Abstract
Background:
Collaborative Chronic Care Models represent an evidence-based way to structure care for chronic conditions, including mental health conditions. Few studies, however, have examined the cost implications of collaborative care for mental health.
Objective:
We aimed to conduct an economic analysis of implementing collaborative care in 9 outpatient general mental health clinics.
Research Design:
Analyses were derived from a stepped wedge hybrid implementation-effectiveness trial. We conducted cost-minimization analyses from the health system perspective, incorporating implementation costs, outpatient costs, and inpatient costs for the year before collaborative care implementation and the implementation year. We used a difference-in-differences approach and conducted 1-way sensitivity analyses to determine the robustness of results to variations ± 15% in model parameters, along with probabilistic sensitivity analysis using Monte Carlo simulation.
Subjects:
Our treatment group included 5507 patients who were initially engaged in care within 9 outpatient general mental health teams that underwent collaborative care implementation. We compared costs for this group to 45,981 control patients who received mental health treatment as usual at the same medical centers.
Results:
Collaborative care implementation cost about $40 per patient and was associated with a significant decrease in inpatient costs and a nonsignificant increase in outpatient mental health costs. This implementation was associated with $78 in cost savings per patient. Monte Carlo simulation suggested that implementation was cost saving in 78% of iterations.
Conclusions:
Collaborative care implementation for mental health teams was associated with significant reductions in mental health hospitalizations, leading to substantial cost savings of about $1.70 for every dollar spent for implementation.
Keywords: collaborative care, Collaborative Chronic Care Model, implementation, mental health, cost-minimization analysis
The Collaborative Chronic Care Model (CCM) represents an evidence-based way to structure health care for chronic conditions.1–3 It consists of up to 6 elements, including work role redesign to support more coordinated care; patient self-management support; clinician decision support; clinical information systems to allow comprehensive data tracking; linkages to community resources; and leadership support.4 These elements are meant to be applied flexibly to align with the priorities and resources of the clinical setting in question.5 While the initial evidence base for CCM-based care focused on chronic medical conditions,4,6 recent work has demonstrated its effectiveness for chronic mental health conditions as well.5,7 That is, CCM-based care appears to deliver better clinical outcomes, on average, than care that is not aligned with the CCM elements.
Randomized trial results suggest that CCM-based mental health care is cost neutral or cost saving.5,8 These trials typically benefit, however, from exogenous research funding to establish CCM-based care (including paying for care coordination staff or infrastructure) that would not be available to real-world clinics attempting to implement CCM-based care. Few existing mental health CCM implementation trials9–15 have accounted for implementation costs themselves16–18 (ie, the costs of getting CCM-based care “up and running”). Other studies make it unclear whether implementation costs were comprehensively collected.19–21 This is problematic because implementation costs are an important consideration for mental health leaders who are deciding whether to deploy limited resources toward CCM-based care.22–25 The logical next step for the field is therefore to determine whether CCM-based care remains cost saving or cost neutral when implementation costs are accounted for.
Thus, in this manuscript, we present economic analyses from the health system perspective for a recent implementation trial26–28 conducted in 9 US Department of Veterans Affairs (VA) outpatient general mental health clinics.13,29 Clinical results from that trial indicated that CCM implementation was associated with lower rates of acute mental health hospitalization, while improvements in mental health–related quality of life for the CCM-treated population were not statistically significant except among patients with 3 or more mental health diagnoses.13 That trial did not determine whether the potential cost savings from reduced hospitalizations counterbalanced the costs of CCM implementation. Our objectives for this manuscript were therefore to quantify implementation costs and differential utilization of health services between patients treated by CCM-enhanced mental health teams and comparable non-CCM patients at the same medical centers. By accounting for implementation costs—something that previous cost analyses of CCM-based care have rarely done—our findings have implications for mental health systems considering CCM implementation.
METHODS
Overview
This combined program evaluation and research project was reviewed by the VA Central Institutional Review Board (IRB). Implementation-related measures were exempt from IRB review, whereas Veteran-level outcome measurement procedures were approved as research. Study procedures took place from 2016 to 2018 and are described briefly below; details of the design and clinical outcomes have been described previously.13,29
We chose cost-minimization analysis (CMA) as our primary analytic approach, quantifying implementation costs, and service use outcomes in dollar values.30 One assumption of CMA is that clinical outcomes between the 2 study arms (in this case, CCM implementation and treatment as usual) are equivalent. Thus, the primary goal of CMA is to determine which of the 2 treatment arms delivers those (equivalent) clinical outcomes at a lower cost. In this case, we were unable to directly compare clinical outcomes between patients in the CCM implementation and treatment as usual arms. However, previous analyses from this trial suggested that mental health–related quality of life did not change substantially for the overall CCM-treated population,13 leading us to tentatively accept the equivalence of clinical outcomes for the 2 arms of the study.
