migration and in vivo osteochondral defect regeneration. The radially oriented scaffolds with aligned channels horizontally and vertically performed better in both cell migration promotion and osteochondral defect healing results [222]. (Fig. 4A).
Fig. 4.
Monophasic scaffolds in osteochondral tissue engineering. A. The radially oriented monophasic collagen scaffolds with aligned channels horizontally and vertically can promote the cell migration and osteochondral defect healing. Reproduced with permission [222]. 2015, Elsevier. B. The silicon-based bioceramic monophasic scaffold has dual-lineage regeneration bioactivities for osteochondral defects. (a) Optical images of the SiCP scaffolds, (b) Scanning electron microscope (SEM) image of the SiCP scaffold, (c) gross images of the osteochondral defects in the three groups at 8 and 16 weeks post-operation, (d) ICRS scores for the three different groups. Reproduced with permission [174]. 2019, Elsevier. C. The dual E7/P15 peptide-functionalized monophasic scaffolds mediated by tannic acid (TA) enhanced the recruitment of BMSCs and promote simultaneously the regeneration of cartilage and subchondral bone. Reproduced with permission [228]. 2020, Elsevier.
In spite of the universally accepted recognition about the poor structural similarity between monophasic scaffolds and the native stratified osteochondral tissues, several recent studies have reported appealing findings about the concurrent regeneration of cartilage and subchondral bone in the rabbit model using these seemingly simple scaffolds [160,161,171,174,177,184,227,228]. The released ions of Mn2+, Sr2+, Si4+, Li+, Cu2+ from the bioceramics were demonstrated to play an important role in the related inflammatory and regeneration process [160,161,171,174,177,184]. Bunpetch et al. fabricated a silicon-based bioceramic (silicon-calcium-phosphate, SiCP) monophasic scaffold with dual-lineage regeneration bioactivities for osteochondral defects. To investigate the efficacy and underlying mechanisms, micro-CT, qPCR, western blot and pathway analysis of RNA-sequencing were conducted, and the significant role of Si ions was ultimately validated [174]. (Fig. 4B) Zhang et al. prepared the dual peptide-functionalized monophasic scaffolds mediated by tannic acid and also presented the satisfactory biological performance in MSCs recruitment and simultaneous regeneration of cartilage and subchondral bone [228]. (Fig. 4C) Although the study of monophasic scaffolds can provide useful information about the effect of a specific composition or structure on tissue reconstruction, the majority of these attempts are far from the requirements to regenerate osteochondral lesions efficaciously.
4.2.2. Biphasic scaffolds
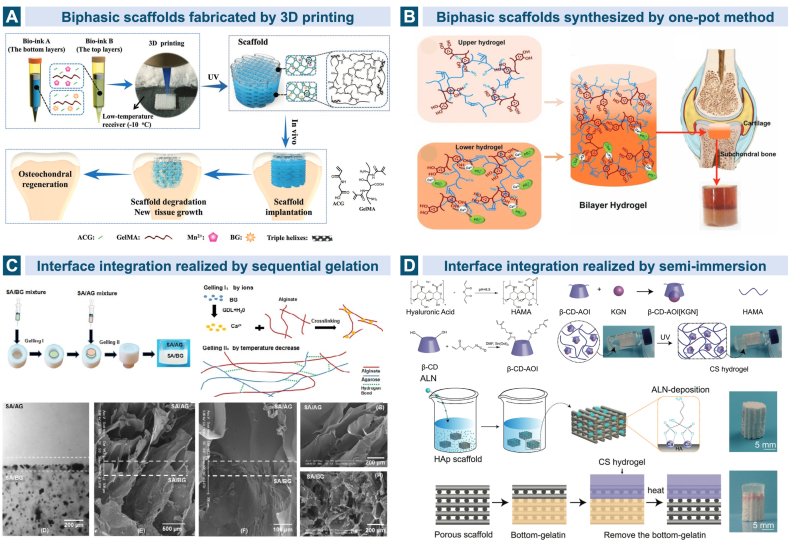
The natural coexistence of the articular cartilage and subchondral bone as the two major lineages in the osteochondral unit accounts for the widely acknowledged bioinspired strategy of biphasic scaffolds with two different regions resembling the stratified anatomical architecture. Numerous biphasic scaffolds have progressed into the preclinical animal studies and a few are even commercially available for clinical utilization now [49]. Getgood et al. compared the in vivo morphological, histological and mechanical performance of a collagen-GAG-calcium phosphate biphasic scaffold (now commercially available as ChondroMimetic®) and a PLGA/PGA/calcium sulfate bilayered scaffold (commercially known as TruFit™). The early stage data after 26 weeks implantation in the osteochondral defects in goats revealed that the former scaffold based on collagen-GAG showed a better environment for tissue regeneration [229]. Generally speaking, the upper cartilaginous layer prefers hydrogels based on natural or synthetic polymers due to their hydrated nature and viscoelastic similarity to the native ECM, while the lower calcified subchondral layer favors more strengthened materials such as bioceramics, metals and harder polymers. A complex mixture of several kinds of biomaterials along with varying resident cells and biological molecules is common in designing both layers [165,170,187,[230], [231], [232], [233], [234]]. Cell-free scaffolds which can promote the chondrogenic and osteogenic differentiation of BMSCs and ECM deposition are popular as well [108,114,166,[235], [236], [237], [238], [239]]. (Fig. 5A and B).
Fig. 5.
Biphasic scaffolds for osteochondral tissue regeneration. A. The bilayered cell-free gelatin scaffold was reinforced by high-strength supramolecular polymer and the top and bottom layers were separately loaded with Mn2+ and bioglass [114]. B. Gelatin methacryloyl was mixed with polydopamine (PDA) in the upper layer, while in the lower layer the HAp was mineralized in situ for subchondral bone repair. Reproduced with permission [236]. 2019, Wiley. C. The stratified biphasic scaffold based on sodium alginate (SA), agarose (AG) and bioglass (BG) formed a continuous interface integration by utilizing the common SA network. Reproduced with persmission [187]. 2018, Elsevier. D. Two stem cell differentiation inducers, kartogenin and alendronate, were incorporated separately into the hyaluronic acid-based cartilage layer and the HAp-based bone layer in order to promote the differentiation of MSCs into chondrocytes and osteoblasts. The two layers were bound by semi-immersion. Reproduced with permission [234]. 2020, Wiley. Triphasic and multiphasic scaffolds involving the calcified cartilage simulation.
Interface integration between the chondral and bony layers in the engineered osteochondral unit is of importance in designing the bilayered but monolithic scaffold. Poor integration may lead to the delamination and final failure in tissue regeneration [169,240]. Zhu et al. used the sodium alginate (SA)/agarose composite hydrogel containing BMSCs and articular chondrocytes as the layer for cartilage reconstruction while fabricated SA/bioglass hydrogel with only BMSCs for subchondral bone regeneration. The continuous SA phase provided a stable integration in the stratified structure through crosslinking. Hyaline cartilage and subchondral bone were observed to be stimulated simultaneously after the injection of the stratified scaffold into the rat model [187]. (Fig. 5C) Liu et al. incorporated two stem cell differentiation inducers, kartogenin and alendronate, separately into the HA-based cartilage layer and the HAp-based bone layer which were bound by semi-immersion. The MSCs in the biomimetic biphasic scaffold can differentiate into both chondrocytes and osteoblasts, promoted by the layer-specific release of the two drugs [234]. (Fig. 5D) However, the calcified cartilage, the natural chondral-osseous interface which is a primary determinant in maintaining the microenvironment of the two distinct tissues is ignored in biphasic scaffolds.
4.2.3. Triphasic and multiphasic scaffolds involving the calcified cartilage simulation
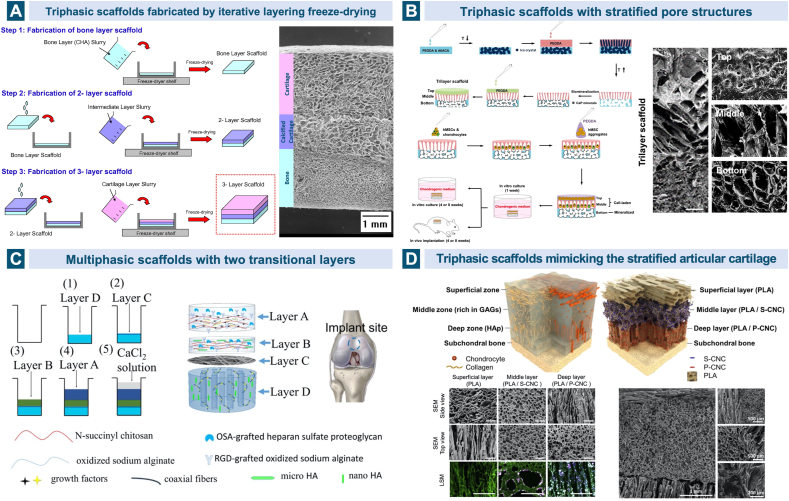
Taking the significance of the calcified interface in native osteochondral tissue into account, researchers have made considerable advances in fabricating scaffolds with a closer resemblance to the osteochondral physiological structure. Over ten years ago, Tampieri et al. developed a biomimetic mineralization process of nucleation of HAp nanocrystals onto the collagen fibers during their self-assembling and then generated a trilayerd scaffold based on HA, type I collagen and HAp. Apart from the upper cartilaginous layer made of hyaluronan-charged collagen and the lower subchondral bone layer composed of the biomineralized collagen (70 wt% HAp), an intermediate region with the same biomineralized collagen but a lower percentage of mineral (40 wt% HAp) was incorporated to resemble the calcified zone [241]. The nano-composite triphasic scaffold was later demonstrated to exhibit good mechanical and biological behavior in treating the chondral and osteochondral lesions in horse and sheep models as well as clinical trials, which is now commercially available as MaioRegen® for clinical applications [[242], [243], [244], [245], [246]]. Levingstone et al. fabricated a triphasic scaffold through a “iterative layering” freeze-drying technique using similar basic materials of HA, type I collagen and HAp, but type II collagen was added in addition in the intermediate and upper layers, imitating the native cartilaginous components. Macroscopic, micro-computed tomographic and histological observations showed that the scaffold was able to restore the osteochondral defects in a relatively long term, 6- and 12-months post implantation in caprine joints [112,247,248]. (Fig. 6A).
Fig. 6.
Triphasic/multiphasic scaffolds in cartilage and osteochondral tissue engineering. A. A triphasic scaffold was fabricated through a “iterative layering” freeze-drying technique using similar basic materials of hyaluronic acid, type I collagen and HAp, but type II collagen was added in addition in the intermediate and upper layers to imitate the native cartilaginous components. Reproduced with permission [247]. 2014, Elsevier. B. The single-unit scaffold based on PEGDA consists three layers with depth-varying pore architecture, mineral and cell environment: the hydrogel top layer, the cryogel middle layer with anisotropic pore structure and the biomineralized bottom layer. Reproduced with permission [250]. 2018, Elsevier. C. Based on composite materials of SA, chitosan and HAp with different micro and nano sizes, an intermediate calcified cartilage layer as well as a subjacent electrospun fibre membrane for cell migration prevention was designed in the four-layered scaffold to repair osteochondral defects. Reproduced with permission [162]. 2018, Elsevier. D. Multiphasic scaffold fabrication strategy was applied in mimicking the complex stratified architecture of articular cartilage structurally, chemically and mechanically. Reproduced with permission [253]. 2015, Elsevier. Multiphasic gradient and continuous gradient scaffolds.
The impact of the intermediate layer in the osteochondral scaffold on tissue regeneration has been studied by Da et al. both in vitro and in vivo. Compared with the biphasic scaffold without the compact intermediate layer fabricated from PLGA and β-TCP, the compact layer-containing scaffold exhibited significantly higher anti-tensile and anti-shear properties as well as better in vivo regeneration results including macroscopic scores, the content of GAG and collagen and the histological properties of the neo-tissue [249]. The compact layer mimicking the calcified cartilage functioned as not only a connector to withstand the stresses within the joint and enhance the integration between the other two zones, but also an insulator to inhibit the adverse infiltration and provide the optimal independent microenvironment [249]. The bone-cartilage interface has developed to be one of the major research projects in designing a biomimetic scaffold for osteochondral tissue engineering, and the adopted materials are always a combination of those used in chondral layer and bone layer with a specific proportion. Stratifications in the content of minerals, and porosity and pore size are common approaches [129,162,[250], [251], [252]]. (Fig. 6B) Chen et al. fabricated a single integrated scaffold with a multi-layered functional structure, concentrating on the transitional zone between the uncalcified cartilage and the subchondral bone. Based on composite materials of SA, chitosan and HAp with different micro and nano sizes, the four-layered scaffold contained an intermediate layer simulating the calcified cartilage as well as a subjacent electrospun fiber membrane to prevent the cell migration and vascular invasion [162]. (Fig. 6C).
Given the fact that the articular hyaline cartilage consists of three distinct zones (the superficial, the middle and the deep zones) with varying structures and compositions, the strategy of triphasic scaffold has also been employed in cartilage tissue engineering [[253], [254], [255]]. Camarero-Espinosa et al. designed a trilayered scaffold that can mimic the mature articular cartilage structurally, chemically and mechanically. PLA was selected as the same basic biopolymer for all three layers but in various pore orientations, while the middle and deep layers were incorporated with sulfated and phosphated cellulose nanocrystals (CNCs) respectively on account of their mechanical reinforcement and cell growth promotion capabilities. The sulfated CNCs in the isotropic porous middle layer were thought to be well representing the native sulfated GAGs and the phosphate modification groups were chosen to promote the localized formation of HAp [253]. (Fig. 6D).
4.2.4. Multiphasic gradient and continuous gradient scaffolds
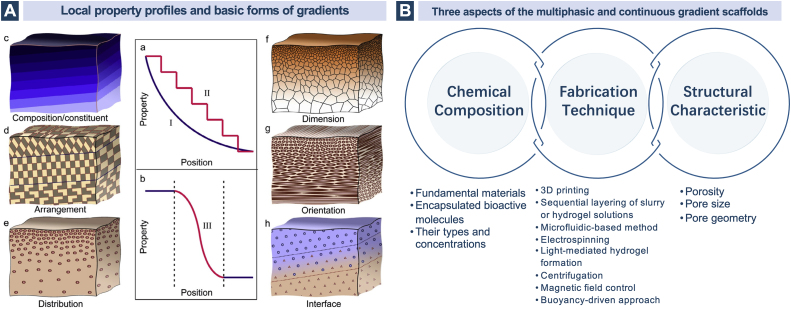
The native osteochondral tissue possesses a much more complicated gradient heterogeneity instead of a straightforward stratification of three separate regions. To imitate this natural paradigm and translate such design motif into bioinspired applications in osteochondral tissue engineering, multiphasic discrete gradient (more than three layers in this section) and continuous gradient scaffolds in a stepwise mode and a gradual manner respectively have been exploited to achieve gradients in a broad scale throughout the entire construct or within a limited interface region. Gradient scaffolds consistently perform superior to monophasic and biphasic ones in regenerating osteochondral defects [256,257]. The gradients can be described in terms of the variations of the basic units in chemical compositions and structural characteristics which further include several basic forms in arrangement, distribution, dimension and orientation [258]. (Fig. 7) The combined incorporation of different patterns of chemical and structural gradients in a monolithic osteochondral scaffold have been explored in orthopedic research as well.
Fig. 7.
A. The local property profiles and basic forms of gradients. Reproduced with permission [258]. 2017, Elsevier. B. The three aspects of the multiphasic and continuous gradient scaffolds.
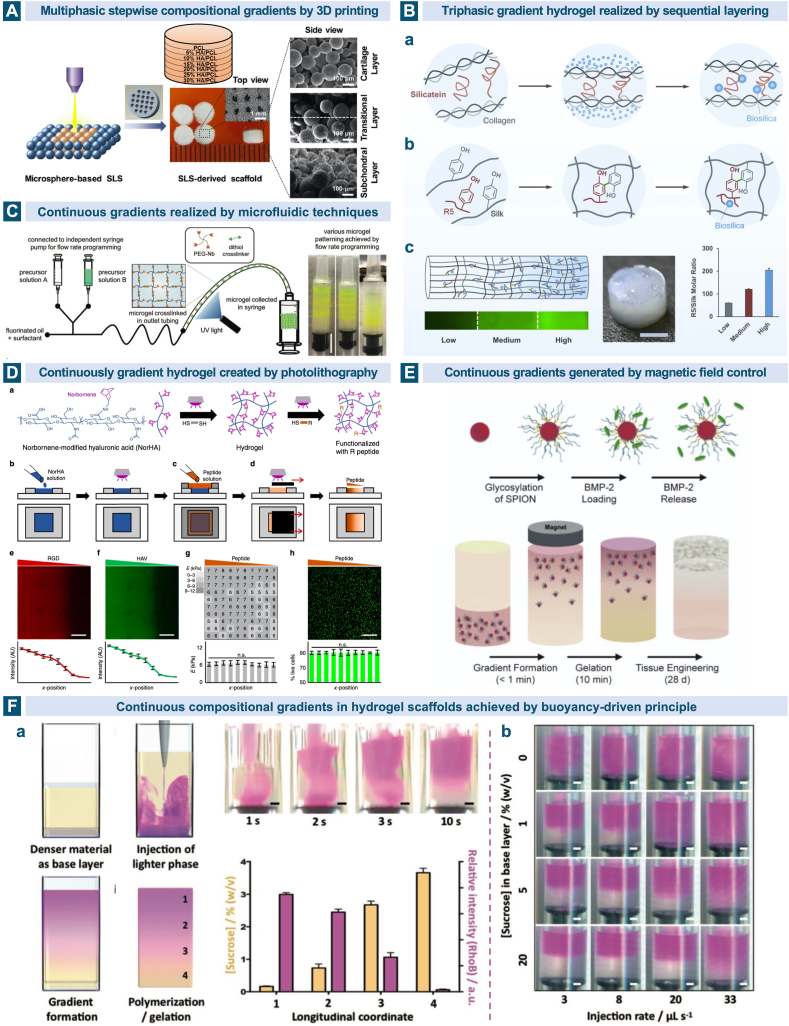
Various techniques have been developed to incorporate multiphasic and continuous gradients into the cartilage and osteochondral scaffolds such as 3D printing especially extrusion printing and selective laser sintering [130,224,259,260], sequential layering of slurry or hydrogel solutions at partial gelation [257,[261], [262], [263]], microfluidic-based method [[264], [265], [266], [267], [268], [269], [270], [271]], electrospinning [272,273], light-mediated hydrogel formation [274,275], centrifugation [[276], [277], [278]], magnetic field control [279] and buoyancy-driven approach [280]. Chemical compositional gradients involve the changes in the fundamental materials and the encapsulated bioactive molecules. An acellular seven-layered gradient scaffold consisting of basic building blocks of PCL and HAp microspheres was prepared through selective laser sintering technique. From the top cartilage layer to the bottom bone layer, the content of HAp was increased from 0 to 30% with 5% increments. The in vitro cellular evaluation and in vivo implantation results verified its capability in inducing the formation of articular cartilage and subchondral bone tissues [130]. (Fig. 8A) With regard to the hydrogel-based scaffolds, sequential addition of different solutions into a cylindrical container layer by layer before the complete gelation allows for the formation of a gradient interface. Utilizing the silk protein-based composites coupled with biosilica selective peptide-R5, Guo and coworkers fabricated a bioinspired gradient protein/biosilica analog by layering three regions with high, medium and low concentrations of the R5 peptide along the longitudinal direction. This gradient silicified silk/R5 system showed continuous transitions in composition, structure and mechanical properties, and could promote the osteogenic differentiation of MSCs in vitro in a gradient manner [262]. (Fig. 8B) Microfluidic techniques for the synthesis of gradient materials on a miniaturized scale rely on their precise control of the fluid flow. Xin et al. produced continuous physicochemical gradients in the microporous annealed particle hydrogels by combining the microfluidic mixing and droplet generator modules. The flow rates of two distinct precursor solutions through two syringe pumps were program-controlled and the continuous gradient profiles were detected in the fluorescent images when the layer thickness was adjusted to 1, 3 and 6 microgels. The findings that MSCs embedded on the hydrogels exhibited differential spreading and proliferation trends along the gradient provided a potential for the hydrogels to facilitate repair of osteochondral defects when a smooth transition from osteogenic to chondrogenic regulations were designed [270]. (Fig. 8C) Gradients created by photolithography can be realized through the manipulation of cross-linker concentration, applied wavelength and duration of irradiation [256]. By moving the opaque sliding mask below the UV light across the exposed hydrogel at a constant rate, continuous peptide gradients were achieved through the thiol-norbornene chemistry in the photocrosslinked HA-based hydrogel. Chondrogenesis of the encapsulated MSCs indicated by the Sox9 and aggrecan expressions showed a spatial variation along the hydrogel's concentration gradient [275]. (Fig. 8D).
