Abstract
Background
Survival in patients with metastatic colorectal cancer (mCRC) has been associated with tumor mutational status, muscle loss, and weight loss. We sought to explore the combined effects of these variables on overall survival.
Materials and Methods
We performed an observational cohort study, prospectively enrolling patients receiving chemotherapy for mCRC. We retrospectively assessed changes in muscle (using computed tomography) and weight, each dichotomized as >5% or ≤5% loss, at 3, 6, and 12 months after diagnosis of mCRC. We used regression models to assess relationships between tumor mutational status, muscle loss, weight loss, and overall survival. Additionally, we evaluated associations between muscle loss, weight loss, and tumor mutational status.
Results
We included 226 patients (mean age 59 ± 13 years, 53% male). Tumor mutational status included 44% wild type, 42% RAS‐mutant, and 14% BRAF‐mutant. Patients with >5% muscle loss at 3 and 12 months experienced worse survival controlling for mutational status and weight (3 months hazard ratio, 2.66; p < .001; 12 months hazard ratio, 2.10; p = .031). We found an association of >5% muscle loss with BRAF‐mutational status at 6 and 12 months. Weight loss was not associated with survival nor mutational status.
Conclusion
Increased muscle loss at 3 and 12 months may identify patients with mCRC at risk for decreased overall survival, independent of tumor mutational status. Specifically, >5% muscle loss identifies patients within each category of tumor mutational status with decreased overall survival in our sample. Our findings suggest that quantifying muscle loss on serial computed tomography scans may refine survival estimates in patients with mCRC.
Implications for Practice
In this study of 226 patients with metastatic colorectal cancer, it was found that losing >5% skeletal muscle at 3 and 12 months after the diagnosis of metastatic disease was associated with worse overall survival, independent of tumor mutational status and weight loss. Interestingly, results did not show a significant association between weight loss and overall survival. These findings suggest that muscle quantification on serial computed tomography may refine survival estimates in patients with metastatic colorectal cancer beyond mutational status.
Keywords: Body composition, Skeletal muscle, Sarcopenia, Colorectal cancer, Survival, Outcomes
Short abstract
Cancer cachexia has traditionally been defined using weight loss; however, loss of skeletal muscle may be a more objective measure. This article reports the results of a retrospective study that assessed whether skeletal muscle loss is associated with overall survival in patients with metastatic colorectal cancer, independent of tumor mutational status and weight loss.
Introduction
Colorectal cancer is the third most common cancer worldwide, accounting for nearly one‐tenth of all cancer‐related deaths [1]. The prognosis for patients with metastatic colorectal cancer (mCRC) is approximately 2.5 to 3 years, with 5‐year survival estimates up to 20% [2]. Notably, mutations in the RAS‐ and BRAF‐oncogenes occur frequently in mCRC, and these oncogenes provide important predictive [3] and prognostic information [4]. RAS mutations can be found in 30%–40% of mCRC tumors, whereas BRAF mutations occur in 5%–10% of these tumors [5]. Prior work suggests that patients with RAS‐ or BRAF‐mutant mCRC experience worse survival than patients with wild‐type (WT) tumors [6]. Therefore, tumor mutational status informs the discussion of treatment options and anticipated outcomes for patients with mCRC [3, 5, 7].
Cancer cachexia is a multifactorial syndrome that has traditionally been defined using weight loss (e.g., >5% weight loss over 6 months) [8]. Although weight is routinely tracked as part of clinical care, basing the diagnosis and management of cachexia solely on weight loss is increasingly criticized by researchers and clinicians [8, 9]. Loss of skeletal muscle measured at the level of the third lumbar vertebral body (L3) on computed tomography (CT) is an objective parameter that can also inform anticipated outcomes in patients with mCRC, and may complement or even outperform weight loss [10, 11, 12]. For example, data demonstrate that low skeletal muscle is associated with adverse postoperative outcomes, prolonged hospitalizations, treatment‐related toxicity, and survival [9, 10, 11, 12, 13, 14, 15, 16]. However, it is unclear whether skeletal muscle loss adds to risk stratification compared with tumor mutational status in patients with mCRC.
We designed this retrospective study to assess whether skeletal muscle loss is associated with overall survival in patients with mCRC, independent of tumor mutational status and weight loss. Our a priori hypothesis was that in patients with mCRC, skeletal muscle loss negatively affects overall survival independent of tumor mutational status and weight loss.
Materials and Methods
Study Design
We conducted a retrospective cohort study of patients who had voluntarily enrolled in a prospective biobanking protocol at the Massachusetts General Hospital Cancer Center between January 2014 and August 2018. All patients provided informed consent for participating in the biobanking protocol. We obtained approval from the Dana‐Farber/Harvard Cancer Center Institutional Review Board for this study (protocol number: 14–046). The study was conducted according to the code of ethics of the world medical association (declaration of Helsinki).
Inclusion/Exclusion Criteria and Scan Selection
To enroll in the prospective biobanking protocol, patients had to be at least 18 years and receive care at the Massachusetts General Hospital Cancer Center for gastrointestinal malignancy. Patients had to be enrolled in the biobanking protocol with a confirmed diagnosis of mCRC and known tumor mutational status to be eligible for the current study.
Patients were excluded if they (a) did not have a baseline abdominal CT scan at metastatic diagnosis ±45 days, (b) did not have a follow‐up scan at 3, 6, or 12 months after the baseline CT scan ±45 days around each time point, or (c) had a BRAF‐mutation other than BRAF V600 (Fig. 1). Patients were not required to have a CT scan at each follow‐up time point. If patients had multiple CT scans around a given time point, we selected the scan obtained closest to the date of interest.
Figure 1.
