Significance
This article reports the discovery of a heretofore unknown icosahedral quasicrystal created by the detonation of the first nuclear device at Alamogordo, NM, on 16 July 1945 (the Trinity test). Like all quasicrystals, the new example violates crystallographic symmetry rules that apply to ordinary (periodic) crystals. It was found in a sample of red trinitite, a combination of glass fused from natural sand and anthropogenic copper from transmission lines used during the test. The new quasicrystal is the oldest extant anthropogenic quasicrystal known, whose place and moment of origin are known from the historic records of the Trinity test. The thermodynamic/shock conditions that formed it are roughly comparable to those that formed natural quasicrystals recently found in meteorites.
Keywords: quasicrystals, trinitite, atomic bomb, Khatyrka, shock
Abstract
The first test explosion of a nuclear bomb, the Trinity test of 16 July 1945, resulted in the fusion of surrounding sand, the test tower, and copper transmission lines into a glassy material known as “trinitite.” Here, we report the discovery, in a sample of red trinitite, of a hitherto unknown composition of icosahedral quasicrystal, Si61Cu30Ca7Fe2. It represents the oldest extant anthropogenic quasicrystal currently known, with the distinctive property that its precise time of creation is indelibly etched in history. Like the naturally formed quasicrystals found in the Khatyrka meteorite and experimental shock syntheses of quasicrystals, the anthropogenic quasicrystals in red trinitite demonstrate that transient extreme pressure–temperature conditions are suitable for the synthesis of quasicrystals and for the discovery of new quasicrystal-forming systems.
The Trinity test—the detonation of a plutonium implosion device known as the “gadget”—occurred at 05:29:45 Mountain War Time on 16 July 1945 on the Alamogordo Bombing Range, about 210 miles south of Los Alamos, NM. The explosion released the equivalent of 21 kilotons of TNT (88 TJ), sufficient to vaporize the 30-m test tower and surrounding miles of copper wires to recording instruments. The explosion produced a large fireball that entrained arkosic sands that rained out as fused crusts and droplets that are now known as trinitite (1–3). We present here evidence of an unintended consequence: the synthesis of a novel icosahedral quasicrystal (empirical formula Si61Cu30Ca7Fe2). The grain (about 10 μm across) was discovered in a copper-rich droplet included in a sample of red trinitite recovered shortly after World War II. This is evidence that the high-temperature, high-pressure conditions created by a nuclear explosion can, like the transient conditions induced by hypervelocity impact in the Khatyrka meteorite (4–8), result in the synthesis of quasicrystals.
The Trinity test created a crater about 1.4 m deep and 80 m wide, vaporizing the experimental infrastructure and fusing the surface sands to a depth of 1 to 2 cm out to a radius of about 300 m. As first reported by Ross (9), trinitite glass was formed by fusing the sand, consisting mostly of quartz and feldspar, and most often is characterized by a pale green color. Later work (1–3) has shown that most of the trinitite formed from sand that was swept up in the fire ball and subjected to the high temperatures and pressures (about 1,500 °C and pressures of 5 to 8 GPa, as explained in Discussion) of the developing cloud, subsequently raining out as glass and fusion crust fragments. Although the vast majority of the trinitite is the classic “green” color, there are numerous fragments characterized as “red” trinitite that are typically rich in metals derived from the test tower and recording equipment.
Results
The trinitite sample investigated in this paper is an example of the rarer oxblood red trinitite (Fig. 1) that Ross (9) reported finding north of the test site, where it was recovered by Lincoln LaPaz just a few months after detonation (3).
Fig. 1.
Incident light images of the red trinitite sample (front and back of the sample).
The red color is attributed to the presence of copper oxide (10) that formed when the copper transmission lines were vaporized. Based on the detection of gamma rays associated with the decay of 152Eu in our sample, the red trinitite appears to have been created about 55 to 60 m from ground zero, close to where the coaxial cable from the top of the tower terminated in a trench.