Study Setting
We conducted this study in 9 VA outpatient general mental health teams, known as Behavioral Health Interdisciplinary Program (BHIP) teams, in partnership with the VA Office of Mental Health and Suicide Prevention. These teams typically consist of 5–8 interdisciplinary staff and provide treatment for a variety of mood, anxiety, and psychotic disorders to a panel of about 1000 Veterans. BHIP clinicians may also treat substance misuse or posttraumatic stress disorder, although specialized services for these conditions are frequently provided in separate clinics. To recruit sites, VA Office of Mental Health and Suicide Prevention distributed information about the project to all BHIP teams nationally through network and facility mental health leaders. Twelve sites expressed interest, of whom 9 were randomized; one of these 9 dropped out before the start of CCM implementation and was replaced by the 10th site.13 Enrolled BHIP teams were located both at VA Medical Centers (n = 5) and smaller Community-Based Outpatient Clinics (n = 4) that are themselves affiliated with a VA Medical Center.
Implementation Support
CCM-based care was implemented via 1 year of implementation facilitation,31 featuring a study-funded external facilitator providing expertise in CCM-based quality improvement, and a facility-funded internal facilitator providing on-site implementation support. Three study authors (C.J.M., B.K., and M.S.B.) served as external facilitators to 3 sites each. We used a structured workbook, the BHIP-CCM Enhancement Guide, to help each BHIP team align their clinical processes with the 6 CCM elements. Facilitation activities for each participating BHIP team included a comprehensive initial assessment, a 1.5-day site visit, weekly conference calls between the internal and external facilitators, and weekly process redesign meetings with the BHIP team and both facilitators. These activities were supplemented by ad hoc phone and e-mail contact between the facilitators for each site.
Trial Design
Implementation support was provided in a stepped wedge,27,28 with all 9 sites receiving CCM implementation support in a staggered fashion over 3 waves; each wave occurred about 4 months apart. We used a balancing algorithm to assign sites to waves while retaining the benefits of randomization.32 Sites assigned to later waves received a copy of the BHIP-CCM Enhancement Guide during the first wave and were invited to attend monthly technical support conference calls while awaiting facilitation.
Population and Sample
For the purposes of conducting CMA, we had 4 populations of interest at each site: the external facilitator, the internal facilitator, BHIP team members, and patients. The external facilitator for each site was study-funded; external facilitators included 1 PhD-level clinical psychologist, 1 nonclinical PhD systems engineer, and 1 MD-level psychiatrist. The internal facilitator was identified and funded by the medical center (typically with a background in mental health or systems redesign; common training backgrounds included social work, clinical psychology, and nursing). Our patient sample consisted of Veterans who were actively engaged in treatment with each participating BHIP, defined as having at least 2 BHIP visits in the year before the start of facilitation at that site, including at least 1 visit in the previous 3 months. We excluded patients with dementia. For comparison, we utilized data from patients treated by other staff at the same outpatient general mental health clinics during the same time frame. The clinician members of the BHIP teams were funded by the local facilities.
Measures
External Facilitator Cost Estimation
We used time-motion tracking to assess external facilitation time,33–35 including meetings, preparation, e-mail, and phone contact. The external facilitator logged all facilitation hours during the initial assessment and site visit, as well as for discrete 2-week periods during the early, middle, and late phases of the facilitation year. On the basis of these results, we estimated external facilitator time to be 2.6 hours per week per site. Combined with salary data and an estimated 30% fringe rate, this allowed us to estimate facilitation costs for each external facilitator. We liberally estimated $1500 per site for external facilitators’ travel costs associated with the 1.5-day face-to-face site visit.
Internal Facilitator Cost Estimation
We estimated that internal facilitators spent an average of 4 hours/week on CCM implementation for each BHIP, based on pilot work using similar procedures.36 To minimize the data collection burden for the internal facilitators, we did not ask them to engage in time-motion tracking. We obtained direct salary information and, as with external facilitators, estimated a 30% fringe rate. In cases where we were unable to obtain direct salary information for internal facilitators, we estimated salary data from the VA Office of Personnel Management (OPM) General Schedule37 based on the facilitator’s disciplinary background, geographic location, title, and years spent working at VA.
