Abstract
Background
Treatment of human papillomavirus‐related oropharyngeal squamous cell carcinoma (HPVOPC) results in unprecedented high survival rates but possibly unnecessary toxicity. We hypothesized that upfront surgery and neck dissection followed by reduced‐dose adjuvant therapy for early and intermediate HPVOPC would ultimately result in equivalent progression‐free survival (PFS) and overall survival while reducing toxicity.
Methods
This study was a nonrandomized phase II trial for early‐stage HPVOPC treated with transoral robotic surgery (TORS) followed by reduced‐dose radiotherapy. Patients with previously untreated p16‐positive HPVOPC and <20 pack years’ smoking history were enrolled. After robotic surgery, patients were assigned to group 1 (no poor risk features; surveillance), group 2 (intermediate pathologic risk factors [perineural invasion, lymphovascular invasion]; 50‐Gy radiotherapy), or group 3 (poor prognostic pathologic factors [extranodal extension [ENE], more than three positive lymph nodes and positive margin]; concurrent 56‐Gy chemoradiotherapy with weekly cisplatin).
Results
Fifty‐four patients were evaluable; there were 25 in group 1, 15 in group 2, and 14 in group 3. Median follow‐up was 43.9 months (9.6–75.8). Disease‐specific survival was 98.1%, and PFS was 90.7%. PFS probability via Kaplan‐Meier was 91.3% for group 1, 86.7% for group 2, and 93.3% for group 3. There were five locoregional failures (LRFs), including one distant metastasis and one contralateral second primary. Average time to LRF was 18.9 months (9.6–59.0); four LRFs were successfully salvaged, and the patients remain disease free (11.0–42.7 months); one subject remains alive with disease.
Conclusion
The results indicate that upfront surgery with neck dissection with reduced‐dose radiation for T1–2, N1 stage (by the eighth edition American Joint Committee on Cancer staging manual) HPVOPC results in favorable survival with excellent function in this population. These results support radiation dose reduction after TORS as a de‐escalation strategy in HPVOPC.
Implications for Practice
Transoral robotic surgery can provide a safe platform for de‐escalation in carefully selected patients with early‐stage human papillomavirus‐related oropharyngeal cancer. In this clinical trial, disease‐specific survival was 100%, over 90% of the cohort had a reduction of therapy from standard of care with excellent functional results, and the five patients with observed locoregional failures were successfully salvaged.
Keywords: Oropharynx cancer, Human papillomavirus, Transoral robotic surgery, De‐escalation
Short abstract
Treatment of human papillomavirus‐related oropharyngeal squamous cell carcinoma results in high survival rates but unnecessary toxicity. The SIRS trial investigated the use of transoral robotic surgery with selective neck dissection, followed by reduced‐dose adjuvant therapy, to reduce toxicity and maintain overall and progression‐free survival rates.
Introduction
The increasing incidence of human papillomavirus‐related oropharyngeal squamous cell carcinoma (HPVOPC) is widely recognized in North America, Europe, and other developed countries [1, 2]. In addition, it is also known that HPVOPC has a substantially more favorable prognosis [3, 4, 5, 6]. Unfortunately, despite the available evidence from phase II trials and phase III clinical trials, national guidelines continue to recommend standard‐dose radiation, or radiation with platinum‐based concurrent chemotherapy, for HPVOPC [7]. As a result, many patients treated for early‐stage HPVOPC will live long lives but may suffer excessive toxicity and long‐term morbidity, primarily because of radiotherapy without clear oncologic benefit [8, 9].
The excellent prognosis of early‐stage HPVOPC highlights the need for clinical trials to address “de‐escalation” [10, 11]. One of the recently proposed de‐escalation strategies is minimally invasive surgery (primarily transoral robotic surgery [TORS] and selective neck dissection) for selected patients with risk‐adjusted adjuvant therapy [12]. This approach has been driven by data indicating excellent oncologic control in HPVOPC with favorable functional results [13, 14, 15, 16]. Low rates of permanent tracheostomy or gastrostomy tube dependence have been reported when adopting this approach [17]. Our hypotheses when designing the Sinai Robotic Surgery (SIRS) trial were that TORS followed by strict pathologic stratification in a cohort of early T‐stage HPVOPC would allow for observation alone in a significant number of patients and reduced‐dose, risk‐adjusted adjuvant therapy for many, and that this approach would achieve equivalent oncologic control with excellent functional outcomes compared with standard of care.
Materials and Methods
Study Design
The SIRS trial (NCT NCT02072148) was a nonrandomized phase II de‐escalation trial approved by the Institutional Review Board of the Icahn School of Medicine. Study endpoints included locoregional control, disease‐specific survival (DSS), disease‐free survival, overall survival (OS), patterns of failure, and salvage outcomes. Eligible patients were presented at the Multidisciplinary Tumor Board, and surgical and nonsurgical standard of care options (TORS with standard‐dose radiation, chemoradiation, etc.) were discussed prior to enrollment. Eligible patients underwent TORS and selective neck dissection. After surgery, patients with early‐stage disease were assigned to surveillance (group 1) or adjuvant therapy, depending on pathologic stratification. Patients with intermediate risk factors (group 2) would receive reduced‐dose radiotherapy (50 Gy). Patients with poor prognostic features (group 3) would receive reduced‐dose concurrent chemoradiotherapy (56 Gy + weekly cisplatin). (The SIRS trial schema is shown in Fig. 1.)
Figure 1.
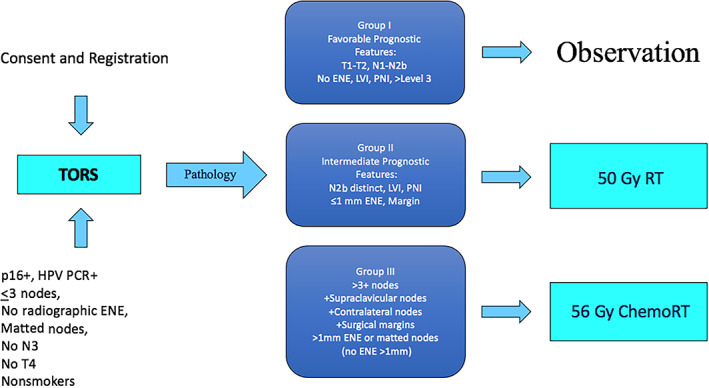
Sinai Robotic Surgery (SIRS) trial schema. Abbreviations: ChemoRT, chemoradiotherapy; ENE, extranodal extension; HPV, human papillomavirus; LVI, lymphovascular invasion; PCR, polymerase chain reaction; PNI, perineural invasion; RT, radiotherapy; TORS, transoral robotic surgery.
