Abstract
Background
Long period of SARS-CoV-2 infection has been associated with psychiatric and cognitive disorders in adolescents and children. SARS-CoV-2 remains dormant in the CNS leading to neurological complications. The wide expression of ACE2 in the brain raises concern for its involvement in SARS-CoV-2 infection. Though, the mechanistic insights about blood-brain barriers (BBB) crossing by SARS-CoV-2 and further brain infection are still not clear. Moreover, the mechanism behind dormant SARS-CoV-2 infections leading to chronic neurological disorders needs to be unveiled. There is an urgent need to find out the risk factor involved in COVID-19-associated neurological disease. Therefore, the role of immune-associated genes in the pathogenesis of COVID-19 associated neurological diseases is presented which could contribute to finding associated genetic risk factors.
Method
The search utilizing multiple databases, specifically, EMBASE, PubMed (Medline), and Google Scholar was performed. Moreover, the literature survey on the involvement of COVID-19, neuropathogenesis, and its consequences was done.
Description
Persistent inflammatory stimuli may promote the progression of neurodegenerative diseases. An increased expression level of cytokine, chemokine, and decreased expression level of immune cells has been associated with the COVID-19 patient. Cytokine storm was observed in severe COVID-19 patients. The nature of SARS-CoV-2 infection can be neuroinflammatory. Genes of immune response could be associated with neurodegenerative diseases.
Conclusion
The present review will provide a useful framework and help in understanding COVID-19-associated neuropathogenesis. Experimental studies on immune-associated genes in COVID-19 patients with neurological manifestations could be helpful to establish its neuropathogenesis.
Keywords: COVID-19 disease, SARS-CoV-2 associated neurological disease, Immune-associated genes, Lung inflammation, Cytokine storm
1. Introduction
SARS-CoV-2 associated neurological complication is an emerging issue [1]. It is associated with the “intracranial cytokine storm” which is an acute hyperinflammatory condition leading to the severity of several viral infections [1]. The “intracranial cytokine storm” commences with the aberrantly enhanced immune cells activities resulting in blood-brain barrier breakdown and involves symmetric, multi-focal lesions. The occurrence of SARS-CoV-2 associated central nervous system (CNS) complications is ~0·04% [2]. The severe and critical COVID-19 patients have a higher chance of SARS-CoV-2 associated neurological complications [3,4]. The viral infiltration in the brainstem increases the chance of CNS pathology [5]. The psychiatric and cognitive disorders in adolescents and children can be developed after a long period of SARS-CoV-2 infection. Genes of immune response play a major function in antiviral immunity, such as cytokines and chemokines involved in the activation of immune cells through the generation of granulocyte-macrophage colony-stimulating factor (GM-CSF) activate the inflammatory process.
SARS-CoV-2 susceptibility and its clinical outcome are influenced by the expression of cytokines and chemokines and other immune regulating genes and the function of these proteins. These factors are associated with the change in synaptic pruning during childhood, adolescence, and adulthood. The dormant persistence of SARS-CoV-2 infection may increase the secretion of inflammatory proteins which can stimulate the development of neurodegenerative diseases.
Following the infection of SARS-CoV-2, the release of cytokines like IL-1 and IL-6 from local tissue may trigger several neuropathogenic phenotypes, such as dementia, cognitive and movement disorders and epileptic seizures [[6], [7], [8]]. However, till now there is no direct evidence of this hypothesis.
ACE2 is present in various organs including skeletal muscles and the nervous system [9]. It is expressed in the endothelial cells of the brain. The SARS-CoV-2 uses ACE2 receptor for cellular entry where S1 protein attaches and binds with ACE2 of endothelial cells and directs viral infection in the brain. Therefore, it may contribute to neurovascular damage among COVID-19 patients [10]. Since ACE2 is expressed in the various brain compartments and binds with the S1 protein, it is involved in SARS-CoV-2 infection through several indirect or direct mechanisms. So far, evidence for SARS-CoV-2 associated CNS complication has not been well documented, however, the SARS-CoV-2 genome isolated from the CSF of COVID-19 patients suggests direct viral neuroinvasion [11].
SARS-CoV-2 infection is associated with disturbances in taste perception and smell, including anosmia [[12], [13], [14], [15], [16], [17]]. In COVID-related anosmia, SARS-CoV-2 persuade transient changes in odour perception due to inflammatory responses [16,18,19]. It often recovers over weeks [16,20,21], but recovery from typical post-viral anosmia often takes months because of direct damage to olfactory sensory neurons (OSNs) [[22], [23], [24]]. This suggests that SARS-CoV-2 may target odour processing by a certain unidentified mechanism different from those used by other viruses.
So far, the range of COVID-19 associated neurologic manifestations has not fully demonstrated [25]. Besides, how SARS-CoV-2 cross the blood-brain barriers (BBB) and infect microglia and astrocytes is still not fully understood [26]. Therefore, the text was compiled to correlate the immune-associated genes with SARS-CoV-2 associated neurological pathogenesis including SARS-CoV-2 entry into the brain cells.
2. Method
The search utilizing multiple databases, specifically, EMBASE, PubMed (Medline), and Google Scholar was performed. Moreover, the literature survey on the involvement of COVID-19, neuropathogenesis, and its consequences was done.
2.1. CNS cells expressing ACE2 and COVID-19-associated neurological disease
ACE2 is expressed in oligodendrocytes, astrocytes, neurons, and monocytes/macrophages [27,28]. It is also expressed in dendritic cells and macrophage, assisting pulmonary invasion by SARS-CoV2, and induces the local and systemic uncontrolled inflammatory responses [29,30]. Host cell protease, Furin and TMPRSS2 are also expressed in macrophages and have a role in SARS virus binding and fusion of the membranes [31], similar to ADAM 17 acting as sheddase of ACE2 [32]. Thus, the virus enters into dendritic cells and macrophages where it replicates in the presence of all these components and activates the abnormal production of proinflammatory chemokines and cytokines [33]. Immediately, after the SARS-CoV-2 infection in macrophages, it increases the expression of proinflammatory chemokines. On the other hand, it reduces the production of antiviral cytokines [34]. The study showed that dendritic cells are susceptible to SARS-CoV2 infection but are not able to support viral replication [35].