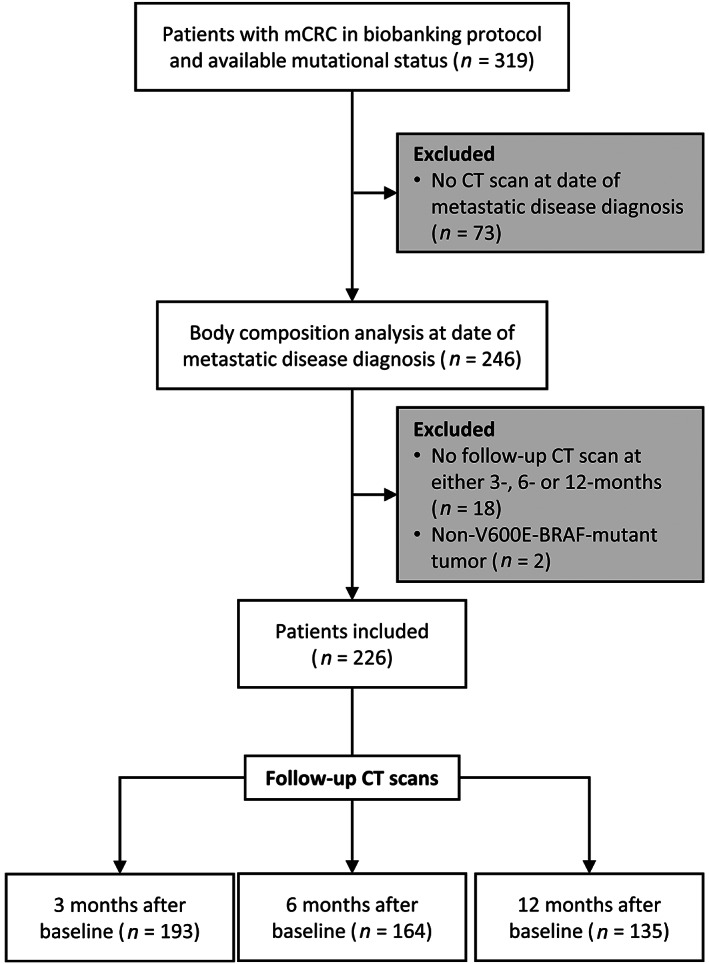
Consort diagram. Flow diagram specifying inclusion and exclusion criteria of patients and computed tomography (CT) scans. All time points ± 45 days. Abbreviations: CT, computed tomography; mCRC, metastatic colorectal cancer.
Body Composition Analysis
We submitted all included CT scans for processing with a previously described fully automated body composition analysis algorithm that can quantify the cross‐sectional area of skeletal muscle, visceral adipose tissue, and subcutaneous adipose tissue on a single axial image at L3 in square centimeters [17]. Briefly, the algorithm consists of convolutional neural networks that were developed using 595 manually segmented axial CT images at the L3 level. Attenuation thresholds used for both training and validation were −29 to +150 Hounsfield units for skeletal muscle and −190 to −30 Hounsfield units for adipose tissue. In the current study, a trained research assistant (T.D.B.) and a board‐certified radiologist (F.J.F.) blinded to clinical outcomes reviewed the visual output of each segmentation performed by the algorithm. Figure 2 illustrates serial body composition analysis on abdominal CT scans.
Figure 2.
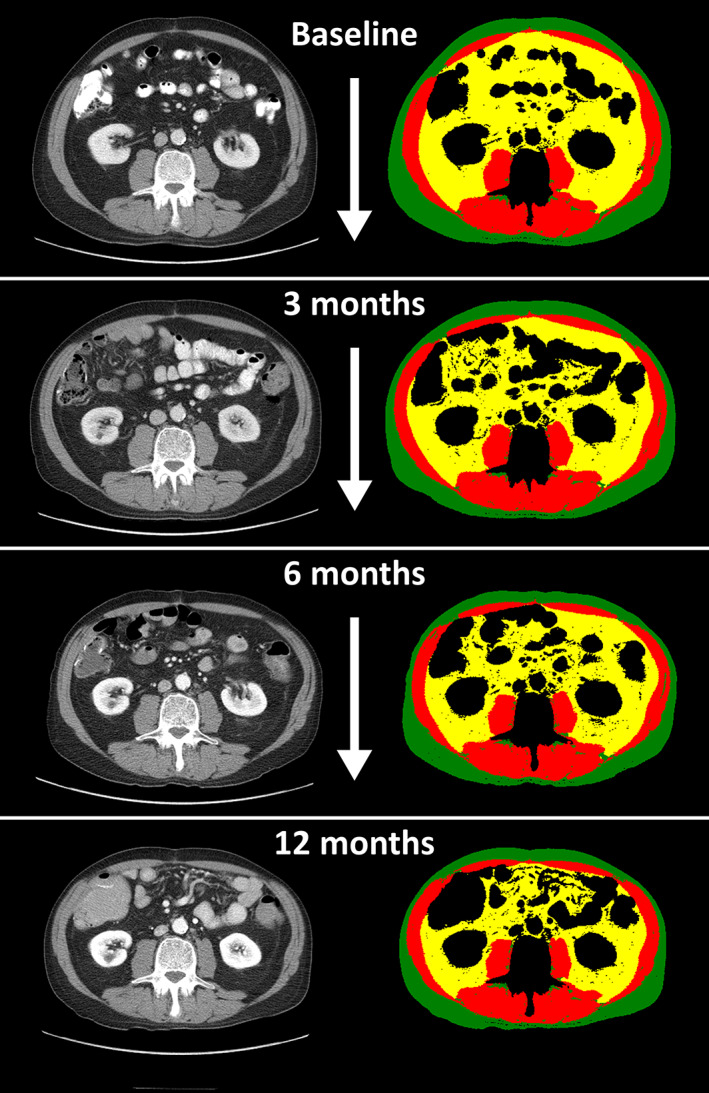
Change in body composition over time is hard to detect with the naked eye. Serial body composition analysis of a 61‐year‐old male patient with metastatic colon cancer. Left column: Serial axial computed tomography images at the level of the third lumbar vertebral body obtained at the time of metastatic disease diagnosis (baseline), and 3‐, 6‐, and 12‐month follow‐up. Right column: Corresponding segmentation of skeletal muscle (red), subcutaneous adipose tissue (green), and visceral adipose tissue (yellow). Twelve months following the diagnosis of metastatic disease, skeletal muscle had decreased by 20% and adipose tissue had reduced by 44% compared with baseline.
