Abstract
Background:
Corticotropin releasing factor (CRF) neural systems are important stress mechanisms in central amygdala (CeA), bed nucleus of stria terminalis (BNST), nucleus accumbens (NAc) and related structures. CRF-containing neural systems are traditionally posited to generate aversive distress states that motivate over-consumption of rewards and relapse in addiction. However, CRF-containing systems may alternatively promote incentive motivation to increase reward pursuit and consumption, without requiring aversive states.
Methods:
We optogenetically stimulated CRF-expressing neurons in CeA, BNST or NAc, using Crh-Cre+ rats (n=37 female, n=34 male) to investigate roles in incentive motivation versus aversive motivation. We paired CRF-expressing neuronal stimulations with earning sucrose rewards in two-choice and progressive ratio tasks and investigated recruitment of distributed limbic circuitry. We further assessed valence with CRF-containing neuron laser self-stimulation tasks.
Results:
Channelrhodopsin excitation of CRF-containing neurons in CeA and NAc amplified and focused incentive motivation and recruited activation of mesocorticolimbic reward circuitry. CRF systems in both CeA and NAc supported laser self-stimulation, amplified incentive motivation for sucrose in a breakpoint test, and focused ‘wanting’ on laser-paired sucrose over a sucrose alternative in a two-choice test. Conversely, stimulation of CRF-containing neurons in BNST produced negative-valence or aversive effects and recruited distress-related circuitry, as stimulation was avoided and suppressed motivation for sucrose.
Conclusions:
CRF-containing systems in NAc and CeA can promote reward consumption by increasing incentive motivation, without involving aversion. By contrast, stimulation of CRF-containing systems in BNST is aversive but suppresses sucrose reward pursuit and consumption, rather than increase as predicted by traditional hedonic self-medication hypotheses.
Keywords: affective valence, incentive motivation, aversive motivation, corticotropin releasing factor, optogenetics, nucleus accumbens, central amygdala, bed nucleus of stria terminalis
Introduction
Corticotropin releasing factor (CRF) is triggered by diverse aversive stressors to initiate behavioral and physiological stress responses (1–11). CRF-expressing neurons are concentrated in the hypothalamic paraventricular nucleus (PVN), but also occur in the nucleus accumbens (NAc), and in extended amygdala components such as the central amygdala (CeA) and bed nucleus of the stria terminalis (BNST)(12–24).
Stress can trigger relapse in addiction or eating disorders (25–27). Traditional views suggest that CRF-containing systems increase reward consumption primarily by mediating the negative-valence of stress, creating unpleasant states that promote drug relapse or eating for hedonic self-medication (27–29). In the opponent-process theory of addiction (30–32) taking addictive drugs activates a pleasant a-process, which is posited to trigger underlying longer-lasting aversive b-processes to create an unpleasant opponent B-state of withdrawal. In particular, opponent-process neuroscience models of addiction have posited that activation of CeA and BNST CRF-containing systems generates unpleasant withdrawal symptoms, again leading to relapse via hedonic self-medication (27–29, 32–35).
However, CRF systems may also activate to changing events that mobilize biobehavioral responses, whether or not stressful (9–11). For example, CRF-containing neurons can be activated by positive reward stimuli (10–15, 36). Some CRF systems may have positively-valenced roles in promoting appetitive incentive motivation without inducing negative distress or withdrawal. For instance, NAc CRF microinjections in rats increase cue-triggered ‘wanting’ for sucrose during Pavlovian Instrumental Transfer testing, comparable to dopamine-stimulating amphetamine microinjections (13). NAc CRF microinjections can also establish positive conditioned place-preference and increase NAc dopamine release in mice, only becoming aversive roles following severe stress (14). Additionally, mice self-stimulate for optogenetic excitation of CeA CRF-expressing neurons, suggesting incentive motivation (12). CRF in rats does mediate stress-induced reinstatement for addictive drugs, but does not require either withdrawal or corticosterone (37–39). Overall, CRF seems not simply tied to an aversive affective dimension, but instead has a larger regulatory role in affective valence and organization of behaviors (40–42).
Here we examined potential positively-valenced versus negatively-valenced roles of CRF-expressing neurons in either NAc, CeA, or BNST, using BAC transgenic Crh-Cre+ rats (21) to optogenetically stimulate CRF-containing neurons in each structure. During two-choice incentive motivation tests, rats could choose between earning sucrose paired with laser stimulations and another sucrose option without laser (43). In progressive ratio breakpoint tests, laser stimulation effects on incentive motivation magnitude for sucrose was assessed. Finally, laser self-stimulation tests assessed whether CRF-containing neuronal stimulation was rewarding on its own. We found that NAc and CeA CRF-containing neuron stimulation enhanced sucrose incentive motivation, was reinforcing, and recruited activation of mesolimbic circuitry. Conversely, BNST CRF-containing neuronal stimulation was avoided, suppressed sucrose pursuit, and recruited pain-related circuitry.
Methods and Materials
Animals
Female (n=37) and male (n=34) Crh-Cre+ Wistar rats (>250g at surgery) (21), were bred and phenotyped in-house. Same-sex groups were housed on a 12-hour reverse light-cycle (~21°C) with ad libitum food (Purina) and water. All experimental procedures were approved by the University of Michigan Institutional Animal Care & Use Committee in accordance with NIH animal care and use guidelines.
Surgery
Surgeries followed previous methods (see Supplementary Methods) (43–45). Bilateral 1.0μl infusions in NAc, CeA, or BNST contained either active AAV-DIO-ChR2-eYFP virus (n=33) or optically-inactive control virus AAV-DIO-eYFP (n=19) to infect only neurons containing Cre-recombinase. We note that the Crh-Cre BAC rats used here express Cre primarily in CRF neurons that are also GABAergic (21). This makes them suitable for our study, given that CeA, BNST, and NAc CRF-expressing neurons predominantly co-express GABA. A separate group received halorhodopsin AAV-DIO-NpHR-eYFP (n=19) virus for CRF-containing neuronal inhibition. NAc shell, lateral CeA, or dorsolateral BNST sites were staggered across individuals (Fig. 1, Table 1) and optic fibers were secured with surgical screws and acrylic.
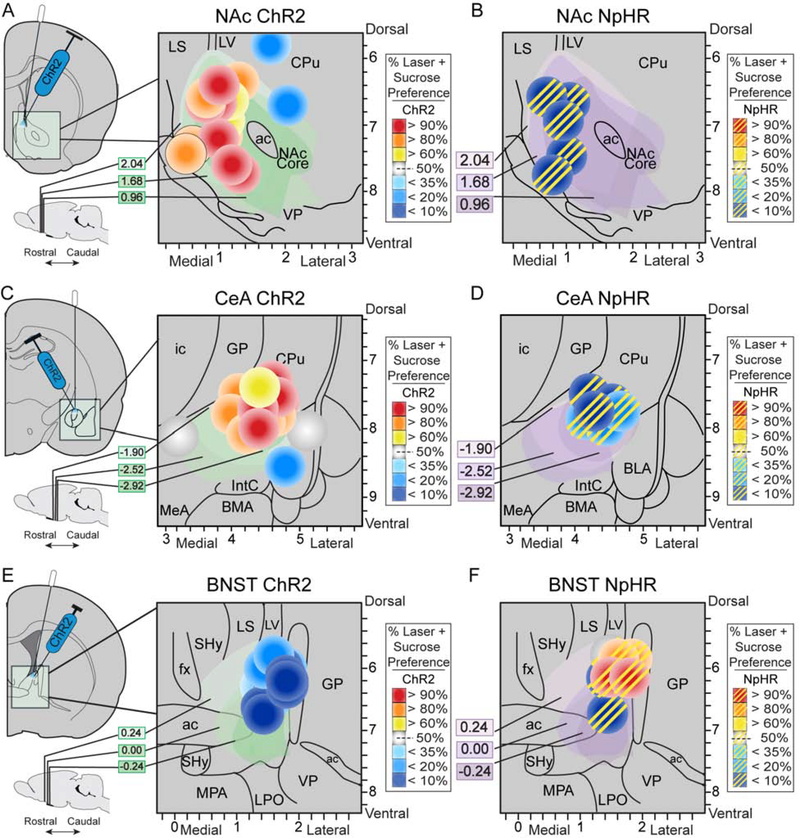
Figure 1. Localization of function maps.
Function maps of effects on sucrose preference in two-choice task of ChR2 stimulation of CRF-expressing neurons in A) NAc, C) CeA, and E) BNST. Maps for inhibitory halorhodopsin effects of laser illumination on CRF-expressing neurons shown in B) NAc, D) CeA, and F) BNST (striped symbols). Symbol sizes reflect size of optogenetic Fos plumes (see Fig. 2 and Supplementary results). Yellow, orange, or red symbol colors show intensity of enhancement of laser-induced preference for Laser+Sucrose option over Sucrose-alone option produced at that site (effects shown for days 6–8 of two-choice task). Conversely, blue colors show intensity of avoidance of Laser+Sucrose (i.e., preference instead for Sucrose-alone). Also see Table 1. CPu, caudate putamen; LV, lateral ventricle; LS, lateral septum; VP, ventral pallidum; ac, anterior commissure; ic, internal capsule; GP, globus pallidus; MeA, medial amygdala; IntC, intercalated amygdala; BMA, basomedial amygdala; BLA, basolateral amygdala; fx, fornix; SHy, septohypothalamic nucleus; MPA, medial preoptic area; LPO, lateral preoptic area.
Table 1. Histological placements of experimental animals.