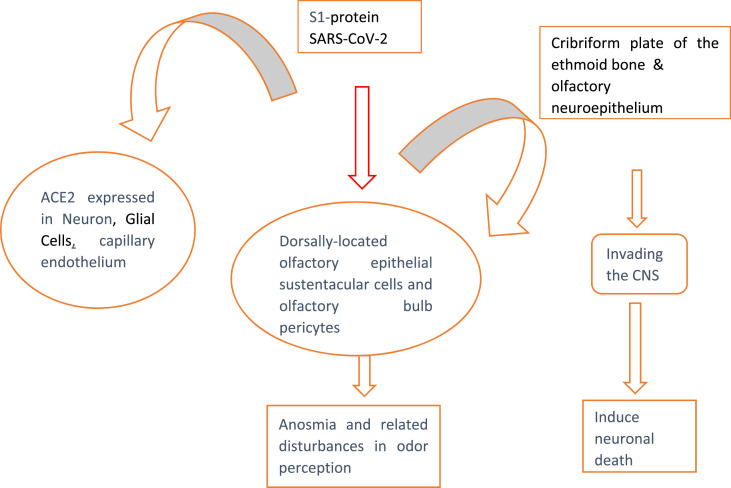
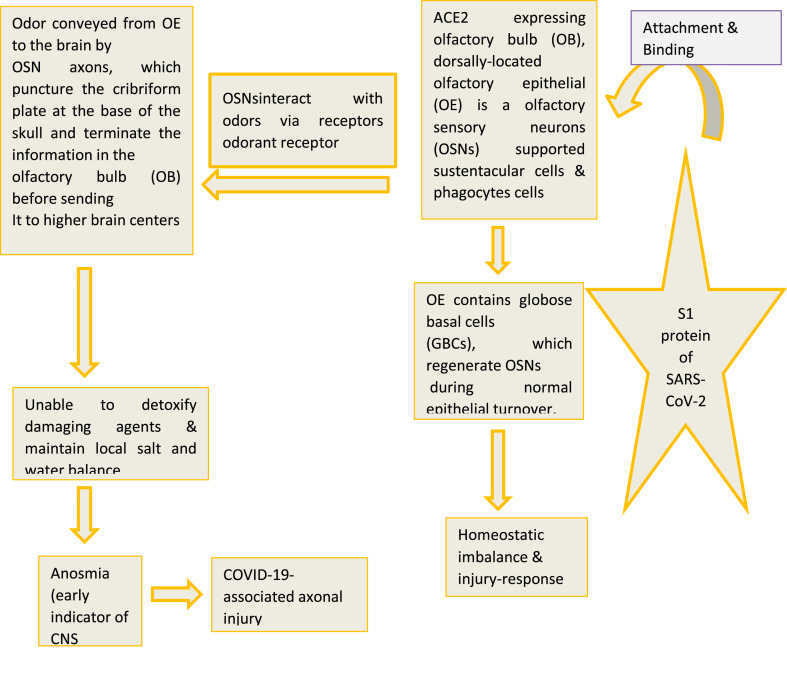
Following SARS-CoV-2 infection, S1 protein binds with ACE2 expressed in neuron, glial and capillary endothelium cells, olfactory bulb pericytes and dorsally-located olfactory epithelial cells leading to anosmia and related disturbances. Besides, S1 protein binds with ACE2 expressed in the olfactory neuroepithelium and cribriform plate of the ethmoid bone leading to its CNS entry and subsequent neuronal death (Fig. 1a, Fig. 1b a and 1b). In the CNS, SARS-CoV-2 gains entry via olfactory bulb and reaches into the brainstem where it causes cytopathy and death of neurons. Thus, SARS-CoV-2 may contribute to the development of COVID-19-associated neurological complications.
Fig. 1a.
ACE2 expression in CNS cells and COVID-19-associated neurological disease.
Fig. 1b.
Epithelial cells and COVID-19-associated axonal injury.
2.2. Association of neuronal and immune cells with COVID-19-associated CNS injury
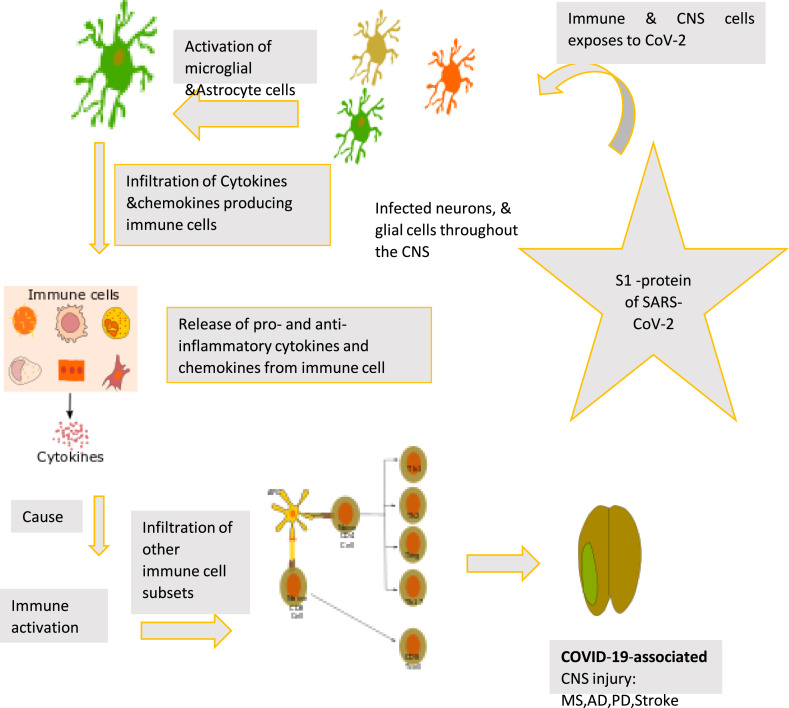
Following the infection of SARS-CoV-2, S1-protein binds with the ACE2 expressing neurons, glial cells, dendritic cells, monocytes, macrophages. Subsequently, the virus activates these cells to get infiltration of other immune cell subsets and replicate. It results in abnormal production of proinflammatory proteins by these cells. On the other hand, it reduces the production of antiviral cytokine. This leads to the induction of local and systemic inflammatory responses in the CNS developing COVID-19-associated CNS injury (Fig. 2 ).
Fig. 2.
Association of neuronal and immune cells with COVID-19-associated CNS Injury.
Innate immune systems have an important role in the response towards external stimuli. An aberrant immune system results in excessive dysregulation of the innate immune response. The defective regulation of immune cells (CD4 and CD8 T cells, CD3, NK cells, CD16, CD56 cells) and continuously increased production of inflammatory proteins (cytokine storm) are associated with the severity of COVID-19 disease [36]. Cytokines (IL-1β, IFN-γ, IL-12, IL-33, IL-6, IL-18, IFN-α), transforming growth factor (TGF-β) and chemokines (CCL2, CCL3, CCL5, CXCL9, CXCL10) are inflammatory mediators, released by immune effector cells [37].
SARS-CoV-2 infection induces increased production of inflammatory proteins which generates an abnormal immune response and modifies the function of immune cells. The persistent abnormal immune response allows the viral infiltration in the brainstem that may contribute to the development of COVID-19-associated neurological complications. Sometimes, individuals may experience neurological complications because of a post-COVID-19 mediated deregulated immune response that can continue as persistent inflammation, immunosuppression, catabolism syndrome (PICS).