Human Papillomavirus Testing
For biopsy and resection specimens, human papillomavirus (HPV) positivity required p16 staining by immunohistochemistry, with confirmation via reverse transcriptase polymerase chain reaction (rtPCR) prior to group assignment [18, 19]. For cytology cell blocks, standalone rtPCR testing was deemed sufficient [20]. (HPV testing is described in supplemental online Appendix 1.)
Smoking Criteria
Active tobacco smoking is an adverse prognostic factor and associated with specific adverse mutational profiles [21, 22, 23, 24]. For these reasons, patients with a history of >20 pack years or recent tobacco use (at least one cigarette or cigarette‐equivalent per day within the last 5 years) were excluded.
Staging Criteria
Patients were enrolled based on the seventh edition of the American Joint Committee on Cancer (AJCC) staging system [25]. Included were stage I, II, and III and intermediate stage IVa (T1N0–2B, T2N0–2B) disease without imaging evidence of extranodal extension (ENE). Per the eighth edition of the AJCC staging system, all subjects were at stage I [6]. Patients with serious medical conditions, immunocompromise, drug/alcohol abuse, or history of malignancy were excluded. Patients who presented with advanced stage III/IV (N2C, N3), clinical or radiographic evidence of ENE, more than three positive cervical nodes, or surgically unresectable disease were excluded.
Surgery
All patients underwent TORS as the initial therapy. This was performed with the DaVinci Si robotic system (Intuitive, Sunnyvale, CA). Radical tonsillectomy was performed with resection of the pharyngeal constrictor muscle as the deep margin. Base‐of‐tongue resection was performed with a 1‐cm margin. Frozen section margins were performed on the main specimen. Surgery proceeded until negative frozen section margins were obtained. Bilateral neck dissection was performed in all cases, with selective neck dissection levels II–IV for ipsilateral N1 and selective neck dissection levels II–III for ipsilateral N0 and contralateral N0 necks. Ipsilateral lingual artery ligation was performed in all tongue resections.
Delivery of Radiotherapy
Assigned patients were treated with intensity‐modulated radiotherapy 5 days per week with 2‐Gy fractions. Total dose was determined based on pathological group assignment. For group 2, radiation dose was 50 Gy in 25 fractions. Clinical target volume (CTV) CTV50 included the primary tumor bed and/or lymph node regions in the neck at risk for harboring microscopic disease. For patients in group 3, 56 Gy was delivered in 28 fractions. CTV56 accounted for areas of highest positive margin. If there was ENE >1 mm, CTV56 encompassed the area that was found to have ENE. During the initial trial design, given the limited data on the impact of pathologic ENE in the population with HPV, the >1 mm ENE cutoff was selected to be a relatively conservative trigger for the high‐risk group. More recent data indicate that these relatively strict cutoffs may be warranted, especially in tobacco users, and that even microscopic ENE may be relevant in this population [26, 27].
Chemoradiotherapy
Group 3 was given cisplatin 40 mg/m2 per week intravenously for 6 weeks during radiotherapy. Doses were reduced for neutropenia or thrombocytopenia. Patients who developed grade 2 hearing loss were switched to carboplatin at an area under the curve of 1.5. Carboplatin was also dose‐adjusted for neutropenia and thrombocytopenia.
Salvage Protocol
All recurrences were presented at multidisciplinary tumor conference, and salvage was planned per standard of care. Details of the salvage cases can be seen in Table 1.
Table 1.
Failures and salvage interventions, with current status
| Failure | Stage/site | Group assignment | Site of failure | Time to failure, mo | Treatment | Status (mo) |
|---|---|---|---|---|---|---|
| 1 | T1N2aM0/right tonsil | Group 1 (observational) | Ipsilateral neck |
11.5 – neck 22.0 – lung metastasis |
Salvage ND + CCRT, RT for lung metastasis | AWD (16) |
| 2 | T1N2aM0/left tonsil | Group 1 (observational) | Local | 3.8 | Salvage CCRT | NED (21) |
| 3 | T2N2bM0/left tonsil | Group 2 (radiation) | Contralateral neck | 9.7 | Salvage ND + CCRT | NED (41) |
| 4 | T1N2aM0/Left tonsil | Group 2 (radiation) | Contralateral right tonsil (second primary) | 59.0 | Salvage TORS + XRT | NED (11) |
| 5 | T2N2aM0/right tonsil | Group 3 (chemoradiation) | Local | 9.8 | Salvage resection, CCRT proton | NED (42) |
Abbreviations: AWD, alive with disease; CCRT, concurrent chemoradiotherapy; ND, neck dissection; NED, no evidence of disease; RT, radiotherapy; TORS, transoral robotic surgery; XRT, radiotherapy for consistency.
Response Evaluation
All subjects underwent surveillance imaging at 4‐month intervals and tracked for time to recurrence, locoregional control, OS, progression‐free survival (PFS), and DSS (see Statistical Analysis section).
Quality of Life Assessment
Quality of life (QOL) was assessed using the MD Anderson Dysphagia Inventory (MDADI), the MD Anderson Symptom Inventory, the University of Michigan Xerostomia Questionnaire, and the European Organization for Research and Treatment of Cancer Questionnaire. Data were collected at baseline and weekly, as well as at 3, 6, 12, 24, 36, and 48 months in the follow‐up period.
Statistical Analysis
Sample Size Calculation
The proposed sample size has been determined to ensure that an upper bound for recurrence may be estimated with adequate precision within of the true recurrence rate. Assuming the true rate of recurrence is between 0.1 to 0.5, with 100 patients, the corresponding upper 95% bound is 0.184 to 0.614. The sample size of 100 for group 1 patients (with surgery only) ensures that the interquartile range for the estimated probability distributions will extend from approximately 10 to 12 percentage points. This range is wider for smaller sample sizes (e.g., 30 or 50) but still less than 20 percentage points.
