Abstract
Typically, progressive supranuclear palsy (PSP) is clinically characterized by slow vertical saccades or supranuclear gaze palsy, levodopa-resistant parkinsonism with predominant axial symptoms, and cognitive executive impairment. Over the past decades, various PSP phenotypes, including PSP with predominant parkinsonism, PSP with corticobasal syndrome, PSP with progressive gait freezing, and PSP with predominant frontal dysfunction, have been identified from pathologically confirmed cases. Expanding knowledge led to new diagnostic criteria for PSP that with increased disease awareness led to increased PSP prevalence estimates. The identification of environmental and modifiable risk factors creates an opportunity to intervene and delay the onset of PSP or slow disease progression. To date, despite the increasing number of publications assessing risk factors for PSP, few articles have focused on environmental and lifestyle risk factors for this disorder. In this article, we reviewed the literature investigating the relationship between PSP and several environmental and other modifiable lifestyle risk factors. In our review, we found that exposures to toxins related to diet, metals, well water, and hypertension were associated with increased PSP risk. In contrast, higher education and statins may be protective. Further case-control studies are encouraged to determine the exact role of these factors in the etiopathogenesis of PSP, which in turn would inform strategies to prevent and reduce the burden of PSP.
Keywords: Environmental exposure, Progressive supranuclear palsy, Risk factors
INTRODUCTION
Typically, progressive supranuclear palsy (PSP) is clinically characterized by vertical slowing of saccades or supranuclear gaze palsy, axial-predominant parkinsonism with poor response to levodopa therapy, bulbar symptoms, and cognitive impairment, specifically, frontal/executive dysfunction [1]. PSP is a primary four-repeat tauopathy that is caused by abnormal accumulation of pathologically altered microtubule-associated protein tau (MAPT) in neurons and glia [2]. Recently, the PSP diagnostic criteria have been revised to increase diagnostic sensitivity and diagnose additional phenotypes. In addition to the classic PSP with Richardson’s syndrome (PSP-RS) described above, phenotypes consisting of PSP with predominant parkinsonism, PSP with progressive gait freezing, PSP with corticobasal syndrome, and PSP with predominant frontal presentation were included [3]. The variants of PSP are distinguished by the predominant clinical features that appear primarily in the early years of the disease, but as the disease progresses, most phenotypes eventually convert to PSP-RS, which allows diagnosis.
The identification of modifiable risk factors for PSP may lead to an improved understanding of the pathophysiology and targeted prevention and therapeutic efforts. In Alzheimer’s disease (AD), another tauopathy, the management of 12 modifiable risk factors, including less education, hypertension, obesity, diabetes, depression, physical inactivity, excessive alcohol consumption, smoking, low social contact, hearing impairment, traumatic brain injury (TBI), and air pollution, might prevent or delay up to 40% of dementias [4]. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study showed that 2-year multidomain interventions for modifiable risk factors could improve cognition in an at-risk elderly population [5], resulting in worldwide harmonizing efforts to reduce modifiable risk factors in AD [6,7]. Similarly, understanding modifiable risk factors in PSP may inform prevention efforts and slow development through interventions. Over the past two decades, tremendous advancements in recognizing environmental and other modifiable risk factors for PSP have been made. Here, we review environmental and modifiable lifestyle risk factors for PSP, including dietary habits, residential areas, education, metal/chemical exposure, hypertension, and drugs.
METHODS
We searched PubMed using the following keywords: “progressive supranuclear palsy,” “epidemiology,” “risk factors,” and “environmental factors.” Subcategory queries were performed with the following terms: “prevalence,” “incidence,” “diet,” “residential areas,” “education,” “metals/chemicals,” “hypertension,” “smoking,” “stress,” “head trauma,” “hormones,” and “drugs.” Publications written in English were included, and the range of years queried was 1964 to 2020. We included all publication types, including original articles, case series, and systematic reviews.
EPIDEMIOLOGY OF PSP
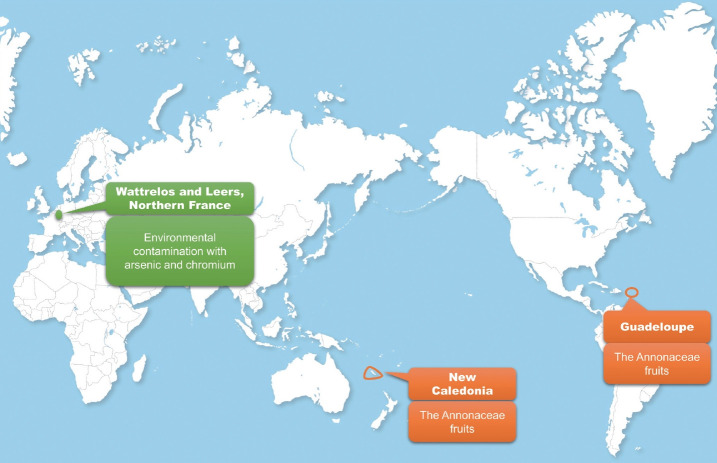
Table 1 shows a summary of studies regarding the prevalence and incidence of PSP. PSP prevalence estimates have increased from 1.39/100,000 to 17.9/100,000 over the past several decades [8,9]. The range in prevalence estimates is likely due to differences in diagnostic knowledge, diagnostic criteria, methodology, and study population. The incidence of PSP is 0.9–1.9/100,000 [10-12], increasing after the age of 60 years [10]. Intriguingly, geographical clusters have been reported in PSP [13-15], highlighting the potential contribution of environmental factors in PSP (Figure 1).
Table 1.
