Abstract
Background/Objectives:
Evaluations of complex models of care for older adults may benefit from simultaneous assessment of intervention implementation. The STRIDE (Strategies To Reduce Injuries and Develop confidence in Elders) pragmatic trial evaluated the effectiveness of a multifactorial intervention to reduce serious fall injuries in older adults. We conducted multi-level stakeholder interviews to identify barriers to STRIDE intervention implementation and understand efforts taken to mitigate these barriers.
Design:
Qualitative interviews with key informants
Setting:
10 clinical trial sites affiliated with practices that provided primary care for persons at increased risk for fall injuries
Participants:
Specially trained registered nurses working as Falls Care Managers who delivered the intervention (n=13 individual interviews), Research Staff who supervised trial implementation locally (n=10 group interviews, 23 included individuals), and members of Central Project Management and the National Patient Stakeholder Council who oversaw national implementation (n=2 group interviews, 6 included individuals).
Measurements:
A semi-structured interview guide derived from the Consolidated Framework for Implementation Research (CFIR)
Results:
We identified 8 key barriers to STRIDE intervention implementation. Falls Care Managers navigated complex relationships with patients and families while working with Research Staff to implement the intervention in primary care practices with limited clinical space, variable provider buy-in, and significant primary care practice staff and provider turnover. The costs of the intervention to individual patients and medical practices amplified these barriers. Efforts to mitigate these barriers varied depending on the needs and opportunities of each primary care setting.
Conclusion:
The many barriers to implementation and the variability in how stakeholders addressed these locally may have affected the overall STRIDE intervention’s effectiveness.
Keywords: fall prevention, pragmatic trial, implementation science, primary care
Future pragmatic trials should incorporate simultaneous implementation aims to better understand how research interventions translate into clinical care that improves the lives of older adults.
Introduction:
Older adults with complicated care needs (e.g., those with multiple chronic conditions, functional impairment, or dementia) may benefit from comprehensive, team-based interventions that can be tailored to each individual’s personal goals and preferences.1,2 Because of the nature of the needs being addressed, such individualized interventions are often complex. Even if found effective in a research setting, these interventions may not translate into meaningful patient outcomes due to behavioral, organizational, payment, or other constraints that lead to subsequent failures in broader implementation.3–6
The field of implementation science examines processes that facilitate the adoption and integration of evidence-based practices and interventions into healthcare and public health settings.7,8 Implementation issues are particularly germane to the field of geriatric medicine not only because of the complexity of care for many older adults, but also because existing evidence-based interventions may require adaptation to meet the heterogeneous needs of an older population, who receive care in a wide variety of settings.9,10 As a result, evaluation of complex interventions in older adults can benefit from an effectiveness-implementation hybrid design, which simultaneously assesses clinical effectiveness and the mechanics of intervention implementation in the healthcare setting.11
The STRIDE (Strategies To Reduce Injuries and Develop confidence in Elders) trial is an example of such a complex intervention. This pragmatic, cluster-randomized trial was funded by the Patient-Centered Outcomes Research Institute and the National Institute on Aging and was conducted in 86 diverse primary care practices across 10 clinical trial sites. The STRIDE trial evaluated whether an evidence-based12–14, multifactorial intervention could prevent serious fall injuries among community-dwelling adults 70 years of age or older who were at increased risk for fall injuries.
Described elsewhere in detail,15,16 the intervention was delivered by specially trained registered nurses (Falls Care Managers or FCMs) embedded in primary care practices. The five components of the intervention were: (1) assessment of seven modifiable risk factors for fall injuries; (2) protocol-driven recommendations for risk factor management that were communicated to participants via motivational interviewing; (3) development of an individualized fall-risk reduction care plan that was approved by the primary care providers; (4) implementation of the care plan (including referrals to community-based programs and providers); and (5) telephonic and in-person follow-up assessment, evaluation and care. Participants’ risk factors for fall injuries were reassessed annually and the care plan was revised as needed.