Fig. 8.
Multiphasic gradient and continuous gradient scaffolds for osteochondral tissue regeneration. A. Through selective laser sintering technique, multilayered gradient scaffold was prepared from the building blocks of PCL and HAp microspheres, with the HAp content increasing from 0 to 5%, 10%, 15%, 20%, 25% and 30% from the top to the bottom. Reproduced with permission [130]. 2017, Elsevier. B. Design strategies of the gradient composite scaffold. (a) The formation of biosilica particles on the fibrous network of collagen, also known as the in vivo silicification, is mediated by the self-assembled silicatein, (b) biosilica selective peptide R5 was utilized to mimic the natural biomineralization process and deposit biosilica particles on the silk templates, (c) the gradient composite scaffold with gradually increasing concentration of the R5 peptide from the top to the bottom. Reproduced with permission [262]. 2017, Elsevier. C. A microfludic device with a Y-shaped mixing module and a T-junction droplet generator module was used to create different patterns of microgels including the continuous physicochemical gradient one. Reproduced with permission [270]. 2019, Wiley. D. Biochemical peptide gradients in the HA based hydrogel was achieved by controlling the UV light exposure time using an opaque sliding mask [275]. E. The glycosylated superparamagnetic iron oxide nanoparticles conjugated with heparin can sequester and release growth factors and finally create a gradient biochemical pattern under an external magnetic field in the agarose hydrogel to form the gradient engineering osteochondral tissue [279]. F. The casting process of the buoyancy-driven gradient scaffold. (a) After the injection of the purple phase at a controlled rate, the system was allowed to equilibrate and form a gradient by polymerization. (b) By changing the injection rate and the sucrose concentration in the base layer, the pattern of the gradient could be easily modulated [280].
Magnetic field has also been exploited as a facile strategy for generating functional gradients in hydrogels. Under the mediation of the external magnetic fields above the mold, the glycosylated superparamagnetic iron oxide nanoparticles loaded with growth factors aligned gradient in the agarose hydrogel in less than 1 min, accounting for the smooth longitudinal gradients of the growth factor concentration. The engineered osteochondral tissue constructs cultured in vitro presented a tidemark transition separating the mineralized subchondral bone/calcified and non-mineralized hyaline cartilage and an interface rich in hypertrophic chondrocytes indicated by type X collagen [279]. (Fig. 8E) Later, the same group of Li et al. developed a more universal approach based on the fundamental physical principle of buoyancy to generate gradient materials in several different systems including gelatin methacryloyl, gellan gum, agarose and acrylate polymers, and a wide range of cargos such as liposomes, nanoparticles and small molecules. A gradient distribution of bone morphogenetic protein 2 in a gelatin methacryloyl hydrogel was then produced as an example to realize integrated osteochondral tissue constructs [280]. (Fig. 8F).
The structural characteristics including porosity, pore size and pore geometry have widely been recognized as important parts of the environmental cues that influence the migration and differentiation of cells as well as the tissue growth within the scaffolds [281,282]. Designing scaffolds with a structural gradient imitating the native osteochondral units is also an appealing strategy for osteochondral defect treatments. By using a centrifugation method, PCL scaffolds with a pore size gradient from 90 μm to 400 μm approximately along the longitudinal direction were prepared. The in vitro study revealed that the sectioned scaffold with the pore size ranging from 370 to 400 μm were more appropriate for the chondrogenic differentiation of adipose stem cells [276]. Di Luca et al. 3D printed a multiphasic pore size gradient scaffold and evaluated the effect on the proliferation and differentiation of the seeded MSCs and the ECM deposition. As the pore size decrease in the gradient scaffold, the gradual increases of chondrogenic markers and ECM deposition were found [259]. They later fabricated the 3D scaffolds presenting a stepwise pore shape gradient by changing the fiber deposition patterns of two consequent layers. Among the four various patterns with separate angles of 15°, 30°, 45° and 90°, squared pores were observed to provide a more favorable environment for chondrogenic differentiation of the embedded MSCs whereas the pores with a rhomboidal shape support a better osteogenic differentiation when the constructs were cultured under osteochondral conditions in vitro [224]. Despite the difficulty in achieving continuous structural variations, the creation of those multiphasic structural gradient scaffolds offers another strategy to obtain an accurate mimicry of native tissues and also serves as a great tool for improving our understanding of the interactions between cells and biomaterials with different pore structures.
The multiphasic gradient and continuous gradient scaffolds fabricated by emerging new technologies and traditional methods have been achieved both in chemical compositions and structural characteristics, as have been summarized in this part. However, the studies on developing gradient scaffolds mimicking the osteochondral heterogeneities in anatomical, biological, physicochemical and mechanical properties are still limited and in the infancy.
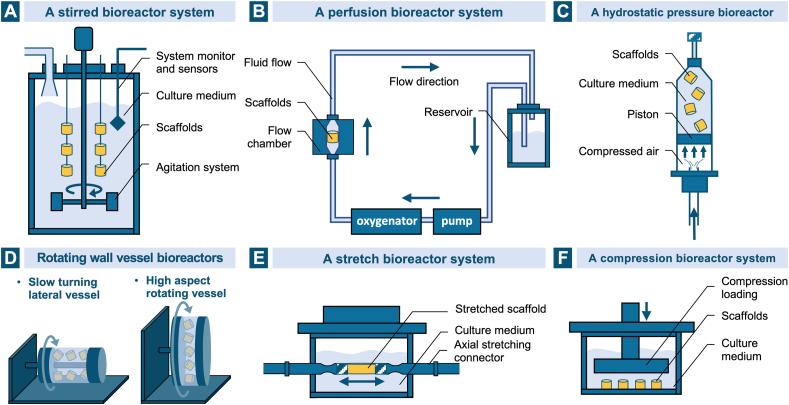
5. Other key elements in cartilage and osteochondral tissue engineering
5.1. Tissue forming cells
The addition of cells with the potential of chondrogenic and/or osteogenic differentiation in the scaffolds is ubiquitous in the field of tissue engineering for the creation of an engineered osteochondral unit. By influencing the interaction between the adjacent native tissue and the artificial scaffold, the incorporated cellular components are universally considered to improve the outcome of ECM deposition and tissue regeneration. Unlike the diversity of biomaterials used to build a biomimetic 3D architecture in osteochondral tissue engineering, limited cell populations are available, including those existed in the host tissue such as chondrocytes, chondroprogenitor cells and osteoblasts, and stem cells with multipotency or pluripotency especially BMSCs, adipose stem cells (ASCs), embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs).
As the main cell type resident in articular cartilage and responsible for its structural and functional maintenance, chondrocytes are one of the most widely used cell types in restoring chondral and osteochondral defects. It has been observed that chondrocytes embedded in a wide range of biomaterials could maintain their morphology and phenotype and promote hyaline cartilage formation [107,117,217,283]. The proliferation and differentiation of chondrocytes not only vary in scaffolds with different compositions, but also can be influenced by the architecture of the scaffolds [284,285], including structural form [286], pore size [287,288], pore geometry [289], fiber orientation [290] and fiber dimensionality [291]. On the other hand, the encapsulation of chondrocytes can also alter the properties of the hydrogel scaffold by interacting with the precursors and then reducing the crosslink density [121]. Nevertheless, chondrocytes are faced with several notable challenges like low isolation efficiency, limited proliferation potential and common dedifferentiation phenomenon in culture [18,292]. For osteochondral defect repair, chondrocytes in the cartilage layer have been used in combination with preosteoblasts in the subchondral layer to mimic the different environment in the two regions [251]. In an effort to solve the limitation of cell isolation from the non-load bearing region of articular cartilage in traditional strategy, nasal septum and auricle have been reported to be alternative cell sources of chondrocytes for articular surface reconstruction in osteochondral lesions [[293], [294], [295]]. Apart from culturing the cells in a three-dimensional scaffold, other effective methods to improve the proliferation capacity and maintain the chondrogenic phenotype in vitro include the addition of bioactive molecules and the utilization of bioreactors, which will be introduced in the following sections.
The use of stem cells is another potential solution to avoid the drawbacks associated with articular chondrocytes. Among the various types of stem cells, MSCs especially those derived from the bone marrow are the most widely studied ones in osteochondral tissue engineering due to their advantages like relative ease of isolation and proliferation, ability to differentiate into cells of both chondrogenic and osteogenic lineages, properties of low immunogenicity and no ethical concerns [296,297]. It is worth mentioning that Caplan, the author of the paper first using this term, and some other researchers have recently called for a name change of mesenchymal stem cells to more precise labels in order to reflect the function more accurately and clear up the mess [298,299]. The encapsulation of BMSCs in the scaffolds have been broadly studied in various materials for cartilage and osteochondral regeneration [149,154,174,190,234,270]. Zhou et al. have evaluated the proliferation and differentiation of BMSCs into chondrocytes and osteoblasts on the two separate layers of the collagen-HAp biphasic scaffold in the presence of chondrogenic and osteogenic chemical factors [165]. The chondrogenesis and hypertrophy of BMSCs are dependent on the type of material [105], and the induced lineages can also be influenced by the scaffold's architecture with different pore sizes, interconnectivities, and the engendered different metabolic environments [300]. Co-culture of BMSCs and chondrocytes in tissue engineering scaffolds has also been explored to enhance the cartilage regeneration, when the chondrogenic phenotype of chondrocytes can be maintained and the chondrogenic differentiation of BMSCs can be promoted [187,301]. Other sources of MSCs include adipose tissue, muscle, umbilical cord and etc. [297], among which ASCs isolated from lipoaspirates are the most readily accessible ones. They possess higher proliferation potential and larger abundance than BMSCs [302] and have showed the ability of both chondrogenesis and osteogenesis in a variety of biomaterial environments in increasing reports [98,[303], [304], [305], [306]]. However, their chondrogenic potential is relatively lower than BMSCs and the optimal culture system as well as the effect of scaffold architectures on the cell differentiation remain to be identified in the future [18,307].
The high hypertrophic tendency of MSCs under the chondrogenic stimuli has urged the investigation of stem cells of other sources for cartilage and osteochondral repair, which include pluripotent ESCs and iPSCs. Although ESCs have drawn a great attention for cartilage tissue engineering due to their capability to offer a virtually unlimited number of chondrogenic cells [308,309], the difficulty in controlling the lineage-specific differentiation, the potential immune rejection and the involved ethical issues have limited their widespread utility [310,311]. To avoid the controversy associated with ESCs, iPSCs isolated from somatic cells such as fibroblasts provide a new way and also show a promise in regenerative medicine because of the similar capabilities of proliferation and differentiation [[312], [313], [314]]. The chondrogenic and osteogenic differentiation of iPSCs have been revealed and utilized for restoring cartilage and bone defect both in vitro and in vivo [[315], [316], [317], [318]]. Ko et al. examined the chondrogenic differences between iPSCs and BMSCs and found that chondro-induced iPSCs exhibited greater GAG contents and better chondrocytic features than the chondro-induced BMSCs in vitro [315]. Several hurdles still need to be overcome such as the optimal protocols of isolation, differentiation and purification ex vivo and the potential tissue malformations in vivo [17].
5.2. Biochemical signaling factors
Apart from the encapsulated tissue forming cells, the selection of biochemical signaling factors is also significant to provide an appropriate microenvironment for the ingrowth of osteochondral tissue. The molecular signals used in cartilage and osteochondral repair consist of growth factors naturally occurring in the body and hormones or small molecular compounds that are synthetic. They are capable to mediate the growth, proliferation and differentiation by activating specific pathways and stimulating the expression of relevant proteins. Transforming growth factor (TGF)-β, bone morphogenetic proteins (BMPs), insulin growth factors (IGFs) and fibroblast growth factors (FGFs) are among the most popular growth factors in cartilage and bone formation, while dexamethasone (DEX) and kartogenin (KGN) are the typical synthetic molecules.
TGF-β is a large superfamily of cell regulatory polypeptides composed of TGF-β, BMPs, activins and inhibins. They can initiate the cell signaling and affect the cell behaviors by binding to two major membrane-bound receptors, type I and type II receptors [19]. Bone is the tissue with the most abundant TGF-β because of the high concentration of TGF-β receptors on the surface of osteoblasts [[319], [320], [321]]. The expression of TGF-β1, 2 and 3 and their receptors has a temporal and spatial variation in different zones of the osteochondral unit and in different growth phases [319]. These three isoforms of TGF-β are generally considered to play key roles in chondrocyte maturation and endochondral ossification by regulating the expression of transcription factors Sex determining region Y box 9 (Sox-9) and Runt-related transcription factor 2 (Runx2). They are able to promote the synthesis of proteoglycans and type II collagen in chondrocytes [319], and at the same time, promote the chondrogenic and osteogenic differentiation of MSCs, dependent on the dosage of TGF-β1 [322,323]. TGF-β2 and TGF-β3 have also been widely used to induce the chondrogenic differentiation of stem cells as indicated by the expression of aggrecan and type II collagen [232,315,324].
Comprising almost one-third of the TGF-β superfamily, BMP family includes a sub-class of 20 polypeptide members (BMP-1–18, BMP-3b and BMP-8b) that are involved in cartilage and bone formation during skeletal morphogenesis [325]. The endogenous BMPs in injured cartilage are important in protecting the cartilage from damage and activating the regenerative processes [326]. BMPs have been shown to be effective tissue engineering growth factors for cartilage and bone regeneration, among which BMP-2, 3, 4, 6, 7 and 9 are the most extensively studied ones [326]. BMP-2 alone or combined with other growth factors has been vastly utilized for the osteogenic and chondrogenic differentiation of chondrocytes or stem cells to regenerate bone, cartilage or osteochondral tissues in vitro and in vivo [231,239,326,327]. BMP-3, also known as osteogenin, is the most abundant type of BMP family that plays an essential role in bone formation. It acts as an antagonist of osteogenic BMPs such as BMP-2 and BMP-4, and may negatively regulate the process of bone morphogenesis and articular cartilage regeneration [326,328]. BMP-4 not only has known roles in bone formation and bone healing, but also is able to accelerate the chondrogenesis of stem cells and the regeneration of hyaline cartilage [329,330]. The endogenous expression of BMP-6 can also be detected in the cartilage of both healthy and osteoarthritic joint [331], and it was reported to drive both the osteogenic and chondrogenic differentiation of various stem cells [332,333]. BMP-7, similar to BMP-2 and BMP-4, is involved in cartilage and bone morphogenesis [325,334,335]. BMP-9 was considered to be potentially the most osteogenic BMP and it was also effective in modulating the cartilage development of chondrocytes and MSCs [326,336,337].
IGF is a single polypeptide named for the similarity of its amino acid sequence with insulin. IGF-1 and IGF-2 are the two included types with close molecular mass. They can be synthesized by wide varieties of cell types and able to regulate the proliferation, apoptosis, differentiation and function of cells. IGF-2 is predominantly expressed during embryonic and fetal development, while IGF-1 is the more relevant form involved in articular cartilage repair [[338], [339], [340], [341]]. IGF-1 functions as a pivotal mediator of cartilage homeostasis by balancing the proteoglycan synthesis and breakdown by chondrocytes and promoting the survival and proliferation of chondrocytes [19,338]. In aged and arthritic articular cartilage, there is a progressive decline in the serum IGF-1 levels and chondrocyte responsiveness to IGF-1, with diminishing capability to maintain structural and functional integrity [338]. In recent years, the incorporation and delivery of IGF-1 has gained wide application in designing osteochondral scaffolds for osteochondral tissue engineering [237,342]. IGF-1 also plays an essential role in stimulating osteoblastic differentiation for bone defect repairing [320]. The overexpression of IGF-1 was found to facilitate the osteogenic capability of aging BMSCs and the biomineralization of the cell clusters [343].
FGF family contains 22 structurally related protein members in human (FGF-1-14, 16–23). Each member can activate one of the four FGF receptors on the cell surface to regulate the proliferation, migration and differentiation of cells [344]. Among them, FGF-2 and FGF-18 are known to be the most important ones in chondrogenesis and skeletal development [345]. FGF-2 is also recognized as the basic fibroblast growth factor that stimulates the matrix synthesis in articular cartilage and acts as a chondrocyte mitogen [346,347]. On the other hand, FGF-2 is expressed in osteoblasts and has been widely employed as a therapeutic agent to promote bone fracture healing [[348], [349], [350]]. FGF-18 is another fibroblast growth factor required in skeletal growth to coordinate the chondrogenesis and osteogenesis. In a monolayer culture system, the round cell morphology and cellular proliferation of chondrocytes obtained from both porcine and human was observed to be promoted by the supplemented FGF-18, while the expression of type I collagen was decreased [351]. During bone regeneration, FGF-18 also serves as an important mediator and has been incorporated in hydrogel scaffold to function as the substitute of BMP-2 for bone defect repair [352,353].
DEX is a kind of synthetic glucocorticoid that has been frequently applied to treat inflammatory diseases clinically [354] and also to treat osteoarthritis [355,356]. Albeit that systemic glucocorticoid therapy is associated with severe complications on metabolic processes and musculoskeletal systems [354], DEX has been found to potentiate the chondrogenic and osteogenic differentiation of several stem cells in vitro, especially when used in combination with other bioactive factors such as TGF-β and BMPs [[357], [358], [359], [360], [361]]. Dosage (pharmacological or physiological levels) and exposure stage during differentiation (early phase or late phase) were considered to be depending elements in deciding the differentiation lineage of MSCs [354]. By comparing the cartilaginous genes and proteins in vitro, it was observed that the influence of DEX on the growth-factor-induced chondrogenesis of MSCs was highly dependent on the tissue origin of MSCs, the culturing microenvironment and the choice of the growth factor [361]. The controlled release of DEX at a relative lower concentration has also been used to promote osteogenesis for bone regeneration. The DEX-loaded composite coatings prepared by Qi et al. showed not only an osteogenic promotion of osteoblasts, which was suggested by the osteogenic gene expression and mineralization, but also an anti-inflammatory effect on macrophages [362].
