Abstract
Phenformin is a drug in the biguanide class that was previously used to treat type 2 diabetes. We have reported the anti-tumor activities of phenformin to enhance the efficacy of BRAF-MEK-ERK pathway inhibition and to inhibit myeloid-derived suppressor cells in various melanoma models. Here we demonstrate that phenformin suppresses tumor growth and promotes keratinocyte differentiation in the DMBA/TPA two stage skin carcinogenesis mouse model. Moreover, phenformin enhances the suspension-induced differentiation of mouse and human keratinocytes. Mechanistically, phenformin induces the nuclear translocation of NFATc1 in keratinocytes in an AMPK-dependent manner. Pharmacological or genetic inhibition of calcineurin/NFAT signaling reverses the effects of phenformin on keratinocyte differentiation. Taken together, our study reveals an anti-tumor activity of phenformin to promote keratinocyte differentiation that warrants future translational efforts to repurpose phenformin for the treatment of cutaneous squamous cell carcinomas.
Keywords: Phenformin, AMPK, calcineurin/NFAT, keratinocyte differentiation, tumorigenesis
INTRODUCTION
The skin is composed of two major layers: the epidermis and the dermis. The stratified squamous epidermis, the outermost layer of the skin, provides a protective epidermal barrier for the human body through a tightly controlled differentiation program of keratinocytes, the most abundant cells in the epidermis (Fuchs, 2016, Watt, 1989, 2001). Keratinocytes located in the basal layer of the epidermis are proliferative and undifferentiated cells, and express cytokeratins 5 and 14 (CK5/K14). Once keratinocytes cease proliferating, they migrate upwards to form the suprabasal layer and express differentiation markers such as cytokeratins 1 and 10 (CK1/CK10). Eventually, keratinocytes reach the outermost layer of the epidermis to form the cornified layer (stratum corneum) and express terminal differentiation markers such as loricrin and filaggrin (Fuchs, 2016, Watt, 2001). The normal homeostasis of the epidermis is maintained by a balance between the proliferation and differentiation of keratinocytes. Multiple signaling pathways, such as the MAPK, EGF, WNT, Notch and calcineurin/NFAT pathways, act in an “integrated” control system to regulate the balance between keratinocyte proliferation and differentiation (Dotto, 1999). Disruptions of this epidermal balance may result in epidermal disorders, such as psoriasis, hyperplasia and skin cancer. Poorly differentiated tumors have been associated with a worse prognosis of cutaneous squamous cell carcinomas (SCCs) (Jennings and Schmults, 2010, Mullen et al., 2006), whereas stimulating keratinocyte differentiation would suppress keratinocyte tumorigenesis (Arnold and Watt, 2001, Wu et al., 2010). Hence, better understanding of the molecular mechanisms involved in keratinocyte differentiation may be conducive to improve strategies for the prevention and treatment of SCCs.
Phenformin is a drug in the biguanide class that was used to treat type 2 diabetes, but was withdrawn in the late 1970s in the United States and replaced by metformin, a related drug with a lower risk of lactic acidosis (DeFronzo et al., 2016). Both phenformin and metformin inhibit mitochondrial complex 1 of the respiratory chain and increase the AMP to ATP ratio, resulting in the activation of AMP-activated protein kinase (AMPK), an important energy sensor in cells (Garcia and Shaw, 2017). Compared to metformin, phenformin is more potent with respect to its antitumor activity, likely due to its lipophilic nature, which makes it less dependent on active transport for cellular uptake. We have previously reported that phenformin, but not metformin, enhances the efficacy of BRAF-MEK-ERK pathway inhibition in cell lines and in mouse models of melanoma (Trousil et al., 2017, Yuan et al., 2013). More recently, we also demonstrated that phenformin selectively reduces the frequency of myeloid-derived suppressor cells (MDSCs) and inhibits their immunosuppressive function (Kim et al., 2017). Importantly, co-treatment with phenformin, but not metformin, enhances the effect of anti-PD-1 to inhibit tumor growth in a melanoma mouse model (Kim et al., 2017). Based on these findings, we have been interested in repurposing phenformin for the treatment of cancers, considering that its toxicity (64 cases of lactate acidosis per 100,000 patient years) is lower than commonly used cancer chemotherapies and adjuvant therapies (Stang et al., 1999). A phase I clinical trial of phenformin with the dabrafenib/trametinib combination in patients with BRAF-mutated melanoma (NCT03026517) is currently underway. To further facilitate the efforts of repurposing phenformin for cancer treatment, it is important to assess its anti-cancer utility beyond melanoma to other cancer types, including non-melanoma skin cancers, such as SCCs.
In this study, we report that phenformin exerts anti-tumor activities and promotes keratinocyte differentiation in the DMBA/TPA two-stage skin carcinogenesis mouse model. Mechanistically, we demonstrate that phenformin activates calcineurin/NFAT signaling in an AMPK-dependent manner to enhance the differentiation of keratinocytes both in vitro and in vivo.
RESULTS
Phenformin suppresses the growth of skin tumors and promotes keratinocyte differentiation in the DMBA-TPA two-stage skin carcinogenesis model
We have previously demonstrated the anti-tumor activity of phenformin in melanoma mouse models (Kim et al., 2017, Trousil et al., 2017, Yuan et al., 2013). To assess the potential anti-tumor activity of phenformin in non-melanoma skin tumors, we used the two-stage DMBA/TPA chemical carcinogenesis mouse model. Eight-week old FVB/N mice were treated with DMBA and TPA to induce skin tumor formation. Sixteen weeks after the TPA treatment, phenformin was added to the drinking water (100 mg/Kg/day) for the treatment group and was maintained up until week 25 (Figure 1a). Interestingly, tumors in mice treated with phenformin failed to grow further from Week 17 to the end of the experiment, in contrast to tumors in the control group (Figure 1b, Supplementary Figure S1a). But there was not a significant difference in the number of tumors per mouse between the two groups (Supplementary Figure S1b). Histological analysis by H&E staining revealed that most tumors in the phenformin-treated group were highly differentiated papillomas with lower cellularity, whereas tumors in the control group were less differentiated with higher cellularity (Figure 1c and d). To further characterize the differentiation status of these skin tumors, the expression of keratinocyte differentiation markers CK1, CK10 and loricrin was analyzed by immunofluorescence (IF) staining (Figure 1e, g and i). Quantification of the staining revealed a significantly stronger expression of all three markers in tumors of the phenformin-treated group compared to those in the control group (Figure 1f, h and j). Taken together, these results demonstrate that phenformin induces tumor cell differentiation and inhibits tumor growth in the DMBA-TPA two-stage chemical skin carcinogenesis model.