Survival Analysis
DSS was calculated by measuring the time from trial entry to cancer‐related death. PFS by calculating the time to biopsy confirmed recurrence or death from any cause. OS from the time of entry to death with any cause. SAS 9.4 software (SAS Institute, Cary, NC) was used to produce the Kaplan‐Meier survival curve (with procedure life‐test) for the study groups. A log‐rank test with Sidak adjustment for multiple comparisons was used to determine statistically significant differences between groups.
Adverse Effects Analysis
The number of adverse effects in patients in each of the three groups were calculated and expressed as a scatter and box plot. Groups were compared with Kruskal‐Wallis analysis of variance followed by Mann‐Whitney U tests with Bonferroni correction of p for multiple comparisons.
QOL Analysis
The primary QOL outcome assessment was dysphagia, the major driver of morbidity after therapy. The MDADI is a 20‐item questionnaire that evaluates swallowing on 5‐point Likert scale. As described by Chen et al. [28], scoring includes two scores: a global score (first question) and composite score (remaining 19 questions). Final results are rescaled to a range of 0–100. Results of questionnaires were collected prior to surgery and at five times subsequently: around 100, 200, 3500, 725, and 1,100 days after surgery. A paired t test was used to compare questionnaire scores. The remainder of the data and analysis related to QOL will be described in a follow‐up manuscript.
Results
Survival Outcomes
Seventy‐five patients were enrolled in the trial; 21 subjects withdrew (see below), leaving 54 subjects evaluable. Three subjects (5.5%) reported a history of tobacco use (<20 pack years, one in each of the groups 1, 2, and 3), a number insufficient for analysis of the impact of tobacco exposure. Twenty‐six percent of the cohort reported consuming light/moderate alcohol on a weekly basis. The majority of the cohort was positive for the HPV16 serotype, but two patients had HPV33, and one had HPV18. The limited number of noncanonical serotypes prohibited analysis regarding serotype.
After TORS and pathologic risk stratification, the 54 subjects were assigned to group 1 (n = 25), group 2 (n = 15), and group 3 (n = 14). Two subjects had positive surgical margins (assigned to group 3). The remainder of the group 3 cohort were assigned because of other adverse pathologic criteria. Rates of perineural invasion and lymphovascular invasion were 6 of 54 (11.1%) patients, and 15 of 54 (27.7%) patients, respectively. Of note, 13 of 54 (24.0%) patients were assigned to group 3 based on ENE >1 mm, although none had ENE identified on preoperative imaging. Matted nodes were documented on final pathology in 3 of 54 (5.5%) patients. The mean nodal yield of the bilateral neck dissections was 39.3 (range 9–113, median 33). The mean number of positive nodes was 1.7 with a median of 2.3 (range 0–8). Eleven of 54 (20.3%) patients had more than three positive nodes. Interestingly, 4 of 54 (7.4%) patients had occult contralateral nodes discovered, two in tonsillar primaries and two in base‐of‐tongue primaries.
Twenty‐one subjects withdrew from the study. Six patients withdrew from the trial prior to surgical intervention. Five patients were lost to follow‐up after TORS. In two patients, TORS was not deemed possible because of progression or extent of the tumor; these patients underwent standard concurrent chemoradiotherapy. One patient declined adjuvant radiation, experienced recurrence in the neck, and was given salvage treatment with neck dissection and radiation. One patient dropped out of the trial after TORS, had recurrence, and underwent salvage concurrent chemoradiotherapy at another institution. An unrelated myocardial infarction was included in the OS analysis. Six patients underwent TORS on protocol, were stratified to group 2 or 3, then withdrew to undergo adjuvant therapy closer to home for logistical reasons. All six patients who had adjuvant therapy at an outside institution were had no evidence of disease at the time of the writing of this article (median follow‐up 34.0 months, range 6–50 months). (The CONSORT diagram is shown in Fig. 2.)
Figure 2.
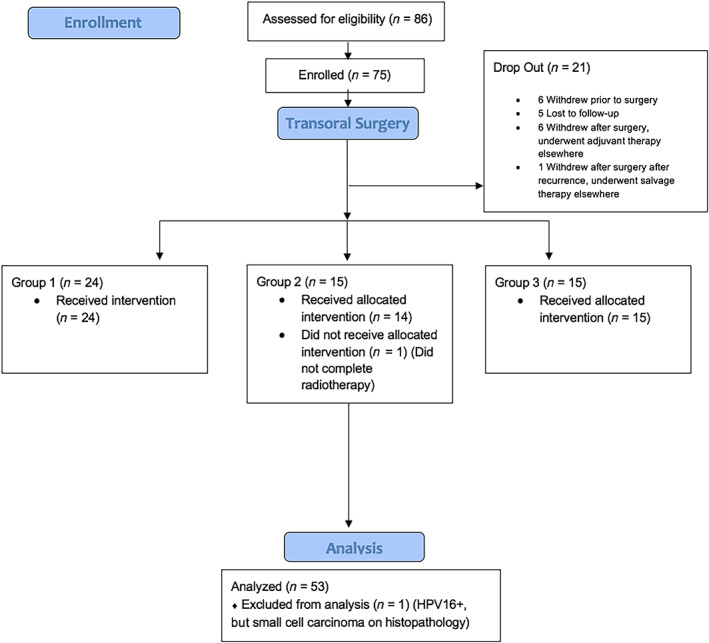
Sinai Robotic Surgery (SIRS) trial CONSORT diagram. Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; HPV16+, positive for the HPV16 serotype.
Median follow‐up was 43.9 months (9.6–75.8). DSS was 100% in the study cohort, disease‐free survival was 98.1%, and PFS was 90.7%. PFS probability via Kaplan‐Meier was 91.3% for group 1, 86.7% for group 2, and 93.3% for group 3 (Fig. 3). There were no statistically significant differences between the survival curves in these three groups (p = .81, log‐rank test). One unrelated death occurred (myocardial infarction) in group 2. Five locoregional failures (LRFs) with one distant metastasis occurred. One of the LRFs was a second primary in the contralateral tonsil (Table 1). Average time to LRF was 18.9 months (9.6–59.0); four LRFs were successfully salvaged, and the patients remain disease free (11.0–42.7 months), with one patient alive with disease (lung metastasis) with an ultimate disease control of 98.1%. OS was 98.1% (53/54) because of the cardiac‐related death.
