Abstract
Background
For evidence-based decision making, primary care physicians need to have specific and reliable information on the pre-test probabilities of underlying diseases and a symptom’s course. We performed a systematic review of symptom-evaluating studies in primary care, following three research questions: (1) What is the prevalence of the symptom cough in children consulting primary care physicians? (2) What are the underlying aetiologies of cough and the respective frequencies? (3) What is the prognosis of children with cough?
Methods
Following a pre-defined algorithm and independent double reviewer ratings we searched MEDLINE and EMBASE. All quantitative original research articles in English, French or German were included if they focused on unselected study populations of children consulting a primary care physician for cough. We used the random effects model for meta-analysis in subgroups, if justifiable in terms of heterogeneity.
Results
We identified 14 eligible studies on prevalence, five on aetiology and one on prognosis. Prevalence estimates varied between 4.7 and 23.3% of all reasons for an encounter, or up to estimates of 60% when related to patients or consultations. Cough in children is more frequent than in adults, with lowest prevalences in adolescents and in summer. Acute cough is mostly caused by upper respiratory tract infections (62.4%) and bronchitis (33.3%); subacute or chronic cough by recurrent respiratory tract infection (27.7%), asthma (up to 50.4% in cough persisting more than 3 weeks), and pertussis (37.2%). Potentially serious diseases like croup, pneumonia or tuberculosis are scarce. In children with subacute and chronic cough the total duration of cough ranged from 24 to 192 days. About 62.3% of children suffering from prolonged cough are still coughing two months after the beginning of symptoms.
Conclusion
Cough is one of the most frequent reasons for an encounter in primary care. Our findings fit in with current guideline recommendations supporting a thoughtful wait-and-see approach in acute cough and a special awareness in chronic cough of the possibility of asthma and pertussis. Further evidence of aetiological pre-test probabilities is needed to assess the diagnostic gain based on patient history and clinical signs for differential diagnoses of cough in children.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-021-02739-4.
Keywords: Cough, Children, Primary care, Prevalence, Prognosis, Aetiology, Symptom evaluation
Background
Cough is a frequent reason for encounters for both children [1, 2] and adults [3] in ambulatory care. It often gives serious concern to parents [4, 5]. Especially when prolonged, cough impairs daily activities or sleep and children’s and caregivers’ quality of life [2, 6, 7]. Therefore, 30 to 40% of coughing children consult a physician [8].
General practitioners (GPs), family physicians or paediatricians triage self-limiting, prolonged, and potentially life-threatening courses. In this respect assumed or research-based pre-test probabilities and prognosis drive GPs’ decision making and action.
Current guideline recommendations are mainly based on secondary or tertiary care studies [9, 10], which do not necessarily conform with the situation in primary care. We therefore performed a systematic literature review, working on the following research questions (1) What is the prevalence of the symptom cough in children consulting primary care practices, or how often do children in general practice or paediatric practices consult for cough? (2) What are the underlying diagnoses and their respective frequencies? (3) What is the usual course of disease or what is the prognosis of these children?
Methods
We performed a systematic review of symptom-evaluating studies. Based on the PRISMA statement [11] (Additional File 1) and the recommendation of Donner-Banzhoff [12] et al., methods were pre-specified in a protocol. Our working group applied the same methods on abdominal pain, tiredness, chest pain, dizziness and dyspnoea [13–17].
Data sources and search strategy
We searched MEDLINE in June 2012, updated our search in 2019, and EMBASE in January 2015, updated in 2020. The reference lists all relevant papers were screened (snowball search). Our search was limited to publications in English, French, and German. The search syntax comprised the term “cough” in all possible wordings in title/abstract OR as MeSH Term, and the term “primary care” in all possible wordings in title/abstract OR in mailing address or in the name of the authors’ institute OR as MeSH Term OR a journal representing primary care research. For this we searched for general practice/family medicine, as well as paediatric primary care. For the entire search syntax see Additional File 2.
Study selection and inclusion/exclusion criteria
We first screened titles and abstracts with respect to (1) original research article, (2) primary care as the study setting, and (3) “cough” as reason for encounter (primary or secondary reason for consultation).
The full text publications were assessed for our inclusion criteria as above plus (4) an unselected study population regarding the likelihood of the underlying aetiology, and (5) data available on incidence, prevalence, underlying diagnoses or prognosis of cough. All criteria had to be fulfilled. We excluded qualitative studies, case reports, reviews, studies without available full text, and studies recruiting in secondary or tertiary care, emergency departments/out-of-hours-services or population-based settings. No studies in which patients were systematically asked about cough were included. To avoid pre-selection, we did not consider studies that excluded patients with chronic diseases, studies, which recruited patients with an increased probability for a particular diagnosis or with cough being part of a required symptom combination (e.g. cough plus fever or expectoration). We included only studies on children. Reasons for exclusion were documented. The selection process was performed by two independently working reviewers: MB/DB or MB/SS (except the search updates 2019/2020). In case of disagreement, reviewers discussed their ratings or, secondly, consulted a third reviewer (AB).
Data extraction
For each publication, we extracted bibliographic information (author, publication year, title, journal), country, inclusion/exclusion criteria, definition of cough, characteristics of physicians and practices, type of recruitment, information on study population (sample size, age, gender distribution) and study duration.
For prevalence/incidence data, we extracted the number of cough cases and the number and type of the reference study sample. For aetiology we registered all diagnostic categories with their relative and absolute frequencies, and we extracted any kind of prognostic data. We analysed all available publications of each study, and in doubt contacted the authors personally (n = 7).
Assessment of methodical quality and risk of bias
Our working group developed a literature-based tool for evaluating risk of bias and clinical heterogeneity in symptom studies [12, 18]. A validation study is still running. Two reviewers (KH, MB) independently assessed 16 items in four key domains (Additional File 3) and rated the risk of bias in patient selection, data collection/patient flow, and in diagnostic and prognostic work-up. The risk of substantial variation/clinical heterogeneity was judged.