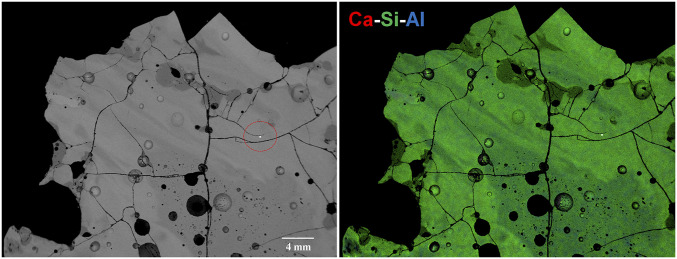
Red trinitite is composed of remnant quartz and feldspar grains which have partially melted to glasses of similar chemistry; Al-Ca-K silicate, Ca-Al silicate, Ca-Al-Fe silicate, and Fe-Ca-Al silicate glasses; and metallic phases comprising 3 to 5% of the material (10). The samples are usually heterogeneous mixtures at the 10- to 100-μm scale. Metallic inclusions (spherules) are common in red trinitite. These include Cu-Pb-Fe and Cu-Fe spherules which frequently show immiscibility and unmixing textures. Fe-Si, Fe-Ti, Cu-S, PbO spherules, and a single grain of a W-Ga-Ta alloy have been found (10). Fig. 2, Left shows a back-scattered scanning electron microscope image of the surface of the red trinitite sample after it was embedded in epoxy and polished. X-ray maps of the polished surface, shown in Fig. 2, Right, indicate the Ca-Si-Al chemical compositional variation. The focus of this paper, though, is on the metallic rounded droplet encircled in Fig. 2, which is embedded in a Na-bearing Al-Ca-K silicate glass (SI Appendix, Fig. S1 and Table S1).
Fig. 2.
General back-scattered scanning electron microscope image of the polished surface of the sample studied (Left). The small bright grain enclosed in the red dashed circle is enlarged in Fig. 3. Combined X-ray maps (Right) of the same portion of the sample.
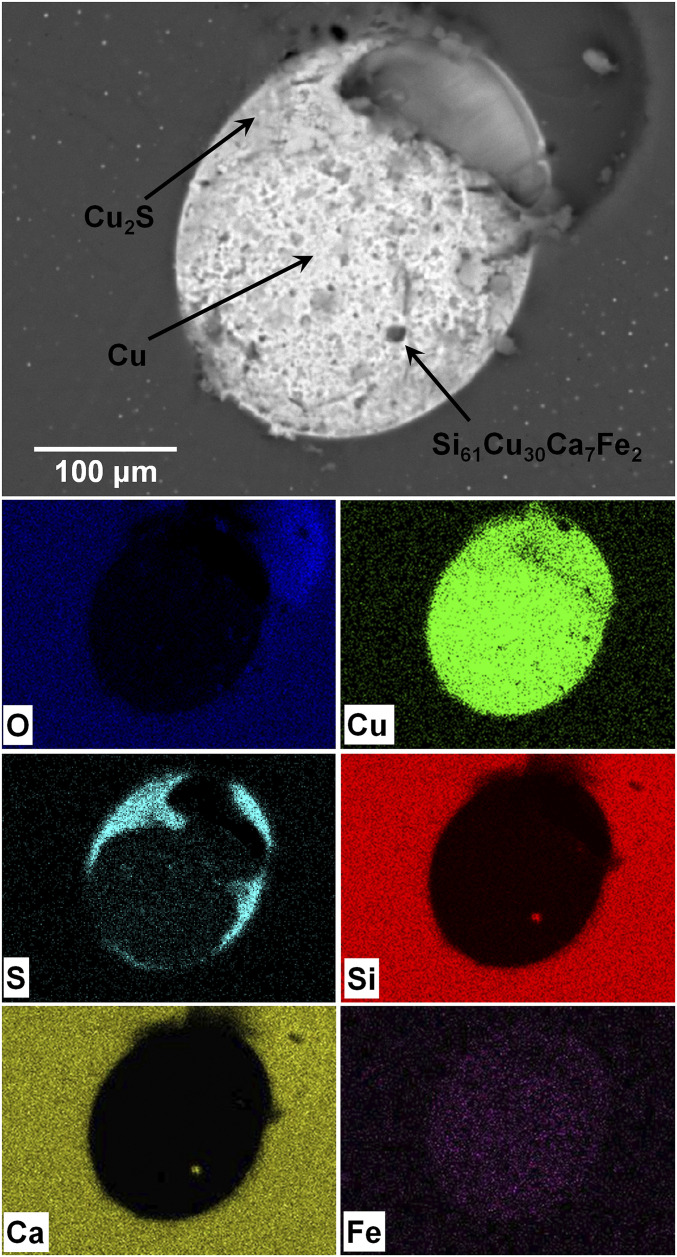
Fig. 3 zooms in on the droplet and shows a back-scattered scanning electron microscope image. The X-ray maps on the right make clear that the droplet is copper-rich, a characteristic of many of the metal droplets in red trinitite (10). Of the dozen or so copper droplets studied in this sample, though, this droplet is unique in that, in addition to the familiar chalcocite (Cu2S), it contains a mostly Si-Cu-Ca phase (about 10 μm in diameter) that has not been reported previously. The average of four electron microprobe analyses yields a composition of Si 42.86(80), Cu 47.70(85), Ca 6.80 (26), Fe 2.65 (19), total 99.80 wt %, corresponding, on the basis of a total of 100 atoms, to Si61Cu30Ca7Fe2.
