Supplemental Digital Content is Available in the Text.
There is evidence that joint and nerve mobilisations compared with sham or no intervention positively influence various neuroimmune responses in animal and human neuromusculoskeletal conditions.
Keywords: Manual therapy, Neural mobilisation, Neurodynamics, Neuropathic pain, Cytokines, Neuroinflammation, Nonpharmacological treatment
Abstract
Several animal and human studies revealed that joint and nerve mobilisations positively influence neuroimmune responses in neuromusculoskeletal conditions. However, no systematic review and meta-analysis has been performed. Therefore, this study aimed to synthesize the effects of joint and nerve mobilisation compared with sham or no intervention on neuroimmune responses in animals and humans with neuromusculoskeletal conditions. Four electronic databases were searched for controlled trials. Two reviewers independently selected studies, extracted data, assessed the risk of bias, and graded the certainty of the evidence. Where possible, meta-analyses using random effects models were used to pool the results. Preliminary evidence from 13 animal studies report neuroimmune responses after joint and nerve mobilisations. In neuropathic pain models, meta-analysis revealed decreased spinal cord levels of glial fibrillary acidic protein, dorsal root ganglion levels of interleukin-1β, number of dorsal root ganglion nonneuronal cells, and increased spinal cord interleukin-10 levels. The 5 included human studies showed mixed effects of spinal manipulation on salivary/serum cortisol levels in people with spinal pain, and no significant effects on serum β-endorphin or interleukin-1β levels in people with spinal pain. There is evidence that joint and nerve mobilisations positively influence various neuroimmune responses. However, as most findings are based on single studies, the certainty of the evidence is low to very low. Further studies are needed.
1. Introduction
Joint mobilisation and nerve mobilisation are common interventions for neuromusculoskeletal conditions, such as back,62 neck,27 knee,93 shoulder,63 or radicular59 pain. Various possible working mechanisms of joint and nerve mobilisations are described, but aggregated evidence about the effects of joint and nerve mobilisations on neuroimmune responses is lacking.9,40,50,58,84,98
Neuroimmune responses are involved in the etiology and pathophysiology of neuromusculoskeletal conditions.2,5,78 The immune system and nervous system communicate using common molecular signaling cues. Neuroimmune responses are defined as processes or substances (such as neuropeptides, cytokines, gene expression, and hormones) involved in interactions between the immune system and nervous system.12 Microglia as main immune cells in the nervous system are responsive to nervous system injury and danger signals.73 They have connections with neuronal cell bodies and influence synaps function.21 After nerve injury, several neuroimmune responses occur within the neuraxis resulting in neuroinflammation and neuromodulation.25,50,84,85 At the compression site, injured axons and resident immune cells release inflammatory mediators, such as cytokines, neuropeptides, neurotrophic factors, reactive oxygen species, and chemokines. These mediators orechstrate local neuroimmune responses, stimulate recruitment of other immune cells, and promote the removal of local debris.35,54,67,89 Remote from the actual lesion site, resident immune cells in the dorsal root ganglion (DRG) and spinal cord react to nerve injury, and their response is reinforced by invading macrophages and T lymphocytes.81,94 Microglia and astrocytes upregulate surface markers and receptors, such as glial fibrillary acidic protein (GFAP), OX-42, and CD11b/c.5,14 Upregulation of immune regulating genes in the DRG and spinal cord reflects the extent of both the recruitment and activity of immune cells.20 The altered gene expression at the DRG results in increased synthesis of peripheral receptors that further sensitise the nociceptors, such as transient receptor potential vanilloid receptor-1 (TRPV1).13
Neuroimmune responses can also be found in supraspinal centres, such as the midbrain, thalamus, nucleus accumbens, and prefrontal cortex, contributing to sensory, affective, and cognitive aspects of neuromusculoskeletal pain.4 Using PET-imaging, neuroinflammation has been revealed in several brain areas of people with chronic low back pain3,43 and in the spinal cord and neuroforamina in people with lumbar radiculopathy.2 There is accumulating evidence that neuroimmune responses not only occur after nerve injury but also in other neuromusculoskeletal conditions, such as knee52 and ankle95 inflammation. In people with spinal pain, increased systemic levels of inflammatory mediators have been demonstrated37,87 with elevated cytokine production after in-vitro whole blood endotoxin stimulation.88
Neuroimmune responses seem to be the main drivers of sensitisation within the neuraxis,14,34,64,78 and there is growing evidence that joint and nerve mobilisations may influence these neuroimmune responses.25,50,76 Nerve mobilisation facilitates movement between the targeted peripheral nerve or nerve root and its surrounding structures.16,17,44 The therapeutic aim of nerve mobilisation is to use movement to restore the altered homeostasis in and around the nerve.7,18 Joint mobilisation is defined as passive movements applied to a joint complex (eg, the joint and all associated soft tissues) with the intent to restore optimal motion, function, and/or to reduce pain.70
Two reviews reported that spinal manipulation in humans increased systemic levels of interleukins and cortisol,38 and triggered the activation of the neuroimmunoendocrine system.15 However, both reviews included studies with healthy participants. This has important limitations as neuroimmune responses may differ in people with pathological conditions. One recent scoping review summarised the physiological responses to manual therapy in pain animal models,42 without critical appraisal of the included studies and with only a narrative description of the results.
Currently, no systematic review is available which summarises the effects of joint mobilisation or nerve mobilisation on neuroimmune responses in animals and humans with neuromusculoskeletal conditions. Therefore, the aim of the present systematic review was to identify, appraise, and synthesise the evidence for neuroimmune responses after joint mobilisation or nerve mobilisation compared with sham or no intervention in animals and humans with neuromusculoskeletal conditions.
2. Methods
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.56 The protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO), CRD42018094090.
2.1. Literature search
The authors designed the literature search strategies together with a research librarian (Appendix A, available at http://links.lww.com/PR9/A104). Medical databases were searched from inception until June 2020 using PubMed, Embase, CINAHL, and Web of Science. Reference lists of included articles, clinical trial registries (clinicaltrials.gov), and open access dissertations were also searched.
2.2. Study selection
The study selection was performed independently by 2 review authors (from a pool of 3 review authors: N.T., I.L.S., and G.S.P.). Differences in study selection between the 2 reviewers were resolved by discussion, but when uncertainty remained, another review author (M.W.C.) was consulted. Standardised forms were used to screen the full text of studies that met the criteria based on title and abstract. Conference articles were excluded.
Animal and human studies in neuromusculoskeletal conditions were eligible when they assessed joint mobilisation (including joint manipulation) or nerve mobilisation compared with a sham intervention or no intervention. Studies which investigated joint mobilisation or nerve mobilisation as part of a multimodal intervention were excluded. At least one outcome measure had to quantify a neuroimmune response, such as levels of neuroinflammatory markers, neurotrophins, neuropeptides, or cytokines.
2.3. Data extraction
Data were independently extracted by 2 review authors (from a pool of 3 reviewers: I.L.S., N.T., and G.S.P.) using the Cochrane Data Extraction Template. A third review author (M.W.C.) was consulted in case of uncertainty. The following data were extracted: (1) methodological information, (2) participant information, (3) information on pathology, (4) information on the intervention(s), (5) primary outcome measures, and (6) secondary outcome measures. We contacted the original authors in case of missing data. If no response could be obtained, an on-screen digitizer (Universal Digitizer 3.8, AVPSoft.com) was used to extract data from graphs.
2.4. Risk of bias assessment
Two review authors (from a pool of 3 reviewers: I.L.S., N.T., and G.S.P.) independently assessed the risk of bias (RoB). For animal studies, we used the RoB tool from the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) (Appendix B, available at http://links.lww.com/PR9/A104).33 This instrument is based on the RoB tool for human studies from the Cochrane Collaboration and has been adapted for animal studies.30,33 The RoB was rated as high, unclear, or low.30 A summary assessment of the RoB was based on the likelihood to seriously alter the results.30 Differences between the 2 review authors were resolved by discussion or with the assistance from a third review author (M.W.C.).
2.5. Data analysis and synthesis
The effects of joint mobilisation and nerve mobilisation on neuroimmune responses are presented using effect sizes. Effect sizes are expressed as standardised mean differences (SMDs) and 95% confidence intervals (95% CIs) for continuous outcomes. Meta-analyses for the animal and human studies were performed when (1) heterogeneity was I2 < 40%23 and (2) the outcome of interest was measured in the same anatomical location. We present the results in forest plots and calculated a pooled estimate if the neuroimmune response of interest was measured in more than one study or in one study with more groups,32 regardless of study population, condition, experimental intervention, and type of control but not anatomical location.
2.6. Certainty of the evidence
Certainty of the evidence was described using GRADE for human studies28,29 and the modified GRADE approach for animal studies31 (Appendix C, available at http://links.lww.com/PR9/A104).
3. Results
3.1. Literature search and selection
The literature search yielded 4801 articles. After removal of duplicates, 2843 articles remained. After screening of titles and abstracts, 39 articles remained. Eighteen articles were included after full-text screening: 13 animal studies22,24,25,49,50,65,68,72,75–77,84,85 and 5 human studies.45,61,74,90,96 Figure 1 presents the flowchart of the selection process. The overall agreement for study inclusion was almost perfect (kappa = 0.95).
Figure 1.
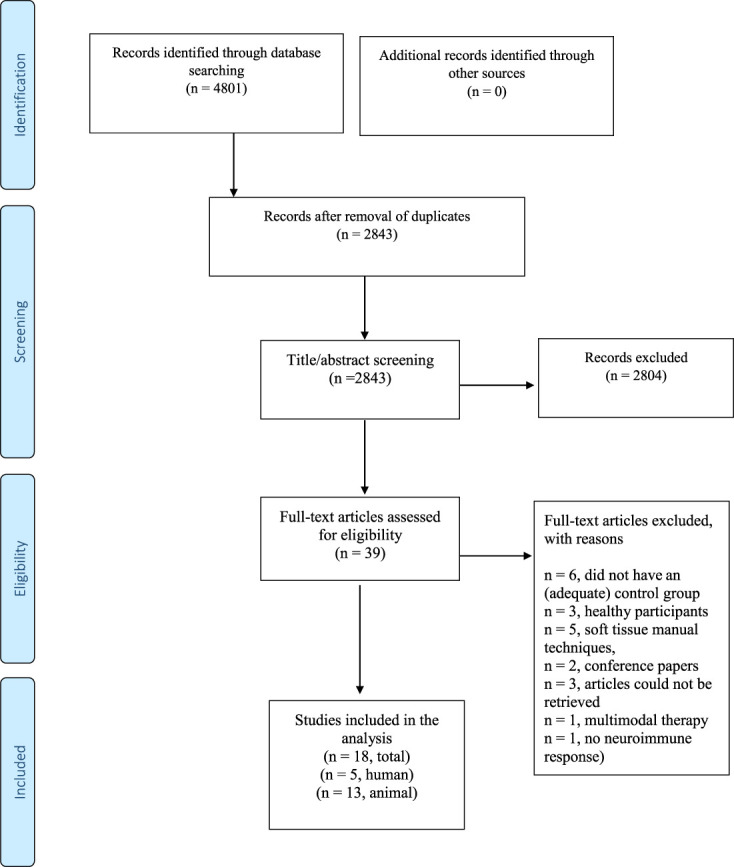
Flowchart of the literature selection.