To help maintain consistency in the implementation of the intervention and adherence to the protocols, a number of strategies were put in place. These included FCM training with a series of online modules and an in-person meeting at the study initiation, two in-person FCM training meetings during the study, chart reviews and site visits by those supervising the trial nationally to ensure fidelity to the intervention protocol, and conference call meetings at least bi-weekly with all FCMs as well as local and national research staff. Local stakeholder committees also met regularly with study staff to discuss ongoing implementation challenges. Given the variation in the resources and other characteristics of the clinical trial sites, flexibility was allowed in the decisions about how to adapt the intervention to address implementation barriers at the local level. While the STRIDE intervention did not significantly reduce the rate of adjudicated serious fall injuries (8% reduction), it was associated with a significant 10% reduction in self-reported fall injuries as compared to enhanced usual care.17
In this paper, we report the results of a qualitative implementation study examining stakeholder perspectives about barriers to implementation of the STRIDE intervention and strategies employed to mitigate those barriers. Together with the previously-published STRIDE trial effectiveness results,17 these reports constitute a hybrid type 1 effectiveness-implementation study (i.e., gathering information on implementation during an effectiveness trial).11 Our study provides important insights that inform interpretation of the STRIDE trial’s effectiveness results and illustrate the importance of considering implementation within studies of complex interventions targeting older adults.
Methods:
Design and Data Collection:
Our study was guided by the Consolidated Framework for Implementation Research (CFIR). The CFIR has been widely used to evaluate the implementation of complex, interacting, and multi-level processes.18 The CFIR describes five key domains relevant to implementation: (1) Intervention Characteristics (i.e., features of the health care intervention itself), (2) Outer Setting (i.e., the broader economic, political, and social context), (3) Inner Setting (i.e., the specific structural, political, and cultural context where implementation happens), (4) Characteristics of Individuals (including individuals who receive, deliver, or facilitate the intervention), and (5) Process (i.e., how implementation of the intervention actively unfolds over time). Each domain has a set of constructs that reflect evidence-based factors associated with implementation across different contexts. The five domains and 39 related constructs are described in Supplemental Table S1.
We conducted 30 to 45-minute long, semi-structured interviews with key stakeholders involved in the implementation of STRIDE between December 2018 and May 2019 (final FCM intervention visits occurred in November 2018). Interview guides were designed to ask about each of the CFIR domains and the majority of CFIR constructs. Guides were similar for all stakeholders with prompts tailored to each stakeholder’s role. Investigators JR, PG, and FK pilot tested the guide and refined it following review of initial interview transcripts using an iterative process. All interviews were conducted by a research coordinator with both MD and MPH degrees who received training and coaching on qualitative interviewing from the investigators (JR and PG). All interviews were audio-recorded and professionally transcribed. The study protocol was approved by the Partner’s Human Research Committee/ IRB and study participants provided verbal informed consent prior to participation.
Participants:
Using purposeful sampling, we identified stakeholders with perspectives on STRIDE intervention implementation at the individual, clinical trial site, and national levels. First, all specially trained nurses working as FCMs (n=14) responsible for the direct delivery of the STRIDE intervention at each primary care practice were approached for interviews. Given both resource limitations and significant turnover among primary care practice staff and providers during the 5 years of the STRIDE trial, we were unable to systematically assess the perspectives of other individuals involved in intervention implementation (e.g., clinicians, primary care practice administrators, older adults, families). Second, the principal investigator at each of the 10 STRIDE clinical trial sites was asked to assemble a team of two to three research staff (e.g., investigator, site clinical director, site coordinator) to participate in a group interview. Finally, the STRIDE Central Project Management (which oversaw the coordination and implementation of STRIDE trial at all sites) and National Patient Stakeholder Council (which consulted both locally and nationally to facilitate patient and stakeholder engagement) were each asked to identify two to three members to participate in separate group interviews.