Identified in 2012, kartogenin is a small molecular organic compound that plays a positive role in promoting the repair of damaged cartilage [363]. This molecule was proven to be able to stimulate the lineage-specific differentiation of MSCs to chondrocytes in a dose-dependent manner and protect the articular chondrocytes under the pathological conditions of osteoarthritis in vitro and in vivo [363]. Since its first discovery, KGN has been attracting an increasing research interest for applications in chondral and osteochondral defect restoration. Strategies to utilize KGN are diverse, such as direct intra-articular injection [364,365], incorporation in particle or thermogel drug delivery systems [[366], [367], [368], [369]], and encapsulation in cartilage and osteochondral tissue engineering scaffolds [233,234,370,371]. By host-guest interactions, Liu and coworkers loaded the KGN to the β-cyclodextrin (β-CD) nanoboxes and then fabricated the HA-based cartilage-regenerating hydrogel embedding both the drug-loaded nanoboxes and MSCs. The β-CD modified with two arms of carbon double bonds can provide the hydrophobic internal cavity for the loading of hydrophobic KGN, and on the other hand form integration with the covalent network of the methacrylated hydrogel under light. KGN could be sustainably released via the molecular detachment from the β-CD and the polymer degradation [233]. The combinational usage of KGN with other growth factors especially TGF-β3 and the synergistic effect have also gained much attention [372,373]. Whereas, studies concerning the potential molecular mechanisms and the in vivo long-term effect of KGN are still extremely limited.
5.3. Physiochemical and physical signaling factors
Apart from the biochemical signaling factors as we have reviewed in the last section, physiochemical ones (oxygen tension, pH and carbon dioxide concentration) and physical ones (temperature, mechanical cues transmitted through liquid and solid medium as well as other non-contactable physical media) can also largely affect the cell fate and the biological processes involved in constructing the artificial cartilage or osteochondral tissues [374,375]. Oxygen tension within articular cartilage shows a range from approximately 7% in the superficial layer to less than 1% in the deep zone close to the subchondral bone, mainly mediated by the hypoxia-inducible factors (HIFs) [375,376]. As a signaling factor known to have significant effects on the development and differentiation of cartilage, the hypoxic condition has also been widely used in combination with scaffold and/or biochemical signaling factors to induce the matrix synthesis of chondrocytes and chondrogenic differentiation of stem cells [[377], [378], [379], [380]].
The device or system which maintains these biologically active conditions for cultivating tissue-engineered constructs is called a bioreactor [20]. The traditional static culture with petri dishes or flasks cultured in an incubator can be considered as the simplest bioreactor which provide the biochemical and physiochemical signaling factors and parts of the physical factors such as pH, carbon dioxide concentration, oxygen tension and temperature [381]. However, in static culture conditions, the mass transport of oxygen, nutrients and wastes based on passive diffusion is too limited to ensure the metabolism. In the native avascular cartilage tissue, the mechanical stimuli during the movement of synovial joints play the role of blood vessels in mass transport which is fundamental in tissue development [374]. Bioreactors employing not only the biochemical and physiochemical stimuli but also the physical ones, especially the mechanical cues, have turned to be appealing strategies to optimize the restoration of damaged cartilage or osteochondral tissues. The configurations of bioreactors mimicking the biomechanical characteristics of articular cartilage are diverse, including stir, perfusion, rotating wall vessel, stretch, compression, hydrostatic pressure and combined ones, etc. [20] (Fig. 9) Although the mechanical cues associated with the physiochemically tunable 3D scaffolds (such as surface topography, matrix stiffness) can also critically impact the cell fate [382], we will focus on those coming from the environmental culturing system in the bioreactor for cartilage and osteochondral tissue engineering in this section. According to the media which transmit the forces, mechanical cues can be classified into those mediated through liquid (hydrostatic pressure, fluid shear stress), solid (compression and its combination with shear stress) and other noncontact media (electromagnetic field, microgravity, ultrasound, etc.) [374,383].
Fig. 9.
Schematic drawing of different configurations of bioreactors mimicking the biomechanical characteristics of articular cartilage. A. A stirred bioreactor system. B. A perfusion bioreactor system. C. A simple low hydrostatic pressure bioreactor. D. Two types of rotating wall vessel bioreactors. E. A stretch bioreactor system. F. A compression bioreactor system.
The hydrostatic pressure (HP) comes from the liquid environment of the articular cartilage including the synovial fluid and the interstitial fluid, while the fluid shear generates with the liquid flow. During joint movement, the cyclical switch of the knee joints between moving and stationary states further exposes the articular cartilage to a cyclic HP [374]. Several types of HP bioreactors have been developed to augment the formation of engineered cartilage [[384], [385], [386], [387]]. Prof. Guangdong Zhou's group designed a highly efficient and stable HP bioreactor and revealed that the provided HP could help improve the mechanical strength, thickness and homogeneity of the engineered cartilage in vitro. They also explored the potential mechanisms of HP regulation comprehensively from tissue level to cell and molecular levels [384]. Correia and coworkers investigated the effects of HP conditions with varying frequency and amplitude on cartilage formation in 3D hydrogels. Compared with the steady HP, the pulsatile HP regime promoted better tissue development in the construct with chondrocytes. On the other hand, greater chondrogenic differentiation and matrix deposition were realized for the ASC group cultured under the HP with a physiologic amplitude (5 MPa) rather than HP with a lower level (0.4 MPa) [385]. Tarng et al. developed a recirculating flow-perfusion bioreactor to simultaneously generate directional HP pressure and oscillatory fluid shear stress. The culture results of cartilage yield, cell morphology and zonal organization showed better resemblance with native cartilage for this combined bioreactor, compared with the bioreactor only offering shear stress or the static culture [388].
There are the two main forms of relative motions between the femoral condyle and the underlying tibia during joint movement, rolling and sliding, exerting compression and shear stress separately on the contacting cartilage. Compression transmitted by solid medium is the most widely studied mechanical stimulus for reconstructing the articular cartilage, especially the cartilage at knee joint, the largest load-bearing joint in human body [[389], [390], [391], [392], [393], [394]]. Wang et al. cultured the multilayer composite scaffold loaded with BMSCs in a bioreactor under a dynamic compression. In vitro assessment and in vivo pig model results presented that the cyclic compression increased the compressive modulus of the tissue-engineered cartilage and showed more favorable effect in repairing the cartilage defects [393]. Combined usage of compression and shear stress is another common strategy in designing bioreactors to improve the performance of engineered cartilage because of their coexistence in knee joints [[395], [396], [397]]. Schätti et al. studied the effect of compression, shear or a combination of both on the chondrogenic differentiation of BMSCs embedded in a composite scaffold. In the absence of any exogenous growth factors, the combination of cyclic axial compression with surface shear induced significant chondrogenic gene expression and GAG and type II collagen synthesis, but groups with compression or shear alone showed insufficient promotion [396].
In the pursuit of more potential mechanical cues, forces transmitted by several types of noncontact media such as electromagnetic field [[398], [399], [400]], microgravity [207,324,401] and ultrasound [[402], [403], [404]] have been explored to optimize cartilage and bone repair. Despite the great advances that have been made in the field of biomechanics in providing appropriate mechanical stimulus through bioreactors for engineered tissue construction, several limitations involved in the design and fabrication of bioreactors remain to be resolved, for example, lack of universal standards, discrepancy in design parameters and the subsequent incomparable experimental results for different apparatuses. Modularization, standardization and personalization would be the possible future tendency [20,374].
6. Commercial products
The last several decades have witnessed the rapid development in tissue-engineered strategies for cartilage and osteochondral repair. Nevertheless, the majority of the advances are far from commercialization, while only a few have made their way into translated medical products and some are still struggling in the regulatory and legal path through clinical trials to get the approval from U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA) in the European Union (EU) or other official organizations in the world. Those products commercially available or on the way to markets for chondral and osteochondral defect repair can be categorized into three types (1) allografts in the state of particulates (e.g. BioCartilage® [405], DeNovo® [406]), or preformed cylinder/disc (e.g. Cartiform® [407] and Chondrofix® [408]), (2) synthetic acellular injectable liquid scaffolds (e.g. CarGel™ [409]) and (3) synthetic acellular cylindrical scaffolds (e.g. TruFit™ [410], MaioRegen™ [411], ChondroMimetic® [412], and Agili-C™ [413]).. (Table 2)
Table 2.
Commercial products for the cartilage and osteochondral repair.
| Product name | Company | Classification | Material and composition | Application | Identifier of clinical trials | References |
|---|---|---|---|---|---|---|
| BioCartilage® | Arthrex | Allografts, particulates | Dehydrated and decellularized native ECM of articular cartilage | The augmentation strategy of microfracture | NCT02203071 (Completed) NCT03696394 (Recruiting) |
[405,414] |
| DeNovo® | Zimmer Biomet | Allografts, particulates | Particulated juvenile cartilage implant | For partial-thickness or full-thickness cartilage restoration; as an option for early intervention | NCT01670617 (Active, not recruiting) NCT01329445 (Active, not recruiting) NCT01347892 (Unknown) |
[406,415] |
| Cartiform® | Arthrex | Allografts, preformed disc or rectangle | Obtained from human donors maintaining chondrocytes, growth factors and ECM proteins | The augmentation strategy of microfracture | Not available | [407,416] |
| Chondrofix® | Zimmer Biomet | Allografts, preformed cylinder | Consisting two layers of decellularized hyaline cartilage and cancellous bone | For full-thickness chondral an osteochondral lesion repair | NCT01410136 (Terminated) | [408,417,418] |
| CarGel™ | Smith & Nephew | Synthetic scaffolds, acellular injectable liquid | Based on chitosan, need to be mixed with the patient's whole blood before surgery | The augmentation strategy of microfracture | NCT01246895 (Completed) NCT00314236 (Completed) |
[409,419] |
| TruFit™ | Smith & Nephew | Synthetic scaffolds, acellular cylinder | Synthetic resorbable biphasic implant including PLGA, calcium sulfate and polyglycolide fibers | For osteochondral lesions involving both the cartilage and the underlying bone | NCT01246635 (Terminated) | [410,420,421] |
| MaioRegen™ | Ficeramica | Synthetic scaffolds, acellular cylinder | Multiphasic gradient with three layers: 1. The cartilaginous layer: deantigenated type I collagen 2. The intermediate layer: a mixture of type I collagen and HAp (60: 40) 3. The subchondral bone layer: a mineralized blend of type I collagen and HAp (30:70) |
Chondral or osteochondral lesions | NCT02345564 (Unknown) NCT01282034 (Completed) |
[242,411,422] |
| ChondroMimetic® | Collagen Solutions Plc | Synthetic scaffolds, acellular cylinder | Biphasic scaffold based on collagen, GAGs and calcium phosphate | Osteochondral lesions | NCT01209390 (Completed) | [229,412,423] |
| Agili-C™ | CartiHeal | Synthetic scaffolds, acellular cylinder | Porous and resorbable biphasic scaffold: aragonite modified with drilled channels and HA (cartilage phase), coralline aragonite (subchondral bone phase) | Osteochondral lesions | NCT03299959 (Active, not recruiting) NCT02423629 (Completed) NCT01471236 (Completed) |
[413,424,425] |
Among those above-mentioned grafts, BioCartilage®, Cartiform® and CarGel™ are the augmentation strategies of traditional microfracture procedures. BioCartilage® is composed of dehydrated and decellularized native ECM of articular cartilage [414] and Cartiform® is an allograft obtained from human donors maintaining chondrocytes, growth factors and ECM proteins but in the preformed shape of disc or rectangle [416]. In contrast, CarGel™ is based on chitosan instead of the native cartilage ECM and it needs to be mixed with the patient's whole blood before surgery, providing the adhesive bond between clot and surrounding cartilage [419]. DeNovo® natural tissue graft from Zimmer Biomet is an FDA-listed particulated juvenile cartilage implant to restore the damaged partial-thickness or full-thickness cartilage in knee, hip, ankle and shoulder as an option for early intervention [415]. Another product of Zimmer Biomet for cartilage repair is Chondrofix®, the first off-the-shelf osteochondral allograft which consists of two layers of decellularized hyaline cartilage and cancellous bone. This biphasic cylindrical allograft is capable to repair full-thickness chondral and osteochondral lesions with great convenience [418]. Nonetheless, a study in 32 patients reported its high rate of failure (72%) within the first 2 years of implantation. Despite the several limitations of this study such as a small sample size, heterogeneous characteristics and poor statistically significant correlations, delamination of the allograft was commonly observed [417].
The deepening understanding of the importance of subchondral bone in the osteochondral unit as well as the booming development in the field of biomaterials and tissue engineering may explain the prevalence of those engineered bilayered and multilayered scaffold products for osteochondral defect repair (TruFit™, MaioRegen™, ChondroMimetic®, Agili-C™). With the approval from FDA and the license to be used in Europe, TruFit™ marketed by Smith & Nephew was once the market leader as a synthetic resorbable biphasic implant to treat osteochondral lesions involving both the cartilage and the underlying bone, with an estimated 8000 patients treated per year. Early preclinical and clinical studies have reported favorable one-year outcomes of TruFit™ implantation in restoring osteochondral defects in the knee joint [426,427]. However, long-term deterioration of the early improvement was observed in several studies, shown as the increasing complaint of the pain and knee swelling, high reoperation rate, lack of evidence for bone ingrowth and limited rate of returning to sport [[428], [429], [430], [431]]. Further in vitro and in vivo investigations are needed to improve the efficacy for osteochondral regeneration. Besides, we notice that TruFit™ is not available now on the official website of Smith & Nephew but the reason has not been figured out.
MaioRegen™ is a multiphasic gradient composite scaffold with three layers mimicking the native osteochondral structures [241,242]. 2-year follow-up of thirty patients implanted with the scaffold in the knee chondral or osteochondral lesions showed a complete defect filling of both bone and cartilage as well as a good integration with the border bone and adjacent cartilage in a majority of the cases (70%) [242]. In a recent multicenter randomized controlled clinical trial with 100 patients, the treatment with MaioRegen™ showed superiority to traditional microfracture at two years [432]. Although it has been approved in European market, a few unsatisfying outcomes were also documented [433,434]. A prospective study in eight patients found that in spite of the good subchondral ossification and cartilage defect filling, the regenerated cartilage tissue had a limited quality, showing slight hypertrophy and fibrous-like cartilage [433]. Considering the relatively small sample size in these studies reporting the unfavorable outcomes, MaioRegen™ can be taken as a suitable option for regenerate the osteochondral defects.
ChondroMimetic® is a biphasic scaffold based on collagen, GAGs and calcium phosphate. A study in a caprine model found the superiority of this biphasic scaffold over PLAG-based biphasic scaffold and the empty control group from morphological, histological and mechanical performance of the restored osteochondral tissues [229]. The efficacy of the biphasic osteochondral scaffold used in combination with cell-based therapy or growth factors has also been explored [423,435]. Recently, the eight-year follow-up clinical study of 15 patients implanted with ChondroMimetic® in the knee demonstrated significant improvement in the clinical symptoms, including pain, function and activity level. These favorable long-term outcomes make it possible for ChondroMimetic® to be considered as an appealing alternative to microfracture to treat small osteochondral and focal chondral defects [412]. To the authors’ knowledge, the process to re-establish the CE Mark for this product is still ongoing.
Agili-C™ is a newly developed porous and resorbable biphasic scaffold from CartiHeal. The subchondral bone phase is made up of coralline aragonite, a biological material with similar 3D structures and crystalline form of calcium carbonate (CaCO3) to human bone, while the cartilage phase based on aragonite is mechanically modified with drilled channels and impregnated with HA to create better environment for hyaline cartilage regeneration [424,436]. Implantation studies in large animal, an ex vivo model and a human case have revealed its ability to promote the deposition of cartilage ECM, stimulate the regeneration of hyaline cartilage, restore both the cartilage and bone tissues and help the patient return to sports [424,[437], [438], [439]]. The implantation has been carried in several places around the world and the early and interim analysis results are quite satisfactory [425,440]. A 12-month follow-up study in 21 patients showed a statistically significant improvement in several types of clinical scores and imaging evaluation. Besides, most of the implants presented good integration of the graft with the adjacent bone and cartilage, as well as a clear border between the two regenerated tissues [425]. Nevertheless, in America, Agili-C™ is an implant device limited for use in the Investigational Device Exemption (IDE) clinical study, not yet available for sale.
Altogether, the clinical products for cartilage and osteochondral defect repair especially the engineered synthetic chondral and osteochondral scaffolds are much limited. Despite the fact that some have obtained the approval for market sale, several hurdles remain to be tackled, such as the delamination of biphasic or multiphasic scaffold [417], the incomplete integration with the adjacent native tissues [429], the limited quality of the regenerated tissue [433] and the deteriorated long-term efficacy of the implant [431], as documented in the clinical reports. More academic research is therefore needed to clarify the relationship between these problems and the scaffold design and to build more theories and knowledge for a better clinical application.
7. Current challenges and future directions
The centuries-old observation by William Hunter in 1742 [441] that ulcerated articular cartilage is a troublesome disease is still true today. Although fully replication of the architectures and functions of articular cartilage is far from success now, the past few decades have witnessed a giant leap in restoring and regenerating the cartilage by palliative, reparative or regenerative treatments, including the tissue engineering approaches such as ACI and MACI. Those encouraging achievements in cartilage repair have motivated researchers to extend their focus to osteochondral defect repair, when cartilage, cartilage-bone interface and subchondral bone are all involved. Considering the spatial hierarchy and complexity of articular cartilage and osteochondral units in composition, structure and functions, there are several key challenges remain to be overcome in reconstructing the damaged cartilage and osteochondral tissues.
The top priority for cartilage and osteochondral tissue engineering is the formation of neo-tissues in the defect, especially the avascular and aneural hyaline cartilage without the self-healing capability. Notwithstanding the reported improvements achieved by some cell-free 3D scaffolds, the hyaline cartilage production with abundant ECM indicated by the type II collagen, aggrecan, GAGs or some related genes is still hard to accomplish. Acknowledging the fact that chondrocytes take the main responsibility for the structure and functions of cartilage, they were the early cells used for generating cartilaginous ECM, implanted directly or encapsulated in the 3D scaffold. However, chondrocytes were found to have some limitations such as low isolation efficiency, poor proliferation capacity and rapid phenotypic drift. The discovery and exploration of several types of stem cells in recent years offered appealing alternatives to chondrocytes for cartilage repair. Further, the phenotypic maintenance of chondrocytes or the chondrogenic differentiation of progenitor cells embedded in the engineered three-dimensional scaffold can be assisted by the utilization of various kinds of biochemical signaling factors and physical stimulations. To satisfy the requirements for both hyaline cartilage and subchondral bone regeneration, the co-culture of different cells [187], the selection of proper growth factors or other bioactive molecules with different concentrations [234,371], the optimization of bioreactors which can produce more complex physical stimulus [391], and the synergistic effect of their combined usage will most likely be the future directions. On the other hand, the study on the articular cartilage development will surely provide more basic knowledge to better comprehend the biology, formation and maintenance of the cartilage and osteochondral units, and subsequently to restore the damaged tissues.
The integration of the grafts and implant to the surrounding tissue is another persisting challenge in cartilage and osteochondral tissue engineering [[442], [443], [444]]. In clinical surgery, the lack of the tissue integration to border zone could cause implant failure and it is also an important evaluation variable post operation. A tissue adhesive is thus always applied to strengthen the interaction. Engineered grafts with the inherent tissue integration capability will ensure their long-term success when implanted into the defect, as the integration can provide stable biologic fixation and appropriate mechanotransduction [445,446]. The high metabolism of the bone drives the readily occurred osteointegration in the bone defects and the vertical integration of cartilage to the subchondral bone in full-thickness chondral defects. Whereas, the limited metabolism and the anti-adhesive ECM in cartilage make it extremely hard to realize the lateral integration between cartilage [447]. Promising strategies to improve this cartilage-to-cartilage integration include the modification or functionalization of the scaffold to form strong covalent bonds or noncovalent interactions with the adjacent cartilage, for instance, chemical interaction between the aldehyde groups in the biomaterial and the amino groups on the cartilage surface [444,448], fiber reinforcement to resist interfacial crack propagation [449], mussel chemistry facilitated by catechol group [447,450]. In addition, constructing implants with similar biomechanical properties to the native tissue is also beneficial for the integration through diminishing the interfacial stress concentration induced by the unmatched mechanical strength [451,452].