Data Collection, Definitions, and Outcome
We obtained patients’ demographics, date of mCRC diagnosis (confirmed by biopsy or imaging), mCRC mutational status, chemotherapy received, and body weight ±14 days around each CT scan from the electronic health record. We categorized mCRC tumor mutational status as WT, RAS‐mutant (KRAS or NRAS), or BRAF‐mutant. The tumor of one patient expressed a double mutation in BRAF/NRAS and was categorized as NRAS‐mutant after expert review (R.B.C.).
We defined total adipose tissue as the sum of visceral adipose tissue and subcutaneous adipose tissue. We calculated the percentage change from baseline for skeletal muscle, total adipose tissue, and weight at 3, 6, and 12 months following the baseline scan. We selected time points based on common imaging intervals in clinical care. We chose to present percent change for skeletal muscle, total adipose tissue, and weight to facilitate clinical interpretation [10, 18]. We used linear interpolation to calculate percent change at 3, 6, and 12 months from the baseline scan date to account for the inclusion windows of CT scans and weight measurements (supplemental online Fig. 1; supplemental online Table 1).
We defined loss of skeletal muscle, total adipose tissue, and weight as a decrease of >5% because this represents a published cutoff value and a practical method for comparison [19, 20, 21, 22, 23, 24]. Our outcome was overall survival, defined from the date of metastatic diagnosis to date of death, and we censored those patients still alive at the date of the last follow‐up.
Statistical Analysis
We used descriptive statistics to estimate frequencies, means, SDs, medians, and interquartile ranges depending on data distribution. We used Cox proportional hazards models to assess whether the dichotomized change in skeletal muscle was associated with survival (independent of tumor mutational status and weight loss) while adjusting for a priori selected covariables age, sex, and total adipose tissue. We plotted Kaplan‐Meier survival curves for skeletal muscle loss and tumor mutational status as well as skeletal muscle loss and weight loss. To account for the left‐truncation in our survival analyses, patients were included in the risk set for an outcome event starting at the time of their follow‐up scan and ending when they died or were censored. We used the Kaplan‐Meier method to estimate median follow‐up, with death as the censoring event. We also used logistic regression models to investigate associations between skeletal muscle loss, weight loss, and tumor mutational status, adjusting for a priori selected covariables age, sex, and baseline skeletal muscle or baseline weight values. We used a significance level of 0.05 for all analyses and did not adjust for multiple testing given this study's exploratory nature. We performed statistical analyses using SAS (version 9.3, SAS Institute Inc., Cary, NC) and R (version 3.5.3, R Foundation for Statistical Computing, Vienna, Austria).
Results
Inclusion and Descriptive Statistics
Of 319 eligible patients, 246 had a baseline CT scan and 228 also had a CT scan at one or more of three possible follow‐up time points. Two patients with BRAF D594G‐mutations were excluded, resulting in 226 included patients (Fig. 1). In our sample, 193 patients had a scan at 3 months, 164 had a scan at 6 months, and 135 had a scan at 12 months after baseline (Fig. 1). Table 1 describes the characteristics of study participants at the time of metastatic diagnosis (baseline). Supplemental online Table 2 shows the baseline characteristics grouped by available scans at each follow‐up time point. Patients had a mean ± SD age of 59 ± 13 years and a mean body mass index of 28 ± 6 kg/m2. Most patients were male (52%) and White (90%) and had left‐sided (66%) colon cancer primaries. Mutational status categories included WT (44%), RAS (42%), and BRAF V600E (14%). Most patients (91%) received standard chemotherapy with a mean of 2 ± 1.5 lines of chemotherapy following their diagnosis of mCRC. At 3 months, we observed a >5% loss of weight in 22% (43/194) of patients whereas 30% (57/194) of patients had lost >5% of skeletal muscle compared with baseline.
Table 1.
Patient characteristics at time of metastatic diagnosis and outcomes (n = 226)
| Characteristic | n = 226 |
|---|---|
| Age, yr | |
| Mean ± SD | 59 ± 13 |
| Median (25th, 75th percentile) | 59 (50, 68) |
| Minimum–maximum | 26–92 |
| Sex, n (%) | |
| Male | 119 (53) |
| Race and ethnicity, n (%) | |
| White | 204 (90) |
| Asian | 10 (4) |
| Black | 2 (1) |
| Hispanic or Latino | 1 (1) |
| Other | 3 (1) |
| Unavailable | 6 (3) |
| Weight at metastatic diagnosis, kg | |
| Mean ± SD | 80 ± 21 |
| Median (25th, 75th percentile) | 77 (66, 91) |
| Minimum–maximum | 43–153 |
| Height, cm | |
| Mean ± SD | 170 ± 11 |
| Median (25th, 75th percentile) | 168 (163, 178) |
| Minimum–maximum | 145–196 |
| Body mass index, kg/m2 | |
| Mean ± SD | 27.5 ± 5.6 |
| Median (25th, 75th percentile) | 26.5 (23.7, 30.5) |
| Minimum–maximum | 17.1–43.8 |
| Organ of cancer origin, n (%) | |
| Colon | 170 (75) |
| Rectum | 56 (25) |
| Location of tumor, n (%) | |
| Left | 149 (66) |
| Right | 64 (28) |
| Transverse | 13 (6) |
| Tumor mutation, n (%) | |
| Wild type | 99 (44) |
| KRAS | 86 (38) |
| NRAS | 9 (4) |
| BRAF (V600 mutations only) | 32 (14) |
| Therapy type of first treatment, n (%) | |
| Standard chemotherapy | 205 (91) |
| Targeted + standard chemotherapy | 21 (9) |
| No. of lines of systemic therapy after metastatic diagnosis | |
| Mean ± SD | 2.4 ± 1.5 |
| Median (25th, 75th percentile) | 2 (1, 3) |
| Minimum–maximum | 0–9 |
| First‐line metastatic chemotherapy regimens n (%) | |
| 5FU/capecitabine | 3 (1) |
| + bevacizumab | 2 (1) |
| FOLFOX | 70 (31) |
| + bevacizumab | 61 (27) |
| + EGFRi | 1 (0.4) |
| FOLFIRI | 13 (6) |
| + bevacizumab | 32 (14) |
| + EGFRi | 14 (6) |
| FOLFOXIRI | 16 (7) |
| + bevacizumab | 5 (2) |
| Irinotecan + bevacizumab | 1 (0.4) |
| None | 8 (4) |
| >5% loss at 3 mo, n (%) | |
| Weight | 43 (22) |
| Skeletal muscle | 57 (30) |
Abbreviations: EGFRi, epidermal growth factor inhibitor; 5FU, 5‐fluorouracil; IQR, interquartile range.
