Abstract
We report a method for the enantioselective hydrogenation of annulated arenes using 4H-pyrido[1,2-a]pyrimidinones as substrates. The method selectively generates multiple stereocenters in adjacent rings leading to architecturally complex motifs, which resemble bioactive molecules. The mechanistic study of the stereochemical outcome revealed that the catalyst is able to overcome substrate stereocontrol providing all-cis-substituted products predominantly. In a sequential protocol, a matching interaction between catalyst and substrate stereocontrol is achieved that facilitates diastereo- and enantioselective access to trans-products.
We report a method for the enantioselective hydrogenation of annulated arenes using 4H-pyrido[1,2-a]pyrimidinones as substrates.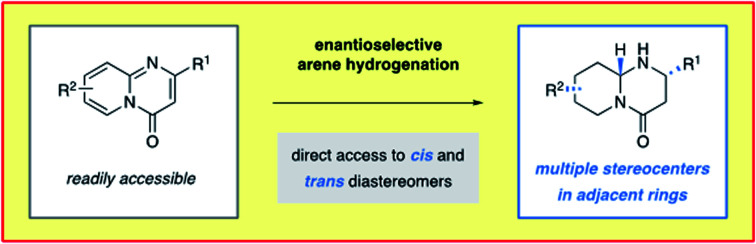
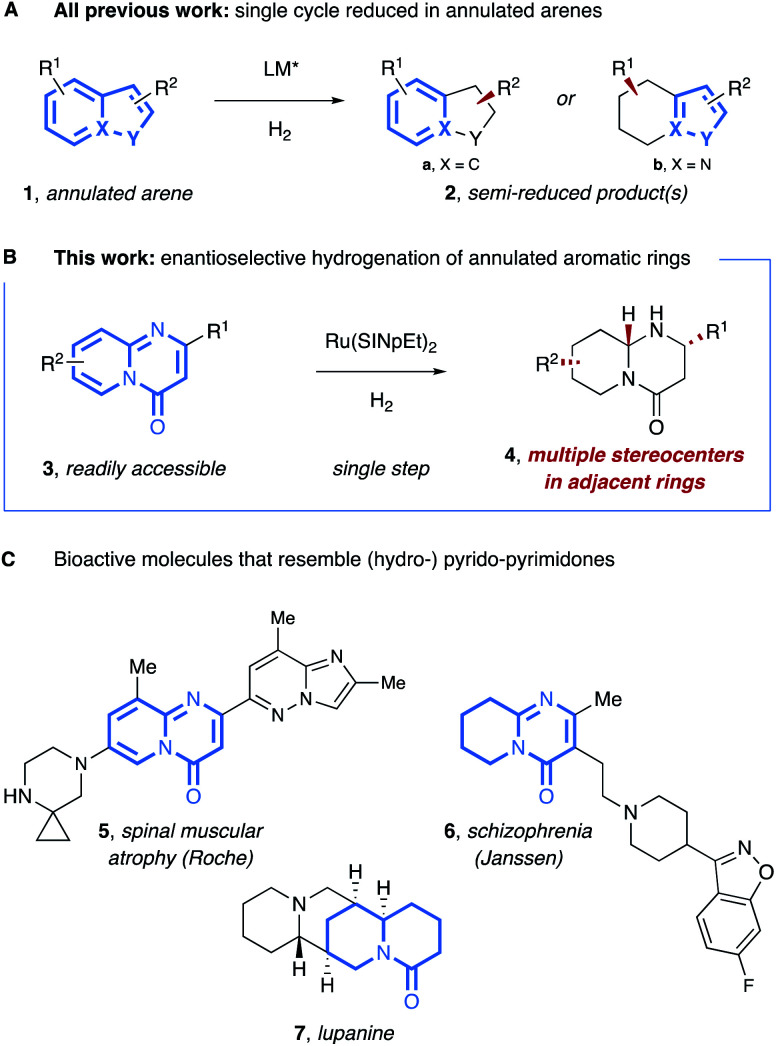
Chiral, saturated carbo- and heterocycles are important structural elements of secondary metabolites and active ingredients in drugs and agrochemicals.1 Compared to classical synthetic routes that utilize prefunctionalized precursors,2 enantioselective arene hydrogenation provides a more direct route to such motifs from readily accessible (hetero-) aromatic substrates.3 With current hydrogenation technologies, a variety of monocyclic arenes such as pyridines,4 furans,5 thiophenes,6 and annulated arenes such as quinolines and isoquinolines,7 indoles,8 and naphthalenes9 can be hydrogenated with high enantioselectivity. However, in all previous reports on the enantioselective hydrogenation of annulated arenes (1) the resulting products (2) contain only a single saturated ring with at least one aromatic sextet being preserved in the product, and consequently, with stereocenter(s) only being formed in one ring (Fig. 1A).10 An enantioselective hydrogenation of multiple annulated aromatic rings would enable the generation of multiple stereocenters at adjacent rings in a single step, leading to desirable, architecturally complex and natural product-like motifs. Herein, we report the first such example using N-bridged 4H-pyrido[1,2-a]pyrimidinone (pyrido-pyrimidinone) substrates (3, Fig. 1B).10a–d Pyrido-pyrimidinones, and their saturated analogs, are featured in bioactive molecules (Fig. 1C). For