Abstract
Pain contributes substantially to reduced quality of life in individuals living with hidradenitis suppurativa (HS). Although improved understanding of HS pathogenesis and treatment has resulted in improved evidence-based HS management guidelines, comprehensive pain management guidelines have yet to be developed. Little HS-specific data exist to guide pharmacologic analgesia, however, recognizing HS pain as either acute or chronic and predominantly nociceptive (aching and gnawing pain due to tissue damage) versus neuropathic (burning type pain due to somatosensory nervous system dysfunction) provides a conceptual framework for applying outside pain management practices to HS management. This manuscript incorporates the best available evidence from the HS and pain literature to propose an HS pain algorithm that integrates psychological, pharmacological, and complementary and alternative treatment modalities.
Keywords: Hidradenitis suppurativa, pain management, treatment, acute pain, chronic pain, nociceptive, neuropathic
Capsule Summary
Pain in hidradenitis suppurativa has far-reaching mental and quality of life impacts. International hidradenitis suppurativa treatment guidelines offer limited guidance for pain management
This article proposes a dermatology-oriented algorithm for hidradenitis suppurativa pain management that is based on the pain’s chronicity and character (classified as nociceptive or neuropathic)
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the hair follicle usually presenting after puberty with painful, deep-seated, inflamed lesions in intertriginous skin.1 HS has one of the most devastating impacts on quality of life (QoL) of any skin disease2–4 due to poor mental health,5 lost work, impaired intimacy,6 chronic pain,7,8 and substance use disorders.9,10
Pain in HS correlates more highly with QoL impairment than even disease severity.7,8 In an international survey of 1299 HS patients, the majority rated their pain as moderate or higher, with 4.5% describing it as the “worst possible.”11 HS pain severity is comparable to chronic post-traumatic headaches and worse than blistering disorders, vulvar lichen sclerosis, vasculitis, and leg ulcers.5,12,13
HS pain is both nociceptive and neuropathic.13,14 Nociceptive pain occurs when signaling molecules at sites of tissue injury induce pain, whereas neuropathic pain arises from somatosensory nervous system dysfunction. In HS, nociceptive pain likely results from inflammation. Neuropathic HS pain may result from chronic inflammation causing peripheral neuroplastic changes and central sensitization.15
Addressing HS pain is critical to improve HS-related QoL and reduce morbidity from opioid and other substance use. Unfortunately, current HS therapies often provide inadequate pain relief, and studies of HS pain-directed therapies are sparse. This, coupled with limited clinical training in pain management among dermatologists, creates an environment where patients’ pain often goes medically untreated and patients may resort to self-management.16 This article reviews evidence-based guidelines for HS pain, incorporating the best available evidence and expert opinion from pain management, psychology, and HS patient advocacy to suggest a rational dermatology-oriented approach to managing HS pain.
Section I: Current Pain Management Guidelines and Evidence
National and international guideline recommendations17–25 for HS pain are summarized in Table 1. Many suggest acknowledging, measuring, and/or treating HS pain, although specific treatment suggestions vary. Intralesional triamcinolone (ILT) 5–10mg/mL19,21,23 and incision and drainage (I&D) are commonly recommended for acutely inflamed lesions and abscesses.23–25 Analgesia with nonsteroidal anti-inflammatory drugs (NSAIDs) and limiting opioid use are commonly suggested.17,25
Table 1:
Summary of major international hidradenitis suppurativa pain management guidelines
| Guideline | Recommendation statements | Specific therapies |
|---|---|---|
| US and Canadian HS Foundations25,a (2019) |
|
Acute Pain:
|
| British Association of Dermatologists21,b (2019) |
|
|
| HS ALLIANCE24,c (2019) |
|
|
| Brazilian Society of Dermatology23 (2019) |
|
|
| Canadian Dermatology Association22 (2018) |
|
|
| Canadian Consensus Guidelines19 (2017) |
|
|
| Swiss Guidelines20 (2017) |
|
|
| European HS Foundation18,d (2016) |
|
|
| European S1 Guidelines17 (2015) |
|
NSAIDS:
|
↑: Weak recommendation for the use of an intervention; GPP: Good Practice Point, derived from informal consensus;21 HS: hidradenitis suppurativa; I&D: incision and drainage; ILT: intralesional triamcinolone; NRS: numeric rating scale; NSAIDs: nonsteroidal anti-inflammatory drugs; PRO: patient-reported outcomes; QoL: quality of life; VAS: visual analogue scale; WHO: World Health Organization
Recommendation level and evidence grade according to Strength of recommendation taxonomy (SORT);120
Recommendations according to British Association of Dermatologists adoption of GRADE methodology;121
Levels of Evidence and Grades of Recommendation according to Oxford Centre for Evidence-based Medicine;122
Recommendations based on GRADE methodology;123
ILT is currently the only pain-directed therapy studied in HS, although these studies generated conflicting data. In one case series of 36 patients, 0.2–2.0 mL (mean 0.75mL) of ILT 10 mg/mL decreased pain from HS nodules and abscesses by 75% at 2 days (from 5.5 to 1.4 on 0–10 scale).26 In a second series of 53 patients with Hurley stage II HS, 0.5 mL of ILT 40 mg/mL significantly reduced pain (from 3.1 to 0.7 on 0–10 scale) and resolved 43.5% of treated lesions.27 However, a randomized controlled trial (RCT) of 67 inflamed lesions <2cm treated with 0.1 mL of ILT 10 mg/ml, 40 mg/ml, or normal saline failed to detect differences in pain reduction or lesion healing time.28
Section II: Review of Pain Management Strategies for Application in Hidradenitis Suppurativa
HS pain is complex and correlates strongly with mood, requiring a multidisciplinary approach that addresses pain and associated psychological distress. This section reviews psychological, pharmacologic, and alternative pain management strategies, although these approaches have not yet been studied specifically in HS.
Psychological Treatments
Depression is common in HS and strongly impacts pain perceptions.13,29 Evidence-based psychological treatments for chronic pain include cognitive behavioral therapy (CBT) and acceptance and commitment therapy (ACT).30–32
CBT is the first-line psychosocial chronic pain treatment.32,33 CBT utilizes problem-solving to reduce pain and psychological distress,33 emphasizing relationships between chronic pain, thoughts, emotions, and behaviors. For instance, pain-related cognitions (e.g., “this pain will last forever”) can impact mood (e.g., irritability, hopelessness, sadness) and behaviors that exacerbate pain (e.g., pushing through or isolating when in pain). Key CBT concepts include cognitive restructuring, relaxation, and activity pacing.34,35
ACT for chronic pain emphasizes psychological flexibility through acceptance, cognitive defusion, present moment awareness, self-as-context, values, and committed action.36 Derived from CBT, ACT helps patients distinguish physical pain and suffering. ACT aims to eliminate the experience of suffering when pain absence is not feasible.
CBT and ACT focus on self-regulation, pain education, and relaxation response, but differ in approach to handling pain-related cognitions.33 In CBT, patients are taught cognitive restructuring techniques to modify their thoughts, whereas ACT emphasizes less reactivity to pain distress.37 ACT may be more advantageous than CBT in poorly controlled HS as physical limitations may impact activity pacing in CBT.
Psychological chronic pain treatments and collaboration with behavioral health experts should be part of an integrated approach to HS pain. No studies have compared psychological treatment in chronic HS pain and further studies are needed to examine CBT and ACT’s efficacy in chronic HS pain.
Pharmacologic Agents
Pharmacologic analgesia may be used when HS-directed therapy fails to relieve HS pain and should be part of a multimodal treatment regimen, although there is minimal data on specific use for HS pain. Where data specific to skin pain are absent, efficacy in other conditions is discussed. Table 2 summarizes analgesics for potential utilization in HS pain.