Skeletal Muscle Loss and Overall Survival
The entire cohort's median overall survival was 40 months (95% confidence interval [CI], 32–48 months). The median follow‐up time was 57 months (95% CI, 47–68 months). Patients with a BRAF‐mutant tumor had the shortest median overall survival (OS) with 19 months (95% CI, 11–28 months), compared with 34 months (95% CI, 26–51 months) for patients with KRAS‐mutant tumors and 50 months (95% CI, 35–62 months) for patients with WT tumors. In patients who had lost >5% skeletal muscle after 3 months, median OS was 27 months (95% CI, 16–42 months), compared with 44 months (95% CI, 32–51 months) for patients who had <5% skeletal muscle. Additional survival estimates, including the 5‐year‐survival rate are presented in supplemental Table 3.
In our multivariable analysis, shorter overall survival was associated with >5% skeletal muscle loss at 3 months (hazard ratio [HR], 2.67; 95% CI, 1.59–4.47; p = .0002) and BRAF‐mutational status (HR, 3.92; 95% CI, 1.84–8.36; p = .0004; Table 2). Figure 3 illustrates survival for patients with >5% skeletal muscle loss at 3‐months compared with patients with ≤5% skeletal muscle at 3 months grouped according to weight loss (Fig. 3A) and tumor mutational status (Fig. 3B). We did not find an association between overall survival and loss of weight, loss of adipose tissue, or sex across all time points.
Table 2.
Multivariable Cox proportional hazard models showing associations between loss of skeletal muscle, weight and total adipose tissue at 3, 6, and 12 months following metastatic diagnosis with overall survival
| Characteristic | 3 mo | 6 mo | 12 mo | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Skeletal muscle loss: >5% vs. ≤5% | 2.667 (1.593–4.466) | .0002 | 1.707 (0.939–3.103) | .0797 | 2.103 (1.068–4.139) | .0314 |
| Weight loss: >5% vs. ≤5% | 0.851 (0.435–1.666) | .6380 | 1.835 (0.839–4.015) | .1287 | 2.096 (0.838–5.244) | .1136 |
| Mutation | .0004 | .0002 | ||||
| BRAF vs. WT | 3.918 (1.837–8.355) | 4.013 (1.914–8.415) | 4.834 (2.030–11.515) | .0004 | ||
| RAS vs. WT | 1.489 (0.857–2.587) | .1580 | 1.618 (0.893–2.932) | .1123 | 1.467 (0.7435–2.894) | .2692 |
| TAT loss: >5% vs. ≤5% | 1.517 (0.836–2.753) | .1705 | 0.660 (0.326–1.334) | .2471 | 0.735 (0.297–1.818) | .5051 |
| Age: ≥70 yr vs. <70 yr | 1.156 (0.650–2.056) | .6208 | 1.325 (0.746–2.354) | .3374 | 2.436 (1.230–4.825) | .0106 |
| Sex: Female vs. male | 1.007 (0.607–1.673) | .9770 | 1.198 (0.679–2.112) | .5335 | 0.785 (0.409–1.508) | .4680 |
For each follow‐up time point, a single model was fit using the CT scans performed at the given time point.
Abbreviations: CI, confidence interval; CT, computed tomography; TAT, total adipose tissue; WT, wild type.
Figure 3.
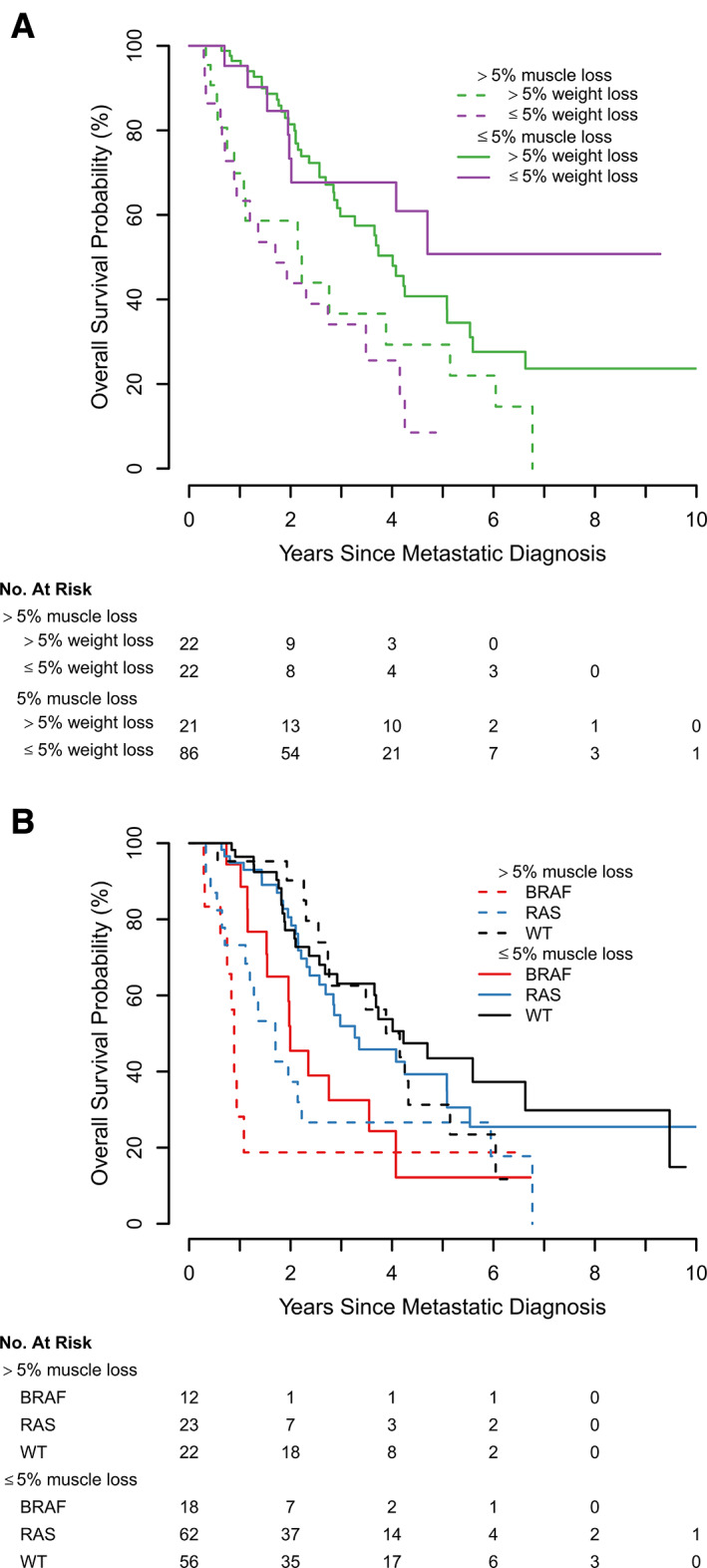
Kaplan‐Meier plots for overall survival. (A) Kaplan‐Meier plots depicting the relationship of the combination of dichotomized skeletal muscle loss with weight loss at 3 months. (B) Relationship of the combination of dichotomized skeletal muscle loss at 3 months with tumor mutational status. Both skeletal muscle and weight loss were dichotomized by >5% versus ≤5%). Abbreviation: WT, wild type.
At 6 months, only BRAF‐mutational status (HR, 4.01; 95% CI, 1.91–8.42; p = .0002) was associated with overall survival. Skeletal muscle loss >5% was not significantly associated with overall survival at this time point (HR 1.71; 95% CI, 0.94–3.10, p = .0797; Table 2). At 12 months, worse survival was significantly associated with >5% skeletal muscle loss (HR, 2.10; 95% CI, 1.07–4.14; p = .0314), BRAF‐mutational status (HR, 4.83; 95% CI, 2.03–11.52; p = .0004), and age ≥ 70 years (HR, 2.44; 95% CI, 1.23–4.83; p = .0106; Table 2).
Associations of Tumor Mutational Status with Skeletal Muscle Loss and Weight Loss