Figure 3.
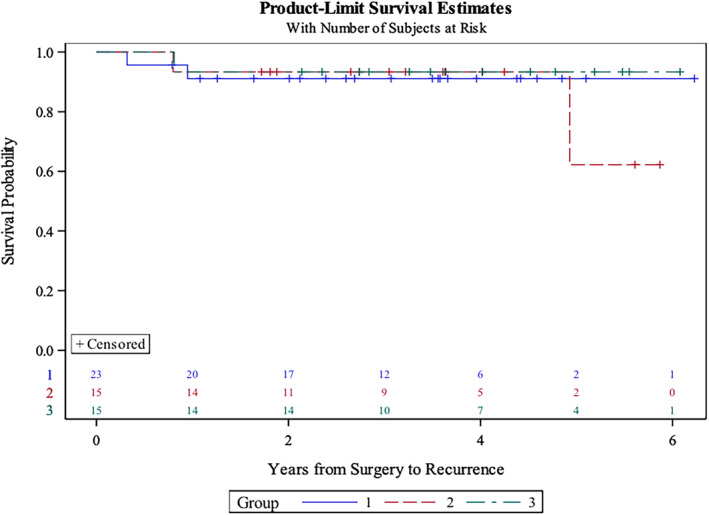
Product limit survival estimate curve (Kaplan‐Meier). Note that there were no statistically significant differences between survival curves in these three groups (p = .81, log‐rank test).
Toxicity and Adverse Events
Fifty‐eight patients were evaluated for adverse events (AEs) and grade 3/4 toxicity, with the additional four patients not included in survival analysis because of recent therapy or insufficient follow‐up time. Scatter box plots indicating the number of adverse events can be viewed in Figure 4.
Figure 4.
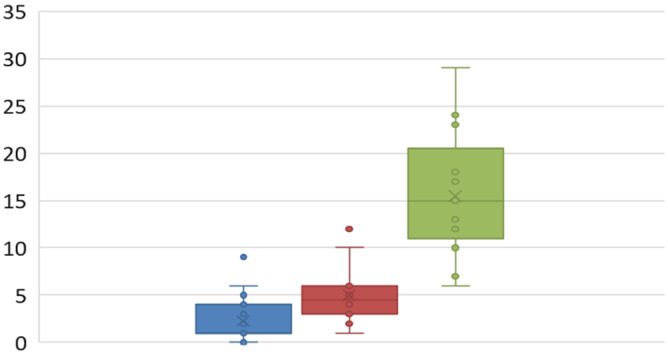
Number of adverse events.
In group 1 (TORS alone), the most commonly reported AEs were dysphagia in 10 of 27 (37%) patients, severe pain in 8 of 27 (29.6%), anxiety in 3 of 27 (11.1%), and, interestingly, xerostomia in 3 of 27 (11.1%). One patient in this group had a postoperative bleed requiring operative management. A single patient reported velopharyngeal insufficiency. Six of 27 (22.2%) patients had complaints related to their neck dissection including numbness, stiffness, or mild pain/swelling.
In group 2 (TORS +50 Gy), the most commonly reported AEs posttherapy were altered taste/dysgeusia in 15 of 15 (100%) patients, xerostomia in 10 of 15 (66.6%), and severe pain in 10 of 15 (66.6%); 3 of 15 (20.0%) patients reported numbness of the neck, and 3 of 15 (20.0%) reported lack of appetite. No grade 3/4 mucositis was reported. Interestingly, 13 of 15 (86.6%) patients in group 2 had neck complaints because of therapy, including numbness or stiffness of the neck or mild pain or swelling.
In group 3 (TORS + cisplatin +56 Gy), the most commonly reported AEs were dysphagia in 16 of 16 (100%) patients, pain in 16 of 16 (100%), dysarthria in 8 of 16 (50.0%), and fatigue in 8 of 16 (50.0%). Eight of 16 (50.0%) patients reported grade 3/4 mucositis in this group, with 15 of 16 (93.7%) reporting some level of mucositis. Only one patient exhibited a > 20‐lb weight loss. There was a pulmonary embolism in a patient in group 3, which was treated and resolved. One patient had a postoperative hemorrhage and one a hematoma, both requiring operative management. Of the 14 patients in group 3, 11 received cisplatin; 4 of these had a dose modification (three for neutropenia and thrombocytopenia and one for dehydration). The median total dose of cisplatin for these 11 patients was 200 mg/m2 (190–240 mg/m2). Three patients received a mixed course of cisplatin and carboplatin for tinnitus (two patients) or a creatinine elevation (one patient).
The incidence of severe postoperative bleeding requiring return to the operating room was 5.1% (3/58) in the cohort. There were no documented neck infections or chyle leaks in the study.
Patterns of Failure
Average time to LRF was 18.9 months (3.8–59.0); however, this time was skewed because of one contralateral second primary tumor in the opposite tonsil; if this case is excluded, average time to recurrence was 16.5 months. Four LRFs were successfully salvaged (Table 1), and the patients remain disease free at the present time (16–42 months postsalvage, except the patient with the contralateral second primary tumor), with one patient alive with disease (single lung metastasis). Two regional failures were single cervical nodes, one ipsilateral previously dissected neck (group 1), and one contralateral neck metastasis (group 2) (Table 2).
Table 2.
Disease‐specific survival and failure rates by group assignment
| Group assignment (n) | PFS, % | Local failure, % | Regional failure, % | Distant failure, % |
|---|---|---|---|---|
| Group 1 (25) | 91.3 | 4.0 | 4.0 | 4.0 |
| Group 2 (15) | 86.7 | 6.6 | 6.6 | 0.0 |
| Group 3 (14) | 93.3 | 7.1 | 0.0 | 0.0 |
| Total (54) | 90.7 (5/54) | 5.5 | 3.7 | 1.9 |
Abbreviation: PFS, progression‐free survival.