Patient-level Cost Estimation
We obtained outpatient and inpatient visit data from the VA Corporate Data Warehouse.38 Specifically, we obtained data regarding the number and type of outpatient visits, and days spent on an inpatient unit, for all Veterans deemed actively engaged in care with the participating BHIPs as described above. We collected these data for all recorded VA-based outpatient visits and inpatient stays (including mental and physical health; a complete list of codes available upon request). For comparison, we obtained similar data for Veterans treated by other clinicians at the same outpatient general mental health clinics. We collected these data for the year before the start of facilitation at each site, as well as the facilitation year (ie, the year following the start of facilitation). Costs were obtained from the VA Health Economics Resource Center Average Cost Databases, which contain patient-level cost estimates for each outpatient visit and inpatient stay based on Medicare reimbursement rates for the current procedural terminology codes associated with the clinical encounter.39
Behavioral Health Interdisciplinary Program Team-level Cost Estimation
As described in more detail below, our primary comparison was between the BHIP teams that underwent CCM implementation and other clinicians at the same outpatient general mental health clinics (treatment as usual). Typically, the weekly process redesign meetings by which CCM implementation occurred took place during existing team meeting times, so we did not account for clinician time spent in these meetings in our primary analyses (as CCM implementation did not involve asking BHIP clinicians to work more hours than other clinicians at their medical centers during the facilitation year). However, participating BHIP clinicians spent 4 hours in implementation-related activities during the initial assessment and site visit, which involved blocking their clinics. We liberally estimated $2000 in salary and fringe for each BHIP team engaged in CCM enhancement to account for this time.
Analytic Plan
Our analysis proceeded in 4 steps. First, we tabulated mean health service utilization (inpatient days, outpatient visits) and BHIP costs (external facilitation costs, internal facilitation costs, outpatient spending, inpatient spending). We stratified utilization and cost estimates for mental health and medical/surgical services for both the CCM-enhanced and non–CCM-enhanced teams for the year preceding facilitation.
We used t tests to compare differences between treatment arms in the preintervention period. Then, we estimated simple difference-in-differences (DID) models, interacting binary indicators for the facilitation year and CCM implementation to assess changes in utilization and costs associated with CCM enhancement. Our unit of analysis was the patient-year.
In sensitivity analyses, we tested the effects of variations in our major cost drivers. We first conducted 1-way sensitivity analyses to check the robustness of our results to plausible changes in internal and external facilitation costs (both ± 15%). Similarly, we used 95% DID confidence intervals (CIs) to include plausible changes in outpatient and inpatient spending. Last, we conducted a probabilistic sensitivity analysis using Monte Carlo simulation to estimate overall model uncertainty, simultaneously conducting 10,000 random draws from probability distributions for each variable (uniform for facilitation costs, normal for health spending) and recalculating CCM implementation costs for each iteration within the model. We a priori determined that all mental health service utilization costs would be included in cost simulations, as they could plausibly be impacted by CCM implementation. In contrast, we planned to only include medical/surgical costs in cost simulations if they differed statistically between treatment arms.
RESULTS
A total of 5507 patients were being treated by the 9 participating BHIP teams when CCM implementation commenced. They were included in the economic analysis, as were 45,891 patients in treatment as usual. Demographic characteristics of this sample have been published previously13: briefly, 15.7% of the Veterans treated by CCM-enhanced teams were female, and the average age was 52.2 ± 14.5 years. Slightly less than half (46.8%) were married; common mental health diagnoses included depressive or anxiety disorders (58.6%), posttraumatic stress disorder (49.9%), and serious mental illnesses such as bipolar spectrum disorders or schizophrenia (34.4%). Patients treated by the CCM-enhanced teams and treatment, as usual, were generally similar in terms of observed characteristics. In the year before the study, however, patients treated by the CCM-enhanced teams had, on average, experienced more inpatient days (0.55 vs. 0.43 d, P = 0.023) for mental health and had had higher outpatient ($4041 vs. $3623, P ≤ 0.001) and inpatient spending ($783 vs. $613, P = 0.028). There were no significant differences in the average number of prior-year outpatient visits between CCM and treatment as usual (16.7 vs. 17.1 visits, P = 0.299).
Table 1 presents the major cost drivers and DID estimates of cost changes associated with CCM enhancement. On the basis of the time-tracking and human resources records described earlier, we estimate $114,743 was spent on internal facilitators and $105,621 was spent on external facilitators across all 9 study sites (per-site implementation cost of $24,485 and per-patient implementation cost of about $40). Inclusion of site visit costs raised the average per-site implementation cost to $27,985. On average, patients in the teams receiving CCM implementation cost the VA $4165 in mental health expenditures each during the facilitation year; $3634 due to outpatient spending and $531 due to inpatient spending. The DID results suggest CCM enhancement was associated with fewer inpatient days (−0.13, 95% CI: −0.23 to −0.02) but more outpatient visits (+1.1, 95% CI: 0.37–1.82) for mental health during the facilitation year. Our analyses showed no significant differences for changes in medical/surgical spending between treatment groups (+$38, 95% CI: −$356 to $433). A priori, we decided to exclude these costs from our later cost analyses if changes in utilization and costs were nonsignificant. VA spending on behavioral health represents a small fraction of total VA spending, and thus random fluctuations in medical/surgical spending has the potential to substantially bias our results. Thus, these expenditures were excluded from our cost simulations.