Statistics
The proportions of prevalence/incidence and underlying aetiologies plus 95% confidence intervals were calculated. Study outcomes vary in nominators and denominators of the frequency measure. For example, some counted consultations for cough in relation to all consultations, while others referred to reasons for encounters or all patients consulting a physician within a certain time frame. Since this had substantial impact on the results, we grouped the identified studies by these pre-specified denominators. Furthermore, we did subgrouping by duration of cough (pre-specified) and regional characteristics (post hoc). Aetiological and prognostic outcomes were analysed descriptively. Probability estimates and variation between studies are visualized with forest plots. For meta-analysis, we used the random-effects model (for distribution across studies) [19].
Study outcomes vary due to methodological (study design and bias) and clinical heterogeneity (study population, inclusion criteria, healthcare system, diagnostic work-up) [19]. We used χ2, p-value and I2: A heterogeneity beyond chance is characterized by high values of χ2 and low p-values; the portion of variability that is not due to chance is marked by I2 [19].
We used the software R (R Foundation for statistical Computing, Vienna, Austria, version 3.4.4) and RStudio V (RStudio, Inc., version 1.1.442).
Results
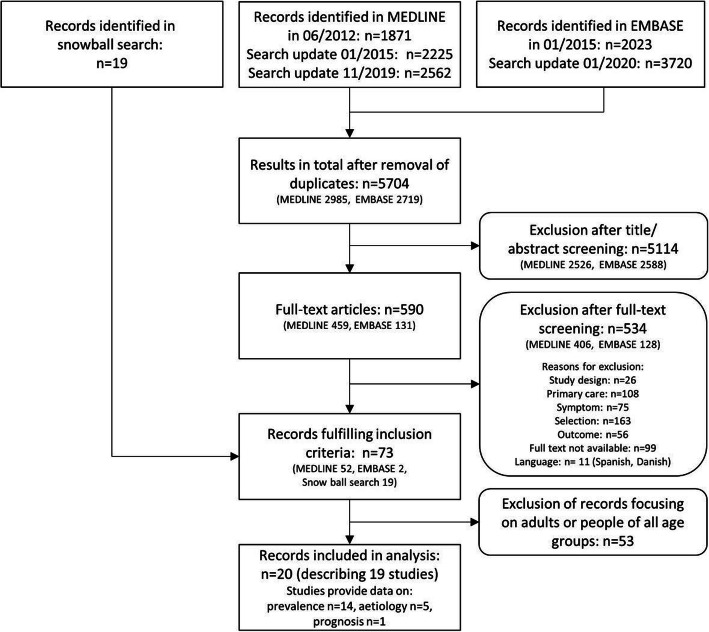
We identified 5704 records (2985 in MEDLINE and 2719 in EMBASE) after removal of duplicates, plus 19 records from snowballing. Seventy-three papers fulfilled our inclusion criteria after full text screening; only 19 of these focused on children. Of the 19 studies, 14 provided data on the prevalence of cough, and five on underlying aetiologies. Only one of these studies reported on prognostic outcomes (see Fig. 1).
Fig. 1.
Search flow
Included studies
Most studies were conducted in Europe (n = 10), followed by North America (n = 3), Australia (n = 1), Africa (n = 4), and Asia (n = 1). Studies were published between 1971 and 2019. Mostly, data was assessed prospectively. The study populations consisted of 121 to 5100 patients, 188 to 92,888 consultations, 1196 to 70,489 reasons for encounters and 3371 episodes of care. Female patients ranged from 45 to 54%, and the mean age varied from 18.4 months to 9.8 years. Only three studies included children of all age groups (one study including some adults consulting paediatric offices). Solely children < 5/< 6/< 7 years were included in five studies, solely children ≥5 years in two studies. Nine studies excluded children > 11/> 14/> 15 years of age. Data was accrued by 1–209 primary care paediatricians or GPs in 1–57 paediatric or general practices. Further details on the included studies are given in Table 1.
Table 1.
Description of the included studies
| Studies | Country | Setting | Time of recruit-ment | Data assess-ment | Study population: Number of females |
Age in study sample (years1) | Inclusion (IN) / Exclusion (EX) criteria | Out-come |
|---|---|---|---|---|---|---|---|---|
| Boyce 2019 [20] | Malawi | 57 health facilities with 250 health surveillance assistants for integrated community case management | n.r. | prospectively |
987 children ♀52% |
Ø23.4 months |
IN: first 4 children, aged 2–59 months, presenting to the health surveillance assistants for an initial consultation of their current illness EX: severely ill children who needed urgent referral to a health facility |
Pre |
| Cazzato 2001 [21] | Italy | 35 family paediatricians in Southern Italy | 04–06/1998 | prospectively |
9917 children ♀50% |
< 12: ≤2: 40.5% 3–6: 33.5% 7–12: 26% |
IN: every patient-doctor contact on an index-day of the week over a 3-month period | Pre |
| Giannattasio 2014 [22] | Italy | 3 primary care paediatric practices in Naples | 12/2011–01/2012 | prospectively |
284 patients 188 consultations due to symptoms ♀ 54% |
Ø 4.8 0–2: 25% 3–5: 36% 6–8: 20% 9–11: 13% 12–14: 6% |
IN: all children aged 0–14 years observed in the index days | Pre |
| Hall 2017 [23] | Australia | 1 Aboriginal-owned and operated comprehensive primary health-care service | 02/2013–10/2015 | prospectively |
121 children ♀ 49% |
0: 32.8% 1: 26.7% 2: 16.1% 3–4: 24.4% Ø18.4 months |
IN: children presenting for any reason, aged < 5 years, registered at the healthcare service and parent willing/able to complete study requirements EX: family was planning to move from the area in the following 12 months |
Pre |
| Harnden 2006 [24] | UK | 18 general practices | 10/2001–05/2005 | prospectively |
172 patients ♀ 45% |
Ø 9.1 (positive pertussis serology) – 9.8 (negative pertussis serology) |
IN: children, aged 5–16 years, with cough ≥14 days EX: refused blood sample |
Aet Prog |
| Krishnan 2019 [25] | USA | 1 predominantly suburban, academic paediatric faculty practice | 1 year | retrospectively |
560 consultations ♀ 47% |
19 days - 18 years Ø 6.6 < 2: 18% 2–5: 41% |
IN: children with completed electronic health record cough template | Aet |
| Leconte 2011 [26] | Belgium | 36 primary care practices | 02–03/2006 | prospectively | 345 patients | n.r. | IN: all consulting children aged 5–17 years | Pre |
| Mash 2012 [27] | South Africa | 83 primary care clinics, 17 mobile clinics, 12 community health centres; nurse-led with support from doctors | 1 year | prospectively | 5545 reasons for encounter | < 1–14 | IN: all ambulatory patients aged 0–14 years seen by health workers | Pre |
| Molony 2016 [28] | Ireland | 1 large general practice with 4 GPs in a primary healthcare centre in North Cork | 10/2010–10/2014 | retrospectively |
5100 patients 52,572 consultations 70,489 RFE |
n.r. |