Fig. 3.
Back-scattered scanning electron microscope image of the studied metal droplet (Top) containing the quasicrystalline phase. X-ray maps of the same area are shown below.
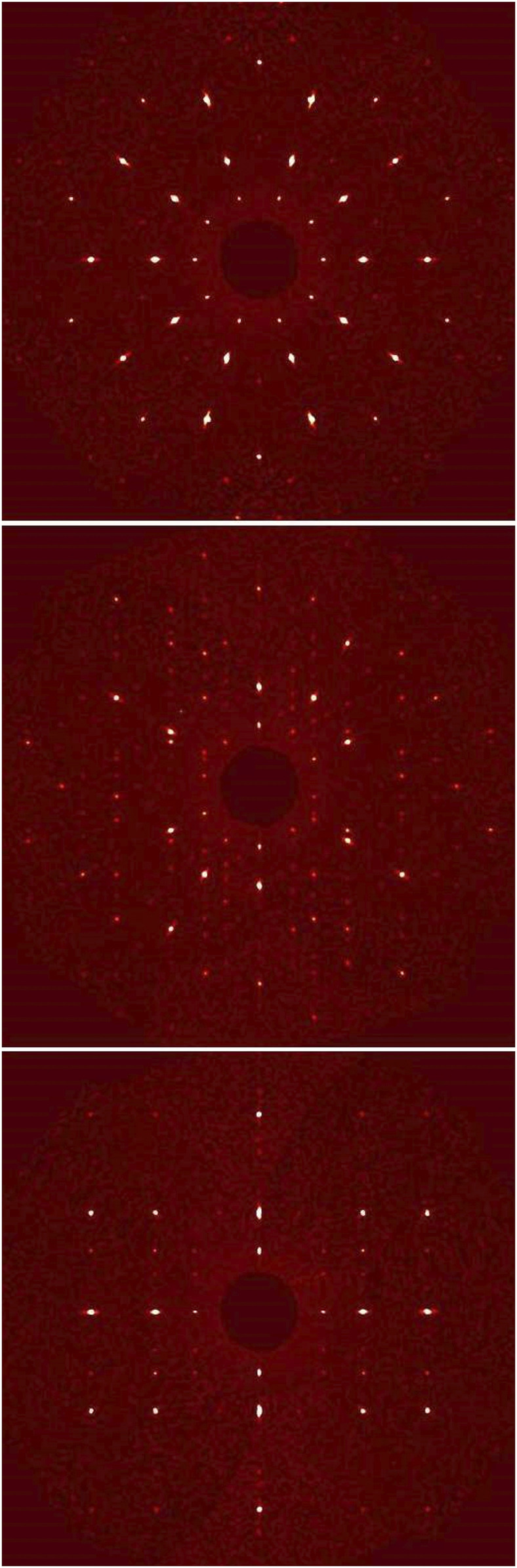
This new phase was extracted and mounted for single-crystal X-ray diffraction. Fig. 4 shows the projections of the single crystal X-ray diffraction data for the Si61Cu30Ca7Fe2 sample formed during the Trinity test along the fivefold (Fig. 4, Top), threefold (Fig. 4, Middle), and twofold symmetry axes (Fig. 4, Bottom) that define the symmetry of an icosahedron. The combination of patterns demonstrates unambiguously that the new phase is an icosahedral quasicrystal, the first to be identified in the remnants of an atomic blast.