TABLE 1.
Changes in Mental Health Treatment Costs Associated With CCM Implementation
| Cost Drivers | Postperiod Costs | Changes in Costs | |||
|---|---|---|---|---|---|
| All CCM-enhanced Sites* | Per Patient† | Per Patient‡ | P | Overall§ | |
| Labor costs ($) | |||||
| Internal facilitator | 114,743 | 21 | 21 | — | 114,743 |
| External facilitator | 105,621 | 19 | 19 | — | 105,621 |
| Outpatient mental health costs ($) | 20,014,100 | 3634 | 50 | 0.455 | 273,257 |
| Inpatient mental health costs ($) | 2,926,957 | 531 | (167) | 0.031 | (921,211) |
| Total ($): | (78) | — | (427,590) | ||
| Savings (%)∥: | −1.86 | — | −1.86 | ||
Numbers in parentheses indicate savings, while other numbers indicate costs.
Total costs for mental health outpatient visits, inpatient stays, and personnel for CCM-enhanced teams.
Total costs divided by the number of patients served by CCM-enhanced teams (N = 5507) to calculate per-patient costs.
DID estimates for the pre/post changes in costs associated with CCM implementation.
DID estimates multiplied by the total number of patients in CCM-enhanced teams.
Percent savings calculated as the estimated changes in cost associated with CCM implementation divided by total postperiod costs among patients treated by CCM-enhanced teams.
CCM indicates Collaborative Chronic Care Model; DID, difference-in-difference.
Regarding spending for mental health services, CCM implementation was associated with a significant decrease in inpatient (−$167, 95% CI: −$319 to −$15) and a nonsignificant increase in outpatient (+$50, 95% CI: −$80 to +$180) costs. Overall, CCM enhancement was associated with a $118 reduction in mental health spending per patient (95% CI: −$329 to $94), and a $78 reduction after the inclusion of implementation costs. This corresponds to about $1.70 saved for every dollar spent on CCM implementation in this sample, such that the average team investing $27,985 in CCM implementation saved about $47,500 during the subsequent year.
Results of 1-way sensitivity analyses are presented in Table 2. Results were robust to changes in implementation costs; increasing the costs of internal and external facilitators by 15% would only decrease the cost savings of BHIP by ~$3 per patient. However, results were sensitive to variations in mental health spending: if we assumed mental health expenditures were at the upper bound of the 95% CI associated with CCM enhancement (ie, $180 per patient-year), then implementation now costs $53 per patient. If CCM enhancement is further assumed to lead to only minor decreases in inpatient mental health spending, it would then cost $74 per patient.
TABLE 2.
One-way Sensitivity Analyses for Cost Changes Associated With CCM Enhancement
| Cost Parameters | Cost Ranges*($) | Sensitivity Analysis Results† ($) | ||||
|---|---|---|---|---|---|---|
| Lower Bound | Base Case | Upper Bound | Lower Bound | Base Case | Upper Bound | |
| Labor costs | ||||||
| Internal facilitator | 17.71 | 20.84 | 23.96 | (80.77) | (77.64) | (74.52) |
| External facilitator | 16.30 | 19.18 | 22.06 | (80.52) | (77.64) | (74.76) |
| Changes in outpatient mental health costs | (80.69) | 49.62 | 179.94 | (207.95) | (77.64) | 52.68 |
| Changes in inpatient mental health costs | (319.34) | (167.28) | (15.23) | (229.70) | (77.64) | 74.41 |
Numbers in parentheses indicate savings, while other numbers indicate costs.
Labor costs were varied ± 15% and divided by the number of patients in the CCM-enhanced teams (N = 5507). The bounds for per-patient outpatient and inpatient mental health costs represents the 95% confidence interval for estimated changes in these costs.
Estimated average per-patient changes in treatment costs associated with CCM implementation.
CCM indicates Collaborative Chronic Care Model.
Figure 1 presents results from the probabilistic sensitivity analyses using Monte Carlo simulation. Overall, CCM implementation was found to be cost saving in 78% of simulations. We further categorized these simulations into 3 groups. We found CCM implementation to be significantly cost saving (cost savings > 2% compared with usual care) in 52% of iterations; cost neutral (absolute cost difference <2% compared with usual care) in a further 42% of iterations; and costlier (cost difference > 2% compared with usual care) in 6% of iterations.