3.2. Description of study characteristics
The 13 animal studies compared nerve mobilisation,22,25,49,75–77 spinal mobilisation,65 spinal manipulation,24,84,85 and knee68 and ankle50,72 mobilisation with no intervention or sham in male Wistar rats (n = 98),22,24,25,49,50,68,75–77 Sprague–Dawley rats (n = 26),84,85 Swiss mice (n = 16),72 and female Wistar rats (n = 6).65 Spinal manipulation and mobilisation was mimicked using an activator-assisted spinal device24,84,85 or computer-controlled feedback motor.65 Several models for neuropathic pain were used, such as chronic constriction injury (CCI) to the sciatic nerve,22,25,75–77 crush injury to the sciatic50 or median47,48,49 nerve, compression-decompression of the DRG,85 and injection of inflammatory mediators within the intervertebral foramen.84 Ankle joint inflammation,68,69,83 chronic postischemia hind paw pain,72 knee joint immobilisation,24 and nerve growth factor (NGF)-induced back pain65 were also used. See Appendix D and Appendix E for further details, available at http://links.lww.com/PR9/A104.
A wide range of outcome measures was evaluated, namely, neurotrophins (NGF,22,49,76 brain-derived neurotrophic factor [BDNF]),25,49 cytokines (tumor necrosis factor [TNF]-α, IL-1β, and IL-10),85 expression of opioid receptors (δ-opioid, κ-opioid, and μ-opioid),75,77 whole-genome expression,68 neuroinflammatory markers (astrocyte marker GFAP,25,50,76 microglial markers CD11b/c,25,50 and/or OX-4225), nonneuronal cell proliferation,84,85 substance-P,77 TRPV1,77 calcitonin gene–related protein (CGRP),65 oxidative stress markers (lipid hydroperoxide,24 nitric oxide metabolites,24 malondialdehyde,72 and carbonyl protein72), and antioxidant enzymes (catalase activity,24,72 superoxide dismutase,24,72 and glutathione peroxidase24). Moreover, the outcomes were measured at different locations, such as in the serum, nerve, DRG, spinal cord, and brain. The on-screen digitizer was used for extracting data regarding cytokines,85 number of nonneuronal cells,84,85 GFAP,50 and CD11b/c50 with an almost perfect overall agreement (kappa = 0.95). Table 1 describes the study characteristics of the animal studies.
Table 1.
Study characteristics included animal trials.
| Author | Study design | Condition | Animals | Groups | Mean age | Male (%) | Treatment | Primary outcome |
|---|---|---|---|---|---|---|---|---|
| Ruhlen 201468 | RCT | Inflammatory ankle injury | Sprague Dawley rats N = 3/group |
E: KJM C: NI |
250–350 g | 100 | 3 × 3 min KJM | L4-L5 spinal cord whole genome expression |
| Giardini 201825 | NCT | CCI | Wistar rats N = 5/group |
E: NM C: NI |
200–220 g | 100 | 10 sessions NM | GFAP Thalamus Midbrain GFAP-IR Thalamus Midbrain OX-42 Thalamus Midbrain OX-42-IR Thalamus Midbrain BDNF Thalamus Midbrain BDNF-IR Thalamus Midbrain |
| Santos 201475 | NCT | CCI | Wistar rats N = 6/group |
E: NM C: NI |
180–220 g | 100 | 10 sessions NM | DOR PAG KOR PAG MOR PAG |
| Santos 201276 | NCT | CCI | Wistar rats N = 6/group |
E: NM C: NI |
180–220 g | 100 | 10 sessions NM | NGF DRG L3-L6 S.C. L3-L6 NGF-IR DRG L4 GFAP DRG L3-L6 S.C. L3-L6 GFAP-IR DRG L4 |
| Da Silva 201522 | NCT | CCI | Wistar rats N = 6/group |
E: NM C: NI |
180–220 g | 100 | 10 sessions NM | NGF Sciatic nerve |
| Santos 201877 | NCT | CCI | Wistar rats N = 6/group |
E: NM C: NI |
180–220 g | 100 | 10 sessions NM | Substance-P DRG L4-L6 TRPV1 DRG L4-L6 DOR DRG L4-L6 KOR DRG L4-L6 MOR DRG L4-L6 |
| Martins 201150 | NCT | Sciatic nerve crush injury | Wistar rats N = 5/group |
E: AJM C: NI |
250–280 g | 100 | 15 sessions AJM | GFAP-IR* Dorsal SC L4-L5 CD11b/c-IR* Dorsal SC L4-L5 |
| Marcioli 201849 | NCT | Median nerve compression | Wistar rats E: N = 12 C: N = 6 |
E: NM C: NI |
14 ± 2 wk | 100 | 1 or 3 minutes NM | NGF-mRNA Median nerve BDNF-mRNA Median nerve |
| Song 201685 | NCT | Compression–decompression of the dorsal root ganglion | Sprague–Dawley rats N = 3–5/group N = 6/group for cytokine analysis |
E: ASMT C: NI |
200–250 g | 100 | 10 sessions ASMT (L5-L6) | Non-neuronal cells* DRG L4-L5 TNF-α* Serum DRG L4-L5 SC L4-L5 IL-1β* Serum DRG L4-L5 SC L4-5 IL-10* Serum DRG L4-5 SC L4-5 |
| Song 200684 | NCT | Intervertebral foramen inflammation | Sprague–Dawley rats N = 4/group |
E: ASMT C: NI |
200–250 g | 100 | 10 sessions ASMT (L5-L6) | Non-neuronal cells* DRG L5 |
| Salgado 201972 | NCT | Chronic postischemia model | Swiss mice N = 8/group |
E: AJM C: NI |
25–35 g | 100 | 10 sessions AJM | Malondialdehyde Hind paw muscle Carbonyls protein Hind paw muscle Superoxide dismutase Hind paw muscle Catalase Hind paw muscle |
| Duarte 201924 | NCT | Knee joint immobilisation | Wistar rats N = 6/group |
E: ASMT C1: ASMT-sham C2: NI |
200–300 g | 100 | 9 sessions ASMT or ASMT-sham (L4-L5) | Lipid hydroperoxides Plasma Nitric oxide Plasma Superoxide dismutase Red blood cells Glutathione peroxidase Red blood cells Catalase Red blood cells |
| Reed 202065 | NCT | NGF-induced trunk hyperalgesia | Sprague–Dawley rats N = 3/group |
E: MSM C: NI |
187–270 g | 0 | 12 sessions of MSM | Calcitonin gene related protein DRG L1-L6 |
*Data extracted using a digital ruler.
AJM, ankle joint mobilisation; ASMT, activator-assisted spinal manipulation (also called mimicked spinal manipulation); BDNF, brain-derived neurotrophic factor; BDNF-IR, BDNF immunoreactivity; C, control group; CCI, chronic constriction injury; CD11b/c, microglial marker; CD11b/c-IR, CD11b/c immunoreactivity; DOR, δ-opioid receptor; E, experimental intervention; g, gram; GFAP, glial fibrillary acidic protein; GFAP-IR, GFAP immunoreactivity; IL-10, interleuking-10; IL-1β, interleukin-1β; KJM, knee joint mobilisation; KOR, κ-opioid receptor; MOR, µ-opioid receptor; mRNA, messenger ribonucleic acid; MSM, motorised spinal mobilisation; NCT, nonrandomised controlled trial; NGF, nerve growth factor; NGF-IR, NGF immunoreactivity; NI, no intervention; NM, nerve mobilisation; OX-42, microglia marker; OX-42-IR, OX-42 immunoreactivity; PAG, periaqueductal gray; RCT, randomised controlled trial; TNF-α, tumor necrosis factor-α.
The 5 included human trials (n = 176) compared (1) spinal manipulation with sham manipulation45,90,96 and with no intervention,61,74 and (2) spinal mobilisation with sham manipulation.90 The conditions were acute nonspecific low back pain,61,74 and acute45 and chronic90,96 nonspecific neck pain. Outcome measures were plasma β-endorphin,74 serum IL-1β,96 serum,45,61 and salivary cortisol levels.90 Table 2 describes the study characteristics of each human study.
Table 2.
Study characteristics included human trials.
| Author | Study design | Population | Numbers | Groups | Mean age (years) | Male (%) | Primary outcome |
|---|---|---|---|---|---|---|---|
| Sanders 199074 | RCT | Acute low back pain | N = 6/group | E: LSM L4/L5/S1 C: NI |
Males 41 ± 13.9 Females 33 ± 8.6 |
Not reported per group | β-endorphin Plasma 5 min after Plasma 20 min after |
| Padayachy 201061 | RCT | Acute low back pain | N = 15/group | E: LSM C: NI |
18–35 y (range) | 100 | Cortisol Serum |
| Lohman 201845 | RCT | Acute nonspecific neck pain | E: N = 13 C: N = 15 |
E: CSM C: sham |
33.4 ± 7.2 | 0 | Cortisol Serum |
| Valera-Calero90 | RCT | Chronic nonspecific neck pain | E1: N = 28 E2: N = 28 C: N = 28 |
E1: CSM E2: CM C: sham |
E1: 35.64 ± 8.11 E2: 37.25 ± 10.54 C: 36.96 ± 8.89 |
E1: 43 E2: 36 C: 36 |
Cortisol Salivary |
| Zemadanis 201996 | RCT | Chronic nonspecific neck pain | E: N = 11 C: N = 11 |
E: TSM C: sham |
E: 40 ± 12 C: 44.7 ± 14 |
E: 73 C: 55 |
Interleukin-1β Serum 20 min after 1 session Serum after 9 sessions in 3 weeks |
C, control group; CM, cervical mobilisation; CSM, cervical spinal manipulation; E, experimental intervention group; LSM, lumbar spinal manipulation; NI, no intervention; RCT, randomized controlled trial; TSM, thoracic spinal manipulation.