Table 2:
Dosing and risk profiles for pharmacologic analgesics in hidradenitis suppurativa
| Refs | ||||
|---|---|---|---|---|
| Intralesional Triamcinolone | ↓ neutrophil extravasation and cytokine signaling | 10–40 mg/mL; 0.2–2.0 mL/lesion Strength and volume determined by lesion type, location, & size | Dermal and subcutaneous atrophy, dyspigmentation | 25–28 |
| Diclofenac topical | ns-COX inhibitor |
Diclofenac sodium gel 1%
|
Application site reaction | 39,43–45,124, 125 |
| Lidocaine topical | Neuronal Na+ channel blockade |
4–5% Cream : Apply to intact skin ≤ 6x daily 4% patch: Apply to intact for ≤ 12 hours/day; Use up to 3 patches simultaneously 2.5% lidocaine with 2.5% prilocaine (EMLA®): Apply max of 20g over a 200cm2 area for ≤ 4h |
Application site reaction | 46,125 |
| Menthol topical | Desensitizes C-type nerves; Na+ channel blockade | 4% ointment or cream: Apply up to 4x daily | Hypersensitivity reaction | 49,51,125–127 |
| Systemic Opioid-Sparing Analgesics | ||||
| Celecoxib | Selective COX-2 inhibition | 100–200 mg q12–24h |
CV events: ↑↑ (higher risk compared to non-selective NSAIDs) GI: ↓ (lower GI bleeding risk compared to non-selective NSAIDs) Renal: AKI |
52,128 |
| Diclofenac | ns-COX inhibitor (acetic acid) | 50 mg q8h |
CV events: ↑ GI: GI bleeding, metabolized via CYP2C9 Renal: AKI |
52,128, 129 |
| Ibuprofen | ns-COX inhibitor (propionic acid) | 400 mg q4–6h |
CV events: ↑ GI: GI bleeding, metabolized via CYP2C9 Renal: AKI; Other: Aseptic meningitis in SLE |
52,128, 129 |
| Naproxen | ns-COX inhibitor (propionic acid) |
Base: 250–500 mgql2h Sodium: † 275–550 mg q12h |
CV events: No association with CV death, NSAID of choice in patients with CV risk GI: GI bleeding Renal: AKI; Other: Aseptic meningitis in SLE |
56 |
| Acetaminophen | Inhibits CNS prostaglandin synthesis | 325–650 mg q4–6 h | Leading cause of liver failure in United States Administration with CYP450 inducers ↑ hepatotoxicity | 57–59,130 |
| Neuromodulatory Analgesics | ||||
| Gabapentin* | Binds α2δ subunit of voltage-dependent calcium channels |
Start: 300 mg × 1d; 300 mg BID × 1d; 300 mg TID × 1d, ↑ dose by 300 mg daily until reach max tolerated dose◊ Max Dose: 3600 daily, divided TID |
CNS: Addictive potential, difficult mentation, dizziness, fatigue, visual disturbances | 61–63,131, 132 |
| Pregabalin | Same as gabapentin |
Start: 150 mg daily divided BID/TID. After 1 week, ↑ to 300 mg daily. Within 2–4 more weeks, may ↑ as tolerated◊ Max Dose: 600 mg daily, divided BID/TID |
CNS: In addition to gabapentin effects, crosses BBB and may induce euphoria | 62 |
| Duloxetine* | SNRI |
Start: 30 mg daily. After 1 week, ↑ to 60 mg daily Max Dose: 120 mg daily (dose >60 mg is rarely more effective) |
Serotonergic (common): Headaches, fatigue, nausea, sexual dysfunction Noradrenergic (dose dependent): night sweats, xerostomia General: Increased suicidality in those <24 years old |
65 |
| Venlafaxine | SNRI |
Start: 37.5 – 75 mg daily, every week, ↑ dose by 75 mg/day Max Dose: 225 mg |
Duloxetine risks plus QTc prolongation | 66 |
| Nortriptyline* | TCA |
Start: 25 mg qHS, every 3–7 days, ↑ dose by 25 mg/day Max Dose: 150 mg |
Anticholinergic: Blurred vision, constipation, tachycardia, urinary retention, xerostomia Alpha-adrenergic: Dizziness, hypotension Histaminergic: Sedation General: Weight gain, QTc prolongation |
133 |
| Desipramine* | TCA | Same as nortriptyline | Similar to nortriptyline | 134 |
| Amitriptyline | TCA | Same as nortriptyline | Compared to other TCAs, ↑ anticholinergic effects, sedation, and weight gain | 67,135, 136 |
AKI: acute kidney injury; BBB: blood brain barrier; BID: two times daily; CNS: central nervous system; COX: cyclooxygenase; CV: cardiovascular; d: day; max: maximum; GI: gastrointestinal; h: hours; Na+: sodium; ns: non-selective; NSAID: nonsteroidal anti-inflammatory drug; qHS: every night at bedtime; SLE: systemic lupus erythematosus; SNRI: serotonin norepinephrine reuptake inhibitor; TCA: tricyclic antidepressant; TID: three times daily
Naproxen sodium has faster onset than base
denotes preferred medication in class due to more favorable side effect profile
Topical Therapies
Topical analgesia is generally well tolerated. Irritation and application site reactions are the most common adverse effects. Topical analgesia is often avoided in areas of skin breakdown to reduce systemic absorption.
Topical NSAIDs
Topical NSAIDs have been suggested for treating HS pain38 and are effective for acute and chronic musculoskeletal pain.39 Topical NSAIDs reach therapeutic levels in tissue at only 0.1–10% the plasma concentration of oral NSAIDs40 and accordingly cause fewer cardiovascular (CV) and gastrointestinal (GI) events than systemic NSAIDs.41,42 Risks of topical NSAIDs include application site reaction.43–45
Topical Lidocaine
Topical lidocaine has been used anecdotally to treat chronic neuropathic pain from post-herpetic, trigeminal, and post-traumatic neuralgia.46 Adverse effects primarily include local irritation.47
Menthol
A small case series suggested topical menthol lowered pain in cancer treatment-related neuropathic pain.48 Given thermal pain descriptions in HS and menthol’s cooling sensation,49–51 menthol may be a useful adjunct.13,16
Non-Opioid Systemic Analgesics
NSAIDs
Oral NSAIDs reduce pain and disability in musculoskeletal and other inflammatory disorders,52,53 making them first-line in HS-related pain. Medical comorbidities and observed effectiveness should be used to tailor NSAID selection.53,54 In patients with elevated GI bleeding risk and low CV risk, cyclooxygenase (COX)-2 selective NSAIDs and/or concomitant proton pump inhibitor administration may reduce adverse GI events compared to non-selective NSAIDs alone.54,55 Naproxen is the preferred NSAID in patients with CV risk factors.56 Avoid NSAIDs in patients with both high CV and high GI risk.55
Acetaminophen
Although popular, data conflict regarding acetaminophen’s use in musculoskeletal pain. High-quality evidence suggests acetaminophen is ineffective for low back pain,57,58 yet significantly decreases osteoarthritis-related hip and knee pain.59 Acetaminophen inhibits prostaglandin synthesis in the central nervous system, modulating pain without anti-inflammatory effects owing to minimal peripheral COX inhibition.60 Acetaminophen’s favorable side effect profile make it a first-line option.38 However, in this group’s experience, few HS patients find acetaminophen helpful.
Neuromodulatory Medications
Gabapentin and Pregabalin
Two small RCTs of anticonvulsants gabapentin and pregabalin demonstrated promise in treating pruritus,61,62 and a systematic review suggests efficacy in post-herpetic neuralgia and painful diabetic neuropathy.63 Gabapentin and pregabalin’s effectiveness in treating neuropathic pain suggest utility for HS pain management.
Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs)
Duloxetine is a first-line treatment for neuropathic pain in adults.64 SNRI effectiveness in neuropathic pain coupled with antidepressant and anxiolytic effects make them attractive options for HS pain.65 Side effects include headaches, nausea, fatigue, sexual dysfunction and serotonin syndrome. Degree of risks differs by agent.66 Venlafaxine may also be beneficial for chronic neuropathic pain but may prolong QTc.
Tricyclic Antidepressants (TCAs)
In a meta-analysis, amitriptyline reduced pain due to peripheral neuropathy, post-herpetic neuralgia, and mixed neuropathy.67 TCAs’ analgesic mechanism is incompletely understood but is independent of mood improvements.68 TCAs have anti-adrenergic, anti-histaminergic, anti-muscarinic, and anti-serotonergic activity causing xerostomia, blurry vision, and constipation. TCAs may also induce arrhythmias, dizziness, and orthostatic hypotension.69 Due to potential side effects, our group recommends experienced providers use TCAs like nortriptyline as second-line options for chronic neuropathic HS pain.
Opioids
Due to their risks, misuse potential, and lack of evidence for superiority compared to many previously discussed agents, this group recommends judicious short course opioid use for severe, acute HS pain.
Despite significantly better pain relief versus placebo, opioids do not outperform other opioid-sparing analgesics, particularly for pain lasting >3 months.70 In a meta-analysis of 96 RCTs, opioids performed similarly to NSAIDs, TCAs, and anticonvulsants in improving pain and physical function scores in neuropathic, nociceptive, central sensitization, and mixed pain.71
HS patients have increased odds of chronic opioid use (OR 1.53; 95% CI: 1.20–1.95)10 with risk factors including increasing age, smoking, and depression.10,71 Other opioid risks include sedation, constipation, cognitive impairment, urinary retention, respiratory suppression and serotonin syndrome in patients receiving certain synthetic opioids and SNRIs.72 Tramadol, with ~10% of morphine’s analgesic potential and SNRI action,73 has lower sedation, constipation, and respiratory depression risk versus other opioids.74,75 Subsets of opiates also possess immunosuppressive properties that may increase infection risk in those receiving concomitant immunomodulatory therapy.76
Low-Dose Naltrexone (LDN)
Naltrexone is a μ-opioid antagonist used off-label at very low doses to treat pain and inflammation in multiple sclerosis, Crohn’s disease, and fibromyalgia.77 Chronic LDN (1–5 mg daily) may increase opioid receptor density, sensitizing the body to exogenous and endogenous opioids.77,78 LDN may decrease pro-inflammatory cytokines implicated in HS, including interleukin (IL)-679 and tumor necrosis factor (TNF)-α.77,80,81 LDN is obtained from compounding pharmacies, its dose should be gradually titrated, and patients must be warned of vivid/disturbing dream risk. LDN should not be combined with opioids, as it may reverse their effects and precipitate withdrawal in patients on chronic opioids.
Complementary and Alternative Medicine (CAM)
Cannabinoids
Data for cannabinoids - tetrahydrocannabinol (THC), cannabidiol (CBD), and synthetic derivatives (nabilone) - in treating pain are mixed. Systematic reviews and meta-analyses of THC and CBD demonstrated ≥30% chronic pain reduction82 and ≥30% improvement in neuropathic pain,83 without statistical significance. Use in cancer and mixed pain conditions (fibromyalgia, rheumatoid arthritis, abdominal pain) failed to show benefit.83 In dermatology, cannabinoids may down-regulate inflammation in pruritic conditions like allergic contact dermatitis,84 and a topical endocannabinoid cream reduced uremic pruritus.85 Cannabis use is common among HS patients.86,87 However, it is unclear whether pain is the primary reason for cannabis use as most patients report using cannabis for pleasure before HS symptom development.87
Most cannabinoid products are unregulated by the United States Food and Drug Administration, complicating safety evaluation, and dosing.88 Cannabinoids may cause weight gain and adverse pulmonary effects when smoked. Prescribing requirements vary by state.
Acupuncture
Acupuncture effectively treats nociceptive pain from osteoarthritis, chronic headaches, and lumbago.89,90 However, a meta-analysis in neuropathic pain showed no benefit.91 Limited evidence in atopic dermatitis suggests that acupuncture improves insomnia but not pruritus or atopic dermatitis severity scores.92 Risks include granuloma formation,93 localized argyria,94 and sclerosing lipogranulomatosis.95 Acupuncture’s role in HS pain management requires further investigation.
Alpha Lipoic Acid (ALA)
ALA improves diabetic peripheral neuropathy symptoms, tendon reflex, and nerve conduction velocity.96 ALA (300–600mg/day) administered intravenously offered maximal relief,96,97 though ALA 600mg/day orally improved burning and stinging.98 ALA may improve blood flow and decrease IL-6 levels.99 Dose-dependent nausea is ALA’s primary side effect.98 ALA interferes with thyroid hormone conversion; thyroid-stimulating hormone should be monitored in patients receiving thyroxine.100 Autoimmune hypoglycemia is a rare potential side effect.101 ALA’s safety profile and efficacy in neuropathic pain make it a low-risk adjunctive option.
Curcumin
Curcumin 1000–1500 mg/day is safe, reduces pain and improves physical function in osteoarthritis.102–104 Curcumin, the dominant anti-inflammatory molecule in turmeric, may also inhibit inflammatory mediators including TNF-α, IL-1, IL-6, and COX-2,105,106 and reduce CV events, added benefits in HS.107
Section III: Proposed Hidradenitis Suppurativa Pain Management Algorithm
Data are lacking to guide pain management in HS; this remains a critical need in HS research. The proposed algorithms (Figure 1) are based on evidence in other diseases and author opinions. They are intended as a guide for practicing dermatologists. Individual patient comorbidities, preference, and provider experience must be weighed when developing an HS pain plan.