Table shows anatomical confirmed placement ranges for experimental animals targeting either lateral central amygdala (CeA), nucleus accumbens (NAc) shell, or dorsolateral bed nucleus of stria terminalis (BNST). Confirmed placement ranges are determined from Paxinos and Watson brain atlas (50) and display anterior/posterior (A/P), medial/lateral (M/L), and dorsal/ventral (D/V) coordinates in mm from bregma. N-values for excitatory channelrhodopsin (ChR2), inactive control eYFP virus, and inhibitory halorhodopsin (NpHR) groups include those with bilateral fiber and virus placements (Bil) or unilateral virus/fiber placements in one hemisphere, with a contralateral miss in the other hemisphere (Uni). For CeA rats (top), contralateral miss sites were located in either basolateral amygdala (BLA), medial amygdala (MeA), or optic tract, with no substantial virus expression found in these missed hemispheres. For NAc rats (middle), placements for unilateral misses were located in dorsal striatum (DS), medial septum (MS), or nucleus accumbens core (NAcC) and no substantial viral expression in these structures was observed. For BNST rats (below) sites of unilateral misses in either the anterior commissure (ac) or globus pallidus (GP) without substantial virus expression. See Fig. 1.
| Target | Confirmed placement ranges (mm from Bregma) | ChR2 N’s | eYFP N’s | NpHR N’s | Contralateral misses, locations | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| A/P | M/L | D/V | Uni | Bil | Uni | Bil | Uni | Bil | ||
| CeA | −2.16 to −3.00 | ± 4.2 to 4.7 | −7.0 to −7.6 | 3 | 7 | 2 | 5 | 3 | 4 | BLA, MeA, optic tract |
| NAc | +1.44 to 0.96 | ± 0.8 to 1.6 | −6.3 to −7.6 | 7 | 5 | 3 | 3 | 3 | 3 | DS, MS, NAcC |
| BNST | +0.24 to −0.24 | ±1.6 to 2.0 | −5.8 to −6.4 | 4 | 7 | 3 | 3 | 2 | 4 | ac, GP |
Stimulation parameters
ChR2 laser-illumination (2–3mW; 473nm) was tested at 10Hz and 40Hz (46–48). Inhibitory halorhodopsin testing used constant-illumination (8–10mW; yellow 592nm) (49).
Two-choice sucrose
An instrumental two-choice task evaluated whether pairing CRF-expressing neuronal stimulation in NAc, CeA, or BNST with one sucrose reward made it more or less desirable than an identical sucrose reward delivered without laser (Supplementary Methods)(43). Briefly, rats learned that presses on one lever earned sucrose pellets plus 8-sec laser illuminations and an 8-sec tone or white-noise (Laser+Sucrose). Presses on a different lever earned sucrose and noise/tone but no laser (Sucrose-alone). Lever and tone/noise assignments were balanced across rats, but remained permanent for each rat.
Reinforcement schedules increased across 8 test days: FR1 (days 1–3), FR4 (4), RR4 (5), RR6 (6–8). Each day rats were required to earn rewards twice from each lever presented alone, before free-choice. The alternate laser frequency (10Hz/40Hz) was tested on three subsequent RR6 days. Separate halorhodopsin rats underwent identical procedures with yellow laser.
Progressive ratio
Progressive ratio tests assessed whether ChR2 stimulation of CRF-containing neurons affects magnitude of sucrose incentive motivation (Supplementary Methods) (43). Briefly, rats were tested one day with only the Laser+Sucrose (10Hz/40Hz) lever available, another day with Sucrose-alone, and a third Laser+Sucrose day with the alternate frequency. Within each session, the responses required to earn the next reward increased after each reward, and breakpoint or ratio reached during 30min sessions was assessed. Separate halorhodopsin rats underwent testing with inhibition.
Spout-touch laser self-stimulation
Incentive properties of laser alone without sucrose were tested in instrumental spout-touch self-stimulation tests. With two empty waterspouts available, each touch on a designated Laser-spout provided stimulation (3-sec; 10Hz/40Hz; 30min). Touches on the other Inactive-spout earned nothing, as a baseline exploration measure. Rats were classified on Day 1 as robust, low, or non-self-stimulators and Days 2–3 evaluated consistency of self-stimulation (Supplementary Methods) (45).
Place-based self-stimulation
In a different place-based self-stimulation test, rats could earn laser self-stimulations by remaining in a designated Laser-delivering chamber within a 3-chamber apparatus (2-major, 1-smaller center, see Supplementary Methods) after an initial session without laser evaluated baseline preference. For 3 test days, Laser-delivering chamber entries triggered laser (3-sec-on/4-sec-off), which continued cycling as long as rats remained, terminating upon exit. Time in Laser-delivering minus time in alternative No-laser chamber difference-scores were assessed.
Histology
Briefly, laser-stimulations preceded lethal doses of sodium pentobarbital and transcardial perfusions for Fos assessment (see Supplementary Methods) (44). Brains were extracted, post-fixed, sectioned into 40μm slices via cryostat (Leica), processed for GFP and cFos immunohistochemistry (Fig. 2), and imaged using a digital camera (Qimaging) and fluorescence microscope (Leica).
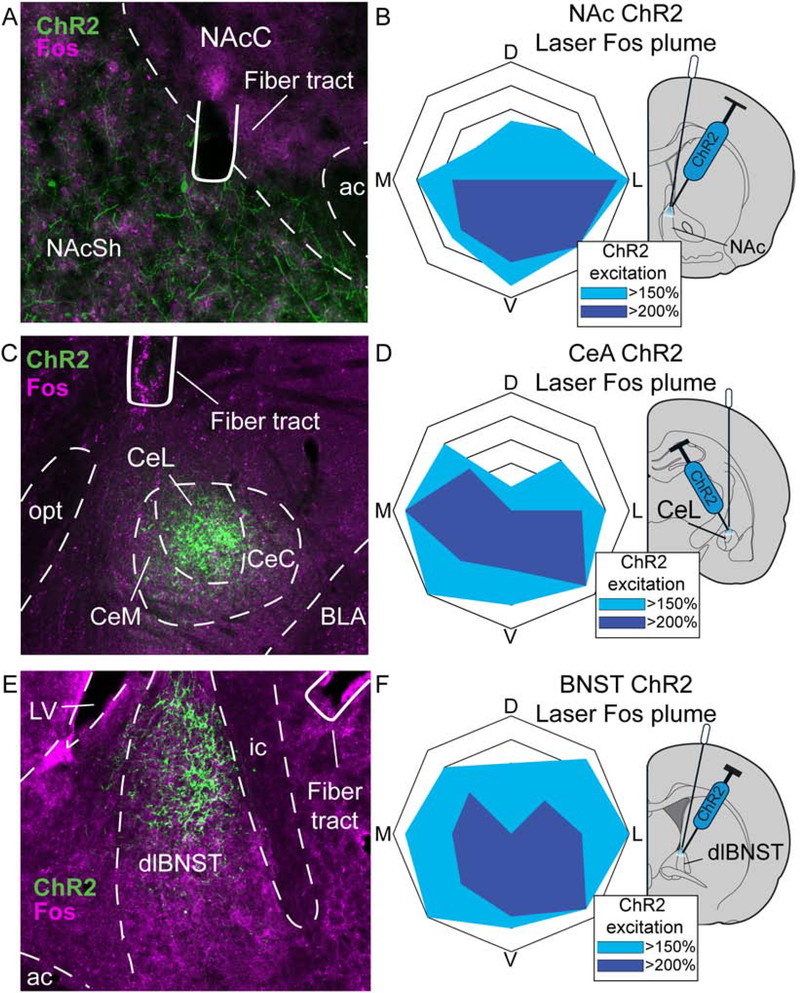
Figure 2. Virus expression and local Fos plumes.
Photomicrograph (×10 magnification) shows channelrhodopsin virus expression (ChR2; green), and neuronal Fos protein expression (magenta) immediately surrounding optic fiber tips for Crh-Cre+ rats in A) nucleus accumbens (NAc) shell, C) lateral division of central nucleus of amygdala (CeA) and E) dorsolateral division of bed nucleus of stria terminalis (BNST). Blue diagrams at right show maps displaying size and intensity of local Fos plumes produced in each structure by laser stimulation of CRF-containing neurons expressing ChR2 (i.e., zones of >150% Fos elevation and >200% Fos elevation over baselines (100%) measured in laser-illuminated eYFP control rats. Average Fos plume diameters are shown for Crh-Cre+ ChR2 rats after laser illumination in B) NAc, D) CeA, and F) BNST CRF-containing neurons. D: dorsal, M: medial, L: lateral, V: ventral to fiber tip; ac, anterior commissure; NAcC, nucleus accumbens core; NAcSh, nucleus accumbens shell; CeC, capsular central amygdala; CeL, lateral central amygdala; CeM, medial central amygdala; dlBNST, dorsolateral bed nucleus of stria terminalis; ic, internal capsule; LV, lateral ventricle.
Coronal sections were imaged (10× magnification) to quantify distributed Fos using Paxinos & Watson atlas (50). Laser-recruited changes in Fos expression in NAc/CeA/BNST groups were compared to eYFP-control levels in several mesocorticolimbic structures (Table 2).
Table 2. Brain regions assessed for laser-recruited changes in Fos expression.
Laser induced enhancements in Fos expression were assessed in the listed mesocorticolimbic brain regions, following CRF-expressing neuronal excitation in NAc, CeA, or BNST. Distant Fos levels in ChR2 animals were compared to levels assessed in inactive eYFP control rats that underwent identical Fos induction procedures. See Fig. 4 and Supplemental Methods.
| Regions | |
|---|---|
| Orbitofrontal cortex (OFC) | Anterior lateral hypothalamus (aLH) |
| Infralimbic cortex (IF) | Posterior lateral hypothalamus (pLH) |
| Nucleus accumbens core (NAcC) | Paraventricular nucleus hypothalamus (PVN) |
| Anterior nucleus accumbens shell (aNAcSh) | Medial amygdala (MeA) |
| Posterior nucleus accumbens shell (pNAcSh) | Central amygdala (CeA) |
| Anterior bed nucleus of stria terminalis (aBNST) | Basolateral amygdala (BLA) |
| Posterior bed nucleus of stria terminalis (pBNST) | Ventral tegmentum (VTA) |
| Anterior ventral pallidum (aVP) | Substantia nigra (SN) |
| Posterior ventral pallidum (pVP) | Midbrain periaqueductal gray (PAG) |
CRF and Cre expression assessed by RNAScope® Fluorescent In Situ Hybridization (FISH)
Colocalization of Cre and CRF in infected neurons was verified with FISH (see Supplementary) (16, 51). Cells containing Cre and Crh mRNAs were manually counted in 100×100×17μm volumes from core samples in NAc, CeA, and BNST (n=6).