FIGURE 1.
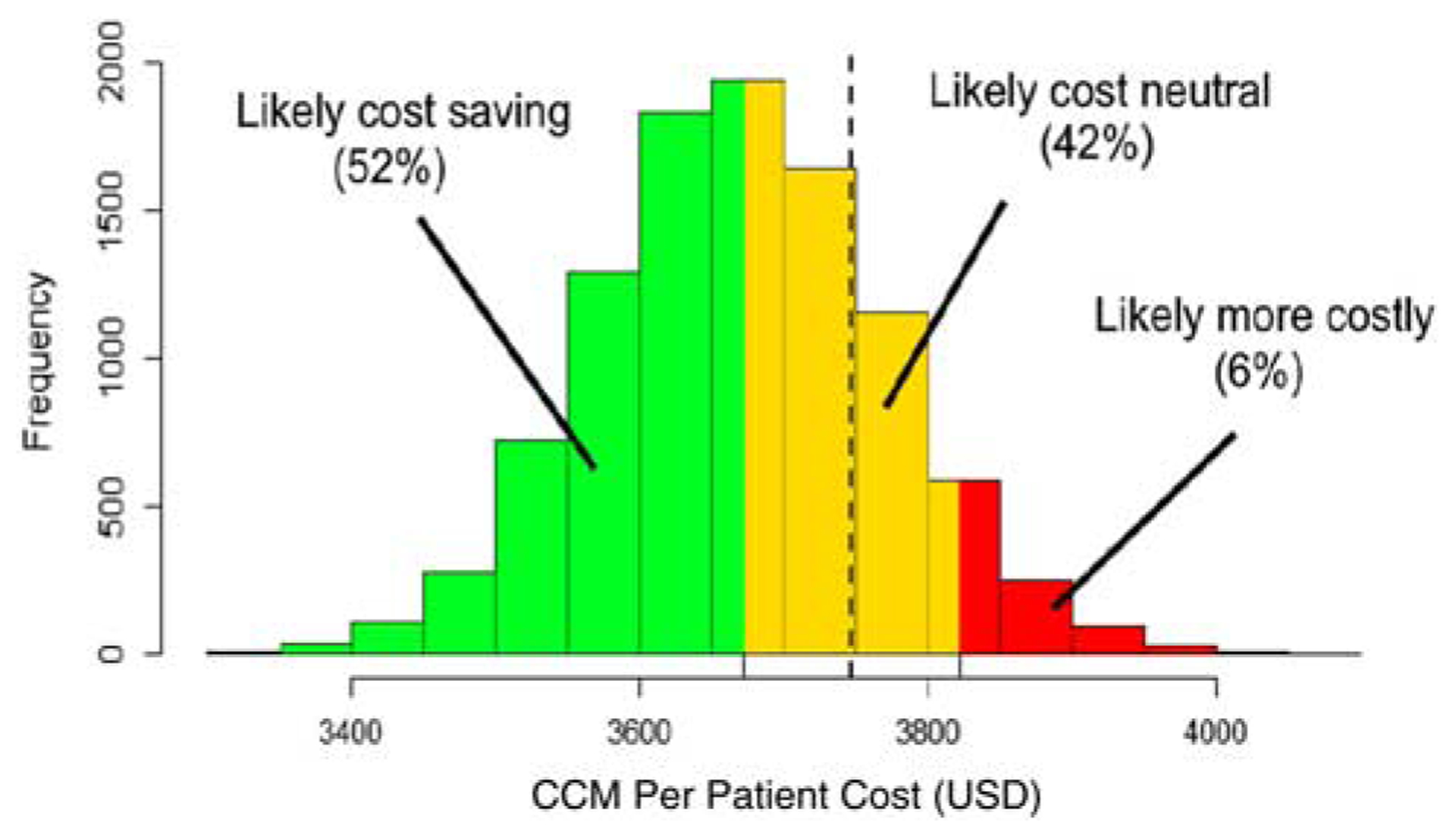
Cost histogram of simulation results for Collaborative Chronic Care Model (CCM) implementation. The figure displays the probabilistic sensitivity analysis results for the per-patient costs of CCM implementation. We used Monte Carlo simulation (n = 10,000 replications) to model the sensitivity of our results to changes in various cost drivers. The dashed vertical line represents the average per-patient costs for care as usual ($3747).