Prevalence and incidence of progressive supranuclear palsy
| Study | Year of investigation | Phenotype | Population denominator | Prevalence (per 100,000) |
Incidence (per 100,000) |
Methods | Region | ||
|---|---|---|---|---|---|---|---|---|---|
| Crude prevalence/sex-specific prevalence | Age- adjusted prevalence | Crude incidence/sex-specific incidence | Age-adjusted incidence | ||||||
| Golbe et al. [8] | 1982–1987 | PSP-RS | 799,022 | 1.39 (95% CI 0.8–2.4) | 7.00 | N/A | N/A | Survey of neurologists and chronic care facilities: mail or telephone | US, New Jersey |
| Men: 1.53, women: 1.23 | |||||||||
| Radhakrishnan et al. [93] | 1983–1986 | PSP-RS | 519,000 | N/A | N/A | 0.3 | - | Part of a survey of different neurological diseases through polyclinics, university hospitals, rehabilitation centers and one neurology center | Libya, Benghazi |
| Bower et al. [12] | 1976–1990 | PSP-RS | 1,424,474 | N/A | N/A | 1.1 | 5.3 | Medical records-linkage system of the Rochester Epidemiology Project | US, Minnesota, Olmsted County |
| Men:1.3 | Men:6.9 | ||||||||
| Women: 0.9 | Women: 4.1 | ||||||||
| Wermuth et al. [94] | 1993–1995 | PSP-RS | 43,709 | 4.6 | - | N/A | N/A | Data from pharmacies, hospitals, general practices, and nursing homes | Faroe Islands |
| Chiò et al. [95] | 1991 | PSP-RS | 61,830 | 3.2 | - | N/A | N/A | Medical records and pharmacies | Northwestern Italy |
| Schrag et al. [32] | 1994–1997 | PSP-RS | 121,608 | 4.9 (95% CI 1.0–10.7) | 6.4 (95% CI 2.3–10.6) | N/A | N/A | Computerized medical records of 15 general practices | UK, London |
| Nath et al. [36] | 1999 | PSP-RS | 59,236,500 | 0.3 (95% CI 0.3–0.4) | 0.3 (95% CI 0.2–0.3) | N/A | N/A | ‘Russian doll’ design | UK, national |
| 2,589,240 | 3.1 (95% CI 2.4–3.8) | 2.4 (95% CI 1.9–3.0) | National study: passive referral mechanisms | UK, regional | |||||
| Men: 2.4 (95% CI 1.3–3.3) | Regional study: collaborative network of neurologists and nonneurologists | ||||||||
| Women: 3.7 (95% CI 2.7–4.8) | |||||||||
| 259,998 | 6.5 (95% CI 3.4–9.7) | 5.0 (95% CI 2.5–7.5) | Community study: computerized records | UK, community | |||||
| Men: 6.2 (95% CI 1.9–10.5) | |||||||||
| Women: 6.9 (95% CI 2.4–11.4) | |||||||||
| Caparros-Lefebvre et al. [96] | 1996–2001 | PSP-RS | 422,000 | 14 | - | N/A | N/A | Patients with parkinsonism referred to the Department of Neurology of the French West Indies University Hospital | France, Guadeloupe |
| Kawashima et al. [18] | 1999–2002 | PSP-RS | 137,420 | 5.82 (95% CI: 1.78–9.86) | 5.03 | N/A | N/A | Medical records of the university hospital, general hospitals, and nursing homes | Japan, Yonago |
| Men: 9.14 (95% CI: 1.82–16.47) | Men: 7.92 | ||||||||
| Women: 2.75 (95% CI: 1.08–6.65) | Women: 2.27 | ||||||||
| Savica et al. [10] | 1991–2005 | PSP-RS | 1,424,474 | N/A | N/A | 0.9 | - | Diagnostic codes and the review of medical records | US, Minnesota, Olmsted County |
| Men: 1.1 | |||||||||
| Women: 0.6 | |||||||||
| Caparros-Lefebvre et al. [15] | 2005–2014 | PSP-RS, PSP-P, PSP-PAGF, PSP-FTD, PSP-AOS | 51,551 | N/A | N/A | The ratio of observed to expected PSP incidence 12.3 (95% CI 4.7–35.9) | - | Patients with parkinsonism in the Centre Hospitalier de Wattrelos | France, Wattrelos and Leers |
| Coyle-Gilchrist et al. [97] | 2013–2014 | FTD, PSP-RS, and CBS | 1,690,000 | 10.8 for FTLDassociated syndrome (95% CI 9.3–12.4) | - | 1.61 for FTLDassociated syndromes (95% CI 1.1–1.9) | - | The PiPPIN (Pick’s Disease and Progressive Supranuclear Palsy: Prevalence and Incidence) | UK, Cambridgeshire and Norfolk |
| Takigawa et al. [9] | 2009–2014 | PSP-RS, PSP-P, PSP-PAGF | 148,271 | 17.9 (95% CI 12.12–26.42) | 17.26 (95% CI 17.03–17.48) | N/A | N/A | Six surveys and medical records | Japan, Yonago |
| Men: 18.05 (95% CI 10.33–31.55) | Men: 18.14 (95% CI 17.81–18.48) | ||||||||
| Women: 17.76 (95% CI 10.38–30.39) | Women: 16.63 (95% CI 16.32–16.95) | ||||||||
| PSP-RS | 14.32 (95% CI 9.27–22.12) | 13.80 (95% CI 13.60–14.01) | |||||||
| Men: 10.53 (95% CI 5.10–21.74) | Men: 10.62 (95% CI 10.37–10.88) | ||||||||
| Women: 17.76 (95% CI 10.38–30.39) | Women: 16.63 (95% CI 16.32–16.95) | ||||||||
| Fluery et al. [11] | 2003–2012 | PSP-RS | 470,512 | 8.3 (95% CI 5.9–11.3) | 8.8 (95% CI 6.1–11.6) | 1.9 (95% CI 1.3–2.6) | 2.0 (95% CI 0.7–3.4) | Medical records from public hospitals, private neurologists and nursing homes | Switzerland, Geneva |
| Men: 9.7 (95% CI 6.1–14.6) | Men: 12.1 (95% CI 7.0–17.2) | Men: 2.4 (95% CI 1.5–3.7) | Men: 3.9 (95% CI 1.0–6.7) | ||||||
| Women: 7.0 (95% CI 4.1–11.2) | Women: 6.7 (95% CI 3.5–9.9) | Women: 1.4 (95% CI 0.7–2.3) | Women: 0.8 (95% CI 0.0–1.8) | ||||||
N/A: not available, CI: confidence interval, FTD: frontotemporal dementia, FTLD: frontotemporal lobar degeneration, PSP: progressive supranuclear palsy, PSP-RS: PSP-Richardson’s syndrome, PSP-P: PSP-parkinsonism, PSP-PAGF: PSP-pure akinesia with gait freezing, PSP-FTD: PSP-frontotemporal dementia, PSP-AOS: PSP-apraxia of speech, CBS: corticobasal syndrome, US: United States, UK: United Kingdom.
Figure 1.
Geographical clusters in progressive supranuclear palsy (PSP). A high prevalence of PSP in Guadeloupe and New Caledonia is associated with high consumption of Annonaceae fruits (orange circle). A cluster in northern France indicates that environmental contamination with metals may play a role in PSP (green circle).
In earlier studies, male preponderance was found to be a distinct feature of PSP [8,16,17]. A higher prevalence of PSP was found in men than in women in other recent studies [11,18]. A male preponderance, in which males account for 68–72% of PSP patients (sex ratio = 2.1–2.6:1), was also shown in Guadeloupe, a location of a geographical cluster of PSP [13,19]. Sex differences in the incidence of PSP were reported, showing a 1.1–1.3/100,000 incidence of PSP in men and a 0.6–0.9/100,000 incidence in women (Table 1) [10,12]. Furthermore, sex differences in PSP were prominent, specifically in pathologically confirmed cases [20]. When we reviewed the results of twelve studies from autopsy-confirmed PSP cases [21-31], we also found a male preponderance in PSP (1.3:1). However, such findings should be interpreted with caution, since selection bias could affect the results of pathological studies. Other studies reported no such preponderenace [9,32]. Some even reported a higher prevalence in women [29,33-36]. Therefore, it is still unclear whether PSP affects men more than women.