IN: doctor-patient face-to-face encounters (children aged < 7 years) on all working days and 146 non-working days with a documentation of diagnostic code in the electronic medical record EX: contacts with practice nurse/ practice’s administrative team, telephone or ‘out-of-hours’ contacts |
Pre |
| Morrell 1971/1972 [29, 30] | UK | 1 general practice with 3 GPs | 1 year | prospectively |
707 patients 4467 consultations ♀ 51.3% |
n.r. |
IN: new patient-initiated consultations with symptoms not presented to any doctor in the previous 12 months, children aged 0–14 years EX: doctor-initiated consultations |
Pre |
| Movsowitz 1987 [31] | South Africa | 1 private paediatric practice in Cape Town | 1984–1985 | prospectively | 256 patients | 3 months −15 years | IN: patients with cough > 3 weeks | Aet |
| NAMCS Schappert 1999 [32] | USA | 195 office-based paediatricians | 01/1995–12/1996 | prospectively |
92,888 consultations ♀ 49.5% |
< 15: 89.6% 15–24 6.2% 25–44: 2.5% 45–64: 1.1% |
IN: office visits to non-federally employed paediatricians occurring during a randomly assigned 1-week reporting period EX: telephone contacts and visits made outside the physician’s office, visits to government-operated facilities and hospital-based outpatient departments |
Pre |
| Nizami 1997 [33] | Pakistan | 65 GPs and 29 paediatricians in Karachi | 04–12/1992 | prospectively | 2433 consultations | n.r. | IN: children aged < 5 years | Pre |
| Njalsson 1992 [34] | Iceland | 12 rural and 4 urban primary care health centres | 01–12/1988 | prospectively | 67,746 RFE | 0–14 | IN: all contacts with children aged 0–14 years, including prescriptions, follow-up visits, tests, procedures and administrative visits | Pre |
| SESAM 2 Study Frese 2011 [35] | Germany | 209 GPs in the federal state of Saxony | 10/1999–09/2000 | prospectively |
805 patients 1196 RFE |
0–4: 13.3% 5–9: 14.7% 10–14: 20.8% 15–19: 51.2% |
IN: randomly selected children, aged 0- ≤ 19 years, presenting in general practice (tenth consultation of the consultation hour) previously known to the practitioner EX: house calls, patients already included in SESAM 2 study |
Pre |
| Simoes 1997 [36] | Ethiopia | 3 primary health centres with 6 outpatient clinic nurses | 3 weeks in August | prospectively |
449 patients ♀ 54% |
2–11 months: 36% | IN: any sick child, aged 2 months – 5 years, presenting during study hours | Pre |
| TRANSITION Okkes 2002 [37] | Netherlands | 54 family physicians in 23 locations in the Netherlands | 1985–1995 | prospectively | 3371 episodes of care | n.r. | IN: episode data for all face-to-face encounters with paediatricians’ listed patients, aged 0–14 years, including encounters for prevention | Aet |
| Usherwood 1991 [38] | UK | 1 general practice in Scotland | 12/1986–01/1988 | prospectively | 466 consultations (including 147 home visits) | n.r. | IN: all health centre consultations of children, aged 2–13 years | Pre |
| Vinson 1993 [39] | USA, Canada | 44 primary care practices in the Ambulatory Sentinel Practice Network (ASPN) | 10/1990–01/1991 | prospectively |
1398 patients ♀ 47% |
infancy - ≤14 Ø 4,8 |
IN: children aged 0–14 years with cough ≤1 month | Aet |
Legend: 1 = unless otherwise stated, aet = aetiology of the symptom cough in primary care, n.r. = not reported, pre = prevalence of the symptom cough in primary care, prog = prognosis of the symptom cough in primary care, resp. = respectively, RFE = reasons for encounter, ♀ = female, Ø = mean
Assessment of methodical quality and risk of bias
We found a high risk of substantial variation/clinical heterogeneity in the majority of studies (n = 11), mostly because certain age groups were excluded (Domain A). The risk of selection bias of patients was low, high and unclear in about a third of studies each. Concerning data collection (Domain B), most studies had a low risk of bias (n = 13), none a high risk. The risk of bias in diagnostic work-up (Domain C) was high in three studies, low in one and unclear in another. There was only one study with prognostic outcomes, showing a low risk of bias in prognostic work-up (domain D). Only five studies showed an overall low risk of bias (in all relevant domains). For details please see Additional File 3 and Additional File 4.
Prevalence
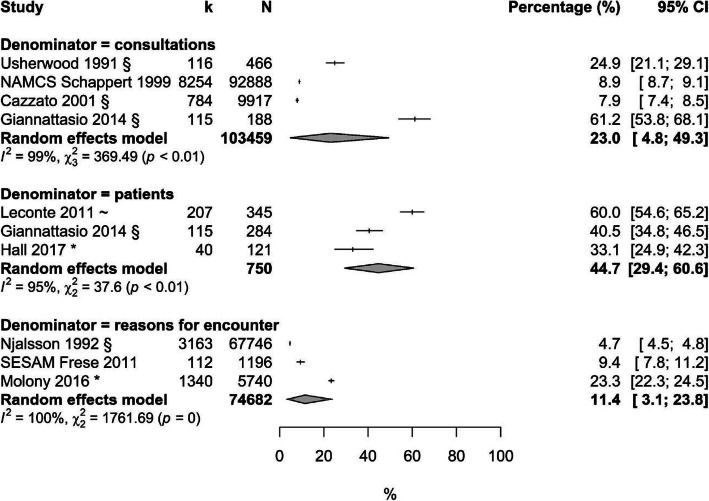
Fourteen studies commented on prevalence or incidence of cough; five of these had an overall low risk of bias [21, 26, 27, 32, 35] (Additional File 4). Five studies describe the number of consultations for cough in relation to all consultations (about 24%). In these, patients consulting their physician repeatedly are counted more than once. This is different to the four studies which found about 35% of all patients seen in consultation complain about cough (in these studies each patient is counted only once). About 11% of all reasons for encounter (including physician consultations as well as consultations for prescriptions, follow-up visits, tests, procedures and administrative visits [34]) refer to the symptom cough. (Additional File 5) Figure 2 visualizes the respective prevalences in Western countries. Seasonal effects can be seen in studies recruiting solely in the European winter season; these show high estimates [22, 26], while studies taking place in Italian spring/summer and Ethiopian August show low estimates [21, 36]. Studies on older children show comparably low prevalences [32, 35]. Morrell et al. found a one-year cough rate of 267 (male) resp. 238 (female) per 1000 patients at risk (0–4 years) and 113 (female) resp. 160 (male) for children aged 5–14 years [30]. Age subgroup analyses didn’t minimize the high heterogeneity across studies.