Figure 1:
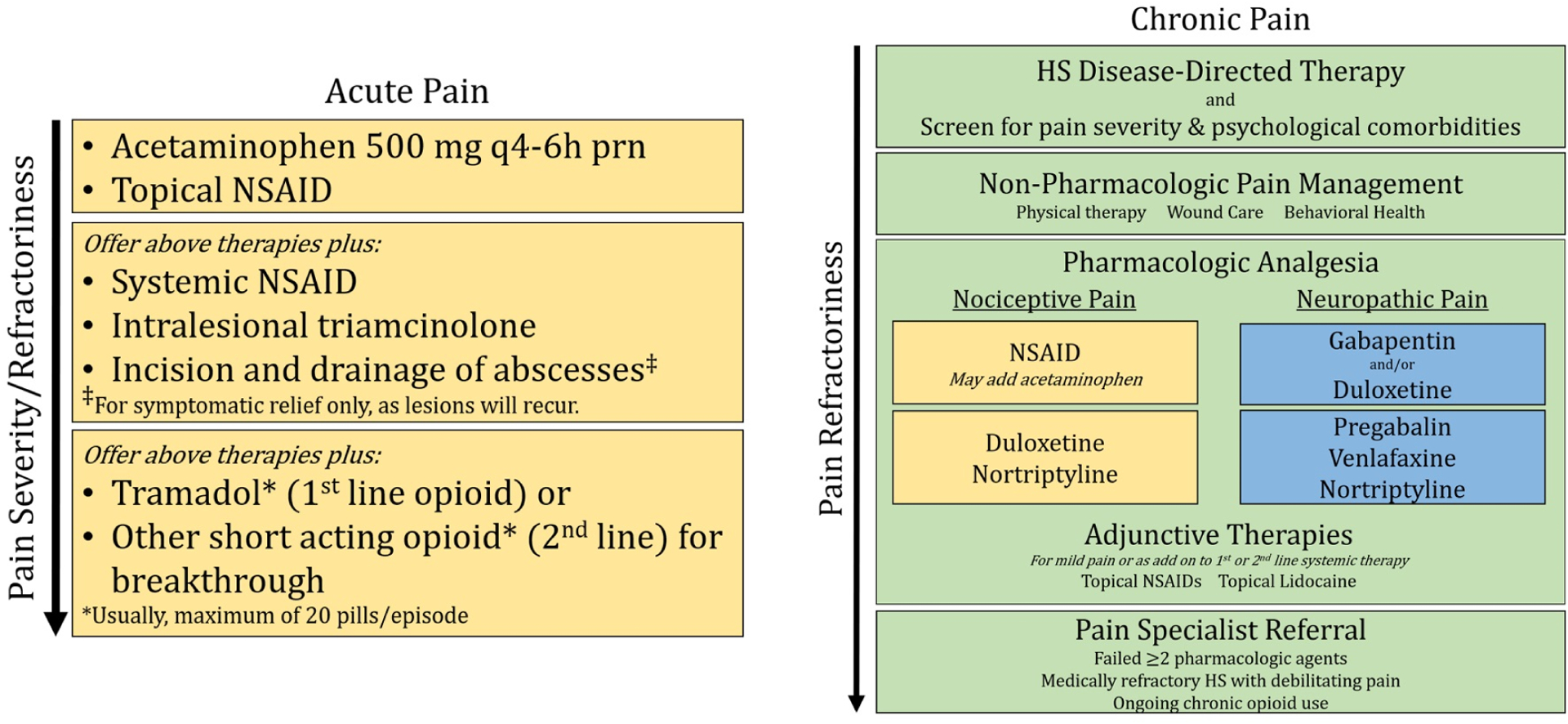
Proposed algorithm for treatment of acute pain and chronic pain in hidradenitis suppurativa.
Acute Pain: Acetaminophen and/or topical NSAIDs may be useful for mild acute pain, which is predominantly nociceptive. If acute pain persists, oral NSAIDs and/or intralesional triamcinolone may be helpful. If acute pain worsens or persists, first-line is tramadol or second-line acting opioid (*both usually maximum of 20 pills/episode). Referral to a pain management specialist is recommended before starting regular opioids.
Chronic Pain: As HS pain is related to disease activity and impact, HS disease-directed therapy and assessment of pain severity and psychological comorbidities is critical to reduce chronic HS pain. Physical therapy, wound care and behavioral health are non-pharmacologic chronic pain management tools. Understanding if the pain is nociceptive or neuropathic can guide the type of pharmacologic analgesia that may be most beneficial. For nociceptive pain (yellow boxes), first-line options include NSAIDs or celecoxib (COX-2 inhibitor), and second-line options include duloxetine (SNRI) or nortriptyline (TCA). For neuropathic pain (blue boxes), first-line options include gabapentin (anticonvulsant) and duloxetine, and second line are pregabablin, venlafaxine (SNRI) and nortriptyline. Anticonvulsants and SNRIs may be safely combined, however SNRIs should not be combined with other serotonergic agents. Adjunctive therapies for all patients with chronic pain include topical NSAIDs and topical lidocaine. Some patients may also be interested in other complementary and alternative medicine (CAM) options such as turmeric (curcumin), α- lipoic acid, acupuncture or medical cannabis. Referral to a pain specialist is recommended if the patient continues to experience chronic pain after utilizing these approaches, and before starting regular opioids.
Clinical evaluation of HS Pain
Pain measurement is vital for evaluating, monitoring, and treating HS pain. The Numeric Rating Scale (NRS) and Visual Analog Scale (VAS) have been widely validated for measuring pain severity,108 and are suggested for clinically assessing HS pain severity. Work is ongoing to suggest pain measurement tools for clinical trials,109 while other modalities including the HIDRAdisk offer rapid QoL assessment, accounting for several factors including pain.110
Analgesic efficacy differs by the pain type; therefore, differentiating nociceptive versus neuropathic pain may be helpful in analgesic selection for HS pain. Several instruments have been used to detect neuropathic pain (LANSS, painDETECT, and PROMIS-PQ-Neuro),111–113 but none are validated in HS. We suggest eliciting the patient’s description to assess pain character. Nociceptive pain usually occurs at HS lesions and may be described as aching, gnawing, pressure-like, stabbing, squeezing, or throbbing.114 Neuropathic pain, by contrast, may radiate and is described as burning, itching, pricking, shock-like, shooting, or tingling.114
Acute Pain
Acute HS pain often results from inflammation during flares; therefore, the acute pain algorithm emphasizes nociceptive pain treatments. Lower risk therapies, including acetaminophen and topical NSAIDs, are first-line for mild pain. For moderate or refractory pain, ILT, I&D, and/or systemic NSAIDs may offer relief. Limited courses of short-acting opiate may be considered for acute, refractory HS pain.
Chronic Pain
Figure 1 incorporates non-cancer pain management guidelines115–117 to propose a dermatologist-oriented algorithm for treating chronic HS pain. Chronic HS pain management begins with disease-directed therapies,25 pain severity evaluation, and identification of psychiatric comorbidities and substance abuse risk factors. If disease-directed therapy is inadequate, a multidisciplinary approach incorporating non-pharmacologic therapies such as physical therapy, wound care, and behavioral health may improve pain outcomes.118
HS causes nociceptive and neuropathic pain,13 and eliciting the predominant pain type may guide analgesic selection. We recommend pharmacologic treatment for chronic nociceptive pain begin with NSAIDs. In patients with low CV event risk, the selective COX-2 inhibitor, celecoxib, is preferred over non-selective NSAIDs due to lower GI event risk.54 Due to the paucity of data supporting acetaminophen for treating inflammatory nociceptive pain, acetaminophen plays a lesser role. Second-line therapies for chronic nociceptive pain include SNRIs and TCAs.
Pharmacologic analgesia for chronic neuropathic HS pain may begin with anticonvulsants or SNRIs. Gabapentin and duloxetine are considered first-line, whereas pregabalin and venlafaxine are recommended second-line given their increased side effect risk. Anticonvulsants and SNRIs may be safely combined. However, SNRIs should not be combined with other serotonergic agents. TCAs may also be beneficial in treating chronic neuropathic HS pain.
Pain specialists may offer higher risk pharmacologic therapy (e.g., muscle relaxants; neurologic agents; chronic opioids) and procedures (e.g., nerve blocks; implantable devices). Referral to pain specialists is warranted when a patient (1) fails at least two pharmacologic therapies, (2) has medically refractory HS and debilitating pain unlikely to improve despite maximal medical therapy, or (3) is using chronic opioids prescribed by generalists.
Conclusion
Pain has an immense impact on HS-related QoL. Effective, evidence-based pain management strategies remain an important unmet need. This article proposes algorithms for multimodal management of HS pain. Further studies are needed to evaluate the effectiveness of individual therapies as well as this algorithmic approach to managing HS pain.