DIETARY HABITS
Chronic exposure to the Annonaceae family, such as Annona muricata (corossol, soursop, guanabana and graviola), Annona squamosa (pomme cannelle, sweepsop, sugar apple, and cherimoya), and Annona reticulata (cachiman, custard-apple and mamon), was reported to be associated with an increased risk of developing PSP-like features [13,19]. In contrast to 20–30% of atypical parkinsonism in other studies [37], a higher occurrence (75%) of atypical parkinsonism, including PSP, than Parkinson’s disease (PD) was reported in Guadeloupe [13]. Guadeloupean PSP developed levodopa-resistant parkinsonism, early postural instability and supranuclear oculomotor dysfunction, which were common features of PSP. However, distinguishing atypical features, including the frequency of tremors, dysautonomia, and the occurrence of hallucinations, were shown in patients with Guadeloupean PSP-like syndrome [19]. Researchers found that the consumption of tropical fruits had a stronger association [odds ratio (OR) = 23.61; 95% confidence interval (95% CI) = 4.19–133.10; p < 0.001) with atypical parkinsonism and PSP than with PD and a strong association (OR = 20.70; 95% CI = 4.64–92.33; p < 0.001) with atypical parkinsonism patients and PSP patients compared with controls after adjusting for age and sex [13]. The leaves of the Annonaceae family are used in traditional medicine as herbal tea for various reasons, such as to maintain general health, to solve digestive problems, or to be sedated [38]. Furthermore, the findings regarding the consumption of tropical fruits were similar to an association between the consumption of herbal tea of the Annonaceae family and atypical parkinsonism and PSP [13]. In addition, there was a high frequency of atypical parkinsonism in Afro-Caribbean and Indian population who lived in the UK. Interestingly, dietary intake of Annonaceae fruits was a common finding in the migrant population living in the UK [39]. In New Caledonia, a French South Pacific Island, where people eat Annonaceae fruits, atypical parkinsonism (46%) was also found to occur more frequently. Higher consumption of Annonaceae fruits was found in patients with atypical parkinsonism (73%) than in those with typical PD (34%) [14]. Recently, one patient with PSP with predominant speech and/or language dysfunction was reported in the Northeast US who was exposed to the same fruits. The clinical features described in this case might be associated with the consumption of 13.5 kg of raw pawpaw fruit over a 10-year period [40]. This was also observed in patients with PSP in a pilot study evaluating dietary and environmental factors in PSP patients and healthy controls conducted in Louisville, KY, and in Atlanta, GA, where Asimina triloba (Annonaceae family, American pawpaw) is eaten (Dr. I. Litvan, personal information; unreferenced).
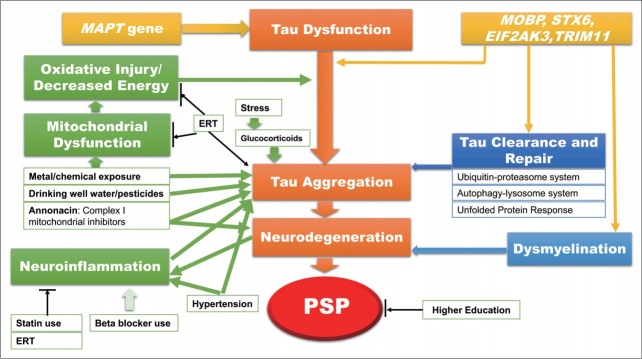
Annonaceae fruits contain three different types of neurotoxins, including benzyl-tetra-isoquinoline reticuline, tetrahydroprotoberberine coreximine, and annonacin [38]. These neurotoxins have detrimental effects on dopaminergic neurons [41-43]. Based on experimental data, the toxic effects of annonacin can be widespread from the nigrostriatal pathway to the striatum and the cortex through the impairment of energy metabolism [42,44] because annonacin is a very potent inhibitor of the mitochondrial respiratory chain at the complex I level [45]. The widespread involvement of annonacin in neurodegenerative changes, not restricted to dopaminergic neurons, could be explained by the fact that annonacin is not a substrate of the dopamine transporter [42]. Tau pathology caused by exposure to annonacin was demonstrated in experimental studies. In a transgenic mouse model, annonacin exposure can induce an increase in tau phosphorylation and in total tau protein levels in western blot analysis, the alteration of tau kinases, and somatodendritic accumulation of hyperphosphorylated tau [46,47]. Evidence regarding the association of annonacin and tau pathology may provide insight into tau pathologies in PSP. Annonacin may play a role in the pathomechanism as a mitochondrial inhibitor and a toxin to induce tau pathology and neurodegeneration (Figure 2).
Figure 2.
Hypothetical model of the pathomechanisms of progressive supranuclear palsy (PSP). The accumulation of pathogenic tau protein (orange box) plays a key role in the pathomechanisms of PSP. 1) Genetic factors (yellow box): Mutations in the MAPT gene, encoding tau protein, are causative in rare autosomal-dominant frontotemporal dementias, some of which present with the PSP phenotype. However, in sporadic PSP, the H1 haplotype of the MAPT gene is associated with sporadic PSP.[90] In view that approximately 78% of controls and approximately 94% of patients exhibit the H1 haplotype, this is likely increasing susceptibility.[90] In addition, variants in MOBP, STX6, EIF2AK3 (also known as PERK) [91] and TRIM11[92] are associated with abnormal tau accumulation and dysmyelination (blue box). 2) Environmental factors (green box): Annonaceae fruits contain a toxic compound, annonacin. Annonacin is a potent inhibitor of the mitochondrial respiratory chain at the complex I level. Mitochondrial dysfunction and oxidative injury are responsible for pathogenic tau accumulation. Metal/chemical exposure and drinking well water/pesticides might be involved in mitochondrial dysfunction and oxidative injury. Furthermore, annonacin induces abnormal tau pathology as well as neurodegeneration. Stress is associated with the development of neurofibrillary tangles via glucocorticoids. Hypertension is associated with an increased risk for PSP by inducing the aggravation of tau pathology and neuroinflammation. Inflammation may be associated with pathological tau accumulation and neurodegeneration. The use of statins and estrogen replacement therapy (ERT) may protect against PSP by reducing inflammation. In contrast, the use of beta blockers may increase the risk for PSP by exacerbating neuroinflammation (light green arrow). High educational attainment is also a protective factor in PSP, but the roles of education in the pathomechanisms remain unclear. Factors that increase the risk of PSP are indicated by green arrows, while those that decrease the risk are indicated by black arrows. The light green arrow represents the possible mechanism of increased risk for PSP with weak certainty.
One case-control study of PSP investigated dietary habits and found that PSP patients ate meat or poultry more frequently than controls (OR = 3.73; 95% CI = 1.57–8.86; p = 0.003) [48]. This finding is supported by the fact that the Mediterranean diet (MedDiet) and Mediterranean-Dietary Approach to Stop Hypertension (DASH) diet Intervention for Neurodegenerative Delay (MIND) diet, indicating high consumption of fresh vegetables and fruits and low consumption of red meats, are associated with a lower risk of AD [49,50]. The beneficial or detrimental effects of specific dietary habits on PSP need to be determined in future case-control studies.
RESIDENTIAL AREAS
Although a case-control study in France did not show an association of PSP with residential areas [48], and a multivariate analysis did not show statistical significance, PSP cases in the Environmental Genetic Progressive Supranuclear Palsy (ENGENE-PSP) study were associated with living in rural areas in a univariate analysis [51]. This finding could be supported by the significant association between drinking well water and PSP (OR = 1.23; 95% CI, 1.02–1.46; p = 0.03) in the ENGENE-PSP study, where the case-control study was much larger in size and the methodology was more robust than that of previous studies [51]. Davis et al. [52] did not find an association between drinking well water and PSP in a case-control study. However, this could be due to the small sample size in their study. Given that drinking water from the well might be associated with living near an agricultural habitat and with exposure to pesticides [51], an association between exposure to pesticides and PSP might be found.
