Abstract
Background
Pressure ulcers (also known as injuries, pressure sores, decubitus ulcers and bed sores) are localised injuries to the skin or underlying soft tissue, or both, caused by unrelieved pressure, shear or friction. Reactive surfaces that are not made of foam or air cells can be used for preventing pressure ulcers.
Objectives
To assess the effects of non‐foam and non‐air‐filled reactive beds, mattresses or overlays compared with any other support surface on the incidence of pressure ulcers in any population in any setting.
Search methods
In November 2019, we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations); Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
We included randomised controlled trials that allocated participants of any age to non‐foam or non‐air‐filled reactive beds, overlays or mattresses. Comparators were any beds, overlays or mattresses used.
Data collection and analysis
At least two review authors independently assessed studies using predetermined inclusion criteria. We carried out data extraction, 'Risk of bias' assessment using the Cochrane 'Risk of bias' tool, and the certainty of the evidence assessment according to Grading of Recommendations, Assessment, Development and Evaluations methodology. If a non‐foam or non‐air‐filled surface was compared with surfaces that were not clearly specified, then the included study was recorded and described but not considered further in any data analyses.
Main results
We included 20 studies (4653 participants) in this review. Most studies were small (median study sample size: 198 participants). The average participant age ranged from 37.2 to 85.4 years (median: 72.5 years). Participants were recruited from a wide range of care settings but were mainly from acute care settings. Almost all studies were conducted in Europe and America. Of the 20 studies, 11 (2826 participants) included surfaces that were not well described and therefore could not be fully classified. We synthesised data for the following 12 comparisons: (1) reactive water surfaces versus alternating pressure (active) air surfaces (three studies with 414 participants), (2) reactive water surfaces versus foam surfaces (one study with 117 participants), (3) reactive water surfaces versus reactive air surfaces (one study with 37 participants), (4) reactive water surfaces versus reactive fibre surfaces (one study with 87 participants), (5) reactive fibre surfaces versus alternating pressure (active) air surfaces (four studies with 384 participants), (6) reactive fibre surfaces versus foam surfaces (two studies with 228 participants), (7) reactive gel surfaces on operating tables followed by foam surfaces on ward beds versus alternating pressure (active) air surfaces on operating tables and subsequently on ward beds (two studies with 415 participants), (8) reactive gel surfaces versus reactive air surfaces (one study with 74 participants), (9) reactive gel surfaces versus foam surfaces (one study with 135 participants), (10) reactive gel surfaces versus reactive gel surfaces (one study with 113 participants), (11) reactive foam and gel surfaces versus reactive gel surfaces (one study with 166 participants) and (12) reactive foam and gel surfaces versus foam surfaces (one study with 91 participants). Of the 20 studies, 16 (80%) presented findings which were considered to be at high overall risk of bias.
Primary outcome: Pressure ulcer incidence
We did not find analysable data for two comparisons: reactive water surfaces versus foam surfaces, and reactive water surfaces versus reactive fibre surfaces. Reactive gel surfaces used on operating tables followed by foam surfaces applied on hospital beds (14/205 (6.8%)) may increase the proportion of people developing a new pressure ulcer compared with alternating pressure (active) air surfaces applied on both operating tables and hospital beds (3/210 (1.4%) (risk ratio 4.53, 95% confidence interval 1.31 to 15.65; 2 studies, 415 participants; I2 = 0%; low‐certainty evidence). For all other comparisons, it is uncertain whether there is a difference in the proportion of participants developing new pressure ulcers as all data were of very low certainty.
Included studies did not report time to pressure ulcer incidence for any comparison in this review.
Secondary outcomes
Support‐surface‐associated patient comfort: the included studies provide data on this outcome for one comparison. It is uncertain if there is a difference in patient comfort between alternating pressure (active) air surfaces and reactive fibre surfaces (one study with 187 participants; very low‐certainty evidence).
All reported adverse events: there is evidence on this outcome for one comparison. It is uncertain if there is a difference in adverse events between reactive gel surfaces followed by foam surfaces and alternating pressure (active) air surfaces applied on both operating tables and hospital beds (one study with 198 participants; very low‐certainty evidence).
We did not find any health‐related quality of life or cost‐effectiveness evidence for any comparison in this review.
Authors' conclusions
Current evidence is generally uncertain about the differences between non‐foam and non‐air‐filled reactive surfaces and other surfaces in terms of pressure ulcer incidence, patient comfort, adverse effects, health‐related quality of life and cost‐effectiveness. Reactive gel surfaces used on operating tables followed by foam surfaces applied on hospital beds may increase the risk of having new pressure ulcers compared with alternating pressure (active) air surfaces applied on both operating tables and hospital beds.
Future research in this area should consider evaluation of the most important support surfaces from the perspective of decision‐makers. Time‐to‐event outcomes, careful assessment of adverse events and trial‐level cost‐effectiveness evaluation should be considered in future studies. Trials should be designed to minimise the risk of detection bias; for example, by using digital photography and adjudicators of the photographs being blinded to group allocation. Further review using network meta‐analysis adds to the findings reported here.
Plain language summary
Do beds, mattresses and mattress toppers that apply constant pressure to the skin and are not air‐filled or made of foam prevent pressure ulcers?
Key messages
Due to a lack of robust evidence, it is unclear whether most types of surface that apply constant pressure to the skin and are not air‐filled or made of foam prevent pressure ulcers.
Lying surgery patients on an operating table with a gel surface that applies constant pressure to the skin and then a hospital bed with a foam surface, rather than using air‐filled surfaces, may increase the risk of developing pressure ulcers.
Future studies should focus on options and effects that are important to decision‐makers, such as:
‐ gel surfaces that apply constant pressure to the skin, compared to air‐filled or foam surfaces; and
‐ whether and when pressure ulcers develop, unwanted effects and costs.
