Abstract
Drug-induced lysosomal storage disease (DILSD) caused by cationic amphiphilic drugs (CADs), which exhibits toxic manifestations and pathological findings mimicking Fabry disease (α-galactosidase A deficiency), has attracted the interests of clinicians and pathologists. Although the affected region is lysosomes in both the diseases, DILSD is characterized by intralysosomal accumulation of phospholipids and Fabry disease that of globotriaosylceramide (Gb3) and globotriaosylsphingosine (Lyso-Gb3). However, it is unknown whether administration of CADs affects the catabolism of Gb3 and Lyso-Gb3 in Fabry disease. In this study, we independently administered hydroxychloroquine/amiodarone to wild-type and Fabry mice and examined the effects of the drugs on the enzyme activity and substrates accumulated in organs and tissues. The results revealed that the administration of the drugs induced accumulation of phosphatidylcholine in both the wild-type and Fabry mice. However, reduction of α-galactosidase A activity in the organs and tissues of the wild-type mice was not found, and the storage of Gb3 and Lyso-Gb3 was not accelerated by these drugs in the Fabry mice. This suggests that hydroxychloroquine/amiodarone do not have any significant impact on the catabolism of Gb3 and Lyso-Gb3 in organs and tissues of both wild-type and Fabry mice.
Keywords: Fabry disease, α-Galactosidase A, Globotriaosylceramide, Globotriaosylsphingosine, Drug-induced lysosomal storage disease, Amiodarone, Hydroxychloroquine, Phospholipid
Abbreviations: α-Gal, α-galactosidase A; Gb3, globotriaosylceramide; Lyso-Gb3, globotriaosylsphingosine; CAD, cationic amphiphilic drug; DILSD, drug-induced lysosomal storage disease; PhC, phosphatidylcholine; LC, liquid chromatography; MS/MS, tandem mass spectrometry; MRM, multiple reaction monitoring; ILV, intralysosomal luminal vesicle
Highlights
-
•
Effects of cationic amphiphilic drugs on the catabolism of Gb3/Lyso-Gb3 were examined.
-
•
The drugs induced phospholipidosis in the wild-type and Fabry mice.
-
•
The drugs did not induce reduction of α-galactosidase A activity in the wild-type mice.
-
•
The drugs did not accelerate accumulation of Gb3/Lyso-gb3 in the Fabry mice.
1. Introduction
Fabry disease (OMIM 301500) is an X-linked genetic disease caused by a deficiency of α-galactosidase A (α-Gal, EC 3.2.1.22) activity. The enzymatic defect leads to impaired catabolism of sphingolipids, resulting in accumulation of globotriaosylceramide (Gb3) and globotriaosylsphingosine (Lyso-Gb3) in lysosomes of various types of cells [1]. Classically affected males with Fabry disease have onset in childhood or adolescence and exhibit acroparesthesia, angiokeratoma, hypohidrosis, and corneal opacities. They develop life-threatening renal, cardiac and cerebrovascuolar disorders in adulthood. Some atypical male patients, who usually have residual α-Gal activity, develop a heart and/or renal disorder without the childhood symptoms [1,2]. The clinical features of Fabry females are heterogeneous, in general milder, according to random X-chromosomal inactivation [3]. The pathological features of biopsied tissues in Fabry patients are characteristic, exhibiting the ultrastructural appearance of lamellar inclusion bodies, zebra bodies, myeloid bodies or concentric electron dense bodies in cells. The existence of such pathological findings had been thought to constitute strong evidence that a patient had Fabry disease [1], although exceptional cases, in which a significant Gb3 deposits could be observed in cardiac tissue before the formation of inclusion bodies, have been reported, suggesting the cardiomyocytes might be affected early on, before the appearance of typical pathological changes during the disease progression [4].
Recently, acquired lysosomal storage diseases caused by cationic amphiphilic drugs (CADs) including chloroquine/hydroxychloroquine and amiodarone have attracted the interests of clinicians and pathologists [[5], [6], [7]]. Chloroquine and hydroxychloroquine are antimalarial drugs, and are also used for treatment of connective tissue diseases such as systemic lupus erythematosus, cutaneous lupus erythematosus, rheumatoid arthritis, and Sjögren syndrome, as an immunomodulator [8,9]. On the other hand, amiodarone is an anti-arrhythmic agent for treatment of ventricular and supraventricular arrhythmia that is difficult to control with other drugs [10,11], and is sometimes used for Fabry patients suffering from severe arrhythmia. It has been reported that patients treated with these drugs sometimes exhibit toxic manifestations and pathological findings that cannot be distinguished from those of Fabry disease [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]]. Furthermore, there is a report that a Fabry male placed on amiodarone for treatment of arrhythmia developed acute decompensated heart failure, although the heart failure resolved after amiodarone was discontinued [27].
A drug-induced lysosomal storage disease (DILSD) caused by CADs is characterized by the accumulation of phospholipids [[5], [6], [7]]. However, the effects of CADs on the metabolism of sphingolipids, especially that on the catabolism of Gb3 and Lyso-Gb3, are unclear. As there would be cases in which chloroquine/hydroxychloroquine or amiodarone are needed to treat patients with Fabry disease, it is important to determine whether these drugs accelerate the storage of Gb3 and Lyso-Gb3, and affect Fabry patients or not in order to avoid inappropriate treatment for them.
In this study, we administered hydroxychloroquine/amiodarone independently to wild-type and Fabry mice, and examined the effects of these drugs on α-Gal activity and the contents of phosphatidylcholine (PhC), as a representative phospholipid, Gb3, and Lyso-Gb3 in their organs and tissues.