Pesticides can disturb the function of mitochondria [53] and mitochondrial dysfunction either by the development of oxidative injury factors that lead to the hyperphosphorylation of tau and/or by decreased metabolism, also leading to tau hyperphosphorylation, and tau aggregation may represent one of the pathomechanisms of PSP (Figure 2). Therefore, it is biologically plausible that exposure to pesticides is associated with an increased risk for PSP. However, case-control studies in France and the US did not demonstrate any association between reported exposure to pesticides and PSP [35,48,51]. Moreover, assigned exposure to pesticide by the industrial hygienist and toxicologist did not find an association with PSP (OR = 0.89; 95% CI = 0.52–1.50; p = 0.82) in the ENGENE-PSP study [51]. Further larger sample size detailed studies are needed to better understand the role of specific pesticides in PSP.
EDUCATION
All studies except one study showed that low education attainment was a risk factor for PSP (Table 2) [35,48,51,54]. The report that high educational background was a risk factor for PSP was based on the results of a case-control study with a small sample size [52]. Furthermore, the opposite finding was shown by the same researchers [35]. They suggested that the discrepant findings might be caused by different referral areas between controls and PSP patients [35].
Table 2.
Association between education and progressive supranuclear palsy in case-control studies
| Study | Cases, n | Controls, n | Education, years | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|---|---|---|
| Davis et al. [52] | 50 | 50 | ≥ 12 | 3.10 | N/A | < 0.050 |
| Golbe et al. [35] | 91 | 104 | ≥ 12 | 0.35 | 0.12–0.95 | 0.022 |
| Vidal et al. [48] | 79 | 79 | < 9 | 2.60 | 1.30–5.60 | 0.010 |
| Litvan et al. [51] | 284 | 284 | ≥ 16 | 0.42 | 0.27–0.64 | < 0.001 |
| Kelly et al. [54] | 67 | 68 | ≥ 12 | 0.30 | N/A | 0.010 |
There are several reasons that may explain these findings. Golbe et al. [35] suggested that lower educational attainment might implicate a poor nutritional state during childhood in association with deficient neurological development. They also suggested that lower educational attainment during childhood might be associated with increased exposure to neurotoxic substances in work or home environments. People with low education may have jobs that are involved in exposure to environmental chemicals, leading to an increased risk for PSP, since it may be possible that there is an association between environmental toxins/chemicals and PSP [52]. However, the ENGENE-PSP case-control study showed that low education is an independent factor from years of use of well water [52]. In AD, in which pathological amyloid and tau abnormally accumulate, the cognitive reserve (CR) hypothesis explains the discrepancy between pathological changes in the brain and clinical symptoms [55]. Subjects with greater CR with higher education or occupational complexity may have reduced risk of developing dementia through protective and compensatory mechanisms. In addition, those with lower CR will show a faster decline after CR depletion as they can no longer compensate for pathological changes in the brain. A meta-analysis of 133 studies showed that low education increased the risk of dementia, including AD, vascular dementia, and dementia in general [56]. It remains uncertain whether the association between educational attainment and PSP could be explained by the CR hypothesis, although one of the important features in PSP is variable cognitive impairment, including frontal dementia.
METAL/CHEMICAL EXPOSURE
Metal exposure is associated with PSP. The ENGENE-PSP study, the largest case-control study of PSP, assigned chemical exposure showed a trend of increased odds (OR = 1.20; 95% CI = 0.99–1.45; p = 0.06), and assigned metal exposure had a trend of increased odds (OR = 1.30; 95% CI = 1.00–1.70; p = 0.05) in the univariate analysis. However, in the multivariate analysis adjusting for confounding factors, there was no association between ever assigned metal exposure and PSP (OR = 0.96; 95% CI = 0.53– 1.74; p = 0.89) [51]. On the other hand, an analysis of the veterans in the ENGENE-PSP study supported metal exposure as a risk factor for PSP. There was an association between firearm exposure during military service and PSP (OR 2.2; 95% CI = 1.0–5.0; p = 0.04), suggesting a possible association of lead with PSP [54]. Notably, metal exposure might be associated with the occurrence of PSP in a report of 92 PSP patients in a cluster occurring in Wattrelos and Leers, France (Figure 1). These regions were contaminated by textile-dyeing and leather tanning plants in which arsenic, a potential neurotoxin, was found in the soil. Residents living in areas near chemical plants might have eaten fruits and vegetables that were contaminated by metals [15]. Alquezar et al. [57] demonstrated experimental evidence of increased total and phosphorylated tau levels by chromium and nickel treatments, implicating metal exposure as a causal factor of tau pathology (Figure 2).
A few anecdotal reports suggested an association between organic solvents and PSP [58-60]. However, the sample size of the case series was too small, from one case to 12 cases. There was no association between organic solvent exposure and PSP (OR = 0.8; 95% CI = 0.89–1.41; p = 0.38) in the ENGENE-PSP study [51].
HYPERTENSION
Observational and case-control studies suggested that hypertension is associated with PSP.61-64 Although a small case-control study with 50 cases did not find an association between hypertension and PSP [52], Dubinsky and Jankovic [61] found that 43.7% of idiopathic PSP patients had hypertension in their observational study. In addition, they also found a 2.5-fold higher frequency of cerebrovascular disease in PSP patients than in PD patients (p < 0.001). An observational study in Switzerland found that up to 81% of PSP patients had presymptomatic, mostly transient and regressive, hypertension [62], while in Italy, only 24.1% of PSP patients had presymptomatic hypertension [65]. The ENGENE-PSP study confirmed a modest association between a history of hypertension (10 years before PSP symptom onset) and PSP (OR = 1.49; 95% CI = 1.05–2.13; p = 0.027) compared with healthy controls in univariate and multivariate analyses [64]. The findings of this study implicated that chronic hypertension itself might play a role in the development of PSP but did not support that hypertension may play a role in the development of cerebrovascular disease and lead to microinfarcts in susceptible neural structures of PSP because brain cerebrovascular disease was an exclusionary criterion for study inclusion. Chronic hypertension induced the aggregation of tau protein in AD and tau mouse models [66] and was associated with neuroinflammation [67,68]. This suggests biological plausibility for the association between hypertension and PSP (Figure 2). The exact pathogenic mechanisms of hypertension in tau pathologies of PSP need to be determined in future large-scale clinical studies and in experimental studies.
DRUGS
The use of beta blockers showed an association with PSP (OR = 2.0; 95% CI = 1.05–3.8; p = 0.034), while other drugs, such as calcium channel blockers, diuretics, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, did not show any association with PSP in the univariate analysis of the ENGENE-PSP study [64]. Several studies also reported an association between the use of beta blockers and an increased risk of PD [69,70], which is intriguing since this finding is common in both PD and PSP. In PD, the use of beta blockers induced an increase in SNCA mRNA and alpha-synuclein protein concentrations [69]. Despite the evidence showing the association between beta blockers and alpha- synuclein, a recent review article concluded that evidence supporting this finding is weak and that this finding might not affect the use of beta blockers in PD [71].