instance, Roche's Risdiplam (5), an inhibitor against spinal muscular atrophy, includes a pyrido-pyrimidinone unit11 and a semi-reduced tetrahydropyrido-pyrimidinone is a part of risperidone (6), a blockbuster drug against schizophrenia, while octahydropyrido-pyrimidinones (4) are structurally closely related to quinolizidine alkaloids such as lupanine (7). To date, only a small number of octahydropyrido-pyrimidinones (4) have been accessed with the majority resulting from the hetero-geneous, racemic hydrogenation of tetrahydropyrido-pyrimidinones (8). To the best of our knowledge, enantioenriched octahydropyrido-pyrimidinones have never been accessed by enantioselective catalysis.12
Fig. 1. (A) Previously: only one ring of annulated arenes reduced by enantioselective hydrogenation. (B) This work: enantioselective hydrogenation of both rings of bicyclic aromatic pyrido-pyrimidinones (3). (C) Biologically active examples/analogues of pyrido-pyrimidinones and their hydrogenated products.
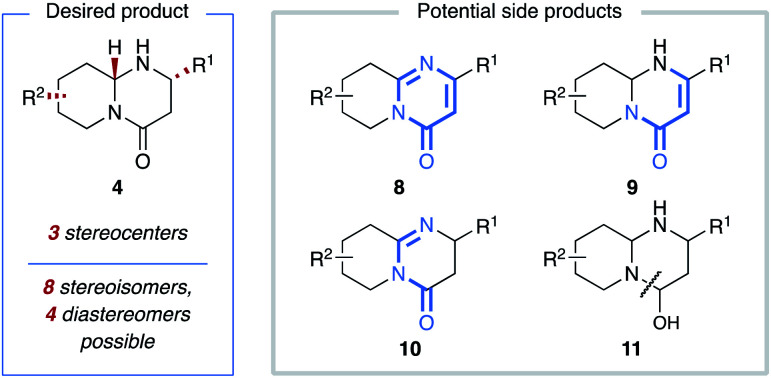
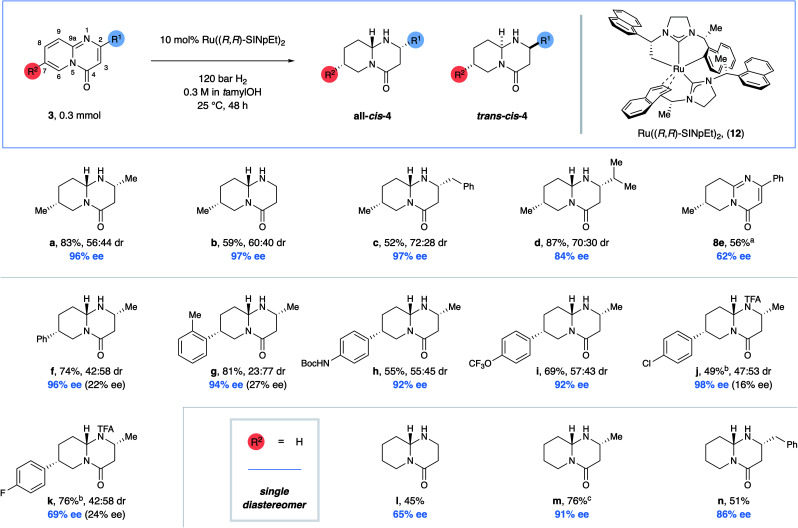
We realized that an enantioselective hydrogenation of the readily accessible pyrido-pyrimidinones would in principle offer the most direct approach towards these valuable product motifs, provided that the chemoselectivity could be controlled and potential side products, such as tetrahydro- (8), hexahydropyrido-pyrimidinones 9 and 10, and the presumably unstable hemiaminal 11 resulting from amide reduction, could be avoided (Fig. 2).13 Furthermore, we rationalized that up to four different diastereomers could result from the creation of three independent stereocenters, making the simultaneous control of chemo-, diastereo-, and enantioselectivity a daunting challenge. Nevertheless, we were ideally positioned to overcome this challenge given the high reactivity, enantioselectivity, and broad tolerance of functional groups shown by the chiral ruthenium-bisNHC catalyst system developed by our group (12).14,15 Indeed, we observed that catalytic hydrogenation of model substrate 2,7-dimethylpyrido-pyrimidinone (3a) proceeded with near-complete chemoselectivity under a variety of reaction conditions (Table S1†). Under the optimized conditions, the desired octahydropyrido-pyrimidinone