2.3. Association of immune-associated genes and COVID-19-associated neurological disease
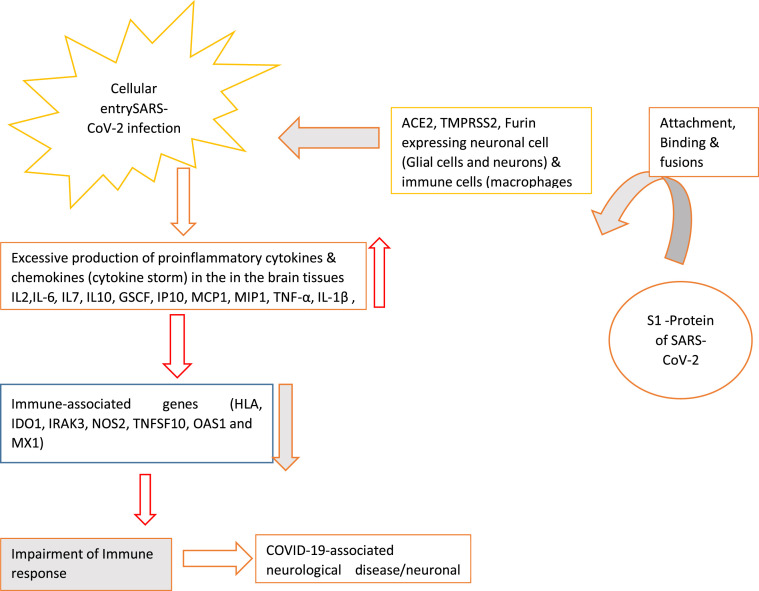
During SARS-CoV-2 infection, S1 protein binds with ACE2 expressed in neuronal cells (glial cells and neurons), immune cells (macrophages, monocytes, and dendritic cells) resulting in reduced production of antiviral cytokine in brain cells. It increases the secretion of proinflammatory chemokines and cytokines in the CSF. On the other hand, it decreases the expression level of immune-responsive genes leading to dysregulated immune response. Following immune-associated genes contribute to the development of COVID-19-associated neurological complications (Fig. 3 ).
Fig. 3.
Immune-associated genes and COVID-19-associated neurological disease.
2.4. Indoleamine-2, 3-dioxygenase 1 (IDO1)
IDO1 (8p11.21) is known as a suppressor of inflammation. Failure of balance between inflammatory response and IDO1-mediated tolerance leads to inflammation and subsequent susceptibility to infection due to decreased immunity against the pathogen. IDO gene is expressed in macrophages, monocytes, dendritic cells, microglia, and several other immune cells and influences neurological complications [38]. IDO is induced by viruses and IFN-inducers, such as lipopolysaccharide (LPS) [39]. SARS-CoV-2 S1 protein binds with ACE2 expressed in dendritic cells, monocytes, macrophages resulting in aberrant IDO1-mediated tolerance and inflammatory response. Therefore, the IDO1 gene may contribute to developing COVID-19-associated neurological complications.
2.5. Nitric oxide (NO) synthase or inducible nitric oxide synthase (iNOS or NOS2)
NOS2 (17q11.2) is present in the glial cells. NOS and NOS2 are inflammatory mediators, with immunopathological and protective capabilities [40]. NOS2 is involved in the production of NO during inflammatory states, and plays an important role during allergic diseases including bronchial asthma [41,42]. Binding of S1 protein with ACE2 expressed in the glial cells interrupts NOS2 mediated protection which abrogates immune homeostasis. These attributes of the NOS2 gene provide several caveats for its contribution to COVID-19-associated neurological complications.
2.6. 2′, 5′-Oligoadenylate synthetase 1 (OAS1)
OAS1 (2q24.13) is induced by type I interferon and has a role in host defense against viral infections [43]. It is expressed in neurons, astrocytes, and oligodendrocytes [44] and converts ATP to 2′,5′-oligoadenylates (2–5As) in presence of dsRNA or ssRNA. Production of 2–5As activates latent RNase L which degrades single-stranded RNAs and inhibits viral replication [43]. Single nucleotide polymorphisms (SNPs) of the OAS1 gene are known to modulate its expression level and enzyme activity resulting in modulated susceptibility and severity of viral diseases [45]. During S1 protein binding with ACE2 expressed in neurons, astrocytes, and oligodendrocytes, OAS1 mediated host defense against infections gets interrupted leading to abrogated immune response. It further supports the contribution of the OAS1 gene in the development of COVID-19-associated neurological complications.
2.7. Human leukocyte antigen (HLA)
HLA [6p21] gene is widely known for its major contribution to the immune response against foreign antigens including viruses [46]. Macrophages or microglia express the histocompatibility glycoprotein (HLA-DR) which is involved in several neurological diseases, such as Pick's and Huntington diseases, Parkinson, Alzheimer, Shy-Drager syndrome, amyotrophic lateral sclerosis, multiple sclerosis, AIDS encephalopathy, and parkinsonism-dementia of Guam [47]. HLA-DR has a role in immune response and facilitates immune surveillance by eliminating foreign antigens. HLA-DR has been mapped to assess the susceptibility or protection in numerous neurological diseases, like neuromyelitis optica, multiple sclerosis, amyotrophic lateral sclerosis, Parkinson, Alzheimer, myasthenia, schizophrenia, and gravis [48]. HLA may increase the binding specificity between the ACE2 receptor and S protein and increases the progression of COVID-19. Several HLA types are associated with the occurrence of SARS infection [49], for instance, the HLA-B*4601 allele is involved in the severity of COVID-19 in Asian populations [49]. Moreover, individuals with HLA-A3.1 allele are susceptible to SARS coronavirus [49]. The ratio of Cw*0801 homozygous and heterozygous alleles was 4.4:1 among individuals with SARS-CoV infection. In contrast, individuals having DRB1*0301 and HLA-Cw*1502 alleles may serve as resistance factors for SARS infection [50]. Also, HLA-B*4601 alleles were significantly correlated with the severity of SARS in the Taiwan population [51]. The frequency of the HLA-B*4601 allele was higher among the suspected SARS infected individuals, and is further increased significantly among the severe patient group [52]. There was a significant association between the HLA-B*0703 allele and SARS development in the Chinese population [53]. Some of the HLA-C variants may trigger neuroinflammation through the release and accumulation of β2microglobulin (β2m) during viral infections.
Interaction of viral S1 with ACE2 expressed on microglia or macrophage cells leads to the interruption of HLA-DR mediated host defense against infections. The major role of HLA-DR in immune response further indicates its contribution towards the development of COVID-19-associated neurological complication.
2.8. Tumor necrosis factor-β (TNFSF1)
TNFSF1 (6p21.3) controls the expression of inflammatory cytokines and the progression of inflammation. TNF-α is produced from astrocytes, microglia, and neurons [54] and it is highly expressed in several clinical conditions, such as spinal cord injury [55], stroke [56], and sciatic nerve injury [57]. TNF-α expression stimulates inflammation and neuronal cell death. Elevated TNFα level is associated with severe COVID-19 patients [58]. TNFα −1031CT/CC and −863 AC genotypes may have risk factors for discharged SARS patients [59]. ACE2 is expressed in oligodendrocytes, neurons, astrocytes, and glial cells (astrocytes and microglia) [27]. During SARS-CoV-2 infection, TNF-mediated immune cell regulation gets disrupted leading to the abrogated immune response and indicates the potential of TNF in the development of COVID-19-related neurological complications.