The possible mechanism of beta blockers in PSP might be associated with the exacerbation of neuroinflammation (Figure 2). A recent animal study showed that beta blockers induced markers of neuroinflammation in both wild-type and amyloid-beta precursor protein (APP) mouse models of AD [72]. The effects of beta blockers on tau pathology were discrepant in AD mouse models [73-75]. An association between the use of beta blockers and PSP as well as the plausible mechanism between the two should be further investigated.
It has been presumed that anti-inflammatory drugs might decrease the risk for PSP since neuroinflammation is correlated with tau aggregation [76]. However, an association between nonsteroidal anti-inflammatory drugs and PSP was not found in case-control studies [48,77]. Experimental evidence showing beneficial effects of statins via anti-inflammatory mechanisms [78] and epidemiological results suggesting a lowered risk of AD with use of statins [79] prompted us to investigate the association between statins and PSP (Figure 2) [80]. In contrast to anti-inflammatory drugs, statin use was inversely correlated with the risk for PSP at a trend level (OR = 0.65; 95% CI = 0.43–1.00; p = 0.05). In addition, it was associated with delayed onset, less severe motor impairment in terms of the PSP rating scale, Unified Parkinson’s Disease Rating Scale (UPDRS) part III, and UPDRS total score [80]. The results of the study indicate that statin use may provide a therapeutic option in the prevention and treatment of PSP, although future studies need to replicate the results. In earlier studies of AD to investigate the role of statins on low-density lipoprotein receptor-related protein 1 (LRP1), treatment with statins increased LRP1 expression, leading to increased clearance of amyloid-beta [81,82]. Intriguingly, a recent study found that LRP1 controls the endocytosis of tau and its subsequent spread [83]. Given that statins may affect the expression of LRP1 and LRP1 may be a key regulator of tau spread, the role of statins in LRP1 in PSP needs to be investigated in future studies.
Exogenous estrogen as well as endogenous estrogen may affect the development and management of a disease as a neuroprotective factor through anti-inflammatory activity, the inhibition of oxidative stress and mitochondrial dysfunction, and increased expression of neurotrophic factors (Figure 2) [84]. Sex differences are associated with chromosomal differences (XX versus XY chromosomes), gonadal differences, or hormonal differences [85]. A recent meta-analysis supported an association between hormone replacement therapy and AD with all-cause dementia [86]. In PSP, only one study from the ENGENE-PSP study investigated an association between lifetime estrogen exposure and PSP, suggesting a potential protective role of estrogen replacement therapy in PSP (OR = 0.52; 95% CI = 0.30–0.92; p = 0.03) [87]. Further studies are needed to determine the effects of sex hormones, specifically exogenous estrogen, in PSP.
OTHERS: STRESS, HEAD TRAUMA, AND SMOKING
Repeated stressful events may increase the risk for PSP. While the total number of life-changing events was not associated with an increased risk of PSP (OR = 0.9; 95% CI = 0.4–2.4; p = 0.76), the total number of highly stressful events increased the risk of PSP (OR = 3.4; 95% CI = 1.1–11.7; p = 0.04). The findings supported the role of glucocorticoids, which are released in response to stress, in the development of neurofibrillary tangles and the association between stress and tauopathies such as AD and PSP [88].
TBI is known to be a risk factor for other tauopathies, such as chronic traumatic encephalopathy and AD. Head trauma was not associated with the development of PSP [52,54]. Although one case-control study did not reach statistical significance, they found a higher occurrence of TBI in PSP cases (38.8%) than in controls (27.9%) [54].
Small case-control studies, consisting of 50 to 79 cases, did not find an association between PSP and smoking habits [48,52,89]. In the ENGENE-PSP study, a higher number of smoking pack-years were associated with PSP cases (OR = 1.15; 95% CI = 1.05–1.27; p = 0.001) in the univariate analysis but did not reach statistical significance in the multivariate analysis (OR = 1.10; 95% CI = 0.99–1.22; p = 0.08) [51].
CONCLUSION
This extensive literature review about environmental and modifiable factors in PSP shows that specific dietary habits, such as high consumption of Annonaceae family fruits, metal/chemical exposure, hypertension, and stress, are risk factors for PSP. High educational level and statin use may be protective. Future studies need to focus on modifiable factors in PSP, especially the effects of any specific dietary habit or physical exercise, through intervention studies along with disease-modifying drugs targeting tau pathology to prevent this devastating disease. Although associations between modifiable factors, including education, hypertension, drugs, stress, and PSP, have been shown, their specific role in the pathomechanism of PSP needs to be determined through experimental and clinical studies. The potential disease-modifying effects of statins should be confirmed in further studies. Prevention will be practicable once we understand the interaction between genes and identified environmental factors.
Acknowledgments
This research was supported by the Original Technology Research Program for Brain Science through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2018M3C7A1057137).
Footnotes
Conflicts of Interest
Dr. Litvan’s research is supported by National Institutes of Health grants 5P50AG005131-33, 2R01AG038791-06A, U01NS090259, U01NS100610, U01NS80818, R25NS098999, P20GM109025, U19 AG063911-1, and 1R21NS114764-01A1; the Parkinson Study Group; the Michael J Fox Foundation; the Parkinson Foundation; the Lewy Body Association; Roche; AbbVie; Biogen; EIP-Pharma; and Biohaven Pharmaceuticals. She was a member of a Lundbeck Advisory Board and Corticobasal Degeneration Solutions. She receives her salary from the University of California San Diego and as Chief Editor of Frontiers in Neurology.
Author Contributions
Conceptualization: Hee Kyung Park. Data curation: Hee Kyung Park. Formal analysis: Hee Kyung Park. Funding acquisition: Hee Kyung Park. Investigation: Hee Kyung Park, Sindana D. Ilango. Methodology: Hee Kyung Park, Sindana D. Ilango. Project administration: Hee Kyung Park, Sindana D. Ilango. Resources: Hee Kyung Park, Irene Litvan. Supervision: Sindana D. Ilango, Irene Litvan. Validation: Sindana D. Ilango, Irene Litvan. Visualization: Sindana D. Ilango, Irene Litvan. Writing—original draft: Hee Kyung Park. Writing—review & editing: Sindana D. Ilango, Irene Litvan.