What are pressure ulcers?
Pressure ulcers are also known as pressure sores or bed sores. They are wounds to the skin and underlying tissue caused by prolonged pressure or rubbing. They often occur on bony parts of the body, such as heels, elbows, hips and the bottom of the spine. People who have mobility problems or who lie in bed for long periods are at risk of developing pressure ulcers.
What did we want to find out?
There are beds, mattresses and mattress toppers specifically designed for people at risk of pressure ulcers. These can be made of a range of materials (such as foam, air cells or water bags) and are divided into two groups:
‐ reactive (static) surfaces that apply a constant pressure to the skin, unless a person moves or is repositioned; and
‐ active (alternating pressure) surfaces that regularly redistribute the pressure under the body.
We wanted to find out if reactive surfaces that are not air‐filled or made of foam:
‐ prevent pressure ulcers;
‐ are comfortable and improve people’s quality of life;
‐ have health benefits that outweigh their costs; and
‐ have any unwanted effects.
What did we do?
We searched the medical literature for studies that evaluated the effects of beds, mattresses and mattress toppers with a reactive surface that was not air‐filled or made of foam. We compared and summarised the results of these studies, and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 20 studies (4653 people, average age: 73 years) that lasted between seven days and six months (average: four weeks). The studies compared reactive surfaces filled with water or gel, or made of fibre, against other active or reactive surfaces.
In general, the studies did not provide sufficiently robust evidence for us to determine if reactive surfaces that are not air‐filled or made of foam prevent pressure ulcers.
Evidence from two studies suggests that people who undergo surgery may be more likely to develop pressure ulcers when they lie on an operating table with a reactive gel surface and then a hospital bed with a foam surface, rather than on active air‐filled surfaces.
The other benefits and risks of gel and other reactive surfaces are unclear. No studies reported information about quality of life and cost.
What limited our confidence in the evidence?
Most studies were small (198 people on average) and used methods likely to introduce errors in their results.
How up‐to‐date is this review?
The evidence in this Cochrane Review is current to November 2019.
Summary of findings
Background
Description of the condition
Pressure ulcers — also known as pressure injuries, pressure sores, decubitus ulcers and bed sores — are localised injuries to the skin or underlying soft tissue (or both) caused by unrelieved pressure, shear or friction (NPIAP 2016). Pressure ulcer severity is generally classified using the National Pressure Injury Advisory Panel (NPIAP) system (NPIAP 2016).
Stage 1: intact skin with a local appearance of non‐blanchable erythema
Stage 2: partial‐thickness skin loss with exposed dermis
Stage 3: full‐thickness skin loss
Stage 4: full‐thickness skin and tissue loss with visible fascia, muscle, tendon, ligament, cartilage or bone
Unstageable pressure injury: full‐thickness skin and tissue loss that is obscured by slough or eschar so that the severity of injury cannot be confirmed
Deep tissue pressure injury: local injury of persistent, non‐blanchable deep red, maroon, purple discolouration or epidermal separation revealing a dark wound bed or blood‐filled blister
The stages described above are consistent with those described in another commonly used system, the International Classification of Diseases for Mortality and Morbidity Statistics (World Health Organization 2019).
Pressure ulcers are complex wounds that are relatively common, affecting people across different care settings. A systematic review found that prevalence estimates for people affected by pressure ulcers in communities of the UK, USA, Ireland, and Sweden ranged from 5.6 to 2300 per 10,000 depending on the nature of the population surveyed (Cullum 2016). A subsequent cross‐sectional survey of people receiving community health services in one city in the UK estimated that 1.8 people per 10,000 have a pressure ulcer (Gray 2018).
Pressure ulcers confer a heavy burden in terms of personal impact and use of health‐service resources. Having a pressure ulcer may impair physical, social and psychological activities (Gorecki 2009). Ulceration impairs health‐related quality of life (Essex 2009); can result in longer institution stays (Theisen 2012); and increases the risk of systemic infection (Espejo 2018). There is also substantial impact on health systems: a 2015 systematic review of 14 studies across a range of care settings in Europe and North America showed that costs related to pressure ulcer treatment ranged from EUR 1.71 to EUR 470.49 per person, per day (Demarré 2015). In the UK, the annual average cost to the National Health Service for managing one person with a pressure ulcer in the community was estimated to be GBP 1400 for a Stage 1 pressure ulcer and more than GPB 8500 for more severe stages (2015/2016 prices; Guest 2018). In Australia, the annual cost of treating pressure ulcers was estimated to be AUD 983 million (95% confidence interval (CI) 815 million to 1151 million) at 2012/2013 prices (Nguyen 2015). The serious consequences of pressure ulceration have led to an intensive focus on their prevention.
Description of the intervention
Pressure ulcers are considered largely preventable. Support surfaces are specialised medical devices designed to relieve or redistribute pressure on the body, or both, in order to prevent pressure ulcers (NPIAP S3I 2007). Types of support surface include, but are not limited to, integrated bed systems, mattresses and overlays (NPIAP S3I 2007).
The NPIAP Support Surface Standards Initiative (S3I) system (NPIAP S3I 2007) can be used to classify types of support surface. According to this system, support surfaces may:
be powered (i.e. require electrical power to function) or non‐powered;
passively redistribute body weight (i.e. reactive pressure redistribution), or mechanically alternate the pressure on the body to reduce the duration of pressure (i.e. active pressure redistribution);
be made of a range of materials, including but not limited to: air cells, foam materials, fibre materials, gel materials, sheepskin for medical use and water‐bags; or
be constructed of air‐filled cells that have small holes on the surface for blowing out air to dry skin (i.e. low‐air‐loss feature) or have fluid‐like characteristics via forcing filtered air through ceramic beads (i.e. air‐fluidised feature), or have neither of these features.