Figure 1. Phenformin suppresses tumor growth and induces tumor cell differentiation in mouse skin.
a: Scheme of the DMBA/TPA two-stage chemical carcinogenesis model; mice were treated with phenformin (Phen) or with the vehicle control starting from week 13. b: Effects of phenformin on tumor progression; average volumes of all tumors on each mouse were calculated; data shown are means ± SEM of all mice (n=10) in each group. c-j: H&E (c) and immunofluorescence analyses (e, g, i) of tumors collected at 25 wks. Staining of the differentiation markers CK1, CK10 and loricrin are shown in red. DAPI (blue) stain identifies nuclei. d: Quantification of highly differentiated (H), medium differentiated (M) and low differentiated (L) tumors based on analysis of H&E staining (c) of tumors in the control and phenformin-treated groups. f, h, j: Quantification of relative expression levels of CK1, CK10 and loricrin, respectively. c-j: Tumors from six mice in each group were used for the quantitative analysis (n=6). * P<0.05, **P<0.01 by two-way ANOVA in b,c, and by Student’s two tailed t-test for others. All scale bars = 100 μm.
Phenformin promotes both human and mouse keratinocyte differentiation in vitro
To examine the effects of phenformin on keratinocyte differentiation in vitro, primary human keratinocytes were cultured in suspension, a widely used approach to induce keratinocyte differentiation, in the presence or absence of phenformin. Keratinocytes were harvested at various time points after suspension to be analyzed for the expression of differentiation markers CK1, CK5, CK10 and loricrin by real time quantitative RT-PCR (qRT-PCR). Phenformin treatment did not affect the mRNA expression level of basal cell marker (undifferentiation) CK5, but significantly enhanced expression levels of suprabasal cell markers (differentiation) CK1, CK10 and loricrin, compared to the control group (Figure 2b, c and d), results that were validated by western-blot analysis (Figure 2e and f). As expected, phenformin increased the level of phospho-AMPK in these cells (Figure 2e and f). In addition, we isolated primary mouse keratinocytes from neonatal C57BL/6 mice to perform suspension assays. Similar effects of phenformin on the expression of differentiation markers were observed as found with human keratinocytes (Figure 2g). To examine whether phenformin induces keratinocyte differentiation through AMPK, we knocked down AMPK expression by transfection with siRNAs against both AMPKα1 and AMPKα2. Knockdown efficiency was confirmed at both the mRNA and protein levels (Supplementary Figure S2a and b). Indeed, qRT-PCR and western blot analyses revealed that the phenformin-induced expression of various differentiation markers was greatly reduced in keratinocytes with siRNA knockdown of AMPKα (Figure 2h and i). Notably, we did not observe significant changes in keratinocyte apoptosis or senescence following phenformin treatment (Supplementary Figure S3). Taken together, these findings demonstrate that phenformin mainly enhances keratinocyte differentiation through the activation of AMPK signaling.
Figure 2. Phenformin enhances human and mouse keratinocyte differentiation induced by suspension assays in vitro.
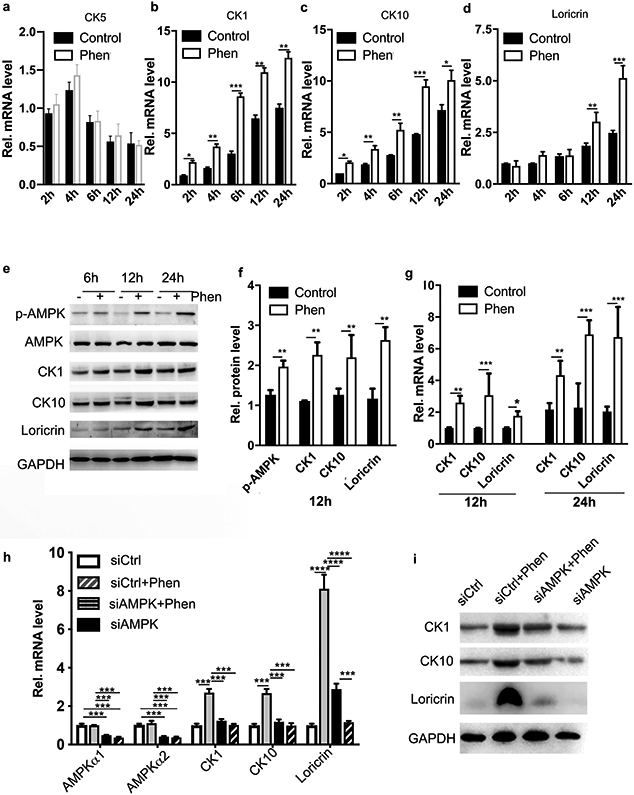
a-d: Human keratinocytes cultured in suspension treated with 1 mM phenformin (Phen) or vehicle were collected at the indicated time points for qRT-PCR analysis of keratinocyte differentiation markers CK5, CK1, CK10 and loricrin as indicated. Expression levels of different markers were calculated relative to the control cells at 2 h in suspension. e: Lysates from human keratinocytes treated as in a-d were analyzed for expression of the markers indicated by western blot. f: Quantification of band densities of samples in e from 12 h after suspension. g: Fresh mouse keratinocytes cultured in suspension with phenformin or vehicle were collected for qRT-PCR analysis. h-i: Human keratinocytes were transfected with siRNAs for AMPK α1 and α2 (siAMPKα) or a scrambled siRNA as a control. Cells were collected for analyses by qRT-PCR (h) and western blot (i) for the indicated mRNAs and proteins after 24 h of treatment with 1 mM phenformin or vehicle. ** P<0.01, *** P<0.005, **** P<0.001 by Student’s two tailed t-test.