Funding:
LAVO’s participation was supported in part by the Building Interdisciplinary Research Careers in Women’s Health of the National Institutes of Health under Award number K12D085850. The funding agency did not have any input in the study design, data collection, data analysis, manuscript publication, or publication decisions. VS’ participation was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award number UL1TR002378 and KL2TR002381. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ACT
Acceptance and Commitment Therapy
- ALA
Alpha Lipoic Acid
- CAM
Complementary and Alternative Medicine
- CBD
Cannabidiol
- CBT
Cognitive Behavioral Therapy
- COX
Cyclooxygenase
- CV
Cardiovascular
- FDA
Food and Drug Administration
- GI
Gastrointestinal
- HS
Hidradenitis Suppurativa
- I&D
Incision and Drainage
- IL
Interleukin
- ILT
Intralesional Triamcinolone
- LDN
Low Dose Naltrexone
- NSAID
Nonsteroidal Anti-inflammatory Drug
- OR
Odds Ratio
- RCT
Randomized Clinical Trial
- SNRI
Serotonin Norepinephrine Reuptake Inhibitor
- TCA
Tricyclic Antidepressant
- THC
Tetrahydrocannabinol
- TNF
Tumor Necrosis Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: LAVO has served as an investigator Chemocentryx; consulted for Chemocentryx, MedEd Consulting, Huron Consulting Group; and received lecture honoraria from Frontline Medical Communications. MAL has served on advisory boards for Abbvie, Janssen and Viela Bio, and consulted for Almirall, BSN, Incyte, Janssen, Kymera, and Xbiotech. VS serves as a consultant for Releviate. AMB, KTS, ZSP and CAY report no disclosures.
Statement on Prior Presentation: Portions of the material has been previously presented at the 2019 Summer AAD and 2019 Symposium for HS Advances.
References
- 1.Kurzen H, Kurokawa I, Jemec GBE, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17(5):455–456; discussion 457–472. doi: 10.1111/j.1600-0625.2008.00712_1.x [DOI] [PubMed] [Google Scholar]
- 2.Wolkenstein P, Loundou A, Barrau K, Auquier P, Revuz J. Quality of life impairment in hidradenitis suppurativa: A study of 61 cases. J Am Acad Dermatol. 2007;56:621–623. doi: 10.1016/j.jaad.2006.08.061 [DOI] [PubMed] [Google Scholar]
- 3.Hamzavi IH, Sundaram M, Nicholson C, et al. Uncovering burden disparity: A comparative analysis of the impact of moderate-to-severe psoriasis and hidradenitis suppurativa. Journal of the American Academy of Dermatology. 2017;77(6):1038–1046. doi: 10.1016/J.JAAD.2017.07.027 [DOI] [PubMed] [Google Scholar]
- 4.Chernyshov PV, Zouboulis CC, Tomas-Aragones L, et al. Quality of life measurement in hidradenitis suppurativa: position statement of the European Academy of Dermatology and Venereology task forces on Quality of Life and Patient-Oriented Outcomes and Acne, Rosacea and Hidradenitis Suppurativa. J Eur Acad Dermatol Venereol. 2019;33(9):1633–1643. doi: 10.1111/jdv.15519 [DOI] [PubMed] [Google Scholar]
- 5.Onderdijk AJ, van der Zee HH, Esmann S, et al. Depression in patients with hidradenitis suppurativa. Journal of the European Academy of Dermatology and Venereology. 2013;27(4):473–478. doi: 10.1111/j.1468-3083.2012.04468.x [DOI] [PubMed] [Google Scholar]
- 6.Janse IC, Deckers IE, van der Maten AD, et al. Sexual health and quality of life are impaired in hidradenitis suppurativa: a multicentre cross-sectional study. British Journal of Dermatology. 2017;176(4):1042–1047. doi: 10.1111/bjd.14975 [DOI] [PubMed] [Google Scholar]
- 7.Patel ZS, Hoffman LK, Buse DC, et al. Pain, Psychological Comorbidities, Disability, and Impaired Quality of Life in Hidradenitis Suppurativa [corrected]. Current pain and headache reports. 2017;21(12):49. doi: 10.1007/s11916-017-0647-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matusiak Ł, Szczęch J, Kaaz K, Lelonek E, Szepietowski JC. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Dermato-Venereologica. 2018;98(2):191–194. doi: 10.2340/00015555-2815 [DOI] [PubMed] [Google Scholar]
- 9.Garg A, Papagermanos V, Midura M, Strunk A, Merson J. Opioid, alcohol, and cannabis misuse among patients with hidradenitis suppurativa: A population-based analysis in the United States. Journal of the American Academy of Dermatology. 2018;79(3):495–500. doi: 10.1016/j.jaad.2018.02.053 [DOI] [PubMed] [Google Scholar]
- 10.Reddy S, Orenstein LAV, Strunk A, Garg A. Incidence of Long-term Opioid Use Among Opioid-Naive Patients With Hidradenitis Suppurativa in the United States. JAMA Dermatol. Published online September 11, 2019. doi: 10.1001/jamadermatol.2019.2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg A, Neuren E, Cha D, et al. Evaluating Patients’ Unmet Needs in Hidradenitis Suppurativa: results from the Global VOICE project. Journal of the American Academy of Dermatology. Published online 2019. doi: 10.1016/j.jaad.2019.06.1301 [DOI] [PubMed] [Google Scholar]
- 12.Balieva F, Kupfer J, Lien L, et al. The burden of common skin diseases assessed with the EQ5D™: a European multicentre study in 13 countries. British Journal of Dermatology. 2017;176(5):1170–1178. doi: 10.1111/bjd.15280 [DOI] [PubMed] [Google Scholar]
- 13.Nielsen RM, Lindsø Andersen P, Sigsgaard V, Theut Riis P, Jemec GB. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol. Published online March 28, 2019. doi: 10.1111/bjd.17935 [DOI] [PubMed] [Google Scholar]
- 14.Huilaja L, Hirvonen MJ, Lipitsä T, et al. Patients with hidradenitis suppurativa may suffer from neuropathic pain: A Finnish multicenter study. J Am Acad Dermatol. Published online November 12, 2019. doi: 10.1016/j.jaad.2019.11.016 [DOI] [PubMed] [Google Scholar]
- 15.van Straalen KR. Chronic Pain in Hidradenitis Suppurativa Explained Through the Process of Central Sensitization. JAMA Dermatol. Published online April 8, 2020. doi: 10.1001/jamadermatol.2020.0225 [DOI] [PubMed] [Google Scholar]
- 16.Ring HC, Theut Riis P, Miller IM, Saunte DM, Jemec GB. Self-reported pain management in hidradenitis suppurativa. Br J Dermatol. 2016;174(4):909–911. doi: 10.1111/bjd.14266 [DOI] [PubMed] [Google Scholar]
- 17.Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. Journal of the European Academy of Dermatology and Venereology. 2015;29(4):619–644. doi: 10.1111/jdv.12966 [DOI] [PubMed] [Google Scholar]
- 18.Gulliver W, Zouboulis CC, Prens E, Jemec GBE, Tzellos T. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016;17(3):343–351. doi: 10.1007/s11154-016-9328-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alavi A, Lynde C, Alhusayen R, et al. Approach to the Management of Patients With Hidradenitis Suppurativa: A Consensus Document. J Cutan Med Surg. 2017;21(6):513–524. doi: 10.1177/1203475417716117 [DOI] [PubMed] [Google Scholar]
- 20.Hunger RE, Laffitte E, Läuchli S, et al. Swiss Practice Recommendations for the Management of Hidradenitis Suppurativa/Acne Inversa. Dermatology (Basel). 2017;233(2–3):113–119. doi: 10.1159/000477459 [DOI] [PubMed] [Google Scholar]
- 21.Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019;180(5):1009–1017. doi: 10.1111/bjd.17537 [DOI] [PubMed] [Google Scholar]
- 22.Gulliver W, Landells IDR, Morgan D, Pirzada S. Hidradenitis Suppurativa: A Novel Model of Care and an Integrative Strategy to Adopt an Orphan Disease. J Cutan Med Surg. 2018;22(1):71–77. doi: 10.1177/1203475417736290 [DOI] [PubMed] [Google Scholar]