Full details of classifications of support surfaces are listed in Appendix 1. Reactive support surfaces cover a spectrum of commonly used beds or mattresses. Reactive air mattresses and reactive foam mattresses are the subject of other, related reviews. This review focuses on non‐foam and non‐air‐filled reactive support surfaces, which includes reactive beds or mattresses made from fibre, gel, sheepskin, water‐bags or other materials (NPIAP S3I 2007). These beds or mattresses are commonly non‐powered and aim to passively redistribute pressure over a larger contact area. Examples of types of alternative reactive mattresses include:
non‐powered reactive fibre mattresses (e.g. Spenco overlay);
non‐powered reactive gel mattresses;
non‐powered reactive sheepskin mattresses (e.g. Australian Medical Sheepskins overlay); and
non‐powered reactive water mattresses.
How the intervention might work
The aim of using support surfaces to prevent pressure ulceration is to redistribute pressure beneath the body, thereby allowing blood to flow to tissues and minimising distortion of the skin and soft tissue (Wounds International 2010). Reactive support surfaces achieve pressure redistribution by passive mechanisms, including immersion (i.e. 'sinking' of the body into a support surface) and envelopment (i.e. conforming of a support surface to the irregularities in the body). These devices distribute the pressure over a greater area, thereby reducing the magnitude of the pressure at specific sites (Clark 2011).
Why it is important to do this review
Support surfaces are widely used for preventing pressure ulcers and are the focus of recommendations in international and national guidelines (EPUAP/NPIAP/PPPIA 2019; NICE 2014). Since the publication of the Cochrane Review, 'Support surfaces for pressure ulcer prevention' (McInnes 2015), there has been a substantial increase in the number of relevant randomised controlled trials published in this area. The NPIAP S3I 2007 support surface‐related terms and definitions have also been internationally recognised, and Cochrane has developed new methodological requirements, such as the use of GRADE assessments (Guyatt 2008). These developments necessitate an update of the evidence base.
In considering this evidence update, we took into account the size and complexity of the published review (McInnes 2015), which included all types of support surface. An alternative approach is to split the review into multiple new titles, each with a narrower focus. We consulted on this splitting option via an international survey in August 2019. The potential new titles suggested were based on clinical use, the new terms and definitions related to support surfaces (NPIAP S3I 2007), a relevant network meta‐analysis (Shi 2018a), and current clinical practice guidelines (EPUAP/NPIAP/PPPIA 2019; NICE 2014). We received responses from 29 health professionals involved in pressure ulcer prevention activity in several countries (Australia, Belgium, China, Italy, the Netherlands and the UK). In total, 83% of respondents supported splitting the review into suggested titles and 17% were unsure (no respondent voted against splitting). The reviews in this series are now:
alternating pressure (active) air surfaces for preventing pressure ulcers;
foam surfaces for preventing pressure ulcers;
reactive air surfaces for preventing pressure ulcers; and
alternative reactive support surfaces (non‐foam and non‐air‐filled) for preventing pressure ulcers (Differences between protocol and review).
We bring the results of these reviews together in an overview with a network meta‐analysis (Salanti 2012), in order to simultaneously compare all support surfaces and to rank them based on the probabilities of each being the most effective for preventing pressure ulcers (Shi 2021).
This particular review compares any type of alternative reactive beds, mattresses or overlays that are non‐foam and non‐air‐filled with any other surface.
Objectives
To assess the effects of non‐foam and non‐air‐filled reactive beds, mattresses or overlays compared with any other support surface on the incidence of pressure ulcers in any population in any setting.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs), including multi‐armed studies, cluster‐RCTs and cross‐over trials, regardless of the language of publication. We excluded studies using quasi‐random allocation methods (e.g. alternation).
Types of participants
We included studies in any population, including those defined as being at risk of ulceration, as well as those with existing pressure ulcers at baseline (when the study measured pressure ulcer incidence).
Types of interventions
The eligible experimental interventions were reactive beds, mattresses or overlays that were non‐foam and non‐air‐filled. These surfaces included, but were not limited to, specific reactive mattresses identified in Shi 2018a, namely:
non‐powered reactive fibre mattresses (e.g. Silicore fibre overlay); or
non‐powered reactive gel mattresses (e.g. a gel pad used on an operating table); or
non‐powered reactive sheepskin mattresses (e.g. Australian Medical Sheepskins overlay); or
non‐powered reactive water mattresses.
We included studies where two or more support surfaces were used sequentially over time or in combination, where the support surface(s) of interest was included in one of the study arms. We included studies comparing eligible non‐foam and non‐air‐filled beds, overlays or mattresses with any comparator defined as a support surface. Comparators could be:
foam‐filled or air‐filled surfaces, including alternating pressure (active) air surfaces such as alternating pressure (or dynamic) air mattresses, reactive air surfaces (e.g. static air overlays, dry flotation mattresses, air‐fluidised beds), and foam mattresses, or
a different type of non‐foam or non‐air‐filled surface.
We included studies in which co‐interventions (e.g. repositioning) were delivered, provided that the co‐interventions were the same in all arms of the study (i.e. interventions randomised were the only systematic difference).
Types of outcome measures
We considered the following primary and secondary outcomes. If a study did not report any review‐relevant outcomes but was otherwise eligible (i.e. eligible study design, participants and interventions), we contacted the study authors (where possible) to clarify whether they measured a relevant outcome but did not report it. We considered the study as 'awaiting classification' if we could not establish whether it measured an outcome or not. We excluded the study if the study authors confirmed that they did not measure any review‐relevant outcomes.
If a study measured an outcome at multiple time points, we considered outcome measures at three months as being of primary interest to this review (Schoonhoven 2007), regardless of the time points specified as being of primary interest by the study. If the study did not report three‐month outcome measures, we considered those closest to three months. Where a study only reported a single time point, we considered these data in this review. Where the study did not specify a time point for outcome measurement, we assumed this was the final duration of follow‐up noted.