The spatiotemporally sophisticated biomechanics of articular cartilage have left a great hurdle on the road to build an engineered functional substitute with high fidelity to native tissue. The mismatches of the mechanical properties will not only lead to the interfacial stress concentrations and decreased integration, but also damage the implant during joint movements and weaken its long-term durability. The lack of knowledge about the components and architectures of cartilage ECM, which are decisive factors in the biomechanical properties, is one of the reasons for the limited progress in structural and functional restoration [453]. Acknowledging the fact that the capability of articular cartilage to withstand the mechanical forces and maintain the resilience is mostly attributed to the intertwined network of proteoglycans and collagen fibers, which are inherently incapable to re-form in mature cartilage, the reconstruction of this collagen network architecture should be considered in the future by exploring the mechanisms of its initial formation [453]. Besides, the supporting subchondral bone assisting the compression resistance of articular cartilage plays an essential role in its functional maintenance, and thus deserves attention in designing engineered grafts for osteoarthritis [41]. Another special biomechanical feature of articular cartilage is the low friction on the surface, unravelling the mechanism of which will shed light on better therapeutic strategies to restore its lubrication [454]. Apart from the complexity in types of mechanical properties of cartilage, the zonal heterogeneity from the cartilaginous superficial region to the deep osseous layer involved in osteochondral units is also a critical issue in studying the biomechanics. Numerous efforts dedicated to construct stratified scaffolds with diverse mechanical strengths in different layers have showed promising results in tackling this problem [129,252].
Restoring the heterogeneities in anatomical, biological, physicochemical and mechanical properties of osteochondral tissues as well as the continuously gradient cartilage-bone interface is still a challenging goal albeit the considerable progress achieved in the field of biomaterials and biological science. Engineered cartilage or bone grafts with more elaborate design and better biomimicry have emerged along with the deepening understanding about the components and architecture, cell biology and biomechanics of cartilage and bone [9,16,383]. Nevertheless, being different from the regeneration of separate cartilage or bone, osteochondral regeneration involves the interface between the two distinct tissues, apart from the underlying bone and the cartilage that includes three regions with different composition, structure and function. Hence, it requires an overall knowledge of both the two tissues and moreover an in-depth comprehension on the osteochondral interface and its complex relationship and interaction with cartilage and bone. The first explored monophasic scaffolds containing a uniform composition and architecture neglect the special stratified feature of the osteochondral unit [78,228]. Later studies developed the biphasic scaffolds that can simultaneously promote the regeneration of both cartilage and bone in the two individual layers without considering the intermediate calcified cartilage [114,234]. The importance of the interface has been taken into account in the triphasic scaffold that can better resembling the osteochondral stratification [112,251]. More recently, efforts have been paid to construct gradient scaffolds with gradient chemical compositions and structural characteristics, whether in multiphasic or continuous mode, which showed promising to imitate the native osteochondral tissues in a more superior way [256,280]. But the research concerning the development of gradient tissue engineering scaffolds is still in its early stage. More exploration in the evolving fabrication technologies and strategies such as 3D printing, microfluidic method, utilization of magnetic field and buoyancy that enable the researchers to better achieve the heterogenous and gradient characteristics is needed.
8. Summary
During the past several decades, the development of tissue engineering strategies in the regeneration of cartilage and osteochondral tissues have gained considerable progress in all the key factors. Based on the anatomical composition of native osteochondral units, the basic materials of the scaffold can be selected from a wide range of natural and synthetic polymers, inorganic materials, metals and those biomaterials modified from ECM. The improving understanding on the stratified and hierarchical architecture of the osteochondral tissue have provided cues for researchers to fabricate layered scaffolds which are different in material compositions, cell encapsulations and biomolecule incorporations. Current efforts paid to scaffolds with compositional or structural gradients are promising to establish superior constructs especially in the osteochondral interface formation. When cultured in bioreactors simulating especially the physical stimuli in native articular cartilage and subchondral bone during joint movement, the engineered constructs can allow for better ECM deposition and tissue regeneration. Among the numbered products commercially available or on the way to markets for osteochondral defect repair, majorities are layered scaffolds. Despite the fact that there are several challenges including the combined efficacy of cells and signaling factors, the integration to the adjacent tissues, the replication of the spatiotemporally sophisticated biomechanical properties, and the restoration of anatomical, biological and physicochemical heterogeneities, we are optimistic that the advancing technologies and further investigations will gradually tackle these problems in osteochondral tissue engineering and eventually reduce the societal and individual burden of joint disease in the musculoskeletal system in the foreseeable future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 51772233), the National Key Research and Development Program of China (2018YFB1105500), the Major Special Projects of Technological Innovation of Hubei Province (No. 2019ACA130), the Application Foundation and Front Research Program of Wuhan (No. 2018010401011273), Foshan Xianhu Laboratory of the Advanced Energy Science and Technology Guangdong Laboratory (XHT2020-008) and the Fundamental Research Funds for the Central Universities (2020-YB-015). The authors would like to thank Prof. Shengmin Zhang, Dr. Yingying Du and Ziyang Lan from Huazhong University of Science and Technology for their help in language polishing. The authors would also like to thank Zhangmancang Xu for the technical support in writing and revising the paper.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., Goldring S.R., Jones G., Teichtahl A.J., Pelletier J.-P. Nature Reviews Disease Primers. 2016;2:1–18. doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 2.Woolf A.D., Pfleger B. Bull. World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 3.Briggs A.M., Woolf A.D., Dreinhöfer K., Homb N., Hoy D.G., Kopansky-Giles D., Åkesson K., March L. Bull. World Health Organ. 2018;96:366–368. doi: 10.2471/BLT.17.204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M., Bridgett L., Williams S., Guillemin F., Hill C.L. Ann. Rheum. Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 5.Hay S.I., Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F., Abdulkader R.S., Abdulle A.M., Abebo T.A., Abera S.F. Lancet. 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long H., Zeng X., Liu Q., Wang H., Vos T., Hou Y., Lin C., Qiu Y., Wang K., Xing D. The Lancet Rheumatology. 2020;2:e164–e172. doi: 10.1016/S2665-9913(19)30145-6. [DOI] [PubMed] [Google Scholar]
- 7.Glyn-Jones S., Palmer A.J.R., Agricola R., Price A.J., Vincent T.L., Weinans H., Carr A.J. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 8.Baumann C.A., Hinckel B.B., Bozynski C.C., Farr J. In: Joint Preservation of the Knee. Yanke A.B., Cole B.J., editors. Springer International Publishing; Cham: 2019. pp. 3–24. [Google Scholar]
- 9.Kwon H., Brown W.E., Lee C.A., Wang D., Paschos N., Hu J.C., Athanasiou K.A. Nat. Rev. Rheumatol. 2019;15:550–570. doi: 10.1038/s41584-019-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosy J.D., Schranz P.J., Toms A.D., Eyres K.S., Mandalia V.I. Arthrosc. J. Arthrosc. Relat. Surg. 2011;27:695–703. doi: 10.1016/j.arthro.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 11.Kreuz P.C., Steinwachs M.R., Erggelet C., Krause S.J., Konrad G., Uhl M., Südkamp N. Osteoarthritis Cartilage. 2006;14:1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Marcacci M., Filardo G., Kon E. Injury. 2013;44:S11–S15. doi: 10.1016/S0020-1383(13)70004-4. [DOI] [PubMed] [Google Scholar]
- 13.Vasiliadis H.S., Danielson B., Ljungberg M., McKeon B., Lindahl A., Peterson L. Am. J. Sports Med. 2010;38:943–949. doi: 10.1177/0363546509358266. [DOI] [PubMed] [Google Scholar]
- 14.Peterson L., Vasiliadis H.S., Brittberg M., Lindahl A. Am. J. Sports Med. 2010;38:1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 15.Langer R., Vacanti J.P. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 16.Koons G.L., Diba M., Mikos A.G. Nature Reviews Materials. 2020;5:584–603. [Google Scholar]
- 17.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Nat. Rev. Rheumatol. 2015;11:21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graceffa V., Vinatier C., Guicheux J., Stoddart M., Alini M., Zeugolis D.I. Biomaterials. 2019;192:199–225. doi: 10.1016/j.biomaterials.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Vinatier C., Mrugala D., Jorgensen C., Guicheux J., Noël D. Trends Biotechnol. 2009;27:307–314. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Bayir E., Sahinler M., Celtikoglu M.M., Sendemir A. In: Biomaterials for Organ and Tissue Regeneration. Vrana N.E., Knopf-Marques H., Barthes J., editors. Woodhead Publishing; UK: 2020. pp. 709–752. [Google Scholar]
- 21.Mescher A.L. fourteenth ed. McGraw-Hill Education; USA: 2016. Junqueira's Basic Histology: Text and Atlas. [Google Scholar]
- 22.Poole A.R., Kojima T., Yasuda T., Mwale F., Kobayashi M., Laverty S. Clin. Orthop. Relat. Res. 2001;391:S26–S33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 23.Chen F.H., Rousche K.T., Tuan R.S. Nat. Clin. Pract. Rheumatol. 2006;2:373–382. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 24.Sophia Fox A.J., Bedi A., Rodeo S.A. Sport Health. 2009;1:461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoulders M.D., Raines R.T. Annu. Rev. Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canelón S.P., Wallace J.M. PloS One. 2016;11:1–13. doi: 10.1371/journal.pone.0166392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautieri A., Vesentini S., Redaelli A., Buehler M.J. Nano Lett. 2011;11:757–766. doi: 10.1021/nl103943u. [DOI] [PubMed] [Google Scholar]
- 28.Li A., Wei Y., Hung C., Vunjak-Novakovic G. Biomaterials. 2018;173:47–57. doi: 10.1016/j.biomaterials.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Symposium B.M.Z.O., Ewing J.W. Arthrosc. J. Arthrosc. Relat. Surg. 1990;6 167-167. [Google Scholar]
- 30.Mow V.C., Holmes M.H., Michael Lai W. J. Biomech. 1984;17:377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 31.Mow V.C., Guo X.E. Annu. Rev. Biomed. Eng. 2002;4:175–209. doi: 10.1146/annurev.bioeng.4.110701.120309. [DOI] [PubMed] [Google Scholar]
- 32.Mow V.C., Kuei S.C., Lai W.M., Armstrong C.G. J. Biomech. Eng. 1980;102:73. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 33.Buckwalter J.A., Mow V.C., Ratcliffe A. J. Am. Acad. Orthop. Surg. 1994;2:192–201. doi: 10.5435/00124635-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Lüssea S., Claassen H., Gehrke T., Hassenpflug J., Schünke M., Heller M., Glüer C.-C. Magn. Reson. Imag. 2000;18:423–430. doi: 10.1016/s0730-725x(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 35.Malda J., Benders K.E.M., Klein T.J., de Grauw J.C., Kik M.J.L., Hutmacher D.W., Saris D.B.F., van Weeren P.R., Dhert W.J.A. Osteoarthritis Cartilage. 2012;20:1147–1151. doi: 10.1016/j.joca.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Redler I., Mow V.C., Zimny M.L., Mansell J. Clin. Orthop. Relat. Res. 1975;112:357–362. [PubMed] [Google Scholar]
- 37.Fawns H.T., Landells J.W. Ann. Rheum. Dis. 1953;12:105–113. doi: 10.1136/ard.12.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldring S.R., Goldring M.B. Nat. Rev. Rheumatol. 2016;12:632–644. doi: 10.1038/nrrheum.2016.148. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y., Wang F., Tan H., Chen G., Guo L., Yang L. Int. J. Med. Sci. 2012;9:353–360. doi: 10.7150/ijms.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arkill K., Winlove C. Osteoarthritis Cartilage. 2008;16:708–714. doi: 10.1016/j.joca.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Li G., Yin J., Gao J., Cheng T.S., Pavlos N.J., Zhang C., Zheng M.H. Arthritis Res. Ther. 2013;15:223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madry H., van Dijk C.N., Mueller-Gerbl M. Knee Surg. Sports Traumatol. Arthrosc. 2010;18:419–433. doi: 10.1007/s00167-010-1054-z. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y., Luo D., Wang T. Small. 2016;12:4611–4632. doi: 10.1002/smll.201600626. [DOI] [PubMed] [Google Scholar]
- 44.Wegst U.G.K., Bai H., Saiz E., Tomsia A.P., Ritchie R.O. Nat. Mater. 2015;14:23–36. doi: 10.1038/nmat4089. [DOI] [PubMed] [Google Scholar]
- 45.Pouran B., Arbabi V., Bleys R.L., René van Weeren P., Zadpoor A.A., Weinans H. J. Biomech. 2017;52:148–154. doi: 10.1016/j.jbiomech.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 46.Curl W.W., Krome J., Gordon E.S., Rushing J., Smith B.P., Poehling G.G. Arthrosc. J. Arthrosc. Relat. Surg. 1997;13:456–460. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 47.Hjelle K., Solheim E., Strand T., Muri R., Brittberg M. Arthrosc. J. Arthrosc. Relat. Surg. 2002;18:730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- 48.Widuchowski W., Widuchowski J., Trzaska T. Knee. 2007;14:177–182. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Lopa S., Madry H. Tissue Eng. 2014;20:2052–2076. doi: 10.1089/ten.tea.2013.0356. [DOI] [PubMed] [Google Scholar]
- 50.Outerbridge R.E. J. Bone Joint Surg. 1961;43B:752–757. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 51.Casscells S.W. Arthrosc. J. Arthrosc. Relat. Surg. 1990;6:253. doi: 10.1016/0749-8063(90)90053-g. [DOI] [PubMed] [Google Scholar]
- 52.Brittberg M., Winalski C.S. J. Bone Joint Surg. 2003;85:58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 53.O'Loughlin P.F., Heyworth B.E., Kennedy J.G. Am. J. Sports Med. 2010;38:392–404. doi: 10.1177/0363546509336336. [DOI] [PubMed] [Google Scholar]
- 54.Katz J.N., Brownlee S.A., Jones M.H. Best Pract. Res. Clin. Rheumatol. 2014;28:143–156. doi: 10.1016/j.berh.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magnuson P.B. Surg. Gynecol. Obstet. 1941;73:1–9. [Google Scholar]
- 56.Upmeier H., Brüggenjürgen B., Weiler A., Flamme C., Laprell H., Willich S.N. Knee Surg. Sports Traumatol. Arthrosc. 2007;15:249–257. doi: 10.1007/s00167-006-0182-y. [DOI] [PubMed] [Google Scholar]
- 57.Cameron-Donaldson M., Holland C., Hungerford D.S., Frondoza C.G. Arthrosc. J. Arthrosc. Relat. Surg. 2004;20:1040–1043. doi: 10.1016/j.arthro.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Felson D.T. Best Pract. Res. Clin. Rheumatol. 2010;24:47–50. doi: 10.1016/j.berh.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lazic S., Boughton O., Hing C., Bernard J. Knee. 2014;21:631–634. doi: 10.1016/j.knee.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 60.Daily R.K. Am. J. Ophthalmol. 1936;19:176. [Google Scholar]
- 61.Johnson L.L. Clin. Orthop. Relat. Res. 2001;391:S306–S317. [PubMed] [Google Scholar]
- 62.Pridie K.H. J. Bone Jt. Surg. Br. Vol. 1959;41:618–619. [Google Scholar]
- 63.Steadman J.R., Rodkey W.G., Rodrigo J.J. Clin. Orthop. Relat. Res. 2001;391:S362–S369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 64.Steadman J.R., Rodkey W.G., Singleton S.B., Briggs K.K. Operat. Tech. Orthop. 1997;7:300–304. [Google Scholar]
- 65.Goyal D., Keyhani S., Lee E.H., Hui J.H.P. Arthrosc. J. Arthrosc. Relat. Surg. 2013;29:1579–1588. doi: 10.1016/j.arthro.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 66.Mithoefer K., McAdams T., Williams R.J., Kreuz P.C., Mandelbaum B.R. Am. J. Sports Med. 2009;37:2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 67.Steadman J.R., Briggs K.K., Rodrigo J.J., Kocher M.S., Gill T.J., Rodkey W.G. Arthrosc. J. Arthrosc. Relat. Surg. 2003;19:477–484. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 68.Bentley G., Greer R.B. Nature. 1971;230:385–388. doi: 10.1038/230385a0. [DOI] [PubMed] [Google Scholar]
- 69.Volkov M. J. Bone Jt. Surg. Br. Vol. 1970;52-B:49–53. [PubMed] [Google Scholar]
- 70.Chesterman P.J., Smith A.U. The journal of bone and joint surgery. British volume. 1968;50-B:184–197. [PubMed] [Google Scholar]
- 71.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. N. Engl. J. Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 72.Batty L., Dance S., Bajaj S., Cole B.J. ANZ J. Surg. 2011;81:18–25. doi: 10.1111/j.1445-2197.2010.05495.x. [DOI] [PubMed] [Google Scholar]
- 73.Gooding C.R., Bartlett W., Bentley G., Skinner J.A., Carrington R., Flanagan A. Knee. 2006;13:203–210. doi: 10.1016/j.knee.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 74.Kon E., Verdonk P., Condello V., Delcogliano M., Dhollander A., Filardo G., Pignotti E., Marcacci M. Am. J. Sports Med. 2009;37:156–166. doi: 10.1177/0363546509351649. [DOI] [PubMed] [Google Scholar]
- 75.Behrens P., Bitter T., Kurz B., Russlies M. Knee. 2006;13:194–202. doi: 10.1016/j.knee.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 76.Cole B.J., Farr J., Winalski C.S., Hosea T., Richmond J., Mandelbaum B., De Deyne P.G. Am. J. Sports Med. 2011;39:1170–1179. doi: 10.1177/0363546511399382. [DOI] [PubMed] [Google Scholar]
- 77.Amini A.R., Laurencin C.T., Nukavarapu S.P. Crit. Rev. Biomed. Eng. 2012;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nukavarapu S.P., Dorcemus D.L. Biotechnol. Adv. 2013;31:706–721. doi: 10.1016/j.biotechadv.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 79.New Scaffolds & Cells | Patient Education, https://cartilage.org/patient/about-cartilage/cartilage-repair/new-scaffolds-cells/.