Fig. 2.
Prevalence of cough in children consulting in primary care of Western countries, sorted by denominators. Legend: * = study included solely children 5–17 years, CI = confidence interval, k = number of consultations because of a cough / reasons for encounter because of a cough / patients in consultation for a cough, N = total number of consultations / reasons for encounter / patients in consultation
Because only one study showed a low overall risk of bias and a low concern of clinical heterogeneity a subgrouping by quality was not possible [35].
Aetiologies
Five studies presented prevalence data on aetiology [24, 25, 31, 37, 39]. Except for Harnden 2006 [24], who excluded children < 5 years, the studies included all age groups. As outcomes referred to different durations of cough, we omitted meta-analysis and presented the data descriptively (see Table 2). The most frequent aetiology for acute cough is upper respiratory tract infection, followed by bronchitis, and, for subacute and chronic cough, the most frequent aetiology is recurrent respiratory tract infection, asthma and pertussis. Estimates of frequencies are lower when related to episodes of care (Transition [37]) where several consultations for the same reason are summarized and counted only once, compared to consultations (Krishnan [25]), when patients may be counted several times. There is a high prevalence of pertussis in children coughing for more than two weeks, confirmed by serological evidence [24]. In all other studies aetiologies based on GPs’ working diagnoses [31, 37, 39] or on the diagnostic work-up were unclear [25], and attended by a high or unclear risk of bias. No study presented with an overall low risk of bias (Domains A, B, and C).
Table 2.
Prevalences of selected aetiologies, referring to children in consultation for a cough in primary care / paediatric practices sorted by duration of cough
| Study Study population |
Vinson 1993 1398 patients |
TRANSITION Okkes 2002 3371 episodes of care |
Krishnan 2019 560 consultations |
Harnden 2006 172 patients |
Movsowitz 1987 256 patients |
|---|---|---|---|---|---|
| Duration of cough Aetiology |
acute | all durations of cough | subacute/chronic | ||
| ≤1 month | ≥2 weeks | > 3 weeks | |||
| Upper respiratory tract infection |
n = 873 62.4% [59.8; 65] viral: 35% (n = 494) bacterial: 27% (n = 379) |
n = 1294 38.4% [36.7; 40.1] |
n = 241 43% [38.9; 47.3] |
n.r. |
n = 71 27.7% [22.4; 33.7] (recurrent upper respiratory tract infection including bronchiolitis and bronchopneumonia) |
| Asthma |
n = 129 9.2% [7.8; 10.9} |
n = 100 3% [2.4; 3.6] |
n = 101 18% [15; 21.5] |
n.r. |
n = 129 50.4% [44.1; 56.7] |
| Pertussis | n.r. |
n = 34 1% [0.7; 1.4] |
n.r. |
n = 64 37,2% [30.1; 44.9] |
n = 56 21.9% [17.1; 27.5] |
| Bronchitis / bronchiolitis |
n = 465 33.3% [30.8; 35.6] |
n = 757 22.5% [21.1; 23.9] (acute bronchitis / bronchiolitis) |
n = 28 5% [3.4; 7.2] |
n.r. | n.r. |
| Pharyngitis | n.r. | n.r. |
n = 45 8% [6; 10.7] |
n.r. | n.r. |
| Sinusitis | n.r. |
n = 55 1.6% [1.2; 2.1] |
n = 45 8% [6; 10.7] |
n.r. | n.r. |
| Laryngitis / tracheitis | n.r. |
n = 245 7.3% [6.4; 8.2] |
n.r. | n.r. | n.r. |
| Croup |
n = 30 2.1% [1.5; 3.1] |
n.r. |
n = 45 8% [6; 10.7%] |
n.r. | n.r. |
| Pneumonia |
n = 78 5.6% [4.5; 6.9] |
n = 73 2.2% [1.7; 2.7] |
n = 39 7% [5.1; 9.5] |
n.r. | n.r. |
| Influenza | n.r. |
n = 43 1.3% [0.9; 1.7] |
n.r. | n.r. | n.r. |
| Otitis | n.r. |
n = 42 1.2% [0.9; 1.7] |
n = 28 5% [3.4; 7.2] |
n.r. | n.r. |
| Other allergic diseases |
n = 52 3.7% [2.8; 4.9] |
n.r. | n.r. | n.r. | n.r. |
| Tonsillitis | n.r. |
n = 54 1.6% [1.2; 2.1] |
n.r. | n.r. | n.r. |
| Hypertrophy tonsils / adenoids | n.r. |
n = 44 1.3% [1.0; 1.8] |
n.r. | n.r. | n.r. |
| Tuberculosis | n.r. | n.r. | n.r. | n.r. |
n = 1 0.4% [0; 2.5] |
| Bronchiectasis following pertussis | n.r. | n.r. | n.r. | n.r. |
n = 1 0.4% [0; 2.5] |
| Persistently atelectatic right middle lobe | n.r. | n.r. | n.r. | n.r. |
n = 1 0.4% [0; 2.5] |
| COPD | n.r. |
n = 8 0.2% [0.1; 0.5] |
n.r. | n.r. | n.r. |
| Heart failure | n.r. |
n = 0 0% [0; 0.1%] |
n.r. | n.r. | n.r. |
| Psychogenic cough | n.r. | n.r. | n.r. | n.r. |
n = 0 0% [0; 1.8%] |
| Cystic fibrosis | n.r. | n.r. | n.r. | n.r. |
n = 0 0% [0; 1.8%] |
| Foreign body nose / larynx / bronchus | n.r. | n.r. | n.r. | n.r. |
n = 0 0% [0; 1.8%] |
Legend: Every cell of table contains the absolute values (n), frequencies (%) and confidence interval [] of the study population with the respective aetiology. COPD = chronic obstructive pulmonary disease, n.r. = not reported
Prognosis
Only one study reported prognostic outcomes. Harnden et al. recruited, from 18 practices in the United Kingdom, 179 children aged 5 to 16 years who had been coughing for 14 days or more [24]. Participants completed a daily cough diary for two weeks, then a weekly diary for the duration of the cough. The total duration of cough ranged from 24 to 192 days (the median duration was 112 days/resp. 58 days for patients with a positive/resp. negative pertussis serology). After two months, 62.3% of children were still coughing (positive pertussis serology: 85%, negative pertussis serology: 49%).