Quality of Life
Global QOL scores improved with time and eventually returned to baseline levels; these will be reported in a future manuscript. Results for composite and global MDADI scores are presented in Figure 5. No score differences existed before and after surgery in group 1. Mean composite score was 89 (on scale of 0 to 100) before surgery and always was above 89 after surgery. In group 2, the score decreased to 76 (p = .027, t test) after 3 months but returned to 85 after 6 months. In group 3, the score substantially decreased to 63 (p = .0001) after 3 months and slowly improved with time. The score was 71 (p = .011) after 6 months, was 78 (p = .11, not significant) after 1 year, and returned to 88 after 2 years. Changes in global score resembled changes in composite scores: no decrease in group 1, decrease after 3 months with recovery at 6 months in group 2, and large decrease after 3 months with slow recovery with time in group 3. In all groups, functional outcomes improved over time, with slower improvement in group 3 as expected. Per study design, no prophylactic gastrostomy tubes were placed. One patient in group 3 required a gastrostomy tube that was subsequently removed. An additional patient required a gastrostomy tube during salvage therapy (removed). No patients required an upfront or late tracheostomy.
Figure 5.
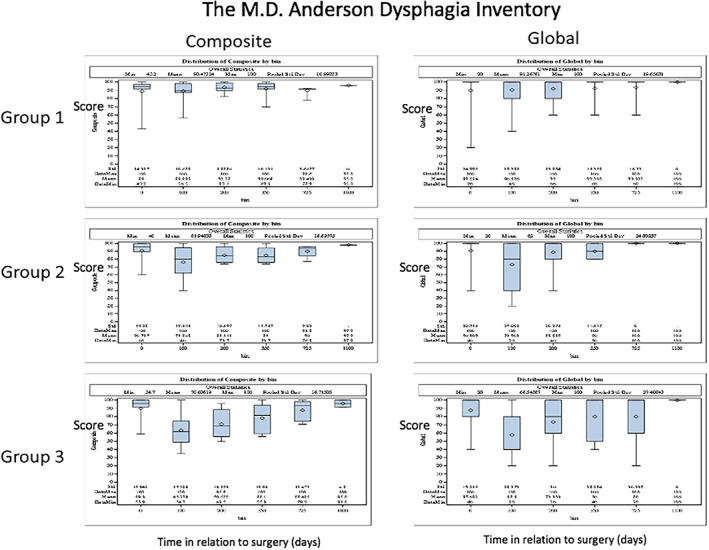
Global and composite swallowing outcomes for groups 1, 2, and 3: MD Anderson Dysphagia Index (MDADI). The y‐axis indicates the score; the x‐axis indicates time (days).
Discussion
Currently it is recognized that HPV‐related oropharyngeal cancer is a distinct disease with an improved prognosis and that the concept of “de‐escalation” therapy may be appropriate for many patients with early‐stage disease [29]. Although the goal of maintaining oncologic outcomes while limiting toxicity is clear, what remains unclear is the appropriate strategy. In addition, there is no clear predictive biomarker to identify patients who would benefit from such strategies [30]. Certainly, for more advanced disease, the current standard of care remains cisplatin‐based chemoradiotherapy [31, 32, 33].
In general, patients with HPVOPC will live for prolonged periods, and they are at high risk for long‐term toxicity from radiotherapy or chemoradiotherapy [34, 35, 36]. Given this, strategies to reduce radiation dose have been tested. ECOG 1308 tested the hypothesis that induction chemotherapy could select patients for reduced‐dose radiation (54 Gy) [37]. The authors reported a 2‐year PFS and OS of 80% and 94%, respectively. Similar trials further support de‐escalation strategies for HPVOPC [38, 39]. Definitive evidence exists that radiation effects are dose dependent and that reduced dosage reduces the risk of toxicity [40, 41, 42].
Although patients treated with chemoradiotherapy alone can be stratified into deintensification groups, pathologic staging information is not obtained, and the potential opportunity to spare them from radiotherapy is lost without that information. In a recent multicenter study, it was reported that 22% of subjects were upstaged and 13% downstaged after surgery indicating the value of pathologic information in this setting [43]. In addition, more recent data indicate that the prognostic impact of the number of cervical lymph nodes appears less important than previously believed [44, 45, 46]. It should, however, be noted that the current staging system is a prognostic tool and was not designed to guide therapy [7].
Radiotherapy alone in the primary management of early‐stage HPVOPC is well established, and its efficacy is supported by several investigations [47, 48, 49]. However, noninferiority of surgery or radiation alone compared with concurrent chemoradiation has not yet been specifically confirmed by randomized clinical trials. As a result, many low‐risk patients with HPVOPC are condemned to morbidity from full‐dose radiation therapy who might otherwise be managed with surgery alone. By selecting an upfront surgical strategy, one could theoretically offer a lower dose or entirely eliminate adjuvant radiation in some individuals, reducing long‐term toxicity.
Presently, minimally invasive surgery with or without adjuvant radiation is considered an acceptable treatment alternative in appropriately selected patients. Despite the significant number of reports, the exact role of surgery still remains to be elucidated [50, 51]. Single‐institution data on omission of chemotherapy for patients have indicated that oncologic results are similar, with improved toxicity [43, 50, 51, 52, 53]. However, these results must be interpreted cautiously, given the careful patient selection in surgical cohorts. Although some functional benefits have been reported in association with surgery, the ORATOR trial failed to show improvement in swallowing‐related outcomes in surgically treated patients. Tracheotomy was performed routinely in the surgical cohort of this trial, which may make the comparison of QOL data with other TORS trials problematic [54, 55]. In light of these data, there is a clear need to evaluate the use of surgery for de‐escalation. Several trials are underway in this setting, including TROG 12.01 and the PATHOS trial. In addition, a recent abstract presented from ECOG 3311 indicates similar outcomes to our trial [56].
The purpose of the SIRS trial was to determine if acceptable oncologic control rates with improved functional outcomes could be realized with TORS followed by reduced‐dose radiation. The results of this trial support upfront surgery followed by pathologic risk stratification to drive adjuvant therapy. Twenty‐one of 54 (38.9%) of patients on this trial did not receive any radiation, and 7 of 25 (28%) patients in group 1 had more than one positive node, traditionally an indication for chemoradiation. In addition, a reduction in the dose of radiation in all groups resulted in excellent oncologic results; overall PFS was 90.7% for the study, and DSS was 98.1%, including salvage cases. The high rate of salvage noted in group 1 is likely related to the strict selection criteria for early‐stage disease. Overall functional outcomes were excellent, with 2 of 54 (3.7%) patients receiving temporary gastrostomy tubes (one after salvage), and no permanent gastrostomy tubes or tracheostomies. Certainly, the authors recognize the issues related to generalizing these findings in the community at large, given the challenges in employing them as strategy in community practices. The role of robotic surgery in standard of care practice for dose de‐escalation remains to be elucidated.
