Abstract
Eighty-seven percent of a large sample of children with autism spectrum disorder (ASD) are at risk for motor impairment (Bhat, Physical Therapy, 2020, 100, 633–644). In spite of the high prevalence for motor impairment in children with ASD, it is not considered among the diagnostic criteria or specifiers within DSM-V. In this article, we analyzed the SPARK study dataset (n = 13,887) to examine associations between risk for motor impairment using the Developmental Coordination Disorder-Questionnaire (DCD-Q), social communication impairment using the Social Communication Questionnaire (SCQ), repetitive behavior severity using the Repetitive Behaviors Scale – Revised (RBS-R), and parent-reported categories of cognitive, functional, and language impairments. Upon including children with ASD with cognitive impairments, 88.2% of the SPARK sample was at risk for motor impairment. The relative risk ratio for motor impairment in children with ASD was 22.2 times greater compared to the general population and that risk further increased up to 6.2 with increasing social communication (5.7), functional (6.2), cognitive (3.8), and language (1.6) impairments as well as repetitive behavior severity (5.0). Additionally, the magnitude of risk for motor impairment (fine- and gross-motor) increased with increasing severity of all impairment types with medium to large effects. These findings highlight the multisystem nature of ASD, the need to recognize motor impairments as one of the diagnostic criteria or specifiers for ASD, and the need for appropriate motor screening and assessment of children with ASD. Interventions must address not only the social communication and cognitive/behavioral challenges of children with ASD but also their motor function and participation.
Lay Abstract:
Eighty-eight percent of the SPARK sample of children with ASD were at risk for motor impairment. The relative risk for motor impairment was 22.2 times greater in children with ASD compared to the general population and the risk increased with more social communication, repetitive behavior, cognitive, and functional impairment. It is important to recognize motor impairments as one of the diagnostic criteria or specifiers for ASD and there is a need to administer appropriate motor screening, assessment, and interventions in children with ASD.
Keywords: autism | motor, cognition, function, language, restricted/repetitive behaviors, social communication
Introduction
Over the last decade, researchers have argued that autism spectrum disorder (ASD) is a complex, neurodevelopmental disorder affecting whole brain connectivity and multiple brain networks leading to various social communication, perceptuo-motor, and cognitive/behavioral impairments [Elsabbagh & Johnson, 2016; Srinivasan & Bhat, 2013; Gepner & Féron, 2009]. However, ASD is primarily characterized by social communication impairments such as poor social reciprocity, verbal and nonverbal communication delays along with the presence of repetitive behaviors and restricted interests [American Psychiatric Association, 2013]. Sensory-perceptual atypicalities and cognitive/behavioral impairments are also considered part of the diagnostic criteria or specifiers of ASD, whereas motor impairments are conspicuous by their absence from either of these categories [American Psychiatric Association, 2013]. There is accumulating evidence for the pervasive nature of motor impairments in children with ASD; however, they are not considered diagnostic due to their presence in other developmental disorders, for example, Developmental Coordination Disorder (DCD) [Caçola, Miller, & Williamson, 2017].
Identification and treatment of motor issues in children with ASD is important because a lack of full movement repertoire affects a child’s play and interaction with peers and caregivers and adds to their social–emotional difficulties [Bhat, Landa, & Galloway, 2011]. Studies have reported a variety of perceptuo-motor comorbidities in young and older children with ASD that relate to children’s concurrent or future social communication, cognitive, language, and daily living/functional skills [Bhat et al., 2011; Bhat, Galloway, & Landa, 2012; Srinivasan & Bhat, 2016; Kaur, Srinivasan, & Bhat, 2018; Lobo, Harbourne, Dusing, & McCoy, 2013; MacDonald, Lord, & Ulrich, 2013a,b; Dziuk et al., 2007; Bedford, Pickles, & Lord, 2015]. Recent analysis of the SPARK study dataset revealed that 87% of a large sample of school-age children with ASD within the United States were at risk for motor impairment and that did not change by 15 years of age [Bhat, 2020]. Only 15% of the sample in the SPARK study dataset held a formal dual diagnosis of DCD and only 31.6% received physical therapy services. Therefore, it appears that a lack of inclusion of motor impairments within ASD diagnostic criteria or specifiers may contribute to a lack of recognition and poor diagnosis and treatment of motor challenges in children with ASD.
For this reason, it is important to conceptualize how motor impairments fit within the broader framework of ASD. Specifically, are motor impairments in ASD domain-specific, domain-general, or transdiagnostic in nature? Motor impairments in ASD could be associated with ASD symptoms such as social communication difficulties and repetitive behaviors (i.e., part of the core features of ASD) and hence, domain-specific. Additionally, motor impairments in ASD could be associated with general cognitive, language, and functional abilities and hence, domain-general (i.e., support the multisystem view of ASD). Motor impairments in ASD could increase in severity with additional comorbid diagnoses reflecting the shared neural mechanisms leading to ASD and other diagnosis(es); hence, transdiagnostic. The present study aimed to better conceptualize the nature of motor impairment in children with ASD and its associations with core and comorbid symptoms/diagnoses to further support the multisystem view of ASD and to move toward shared diagnostic and treatment approaches that cross diagnostic boundaries.
Multiple literature reviews provide evidence for the widespread presence of motor impairments in children and adolescents with ASD [Fournier, Hass, Naik, Lodha, & Cauraugh, 2010; Bhat et al., 2011; Downey & Rapport, 2012; Floris & Howells, 2018,] whereas fewer studies have examined relationships between motor, social communication, cognitive, and functional performance in this population. Children with ASD have gross-motor impairments in whole-body coordination [Jansiewicz et al., 2006; Dewey, Cantell, & Crawford, 2007; Green et al., 2009; McPhillips, Finlay, Bejerot, & Hanley, 2014; Kaur et al., 2018], visuo-motor coordination such as ball skills [Dewey et al., 2007; Kushki, Chau, & Anagnostou, 2011; Fleury, Kushki, Tanel, Anagnostou, & Chau, 2013; McPhillips et al., 2014; Ament et al., 2014; Kaur et al., 2018], balance [Green et al., 2009; Jansiewicz et al., 2006; Green et al., 2009; McPhillips et al., 2014; Ament et al., 2014; Kaur et al., 2018] as well as fine-motor coordination problems such as handwriting/drawing difficulties [Dewey et al., 2007; Kushki et al., 2011; Fleury et al., 2013; Kaur et al., 2018] and manual dexterity problems [Jansiewicz et al., 2006; Fleury et al., 2013; Biscaldi et al., 2013; Kaur et al., 2018] compared to typically developing (TD) children. A variety of the aforementioned motor skills are either more affected [McPhillips et al., 2014; Ament et al., 2014] or equally affected [Macneil & Mostofsky, 2012; Dewey et al., 2007] in children with ASD compared to other special populations such as children with Developmental Coordination Disorder (DCD), Attention Deficit Hyperactivity Disorder (ADHD), and Specific Language Impairments (SLI). A meta-analysis conducted by Fournier et al. [2010] found motor impairments to be a cardinal feature of ASD in individuals with differing levels of impairment as well as ages.
In terms of motor-language relations, early gross and fine-motor development within the first year of life predicts the rate of language development in children with ASD between 2 and 9 years as well as a future outcome of ASD [Bedford et al., 2015; Leonard, Bedford, Pickles, & Hill, 2015; Choi, Leech, Tager-Flusberg, & Nelson, 2018; LeBarton & Landa, 2019]. In terms of motor-cognition relations, children diagnosed with ASD with lower IQ have greater severity of motor impairment compared to those with higher IQ [Ghaziuddin & Butler, 1998; Licari et al., 2019; Kaur et al., 2018]. Motor impairments in children with ASD also predict their levels of adaptive functioning [MacDonald et al., 2013a; Licari et al., 2019]. Studies have also found that basic motor skill performance is associated with IQ scores, whereas praxis/motor planning skills were more related to ASD severity based on social communication performance and presence of repetitive behaviors [Mari, Castiello, Marks, Marraffa, & Prior, 2003; Dziuk et al., 2007; Stieglitz Ham et al., 2010; Gizzonio et al., 2015; Bhat, Srinivasan, Woxholdt, & Shield, 2016; Shield, Knapke, Henry, Srinivasan, & Bhat, 2017; Kaur et al., 2018]. In terms of transdiagnostic continuity, there are conflicting findings in that some studies report similar levels of motor impairment across various diagnoses including children with ASD, DCD, ADHD, Learning Disabilities, and SLI, whereas others report that children with ASD have more severe motor impairments compared to children with ADHD, SLI, and DCD [Miyahara et al., 1997; Jansiewicz et al., 2006; Dewey et al., 2007; Ament et al., 2014; Macneil & Mostofsky, 2012; McPhillips et al., 2014]. Moreover, some studies have found that children with ASD have specific impairments within praxis, gestural, or visuomotor tasks such as ball skills and balance; whereas children with ADHD, SLI, and DCD have more generalized patterns of motor impairment [Dewey et al., 2007; Whyatt & Craig, 2011; Ament et al., 2014; Macneil & Mostofsky, 2012; McPhillips et al., 2014].
Overall, studies in the current literature have reported relations between motor, social communication, cognitive, language, and functional impairments of children with ASD. However, these studies are limited by small or biased samples of children with ASD with specific levels of functioning, language, or cognitive abilities. In contrast, the SPARK study has obtained data from a large group of parents (n = 13,887) of school-age children and adolescents with ASD within the United States using the Developmental Coordination Disorder Questionnaire (DCD-Q, Schoemaker et al., 2006), a parent screener for motor impairment, the Social Communication Questionnaire – Lifetime [SCQ; Berument, Rutter, Lord, Pickles & Bailey, 1999], a parent screener for social communication delay, and the Repetitive Behaviors Scale (RBS-R, Lam & Aman, 2006), a parent-reported measure of frequency, intensity, and variety of repetitive behaviors and restricted interests. Additionally, parents were asked to provide information on their child’s current language, functional, and cognitive abilities compared to same-age peers. This rich dataset also includes basic demographic, birth history, and diagnostic information.