Phenformin promotes the nuclear translocation of NFATc1 in keratinocytes
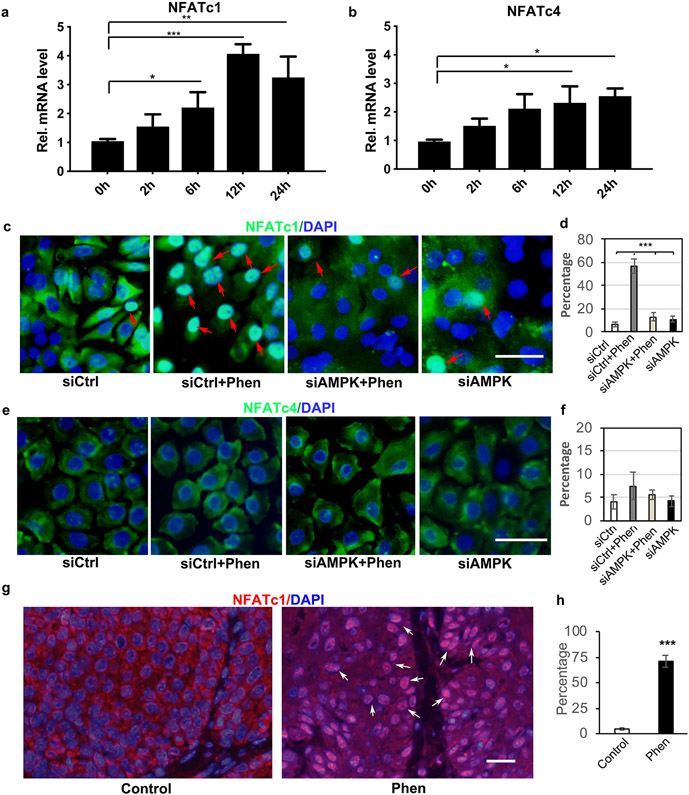
Next, we investigated the underlying molecular mechanism of phenformin to promote keratinocyte differentiation. We performed real-time PCR analysis to examine several well-known pathways involved in regulating keratinocyte differentiation, including the NOTCH, WNT/β-Catenin and calcineurin/NFAT pathways, during the course of human keratinocyte suspension culture. Interestingly, we found that suspension culture of keratinocytes significantly induces the expression of NFATc1 and NFATc4 (Figure 3a and b), in a pattern similar to CK1 and CK10 (Figure 2b and c). Although some markers in the NOTCH and WNT pathways increased at certain time points during the suspension culture, their overall patterns were different (Supplementary Figure S4). Nuclear translocation of the transcription factor NFAT is known to be a critical regulatory mechanism for its activation (Mammucari et al., 2005, Santini et al., 2001). Hence, we examined the effects of phenformin on the subcellular localization of NFATc1 and NFATc4. Interestingly, we found that phenformin treatment dramatically promoted the nuclear translocation of NFATc1 as indicated by IF analysis (red arrows, Figure 3c and d, Supplementary Figure S5). We also detected a slightly increased nuclear staining of NFATc4 in phenformin-treated keratinocytes, but that was not statistically significant compared to the control (Figure 3e and f, Supplementary Figure S6). In addition to keratinocytes in vitro, we also found higher levels of nuclear staining of NFATc1 in mouse skin tumor cells treated with phenformin, compared to the control group, in the DMBA-TPA mouse model (arrows, Figure 3g and h, Supplementary Figure S7).
Figure 3. Phenformin promotes keratinocyte differentiation via the activation of CnB/NFAT signaling.
a-b: Human keratinocytes in suspension assays were collected at the indicated time points for qRT-PCR analysis of NFATc1 (a) and NFATc4 (b). c-f: Human keratinocytes transfected with siRNAs against AMPKα1/α2 (siAMPK) or a scrambled control siRNA (siCtrl), were cultured in the presence or absence of 1 mM phenformin (Phen), followed by IF analyses with anti-NFATc1 (green, c) or anti-NFATc4 antibody (green, e) together with DAPI staining for nuclei (blue). Red arrows indicate nuclear staining of NAFTc1. Percentages of nuclear positive staining of NFATc1 (d) or NFATc4 (f) in 200 cells were quantified from the images shown in c and e, respectively. g-h: Tumor sections from Figure 1 were used for IF analyses of NFATc1 (red) together with DAPI staining for nuclei (blue) (g) and were quantified (h). c, e: bar = 50 μm; g: bars = 100 μm. * P<0.05, **P<0.01, ***P<0.005. by Student’s two tailed t-test.
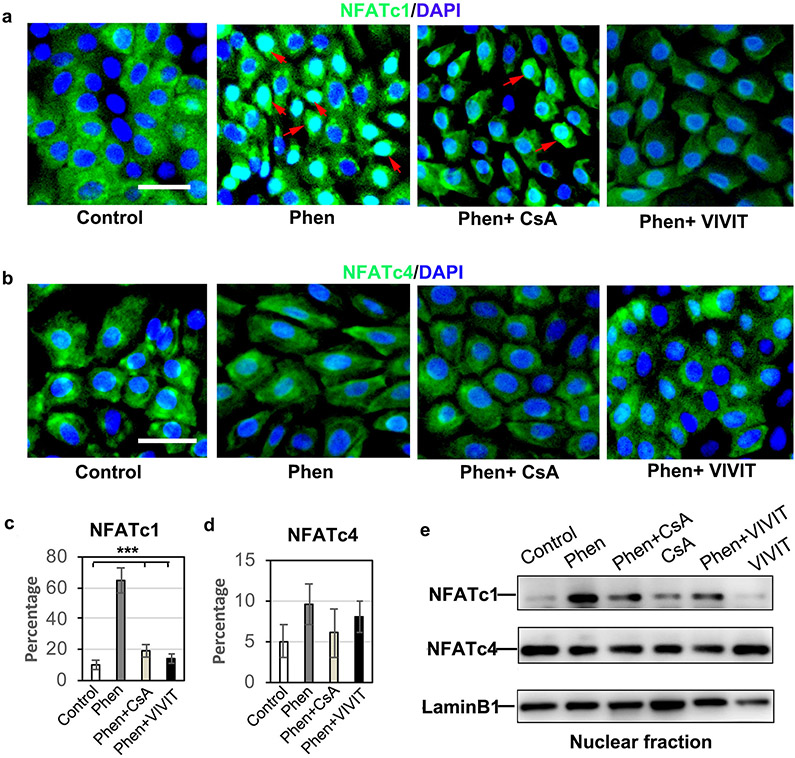
To further test whether phenformin induces the activation of NFAT through AMPK, we performed IF staining of NFATc1/c4 in keratinocytes transfected with AMPKα siRNA in the presence of phenformin. We observed that the increased nuclear staining of NFATc1 elicited in keratinocytes by phenformin was largely inhibited by double knockdown of AMPKα1/α2 (Figure 3c and d), suggesting that phenformin promotes the nuclear translocation of NFATc1 through AMPK activation. Moreover, the phenformin-induced nuclear translocation of NFATc1 was blocked by the calcineurin inhibitor CsA and by the NFAT inhibitor VIVIT (Figure 4a and c). Similar effects of siAMPK (Figure 3c), CsA and VIVIT on the nuclear translocation of NFATc1 were observed when NFATc1 protein levels in the nuclear fraction were analyzed by western blot (Figure 4e). Consistent with the IF staining and western blot results, the level of NFATc4 in the nuclear fraction did not change significantly under the various conditions tested (Figure 4b, d and e). Taken together, these data demonstrate that phenformin promotes the nuclear translocation of NFATc1 in keratinocytes via the AMPK pathway.