- 80.Osteochondral Tissue Engineering: Nanotechnology, Scaffolding-Related Developments and Translation. Springer International Publishing; Cham: 2018. [Google Scholar]
- 81.Yang J., Zhang Y.S., Yue K., Khademhosseini A. Acta Biomater. 2017;57:1–25. doi: 10.1016/j.actbio.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Armiento A.R., Stoddart M.J., Alini M., Eglin D. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 83.Jeuken R., Roth A., Peters R., van Donkelaar C., Thies J., van Rhijn L., Emans P. Polymers. 2016;8:219. doi: 10.3390/polym8060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh Y.P., Bhardwaj N., Mandal B.B. ACS Appl. Mater. Interfaces. 2016;8:21236–21249. doi: 10.1021/acsami.6b08285. [DOI] [PubMed] [Google Scholar]
- 85.Jeon O., Song S.J., Lee K.-J., Park M.H., Lee S.-H., Hahn S.K., Kim S., Kim B.-S. Carbohydr. Polym. 2007;70:251–257. [Google Scholar]
- 86.Chen J., Yang J., Wang L., Zhang X., Heng B.C., Wang D.-A., Ge Z. Bioactive Materials. 2021;6:1689–1698. doi: 10.1016/j.bioactmat.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamanlar S., Sant S., Boudou T., Picart C., Khademhosseini A. Biomaterials. 2011;32:5590–5599. doi: 10.1016/j.biomaterials.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi B., Kim S., Lin B., Wu B.M., Lee M. ACS Appl. Mater. Interfaces. 2014;6:20110–20121. doi: 10.1021/am505723k. [DOI] [PubMed] [Google Scholar]
- 89.Hochberg M.C., Martel-Pelletier J., Monfort J., Möller I., Castillo J.R., Arden N., Berenbaum F., Blanco F.J., Conaghan P.G., Doménech G. Ann. Rheum. Dis. 2016;75:37–44. doi: 10.1136/annrheumdis-2014-206792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valcarcel J., Novoa-Carballal R., Pérez-Martín R.I., Reis R.L., Vázquez J.A. Biotechnol. Adv. 2017;35:711–725. doi: 10.1016/j.biotechadv.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Yang J., Shen M., Wen H., Luo Y., Huang R., Rong L., Xie J. Carbohydr. Polym. 2020;230:115650. doi: 10.1016/j.carbpol.2019.115650. [DOI] [PubMed] [Google Scholar]
- 92.Zhou F., Zhang X., Cai D., Li J., Mu Q., Zhang W., Zhu S., Jiang Y., Shen W., Zhang S. Acta Biomater. 2017;63:64–75. doi: 10.1016/j.actbio.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Arno M.C., Inam M., Weems A.C., Li Z., Binch A.L.A., Platt C.I., Richardson S.M., Hoyland J.A., Dove A.P., O'Reilly R.K. Nat. Commun. 2020;11:1–9. doi: 10.1038/s41467-020-15206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Balakrishnan B., Joshi N., Jayakrishnan A., Banerjee R. Acta Biomater. 2014;10:3650–3663. doi: 10.1016/j.actbio.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 95.Markstedt K., Mantas A., Tournier I., Martínez Ávila H., Hägg D., Gatenholm P. Biomacromolecules. 2015;16:1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 96.Pawar S.N., Edgar K.J. Biomaterials. 2012;33:3279–3305. doi: 10.1016/j.biomaterials.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 97.Lee K.Y., Mooney D.J. Prog. Polym. Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Awad H.A., Quinn Wickham M., Leddy H.A., Gimble J.M., Guilak F. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 99.Mauck R.L., Yuan X., Tuan R.S. Osteoarthritis Cartilage. 2006;14:179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 100.Rennerfeldt D.A., Renth A.N., Talata Z., Gehrke S.H., Detamore M.S. Biomaterials. 2013;34:8241–8257. doi: 10.1016/j.biomaterials.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zarrintaj P., Manouchehri S., Ahmadi Z., Saeb M.R., Urbanska A.M., Kaplan D.L., Mozafari M. Carbohydr. Polym. 2018;187:66–84. doi: 10.1016/j.carbpol.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 102.Francis Suh J.-K., Matthew H.W.T. Biomaterials. 2000;21:2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 103.Kim I.-Y., Seo S.-J., Moon H.-S., Yoo M.-K., Park I.-Y., Kim B.-C., Cho C.-S. Biotechnol. Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 104.Liu J., Yang B., Li M., Li J., Wan Y. Carbohydr. Polym. 2020;227:115335. doi: 10.1016/j.carbpol.2019.115335. [DOI] [PubMed] [Google Scholar]
- 105.Sheehy E.J., Mesallati T., Vinardell T., Kelly D.J. Acta Biomater. 2015;13:245–253. doi: 10.1016/j.actbio.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 106.Waibel K.H., Haney B., Moore M., Whisman B., Gomez R. Mil. Med. 2011;176:1153–1156. doi: 10.7205/milmed-d-11-00150. [DOI] [PubMed] [Google Scholar]
- 107.Heo D.N., Kim H.-J., Lee D., Kim H., Lee S.J., Lee H.-R., Kwon I.K., Do S.H. Int. J. Biol. Macromol. 2020;146:922–930. doi: 10.1016/j.ijbiomac.2019.09.215. [DOI] [PubMed] [Google Scholar]
- 108.Pereira D.R., Canadas R.F., Silva-Correia J., da Silva Morais A., Oliveira M.B., Dias I.R., Mano J.F., Marques A.P., Reis R.L., Oliveira J.M. Applied Materials Today. 2018;12:309–321. [Google Scholar]
- 109.Stevens L.R., Gilmore K.J., Wallace G.G., Panhuis M.i. h. Biomaterials Science. 2016;4:1276–1290. doi: 10.1039/c6bm00322b. [DOI] [PubMed] [Google Scholar]
- 110.Zia K.M., Tabasum S., Khan M.F., Akram N., Akhter N., Noreen A., Zuber M. Int. J. Biol. Macromol. 2018;109:1068–1087. doi: 10.1016/j.ijbiomac.2017.11.099. [DOI] [PubMed] [Google Scholar]
- 111.Zhou H., Chen R., Wang J., Lu J., Yu T., Wu X., Xu S., Li Z., Jie C., Cao R. Mater. Des. 2020;195:108947. [Google Scholar]
- 112.Levingstone T.J., Ramesh A., Brady R.T., Brama P.A.J., Kearney C., Gleeson J.P., O'Brien F.J. Biomaterials. 2016;87:69–81. doi: 10.1016/j.biomaterials.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 113.Parenteau-Bareil R., Gauvin R., Berthod F. Materials. 2010;3:1863–1887. [Google Scholar]
- 114.Gao F., Xu Z., Liang Q., Li H., Peng L., Wu M., Zhao X., Cui X., Ruan C., Liu W. Advanced Science. 2019;6:1900867. doi: 10.1002/advs.201900867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xiao S., Zhao T., Wang J., Wang C., Du J., Ying L., Lin J., Zhang C., Hu W., Wang L. Stem Cell Reviews and Reports. 2019;15:664–679. doi: 10.1007/s12015-019-09893-4. [DOI] [PubMed] [Google Scholar]
- 116.Yue K., Trujillo-de Santiago G., Alvarez M.M., Tamayol A., Annabi N., Khademhosseini A. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hong H., Seo Y.B., Kim D.Y., Lee J.S., Lee Y.J., Lee H., Ajiteru O., Sultan M.T., Lee O.J., Kim S.H. Biomaterials. 2020;232:119679. doi: 10.1016/j.biomaterials.2019.119679. [DOI] [PubMed] [Google Scholar]
- 118.Rockwood D.N., Preda R.C., Yücel T., Wang X., Lovett M.L., Kaplan D.L. Nat. Protoc. 2011;6:1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vepari C., Kaplan D.L. Prog. Polym. Sci. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yodmuang S., McNamara S.L., Nover A.B., Mandal B.B., Agarwal M., Kelly T.-A.N., Chao P.-h. G., Hung C., Kaplan D.L., Vunjak-Novakovic G. Acta Biomater. 2015;11:27–36. doi: 10.1016/j.actbio.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chu S., Maples M.M., Bryant S.J. Acta Biomater. 2020;109:37–50. doi: 10.1016/j.actbio.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sharma B., Fermanian S., Gibson M., Unterman S., Herzka D.A., Cascio B., Coburn J., Hui A.Y., Marcus N., Gold G.E. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3004838. 167ra166-167ra166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spang M.T., Christman K.L. Acta Biomater. 2018;68:1–14. doi: 10.1016/j.actbio.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang J., Zhang F., Tsang W.P., Wan C., Wu C. Biomaterials. 2017;120:11–21. doi: 10.1016/j.biomaterials.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 125.Critchley S., Sheehy E.J., Cunniffe G., Diaz-Payno P., Carroll S.F., Jeon O., Alsberg E., Brama P.A.J., Kelly D.J. Acta Biomater. 2020;113:130–143. doi: 10.1016/j.actbio.2020.05.040. [DOI] [PubMed] [Google Scholar]
- 126.Niederauer G.G., Slivka M.A., Leatherbury N.C., Korvick D.L., Hugh Harroff J., Ehler H.W.C., Dunn C.J., Kieswetter K. Biomaterials. 2000;21:2561–2574. doi: 10.1016/s0142-9612(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 127.Okamoto M., John B. Prog. Polym. Sci. 2013;38:1487–1503. [Google Scholar]
- 128.Yao Q., Cosme J.G.L., Xu T., Miszuk J.M., Picciani P.H.S., Fong H., Sun H. Biomaterials. 2017;115:115–127. doi: 10.1016/j.biomaterials.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bittner S.M., Smith B.T., Diaz-Gomez L., Hudgins C.D., Melchiorri A.J., Scott D.W., Fisher J.P., Mikos A.G. Acta Biomater. 2019;90:37–48. doi: 10.1016/j.actbio.2019.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Du Y., Liu H., Yang Q., Wang S., Wang J., Ma J., Noh I., Mikos A.G., Zhang S. Biomaterials. 2017;137:37–48. doi: 10.1016/j.biomaterials.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kang H.-W., Lee S.J., Ko I.K., Kengla C., Yoo J.J., Atala A. Nat. Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 132.Woodruff M.A., Hutmacher D.W. Prog. Polym. Sci. 2010;35:1217–1256. [Google Scholar]
- 133.Baker M.I., Walsh S.P., Schwartz Z., Boyan B.D. J. Biomed. Mater. Res. B Appl. Biomater. 2012;100B:1451–1457. doi: 10.1002/jbm.b.32694. [DOI] [PubMed] [Google Scholar]
- 134.Grant C., Twigg P., Egan A., Moody A., Smith A., Eagland D., Crowther N., Britland S. Biotechnol. Prog. 2006;22:1400–1406. doi: 10.1021/bp060181u. [DOI] [PubMed] [Google Scholar]
- 135.Kobayashi M., Chang Y.-S., Oka M. Biomaterials. 2005;26:3243–3248. doi: 10.1016/j.biomaterials.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 136.Zhao Y., Li M., Liu B., Xiang J., Cui Z., Qu X., Qiu D., Tian Y., Yang Z. J. Mater. Chem. B. 2018;6:1351–1358. doi: 10.1039/c7tb03177g. [DOI] [PubMed] [Google Scholar]
- 137.Qin Y., Li G., Wang C., Zhang D., Zhang L., Fang H., Yan S., Zhang K., Yin J. ACS Biomater. Sci. Eng. 2020;6:3070–3080. doi: 10.1021/acsbiomaterials.0c00200. [DOI] [PubMed] [Google Scholar]
- 138.Yan S., Wang T., Feng L., Zhu J., Zhang K., Chen X., Cui L., Yin J. Biomacromolecules. 2014;15:4495–4508. doi: 10.1021/bm501313t. [DOI] [PubMed] [Google Scholar]
- 139.Yan S., Zhang X., Zhang K., Di H., Feng L., Li G., Fang J., Cui L., Chen X., Yin J. J. Mater. Chem. B. 2016;4:947–961. doi: 10.1039/c5tb01488c. [DOI] [PubMed] [Google Scholar]
- 140.Zhang K., Zhang Y., Yan S., Gong L., Wang J., Chen X., Cui L., Yin J. Acta Biomater. 2013;9:7276–7288. doi: 10.1016/j.actbio.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 141.Cai Z., Wan Y., Becker M.L., Long Y.-Z., Dean D. Biomaterials. 2019;208:45–71. doi: 10.1016/j.biomaterials.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 142.Liu X., George M.N., Park S., Miller II A.L., Gaihre B., Li L., Waletzki B.E., Terzic A., Yaszemski M.J., Lu L. Acta Biomater. 2020;111:129–140. doi: 10.1016/j.actbio.2020.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ma C., Ma Z., Yang F., Wang J., Liu C. Biomed. Mater. 2019;14 doi: 10.1088/1748-605X/ab12ae. [DOI] [PubMed] [Google Scholar]
- 144.Yaszemski M.J., Payne R.G., Hayes W.C., Langer R., Mikos A.G. Biomaterials. 1996;17:2127–2130. doi: 10.1016/0142-9612(96)00008-7. [DOI] [PubMed] [Google Scholar]
- 145.Atoufi Z., Kamrava S.K., Davachi S.M., Hassanabadi M., Saeedi Garakani S., Alizadeh R., Farhadi M., Tavakol S., Bagher Z., Hashemi Motlagh G. Int. J. Biol. Macromol. 2019;139:1168–1181. doi: 10.1016/j.ijbiomac.2019.08.101. [DOI] [PubMed] [Google Scholar]
- 146.Bordat A., Boissenot T., Nicolas J., Tsapis N. Adv. Drug Deliv. Rev. 2019;138:167–192. doi: 10.1016/j.addr.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 147.Ren Z., Wang Y., Ma S., Duan S., Yang X., Gao P., Zhang X., Cai Q. ACS Appl. Mater. Interfaces. 2015;7:19006–19015. doi: 10.1021/acsami.5b02821. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Y., Yu J., Ren K., Zuo J., Ding J., Chen X. Biomacromolecules. 2019;20:1478–1492. doi: 10.1021/acs.biomac.9b00043. [DOI] [PubMed] [Google Scholar]
- 149.Vainieri M.L., Lolli A., Kops N., D'Atri D., Eglin D., Yayon A., Alini M., Grad S., Sivasubramaniyan K., van Osch G.J.V.M. Acta Biomater. 2020;101:293–303. doi: 10.1016/j.actbio.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 150.Pérez-Madrigal M.M., Shaw J.E., Arno M.C., Hoyland J.A., Richardson S.M., Dove A.P. Biomaterials Science. 2020;8:405–412. doi: 10.1039/c9bm01494b. [DOI] [PubMed] [Google Scholar]
- 151.Broguiere N., Isenmann L., Zenobi-Wong M. Biomaterials. 2016;99:47–55. doi: 10.1016/j.biomaterials.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 152.Jooybar E., Abdekhodaie M.J., Alvi M., Mousavi A., Karperien M., Dijkstra P.J. Acta Biomater. 2019;83:233–244. doi: 10.1016/j.actbio.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 153.Li X., Teng Y., Liu J., Lin H., Fan Y., Zhang X. J. Mater. Chem. B. 2017;5:5109–5119. doi: 10.1039/c7tb01020f. [DOI] [PubMed] [Google Scholar]
- 154.Meghdadi M., Pezeshki-Modaress M., Irani S., Atyabi S.M., Zandi M. Int. J. Biol. Macromol. 2019;136:616–624. doi: 10.1016/j.ijbiomac.2019.06.061. [DOI] [PubMed] [Google Scholar]
- 155.Ding Z., Lu G., Cheng W., Xu G., Zuo B., Lu Q., Kaplan D.L. ACS Biomater. Sci. Eng. 2020;6:2357–2367. doi: 10.1021/acsbiomaterials.0c00143. [DOI] [PubMed] [Google Scholar]
- 156.Liu X., Jin X., Ma P.X. Nat. Mater. 2011;10:398–406. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma H., Feng C., Chang J., Wu C. Acta Biomater. 2018;79:37–59. doi: 10.1016/j.actbio.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 158.Ribas R.G., Schatkoski V.M., Montanheiro T.L.d.A., de Menezes B.R.C., Stegemann C., Leite D.M.G., Thim G.P. Ceram. Int. 2019;45:21051–21061. [Google Scholar]
- 159.Zhou Y., Wu C., Chang J. Mater. Today. 2019;24:41–56. [Google Scholar]
- 160.Chen L., Deng C., Li J., Yao Q., Chang J., Wang L., Wu C. Biomaterials. 2019;196:138–150. doi: 10.1016/j.biomaterials.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 161.Deng C., Zhu H., Li J., Feng C., Yao Q., Wang L., Chang J., Wu C. Theranostics. 2018;8:1940–1955. doi: 10.7150/thno.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen T., Bai J., Tian J., Huang P., Zheng H., Wang J. Colloids Surf. B Biointerfaces. 2018;167:354–363. doi: 10.1016/j.colsurfb.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 163.Ding Z., Han H., Fan Z., Lu H., Sang Y., Yao Y., Cheng Q., Lu Q., Kaplan D.L. ACS Appl. Mater. Interfaces. 2017;9:16913–16921. doi: 10.1021/acsami.7b03932. [DOI] [PubMed] [Google Scholar]
- 164.Zou L., Zhang Y., Liu X., Chen J., Zhang Q. Colloids Surf. B Biointerfaces. 2019;178:222–229. doi: 10.1016/j.colsurfb.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 165.Zhou J., Xu C., Wu G., Cao X., Zhang L., Zhai Z., Zheng Z., Chen X., Wang Y. Acta Biomater. 2011;7:3999–4006. doi: 10.1016/j.actbio.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 166.Gotterbarm T., Richter W., Jung M., Berardi Vilei S., Mainil-Varlet P., Yamashita T., Breusch S.J. Biomaterials. 2006;27:3387–3395. doi: 10.1016/j.biomaterials.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 167.Kosik-Kozioł A., Costantini M., Mróz A., Idaszek J., Heljak M., Jaroszewicz J., Kijeńska E., Szöke K., Frerker N., Barbetta A. Biofabrication. 2019;11 doi: 10.1088/1758-5090/ab15cb. [DOI] [PubMed] [Google Scholar]
- 168.Li W., Kang J., Yuan Y., Xiao F., Yao H., Liu S., Lu J., Wang Y., Wang Z., Ren L. Compos. Sci. Technol. 2016;128:58–64. [Google Scholar]
- 169.Zhang W., Lian Q., Li D., Wang K., Hao D., Bian W., Jin Z. Mater. Sci. Eng. C. 2015;46:10–15. doi: 10.1016/j.msec.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 170.Ribeiro V.P., Pina S., Costa J.B., Cengiz I.F., García-Fernández L., Fernández-Gutiérrez M.d.M., Paiva O.C., Oliveira A.L., San-Román J., Oliveira J.M. ACS Appl. Mater. Interfaces. 2019;11:3781–3799. doi: 10.1021/acsami.8b21259. [DOI] [PubMed] [Google Scholar]
- 171.Deng C., Yao Q., Feng C., Li J., Wang L., Cheng G., Shi M., Chen L., Chang J., Wu C. Adv. Funct. Mater. 2017;27:1703117. [Google Scholar]
- 172.Li H., Chang J. Acta Biomater. 2013;9:5379–5389. doi: 10.1016/j.actbio.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 173.Li H., Chang J. Acta Biomater. 2013;9:6981–6991. doi: 10.1016/j.actbio.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 174.Bunpetch V., Zhang X., Li T., Lin J., Maswikiti E.P., Wu Y., Cai D., Li J., Zhang S., Wu C. Biomaterials. 2019;192:323–333. doi: 10.1016/j.biomaterials.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 175.Yu Q., Chang J., Wu C. J. Mater. Chem. B. 2019;7:5449–5460. doi: 10.1039/c9tb01467e. [DOI] [PubMed] [Google Scholar]