Primary outcomes
Our primary outcome was pressure ulcer incidence. We recorded two outcome measures (the proportion of participants developing a new pressure ulcer; and time to pressure ulcer incidence), where available. However, we considered the proportion of participants developing a new pressure ulcer as the primary outcome for this review. Our preferred measure was time to pressure ulcer incidence; however, we did not expect it to be reported in many studies. We extracted and analysed time‐to‐event data but focused on the binary outcome in our conclusions. We accepted the study authors' definitions of an incident ulcer regardless of which pressure ulcer severity classification system was used to measure or grade new pressure ulcers. We also considered the outcome of pressure ulcer incidence irrespective of whether studies reported ulcers by stages or as a non‐stratified value.
We did not consider subjective outcome measures (e.g. 'better' or 'worse' skin condition) as measures of pressure ulcer incidence.
Secondary outcomes
Support‐surface‐associated patient comfort. We considered patient comfort outcome data in this review only if the evaluation of patient comfort was pre‐planned and was systematically conducted across all participants in the same way in a study. The definition and measurement of this outcome varied from one study to another; for example, the proportion of participants who report comfort, or comfort measured by a scale with continuous (categorical) numbers. We planned to include these data with different measurements in separate meta‐analyses when possible.
All reported adverse events (measured using surveys or questionnaires, other data capture process or visual analogue scale). We included data where study authors specified a clear method for collecting adverse event data. Where available, we extracted data on all serious and all non‐serious adverse events as an outcome. We recorded where it was clear that events were reported at the participant level or whether multiple events per person were reported, in which case appropriate adjustments were required for data clustering (Peryer 2019). We considered the assessment of any event in general defined as adverse by participants, health professionals, or both.
Health‐related quality of life (measured using a standardised generic questionnaire such as EQ‐5D (Herdman 2011), 36‐item Short Form (SF‐36; Ware 1992), or pressure ulcer‐specific questionnaires such as the PURPOSE Pressure Ulcer Quality of Life (PU‐QOL) questionnaire (Gorecki 2013), at noted time points. We did not include ad hoc measures of quality of life or qualitative interviews of quality of life because these measures were unlikely to be validated.
Cost‐effectiveness: within‐trial cost‐effectiveness analysis comparing mean differences in effects with mean cost differences between the two arms. We extracted data on incremental mean cost per incremental gain in benefit (incremental cost‐effectiveness ratio (ICER)). We also considered other measures of relative cost‐effectiveness (e.g. net monetary benefit, net health benefit).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
the Cochrane Wounds Specialised Register (searched 14 November 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 10) in the Cochrane Library (searched 14 November 2019);
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 14 November 2019);
Ovid Embase (1974 to 14 November 2019);
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to November 14 2019).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2019). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2019). We combined the CINAHL Plus search with the trial filter developed by Glanville 2019. There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov) (searched 20 November 2019);
World Health Organization (WHO) International Clinical Trials Registry Platform (https://www.who.int/clinical-trials-registry-platform) (searched 20 November 2019).
Search strategies for clinical trials registries can be found in Appendix 2.
Searching other resources
For previous versions of McInnes 2015, the review authors of McInnes 2015 contacted experts in the field of wound care to enquire about potentially relevant studies that are ongoing or recently published. In addition, the review authors of McInnes 2015 contacted manufacturers of support surfaces for details of any studies manufacturers were conducting. This approach did not yield any additional studies, therefore we did not repeat it for this review.
We identified other potentially eligible studies or ancillary publications by searching the reference lists of retrieved included studies, as well as relevant systematic reviews, meta‐analyses and health technology assessment reports.
When necessary, we contacted authors of key papers and abstracts to request further information about their trials.
We did not perform a separate search for adverse effects of interventions used; we considered adverse effects described in included studies only.
Data collection and analysis
We carried out data collection and analysis according to the methods stated in the published protocol (Shi 2020), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Li 2019). Changes from the protocol or previous published versions of the review are documented in Differences between protocol and review.
Selection of studies
One review author re‐checked the RCTs included in McInnes 2015 for eligibility (CS). Two review authors or researchers (CS and Asmara Jammali‐Blasi, or JCD) independently assessed the titles and abstracts of the new search results for relevance using Rayyan (Ouzzani 2016) (Differences between protocol and review), and then independently inspected the full text of all potentially eligible studies. The two review authors or researchers (CS and Asmara Jammali‐Blasi, or JCD) resolved disagreements through discussion or by involving another review author if necessary.
Data extraction and management
One review author checked data from the studies included in McInnes 2015 and extracted additional data where necessary (CS). A second review author or researcher (SR, EM, Zhenmi Liu, Gill Norman, or Melanie Stephens) checked any new data extracted. For new included studies, one review author (CS) independently extracted data and another review author or researcher (SR, EM, Zhenmi Liu, Gill Norman, or Melanie Stephens) checked all data (Differences between protocol and review). Any disagreements were resolved through discussion and, if necessary, with the involvement of another review author. Where necessary, we contacted the authors of included studies to clarify data.
We extracted these data using a pre‐prepared data extraction form:
basic characteristics of studies (first author, publication type, publication year and country);
funding sources;
care setting;
characteristics of participants (trial eligibility criteria, average age in each arm or in a study, proportions of participants by gender and participants’ baseline skin status);
support surfaces being compared (including their descriptions);
details on any co‐interventions;
duration of follow‐up;
the number of participants enrolled;
the number of participants randomised to each arm;
the number of participants analysed;
participant withdrawals with reasons;
the number of participants developing new ulcers (by ulcer stages where possible);
data on time to pressure ulceration;
support‐surface‐associated patient comfort;
adverse event outcome data;
health‐related quality of life outcome data; and
cost‐effectiveness outcome data.