Patients on trial experienced a small number of surgical complications, the majority short term, and the rate of major surgical complications was low (5.1%). Certainly, surgery is not without sequelae; it is notable that 22.2% of patients reported issues related to neck dissection, although many were in the radiated groups. In contrast to our study design, recent data indicate that bilateral neck dissection is likely not warranted in this population [57].
This trial has several limitations, most notably that this is a single‐center prospective trial stratified into three arms, resulting in small numbers in each arm, limiting the statistical power of the available data. The small sample size within each group certainly limited the generalizability of the reported results. Furthermore, although this was a function of the study design, the trial was nonrandomized. Randomization between nonsurgical and surgical management would present significant ethical issues, and recruitment challenges and would likely not be appropriate, as 21 of 54 (38.9%) of patients ultimately did not require any adjuvant therapy. This observation highlights the importance of careful patient selection and inclusion criteria for de‐escalation trials, to ensure both that patients with early disease amenable to surgery are not enrolled in trials without a surgical arm and, conversely, that patients with significant locoregional disease burden are not enrolled in trials with a “surgery alone” arm. An additional limitation is that a large number of patients enrolled in the trial subsequently withdrew from the study, limiting the available data for analysis. This was in a large part likely to conducting a clinical trial in the New York City area, which has several geographically close cancer centers and challenging transportation issues for patients who require daily radiation therapy. Finally, the mean length of follow‐up (43.8 months) is shorter than the standard 5‐year reporting criteria. Longer follow‐up with late QOL data will be reported at a later date.
Conclusion
The findings of the SIRS trial indicate that TORS with selective neck dissection is a reasonable primary modality for early‐stage HPV‐related oropharyngeal cancer and permits successful de‐escalation of radiation therapy dose. Oncologic and functional outcomes observed in the trial were excellent. The value of obtaining pathologic information to stratify risk and determine appropriate adjuvant therapy should not be underestimated. This approach offers significant numbers of patients the opportunity to reduce long‐term toxicity. The field anxiously awaits the results of other ongoing clinical trials, and certainly confirmation of these findings with sufficiently powered phase III efforts is required to confirm our findings.
Author Contributions
Conception/design: Brett A. Miles, Marshall R. Posner, Vishal Gupta, Richard L. Bakst, Kryzsztof J. Misiukiewicz, Andrew G. Sikora, Eric M. Genden
Provision of study material or patients: Brett A. Miles, Marshall R. Posner, Vishal Gupta, Marita S. Teng, Richard L. Bakst, Mike Yao, Kryzsztof J. Misiukiewicz, Raymond L. Chai, Sonam Sharma, William H. Westra, Eric M. Genden
Collection and/or assembly of data: Brett A. Miles, Marshall R. Posner, Vishal Gupta, Richard L. Bakst, William H. Westra, Seunghee Kim‐Schulze, Bheesham Dayal
Data analysis and interpretation: Brett A Miles, Marshall R. Posner, Vishal Gupta, Richard L. Bakst, William H. Westra, Seunghee Kim‐Schulze, Bheesham Dayal, Stanislaw Sobotka, Andrew G. Sikora
Manuscript writing: Brett A. miles, Marshall R. Posner, Vishal Gupta, Marita S. Teng, Richard L. Bakst, Kryzsztof J. Misiukiewicz, Raymond L. Chai, Sonam Sharma, William H. Westra, Stanislaw Sobotka, Andrew G. Sikora, Eric M. Genden
Final approval of manuscript: Brett A. miles, Marshall R. Posner, Vishal Gupta, Marita S. Teng, Richard L. Bakst, Mike Yao, Kryzsztof J. Misiukiewicz, Raymond L. Chai, Sonam Sharma, William H. Westra, Seunghee Kim‐Schulze, Stanislaw Sobotka, Bheesham Dayal, Andrew G. Sikora, Peter M. Som, Eric M. Genden
Disclosures
Andrew G. Sikora: SQZ Therapeutics, Tessa Therapeutics, Pelican Therapeutics (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information
Acknowledgments
We acknowledge the support of the Tisch Cancer Institute (Icahn School of Medicine at Mount Sinai, New York, NY). Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number P30 CA196521. This work was presented at the Poster Theater Session of the 2020 Multidisciplinary Head and Neck Cancer Symposium, February 27, Scottsdale, AZ.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com.
Footnotes
For Further Reading: Ari J. Rosenberg, Everett E. Vokes. Optimizing Treatment De‐Escalation in Head and Neck Cancer: Current and Future Perspectives. The Oncologist 2021;26:40–48.
Implications for Practice: The toxicity associated with standard multimodality treatment for head and neck cancer underscores the need to seek less‐intensive therapies with a reduced long‐term symptom burden through de‐escalated treatment paradigms that minimize toxicity while maintaining oncologic control in appropriately selected patients. Controversy regarding the optimal de‐escalation strategy and criteria for patient selection for de‐escalated therapy has led to multiple parallel strategies undergoing clinical investigation. Well‐designed trials that optimize multimodal strategies are needed. Given the absence of positive randomized trials testing de‐escalated therapy to date, practicing oncologists should exercise caution and administer established standard‐of‐care therapy outside the context of a clinical trial.