Statistical Analyses
Mixed-model ANOVAs evaluated within-group (e.g., laser-pairings) and between-group effects (e.g., ChR2/eYFP) followed by post-hoc comparisons with Bonferroni corrections. Distant Fos was evaluated by unpaired t-tests. Effect sizes are Cohen’s D. For all analyses, significance level was p=0.05, two-tailed.
Results
Cre and CRF colocalization
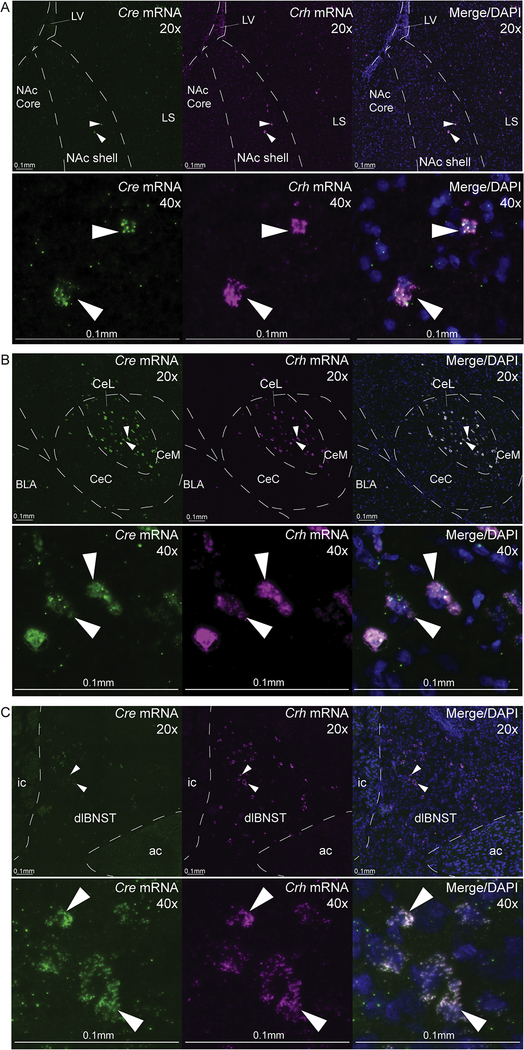
Crh and Cre mRNAs were visualized using FISH in slices from Crh-Cre+ rats (n=6) and found to typically occur together in the same neurons. CRF+ and Cre+ co-expressing neurons were densely concentrated within the lateral CeA (10.1±0.9 co-labeled neurons per 100×100×17μm volume) and dorsolateral BNST (10.0±0.7). In NAc, CRF+ neurons were sparsely distributed throughout medial shell (6.0±0.7 co-labeled neurons, or nearly one-half CeA/BNST density; Fig. 3, Supplementary results).
Figure 3. CRF and Cre colocalization verification through fluorescence in situ hybridization.
Representative images for Cre mRNA expression and Crh mRNA expression in A) nucleus accumbens (NAc) shell, B) lateral division of central amygdala (CeL), and C) dorsolateral division of bed nucleus of stria terminalis (dlBNST) in Crh-Cre+ rats (n=6). Low-power (20×) and high-power (40×) images show localization of neurons expressing Cre mRNA (green) or Crh mRNA (magenta), and Cre/CRF colocalization with cell bodies stained with DAPI (blue). Arrows point to examples of cells coexpressing Cre and Crh mRNAs. Scale bars denote 0.1mm at both 20× and 40×. See Supplementary methods and results. LV, lateral ventricle; LS, lateral septum; BLA, basolateral amygdala; CeC, capsular central amygdala, medial central amygdala; ic, internal capsule; ac, anterior commissure.
NAc and CeA CRF-expressing neurons recruit similar structures, BNST shows distinct activation
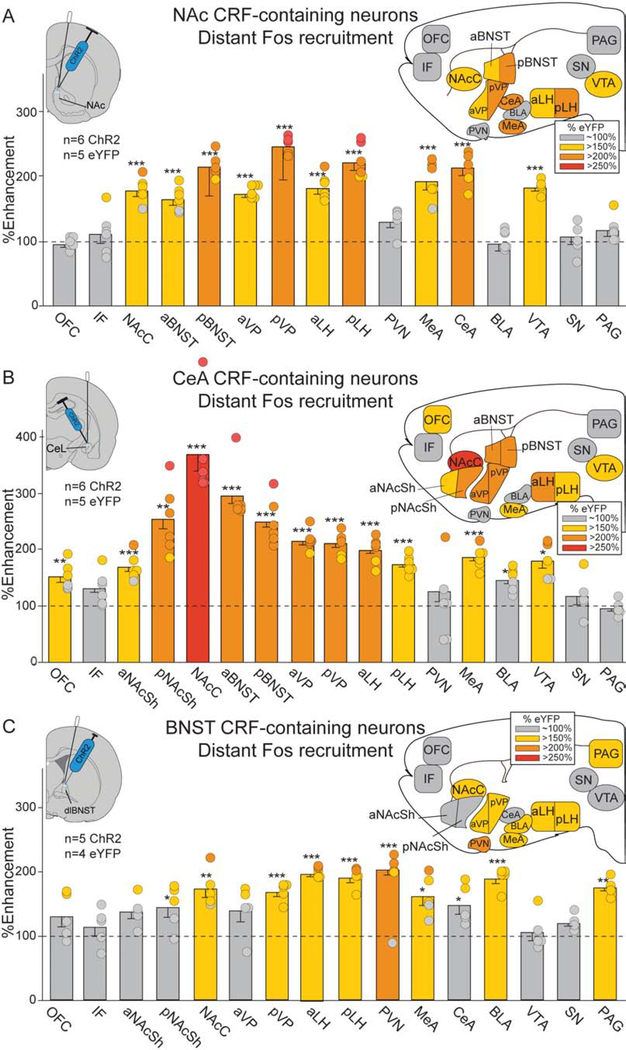
Recruitment of Fos elevation in distant brain circuitry was assessed following CRF-expressing neuron excitation in NAc, CeA or BNST (Tables 2, S1).
Laser ChR2 excitation of CRF-containing neurons in NAc shell (NAcSh) recruited 150–200% increases in distant Fos expression over eYFP control levels in reward-related mesocorticolimbic structures including NAc core, CeA, ventral tegmentum (VTA), ventral pallidum (VP), lateral hypothalamus (LH), etc. (Fig. 4A). Similarly, CeA stimulation of CRF-expressing neurons excitation increased Fos expression 150–250% in NAcSh, VTA, VP, LH, etc. (Fig. 4B).
Figure 4. Laser-enhancements in distant Fos expression.
Brain maps show recruitment of distant Fos elevation in mesocorticolimbic structures following CRF-containing neuron ChR2 stimulation in NAc, CeA or BNST (colors denote percent Fos elevation vs. eYFP controls, all two-way unpaired t-tests). A) NAc ChR2 stimulation (n=3 female, n=3 male): NAc core (NAcC), ventral tegmentum (VTA), anterior ventral pallidum (aVP), posterior VP (pVP), anterior lateral hypothalamus (aLH), pLH, medial amygdala (MeA), CeA, aBNST, pBNST. B) CeA ChR2 stimulation (n=3 female, n=3 male): orbitofrontal cortex (OFC), aNAcSh, pNAcSh, NAcC, aVP, pVP, aLH, pLH, MeA, VTA, aBNST, pBNST, and minor increases in basolateral amygdala (BLA; <150%). C) BNST ChR2 stimulation (n=2 female, n=3 male): BLA, periaqueductal gray (PAG), hypothalamic paraventricular nucleus (PVN), NAcC, pVP, aLH, pLH, MeA, and minor increases in pNAcSh (<150%) and CeA (<150%). See Table S1. infralimbic cortex (IF); substantia nigra (SN). Means and SEM reported. *p<0.05,**p<0.01,***p<0.001
Conversely in BNST ChR2 rats, CRF-containing neuron excitation recruited distant Fos 150–200% elevation in several structures related to pain, aversion, fear, or satiety: midbrain periaqueductal gray (PAG), PVN, and basolateral amygdala (BLA), in addition to 150% elevation in some mesocorticolimbic structures (Fig. 4C).
NAc and CeA CRF-expressing neuronal stimulation enhances paired-sucrose value
NAc CRF-containing neuron incentive enhancement.
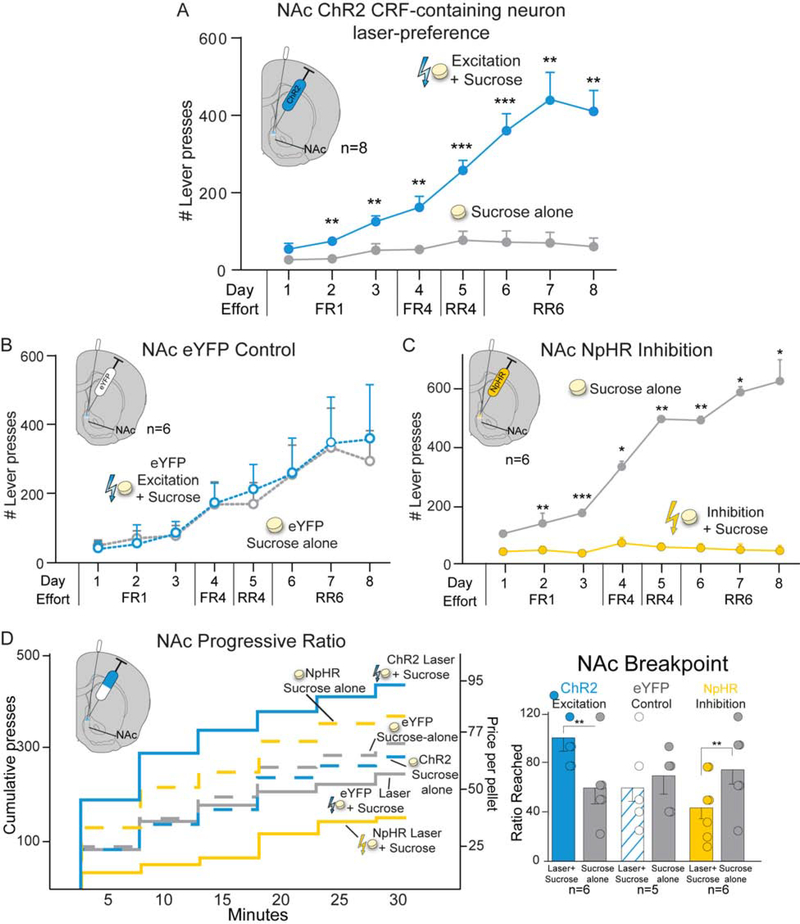
Pairing ChR2 stimulation of CRF-containing neurons in NAc (n=8) with earning sucrose rewards in the two-choice tasks caused rats to pursue that paired Laser+Sucrose option nearly exclusively over the other identical Sucrose-alone option without laser (F1,6=46.700, p<0.001; Fig. 5A). Rats reached a 7:1 ratio preference by final day 8 (t7=5.846, p=0.001, 95%CI:[208,491], d=2.66). Both female and male ChR2 Crh-cre+ rats showed strong preferences for NAc Laser+Sucrose lever over Sucrose-alone lever (females: 5:1±1 ratio, males: 7:1±1; Fig. S1A). Both 10Hz (n=5; F1,4=24.540, p=0.008) and 40Hz frequencies of NAc laser excitation (n=7; F1,6=39.209, p=0.001) supported similar Laser+Sucrose preference, with no difference between them (F1,10=1.186, p=0.302; Fig. S2C). By contrast, NAc eYFP controls with inactive virus chose randomly between Laser+Sucrose and Sucrose-alone options (n=6; F1,5=0.014, p=0.911; Fig. 5B).