Figure 4. Phenformin induces the nuclear translocation of NFATc1 in keratinocytes.
a-d: Human keratinocytes were cultured in the presence or absence of 1 mM phenformin (Phen) together with 5 μM CsA or VIVIT, followed by IF analyses with anti-NFATc1 (green, a) or anti-NFATc4 antibody (green, b). Percentages of nuclear positive staining of NFATc1 (c) and NFATc4 (d) in 200 nuclei (c, d) were calculated. Blue indicates DAPI staining of nuclei. Red arrows indicate nuclear staining of NFATc1. e: Nuclear fractions prepared from human keratinocytes under various drug treatment conditions were used for western blot analyses with the indicated antibodies. Laminin B1 was used as a loading control. a-b: bars = 50 μm. c: ***P<0.005. by Student’s two tailed t-test.
Phenformin enhances keratinocyte differentiation via the activation of calcineurin/NFAT signaling
To investigate the contribution of NFAT activation to the effects of phenformin on keratinocyte differentiation, we used the calcineurin inhibitor CsA and the NFAT inhibitor VIVIT together with phenformin in keratinocyte suspension-dependent differentiation assays. We found that both CsA and VIVIT blocked the phenformin-enhanced keratinocyte differentiation, as indicated by changes in the expression of CK1, CK10 and Loricrin at both the protein and mRNA levels (Figure 5a and b). Similar effects were observed when we used a siRNA to knockdown the expression of calcineurin B1 (CnB1) (Figure 5c and d, Supplementary Figure S8), which is the only isoform of calcineurin expressed in keratinocytes (Dotto, 2011).
Figure 5. Phenformin promotes keratinocyte differentiation through the activation of CnB/NFAT signaling.
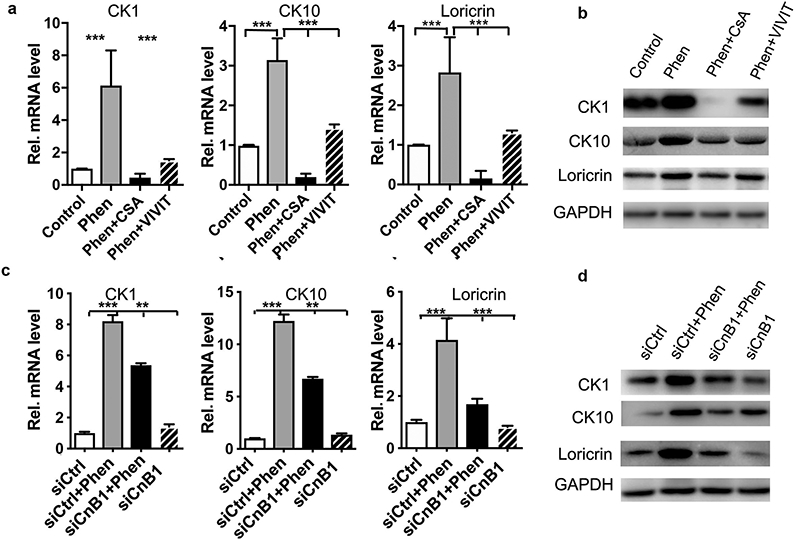
a-b: Human keratinocytes cultured in suspension in the presence of phenformin (Phen) or DMSO control, combined with 5 μM CsA or VIVIT for 24 h were collected for qRT-PCR (a) and western-blot (b) analysis of the keratinocyte differentiation markers CK1, CK10 and loricrin as indicated. c-d: Human keratinocytes transfected with a siRNA for calcineurin B1 (siCnB1) or a scrambled siRNA as a control (siCtrl) were cultured in suspension in the absence or presence of 1 mM phenformin for 24 h, then were lysed for qRT-PCR (c) and western blot (d) analysis of the keratinocyte differentiation markers CK1, CK10 and loricrin as indicated. a, c: **P<0.01; ***P<0.005. by Student’s two tailed t-test.