The present study examined the SPARK study dataset, version 3 to evaluate the domain-specific, domain-general, and transdiagnostic nature of motor impairments in children with ASD using the DCD-Q, SCQ, RBS-R, and parent-reported current abilities data. It is hypothesized that ASD is a multisystem disorder impacting motor, social communication, cognitive, and functional abilities as well as repetitive behavior severity; hence, the relative risk for motor impairment in children with ASD will increase as a function of social communication impairment and repetitive behavior severity (i.e., motor impairment in ASD is domain-specific and increases with ASD severity). Next, the relative risk for motor impairment in children with ASD will increase as a function of cognitive, language, and functional impairments (i.e., motor impairment in ASD is domain-general and supports the multisystem view of ASD). Additionally, the relative risk of motor impairment will increase with additional comorbid diagnoses reported in the literature such as ADHD, Language Impairments, Intellectual Disability, and DCD (i.e., motor impairment in ASD is transdiagnostic and supports a cross-diagnostic clinical approach). Lastly, it is also hypothesized that the DCD-Q total scores (i.e., risk for motor impairment) and the subscale scores (i.e., finemotor, gross-motor, and general coordination scores) will be significantly lower in children with ASD with higher SCQ and RBS-R scores as well as greater language, cognitive, and functional impairments.
Methods
SPARK Study Procedures and Data Access
Families throughout the United States with one or more children with ASD were recruited in the SPARK study through 21 clinical sites across the United States using a multipronged social media strategy [Feliciano et al., 2018]. Families voluntarily signed up for this study by completing the online questionnaires (https://sparkforautism.org/registration/account_information/) on the SPARK website. They also received information on studies in their nearby community to volunteer for local research studies. This author signed up with the SPARK study to utilize their study recruitment resources (i.e., SPARK Participant Match Resource) for her lab’s ongoing research studies approved by the University of Delaware (UD)’s Human Subjects Review Board. UD also signed an authorization agreement with the Simons Foundation; after which the author was given access to version 3 of the SPARK study database (release date: February 2019).
SPARK Forms and Measures
The SPARK database comprised of multiple parent questionnaires such as the basic medical screening form, individual data form, and background history form. The basic medical screening form includes demographic information, birth history, professional diagnosis of ASD and other disorders, as well as other general medical conditions. The individual data form provides details on when the ASD diagnosis was made, which professional provided the diagnoses, whether there is a presence of a cognitive impairment, whether there is an Individualized Education Plan (IEP) for the child, and whether the child receives ASD services. The background history form lists the various intervention services received by the child as well as information regarding cognitive, language, and functional abilities of each participant (i.e., above, at, slightly below, or significantly below same-age peers). Table 1 summarizes the type of SPARK study data used for our analysis. Apart from these participant details, three parent questionnaires including the Developmental Coordination Disorder Questionnaire [DCD-Q; Schoemaker et al., 2006], the Social Communication Questionnaire – Lifetime [SCQ; Berument, Rutter, Lord, Pickles, & Bailey, 1999], and the Repetitive Behaviors Scale – Revised [RBS-R; Lam & Aman, 2006] have also been examined.
Table 1.
Type of Data Used From the SPARK Study Database
| Demographic data/birth/developmental history | |
|---|---|
| Age in months and years | |
| Gender | Male/Female |
| • White | |
| • African American | |
| • Asian | |
| • Native American | |
| • Native Hawaiian | |
| • Other | |
| • Multi-racial | |
| Race | • Not reported |
| Ethnicity | Hispanic/Not Hispanic/Not reported |
| • Hollingshead variables: Parent’s occupations and level of education | |
| Socio-economic status (SES) | • Household income ranges |
| Birth/developmental history | • CNS malformations in the brain and spinal cord • Prenatal alcohol or drug exposure • Bleed in the brain • Preterm birth • Perinatal infection • Insufficient oxygen at birth with NICU stay • Cognitive delays due to brain injury, stroke, lead poisoning, Fetal Alcohol Syndrome, HIV, radiation, hydrocephalus, brain tumor, drug effects, and so on |
| Parent questionnaires DCD-Q |
Item (15), sub-scale (3), and total scores Yes/No DCD |
| SCQ | Item (40) and total scores |
| RBS-R Parent-reported curren Language |
Item (43), sub-scale (6), total scores Total number of items endorsed Scores summed into Repetitive Sensory Motor (RSM, sum of subscales I + VI), Insistence on Sameness (IS, sum of subscales III + IV + V), and Self-Injury subscales (II) IS is related to ASD symptoms and RSM is related to intellectual disability t abilities Above/at/slightly below/significantly below age level |
| Cognition | Above/at/slightly below/significantly below age level |
| Function | Above/at/slightly below/significantly below age level |
DCD-Q
The DCD-Q is a 15-item parent questionnaire used to assess a child’s gross- and fine-motor coordination during everyday functional/play skills within their natural environment [Schoemaker et al., 2006]. The questionnaire focuses on various motor skills such as ball skills (e.g., hitting or catching a ball), complex body coordination skills (e.g., jumping, running, etc.), fine motor skills (e.g., writing, cutting, etc.), and general motor control abilities (e.g., quickness, clumsiness, fatigability, etc.). These skills are categorized into three subscales: control during movement, fine motor coordination, and general coordination. The total final score is calculated as a sum of the individual subscale scores. Definite motor impairment or suspect DCD (<10th percentile) is determined based on the final score cutoffs, which differ for different age groups. For example, these cutoffs include a score < 47 for children between 5 years and <8 years, a score below 56 for children between 8 years and <10 years, and a score < 58 for children between 10 and 15 years. Based on these criteria, an assignment of risk for motor impairment or DCD (1 = Yes, 0 = No) is provided for each participant. A motor impairment or DCD diagnosis is typically confirmed with a follow-up, standardized motor assessment, and clinical judgment of a trained movement clinician. Note that the positive predictive value of the DCD-Q with a clinical motor assessment such as the Movement ABC [Green et al., 2009] is 92%; which means 92% of the children who are at-risk for a motor impairment using the DCD-Q will most likely perform poorly on the M-ABC standard motor assessment.
SCQ
The SCQ is a widely used, 40-item parent questionnaire (Yes/No format) to screen for autistic traits in children above 4 years of age with a mental age of at least 2 years [Berument et al., 1999]. It is based on a well-validated diagnostic interview, the Autism Diagnostic Interview-Revised (ADI-R). The SCQ has two versions—Lifetime which is used to support a diagnosis and—Current which is used to support an evaluation of current difficulties. The Lifetime version provides a total SCQ score. If the total score is ≥12, it indicates a social communication delay and higher likelihood to be on the autism spectrum. The cut-off of 12 used in this study is a research recommended, more sensitive cut-off score [Lee et al., 2010; Daniels et al., 2011; Zwaigenbaum et al., 2015; Marvin, Marvin, Lipkin, & Law, 2017].
RBS-R
The RBS-R is a widely used 43-item parent-reported measure to characterize the repetitive behaviors of children with ASDs. It has high internal consistency and medium reliability (Lam & Aman, 2006). Each item/question is scored on a 4-point scale: 0 (no such behavior), 1 (mild problem), 2 (moderate problem), and 3 (severe problem); therefore, the total score ranges between 0 and 129 with higher score indicating more repetitive behavior. It has six subscales on the child’s stereotyped (I), self-injurious (II), compulsive behaviors (III), ritualistic (IV), sameness behaviors (V), and restricted interests (VI).
Inclusion/Exclusion Criteria
Out of the total 150,064 individuals in the SPARK database, which include children with ASD and their family members, parents of 16,705 children with ASD completed the DCDQ form; hence that was our base sample. Table 2 shows the subsequent filters applied in this work to further exclude some children. Parents of some children did not complete certain items on the questionnaire; therefore, final DCDQ scores and assignment of risk for motor impairment (MI)/DCD (Yes/No) were not assigned to them according to publisher guidelines. Children below the age of 5 years and above the age of 15 years were excluded. After these filters, there were 16,338 individuals remaining with a valid DCDQ administration. Next, the children who were truly affected by ASD were confirmed. Here, two robust criteria for ASD diagnosis (i.e., diagnosis age >18 months or has an Individualized Education Plan (IEP) for ASD) reduced the sample to 16,287 children. From this sample, participants who had a valid (<3 items missing) Social Communication Questionnaire – Lifetime (SCQ) form were identified, which decreased the sample to 15,888 children. As discussed earlier, participants who met the SCQ cut-off of score ≥ 12 were included, which reduced the sample to 14,745 children. Finally, it was also confirmed that the children with ASD did not have general neuromotor injuries unrelated to ASD. Specifically, children with medical conditions/birth injuries (i.e., brain and spinal cord malformations, prenatal alcohol/drug exposure, and brain bleed) were excluded. After applying these criteria, the final sample included 13,887 children. Compared to the sample analyzed in the past publication [Bhat, 2020], the current study sample includes participants with cognitive impairment/intellectual disability as this analysis focuses on how the risk for motor impairment differs in children with ASD as a function of cognitive abilities.
Table 2.
Filters Used to Remove Samples Based on DCDQ, SCQ, and Birth Injury Criteria. The Final Dataset Includes Samples for Which Valid DCDQ and SCQ Scores Are Available
| Criterion for removing samples | Number of samples removed | Number of samples retained |
|---|---|---|
| Original dataset | 16,705a | |
| DCDQ final score was not assigned due to missing items | 364 | 16,341 |
| Age at DCDQ evaluation was <60 months and >191 months | 3 | 16,338 |
| No professional diagnosis of ASD | 0 | 16,338 |
| Diagnosis age < 18 months and no IEP | 51 | 16,287 |
| Inclusion criteria | ||
| Assigned as invalid using SCQ validity column | 4 | 16,283 |
| SCQ test was not completed | 171 | 16,112 |
| SCQ final score was not assigned due to missing items | 224 | 15,888 |
| SCQ final score < 12 | 1143 | 14,745 |
| Exclusion criteria | ||
| CNS malformations (brain, spinal cord) | 75 | 14,670 |
| Prenatal alcohol or drug exposure | 118 | 14,552 |
| Intraventricular hemorrhage | 120 | 14,432 |
| Delays or impairment due to brain injury, stroke, lead poisoning, FAS, HIV, radiation, hydrocephalus, brain tumor, drug effects, and so on | 545 | 13,887 |
| Final dataset | 13,887 | |
This corresponds to the number of completed DCDQ questionnaires.
Categories of Language, Cognition, and Functional Abilities
To study the risk for motor impairment as a function of cognition, language, and function, the eligible sample was categorized based on parent reports of their child’s current language, cognitive, and functional impairments. For each type of impairment, a child’s ability was reported as above age, at age, slightly below age, or significantly below age, compared to same-age peers. The eligible sample was divided into three categories based on current cognitive abilities:
Category 1—No cognitive delay (ability at or above age, N = 6,080).
Category 2—Some cognitive delay (ability slightly below age, N = 3,756).
Category 3—Significant cognitive delay (ability significantly below peers, N = 3,361).
Six-hundred and ninety children (5.0%) with missing data were excluded.