Figure 5. CRF-containing neuron stimulation in NAc biases and amplifies sucrose motivation.
ChR2 excitation of CRF-containing neurons in NAc shell caused preference for paired Laser+Sucrose over Sucrose Alone in A) two-choice test (n=3 female, n=5 male), reaching a 7:1 ratio by day 8. By contrast B) control NAc eYFP rats chose equally between options. C) NpHR inhibition of CRF-containing neurons in NAc shell (n=3 female, n=3 male) caused avoidance of Laser+Sucrose and Sucrose-alone preference. D) In a progressive ratio test, NAc ChR2 CRF-containing neuron excitation enhanced incentive motivation breakpoint Laser+Sucrose over Sucrose Alone (n=3 female, n=3 male). ChR2 rats had higher Laser+Sucrose breakpoints than eYFP controls (n=5). Laser did not affect NAc eYFP control breakpoint between progressive ratio test days. NAc NpHR inhibition of CRF-containing neurons reduced Laser+Sucrose breakpoint motivation (n=3 female, n=3 male). Means and SEM reported. *p<0.05,**p<0.01,***p<0.001
NAc CRF-containing neuron inhibition paired-avoidance.
Separate inhibition rats, with halorhodopsin (NpHR) in NAc, developed strong avoidance of the paired Laser+Sucrose option and instead preferred Sucrose-alone by a 20:1 ratio (n=6; F1,5=25.741, p=0.004; Fig. 5C).
CeA CRF-containing neuron incentive enhancement.
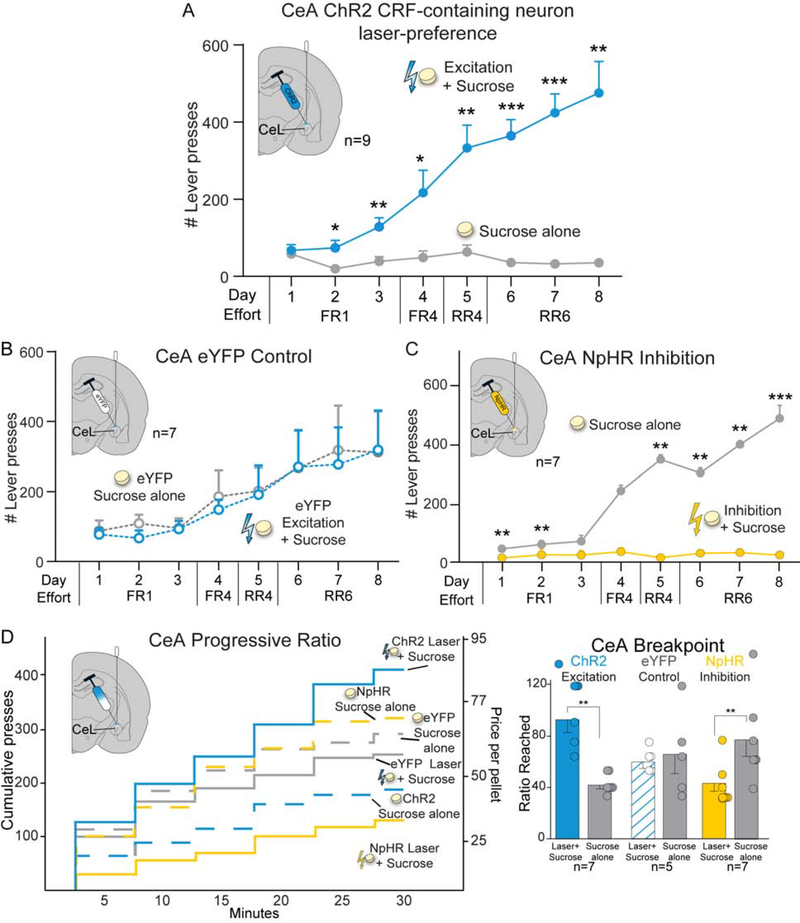
In CeA, ChR2 stimulation of CRF-containing neurons induced similar near-exclusive pursuit of the paired Laser+Sucrose option (n=9; F1,7=19.227, p=0.003; Fig. 6A), growing to a >10:1 ratio over Sucrose-alone by day 8 (t8=5.110, p=0.001, 95%CI:[241,638], d=3.09). Female and male ChR2 Crh-Cre+ rats had similar preference ratios for CeA Laser+Sucrose over Sucrose-alone (females: 13:1±2, males: 10:1±2; Fig. S1A). Both 10Hz (n=9; F1,8=59.101, p<0.001; Fig. S2D) and 40Hz frequencies of CeA laser excitation (n=5; F1,4=90.572, p=0.001) supported comparable levels of preference (F1,12=0.534, p=0.479). By contrast, control CeA eYFP rats chose equally between sucrose options (n=7; F1,6=0.003, p=0.959) and so differed significantly from CeA ChR2 rats (F1,14=4.853, p=0.045; Fig. 6B).
Figure 6. CRF-containing neuron stimulation in CeA biases and amplifies sucrose motivation.
CeA ChR2 excitation of CRF-containing neurons caused near-exclusive preference for Laser+Sucrose over Sucrose-alone rewards in two-choice test (n=4 female, n=5 male), A) reaching a 10:1 ratio by day 8. B) CeA eYFP controls chose equally between sucrose options (n=7) and differed from CeA ChR2 rats. C) CeA NpHR inhibition of CRF-containing neurons caused Laser+Sucrose avoidance and Sucrose-alone preference (n=2 female, n=5 male). D) In progressive ratio test, CeA CRF-containing neuron excitation enhanced incentive motivation for sucrose breakpoint (n=3 female, n=4 male). Laser did not affect CeA eYFP control breakpoint (n=5), which differed from ChR2 rats. CeA NpHR inhibition of CRF-containing neurons reduced Laser+Sucrose breakpoint (n=2 female, n=5 male). Means and SEM reported. *p<0.05,**p<0.01,***p<0.001
CeA CRF-containing neuron inhibition paired-avoidance.
NpHR CeA inhibition of CRF-containing neurons (n=7) produced avoidance of the laser-paired sucrose option, instead causing a 10:1 ratio preference for Sucrose-alone (F1,6=72.960, p<0.001; Fig. 6C).
NAc and CeA CRF-expressing neuronal excitation increases breakpoint
Progressive ratio (PR) breakpoint tests assessed whether CRF-containing neuron stimulation changed the intensity of incentive motivation to obtain sucrose reward. NAc ChR2 rats (n=6) worked twice as hard in the PR task on their Laser+Sucrose day, and achieved 200% higher effort breakpoints, than on the Sucrose-alone day (t5=6.010, p=0.002, 95%CI:[23,58], d=2.6; Fig. 5D). Both female (210±16%) and male rats doubled their breakpoints in Laser+Sucrose condition (170±24%; Fig. S1B). Similarly, 10Hz (t3=4.841, p=0.017, n=4) and 40Hz (t5=6.010, p=0.002, n=6; Fig. S3D) laser frequencies supported similar doubling of breakpoint. NAc eYFP controls showed no breakpoint differences between Laser+Sucrose and Sucrose-alone days (n=5; t4=0.533, p=0.62; Fig. 5D), and so differed significantly from ChR2 rats (F1,9=6.689, p=0.029).
In CeA, excitation of CRF-containing neurons also increased Laser+Sucrose breakpoint by >200% over Sucrose-alone (n=7; t6=6.712, p=0.001, 95%CI:[34,73], d=3.58; Fig. 6D). CeA stimulation doubled breakpoint in both females (250±56%) and males (250 ±25% males; Fig. S1B), and at both 10Hz (n=7, t6=4.992, p=0.002) and 40Hz frequencies (n=5, t4=4.3981, p=0.012; Fig. S3D). Control CeA eYFP rats (n=5) showed no laser effect on breakpoint (t4=0.314, p=0.769; Fig. 6D), and significantly differed from ChR2 rats (F1,10=9.590, p=0.011).