Fig. 4.
Reconstructed precession images along the fivefold (Top), threefold (Middle), and twofold symmetry axis (Bottom) obtained using the collected single-crystal X-ray dataset (MoKα radiation) on the extracted quasicrystalline fragment.
Discussion
Quasicrystals, as first introduced by Levine and Steinhardt (11), are defined by a quasiperiodic distribution of atoms arranged in a pattern that violates the crystallographic symmetry rules that apply to ordinary (periodic) crystals. The first report of an icosahedral alloy was by Shechtman et al. (12), who described a metastable phase of Al and Mn. Since then, over a hundred other quasicrystal compositions have been identified that have icosahedral symmetry or other symmetries forbidden to periodic crystals, such as 8-, 10-, or 12-fold symmetry (13, 14). Many have a thermodynamic stability field on the liquidus (13).
The icosahedral Si61Cu30Ca7Fe2 phase reported here is unique in several respects. It has been discovered first in the fused remnants from an atomic blast and has yet to be synthesized in the laboratory. In fact, there are currently no other known compositional mixtures of Si, Cu, and Ca of any type that have been shown to be quasicrystalline, nor is there any other currently known quasicrystal that is dominantly composed of Si. Ca-bearing quasicrystals containing Au or rare earth elements have been reported (13, 14), but none containing Cu or Si.
The icosahedral Si61Cu30Ca7Fe2 phase is also the oldest extant anthropogenic quasicrystal known. The first reported example of a laboratory-synthesized icosahedral phase or a quasicrystal of any type is the spin-quenched sample of Al6Mn described by Shechtman et al. in 1984 (12). Bradley and Goldschmidt (15) studied the X-ray powder diffraction patterns of the stable phases of Al-Fe-Cu alloys that included a phase ψ, which was shown 50 y later to be a stable icosahedral quasicrystal phase (16). However, in 1939, Bradley and Goldschmidt (15) could not determine the symmetries of the ψ phase from their powder data and the original samples do not exist for verification. Other known near-misses for discovery of the first quasicrystal (13, 14) include phases studied by Hardy and Silcock (17), Palenzona (18), and Bruzzone (19), whose alloys were later resynthesized and found to be quasicrystalline.
Unlike the other historic examples, the icosahedral quasicrystal phase reported here exists and its symmetries can be verified today. Furthermore, because of its unique mode of creation, its time of synthesis is known to within a few seconds. Although we do not know if this composition of icosahedral quasicrystal is thermodynamically stable or metastable, it has persisted in the quasicrystalline state for 75 y. The only known examples of older quasicrystals are the naturally formed quasicrystals discovered in the Khatyrka meteorite that date back at least hundreds of millions of years and perhaps to the beginning of the Solar System (6, 20–23). Curiously, neither the oldest extant natural nor the oldest extant anthropogenic quasicrystal was made under controlled laboratory conditions.