3.3. Effects of joint mobilisation and nerve mobilisation in animal studies
3.3.1. Neuroinflammation markers
3.3.1.1. Microglia
After sciatic crush injury, ankle mobilisation resulted in a decrease of CD11b/c in the spinal cord (1 study, n = 10 animals, SMD: −1.68, 95% CI −0.12 to −3.23) compared with no intervention.50 One study measured the effects of nerve mobilisation in the CCI model and revealed that nerve mobilisation decreased OX-42 protein levels in the thalamus (ventral posterolateral nucleus [VPL]) (n = 10 animals, SMD: −3.69, 95% CI −1.27 to −6.10) and in the midbrain (periaqueductal gray [PAG]) (n = 10 animals, SMD: −6.47, 95% CI −10.32 to −2.62) (Fig. 2A).25
Figure 2.
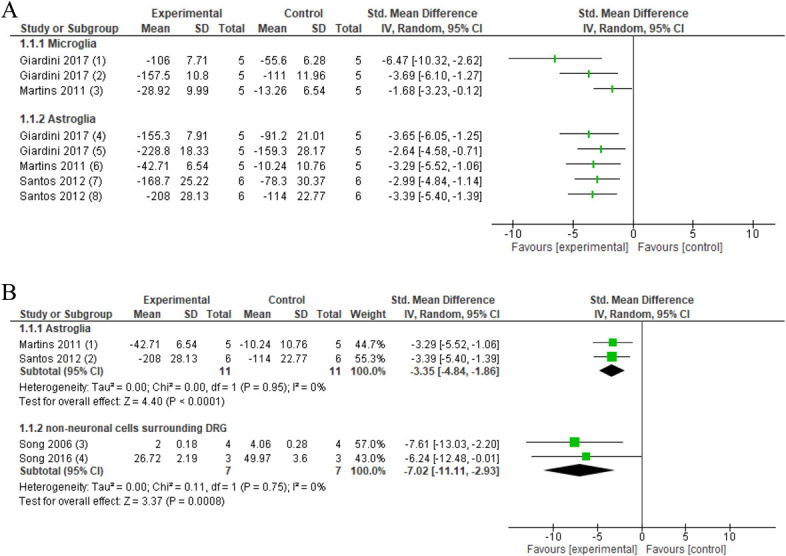
Forest plot for neuroinflammatory markers. 2A. Forest plot for microglia markers OX-42 and CD11b/c and astroglia marker GFAP. Favours experimental implies a reduction in microglia markers. (1) Number of OX-42 levels in PAG in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). (2) Number of OX-42 levels in the thalamus in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). (3) CD11b/c immunoreactivity in the spinal cord L4-5 in crush injury after several sessions of ankle mobilisation (experimental) compared with no intervention (control). Favours experimental implies a reduction in astrocyte GFAP. (4) GFAP protein levels in PAG in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). (5) GFAP protein levels in the thalamus in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). (6) GFAP immunoreactivity in the spinal cord L4-5 in crush injury after several sessions of ankle mobilisation (experimental) compared with no intervention (control). (7) GFAP protein levels in the spinal cord after several sessions neural mobilisation (experimental) compared with no intervention (control). (8) GFAP protein levels in DRG after several sessions neural mobilisation (experimental) compared with no intervention (control). 2B: Forest plot for GFAP and number of nonneuronal cells surrounding the DRG. Favours experimental implies that astrocyte marker GFAP in the spinal cord of these animal models of nerve injury is reduced after joint and nerve mobilisations (experimental) compared with no intervention (control). (1) GFAP immunoreactivity in the spinal cord L4-5 in crush injury after several sessions of ankle mobilisation (experimental) compared with no intervention (control). (2) GFAP protein levels in the spinal cord after several sessions of neural mobilisation (experimental) compared with no intervention (control). Favours experimental implies a reduction in the number of nonneuronal cells surrounding the DRG. (3) Number of nonneuronal cells surrounding DRG in intervertebral foramen inflammation. Activator-assisted spinal manipulation (ASMT; experimental) compared with no intervention (control). (4) Number of nonneuronal cells surrounding the DRG in compression–decompression of the dorsal root ganglion model after ASMT (experimental) compared with no intervention (control). CCI, chronic constriction injury; DRG, dorsal root ganglion; GFAP, glial fibrillary acidic protein.
3.3.1.2. Astroglia
Pooled data revealed that joint and nerve mobilisations compared with no intervention decreased astrocyte marker GFAP in the spinal cord in neuropathic pain models (pooled data, 2 studies, n = 22 animals, SMD: −3.35, 95% CI −4.84 to −1.86) (Fig. 2B).50,76 Two studies investigated the effects of nerve mobilisations in the CCI model and found reduced protein levels of GFAP in the midbrain (PAG) (1 study, n = 10 animals, SMD: −3.65, 95% CI −6.05 to −1.25), thalamus (VPL) (1 study, n = 10 animals, SMD: −2.64, 95% CI −0.71 to −4.58), and DRG (1 study, n = 10 animals, SMD: −2.99, 95% CI −1.14 to −4.84) (Fig. 2A).25,76
3.3.1.3. Nonneuronal cells at the level of the dorsal root ganglion
Pooled data revealed that spinal manipulation, compared with no intervention, decreased the amount of nonneuronal cells surrounding inflamed DRG in a neuropathic pain model (pooled data, 2 studies, n = 14 animals, SMD: −7.02, 95% CI −11.11 to −2.93) (Fig. 2B).84,85
3.3.2. Neurotrophins
3.3.2.1. Nerve growth factor
Nerve mobilisation increased NGF protein levels in the sciatic nerve22 (1 study, n = 12 animals, SMD: 6.26, 95% CI 3.00–9.51) decreased NGF levels in the DRG (L3-6)76 (1 study, n = 12 animals, SMD: −2.55, 95% CI −4.24 to −0.87) and did not affect NGF levels at the spinal cord76 (1 study, n = 12 animals, SMD: −0.45, 95% CI −1.60–0.70) in a CCI model (Fig. 3). In the median nerve compression model, differences in expression of median nerve NGF mRNA could not be detected after median nerve mobilisation compared with a no intervention group.49
Figure 3.
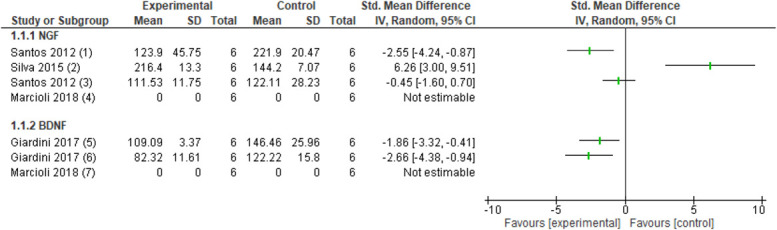
Forest plot for neurotrophins. Favours experimental implies a reduction in NGF levels. (1) Number of NGF protein levels in the DRG in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). (2) Number of NGF protein levels in the sciatic nerve in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). (3) Number of NGF protein levels in the spinal cord in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). (4) Number of NGF mRNA levels in the median nerve in median nerve compression model after several sessions of neural mobilisation (experimental) compared with no intervention (control). Favours experimental implies a reduction in BDNF levels. (5) Number of BDNF protein levels in the thalamus in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). (6) Number of BDNF protein levels in the PAG in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). (7) Number of BDNF mRNA levels in the median nerve in the median nerve compression model after several sessions of neural mobilisation (experimental) compared with no intervention (control). BDNF, brain-derived neurotrophic factor; CCI, chronic constriction injury; DRG, dorsal root ganglion; NGF, nerve growth factor.
3.3.2.2. Brain-derived neurotrophic factor
After treatment with nerve mobilisation, BDNF protein levels were reduced in the midbrain (PAG) (1 study, n = 10 animals, SMD: −2.66, 95% CI −4.38 to −0.94) and thalamus (VPL) (1 study, n = 10 animals, SMD: −1.86, 95% CI −3.32 to −0.41) in a CCI model (Fig. 3).25 Another study did not detect nerve BDNF mRNA in a median nerve compression model in the nerve mobilisation and control groups.49
3.3.3. Neuropeptides
3.3.3.1. Substance P
Nerve mobilisation compared with no intervention resulted in a reduction in substance-P levels at the DRG (1 study, n = 12 animals, SMD: −3.27, 95% CI −5.22 to −1.31) in a CCI model.77
3.3.3.2. Calcitonin gene–related protein
Lumbar spinal mobilisation compared with no intervention resulted in a significant reduction in L1 and L2 CGRP positive DRG neurons (1 study, L1 n = 6 animals, SMD: −2.30, 95% CI −5.04 to 0.44; L2 n = 6 animals, SMD: −2.94, 95% CI −6.21 to 0.32) but not at R1 and R2 CGRP positive DRG neurons (1 study, R1, n = 6 animals, SMD: −1.71, 95% CI −4.00 to 0.59; R2 n = 6 animals, SMD −0.79, 95% CI −2.56 to 0.98) in a low back pain model.65
3.3.4 Receptors
3.3.4.1. Opioid receptor expression
Nerve mobilisation increased protein levels of μ-opioid receptors in the DRG77 (1 study, n = 12 animals, SMD: 18.60, 95% CI 9.45–27.74), but no effect was observed in the PAG75 (1 study, n = 12 animals, SMD: −1.27, 95% CI −2.56 to 0.02) in a CCI model (Fig. 4). After nerve mobilisation, in a CCI model, increased κ-opioid and δ-opioid receptor protein levels were observed in the PAG75 (1 study, κ-opioid n = 12 animals, SMD: 5.07, 95% CI 2.35–7.79; δ-opioid n = 12 animals, SMD: 16.12, 95% CI 8.17–24.06), but protein levels could not be detected in the DRG (Fig. 4).77
Figure 4.
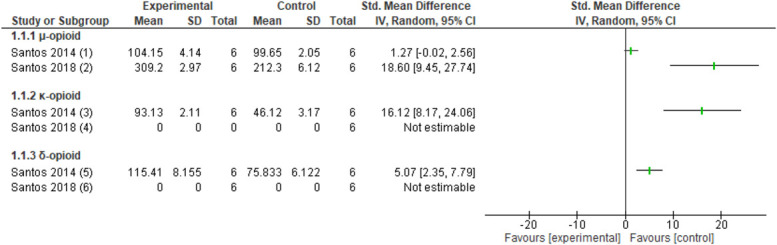
Forest plot for opioid receptor levels. Favours experimental implies an increase in µ-opioid receptor. (1) Number of µ-opioid receptor protein levels in the PAG in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). (2) Number of µ-opioid receptor protein levels in the DRG in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). Favours experimental implies an increase in κ-opioid receptor. (3) Number of κ-opioid receptor protein levels in the PAG in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). (4) κ-opioid receptor protein levels could not be detected in the DRG in the CCI model after several sessions of neural mobilisation (experimental) and no intervention (control). Favours experimental implies an increase in δ-opioid receptor. (5) Number of δ-opioid receptor protein levels in the PAG in the CCI model after several sessions of neural mobilisation (experimental) compared with no intervention (control). (6) δ-opioid receptor protein levels could not be detected in the DRG in the CCI model after several sessions of neural mobilisation (experimental) and no intervention (control). CCI, chronic constriction injury; DRG, dorsal root ganglion; PAG, periaqueductal gray.