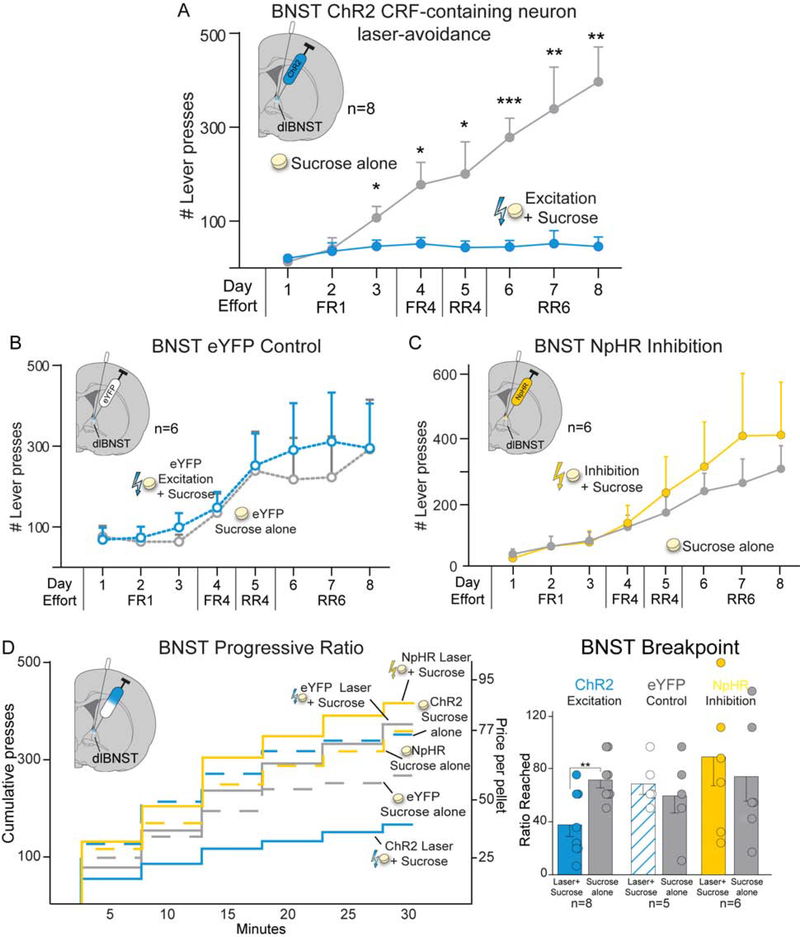
BNST CRF-containing neuron excitation induces laser-paired sucrose avoidance.
In the two-choice task, BNST ChR2 rats avoided their Laser+Sucrose option and instead preferred Sucrose-alone (n=8; F1,6=13.927, p=0.010; Fig. 7A), reaching an 8:1 Sucrose-alone preference by day 8 (n=8; t7=6.059, p=0.001, 95%CI:[214,488], d=4.72). ChR2 males showed numerically stronger avoidance of BNST Laser+Sucrose, (10:1±3 preference for Sucrose-alone) than females (5:1±1), but the small group sizes were not adequately powered to statistically evaluate sex differences here (Fig. S1). Both 10Hz (n=7; F1,6=30.241, p=0.002) and 40Hz frequencies supported similar Laser+Sucrose avoidance (n=5; F1,4=9.474, p=0.037), with no difference in magnitude (F1,10=0.996, p=0.342). In contrast, BNST eYFP control rats chose equally between the two sucrose options (n=6; F1,5=0.054, p=0.826; Fig. 7B).
Figure 7. CRF-containing neuron stimulation in BNST is avoided and suppresses sucrose motivation.
ChR2 rats avoided Laser+Sucrose that stimulated CRF-expressing neurons in BNST in A) two-choice test (n=5 female, n=3 male). BNST ChR2 Laser+Sucrose avoidance rose to an 8:1 opposite preference for Sucrose-alone by day 8. B) Control eYFP BNST rats chose equally between sucrose options (n=6). C) BNST NpHR rats (n=3 female, n=3 male) showed no significant difference between inhibitory Laser+Sucrose and Sucrose-alone. D) BNST ChR2 excitation of CRF-containing neurons suppressed breakpoint effort for sucrose in progressive ratio tests (n=5 female, n=3 male). Laser did not affect BNST eYFP control breakpoint, and so eYFP rats significantly differed from BNST ChR2 rats in laser effects on breakpoint. NpHR inhibition of BNST CRF-containing neurons did not statistically alter sucrose breakpoint, despite a nonsignificant trend toward increased motivation (n=3 female, n=3 male). Means and SEM reported. *p<0.05,**p<0.01,***p<0.001
BNST NpHR two-choice.
BNST NpHR rats (n=6) showed no statistical difference in choice between sucrose options (F1,5=0.167, p=0.700; Fig. 7C), although there was a nonsignificant trend toward preferring the Laser+Sucrose option paired with halorhodopsin inhibition.
BNST CRF-containing neuron excitation suppresses sucrose incentive motivation.
Excitation of BNST CRF-containing neurons suppressed incentive motivation to earn sucrose, reducing Laser+Sucrose breakpoint effort to half that of Sucrose-alone (t7=5.492, p=0.001, 95%CI:[20,49], d=2.25; Fig. 7D). Both female (49±27%) and male rats (54±14%) showed similar breakpoint reductions, and both 10Hz (t7=6.178, p<0.001, n=8) and 40Hz frequencies were comparably effective (t3=5.333, p=0.013, n=4). By contrast, BNST eYFP controls showed no breakpoint laser-effects (n=5; t4=0.441, p=0.682; Fig. 7D), and so differed from BNST ChR2 rats (F1,11=5.874, p=0.034).
Opposite breakpoint effects for CRF-expressing neuronal inhibition
Halorhodopsin inhibition of CRF-containing neurons in NAc (n=6, Fig. 5D) or CeA (n=7; Fig. 6D) suppressed Laser+Sucrose breakpoint to ~50% that of Sucrose-alone (NAc: t5=5.308, p=0.003, 95%CI:[19,53], d=2.58; CeA: t6=4.032, p=0.007, 95%CI:[13,55], d=2.33). BNST CRF-containing neuronal inhibition did not significantly alter breakpoint effort, though there was a nonsignificant trend toward a higher breakpoint for Laser+Sucrose (n=6; t5=0.717, p=0.506; Fig. 7D).
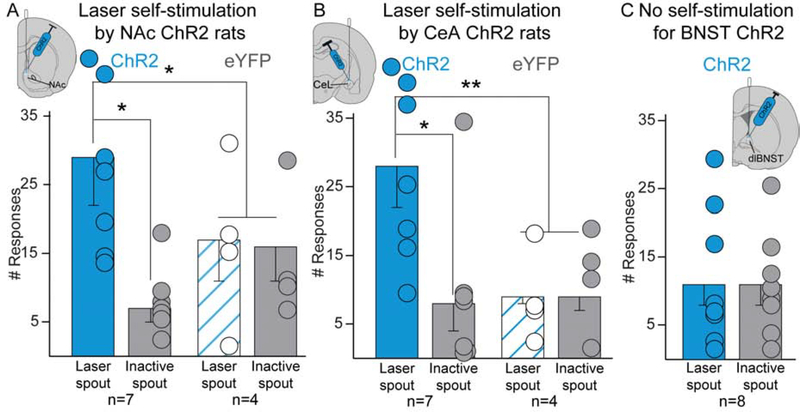
Spout-touch self-stimulation: NAc and CeA stimulation of CRF-expressing neurons by itself is a moderate reward
In the instrumental self-stimulation task, each touch on the designated Laser-spout earned 3-sec of laser excitation, while Inactive-spout touches delivered nothing. No NAc ChR2 rats met criterion for robust self-stimulation of >50 touches on Laser-spout on Day 1 (45). However, 7 of 8 NAc ChR2 rats demonstrated low-level self-stimulation, meeting a lesser criterion of only >10 Laser-spout touches and >2× touches on Laser-spout as on Inactive-spout. On Days 2–3, those 7 NAc rats achieved 25–35 self-stimulations per 30min session, roughly 4× more than Inactive-spout touches (n=7; F1,5=7.823, p=0.038; Fig. 8A), and~1.5× more Laser-spout touches than eYFP control rats (F1,9=9.949, p=0.012). Female and male NAc ChR2 rats showed similar levels of self-stimulation (males: 29±16 illuminations; females: 29±8), and 10Hz and 40Hz frequencies both supported self-stimulation (10Hz: 25±10, n=3; 40Hz: 32±10, n=4).
Figure 8. Laser is self-stimulated by NAc Crh-Cre+ rats and CeA Crh-Cre+ rats in spout-touch task, but not by BNST Crh-Cre+ rats.
A) NAc ChR2 rats self-stimulated ~25–35 times on average (n=5 female, n=2 male), whereas NAc eYFP control rats (n=4) touched both spouts equally about 15 times. B) CeA ChR2 rats similarly self-stimulated (n=2 female, n=5 male), whereas eYFP control rats did not (n=4). C) BNST ChR2 rats failed to self-stimulate for laser in BNST CRF-containing neurons (n=3 female, n=5 male). Also see Fig. S4 and Fig. S6D. Means ± SEM, and individual scores shown. *p<0.05,**p<0.01
CeA self-stimulation.
Two of eight CeA ChR2 rats met the >50 illuminations criterion for robust self-stimulation, while 7 met the lower >10 self-stimulation criterion. These 7 CeA ChR2 rats self-stimulated ~25–35 times on days 2–3, >3× more than Inactive-spout (F1,5=12.009, p=0.018; Fig. 8B), and earned >3× more illuminations than eYFP control rats (F1,9=17.576, p=0.002). The 2 most robust self-stimulators were both females and reached 40±3 self-stimulations per day (males n=5, 23±7). Both 10Hz (n=4) and 40Hz (n=3) frequencies supported similar levels of CeA self-stimulation (10Hz: 27±10 self-stimulations; 40Hz: 29±8).
BNST fails to support self-stimulation.
No BNST ChR2 rats met any criteria for self-stimulation of CRF-containing neurons, responding equally at low rates on both spouts (n=8, F1,6=0.006, p=0.939, Fig. 8C).
CeA and NAc self-stimulation does not account for laser effects on sucrose motivation.
Did laser self-stimulation in CeA and NAc substantially drive laser’s ability to control sucrose pursuit in two-choice or PR tasks? The answer appears to be ‘no’: there was no correlation between self-stimulation values, which were generally low, and control of sucrose pursuit in the two-choice test (n=6 NAc: r=0.624, p=0.098; n=7 CeA: r=−0.024, p=0.926). Nor was there a correlation between self-stimulation and enhancement of PR breakpoint, which was relatively strong in most NAc or CeA ChR2 rats (Pearson’s correlation, n=6 NAc: r=−0.349, p=0.498; n=7 CeA: r=0.605, p=0.280). Finally, even CeA (n=1) and NAc (n=1) rats that failed to self-stimulate showed ~200% laser-induced enhancements of breakpoint, and control of Laser+Sucrose preference (11:1 ratio) as strong as in self-stimulators (~200%, 9:1).