- 176.Lin K., Xia L., Li H., Jiang X., Pan H., Xu Y., Lu W.W., Zhang Z., Chang J. Biomaterials. 2013;34:10028–10042. doi: 10.1016/j.biomaterials.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 177.Wang C., Chen B., Wang W., Zhang X., Hu T., He Y., Lin K., Liu X. Mater. Sci. Eng. C. 2019;103:109833. doi: 10.1016/j.msec.2019.109833. [DOI] [PubMed] [Google Scholar]
- 178.Fiocco L., Li S., Stevens M.M., Bernardo E., Jones J.R. Acta Biomater. 2017;50:56–67. doi: 10.1016/j.actbio.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 179.Yu X., Zhao T., Qi Y., Luo J., Fang J., Yang X., Liu X., Xu T., Yang Q., Gou Z. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-018-36200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhai W., Lu H., Chen L., Lin X., Huang Y., Dai K., Naoki K., Chen G., Chang J. Acta Biomater. 2012;8:341–349. doi: 10.1016/j.actbio.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 181.Hench L.L. J. Am. Ceram. Soc. 1991;74:1487–1510. [Google Scholar]
- 182.Hench L.L. Science. 2002;295:1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 183.Jones J.R. Acta Biomater. 2013;9:4457–4486. doi: 10.1016/j.actbio.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 184.Lin R., Deng C., Li X., Liu Y., Zhang M., Qin C., Yao Q., Wang L., Wu C. Theranostics. 2019;9:6300–6313. doi: 10.7150/thno.36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Patel K.D., Buitrago J.O., Parthiban S.P., Lee J.-H., Singh R.K., Knowles J.C., Kim H.-W. ACS Applied Bio Materials. 2019;2:5190–5203. doi: 10.1021/acsabm.9b00859. [DOI] [PubMed] [Google Scholar]
- 186.Ryan E.J., Ryan A.J., González-Vázquez A., Philippart A., Ciraldo F.E., Hobbs C., Nicolosi V., Boccaccini A.R., Kearney C.J., O'Brien F.J. Biomaterials. 2019;197:405–416. doi: 10.1016/j.biomaterials.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 187.Zhu Y., Kong L., Farhadi F., Xia W., Chang J., He Y., Li H. Biomaterials. 2019;192:149–158. doi: 10.1016/j.biomaterials.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 188.Bandyopadhyay A., Shivaram A., Isik M., Avila J.D., Dernell W.S., Bose S. Additive Manufacturing. 2019;28:312–324. doi: 10.1016/j.addma.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tong X., Shi Z., Xu L., Lin J., Zhang D., Wang K., Li Y., Wen C. Acta Biomater. 2020;102:481–492. doi: 10.1016/j.actbio.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 190.Wang H., Su K., Su L., Liang P., Ji P., Wang C. Mater. Sci. Eng. C. 2019;104:109908. doi: 10.1016/j.msec.2019.109908. [DOI] [PubMed] [Google Scholar]
- 191.Wang S., Liu L., Li K., Zhu L., Chen J., Hao Y. Mater. Des. 2019;168:107643. [Google Scholar]
- 192.Wang X., Xu S., Zhou S., Xu W., Leary M., Choong P., Qian M., Brandt M., Xie Y.M. Biomaterials. 2016;83:127–141. doi: 10.1016/j.biomaterials.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 193.Castañeda S., Roman-Blas J.A., Largo R., Herrero-Beaumont G. Biochem. Pharmacol. 2012;83:315–323. doi: 10.1016/j.bcp.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 194.Bal B.S., Rahaman M.N., Jayabalan P., Kuroki K., Cockrell M.K., Yao J.Q., Cook J.L. J. Biomed. Mater. Res. B Appl. Biomater. 2010;93B:164–174. doi: 10.1002/jbm.b.31571. [DOI] [PubMed] [Google Scholar]
- 195.Díaz Lantada A., Alarcón Iniesta H., García-Ruíz J.P. Mater. Sci. Eng. C. 2016;59:218–227. doi: 10.1016/j.msec.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 196.Duan X., Zhu X., Dong X., Yang J., Huang F., Cen S., Leung F., Fan H., Xiang Z. Mater. Sci. Eng. C. 2013;33:3951–3957. doi: 10.1016/j.msec.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 197.Nover A.B., Lee S.L., Georgescu M.S., Howard D.R., Saunders R.A., Yu W.T., Klein R.W., Napolitano A.P., Ateshian G.A., Hung C.T. Acta Biomater. 2015;27:286–293. doi: 10.1016/j.actbio.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhao C., Zhang H., Cai B., Wang G., Fan H., Zhang X. Biomaterials Science. 2013;1:703–710. doi: 10.1039/c3bm00199g. [DOI] [PubMed] [Google Scholar]
- 199.Benders K.E.M., Weeren P.R.v., Badylak S.F., Saris D.B.F., Dhert W.J.A., Malda J. Trends Biotechnol. 2013;31:169–176. doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 200.Cheng C.W., Solorio L.D., Alsberg E. Biotechnol. Adv. 2014;32:462–484. doi: 10.1016/j.biotechadv.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hussey G.S., Dziki J.L., Badylak S.F. Nature Reviews Materials. 2018;3:159–173. [Google Scholar]
- 202.Kim Y.S., Majid M., Melchiorri A.J., Mikos A.G. Bioengineering & Translational Medicine. 2019;4:83–95. doi: 10.1002/btm2.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Crapo P.M., Gilbert T.W., Badylak S.F. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Luo L., Chu J.Y.J., Eswaramoorthy R., Mulhall K.J., Kelly D.J. ACS Biomater. Sci. Eng. 2017;3:1933–1943. doi: 10.1021/acsbiomaterials.6b00020. [DOI] [PubMed] [Google Scholar]
- 205.Novak T., Seelbinder B., Twitchell C.M., Voytik‐Harbin S.L., Neu C.P. Adv. Funct. Mater. 2016;26:5427–5436. doi: 10.1002/adfm.201601877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Teng Y., Li X., Chen Y., Cai H., Cao W., Chen X., Sun Y., Liang J., Fan Y., Zhang X. J. Mater. Chem. B. 2017;5:3283–3292. doi: 10.1039/c7tb00640c. [DOI] [PubMed] [Google Scholar]
- 207.Yin H., Wang Y., Sun X., Cui G., Sun Z., Chen P., Xu Y., Yuan X., Meng H., Xu W. Acta Biomater. 2018;77:127–141. doi: 10.1016/j.actbio.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 208.Lin X., Chen J., Qiu P., Zhang Q., Wang S., Su M., Chen Y., Jin K., Qin A., Fan S. Osteoarthritis Cartilage. 2018;26:433–444. doi: 10.1016/j.joca.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 209.Kwon J.S., Yoon S.M., Shim S.W., Park J.H., Min K.J., Oh H.J., Kim J.H., Kim Y.J., Yoon J.J., Choi B.H. Int. J. Pharm. 2013;454:183–191. doi: 10.1016/j.ijpharm.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 210.Rothrauff B.B., Coluccino L., Gottardi R., Ceseracciu L., Scaglione S., Goldoni L., Tuan R.S. Journal of Tissue Engineering and Regenerative Medicine. 2018;12:e159–e170. doi: 10.1002/term.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wu J., Ding Q., Dutta A., Wang Y., Huang Y.-h., Weng H., Tang L., Hong Y. Acta Biomater. 2015;16:49–59. doi: 10.1016/j.actbio.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 212.Chen W., Xu Y., Li Y., Jia L., Mo X., Jiang G., Zhou G. Chem. Eng. J. 2020;382:122986. [Google Scholar]
- 213.Jang J., Kim T.G., Kim B.S., Kim S.-W., Kwon S.-M., Cho D.-W. Acta Biomater. 2016;33:88–95. doi: 10.1016/j.actbio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 214.Kim D., Cho H.H., Thangavelu M., Song C., Kim H.S., Choi M.J., Song J.E., Khang G. Int. J. Biol. Macromol. 2020;149:381–394. doi: 10.1016/j.ijbiomac.2020.01.191. [DOI] [PubMed] [Google Scholar]
- 215.Pati F., Jang J., Ha D.-H., Won Kim S., Rhie J.-W., Shim J.-H., Kim D.-H., Cho D.-W. Nat. Commun. 2014;5:1–11. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Skardal A., Devarasetty M., Kang H.-W., Mead I., Bishop C., Shupe T., Lee S.J., Jackson J., Yoo J., Soker S. Acta Biomater. 2015;25:24–34. doi: 10.1016/j.actbio.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 217.Li Y., Liu Y., Xun X., Zhang W., Xu Y., Gu D. ACS Appl. Mater. Interfaces. 2019;11:36359–36370. doi: 10.1021/acsami.9b12206. [DOI] [PubMed] [Google Scholar]
- 218.Saeedi Garakani S., Khanmohammadi M., Atoufi Z., Kamrava S.K., Setayeshmehr M., Alizadeh R., Faghihi F., Bagher Z., Davachi S.M., Abbaspourrad A. Int. J. Biol. Macromol. 2020;143:533–545. doi: 10.1016/j.ijbiomac.2019.12.040. [DOI] [PubMed] [Google Scholar]
- 219.Beachley V., Ma G., Papadimitriou C., Gibson M., Corvelli M., Elisseeff J. J. Biomed. Mater. Res. 2018;106:147–159. doi: 10.1002/jbm.a.36218. [DOI] [PubMed] [Google Scholar]
- 220.Khanarian N.T., Haney N.M., Burga R.A., Lu H.H. Biomaterials. 2012;33:5247–5258. doi: 10.1016/j.biomaterials.2012.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang W., Sun L., Zhang P., Song J., Liu W. Acta Biomater. 2014;10:4983–4995. doi: 10.1016/j.actbio.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 222.Chen P., Tao J., Zhu S., Cai Y., Mao Q., Yu D., Dai J., Ouyang H. Biomaterials. 2015;39:114–123. doi: 10.1016/j.biomaterials.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 223.Coburn J.M., Gibson M., Monagle S., Patterson Z., Elisseeff J.H. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109:10012–10017. doi: 10.1073/pnas.1121605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Di Luca A., Lorenzo-Moldero I., Mota C., Lepedda A., Auhl D., Van Blitterswijk C., Moroni L. Advanced Healthcare Materials. 2016;5:1753–1763. doi: 10.1002/adhm.201600083. [DOI] [PubMed] [Google Scholar]
- 225.Malda J., Woodfield T.B.F., van der Vloodt F., Wilson C., Martens D.E., Tramper J., van Blitterswijk C.A., Riesle J. Biomaterials. 2005;26:63–72. doi: 10.1016/j.biomaterials.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 226.Zhang Y., Liu X., Zeng L., Zhang J., Zuo J., Zou J., Ding J., Chen X. Adv. Funct. Mater. 2019;29:1903279. [Google Scholar]
- 227.Dai Y., Gao Z., Ma L., Wang D., Gao C. Macromol. Biosci. 2016;16:1632–1642. doi: 10.1002/mabi.201600218. [DOI] [PubMed] [Google Scholar]
- 228.Zhang W., Ling C., Liu H., Zhang A., Mao L., Wang J., Chao J., Backman L.J., Yao Q., Chen J. Chem. Eng. J. 2020;396:125232. [Google Scholar]
- 229.Getgood A.M.J., Kew S.J., Brooks R., Aberman H., Simon T., Lynn A.K., Rushton N. Knee. 2012;19:422–430. doi: 10.1016/j.knee.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 230.Gao F., Xu Z., Liang Q., Liu B., Li H., Wu Y., Zhang Y., Lin Z., Wu M., Ruan C. Adv. Funct. Mater. 2018;28:1706644. [Google Scholar]
- 231.Gonzalez-Fernandez T., Rathan S., Hobbs C., Pitacco P., Freeman F.E., Cunniffe G.M., Dunne N.J., McCarthy H.O., Nicolosi V., O'Brien F.J. J. Contr. Release. 2019;301:13–27. doi: 10.1016/j.jconrel.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 232.Lam J., Lu S., Meretoja V.V., Tabata Y., Mikos A.G., Kasper F.K. Acta Biomater. 2014;10:1112–1123. doi: 10.1016/j.actbio.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu X., Chen Y., Mao A.S., Xuan C., Wang Z., Gao H., An G., Zhu Y., Shi X., Mao C. Biomaterials. 2019;232:119644. doi: 10.1016/j.biomaterials.2019.119644. [DOI] [PubMed] [Google Scholar]
- 234.Liu X., Wei Y., Xuan C., Liu L., Lai C., Chai M., Zhang Z., Wang L., Shi X. Adv. Healthc. Mater. 2020;9 doi: 10.1002/adhm.202000076. 2000076. [DOI] [PubMed] [Google Scholar]
- 235.Duan P., Pan Z., Cao L., He Y., Wang H., Qu Z., Dong J., Ding J. J. Biomed. Mater. Res. 2014;102:180–192. doi: 10.1002/jbm.a.34683. [DOI] [PubMed] [Google Scholar]
- 236.Gan D., Wang Z., Xie C., Wang X., Xing W., Ge X., Yuan H., Wang K., Tan H., Lu X. Advanced Healthcare Materials. 2019;8:1901103. doi: 10.1002/adhm.201901103. [DOI] [PubMed] [Google Scholar]
- 237.Lu S., Lam J., Trachtenberg J.E., Lee E.J., Seyednejad H., van den Beucken J.J.J.P., Tabata Y., Wong M.E., Jansen J.A., Mikos A.G. Biomaterials. 2014;35:8829–8839. doi: 10.1016/j.biomaterials.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pan Z., Duan P., Liu X., Wang H., Cao L., He Y., Dong J., Ding J. Regenerative Biomaterials. 2015;2:9–19. doi: 10.1093/rb/rbv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zheng L., Li D., Wang W., Zhang Q., Zhou X., Liu D., Zhang J., You Z., Zhang J., He C. ACS Biomater. Sci. Eng. 2019;5:4564–4573. doi: 10.1021/acsbiomaterials.9b00513. [DOI] [PubMed] [Google Scholar]
- 240.Harris J.D., Siston R.A., Brophy R.H., Lattermann C., Carey J.L., Flanigan D.C. Osteoarthritis Cartilage. 2011;19:779–791. doi: 10.1016/j.joca.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 241.Tampieri A., Sandri M., Landi E., Pressato D., Francioli S., Quarto R., Martin I. Biomaterials. 2008;29:3539–3546. doi: 10.1016/j.biomaterials.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 242.Kon E., Delcogliano M., Filardo G., Busacca M., Di Martino A., Marcacci M. Am. J. Sports Med. 2011;39:1180–1190. doi: 10.1177/0363546510392711. [DOI] [PubMed] [Google Scholar]
- 243.Kon E., Delcogliano M., Filardo G., Fini M., Giavaresi G., Francioli S., Martin I., Pressato D., Arcangeli E., Quarto R. J. Orthop. Res. 2009;28:116–124. doi: 10.1002/jor.20958. [DOI] [PubMed] [Google Scholar]
- 244.Kon E., Filardo G., Delcogliano M., Fini M., Salamanna F., Giavaresi G., Martin I., Marcacci M. BMC Muscoskel. Disord. 2010;11:1–12. doi: 10.1186/1471-2474-11-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kon E., Mutini A., Arcangeli E., Delcogliano M., Filardo G., Nicoli Aldini N., Pressato D., Quarto R., Zaffagnini S., Marcacci M. Journal of Tissue Engineering and Regenerative Medicine. 2010;4:300–308. doi: 10.1002/term.243. [DOI] [PubMed] [Google Scholar]
- 246.Marcacci M., Zaffagnini S., Kon E., Delcogliano M., Di Martino A., Filardo G., Altadonna G., Balbonie F. Arch. Ortop. Reumatol. 2009;120:35–37. [Google Scholar]
- 247.Levingstone T.J., Matsiko A., Dickson G.R., O'Brien F.J., Gleeson J.P. Acta Biomater. 2014;10:1996–2004. doi: 10.1016/j.actbio.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 248.Levingstone T.J., Thompson E., Matsiko A., Schepens A., Gleeson J.P., O'Brien F.J. Acta Biomater. 2016;32:149–160. doi: 10.1016/j.actbio.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 249.Da H., Jia S.-J., Meng G.-L., Cheng J.-H., Zhou W., Xiong Z., Mu Y.-J., Liu J. PloS One. 2013;8 doi: 10.1371/journal.pone.0054838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kang H., Zeng Y., Varghese S. Acta Biomater. 2018;78:365–377. doi: 10.1016/j.actbio.2018.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Korpayev S., Kaygusuz G., Şen M., Orhan K., Oto Ç., Karakeçili A. Int. J. Biol. Macromol. 2020;156:681–690. doi: 10.1016/j.ijbiomac.2020.04.109. [DOI] [PubMed] [Google Scholar]
- 252.Liu J., Li L., Suo H., Yan M., Yin J., Fu J. Mater. Des. 2019;171:107708. [Google Scholar]
- 253.Camarero-Espinosa S., Rothen-Rutishauser B., Weder C., Foster E.J. Biomaterials. 2016;74:42–52. doi: 10.1016/j.biomaterials.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 254.Girão A.F., Semitela Â., Ramalho G., Completo A., Marques P.A.A.P. Compos. B Eng. 2018;154:99–107. [Google Scholar]
- 255.Klein T.J., Rizzi S.C., Reichert J.C., Georgi N., Malda J., Schuurman W., Crawford R.W., Hutmacher D.W. Macromol. Biosci. 2009;9:1049–1058. doi: 10.1002/mabi.200900176. [DOI] [PubMed] [Google Scholar]
- 256.Lowen J.M., Leach J.K. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201909089. 1909089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Radhakrishnan J., Manigandan A., Chinnaswamy P., Subramanian A., Sethuraman S. Biomaterials. 2018;162:82–98. doi: 10.1016/j.biomaterials.2018.01.056. [DOI] [PubMed] [Google Scholar]
- 258.Liu Z., Meyers M.A., Zhang Z., Ritchie R.O. Prog. Mater. Sci. 2017;88:467–498. [Google Scholar]