REFERENCES
- 1.Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009;8:270–279. doi: 10.1016/S1474-4422(09)70042-0. [DOI] [PubMed] [Google Scholar]
- 2.Rösler TW, Tayaranian Marvian A, Brendel M, Nykänen NP, Höllerhage M, Schwarz SC, et al. Four-repeat tauopathies. Prog Neurobiol. 2019;180:101644. doi: 10.1016/j.pneurobio.2019.101644. [DOI] [PubMed] [Google Scholar]
- 3.Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32:853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 6.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg A, Mangialasche F, Ngandu T, Solomon A, Kivipelto M. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from FINGER to World-Wide FINGERS. J Prev Alzheimers Dis. 2020;7:29–36. doi: 10.14283/jpad.2019.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golbe LI, Davis PH, Schoenberg BS, Duvoisin RC. Prevalence and natural history of progressive supranuclear palsy. Neurology. 1988;38:1031–1034. doi: 10.1212/wnl.38.7.1031. [DOI] [PubMed] [Google Scholar]
- 9.Takigawa H, Kitayama M, Wada-Isoe K, Kowa H, Nakashima K. Prevalence of progressive supranuclear palsy in Yonago: change throughout a decade. Brain Behav. 2016;6:e00557. doi: 10.1002/brb3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol. 2013;70:859–866. doi: 10.1001/jamaneurol.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleury V, Brindel P, Nicastro N, Burkhard PR. Descriptive epidemiology of parkinsonism in the Canton of Geneva, Switzerland. Parkinsonism Relat Disord. 2018;54:30–39. doi: 10.1016/j.parkreldis.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology. 1997;49:1284–1288. doi: 10.1212/wnl.49.5.1284. [DOI] [PubMed] [Google Scholar]
- 13.Caparros-Lefebvre D, Elbaz A. Possible relation of atypical parkinsonism in the French West Indies with consumption of tropical plants: a casecontrol study. Lancet. 1999;354:281–286. doi: 10.1016/s0140-6736(98)10166-6. [DOI] [PubMed] [Google Scholar]
- 14.Angibaud G, Gaultier C, Rascol O. Atypical parkinsonism and Annonaceae consumption in New Caledonia. Mov Disord. 2004;19:603–604. doi: 10.1002/mds.20104. [DOI] [PubMed] [Google Scholar]
- 15.Caparros-Lefebvre D, Golbe LI, Deramecourt V, Maurage CA, Huin V, Buée-Scherrer V, et al. A geographical cluster of progressive supranuclear palsy in northern France. Neurology. 2015;85:1293–1300. doi: 10.1212/WNL.0000000000001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steele JC. Progressive supranuclear palsy. Brain. 1972;95:693–704. [PubMed] [Google Scholar]
- 17.Kristensen MO. Progressive supranuclear palsy--20 years later. Acta Neurol Scand. 1985;71:177–189. doi: 10.1111/j.1600-0404.1985.tb03186.x. [DOI] [PubMed] [Google Scholar]
- 18.Kawashima M, Miyake M, Kusumi M, Adachi Y, Nakashima K. Prevalence of progressive supranuclear palsy in Yonago, Japan. Mov Disord. 2004;19:1239–1240. doi: 10.1002/mds.20149. [DOI] [PubMed] [Google Scholar]
- 19.Lannuzel A, Höglinger GU, Verhaeghe S, Gire L, Belson S, Escobar-Khondiker M, et al. Atypical parkinsonism in Guadeloupe: a common risk factor for two closely related phenotypes? Brain. 2007;130:816–827. doi: 10.1093/brain/awl347. [DOI] [PubMed] [Google Scholar]
- 20.Santacruz P, Uttl B, Litvan I, Grafman J. Progressive supranuclear palsy: a survey of the disease course. Neurology. 1998;50:1637–1647. doi: 10.1212/wnl.50.6.1637. [DOI] [PubMed] [Google Scholar]
- 21.Litvan I, Mangone CA, McKee A, Verny M, Parsa A, Jellinger K, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry. 1996;60:615–620. doi: 10.1136/jnnp.60.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins SJ, Ahlskog JE, Parisi JE, Maraganore DM. Progressive supranuclear palsy: neuropathologically based diagnostic clinical criteria. J Neurol Neurosurg Psychiatry. 1995;58:167–173. doi: 10.1136/jnnp.58.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniel SE, de Bruin VM, Lees AJ. The clinical and pathological spectrum of Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy): a reappraisal. Brain. 1995;118:759–770. doi: 10.1093/brain/118.3.759. [DOI] [PubMed] [Google Scholar]
- 24.Birdi S, Rajput AH, Fenton M, Donat JR, Rozdilsky B, Robinson C, et al. Progressive supranuclear palsy diagnosis and confounding features: report on 16 autopsied cases. Mov Disord. 2002;17:1255–1264. doi: 10.1002/mds.10211. [DOI] [PubMed] [Google Scholar]
- 25.Colosimo C, Osaki Y, Vanacore N, Lees AJ. Lack of association between progressive supranuclear palsy and arterial hypertension: a clinicopathological study. Mov Disord. 2003;18:694–697. doi: 10.1002/mds.10392. [DOI] [PubMed] [Google Scholar]
- 26.Schofield EC, Caine D, Kril JJ, Cordato NJ, Halliday GM. Staging disease severity in movement disorder tauopathies: brain atrophy separates progressive supranuclear palsy from corticobasal degeneration. Mov Disord. 2005;20:34–39. doi: 10.1002/mds.20286. [DOI] [PubMed] [Google Scholar]
- 27.Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSPparkinsonism. Brain. 2005;128:1247–1258. doi: 10.1093/brain/awh488. [DOI] [PubMed] [Google Scholar]
- 28.O’Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131:1362–1372. doi: 10.1093/brain/awn065. [DOI] [PubMed] [Google Scholar]
- 29.Respondek G, Stamelou M, Kurz C, Ferguson LW, Rajput A, Chiu WZ, et al. The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord. 2014;29:1758–1766. doi: 10.1002/mds.26054. [DOI] [PubMed] [Google Scholar]
- 30.Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141:2181–2193. doi: 10.1093/brain/awy146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovacs GG, Lukic MJ, Irwin DJ, Arzberger T, Respondek G, Lee EB, et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020;140:99–119. doi: 10.1007/s00401-020-02158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet. 1999;354:1771–1775. doi: 10.1016/s0140-6736(99)04137-9. [DOI] [PubMed] [Google Scholar]
- 33.Maher ER, Lees AJ. The clinical features and natural history of the Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1986;36:1005–1008. doi: 10.1212/wnl.36.7.1005. [DOI] [PubMed] [Google Scholar]
- 34.Nath U, Ben-Shlomo Y, Thomson RG, Lees AJ, Burn DJ. Clinical features and natural history of progressive supranuclear palsy: a clinical cohort study. Neurology. 2003;60:910–916. doi: 10.1212/01.wnl.0000052991.70149.68. [DOI] [PubMed] [Google Scholar]
- 35.Golbe LI, Rubin RS, Cody RP, Belsh JM, Duvoisin RC, Grosmann C, et al. Follow-up study of risk factors in progressive supranuclear palsy. Neurology. 1996;47:148–154. doi: 10.1212/wnl.47.1.148. [DOI] [PubMed] [Google Scholar]
- 36.Nath U, Ben-Shlomo Y, Thomson RG, Morris HR, Wood NW, Lees AJ, et al. The prevalence of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) in the UK. Brain. 2001;124:1438–1449. doi: 10.1093/brain/124.7.1438. [DOI] [PubMed] [Google Scholar]