NAc and CeA place-based self-stimulation, BNST place-avoidance
Rats were additionally tested for self-stimulation using a second place-based task, where entering and staying in a designated chamber earned laser (cycling 3-sec-on/4-sec-off; 15min).
NAc and CeA place-based self-stimulation.
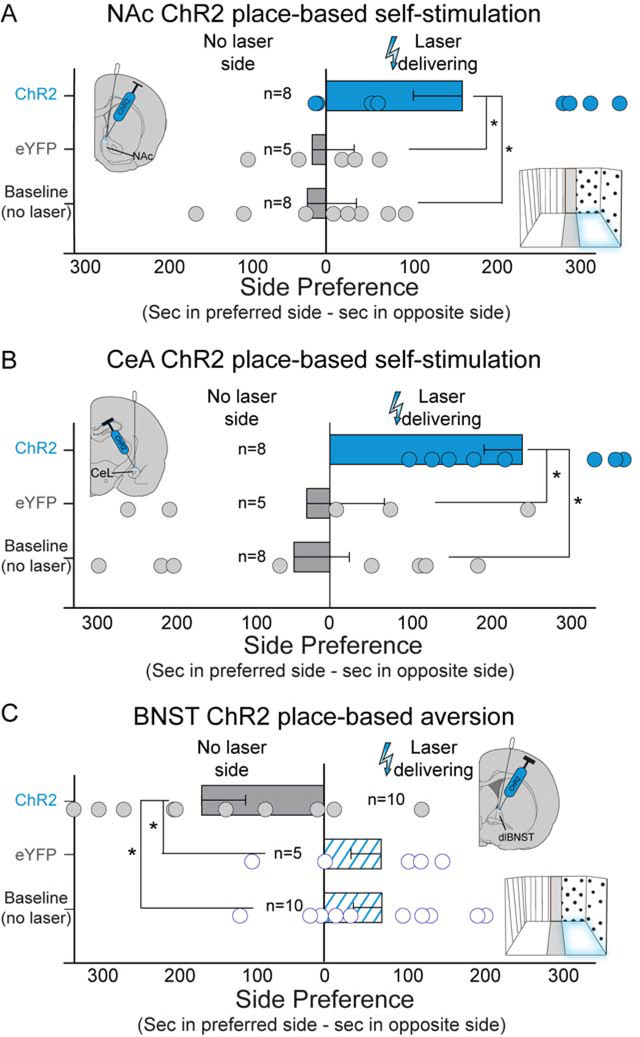
NAc ChR2 rats spent >150% more time in Laser-delivering chamber than in No-laser chamber (F1,6=6.664, p=0.042; Fig. 9a). NAc ChR2 rats also spent 150% longer in Laser-delivering chamber than they had during previous baseline tests without laser (t7=3.376, p=0.012, 95%CI:[56,318], d=1.21), and more time in their Laser-delivering chamber than inactive eYFP controls (t11=2.318, p=0.041, 95%CI: [9,353], d=1.05). Both female (n=2) and male (n=6) NAc ChR2 rats spent comparably more time in the Laser-delivering chamber (female: 160±20%; males: 140±10%), and both 10Hz (n=3; 160±20%) and 40Hz (n=5; 160±20%) frequencies were equally effective.
Figure 9. Place-based self-stimulation and place-based aversion following CRF-containing neuron stimulation.
A) NAc supported ChR2 place-based laser self-stimulation of CRF-containing neurons. NAc ChR2 rats (n=2 female, n=6 male) spent more time in Laser-delivering chamber than in No-laser chamber, more time in Laser-delivering chamber than NAc eYFP controls (n=5), and more than they previously spent in same chamber during no-laser baseline preference tests. B) CeA also supported place-based self-stimulation of CRF-containing neurons. CeA ChR2 rats (n=5 female, n=3 male) spent more time in Laser-delivering chamber than in no-laser chamber, more time in Laser-delivering chamber than CeA eYFP controls (n=5), and more than they previously spent in identical chamber during no-laser baseline tests. C) Conversely, BNST produced avoidance the Laser-delivering chamber. BNST ChR2 rats (n=5 female, n=5 male) spent less time in Laser-delivering chamber than eYFP controls (n=5), and less time than they spent in the same chamber during no-laser baseline tests. Also see Fig. S5 and Fig. S6E. Means and SEM reported. *p<0.05
CeA ChR2 rats (n=8) demonstrated robust place-based self-stimulation of CeA CRF-containing neurons, spending ~200% more time in Laser-delivering than the No-laser chamber (F1,6=21.085, p=0.004). CeA ChR2 rats also spent 200% longer in Laser-delivering chamber than they had during previous baseline tests without laser (t7=3.038, p=0.019, 95%CI:[63,509], d=1.41), and more than CeA eYFP controls (t11=2.062, p=0.011, 95%CI:[57,484], d=1.20). Both female (n=2; 160±20% more Laser-delivering time) and male (n=3; 200±20%) CeA ChR2 rats showed place-based self-stimulation, and 10Hz (n=5) and 40Hz (n=3) laser frequencies were both effective (10Hz: 200±10%; 40Hz: 150±30%).
BNST induces place-avoidance.
BNST ChR2 rats mildly avoided the Laser-delivering chamber that stimulated CRF-containing neurons in BNST, spending only <75% as much time there as in the No-laser chamber (n=10; F1,8=6.593, p=0.033; Fig. 9C). ChR2 BNST rats also spent less time in their Laser-delivering chamber than they had during baseline tests without laser (t9=3.188, p=0.011, 95%CI:[67,397], d=1.25), and less time than eYFP control rats (t13=2.737, p=0.017, 95%CI:[49,415], d=1.76). Both female (n=5) and male (n=5) BNST ChR2 rats showed avoidance of the Laser-delivering chamber (female: <85±10%; males: <50±10%), and both 10Hz (n=6; <65±10%) and 40Hz (n=4; <70±10%) frequencies induced place-based avoidance.
Discussion
Our results demonstrate that optogenetic excitation of CRF-containing neural systems in both CeA and NAc shell focused and increased incentive motivation for sucrose and carried positive valence by itself. ChR2 stimulation of CRF-containing neurons in CeA and NAc 1) focused intense incentive motivation on the Laser+Sucrose option over an alternative Sucrose-alone option in the two-choice task, 2) amplified incentive motivation and breakpoint effort for sucrose reward, and 3) was actively sought by itself as laser self-stimulation. Simultaneously, ChR2 stimulation of CRF-expressing neurons in CeA and NAc recruited reward-related mesolimbic circuitry, reflected as Fos increases in VTA, NAc, VP, LH, etc.
By contrast, only BNST optogenetic excitation of CRF-containing neurons produced aversive motivation. BNST CRF-containing neuronal excitation here caused avoidance of the Laser+Sucrose option and of laser by itself, suppressed breakpoint of sucrose motivation, and recruited increased Fos in PVN and PAG, structures associated with negative-avoidance or distress.
Our NAc and CeA incentive effects are consistent with previous reports that CRF systems in CeA or NAc can contribute positively to reward motivation (11–15, 36). NAc CRF microinjections increase bursts of cue-triggered ‘wanting’ for sucrose rewards in rats, and cause conditioned place-preference and increase NAc dopamine release in non-stressed mice (13–15).
CRF systems mobilize bio/behavioral responses to changing events (9–11), and can be responsive to either positive or negative events. For instance, it has long been known that CRF systems in CeA respond to positive reward stimuli such as food cues, not only to aversive stimuli (11). Indicating positively-valenced roles, mice optogenetically self-stimulate CRF-containing neurons in CeA (12). Our results confirm CeA CRF-containing neuronal self-stimulation in rats and extend CRF neuronal self-stimulation to NAc. They further demonstrate that NAc and CeA activations potentiate and focus incentive motivation for natural sucrose reward. Conversely, CRF-containing neuronal stimulation in BNST produced opposite negative motivational effects.
Future studies could identify the specific projections from CeA, NAc and BNST that mediate these effects. For example, CeA CRF-containing neurons project to LH, VP, VTA, and BNST (21, 52–56). CeA-BNST CRF-containing projections may reliably mediate aversive motivation (35, 53–55), implying that projections to LH, VP, VTA or elsewhere may mediate incentive motivation effects. ChR2 stimulation here likely activated these CeA-BNST projections too, implying that other positively-valenced CeA-outputs may overpower BNST aversive effects when simultaneously activated. For NAc, local connections of CRF-containing neurons may mediate incentive motivation effects, such as intra-NAc connections to cholinergic interneurons, which may modulate dopamine release in NAc (14–16). Neuroanatomically, it would be of interest to additionally investigate the motivational effects of dense CRF-containing neuronal projections from hypothalamic PVN. However, PVN CRF-containing neurons may co-release glutamate, whereas the Crh-Cre rat line used here may primarily target CRF-expressing neurons that co-release GABA (21, 57).
Neurochemically, it would be useful in future studies to examine the roles in these motivational effects of CRF release versus other neurotransmitters co-released by CRF-expressing neurons, such as GABA, dynorphin, neurotensin, and somatostatin (21, 47, 58–60). Co-release might be related to why CRFR1 antagonists may fail to block stress-induced craving in clinical models (61–64).
Positive NAc and CeA vs negative BNST: Anatomical differences in motivational valence
Why did CRF-containing neuron activations have positively-valenced effects in NAc and CeA but negatively-valenced effects in BNST? NAc and CeA are both striatal-level structures in cortico-striatal-pallidal macrosystem frameworks of telencephalon organization, having neuronal, connectivity, neurochemical, and embryological features shared with neostriatum (65–67). For example, CeA and NAc contain mostly GABAergic neurons that receive descending cortical-type glutamatergic inputs and ascending mesotelencephalic dopaminergic inputs, and both send GABAergic outputs to pallidal-level structures of BNST or VP (65–68). In the same frameworks, BNST is a pallidal-level structure with descending outputs to hypothalamus and brainstem, plus ascending re-entrant projections back to thalamo-cortico-striatal-pallidal loops (22–24, 65–68).