- 23.Magalhães RF, Rivitti-Machado MC, Duarte GV, et al. Consensus on the treatment of hidradenitis suppurativa - Brazilian Society of Dermatology. An Bras Dermatol. 2019;94(2 Suppl 1):7–19. doi: 10.1590/abd1806-4841.20198607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019;33(1):19–31. doi: 10.1111/jdv.15233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81(1):76–90. doi: 10.1016/j.jaad.2019.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): A case series. J Am Acad Dermatol. 2016;75(6):1151–1155. doi: 10.1016/j.jaad.2016.06.049 [DOI] [PubMed] [Google Scholar]
- 27.Álvarez P, García-Martínez FJ, Poveda I, Pascual JC. Intralesional Triamcinolone for Fistulous Tracts in Hidradenitis Suppurativa: An Uncontrolled Prospective Trial with Clinical and Ultrasonographic Follow-Up. Dermatology (Basel). Published online May 29, 2019:1–6. doi: 10.1159/000499934 [DOI] [PubMed] [Google Scholar]
- 28.Fajgenbaum K, Crouse L, Dong L, Zeng D, Sayed C. Intralesional Triamcinolone May Not Be Beneficial for Treating Acute Hidradenitis Suppurativa Lesions: A Double-Blind, Randomized, Placebo-Controlled Trial. Dermatol Surg. Published online September 2, 2019. doi: 10.1097/DSS.0000000000002112 [DOI] [PubMed] [Google Scholar]
- 29.Machado MO, Stergiopoulos V, Maes M, et al. Depression and Anxiety in Adults With Hidradenitis Suppurativa: A Systematic Review and Meta-analysis. JAMA Dermatol. Published online June 5, 2019. doi: 10.1001/jamadermatol.2019.0759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J, Klatt L-I, McCracken LM, Baumeister H. Psychological flexibility mediates the effect of an online-based acceptance and commitment therapy for chronic pain: an investigation of change processes. Pain. 2018;159(4):663–672. doi: 10.1097/j.pain.0000000000001134 [DOI] [PubMed] [Google Scholar]
- 31.Veehof MM, Oskam M-J, Schreurs KMG, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533–542. doi: 10.1016/j.pain.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 32.Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol. 2014;69(2):153–166. doi: 10.1037/a0035747 [DOI] [PubMed] [Google Scholar]
- 33.Darnall B Psychological Treatment for Patients with Chronic Pain. 1st Edition. American Psychological Association; 2019. [Google Scholar]
- 34.Barron BA. Otis JD, Managing chronic pain: a cognitive-behavioral therapy approach. Therapist guide. J Occup Rehabil. 2009;19(1):113. doi:J Occup Rehabil (2009) 19:113 [Google Scholar]
- 35.Beehler GP, Murphy JL, King PR, et al. Brief Cognitive Behavioral Therapy For Chronic Pain: Results From a Clinical Demonstration Project in Primary Care Behavioral Health. Clin J Pain. 2019;35(10):809–817. doi: 10.1097/AJP.0000000000000747 [DOI] [PubMed] [Google Scholar]
- 36.McCracken LM, Vowles KE. Acceptance and commitment therapy and mindfulness for chronic pain: model, process, and progress. Am Psychol. 2014;69(2):178–187. doi: 10.1037/a0035623 [DOI] [PubMed] [Google Scholar]
- 37.Dahl J, Lundgren T. Living Beyond Your Pain: Using Acceptance and Commitment Therapy to Ease Chronic Pain. 1st Edition. New Harbinger Publications, Inc; 2006. [Google Scholar]
- 38.Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 Suppl 1):S47–51. doi: 10.1016/j.jaad.2015.07.046 [DOI] [PubMed] [Google Scholar]
- 39.Derry S, Moore RA, Gaskell H, McIntyre M, Wiffen PJ. Topical NSAIDs for acute musculoskeletal pain in adults. Cochrane Database Syst Rev. 2015;2015(6). doi: 10.1002/14651858.CD007402.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rother M, Lavins BJ, Kneer W, Lehnhardt K, Seidel EJ, Mazgareanu S. Efficacy and safety of epicutaneous ketoprofen in Transfersome (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: multicentre randomised controlled trial. Ann Rheum Dis. 2007;66(9):1178–1183. doi: 10.1136/ard.2006.065128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31(10):2002–2012. [PubMed] [Google Scholar]
- 42.Lin T-C, Solomon DH, Tedeschi SK, Yoshida K, Kao Yang Y-H. Comparative Risk of Cardiovascular Outcomes Between Topical and Oral Nonselective NSAIDs in Taiwanese Patients With Rheumatoid Arthritis. J Am Heart Assoc. 2017;6(11). doi: 10.1161/JAHA.117.006874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novartis. Diclofenac Sodium Topical Gel (Voltaren Gel) Package Labeling. Published online 2007.
- 44.Heyneman CA, Lawless-Liday C, Wall GC. Oral versus topical NSAIDs in rheumatic diseases: a comparison. Drugs. 2000;60(3):555–574. doi: 10.2165/00003495-200060030-00004 [DOI] [PubMed] [Google Scholar]
- 45.Haroutiunian S, Drennan DA, Lipman AG. Topical NSAID therapy for musculoskeletal pain. Pain Med. 2010;11(4):535–549. doi: 10.1111/j.1526-4637.2010.00809.x [DOI] [PubMed] [Google Scholar]
- 46.Derry S, Wiffen PJ, Moore RA, Quinlan J. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(7):CD010958. doi: 10.1002/14651858.CD010958.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran AN, Koo JY. Risk of systemic toxicity with topical lidocaine/prilocaine: a review. J Drugs Dermatol. 2014;13(9):1118–1122. [PubMed] [Google Scholar]
- 48.Fallon MT, Storey DJ, Krishan A, et al. Cancer treatment-related neuropathic pain: proof of concept study with menthol—a TRPM8 agonist. Support Care Cancer. 2015;23(9):2769–2777. doi: 10.1007/s00520-015-2642-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel T, Ishiuji Y, Yosipovitch G. Menthol: a refreshing look at this ancient compound. J Am Acad Dermatol. 2007;57(5):873–878. doi: 10.1016/j.jaad.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 50.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719 [DOI] [PubMed] [Google Scholar]
- 51.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/s0092-8674(02)00652-9 [DOI] [PubMed] [Google Scholar]
- 52.Machado GC, Maher CG, Ferreira PH, Day RO, Pinheiro MB, Ferreira ML. Non-steroidal anti-inflammatory drugs for spinal pain: a systematic review and meta-analysis. Ann Rheum Dis. 2017;76(7):1269–1278. doi: 10.1136/annrheumdis-2016-210597 [DOI] [PubMed] [Google Scholar]
- 53.Enthoven WTM, Roelofs PDDM, Deyo RA, van Tulder MW, Koes BW. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087. doi: 10.1002/14651858.CD012087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burmester G, Lanas A, Biasucci L, et al. The appropriate use of non-steroidal anti-inflammatory drugs in rheumatic disease: opinions of a multidisciplinary European expert panel. Ann Rheum Dis. 2011;70(5):818–822. doi: 10.1136/ard.2010.128660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scarpignato C, Lanas A, Blandizzi C, Lems WF, Hermann M, Hunt RH. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis – an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015;13. doi: 10.1186/s12916-015-0285-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342. doi: 10.1136/bmj.c7086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saragiotto BT, Machado GC, Ferreira ML, Pinheiro MB, Shaheed CA, Maher CG. Paracetamol for low back pain. Cochrane Database of Systematic Reviews. 2016;(6). doi: 10.1002/14651858.CD012230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384(9954):1586–1596. doi: 10.1016/S0140-6736(14)60805-9 [DOI] [PubMed] [Google Scholar]