The eligible sample was divided into three categories based on current language abilities:
Category 1—No language delay (ability at or above age, N = 5,143).
Category 2—Some language delay (ability slightly below age, N = 3,716).
Category 3—Significant language delay (ability significantly below peers, N = 4,564).
Four hundred and sixty-four children (3.3%) with missing data were excluded.
The eligible sample was divided into three categories based on current functional abilities:
Category 1—No functional delay (ability at or above age, N = 3,044).
Category 2—Some functional delay (ability slightly below age, N = 6,566).
Category 3—Significant functional delay (ability significantly below peers, N = 3,839).
Four hundred and thirty-eight children (3.2%) with missing data were excluded.
In order to categorize the sample based on SCQ and RBS-R scores, the score mean (μ) and standard deviation (δ) were used to divide the entire sample into following ranges: < μ-δ (very low), μ-δ to μ (low), μ to μ + δ (high), μ + δ to μ + 2δ (very high), and > μ + 2δ (extremely high impairment). Using the sample SCQ score mean (μ = 23.4) and standard deviation (δ = 6.1), the entire sample was divided into the following five categories of Social Communication Impairment (SCI):
Category 1—Very low SCI (SCQ score range = 0 to ≤17, N = 2,705).
Category 2—Low SCI (SCQ score range = 18 to ≤23, N = 4,276).
Category 3—High SCI (SCQ score range = 24 to ≤29, N = 4,448).
Category 4—Very high SCI (SCQ score range = 30 to ≤35, N = 2,188).
Category 5—Extremely high SCI (SCQ score > 35, N = 259).
No children were excluded from the grouping based on SCI as all data were available.
Based on sample RBS-R score mean (μ = 36.1) and standard deviation (δ = 20.2), the entire sample was divided into the following five categories of repetitive behavior severity (RBS):
Category 1—Very low RBS (RBS-R score range = 0 to ≤15, N = 1,833).
Category 2—Low RBS (RBS-R score range = 16 to ≤36, N = 6,169).
Category 3—High RBS (RBS-R score range = 37 to ≤56, N = 3,682).
Category 4—Very high RBS (RBS-R score = 57 to ≤76, N = 1,505).
Category 5—Extremely high RBS (RBS-R score > 76, N = 614).
Eighty-four children (0.6%) with missing data were excluded.
Statistical Analysis
Relative risk ratios were used to calculate the risk for motor impairment using overall, fine-motor, gross-motor, and general coordination scores from the DCD-Q data. Relative risk ratio is a measure of association used to assess the risk of developing a better or worse outcome in relation to certain factors and is often used for large-sized samples [Di Lorenzo, 2014]. For control group comparisons, the reported risk for motor impairment/DCD prevalence rate of ∼4% in school-age children from a general population using the DCD-Q measure was used [Rivard, Missiuna, McCauley, & Cairney, 2012]. The relative risk ratio for a risk of motor impairment in each sub-category of children with ASD based on their SCQ/RBS-R scores and language, cognitive, functional impairments was obtained by comparing the proportion of risk for motor impairment in children with ASD compared to the proportion in the general population (∼4%). The 95% confidence intervals (CIs) are also calculated for each relative risk ratio. Risk difference is also reported based on how the proportion of motor impairment differs compared to the general population. Pearson or Spearman rank correlations have been reported across SCQ, RBS-R scores and language, cognitive, and functional ability categories. One-way Analysis of Variances (ANOVAs) of DCD-Q including total and subscale (i.e., fine-motor, gross-motor, and general coordination) scores were carried out with impairment categories as a factor for SCQ and RBS-R categories as well as language, cognitive, and functional impairments. Statistically significant differences are being reported based on significant Tukey post hoc testing along with confidence intervals that do not include a zero value. The magnitude of between-group differences is being reported using effect size estimates such as Cohen’s d (small: <0.45, medium: >0.45 but <0.8, large: >0.8).
Results
Risk for Motor Impairment as a Function of Social
Communication Impairment
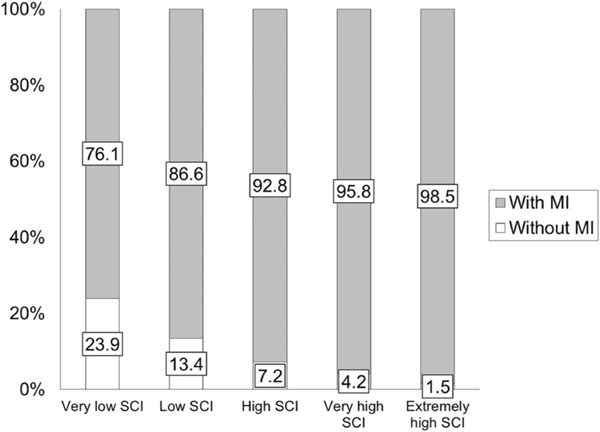
Using Pearson’s correlation, DCD-Q total, and SCQ total scores had a small correlation of −0.31 (P < 0.0001, Table 3). The overall relative risk for motor impairment in all children with ASD compared to the general population is 22.2 and overall proportion of children with ASD with a risk for motor impairment (i.e., those who failed on DCD-Q) is 88.2% (Table 4). With increasing SCI, the relative risk increased from 19.2 to 24.8 (i.e., a further increase by 5.6 with more SCI, Table 4). The proportion of children with ASD that are at a risk for motor impairment increased from 76.1 to 98.5 as the severity of SCI increases (Fig. 1 and Table 4). Further analysis using a one-way ANOVA on DCD-Q total and sub-scale scores and subsequent post hoc t-tests revealed that the DCD-Q total and sub-scale scores significantly differed across all SCI categories (Fig. S1, Table 5, and Tables S1-S3 in the Supporting Information, Ps < 0.0074) with one exception. The C4-C5 category pair did not differ for fine motor handwriting (P > 0.1). The effect sizes for all comparisons of DCD-Q total scores ranged from 0.21 to 1.15 (6 out of 10 medium to large effects), for fine-motor (FM) scores ranged from 0.07 to 0.79 (4 out of 10 medium to large effects), for gross-motor/control during movement (CDM) scores ranged from 0.20 to 1.07 (6 out of 10 medium to large effects), and for general coordination (GC) scores ranged from 0.16 to 0.95 (4 out of 10 medium to large effects, Table S4 in the Supporting Information).
Table 3.
Spearman Rank Correlations Between Measures Used in This Analysis
| DCDQ score | SCQ score | RBS-R score | Cognitive ability | Language ability | Functional ability | |
|---|---|---|---|---|---|---|
| DCDQ score | 1 | −0.31 | −0.26 | −0.34 | −0.25 | −0.44 |
| SCQ score | 1 | 0.39 | 0.24 | 0.24 | 0.29 | |
| RBS-R score | 1 | 0.15 | 0.10 | 0.24 | ||
| Cognitive ability | 1 | 0.45 | 0.37 | |||
| Language ability | 1 | 0.35 | ||||
| Functional ability | 1 | |||||
Note. ps < 0.0001 for all correlations reported in this table. Note that the variability/subgrouping of the SPARK sample is enormous and simple correlational analyses do not show high correlations. Hence, relative risk ratios are being studied for this sample as is often reported for large-sized samples.
Table 4.
Relative Risk Ratio for Motor Impairment (MI) With Increasing Social Communication, Language, Cognitive, and Functional Impairment As Well As Repetitive Behavior Severity
| With MI (%) | Without MI (%) | Risk ratio and CI | Difference in risk ratio between consecutive categories | Risk difference (%) | |
|---|---|---|---|---|---|
| General population [Rivard et al., 2012] | 4.0 | 96.0 | — | — | — |
| Children with ASD in the SPARK dataset | 88.2 | 11.8 | 22.2 [18.6, 26.4] | — | 84.2 |
| Social communication impairment (SC), Mean and ndard deviation of total score | |||||
| Very low SCI, ASD (14.9, 1.7) | 76.1 | 23.9 | 19.2 [16.1, 22.8] | 0.0 | 72.1 |
| Low SCI, ASD (20.6, 1.7) | 86.6 | 13.4 | 21.8 [18.3, 25.9] | 2.6 | 82.2 |
| High SCI, ASD (26.4, 1.6) | 92.8 | 7.2 | 23.3 [19.6, 27.8] | 4.1 | 88.8 |
| Very high SCI, ASD (31.9, 1.6) | 95.8 | 4.2 | 24.1 [20.3, 28.7] | 4.9 | 91.8 |
| Extremely high SCI, ASD (36.7, 0.9) |
98.5 | 1.5 | 24.8 [20.8, 29.5] | 5.6 | 94.5 |
| Repetitive behaviors (RB), Mean, and Standard deviation of total score | |||||
| Very low RB, ASD (10.5, 3.7) | 75.4 | 24.6 | 19.0 [15.9, 22.6] | 0.0 | 71.4 |
| Low RB, ASD (26, 5.9) | 87.4 | 12.6 | 22.0 [18.5, 26.2] | 3.0 | 83.4 |
| High RB, ASD (45.3, 5.6) | 92.6 | 7.4 | 23.3 [19.6, 27.7] | 4.3 | 88.6 |
| Very high RB, ASD (65.1, 5.5) | 93.4 | 6.6 | 23.5 [19.7, 28.0] | 4.5 | 89.4 |
| Extremely high RB, ASD (88.9, 10.7) | 95.3 | 4.7 | 24.0 [20.1, 28.6] | 5.0 | 91.3 |
| Cognitive ability | |||||
| No cognition delay, ASD | 81.8 | 18.2 | 20.6 [17.3, 24.5] | 0.0 | 77.8 |
| Some cognition delay, ASD | 90.6 | 9.4 | 22.8 [19.2, 27.1] | 2.2 | 86.6 |
| Significant cognition delay, ASD | 96.9 | 3.1 | 24.4 [20.5, 29.0] | 3.8 | 92.9 |
| Language ability | |||||
| No language delay, ASD | 85.9 | 14.1 | 21.6 [18.2, 25.7] | 0.0 | 81.9 |
| Some language delay, ASD | 86.9 | 13.1 | 21.9 [18.4, 26.0] | 0.3 | 82.9 |
| Significant language delay, ASD | 92.1 | 7.9 | 23.2 [19.5, 27.6] | 1.6 | 88.1 |
| Functional ability | |||||
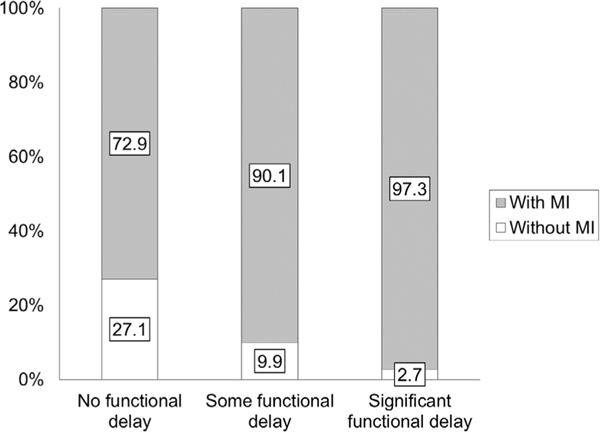
| No functional delay, ASD | 72.9 | 27.1 | 18.3 [15.4, 21.9] | 0.0 | 68.9 |
| Some functional delay, ASD | 90.1 | 9.9 | 22.7 [19.1, 27.0] | 4.4 | 86.1 |
| Significant functional delay, ASD | 97.3 | 2.7 | 24.5 [20.6, 29.1] | 6.2 | 93.3 |
Note. Ps < 0.0001 for all relative risk ratios reported in this table. An increase in risk magnitude of 1 or more is considered substantial. There was an increase in the relative risk of motor impairment up to 6.2 across the three to five categories of social communication, cognitive, language, and functional ability as well as repetitive behavior severity.