3.3.4.2. Transient receptor potential vanilloid 1 expression
Nerve mobilisations in a CCI model decreased DRG TRPV1 levels compared with no intervention (one study, n = 12 animals, SMD: −6.17, 95% CI −9.39 to −2.95).77
3.3.5. Cytokines
3.3.5.1. Serum levels of tumor necrosis factor-α, IL-1β, and IL-10
Serum levels did not change significantly after spinal manipulation (pooled data, 1 study, 2 interventions, n = 18 animals) for TNF-α (SMD: 0.09, 95% CI −0.89 to 1.07), IL-1β (SMD: 0.24, 95% CI −0.75 to 1.23), and IL-10 (SMD: 0.36, 95% CI −0.63 to 1.36) (Fig. 5A) compared with no intervention in a compression-decompression DRG model.85
Figure 5.
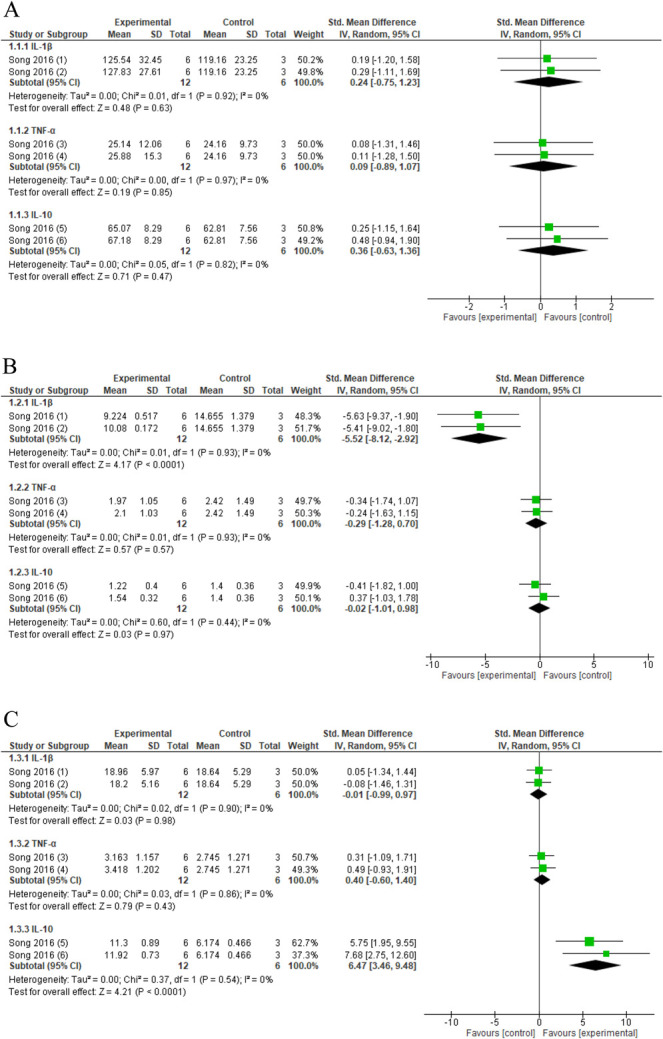
Forest plot for cytokines. (A) Forest plot for serum cytokine levels. Favours experimental implies a reduction in serum cytokines levels. (1-3-4) Serum cytokine levels in compression–decompression of the dorsal root ganglion model after several sessions of activator-assisted spinal manipulation (ASMT-1: force setting 1; experimental) compared with no intervention (control). (2-4-6) Serum cytokine levels in compression–decompression of the dorsal root ganglion model after several sessions of activator-assisted spinal manipulation (ASMT-2: force setting 2; experimental) compared with no intervention (control). (B) Forest plot for DRG cytokine levels. Favours experimental implies a reduction in DRG cytokines levels. (1-3-4) DRG cytokine levels in compression–decompression of the dorsal root ganglion model after several sessions of activator-assisted spinal manipulation (ASMT-1: force setting 1; experimental) compared with no intervention (control). (2-4-6) DRG cytokine levels in compression–decompression of the dorsal root ganglion model after several sessions of activator-assisted spinal manipulation (ASMT-2: force setting 2; experimental) compared with no intervention (control). (C) Forest plot for spinal cord cytokine levels. Favours experimental implies an increase in cytokines levels. (1-3-4) Spinal cord cytokine levels in compression–decompression of the dorsal root ganglion model after several sessions of activator-assisted spinal manipulation (ASMT-1: force setting 1; experimental) compared with no intervention (control). (2-4-6) Spinal cord cytokine levels in compression–decompression of the dorsal root ganglion model after several sessions of activator-assisted spinal manipulation (ASMT-2: force setting 2; experimental) compared with no intervention (control). DRG, dorsal root ganglion.
3.3.5.2. DRG levels of tumor necrosis factor-α, IL-1β, and IL-10
Treatment with spinal manipulation (pooled data, 1 study, 2 interventions, n = 18 animals) showed reduced levels of the proinflammatory cytokine IL-1β (SMD: −5.52, 95% CI −8.12 to −2.92) in the DRG compared with no intervention in a compression–decompression DRG model and no significant changes in TNF-α (SMD: −0.29, 95% CI −1.28 to 0.70) or IL-10 (SMD: 0.02, 95% CI −1.01 to 0.98) (Fig. 5B).85
3.3.5.3. Spinal cord levels of tumor necrosis factor-α, IL-1β, and IL-10
Spinal manipulation (pooled data, 1 study, 2 interventions, n = 18 animals) increased spinal cord IL-10 levels (SMD: 6.47, 95% CI 3.46–9.48) in a compression–decompression DRG model compared with no intervention but did not change TNF-α (SMD: 0.40, 95% CI −0.60 to 1.40) and IL-1β (SMD: −0.01, 95% CI −0.99 to 0.97) (Fig. 5C) levels at the spinal cord.85
3.3.6. Whole-genome expression
No significant differences were found in whole-genome expression at the L4-L5 spinal cord in rats with an inflammatory ankle injury after knee joint mobilisation compared with no intervention.68
3.3.7. Oxidative stress markers
Spinal manipulation compared with no intervention and sham spinal manipulation did not resulted in reduced levels of lipid hydroperoxide (pooled data, 1 study, 2 control groups, n = 18 animals, SMD: −1.08, 95% CI −2.19 to 0.03) and nitric oxide metabolites (pooled data, 1 study, 2 control groups, n = 18 animals, SMD: −0.25, 95% CI −1.24 to 0.73) (Fig. 6) in a knee joint immobilisation model.24 The levels of hind paw muscle malondialdehyde (1 study, n = 16 animals, SMD: −1.23, 95% CI −2.32 to −0.13) and carbonyl proteins (1 study, n = 16 animals, SMD: 1.44, 95% CI −2.58 to −0.30) were reduced in a chronic postischemia model after ankle joint mobilisation compared with no intervention.72
Figure 6.
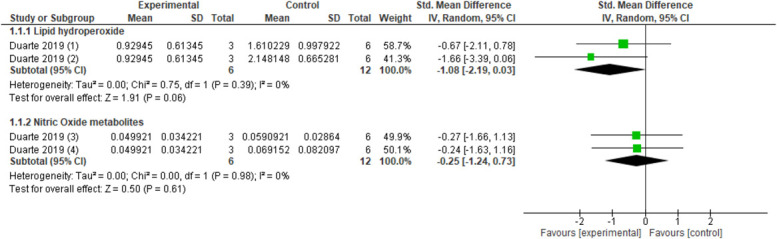
Forest plot for oxidative stress markers. Favours experimental implies a reduction in lipid hydroperoxides. (1) Lipid hydroperoxides activity in red blood cells after several sessions of spinal manipulation (experimental) compared with sham (control). (2) Lipid hydroperoxides activity in red blood cells after several sessions of spinal manipulation (experimental) compared with no intervention (control). Favours experimental implies a reduction in nitric oxide metabolites. (3) Nitric oxide metabolites levels in plasma after several sessions of spinal manipulation (experimental) compared with sham (control). (4) Nitric oxide metabolites levels in plasma after several sessions of spinal manipulation (experimental) compared with no intervention (control).
3.3.8. Antioxidant enzymes
Ankle mobilisation compared with no intervention resulted in increased muscle catalase activity (1 study, n = 16 animals, SMD: 1.58, 95% CI 0.41–2.74) but did not influence muscle SOD activity (1 study, n = 16 animals, SMD: 0.99, 95%CI −0.07 to 2.05) in a chronic postischemia model.72 Catalase activity in red blood cells (pooled data, 1 study, 2 control groups, n = 18 animals, SMD: −1.34, 95% CI −2.50 to −0.18) (Fig. 7) were reduced after spinal manipulation compared with no intervention and sham spinal manipulation.24 Superoxide dismutase (pooled data, 1 study, 2 control groups, n = 18 animals, SMD −0.24, 95% CI −1.23 to 0.75) and glutathione peroxidase (pooled data, 1 study, 2 control groups, n = 18 animals, SMD −0.86, 95% CI −2.10 to 0.38) (Fig. 7) in red blood cells were not changed after spinal manipulation compared with no intervention and sham spinal manipulation in a knee joint immobilisation model.24
Figure 7.