Analysis:
After review of the initial transcribed interviews, JR, PG, and FK developed and iteratively refined a codebook, a list of defined themes (i.e., “codes”) that were discussed in the interviews. While the codebook placed emphasis on deductive or a priori codes corresponding to the defined CFIR domains and constructs, we also used inductive approaches to create codes that described new themes not adequately captured in the CFIR framework. The refined codebook was applied to all transcripts using NVivo (version 11; QRS International). The initial transcripts were coded by a research assistant and at least one investigator (JR, PG, and/or FK) to ensure fidelity to the codebook and disagreements in coding were resolved by consensus. The remaining transcripts were coded by a research assistant, with random transcripts spot-checked by an additional researcher (JR and PG) to confirm coding accuracy. Coded data were then analyzed and summarized by the full research team, who identified the most commonly described CFIR constructs. For each predominant construct, the team identified corresponding implementation barriers and illustrative examples describing the experiences of participants as they worked to mitigate these barriers.
Results:
Table 1 presents key characteristics of stakeholders. One eligible FCM did not participate in an interview. The group interviews with the research study staff at each site most often included the site principal investigator and the site coordinator.
Table 1:
Characteristics of Stakeholders
| Practice Level | Clinical Trial Site Level | National Level | ||
|---|---|---|---|---|
| Falls Care Managers | Research Staff | Central Project Management | National Patient Stakeholder Council | |
| Number of Individuals | 13 individuals | 23 individuals | 3 individuals | 3 individuals |
| Number of Interviews | 13 interviews | 10 interviews | 1 interview | 1 interview |
| Age Range, Years | 38–66 | 27–77* | 51–68 | 42–71 |
| Female | 13/13 | 11/20* | 2/3 | 3/3 |
| White non-Hispanic | 7/10** | 16/20* | 3/3 | 3/3 |
| Educational Attainment | Associates: 1 Bachelors: 9 Masters: 3 Doctoral: 0 |
Associates: 0 Bachelors: 7 Masters: 4 Doctoral: 9* |
Associates: 0 Bachelors: 0 Masters: 0 Doctoral: 3 |
Associates: 1 Bachelors: 1 Masters: 0 Doctoral: 1 |
3 participants responses missing on this item
4 participants responses missing on this item
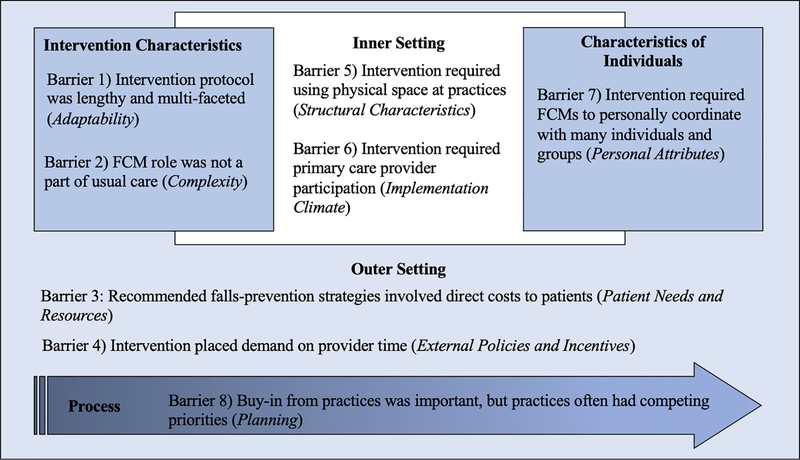
We identified 8 key barriers to the implementation of the STRIDE intervention. Figure 1 illustrates how each of the barriers is related to the CFIR domains and constructs: (1) Intervention protocol was lengthy and multi-faceted (Intervention Characteristics domain, Adaptability construct), (2) FCM role was not a part of usual care in the primary care practice sites (Intervention Characteristics domain, Complexity construct), (3) Recommended falls-prevention strategies involved direct costs to patients (Outer Setting domain, Patient Needs and Resources construct), (4) Intervention placed demand on provider time (Outer Setting domain, External Policies and Incentives construct), (5) Intervention required using physical spaces at practices (Inner Setting domain, Structural Characteristics construct), (6) Intervention required primary care provider participation (Inner Setting domain, Implementation Climate construct), (7) Intervention required FCMs to personally coordinate with many individuals and groups (Characteristics of Individuals domain, Personal Attributes construct), and (8) Buy-in from practices was important but practices often had competing priorities (Process domain, Planning construct). As acknowledged by those who developed the CFIR framework, boundaries between domains often blur and barriers may at times reflect multiple domains. For example, the intervention placed demands on the time of providers in primary care practices, affecting both productivity in the larger fee-for-service medical reimbursement environment (Outer Setting domain, External Policies and Incentives construct,) as well as provider receptivity to having their patients participate in the intervention (Inner Setting domain, Implementation Climate construct).