Figure 1.
Proportion of children with ASD with risk for motor impairment (MI, i.e., who failed on the DCD-Q) across the various SCI categories.
Table 5.
Sample Size, Mean, Standard Error, and Confidence Intervals for DCD-Q Total Scores Across SCQ, RBS-R, Cognitive, Language, and Functional Impairment Categories in Children With ASD
| Categories | N | Mean | Std. Error | Lower 95% | Upper 95% |
|---|---|---|---|---|---|
| Final DCD-Q score as a function of social communication (SC) impairment | |||||
| Very low SCI | 2,716 | 42.4 | 0.22 | 42.0 | 42.9 |
| Low SCI | 4,276 | 38.0 | 0.18 | 37.7 | 38.4 |
| High SCI | 4,448 | 34.3 | 0.17 | 33.9 | 34.6 |
| Very high SCI | 2,188 | 31.9 | 0.25 | 31.4 | 32.4 |
| Extremely high SCI | 259 | 29.1 | 0.72 | 27.6 | 30.5 |
| Final DCD-Q score as a function of repetitive behavior (RB) severity | |||||
| Very low RB | 1,833 | 43.1 | 0.27 | 42.6 | 43.7 |
| Low RB | 6,169 | 37.5 | 0.15 | 37.2 | 37.8 |
| High RB | 3,682 | 34.3 | 0.19 | 34.0 | 34.7 |
| Very high RB | 1,505 | 32.7 | 0.30 | 32.2 | 33.3 |
| Extremely high RB | 614 | 30.4 | 0.47 | 29.5 | 31.4 |
| Final DCD-Q score as a function of cognitive ability | |||||
| No cognition delay, ASD | 6,080 | 40.3 | 0.15 | 40.0 | 40.6 |
| Some cognition delay, ASD | 3,756 | 36.4 | 0.19 | 36.0 | 36.7 |
| Significant cognition delay, ASD | 3,361 | 30.0 | 0.20 | 29.6 | 30.4 |
| Final DCD-Q score as a function of language ability | |||||
| No language delay, ASD | 5,143 | 39.5 | 0.16 | 39.2 | 39.8 |
| Some language delay, ASD | 3,716 | 37.4 | 0.19 | 37.1 | 37.8 |
| Significant language delay, ASD | 4,564 | 32.4 | 0.17 | 32.1 | 32.8 |
| Final DCD-Q score as a function of functional ability | |||||
| No functional delay, ASD | 3,044 | 44.6 | 0.20 | 44.2 | 44.9 |
| Some functional delay, ASD | 6,566 | 36.8 | 0.14 | 36.5 | 37.1 |
| Significant functional delay, ASD | 3,839 | 29.6 | 0.18 | 29.3 | 30.0 |
Note. DCD-Q final scores worsened with increasing SC impairment, increasing RB severity, decreasing cognitive ability, decreasing language ability, and decreasing functional ability, with each category significantly differing from the other (ps < 0.0001).
Risk for Motor Impairment as a Function of Repetitive Behavior Severity
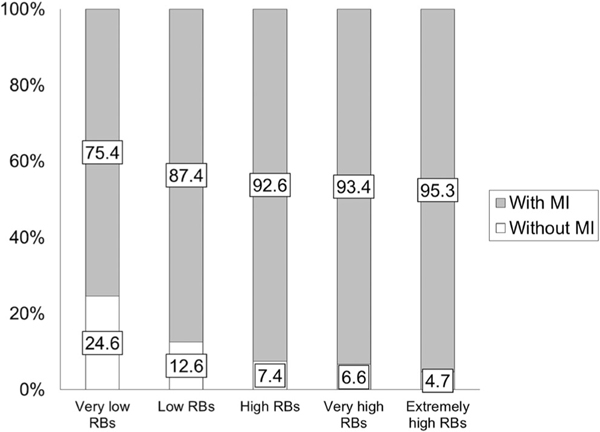
Using Pearson’s correlation, DCD-Q total and RBS-R total −0.26 (P < 0.0001, Table 3). The overall relative risk for motor impairment in all children with ASD compared to the general population is 22.2. With increasing repetitive behavior severity, this relative risk increased from 19.0 to 24.0 (i.e., a further increase of 5.0 with more repetitive behavior severity, Table 4). The proportion of children with ASD that are at a risk for motor impairment increased from 75.4 to 95.3 as repetitive behavior severity increased (Fig. 2 and Table 4). Further analysis using a one-way ANOVA on DCD-Q total and sub-scale scores and subsequent post hoc t-tests revealed that DCD-Q total and sub-scale scores significantly differed across all RBS-R categories (Supporting Information Fig. S1, Table 5, and Tables S1–S3, Ps < 0.0007) with some exceptions. The C4-C5 category pair showed a trend for lowering of fine motor handwriting scores with more RB severity (P = 0.05) and did not differ for general coordination (P > 0.1). The C3-C4 category pair showed a trend for lowering of “control during movement” scores with more repetitive behavior severity (P = 0.02). The effect sizes for all comparisons of DCD-Q total scores ranged from 0.14 to 1.08 (5 out of 10 medium to large effects), for fine-motor (FM) scores ranged from 0.12 to 0.92 (4 out of 10 medium to large effects), for gross-motor/control during movement (CDM) scores ranged from 0.10 to 0.85 (4 out of 10 medium to large effects), and for general coordination (GC) scores ranged from 0.11 to 0.92 (5 out of 10 medium to large effects, Supporting Information Table S4).
Figure 2.
Proportion of children with ASD with risk for motor impairment (MI, i.e., who failed on the DCD-Q) across the various RBS-R categories.
Risk for Motor Impairment as a Function of Cognitive Abilities
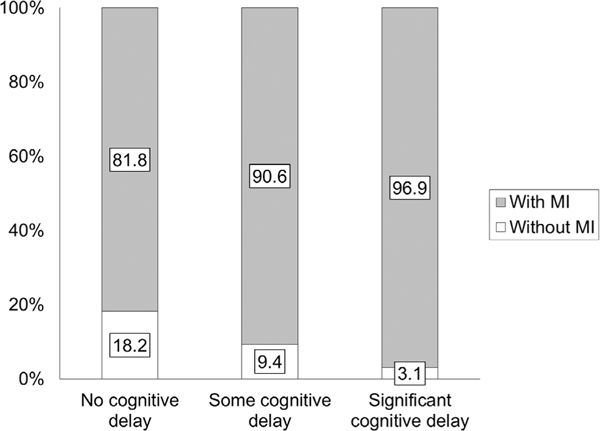
Using Spearman’s rank correlation, DCD-Q total and functional ability categories had a small correlation of −0.34 (P < 0.0001, Table 3). The overall relative risk for motor impairment in all children with ASD compared to the general population is 22.2. With increasing cognitive impairment, this relative risk increased from 20.6 to 24.4 (i.e., a further increase of 3.8 with more cognitive impairment, Table 4). The proportion of children with ASD at risk for motor impairment increased from 81.8 to 96.9 as cognitive impairment increased (Fig. 3 and Table 4). Further analysis using a one-way ANOVA on DCD-Q total and sub-scale scores and subsequent post hoc t-tests revealed that DCD-Q total and sub-scale scores significantly differed across the three cognitive impairment categories (Supporting Information Fig. S1, Table 5 and Tables S1–S3, Ps < 0.0001) with no exceptions. The effect sizes for all comparisons of DCD-Q total scores ranged from 0.34 to 0.90 (2 out of 3 medium to large effects), for fine-motor (FM) scores ranged from 0.45 to 0.99 (3 out of 3 medium to large effects), for gross-motor/control during movement (CDM) scores ranged from 0.27 to 0.75 (2 out of 3 medium to large effects), and for general coordination (GC) scores ranged from 0.14 to 0.46 (1 out of 3 had medium to large effects, Supporting Information Table S4).
Figure 3.
Proportion of children with ASD with risk for motor impairment (MI, i.e., who failed on the DCD-Q) across the three categories of cognitive abilities.
Risk for Motor Impairment as a Function of Language Abilities
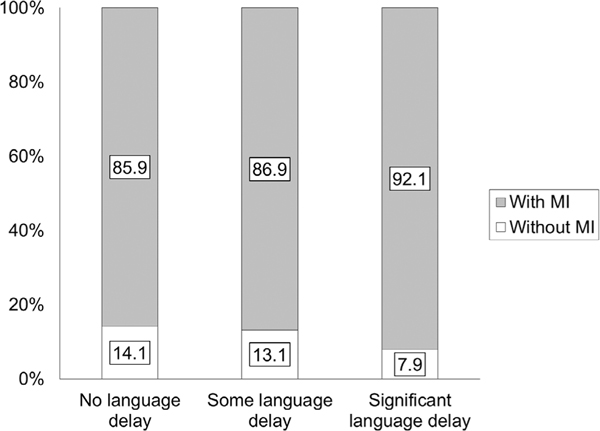
Using Spearman’s rank correlation, DCD-Q total and language ability categories had a small correlation of −0.25 (P < 0.0001, Table 3). The relative risk for motor impairment in all children with ASD compared to the general population is 22.2. With increasing language impairment, this relative risk increased from 21.6 to 23.2 (i.e., a further increase of 1.6 with more language impairment, Table 4). The proportion of children with ASD that are at a risk for motor impairment did not change much from 85.9 to 92.1 as language impairments increased (Fig. 4 and Table 4). Further analysis using a one-way ANOVA on DCD-Q total and sub-scale scores and subsequent post hoc t-tests revealed that DCD-Q total and sub-scale scores significantly differed across the three language impairment categories (Fig. S1, Table 5, and Tables S1–S3, Ps < 0.0001) with one exception. The C1-C2 category pair did not differ for general coordination (P > 0.1). The effect sizes for all comparisons of DCD-Q total scores ranged from 0.18 to 0.60 (1 out of 3 small to medium effects), for fine-motor (FM) scores ranged from 0.26 to 0.80 (2 out of 3 medium to large effects), for gross-motor/control during movement (CDM) scores ranged from 0.19 to 0.54 (1 out of 3 having a medium effect), and for general coordination (GC) scores ranged from 0.03 to 0.17 (no medium to large effects, Supporting Information Table S4).