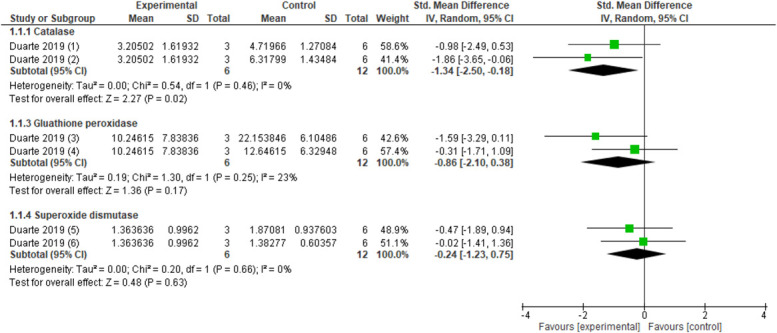
Forest plot for antioxidant enzymes. Favours experimental implies a reduction in catalase. (1) Catalase activity in red blood cells after several sessions of spinal manipulation (experimental) compared with sham (control). (2) Catalase activity in red blood cells after several sessions of spinal manipulation (experimental) compared with no intervention (control). Favours experimental implies a reduction in glutathione peroxidase. (3) Glutathione peroxidase activity in red blood cells after several sessions of spinal manipulation (experimental) compared with sham (control). (4) Glutathione peroxidase activity in red blood cells after several sessions of spinal manipulation (experimental) compared with no intervention (control). Favours experimental implies a reduction in superoxide dismutase. (5) Superoxide dismutase activity in red blood cells after several sessions of spinal manipulation (experimental) compared with sham (control). (6) Superoxide dismutase activity in red blood cells after several sessions of spinal manipulation (experimental) compared with no intervention (control).
3.3.9. Secondary outcomes
Among the 13 animal studies, 9 studies22,24,25,50,65,72,76,84,85 described joint and/or nerve mobilisation-induced morphological and behavioural changes (Appendix G, available at http://links.lww.com/PR9/A104). In neuropathic pain models, nerve morphology parameters (nerve and axon diameter and myelin sheath thickness)22,25,50,84,85 and myelin protein zero22 were increased after joint and nerve mobilisations. Moreover, there was an increase in nociceptive withdrawal thresholds,50,76,84,85 reduced DRG neuron excitability,84,85 and markers of neural activity (c-Fos, PKC-γ)85 were reduced. Finally, nerve mobilisation compared with no intervention resulted in higher scores on functional measurements (tetanic muscle force, sciatic functional index, and static functional index).22,50,85 In the other neuromusculoskeletal conditions (postischemia and low back pain), joint mobilisation increased the nociceptive withdrawal thresholds.65,72 The mechanical withdrawal threshold did not differ between spinal manipulation compared with sham manipulation and with no intervention in the knee joint immobilisation model, although functional measurements improved.24
3.4. Effects of joint mobilisation and nerve mobilisation in human studies
3.4.1. Cortisol
Three studies (n = 140 patients) assessed the change in cortisol immediately after joint mobilisation or manipulation in people with back and/or neck pain (Fig. 8).45,61,90 However, data could not be pooled because of high heterogeneity. A study in people with acute neck pain did not find a significant effect of cervical spinal manipulation on serum cortisol compared with a sham manipulation (1 study, n = 28 patients, SMD: 0.001, 95% CI −0.74 to 0.74).45 In people with acute back pain, lumbar spinal manipulation increased the levels of serum cortisol to no intervention (data could not be retrieved).61 In people with chronic neck pain, cervical manipulation (1 study, n = 54 patients, SMD: 14.86, 95% CI 11.9–17.82) and cervical mobilisation (1 study, n = 54 patients, SMD: 9.36, 95% CI 7.45–11.27) increased salivary cortisol immediately after treatment compared with sham treatment.90
Figure 8.
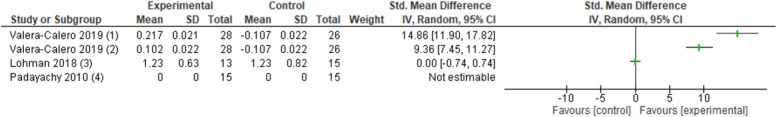
Forest plot for human cortisol. Favours experimental implies an increase in cortisol levels. (1) Levels of serum cortisol levels in acute nonspecific mechanical neck pain after a single cervical spinal manipulation (experimental) compared with a sham cervical manipulation (control). (2) Levels of salivary cortisol levels in chronic nonspecific mechanical neck pain after a single cervical spinal mobilisation (experimental) compared with a sham cervical manipulation (control). (3) Levels of salivary cortisol levels in chronic nonspecific mechanical neck pain after a single cervical spinal manipulation (experimental) compared with a sham cervical manipulation (control). (4) Levels of serum cortisol levels in acute nonspecific mechanical low back pain after a single lumbar spinal manipulation (experimental) compared with no intervention (control) (data could not be retrieved).
3.4.2. Plasma β-endorphin
Lumbar spine manipulation did not change plasma β-endorphins levels in people with acute back pain.74
3.4.3. Serum interleukin- 1β
The levels of IL-1β did not differ 20-minutes after a single session (1 study, n = 22 patients, SMD: −0.36, 95% CI −1.20 to 0.49) of spinal manipulation compared with sham in chronic neck pain patients.96 However, a trend was observed that spinal manipulation reduced the levels of IL-1β (1 study, n = 22 patients, SMD: −0.80, 95% CI −1.68 to 0.07) compared with sham after an intervention period of 3 weeks.96
3.4.4. Secondary outcome
Two of 3 studies did find a reduction in pain intensity after joint manipulation compared with control.74,96 Another study did not find significant differences in pain intensity and pressure pain thresholds after joint manipulation compared with sham intervention.46
A full description of the quantitative results for the animal and human neuroimmune responses after joint and nerve mobilisations in comparison with the control intervention can be found in Appendix G (available at http://links.lww.com/PR9/A104).
3.5. Adverse events
None of the animal or human studies reported adverse events.
3.6. Risk of bias
Risk of bias for all animal studies was unclear because of lack of reporting or performance (Fig. 9). Only 3 animal studies reported that the outcome assessor was blinded for group assignment and were therefore graded as low risk of detection bias.50,65,85 There was a high risk of performance bias because of lack of blinding of those who provided the treatment.22,24,25,49,50,65,68,72,75–77,84,85 Five studies were graded as high risk of other bias as the control group did not receive anesthesia whereas the experimental group did.22,25,75–77 Five other studies were graded as unclear risk of other bias as the control and experimental group received anesthesia during the intervention, a possible example of cointervention bias.49,50,65,68,72
Figure 9.
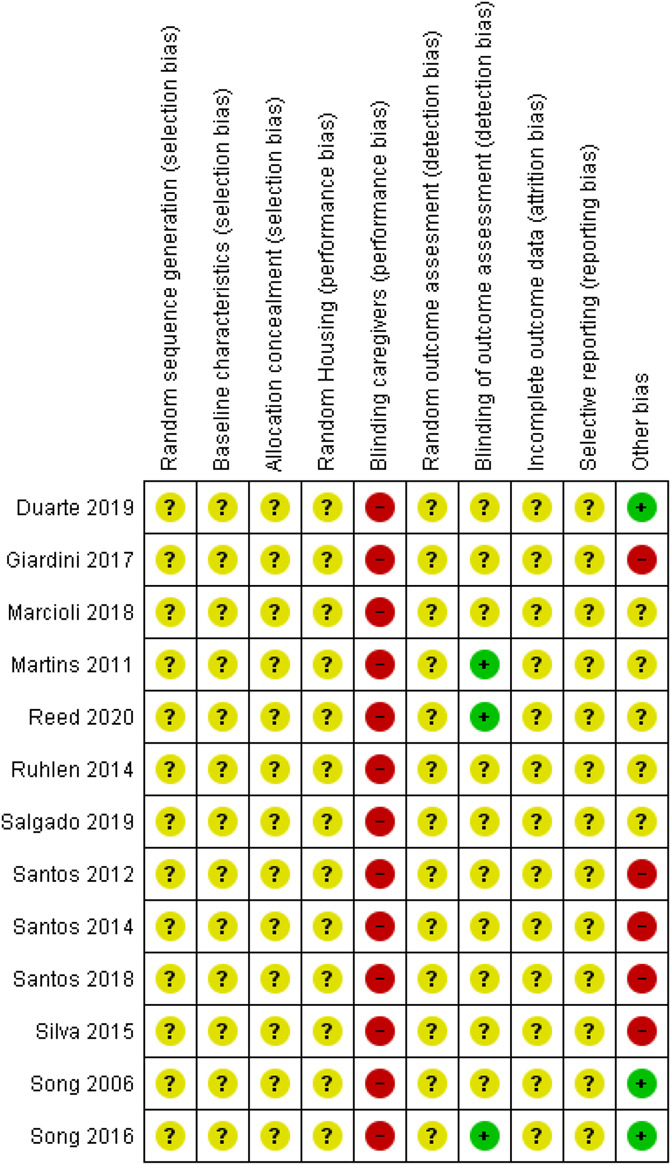
Risk of bias overview for the animal studies. Symbols: ?: unclear risk of bias, −: high risk of bias, and +: low risk of bias.
Four human studies had low RoB,61,74,90,96 and 1 was unclear45 (Fig. 10). All human studies had high risk of performance bias as the health care practitioner could not be blinded. Information regarding allocation concealment, sample size calculation, and trial registration was unclear and potentially resulted in high levels of bias.45,61,74,96 The interrater agreement for the RoB assessment was (nearly) perfect (kappa = 0.93 for the animal studies and kappa = 1.0 for the human studies).
Figure 10.
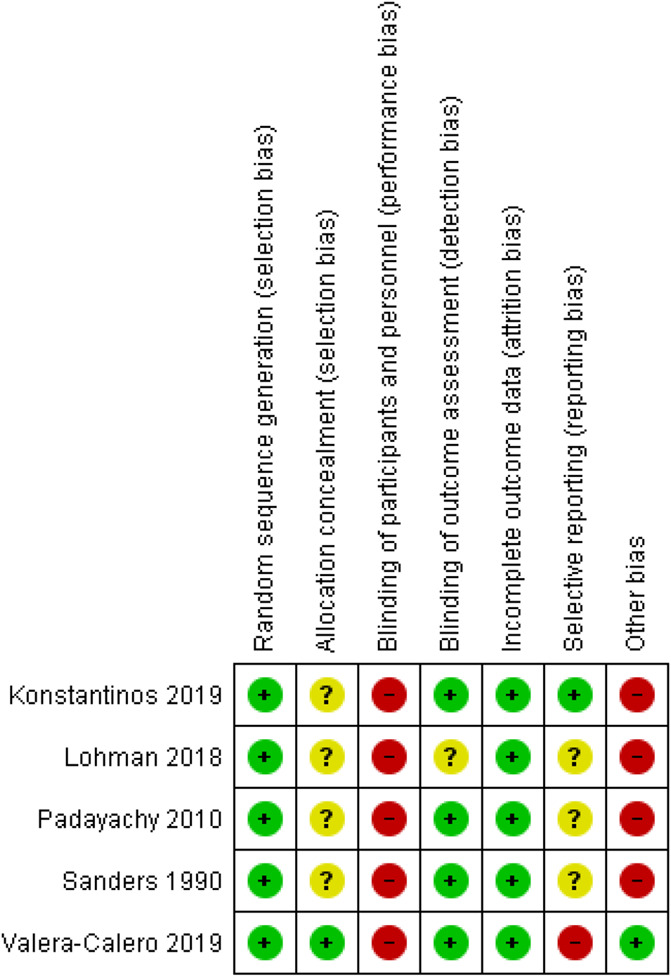
Risk of bias overview for the human studies. Symbols: ?: unclear risk of bias, − high risk of bias, and +: low risk of bias.