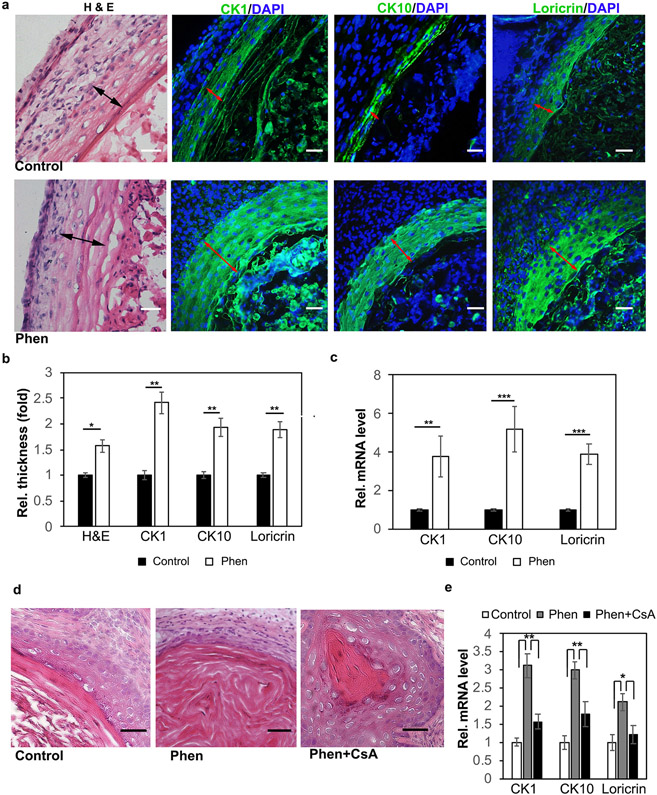
Lastly, we examined whether phenformin induces human keratinocyte differentiation in vivo using the cyst formation assay, a previously described, convenient approach to reconstitute human skin in mice (Wu et al., 2010). For this purpose, human dermal and epidermal cells were injected into the epidermal-dermal junction of nu/nu mice, followed by treatment with phenformin for 2 weeks. Grafted tissues were then collected for histological analyses and IF staining of the differentiation markers CK1, CK10 and loricrin. Those analyses showed that the keratinization areas (red arrows) were thicker in the phenformin-treated group compared to the control group (Figure 6a and b). We also collected the grafts for qRT-PCR analyses of various differentiation markers, and found that phenformin significantly induced higher expression levels of CK1, CK10 and loricrin in vivo (Figure 6c). Importantly, these inductions were attenuated by treatment with the calcineurin inhibitor CsA (Figure 6d and e). Taken together, these findings show that phenformin induces keratinocyte differentiation in vitro and in vivo through the activation of calcineurin/NFAT signaling.
Figure 6. Phenformin induces human keratinocyte differentiation in vivo.
a: Human keratinocytes and dermal fibroblasts were injected into nu/nu mice, which were treated with phenformin (Phen) or with PBS (control). At 2 weeks, the grafts were collected for H&E stains, and IF staining of the differentiation markers CK1, CK10 and loricrin (green) as indicated. Black arrows indicate the keratinization zone of the epidermis in HE staining; red arrows indicate the positive staining zone of the epidermis. b: Quantification of area size indicated by black arrows in (a). c: Grafts collected from a were subjected to qRT-PCR analysis. d-e: Human skin cells prepared and grafted as described in (a); after grafting, mice were treated with drugs as indicated. At two weeks, the grafts were collected for H&E (d) or for RT-PCR analysis of differentiation markers (e). Bars = 100 μm. b, c, e: * P<0.05, **P<0.01, ***P<0.005. by Student’s two tailed t-test.
DISCUSSION
The results of this study demonstrate that phenformin, a mitochondrial complex I inhibitor and AMPK activator, promotes keratinocyte differentiation both in vitro and in vivo. Interestingly, phenformin-induced keratinocyte differentiation was significantly blocked by the calcineurin inibitor CsA, by the NFAT inhibtor VIVIT and by siRNAs against calcineurin B1, which suggests the involvement of calcineurin/NFAT signaling in that process. Calcineurin is a serine/threonine phosphatase that is directly controlled by calcium/calmodulin. Upon activation, calcineurin dephoshorylates the transcription factor NFAT to unmask its nuclear localization signal, allowing its import into the nucleus and the subsequent transcriptional induction of specific genes with NFAT-responsive elements (Pan et al., 2013, Wu et al., 2007). The calcineurin/NFAT pathway has been shown to play an important role in regulating keratinocyte differentiation (Dotto, 2011, Mammucari et al., 2005, Santini et al., 2001, Wu et al., 2010). All four NFAT family members (c1 to c4) are expressed in skin keratinocytes, but NFATc1 is the most extensively studied member. We have previously reported that the inhibition of calcineurin/NFATc1 by CsA or by VIVIT downregulates p53 expression to block keratinocyte senescence and differentiation, which promotes keratinocyte tumorigenesis (Wu et al., 2010). Those previous findings together with our current results on the effects of phenformin to activate NFATc1 and inhibit tumor growth in the DMBA-TPA tumorigenesis model are consistent with an anti-tumor role of NFATc1. It is noteworthy that NFATc1 may also have a pro-tumor role in certain contexts. For example, mice overexpressing a constitutively active NFATc1 isoform driven by the Hoxb7 promoter in the skin epithelium developed spontaneous skin SCCs (Tripathi et al., 2014). Interestingly, mouse NFATc1 was also reported to be localized in follicle stem cells (Goldstein et al., 2015, Horsley et al., 2008). It is conceivable NFATc1 may have different roles in follicle stem cells versus interfollicular keratinocytes, which may help to explain its different effects on skin tumorigenesis.
AMPK is an essential metabolic sensor found in all eukaryotes that regulates energy homeostasis by monitoring changes in intracellular AMP or ADP to ATP ratios (Garcia and Shaw, 2017). AMPK is involved in regulating various cellular processes in response to energy changes, including cell growth, proliferation, survival, cell polarity, autophagy, cell motility, and so on (Arkwright et al., 2015, Hu et al., 2016, Jacquel et al., 2018). It is also well known that AMPK signaling plays important roles in cell differentiation. For example, AMPK controls the differentiation of hematopoietic stem cells (HSCs), progenitors and mature hematopoietic cells by regulating transcription programs necessary for the completion of cellular differentiation (Jacquel et al., 2018, Nakada et al., 2010). Recent studies also showed that AMPK promotes gut epithelial differentiation to enhance intestinal barrier function, through promoting the expression of CDX2, a key transcription factor regulating epithelial differentiation (Sun et al., 2017, Zhu et al., 2018). Here we show that phenformin promotes the nuclear translocation of NFATc1 to enhance skin epidermal differentiation, which was blocked by knockdown of AMPK catalytic α-subunits α1 and α2, supporting the role of AMPK in controlling keratinocyte differentiation. Future efforts will be devoted to elucidating the detailed mechanism underlying the regulation of calcineurin/NFAT signaling by AMPK in the regulation of keratinocyte differentiation.