We found a significant relationship between BRAF‐mutational status and increased odds of >5% skeletal muscle loss at 6 months (OR, 3.37; 95% CI:,1.27–8.90; p = .0144) and at 12 months (OR, 5.53; 95% CI, 1.60–19.09; p = .0068; Table 3, supplemental online Table 4). We did not find an association between >5% weight loss and tumor mutational status across all time points. However, female sex was consistently associated with >5% weight loss (supplemental online Table 4). Additionally, we found a significant relationship between baseline weight and > 5% weight loss at 3 and 6 months (OR, 1.03; 95% CI, 1.01–1.05; p = .0159) but not at 12 months (supplemental online Table 4).
Table 3.
Multivariable model demonstrating the relationship of tumor mutation status with >5% loss of skeletal muscle and >5% loss of weight
| Time point, mutation | >5% weight loss | >5% skeletal muscle loss | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| 3 mo | ||||
| WT | Ref | Ref | ||
| BRAF | 1.543 (0.521 – 4.568) | .4332 | 1.786 (0.727 – 4.387) | .2057 |
| RAS | 0.840 (0.366 – 1.926) | .6801 | 0.972 (0.483 – 1.975) | .9355 |
| 6 mo | ||||
| WT | Ref | Ref | ||
| BRAF | 2.379 (0.778 – 7.274) | .1284 | 3.372 (1.273 – 8.933) | .0144 |
| RAS | 1.188 (0.493 – 2.862) | .7016 | 1.144 (0.538 – 2.435) | .7261 |
| 12 mo | ||||
| WT | Ref | Ref | ||
| BRAF | 4.426 (0.982 – 19.957) | .0529 | 5.533 (1.604 – 19.091) | .0068 |
| RAS | 2.217 (0.717 – 6.853) | .1666 | 1.577 (0.667 – 3.727) | .2991 |
Body composition change was evaluated on computed tomography scans obtained 3, 6, and 12 months following the date of metastatic diagnosis. Estimates are adjusted for potential confounders, including age, sex, and baseline skeletal muscle or baseline weight. For the values of all covariables see supplemental online Table 4.
Abbreviations: CI, confidence interval; OR, odds ratio; Ref, reference; WT, wild type.
Discussion
In this study of patients with mCRC, we provide evidence that skeletal muscle loss may identify patients at risk for decreased survival as early as 3 months following the diagnosis of metastatic disease, independent of tumor mutational status and weight. Additionally, we found associations between skeletal muscle loss and BRAF‐mutational status. Notably, our finding that weight loss was not associated with survival nor tumor mutational status supports the concept that weight loss alone cannot reliably predict outcomes in patients with mCRC.
Collectively, our findings suggest that skeletal muscle derived from serial CT scans is an imaging biomarker with the potential to refine survival estimates for patients with mCRC beyond tumor mutational status and weight. Within each mutational status category (WT, RAS, BRAF), skeletal muscle loss identified patients who experience worse overall survival than patients with the same tumor mutational status and ≤5% skeletal muscle loss (Fig. 3B). Interestingly, in our sample, a new prognostic pattern of poor overall survival emerges, as demonstrated by patients with >5% skeletal muscle loss and RAS‐mutation experiencing similar overall survival compared with patients with BRAF‐mutant tumors. In contrast, we observed that patients with BRAF‐mutant mCRC experience significantly more skeletal muscle loss compared with patients with WT tumors, supporting previously published data describing more aggressive biology of BRAF‐mutant mCRC. Although we found a significant association between >5% skeletal muscle loss and overall survival at 3 and 12 months, the absence of statistical significance at 6 months merits additional investigation. We believe this is due to a lack of power in the 6‐months subgroup as the overall direction of effect and magnitude of effect size is similar to that of the 12‐months subgroup.