3.7. Certainty in the evidence
The neuroimmune responses (GFAP and nonneuronal cells) used in the meta-analysis were graded as consistent and precise based on the overlap between confidence intervals, magnitude, and direction of effect. All other animal neuroimmune responses were graded as inconsistent and imprecise because these were studied in single trials. Indirectness was graded as a serious limitation in most studies,22,24,25,50,65,72,75–77,84,85 mostly because the used animal models were likely to be more severe (eg, more axonal damage than human compression neuropathies).79,80 All other items for the certainty assessment were graded as unclear.
For the human studies, there was high heterogeneity between studies45,61,90 for cortisol (I2 > 90), so we decided not to pool results.29 All human neuroimmune responses were studied in single trials and could therefore be labeled as inconsistent and imprecise, resulting in very low certainty in the evidence.45,61,74,90,96 GRADE results are summarised in Appendix F (available at http://links.lww.com/PR9/A104).
4. Discussion
Most studies22,24,25,50,61,65,72,75–77,84,85,90,96 assessed the effects of joint mobilisation and nerve mobilisation on distinct biomarkers, providing a broad description of possible neuroimmune responses primarily in animal models of neuropathic pain and human spinal pain (Appendix D, available at http://links.lww.com/PR9/A104). Eleven22,24,25,50,65,72,75–77,84,85 of the 13 animal studies identified significant changes in at least one neuroimmune response after joint and nerve mobilisations compared with the control intervention. For the human studies, 261,90 of 5 studies reported an increase in cortisol after joint mobilisation. Fourteen22,24,25,45,49,65,68,72,75,77,85,90,96 of 18 studies were published in the last 5 years. This reflects the growing interest in the effects of joint mobilisation and nerve mobilisation on neuroimmune responses in recent years.
The first important finding of this systematic review was that joint mobilisation and nerve mobilisation may attenuate DRG neuroinflammation as observed by reduced levels of proinflammatory cytokine IL-1β and a reduction in nonneuronal cells surrounding the DRG.84,85 Interleukin-1β can be considered as a key mediator in the crosstalk between glial cells and neurons in neuropathic pain, as production of Il-1β is part of complex signalling cascades resulting in hyperalgesia and enhanced neuronal responses.6,66 The proliferation of nonneuronal cells surrounding the DRG is together with the activation of other glial cells and the production of inflammatory mediators a hallmark of DRG neuroinflammation.34,94 Therefore, the reduction in nonneuronal cells surrounding the DRG can be considered as an attenuation in DRG neuroinflammation. Yet, it is currently unclear how DRG neuroinflammation represents changes in the supraspinal encoding of pain.13
The second important finding of this systematic review was at the level of the spinal cord, where there is a reduction in astrocyte marker GFAP and an increase of anti-inflammatory IL-10 after joint and nerve mobilisations compared with no intervention.50,76,85 Astrocytes perform numerous functions, such as neurotransmitter recycling, contributing to the formation of the blood–brain barrier, regulation of extracellular ion concentration, and modulation of synaptic transmission, among many others.34 Nerve injury may induce reactive astrogliosis that leads to enhanced nociception.41 For example, after nerve injury, astrocytes lose their ability to maintain the homeostatic concentration of extracellular potassium (K+) and glutamate, leading to neuronal hyperexcitability.35 A reduction of astrocyte GFAP might reinstate the normal function of spinal astrocytes and a reduction of astrogliosis and spinal neuroinflammation. An increase of the endogenous cytokine IL-10 in the spinal cord was found after spinal manipulation compared with no intervention.85 Anti-inflammatory Il-10 exerts a wide spectrum of regulatory activities in neuroimmune crosstalk after nerve injury and plays an important role in controlling glial proinflammatory products that act to enhance nociceptive transmission.53,57,82,97
In the human studies, an increase in serum and salivary cortisol concentration was revealed directly after joint mobilisation and/or manipulation in patients with chronic neck90 and back61 pain. In acute neck pain, cervical spinal manipulation did not reveal significant differences in serum cortisol compared with sham treatment.45 These findings are in contrast with a recent review which concluded that there was moderate evidence that cortisol levels were higher after spinal manipulation compared with control immediately after intervention.38 These differences could be explained by the included study populations (healthy participants vs patients with musculoskeletal disorders) and differences in treatment instruction.39,46 Patients might respond differently compared with healthy participants because of hypothalamic–pituitary–adrenal (HPA) axis dysfunction.91 In acute pain, higher cortisol levels may be associated with lower pain intensity.1 HPA-axis function in chronic pain, including the direction (hyperexpression or hypoexpression) of cortisol is however still unclear.60 In addition, the interpretation of an immediate increase in cortisol after joint mobilisation or manipulation is still unclear.71
Ten22,24,49,50,65,72,75,76,84,85 of the 13 animal studies and 374,90,96 of 5 human studies revealed that the neuroimmune responses were accompanied by several morphological, behavioural, and functional improvements. These improvements could imply tissue healing, functional recovery, and reduced pain intensity after joint and nerve mobilisations in animal neuropathic pain conditions.
4.1. Limitations and recommendations
Several limitations should be noted when interpreting the findings of the current systematic review and meta-analyses. Neuroimmune responses seem to be the main drivers of altered homeostasis in joint and nerve pathology.12,14,34,51,64 The review focused on treatment approaches that aim to directly target these structures, such as joint mobilisation and nerve mobilisation. Therefore, other soft-tissue techniques which are not directly aimed at these structures were excluded, although they may have neuroimmunomodulatory effects.8,10,11,19 To gain insight into the mechanisms of action, the search and selection criteria for the study design were stringent, and only controlled trials for neuromusculoskeletal conditions were included. Broad search and selection criteria were formulated for neuroimmune responses to ensure all studied neuroimmune responses were included.
A limited number of animal and human studies were included, and these trials studied a wide range of neuroimmune responses. Summarising the data quantitatively in a meta-analysis was therefore difficult for most neuroimmune responses. We used SYRCLE's RoB tool to assess the quality of the studies.33 The RoB was unclear for most studies. Methodological weaknesses in the included animal studies were observed, such as the experimental design, performance, and reporting methods. Most neuroimmune responses were investigated in single trials resulting in limitations such as inconsistency and imprecision.22,24,25,50,65,68,72,75–77,84,85 For the animal neuroimmune responses, an overall judgement in rating the certainty of the evidence was not possible because it is currently unknown how the 8 factors of GRADE should be weighted in the overall rating in the evidence.31
Future studies need to be more transparent in their methodology and adequately report study details conform the ARRIVE guidelines.36 In addition to improved study designs, future trials need to take into account potential confounding effects of anesthetic drugs during the intervention. In most of the included animal studies,22,25,50,65,68,72,75–77 the animals in the experimental group inhaled anesthetic drugs during the intervention which is known to have immunomodulatory actions.26,92 One study reported that the induced nerve injury combined with the anesthetic isoflurane resulted in an upregulation of CD11b/c and GFAP in the spinal cord compared with the nerve injury condition without isoflurane.50 Two studies used isoflurane,75,77 and 3 studies used halothane22,25,76 as an anesthetic drug during joint mobilisation and nerve mobilisation without administering the anesthesia to the control group, which might have confounded the results. The lack of long-term effects of joint and nerve mobilisations on neuroimmune responses can be considered as a limitation. Results of long-term neuroimmune effects may increase extrapolation to human conditions. Finally, 12 animal studies22,24,25,49,50,68,72,75–77,84,85 and 1 human study61 included only males. Consequently, the results may not be extrapolated to females, thereby limiting the translational potential.55 In particular, because sex differences in the immune system might be related to hypersensitivity and pain.86
Disclosures
The authors have no conflicts of interest to declare.
This study was supported by ZonMw grant synthesis of evidence in practice, within the program More Knowledge with Fewer Animals, (grant nr. 114024120; in Dutch: Meer Kennis met Minder Dieren—Module Kennisinfrastructuur “De praktijk van een Synthesis of Evidence” voor dierexperimenteel onderzoek) and the Dutch Association for Manual Therapy (NVMT, grant nr. 2015-10).
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A104.
Acknowledgements
The authors thank Alice Tillema from the Medical Library of the Radboud Medical Center and Prof. Robert Dantzer from the laboratories of Neuroimmunology, Department of Symptom Research, The University of Texas, MD Anderson Cancer Center, for their assistance with the literature search.
Authorship contribution: all authors were involved in the design of the work. I. J. Lutke Schipholt, NT and G. G. M. Scholten-Peeters retrieved the data. All authors were involved in the data analysis and interpretation. I. J. Lutke Schipholt, G. G. M. Scholten-Peeters, M. W. Coppieters, and O. G. Meijer drafted the article. All authors critically revised the article and approved the final version.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Ivo J. Lutke Schipholt, Email: i.j.m.lutkeschipholt@vu.nl.
Michel W. Coppieters, Email: m.coppieters@griffith.edu.au.
Onno G. Meijer, Email: ogmeijer@yahoo.com.
Nefeli Tompra, Email: n.tompra@gmail.com.
Rob B. M. de Vries, Email: Rob.deVries@radboudumc.nl.