Discussion
Summary
Our systematic review identified 19 eligible studies. Prevalence estimates in Western countries varied widely between 12% of all reasons for encounter and up to 45% of patients consulting their physician. We found differential effects with lower prevalences in summer and in older children. Acute cough is mostly caused by infectious diseases like upper respiratory tract infection (RTI) or bronchitis; in chronic cough the most important diagnoses are RTI, asthma, and pertussis. Potentially serious diseases like pneumonia or tuberculosis are scarce. Duration of cough varies widely; spontaneous relief within a short time seems unlikely in subacute/chronic cough, with 62.3% of children still coughing after two months.
Strengths and limitations
Sources of potential bias in systematic reviews are (1) criteria affecting the internal validity of studies (imprecise inclusion criteria and incomplete recruitment of study population), (2) limitations to the external validity of studies (setting characteristics and recruitment practice compromising the generalizability and applicability of the results), (3) methodological factors affecting the review’s internal validity (accuracy in literature search, screening process and data analysis), and (4) limitations to the review’s external validity [16, 17, 40].
To control the internal validity (1), we performed a substantial search and stated clear inclusion and exclusion criteria, but we omitted specialised paediatric journals or the term” paediatric practice” in our syntax. Still, we expect the misclassification to be low due to the comprehensive search of primary care settings including primary care paediatricians. As for the external validity (2) we did a double reviewer screening. Selection bias was minimized by considering only unselected study populations: In case of missing data regarding eligibility criteria we contacted study authors, although in some cases uncertainty remained. For (3) we performed a strict and standardized assessment of methodical quality, clinical heterogeneity and risk of bias [12]. Given the small number of included studies, we didn’t control for risk of bias across studies. However, publication bias seems unlikely, since there is no reason why prevalences, aetiologies or prognosis wouldn’t be published.
We found substantial methodological and clinical heterogeneity across age groups, study settings, healthcare systems, duration of cough, outcomes and reference parameters, which limits the external validity of our review (4). Cultural variables or gatekeeping influence the threshold to consulting a doctor, which is why we included only studies which had recruited in primary care settings. Still, age distribution in study samples may affect results: in German general practices over 50% of the study population were 15–19 years of age [35], while in two Italian family paediatricians’ offices 61–73% of children were < 6 years [21, 22]. In fact, the impact of cultural variables seems to be low, since heterogeneity was not minimized by age-related subgroups. The biggest limit to our study probably is the scarcity of high quality studies.
Comparison with existing literature
Indeed, reviews report coughing as one of the most common reasons for consultation in routine paediatric and family practice [2, 41]. The majority of children experience 5 to 8 episodes of one week of cough throughout the year [41]. However, these studies are mostly based on secondary/tertiary care data [42, 43] or are population-based [44]. Age influences the development of the respiratory system in general [45], which explains the change of prevalences over lifetime, and distinctive age-related patterns [44], as shown in our study, with the lowest cough prevalences mainly in studies on older children (51.2% of children aged 15 to 19 years [35]). This is in accordance with the guidelines of the American College of Chest Physicians, who set the cut-off age for applying adult protocols at 14 years of age [11, 46].
The distinction between acute, subacute and chronic cough differs from what is applied in adults [46–49]. The US and Australian-New Zealand guidelines define acute cough in children to last < 2 weeks, subacute cough 2–4 weeks and chronic cough > 4 weeks. This is based on the natural course of upper RTI in children [9, 50] differing from the course in adults (< 3 weeks, 3–8 weeks and > 8 weeks) [7]. Triaging patients according the duration of cough is the first step in the diagnostic process, which is why aetiological data for both acute and chronic cough are required. However, the categorizations in the identified studies differed from those suggested in the cough guidelines [24, 31], which limits the impact of these studies for guideline development or validation.
Acute cough in children is mostly caused by upper RTI and bronchitis, which is confirmed by the current literature [2, 7, 51]. Its self-limiting course justifies a “wait-and-see” strategy, if no warning signs are present. Transient RTI is still a frequent cause of disease in chronic cough (despite the high share of prolonged courses as outlined above). Therefore, primary care guidelines recommend a 3–8 weeks’ observational period (as long as no signs of specific aetiologies are present) [7, 9]. In contrast to chronic cough in adults, the other two big causes of disease in children are asthma and pertussis. Their importance is confirmed by studies conducted on chronic cough in hospitals [42, 43, 52]. However, potential overdiagnosis and unnecessary long-term medication in children seem to occur frequently, since, especially in younger children, spirometry anti-asthma therapy trials are not sufficiently valid [7]. A frequency of 1 in 2 for asthma, as shown by Movsowith et al. (a study with a high risk of bias) should not tempt us to be less critical before initiating anti-asthma therapy for children. Instead, working with a category of “chronic non-specific persistent cough” is recommended as a more adequate way of facing the diagnostic uncertainty. Any cough in children with no signs of serious diseases can be summarized in this category [7, 9]. This supports primary care physicians in keeping awareness combined with regular re-evaluation, instead of jumping into hasty therapeutic processes. A more valid outcome is the high prevalence of pertussis found in a multicentre study in the UK with a low risk of bias [24]. In contrast to asthma, underdiagnosis of pertussis seems likely. This is especially relevant within the first 14 days, since antibiotics can reduce spread and school exclusion when given at an early stage of the disease – after that no specific treatment has been shown to be effective [7]. For evaluation of chronic cough, guidelines recommend relying on signs (“pointers”) in patient history or clinical examination for specific causes, like wheezing for asthma or a paroxysmal spasmodic cough for pertussis-like illness [7, 9]. Still, we need more aetiological evidence, because the diagnostic gain of all signs depends on setting specific pre-test probabilities.