2. Material and methods
2.1. Reagents
Hydroxychloroquine sulfate and amiodarone hydrochloride (Ancaron®) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and Sanofi Co., (Tokyo, Japan), respectively. PhC (C24:0) was purchased from Wako Pure Chemical Co., Ltd. (Osaka, Japan). 4-Methylumbelliferyl α-D-galactopyranoside and N-acetyl-D-galactosamine were purchased from Calbiochem (La Jolla, CA) and Sigma-Aldrich (St. Louis, MO), respectively. A mixture of various Gb3 isoforms, Gb3 (C17:0), and Lyso-Gb3 were purchased from Matreya LLC. (Pleasant Gap, PA). Stable isotope-labelled Lyso-Gb3 having one 13C and three deuterium atoms was synthesized by Nard Institute, Ltd. (Kobe, Japan). All other reagents used in this study were of analytical grade.
2.2. Animals
Four-month-old male Gla-knockout (Fabry model) mice donated by A.B. Kulkarni and T. Oshima (Gene Targeting Research and Core Facility, National Institute of Dental Research, National Institute of Health), and C57BL/6 J wild-type ones were used for animal experiments in this study. The study involving mice was approved by the Animal Care and Use Committee of Meiji Pharmaceutical University.
2.3. Animal experiments
To examine effects of hydroxychloroquine and amiodarone on the cabolism of Gb3 and Lyso-Gb3 in Fabry disease, 50 mg/kg body weight of these drugs was independently administered intraperitoneally to Fabry mice and wild-type ones every day for 14 days, the mice being sacrificed on the day after the last injection. The mice were perfused then with phosphate-buffered saline (PBS), pH 7.4. Then, their livers, kidneys and hearts were harvested, homogenized in 20 mmol/L MES buffer, pH 6.0, containing cOmplete™ mini (Sigma-Aldrich), and used for biochemical analyses. Each group consisted of four mice.
2.4. Protein determination
The protein concentrations of the homogenates were determined with a Micro BCA protein assay kit (PIERCE, Rockford, IL), using bovine serum albumin as the standard.
2.5. Measurement of PhC in livers of mice
To confirm the occurrence of phospholipidosis induced by hydroxychloroquine/amiodarone, the contents of PhC isoforms in the liver of mice were measured basically according to the method of Sanoh et al. [28]. Briefly, the lipid fraction of a liver homogenate, containing PhC (C24:0) as an internal standard (PhC IS), was extracted by adding methanol:chloroform:water (2:2:1). Then, the chloroform layer was separated by centrifugation, followed by second extraction with chloroform. Then, the lower extracts were evaporated and the residue was reconstituted in isopropanol:methanol (2:1). For liquid chromatography (LC), a LC system (Shimadzu, Kyoto, Japan) and an Inertsil ODS-3 column (125 × 2.1 mm ID., 5 μm; GL Science Ltd., Tokyo, Japan) were used. The mobile phases were mixtures of 0.1% formic acid (solvent A) and methanol (solvent B) flowing at 300 μL/min at 40 °C. The gradient conditions were as follows: 0–1.5 min, 70% B; 1.5–6.0 min, 70–100% B; 6.0–15.0 min, 100% B; 15.0–17.0 min, 100–70% B; and 17.0–20.0 min, 70% B for re-equilibration. For tandem mass spectrometry (MS/MS) to detect PhC isoforms, an LCMS-8040 triple quadrupole mass spectrometer (Shimadzu) equipped with an electrospray ionization interface was used. The multiple reaction monitoring (MRM) transitions of the target PhC isoforms (C32:0-C40:7) are shown in Table 1.
Table 1.
The MRM transitions for PhCs, Gb3 isoforms and Lyso-Gb3.
| Precursor ion [m/z] | Product ion [m/z] | Collision energy [v] | |
|---|---|---|---|
| PhC (C24:0) IS | 622.3 | 86.2 | 61 |
| PhC (C32:0) | 734.5 | 184.0 | 30 |
| PhC (C34:1) | 760.6 | 183.9 | 28 |
| PhC (C36:2) | 786.4 | 184.0 | 31 |
| PhC (C36:4) | 782.5 | 184.0 | 30 |
| PhC (C38:0) | 818.4 | 184.0 | 35 |
| PhC (C38:3) | 812.4 | 183.9 | 32 |
| PhC (C40:7) | 832.2 | 184.1 | 41 |
| Gb3 (C16:0) | 1046.7 | 884.6 | 66 |
| Gb3 (C17:0) IS | 1060.7 | 898.6 | 64 |
| Gb3 (C18:0) | 1074.7 | 912.6 | 71 |
| Gb3 (C20:0) | 1102.7 | 940.7 | 66 |
| Gb3 (C22:0) | 1130.8 | 968.7 | 67 |
| Gb3 (C22:1) | 1128.7 | 966.7 | 66 |
| Gb3 (C24:0) | 1158.8 | 996.7 | 70 |
| Gb3 (C24:1) | 1156.8 | 994.7 | 70 |
| Gb3 (C24OH) | 1174.8 | 1012.7 | 69 |
| Lyso-Gb3 | 786.5 | 282.4 | 39 |
| Lyso-Gb3 IS | 790.3 | 286.4 | 38 |
2.6. Measurement of α-Gal activity in organs and tissues of mice
α -Gal activity in organs and tissues in mice was measured by assaying with an artificial substrate, as described previously [29]. Ten μl of a tissue homogenate was mixed with the substrate solution comprising 5 mmol/L 4-methylumbelliferyl α -D-galactopyranoside, as a substrate, and 117 mmol/L N-acetyl-D-galactosamine, as an inhibitor of α -galactosidase B (α -N-acetylgalactosaminidase), in 0.1 mol/L citrate-phosphate buffer, pH 4.6, in a 1.5 mL micro-tube, followed by incubation at 37 °C for 30 min. Then, the reaction was stopped by adding 950 μL of 0.2 mol/L glycine buffer, pH 10.7. Then, the released 4-methylumberiferone was measured using a fluorometer (Varioskan LUX; Thermo Fisher Scientific, Waltham, MA) at excitation and emission wavelengths of 365 nm and 450 nm, respectively.