Figure 4.
Proportion of children with ASD with risk for motor impairment (MI, i.e., who failed on the DCD-Q) across the three categories of language abilities.
Risk for Motor Impairment as a Function of Functional Abilities
Using Spearman’s rank correlation, DCD-Q total and functional ability categories had a moderate correlation of −0.44 (P < 0.0001, Table 3). The relative risk for motor impairment in all children with ASD compared to the general population is 22.2. With increasing functional impairment, this relative risk increased from 18.3 to 24.5 (i.e., a further increase of 6.2 with more functional impairment, Table 4). The proportion of children with ASD that are at a risk for motor impairment increased from 72.9 to 97.3 as functional impairment increased (Fig. 5 and Table 4). Further analysis using a one-way ANOVA on DCD-Q total and sub-scale scores and subsequent post hoc t-tests revealed that DCD-Q total and sub-scale scores significantly differed across the three functional impairment categories (Fig. S1, Table 5, and Tables S1–S3, Ps < 0.0001) with no exceptions. The effect sizes for all comparisons of DCD-Q total scores ranged from 0.66 to 1.36 (3 out of 3 medium to large effects), for fine-motor (FM) scores ranged from 0.59 to 1.20 (3 out of 3 medium to large effects), for gross-motor/control during movement (CDM) scores ranged from 0.53 to 1.09 (3 out of 3 medium to large effects), and for general coordination (GC) scores ranged from 0.42 to 1.01 (2 out of 3 had medium to large effects, Supporting Information Table S4).
Figure 5.
Proportion of children with ASD with risk for motor impairment (i.e., who failed on the DCD-Q) across the three categories of functional abilities.
Risk for Motor Impairment as a Function of Comorbid Diagnoses
The relative risk for motor impairment in children with ASD with and without various comorbid diagnoses were compared. It increased by a magnitude of 1 or more for children with additional diagnoses of ADHD, Learning Disability, Anxiety Disorder, Intellectual Disability, Developmental Coordination Disorder, and Depression but not in children with comorbid Language disorder, Social anxiety/phobia, Obsessive Compulsive Disorder, and Oppositional Defiant Disorder (Table 6). The proportion of children with ASD with a risk for motor impairment increased (i.e., risk difference) due to various comorbid diagnoses. Specifically, the risk difference was 4.4% for comorbid ADHD, 7.7% for comorbid Learning Disability, 4.3% for comorbid Anxiety Disorder, 8.6% for comorbid Intellectual Disability, 9.4% for comorbid DCD, and 4.6% for comorbid Depression.
Table 6.
Relative Risk Ratio for Motor Impairment (MI) With Increasing Various Comorbid Diagnoses in Children With ASD
| Additional comorbid diagnoses in children with ASD | Yes/No MI | Proportion of sample (%) | DCD prevalence rate (%) | Risk difference for DCD [Yes – No] | Risk ratio |
|---|---|---|---|---|---|
| General population [Rivard et al., 2012] | — | — | 4 | — | — |
| Missing information | 0.5 | — | — | — | |
| Language disorder | Y N |
61.4 38.1 |
89.1 86.8 |
2.2 | 22.4 21.9 |
| Attention deficit hyperactivity disorder | Y N |
41.4 58.2 |
90.8 86.4 |
4.4 | 22.9a 21.8 |
| Learning disability | Y N |
22.5 77.0 |
94.2 86.5 |
7.7 | 23.7a 21.8 |
| Anxiety disorder | Y N |
20.9 78.6 |
91.6 87.3 |
4.3 | 23.1a 22.0 |
| Intellectual disability | Y N |
18.5 81.1 |
95.2 86.6 |
8.6 | 24.0a 21.8 |
| Developmental coordination disorder | Y N |
17.5 82.0 |
95.9 86.6 |
9.4 | 24.2a 21.8 |
| Social anxiety/Phobia | Y N |
9.3 90.3 |
91.1 87.9 |
3.2 | 22.9 22.1 |
| Obsessive compulsive disorder | Y N |
8.5 91.0 |
91.2 87.9 |
3.2 | 23.0 22.1 |
| Oppositional defiant disorder | Y N |
8.5 91.1 |
91.2 87.9 |
3.2 | 23.0 22.1 |
| Depression | Y N |
6.7 92.8 |
92.5 87.9 | 4.6 | 23.3a 22.1 |
Indicates an increase in risk ratio of greater than 1 following a comorbid developmental/psychiatric diagnosis in children with ASD.
Discussion
In the past SPARK study report, 87% of school-age children with ASD were at-risk for a motor impairment [Bhat, 2020], whereas in the current study, the prevalence was 88.2% upon including children with ASD with cognitive impairments. There was an association between risk for motor impairment using the DCD-Q and severity of social communication impairment using the SCQ, repetitive behavior severity using the RBS-R, and parent-report of cognitive, language, and functional abilities. The associations with SCQ and RBS-R highlight the domain-specific nature of motor impairments. The associations with cognition, language, and functional impairments highlight the domain-general nature of motor impairments and support a multisystem view of ASD. The associations with comorbid diagnoses highlight the transdiagnostic nature of motor impairments and how motor impairments may cross diagnostic boundaries and could be explained by shared neural mechanisms and may benefit from shared assessments and treatments across disorders. Together, these findings highlight the pervasive nature of motor impairment in ASD as well as how ASD is multisystem in nature and affects various developing systems. There were small to moderate correlations between the various aforementioned measures given the high variability of this large sample; hence, a regression approach was not pursued and instead relative risk ratios for risk of motor impairment were calculated in relation to ASD symptoms, cognitive, functional, and language impairments, as well as comorbid diagnoses; an approach often used to study large-sized samples [Di Lorenzo, 2014]. Second, the risk for motor impairment was 22 times greater in children with ASD compared to the general population. Third, the risk for motor impairment increased across all impairment types (i.e., social communication, repetitive behavior severity, cognitive, language, and functional impairments). Specifically, the risk for motor impairment increased the most with functional impairments (change in risk ratio = 6.2), then social communication impairments (5.7), then repetitive behavior severity (5.0), then cognitive impairments (3.8), and lastly, language impairments (1.6). Moreover, the change in magnitude of motor impairment for all motor scores (overall, fine-motor, gross-motor, and general coordination) had large effects for the extreme category comparisons (C1 v. C5 or C1 v. C3) across all impairment types—functional impairments (11 out of 12 medium to large effects), cognitive impairments (8 out of 12 medium to large effects), social communication impairments (20 out of 40 medium to large effects), repetitive behavior severity (18 out of 40 medium to large effects), and language impairments (4 out of 12 medium to large effects). Below these findings are placed within the context of the current literature and clinical implications for assessment and treatment of children and adolescents with ASD are discussed.
Motor Impairment is Associated With ASD Severity
The risk for motor impairment is 22 times greater in children and adolescents with ASD than the general population and further increased 5.7 times more as the severity of social communication impairment increased from very low to extremely high and 5 times more as repetitive behavior severity increased from very low to extremely high. The magnitude of all DCD-Q scores including overall, fine-motor, gross-motor, and general coordination scores worsened with increasing social communication impairment and repetitive behavior severity and both fine and gross-motor subdomains were equally impaired across both impairment types. These SPARK study findings confirm that the risk for motor impairment in children with ASD is associated with their ASD severity and hence, meets the standard for what the field considers ASD specific. These findings fit with the literature reporting the pervasive nature of motor impairments in children and adolescents with ASD [Green et al., 2009; Fournier et al., 2010; Bhat et al., 2011; Hilton, Zhang, Whilte, Klohr, & Constantino, 2011; Bhat, 2020]. Gross- and fine-motor delays are one of the earliest markers of ASD; however, early delays are milder in nature and may not reliably predict an ASD outcome [Iverson, 2018]. There is more recent contrasting evidence on how early gross and fine-motor motor delays were predictive of a 3-year ASD outcome in young children with ASD [Choi et al., 2018; LeBarton & Landa, 2019]. Furthermore, certain subdomains of motor performance in school-age children and adolescents with ASD are considered more ASD-specific than others, specifically, visuomotor skills, fine-motor skills, and/or motor planning/praxis skills [Nebel et al., 2016; Whyatt & Craig, 2011; Macneil & Mostofsky, 2012; Dziuk et al., 2007; Choi et al., 2018]. The present study encompassed a much larger sample of children with ASD with widely ranging social communication, cognitive, and language abilities and the overall risk for motor impairment/incoordination including gross-motor and fine-motor impairment worsened equally with increasing social communication impairment and repetitive behavior severity. Using the large SPARK study sample, broader population trends in a highly variable sample of children with ASD were evaluated. These findings join other recent studies supporting the ASD-specific nature of motor impairments in children and adolescents with ASD and call for motor incoordination to be recognized within the diagnostic criteria for ASD [Hilton et al., 2011; Licari et al., 2019; Bhat, 2020]. The variation in motor impairment across children with ASD is more likely a reflection of the severity of ASD neuropathology as well as movement experiences received to outgrow motor impairments throughout childhood. However, based on the current and past findings from the SPARK study, majority of the adolescents with ASD have not outgrown their motor coordination/planning difficulties by 15 years of age [Bhat, 2020].