was obtained in 92% yield as a mixture of just two from the possible four diastereomers (63 : 37 diastereomeric ratio (dr)) and with excellent 96% enantiomeric excess (ee) for the major diastereomer (Table S1,† entry 4). The evaluation of other substitution patterns of the pyrido- pyrimidinone using the respective dimethyl-substituted substrates revealed that the 2,8-disubstituted product can also be obtained with good chemo-, enhanced diastereo-, and moderate enantioselectivity. However, substrates with 2,6- and 2,9-substitution patterns were less reactive. Only the eastern pyridine ring was reduced in 48% and 82% yield, respectively (Table S2†). As such we decided to focus on substrates with a 2,7-substitution pattern when exploring the scope of this reaction (Fig. 3). Next, the scope was evaluated (Fig. 3). Substrates without a substituent in the C2-position (3b), and substrates containing sterically more hindering substituents such as a benzyl or an iso-propyl group (3c,d) in the C2-position all delivered the products in high yields as a mixture of just two diastereomers with moderate dr and excellent ee values for the major diastereomer. The dr values increase with the steric size of the C2-substituent. A phenyl substituent in the C2-position rendered the (eastern) pyrimidinone ring inactive, thus chemoselectively providing tetrahydropyrido-pyrimidinone 8e with moderate ee under the standard reaction conditions including the reaction time of 48 h. Contrastingly, the introduction of aryl groups in the 7-position of the (western) pyridine ring was well-tolerated. Consequently, octahydropyrido-pyrimidinones carrying aryl groups such as phenyl (4f), sterically demanding ortho-tolyl (4g), and other aryl groups bearing various synthetically useful functional groups such as NHBoc, OCF3, or Cl in the para-position (4h–j) can all be accessed in high yields and ee values. Curiously, while 7-para-fluorophenyl-substituted product 4k was obtained in high yield, the enantioselectivity was reduced in comparison to its chlorine-containing counterpart 4j. Products without a substituent in the 7-position (4l–n) were isolated in good yields as single diastereomers with good to high ee.
Fig. 2. Chemo- and stereoselectivity challenges.