2.7. Measurement of Gb3 and Lyso-Gb3 in organs and tissues of mice
The contents of Gb3 and Lyso-Gb3 in organs and tissues were determined by LC and MS/MS, as described previously [30]. Briefly, for extraction of Gb3 and Lys-Gb3 from organs and tissues, a 10 μL aliquot of a homogenate was mixed with a 70 μL aliquot of chloroform:methanol (1:2), and then a 10 μL aliquot of 5 μg/mL Gb3 (C17:0) (Gb3 IS) and a 10 μL aliquot of 500 nmol/L stable isotope-labelled Lyso-Gb3 (Lyso-Gb3 IS) were added as the internal standards. The mixture was centrifuged and the supernatant was transferred to a LC vial. For LC, an Unison UK-C8 column (20 × 3 mm ID., 3 μm; Imtakt Co.) was used, the column oven being kept at 30 °C. Chromatographic separation was performed with a binary gradient comprising a mobile phase of water containing 0.1% acetic acid and 2 mmol/L ammonium acetate (solution A), and methanol containing 0.1% acetic acid and 2 mmol/L ammonium acetate (solution B). The gradient conditions were as follows: 0–0.5 min, 50–100% B; 0.5–5.5 min, 100% B; and 5.5–13 min, 50% B. The flow rate was 0.25 mL/min and the injection volume was 2 μL. Then, Gb3 isoforms and Lyso-Gb3 in the samples were detected by MS/MS using a LCMS-8040 triple quadrupole mass spectrometer (Shimadzu) equipped with an electrospray ionization interface in the positive-ion mode. The MRM transitions for Gb3 isoforms and Lyso-Gb3 are shown in Table 1. The total Gb3 contents of the organs and tissues were calculated from the sums of the Gb3 isoforms.
2.8. Statistical analysis
Data are expressed as means ± standard deviation (SD) [n: number of trials]. The differences among the targeted groups were assessed by means of Welch's t-test, it being taken that there was a significant difference if p < 0.05.
3. Results
3.1. Levels of PhCs after administration of hydroxychloroquine/amiodarone in livers of wild-type and Fabry mice
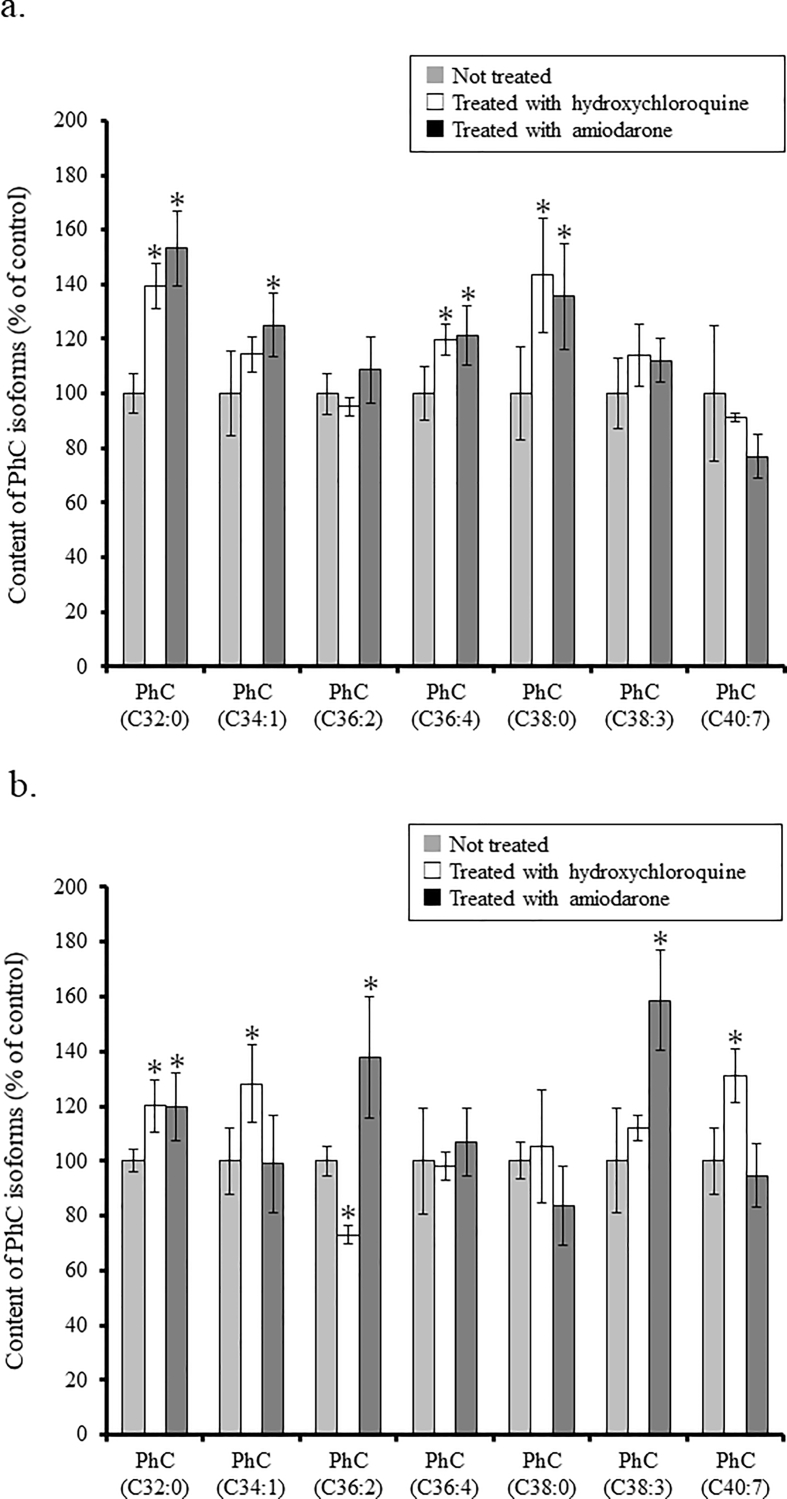
To examine the effects of hydroxychloroquine/amiodarone on the metabolism of phospholipids in the livers of wild-type and Fabry mice, the levels of PhC isoforms in their livers were measured by means of LC-MS/MS, and the results are summarized in Fig. 1. When hydroxychloroquine was administered, increases in the contents of PhC (C32:0), PhC (C36:4), and PhC (C38:0), and those of PhC (C32:0), PhC (C34:1), and PhC (C40:7) were observed in the wild-type and Fabry mice, respectively, although the level of PhC (C36:2) in the hydroxychloroquine-treated Fabry mice was decreased, compared with in non-treated cases (treated vs. not treated, p < 0.05). The reason why it decreased is unknown, but it was confirmed that the content of whole PhC isoforms apparently increased in hydroxychloroquine-administered Fabry mice compared with in non-treated cases. On the other hand, when amiodarone was administered, increases in the contents of PhC (32:0), PhC (C34:1), PhC (C36:4), and PhC (38:0), and those of PhC (32:0), PhC (36:2), and PhC (38:3) were found in the wild-type and Fabry mice, respectively (treated vs. not treated, p < 0.05).