Motor Impairments are Pervasive in Children With ASD Across Various Categories of Cognitive, Functional, and Language Impairments
The risk for motor impairment increased 6 times between children with ASD with none to significant functional impairments, 4 times between children with none to significant cognitive impairments, and 1.6 times between children with ASD with none to significant language impairments. The magnitude of risk for overall motor impairment as well as fine-motor or gross-motor performance equally worsened with growing functional, cognitive, or language impairments suggesting that not one specific motor subdomain was more affected than the other. The association between risk for motor impairment and functional impairments in children with ASD are a reasonable finding because a range of daily living skills require motor coordination and planning and incoordination/dyspraxia may contribute to functional impairments and poor physical fitness in children with ASD [Bremer & Cairney, 2019]. Fine and gross motor skill performance of young children with ASD between 1 and 3 years of age predicted daily living skills performance on the Vineland Adaptive Behavioral Scales [Macdonald et al., 2013a]. Recently, Licari et al. [2019] found that motor performance in a larger sample of ∼2000 children with ASD below 6 years of age correlated with daily living skills the most (r = 0.6) then social skills (r = 0.5) and lastly communication skills (r = 0.3). The proportion of children with motor impairment was significantly greater in children with ASD with a cognitive impairment compared to those without a cognitive impairment, even though both subgroups had a substantial prevalence of motor impairment. The relationships between cognitive and motor impairments have been well studied over the last two decades and the aforementioned recent findings confirm that children with ASD with varying levels of cognition present with motor impairments and the magnitude/severity of motor impairment increases with greater cognitive impairment. There was an increasing risk for motor impairment in children with ASD with greater language impairments although this finding is slightly weaker compared to other associations with function, cognition, social communication abilities, and repetitive behavior severity. Nevertheless, there is considerable evidence on how early gross- and fine-motor motor delays in young children with ASD predict language outcomes as well as rate of language development at later ages between 2 and 9 years [Gernsbacher, Sauer, Geye, Schweigert, & Hill Goldsmith, 2008; Lloyd, MacDonald, & Lord, 2011; Bedford et al., 2015; Leonard et al., 2015; Choi et al., 2018; LeBarton & Landa, 2019]. In the past, associations between motor and cognition/language performance have been considered evidence for domain-generality and evidence against domain-specificity; however, findings reported in this article show the pervasive nature of motor impairments across the autism spectrum including those with less severe cognitive, functional, and language impairments. In fact, the increasing severity of motor impairment as a function of cognitive, language, and functional impairments in children with ASD shows that motor impairment could be an indicator of how severe the original neuropathology is.
Motor Impairment Increases With Comorbid Diagnoses and Crosses Diagnostic Boundaries
The risk for motor impairment in children with ASD increased with certain comorbid developmental disorders with risk difference increasing by 4.4% for comorbid ADHD, 7.7% for comorbid Learning Disability, 4.3% for comorbid Anxiety Disorder, 8.6% for comorbid Intellectual Disability, 9.4% for comorbid DCD, and 4.6% for comorbid Depression in comparison to children with ASD without the aforementioned diagnoses. These findings show the transdiagnostic nature of motor impairments across various neurodevelopmental/psychiatric disorders (i.e., ASD, ADHD, AD, ID, DCD, and Depression) and add to the current literature reporting more severe motor impairments in children with ASD compared to that of other disorders such as ADHD, DCD, SLI, and Learning disabilities [Miyahara et al., 1997; Jansiewicz et al., 2006; Dewey et al., 2007; Ament et al., 2014; Macneil & Mostofsky, 2012; McPhillips et al., 2014]. Our findings suggest an additive effect of comorbid diagnoses in children with ASD with potentially more substantial motor impairments in children with dual diagnoses. NIMH’s research domain criteria (RDoC) framework recently added the sensori-motor domain to its matrix of criteria to better understand the sensorimotor symptoms occurring from shared pathophysiology across various neurodevelopmental disorders [Hirjak et al., 2018; Anttila et al., 2018]. Clinicians and scientists must consider using a transdiagnostic approach to investigate shared neural/pathophysiological frameworks to explain motor impairments and implement common motor assessment and treatment approaches that can be shared across developmental disorders.
The Multisystem Nature of ASD
The current analysis of the SPARK dataset confirmed that ASD is a complex, multisystem, disorder leading to varying levels of social communication, sensori-motor, and cognitive-behavioral symptoms. Motor symptoms present in children and adolescents with ASD were specific to their social communication impairments and repetitive behavior severity. Furthermore, motor symptoms were associated with co-occurring cognitive, functional, and language impairments of children with ASD. These domain-specific and domain-general patterns of motor impairment are best explained by the neural connectivity theory of ASD [Courchesne et al., 2007; Just, Keller, Malave, Kana, & Varma, 2012]. Studies in children and adults with ASD report reduced long-range connectivity between cortices (e.g., reduced fronto-temporal or frontoparietal connectivity) as well as excessive/reduced short-range connectivity within the frontal, parietal, temporal, and visual cortices [Vasa, Mostofsky, & Ewen, 2016; Turner, Frost, Linsenbardt, McIlroy, & Müller, 2006; Nair, Treiber, Shukla, Shih, & Müller, 2013; Frazier, Keshavan, Minshew, & Hardan, 2012; Hanaie et al., 2018; Chen et al., 2018; Li et al., 2019]. Additionally, disordered or poor long-range connectivity has been reported in cortico-subcortical networks such as cortical-cerebellar [Macneil & Mostofsky, 2012; Vasa et al., 2016], corticostriatal [Turner et al., 2006] cortico-thalamic [Nair et al., 2013] connections as well as inter-hemispheric, callosal connectivity [Frazier et al., 2012]. These widespread abnormalities encompass multiple brain structures important for sensori-motor, social communication, and cognitive-behavioral functions and hence, explain the multisystem impairments of children with ASD. In fact, abnormal callosal, cortico-cortical and cortico-subcortical connectivity are known to correlate with motor, social communication skills, and executive functions of children and adolescents with ASD [Carper, Solders, Treiber, Fishman, & Müller, 2015; Chen et al., 2018; Hanaie et al., 2018]. Findings from the SPARK study, specifically, the associations between motor and social communication impairments as well as repetitive behavior severity indicate that motor impairments could be considered a fundamental feature of ASD.
The transdiagnostic nature of motor symptoms with additive motor impairments occurring in children with ASD with comorbid DCD, ADHD, Anxiety, and Depression confirm that ASD shares abnormal brain networks with other neurodevelopmental disorders. Few studies have compared connectivity patterns between children with ASD and other developmental disorders, namely ADHD and DCD [Kern, Geier, Sykes, Geier, & Deth, 2012; Caeyenberghs et al., 2016]. Specifically, the pattern of long-range underconnectivity and short-range overconnectivity has been reported in all three diagnoses—children with ASD, ADHD, and DCD; however, brain abnormalities were much more widespread in children with ASD [Kern et al., 2012; Caeyenberghs et al., 2016]. The associations between motor and other systems found in this study as well as the increased risk for motor impairment with comorbid diagnoses in children with ASD support the possibility of a more severe and widespread neuropathology leading to a range of impairments in children with ASD that are shared across multiple neurodevelopmental disorders.
Clinical Implications
Findings from the SPARK study confirm that motor impairments are associated with ASD severity/diagnostic criteria as well as ASD specifiers (i.e., cognitive and language abilities) as defined in the DSM-V. These findings support a multisystem view of ASD and how it impacts various systems including social communication, sensorimotor, and cognitive-behavioral systems. This evidence also supports the inclusion of motor impairment/incoordination as part of the diagnostic criteria or as a specifiers for ASD. Future studies must differentiate whether certain aspects of motor performance such as motor incoordination/dyspraxia and visuo-motor incoordination are better placed within diagnostic criteria whereas general motor issues such as slowed movement could be part of ASD specifiers.
Recognizing motor impairment within ASD criteria or specifiers will bring it within the radar of diagnostic clinicians (i.e., pediatricians, neurologists, and psychologists) who need to screen for motor impairments and refer to movement clinicians (i.e., OTs and PTs) for further evaluation of fine- and gross-motor impairments. The original paper on the SPARK DCD-Q dataset reported that only 15% of children with ASD are diagnosed with DCD indicating a lack of recognition for motor impairments among diagnostic clinicians. While 80% of children with ASD were receiving occupational therapy (OT) services to address their fine-motor and sensory integration problems, only 32% were receiving physical therapy (PT) services to address gross-motor issues. Overall, there is an urgent need to recommend motor screening for all children with ASD in early intervention and school settings with further evaluations by OTs and PTs to address fine- and gross-motor impairments.
In terms of motor interventions, movement clinicians such as OTs, PTs, and adaptive physical educators should be advocates for motor problems of children with ASD beyond fundamental skills of reaching and walking. Older children with ASD appear to have an enormous motor gap created throughout development that is not being closed by 15 years of age. There is a dearth of evidence-based motor interventions to promote a variety of motor and daily functions in young and older children with ASD. Motor interventions for ASD cannot be limited to fundamental motor skills and should encompass complex motor skills requiring bilateral, multilimb, and whole-body coordination as well as physical activity through play, sport, martial arts, or creative movement such as musical, yoga, and dance activities conducted in community or home settings within appropriately sized social groups [Srinivasan & Bhat, 2013; Srinivasan, Pescatello, & Bhat, 2014]. Recent studies assessing the effects of creative movement approaches using music and movement, and yoga have reported cascading effects of socially embedded motor interventions on the affective, communication, and behavioral skills of children with ASD [Srinivasan et al., 2016,b; Srinivasan et al., 2015,b; Kaur & Bhat, 2019].
In addition to sensori-motor goals, ASD interventions must meet the needs of the family and encompass functional, social communication, and cognitive-behavioral goals [Lobo et al., 2013]. Trainer–child interactions during movement interventions are embodied, that is, social communication and cognitive needs are naturally addressed while learning a variety of movement patterns. By providing motor interventions across multiple studies, it is clear that motor learning is inextricably linked to natural social interactions and communication between the child and the trainer [Srinivasan et al., 2016,b; Srinivasan et al., 2015,b; Kaur & Bhat, 2019]. Performing complex limb and body movements inherently require planning and executive functioning. It would be ideal to move away from seated play to promoting social communication and cognitive skills during motor learning tasks. Real-world interactions require us to move through space as we look around, speak, think, and learn, for example, playing a sport, shopping, cleaning, cooking, organizing things in your house, and so on. Hence, ASD interventions also need to be embodied and inclusive of movement while promoting a variety of social communication and cognitive-behavioral skills.