Figure 1: Key Barriers Affecting STRIDE Implementation and Their Relationship with the Domains and Constructs of the Consolidated Framework for Implementation Research (CFIR) Framework.
Footnotes:
Bold text denotes CFIR domains
Italicized text donates CFIR constructs
In general, implementation barriers were described by multiple stakeholder groups and representatives of multiple clinical trial sites. For example, FCMs, Research Staff, and Central Project Management representatives from nearly all clinical trial sites discussed that finding space for FCMs to work within the primary care practices was an ongoing challenge and available space directly impacted STRIDE intervention implementation. Yet despite the common barriers, experiences of stakeholders working to mitigate barriers varied substantially across clinical sites. Furthermore, even stakeholders from a single clinical trial site often described very different implementation environments at the individual practices where the STRIDE intervention was implemented. Table 2 highlights this range of experiences. As the included quotations exemplify, barriers that seemed insurmountable to some stakeholders were quite minor for others. For example, the FCM role was not a part of usual care in the primary care practice sites (Barrier 2). Some stakeholders described the challenges of integrating this role into routine practice: “Sometimes people are getting over bombarded with case management and chronic care management and fall care management and I just think that it can confuse the patient.” (FCM 3). Meanwhile, others were able to more seamlessly integrate FCMs in primary care practices by creating workflows that paralleled existing practices: “STRIDE was more integrated into the operation of our site than I would say than any other site just because we [already] had clinic-based screenings going on.” (RS 10).
Table 2:
Range of Experiences Confronting and Mitigating Implementation Barriers
| Implementation Barrier | Experiences Confronting and Mitigating Implementation Barriers |
|---|---|
| 1) Intervention protocol was lengthy and multifaceted: “The protocol was detailed… But it was also flexible enough to be adopted in different settings.” (CPM) |
“The flexibility to do home care for patients who wanted it was big here, and I think it really helped with implementation in what otherwise would’ve been a very challenging winter with all that snow.” (RS 6) |
| “I think the resources were different across sites too. Here … PharmDs have prescriptive authority, and they can bill. They can see patients who have complex medication regimens, and it’s a common practice.” (CPM) | |
| “We identified some cultural challenges with the sequencing… like getting the content of question very early in the script. That was perceived negatively by people… Here in Texas, you don’t start trusting people that early in the conversation before you said, “Howdy!” (NPSC) | |
| 2) FCM role was not a part of usual care in the primary care practice sites: “It was, in a sense, disruptive, and there were some difficulties along the way.” (CPM) |
“Sometimes people are getting over bombarded with case management and chronic care management and fall care management and I just think that it can confuse the patient.” (FCM 3) |
| “STRIDE was more integrated into the operation of our site than I would say than any other site just because we [already] had clinic-based screenings going on.” (RS 10) | |
| “I think it fit okay. Our site has health coaches which are like care managers… so I do feel like… it fits in the workflow.” (FCM 6) | |
| 3) Recommended falls-prevention strategies involved direct costs to patients: “It’s not only expensive for insurance, [it’s] expensive for patients when you look at copays and things like that.” (FCM 8) |
“I did occasionally have patients who were not interested in physical therapy, but more of the problem was that physical therapy, for some people, has a very high copay. So, they wanted it, but couldn’t afford it.” (FCM 4) |
| “[There was] cost for some of the exercise [programs]… Our research assistants have done an excellent job of researching all the available… exercise-type places. And we found some that had free yoga for patients… there was even balance ones they had free too as long as you went. So, that really helped break down that barrier for the patient.” (FCM 9) | |
| 4) Intervention placed demand on provider time: “If you even disrupt minimally [providers] income stream it really can bite them hard.” (RS 8) |