Our finding that weight loss was not associated with overall survival or tumor mutational status is of particular clinical interest. First, this further supports a more focused definition of cancer cachexia based on skeletal muscle loss rather than a reliance on weight loss alone [8, 9]. By focusing only on weight loss rather than loss of skeletal muscle, oncologists may limit recognition of cachexia to its later stages [9]. This is particularly true when third‐spacing of fluid (e.g., ascites, lower extremity edema) leads to stable or increased weight, thereby obscuring skeletal muscle loss [21, 25]. For example, 30% of patients in our study lost >5% skeletal muscle within 3 months following the diagnosis of mCRC, whereas only 22% of patients lost >5% weight in the same period. Second, skeletal muscle loss represents a viable target for prehabilitation and interventions such as exercise, nutrition, and drug therapy [20, 26]. By showing that patients with BRAF‐mutant mCRC are particularly vulnerable to skeletal muscle loss, we define an enriched cohort of patients likely to benefit from prospective studies examining causality between muscle loss and overall survival.
This study relies on an innovative method to obtain body composition data for translational research. We demonstrate that machine learning enables a fully automated workflow to analyze body composition on serial CT scans obtained as part of routine cancer care [17, 27, 28, 29, 30]. Without segmentation tools, change in skeletal muscle and adipose tissue is difficult to appreciate as illustrated in Figure 2. For example, this patient lost 20% of his skeletal muscle and 44% of his total adipose tissue between baseline and 12‐month follow‐up. However, manual segmentation of skeletal muscle and adipose tissue is time‐consuming and requires trained experts, which were not necessary in this study [31, 32]. Therefore, we believe that machine learning‐based body composition analysis will increasingly make body composition data available across clinical care settings [17, 27, 33, 34].
Limitations
We note several limitations of this study, including its retrospective, single‐center design in a population with limited sociodemographic diversity. These factors limit the generalizability to more diverse populations. Furthermore, we note that our findings are explorative due to the small sample size in the strata of tumor mutational status. In this sample, we did not observe associations between overall survival and changes in adipose tissue, possibly due to sample size, and should be further explored in larger cohorts. We also lack information about metabolism, diet, and exercise measures, which may impact skeletal muscle, weight, and survival [35, 36, 37, 38]. Additionally, we lack data on other important clinical outcomes, such as chemotherapy treatment intensity, toxicities, and patient‐reported outcomes.
Conclusion
In this study, we found that early skeletal muscle loss may identify patients with mCRC at risk for decreased overall survival, independent of mutational status and weight loss. We also discovered a new survival pattern within each tumor mutational category with >5% skeletal muscle loss demarcating high‐risk subgroups. As expected, patients with BRAF‐mutant tumors experience greater skeletal muscle loss and worse survival than those with WT or RAS‐mutant tumors. Weight loss was not significantly associated with survival, further underscoring that weight loss alone provides incomplete prognostic information. Additionally, the use of automated body composition analysis demonstrated in this study represents a viable mechanism to integrate longitudinal body composition data more readily into routine cancer care. Ultimately, our findings offer novel information highlighting the importance of serial muscle assessment as a reliable, practical method that may further refine survival estimates in patients with mCRC.
Author Contributions
Conception/design: Till Dominik Best, Eric J. Roeland, Nora K. Horick, Emily E. Van Seventer, Areej El‐Jawahri, Jennifer S. Temel, Ryan B. Corcoran, Ryan D. Nipp, Florian J. Fintelmann
Provision of study material or patients: Till Dominik Best, Eric J. Roeland, Emily E. Van Seventer, Amelie S. Troschel, Patrick C. Johnson, Katie N. Kanter, Madeleine G. Fish, J. Peter Marquardt, Jennifer S. Temel, Ryan B. Corcoran, Ryan D. Nipp, Florian J. Fintelmann
Collection and/or assembly of data: Till Dominik Best, Eric J. Roeland, Emily E. Van Seventer, Amelie S. Troschel, Patrick C. Johnson, Katie N. Kanter, Madeleine G. Fish, J. Peter Marquardt, Christopher P. Bridge, Ryan B. Corcoran, Ryan D. Nipp, Florian J. Fintelmann
Data analysis and interpretation: Till Dominik Best, Eric J. Roeland, Nora K. Horick, Emily E. Van Seventer, Areej El‐Jawahri, J. Peter Marquardt, Jennifer S. Temel, Ryan D. Nipp, Florian J. Fintelmann
Manuscript writing: Till Dominik Best, Eric J. Roeland, Nora K. Horick, Emily E. Van Seventer, Areej El‐Jawahri, Amelie S. Troschel, Patrick C. Johnson, Katie N. Kanter, Madeleine G. Fish, J. Peter Marquardt, Christopher P. Bridge, Jennifer S. Temel, Ryan B. Corcoran, Ryan D. Nipp, Florian J. Fintelmann
Final approval of manuscript: Till Dominik Best, Eric J. Roeland, Nora K. Horick, Emily E. Van Seventer, Areej El‐Jawahri, Amelie S. Troschel, Patrick C. Johnson, Katie N. Kanter, Madeleine G. Fish, J. Peter Marquardt, Christopher P. Bridge, Jennifer S. Temel, Ryan B. Corcoran, Ryan D. Nipp, Florian J. Fintelmann
Disclosures
Eric J. Roeland: Mitobridge Inc., Asahi Kasei Pharmaceuticals, DRG Consulting, Napo Pharmaceuticals, American Imaging Management, Immuneering Corporation, Prime Oncology (C/A), Heron Pharmaceuticals, Helsinn Pharmaceuticals, Vector Oncology (SAB), Oragenics, Inc, Galera Pharmaceuticals, Enzychem Lifesciences Pharmaceutical Company (Other‐data safety monitoring); Christopher P. Bridge: GE Healthcare, Nuance Communications, Nvidia Corporation, Fujifilm Sonosite (RF‐institutional); Ryan B. Corcoran: Abbvie, Amgen, Array Biopharma/Pfizer, Asana Biosciences, Astex Pharmaceuticals, AstraZeneca, Avidity Biosciences, Bristol‐Myers Squibb, C4 Therapeutics, Chugai, Elicio, Fog Pharma, Fount Therapeutics/Kinnate Biopharma, Genentech, Guardant Health, Ipsen, LOXO, Merrimack, Mirati Therapeutics, Natera, N‐of‐one/Qiagen, Novartis, nRichDx, Revolution Medicines, Roche, Roivant, Shionogi, Shire, Spectrum Pharmaceuticals, Symphogen, Tango Therapeutics, Taiho, Warp Drive Bio, Zikani Therapeutics (C/A), Avidity Biosciences, C4 Therapeutics, Fount Therapeutics/Kinnate Biopharma, nRichDx, and Revolution Medicines (OI), Asana, AstraZeneca, Eli Lilly & Co, Sanofi (RF); Florian J. Fintelmann: IP related patent pending, American Roentgen Ray Society Scholarship (RF‐career development award). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1 Timing of Follow‐up Scans.