We (CS and NC) classified specific support surfaces in the included studies into intervention groups using the NPIAP S3I support surface‐related terms and definitions (NPIAP S3I 2007). Therefore, to accurately assign specific support surfaces to intervention groups, we extracted full descriptions of support surfaces from included studies, and when necessary, supplemented the information with that from external sources such as other publications about the same support surface, manufacturers’ or product websites, and expert clinical opinion (Shi 2018b). If we were unable to define any of specific support surfaces evaluated in an included study, we extracted available data and reported these as additional data outside the main review results.
Assessment of risk of bias in included studies
Two review authors or researchers (CS and SR, EM, Zhenmi Liu, Gill Norman, or Melanie Stephens) independently assessed risk of bias of each included study using the Cochrane 'Risk of bias' tool (see Appendix 3). This tool has seven specific domains: sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete data (attrition bias), selective outcome reporting (reporting bias), and other issues (Higgins 2017). We assessed performance bias, detection bias and attrition bias separately for each of the review outcomes (Higgins 2017). We noted that it is often impossible to blind participants and personnel in device trials. In this case, performance bias may be introduced if knowledge of treatment allocation results in deviations from intended interventions, differential use of co‐interventions or care between groups not specified in the study protocol that may influence outcomes. We attempted to understand if, and how, included studies compensated for challenges in blinding; for example, implementing strict protocols to maximise consistency of co‐interventions between groups to reduce the risk of performance bias. We also noted that pressure ulcer incidence is a subjective outcome. Compared with blinded assessment, non‐blinded assessment of subjective outcomes tends to be associated with more optimistic effect estimates of experimental interventions in RCTs (Hróbjartsson 2012). Therefore, we judged non‐blinded outcome assessment as being at high risk of detection bias. In this review, we included the issues of differential diagnostic activity and unit of analysis under the domain of 'other issues'. For example, unit of analysis issues occurred where a cluster‐randomised trial had been undertaken but analysed at the individual level in the study report.
For the studies included in McInnes 2015, one review author (CS) checked the 'Risk of bias' judgements and, where necessary, updated them. A second review author or researcher (SR, EM, Zhenmi Liu, Gill Norman, or Melanie Stephens) checked any updated judgement. We assigned each 'Risk of bias' domain a judgement of high, low, or unclear risk of bias. We resolved any discrepancy through discussion and by involving another review author where necessary. Where possible, useful and feasible, when a lack of reported information resulted in a judgement of unclear risk of bias, we planned to contact study authors for clarification.
We present our assessment of risk of bias for the proportion of participants developing a new pressure ulcer outcome using two 'Risk of bias' summary figures: one is a summary of bias for each item across all studies, and the second shows a cross‐tabulation of each study by all of the 'Risk of bias' items.
Once we had given our judgements for all 'Risk of bias' domains, we judged the overall risk of bias for each outcome across studies as:
low risk of bias, if we judged all domains to be at low risk of bias;
unclear risk of bias, if we judged one or more domains to be at unclear risk of bias and other domains were at low risk of bias but no domain was at high risk of bias; or
high risk of bias, as long as we judged one or more domains as being at high risk of bias, or all domains had unclear 'Risk of bias' judgements, as this could substantially reduce confidence in the result.
We resolved any discrepancy between two review authors through discussion and by involving another review author where necessary. For studies using cluster randomisation, we planned to consider the risk of bias in relation to recruitment bias, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually randomised studies (Eldridge 2019; Higgins 2019) (Appendix 3). However, we did not include any studies with a cluster design.
Measures of treatment effect
For meta‐analysis of pressure ulcer incidence data, we present the risk ratio (RR) with its 95% confidence interval (CI). For continuous outcome data, we present the mean difference (MD) with 95% CIs for studies that use the same assessment scale. If studies reporting continuous data used different assessment scales, we planned to report the standardised mean difference (SMD) with 95% CIs. However, this was not undertaken in the review.
For time‐to‐event data (time to pressure ulcer incidence), we present the hazard ratio (HR) with its 95% CI. If included studies reporting time‐to‐event data did not report an HR, when feasible, we estimated this using other reported outcomes (such as numbers of events) through employing available statistical methods (Parmar 1998; Tierney 2007).
Unit of analysis issues
We noted whether studies presented outcomes at the level of cluster (e.g. ward, research site) or at the level of participants. We also recorded whether the same participant was reported as having multiple pressure ulcers.
Unit of analysis issues may occur if studies randomise at the cluster level but the incidence of pressure ulcers is observed and data are presented and analysed at the level of participants (clustered data). We noted whether data regarding participants within a cluster were (incorrectly) treated as independent within a study, or were analysed using within‐cluster analysis methods. If clustered data were incorrectly analysed, we recorded this as part of the 'Risk of bias' assessment.
If a cluster‐RCT was not correctly analysed, we planned to use the following information to adjust for clustering ourselves, where possible, in accordance with guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
The number of clusters randomly assigned to each intervention, or the average (mean) number of participants per cluster.
Outcome data, ignoring the cluster design for the total number of participants.
Estimate of the intra‐cluster (or intra‐class) correlation coefficient (ICC).
Cross‐over trials
For cross‐over trials, we only considered outcome data at the first intervention phase (i.e. prior to cross‐over) as eligible.
Studies with multiple treatment groups
If a study had more than two eligible study groups, where appropriate, we combined results across these arms to make single pair‐wise comparisons (Higgins 2019).
Dealing with missing data
Data are commonly missing from study reports. Reasons for missing data could be the exclusion of participants after randomisation, withdrawal of participants from a study, or loss to follow‐up. The exclusion of these data from analysis may break the randomisation and potentially introduces bias.