We know from secondary care studies that acute cough caused by upper RTI lasts about 5.18 days (follow-up 6 days) in children [53]. In the primary care setting, acute cough seems to resolve in half of children within one week, and in 10–20% of children by three weeks [51, 54]. The methodological quality of these studies is low [51, 54]. Terms like “acute cough”, “acute bronchitis” or “chest infection” are often used simultaneously for different signs and symptoms [55]. To improve evidence regarding a wait-and-see strategy or observation phase authors advocate for more prognostic studies in primary care based on symptoms [54, 55], with a sufficiently long follow-up period and an unselected patient population.
Conclusions
The prevalence of cough is higher in younger children than in adolescents, and lowest in summer. The high prevalence of upper RTI as an underlying disease and the low prevalence of potential serious illnesses seems to justify a “wait and see” approach to acute cough. Evidence on prolonged cough is scarce, but the prevalence of asthma and pertussis seems to rise substantially in subacute or chronic cough. Pertussis is especially prone to underdiagnosis. Other serious diseases like pneumonia or tuberculosis have a prevalence rate of less than 0.5%. There is hardly any data on prognosis of cough of children. Accordingly, thorough history taking and clinical examinations are mandatory to distinguish among differential diagnoses in coughing children. Further clarification of aetiological prevalences is needed to assess pre-test probabilities and the diagnostic gain from clinical signs which, once found to be valid, should be part of the standardized evaluation of cough.
Supplementary Information
Additional file 2. Search strategy. Detailed search strategy.
Additional file 3. Tool for assessment of methodical quality, risk of bias and clinical heterogeneity. Instrument used to assess the quality of the studies and the risk of bias.
Additional file 4. Quality, risk of bias and clinical heterogeneity of included studies. Quality review results for all included studies.
Additional file 5. Prevalence / incidence of cough of children consulting in primary care (all studies). Forrest plots of prevalences and incidences of cough related to all studies that provided information on this.
Acknowledgements
We would like to thank Thomas Frese for providing information and data.
Abbreviations
- GP
General Practitioner
- RTI
Respiratory tract infection
Authors’ contributions
MB, JH, DB, SSch, KH, SB, PG, LS, AV, NDB, and AB participated in the study design and analyses. MB AB, and JH performed and wrote a first draft of the manuscript. JH, SSch, PG, LS, KH, SB, DB, NDB, and AV commented on this draft and performed critical revisions. All authors have read and approved the manuscript.
Funding
This work was supported by the own resources of the Department of Primary Care, University of Marburg.
Availability of data and materials
All data analysed during this study are drawn from published articles. The respective references and extracted numbers are all included in this article and its supplementary data files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fegeler U, Jager-Roman E, Martin R, Nentwich H-J. Pediatric primary healthcare: health services study of the German academy for child and adolescent medicine. [German] Monatsschrift fur Kinderheilkunde. 2014;162(12):1117–1130. doi: 10.1007/s00112-014-3258-7. [DOI] [Google Scholar]
- 2.Lamas A, Ruiz de Valbuena M, Máiz L. Cough in Children. Arch Bronconeumol. 2014;50(7):294–300. doi: 10.1016/j.arbres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Hsiao C-J, Cherry DK, Beatty PC, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2007 Summary. Nat Health Stat Report 2010:1–32. [PubMed]
- 4.Cornford CS, Morgan M, Ridsdale L. Why do mothers consult when their children cough? Fam Pract. 1993;10(2):193–196. doi: 10.1093/fampra/10.2.193. [DOI] [PubMed] [Google Scholar]
- 5.Ramanuja S, Kelkar PS. The approach to pediatric cough. Ann Allergy Asthma Immunol. 2010;105:3–8; quiz 9–11, 42. [DOI] [PubMed]
- 6.Marchant JM, Newcombe PA, Juniper EF, Sheffield JK, Stathis SL, Chang AB. What is the burden of chronic cough for families? Chest. 2008;134(2):303–309. doi: 10.1378/chest.07-2236. [DOI] [PubMed] [Google Scholar]
- 7.Shields MD, Bush A, Everard ML, McKenzie S, Primhak R. Recommendations for the assessment and management of cough in children. Thorax. 2008;63(Suppl 3):iii1–iii15. doi: 10.1136/thx.2007.077370. [DOI] [PubMed] [Google Scholar]
- 8.Hay AD, Heron J, Ness A. The prevalence of symptoms and consultations in pre-school children in the Avon longitudinal study of parents and children (ALSPAC): a prospective cohort study. Fam Pract. 2005;22(4):367–374. doi: 10.1093/fampra/cmi035. [DOI] [PubMed] [Google Scholar]
- 9.Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics. Chest. 2006;129(1):260S–283S. doi: 10.1378/chest.129.1_suppl.260S. [DOI] [PubMed] [Google Scholar]
- 10.Chang AB, Oppenheimer JJ, Weinberger M, Grant CC, Rubin BK, Irwin RS, Altman KW, Azoulay E, Barker AF, Birring SS, Blackhall F, Bolser DC, Brightling, Callahan-Lyon P, Chang AB, Davenport P, Ebihara S, el Solh AA, Escalante P, Field SK, Fisher D, French CT, Harding SM, Gibson P, Gold P, Harnden A, Hill AT, Irwin RS, Kavanagh J, Keogh KA, Lai K, Lane AP, Madison JM, Malesker MA, Mazzone S, Molassoitis A, Murad MH, Narasimhan M, Nguyen HQ, Newcombe P, Oppenheimer J, Restrepo MI, Rosen M, Rubin B, Ryu JH, Tarlo SM, Turmel J, Vertigan AE, Weinberger M, Weir K. Etiologies of chronic cough in pediatric cohorts: CHEST guideline and expert panel report. Chest. 2017;152(3):607–617. doi: 10.1016/j.chest.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donner-Banzhoff N, Kunz R, Rosser W. Studies of symptoms in primary care. Fam Pract. 2001;18(1):33–38. doi: 10.1093/fampra/18.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Viniol A, Keunecke C, Biroga T, Stadje R, Dornieden K, Bösner S, et al. Studies of the symptom abdominal pain—a systematic review and meta-analysis. Fam Pract. 2014;31(5):517–529. doi: 10.1093/fampra/cmu036. [DOI] [PubMed] [Google Scholar]