Although the maximum instantaneous temperature reached during the Trinity test may have been as high as 8,000 °C, Eby et al. (10) present mineralogical and chemical data indicating that trinitite formed at temperatures of about 1,500 °C and pressures of 5 to 8 GPa. Since Si61Cu30Ca7Fe2 has not yet been synthesized in the laboratory, it is not known if a stability field exists and, if so, over what temperature–pressure range. Coincidentally, the temperature–pressure range estimated for red trinitite is comparable to what was reported (21) in the study of the high-velocity impact shock experienced by the Khatyrka meteorite (about 1,200 °C and >5 GPa), which—given a completely different mix of starting materials—led to the formation of quasicrystals with completely different compositions. Inspired by Khatyrka, Asimow et al. (24) performed a series of plate impact shock recovery experiments at somewhat lower temperatures (about 400 °C) and higher pressures (14 to 21 GPa) that led to the formation of quasicrystalline alloys similar to those in Khatyrka. However, in the case of Al-dominant shock-synthesized quasicrystals in Khatyrka and in experiments, reduced Al metal was already present in the starting materials. Metallic Cu was also present as well as both oxidized and metallic Fe; Oppenheim et al. (25) showed that metallic Fe precursor was apparently necessary for the synthesis. The Si-dominant quasicrystal in the trinitite case differs in that the original source of the major element, Si, was in oxidized form as quartz or plagioclase in the sand at the test site. Reducing Si is challenging, but it occurs in shock and release events, both at very high temperatures according to thermodynamic calculations (26) and at moderate temperatures at metal–silicate interfaces in recovery experiments (27), even in the absence of strong reducing agents. We emphasize that at this time we do not know the detailed sequence of events that led to the formation of a reduced Si-bearing quasicrystal included in a Cu-Cu2S droplet embedded in red trinitite. Forensically demonstrating that it is present is a first step; further detailed work toward a mechanistic understanding of its origin must consider the various thermodynamic and kinetic processes that may have occurred along possible pathways through time, temperature, pressure, and oxygen fugacity in the nuclear test environment.
The plate impact shock experiments, the high-velocity meteoroid shock impacts, and now an atomic blast shock event have not only led to the formation of icosahedral quasicrystals, but also each case has formed quasicrystal phases that were previously unknown, despite over three decades of systematic laboratory synthesis searches. These findings suggest that examining the remnants of these and other shock phenomena may prove to be surprisingly successful in identifying new quasicrystal-forming compositions and studying their kinetic stability. One reason may be that the shock phenomena often mix four or more elements in combinations not normally explored in the laboratory and quasicrystals may be more common when there are more components (25, 28). Also, for some quasicrystals, such as those in the Al-Fe-Cu alloy system (29), the stability field is known to be enlarged under static high-pressure conditions. It remains to be seen if the newly discovered icosahedral Si61Cu30Ca7Fe2 phase can be synthesized under ordinary laboratory conditions and what its thermodynamic stability field may be.
The work presented here thus motivates several new directions of research. Studying remnants from other nuclear test sites may yield yet other novel quasicrystalline phases and, by understanding their thermodynamic properties, provide a new tool for nuclear forensics. Fulgurites resulting from lightning strikes (30) may be a source of either natural or semianthropogenic quasicrystals depending on what materials are struck. Material from Meteor Crater and other meteor impact structures as well as some tektites (31) and lunar surface samples (32) provide further sources of material that experienced comparable high-temperature, short-duration shock events. All of these may be useful for discovering new quasicrystal phases, shedding light on why quasicrystals form, and learning how ubiquitous they are.
Materials and Methods
Samples.
The red trinitite sample described in this paper and shown in Fig. 1 is one of six samples of roughly the same size examined for this study, all of which were provided by one of the authors (W.K.) and are among samples collected in late 1945 by Lincoln LaPaz from a location north of ground zero. Red (rather than green) trinitite samples were selected because they were reported by Eby et al. (10) to incorporate blobs with rich inhomogeneous combinations of metallic phases (most currently known quasicrystals are metallic alloys).
The samples were embedded in epoxy resin and prepared as polished thick sections. Twelve metallic blob candidates that appeared promising in terms of chemical composition and suitable size for being tested by single-crystal X-ray diffraction were removed by hand (using fine needles) from the polished sections. Each was about 20 μm across. All grains were found to have relatively good diffraction quality: Eight turned out to be cubic, three hexagonal, and one icosahedral (quasicrystal), the last of which is the subject of this paper. Even in hindsight we cannot identify characteristics that allow one to distinguish the quasicrystals from crystals without diffraction data; finding more samples relies on systematic search.
The results presented here are from scanning electron microscopy, electron microprobe, wavelength-dispersive spectrometry (WDS), and single-crystal X-ray diffraction techniques.
Radioactivity Measurements.