References
- [1].al'Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. PAIN 2002;96:197–204. [DOI] [PubMed] [Google Scholar]
- [2].Albrecht DS, Ahmed SU, Kettner NW, Borra RJH, Cohen-Adad J, Deng H, Houle TT, Opalacz A, Roth SA, Melo MFV, Chen L, Mao J, Hooker JM, Loggia ML, Zhang Y. Neuroinflammation of the spinal cord and nerve roots in chronic radicular pain patients. PAIN 2018;159:968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Albrecht DS, Kim M, Akeju O, Torrado-Carvajal A, Edwards RR, Zhang Y, Bergan C, Protsenko E, Kucyi A, Wasan AD, Hooker JM, Napadow V, Loggia ML. The neuroinflammatory component of negative affect in patients with chronic pain. Mol Psychiatry 2021;26:864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Austin PJ, Fiore NT. Supraspinal neuroimmune crosstalk in chronic pain states. Curr Opin Physiol 2019;11:7–15. [Google Scholar]
- [5].Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunology 2010;229:26–50. [DOI] [PubMed] [Google Scholar]
- [6].Baral P, Udit S, Chiu IM. Pain and immunity: implications for host defence. Nat Rev Immunol 2019;19:433–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Basson A, Olivier B, Ellis R, Coppieters M, Stewart A, Mudzi W. The effectiveness of neural mobilization for neuromusculoskeletal conditions: a systematic review and meta-analysis. J Orthop Sports Phys Ther 2017;47:593–615. [DOI] [PubMed] [Google Scholar]
- [8].Berrueta L, Muskaj I, Olenich S, Butler T, Badger GJ, Colas RA, Spite M, Serhan CN, Langevin HM. Stretching impacts inflammation resolution in connective tissue. J Cell Physiol 2016;231:1621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bialosky JE, Beneciuk JM, Bishop MD, Coronado RA, Penza CW, Simon CB, George SZ. Unraveling the mechanisms of manual therapy: modeling an approach. J Orthop Sports Phys Ther 2018;48:8–18. [DOI] [PubMed] [Google Scholar]
- [10].Bove GM, Delany SP, Hobson L, Cruz GE, Harris MY, Amin M, Chapelle SL, Barbe MF. Manual therapy prevents onset of nociceptor activity, sensorimotor dysfunction, and neural fibrosis induced by a volitional repetitive task. PAIN 2019;160:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bove GM, Harris MY, Zhao H, Barbe MF. Manual therapy as an effective treatment for fibrosis in a rat model of upper extremity overuse injury. J Neurol Sci 2016;361:168–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain 2008;9:122–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chapman CR, Vierck CJ. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain 2017;18:359.e351–e338. [DOI] [PubMed] [Google Scholar]
- [14].Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018;100:1292–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Colombi A, Testa M. The effects induced by spinal manipulative therapy on the immune and endocrine systems. Medicina 2019;55:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Coppieters MW, Alshami AM. Longitudinal excursion and strain in the median nerve during novel nerve gliding exercises for carpal tunnel syndrome. J Orthopaedic Res 2007;25:972–80. [DOI] [PubMed] [Google Scholar]
- [17].Coppieters MW, Andersen LS, Johansen R, Giskegjerde PK, Høivik M, Vestre S, Nee RJ. Excursion of the sciatic nerve during nerve mobilization exercises: an in vivo cross-sectional study using dynamic ultrasound imaging. J Orthop Sports Phys Ther 2015;45:731–7. [DOI] [PubMed] [Google Scholar]
- [18].Coppieters MWN R. Neurodynamic management of the peripheral nervous system In: GM Jull A, Falla D, Lewis J, McCarthy C, Sterling M, editors. Grieve's Modern Musculoskeletal Physiotherapy, Vol. 4. Edinburgh: Elsevier, 2015. pp. 287–97. [Google Scholar]
- [19].Corey SM, Vizzard MA, Bouffard NA, Badger GJ, Langevin HM. Stretching of the back improves gait, mechanical sensitivity and connective tissue inflammation in a rodent model. PLoS One 2012;7:e29831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Costigan M, Befort K, Karchewski L, Griffin RS, D'Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci 2002;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cserep C, Posfai B, Lenart N, Fekete R, Laszlo ZI, Lele Z, Orsolits B, Molnar G, Heindl S, Schwarcz AD, Ujvari K, Kornyei Z, Toth K, Szabadits E, Sperlagh B, Baranyi M, Csiba L, Hortobagyi T, Magloczky Z, Martinecz B, Szabo G, Erdelyi F, Szipocs R, Tamkun MM, Gesierich B, Duering M, Katona I, Liesz A, Tamas G, Denes A. Microglia monitor and protect neuronal function via specialized somatic purinergic junctions. Science 2020;367:528–37. [DOI] [PubMed] [Google Scholar]
- [22].da Silva JT, Santos FM, Giardini AC, Martins Dde O, de Oliveira ME, Ciena AP, Gutierrez VP, Watanabe IS, Britto LR, Chacur M. Neural mobilization promotes nerve regeneration by nerve growth factor and myelin protein zero increased after sciatic nerve injury. Growth Factors 2015;33:8–13. [DOI] [PubMed] [Google Scholar]
- [23].Deeks JJ, Higgins JPT, Altman DG. Chaptor 10: analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 61 (updated September 2020) Cochrane, 2020.
- [24].Duarte FCK, Kolberg C, Riffel APK, Souza JA, Bello-Klein A, Partata WA. Spinal manipulation therapy improves tactile allodynia and peripheral nerve functionality and modulates blood oxidative stress markers in rats exposed to knee-joint immobilization. J Manipulative Physiol Ther 2019;42:385–98. [DOI] [PubMed] [Google Scholar]
- [25].Giardini AC, Dos Santos FM, da Silva JT, de Oliveira ME, Martins DO, Chacur M. Neural mobilization treatment decreases glial cells and brain-derived neurotrophic factor expression in the central nervous system in rats with neuropathic pain induced by CCI in rats. Pain Res Manag 2017;2017:7429761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Giraud O, Seince PF, Rolland C, Lecon-Malas V, Desmonts JM, Aubier M, Dehoux M. Halothane reduces the early lipopolysaccharide-induced lung inflammation in mechanically ventilated rats. Am J Respir Crit Care Med 2000;162:2278–86. [DOI] [PubMed] [Google Scholar]
- [27].Gross A, Langevin P, Burnie SJ, Bedard-Brochu MS, Empey B, Dugas E, Faber-Dobrescu M, Andres C, Graham N, Goldsmith CH, Bronfort G, Hoving JL, LeBlanc F. Manipulation and mobilisation for neck pain contrasted against an inactive control or another active treatment. Cochrane Database Syst Rev 2015:Cd004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, Atkins D, Kunz R, Montori V, Jaeschke R, Rind D, Dahm P, Akl EA, Meerpohl J, Vist G, Berliner E, Norris S, Falck-Ytter Y, Schunemann HJ. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013;66:151–7. [DOI] [PubMed] [Google Scholar]
- [29].Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Higgins JPTAD, Sterne JAC. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at: www.handbook.cochrane.org. Accessed 24 July, 2020. [Google Scholar]
- [31].Hooijmans CR, de Vries RBM, Ritskes-Hoitinga M, Rovers MM, Leeflang MM, IntHout J, Wever KE, Hooft L, de Beer H, Kuijpers T, Macleod MR, Sena ES, Ter Riet G, Morgan RL, Thayer KA, Rooney AA, Guyatt GH, Schunemann HJ, Langendam MW. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS one 2018;13:e0187271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hooijmans CR, IntHout J, Ritskes-Hoitinga M, Rovers MM. Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. ILAR J 2014;55:418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ji R-R, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science 2016;354:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? PAIN 2013;154(Suppl 1):S10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kilkenny C, Browne WJ, Cuthi I, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Vet Clin Pathol 2012;41:27–31. [DOI] [PubMed] [Google Scholar]
- [37].Klyne DM, Barbe MF, Hodges PW. Systemic inflammatory profiles and their relationships with demographic, behavioural and clinical features in acute low back pain. Brain Behav Immun 2017;60:84–92. [DOI] [PubMed] [Google Scholar]
- [38].Kovanur-Sampath K, Mani R, Cotter J, Gisselman AS, Tumilty S. Changes in biochemical markers following spinal manipulation-a systematic review and meta-analysis. Musculoskelet Sci Pract 2017;29:120–31. [DOI] [PubMed] [Google Scholar]
- [39].Kudielka BM, Gierens A, Hellhammer DH, Wust S, Schlotz W. Salivary cortisol in ambulatory assessment-some dos, some don'ts, and some open questions. Psychosom Med 2012;74:418–31. [DOI] [PubMed] [Google Scholar]
- [40].Kunanusornchai W, Muanprasat C, Chatsudthipong V. Adenosine monophosphate-activated protein kinase activation and suppression of inflammatory response by cell stretching in rabbit synovial fibroblasts. Mol Cell Biochem 2016;423:175–85. [DOI] [PubMed] [Google Scholar]
- [41].Li T, Chen X, Zhang C, Zhang Y, Yao W. An update on reactive astrocytes in chronic pain. J neuroinflammation 2019;16:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lima CR, Martins DF, Reed WR. Physiological responses induced by manual therapy in animal models: a scoping review. Front Neurosci 2020;14:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, Riley M, Wasan AD, Zurcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM. Evidence for brain glial activation in chronic pain patients. Brain 2015;138:604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lohman CM, Gilbert KK, Sobczak S, Brismée JM, James CR, Day M, Smith MP, Taylor L, Dugailly PM, Pendergrass T, Sizer PJ. Young investigator award winner: cervical nerve root displacement and strain during upper limb neural tension testing: Part 1: a minimally invasive assessment in unembalmed cadavers. Spine 2015;40:793–800. [DOI] [PubMed] [Google Scholar]
- [45].Lohman EB, Pacheco GR, Gharibvand L, Daher N, Devore K, Bains G, AlAmeri M, Berk LS. The immediate effects of cervical spine manipulation on pain and biochemical markers in females with acute non-specific mechanical neck pain: a randomized clinical trial. J Man Manipulative Ther 2019;27:186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Malfliet A, Lluch Girbes E, Pecos-Martin D, Gallego-Izquierdo T, Valera-Calero A. The influence of treatment expectations on clinical outcomes and cortisol levels in patients with chronic neck pain: an experimental study. Pain Pract 2019;19:370–81. [DOI] [PubMed] [Google Scholar]
- [47].Marcioli M, Meireles A, Silva L, Rosa C, Bertolini G. Grip strength evaluation of Wistar rats submitted to a model of median nerve compression treated with neural mobilization. Int J of Ther Rehab Res 2012;1:29. doi: 10.5455/ijtrr.00000012. [DOI] [Google Scholar]