Other than differentiation, AMPK activation has previously been shown to inhibit the proliferation of keratinocytes (Bridgeman et al., 2016, Li et al., 2014, Nguyen et al., 2013, Saha et al., 2006, Shen et al., 2013). AMPK was reported to inhibit UV-induced mTOR activation and DNA repair in keratinocytes (Bridgeman et al., 2016, Cao et al., 2008, Wu et al., 2013) and to delay the onset of UVB-induced skin tumorigenesis in the SKH1 hairless mouse model by increasing expression of the DNA repair protein xeroderma pigmentosum C (XPC) during UVB-induced DNA repair (Bridgeman et al., 2016, Wu et al., 2013). Our laboratory has demonstrated that AMPK activation attenuates ERK signaling in keratinocytes and epidermal hyperplasia in mouse skin (Shen et al., 2013). Consistent with the anti-tumor function of AMPK, metformin, another AMPK activator, was shown to inhibit TPA-induced tumor development in obese or diabetic mice in a preventive setting, which was associated with attenuation in mTOR signaling (Checkley et al., 2014). Here we showed that, phenformin, a more potent biguanide drug than metformin, inhibits DMBA-TPA induced skin tumor development and promotes tumor cell differentiation. Unlike the prior study using metformin (Checkley et al., 2014), which was administered to mice together with TPA after DMBA initiation, in this study we treated the mice with phenformin at week 15 after the TPA induction in an experimental setting of treatment, rather than prevention. Importantly, we demonstrated that phenformin activates calcineurin/NFAT signaling to promote keratinocyte differentiation in an AMPK-dependent manner. Our results suggest that AMPK can exert its anti-tumor function through a mechanism, to our knowledge previously unreported, i.e. promoting keratinocyte differentiation, in addition to its effect on keratinocyte proliferation. Furthermore, our findings also provide an important rational basis for future translational efforts to explore the potential utility of phenformin in the treatment of SCCs.
MATERIALS AND METHODS
Cell culture
Isolation and culture of human keratinocytes and dermal fibroblasts were performed as previously described (Liu et al., 2018, Qian et al., 2018, Wen et al., 2018). Human keratinocytes were derived from neonatal foreskin tissues obtained from discarded hospital specimens. The protocol was reviewed and approved by the Medical Ethical Committee of the School of Stomatology, Shandong University (Protocol NO: 2015120401, Date: 12-05-2015). Specimens were analyzed anonymously, and no patient consent was required. Mouse keratinocytes were isolated from newborn C57BL/6 mice according to a previously published method (Li et al., 2017). Dermal fibroblasts were cultured in DMEM/F12 (3:1) containing 0.1% penicillin/streptomycin, 40 μg/ml fungizone, 40 ng /ml FGF2, 20 ng/ ml EGF, 2% B27 supplement with 5% FBS with medium changes every 5 days. K-SFM (10744019, Gibco, Grand Island, NY, USA) supplemented with 100 μg/mL streptomycin and 100 units/mL penicillin was used to culture human and mouse keratinocytes at 37°C in a humidified incubator containing 5% CO2. For human keratinocytes, passage 3 cells were used for these experiments, while passage 0 mouse keratinocytes were used. For suspension-induced keratinocyte differentiation assays, human or mouse cells were seeded in ultra-low attachment 6-well plates (Catalog# 3471, Corning, Kennebunk, ME, USA) with K-SFM in the presence or absence of 1 mM phenformin (P7045, Sigma-Aldrich, St. Louis, MO, USA) for various times as indicated.
siRNA transfection
Transfection of siRNAs into keratinocytes was carried out as previously described (Wu et al., 2018). Briefly, keratinocytes were seeded in 6-well plates, grown to 70% confluence, and transfected with various siRNAs using lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). Seventy-two h later, cells were collected for analysis by RT-PCR and western blot to determine the knockdown efficiency. siRNA oligos that target AMPKα, CnB1 or a negative control scrambled siRNA were purchased from Gene Pharma (Shanghai, China). Three independent siRNAs for each gene were tested for knockdown efficiency. The oligo sequences of all siRNAs are shown in Supplemental Table 1.
Quantitative real time RT-PCR and immunodetection techniques
Procedures for real time RT-PCR analysis, immunoblotting and IF analysis were carried out as previously described (Hnasko and Hnasko, 2015, Kim, 2017). Nuclear and cytoplasmic proteins were extracted using a Cytoplasmic and Nuclear Extraction kit (SC-003, Invent Biotech, Plymouth, MN, USA). The band densities in western blots were quantified using ImageJ software (NIH, Rockville, MD, USA). The list of gene-specific primers used for PCR is provided in Supplemental Table 2. Relative mRNA expression levels of all tested genes were normalized to the mRNA level of the housekeeping gene 36B4. The following primary antibodies were used for immunoblotting and IF staining: anti-CK1 (Cytokeratin 1, ab93652), anti-CK10 (Cytokeratin 10, ab76318), anti-Loricrin (ab85679), anti-NFATc1 (ab25916) and anti-NFATc4 (ab62613) were from Abcam (Cambridge, MA, USA); anti-p-AMPKαThr172 (#50081) and anti-AMPK (#5831) were from Cell Signaling Technology (Danvers, MA, USA); GAPDH (#607904) was from Biolegend (San Diego, CA, USA) and LaminB1 (10-P1048) was from American Research Products (Waltham, MA, USA). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; ab104139, abcam). Secondary antibodies HRP-goat anti-rabbit IgG (H+L) (SA00001-2) and HRP-goat anti-mouse IgG (H+L) (SA00001-1) were from Proteintech (Chicago, IL, USA). Quantification analysis of staining was done as previously described (Wu et al., 2018). Briefly, IF staining for each marker was carried out on 6 mice from each group (n=6), and the staining was evaluated blindly twice by two independent persons. Evaluations were based on arbitrary units as follows: 1, no or weak staining; 2, intermediate staining; and 3, strong staining. For relative quantification of differentiation marker expression levels, the mean value of independent measurements was taken as the final score.