Limitations
Reliance on parent-reported questionnaires is a clear limitation of this study. Parent reports of their child’s abilities could be influenced by a reporting bias such as the Horn effect, that is, parents of children with greater impairments may have rated their child worse across all measures. However, parent reports as opposed to clinical evaluations are the best way to obtain information from a large sample to help elucidate the broader population patterns of a disorder. Fine-motor and gross-motor subdomains were broadly assessed without differentiating subdomains such as visuomotor or bilateral coordination, or balance skills as is often done using standard assessments with multiple subscales. Parent questionnaires provide limited information, hence, standardized and functional motor assessments using large-sized samples are important to further confirm whether certain motor behaviors are associated with the core features of ASD (e.g., multilimb/visuo-motor incoordination or dyspraxia, etc.) vs. motor behaviors that are more generalized (e.g., slow movement speeds) and better used as specifiers. Lastly, in spite of the large sized-sample, there could still be an ascertainment bias for urban/higher household income families due to the online nature of the study and inclusion of participants from autism centers within large cities in the United States.
Conclusions
Motor impairments in ASD were clearly associated with its core symptoms and are present in children with ASD with varying cognitive, language, and functional abilities. These findings support a multisystem view of ASD wherein motor impairments are a manifestation of a broader pattern of connectivity impairments involving multiple brain networks. Motor impairments should be included within the future DSM criteria or specifiers for ASD. Diagnostic clinicians must conduct motor screening and evaluations in children with ASD as well as recommend complete evaluations by movement clinicians to better address children’s motor problems. Movement clinicians must evaluate and offer appropriate interventions that address not only motor impairments but also social communication and cognitive-behavioral impairments to provide wholistic interventions for a multisystem disorder such as ASD. Clinical teams must utilize an interdisciplinary approach wherein co-treatment and role release is often practiced by a team of special educators, OTs, PTs, speech therapists to address the various needs of children with ASD. Movement clinicians such as OTs, PTs, and adaptive physical educators will need to look beyond basic motor delays/impairments to truly address the gap in motor performance, functions, play behaviors, and physical activity in children and adolescents with ASD.
Supplementary Material
Acknowledgments
A.B. is grateful to all SPARK families, SPARK clinical sites, and SPARK staff. Specifically, A.B. would like to thank Dr. LeeAnne Green-Snyder and Dr. Kiely Law for their prompt and detailed responses to all email communications regarding this dataset. A.B. truly appreciates obtaining access to the phenotypic data on SFARI Base. Approved researchers can obtain the SPARK population dataset described in this study at https://base.sfari.org/ordering/phenotype/sfari-phenotype by applying for the same at the following website: https://base.sfari.org. A.B.’s research during the period of writing this manuscript was supported by the National Institute of General Medical Sciences of the National Institutes of Health through a DE-INBRE Pilot Award (Project PI) through Institutional Development Award (IdeA) funding (Grant #: P20-GM103446, Site PI: Stanhope, S.) and the Dana Foundation’s clinical neuroscience grant. AB’s research is also supported by the National Institutes of Mental Health (Grant#: R01MH125823).
Footnotes
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Appendix S1: Supporting Information
Conflict of Interest
The author has no conflicts of interest to report.
References
- Ament K, Mejia A, Buhlman R, Erklin S, Caffo B, Mostofsky S, & Wodka E. (2014). Evidence for specificity of motor impairments in catching and balance in children with autism. Journal of Autism and Developmental Disorders, 45(3), 742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders, 5th Edition: DSM-5. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, … Neale BM (2018). Analysis of shared heritability in common disorders of the brain. Science, 360 (6395), eaap8757. 10.1126/science.aap8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford R, Pickles A, & Lord C. (2015). Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder. Autism Research, 9(9), 993–1001. 10.1002/aur.1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, & Bailey A. (1999). Autism screening questionnaire: Diagnostic validity. British Journal of Psychiatry, 175(5), 444–451. 10.1192/bjp.175.5.444 [DOI] [PubMed] [Google Scholar]
- Bhat AN (2020). Is motor impairment in autism spectrum disorder distinct from developmental coordination disorder? A report from the SPARK study. Physical Therapy, 100(4), 633–644. 10.1093/ptj/pzz190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, & Landa RJ (2012). Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior and Development, 35(4), 838–846. 10.1016/j.infbeh.2012.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Landa RJ, & Galloway JCC (2011). Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical Therapy, 91 (7), 1116–1129. 10.2522/ptj.20100294 [DOI] [PubMed] [Google Scholar]
- Bhat AN, Srinivasan SM, Woxholdt C, & Shield A. (2016). Differences in praxis performance and receptive language during fingerspelling between deaf children with and without autism spectrum disorder. Autism, 22(3), 271–282. 10.1177/1362361316672179 [DOI] [PubMed] [Google Scholar]
- Biscaldi M, Rauh R, Irion L, Jung NH, Mall V, Fleischhaker C, & Klein C. (2013). Deficits in motor abilities and developmental fractionation of imitation performance in high-functioning autism spectrum disorders. European Child & Adolescent Psychiatry, 23(7), 599–610. 10.1007/s00787-013-0475-x [DOI] [PubMed] [Google Scholar]
- Bremer E, & Cairney J. (2019). Adaptive behavior moderates health-related pathways in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 50 (2), 491–499. [DOI] [PubMed] [Google Scholar]
- Caçola P, Miller HL, & Williamson PO (2017). Behavioral comparisons in Autism spectrum disorder and developmental coordination disorder: A systematic literature review. Research in Autism Spectrum Disorders, 38, 6–18. 10.1016/j.rasd.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K, Taymans T, Wilson PH, Vanderstraeten G, Hosseini H, & van Waelvelde H. (2016). Neural signature of developmental coordination disorder in the structural connectome independent of comorbid autism. Developmental Science, 19(4), 599–612. 10.1111/desc.12424 [DOI] [PubMed] [Google Scholar]
- Carper RA, Solders S, Treiber JM, Fishman I, & Müller RA (2015). Corticospinal tract anatomy and functional connectivity of primary motor cortex in autism. Journal of the American Academy of Child & Adolescent Psychiatry, 54(10), 859–867. 10.1016/j.jaac.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wang J, Uddin LQ, Wang X, Guo X, Lu F, … Chen H. (2018). Aberrant functional connectivity of neural circuits associated with social and sensorimotor deficits in young children with autism spectrum disorder. Autism Research, 11 (12), 1643–1652. 10.1002/aur.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B, Leech KA, Tager-Flusberg H, & Nelson CA (2018). Development of fine motor skills is associated with expressive language outcomes in infants at high and low risk for autism spectrum disorder. Journal of Neurodevelopmental Disorders, 10(14), 1–11. 10.1186/s11689-018-9231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, & Morgan J. (2007). Mapping early brain development in autism. Neuron, 56(2), 399–413. 10.1016/j.neuron.2007.10.016 [DOI] [PubMed] [Google Scholar]
- Daniels AM, Rosenberg RE, Anderson C, Law JK, Marvin AR, & Law PA (2011). Verification of parent-report of child autism spectrum disorder diagnosis to a web-based autism registry. Journal of Autism and Developmental Disorders, 42(2), 257–265. 10.1007/s10803-011-1236-7 [DOI] [PubMed] [Google Scholar]
- Dewey D, Cantell M, & Crawford S. (2007). Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society, 13(02), 246–256. 10.1017/s1355617707070270 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo L. (2014). The use of odds ratio in the large population-based studies: Warning to readers. Muscle, Ligaments and Tendons Journal, 4(1), 90–92. 10.11138/mltj/2014.4.1.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey R, & Rapport MJK (2012). Motor activity in children with autism. Pediatric Physical Therapy, 24(1), 2–20. 10.1097/pep.0b013e31823db95f [DOI] [PubMed] [Google Scholar]
- Dziuk MA, Larson JCG, Apostu A, Mahone EM, Denckla MB, & Mostofsky SH (2007). Dyspraxia in autism: Association with motor, social, and communicative deficits. Developmental Medicine & Child Neurology, 49(10), 734–739. 10.1111/j.1469-8749.2007.00734.x [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, & Johnson MH (2016). Autism and the social brain: The first-year puzzle. Biological Psychiatry, 80(2), 94–99. 10.1016/j.biopsych.2016.02.019 [DOI] [PubMed] [Google Scholar]
- Feliciano P, Daniels AM, Green Snyder L, Beaumont A, Camba A, Esler A, … Chung WK (2018). SPARK: A US cohort of 50,000 families to accelerate autism research. Neuron, 97(3), 488–493. 10.1016/j.neuron.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury A, Kushki A, Tanel N, Anagnostou E, & Chau T. (2013). Statistical persistence and timing characteristics of repetitive circle drawing in children with ASD. Developmental Neurorehabilitation, 16(4), 245–254. 10.3109/17518423.2012.758184 [DOI] [PubMed] [Google Scholar]
- Floris DL, & Howells H. (2018). Atypical structural and functional motor networks in autism. Progress in Brain Research, 238, 207–248. 10.1016/bs.pbr.2018.06.010 [DOI] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, & Cauraugh JH (2010). Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders, 40(10), 1227–1240. 10.1007/s10803-010-0981-3 [DOI] [PubMed] [Google Scholar]
- Frazier TW, Keshavan MS, Minshew NJ, & Hardan AY (2012). A two-year longitudinal MRI study of the corpus callosum in autism. Journal of Autism and Developmental Disorders, 42(11), 2312–2322. 10.1007/s10803-012-1478-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner B, & Féron F. (2009). Autism: A world changing too fast for a mis-wired brain? Neuroscience & Biobehavioral Reviews, 33(8), 1227–1242. 10.1016/j.neubiorev.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Sauer EA, Geye HM, Schweigert EK, & Hill Goldsmith H. (2008). Infant and toddler oral- and manual-motor skills predict later speech fluency in autism. Journal of Child Psychology and Psychiatry, 49(1), 43–50. 10.1111/j.1469-7610.2007.01820.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaziuddin M, & Butler E. (1998). Clumsiness in autism and Asperger syndrome: A further report. Journal of Intellectual Disability Research, 42(1), 43–48. 10.1046/j.1365-2788.1998.00065.x [DOI] [PubMed] [Google Scholar]
- Gizzonio V, Avanzini P, Campi C, Orivoli S, Piccolo B, Cantalupo G, … Fabbri-Destro M. (2015). Failure in pantomime action execution correlates with the severity of social behavior deficits in children with autism: A Praxis study. Journal of Autism and Developmental Disorders, 45(10), 3085–3097. 10.1007/s10803-015-2461-2 [DOI] [PubMed] [Google Scholar]
- Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, & Baird G. (2009). Impairment in movement skills of children with autistic spectrum disorders. Developmental Medicine & Child Neurology, 51(4), 311–316. 10.1111/j.1469-8749.2008.03242.x [DOI] [PubMed] [Google Scholar]
- Hanaie R, Mohri I, Kagitani-Shimono K, Tachibana M, Matsuzaki J, Hirata I, … Taniike M. (2018). Aberrant Cerebellar–Cerebral Functional Connectivity in Children and Adolescents With Autism Spectrum Disorder. Frontiers in Human Neuroscience, 12, 454. 10.3389/fnhum.2018.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton CL, Zhang Y, Whilte MR, Klohr CL, & Constantino J. (2011). Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism, 16(4), 430–441. 10.1177/1362361311423018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirjak D, Meyer-Lindenberg A, Fritze S, Sambataro F, Kubera KM, & Wolf RC (2018). Motor dysfunction as research domain across bipolar, obsessive-compulsive and neurodevelopmental disorders. Neuroscience Biobehavioral Reviews., 95, 315–335. 10.1016/j.neubiorev.2018.09.009.Epub 2018 Sep 17 [DOI] [PubMed] [Google Scholar]
- Iverson JM (2018). Early motor and communicative development in infants with an older sibling with autism spectrum disorder. Journal of Speech, Language, and Hearing Research, 61(11), 2673–2684. 10.1044/2018_jslhr-l-rsaut-18-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, & Mostofsky SH (2006). Motor Signs Distinguish Children with High Functioning Autism and Asperger’s Syndrome from Controls. Journal of Autism and Developmental Disorders, 36(5), 613–621. 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, & Varma S. (2012). Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience & Biobehavioral Reviews, 36(4), 1292–1313. 10.1016/j.neubiorev.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, & Bhat A. (2019). Creative yoga intervention improves motor and imitation skills of children with autism spectrum disorder. Physical Therapy, 99(11), 1520–1534. 10.1093/ptj/pzz115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Srinivasan M, & Bhat N. (2018). Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without Autism Spectrum Disorder (ASD). Research in Developmental Disabilities, 72, 79–95. 10.1016/j.ridd.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Sykes LK, Geier MR, & Deth RC (2012). Are ASD and ADHD a continuum? A comparison of pathophysiological similarities between the Ddsorders. Journal of Attention Disorders, 19(9), 805–827. 10.1177/1087054712459886 [DOI] [PubMed] [Google Scholar]
- Kushki A, Chau T, & Anagnostou E. (2011). Handwriting difficulties in children with autism spectrum disorders: A scoping review. Journal of Autism and Developmental Disorders, 41(12), 1706–1716. 10.1007/s10803-011-1206-0 [DOI] [PubMed] [Google Scholar]
- Lam KSL, & Aman MG (2006). The repetitive behavior scale-revised: Independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(5), 855–866. 10.1007/s10803-006-0213-z [DOI] [PubMed] [Google Scholar]
- LeBarton ES, & Landa RJ (2019). Infant motor skill predicts later expressive language and autism spectrum disorder diagnosis. Infant Behavior and Development, 54, 37–47. 10.1016/j.infbeh.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Lee H, Marvin AR, Watson T, Piggot J, Law JK, Law PA, … Nelson SF (2010). Accuracy of phenotyping of autistic children based on internet implemented parent report. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 153B(6), 1119–1126. 10.1002/ajmg.b.31103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard HC, Bedford R, Pickles A, & Hill EL (2015). Predicting the rate of language development from early motor skills in at-risk infants who develop autism spectrum disorder. Research in Autism Spectrum Disorders, 13–14, 15–24. 10.1016/j.rasd.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licari MK, Alvares GA, Varcin K, Evans KL, Cleary D, Reid SL, … Whitehouse AJO (2019). Prevalence of motor difficulties in autism spectrum disorder: Analysis of a population-based cohort. Autism Research, 13(2), 298–306. 10.1002/aur.2230 [DOI] [PubMed] [Google Scholar]
- Li Q, Becker B, Jiang X, Zhao Z, Zhang Q, Yao S, & Kendrick KM (2019). Decreased interhemispheric functional connectivity rather than corpus callosum volume as a potential biomarker for autism spectrum disorder. Cortex, 119, 258–266. 10.1016/j.cortex.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Lloyd M, MacDonald M, & Lord C. (2011). Motor skills of toddlers with autism spectrum disorders. Autism, 17(2), 133–146. 10.1177/1362361311402230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MA, Harbourne RT, Dusing SC, & McCoy SW (2013). Grounding early intervention: Physical therapy cannot just be about motor skills anymore. Physical Therapy, 93 (1), 94–103. 10.2522/ptj.20120158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M, Lord C, & Ulrich D. (2013a). The relationship of motor skills and adaptive behavior skills in young children with autism spectrum disorders. Research in Autism Spectrum Disorders, 7(11), 1383–1390. 10.1016/j.rasd.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M, Lord C, & Ulrich DA (2013b). The relationship of motor skills and social communicative skills in school-aged children with autism spectrum disorder. Adapted Physical Activity Quarterly, 30(3), 271–282. 10.1123/apaq.30.3.271 [DOI] [PubMed] [Google Scholar]
- MacNeil LK, & Mostofsky SH (2012). Specificity of dyspraxia in children with autism. Neuropsychology, 26(2), 165–171. 10.1037/a0026955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari M, Castiello U, Marks D, Marraffa C, & Prior M. (2003). The reach–to–grasp movement in children with autism spectrum disorder. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 358 (1430), 393–403. 10.1098/rstb.2002.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin AR, Marvin DJ, Lipkin PH, & Law JK (2017). Analysis of social communication questionnaire (SCQ) screening for children less than age 4. Current Developmental Disorders Reports, 4(4), 137–144. 10.1007/s40474-017-0122-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhillips M, Finlay J, Bejerot S, & Hanley M. (2014). Motor deficits in children with autism spectrum disorder: A cross-syndrome study. Autism Research, 7(6), 664–676. 10.1002/aur.1408 [DOI] [PubMed] [Google Scholar]
- Miyahara M, Tsujii M, Hori M, Nakanishi K, Kageyama H, & Sugiyama T. (1997). Brief report: Motor incoordination in Children with Asperger syndrome and learning disabilities. Journal of Autism and Developmental Disorders, 27(5), 595–603. 10.1023/a:1025834211548 [DOI] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, & Müller R-A (2013). Impaired thalamocortical connectivity in autism spectrum disorder: A study of functional and anatomical connectivity. Brain, 136(6), 1942–1955. 10.1093/brain/awt079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel MB, Eloyan A, Nettles CA, Sweeney KL, Ament K, Ward RE, … Mostofsky SH (2016). Intrinsic visual-motor synchrony correlates with social deficits in autism. Biological Psychiatry, 79(8), 633–641. 10.1016/j.biopsych.2015.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivard L, Missiuna C, McCauley D, & Cairney J. (2012). Descriptive and factor analysis of the Developmental Coordination Disorder Questionnaire (DCDQ’07) in a population-based sample of children with and without Developmental Coordination Disorder. Child: Care, Health and Development, 40(1), 42–49. 10.1111/j.1365-2214.2012.01425.x [DOI] [PubMed] [Google Scholar]
- Schoemaker MM, Flapper B, Verheij NP, Wilson BN, Reinders-Messelink HA, & de Kloet A. (2006). Evaluation of the Developmental Coordination Disorder Questionnaire as a screening instrument. Developmental Medicine & Child Neurology, 48(08), 668. 10.1017/s001216220600140x [DOI] [PubMed] [Google Scholar]
- Shield A, Knapke K, Henry M, Srinivasan S, & Bhat A. (2017). Impaired praxis in gesture imitation by deaf children with autism spectrum disorder. Autism & Developmental Language Impairments, 2, 239694151774567. 10.1177/2396941517745674 [DOI] [Google Scholar]
- Srinivasan SM, & Bhat AN (2013). A review of “music and movement” therapies for children with autism: Embodied interventions for multisystem development. Frontiers in Integrative Neuroscience, 7, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, & Bhat AN (2016). Differences in object sharing between infants at risk for autism and typically developing infants from 9 to 15 months of age. Infant Behavior and Development, 42, 128–141. 10.1016/j.infbeh.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Eigsti I-M, Gifford T, & Bhat AN (2016). The effects of embodied rhythm and robotic interventions on the spontaneous and responsive verbal communication skills of children with Autism Spectrum Disorder (ASD): A further outcome of a pilot randomized controlled trial. Research in Autism Spectrum Disorders, 27, 73–87. 10.1016/j.rasd.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Eigsti I-M, Neelly L, & Bhat AN (2016). The effects of embodied rhythm and robotic interventions on the spontaneous and responsive social attention patterns of children with autism spectrum disorder (ASD): A pilot randomized controlled trial. Research in Autism Spectrum Disorders, 27, 54–72. 10.1016/j.rasd.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Kaur M, Park IK, Gifford TD, Marsh KL, & Bhat AN (2015). The Effects of rhythm and robotic interventions on the imitation/Praxis, interpersonal synchrony, and motor performance of children with autism spectrum disorder (ASD): A pilot randomized controlled trial. Autism Research and Treatment, 2015, 1–18. 10.1155/2015/736516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Park IK, Neelly LB, & Bhat AN (2015). A comparison of the effects of rhythm and robotic interventions on repetitive behaviors and affective states of children with autism spectrum disorder (ASD). Research in Autism Spectrum Disorders, 18, 51–63. 10.1016/j.rasd.2015.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Pescatello LS, & Bhat AN (2014). Current perspectives on physical activity and exercise recommendations for children and adolescents with autism spectrum disorders. Physical Therapy, 94(6), 875–889. 10.2522/ptj.20130157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz Ham H, Bartolo A, Corley M, Rajendran G, Szabo A, & Swanson S. (2010). Exploring the relationship between gestural recognition and imitation: Evidence of dyspraxia in autism spectrum disorders. Journal of Autism and Developmental Disorders, 41(1), 1–12. 10.1007/s10803-010-1011-1 [DOI] [PubMed] [Google Scholar]
- Turner KC, Frost L, Linsenbardt D, McIlroy JR, & Müller R-A (2006). Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behavioral and Brain Functions, 2(1), 34. 10.1186/1744-9081-2-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa RA, Mostofsky SH, & Ewen JB (2016). The disrupted connectivity hypothesis of autism spectrum disorders: Time for the next phase in research. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(3), 245–252. 10.1016/j.bpsc.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt CP, & Craig CM (2011). Motor skills in children aged 7–10 years, diagnosed with autism spectrum disorder. Journal of Autism and Developmental Disorders, 42(9),1799–1809. 10.1007/s10803-011-1421-8 [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bauman ML, Stone WL, Yirmiya N, Estes A, Hansen RL, … Wetherby A. (2015). Early identification of autism spectrum disorder: Recommendations for practice and research. Pediatrics, 136(Supplement), S10–S40. 10.1542/peds.2014-3667c [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.