Bee plot overlaid with boxplots showing the distribution of CT scan dates around each of the three follow‐up time points. The x‐axis is relative to the baseline CT scan. Therefore, all baseline scans are exactly on day 0 (n = 226). Red vertical lines indicate the exact date, and gray dashed lines indicate the 45‐day range. To account for the fluctuation of scan intervals, we performed linear interpolation to calculate percent change from baseline to exactly 3‐, 6‐ and 12‐ month follow‐up (see Patients and Methods).
CT, computed tomography.
Supplemental Table 1 Days Between Baseline Scan and 3‐, 6‐, and 12‐month Follow‐up Scans. To account for the fluctuation of scan intervals we performed linear interpolation to calculate percent change from baseline to exactly 3‐, 6‐ and 12‐month follow‐up (see Patients and Methods).
CT, computed tomography; SD, standard deviation.
Supplemental Table 2. Patient Characteristics at Time of Metastatic Diagnosis by Availability of Scans at Follow‐up Time Points.
n, number; SD, standard deviation.
Supplemental Table 3. Univariate survival estimates for skeletal muscle loss at 3 months, weight loss at 3 months, and tumor mutational status.
CI, confidence interval; n, number; OS, overall survival; SD, standard deviation.
Supplemental Table 4. Multivariable Model Demonstrating the Relationship Between > 5% Loss of Body Composition and Tumor Mutation Status.
Body composition change was evaluated at scans obtained 3, 6, and 12 months from metastatic diagnosis. Estimates are adjusted for potential confounders including age, sex, and baseline skeletal muscle or baseline body weight. Table 3 is a shortened version of this table.
CI, confidence interval; OR, odds ratio; WT, wild type.
Acknowledgments
We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin.
Open access funding enabled and organized by Projekt DEAL.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Horner MJ, Ries LAG, Krapcho M et al. SEER Cancer Statistics Review, 1975‐2006. Edwards BK Betheseda, MD: National Cancer Institute; 2009. https://seer.cancer.gov/csr/1975_2006/. [Google Scholar]
- 3. Di Nicolantonio F, Martini M, Molinari F et al. Wild‐type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705–5712. [DOI] [PubMed] [Google Scholar]
- 4. Siena S, Rivera F, Taieb J et al. Survival outcomes in patients With RAS wild type metastatic colorectal cancer classified according to Köhne prognostic category and BRAF mutation status. Clin Colorectal Cancer 2018;17:50–57. [DOI] [PubMed] [Google Scholar]
- 5. Lievre A, Bachet JB, Le Corre D et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006;66:3992–3995. [DOI] [PubMed] [Google Scholar]
- 6. Foltran L, Maglio DG, Pella N et al. Progonostic role of KRAS, NRAS, BRAF and PIK3CA mutations in advanced colorectal cancer. Future Oncol 2015;11:629–640. [DOI] [PubMed] [Google Scholar]
- 7. Falcone A, Ricci S, Brunetti I et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first‐line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol 2007;25:1670–1676. [DOI] [PubMed] [Google Scholar]
- 8. Fearon K, Strasser F, Anker SD et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 9. Bruggeman AR, Kamal AH, LeBlanc TW et al. Cancer cachexia: Beyond weight loss. J Oncol Pract. 2016;12:1163–1171. [DOI] [PubMed] [Google Scholar]
- 10. Lieffers JR, Mourtzakis M, Hall KD et al. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: Contributions of organ and tumor mass to whole‐body energy demands. Am J Clin Nutr 2009;89:1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barret M, Antoun S, Dalban C et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 2014;66:583–589. [DOI] [PubMed] [Google Scholar]
- 12. Blauwhoff‐Buskermolen S, Versteeg KS, de van der Schueren MA et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 2016;34:1339–1344. [DOI] [PubMed] [Google Scholar]
- 13. Tsaousi G, Kokkota S, Papakostas P et al. Body composition analysis for discrimination of prolonged hospital stay in colorectal cancer surgery patients. Eur J Cancer Care (Engl) 2017;26:e12491. [DOI] [PubMed] [Google Scholar]
- 14. Lieffers J, Bathe O, Fassbender K et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsaousi G, Kokkota S, Papakostas P et al. Body composition analysis for discrimination of prolonged hospital stay in colorectal cancer surgery patients. Eur J Cancer Care (Engl) 2017;26:e12491. 10.1111/ecc.12491. [DOI] [PubMed] [Google Scholar]
- 16. Caan B, Meyerhardt JA, Kroenke CH et al. Explaining the obesity paradox: The association between body composition and colorectal cancer survival (C‐SCANS study). Cancer Epidemiol Biomarkers Prev 2017;26:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bridge CP, Rosenthal M, Wright B et al. Fully‐automated analysis of body composition from CT in cancer patients using convolutional neural networks. In: OR 2.0 Context‐Aware Operating Theaters, Computer Assisted Robotic Endoscopy, Clinical Image‐Based Procedures, and Skin Image Analysis. Cham, Switzerland: Springer; 2018:204–213.