Fig. 3. Scope. Combined isolated yields of both diastereomers are reported. The dr was determined by NMR-spectroscopy. The major diastereomer (and enantiomer) was assigned by X-ray crystallographic analysis of both diastereomers of 4a. The assignment for the other products was conducted by analogy. The ee of the minor diastereomer was measured in all cases (see ESI†). aTraces of an unknown impurity could not be separated from the product. bProtection with trifluoroacetic anhydride. c80 bar H2 used. SINpEt: 1,3-bis(1-(naphthalene-1-yl)ethyl)-4,5-dihydroimidazolylidene; TFA: trifluoroacetate.
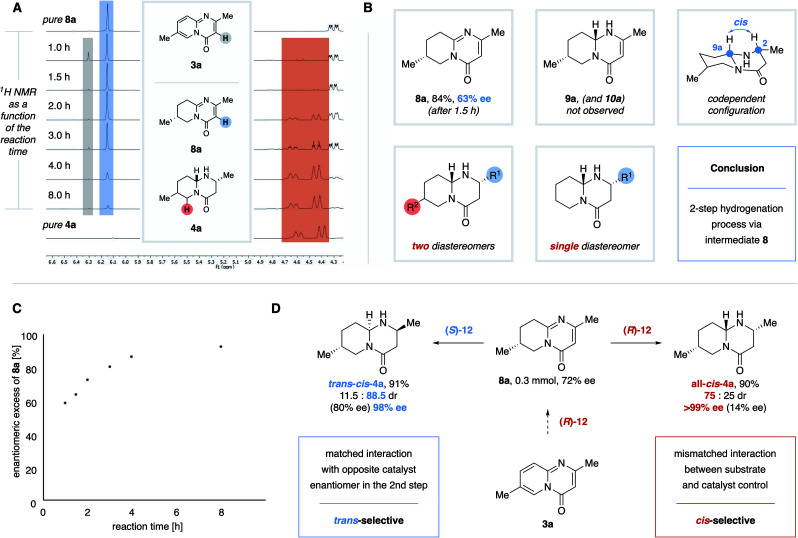
Intrigued by those observations, we proceeded to study the stereochemical mechanism of the reaction. First, we determined the reaction progress of the hydrogenation of model substrate 3a over time (Fig. 4A). After a reaction time of 1.5 h, tetrahydropyrido-pyrimidinone 8a is almost the exclusive species before its relative abundance decreases with a coinciding increase of the proportion of octahydropyrido-pyrimidinone 4a. Notably, 7-methyl- and 7-phenyl-substituted intermediates 8a and 8f were isolated in good yields and with moderate enantiomeric excess (8a: 84% yield, 63% ee; 8f: 50% yield, 62% ee). The reaction is complete after 8 h, leaving octahydrogenated product 4a as the exclusive species. Hence 8a must be an intermediate in the hydrogenation of 3a to 4a. No further signals, other than those allocated to the substrate 3a and tetrahydrointermediate 8a, were observed in the enone or α-ketone regions at any of the time points. Furthermore, the C2 and C9a C–H bonds are cis-aligned in both diastereomers of the 2,7-dimethylproduct 4a (confirmed by X-ray crystallography, see ESI†). A cis alignment would be enforced by a non-interrupted coordination of the catalyst during the hydrogenation of the (eastern) pyrimidinone ring. The codependence of the configurations of C2 and C9a would result in the formation of only two diastereomers in the hydrogenation of 7-substituted substrates and only a single diastereomer in the hydrogenation of 7-unsubstituted substrates, which is in agreement with the observations made during the exploration of the scope (Fig. 4B).16 Both observations suggest that enone 9a and imine 10a are not intermediates in this reaction. Finally, we determined that two separate effects cause the (relatively low) diastereomeric ratios of 7-substituted octahydropyrido-pyrimidinones. Firstly, the enantiocontrol over the initially formed C7-stereocenter is only moderate (8a: 63% ee after 1.5 h, Fig. 4B). Secondly, the ee of the intermediates 8 increases with the reaction progress of further hydrogenation towards the octahydroproducts 4 (Fig. 4C). It follows that the minor enantiomer of chiral intermediate 8 is converted into octahydro-product 4 more rapidly than the major enantiomer, indicating a mismatched interaction between substrate and catalyst control. To further test this conclusion, isolated intermediate 8a was submitted to the enantioselective hydrogenation using the opposite enantiomer of the chiral catalyst in the second hydrogenation step (Fig. 4D).16 In this case, hydrogenation proceeds with a matched interaction between catalyst and substrate control and provides the opposite diastereomer trans–cis-4a with excellent diastereo- and enantiocontrol. Notably even racemic arene hydrogenation rarely produces trans-products.18 The dr of all–cis-4a derived from the two-step hydrogenation (Fig. 4D, red equation) is higher than that of the standard one-pot protocol (75 : 25 vs 56 : 44 dr). This is a result of the increase of the ee of intermediate 8a as a function of the reaction time (the minor enantiomer is consumed faster to form 4a). The effective ee of 8a in the two-step protocol (isolated after 2 h) was higher than that of 8a in the standard protocol resulting in a higher dr of 4a.