DMBA-TPA carcinogenesis model
Eight-week-old FVB/N female mice (Jackson Laboratories, Bar Harbor, ME, USA) were used for in vivo tumor formation studies. The two-stage chemical carcinogenesis was carried out following the standard protocol (Filler et al., 2007). Briefly, the dorsal skin of each mouse was shaved and 48 h later, was treated with 7,12-dimethylbenz[a]anthracene (DMBA; 25 nM/100 μL in acetone) once. A week later, 12-O-tetradecanoylphorbol-13-acetate (TPA; 1.7 nM/100 μL acetone) was applied on the dorsal skin twice a week. At 16 weeks, mice were randomly divided into the phenformin-treatment group, to which phenformin in PBS (100mg/kg/day, intragastric administration, i.g.) was added, and the control group, to which the same volume of PBS only by i.g. was added. Both groups were continuously treated with TPA until 25 wks. Mice were monitored for tumor development and the total number of tumors on each mouse was counted every week. Tumor volumes were calculated from caliper measurements using the following formula: (D × d2)/2, where D represents the large and d the small diameter of the tumor. For tumor size data, we first measured and calculated the average size of all tumors in each mouse. We then calculated the mean and SEM of tumor size of all mice in each group at different time points and plotted. At 25 weeks, mice were sacrificed, and skin tumors were processed for histological analysis and IF staining for various differentiation markers. TPA (P8139) and DMBA (D3254) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The Research Randomizer (http://www.randomizer.org) was used to assign mice to different treatment groups and to generate random numbers assigned to samples for the purpose of blinding. These experiments were performed by following the guidelines from Columbia University’s Institutional Animal Care and Use Committee.
Reconstituted human skin model
Eight-week-old female nu/nu mice (Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) were used to reconstitute human skin models in vivo as previously described (Wu et al., 2010, Zhang et al., 2017). Briefly, 2 x 106 passage 3 human keratinocytes combined with 1 x 106 passage 3 dermal fibroblasts were subcutaneously injected into the dorsal skin of nu/nu mice. Mice were treated with phenformin (10 mg/kg, PBS as a control) with or without CsA (20 μg/g, DMSO as a control) by i.p. injections every other day. Two weeks later, the mice were sacrificed, and the reconstituted skins were collected for histological analysis and IF analysis of various differentiation markers. These experiments were performed following the guidelines from the Ethics Committee of Stomatological Hospital Shandong University.
Apoptosis and senescence assays
Approximately 2 × 105 human keratinocytes were seeded per well in six-well plates with vehicle (control) or with 1 mM phenformin (Phen) for 24 h. Cells were collected and stained with Annexin V-FITC and propidium iodide (PI) from an Apoptosis Detection kit (Biolegend, 640914), followed by flow cytometry analysis (FACS) for apoptotic cells. Alternatively, cells were fixed with 4% formaldehyde and assayed for senescent cells using a Senescence β-Galactosidase Staining kit (Beyotime, Shanghai, China, C0602).
STATISTICAL ANALYSIS
Statistical analyses were carried out using GraphPad Prism 8, and Student’s t-test was used to analyze differences between two groups where indicated. Comparisons of more than 2 groups used either One-way or Two-way ANOVA with correction for multiple pairwise comparisons. P values < 0.05 are considered statistically significant. All experiments were repeated at least three times. Standard error bars are shown in the Figures and P values are indicated in the Figures as *P < 0.05, ** P < 0.01, ***P < 0.005 and **** P < 0.001.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Zheng Lab and the Wu lab for helpful discussion and Gabriella Stephenson for technical assistance. This work is supported by the National Key Research and Development Program of China (2017YFA0104604), the General Program of National Natural Science Foundation of China (81772093 and 81572681) , the Key Program of Shandong Province Natural Science Foundation (ZR2019ZD36) and The Key Research and Development Program of Shandong Province (2019GSF108107) (X.W); and a grant from the Alexander and Margaret Stewart Trust (B.Z.)
Abbreviations used:
- AMPK
AMP-activated protein kinase
- CnB1
calcineurin B1
- CsA
cyclosporine A
- CK
cytokeratin
- DMBA
7,12-Dimethylbenz[a]anthracene
- EGF
epidermal growth factor
- FACS
flow cytometry analysis
- IF
immunofluorescence
- PBS
phosphate buffered saline
- SCC
squamous cell carcinomas
- MAPK
mitogen-activated protein kinase
- MDSC
myeloid-derived suppressor cells
- NFAT
nuclear factor of activated T-cells
- TPA
12-O-tetradecanoylphorbol-13-acetate
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
DATA AVAILABILITY
No datasets were generated or analyzed during the current study.
REFERENCES
- Arkwright RT, Deshmukh R, Adapa N, Stevens R, Zonder E, Zhang Z, et al. Lessons from Nature: Sources and Strategies for Developing AMPK Activators for Cancer Chemotherapeutics. Anticancer Agents Med Chem 2015;15(5):657–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol 2001;11(8):558–68. [DOI] [PubMed] [Google Scholar]
- Bridgeman BB, Wang P, Ye B, Pelling JC, Volpert OV, Tong X. Inhibition of mTOR by apigenin in UVB-irradiated keratinocytes: A new implication of skin cancer prevention. Cell Signal 2016;28(5):460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Lu S, Kivlin R, Wallin B, Card E, Bagdasarian A, et al. AMP-activated protein kinase contributes to UV- and H2O2-induced apoptosis in human skin keratinocytes. J Biol Chem 2008;283(43):28897–908. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Checkley LA, Rho O, Angel JM, Cho J, Blando J, Beltran L, et al. Metformin inhibits skin tumor promotion in overweight and obese mice. Cancer Prev Res (Phila) 2014;7(1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016;65(2):20–9. [DOI] [PubMed] [Google Scholar]
- Dotto GP. Signal transduction pathways controlling the switch between keratinocyte growth and differentiation. Crit Rev Oral Biol Med 1999;10(4):442–57. [DOI] [PubMed] [Google Scholar]
- Dotto GP. Calcineurin signaling as a negative determinant of keratinocyte cancer stem cell potential and carcinogenesis. Cancer Res 2011;71(6):2029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler RB, Roberts SJ, Girardi M. Cutaneous two-stage chemical carcinogenesis. CSH Protoc 2007;2007:pdb prot4837. [DOI] [PubMed] [Google Scholar]