DISCUSSION
Major Findings in Context
The CCM is effective for mental health conditions,7 and randomized trials have found it to be cost-effective to cost neutral.5 However, many earlier economic analyses were conducted in the context of a randomized controlled trial, typically with relatively small sample sizes, and with short (< 6 mo) follow-up periods for cost outcomes, which may have been insufficient to observe differences in service utilization.40 We are also aware of a few studies to date that have accounted for the initial resources needed to implement CCM-based care in real-world clinical settings.16–18 We, therefore, conducted CMA using data from our recent stepped wedge trial conducted in 9 outpatient mental health clinics.13,29 We found that using implementation facilitation31 to align outpatient mental health teams’ clinical processes with the CCM elements cost an average of $27,985 (including site visit costs). Our base case analysis suggested that each dollar spent on CCM implementation was associated with savings of about $1.70 during the implementation year, such that this up-front investment in implementation was associated with savings of about $47,500 per site during the subsequent year. This savings came primarily from significantly decreased acute mental health hospitalization costs for patients treated by CCM-enhanced teams that more than counterbalanced implementation costs and a nonsignificant increase in outpatient mental health service costs. Monte Carlo simulation—incorporating variations in implementation costs, inpatient mental health costs, and outpatient mental health costs—found CCM implementation to be significantly cost saving in 52% of iterations, and cost neutral in an additional 42% of iterations. This study, therefore, strengthens the business case for CCM-based care, demonstrating that the cost savings (or, at worst, cost neutrality) associated with the CCM still hold after consideration of up-front implementation costs.
Limitations
These findings should be considered in the context of several limitations. First, while we used a balancing algorithm to assign sites to waves of CCM implementation that maintained some benefits of randomization in our stepped wedge,32 our control group for these analyses consisted of patients treated in other outpatient mental health clinics at the same medical centers. It is, therefore, possible that the reduced rate of acute mental health hospitalization achieved in our CCM implementation sites was due to unmeasured confounders (eg, differential rates of homelessness) instead of CCM implementation itself. Initial analyses, however, suggested that CCM and control patients had similar patterns of service utilization during the preimplementation year. Second, because control patients were drawn from the same sites as CCM patients, it is possible that CCM implementation led to contamination (ie, that other clinicians at the same medical centers, in interacting with their colleagues who were incorporating the CCM elements into their clinical processes, might have begun adopting some version of CCM-based care themselves). If this occurred, however, it would likely reduce rather than inflate our DID findings: thus, in the presence of contamination, our findings should be considered conservative, and may, therefore, have underestimated CCM-based cost savings. Third, an assumption of CMA is that the 2 conditions whose arms are being compared—in our case, CCM implementation and treatment as usual—result in equivalent clinical outcomes. We were, unfortunately, unable to collect clinical outcomes data on our control sample. However, the fact that CCM-based care was associated with a robust drop in acute mental health hospitalizations (counter-balanced with only a modest increase in outpatient service utilization) suggests that CCM-based care would be at least equivalent to a treatment as usual in terms of clinical outcomes in these settings. Fourth, we were unable to account for health care costs incurred by patients in our sample outside of the VA system. More than 92% of Veterans treated by our CCM-enhanced teams continued with VA care during the facilitation year, however.13 Furthermore, given the requirements for VA coverage of non-VA services when this study took place, it is unlikely that non-VA care would be differentially sought by different teams within the same facility. These factors make it unlikely that incorporating costs from non-VA health care services would substantially change our results.
More broadly, it is important to consider the extent to which our findings are applicable to different health care systems. We conducted this study in the VA, an integrated capitated care system, and chose the health system perspective for our CMA (meaning we aggregated outpatient and inpatient costs). But even within such a system, realizing CCM-based cost savings would require redirecting staffing and resources from inpatient to outpatient settings. Systems without this flexibility—for example, due to mandates regarding inpatient service capacity independent of demand—may instead find that CCM implementation in outpatient settings increases costs. Furthermore, our findings say nothing about the costs that such a redirection of resources (eg, closing inpatient units, retraining inpatient staff) would entail. Our findings of CCM-based cost savings would also not be expected to hold within fee-for-service systems (in which decreased inpatient service utilization is associated with decreased revenue rather than decreased costs).
CONCLUSIONS AND FUTURE DIRECTIONS
Chronic health conditions, including mental health conditions such as major depression and anxiety disorders, are responsible for an increasing share of health care expenditures.41 Treatment for these conditions frequently occurs in outpatient settings and requires coordination of multiple providers. The CCM is an evidence-based way to structure care in these circumstances,1,2,4 but previous analyses of the cost-effectiveness of CCM-based care have typically not accounted for the up-front implementation costs that may serve as a deterrent to implementation for many health systems.9–15,25 Our findings suggest that relatively modest implementation resources (on the order of $25,000–$30,000 for a multidisciplinary team of 5–8 staff providing general mental health care to a panel of about 1000 Veterans) achieved a return of about $1.70 for every dollar spent, primarily by reducing costly acute mental health inpatient stays.