Where there were missing data, and where relevant, we contacted study authors to pose specific queries about these data. In the absence of other information, for pressure ulcer incidence, we assumed that participants with missing data did not develop new pressure ulcers for the main analysis (i.e. we added missing data to the denominator but not the numerator). We examined the impact of this assumption through undertaking a sensitivity analysis (see Sensitivity analysis). When a study did not specify the number of randomised participants prior to dropout, we used the available number of participants as the number randomised.
Assessment of heterogeneity
Assessing heterogeneity can be a complex, multifaceted process. Firstly, we considered clinical and methodological heterogeneity; that is, the extent to which the included studies varied in terms of participant, intervention, outcome and other characteristics including duration of follow‐up, clinical settings and overall study‐level 'Risk of bias' judgement (Deeks 2019). In terms of the duration of follow‐up, in order to assess the relevant heterogeneity, we recorded and categorised assessment of outcome measures as follows:
up to eight weeks (short‐term);
more than eight weeks to 16 weeks (medium‐term); and
more than 16 weeks (long‐term).
We supplemented this assessment of clinical and methodological heterogeneity with information regarding statistical heterogeneity assessed using the Chi2 test. We considered a P value of less than 0.10 to indicate statistically significant heterogeneity given that the Chi2 test has low power, particularly in the case where studies included in a meta‐analysis have small sample size. We carried out this statistical assessment in conjunction with the I2 statistic (Higgins 2003), and the use of prediction intervals for random‐effects meta‐analyses (Borenstein 2017; Riley 2011).
The I2 statistic is the percentage of total variation across studies due to heterogeneity rather than chance (Higgins 2003). Very broadly, we considered that I2 values of 25% or less may indicate a low level of heterogeneity and values of 75% or more may indicate very high heterogeneity (Higgins 2003). For random‐effects models where the meta‐analysis had more than 10 included studies and no clear funnel plot asymmetry, we also planned to present 95% prediction intervals (Deeks 2019). We planned to calculate prediction intervals following methods proposed by Borenstein 2017.
Random‐effects analyses produce an average treatment effect, with 95% confidence intervals indicating where the true population average value is likely to lie. Prediction intervals quantify variation away from this average due to between‐study heterogeneity. The interval conveys where a future study treatment effect estimate is likely to fall based on the data analysed to date (Riley 2011). Prediction intervals are always wider than confidence intervals (Riley 2011).
It is important to note that prediction intervals reflect heterogeneity of any source, including from methodological issues as well as clinical variation. For this reason some authors have suggested that prediction intervals are best calculated for studies at low risk of bias to ensure intervals that have meaningful clinical interpretation (Riley 2011). We had planned to calculate prediction intervals for all analyses to assess heterogeneity and then to explore the impact of risk of bias in subgroup analysis stratified by study risk of bias assessment as detailed below. However, we did not calculate any prediction intervals because all conducted meta‐analyses contained fewer than 10 studies.
Assessment of reporting biases
We followed the systematic framework recommended by Page 2019 to assess risk of bias due to missing results (non‐reporting bias) in the meta‐analysis of pressure ulcer incidence data. To make an overall judgement about risk of bias due to missing results, we did the following.
Identified whether pressure ulcer incidence data were unavailable by comparing the details of outcomes in trials registers, protocols or statistical analysis plans (if available) with reported results. If the above information sources were unavailable, we compared outcomes in the conference abstracts or in the methods section of the publication, or both, with the reported results. If we found non‐reporting of study results, we then judged whether the non‐reporting was associated with the nature of findings by using the 'Outcome Reporting Bias In Trials' (ORBIT) system (Kirkham 2018).
Assessed the influence of definitely missing pressure ulcer incidence data on meta‐analysis.
Assessed the likelihood of bias where a study had been conducted but not reported in any form. For this assessment, we considered whether the literature search was comprehensive and planned to produce a funnel plot for meta‐analysis for seeking more evidence about the extent of missing results, provided there were at least 10 included studies (Peters 2008; Salanti 2014).
However, we did not produce a funnel plot for any meta‐analysis because all analyses in this review had fewer than 10 included studies.
Data synthesis
We summarised the included studies narratively and synthesised included data by using meta‐analysis where applicable. We structured comparisons according to type of comparator and then by outcomes, ordered by follow‐up period.
We considered clinical and methodological heterogeneity and undertook pooling when studies appeared appropriately similar in terms of participants, support surfaces and outcome type. Where statistical synthesis of data from more than one study was not possible or considered inappropriate, we conducted a narrative review of eligible studies.
Once the decision to pool was made, we used a random‐effects model, which estimated an underlying average treatment effect from studies. Conducting meta‐analysis with a fixed‐effect model in the presence of even minor heterogeneity may provide overly narrow confidence intervals. We used the Chi2 test and I2 statistic to quantify heterogeneity but not to guide choice of model for meta‐analysis (Borenstein 2009). We exercised caution when meta‐analysed data were at risk of small‐study effects because use of a random‐effects model may be unsuitable in this situation. In this case, or where there were other reasons to question the choice of a fixed‐effect or random‐effects model, we assessed the impact of the approach using sensitivity analyses to compare results from alternate models (Thompson 1999).
We performed meta‐analyses largely using Review Manager 5.4 (Review Manager 2020). We presented data using forest plots where possible. For dichotomous outcomes, we presented the summary estimate as a RR with 95% CIs. Where continuous outcomes were measured, we presented the MD with 95% CIs. We planned to report SMD estimates where studies measured the same outcome using different methods. For time‐to‐event data, we presented the summary estimates as HRs with 95% CIs.
Subgroup analysis and investigation of heterogeneity
Investigation of heterogeneity
When important heterogeneity occurred, we planned to follow steps proposed by Cipriani 2013 and Deeks 2019 to investigate further:
check the data extraction and data entry for errors and possible outlying studies;
if outliers existed, perform sensitivity analysis by removing them; and
if heterogeneity was still present, we planned to perform subgroup analyses for study‐level characteristics (see below) in order to explain heterogeneity as far as possible. However, we did not undertake any subgroup analysis because meta‐analyses in this review included fewer than 10 studies.