2.9. Inflammatory cytokine genes and pathogenesis of COVID-19-associated neurological complications
2.9.1. Interleukin-2 (IL-2)
IL-2 (4q27) is known as an inflammatory cytokine with both pro and anti-inflammatory activity. It is involved in the regulation of the immune system and the pathogenesis of asthma. IL-2 or IL-2R molecules are found in the cerebellum, hippocampal formation, septum, frontal cortex, hypothalamus and pituitary fibre tracts such as the corpus callosum striatum, and locus coeruleus. ACE2 and IL-2R are expressed in both neuronal and glial cells. IL-2R (IL-2R alpha) serves as receptor for IL-2.Further, after binding of IL-2 with its receptor (IL-2R alpha), IL-2 penetrates the BBB and controls the communications between the CNS and peripheral tissues. The functional and pathological changes in the brain are affected by communication between IL-2/IL-2R. Enhanced serum IL-2 level was associated with severe COVID-19 patients [58]. As mentioned above the involvement of IL-2 in SARS-CoV-2 infection indicates its contribution to the development of COVID-19-related neurological complications.
2.9.2. Interleukin 10 (IL-10)
IL-10 (1q32.1) is an immunosuppressive cytokine produced within the CNS and acts as an anti-inflammatory factor. IL-10 regulates inflammatory response to infections under various conditions including, glial inflammatory responses, CNS catheter infection, and peripheral inflammation [60,61]. Clinical evidence indicated association of elevated IL-10 level with ICU admitted COVID‐19 patients [59]. It is suggested in the literature that, IL-10 -1082G/A and −592A/C polymorphism could be associated with SARS infection [62]. As ACE2 is highly expressed in neurons and glial cells, CNS could be a potential target for SARS-CoV-2 [63] and IL-10 can play a major role in this process through regulation of inflammatory response.
2.9.3. Interleukin 6 (IL-6)
IL-6 (7p15.3) is an inflammatory cytokine implicated in both pro and anti-inflammatory activities and has an important role in response to acute lung injury. A higher plasma IL‐6 level was correlated with the risk of severe respiratory failure in COVID-19 patients [64]. Another study indicated increased levels of IL-6, and ACE in CSF of SARS-CoV-2–associated encephalitis patients [65]. Clinical studies indicated that IL-6 174G/C polymorphism influences IL-6 levels and higher plasma IL-6 levels are associated with severe COVID-19 among the study group [66]. IL-6 polymorphism is suggested to be used as an indicator of severity in COVID-19 patients in the Korean population [67]. IL-6 depends on GM-CSF and JAK2 signaling and SARS-CoV-2 can play an important role in this process. Hence IL-6 could be a potential therapeutic target for hyperinflammatory response [68]. In COVID-19 patients, IL-6 could be important for fibrogenesis and endothelial cell dysfunction [67]. IL-6 is produced by both astrocytes and microglia [68] and its neuronal expression contributes to the glial cell activation [69]. ACE2 is also expressed in neurons, astrocytes, and glial cells [27,70], and therefore the S1-ACE2 interaction can be affected through IL-6 contributing to the development of COVID-19-associated neurological complications. Moreover, the inhibitors of IL-6 are suggested to reduce the COVID-19 associated mortality without increasing secondary infections [71].
2.9.4. Interleukin 7 (IL-7)
IL-7 (8q21.13) is an inflammatory cytokine involved in lymphoid cell survival and maintenance of naive and memory T cells. The study indicated the presence of IL-7 in the CSF and its high level is associated with inflammatory CNS disease [72]. Increased IL-7 mRNA expression was detected in the CNS [73] and elevated serum IL-7 levels, along with other circulating cytokines and chemokines is associated with the severity of COVID-19 disease [58]. ACE2 is widely expressed throughout the CNS indicating the possible influence of IL-7 on S1-ACE2 mediated interaction and subsequent SARS-CoV-2 infection [62]. Therefore, disruption of IL-7 mediated maintenance of T cells and immune function may contribute to COVID-19-associated neurological complications and needs further investigations.
2.9.5. Interleukin 15 (IL-15)
IL-15(4q31) cytokine is involved in defense against infections including antiviral response and protection against allograft rejection and autoimmune diseases. SARS-CoV-2 induces IL-15 cytokine [74] and the viral clearance depends on several factors including, type I interferons, IL-15 and IFNγ. The clearance of SARS-CoV-2 could be inhibited by JAK1/JAK3 inhibitors, moreover, viral entry could be blocked by JAK2 inhibition [75]. IL-15 is expressed by multiple types of brain cells, including astrocytes, and neurons. Similarly, ACE2 is also expressed in astrocytes, oligodendrocytes, and neurons [27], and it indicates the potential of IL-15 in the development of COVID-19-associated neurological complications.
2.9.6. Interleukin-1β (IL-1β)
‘IL-1β (2q13) is a pro-inflammatory cytokine released by immune effector cells and play an important role as an inflammatory mediator after binding to the IL-1 receptor [36]. IL-1 is expressed in microglia and acts as a mediator of microglial activation and proliferation. An inappropriate expression of the IL-1 gene could lead the CNS dysfunction. IL-1 stimulates a variety of factors like adhesion molecules and endothelial cells [76] and eicosanoids [77]. The variations in these factors increase the BBB permeability and simultaneous ACE2 expression on the neurons and glial cells leaves several caveats [70,78]. In disease condition, microglia cells are significantly activated which can contribute to neuropathology [79]. An increase in the secretion of IL-1β induces severe inflammation and therefore IL-1β gene may contribute to COVID-19-associated CNS dysfunction. In COVID-19 associated CNS disease, plasma IL-1 or CNS levels were correlated with the severities [[80], [81], [82], [83], [84], [85]]. An increased level of IL-1 expression and activated microglial cells can contribute to CNS pathogenesis [86]. In neuropathology and neuroinflammation, a higher IL-1 expression was observed in the brain tissue along with morphological changes in microglia [81,[87], [88], [89], [90], [91]]. Due to the major involvement of IL-1β in several neurological conditions and its already known role in COVID-19, it needs critical investigation to evaluate its role in COVID-19 associated neurological complications.
2.9.7. Interferon-gamma (IFNγ)
IFNγ (ch12) is a pleiotropic cytokine with pathological and protective roles in CNS diseases. It is involved in Th1 responses and immunity [92] resulting in susceptibility to several inflammatory and infectious diseases. IFNγ induces proinflammatory cytokines (IL-6, IL-1, TNF-α). Individuals with IFN-γ +874A allele were susceptible to SARS infection in a previous report [93]. IFNγ is also expressed in neuroinflammatory and neurodegenerative conditions and provides protection during viral brain infections, however in infected neurons, IFNγ mediates a non-cytolytic viral control. IFN-γ and ACE2 are expressed in the astrocytes and microglia [70,92] and therefore SARS-CoV-2 infection can affect IFN-γ mediated protective mechanisms in the brain. Therefore, the IFN-γ gene may be a potential candidate for its contribution to COVID-19 associated CNS dysfunction.