References
- 1. Van Dyne EA, Henley SJ, Saraiya M et al. Trends in human papillomavirus‐associated cancers ‐ United States, 1999‐2015. MMWR Morb Mortal Wkly Rep 2018;67:918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saraiya M, Unger ER, Thompson TD et al. US assessment of HPV types in cancers: Implications for current and 9‐valent HPV vaccines. J Natl Cancer Inst 2015;107:djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson KS, Wong J, D'Souza G et al. Serum antibodies to the HPV16 proteome as biomarkers for head and neck cancer. Br J Cancer 2011;104:1896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ang KK, Harris J, Wheeler R et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gillison ML. Human papillomavirus and prognosis of oropharyngeal squamous cell carcinoma: Implications for clinical research in head and neck cancers. J Clin Oncol 2006;24:5623–5625. [DOI] [PubMed] [Google Scholar]
- 6. Panwar A, Interval E, Lydiatt WM. Emergence of a novel staging system for oropharyngeal squamous cell carcinoma based on HPV status. Oncology (Williston Park) 2017;31:e33–e40. [PubMed] [Google Scholar]
- 7. Adelstein DJ, Ismaila N, Ku JA et al. Role of treatment deintensification in the management of p16+ oropharyngeal cancer: ASCO provisional clinical opinion. J Clin Oncol 2019;37:1578–1589. [DOI] [PubMed] [Google Scholar]
- 8. Forastiere AA, Adelstein DJ, Manola J. Induction chemotherapy meta‐analysis in head and neck cancer: Right answer, wrong question. J Clin Oncol 2013;31:2844–2846. [DOI] [PubMed] [Google Scholar]
- 9. Eisbruch A, Schwartz M, Rasch C et al. Dysphagia and aspiration after chemoradiotherapy for head‐and‐neck cancer: Which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys 2004;60:1425–1439. [DOI] [PubMed] [Google Scholar]
- 10. Licitra L, Perrone F, Bossi P et al. High‐risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 2006;24:5630–5636. [DOI] [PubMed] [Google Scholar]
- 11. Misiukiewicz K, Gupta V, Miles BA et al. Standard of care vs reduced‐dose chemoradiation after induction chemotherapy in HPV+ oropharyngeal carcinoma patients: The Quarterback trial. Oral Oncol 2019;95:170–177. [DOI] [PubMed] [Google Scholar]
- 12. Cramer JD, Ferris RL, Kim S et al. Primary surgery for human papillomavirus‐associated oropharyngeal cancer: Survival outcomes with or without adjuvant treatment. Oral Oncol 2018;87:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinstein GS, O'Malley BW Jr, Cohen MA et al. Transoral robotic surgery for advanced oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 2010;136:1079–1085. [DOI] [PubMed] [Google Scholar]
- 14. Hurtuk A, Agrawal A, Old M et al. Outcomes of transoral robotic surgery: A preliminary clinical experience. Otolaryngol Head Neck Surg 2011;145:248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore EJ, Olsen KD, Kasperbauer JL. Transoral robotic surgery for oropharyngeal squamous cell carcinoma: A prospective study of feasibility and functional outcomes. Laryngoscope 2009;119:2156–2164. [DOI] [PubMed] [Google Scholar]
- 16. Genden EM, Kotz T, Tong CC et al. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope 2011;121:1668–1674. [DOI] [PubMed] [Google Scholar]
- 17. Van Abel KM, Quick MH, Graner DE et al. Outcomes following TORS for HPV‐positive oropharyngeal carcinoma: PEGs, tracheostomies, and beyond. Am J Otolaryngol 2019;40:729–734. [DOI] [PubMed] [Google Scholar]
- 18. Agoston ES, Robinson SJ, Mehra KK et al. Polymerase chain reaction detection of HPV in squamous carcinoma of the oropharynx. Am J Clin Pathol 2010;134:36–41. [DOI] [PubMed] [Google Scholar]
- 19. Chaturvedi A, Engels EA, Pfeiffer RM et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El‐Salem F, Mansour M, Gitman M et al. Real‐time PCR HPV genotyping in fine needle aspirations of metastatic head and neck squamous cell carcinoma: Exposing the limitations of conventional p16 immunostaining. Oral Oncol 2019;90:74–79. [DOI] [PubMed] [Google Scholar]
- 21. Villepelet A, Hugonin S, Atallah S et al. Effects of tobacco abuse on major chromosomal instability in human papilloma virus 16‐positive oropharyngeal squamous cell carcinoma. Int J Oncol 2019;55:527–535. [DOI] [PubMed] [Google Scholar]
- 22. Maxwell JH, Kumar B, Feng FY et al. Tobacco use in human papillomavirus‐positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res 2010;16:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vawda N, Banerjee RN, Debenham BJ. Impact of smoking on outcomes of HPV‐related oropharyngeal cancer treated with primary radiation or surgery. Int J Radiat Oncol Biol Phys 2019;103:1125–1131. [DOI] [PubMed] [Google Scholar]
- 24. Platek AJ, Jayaprakash V, Merzianu M et al. Smoking cessation is associated with improved survival in oropharynx cancer treated by chemoradiation. Laryngoscope 2016;126:2733–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. American Joint Committee on Cancer . AJCC Cancer Staging Manual. 7th ed. Chicago, IL: American Joint Committee on Cancer, 2010;41–56. [Google Scholar]
- 26. Kompelli AR, Morgan P, Li H et al. Prognostic impact of high‐risk pathologic features in HPV‐related oropharyngeal squamous cell carcinoma and tobacco use. Otolaryngol Head Neck Surg 2019;160:855–861. [DOI] [PubMed] [Google Scholar]
- 27. Bauer E, Mazul A, Chernock R et al. Extranodal extension is a strong prognosticator in HPV‐positive oropharyngeal squamous cell carcinoma. Laryngoscope 2020;130:939–945. [DOI] [PubMed] [Google Scholar]
- 28. Chen AY, Frankowski R, Bishop‐Leone J et al. The development and validation of a dysphagia‐specific quality‐of‐life questionnaire for patients with head and neck cancer: The M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg 2001;127:870–876. [PubMed] [Google Scholar]
- 29. Peres J. HPV‐positive oropharyngeal cancer: Data may justify new approach. J Natl Cancer Inst 2010;102:1456–1459. [DOI] [PubMed] [Google Scholar]
- 30. Wirth LJ, Burtness B, Nathan CO et al. Point/counterpoint: Do we de‐escalate treatment of HPV‐associated oropharynx cancer now? And how? Am Soc Clin Oncol Educ Book 2019;39:364–372. [DOI] [PubMed] [Google Scholar]