- Fuchs E Epithelial Skin Biology: Three Decades of Developmental Biology, a Hundred Questions Answered and a Thousand New Ones to Address. Curr Top Dev Biol 2016;116:357–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D, Shaw RJ. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol Cell 2017;66(6):789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J, Roth E, Roberts N, Zwick R, Lin S, Fletcher S, et al. Loss of endogenous Nfatc1 reduces the rate of DMBA/TPA-induced skin tumorigenesis. Mol Biol Cell 2015;26(20):3606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hnasko RM. The Western Blot. Methods Mol Biol 2015;1318:87–96. [DOI] [PubMed] [Google Scholar]
- Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 2008;132(2):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Fan L, Cen P, Chen E, Jiang Z, Li L. Energy Metabolism Plays a Critical Role in Stem Cell Maintenance and Differentiation. Int J Mol Sci 2016;17(2):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquel A, Luciano F, Robert G, Auberger P. Implication and Regulation of AMPK during Physiological and Pathological Myeloid Differentiation. Int J Mol Sci 2018;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings L, Schmults CD. Management of high-risk cutaneous squamous cell carcinoma. J Clin Aesthet Dermatol 2010;3(4):39–48. [PMC free article] [PubMed] [Google Scholar]
- Kim B Western Blot Techniques. Methods Mol Biol 2017;1606:133–9. [DOI] [PubMed] [Google Scholar]
- Kim SH, Li M, Trousil S, Zhang Y, Pasca di Magliano M, Swanson KD, et al. Phenformin Inhibits Myeloid-Derived Suppressor Cells and Enhances the Anti-Tumor Activity of PD-1 Blockade in Melanoma. J Invest Dermatol 2017;137(8):1740–8. [DOI] [PubMed] [Google Scholar]
- Li F, Adase CA, Zhang LJ. Isolation and Culture of Primary Mouse Keratinocytes from Neonatal and Adult Mouse Skin. J Vis Exp 2017(125). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ma W, Zhong H, Liu W, Sun Q. Metformin inhibits proliferation of human keratinocytes through a mechanism associated with activation of the MAPK signaling pathway. Exp Ther Med 2014;7(2):389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wen J, Leng X, Zhou Q, Zhou C, Zhao H, et al. A Simplified and Efficient Method to Isolate Primary Human Keratinocytes from Adult Skin Tissue. J Vis Exp 2018(138). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, Tommasi di Vignano A, Sharov AA, Neilson J, Havrda MC, Roop DR, et al. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell 2005;8(5):665–76. [DOI] [PubMed] [Google Scholar]
- Mullen JT, Feng L, Xing Y, Mansfield PF, Gershenwald JE, Lee JE, et al. Invasive squamous cell carcinoma of the skin: defining a high-risk group. Ann Surg Oncol 2006;13(7):902–9. [DOI] [PubMed] [Google Scholar]
- Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 2010;468(7324):653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HB, Babcock JT, Wells CD, Quilliam LA. LKB1 tumor suppressor regulates AMP kinase/mTOR-independent cell growth and proliferation via the phosphorylation of Yap. Oncogene 2013;32(35):4100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan MG, Xiong Y, Chen F. NFAT gene family in inflammation and cancer. Curr Mol Med 2013;13(4):543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Leng X, Wen J, Zhou Q, Xu X, Wu X. One-Step Simple Isolation Method to Obtain Both Epidermal and Dermal Stem Cells from Human Skin Specimen. Methods Mol Biol 2018. [DOI] [PubMed] [Google Scholar]
- Saha AK, Persons K, Safer JD, Luo Z, Holick MF, Ruderman NB. AMPK regulation of the growth of cultured human keratinocytes. Biochem Biophys Res Commun 2006;349(2):519–24. [DOI] [PubMed] [Google Scholar]
- Santini MP, Talora C, Seki T, Bolgan L, Dotto GP. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation. Proc Natl Acad Sci U S A 2001;98(17):9575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CH, Yuan P, Perez-Lorenzo R, Zhang Y, Lee SX, Ou Y, et al. Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation. Mol Cell 2013;52(2):161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang M, Wysowski DK, Butler-Jones D. Incidence of lactic acidosis in metformin users. Diabetes Care 1999;22(6):925–7. [DOI] [PubMed] [Google Scholar]
- Sun X, Yang Q, Rogers CJ, Du M, Zhu MJ. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ 2017;24(5):819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P, Wang Y, Coussens M, Manda KR, Casey AM, Lin C, et al. Activation of NFAT signaling establishes a tumorigenic microenvironment through cell autonomous and non-cell autonomous mechanisms. Oncogene 2014;33(14):1840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trousil S, Chen S, Mu C, Shaw FM, Yao Z, Ran Y, et al. Phenformin Enhances the Efficacy of ERK Inhibition in NF1-Mutant Melanoma. J Invest Dermatol 2017;137(5):1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM. Terminal differentiation of epidermal keratinocytes. Curr Opin Cell Biol 1989;1(6):1107–15. [DOI] [PubMed] [Google Scholar]
- Watt FM. Stem cell fate and patterning in mammalian epidermis. Curr Opin Genet Dev 2001;11(4):410–7. [DOI] [PubMed] [Google Scholar]
- Wen J, Zu T, Zhou Q, Leng X, Wu X. Y-27632 simplifies the isolation procedure of human primary epidermal cells by selectively blocking focal adhesion of dermal cells. J Tissue Eng Regen Med 2018;12(2):e1251–e5. [DOI] [PubMed] [Google Scholar]
- Wu CL, Qiang L, Han W, Ming M, Viollet B, He YY. Role of AMPK in UVB-induced DNA damage repair and growth control. Oncogene 2013;32(21):2682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Peisley A, Graef IA, Crabtree GR. NFAT signaling and the invention of vertebrates. Trends Cell Biol 2007;17(6):251–60. [DOI] [PubMed] [Google Scholar]
- Wu X, Nguyen BC, Dziunycz P, Chang S, Brooks Y, Lefort K, et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature 2010;465(7296):368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Tommasi di Vignano A, Zhou Q, Michel-Dziunycz PJ, Bai F, Mi J, et al. The ARE-binding protein Tristetraprolin (TTP) is a novel target and mediator of calcineurin tumor suppressing function in the skin. PLoS Genet 2018;14(5):e1007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Ito K, Perez-Lorenzo R, Del Guzzo C, Lee JH, Shen CH, et al. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc Natl Acad Sci U S A 2013;110(45):18226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zu T, Zhou Q, Wen J, Leng X, Wu X. The patch assay reconstitutes mature hair follicles by culture-expanded human cells. Regen Med 2017;12(5):503–11. [DOI] [PubMed] [Google Scholar]
- Zhu MJ, Sun X, Du M. AMPK in regulation of apical junctions and barrier function of intestinal epithelium. Tissue Barriers 2018;6(2):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.