- 59.Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dietrich E, Talana A. 38: Anti-inflammatory, Antipyretic, and Analgesic Agents. In: Whalen K, Feild C, Radhakrishnan R, eds. Lippincott Illustrated Reviews: Pharmacology. 7th ed. Wolters Kluwer; 2019:508–526. [Google Scholar]
- 61.Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H. Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant. 2004;19(12):3137–3139. doi: 10.1093/ndt/gfh496 [DOI] [PubMed] [Google Scholar]
- 62.Matsuda KM, Sharma D, Schonfeld AR, Kwatra SG. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75(3):619–625.e6. doi: 10.1016/j.jaad.2016.02.1237 [DOI] [PubMed] [Google Scholar]
- 63.Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;2017(6). doi: 10.1002/14651858.CD007938.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lunn MPT, Hughes RAC, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115. doi: 10.1002/14651858.CD007115.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sansone RA, Sansone LA. Serotonin Norepinephrine Reuptake Inhibitors: A Pharmacological Comparison. Innov Clin Neurosci. 2014;11(3–4):37–42. [PMC free article] [PubMed] [Google Scholar]
- 67.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;(7):CD008242. doi: 10.1002/14651858.CD008242.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sansone RA, Sansone LA. Pain, Pain, Go Away. Psychiatry (Edgmont). 2008;5(12):16–19. [PMC free article] [PubMed] [Google Scholar]
- 69.Rey J. 10: Antidepressants. In: Whalen K, Feild C, Radhakrishnan R, eds. Lippincott Illustrated Reviews: Pharmacology, 7e. 7th ed. Wolters Kluwer; 2019. [Google Scholar]
- 70.Krebs EE, Gravely A, Nugent S, et al. Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain: The SPACE Randomized Clinical Trial. JAMA. 2018;319(9):872–882. doi: 10.1001/jama.2018.0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Busse JW, Wang L, Kamaleldin M, et al. Opioids for Chronic Noncancer Pain: A Systematic Review and Meta-analysis. JAMA. 2018;320(23):2448–2460. doi: 10.1001/jama.2018.18472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baldo BA. Opioid analgesic drugs and serotonin toxicity (syndrome): mechanisms, animal models, and links to clinical effects. Arch Toxicol. 2018;92(8):2457–2473. doi: 10.1007/s00204-018-2244-6 [DOI] [PubMed] [Google Scholar]
- 73.Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. Trends in Tramadol: Pharmacology, Metabolism, and Misuse. Anesth Analg. 2017;124(1):44–51. doi: 10.1213/ANE.0000000000001683 [DOI] [PubMed] [Google Scholar]
- 74.Schug SA. The role of tramadol in current treatment strategies for musculoskeletal pain. Ther Clin Risk Manag. 2007;3(5):717–723. [PMC free article] [PubMed] [Google Scholar]
- 75.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923. doi: 10.2165/00003088-200443130-00004 [DOI] [PubMed] [Google Scholar]
- 76.Wiese AD, Griffin MR, Schaffner W, et al. Long-acting Opioid Use and the Risk of Serious Infections: A Retrospective Cohort Study. Clin Infect Dis. 2019;68(11):1862–1869. doi: 10.1093/cid/ciy809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patten DK, Schultz BG, Berlau DJ. The Safety and Efficacy of Low-Dose Naltrexone in the Management of Chronic Pain and Inflammation in Multiple Sclerosis, Fibromyalgia, Crohn’s Disease, and Other Chronic Pain Disorders. Pharmacotherapy. 2018;38(3):382–389. doi: 10.1002/phar.2086 [DOI] [PubMed] [Google Scholar]
- 78.Tempel A, Gardner EL, Zukin RS. Neurochemical and functional correlates of naltrexone-induced opiate receptor up-regulation. J Pharmacol Exp Ther. 1985;232(2):439–444. [PubMed] [Google Scholar]
- 79.Witte-Händel E, Wolk K, Tsaousi A, et al. The IL-1 pathway is hyperactive in Hidradenitis suppurativa and contributes to skin infiltration and destruction. J Invest Dermatol. Published online December 5, 2018. doi: 10.1016/j.jid.2018.11.018 [DOI] [PubMed] [Google Scholar]
- 80.Hutchinson MR, Zhang Y, Brown K, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur J Neurosci. 2008;28(1):20–29. doi: 10.1111/j.1460-9568.2008.06321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vossen ARJV, Ardon CB, van der Zee HH, Lubberts E, Prens EP. The anti-inflammatory potency of biologics targeting tumour necrosis factor-α, interleukin (IL)-17A, IL-12/23 and CD20 in hidradenitis suppurativa: an ex vivo study. Br J Dermatol. 2019;181(2):314–323. doi: 10.1111/bjd.17641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015;313(24):2456–2473. doi: 10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- 83.Nugent SM, Morasco BJ, O’Neil ME, et al. The Effects of Cannabis Among Adults With Chronic Pain and an Overview of General Harms: A Systematic Review. Ann Intern Med. 2017;167(5):319–331. doi: 10.7326/M17-0155 [DOI] [PubMed] [Google Scholar]
- 84.Petrosino S, Cristino L, Karsak M, et al. Protective role of palmitoylethanolamide in contact allergic dermatitis. Allergy. 2010;65(6):698–711. doi: 10.1111/j.1398-9995.2009.02254.x [DOI] [PubMed] [Google Scholar]
- 85.Szepietowski JC, Szepietowski T, Reich A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: a preliminary study. Acta Dermatovenerol Croat. 2005;13(2):97–103. [PubMed] [Google Scholar]
- 86.Garg A, Papagermanos V, Midura M, Strunk A, Merson J. Opioid, alcohol, and cannabis misuse among patients with hidradenitis suppurativa: A population-based analysis in the United States. Journal of the American Academy of Dermatology. 2018;79(3):495–500.e1. doi: 10.1016/j.jaad.2018.02.053 [DOI] [PubMed] [Google Scholar]
- 87.Lesort C, Villani AP, Giai J, et al. High prevalence of cannabis use among patients with hidradenitis suppurativa: results from the VERADDICT survey. Br J Dermatol. 2019;181(4):839–841. doi: 10.1111/bjd.17930 [DOI] [PubMed] [Google Scholar]
- 88.Grinspoon P. Cannabidiol (CBD) - what we know and what we don’t. Harvard Health Publishing. Published August 24, 2018. Accessed March 17, 2020. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-we-dont-2018082414476 [Google Scholar]
- 89.Vickers AJ, Vertosick EA, Lewith G, et al. Acupuncture for chronic pain: update of an individual patient data meta-analysis. J Pain. 2018;19(5):455–474. doi: 10.1016/j.jpain.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005;(1):CD001351. doi: 10.1002/14651858.CD001351.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ju ZY, Wang K, Cui HS, et al. Acupuncture for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;12:CD012057. doi: 10.1002/14651858.CD012057.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kang S, Kim Y-K, Yeom M, et al. Acupuncture improves symptoms in patients with mild-to-moderate atopic dermatitis: A randomized, sham-controlled preliminary trial. Complement Ther Med. 2018;41:90–98. doi: 10.1016/j.ctim.2018.08.013 [DOI] [PubMed] [Google Scholar]
- 93.Alani RM, Busam K. Acupuncture granulomas. J Am Acad Dermatol. 2001;45(6 Suppl):S225–226. doi: 10.1067/mjd.2001.103993 [DOI] [PubMed] [Google Scholar]
- 94.Park MY, Lee JS, Jin HJ, et al. Localized argyria: troublesome side-effect of acupuncture. J Eur Acad Dermatol Venereol. 2018;32(2):e62–e65. doi: 10.1111/jdv.14526 [DOI] [PubMed] [Google Scholar]
- 95.Bashey S, Lee DS, Kim G. Extensive facial sclerosing lipogranulomatosis as a complication of cosmetic acupuncture. Dermatol Surg. 2015;41(4):513–516. doi: 10.1097/DSS.0000000000000318 [DOI] [PubMed] [Google Scholar]