- 259.Di Luca A., Szlazak K., Lorenzo-Moldero I., Ghebes C.A., Lepedda A., Swieszkowski W., Van Blitterswijk C., Moroni L. Acta Biomater. 2016;36:210–219. doi: 10.1016/j.actbio.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 260.Diloksumpan P., Bolaños R.V., Cokelaere S., Pouran B., Grauw J.d., Rijen M.v., Weeren R.v., Levato R., Malda J. Advanced Healthcare Materials. 2020;9 doi: 10.1002/adhm.201901807. 1901807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cross L.M., Shah K., Palani S., Peak C.W., Gaharwar A.K. Nanomed. Nanotechnol. Biol. Med. 2018;14:2465–2474. doi: 10.1016/j.nano.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Guo J., Li C., Ling S., Huang W., Chen Y., Kaplan D.L. Biomaterials. 2017;145:44–55. doi: 10.1016/j.biomaterials.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Parisi C., Salvatore L., Veschini L., Serra M.P., Hobbs C., Madaghiele M., Sannino A., Silvio L.D. J. Tissue Eng. 2020;11:1–13. doi: 10.1177/2041731419896068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Dormer N.H., Singh M., Wang L., Berkland C.J., Detamore M.S. Ann. Biomed. Eng. 2010;38:2167–2182. doi: 10.1007/s10439-010-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Dormer N.H., Singh M., Zhao L., Mohan N., Berkland C.J., Detamore M.S. J. Biomed. Mater. Res. 2012;100A:162–170. doi: 10.1002/jbm.a.33225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Jeon O., Alt D.S., Linderman S.W., Alsberg E. Adv. Mater. 2013;25:6366–6372. doi: 10.1002/adma.201302364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Singh M., Dormer N., Salash J.R., Christian J.M., Moore D.S., Berkland C., Detamore M.S. J. Biomed. Mater. Res. 2010;94A:870–876. doi: 10.1002/jbm.a.32765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Singh M., Morris C.P., Ellis R.J., Detamore M.S., Berkland C. Tissue Eng. C Methods. 2008;14:299–309. doi: 10.1089/ten.tec.2008.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wang X., Wenk E., Zhang X., Meinel L., Vunjak-Novakovic G., Kaplan D.L. J. Contr. Release. 2009;134:81–90. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Xin S., Dai J., Gregory C.A., Han A., Alge D.L. Adv. Funct. Mater. 2020;30:1907102. doi: 10.1002/adfm.201907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhu D., Tong X., Trinh P., Yang F. Tissue Eng. 2018;24:1–10. doi: 10.1089/ten.tea.2016.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Liu J., Fang Q., Yu X., Wan Y., Xiao B. Int. J. Mol. Sci. 2018;19:2330. doi: 10.3390/ijms19082330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sundararaghavan H.G., Burdick J.A. Biomacromolecules. 2011;12:2344–2350. doi: 10.1021/bm200415g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Hadden W.J., Young J.L., Holle A.W., McFetridge M.L., Kim D.Y., Wijesinghe P., Taylor-Weiner H., Wen J.H., Lee A.R., Bieback K. Proc. Natl. Acad. Sci. Unit. States Am. 2017;114:5647–5652. doi: 10.1073/pnas.1618239114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Vega S.L., Kwon M.Y., Song K.H., Wang C., Mauck R.L., Han L., Burdick J.A. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-018-03021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Oh S.H., Kim T.H., Im G.I., Lee J.H. Biomacromolecules. 2010;11:1948–1955. doi: 10.1021/bm100199m. [DOI] [PubMed] [Google Scholar]
- 277.Oh S.H., Kim T.H., Lee J.H. Biomaterials. 2011;32:8254–8260. doi: 10.1016/j.biomaterials.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 278.Xiao H., Huang W., Xiong K., Ruan S., Yuan C., Mo G., Tian R., Zhou S., She R., Ye P. Int. J. Nanomed. 2019;14:2011–2027. doi: 10.2147/IJN.S191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Li C., Armstrong J.P., Pence I.J., Kit-Anan W., Puetzer J.L., Correia Carreira S., Moore A.C., Stevens M.M. Biomaterials. 2018;176:24–33. doi: 10.1016/j.biomaterials.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Li C., Ouyang L., Pence I.J., Moore A.C., Lin Y., Winter C.W., Armstrong J.P.K., Stevens M.M. Adv. Mater. 2019;31:1900291. doi: 10.1002/adma.201900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sobral J.M., Caridade S.G., Sousa R.A., Mano J.F., Reis R.L. Acta Biomater. 2011;7:1009–1018. doi: 10.1016/j.actbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 282.Woodfield T.B.F., Malda J., de Wijn J., Péters F., Riesle J., van Blitterswijk C.A. Biomaterials. 2004;25:4149–4161. doi: 10.1016/j.biomaterials.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 283.Camarero-Espinosa S., Calore A., Wilbers A., Harings J., Moroni L. Acta Biomater. 2020;102:192–204. doi: 10.1016/j.actbio.2019.11.041. [DOI] [PubMed] [Google Scholar]
- 284.Miot S., Woodfield T., Daniels A.U., Suetterlin R., Peterschmitt I., Heberer M., van Blitterswijk C.A., Riesle J., Martin I. Biomaterials. 2005;26:2479–2489. doi: 10.1016/j.biomaterials.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 285.Nuernberger S., Cyran N., Albrecht C., Redl H., Vécsei V., Marlovits S. Biomaterials. 2011;32:1032–1040. doi: 10.1016/j.biomaterials.2010.08.100. [DOI] [PubMed] [Google Scholar]
- 286.Zhang J., Yang Z., Li C., Dou Y., Li Y., Thote T., Wang D.-a., Ge Z. Tissue Eng. 2013;19:2166–2175. doi: 10.1089/ten.tea.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Lien S.-M., Ko L.-Y., Huang T.-J. Acta Biomater. 2009;5:670–679. doi: 10.1016/j.actbio.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 288.Zhang Q., Lu H., Kawazoe N., Chen G. Acta Biomater. 2014;10:2005–2013. doi: 10.1016/j.actbio.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 289.Mačiulaitis J., Deveikytė M., Rekštytė S., Bratchikov M., Darinskas A., Šimbelytė A., Daunoras G., Laurinavičienė A., Laurinavičius A., Gudas R. Biofabrication. 2015;7 doi: 10.1088/1758-5090/7/1/015015. [DOI] [PubMed] [Google Scholar]
- 290.Accardi M.A., McCullen S.D., Callanan A., Chung S., Cann P.M., Stevens M.M., Dini D. Tissue Eng. 2013;19:2300–2310. doi: 10.1089/ten.tea.2012.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Li W.-J., Jiang Y.J., Tuan R.S. Tissue Eng. 2006;12:1775–1785. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 292.Darling E.M., Athanasiou K.A. J. Orthop. Res. 2005;23:425–432. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 293.Barandun M., Iselin L.D., Santini F., Pansini M., Scotti C., Baumhoer D., Bieri O., Studler U., Wirz D., Haug M. J. Orthop. Res. 2015;33:1111–1119. doi: 10.1002/jor.22865. [DOI] [PubMed] [Google Scholar]
- 294.Lohan A., Marzahn U., El Sayed K., Haisch A., Müller R.D., Kohl B., Stölzel K., Ertel W., John T., Schulze-Tanzil G. Annals of Anatomy - Anatomischer Anzeiger. 2014;196:317–326. doi: 10.1016/j.aanat.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 295.Mumme M., Barbero A., Miot S., Wixmerten A., Feliciano S., Wolf F., Asnaghi A.M., Baumhoer D., Bieri O., Kretzschmar M. Lancet. 2016;388:1985–1994. doi: 10.1016/S0140-6736(16)31658-0. [DOI] [PubMed] [Google Scholar]
- 296.Caplan Arnold I., Correa D. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wei X., Yang X., Han Z.-p., Qu F.-f., Shao L., Shi Y.-f. Acta Pharmacol. Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Caplan A.I. STEM CELLS Translational Medicine. 2017;6:1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sipp D., Robey P.G., Turner L. Nature. 2018;561:455–457. doi: 10.1038/d41586-018-06756-9. [DOI] [PubMed] [Google Scholar]
- 300.Zhang J., Wu Y., Thote T., Lee E.H., Ge Z., Yang Z. Biomed. Mater. 2014;9 doi: 10.1088/1748-6041/9/3/035011. [DOI] [PubMed] [Google Scholar]
- 301.Meretoja V.V., Dahlin R.L., Kasper F.K., Mikos A.G. Biomaterials. 2012;33:6362–6369. doi: 10.1016/j.biomaterials.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Peng L., Jia Z., Yin X., Zhang X., Liu Y., Chen P., Ma K., Zhou C. Stem Cell. Dev. 2008;17:761–774. doi: 10.1089/scd.2007.0217. [DOI] [PubMed] [Google Scholar]
- 303.Estes B.T., Diekman B.O., Gimble J.M., Guilak F. Nat. Protoc. 2010;5:1294–1311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Mellor L.F., Mohiti-Asli M., Williams J., Kannan A., Dent M.R., Guilak F., Loboa E.G. Tissue Eng. 2015;21:2323–2333. doi: 10.1089/ten.tea.2014.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Oshima T., Nakase J., Toratani T., Numata H., Takata Y., Nakayama K., Tsuchiya H. Arthrosc. J. Arthrosc. Relat. Surg. 2019;35:583–593. doi: 10.1016/j.arthro.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 306.Zhang K., He S., Yan S., Li G., Zhang D., Cui L., Yin J. J. Mater. Chem. B. 2016;4:2628–2645. doi: 10.1039/c5tb02113h. [DOI] [PubMed] [Google Scholar]
- 307.Perdisa F., Gostyńska N., Roffi A., Filardo G., Marcacci M., Kon E. Stem Cell. Int. 2015;2015:597652. doi: 10.1155/2015/597652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Cheng A., Kapacee Z., Peng J., Lu S., Lucas R.J., Hardingham T.E., Kimber S.J. STEM CELLS Translational Medicine. 2014;3:1287–1294. doi: 10.5966/sctm.2014-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Toh W.S., Lee E.H., Guo X.-M., Chan J.K.Y., Yeow C.H., Choo A.B., Cao T. Biomaterials. 2010;31:6968–6980. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 310.Murry C.E., Keller G. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 311.Okita K., Ichisaka T., Yamanaka S. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 312.Takahashi K., Yamanaka S. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 313.Yamanaka S. Cell Stem Cell. 2012;10:678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 314.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 315.Ko J.-Y., Kim K.-I., Park S., Im G.-I. Biomaterials. 2014;35:3571–3581. doi: 10.1016/j.biomaterials.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 316.Mahboudi H., Soleimani M., Enderami S.E., Kehtari M., Ahvaz H.H.-, Ghanbarian H., Bandehpour M., Nojehdehi S., Mirzaei S., Kazemi B. Artificial cells. Nanomedicine, and Biotechnology. 2018;46:1948–1956. doi: 10.1080/21691401.2017.1396998. [DOI] [PubMed] [Google Scholar]
- 317.Nguyen D., Hägg D.A., Forsman A., Ekholm J., Nimkingratana P., Brantsing C., Kalogeropoulos T., Zaunz S., Concaro S., Brittberg M. Sci. Rep. 2017;7:658. doi: 10.1038/s41598-017-00690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Park S., Im G.-I. Tissue Eng. B Rev. 2013;20:381–391. doi: 10.1089/ten.TEB.2013.0530. [DOI] [PubMed] [Google Scholar]
- 319.Grimaud E., Heymann D., Rédini F. Cytokine Growth Factor Rev. 2002;13:241–257. doi: 10.1016/s1359-6101(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 320.Linkhart T.A., Mohan S., Baylink D.J. Bone. 1996;19:S1–S12. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 321.Travis M.A., Sheppard D. Annu. Rev. Immunol. 2014;32:51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Guo X., Park H., Young S., Kretlow J.D., van den Beucken J.J., Baggett L.S., Tabata Y., Kasper F.K., Mikos A.G., Jansen J.A. Acta Biomater. 2010;6:39–47. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Worster A.A., Nixon A.J., Brower-Toland B.D., Williams J. Am. J. Vet. Res. 2000;61:1003–1010. doi: 10.2460/ajvr.2000.61.1003. [DOI] [PubMed] [Google Scholar]
- 324.Zhu Y., Song K., Jiang S., Chen J., Tang L., Li S., Fan J., Wang Y., Zhao J., Liu T. Appl. Biochem. Biotechnol. 2017;181:250–266. doi: 10.1007/s12010-016-2210-9. [DOI] [PubMed] [Google Scholar]
- 325.Bessa P.C., Casal M., Reis R.L. Journal of Tissue Engineering and Regenerative Medicine. 2008;2:1–13. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 326.Deng Z.H., Li Y.S., Gao X., Lei G.H., Huard J. Osteoarthritis Cartilage. 2018;26:1153–1161. doi: 10.1016/j.joca.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 327.Ma C., Ma Z., Yang F., Wang J., Liu C. Biomed. Mater. 2019;14 doi: 10.1088/1748-605X/ab12ae. [DOI] [PubMed] [Google Scholar]
- 328.Daluiski A., Engstrand T., Bahamonde M.E., Gamer L.W., Agius E., Stevenson S.L., Cox K., Rosen V., Lyons K.M. Nat. Genet. 2001;27:84–88. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 329.Wu M., Chen G., Li Y.-P. Bone Research. 2016;4:1–21. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zhang X., Zheng Z., Liu P., Ma Y., Lin L., Lang N., Fu X., Zhang J., Ma K., Chen P. Biomaterials. 2008;29:4616–4629. doi: 10.1016/j.biomaterials.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 331.Bobacz K., Gruber R., Soleiman A., Erlacher L., Smolen J.S., Graninger W.B. Arthritis Rheum. 2003;48:2501–2508. doi: 10.1002/art.11248. [DOI] [PubMed] [Google Scholar]
- 332.Dvořáková J., Kučera L., Kučera J., Švík K., Foglarová M., Muthný T., Pravda M., Němcová M., Velebný V., Kubala L. J. Biomed. Mater. Res. 2014;102:3523–3530. doi: 10.1002/jbm.a.35033. [DOI] [PubMed] [Google Scholar]
- 333.Kemmis C.M., Vahdati A., Weiss H.E., Wagner D.R. Biochem. Biophys. Res. Commun. 2010;401:20–25. doi: 10.1016/j.bbrc.2010.08.135. [DOI] [PubMed] [Google Scholar]
- 334.Kuo A.C., Rodrigo J.J., Reddi A.H., Curtiss S., Grotkopp E., Chiu M. Osteoarthritis Cartilage. 2006;14:1126–1135. doi: 10.1016/j.joca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 335.Shen B., Wei A., Whittaker S., Williams L.A., Tao H., Ma D.D.F., Diwan A.D. J. Cell. Biochem. 2010;109:406–416. doi: 10.1002/jcb.22412. [DOI] [PubMed] [Google Scholar]
- 336.Jain A.P., Pundir S., Sharma A. J. Indian Soc. Periodontol. 2013;17:583–586. doi: 10.4103/0972-124X.119275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ren X., Weisgerber D.W., Bischoff D., Lewis M.S., Reid R.R., He T.-C., Yamaguchi D.T., Miller T.A., Harley B.A.C., Lee J.C. Advanced Healthcare Materials. 2016;5:1821–1830. doi: 10.1002/adhm.201600187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Schmidt M.B., Chen E.H., Lynch S.E. Osteoarthritis Cartilage. 2006;14:403–412. doi: 10.1016/j.joca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 339.Jones J.I., Clemmons D.R. Endocr. Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 340.Uebersax L., Merkle H.P., Meinel L. J. Contr. Release. 2008;127:12–21. doi: 10.1016/j.jconrel.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 341.Wang L., Detamore M.S. J. Orthop. Res. 2009;27:1109–1115. doi: 10.1002/jor.20848. [DOI] [PubMed] [Google Scholar]
- 342.Kim K., Lam J., Lu S., Spicer P.P., Lueckgen A., Tabata Y., Wong M.E., Jansen J.A., Mikos A.G., Kasper F.K. J. Contr. Release. 2013;168:166–178. doi: 10.1016/j.jconrel.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Chen C.-Y., Tseng K.-Y., Lai Y.-L., Chen Y.-S., Lin F.-H., Lin S. Theranostics. 2017;7:1598–1611. doi: 10.7150/thno.16637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ornitz D.M., Itoh N. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. reviews3005.1-3005.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Ornitz D.M., Marie P.J. Genes Dev. 2015;29:1463–1486. doi: 10.1101/gad.266551.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Li X., Su G., Wang J., Zhou Z., Li L., Liu L., Guan M., Zhang Q., Wang H. Osteoarthritis Cartilage. 2013;21:1567–1575. doi: 10.1016/j.joca.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 347.Yang W., Cao Y., Zhang Z., Du F., Shi Y., Li X., Zhang Q. Acta Biomater. 2018;69:170–182. doi: 10.1016/j.actbio.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 348.Komaki H., Tanaka T., Chazono M., Kikuchi T. Biomaterials. 2006;27:5118–5126. doi: 10.1016/j.biomaterials.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 349.Montero A., Okada Y., Tomita M., Ito M., Tsurukami H., Nakamura T., Doetschman T., Coffin J.D., Hurley M.M. J. Clin. Invest. 2000;105:1085–1093. doi: 10.1172/JCI8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Yonemitsu R., Tokunaga T., Shukunami C., Ideo K., Arimura H., Karasugi T., Nakamura E., Ide J., Hiraki Y., Mizuta H. Am. J. Sports Med. 2019;47:1701–1712. doi: 10.1177/0363546519836959. [DOI] [PubMed] [Google Scholar]
- 351.Gigout A., Guehring H., Froemel D., Meurer A., Ladel C., Reker D., Bay-Jensen A.C., Karsdal M.A., Lindemann S. Osteoarthritis Cartilage. 2017;25:1858–1867. doi: 10.1016/j.joca.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 352.Behr B., Sorkin M., Manu A., Lehnhardt M., Longaker M.T., Quarto N. Tissue Eng. 2011;17:2061–2069. doi: 10.1089/ten.tea.2010.0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Sivashanmugam A., Charoenlarp P., Deepthi S., Rajendran A., Nair S.V., Iseki S., Jayakumar R. ACS Appl. Mater. Interfaces. 2017;9:42639–42652. doi: 10.1021/acsami.7b15845. [DOI] [PubMed] [Google Scholar]
- 354.Hartmann K., Koenen M., Schauer S., Wittig-Blaich S., Ahmad M., Baschant U., Tuckermann J.P. Physiol. Rev. 2016;96:409–447. doi: 10.1152/physrev.00011.2015. [DOI] [PubMed] [Google Scholar]
- 355.Huebner K.D., Shrive N.G., Frank C.B. J. Orthop. Res. 2014;32:566–572. doi: 10.1002/jor.22568. [DOI] [PubMed] [Google Scholar]
- 356.Zhao Y., Wei C., Chen X., Liu J., Yu Q., Liu Y., Liu J. ACS Appl. Mater. Interfaces. 2019;11:11587–11601. doi: 10.1021/acsami.8b20372. [DOI] [PubMed] [Google Scholar]
- 357.Awad H.A., Halvorsen Y.-D.C., Gimble J.M., Guilak F. Tissue Eng. 2003;9:1301–1312. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- 358.Langenbach F., Handschel J. Stem Cell Res. Ther. 2013;4:117. doi: 10.1186/scrt328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Morsczeck C., Moehl C., Götz W., Heredia A., Schäffer T.E., Eckstein N., Sippel C., Hoffmann K.H. Cell Biol. Int. 2005;29:567–575. doi: 10.1016/j.cellbi.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 360.Nuttelman C.R., Tripodi M.C., Anseth K.S. J. Biomed. Mater. Res. 2006;76A:183–195. doi: 10.1002/jbm.a.30537. [DOI] [PubMed] [Google Scholar]
- 361.Shintani N., Hunziker E.B. Eur. Cell. Mater. 2011;22:302–320. doi: 10.22203/ecm.v022a23. [DOI] [PubMed] [Google Scholar]
- 362.Qi H., Chen Q., Ren H., Wu X., Liu X., Lu T. Colloids Surf. B Biointerfaces. 2018;169:249–256. doi: 10.1016/j.colsurfb.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 363.Johnson K., Zhu S., Tremblay M.S., Payette J.N., Wang J., Bouchez L.C., Meeusen S., Althage A., Cho C.Y., Wu X. Science. 2012;336:717–721. doi: 10.1126/science.1215157. [DOI] [PubMed] [Google Scholar]