Fig. 1.
Contents of PhC isoforms in the liver of the wild-type (a) and Fabry mice (b). The level of each PhC isoforms in the liver of the mice administered with hydroxychloroquine/amiodarone is exhibited as a value (%) compared to that of the non-treated ones (controls). Gray column: not treated; white column: treated with hydroxychloroquine; and black column: treated with amiodarone. Data are expressed as means ± SD (n = 4), *treated vs. not treated, p < 0.05.
3.2. α-Gal activity in organs and tissues of wild-type and Fabry mice after administration of hydroxychloroquine/amiodarone
To examine the effects of hydroxychloroquine/amiodarone on α-Gal, the enzyme responsible for Fabry disease, the enzyme activity in the liver, kidneys, and heart of wild-type and Fabry mice was measured, and the results are summarized in Table 2. The α-Gal activity in the organs and tissues of both the wild-type and Fabry mice was not affected by the administration of the drugs (treated vs. not treated, p ≥ 0.05), the wild-type mice exhibiting sufficient α-Gal activity and the Fabry mice a deficiency of it.
Table 2.
α-Gal activity in organs and tissues of wild-type and Fabry mice.
| Wild-type [n = 4] |
Fabry [n = 4] |
|
|---|---|---|
| (nmol/h/mg/protein) | (nmol/h/mg/protein) | |
| Liver | ||
| Not treated | 26 ± 4 | < 1 |
| Hydroxychloroquine | 28 ± 2 | < 1 |
| Amiodarone | 28 ± 3 | < 1 |
| Kidneys | ||
| Not treated | 17 ± 1 | < 1 |
| Hydroxychloroquine | 17 ± 1 | < 1 |
| Amiodarone | 16 ± 1 | < 1 |
| Heart | ||
| Not treated | 4 ± 0 | < 1 |
| Hydroxychloroquine | 4 ± 1 | < 1 |
| Amiodarone | 3 ± 0 | < 1 |
Data are expressed as means ± SD.
3.3. Contents of Gb3 and Lyso-Gb3 in organs and tissues of wild-type and Fabry mice after administration of hydroxychloroquine/amiodarone
To examine the impact of hydroxychloroquine/amiodarone on the metabolism of Gb3 and Lyso-Gb3 in various organs and tissues of wild-type and Fabry mice, the contents of Gb3 and Lyso-Gb3 in the liver, kidneys, and heart were measured by means of LC-MS/MS. The results of the measurements are summarized in Table 3. The contents of Gb3 and Lyso-Gb3 in the liver, kidneys, and heart of Fabry mice were apparently higher than those in the wild-type ones (wild-type vs. Fabry, p < 0.05). However, there were no statistical differences in the contents of Gb3 and Lyso-Gb3 in the organs and tissues between the hydroxychloroquine/amiodarone administered group and non-treated one (treated vs. not treated, p ≥ 0.05) in both the wild-type and Fabry mice.
Table 3.