- 14.Stadje R, Dornieden K, Baum E, Becker A, Biroga T, Bösner S, Haasenritter J, Keunecke C, Viniol A, Donner-Banzhoff N. The differential diagnosis of tiredness: a systematic review. BMC Fam Pract. 2016;17(1):147. doi: 10.1186/s12875-016-0545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haasenritter J, Biroga T, Keunecke C, Becker A, Donner-Banzhoff N, Dornieden K, Stadje R, Viniol A, Bösner S. Causes of chest pain in primary care—a systematic review and meta-analysis. Croat Med J. 2015;56(5):422–430. doi: 10.3325/cmj.2015.56.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bösner S, Schwarm S, Grevenrath P, Schmidt L, Hörner K, Beidatsch D, Bergmann M, Viniol A, Becker A, Haasenritter J. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19(1):33. doi: 10.1186/s12875-017-0695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viniol A, Beidatsch D, Frese T, Bergmann M, Grevenrath P, Schmidt L, Schwarm S, Haasenritter J, Bösner S, Becker A. Studies of the symptom dyspnoea: a systematic review. BMC Fam Pract. 2015;16(1):152. doi: 10.1186/s12875-015-0373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson WS, Wilson MC, Guyatt GH, Cook DJ, Nishikawa J. Users’ guides to the medical literature: XV. How to use an article about disease probability for differential diagnosis. Evidence-Based Medicine Working Group JAMA 1999;281:1214–1219. [DOI] [PubMed]
- 19.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al. Cochrane handbook for systematic reviews of interventions. 2019. Available at: www.training.cochrane.org/handbook. Accessed 24 Jan 2021, 10.1002/9781119536604. [DOI] [PMC free article] [PubMed]
- 20.Boyce SP, Nyangara F, Kamunyori J. A mixed-methods quasi-experimental evaluation of a mobile health application and quality of care in the integrated community case management program in Malawi. J Glob Health. 2019;9(1):10811. doi: 10.7189/jogh.09.010811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cazzato T, Pandolfini C, Campi R, Bonati M. Drug prescribing in out-patient children in southern Italy. Eur J Clin Pharmacol. 2001;57(8):611–616. doi: 10.1007/s002280100356. [DOI] [PubMed] [Google Scholar]
- 22.Giannattasio A, Lo Vecchio A, Napolitano C, Di Florio L, Guarino A. A prospective study on ambulatory care provided by primary care pediatricians during influenza season. Ital J Pediatr. 2014;40(1):38. doi: 10.1186/1824-7288-40-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall KK, Chang AB, Anderson J, Dunbar M, Arnold D, O’Grady K-AF. Characteristics and respiratory risk profile of children aged less than 5 years presenting to an urban, Aboriginal-friendly, comprehensive primary health practice in Australia. J Paediatr Child Health. 2017;53(7):636–643. doi: 10.1111/jpc.13536. [DOI] [PubMed] [Google Scholar]
- 24.Harnden A, Grant C, Harrison T, Perera R, Brueggemann AB, Mayon-White R, Mant D. Whooping cough in school age children with persistent cough: prospective cohort study in primary care. BMJ. 2006;333(7560):174–177. doi: 10.1136/bmj.38870.655405.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnan S, Ianotti V, Welter J, Gallagher MM, Ndjatou T, Dozor AJ. Bronchodilators, Antibiotics, and Oral Corticosteroids Use in Primary Care for Children With Cough. Global Pediatric Health. 2019;6:2333794X19831296. [DOI] [PMC free article] [PubMed]
- 26.Leconte S, Degryse J. Prolonged cough in children in the primary care office. Rev Med Brux. 2011;32:5–9. [PubMed] [Google Scholar]
- 27.Mash B, Fairall L, Adejayan O, Ikpefan O, Kumari J, Matheel S, et al. A morbidity survey of south African primary care. PLoS One. 2012;7(3):e32358. doi: 10.1371/journal.pone.0032358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molony D, Beame C, Behan W, Crowley J, Dennehy T, Quinlan M, Cullen W. 70,489 primary care encounters: retrospective analysis of morbidity at a primary care Centre in Ireland. Ir J Med Sci. 2016;185(4):805–811. doi: 10.1007/s11845-015-1367-5. [DOI] [PubMed] [Google Scholar]
- 29.Morrell DC, Gage HG, Robinson NA. Symptoms in general practice. J R Coll Gen Pract. 1971;21(102):32–43. [PMC free article] [PubMed] [Google Scholar]
- 30.Morrell DC. Symptom interpretation in general practice. J R Coll Gen Pract. 1972;22(118):297–309. [PMC free article] [PubMed] [Google Scholar]
- 31.Movsowitz L. Chronic cough and cough mixtures in a private paediatric practice. S Afr Med J. 1987;71(9):573–574. [PubMed] [Google Scholar]
- 32.Schappert SM, Nelson C. National Ambulatory Medical Care Survey: 1995-96 summary. Vital Health Stat 13. 1999; series 13, data from the National Health Survey (142):i-vi, 1-122. [PubMed]
- 33.Nizami SQ, Khan IA, Bhutta ZA. Paediatric prescribing in Karachi. J Pak Med Assoc. 1997;47(1):29–32. [PubMed] [Google Scholar]
- 34.Njalsson T, McAuley RG. Reasons for contact in family practice. An Icelandic multicentre study on content of practice. Scand J Prim Health Care. 1992;10(4):250–256. doi: 10.3109/02813439209014070. [DOI] [PubMed] [Google Scholar]
- 35.Frese T, Klauss S, Herrmann K, Sandholzer H. Children and adolescents as patients in general practice - the reasons for encounter. J Clin Med Res. 2011;3:177–182. doi: 10.4021/jocmr597w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simoes EA, Desta T, Tessema T, Gerbresellassie T, Dagnew M, Gove S. Performance of health workers after training in integrated management of childhood illness in Gondar, Ethiopia. Bull World Health Organ. 1997;75(Suppl 1):43–53. [PMC free article] [PubMed] [Google Scholar]
- 37.Okkes IM, Oskam SK, Lamberts H. The probability of specific diagnoses for patients presenting with common symptoms to Dutch family physicians. J Fam Pract. 2002;51(1):31–36. [PubMed] [Google Scholar]
- 38.Usherwood TP. Development and randomized controlled trial of a booklet of advice for parents. Br J Gen Pract. 1991;41(343):58–62. [PMC free article] [PubMed] [Google Scholar]
- 39.Vinson DC, Lutz LJ. The effect of parental expectations on treatment of children with a cough: a report from ASPN. J Fam Pract. 1993;37(1):23–27. [PubMed] [Google Scholar]
- 40.Cochrane Deutschland, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften - Institut für Medizinisches Wissensmanagement. Bewertung des Biasrisikos (Risiko systematischer Fehler) in klinischen Studien: ein Manual für die Leitlinienerstellung. [Evaluation of risk of bias (ris of systematic bias) in clinical studies: a manual for guideline development] 2016. http://www.cochrane.de/de/rob-manual; AWMF: http://www.awmf.org/leitlinien/awmf-regelwerk/ll-entwicklung.html. Accessed 24 Jan 2021.