- 37.de Rijk MC, Tzourio C, Breteler MM, Dartigues JF, Amaducci L, Lopez-Pousa S, et al. Prevalence of parkinsonism and Parkinson’s disease in Europe: the EUROPARKINSON collaborative study. J Neurol Neurosurg Psychiatry. 1997;62:10–15. doi: 10.1136/jnnp.62.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lannuzel A, Ruberg M, Michel PP. Atypical parkinsonism in the Caribbean island of Guadeloupe: etiological role of the mitochondrial complex I inhibitor annonacin. Mov Disord. 2008;23:2122–2128. doi: 10.1002/mds.22300. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhuri KR, Hu MT, Brooks DJ. Atypical parkinsonism in Afro-Caribbean and Indian origin immigrants to the UK. Mov Disord. 2000;15:18–23. doi: 10.1002/1531-8257(200001)15:1<18::aid-mds1005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 40.Kaas B, Hillis AE, Pantelyat A. Progressive supranuclear palsy and pawpaw. Neurol Clin Pract. 2020;10:e17–e18. doi: 10.1212/CPJ.0000000000000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lannuzel A, Michel PP, Caparros-Lefebvre D, Abaul J, Hocquemiller R, Ruberg M. Toxicity of Annonaceae for dopaminergic neurons: potential role in atypical parkinsonism in Guadeloupe. Mov Disord. 2002;17:84–90. doi: 10.1002/mds.1246. [DOI] [PubMed] [Google Scholar]
- 42.Lannuzel A, Michel PP, Höglinger GU, Champy P, Jousset A, Medja F, et al. The mitochondrial complex I inhibitor annonacin is toxic to mesencephalic dopaminergic neurons by impairment of energy metabolism. Neuroscience. 2003;121:287–296. doi: 10.1016/s0306-4522(03)00441-x. [DOI] [PubMed] [Google Scholar]
- 43.Champy P, Höglinger GU, Féger J, Gleye C, Hocquemiller R, Laurens A, et al. Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: possible relevance for atypical parkinsonism in Guadeloupe. J Neurochem. 2004;88:63–69. doi: 10.1046/j.1471-4159.2003.02138.x. [DOI] [PubMed] [Google Scholar]
- 44.Potts LF, Luzzio FA, Smith SC, Hetman M, Champy P, Litvan I. Annonacin in Asimina triloba fruit: implication for neurotoxicity. NeuroToxicology. 2012;33:53–58. doi: 10.1016/j.neuro.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Alali FQ, Liu XX, McLaughlin JL. Annonaceous acetogenins: recent progress. J Nat Prod. 1999;62:504–540. doi: 10.1021/np980406d. [DOI] [PubMed] [Google Scholar]
- 46.Yamada ES, Respondek G, Müssner S, de Andrade A, Höllerhage M, Depienne C, et al. Annonacin, a natural lipophilic mitochondrial complex I inhibitor, increases phosphorylation of tau in the brain of FTDP-17 transgenic mice. Exp Neurol. 2014;253:113–125. doi: 10.1016/j.expneurol.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 47.Rottscholl R, Haegele M, Jainsch B, Xu H, Respondek G, Höllerhage M, et al. Chronic consumption of Annona muricata juice triggers and aggravates cerebral tau phosphorylation in wild-type and MAPT transgenic mice. J Neurochem. 2016;139:624–639. doi: 10.1111/jnc.13835. [DOI] [PubMed] [Google Scholar]
- 48.Vidal JS, Vidailhet M, Derkinderen P, de Gaillarbois TD, Tzourio C, Alpérovitch A. Risk factors for progressive supranuclear palsy: a case-control study in France. J Neurol Neurosurg Psychiatry. 2009;80:1271–1274. doi: 10.1136/jnnp.2008.149849. [DOI] [PubMed] [Google Scholar]
- 49.Wu L, Sun D. Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci Rep. 2017;7:41317. doi: 10.1038/srep41317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hosking DE, Eramudugolla R, Cherbuin N, Anstey KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement. 2019;15:581–589. doi: 10.1016/j.jalz.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 51.Litvan I, Lees PS, Cunningham CR, Rai SN, Cambon AC, Standaert DG, et al. Environmental and occupational risk factors for progressive supranuclear palsy: case-control study. Mov Disord. 2016;31:644–652. doi: 10.1002/mds.26512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis PH, Golbe LI, Duvoisin RC, Schoenberg BS. Risk factors for progressive supranuclear palsy. Neurology. 1988;38:1546–1552. doi: 10.1212/wnl.38.10.1546. [DOI] [PubMed] [Google Scholar]
- 53.Farkhondeh T, Mehrpour O, Forouzanfar F, Roshanravan B, Samarghandian S. Oxidative stress and mitochondrial dysfunction in organophosphate pesticide-induced neurotoxicity and its amelioration: a review. Environ Sci Pollut Res Int. 2020;27:24799–24814. doi: 10.1007/s11356-020-09045-z. [DOI] [PubMed] [Google Scholar]
- 54.Kelley KD, Checkoway H, Hall DA, Reich SG, Cunningham C, Litvan I. Traumatic brain injury and firearm use and risk of progressive supranuclear palsy among veterans. Front Neurol. 2018;9:474. doi: 10.3389/fneur.2018.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One. 2012;7:e38268. doi: 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alquezar C, Felix JB, McCandlish E, Buckley BT, Caparros-Lefebvre D, Karch CM, et al. Heavy metals contaminating the environment of a progressive supranuclear palsy cluster induce tau accumulation and cell death in cultured neurons. Sci Rep. 2020;10:569. doi: 10.1038/s41598-019-56930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Umeda Y, Sakata E. A case of progressive supranuclear palsy--neurotological findings and etiology. J Otolaryngol. 1978;7:409–414. [PubMed] [Google Scholar]
- 59.McCrank E, Rabheru K. Four cases of progressive supranuclear palsy in patients exposed to organic solvents. Can J Psychiatry. 1989;34:934–936. doi: 10.1177/070674378903400916. [DOI] [PubMed] [Google Scholar]
- 60.McCrank E. PSP risk factors. Neurology. 1990;40:1637. doi: 10.1212/wnl.40.10.1637-a. [DOI] [PubMed] [Google Scholar]
- 61.Dubinsky RM, Jankovic J. Progressive supranuclear palsy and a multi-infarct state. Neurology. 1987;37:570–576. doi: 10.1212/wnl.37.4.570. [DOI] [PubMed] [Google Scholar]
- 62.Ghika J, Bogousslavsky J. Presymptomatic hypertension is a major feature in the diagnosis of progressive supranuclear palsy. Arch Neurol. 1997;54:1104–1108. doi: 10.1001/archneur.1997.00550210038010. [DOI] [PubMed] [Google Scholar]
- 63.Papapetropoulos S, Singer C, McCorquodale D, Gonzalez J, Mash DC. Cause, seasonality of death and co-morbidities in progressive supranuclear palsy (PSP) Parkinsonism Relat Disord. 2005;11:459–463. doi: 10.1016/j.parkreldis.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 64.Rabadia SV, Litvan I, Juncos J, Bordelon Y, Riley DE, Standaert D, et al. Hypertension and progressive supranuclear palsy. Parkinsonism Relat Disord. 2019;66:166–170. doi: 10.1016/j.parkreldis.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 65.Fabbrini G, Vanacore N, Bonifati V, Colosimo C, Meco G. Presymptomatic hypertension in progressive supranuclear palsy. Arch Neurol. 1998;55:1153–1155. doi: 10.1001/archneur.55.8.1153. [DOI] [PubMed] [Google Scholar]