Contents of Gb3 and Lyso-Gb3 in organs and tissues of wild-type and Fabry mice.
| Wild-type [n = 4] |
Fabry [n = 4] |
|||
|---|---|---|---|---|
| Gb3 (μg/g) | Lyso-Gb3 (nmol/g) | Gb3 (μg/g) | Lyso-Gb3 (nmol/g) | |
| Liver | ||||
| Not treated | 1.0 ± 0.0 | 0.08 ± 0.01 | 155 ± 15 | 7.3 ± 0.6 |
| Hydroxychloroquine | 1.0 ± 0.0 | 0.06 ± 0.01 | 145 ± 12 | 6.7 ± 0.3 |
| Amiodarone | 1.0 ± 0.0 | 0.06 ± 0.01 | 169 ± 9 | 7.6 ± 0.9 |
| Kidneys | ||||
| Not treated | 15 ± 3 | 0.08 ± 0.01 | 165 ± 12 | 2.0 ± 0.3 |
| Hydroxychloroquine | 13 ± 2 | 0.08 ± 0.01 | 156 ± 10 | 1.9 ± 0.1 |
| Amiodarone | 14 ± 1 | 0.09 ± 0.02 | 160 ± 16 | 2.1 ± 0.2 |
| Heart | ||||
| Not treated | 0.5 ± 0.0 | 0.09 ± 0.02 | 37 ± 6 | 0.59 ± 0.11 |
| Hydroxychloroquine | 0.5 ± 0.0 | 0.10 ± 0.01 | 38 ± 3 | 0.58 ± 0.55 |
| Amiodarone | 0.5 ± 0.0 | 0.08 ± 0.01 | 40 ± 3 | 0.59 ± 0.07 |
Data are expressed as means ± SD.
4. Discussion
CADs including hydroxychloroquine/amiodarone have both a hydrophobic ring structure and a hydrophilic side chain with charged cationic amines, being partly soluble in nonpolar solvents and partly soluble in water. Due to these characteristics, they are incorporated into various cells and induce accumulation of phospholipids such as PhC, sphingomyelin, phosphatidylserine, and phosphatidylethanolamine in lysosomes, leading to the formation of lamellar inclusion bodies [31]. As the morphological findings and toxic manifestations are similar to those in Fabry disease, DILSD caused by CADs sometimes leads to initial erroneous interpretation of Fabry disease [22].
Although the molecular mechanism underlying CAD-induced phospholipidosis remains to be resolved, it has been reported that CADs directly inhibit the activities of phospholipases responsible for phospholipid catabolism and bind to phospholipids, followed by the formation of a complex more resistant to degradation [6,31,32]. There have also been reports that CADs inhibit the function of saposin B [33], an activator of some lysosomal enzymes including α-Gal, and disturb autophagy [34]. Furthermore, CADs are thought to cause redistribution of mannose 6-phosphate receptors in cultured cells, resulting in increased secretion and an intracellular decline of lysosomal enzymes [35]. In cultured cells, CADs incorporated into cells from the culture medium are integrated into the lysosomes, and increase the pH value, leading to inhibition of lysosomal enzymes, especially α-Gal, although their direct inhibitory effect on the enzymes is not so strong [36]. Inagaki et al. reported that treatment of transformed endothelial cells with chloroquine caused specific reduction of α-Gal activity in cultured cells and treatment of cells with chloroquine and glycosphingolipid mixture induced lamellar structures [37].
Thus, considering that both Fabry disease and DILSD caused by CADs affect lysosomes and exhibit similar pathological findings, it is important for clinicians involved in treatment of Fabry patients to know whether hydroxychloroquine/amiodarone affect the metabolism of glycosphingolipids including Gb3 and Lyso-Gb3 and thereby exacerbate the disease or not.
In this study, we intraperitoneally administered hydroxychloroquine/amiodarone to wild-type and Fabry mice, and measured α-Gal activity and the contents of Gb3 and Lyso-Gb3 in their organs and tissues. The doses of the drugs were determined according to the papers describing experiments involving rats previously published [[38], [39], [40]]. According to a report by Kumamoto, et al. [40], administration of 50 mg/kg body weight of chloroquine induced pathological changes such as inclusion bodies in the organs and tissues. The results of our experiment confirmed that administration of hydroxychloroquine/amiodarone under experimental conditions induced accumulation of PhCs in the liver in both the wild-type and Fabry mice. However, a decrease in α-Gal activity in the organs and tissues of the wild-type mice was not found. As it is known that hydroxychloroquine/amiodarone are essentially catabolized in the liver via various metabolic pathways and excreted in urine and feces (Japan Pharmaceutical Interview Form on hydroxychloroquine sulfate/amiodarone hydrochrolide), these drugs may not be concentrated in the lysosomes of cells of organs and tissues under experimental conditions, different from in the case of cultured cells.
Recently, Breiden and Sandhoff [41] proposed intralysosomal luminal vesicles (ILVs) as major platforms for degradation of lipids and phospholipids. They reported that CADs possibly affect ILVs and thereby inhibit the lysosomal degradation of major lipids, sphingolipids and phospholipids although the resulting lysosomal storage is dominated by the accumulation of phospholipids as their normal turnover exceeds that of sphingolipids. The results of our experiments suggested that hydroxychloroquine/amiodarone do not have any significant impact on the catabolism of Gb3 and Lyso-Gb3, at least they did not induce acceleration of the storage of Gb3 and Lyso-Gb3 in the organs and tissues of Fabry mice, although it has not been completely determined whether the defects of lysosomal functions induced by the CADs lead to progression of Fabry disease. More detailed analysis is required in future.