- 41.Ptak K, Cichocka-Jarosz E, Kwinta P. Chronic cough in children. Developmental Period Medicine. 2018;22. [DOI] [PMC free article] [PubMed]
- 42.Marchant JM, Masters IB, Taylor SM, Cox NC, Seymour GJ, Chang AB. Evaluation and outcome of young children with chronic cough. Chest. 2006;129(5):1132–1141. doi: 10.1378/chest.129.5.1132. [DOI] [PubMed] [Google Scholar]
- 43.Chang AB, Robertson CF, van Asperen PP, Glasgow NJ, Mellis CM, Masters IB, Teoh L, Tjhung I, Morris PS, Petsky HL, Willis C, Landau LI. A multicenter study on chronic cough in children. Chest. 2012;142(4):943–950. doi: 10.1378/chest.11-2725. [DOI] [PubMed] [Google Scholar]
- 44.Jurca M, Ramette A, Dogaru CM, Goutaki M, Spycher BD, Latzin P, Gaillard EA, Kuehni CE. Prevalence of cough throughout childhood: a cohort study. PLoS One. 2017;12(5):e0177485. doi: 10.1371/journal.pone.0177485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang AB, Widdicombe JG. Cough throughout life: children, adults and the senile. Pulm Pharmacol Ther. 2007;20(4):371–382. doi: 10.1016/j.pupt.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Irwin RS, French CL, Chang AB, Altman KW. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest. 2018;153(1):196–209. doi: 10.1016/j.chest.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, Brown KK, Canning BJ, Chang AB, Dicpinigaitis PV, Eccles R, Glomb WB, Goldstein LB, Graham LRM, Hargreave FE, Kvale PA, Lewis SZ, McCool FD, McCrory DC, Prakash UBS, Pratter MR, Rosen MJ, Schulman E, Shannon JJ, Hammond CS, Tarlo SM. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(Suppl 1):1S–23S. doi: 10.1378/chest.129.1_suppl.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohno S, Ishida T, Uchida Y, Yoshyuki KH, Sasaki H, Shioya T, et al. The Japanese respiratory society guidelines for management of cough. Respirology. 2006;11(Suppl 4):S135–S186. doi: 10.1111/j.1440-1843.2006.00920_1.x. [DOI] [PubMed] [Google Scholar]
- 49.Lai K, Shen H, Zhou X, Qiu Z, Cai S, Huang K, Wang Q, Wang C, Lin J, Hao C, Kong L, Zhang S, Chen Y, Luo W, Jiang M, Xie J, Zhong N. Clinical practice guidelines for diagnosis and Management of Cough-Chinese Thoracic Society (CTS) asthma consortium. J Thorac Dis. 2018;10(11):6314–6351. doi: 10.21037/jtd.2018.09.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang AB, Landau LI, Van Asperen PP, Glasgow NJ, Robertson CF, Marchant JM, et al. Cough in children: definitions and clinical evaluation. Med J Aust. 2006;184(8):398–403. doi: 10.5694/j.1326-5377.2006.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 51.Hay AD, Wilson AD. The natural history of acute cough in children aged 0 to 4 years in primary care: a systematic review. Br J Gen Pract. 2002;52(478):401–409. [PMC free article] [PubMed] [Google Scholar]
- 52.Asilsoy S, Bayram E, Agin H, Apa H, Can D, Gulle S, Altinoz S. Evaluation of chronic cough in children. Chest. 2008;134(6):1122–1128. doi: 10.1378/chest.08-0885. [DOI] [PubMed] [Google Scholar]
- 53.Oduwole O, Udoh EE, Oyo-Ita A, Meremikwu MM. Honey for acute cough in children. The Cochrane database of systematic reviews 2018;4. [DOI] [PMC free article] [PubMed]
- 54.Hay AD, Wilson A, Fahey T, Peters TJ. The duration of acute cough in pre-school children presenting to primary care: a prospective cohort study. Fam Pract. 2003;20(6):696–705. doi: 10.1093/fampra/cmg613. [DOI] [PubMed] [Google Scholar]
- 55.Stocks N, Fahey T. Labelling of acute respiratory illness: evidence of between-practitioner variation in the UK. Fam Pract. 2002;19(4):375–377. doi: 10.1093/fampra/19.4.375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2. Search strategy. Detailed search strategy.
Additional file 3. Tool for assessment of methodical quality, risk of bias and clinical heterogeneity. Instrument used to assess the quality of the studies and the risk of bias.
Additional file 4. Quality, risk of bias and clinical heterogeneity of included studies. Quality review results for all included studies.
Additional file 5. Prevalence / incidence of cough of children consulting in primary care (all studies). Forrest plots of prevalences and incidences of cough related to all studies that provided information on this.
Data Availability Statement
All data analysed during this study are drawn from published articles. The respective references and extracted numbers are all included in this article and its supplementary data files.