Hypothesized roles of CRF-containing systems in addiction
Traditionally, CRF-containing neurons have been hypothesized to generate aversive states like anxiety and drug withdrawal, although CRF systems also have wider roles in affective appraisals of incentives that mobilize motivational states (9–11). Our study helps puts this in perspective.
Regarding the role of CRF in anxiety and addiction, the allostatic theory of addiction posits that CRF-containing neuronal activation in CeA and BNST components of extended amygdala cause aversive drug withdrawal, which is hypothesized to promote relapse through efforts to hedonically self-medicate via consumption of drug rewards (27–29, 32–34).
Our results call into question some of these assumptions. Indeed, the hypothesis that CRF-containing neurons in CeA and BNST necessarily generate negatively-valenced states may not apply to CeA. Instead, our results indicate that CRF-expressing neuronal activation in both CeA and NAc increases reward pursuit and produces positively-valenced incentive states which rats actively worked to induce. Conversely, in partial support of the allostatic model, BNST CRF-containing neural activation did cause aversive motivational states. However, the aversive state induced by stimulating BNST CRF-expressing neurons failed to increase reward-seeking, instead suppressing sucrose pursuit.
This suggests that hedonic self-medication of aversion may not be the primary mechanism by which CRF-containing neurons promote reward pursuit and consumption for any of these structures. Instead, CRF-expressing neurons in CeA and NAc amplify ‘wanting’ to pursue and consume rewards without aversive states, while BNST CRF-expressing neuronal excitation may actually impede reward pursuit and consumption. This may be why drug withdrawal is not as effective for reinstatement of drug taking as stress or drug priming (25, 37–39). Although brain-wide CRF activation may cause aversive withdrawal states through BNST CRF-containing neurons, our results suggest that any accompanying increases in reward pursuit or addictive relapse might predominantly be due to co-activation of CRF incentive motivation systems in NAc and CeA.
Valence flips
Motivational valence induced by CeA optogenetic stimulation can switch depending on environmental situation, and therefore the valence of our CRF-containing neuron stimulation could potentially switch in certain circumstances (14, 45). If so, CRF systems could be quite labile in their functional role in motivated behaviors depending upon context and need, which deserves further investigation.
Clinical implications
Activation of CRF systems during stress or emotional excitement may promote relapse in addiction, binge-eating, and other excessive consumption. The dominant perspective relied solely on the postulated aversiveness of CRF-expressing neural activation. However, our results indicate that incentive motivation roles of CRF-containing neurons in NAc and CeA predominate under tested conditions, and promote intense reward pursuit without aversive distress (12–14). This could explain why even positively-valenced stressors (i.e., new relationships, winning the lottery) can be triggers of addictive relapse and binge-eating (69–72). Conversely, aversive motivation induced by BNST CRF-containing neurons contributed little to reward pursuit. Ultimately, CRF-containing systems have diverse motivational roles. Further clarification of negatively-valenced versus positively-valenced motivation roles of CRF systems will be important to understand how they promote excessive consumption in addiction and related disorders.
Supplementary Material
KEY RESOURCES TABLE.
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | rabbit anti-cFos | Synaptic Systems, Göttingen, Germany | Catalog#: 226 003; Lot #: 4–63; RRID:AB_2231974 | 1:2500 dilution |
| Antibody | chicken anti-GFP | Abcam, Cambridge, MA | Catalog#: AB13970; Lot #: GR3190550–30; RRID:AB_300798 | 1:2000 dilution |
| Antibody | Biotin-SP donkey anti-rabbit | Jackson Immunoresearch, West Grove, PA | Catalog #: AB2340593; Lot #: 128703; RRID:AB_2340593 | 1:300 dilution |
| Antibody | donkey anti-chicken AlexaFluor 488 | Jackson Immunoresearch, West Grove, PA | Code #: AB2340375; Lot #: 144438; RRID:AB_2340375 | 1:300 dilution |
| Antibody | Streptavidin Cy3 | Jackson Immunoresearch, West Grove, PA | Catalog #: AB2337244, Lot #: 141873, RRID:AB_2337244 | 1:300 dilution |
| Bacterial or Viral Strain | AAV-EF1a-DIO-hChR2(H134R)-EYFP | University of North Carolina Vector Core | N/A | |
| Bacterial or Viral Strain | AAV-EF1a-DIO-EYFP | University of North Carolina Vector Core | N/A | |
| Bacterial or Viral Strain | AAV-EF1a-DIO-eNpHR3.0-EYFP | University of North Carolina Vector Core | N/A | |
| Chemical Compound or Drug | Atropine | Henry Schein | N/A | 0.05mg/kg; i.p. |
| Chemical Compound or Drug | Cefazolin | Henry Schein | N/A | 75mg/kg, s.c. |
| Chemical Compound or Drug | Carprofen | Henry Schein | N/A | 5mg/kg, s.c. |
| Commercial Assay Or Kit | RNAscope® Multiplex Fluorescent Reagent Kit v2 | Advanced Cellular Diagnostics | Cat. No. 323100 | |
| Organism/Strain | Rat: Crh-Cre, male and female | Messing Lab, University of Texas | RRRC#: 00852 | |
| Sequence-Based Reagent | Rn-Crem-03 probe | Advanced Cellular Diagnostics | Cat. No. 530001 | |
| Sequence-Based Reagent | Rn-Crh-C3 | Advanced Cellular Diagnostics | Cat. No. 318931-C3 | |
| Software; Algorithm | Matlab | Mathworks | RRID:SCR_001622 | |
| Software; Algorithm | Surveyor with Turboscan | Objective Imaging LTD. | RRID:SCR_014433 | |
| Software; Algorithm | MED-PC IV | MedAssociates | RRID:SCR_012156 | |
| Software; Algorithm | Adobe Illustrator 23.0 | Adobe Inc. | RRID:SCR_010279 | |
| Software; Algorithm | Observer XT 12 | Noldus | RRID:SCR_004074 |
Acknowledgements:
This research was supported by National Institutes of Health grants MH063649 and DA015188 to KCB, and NIH grants F31 DA047738 and T32 DA007281 for HMB. The project described was also supported by Grant Number P30DK020572 (MDRC) from the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Michigan In Situ Hybridization Laboratory. We are especially grateful to Dr. Robert Messing for his generosity in providing us with breeder Crh-Cre rats, which were developed in his laboratory at the University of Texas at Austin, as well as for feedback on a previous version of the manuscript. We thank Marco Liera and Madeliene Ayoub for technical assistance and Laura Huerta Sanchez and Tayah Schuette for behavioral testing and histology. We also thank Erin Naffziger and Ileana Morales for feedback on the manuscript.
Footnotes
Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Code availability:
Code for MedPC software for two-choice, progressive ratio, and spout self-stimulation tasks is available through NIH figshare public repository, doi: https://doi.org/10.6084/m9.figshare.12762389.v1
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability:
The data that support the findings of this study (Figures 1–9 and Supplemental information) are made available through NIH figshare public repository, doi: https://doi.org/10.6084/m9.figshare.12762383
References:
- 1.Vale W, Spiess J, Rivier C, Rivier J (1981): Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 213(4514): 1394–1397. [DOI] [PubMed] [Google Scholar]
- 2.Hupalo S, Bryce CA, Bangasser DA, Berridge CW, Valentino RJ, Floresco SB (2019): Corticotropin-releasing factor (crf) circuit modulation of cognition and motivation. Neurosci. Biobehav. Rev. 103: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McEwen BS, Akil H (2020): Revisiting the stress concept: implications for affective disorders. J. Neurosci. 40(1): 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart J (2000): Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J. Psychiatry Neurosci. 25(2): 125–136. [PMC free article] [PubMed] [Google Scholar]
- 5.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. (2003): Chronic stress and obesity: a new view of “comfort food”. Proc. Natl. Acad. Sci. USA. 100(20): 11696–11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koob GF, Bloom FE (1985): Corticotropin-releasing factor and behavior. Fed Proc. 44(1 Pt 2): 259–263. [PubMed] [Google Scholar]
- 7.Dunn AJ, Berridge CW (1990): Physiological and behavioral responses to corticotropin-releasing factor administration: is crf a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 15(2): 71–100. [DOI] [PubMed] [Google Scholar]
- 8.Bale TL, Vale WW (2003): Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. J. Neurosci. 23(12): 5295–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulkin J (2017): The CRF Signal: Uncovering an Information Molecule, Oxford University Press, [Google Scholar]
- 10.Merali Z, McIntosh J, Anisman H (2004): Anticipatory cues differentially provoke in vivo peptidergic and monoaminergic release at the medial prefrontal cortex. Neuropsychopharmacology. 29(8): 1409–1418. [DOI] [PubMed] [Google Scholar]
- 11.Merali Z, McIntosh J, Kent P, Michaud D, Anisman H (1998): Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J. Neurosci. 18(12): 4758–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S (2017): Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron. 93(6): 1464–1479.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peciña S, Schulkin J, Berridge KC (2006): Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemos JC, Wanat MJ, Smith JS, Reyes BAS, Hollon NG, Van Bockstaele EJ, et al. (2012): Severe stress switches crf action in the nucleus accumbens from appetitive to aversive. Nature. 490(7420): 402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemos JC, Alvarez VA (2020): The upside of stress: a mechanism for the positive motivational role of corticotropin releasing factor. Neuropsychopharmacology. 45(1): 219–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemos JC, Shin JH, Alvarez VA (2019): Striatal cholinergic interneurons are a novel target of corticotropin releasing factor. J. Neurosci. 39(29): 5647–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swanson LW, Simmons DM (1989): Differential steroid hormone and neural influences on peptide mrna levels in crh cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J. Comp. Neurol. 285(4): 413–435. [DOI] [PubMed] [Google Scholar]
- 18.Makino S, Gold PW, Schulkin J (1994): Effects of corticosterone on crh mrna and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res. 657(1–2): 141–149. [DOI] [PubMed] [Google Scholar]
- 19.Makino S, Gold PW, Schulkin J (1994): Corticosterone effects on corticotropin-releasing hormone mrna in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 640(1–2): 105–112. [DOI] [PubMed] [Google Scholar]