- 18. Shen W, Punyanitya M, Wang Z et al. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross‐sectional image. J Appl Physiol (1985). 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 19. Cunningham D, Pyrhönen S, James RD et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 1998;352:1413–1418. [DOI] [PubMed] [Google Scholar]
- 20. Temel JS, Abernethy AP, Currow DC et al. Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomised, double‐blind, phase 3 trials. Lancet Oncol 2016;17:519‐531. [DOI] [PubMed] [Google Scholar]
- 21. Roeland E, Nelson S, Campillo A et al. Inclusion criteria for cancer cachexia clinical trials: CT‐defined skeletal muscle loss versus body weight loss. J Clin Oncol 2015;33(suppl 29):67a. [Google Scholar]
- 22. Miyamoto Y, Baba Y, Sakamoto Y et al. Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS One 2015;10:e0129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hickish T, Andre T, Wyrwicz L et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: A randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Oncol 2017;18:192–201. [DOI] [PubMed] [Google Scholar]
- 24. Golan T, Geva R, Richards D et al. LY2495655, an antimyostatin antibody, in pancreatic cancer: A randomized, phase 2 trial. J Cachexia Sarcopenia Muscle 2018;9:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Becker G, Galandi D, Blum HE. Malignant ascites: Systematic review and guideline for treatment. Eur J Cancer 2006;42:589–597. [DOI] [PubMed] [Google Scholar]
- 26. Silver JK, Baima J. Cancer prehabilitation: An opportunity to decrease treatment‐related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil 2013;92:715–727. [DOI] [PubMed] [Google Scholar]
- 27. Lee H, Troschel FM, Tajmir S et al. Pixel‐level deep segmentation: Artificial intelligence quantifies muscle on computed tomography for body morphometric analysis. J Digit Imaging 2017;30:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nowak S, Faron A, Luetkens JA et al. Fully automated segmentation of connective tissue compartments for CT‐based body composition analysis: A deep learning approach. Invest Radiol 2020;55:357–366. [DOI] [PubMed] [Google Scholar]
- 29. Liu T, Pan J, Torigian DA et al. ABCNet: A new efficient 3D dense‐structure network for segmentation and analysis of body tissue composition on body‐torso‐wide CT images. Med Phys 2020;47:2986–2999. [DOI] [PubMed] [Google Scholar]
- 30. Pickhardt PJ, Graffy PM, Zea R et al. Automated CT biomarkers for opportunistic prediction of future cardiovascular events and mortality in an asymptomatic screening population: A retrospective cohort study. Lancet Digit Health 2020;2:e192–e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mourtzakis M, Prado CM, Lieffers JR et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr and Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 32. Troschel AS, Troschel FM, Best TD et al. Computed tomography–based body composition analysis and its role in lung cancer care. J Thorac Imaging 2020;35:91–100. [DOI] [PubMed] [Google Scholar]
- 33. Hu P, Huo Y, Kong D et al. Automated characterization of body composition and frailty with clinically acquired CT. Comput Methods Clin Appl Musculoskelet Imaging (2017) 2018;10734:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burns JE, Yao J, Chalhoub D et al. A machine learning algorithm to estimate sarcopenia on abdominal CT. Acad Radiol 2020;27:311–320. [DOI] [PubMed] [Google Scholar]
- 35. Shen Z, Ye Y, Bin L et al. Metabolic syndrome is an important factor for the evolution of prognosis of colorectal cancer: Survival, recurrence, and liver metastasis. Am J Surg 2010;200:59–63. [DOI] [PubMed] [Google Scholar]
- 36. Dray X, Boutron‐Ruault MC, Bertrais S et al. Influence of dietary factors on colorectal cancer survival. Gut 2003;52:868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meyerhardt JA, Giovannucci EL, Ogino S, et al. Physical activity and male colorectal cancer survival. Arch Intern Med 2009;169:2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laiyemo AO, Doubeni C, Pinsky PF et al. Race and colorectal cancer disparities: Health‐care utilization vs different cancer susceptibilities. J Natl Cancer Inst 2010;102:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1 Timing of Follow‐up Scans.
Bee plot overlaid with boxplots showing the distribution of CT scan dates around each of the three follow‐up time points. The x‐axis is relative to the baseline CT scan. Therefore, all baseline scans are exactly on day 0 (n = 226). Red vertical lines indicate the exact date, and gray dashed lines indicate the 45‐day range. To account for the fluctuation of scan intervals, we performed linear interpolation to calculate percent change from baseline to exactly 3‐, 6‐ and 12‐ month follow‐up (see Patients and Methods).
CT, computed tomography.
Supplemental Table 1 Days Between Baseline Scan and 3‐, 6‐, and 12‐month Follow‐up Scans. To account for the fluctuation of scan intervals we performed linear interpolation to calculate percent change from baseline to exactly 3‐, 6‐ and 12‐month follow‐up (see Patients and Methods).
CT, computed tomography; SD, standard deviation.
Supplemental Table 2. Patient Characteristics at Time of Metastatic Diagnosis by Availability of Scans at Follow‐up Time Points.
n, number; SD, standard deviation.
Supplemental Table 3. Univariate survival estimates for skeletal muscle loss at 3 months, weight loss at 3 months, and tumor mutational status.
CI, confidence interval; n, number; OS, overall survival; SD, standard deviation.
Supplemental Table 4. Multivariable Model Demonstrating the Relationship Between > 5% Loss of Body Composition and Tumor Mutation Status.
Body composition change was evaluated at scans obtained 3, 6, and 12 months from metastatic diagnosis. Estimates are adjusted for potential confounders including age, sex, and baseline skeletal muscle or baseline body weight. Table 3 is a shortened version of this table.
CI, confidence interval; OR, odds ratio; WT, wild type.