- 96.Han T, Bai J, Liu W, Hu Y. A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur J Endocrinol. 2012;167(4):465–471. doi: 10.1530/EJE-12-0555 [DOI] [PubMed] [Google Scholar]
- 97.Bartkoski S, Day M. Alpha-Lipoic Acid for Treatment of Diabetic Peripheral Neuropathy. Am Fam Physician. 2016;93(9):786. [PubMed] [Google Scholar]
- 98.Ziegler D, Ametov A, Barinov A, et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29(11):2365–2370. doi: 10.2337/dc06-1216 [DOI] [PubMed] [Google Scholar]
- 99.Sola S, Mir MQS, Cheema FA, et al. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005;111(3):343–348. doi: 10.1161/01.CIR.0000153272.48711.B9 [DOI] [PubMed] [Google Scholar]
- 100.Segermann J, Hotze A, Ulrich H, Rao GS. Effect of alpha-lipoic acid on the peripheral conversion of thyroxine to triiodothyronine and on serum lipid-, protein- and glucose levels. Arzneimittelforschung. 1991;41(12):1294–1298. [PubMed] [Google Scholar]
- 101.Veltroni A, Zambon G, Cingarlini S, Davì MV. Autoimmune hypoglycaemia caused by alpha-lipoic acid: a rare condition in Caucasian patients. Endocrinol Diabetes Metab Case Rep. 2018;2018. doi: 10.1530/EDM-18-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu X, Machado GC, Eyles JP, Ravi V, Hunter DJ. Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br J Sports Med. 2018;52(3):167–175. doi: 10.1136/bjsports-2016-097333 [DOI] [PubMed] [Google Scholar]
- 103.Soleimani V, Sahebkar A, Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother Res. 2018;32(6):985–995. doi: 10.1002/ptr.6054 [DOI] [PubMed] [Google Scholar]
- 104.Hewlings SJ, Kalman DS. Curcumin: A Review of Its’ Effects on Human Health. Foods. 2017;6(10). doi: 10.3390/foods6100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. Biofactors. 2013;39(1):69–77. doi: 10.1002/biof.1066 [DOI] [PubMed] [Google Scholar]
- 106.Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim). 2010;343(9):489–499. doi: 10.1002/ardp.200900319 [DOI] [PubMed] [Google Scholar]
- 107.Li C, Miao X, Li F, et al. Curcuminoids: Implication for inflammation and oxidative stress in cardiovascular diseases. Phytother Res. 2019;33(5):1302–1317. doi: 10.1002/ptr.6324 [DOI] [PubMed] [Google Scholar]
- 108.Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101(1):17–24. doi: 10.1093/bja/aen103 [DOI] [PubMed] [Google Scholar]
- 109.Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179(3):642–650. doi: 10.1111/bjd.16672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fabbrocini G, Marasca C, Megna M, Peris K. Age and gender influence on HIDRAdisk outcomes in adalimumab-treated hidradenitis suppurativa patients. Journal of the European Academy of Dermatology and Venereology. 2019;33(S6):25–27. doi: 10.1111/jdv.15821 [DOI] [PubMed] [Google Scholar]
- 111.Bennett M The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92(1–2):147–157. doi: 10.1016/s0304-3959(00)00482-6 [DOI] [PubMed] [Google Scholar]
- 112.Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. doi: 10.1185/030079906X132488 [DOI] [PubMed] [Google Scholar]
- 113.Askew RL, Cook KF, Keefe FJ, et al. A PROMIS Measure of Neuropathic Pain Quality. Value Health. 2016;19(5):623–630. doi: 10.1016/j.jval.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilkie DJ, Huang HY, Reilly N, Cain KC. Nociceptive and neuropathic pain in patients with lung cancer: a comparison of pain quality descriptors. J Pain Symptom Manage. 2001;22(5):899–910. doi: 10.1016/s0885-3924(01)00351-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jongen JLM, Hans G, Benzon HT, Huygen F, Hartrick CT. Neuropathic Pain and harmacological Treatment. Pain Practice. 2014;14(3):283–295. doi: 10.1111/papr.12085 [DOI] [PubMed] [Google Scholar]
- 116.Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35–42. doi: 10.1016/j.pain.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 117.Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet (London, England). 2011;377(9784):2226–2235. doi: 10.1016/S0140-6736(11)60402-9 [DOI] [PubMed] [Google Scholar]
- 118.Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ (Online). 2015;350. doi: 10.1136/bmj.h444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.World Health Organization. Cancer pain relief. Published online 1996.
- 120.Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Am Board Fam Pract. 2004;17(1):59–67. doi: 10.3122/jabfm.17.1.59 [DOI] [PubMed] [Google Scholar]
- 121.Mohd Mustapa MF, Exton LS, Bell HK, et al. Updated guidance for writing a British Association of Dermatologists clinical guideline: the adoption of the GRADE methodology 2016. Br J Dermatol. 2017;176(1):44–51. doi: 10.1111/bjd.15201 [DOI] [PubMed] [Google Scholar]
- 122.Oxford Centre for Evidence-based Medicine - Levels of Evidence (March 2009). CEBM. Published June 11, 2009. Accessed March 25, 2020. https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/
- 123.Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ. 2008;336(7652):1049–1051. doi: 10.1136/bmj.39493.646875.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Derry S, Conaghan P, Da Silva JAP, Wiffen PJ, Moore RA. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;4:CD007400. doi: 10.1002/14651858.CD007400.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–D1082. doi: 10.1093/nar/gkx1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cliff MA, Green BG. Sensory irritation and coolness produced by menthol: evidence for selective desensitization of irritation. Physiol Behav. 1994;56(5):1021–1029. doi: 10.1016/0031-9384(94)90338-7 [DOI] [PubMed] [Google Scholar]
- 127.Haeseler G, Maue D, Grosskreutz J, et al. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur J Anaesthesiol. 2002;19(8):571–579. doi: 10.1017/s0265021502000923 [DOI] [PubMed] [Google Scholar]
- 128.Enthoven WTM, Roelofs PDDM, Deyo RA, van Tulder MW, Koes BW. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087. doi: 10.1002/14651858.CD012087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mamdani M, Rochon PA, Juurlink DN, et al. Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs. BMJ. 2002;325(7365):624. doi: 10.1136/bmj.325.7365.624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–1372. doi: 10.1002/hep.20948 [DOI] [PubMed] [Google Scholar]
- 131.Hah J, Mackey SC, Schmidt P, et al. Effect of Perioperative Gabapentin on Postoperative Pain Resolution and Opioid Cessation in a Mixed Surgical Cohort: A Randomized Clinical Trial. JAMA Surg. 2018;153(4):303–311. doi: 10.1001/jamasurg.2017.4915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhai LL, Savage KT, Qiu CC, Jin A, Valdes-Rodriguez R, Mollanazar NK. Chronic Pruritus Responding to Dupilumab—A Case Series. Medicines (Basel). 2019;6(3). doi: 10.3390/medicines6030072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Derry S, Wiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209. doi: 10.1002/14651858.CD011209.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;(9):CD011003. doi: 10.1002/14651858.CD011003.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of Low-Dose Amitriptyline for Chronic Low Back Pain: A Randomized Clinical Trial. JAMA Intern Med. 2018;178(11):1474–1481. doi: 10.1001/jamainternmed.2018.4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;12:CD008242. doi: 10.1002/14651858.CD008242.pub2 [DOI] [PubMed] [Google Scholar]