An Australian product information document on Replagal® (agalsidase alfa, therapeutic recombinant human α-Gal produced in human fibroblasts, Shire, Lexington, MA) describes that this drug should not be co-administered with chloroquine, amiodarone, benoquin or gentamicin to Fabry patients who require treatment with these drugs because it has the potential to inhibit intracellular α-Gal activity. As the next study, it is necessary to examine whether CADs affect the therapeutic efficacy of recombinant α-Gal or not.
This study has several limitations. First, it was performed to examine the effects of CADs on organs and tissues using mice, as an experimental target, not humans. Based on the results of this animal experiment, to translate this evidence from animal experiment to humans, it will be necessary to measure Gb3 and Lyso-Gb3 in clinical samples (i.e., blood and urine) of Fabry patients treated with hydroxychloroquine and amiodarone in the future. Second, the experiments were performed under the fixed conditions (i.e., doses and method of administration of the drugs) described. Third, we focused on the effect of CADs on the catabolism of Gb3/Lyso-Gb3 in the organs and tissues in this study. Additional biochemical and functional experiments are required to determine the influence of the drugs on Fabry disease.
5. Conclusion
In this study, we examined the impact of administration of hydroxychloroquine/amiodarone on the content of PhC in the liver, and the α-Gal activity and contents of Gb3/Lyso-Gb3 in the liver, kidneys, and heart of wild-type and Fabry mice. The results revealed that these drugs induced phospholipidosis but did not cause reduction of the α-Gal activity in the wild-type mice or acceleration of Gb3/Lyso-Gb3 accumulation in the organs and tissues of Fabry mice.
Funding
Hitoshi Sakuraba received grants from Sumitomo Dainippon Pharma Co., Ltd. and Sanofi Japan Co. Tadayasu Togawa received a grant from Sanofi Japan Co. Takahiro Tsukimura received a grant, JSPS KAKENHI (JP18K15071). The funders played no role in the study design, data collection or analysis. Tomoko Shiga, Koki Saito, and Yasuhiro Ogawa have nothing to disclose.
Author statement
T. Tsukimura: Conceptualization, Investigation, Formal analysis, Writing-original draft, Writing- Reviewing and Editing. H. Sakuraba: Conceptualization, Formal analysis, Writing-original draft, Writing- Reviewing and Editing. T. Togawa: Conceptualization, Formal analysis, Writing- Reviewing and Editing. T. Shiga: Investigation. K. Saito: Investigation. Y. Ogawa: Investigation.
Declaration of Competing Interest
We declare that none of the authors have any competing interests.
Acknowledgments
Acknowledgement
We wish to thank Ms. M. Tanaka for collecting the materials and typing the manuscript.
References
- 1.Germain D.P. Fabry disease. Orphanet. J. Rare Dis. 2010;5:30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Germain D.P., Oliveira J.P., Bichet D.G., Yoo H.W., Hopkin R.J., Lemay R., Politei J., Wanner C., Wilcox W.R., Warnock D.G. Use of a rare disease registry for establishing phenotypic classification of previously unassigned GLA variants: a consensus classification system by a multispecialty Fabry disease genotype-phenotype workgroup. J. Med. Genet. 2020;57:542–551. doi: 10.1136/jmedgenet-2019-106467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Echevarria L., Benistan K., Toussaint A., Dubourg O., Hagege A.A., Eladari D., Jabbour F., Beldjord C., De Mazancourt P., Germain D.P. X-chromosome inactivation in female patients with Fabry disease. Clin. Genet. 2016;89:44–54. doi: 10.1111/cge.12613. [DOI] [PubMed] [Google Scholar]
- 4.Hsu M.-J., Chang F.-P., Lu Y.-H., Hung S.-C., Wang Y.-C., Yang A.-H., Lee H.-J., Sung S.-H., Wang Y.-F., Yu W.-C., Hsu T.-R., Huang P.-H., Chang S.-K., Dzhagalov I., Hsu C.-L., Niu D.-M. Identification of lysosomal and extralysosomal globotriaosylceramide (Gb3) accumulations before the occurrence of typical pathological changes in the endomyocardial biopsies of Fabry disease patients. Genet. Med. 2019;21:224–232. doi: 10.1038/s41436-018-0010-z. 29875425 [DOI] [PubMed] [Google Scholar]
- 5.Shayman J.A., Abe A. Drug-induced phospholipidosis: an acquired lysosomal storage disorder. Biochim. Biophys. Acta. 2013;1831:602–611. doi: 10.1016/j.bbalip.2012.08.013. 22960355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reasor M.J., Kacew S. Drug-induced phospholipidosis: are there functional consequences? Exp. Biol. Med. (Maywood). 2001;226:825–830. doi: 10.1177/153537020122600903. 11568304 [DOI] [PubMed] [Google Scholar]
- 7.Reasor M.J., Hastings K.L., Ulrich R.G. Drug-induced phospholipidosis: issues and future directions. Expert Opin. Drug Saf. 2006;5:567–583. doi: 10.1517/14740338.5.4.567. 16774494 [DOI] [PubMed] [Google Scholar]