2.9.8. Interleukin-17 (IL-17)
IL-17 (6p12.2) is a pro-inflammatory cytokine secreted by Th17 and CD8 cells, involved in many physiological and pathophysiological conditions. IL-17 expression is associated with the severity of COVID-19 patients in several studies [75,[94], [95], [96]]. A higher number of CCR6 + Th17 cells are found among severe COVID-19 patients [97]. Increased IL-17 signaling may affect neuronal toxicity by activating NFκB mediated inflammatory pathways [98]. IL-17 is mainly expressed in spinal cord astrocytes while IL-17RA is expressed in both astrocytes and microglia while ACE2 is also expressed in oligodendrocytes, astrocytes, neurons [27]. It indicates that SARS-CoV-2 may affect IL-17 mediated protective immunity to pathogens, and therefore, the IL-17 gene may contribute to neuronal toxicity and COVID-19-associated CNS dysfunction.
2.10. Endothelial cells and COVID-19-associated neurovascular damage
Endothelial cells, astrocyte and pericytes constituting the BBB and peripherally derived immune cells express ACE2. The presence of ACE2 makes them an important target for SARS-CoV-2 through ACE2-S1 interaction. CNS endothelial cells (EC) and polymorphonuclear cells (PMNs) may contribute to inflammation after virus-mediated cytokine induction, consequently disrupting the blood-brain barrier. Therefore, it may contribute to early CNS toxicity or COVID-19-associated neurovascular damage (Fig. 4 ).
Fig. 4.
Association of endothelial cells and COVID-19-associated Neurovascular damage.
3. Summary
Four possible ways of CNS dysfunction and the development of COVID-19-associated disease has been discussed. It is possible that SARS-CoV-2 can manifest neurological complications through interaction with several cell types and regulators, such as [1] ACE2 expression in CNS cells make them vulnerable for COVID -19 associated neurological damage. 2) ACE2 expressing immune cells can mediate COVID-19 associated CNS injury. 3) Immune associated genes may mediate mechanisms of COVID -19 associated neurological disease 4) Endothelial cells expressing ACE2 can lead to COVID-19 associated neurological damage. Further laboratory and clinical studies related to immune-associated genes in patients with COVID-19-associated CNS dysfunction should be conducted to understand its neuropathogenesis.
4. Future
Neurological complications of COVID-19 are not completely understood. Prospective multicentric studies should be carried out to collect cognitive, psychiatric, virological, and neurophysiological data from patients surviving severe COVID-19. This review will provide a useful insight and framework to understand COVID-19-associated neuropathogenesis. Present review could be helpful to find out the susceptible cell types to CoV-2 infection which may provide a better understanding of altered smell perception in COVID-19 disease. Furthermore, this review will be useful to design the expression studies related to the above-mentioned genes in patients with COVID-19 associated neurologic complications for better understanding of neuropathogenesis caused by COVID-19 disease.
Ethical approval
Not required.
Funding source
Not required.
Conflicts of interest
No.
Data availability statement
Data will be available on request by email.
Authors contribution
HariOm Singh: Overall supervision, Amita Singh: Manuscript writing, Abdul Arif Khan: Review of manuscript, Vivek Gupta: Manuscript Review.
Declaration of competing interest
There are no conflict among the Authors.
Acknowledgements
The Authors acknowledge the ICMR-National AIDS Research Institute, Pune for providing necessary facilities. We are highly thankful to Mr Chandrashekhar Jori, JRF and Kishore Dhotre, JRF-ICMR-NARI, Pune for editing and grammar checking of the manuscript.
References
- 1.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020 Aug;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020 Sep;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M., Klein M., Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020 Jul;146(1):128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirtipal N., Bharadwaj S., Kang S.G. From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect. Genet. Evol. 2020 Nov;85:104502. doi: 10.1016/j.meegid.2020.104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. China medical treatment expert group for covid-19. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aarli J.A. Role of cytokines in neurological disorders. Curr. Med. Chem. 2003 Oct;10(19):1931–1937. doi: 10.2174/0929867033456918. [DOI] [PubMed] [Google Scholar]
- 7.Rothaug M., Becker-Pauly C., Rose-John S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta. 2016 Jun;1863(6 Pt A):1218–1227. doi: 10.1016/j.bbamcr.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Erta M., Quintana A., Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012;8(9):1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004 Jun;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nath A. Neurologic complications of coronavirus infections. Neurology. 2020 May 12;94(19):809–810. doi: 10.1212/WNL.0000000000009455. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L., Zhang M., Wang J., Gao J. Sars-Cov-2: underestimated damage to nervous system. Travel Med Infect Dis. 2020 Jul-Aug;36:101642. doi: 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagheri S.H., Asghari A., Farhadi M., Shamshiri A.R., Kabir A., Kamrava S.K., Jalessi M., Mohebbi A., Alizadeh R., Honarmand A.A., Ghalehbaghi B., Salimi A., Dehghani Firouzabadi F. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med. J. Islam. Repub. Iran. 2020 Jun 15;34:62. doi: 10.34171/mjiri.34.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A.L., Rizzardini G., Antinori S., Galli M. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin. Infect. Dis. 2020 Jul 28;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 May;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 15.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., Huet K., Plzak J., Horoi M., Hans S., Rosaria Barillari M., Cammaroto G., Fakhry N., Martiny D., Ayad T., Jouffe L., Hopkins C., Saussez S., Covid-19 Task Force of Yo-Ifos Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J. Intern. Med. 2020 Sep;288(3):335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A., Ganesh S., Varsavsky T., Cardoso M.J., El-Sayed Moustafa J.S., Visconti A., Hysi P., Bowyer R.C.E., Mangino M., Falchi M., Wolf J., Ourselin S., Chan A.T., Steves C.J., Spector T.D. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020 Jul;26(7):1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spinato G., Fabbris C., Polesel J., Cazzador D., Borsetto D., Hopkins C., Boscolo-Rizzo P. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. J. Am. Med. Assoc. 2020 May 26;323(20):2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eliezer M., Hautefort C., Hamel A.L., Verillaud B., Herman P., Houdart E., Eloit C. Sudden and complete olfactory loss of function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. 