- 66.Díaz-Ruiz C, Wang J, Ksiezak-Reding H, Ho L, Qian X, Humala N, et al. Role of hypertension in aggravating abeta neuropathology of AD type and tau-mediated motor impairment. Cardiovasc Psychiatry Neurol. 2009;2009:107286. doi: 10.1155/2009/107286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fernández-Botrán R, Ahmed Z, Crespo FA, Gatenbee C, Gonzalez J, Dickson DW, et al. Cytokine expression and microglial activation in progressive supranuclear palsy. Parkinsonism Relat Disord. 2011;17:683–688. doi: 10.1016/j.parkreldis.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014:406960. doi: 10.1155/2014/406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mittal S, Bjørnevik K, Im DS, Flierl A, Dong X, Locascio JJ, et al. β2- Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease. Science. 2017;357:891–898. doi: 10.1126/science.aaf3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gronich N, Abernethy DR, Auriel E, Lavi I, Rennert G, Saliba W. β2- adrenoceptor agonists and antagonists and risk of Parkinson’s disease. Mov Disord. 2018;33:1465–1471. doi: 10.1002/mds.108. [DOI] [PubMed] [Google Scholar]
- 71.Hopfner F, Höglinger GU, Kuhlenbäumer G, Pottegård A, Wod M, Christensen K, et al. β-adrenoreceptors and the risk of Parkinson’s disease. Lancet Neurol. 2020;19:247–254. doi: 10.1016/S1474-4422(19)30400-4. [DOI] [PubMed] [Google Scholar]
- 72.Evans AK, Ardestani PM, Yi B, Park HH, Lam RK, Shamloo M. Betaadrenergic receptor antagonism is proinflammatory and exacerbates neuroinflammation in a mouse model of Alzheimer’s Disease. Neurobiol Dis. 2020;146:105089. doi: 10.1016/j.nbd.2020.105089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gliebus G, Lippa CF. The influence of β-blockers on delayed memory function in people with cognitive impairment. Am J Alzheimers Dis Other Demen. 2007;22:57–61. doi: 10.1177/1533317506295889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dobarro M, Orejana L, Aguirre N, Ramírez MJ. Propranolol restores cognitive deficits and improves amyloid and tau pathologies in a senescence-accelerated mouse model. Neuropharmacology. 2013;64:137–144. doi: 10.1016/j.neuropharm.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 75.Branca C, Wisely EV, Hartman LK, Caccamo A, Oddo S. Administration of a selective β2 adrenergic receptor antagonist exacerbates neuropathology and cognitive deficits in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2014;35:2726–2735. doi: 10.1016/j.neurobiolaging.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dani M, Wood M, Mizoguchi R, Fan Z, Walker Z, Morgan R, et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain. 2018;141:2740–2754. doi: 10.1093/brain/awy188. [DOI] [PubMed] [Google Scholar]
- 77.Marras C, Cunningham CR, Hou J, Proudfoot J, Standaert DG, Juncos J, et al. Anti-inflammatory drug use and progressive supranuclear palsy. Parkinsonism Relat Disord. 2018;48:89–92. doi: 10.1016/j.parkreldis.2017.11.346. [DOI] [PubMed] [Google Scholar]
- 78.Boimel M, Grigoriadis N, Lourbopoulos A, Touloumi O, Rosenmann D, Abramsky O, et al. Statins reduce the neurofibrillary tangle burden in a mouse model of tauopathy. J Neuropathol Exp Neurol. 2009;68:314–325. doi: 10.1097/NEN.0b013e31819ac3cb. [DOI] [PubMed] [Google Scholar]
- 79.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016;(1):CD003160. doi: 10.1002/14651858.CD003160.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bayram E, Marras C, Standaert DG, Kluger BM, Bordelon YM, Shprecher DR, et al. Progressive supranuclear palsy and statin use. Mov Disord. 2020;35:1253–1257. doi: 10.1002/mds.28038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deane R, Wu Z, Zlokovic BV. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke. 2004;35(11 Suppl 1):2628–2631. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- 82.Shinohara M, Sato N, Kurinami H, Takeuchi D, Takeda S, Shimamura M, et al. Reduction of brain β-amyloid (Aβ) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and Aβ clearance. J Biol Chem. 2010;285:22091–22102. doi: 10.1074/jbc.M110.102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rauch JN, Luna G, Guzman E, Audouard M, Challis C, Sibih YE, et al. LRP1 is a master regulator of tau uptake and spread. Nature. 2020;580:381–385. doi: 10.1038/s41586-020-2156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16:17–29. doi: 10.1038/nrn3856. [DOI] [PubMed] [Google Scholar]
- 85.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu M, Li M, Yuan J, Liang S, Chen Z, Ye M, et al. Postmenopausal hormone therapy and Alzheimer’s disease, dementia, and Parkinson’s disease: a systematic review and time-response meta-analysis. Pharmacol Res. 2020;155:104693. doi: 10.1016/j.phrs.2020.104693. [DOI] [PubMed] [Google Scholar]
- 87.Park HK, Ilango S, Charriez CM, Checkoway H, Riley D, Standaert DG, et al. Lifetime exposure to estrogen and progressive supranuclear palsy: environmental and Genetic PSP study. Mov Disord. 2018;33:468–472. doi: 10.1002/mds.27336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kelley KD, Peavy G, Edland S, Rogers W, Riley DE, Bordelon Y, et al. The role of stress as a risk factor for progressive supranuclear palsy. J Parkinsons Dis. 2017;7:377–383. doi: 10.3233/JPD-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vanacore N, Bonifati V, Fabbrini G, Colosimo C, Marconi R, Nicholl D, et al. Smoking habits in multiple system atrophy and progressive supranuclear palsy. Neurology. 2000;54:114–119. doi: 10.1212/wnl.54.1.114. [DOI] [PubMed] [Google Scholar]
- 90.Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- 91.Höglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang LS, Klei L, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jabbari E, Woodside J, Tan MMX, Shoai M, Pittman A, Ferrari R, et al. Variation at the TRIM11 locus modifies progressive supranuclear palsy phenotype. Ann Neurol. 2018;84:485–496. doi: 10.1002/ana.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Radhakrishnan K, Thacker AK, Maloo JC, Gerryo SE, Mousa ME. Descriptive epidemiology of some rare neurological diseases in Benghazi, Libya. Neuroepidemiology. 1988;7:159–164. doi: 10.1159/000110150. [DOI] [PubMed] [Google Scholar]
- 94.Wermuth L, Joensen P, Bünger N, Jeune B. High prevalence of Parkinson’s disease in the Faroe Islands. Neurology. 1997;49:426–432. doi: 10.1212/wnl.49.2.426. [DOI] [PubMed] [Google Scholar]
- 95.Chiò A, Magnani C, Schiffer D. Prevalence of Parkinson’s disease in Northwestern Italy: comparison of tracer methodology and clinical ascertainment of cases. Mov Disord. 1998;13:400–405. doi: 10.1002/mds.870130305. [DOI] [PubMed] [Google Scholar]
- 96.Caparros-Lefebvre D, Sergeant N, Lees A, Camuzat A, Daniel S, Lannuzel A, et al. Guadeloupean parkinsonism: a cluster of progressive supranuclear palsy-like tauopathy. Brain. 2002;125(Pt 4):801–811. doi: 10.1093/brain/awf086. [DOI] [PubMed] [Google Scholar]
- 97.Coyle-Gilchrist IT, Dick KM, Patterson K, Vázquez Rodríquez P, Wehmann E, Wilcox A, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86:1736–1743. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]