“We used Epic messaging documentation and it was a simple sign-off. It worked fine. Because we have the electronic medical record, I was able to send messages, order the required consults or request a PharmD evaluation or… consults to ophthalmology or optometry and podiatry. It was very easy and it was a co-signed required so just a physician would sign-off on it and it was easy for both of us. (FCM 3) |
| “We found that… a lot of the docs didn’t answer their email in general. They’re put upon. They’re very busy. So, we established an alternative line of communication. (RS 5) | |
| 5) Intervention required using physical space at practices: "There was a tremendous variation [in space availability] across the sites.” (CPM) |
“Some clinics I never got a room. I had to make do in a corner of the waiting room or in a room that was more like an office. I had to make sure I had a laptop with me… I needed my cell phone to act as a mobile hotspot… Then on times where I couldn’t get cell phone reception, I needed to make sure I had a hard copy of my notes so when I was talking to them, I could fill that in.” (FCM 5) |
| “In terms of the issue of scheduling and space… this was not an intervention that could be done in the context of an existing and scheduled visit… [which contributed to] the need to do home visits.” (RS 1) | |
| 6) Intervention required primary care provider participation: “Commitment to the project varied among the providers.” (FCM 10) |
“There were some places that said, “We just don’t believe the literature.” At one point, we needed to actually have a conference call with an osteoporosis expert… to talk to the different clinical trial site directors.” (CPM) |
| “In the beginning… there were some delays with [providers] getting back to their falls care managers… We just collected data about that and then we followed up with the… medical directors in each of the different practices… Once we started giving them feedback that issue resolved very quickly.” (RS 6) | |
| 7) Intervention required FCMs to personally coordinate with many individuals and groups: “The [FCM] was really was an advocate for the patient in making sure that they got the care that they needed. (RS 4) |
“When we started with the pilot… we had an FCM in mind. It’s just that it didn’t work out. It wasn’t the right fit for our team. It wasn’t the right fit for the patients either… But it wasn’t until we found our main FCM... then it was her personality, her commitment to the program… It was like night and day with the other FCMs that were before her.” (RS 1) |
| “I couldn’t get my bearings on which exercise program is right for them, which physician, which different services where I could send them. I never had a firm grasp on that because it was just such a massive demographic there.” (FCM 5) | |
| 8) Buy-in from practices was important, but practices often had competing priorities: “From the beginning, it was really getting the sites engaged.” (RS 5) |
“We had a lot of turnover of [primary care practice] staff… Maybe even every six to eight months there were some sites that you either [had a new] practice manager or medical director… We had to go back and speak again, because they knew nothing about the program.” (RS 1) |
| “Initially, we met with the clinic administrator and the medical director for the site and the faculty, but then we would go back also to the sites over time… and when they would have monthly meetings, we would go and update them on progress or any changes that had occurred.” (RS 2) |
FCM=Falls Care Manager, RS=Research Staff, CPM= Central Project Management, NPSC=National Patient Stakeholder Council
Discussion:
This implementation evaluation provides insights from multiple stakeholders about barriers to implementing the multifactorial STRIDE intervention in primary care practices throughout the country. While the challenges facing intervention implementation were shared by all, experiences working to mitigate these barriers varied by clinical trial and practice site. This variability may have impacted the effectiveness of the STRIDE intervention overall and highlights the importance of considering implementation evaluation during pragmatic trials among older adults.
The number of implementation barriers elucidated in this study are evidence of the complexity of the STRIDE intervention itself. In order to enact treatment plans, the FCMs often had to navigate complex relationships with patients and families. At the same time, they worked side by side with Research Staff to coordinate with individual primary care practices and implement the intervention despite limited clinical space, variable provider buy-in, and frequent primary care practice staff and provider turnover. The costs of the intervention for both the individual patients (e.g., copays, travel costs) and medical practices (e.g., provider time, physical space) may have amplified these barriers.