2020 Jul 1;146(7):674–675. doi: 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- 19.Parma V., Ohla K., Veldhuizen M.G., Niv M.Y., Kelly C.E., Bakke A.J., Cooper K.W., Bouysset C., Pirastu N., Dibattista M., Kaur R., Liuzza M.T., Pepino M.Y., Schöpf V., Pereda-Loth V., Olsson S.B., Gerkin R.C., Rohlfs Domínguez P., Albayay J., Farruggia M.C., Bhutani S., Fjaeldstad A.W., Kumar R., Menini A., Bensafi M., Sandell M., Konstantinidis I., Di Pizio A., Genovese F., Öztürk L., Thomas-Danguin T., Frasnelli J., Boesveldt S., Ö Saatci, Saraiva L.R., Lin C., Golebiowski J., Hwang L.D., Ozdener M.H., Guàrdia M.D., Laudamiel C., Ritchie M., Havlícek J., Pierron D., Roura E., Navarro M., Nolden A.A., Lim J., Whitcroft K.L., Colquitt L.R., Ferdenzi C., Brindha E.V., Altundag A., Macchi A., Nunez-Parra A., Patel Z.M., Fiorucci S., Philpott C.M., Smith B.C., Lundström J.N., Mucignat C., Parker J.K., van den Brink M., Schmuker M., Fischmeister F.P.S., Heinbockel T., Shields V.D.C., Faraji F., Santamaría E., Fredborg W.E.A., Morini G., Olofsson J.K., Jalessi M., Karni N., D'Errico A., Alizadeh R., Pellegrino R., Meyer P., Huart C., Chen B., Soler G.M., Alwashahi M.K., Welge-Lüssen A., Freiherr J., de Groot J.H.B., Klein H., Okamoto M., Singh P.B., Hsieh J.W., Gccr Group Author, Reed D.R., Hummel T., Munger S.D., Hayes J.E. More than smell-COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem. Senses. 2020 Oct 9;45(7):609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y., Min P., Lee S., Kim S.W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J. Kor. Med. Sci. 2020 May 11;35(18):e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan C.H., Faraji F., Prajapati D.P., Boone C.E., DeConde A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020 Jul;10(7):806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welge-Lüssen A., Wolfensberger M. Olfactory disorders following upper respiratory tract infections. Adv. Oto-Rhino-Laryngol. 2006;63:125–132. doi: 10.1159/000093758. [DOI] [PubMed] [Google Scholar]
- 23.Duncan H.J., Seiden A.M. Long-term follow-up of olfactory loss secondary to head trauma and upper respiratory tract infection. Arch. Otolaryngol. Head Neck Surg. 1995 Oct;121(10):1183–1187. doi: 10.1001/archotol.1995.01890100087015. [DOI] [PubMed] [Google Scholar]
- 24.Cavazzana A., Larsson M., Münch M., Hähner A., Hummel T. Postinfectious olfactory loss: a retrospective study on 791 patients. Laryngoscope. 2018 Jan;128(1):10–15. doi: 10.1002/lary.26606. [DOI] [PubMed] [Google Scholar]
- 25.Pleasure S.J., Green A.J., Josephson S.A. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection: neurologists move to the frontlines. JAMA Neurol. 2020 Jun 1;77(6):679–680. doi: 10.1001/jamaneurol.2020.1065. [DOI] [PubMed] [Google Scholar]
- 26.Serrano-Castro P.J., Estivill-Torrús G., Cabezudo-García P., Reyes-Bueno J.A., Ciano Petersen N., Aguilar-Castillo M.J., Suárez-Pérez J., Jiménez-Hernández M.D., Moya-Molina M.Á., Oliver-Martos B., Arrabal-Gómez C., Rodríguez de Fonseca F. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: a delayed pandemic? Neurologia. 2020 May;35(4):245–251. doi: 10.1016/j.nrl.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R., Wang K., Yu J., Chen Z., Wen C., Xu Z. 2020; May 18. The Spatial and Cell-type Distribution of SARS-CoV-2 Receptor ACE2 in Human and Mouse Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keidar S., Gamliel-Lazarovich A., Kaplan M., Pavlotzky E., Hamoud S., Hayek T., Karry R., Abassi Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ. Res. 2005 Oct 28;97(9):946–953. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- 29.Abassi Z., Knaney Y., Karram T., Heyman S.N. The lung macrophage in SARS-CoV-2 infection: a friend or a foe? Front. Immunol. 2020 Jun 5;11:1312. doi: 10.3389/fimmu.2020.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020 May 1;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 31.Gagnon H., Refaie S., Gagnon S., Desjardins R., Salzet M., Day R. Proprotein convertase 1/3 (PC1/3) in the rat alveolar macrophage cell line NR8383: localization, trafficking and effects on cytokine secretion. PloS One. 2013 Apr 24;8(4) doi: 10.1371/journal.pone.0061557. e61557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikolaidis N.M., Gray J.K., Gurusamy D., Fox W., Stuart W.D., Huber N., Waltz S.E. Ron receptor tyrosine kinase negatively regulates TNFalpha production in alveolar macrophages by inhibiting NF-kappaB activity and Adam17 production. Shock. 2010 Feb;33(2):197–204. doi: 10.1097/SHK.0b013e3181ae8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J., Chu H., Chan J.F., Yuen K.Y. Middle East respiratory syndrome coronavirus infection: virus-host cell interactions and implications on pathogenesis. Virol. J. 2015 Dec 22;12:218. doi: 10.1186/s12985-015-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tseng C.T., Perrone L.A., Zhu H., Makino S., Peters C.J. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J. Immunol. 2005 Jun 15;174(12):7977–7985. doi: 10.4049/jimmunol.174.12.7977. [DOI] [PubMed] [Google Scholar]
- 35.Law H.K., Cheung C.Y., Ng H.Y., Sia S.F., Chan Y.O., Luk W., Nicholls J.M., Peiris J.S., Lau Y.L. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005 Oct 1;106(7):2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017 Jul;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mándi Y., Vécsei L. The kynurenine system and immunoregulation. J. Neural. Transm. 2012 Feb;119(2):197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- 39.Musso T., Gusella G.L., Brooks A., Longo D.L., Varesio L. Interleukin-4 inhibits indoleamine 2,3-dioxygenase expression in human monocytes. Blood. 1994 Mar 1;83(5):1408–1411. [PubMed] [Google Scholar]
- 40.Nathan C., Xie Q.W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 41.Garme Y., Moudi M., Saravani R., Galavi H. Nitric oxide synthase 2 polymorphisms (rs2779248T/C and rs1137933C/T) and the risk of type 2 diabetes in zahedan, southeastern Iran. Iran. J. Public Health. 2018 Nov;47(11):1734–1741. [PMC free article] [PubMed] [Google Scholar]
- 42.Topchieva L.V., Balan O.V., Korneeva V.A., Malysheva I.E. The role of inducible NOS2 gene polymorphism in the development of essential arterial hypertension. Bull. Exp. Biol. Med. 2019 Nov;168(1):79–83. doi: 10.1007/s10517-019-04652-4. [DOI] [PubMed] [Google Scholar]