Subgroup analysis
We investigated heterogeneity using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019). We planned to perform subgroup analyses for binary and categorical factors (or meta‐regression for continuous factors) to determine whether the size of treatment effects was influenced by these four study‐level characteristics:
risk of bias (binary: low or unclear risk of bias; and high risk of bias (Schulz 1995));
settings (categorical: acute care and other hospital settings; long‐term care settings; operating theatre setting; and intensive care unit);
baseline skin status (categorical: participants at risk, of mixed skin status or non‐reporting; non‐blanchable erythema; existing ulcers of Stage 2 or serious (Shi 2018c)); and
follow‐up duration (continuous).
We planned to compare subgroup findings using the 'Test for Subgroup Differences’ in Review Manager 5.4 (Review Manager 2020). We did not perform subgroup analysis/meta‐regression when the number of studies included in the meta‐analysis was not reasonable (i.e. fewer than 10).
Sensitivity analysis
We conducted sensitivity analyses for the following factors, to assess the robustness of meta‐analysis of data on pressure ulcer incidence.
Impact of the selection of pressure ulcer incidence outcome measure. The proportion of participants developing a new pressure ulcer was the primary outcome measure for this review but we also analysed time to pressure ulcer incidence, where data were available.
Impact of missing data. The primary analysis assumed that participants with missing data did not develop new pressure ulcers. We also analysed pressure ulcer incidence by only including data for the participants for whom we had endpoint data (complete cases). We noted that when a study only had complete case data (i.e. missing data or the numbers of participants randomised were not reported), complete case data were considered in the related main analysis (Differences between protocol and review).
Impact of using a fixed‐effect model instead of a random‐effects model.
Summary of findings and assessment of the certainty of the evidence
We presented the main, pooled results of the review in 'Summary of findings' tables, which we created using GRADEpro GDT software. These tables present key information concerning the certainty of evidence, the magnitude of the effects of the interventions examined and the sum of available data for the main outcomes (Schünemann 2019). The tables also include an overall grading of the certainty of the evidence associated with each of the main outcomes that we assessed using the GRADE approach. The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest.
The GRADE assessment involves consideration of five factors: within‐trial risk of bias, directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias (Schünemann 2019). The certainty of evidence can be assessed as being high, moderate, low or very low; RCT evidence has the potential to be high‐certainty. We did not downgrade the certainty of evidence for the risk of bias factor in a specific circumstance. That is, if the blinding of participants and personnel was the only domain resulting in our judgement of overall high risk of bias for the included studies; however for these studies it was impossible to blind participants and personnel.
When downgrading for imprecision, we followed the methods described in Guyatt 2011: either considering both the optimal information size (OIS) and the 95% CI of each meta‐analysis if they were estimable; or considering the sample size, the number of events and other effectiveness indicators if the calculation of OIS and undertaking a meta‐analysis were not applicable. Where necessary, we used the GRADE 'default' minimum important difference values (e.g. RR = 1.25 and 0.75) as the thresholds to judge if a 95% CI was wide (imprecise) so as to include the possibility of clinically important harm and benefit (Guyatt 2011).
We presented a separate 'Summary of findings' table for all key comparisons evaluated in this review. Six comparisons had no analysis and we did not present 'Summary of findings' tables for these. These comparisons were: reactive water surfaces versus foam surfaces, reactive water surfaces versus reactive fibre surfaces, reactive gel surfaces versus reactive gel surfaces, reactive gel surfaces versus foam surfaces, reactive foam and gel surfaces versus reactive gel surfaces, and reactive foam and gel surfaces versus foam surfaces (Differences between protocol and review). We presented these outcomes in the 'Summary of findings' tables:
proportion of participants developing a new pressure ulcer;
time to pressure ulcer incidence;
support‐surface‐associated patient comfort;
all reported adverse events;
health‐related quality of life; and
cost‐effectiveness.
We prioritised the time points and method of outcome measurement specified in Types of outcome measures for presentation in ‘Summary of findings’ tables. Where we did not pool data for some outcomes within a comparison, we conducted a GRADE assessment for each of these outcomes and presented these assessments in a narrative format in 'Summary of findings' tables (Differences between protocol and review).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The electronic searches identified 1624 records, including 1164 from electronic databases and 460 from trials registries. We excluded 218 duplicate records and screened 1412 records, of which 234 were identified as potentially eligible and obtained as full‐text. Following full‐text screening, we considered 34 records of 20 studies eligible for inclusion in this review (Andersen 1982; Aronovitch 1999; Bliss 1995a; Cassino 2013a; Conine 1990; Daechsel 1985; Ewing 1964; Hoshowsky 1994; IRCT2015110619919N3; Jolley 2004; Lazzara 1991; McGowan 2000; Mistiaen 2010; Nixon 1998; Ricci 2013; Russell 2000; Sideranko 1992; Stapleton 1986; Van Leen 2018; Vermette 2012).
We identified no additional studies from other resources. Of the 20 studies, IRCT2015110619919N3 was a trials registry record. See Figure 1.
1.