The sample was measured on a 5-inch NaI(Tl) well detector to estimate the Eu-152 and Cs-137 content. The 4-g sample contained ∼6 Bq of Eu-152 and 100 Bq of Cs-137 which are typical in red trinitite. Eu-152 is a soil activation product and gives an indication of the distance from ground zero (33). The specific activity in this sample is consistent with trinitite found 55 to 60 m from the explosion hypocenter.
Scanning Electron Microscopy.
The instrument used was a Zeiss EVO MA15 scanning electron microscope coupled with an Oxford INCA250 energy-dispersive spectrometer, operating at 25 kV accelerating potential, 500-pA probe current, 2,500 counts per second as average count rate on the whole spectrum, and a counting time of 500 s. Sample was sputter-coated with 30-nm-thick carbon film.
Electron Microprobe.
Quantitative analyses were carried out using a JEOL JXA 8200 microprobe (WDS mode, 15 kV, 10 nA, 1-μm beam size, counting times 20 s for peak and 10 s for background). For the WDS analyses the Kα lines for all the elements were used. The quasicrystal fragment was found to be homogeneous within analytical error. The standards used were Si metal, synthetic CaSi2 (Ca), Cu metal, and Fe metal. Four point analyses on different spots on the single reported ∼10-mm quasicrystal were collected.
Single-Crystal X-Ray Diffraction.
Single-crystal X-ray studies were carried out using a Bruker D8 Venture diffractometer equipped with a Photon III CCD detector, with graphite-monochromatized MoKα radiation (λ = 0.71073 Å) and with 30-s exposure time per frame; the detector-to-sample distance was 7 cm.
Supplementary Material
Acknowledgments
L.B. is funded by the MIUR-PRIN2017 project “TEOREM - deciphering geological processes using Terrestrial and Extraterrestrial ORE Minerals”, prot. 2017AK8C32 (Principal Investigator: L.B.). P.J.S. was supported in part by the Princeton University Innovation Fund for New Ideas in the Natural Sciences; and P.D.A. was supported in part by NSF Award 1725349. We thank Walter Steurer, William Steinhardt, and Bill Press for useful exchanges and Teresa Salvatici for the high-resolution pictures of the studied sample.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2101350118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Hermes R. E., Strickfaden W. B., A new look at trinitite. Nucl. Weapons J. 2, 2–7 (2005). [Google Scholar]
- 2.Koeman E. C., Simonetti A., Burns P. C., Sourcing of copper and lead within red inclusions from trinitite postdetonation material. Anal. Chem. 87, 5380–5386 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Kolb W., Trinitite: The Atomic Age Mineral (Syntec, West Village, CA, ed. 2, 2018). [Google Scholar]
- 4.Bindi L., Steinhardt P. J., Yao N., Lu P. J., Natural quasicrystals. Science 324, 1306–1309 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Bindi L., Steinhardt P. J., Yao N., Lu P. J., Icosahedrite, Al63Cu24Fe13, the first natural quasicrystal. Am. Mineral. 96, 928–931 (2011). [Google Scholar]
- 6.Bindi L., et al., Evidence for the extraterrestrial origin of a natural quasicrystal. Proc. Natl. Acad. Sci. U.S.A. 109, 1396–1401 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bindi L., et al., Natural quasicrystal with decagonal symmetry. Sci. Rep. 5, 9111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bindi L., et al., Decagonite, Al71Ni24Fe5, a quasicrystal with decagonal symmetry from the Khatyrka CV3 carbonaceous chondrite. Am. Mineral. 100, 2340–2343 (2015). [Google Scholar]
- 9.Ross C., Optical properties of glass from Alamogordo, New Mexico. Am. Mineral. 33, 360–361 (1948). [Google Scholar]
- 10.Eby G. N., et al., Trinitite redux: Mineralogy and petrology. Am. Mineral. 100, 427–441 (2015). [Google Scholar]
- 11.Levine D., Steinhardt P. J., Quasicrystals: A new class of ordered structures. Phys. Rev. Lett. 53, 2477 (1984). [Google Scholar]