- 31. Gillison ML, Trotti AM, Harris J et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus‐positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non‐inferiority trial. Lancet 2019;393:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suton P, Skelin M, Rakusic Z et al. Cisplatin‐based chemoradiotherapy vs. cetuximab‐based bioradiotherapy for p16‐positive oropharyngeal cancer: An updated meta‐analysis including trials RTOG 1016 and De‐ESCALaTE. Eur Arch Otorhinolaryngol 2019;276:1275–1281. [DOI] [PubMed] [Google Scholar]
- 33. Mehanna H, Robinson M, Hartley A et al. Radiotherapy plus cisplatin or cetuximab in low‐risk human papillomavirus‐positive oropharyngeal cancer (De‐ESCALaTE HPV): An open‐label randomised controlled phase 3 trial. Lancet 2019;393:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Machtay M, Moughan J, Trotti A et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol 2008;26:3582–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adelstein D, Li Y, Adams G et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003;21:92–98. [DOI] [PubMed] [Google Scholar]
- 36. Forastiere A, Maor M, Weber R et al. Long‐term results of Intergroup RTOG 91‐11: A phase III trial to preserve the larynx—Induction cisplatin/5‐FU and radiation therapy versus concurrent cisplatin and radiation therapy versus radiation therapy. J Clin Oncol 2006;24(suppl 18):5517a. [Google Scholar]
- 37. Marur S, Li S, Cmelak AJ et al. E1308: Phase II trial of induction chemotherapy followed by reduced‐dose radiation and weekly cetuximab in patients with HPV‐associated resectable squamous cell carcinoma of the oropharynx—ECOG‐ACRIN Cancer Research Group. J Clin Oncol 2017;35:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen AM, Felix C, Wang PC et al. Reduced‐dose radiotherapy for human papillomavirus‐associated squamous‐cell carcinoma of the oropharynx: A single‐arm, phase 2 study. Lancet Oncol 2017;18:803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seiwert TY, Foster CC, Blair EA et al. OPTIMA: A phase II dose and volume de‐escalation trial for human papillomavirus‐positive oropharyngeal cancer. Ann Oncol 2019;30:1673. [DOI] [PubMed] [Google Scholar]
- 40. Roe JW, Carding PN, Dwivedi RC et al. Swallowing outcomes following intensity modulated radiation therapy (IMRT) for head & neck cancer ‐ a systematic review. Oral Oncol 2010;46:727–733. [DOI] [PubMed] [Google Scholar]
- 41. Caudell JJ, Schaner PE, Desmond RA et al. Dosimetric factors associated with long‐term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2010;76:403–409. [DOI] [PubMed] [Google Scholar]
- 42. Eisbruch A, Levendag PC, Feng FY et al. Can IMRT or brachytherapy reduce dysphagia associated with chemoradiotherapy of head and neck cancer? The Michigan and Rotterdam experiences. Int J Radiat Oncol Biol Phys 2007;69:S40–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Su W, Liu J, Miles BA et al. Adjuvant radiation therapy alone for HPV related oropharyngeal cancers with high risk features. PLoS One 2016;11:e0168061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Malm IJ, Fan CJ, Yin LX et al. Evaluation of proposed staging systems for human papillomavirus‐related oropharyngeal squamous cell carcinoma. Cancer 2017. 123:1768–1777. [DOI] [PubMed] [Google Scholar]
- 45. Mizumachi T, Homma A, Sakashita T et al. Confirmation of the eighth edition of the AJCC/UICC TNM staging system for HPV‐mediated oropharyngeal cancer in Japan. Int J Clin Oncol 2017;22:682–689. [DOI] [PubMed] [Google Scholar]
- 46. Lydiatt WM, Patel SG, O'Sullivan B et al. Head and Neck cancers‐major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:122–137. [DOI] [PubMed] [Google Scholar]
- 47. Mavroidis P, Price A, Fried D et al. Dose‐volume toxicity modeling for de‐intensified chemo‐radiation therapy for HPV‐positive oropharynx cancer. Radiother Oncol 2017;124:240–247. [DOI] [PubMed] [Google Scholar]
- 48. Garden AS, Fuller CD, Rosenthal DI et al. Radiation therapy (with or without neck surgery) for phenotypic human papillomavirus‐associated oropharyngeal cancer. Cancer 2016;122:1702–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hall SF, Liu FF, O'Sullivan B et al. Did the addition of concurrent chemotherapy to conventional radiotherapy improve survival for patients with HPV+ve and HPV‐ve Oropharynx cancer? A population‐based study. Br J Cancer 2017;117:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cohen MA, Weinstein GS, O'Malley BW Jr et al. Transoral robotic surgery and human papillomavirus status: Oncologic results. Head Neck 2011;33:573–580. [DOI] [PubMed] [Google Scholar]
- 51. White HN, Moore EJ, Rosenthal EL et al. Transoral robotic‐assisted surgery for head and neck squamous cell carcinoma: One‐ and 2‐year survival analysis. Arch Otolaryngol Head Neck Surg 2010;136:1248–1252. [DOI] [PubMed] [Google Scholar]
- 52. Chin RI, Spencer CR, DeWees T et al. Reevaluation of postoperative radiation dose in the management of human papillomavirus‐positive oropharyngeal cancer. Head Neck 2016;38:1643–1649. [DOI] [PubMed] [Google Scholar]
- 53. Sanguineti G, Gunn GB, Endres EJ et al. Patterns of locoregional failure after exclusive IMRT for oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2008;72:737–746. [DOI] [PubMed] [Google Scholar]
- 54. Nichols AC, Yoo J, Hammond JA et al. Early‐stage squamous cell carcinoma of the oropharynx: Radiotherapy vs. trans‐oral robotic surgery (ORATOR)‐‐study protocol for a randomized phase II trial. BMC Cancer 2013;13:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wilkie MD, Upile NS, Lau AS et al. Transoral laser microsurgery for oropharyngeal squamous cell carcinoma: A paradigm shift in therapeutic approach. Head Neck 2016;38:1263–1270. [DOI] [PubMed] [Google Scholar]
- 56. Ferris RL Flamand Y, Weinstein GS et al. Transoral robotic surgical resection followed by randomization to low‐ or standard‐dose IMRT in resectable p16+ locally advanced oropharynx cancer: A trial of the ECOG‐ACRIN Cancer Research Group (E3311). J Clin Oncol 2020;38(suppl 15):6500a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McMullen CP, Garneau J, Weimar E et al. Occult contralateral nodal disease in oropharyngeal squamous cell carcinoma patients undergoing primary TORS with bilateral neck dissection. Oral Oncol 2019;93:96–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information