Fig. 4. Mechanistic studies. (A) Excerpt of the 1H NMR spectrum of the reaction mixture as a function of the reaction time. (B) Key observations that suggest a two-step hydrogenation process. (C) ee of 8a as a function of the reaction time. (D) Sequential enantioselective hydrogenation of 3a using opposite enantiomers of the chiral catalyst.17.
Conclusions
In conclusion, we disclose the first method for the enantioselective hydrogenation of multiple annulated arenes forming stereocenters in adjacent rings. Using pyrido-pyrimidones as substrates, the method produces architecturally complex, natural product-like motifs and routinely forms three stereocenters at adjacent rings selectively in a single operation. Mechanistic investigation of the stereochemical outcome suggests a two-step hydrogenation pathway involving a catalyst dissociation/reassociation step. The catalyst is able to overcome the substrate stereocontrol in the second hydrogenation step leading predominantly to all-cis-substituted products. This mis-matched interaction can be converted into a matched interaction by switching the enantiomer of the chiral catalyst in the second step in a two-pot operation that provides the opposite, trans-diastereomer with excellent diastereo- and enantiocontrol.
Author contributions
M. P. W., D. M., and D. P. performed the synthetic experiments and developed the project. F. G. supervised the research and M. P. W., D. M., D. P., and F. G. wrote the manuscript.
Conflicts of interest
The authors declare no competing financial interests.
Supplementary Material
Acknowledgments
We are grateful to the Studienstiftung des Deutschen Volkes, the Deutsche Akademie der Naturforscher Leopoldina (both M. P. W.), the Fonds der Chemischen Industrie (D. M.), the Deutsche Forschungsgemeinschaft (Leibniz Award, F. G.), and the European Research Council (ERC Advanced Grant Agreement no. 788558) for generous financial support. We sincerely thank C. Schlepphorst, M. v. Gemmeren, and D. Janssen-Müller for many helpful discussions, C. Gelis for experimental support, T. Dalton for proofreading of the manuscript, and C. G. Daniliuc for X-ray crystallographic analysis.