Future work will ideally investigate the cost-effectiveness of CCM implementation outside of the VA system, and over longer time periods (ie, beyond the 1-y time frame of this study). Cost comparisons between different implementation strategies (eg, implementation facilitation vs. “lighter touch” interventions such as technical assistance) are also warranted. Ideally, future research will also look more carefully at whether the potential cost savings described in this study can actually be achieved in real-world settings. For example, is it possible to implement CCM-based care broadly enough that health systems can shift resources from inpatient to outpatient settings as a result? Answering these types of questions will likely require larger-scale studies than the one conducted here. Along similar lines, the field would also benefit from studies of how best to spread CCM-based care within medical centers, as the current study focused on just one team per facility. These types of studies will not only advance implementation science, but will also expand the evidence base regarding the clinical effectiveness and potential cost savings of outpatient mental health care that—consistent with the CCM elements—is more coordinated, anticipatory, and evidence-based.
Acknowledgments
Supported by the US Department of Veterans Affairs (VA) Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) grant #QUE 15-289 (PI: M.S.B.). The views expressed in this article are those of the authors and do not necessarily reflect the views of VA or the US government.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the Chronic Care Model, part 2. JAMA. 2002;288:1909–1914. [DOI] [PubMed] [Google Scholar]
- 2.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775–1779. [DOI] [PubMed] [Google Scholar]
- 3.Coleman K, Austin BT, Brach C, et al. Evidence on the Chronic Care Model in the new millennium. Health Aff (Millwood). 2009;28:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74:511–544. [PubMed] [Google Scholar]
- 5.Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of Collaborative Chronic Care Models for mental health conditions across primary, specialty, and behavioral health care settings: Systematic review and meta-analysis. Am J Psychiatry. 2012;169:790–804. [DOI] [PubMed] [Google Scholar]
- 6.Von Korff M, Gruman J, Schaefer J, et al. Collaborative management of chronic illness. Ann Intern Med. 1997;127:1097–1102. [DOI] [PubMed] [Google Scholar]
- 7.Miller CJ, Grogan-Kaylor A, Perron BE, et al. Collaborative Chronic Care Models for mental health conditions: cumulative meta-analysis and metaregression to guide future research and implementation. Med Care. 2013;51:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavelle TA, Kommareddi M, Jaycox LH, et al. Cost-effectiveness of collaborative care for depression and PTSD in military personnel. Am J Manag Care. 2018;24:91–98. [PubMed] [Google Scholar]
- 9.Bauer AM, Azzone V, Goldman HH, et al. Implementation of collaborative depression management at community-based primary care clinics: an evaluation. Psychiatr Serv. 2011;62:1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossom RC, Solberg LI, Magnan S, et al. Impact of a national collaborative care initiative for patients with depression and diabetes or cardiovascular disease. Gen Hosp Psychiatry. 2017;44:77–85. [DOI] [PubMed] [Google Scholar]
- 11.Sowa NA, Jeng P, Bauer AM, et al. Psychiatric case review and treatment intensification in collaborative care management for depression in primary care. Psychiatr Serv. 2018;69:549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann HP, Bender BU, Schultze-Seemann W, et al. The kidney and von Hippel-Lindau disease: impact of molecular genetic analysis of the VHL gene for clinical management. Contrib Nephrol. 1997;122:102–108. [DOI] [PubMed] [Google Scholar]
- 13.Bauer MS, Miller CJ, Kim B, et al. Effectiveness of implementing a Collaborative Chronic Care Model for clinician teams on patient outcomes and health status in mental health: a randomized clinical trial. JAMA Netw Open. 2019;2:e190230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waxmonsky J, Kilbourne AM, Goodrich DE, et al. Enhanced fidelity to treatment for bipolar disorder: results from a randomized controlled implementation trial. Psychiatr Serv. 2014;65:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilbourne AM, Goodrich DE, Nord KM, et al. Long-term clinical outcomes from a randomized controlled trial of two implementation strategies to promote collaborative care attendance in community practices. Adm Policy Ment Health. 2015;42:642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeft TJ, Wilcox H, Hinton L, et al. Costs of implementing and sustaining enhanced collaborative care programs involving community partners. Implement Sci. 2019;14:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer MS, McBride L, Williford WO, et al. Collaborative care for bipolar disorder: part I. Intervention and implementation in a randomized effectiveness trial. Psychiatr Serv. 2006;57:927–936. [DOI] [PubMed] [Google Scholar]