- 43.Choi U.Y., Kang J.S., Hwang Y.S., Kim Y.J. Oligoadenylate synthase-like (OASL) proteins: dual functions and associations with diseases. Exp. Mol. Med. 2015 Mar 6;47(3):e144. doi: 10.1038/emm.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao L., Birdwell L.D., Wu A., Elliott R., Rose K.M., Phillips J.M., Li Y., Grinspan J., Silverman R.H., Weiss S.R. Cell-type-specific activation of the oligoadenylate synthetase-RNase L pathway by a murine coronavirus. J. Virol. 2013 Aug;87(15):8408–8418. doi: 10.1128/JVI.00769-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonnevie-Nielsen V., Field L.L., Lu S., Zheng D.J., Li M., Martensen P.M., Nielsen T.B., Beck-Nielsen H., Lau Y.L., Pociot F. Variation in antiviral 2',5'-oligoadenylate synthetase (2'5'AS) enzyme activity is controlled by a single-nucleotide polymorphism at a splice-acceptor site in the OAS1 gene. Am. J. Hum. Genet. 2005 Apr;76(4):623–633. doi: 10.1086/429391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meuer S.C., Hussey R.E., Hodgdon J.C., Hercend T., Schlossman S.F., Reinherz E.L. Surface structures involved in target recognition by human cytotoxic T lymphocytes. Science. 1982 Oct 29;218(4571):471–473. doi: 10.1126/science.6981845. [DOI] [PubMed] [Google Scholar]
- 47.McGeer P.L., Itagaki S., McGeer E.G. Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 1988;76(6):550–557. doi: 10.1007/BF00689592. [DOI] [PubMed] [Google Scholar]
- 48.Misra M.K., Damotte V., Hollenbach J.A. The immunogenetics of neurological disease. Immunology. 2018 Apr;153(4):399–414. doi: 10.1111/imm.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomita Y., Ikeda T., Sato R., Sakagami T. Association between HLA gene polymorphisms and mortality of COVID-19: an in silico analysis. Immun Inflamm Dis. 2020 Dec;8(4):684–694. doi: 10.1002/iid3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Yuying, Xi Yongzhi. Association between HLA gene polymorphism and the genetic susceptibility of SARS infection. HLA and Associated Important Diseases, Yongzhi Xi, IntechOpen. March 19th 2014 doi: 10.5772/57561. [DOI] [Google Scholar]
- 51.Lin M., Tseng H.K., Trejaut J.A., Lee H.L., Loo J.H., Chu C.C., Chen P.J., Su Y.W., Lim K.H., Tsai Z.U., Lin R.Y., Lin R.S., Huang C.H. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med. Genet. 2003 Sep 12;4:9. doi: 10.1186/1471-2350-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Margaret H.L., Ng, Lau Kin-Mang, Li Libby, Cheng Suk-Hang, Chan Wing Y., Hui Pak K., Zee Benny, Leung Chi-Bon, Joseph J., Sung Y. Association of human-leukocyte-antigen class I (B*0703) and class II (DRB1*0301) genotypes with susceptibility and resistance to the development of severe acute respiratory syndrome. J. Infect. Dis. 2004;190(3):515–518. doi: 10.1086/421523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strohmeyer R., Rogers J. Molecular and cellular mediators of Alzheimer's disease inflammation. J Alzheimers Dis. 2001 Feb;3(1):131–157. doi: 10.3233/jad-2001-3118. [DOI] [PubMed] [Google Scholar]
- 54.Ohtori S., Takahashi K., Moriya H., Myers R.R. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine (Phila Pa. 1976;29(10):1082–1088. doi: 10.1097/00007632-200405150-00006. 2004 May 15. [DOI] [PubMed] [Google Scholar]
- 55.Liu T., Clark R.K., McDonnell P.C., Young P.R., White R.F., Barone F.C., Feuerstein G.Z. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994 Jul;25(7):1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 56.Schäfers M., Geis C., Svensson C.I., Luo Z.D., Sommer C. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur. J. Neurosci. 2003 Feb;17(4):791–804. doi: 10.1046/j.1460-9568.2003.02504.x. [DOI] [PubMed] [Google Scholar]
- 57.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S., Wei M., Han Y., Zhang K., He L., Yang Z., Su B., Zhang Z., Hu Y., Hui W. Roles of TNF-alpha gene polymorphisms in the occurrence and progress of SARS-Cov infection: a case-control study. BMC Infect. Dis. 2008 Feb 29;8:27. doi: 10.1186/1471-2334-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gutierrez-Murgas Y.M., Skar G., Ramirez D., Beaver M., Snowden J.N. IL-10 plays an important role in the control of inflammation but not in the bacterial burden in S. epidermidis CNS catheter infection. J. Neuroinflammation. 2016 Oct 13;13(1):271. doi: 10.1186/s12974-016-0741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutchins A.P., Diez D., Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics. 2013 Nov;12(6):489–498. doi: 10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020 Apr 1;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 62.Anastassopoulou C., Gkizarioti Z., Patrinos G.P., Tsakris A. Human genetic factors associated with susceptibility to SARS-CoV-2 infection and COVID-19 disease severity. Hum. Genom. 2020 Oct 22;14(1):40. doi: 10.1186/s40246-020-00290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barrantes F.J. Central nervous system targets and routes for SARS-CoV-2: current views and new hypotheses. ACS Chem. Neurosci. 2020;11(18):2793–2803. doi: 10.1021/acschemneuro.0c00434. [DOI] [PubMed] [Google Scholar]
- 64.Herold T., Jurinovic V., Arnreich C., et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020;146(1):128–136. doi: 10.1016/j.jaci.2020.05.008. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bodro M., Compta Y., Llansó L., Esteller D., Doncel-Moriano A., Mesa A., Rodríguez A., Sarto J., Martínez-Hernandez E., Vlagea A., Egri N., Filella X., Morales-Ruiz M., Yagüe J., Soriano Á., Graus F., García F. “Hospital clínic infecto-COVID-19” and “hospital clínic neuro-COVID-19” groups. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020 Jul 1;7(5) doi: 10.1212/NXI.0000000000000821. e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gupta K.K., Khan M.A., Singh S.K. Constitutive inflammatory cytokine storm: a major threat to human health. J. Interferon Cytokine Res. 2020 Jan;40(1):19–23. doi: 10.1089/jir.2019.0085. [DOI] [PubMed] [Google Scholar]
- 67.Kirtipal N., Bharadwaj S. Interleukin 6 polymorphisms as an indicator of COVID-19 severity in humans. J. Biomol. Struct. Dyn. 2020 Jun 12:1–3. doi: 10.1080/07391102.2020.1776640. [DOI] [PubMed] [Google Scholar]
- 68.Schönrock L.M., Gawlowski G., Brück W. Interleukin-6 expression in human multiple sclerosis lesions. Neurosci. Lett. 2000 Nov 10;294(1):45–48. doi: 10.1016/s0304-3940(00)01543-3. [DOI] [PubMed] [Google Scholar]