Many of these barriers reflect the difficulty of changing care processes in primary care practices that are often already overburdened. Our findings of implementation barriers in the primary care setting are similar to those described in other studies.19–22 Policies that support alternatives to fee-for-service reimbursement may allow primary care practices more flexibility in how needed services can be provided (e.g., increased utilization of registered nurses in primary care, increased use of non-face-to-face and asynchronous communication). This could, in turn, improve the effectiveness and sustainability of innovative models of care for complex older adults like STRIDE.
The variable experiences of stakeholders confronting and mitigating barriers suggest that implementation of the STRIDE intervention was likely easier at some sites or practices than others. Primary care practices throughout the country vary greatly in terms of structure, size, and available resources23 and the practices participating in the STRIDE intervention were no exception. While steps were taken throughout the study to ensure that basic elements of the intervention were delivered at each intervention site, this variation may have impacted the STRIDE study’s effects at individual sites and the consistency of the intervention. Implementation scientists have suggested that this “fit” between an intervention and the unique primary care context where it is implemented impacts the intervention’s ultimate success.24 Given this, early assessment of potential practice-specific implementation barriers and proactive modification of intervention workflows to match practice needs may improve future intervention implementation.
A limitation of our study is that it was not designed to individually evaluate implementation at each of the 43 primary care practices randomized to receive the STRIDE intervention. Of note, we did not interview clinicians, administrators, or older adults themselves who could have offered more nuanced perspectives about how the intervention was implemented at their primary care practice. As a result, we cannot say implementation at any given practice site impacted the experience of study participants or STRIDE study outcomes. An additional limitation is that stakeholder reflections on intervention implementation included in this study came at the end of a long trial. Although observations at the end may have reflected the cumulative experience, they may not capture the more nuanced adaptations of the STRIDE protocol that occurred as a result of the STRIDE team’s regular meetings.
In summary, this multi-level implementation evaluation provides unique insights into the barriers encountered during implementation of an individualized, multifactorial interventions to prevent fall injuries at 10 clinical trial sites throughout the country. Although adaptability was an intentional feature of the intervention design, the wide variety of experiences of implementing the intervention in the busy primary care setting may have led to practice-specific differences in intervention success and contributed to a lower than hypothesized treatment effect. In conjunction with results from an ongoing evaluation of patient-level factors that influenced intervention effectiveness, our findings will help identify ways to make future iterations of the STRIDE intervention more effective. In addition, our findings also make clear that future pragmatic trials of complex, evidence-based interventions for older adults should both consider implementation challenges during intervention develop and incorporate formal implementation aims. This is needed to ensure that research interventions translate into clinical care that improves the lives of older adults.
Supplementary Material
Supplemental Table S1: Consolidated Framework for Implementation Research (CFIR) Domains and Constructs*
Key Points:
Key Points
The many barriers to implementation and the variability in how stakeholders addressed these locally may have affected the overall STRIDE intervention’s effectiveness.
Future pragmatic trials should incorporate simultaneous implementation aims to better understand how research interventions translate into clinical care that improves the lives of older adults.
Why does this paper matter?
Our finding of variability in how the multicomponent STRIDE intervention was implemented in the busy primary care setting underscores the importance of considering potential implementation challenges when developing and evaluating interventions that target high-risk older adults.
Acknowledgements:
The authors would like to thank Elisse Catalan for conducting study interviews and Gabrielle Schiller and Yuran Cai for assisting with transcript coding.
Funding Sources:
NIA (P30AG028741 to AS; K08AG050808 to FK; K23AG066930 to JR)
PCORI (U01AG048270 to DR)
Sponsor’s Role:
The sponsors had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of the paper.
Footnotes
Conflict of Interest:
The authors have no conflicts.