Electronic supplementary information (ESI) available. CCDC 2006231 and 2006232. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc07099h
Notes and references
- (a) Finefield J. F. Sherman D. H. Kreitman M. Williams R. M. Angew. Chem., Int. Ed. 2012;51:4802. doi: 10.1002/anie.201107204. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Michael P. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 1994;11:639. doi: 10.1039/np9941100639. [DOI] [PubMed] [Google Scholar]
- Classical syntheses of saturated N-heterocycles: ; (a) Royer J., Asymmetric Synthesis of Nitrogen Heterocycles, Wiley-VCH, Weinheim, 2009 [Google Scholar]; (b) Vo C.-V. T. Bode J. W. J. Org. Chem. 2014;79:2809. doi: 10.1021/jo5001252. [DOI] [PubMed] [Google Scholar]
- Selected recent reviews: ; (a) Wang D.-S. Chen Q.-A. Lu S.-M. Zhou Y.-G. Chem. Rev. 2012;112:2557. doi: 10.1021/cr200328h. [DOI] [PubMed] [Google Scholar]; (b) He Y.-M. Song F.-T. Fan Q.-H. Top. Curr. Chem. 2014;343:145. doi: 10.1007/128_2013_480. [DOI] [PubMed] [Google Scholar]; (c) Wiesenfeldt M. P. Nairoukh Z. Dalton T. Glorius F. Angew. Chem., Int. Ed. 2019;58:10460. doi: 10.1002/anie.201814471. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kim A. N. Stoltz B. M. ACS Catal. 2020;10:13834. doi: 10.1021/acscatal.0c03958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selected methods: ; (a) Legault C. Y. Charette A. B. J. Am. Chem. Soc. 2005;127:8966. doi: 10.1021/ja0525298. [DOI] [PubMed] [Google Scholar]; (b) Ye Z.-S. Chen M.-W. Shi L. Duan Y. Zhou Y.-G. Angew. Chem., Int. Ed. 2012;51:10181. doi: 10.1002/anie.201205187. [DOI] [PubMed] [Google Scholar]; (c) Chang M. Huang Y. Liu S. Chen Y. Krska S. W. Davies I. W. Zhang X. Angew. Chem., Int. Ed. 2014;53:12761. doi: 10.1002/anie.201406762. [DOI] [PubMed] [Google Scholar]
- Selected methods: ; (a) Kaiser S. Smidt S. P. Pfaltz A. Angew. Chem., Int. Ed. 2006;45:5194. doi: 10.1002/anie.200601529. [DOI] [PubMed] [Google Scholar]; (b) Wysocki J. Ortega N. Glorius F. Angew. Chem., Int. Ed. 2014;53:8751. doi: 10.1002/anie.201310985. [DOI] [PubMed] [Google Scholar]
- Urban S. Beiring B. Ortega N. Paul D. Glorius F. J. Am. Chem. Soc. 2012;134:15241. doi: 10.1021/ja306622y. [DOI] [PubMed] [Google Scholar]
- Selected methods for quinolines: ; (a) Wang W.-B. Lu S.-M. Yang P.-Y. Han X.-W. Zhou Y.-G. J. Am. Chem. Soc. 2003;125:10536. doi: 10.1021/ja0353762. [DOI] [PubMed] [Google Scholar]; (b) Zhou H. Li Z. Wang Z. Xu L. He Y. Fan Q.-H. Pan J. Gu L. Chan A. S. C. Angew. Chem., Int. Ed. 2008;47:8464. doi: 10.1002/anie.200802237. [DOI] [PubMed] [Google Scholar]; Isoquinolines: ; (c) Lu S.-M. Wang Y.-Q. Han X.-W. Zhou Y.-G. Angew. Chem., Int. Ed. 2006;45:2260. doi: 10.1002/anie.200503073. [DOI] [PubMed] [Google Scholar]; (d) Iimuro A. Yamaji K. Kandula S. Nagano T. Kita Y. Mashima K. Angew. Chem., Int. Ed. 2013;52:2046. doi: 10.1002/anie.201207748. [DOI] [PubMed] [Google Scholar]; (e) Wen J. Tan R. Liu S. Zhao Q. Zhang X. Chem. Sci. 2016;7:3047. doi: 10.1039/c5sc04712a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Kim A. N. Ngamnithiporn A. Welin E. R. Daiger M. Grünanger C. Bartberger M. D. Virgil S. C. Stoltz B. M. ACS Catal. 2020;10:3241. doi: 10.1021/acscatal.0c00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selected methods: ; (a) Kuwano R. Sato K. Kurokawa T. Karube D. Ito Y. J. Am. Chem. Soc. 2000;122:7614. [Google Scholar]; (b) Baeza A. Pfaltz A. Chem.–Eur. J. 2010;16:2036. doi: 10.1002/chem.200903105. [DOI] [PubMed] [Google Scholar]