- 69.Klein M.A., Möller J.C., Jones L.L., Bluethmann H., Kreutzberg G.W., Raivich G. Impaired neuroglial activation in interleukin-6 deficient mice. Glia. 1997 Mar;19(3):227–233. doi: 10.1002/(sici)1098-1136(199703)19:3<227::aid-glia5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 70.Xia H., Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J. Neurochem. 2008 Dec;107(6):1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han Q., Guo M., Zheng Y., Zhang Y., De Y., Xu C., Zhang L., Sun R., Lv Y., Liang Y., Xu F., Pang J., Chen Y. Current evidence of interleukin-6 signaling inhibitors in patients with COVID-19: a systematic review and meta-analysis. Front. Pharmacol. 2020 Dec 15;11:615972. doi: 10.3389/fphar.2020.615972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lundmark F., Duvefelt K., Iacobaeus E., Kockum I., Wallström E., Khademi M., Oturai A., Ryder L.P., Saarela J., Harbo H.F., Celius E.G., Salter H., Olsson T., Hillert J. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat. Genet. 2007 Sep;39(9):1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- 73.Arbelaez C.A., Glatigny S., Duhen R., Eberl G., Oukka M., Bettelli E. IL-7/IL-7 receptor signaling differentially affects effector CD4+ T cell subsets involved in experimental autoimmune encephalomyelitis. J. Immunol. 2015 Sep 1;195(5):1974–1983. doi: 10.4049/jimmunol.1403135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andrisani G., Frasca D., Romero M., Armuzzi A., Felice C., Marzo M., Pugliese D., Papa A., Mocci G., De Vitis I., Rapaccini G.L., Blomberg B.B., Guidi L. Immune response to influenza A/H1N1 vaccine in inflammatory bowel disease patients treated with anti TNF-α agents: effects of combined therapy with immunosuppressants. J Crohns Colitis. 2013 May;7(4):301–307. doi: 10.1016/j.crohns.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020 Jun;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong D., Dorovini-Zis K. Upregulation of intercellular adhesion molecule-1 (ICAM-1) expression in primary cultures of human brain microvessel endothelial cells by cytokines and lipopolysaccharide. J. Neuroimmunol. 1992 Jul;39(1–2):11–21. doi: 10.1016/0165-5728(92)90170-p. [DOI] [PubMed] [Google Scholar]
- 77.de Vries H.E., Hoogendoorn K.H., van Dijk J., Zijlstra F.J., van Dam A.M., Breimer D.D., van Berkel T.J., de Boer A.G., Kuiper J. Eicosanoid production by rat cerebral endothelial cells: stimulation by lipopolysaccharide, interleukin-1 and interleukin-6. J. Neuroimmunol. 1995 Jun;59(1–2):1–8. doi: 10.1016/0165-5728(95)00009-q. [DOI] [PubMed] [Google Scholar]
- 78.Doobay M.F., Talman L.S., Obr T.D., Tian X., Davisson R.L., Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007 Jan;292(1):R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salter M.W., Stevens B. Microglia emerge as central players in brain disease. Nat. Med. 2017 Sep 8;23(9):1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 80.Hewett S.J., Jackman N.A., Claycomb R.J. Interleukin-1β in central nervous system injury and repair. Eur J Neurodegener Dis. 2012 Aug;1(2):195–211. [PMC free article] [PubMed] [Google Scholar]
- 81.Gui W.S., Wei X., Mai C.L., Murugan M., Wu L.J., Xin W.J., Zhou L.J., Liu X.G. Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol. Pain. 2016 May 12;12 doi: 10.1177/1744806916646784. 1744806916646784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mrak R.E., Griffin W.S. Interleukin-1 and the immunogenetics of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2000 Jun;59(6):471–476. doi: 10.1093/jnen/59.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bessler H., Djaldetti R., Salman H., Bergman M., Djaldetti M. IL-1 beta, IL-2, IL-6 and TNF-alpha production by peripheral blood mononuclear cells from patients with Parkinson's disease. Biomed. Pharmacother. 1999 Apr;53(3):141–145. doi: 10.1016/S0753-3322(99)80079-1. [DOI] [PubMed] [Google Scholar]
- 84.Qin X.Y., Zhang S.P., Cao C., Loh Y.P., Cheng Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol. 2016 Nov 1;73(11):1316–1324. doi: 10.1001/jamaneurol.2016.2742. [DOI] [PubMed] [Google Scholar]
- 85.Careaga M., Rogers S., Hansen R.L., Amaral D.G., Van de Water J., Ashwood P. Immune endophenotypes in children with autism spectrum disorder. Biol. Psychiatr. 2017 Mar 1;81(5):434–441. doi: 10.1016/j.biopsych.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schultz R.M. Interleukin 1 and interferon-gamma: cytokines that provide reciprocal regulation of macrophage and T cell function. Toxicol. Pathol. 1987;15(3):333–337. doi: 10.1177/019262338701500311. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Q.S., Heng Y., Yuan Y.H., Chen N.H. Pathological α-synuclein exacerbates the progression of Parkinson's disease through microglial activation. Toxicol. Lett. 2017 Jan 4;265:30–37. doi: 10.1016/j.toxlet.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 88.Sznejder-Pachołek A., Joniec-Maciejak I., Wawer A., Ciesielska A., Mirowska-Guzel D. The effect of α-synuclein on gliosis and IL-1α, TNFα, IFNγ, TGFβ expression in murine brain. Pharmacol. Rep. 2017 Apr;69(2):242–251. doi: 10.1016/j.pharep.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Alyu F., Dikmen M. Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr. 2017 Feb;29(1):1–16. doi: 10.1017/neu.2016.47. [DOI] [PubMed] [Google Scholar]
- 90.Stojakovic A., Paz-Filho G., Arcos-Burgos M., Licinio J., Wong M.L., Mastronardi C.A. Role of the IL-1 pathway in dopaminergic neurodegeneration and decreased voluntary movement. Mol. Neurobiol. 2017 Aug;54(6):4486–4495. doi: 10.1007/s12035-016-9988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hou Y., Xie G., Liu X., Li G., Jia C., Xu J., Wang B. Minocycline protects against lipopolysaccharide-induced cognitive impairment in mice. Psychopharmacology (Berl) 2016 Mar;233(5):905–916. doi: 10.1007/s00213-015-4169-6. [DOI] [PubMed] [Google Scholar]
- 92.Kulkarni A., Ganesan P., O'Donnell L.A. Interferon gamma: influence on neural stem cell function in neurodegenerative and neuroinflammatory disease. Clin. Med. Insights Pathol. 2016 Oct 13;9(Suppl 1):9–19. doi: 10.4137/CPath.S40497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chong W.P., Ip W.K., Tso G.H., Ng M.W., Wong W.H., Law H.K., Yung R.W., Chow E.Y., Au K.L., Chan E.Y., Lim W., Peiris J.S., Lau Y.L. The interferon gamma gene polymorphism +874 A/T is associated with severe acute respiratory syndrome. BMC Infect. Dis. 2006 May 4;6:82. doi: 10.1186/1471-2334-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cafarotti S. Severe acute respiratory syndrome-coronavirus-2 infection and patients with lung cancer: the potential role of interleukin-17 target therapy. J. Thorac. Oncol. 2020 Jul;15(7):e101–e103. doi: 10.1016/j.jtho.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tufan A., Avanoğlu Güler A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020 Apr 21;50(SI-1):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Yingxia, Zhang Cong, Huang Fengming, Yang Yang, Wang Fuxiang, Yuan Jing, Zhang Zheng, Qin Yuhao, Li Xiaoyun, Zhao Dandan, Li Shunwang, Tan Shuguang, Wang Zhaoqin, Li Jinxiu, Shen Chenguang, Li Jianming, Peng Ling, Wu Weibo, Cao Mengli, Xing Li, Xu Zhixiang, Chen Li, Zhou Congzhao, Liu William J., Liu Lei, Jiang Chengyu. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. National Science Review. 2020;7(6):1003–1011. doi: 10.1093/nsr/nwaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sommer A., Marxreiter F., Krach F., Fadler T., Grosch J., Maroni M., Graef D., Eberhardt E., Riemenschneider M.J., Yeo G.W., Kohl Z., Xiang W., Gage F.H., Winkler J., Prots I., Winner B. Th17 lymphocytes induce neuronal cell death in a human iPSC-based model of Parkinson's disease. Cell Stem Cell. 2018 Jul 5;23(1):123–131. doi: 10.1016/j.stem.2018.06.015. e6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available on request by email.