- 18.Bauer MS, McBride L, Williford WO, et al. Collaborative care for bipolar disorder: part II. Impact on clinical outcome, function, and costs. Psychiatr Serv. 2006;57:937–945. [DOI] [PubMed] [Google Scholar]
- 19.Soi C, Babigumira JB, Chilundo B, et al. Implementation strategy and cost of Mozambique’s HPV vaccine demonstration project. BMC Public Health. 2019;19:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noain A, Garcia-Cardenas V, Gastelurrutia MA, et al. Cost analysis for the implementation of a medication review with follow-up service in Spain. Int J Clin Pharm. 2017;39:750–758. [DOI] [PubMed] [Google Scholar]
- 21.Idrisov B, Murphy SM, Morrill T, et al. Implementation of methadone therapy for opioid use disorder in Russia—a modeled cost-effectiveness analysis. Subst Abuse Treat Prev Policy. 2017;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisman AB, Kilbourne AM, Dopp AR, et al. Economic evaluation in implementation science: making the business case for implementation strategies. Psychiatry Res. 2020;283:112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roundfield KD, Lang JM. Costs to community mental health agencies to sustain an evidence-based practice. Psychiatr Serv. 2017;68:876–882. [DOI] [PubMed] [Google Scholar]
- 24.Bond GR, Drake RE, McHugo GJ, et al. Long-term sustainability of evidence-based practices in community mental health agencies. Adm Policy Ment Health. 2014;41:228–236. [DOI] [PubMed] [Google Scholar]
- 25.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown CA, Lilford RJ. The stepped wedge trial design: a systematic review. BMC Med Res Methodol. 2006;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davey C, Hargreaves J, Thompson JA, et al. Analysis and reporting of stepped wedge randomised controlled trials: synthesis and critical appraisal of published studies, 2010 to 2014. Trials. 2015;16:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer MS, Miller C, Kim B, et al. Partnering with health system operations leadership to develop a controlled implementation trial. Implement Sci. 2016;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann PJ, Sanders GD, Russell LB, et al. Cost-effectiveness in Health and Medicine. New York, NY: Oxford University Press; 2016. [Google Scholar]
- 31.Kirchner J, Edlund CN, Henderson K, et al. Using a multi-level approach to implement a primary care mental health (PCMH) program. Fam Syst Health. 2010;28:161–174. [DOI] [PubMed] [Google Scholar]
- 32.Lew RA, Miller CJ, Kim B, et al. A method to reduce imbalance for site-level randomized stepped wedge implementation trial designs. Implement Sci. 2019;14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glick HA, Kinosian B, McBride L, et al. Clinical nurse specialist care managers’ time commitments in a disease-management program for bipolar disorder. Bipolar Disord. 2004;6:452–459. [DOI] [PubMed] [Google Scholar]
- 34.Kim B, Miller CJ, Ritchie M, et al. Time-motion analysis of implementing the Collaborative Chronic Care Model in general mental health clinics: assessing external facilitation effort over time using continuous and interval-based data collection approaches. Annual Conference on the Science of Dissemination and Implementation in Health; December 3–5, 2018. Washington, DC: National Institutes of Health and AcademyHealth; 2018. [Google Scholar]
- 35.Ritchie MJ, Liu CF, Townsend JC, et al. Time and cost of “extreme” implementation facilitation to address challenging clinical contexts. 4th Biennial Society for Implementation Research Collaboration (SIRC) Conference; Seattle, WA, 2017. [Google Scholar]
- 36.Kim B, Miller CJ, Elwy AR, et al. Staff perceptions implementing interprofessional team-based behavioural healthcare. J Interprof Care. 2017;31:360–367. [DOI] [PubMed] [Google Scholar]
- 37.VA Office of Personnel Management (OPM). 2019 General Schedule (GS) Locality Pay Tables. Washington, DC; 2019. Available at: www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/2019/general-schedule/. Accessed June 1, 2020. [Google Scholar]
- 38.US Department of Veterans Affairs. Veterans Health Administration Corporate Data Warehouse; 2018.
- 39.US Department of Veterans Affairs. Health Economics Resource Center (HERC); 2019. Available at: www.herc.research.va.gov/include/page.asp?id=home. Accessed June 1, 2020.
- 40.van Steenbergen-Weijenburg KM, van der Feltz-Cornelis CM, Horn EK, et al. Cost-effectiveness of collaborative care for the treatment of major depressive disorder in primary care. A systematic review. BMC Health Serv Res. 2010;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emanuel EJ. Where are the health care cost savings? JAMA. 2012;307:39–40. [DOI] [PubMed] [Google Scholar]