- [48].Marcioli MA, Coradini JG, Kunz RI, Ribeiro Lde F, Brancalhao RM, Bertolini GR. Nociceptive and histomorphometric evaluation of neural mobilization in experimental injury of the median nerve. ScientificWorldJournal 2013;2013:476890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Marcioli MAR, Silva J, Ribeiro LFC, Brancalhao RMC, Bertolini GRF. Neurotrophin expression and histomorphometric evaluation in Wistar rats subjected to neural mobilization after compression of the median nerve. Rev Bras Ortop 2018;53:276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Martins DF, Mazzardo-Martins L, Gadotti VM, Nascimento FP, Lima DA, Speckhann B, Favretto GA, Bobinski F, Cargnin-Ferreira E, Bressan E, Dutra RC, Calixto JB, Santos AR. Ankle joint mobilization reduces axonotmesis-induced neuropathic pain and glial activation in the spinal cord and enhances nerve regeneration in rats. PAIN 2011;152:2653–61. [DOI] [PubMed] [Google Scholar]
- [51].Meijer OG, Barbe MF, Prins MR, Schipholt IJL, Hu H, Daffertshofer A. The Pelvic Girdle Pain deadlock: 2. Topics that, so far, have remained out of focus. Musculoskelet Sci Pract 2020;48:102166. [DOI] [PubMed] [Google Scholar]
- [52].Miller TR, Wetter JB, Jarvis MF, Bitner RS. Spinal microglial activation in rat models of neuropathic and osteoarthritic pain: an autoradiographic study using [3H]PK11195. Eur J Pain 2013;17:692–703. [DOI] [PubMed] [Google Scholar]
- [53].Milligan ED, Penzkover KR, Soderquist RG, Mahoney MJ. Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation 2012;15:520–6; discussion 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014;20:1126–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 2020;21:353–65. [DOI] [PubMed] [Google Scholar]
- [56].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- [58].Nee RJ, Butler D. Management of peripheral neuropathic pain: integrating neurobiology, neurodynamics, and clinical evidence. Phys Ther Sport 2006;7:36–49. [Google Scholar]
- [59].Nee RJ, Vicenzino B, Jull GA, Cleland JA, Coppieters MW. Neural tissue management provides immediate clinically relevant benefits without harmful effects for patients with nerve-related neck and arm pain: a randomised trial. J Physiother 2012;58:23–31. [DOI] [PubMed] [Google Scholar]
- [60].Nees F, Löffler M, Usai K, Flor H. Hypothalamic-pituitary-adrenal axis feedback sensitivity in different states of back pain. Psychoneuroendocrinol 2019;101:60–6. [DOI] [PubMed] [Google Scholar]
- [61].Padayachy K, Vawda GHM, Shaik J, McCarthy PW. The immediate effect of low back manipulation on serum cortisol levels in adult males with mechanical low back pain. Clin Chiropractic 2010;13:246–52 247p. [Google Scholar]
- [62].Paige NM, Miake-Lye IM, Booth MS, Beroes JM, Mardian AS, Dougherty P, Branson R, Tang B, Morton SC, Shekelle PG. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain: systematic review and meta-analysis. Jama 2017;317:1451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pieters L, Lewis J, Kuppens K, Jochems J, Bruijstens T, Joossens L, Struyf F. An update of systematic reviews examining the effectiveness of conservative physiotherapy interventions for subacromial shoulder pain. J Orthopaedic Sports Phys Ther 2019;50:1–33. [DOI] [PubMed] [Google Scholar]
- [64].Pinho-Ribeiro FA, Verri WA, Jr, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunology 2017;38:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Reed WR, Little JW, Lima CR, Sorge RE, Yarar-Fisher C, Eraslan M, Hurt CP, Ness TJ, Gu JG, Martins DF, Li P. Spinal mobilization prevents NGF-induced trunk mechanical hyperalgesia and attenuates expression of CGRP. Front Neurosci 2020;14:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ren K, Torres R. Role of interleukin-1β during pain and inflammation. Brain Res Rev 2009;60:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Roy A, Jana A, Yatish K, Freidt MB, Fung YK, Martinson JA, Pahan K. Reactive oxygen species up-regulate CD11b in microglia via nitric oxide: implications for neurodegenerative diseases. Free Radic Biol Med 2008;45:686–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ruhlen RL, Singh VK, Pazdernik VK, Towns LC, Snider EJ, Sargentini NJ, Degenhardt BF. Changes in rat spinal cord gene expression after inflammatory hyperalgesia of the joint and manual therapy. J Am Osteopathic Assoc 2014;114:768–76. [DOI] [PubMed] [Google Scholar]
- [69].Ruhlen RL, Snider EJ, Sargentini NJ, Worthington BD, Singh VK, Pazdernik VK, Johnson JC, Degenhardt BF. Influence of manual therapy on functional mobility after joint injury in a rat model. J Am Osteopathic Assoc 2013;113:738–52, e748-739. [DOI] [PubMed] [Google Scholar]
- [70].Rushton A, Beeton K, Jordaan R, Langendoen J, Levesque L, Maffey L, Pool J. Educational standards in orthopaedic manipulative therapy. IFOMPT, 2016. [Google Scholar]
- [71].Russell G, Lightman S. The human stress response. Nat Rev Endocrinol 2019;15:525–34. [DOI] [PubMed] [Google Scholar]
- [72].Salgado ASI, Stramosk J, Ludtke DD, Kuci ACC, Salm DC, Ceci LA, Petronilho F, Florentino D, Danielski LG, Gassenferth A, Souza LR, Rezin GT, Santos ARS, Mazzardo-Martins L, Reed WR, Martins DF. Manual therapy reduces pain behavior and oxidative stress in a murine model of complex regional pain syndrome type I. Brain Sci 2019;9:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Salter MW, Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell 2014;158:15–24. [DOI] [PubMed] [Google Scholar]
- [74].Sanders GE, Reinert O, Tepe R, Maloney P. Chiropractic adjustive manipulation on subjects with acute low back pain: visual analog pain scores and plasma beta-endorphin levels. J Manipulative Physiol Ther 1990;13:391–5. [PubMed] [Google Scholar]
- [75].Santos FM, Grecco LH, Pereira MG, Oliveira ME, Rocha PA, Silva JT, Martins DO, Miyabara EH, Chacur M. The neural mobilization technique modulates the expression of endogenous opioids in the periaqueductal gray and improves muscle strength and mobility in rats with neuropathic pain. Behav Brain Funct 2014;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Santos FM, Silva JT, Giardini AC, Rocha PA, Achermann AP, Alves AS, Britto LR, Chacur M. Neural mobilization reverses behavioral and cellular changes that characterize neuropathic pain in rats. Mol pain 2012;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Santos FM, Silva JT, Rocha IRC, Martins DO, Chacur M. Non-pharmacological treatment affects neuropeptide expression in neuropathic pain model. Brain Res 2018;1687:60–5. [DOI] [PubMed] [Google Scholar]
- [78].Sawicki CM, Humeidan ML, Sheridan JF. Neuroimmune interactions in pain and stress: an interdisciplinary approach. Neuroscientist 2021;27:113–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Schmid AB, Coppieters MW, Ruitenberg MJ, McLachlan EM. Local and remote immune-mediated inflammation after mild peripheral nerve compression in rats. J Neuropathol Exp Neurol 2013;72:662–80. [DOI] [PubMed] [Google Scholar]
- [80].Schmid AB, Nee RJ, Coppieters MW. Reappraising entrapment neuropathies--mechanisms, diagnosis and management. Man Ther 2013;18:449–57. [DOI] [PubMed] [Google Scholar]
- [81].Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007;10:1361–8. [DOI] [PubMed] [Google Scholar]
- [82].Shen KF, Zhu HQ, Wei XH, Wang J, Li YY, Pang RP, Liu XG. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp Neurol 2013;247:466–75. [DOI] [PubMed] [Google Scholar]
- [83].Sluka KA, Wright A. Knee joint mobilization reduces secondary mechanical hyperalgesia induced by capsaicin injection into the ankle joint. Eur J Pain 2001;5:81–7. [DOI] [PubMed] [Google Scholar]
- [84].Song XJ, Gan Q, Cao JL, Wang ZB, Rupert RL. Spinal manipulation reduces pain and hyperalgesia after lumbar intervertebral foramen inflammation in the rat. J Manipulative Physiol Ther 2006;29:5–13. [DOI] [PubMed] [Google Scholar]
- [85].Song XJ, Huang ZJ, Song WB, Song XS, Fuhr AF, Rosner AL, Ndtan H, Rupert RL. Attenuation effect of spinal manipulation on neuropathic and postoperative pain through activating endogenous anti-inflammatory cytokine interleukin 10 in rat spinal cord. J manipulative Physiol Ther 2016;39:42–53. [DOI] [PubMed] [Google Scholar]
- [86].Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015;18:1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sterling M, Elliott JM, Cabot PJ. The course of serum inflammatory biomarkers following whiplash injury and their relationship to sensory and muscle measures: a longitudinal cohort study. PLoS One 2013;8:e77903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Teodorczyk-Injeyan JA, Triano JJ, Injeyan HS. Nonspecific low back pain: inflammatory profiles of patients with acute and chronic pain. Clin J Pain 2019;35:818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Twining CM, Sloane EM, Milligan ED, Chacur M, Martin D, Poole S, Marsh H, Maier SF, Watkins LR. Peri-sciatic proinflammatory cytokines, reactive oxygen species, and complement induce mirror-image neuropathic pain in rats. PAIN 2004;110:299–309. [DOI] [PubMed] [Google Scholar]
- [90].Valera-Calero A, Lluch E, Gallego-Izquierdo T, Malfliet A, Pecos-Martin D. Endocrine response after cervical manipulation and mobilization in people with chronic mechanical neck pain: a randomized controlled trial. Eur J Phys Rehabil Med 2019;55:792–805. [DOI] [PubMed] [Google Scholar]
- [91].Woda A, Picard P, Dutheil F. Dysfunctional stress responses in chronic pain. Psychoneuroendocrinol 2016;71:127–35. [DOI] [PubMed] [Google Scholar]
- [92].Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-alpha, IL-6, and IL-1beta. Neurobiol Aging 2012;33:1364–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Xu Q, Chen B, Wang Y, Wang X, Han D, Ding D, Zheng Y, Cao Y, Zhan H, Zhou Y. The effectiveness of manual therapy for relieving pain, stiffness, and dysfunction in knee osteoarthritis: a systematic review and meta-analysis. Pain physician 2017;20:229–43. [PubMed] [Google Scholar]
- [94].Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, Basbaum AI. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 2020;11:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yukhananov R, Kissin I. Persistent changes in spinal cord gene expression after recovery from inflammatory hyperalgesia: a preliminary study on pain memory. BMC Neurosci 2008;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zemadanis K, Betsos T, Philippou A, Koutsilieris M. The impact of manual therapy techniques on pain, disability and il-1b levels in patients with chronic cervical pain. Int J Physiother 2019;6. doi: 10.15621/ijphy/2019/v6i6/190224. [DOI] [Google Scholar]
- [97].Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. Interleukin-10 provides direct trophic support to neurons. J Neurochem 2009;110:1617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zusman M. There's something about passive movement. Med hypotheses 2010;75:106–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A104.