- Kuwano R. Morioka R. Kashiwabara M. Kameyama N. Angew. Chem., Int. Ed. 2012;51:4136. doi: 10.1002/anie.201201153. [DOI] [PubMed] [Google Scholar]
- See comprehensive reviews (3a,c). Preservation of an aromatic sextet in N-bridged heteroarenes: ; (a) Huang W.-X. Yu C.-B. Shi L. Zhou Y.-G. Org. Lett. 2014;16:3324. doi: 10.1021/ol5013313. [DOI] [PubMed] [Google Scholar]; (b) Makida Y. Saita M. Kuramoto T. Ishizuka K. Kuwano R. Angew. Chem., Int. Ed. 2016;55:11859. doi: 10.1002/anie.201606083. [DOI] [PubMed] [Google Scholar]; (c) Ortega N. Tang D.-T. D. Urban S. Zhao D. Glorius F. Angew. Chem., Int. Ed. 2013;52:9500. doi: 10.1002/anie.201302218. [DOI] [PubMed] [Google Scholar]; (d) Schlepphorst C. Wiesenfeldt M. P. Glorius F. Chem.–Eur. J. 2017;24:356. doi: 10.1002/chem.201705370. [DOI] [PubMed] [Google Scholar]; Construction of up to three stereocenters in one ring: ; (e) Kuwano R. Kashiwabara M. Ohsumi M. Kusano H. J. Am. Chem. Soc. 2008;130:808. doi: 10.1021/ja7102422. [DOI] [PubMed] [Google Scholar]
- Naryshkin N. A. et al. . Science. 2014;345:688. doi: 10.1126/science.1250127. [DOI] [PubMed] [Google Scholar]
- Heterogeneous hydrogenation: ; (a) Yale H. L. Spitzmiller E. R. J. Heterocycl. Chem. 1976;13:797. [Google Scholar]; (b) Hermecz I. Toth G. Ungvary F. Meszaros Z. J. Org. Chem. 1982;47:4780. [Google Scholar]; Synthesis of one closely related achiral structure containing a quaternary stereocenter: ; (c) Unsworth W. P. Coulthard G. Kitsiou C. Taylor R. J. K. J. Org. Chem. 2014;79:1368. doi: 10.1021/jo402768r. [DOI] [PubMed] [Google Scholar]; ; Synthesis of one enantioenriched motif starting from enantioenriched dipeptides: ; (d) Mizutani N. Chiou W.-H. Ojima I. Org. Lett. 2002;4:4575. doi: 10.1021/ol026782d. [DOI] [PubMed] [Google Scholar]; (e) Chiou W.-H. Mizutani N. Ojima I. J. Org. Chem. 2007;72:1871. doi: 10.1021/jo061692y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a).Related β-amino-enone resulting from arene hydrogenation: ; Wang X.-B. Zeng W. Zhou Y.-G. Tetrahedron Lett. 2008;49:4922. [Google Scholar]; (Ref. 12a and b) showed that the pyrimidone ring is less reactive than the pyridine ring
- (a) Urban S. Ortega N. Glorius F. Angew. Chem., Int. Ed. 2011;50:3803. doi: 10.1002/anie.201100008. [DOI] [PubMed] [Google Scholar]; (b) Paul D. Beiring B. Plois M. Ortega N. Kock S. Schlüns D. Neugebauer J. Wolf R. Glorius F. Organometallics. 2016;35:3641. [Google Scholar]
- The NHC precursor is commercially available at Merck Millipore. Product number: 905542
- The hydrogenation of 3e provided a mixture of a hexahydropyrido-pyrimidinone and 4 diastereomers of an octahydropyridopyrimidinone 8e as byproducts.
- The absolute configuration of all-cis-4a was determined by X-ray crystallography. Subsequently, the absolute configuration of the major enantiomers of 8a and trans–cis-4a were assigned by considering that the major enantiomer of 8a must lead to the major enantiomer of both diastereomers (provided that there is no interconversion of the two enantiomers of 8a)
- Wollenburg M. Heusler A. Bergander K. Glorius F. ACS Catal. 2020;10:11365. doi: 10.1021/acscatal.0c03423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.