- 8.Shukla A.M., Shukla A.W. Expanding horizons for clinical applications of chloroquine, hydroxychloroquine, and related structural analogues. Drugs Context. 2019;8 doi: 10.7573/dic.2019-9-1. 2019-9-1. 31844421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu C., Lu L., Wan J.-P., Wen C. The pharmacological mechanisms and therapeutic activities of hydroxychloroquine in rheumatic and related diseases. Curr. Med. Chem. 2017;24:2241–2249. doi: 10.2174/0929867324666170316115938. 28302011. [DOI] [PubMed] [Google Scholar]
- 10.Biancatelli R.M.L.C., Congedo V., Calvosa L., Ciacciarelli M., Polidoro A., Iuliano L. Adverse reactions of amiodarone. J. Geriatr. Cardiol. 2019;16:552–566. doi: 10.11909/j.issn.1671-5411.2019.07.004. 31447894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein A.E., Olshansky B., Naccarelli G.V., Kennedy J.I., Jr., Murphy E.J., Goldschlager N. Practical management guide for clinicians who treat patients with amiodarone. Am. J. Med. 2016;129:468–475. doi: 10.1016/j.amjmed.2015.08.039. 26497904 [DOI] [PubMed] [Google Scholar]
- 12.Yogasundaram H., Hung W., Paterson I.D., Sergi C., Oudit G.Y. Chloroquine-induced cardiomyopathy: a reversible cause of heart failure. ESC Heart Fail. 2018;5:372–375. doi: 10.1002/ehf2.12276. 29460476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serre J., Buob D., Boffa J.J. Hydroxychloroquine-induced podocytopathy mimicking Fabry disease. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2018-228876. 31088818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla R., Jowett N.I., Thompson D.R., Pohl J.E. Side effects with amiodarone therapy. Postgrad. Med. J. 1994;70:492–498. doi: 10.1136/pgmj.70.825.492. 7937427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller-Höcker J., Schmid H., Weiss M., Dendorfer U., Braun G.S. Chloroquine-induced phospholipidosis of the kidney mimicking Fabry's disease: case report and review of the literature. Hum. Pathol. 2003;34:285–289. doi: 10.1053/hupa.2003.36. 12673565 [DOI] [PubMed] [Google Scholar]
- 16.Roos J.M., Aubry M.C., Edwards W.D. Chloroquine cardiotoxicity: clinicopathologic features in three patients and comparison with three patients with Fabry disease. Cardiovasc. Pathol. 2002;11:277–283. doi: 10.1016/S1054-8807(02)00118-7. 12361838. [DOI] [PubMed] [Google Scholar]
- 17.Tönnesmann E., Kandolf R., Lewalter T. Chloroquine cardiomyopathy - a review of the literature. Immunopharmacol. Immunotoxicol. 2013;35:434–442. doi: 10.3109/08923973.2013.780078. [DOI] [PubMed] [Google Scholar]
- 18.Khubchandani S.R., Bichle L.S. Hydroxychloroquine-induced phospholipidosis in a case of SLE: the wolf in zebra clothing. Ultrastruct. Pathol. 2013;37:146–150. doi: 10.3109/01913123.2012.751950. 23573895 [DOI] [PubMed] [Google Scholar]
- 19.Jones C.J., Salisbury R.S., Jayson M.I. The presence of abnormal lysosomes in lymphocytes and neutrophils during chloroquine therapy: a quantitative ultrastructural study. Ann. Rheum. Dis. 1984;43:710–715. doi: 10.1136/ard.43.5.710. 6093716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albay D., Adler S.G., Philipose J., Calescibetta C.C., Romansky S.G., Cohen A.H. Chloroquine-induced lipidosis mimicking Fabry disease. Mod. Pathol. 2005;18:733–738. doi: 10.1038/modpathol.3800344. 15605079 [DOI] [PubMed] [Google Scholar]
- 21.Costa R.M., Martul E.V., Reboredo J.M., Cigarrán S. Curvilinear bodies in hydroxychloroquine-induced renal phospholipidosis resembling Fabry disease. Clin. Kidney J. 2013;6:533–536. doi: 10.1093/ckj/sft089. 26120446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Menezes Neves P.D.M., Machado J.R., Custódio F.B., Dos Reis Monteiro M.L.G., Iwamoto S., Freire M., Ferreira M.F., Dos Reis M.A. Ultrastructural deposits appearing as “zebra bodies” in renal biopsy: Fabry disease? Comparative case reports. BMC Nephrol. 2017;18:157. doi: 10.1186/s12882-017-0571-0. 28499424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S.-Z., Liang X., Geng J., Zhang M.-B., Xie N., Su X.-Y. Hydroxychloroquine-induced renal phospholipidosis resembling Fabry disease in undifferentiated connective tissue disease: A case report. World J. Clin. Cases. 2019;7:4377–4383. doi: 10.12998/wjcc.v7.i24.4377. 31911921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bracamonte E.R., Kowalewska J., Starr J., Gitomer J., Alpers C.E. Iatrogenic phospholipidosis mimicking Fabry disease. Am. J. Kidney Dis. 2006;48:844–850. doi: 10.1053/j.ajkd.2006.05.034. 17060007 [DOI] [PubMed] [Google Scholar]