- 364.Mohan G., Magnitsky S., Melkus G., Subburaj K., Kazakia G., Burghardt A.J., Dang A., Lane N.E., Majumdar S. J. Orthop. Res. 2016;34:1780–1789. doi: 10.1002/jor.23197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Zhang J., Wang J.H.-C. Bone Research. 2014;2:1–10. doi: 10.1038/boneres.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Kang M.L., Ko J.-Y., Kim J.E., Im G.-I. Biomaterials. 2014;35:9984–9994. doi: 10.1016/j.biomaterials.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 367.Lee S.S., Choi G.E., Lee H.J., Kim Y., Choy J.-H., Jeong B. ACS Appl. Mater. Interfaces. 2017;9:42668–42675. doi: 10.1021/acsami.7b17173. [DOI] [PubMed] [Google Scholar]
- 368.Li X., Ding J., Zhang Z., Yang M., Yu J., Wang J., Chang F., Chen X. ACS Appl. Mater. Interfaces. 2016;8:5148–5159. doi: 10.1021/acsami.5b12212. [DOI] [PubMed] [Google Scholar]
- 369.Maudens P., Seemayer C.A., Thauvin C., Gabay C., Jordan O., Allémann E. Small. 2018;14:1703108. doi: 10.1002/smll.201703108. [DOI] [PubMed] [Google Scholar]
- 370.Xu J., Feng Q., Lin S., Yuan W., Li R., Li J., Wei K., Chen X., Zhang K., Yang Y. Biomaterials. 2019;210:51–61. doi: 10.1016/j.biomaterials.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 371.Xuan H., Hu H., Geng C., Song J., Shen Y., Lei D., Guan Q., Zhao S., You Z. Acta Biomater. 2020;105:97–110. doi: 10.1016/j.actbio.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 372.Fan W., Yuan L., Li J., Wang Z., Chen J., Guo C., Mo X., Yan Z. Mater. Sci. Eng. C. 2020;110:110705. doi: 10.1016/j.msec.2020.110705. [DOI] [PubMed] [Google Scholar]
- 373.Zhao Y., Teng B., Sun X., Dong Y., Wang S., Hu Y., Wang Z., Ma X., Yang Q. Orthop. Surg. 2020;12:938–945. doi: 10.1111/os.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Li K., Zhang C., Qiu L., Gao L., Zhang X. Tissue Eng. B Rev. 2017;23:399–411. doi: 10.1089/ten.TEB.2016.0427. [DOI] [PubMed] [Google Scholar]
- 375.Stegen S., Laperre K., Eelen G., Rinaldi G., Fraisl P., Torrekens S., Van Looveren R., Loopmans S., Bultynck G., Vinckier S. Nature. 2019;565:511–515. doi: 10.1038/s41586-019-0874-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Henrotin Y., Kurz B., Aigner T. Osteoarthritis Cartilage. 2005;13:643–654. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 377.Duval E., Baugé C., Andriamanalijaona R., Bénateau H., Leclercq S., Dutoit S., Poulain L., Galéra P., Boumédiene K. Biomaterials. 2012;33:6042–6051. doi: 10.1016/j.biomaterials.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 378.Legendre F., Ollitrault D., Hervieu M., Baugé C., Maneix L., Goux D., Chajra H., Mallein-Gerin F., Boumediene K., Galera P. Tissue Eng. C Methods. 2012;19:550–567. doi: 10.1089/ten.TEC.2012.0508. [DOI] [PubMed] [Google Scholar]
- 379.Meretoja V.V., Dahlin R.L., Wright S., Kasper F.K., Mikos A.G. Biomaterials. 2013;34:4266–4273. doi: 10.1016/j.biomaterials.2013.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Zubillaga V., Alonso-Varona A., Fernandes S.C.M., Salaberria A.M., Palomares T. Int. J. Mol. Sci. 2020;21:1004. doi: 10.3390/ijms21031004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Oragui E., Nannaparaju M., Khan W.S. Open Orthop. J. 2011;5:267–270. doi: 10.2174/1874325001105010267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Ma Y., Lin M., Huang G., Li Y., Wang S., Bai G., Lu T.J., Xu F. Adv. Mater. 2018;30:1705911. doi: 10.1002/adma.201705911. [DOI] [PubMed] [Google Scholar]
- 383.Du Y., Guo J.L., Wang J., Mikos A.G., Zhang S. Biomaterials. 2019;218:119334. doi: 10.1016/j.biomaterials.2019.119334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Chen J., Yuan Z., Liu Y., Zheng R., Dai Y., Tao R., Xia H., Liu H., Zhang Z., Zhang W. STEM CELLS Translational Medicine. 2017;6:982–991. doi: 10.5966/sctm.2016-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Correia C., Pereira A.L., Duarte A.R.C., Frias A.M., Pedro A.J., Oliveira J.T., Sousa R.A., Reis R.L. Tissue Eng. 2012;18:1979–1991. doi: 10.1089/ten.tea.2012.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Elder B.D., Athanasiou K.A. Tissue Eng. B Rev. 2009;15:43–53. doi: 10.1089/ten.teb.2008.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Schröder C., Hölzer A., Zhu G., Woiczinski M., Betz O.B., Graf H., Mayer-Wagner S., Müller P.E. J. Mech. Med. Biol. 2015;16:1650025. [Google Scholar]
- 388.Tarng Y.-W., Huang B.-F., Su F.-C. Int. Orthop. 2012;36:863–868. doi: 10.1007/s00264-011-1291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Correia V., Panadero J.A., Ribeiro C., Sencadas V., Rocha J.G., Gomez Ribelles J.L., Lanceros-Méndez S. Biomech. Model. Mechanobiol. 2016;15:471–478. doi: 10.1007/s10237-015-0698-5. [DOI] [PubMed] [Google Scholar]
- 390.Occhetta P., Mainardi A., Votta E., Vallmajo-Martin Q., Ehrbar M., Martin I., Barbero A., Rasponi M. Nature Biomedical Engineering. 2019;3:545–557. doi: 10.1038/s41551-019-0406-3. [DOI] [PubMed] [Google Scholar]
- 391.Paggi C.A., Venzac B., Karperien M., Leijten J.C.H., Le Gac S. Sensor. Actuator. B Chem. 2020;315:127917. [Google Scholar]
- 392.Steinmetz N.J., Aisenbrey E.A., Westbrook K.K., Qi H.J., Bryant S.J. Acta Biomater. 2015;21:142–153. doi: 10.1016/j.actbio.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 393.Wang W., Wan Y., Fu T., Zhou T., Tang X., Wu H., Liu C., Jagodzinski M. J. Biomed. Mater. Res. 2019;107:1294–1302. doi: 10.1002/jbm.a.36642. [DOI] [PubMed] [Google Scholar]
- 394.Zhang C., Qiu L., Gao L., Guan Y., Xu Q., Zhang X., Chen Q. Mater. Sci. Eng. C. 2016;69:262–267. doi: 10.1016/j.msec.2016.06.080. [DOI] [PubMed] [Google Scholar]
- 395.Huang A.H., Baker B.M., Ateshian G.A., Mauck R.L. Eur. Cell. Mater. 2012;24:29–45. doi: 10.22203/ecm.v024a03. [DOI] [PubMed] [Google Scholar]
- 396.Schätti O., Grad S., Salzmann G., Li Z., Alini M., Stoddart M. Eur. Cell. Mater. 2011;22:214–225. doi: 10.22203/ecm.v022a17. [DOI] [PubMed] [Google Scholar]
- 397.Vainieri M.L., Wahl D., Alini M., van Osch G.J.V.M., Grad S. Acta Biomater. 2018;81:256–266. doi: 10.1016/j.actbio.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 398.Assiotis A., Sachinis N.P., Chalidis B.E. J. Orthop. Surg. Res. 2012;7:24. doi: 10.1186/1749-799X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Hilz F.M., Ahrens P., Grad S., Stoddart M.J., Dahmani C., Wilken F.L., Sauerschnig M., Niemeyer P., Zwingmann J., Burgkart R. Bioelectromagnetics. 2014;35:116–128. doi: 10.1002/bem.21822. [DOI] [PubMed] [Google Scholar]
- 400.Stefani R.M., Barbosa S., Tan A.R., Setti S., Stoker A.M., Ateshian G.A., Cadossi R., Vunjak‐Novakovic G., Aaron R.K., Cook J.L. Biotechnol. Bioeng. 2020;117:1584–1596. doi: 10.1002/bit.27287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Freed L.E., Langer R., Martin I., Pellis N.R., Vunjak-Novakovic G. Proc. Natl. Acad. Sci. Unit. States Am. 1997;94:13885–13890. doi: 10.1073/pnas.94.25.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Guha Thakurta S., Kraft M., Viljoen H.J., Subramanian A. Acta Biomater. 2014;10:4798–4810. doi: 10.1016/j.actbio.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Subramanian A., Turner J.A., Budhiraja G., Thakurta S.G., Whitney N.P., Nudurupati S.S. Tissue Eng. C Methods. 2013;19:244–255. doi: 10.1089/ten.tec.2012.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wang X., Lin Q., Zhang T., Wang X., Cheng K., Gao M., Xia P., Li X. Stem Cell Res. Ther. 2019;10:41. doi: 10.1186/s13287-019-1142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Arthrex, Arthrex - BioCartilage® Extracellular Matrix, (accessed July, 2020). https://www.arthrex.com/orthobiologics/biocartilage-extracellular-matrix/resources.
- 406.Z. Biomet, DeNovo® NT Natural Tissue Graft, (accessed July, 2020). https://www.zimmerbiomet.com/medical-professionals/biologics/product/denovo-nt-natural-tissue.html.
- 407.Arthrex, Arthrex - Cartiform® Viable Osteochondral Allograft, (accessed July, 2020). https://www.arthrex.com/orthobiologics/cartiform.
- 408.Z. Biomet, Chondrofix® Osteochondral Allograft, (accessed July, 2020). https://www.zimmerbiomet.com/medical-professionals/biologics/product/chondrofix.html.
- 409.Smith+Nephew, CarGel Bioscaffold, (accessed July, 2020). https://www.smith-nephew.com/key-products/sports-medicine/bst-cargel/.
- 410.Wang D., Nawabi D.H., Krych A.J., Jones K.J., Nguyen J., Elbuluk A.M., Farshad-Amacker N.A., Potter H.G., Riley I.I.I., Williams J. CARTILAGE. 2020:1–12. doi: 10.1177/1947603520903418. [DOI] [Google Scholar]
- 411.Finceramica, Maioregen Osteochondral Substitute, (accessed July, 2020). http://www.finceramica.it/en/prodotti_servizi/chirurgia_ortopedica_e_spianle/maioregen_sostituto_osteocondrale.
- 412.C. Solutions, Collagen Solutions Plc | Positive Eight-Year Results of ChondroMimetic® Cartilage Repair Clinical Study, (accessed July, 2020). https://ir.collagensolutions.com/content/news/2018_ir_news/210218.
- 413.CartiHeal, Agili-C™, (accessed July, 2020). https://www.cartiheal.com/agili-c/.
- 414.Cheng N.-C., Estes B.T., Awad H.A., Guilak F. Tissue Eng. 2008;15:231–241. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Farr J., Tabet S.K., Margerrison E., Cole B.J. Am. J. Sports Med. 2014;42:1417–1425. doi: 10.1177/0363546514528671. [DOI] [PubMed] [Google Scholar]
- 416.Geraghty S., Kuang J.-Q., Yoo D., LeRoux-Williams M., Vangsness C.T., Danilkovitch A. J. Orthop. Surg. Res. 2015;10:66. doi: 10.1186/s13018-015-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Farr J., Gracitelli G.C., Shah N., Chang E.Y., Gomoll A.H. Am. J. Sports Med. 2016;44:2015–2022. doi: 10.1177/0363546516645086. [DOI] [PubMed] [Google Scholar]
- 418.Reynolds K.L., Bishai S.K. Osteoarthritis Cartilage. 2014;22:S155–S156. [Google Scholar]
- 419.Rhee C., Amar E., Glazebrook M., Coday C., Wong I.H. Orthopaedic Journal of Sports Medicine. 2018;6 doi: 10.1177/2325967118789871. 2325967118789871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Melton J.T., Wilson A.J., Chapman-Sheath P., Cossey A.J. Expet Rev. Med. Dev. 2010;7:333–341. doi: 10.1586/erd.10.15. [DOI] [PubMed] [Google Scholar]
- 421.Verhaegen J., Clockaerts S., Van Osch G.J.V.M., Somville J., Verdonk P., Mertens P. CARTILAGE. 2015;6:12–19. doi: 10.1177/1947603514548890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Kon E., Filardo G., Di Martino A., Busacca M., Moio A., Perdisa F., Marcacci M. Am. J. Sports Med. 2014;42:158–165. doi: 10.1177/0363546513505434. [DOI] [PubMed] [Google Scholar]
- 423.Getgood A., Henson F., Skelton C., Herrera E., Brooks R., Fortier L.A., Rushton N. CARTILAGE. 2012;3:351–363. doi: 10.1177/1947603512444597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Kon E., Filardo G., Robinson D., Eisman J.A., Levy A., Zaslav K., Shani J., Altschuler N. Knee Surg. Sports Traumatol. Arthrosc. 2014;22:1452–1464. doi: 10.1007/s00167-013-2467-2. [DOI] [PubMed] [Google Scholar]
- 425.Kon E., Robinson D., Verdonk P., Drobnic M., Patrascu J.M., Dulic O., Gavrilovic G., Filardo G. Injury. 2016;47:S27–S32. doi: 10.1016/S0020-1383(16)30836-1. [DOI] [PubMed] [Google Scholar]
- 426.Williams R.J., Gamradt S.C. Instr. Course Lect. 2008;57:563–571. [PubMed] [Google Scholar]
- 427.Bekkers J.E.J., Bartels L.W., Vincken K.L., Dhert W.J.A., Creemers L.B., Saris D.B.F. Am. J. Sports Med. 2013;41:1290–1295. doi: 10.1177/0363546513483536. [DOI] [PubMed] [Google Scholar]
- 428.Azam A., Forster M., Robertson A. J. Orthop. 2018;15:47–51. doi: 10.1016/j.jor.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Gelber P.E., Batista J., Millan-Billi A., Patthauer L., Vera S., Gomez-Masdeu M., Monllau J.C. Knee. 2014;21:827–832. doi: 10.1016/j.knee.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 430.Hindle P., Hendry J.L., Keating J.F., Biant L.C. Knee Surg. Sports Traumatol. Arthrosc. 2014;22:1235–1240. doi: 10.1007/s00167-013-2493-0. [DOI] [PubMed] [Google Scholar]
- 431.Joshi N., Reverte-Vinaixa M., Díaz-Ferreiro E.W., Domínguez-Oronoz R. Am. J. Sports Med. 2012;40:1289–1295. doi: 10.1177/0363546512441585. [DOI] [PubMed] [Google Scholar]
- 432.Kon E., Filardo G., Brittberg M., Busacca M., Condello V., Engebretsen L., Marlovits S., Niemeyer P., Platzer P., Posthumus M. Knee Surg. Sports Traumatol. Arthrosc. 2018;26:2704–2715. doi: 10.1007/s00167-017-4707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Brix M., Kaipel M., Kellner R., Schreiner M., Apprich S., Boszotta H., Windhager R., Domayer S., Trattnig S. Int. Orthop. 2016;40:625–632. doi: 10.1007/s00264-016-3118-2. [DOI] [PubMed] [Google Scholar]
- 434.Christensen B.B., Foldager C.B., Jensen J., Jensen N.C., Lind M. Knee Surg. Sports Traumatol. Arthrosc. 2016;24:2380–2387. doi: 10.1007/s00167-015-3538-3. [DOI] [PubMed] [Google Scholar]
- 435.Hopper N., Wardale J., Brooks R., Power J., Rushton N., Henson F. PloS One. 2015;10 doi: 10.1371/journal.pone.0133937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Matta C., Szűcs-Somogyi C., Kon E., Robinson D., Neufeld T., Altschuler N., Berta A., Hangody L., Veréb Z., Zákány R. Differentiation. 2019;107:24–34. doi: 10.1016/j.diff.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 437.Chubinskaya S., Di Matteo B., Lovato L., Iacono F., Robinson D., Kon E. Knee Surg. Sports Traumatol. Arthrosc. 2019;27:1953–1964. doi: 10.1007/s00167-018-5263-1. [DOI] [PubMed] [Google Scholar]
- 438.Kon E., Drobnic M., Davidson P.A., Levy A., Zaslav K., Robinson D. J. Sport Rehabil. 2014;23:270–275. doi: 10.1123/jsr.2013-0054. [DOI] [PubMed] [Google Scholar]
- 439.Kon E., Filardo G., Shani J., Altschuler N., Levy A., Zaslav K., Eisman J.E., Robinson D. J. Orthop. Surg. Res. 2015:10. doi: 10.1186/s13018-015-0211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Natasha N. The Future of Joint Repair. 2019 [Google Scholar]
- 441.Hunter W. Phil. Trans. 1742;42:514–521. 1683-1775. [Google Scholar]
- 442.Lim K.S., Abinzano F., Bernal P.N., Sanchez A.A., Atienza‐Roca P., Otto I.A., Peiffer Q.C., Matsusaki M., Woodfield T.B.F., Malda J. Advanced Healthcare Materials. 2020;9 doi: 10.1002/adhm.201901792. 1901792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Wang D.-A., Varghese S., Sharma B., Strehin I., Fermanian S., Gorham J., Fairbrother D.H., Cascio B., Elisseeff J.H. Nat. Mater. 2007;6:385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Zhou F., Hong Y., Zhang X., Yang L., Li J., Jiang D., Bunpetch V., Hu Y., Ouyang H., Zhang S. Applied Materials Today. 2018;13:32–44. [Google Scholar]
- 445.Rogers B.A., Chahal J., Gross A.E. In: Articular Cartilage of the Knee: Health, Disease and Therapy. Gahunia H.K., Gross A.E., Pritzker K.P.H., Babyn P.S., Murnaghan L., editors. Springer; New York, NY: 2020. pp. 427–443. [Google Scholar]
- 446.Huey D.J., Hu J.C., Athanasiou K.A. Science. 2012;338:917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Han L., Wang M., Li P., Gan D., Yan L., Xu J., Wang K., Fang L., Chan C.W., Zhang H. ACS Appl. Mater. Interfaces. 2018;10:28015–28026. doi: 10.1021/acsami.8b05314. [DOI] [PubMed] [Google Scholar]
- 448.Zhang J., Zhang X., Hong Y., Fu Q., He Q., Mechakra A., Zhu Q., Zhou F., Liang R., Li C. ACS Appl. Mater. Interfaces. 2020;12:22467–22478. doi: 10.1021/acsami.0c01776. [DOI] [PubMed] [Google Scholar]
- 449.Karami P., Wyss C.S., Khoushabi A., Schmocker A., Broome M., Moser C., Bourban P.-E., Pioletti D.P. ACS Appl. Mater. Interfaces. 2018;10:38692–38699. doi: 10.1021/acsami.8b10735. [DOI] [PubMed] [Google Scholar]
- 450.Learmonth D.A., Costa P.M., Veloso T.R., Cunha C.B., Cautela M.P., Correia C., Vallejo M.C., Sousa R.A. Biomaterials Science. 2020;8:3697–3711. doi: 10.1039/d0bm00286k. [DOI] [PubMed] [Google Scholar]
- 451.Khan I.M., Gilbert S.J., Singhrao S.K., Duance V.C., Archer C.W. Eur. Cell. Mater. 2008;16:26–39. doi: 10.22203/ecm.v016a04. [DOI] [PubMed] [Google Scholar]
- 452.Yodmuang S., Guo H., Brial C., Warren R.F., Torzilli P.A., Chen T., Maher S.A. J. Orthop. Res. 2019;37:845–854. doi: 10.1002/jor.24238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Malda J., Groll J., van Weeren P.R. Nat. Rev. Rheumatol. 2019;15:571–572. doi: 10.1038/s41584-019-0278-7. [DOI] [PubMed] [Google Scholar]
- 454.Sun Z., Feeney E., Guan Y., Cook S.G., Gourdon D., Bonassar L.J., Putnam D. Proc. Natl. Acad. Sci. Unit. States Am. 2019;116:12437–12441. doi: 10.1073/pnas.1900716116. [DOI] [PMC free article] [PubMed] [Google Scholar]