- 20.Itoga CA, Chen Y, Fateri C, Echeverry PA, Lai JM, Delgado J, et al. (2019): New viral-genetic mapping uncovers an enrichment of corticotropin-releasing hormone-expressing neuronal inputs to the nucleus accumbens from stress-related brain regions. J. Comp. Neurol. 527(15): 2474–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, et al. (2015): A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits. Front. Neurosci. 9: 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray TS, Magnuson DJ (1992): Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 13(3): 451–460. [DOI] [PubMed] [Google Scholar]
- 23.Giardino WJ, Eban-Rothschild A, Christoffel DJ, Li S-B, Malenka RC, de Lecea L (2018): Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat. Neurosci. 21(8): 1084–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dabrowska J, Martinon D, Moaddab M, Rainnie DG (2016): Targeting corticotropin-releasing factor projections from the oval nucleus of the bed nucleus of the stria terminalis using cell-type specific neuronal tracing studies in mouse and rat brain. J. Neuroendocrinol. 28(12): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y (2016): Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 41(1): 335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grilo CM, Pagano ME, Stout RL, Markowitz JC, Ansell EB, Pinto A, et al. (2012): Stressful life events predict eating disorder relapse following remission: six-year prospective outcomes. Int. J. Eat. Disord. 45(2): 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koob GF, Schulkin J (2019): Addiction and stress: an allostatic view. Neurosci. Biobehav. Rev. 106: 245–262. [DOI] [PubMed] [Google Scholar]
- 28.Koob GF (2013): Addiction is a reward deficit and stress surfeit disorder. Front. Psychiatry. 4: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberto M, Spierling SR, Kirson D, Zorrilla EP (2017): Corticotropin-releasing factor (crf) and addictive behaviors. Int Rev Neurobiol. 136: 5–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon RL, Corbit JD (1978): An opponent-process theory of motivation. The American Economic Review [Google Scholar]
- 31.Solomon RL (1980): The opponent-process theory of acquired motivation: the costs of pleasure and the benefits of pain. Am. Psychol. 35(8): 691–712. [DOI] [PubMed] [Google Scholar]
- 32.Koob GF, Le Moal M (1997): Drug abuse: hedonic homeostatic dysregulation. Science. 278(5335): 52–58. [DOI] [PubMed] [Google Scholar]
- 33.Funk CK, O’Dell LE, Crawford EF, Koob GF (2006): Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J. Neurosci. 26(44): 11324–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zorrilla EP, Logrip ML, Koob GF (2014): Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol. 35(2): 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Guglielmo G, Kallupi M, Pomrenze MB, Crawford E, Simpson S, Schweitzer P, et al. (2019): Inactivation of a crf-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat. Commun. 10(1): 1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z, Young LJ (2007): Crf receptors in the nucleus accumbens modulate partner preference in prairie voles. Horm. Behav. 51(4): 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J (1997): Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J. Neurosci. 17(7): 2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaham Y, Stewart J (1995): Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 119(3): 334–341. [DOI] [PubMed] [Google Scholar]
- 39.Erb S, Petrovic A, Yi D, Kayyali H (2006): Central injections of crf reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context. Psychopharmacology. 187(1): 112–120. [DOI] [PubMed] [Google Scholar]
- 40.Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, et al. (2011): Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of crhr1. Science. 333(6051): 1903–1907. [DOI] [PubMed] [Google Scholar]
- 41.Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You Z-B (2005): Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J. Neurosci. 25(22): 5389–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vranjkovic O, Van Newenhizen EC, Nordness ME, Blacktop JM, Urbanik LA, Mathy JC, et al. (2018): Enhanced crfr1-dependent regulation of a ventral tegmental area to prelimbic cortex projection establishes susceptibility to stress-induced cocaine seeking. J. Neurosci. 38(50): 10657–10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson MJF, Warlow SM, Berridge KC (2014): Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another. J. Neurosci. 34(50): 16567–16580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baumgartner HM, Cole SL, Olney JJ, Berridge KC (2020): Desire or dread from nucleus accumbens inhibitions: reversed by same-site optogenetic excitations. J. Neurosci. 40(13): 2737–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warlow SM, Naffziger EE, Berridge KC (2020): The central amygdala recruits mesocorticolimbic circuitry for pursuit of reward or pain. Nat. Commun. 11(1): 2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, et al. (2017): A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 542(7639): 96–100. [DOI] [PubMed] [Google Scholar]
- 47.Torruella-Suárez ML, Vandenberg JR, Cogan ES, Tipton GJ, Teklezghi A, Dange K, et al. (2020): Manipulations of central amygdala neurotensin neurons alter the consumption of ethanol and sweet fluids in mice. J. Neurosci. 40(3): 632–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, et al. (2016): Activation of d2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat. Commun. 7: 11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warlow SM, Robinson MJF, Berridge KC (2017): Optogenetic central amygdala stimulation intensifies and narrows motivation for cocaine. J. Neurosci. 37(35): 8330–8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paxinos G, Watson C (2007): The rat brain in stereotaxic coordinates, ed 6. amsterdam: academic. [DOI] [PubMed] [Google Scholar]
- 51.Wang F, Flanagan J, Su N, Wang L-C, Bui S, Nielson A, et al. (2012): Rnascope: a novel in situ rna analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 14(1): 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodaros D, Caruana DA, Amir S, Stewart J (2007): Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 150(1): 8–13. [DOI] [PubMed] [Google Scholar]
- 53.Asok A, Draper A, Hoffman AF, Schulkin J, Lupica CR, Rosen JB (2018): Optogenetic silencing of a corticotropin-releasing factor pathway from the central amygdala to the bed nucleus of the stria terminalis disrupts sustained fear. Mol. Psychiatry. 23(4): 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pomrenze MB, Tovar-Diaz J, Blasio A, Maiya R, Giovanetti SM, Lei K, et al. (2019): A corticotropin releasing factor network in the extended amygdala for anxiety. J. Neurosci. 39(6): 1030–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ventura-Silva AP, Borges S, Sousa N, Rodrigues AJ, Pêgo JM (2020): Amygdalar corticotropin-releasing factor mediates stress-induced anxiety. Brain Res. 1729: 146622. [DOI] [PubMed] [Google Scholar]
- 56.Erb S, Salmaso N, Rodaros D, Stewart J (2001): A role for the crf-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 158(4): 360–365. [DOI] [PubMed] [Google Scholar]
- 57.Dabrowska J, Hazra R, Guo J-D, Dewitt S, Rainnie DG (2013): Central crf neurons are not created equal: phenotypic differences in crf-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front. Neurosci. 7: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pomrenze MB, Giovanetti SM, Maiya R, Gordon AG, Kreeger LJ, Messing RO (2019): Dissecting the roles of gaba and neuropeptides from rat central amygdala crf neurons in anxiety and fear learning. Cell Rep. 29(1): 13–21.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Partridge JG, Forcelli PA, Luo R, Cashdan JM, Schulkin J, Valentino RJ, et al. (2016): Stress increases gabaergic neurotransmission in crf neurons of the central amygdala and bed nucleus stria terminalis. Neuropharmacology. 107: 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimada S, Inagaki S, Kubota Y, Ogawa N, Shibasaki T, Takagi H (1989): Coexistence of peptides (corticotropin releasing factor/neurotensin and substance p/somatostatin) in the bed nucleus of the stria terminalis and central amygdaloid nucleus of the rat. Neuroscience. 30(2): 377–383. [DOI] [PubMed] [Google Scholar]
- 61.Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, et al. (2016): The crf1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology. 41(12): 2818–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, et al. (2015): The corticotropin releasing hormone-1 (crh1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 40(5): 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grillon C, Hale E, Lieberman L, Davis A, Pine DS, Ernst M (2015): The crh1 antagonist gsk561679 increases human fear but not anxiety as assessed by startle. Neuropsychopharmacology. 40(5): 1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaham Y, de Wit H (2016): Lost in translation: crf1 receptor antagonists and addiction treatment. Neuropsychopharmacology. 41(12): 2795–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swanson LW (2005): Anatomy of the soul as reflected in the cerebral hemispheres: neural circuits underlying voluntary control of basic motivated behaviors. J. Comp. Neurol. 493(1): 122–131. [DOI] [PubMed] [Google Scholar]
- 66.Heimer L, Van Hoesen GW, Trimble M, Zahm DS (2007): Anatomy of Neuropsychiatry: The New Anatomy of the Basal Forebrain and Its Implications for Neuropsychiatric Illness, Academic Press, [Google Scholar]
- 67.Zahm DS (2006): The evolving theory of basal forebrain functional-anatomical “macrosystems”. Neurosci. Biobehav. Rev. 30(2): 148–172. [DOI] [PubMed] [Google Scholar]
- 68.Heimer L, Van Hoesen GW (2006): The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci. Biobehav. Rev. 30(2): 126–147. [DOI] [PubMed] [Google Scholar]
- 69.Larimer ME, Palmer RS, Marlatt GA (1999): Relapse prevention. an overview of marlatt’s cognitive-behavioral model. Alcohol Res. Health. 23(2): 151–160. [PMC free article] [PubMed] [Google Scholar]
- 70.Maisto SA, O’Farrell TJ, Connors GJ, McKay JR, Pelcovits M (1988): Alcoholics’ attributions of factors affecting their relapse to drinking and reasons for terminating relapse episodes. Addict. Behav. 13(1): 79–82. [DOI] [PubMed] [Google Scholar]
- 71.Kaundal P, Sharma I, Jha T (2016): Assessment of psychosocial factors associated with relapse in patients with alcohol dependence: a retrospective observational study. Int. J. Basic Clin. Pharmacol. [Google Scholar]
- 72.Annis HM, Graham JM (1995): Profile types on the inventory of drinking situations: implications for relapse prevention counseling. Psychol Addict Behav. 9(3): 176–182. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study (Figures 1–9 and Supplemental information) are made available through NIH figshare public repository, doi: https://doi.org/10.6084/m9.figshare.12762383