- 12.Shechtman D., Blech I., Gratias D., Cahn J. W., Metallic phase with long-range orientational order and no translational symmetry. Phys. Rev. Lett. 53, 1951 (1984). [Google Scholar]
- 13.Steurer W., Deloudi S., Crystallography of Quasicrystals: Concepts, Methods and Structures (Springer Science & Business Media, 2009). [Google Scholar]
- 14.Steurer W., Quasicrystals: What do we know? What do we want to know? What can we know? Acta Crystallogr. A Found. Adv. 74, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley A. J., Goldschmidt H. J., An X-ray study of slowly cooled iron-copper-aluminium alloys. J. Inst. Met. 6, 157–210 (1939). [Google Scholar]
- 16.Tsai A.-P., Inoue A., Masumoto T., A stable quasicrystal in Al-Cu-Fe system. Jpn. J. Appl. Phys. 26, L1505–L1507 (1987). [Google Scholar]
- 17.Hardy H. K., Silcock J. M., The phase sections at 500 and 350°C of Al rich Al-Cu-Li alloys. J. Inst. Met. 84, 423–428 (1956). [Google Scholar]
- 18.Palenzona A., The ytterbium-cadmium system. J. Less Common Met. 25, 367–372 (1971). [Google Scholar]
- 19.Bruzzone G., Ca-Cd and Ba-Cd systems. Gazz. Chim. Ital. 102, 234–242 (1972). [Google Scholar]
- 20.MacPherson G. J., et al., Khatyrka, a new CV3 find from the Koryak Mountains, Eastern Russia. Meteorit. Planet. Sci. 48, 1499–1514 (2013). [Google Scholar]
- 21.Hollister L. S., et al., Impact-induced shock and the formation of natural quasicrystals in the early solar system. Nat. Commun. 5, 4040 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Lin C., et al., Evidence of cross-cutting and redox reaction in Khatyrka meteorite reveals metallic-Al minerals formed in outer space. Sci. Rep. 7, 1637 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meier M. M. M., et al., Cosmic history and a candidate parent asteroid for the quasicrystal-bearing meteorite Khatyrka. Earth Planet. Sci. Lett. 490, 122–131 (2018). [Google Scholar]
- 24.Asimow P. D., et al., Shock synthesis of quasicrystals with implications for their origin in asteroid collisions. Proc. Natl. Acad. Sci. U.S.A. 113, 7077–7081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oppenheim J., et al., Shock synthesis of five-component icosahedral quasicrystals. Sci. Rep. 7, 15629 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melosh H. J., A hydrocode equation of state for SiO2. Meteorit. Planet. Sci. 42, 2079–2098 (2007). [Google Scholar]
- 27.Tschauner O., et al., Ultrafast growth of wadsleyite in shock-produced melts and its implications for early solar system impact processes. Proc. Natl. Acad. Sci. U.S.A. 106, 13691–13695 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oppenheim J., et al., Shock synthesis of decagonal quasicrystals. Sci. Rep. 7, 15628 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stagno V., et al., Quasicrystals at extreme conditions: The role of pressure in stabilizing icosahedral Al63Cu24Fe13 at high temperature. Am. Mineral. 100, 2412–2418 (2015). [Google Scholar]
- 30.Essene E. J., Fisher D. C., Lightning strike fusion: Extreme reduction and metal-silicate liquid immiscibility. Science 234, 189–193 (1986). [DOI] [PubMed] [Google Scholar]
- 31.Belkin H. E., Horton J. W. Jr., “Silicate glasses and sulphide melts in the ICDP-USGS Eyreville B core, Chesapeake Bay impact structure, Virginia, U.S.A.” in The ICDP-USGS Deep Drilling Project in the Chesapeake Bay Impact Structure: Results from the Eyreville Core Hole, Gohn G. S., Koeberl C., Miller K. G., Reimold W. U., Eds. (Geological Society of America, Boulder, CO, 2009), Special Paper 458, pp. 447–468 (2009). [Google Scholar]
- 32.Warren P. H., Lunar rock-rain: Diverse silicate impact-vapor condensates in an Apollo-14 regolith breccia. Geochim. Cosmochim. Acta 72, 3562–3585 (2008). [Google Scholar]
- 33.Parekh P. P., et al., Radioactivity in trinitite six decades later. J. Environ. Radioact. 85, 103–120 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.