- 25.Shaikh N.A., Downar E., Butany J. Amiodarone-an inhibitor of phospholipase activity: a comparative study of the inhibitory effects of amiodarone, chloroquine and chlorpromazine. Mol. Cell. Biochem. 1987;76:163–172. doi: 10.1007/BF00223481. 2823097 [DOI] [PubMed] [Google Scholar]
- 26.Poucell S., Ireton J., Valencia-Mayoral P., Downar E., Larratt L., Patterson J., Blendis L., Phillips M.J. Amiodarone-associated phospholipidosis and fibrosis of the liver. Light, immunohistochemical, and electron microscopic studies. Gastroenterology. 1984;86:926–936. (PMID: 6706074) [PubMed] [Google Scholar]
- 27.Fine N.M., Wang Y., Khan A. Acute decompensated heart failure after initiation of amiodarone in a patient with Anderson-Fabry Disease. Can. J. Cardiol. 2019;35 doi: 10.1016/j.cjca.2018.10.004. 104. e5–104.e7. 30595173. [DOI] [PubMed] [Google Scholar]
- 28.Sanoh S., Yamachika Y., Tamura Y., Kotake Y., Yoshizane Y., Ishida Y., Tateno C., Ohta S. Assessment of amiodarone-induced phospholipidosis in chimeric mice with a humanized liver. J. Toxicol. Sci. 2017;42:589–596. doi: 10.2131/jts.42.589. 28904294 [DOI] [PubMed] [Google Scholar]
- 29.Tajima Y., Kawashima I., Tsukimura T., Sugawara K., Kuroda M., Suzuki T., Togawa T., Chiba Y., Jigami Y., Ohno K., Fukushige T., Kanekura T., Itoh K., Ohashi T., Sakuraba H. Use of a modified alpha-N-acetylgalactosaminidase in the development of enzyme replacement therapy for Fabry disease. Am. J. Hum. Genet. 2009;85:569–580. doi: 10.1016/j.ajhg.2009.09.016. 19853240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kodama T., Tsukimura T., Kawashima I., Sato A., Sakuraba H., Togawa T. Differences in cleavage of globotriaosylceramide and its derivatives accumulated in organs of young Fabry mice following enzyme replacement therapy. Mol. Genet. Metab. 2017;120:116–120. doi: 10.1016/j.ymgme.2016.10.003. 27756537 [DOI] [PubMed] [Google Scholar]
- 31.Halliwell W.H. Cationic amphiphilic drug-induced phospholipidosis. Toxicol. Pathol. 1997;25:53–60. doi: 10.1177/019262339702500111. 9061852 [DOI] [PubMed] [Google Scholar]
- 32.Heath M.F., Costa-Jussà F.R., Jacobs J.M., Jacobson W. The induction of pulmonary phospholipidosis and the inhibition of lysosomal phospholipases by amiodarone. Br. J. Exp. Pathol. 1985;66:391–397. 2992568 [PMC free article] [PubMed] [Google Scholar]
- 33.Huta B.P., Mehlenbacher M.R., Nie Y., Lai X., Zubieta C., Bou-Abdallah F., Doyle R.P. The lysosomal protein saposin B binds chloroquine. ChemMedChem. 2016;11:277–282. doi: 10.1002/cmdc.201500494. 26616259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon I., Lee Y., Cosio-Lima L.M., Cho J.-Y., Yeom D.-C. Effects of long-term resistance exercise training on autophagy in rat skeletal muscle of chloroquine-induced sporadic inclusion body myositis. J. Exerc. Nutr. Biochem. 2015;19:225–234. doi: 10.5717/jenb.2015.15090710. 26525066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda K., Hirayama M., Hirota Y., Asa E., Seki J., Tanaka Y. Drug-induced phospholipidosis is caused by blockade of mannose 6-phosphate receptor-mediated targeting of lysosomal enzymes. Biochem. Biophys. Res. Commun. 2008;377:268–274. doi: 10.1016/j.bbrc.2008.09.121. 18840403 [DOI] [PubMed] [Google Scholar]
- 36.De Groot P.G., Elferink R.O., Hollemans M., Strijland A., Westerveld A., Khan P.M., Tager J.M. Inactivation by chloroquine of alpha-galactosidase in cultured human skin fibroblasts. Exp. Cell Res. 1981;136:327–333. doi: 10.1016/0014-4827(81)90011-2. 6273196 [DOI] [PubMed] [Google Scholar]
- 37.Inagaki M., Katsumoto T., Nanba E., Ohno K., Suehiro S., Takeshita K. Lysosomal glycosphingolipid storage in chloroquine-induced alpha-galactosidase-deficient human endothelial cells with transformation by simian virus 40: in vitro model of Fabry disease. Acta Neuropathol. 1993;85:272–279. doi: 10.1007/BF00227722. 8384772 [DOI] [PubMed] [Google Scholar]
- 38.Kannan R., Sarma J.S., Guha M., Venkataraman K. Tissue drug accumulation and ultrastructural changes during amiodarone administration in rats. Fundam. Appl. Toxicol. 1989;13:793–803. doi: 10.1016/0272-0590(89)90334-5. 2620796 [DOI] [PubMed] [Google Scholar]
- 39.Snyder V.L., Bandyopadhyay S., Collins J., Gross S.A., Somani P., Didio L.J. Subcellular changes of rat myocardium after treatment with amiodarone or desethylamiodarone, studied with electron microscopy. J. Submicrosc. Cytol. Pathol. 1990;22:71–78. 2155704 [PubMed] [Google Scholar]
- 40.Kumamoto T., Araki S., Watanabe S., Ikebe N., Fukuhara N. Experimental chloroquine myopathy: morphological and biochemical studies. Eur. Neurol. 1989;29:202–207. doi: 10.1159/000116412. 2759144 [DOI] [PubMed] [Google Scholar]
- 41.Breiden B., Sandhoff K. Emerging mechanisms of drug-induced phospholipidosis. Biol. Chem. 2020;401:31–46. doi: 10.1515/hsz-2019-0270. 31408430 [DOI] [PubMed] [Google Scholar]