References:
- 1.Boult C, Green AF, Boult LB, Pacala JT, Snyder C, Leff B. Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine’s “retooling for an aging America” report. J Am Geriatr Soc 2009;57:2328–37. [DOI] [PubMed] [Google Scholar]
- 2.Guiding principles for the care of older adults with multimorbidity: an approach for c. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc 2012;60:E1–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine (U.S.). Committee on the Future Health Care Workforce for Older Americans. Retooling for an aging America : building the health care workforce. Washington, D.C.: National Academies Press; 2008. [PubMed] [Google Scholar]
- 4.Chodosh J, Weiner M. Implementing Models of Geriatric Care-Behind the Scenes. J Am Geriatr Soc 2018;66:364–6. [DOI] [PubMed] [Google Scholar]
- 5.Burnes B Emergent change and planned change – competitors or allies? International Journal of Operations & Production Management 2004;24:886–902. [Google Scholar]
- 6.Gitlin LN, Baier RR, Jutkowitz E, et al. Dissemination and Implementation of Evidence-Based Dementia Care Using Embedded Pragmatic Trials. J Am Geriatr Soc 2020;68 Suppl 2:S28–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National Institutes of Health approaches to dissemination and implementation science: current and future directions. Am J Public Health 2012;102:1274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wensing M, Grol R. Knowledge translation in health: how implementation science could contribute more. BMC Med 2019;17:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers DA, Norton WE. The Adaptome: Advancing the Science of Intervention Adaptation. Am J Prev Med 2016;51:S124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrera AP, Snipes SA, King DW, Torres-Vigil I, Goldberg DS, Weinberg AD. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health 2010;100 Suppl 1:S105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guirguis-Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL. Interventions to Prevent Falls in Older Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018;319:1705–16. [DOI] [PubMed] [Google Scholar]
- 13.Panel on Prevention of Falls in Older Persons AGS, British Geriatrics S. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc 2011;59:148–57. [DOI] [PubMed] [Google Scholar]
- 14.Ganz DA, Latham NK. Prevention of Falls in Community-Dwelling Older Adults. N Engl J Med 2020;382:734–43. [DOI] [PubMed] [Google Scholar]
- 15.Bhasin S, Gill TM, Reuben DB, et al. Strategies to Reduce Injuries and Develop Confidence in Elders (STRIDE): A Cluster-Randomized Pragmatic Trial of a Multifactorial Fall Injury Prevention Strategy: Design and Methods. J Gerontol A Biol Sci Med Sci 2018;73:1053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill TM, McGloin JM, Latham NK, et al. Screening, Recruitment, and Baseline Characteristics for the Strategies to Reduce Injuries and Develop Confidence in Elders (STRIDE) Study. J Gerontol A Biol Sci Med Sci 2018;73:1495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhasin S, Gill TM, Reuben DB, et al. A Randomized Trial of a Multifactorial Strategy to Prevent Serious Fall Injuries. N Engl J Med 2020;383:129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation Science 2009;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau R, Stevenson F, Ong BN, et al. Achieving change in primary care--effectiveness of strategies for improving implementation of complex interventions: systematic review of reviews. BMJ Open 2015;5:e009993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryce C, Fleming J, Reeve J. Implementing change in primary care practice: lessons from a mixed-methods evaluation of a frailty initiative. BJGP Open 2018;2:bjgpopen18X101421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callahan CM, Boustani MA, Weiner M, et al. Implementing dementia care models in primary care settings: The Aging Brain Care Medical Home. Aging Ment Health 2011;15:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritchie C, Andersen R, Eng J, et al. Implementation of an Interdisciplinary, Team-Based Complex Care Support Health Care Model at an Academic Medical Center: Impact on Health Care Utilization and Quality of Life. PLoS One 2016;11:e0148096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine DM, Linder JA, Landon BE. Characteristics and Disparities among Primary Care Practices in the United States. J Gen Intern Med 2018;33:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau R, Stevenson F, Ong BN, et al. Achieving change in primary care--causes of the evidence to practice gap: systematic reviews of reviews. Implement Sci 2016;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: Consolidated Framework for Implementation Research (CFIR) Domains and Constructs*