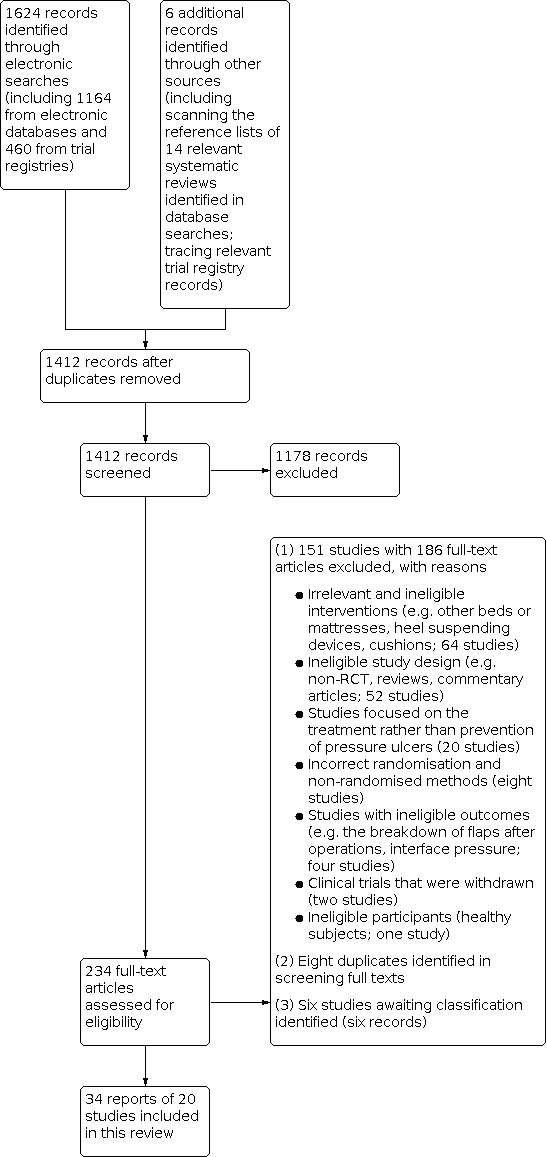
Study flow diagram
Included studies
Types of studies
Of the 20 included RCTs, 18 had a parallel group design: 15 with two arms, and three with three arms. Two studies had particular design features:
Bliss 1995a appeared to be a multi‐arm, multi‐stage trial design with eight arms, of which seven were randomised and eligible for this review;
Hoshowsky 1994 was a split body design (that is, it randomly allocated different support surfaces to either the right or left half of the body of the same person) and three of its six arms included foam surfaces.
Six of 20 studies were conducted at more than one research site (Cassino 2013a; McGowan 2000; Mistiaen 2010; Nixon 1998; Ricci 2013; Van Leen 2018). Except for one study conducted in Iran (IRCT2015110619919N3), and three in Australia (Ewing 1964; Jolley 2004; McGowan 2000), all of the included studies were conducted in high‐income and upper‐middle‐income economies in Europe and North America, including: Canada (Conine 1990; Daechsel 1985; Russell 2000; Vermette 2012), Denmark (Andersen 1982), Italy (Cassino 2013a; Ricci 2013), the Netherlands (Mistiaen 2010; Van Leen 2018), the UK (Bliss 1995a; Nixon 1998; Stapleton 1986), and the USA (Aronovitch 1999; Hoshowsky 1994; Lazzara 1991; Sideranko 1992).
In the 16 studies that clearly stated duration of follow‐up, the median was four weeks (range: seven days to six months).
Types of participants
Age and sex at baseline
Of the 20 studies, 19 enrolled a total of 4653 participants (median study sample size: 198 participants; range: 32.0 to 588.0) whilst one (IRCT2015110619919N3) did not specify the number of participants. The average participant age was specified for 17 studies and ranged between 37.2 and 85.4 years (median: 72.5 years). The sex of participants was specified for 17 studies; and within these, 1708 (43.0%) of participants were male and 2262 (57.0%) were female.
Skin status at baseline
Of the 20 studies, 16 (4040 participants) recruited people at risk of having a new ulcer with risk assessed largely using the Waterlow, Norton or Braden scales. In 13 of these studies, 3087 (76.4%) participants were free of pressure ulcers at baseline. In three studies, 953 (23.6%) participants with superficial ulcers were enrolled (Bliss 1995a; Nixon 1998; Ricci 2013). In one study (Cassino 2013a), people with severe full‐thickness pressure ulcers were enrolled. Three studies did not specify participants' skin status at baseline (Ewing 1964; Hoshowsky 1994; IRCT2015110619919N3).
Care settings
Participants were from a variety of settings, including:
acute care settings (including accident and emergency departments and hospitals in general; Andersen 1982; Aronovitch 1999; Bliss 1995a; Ewing 1964; Hoshowsky 1994; Jolley 2004; McGowan 2000; Russell 2000; Stapleton 1986; Vermette 2012);
intensive care units (Sideranko 1992);
operating rooms (IRCT2015110619919N3; Nixon 1998); and
long‐term care settings (including nursing homes, extended care facilities and long‐term care hospitals; Cassino 2013a; Conine 1990; Daechsel 1985; Lazzara 1991; Mistiaen 2010; Ricci 2013; Van Leen 2018).
Types of interventions
The 20 included studies investigated a wide range of non‐air and non‐foam‐filled surfaces, including:
reactive water surfaces (Andersen 1982; Bliss 1995a; Sideranko 1992);
reactive fibre surfaces (Bliss 1995a; Conine 1990; Daechsel 1985; Stapleton 1986);
reactive gel surfaces (Aronovitch 1999; Cassino 2013a; Hoshowsky 1994; IRCT2015110619919N3; Lazzara 1991; Nixon 1998; Ricci 2013; Russell 2000);
reactive foam and gel surfaces (Hoshowsky 1994);
reactive sheepskin surfaces (Ewing 1964; Jolley 2004; McGowan 2000; Mistiaen 2010); and
two types of non‐air and non‐foam‐filled surfaces that we could not define using NPIAP S3I support surfaces terms and definitions: the Bedcare (Sense Textile, 's‐Hertogenbosch) multilayer mattress system used in Van Leen 2018 and the RIK® microfluid static overlays used in Vermette 2012.
In terms of control surfaces, we could classify the surfaces used in 11 of the 20 studies using the NPIAP S3I support surfaces terms and definitions. The following control surfaces in the remaining nine studies could not be classified further: the Aiartex® overlays evaluated in two studies (122 participants; Cassino 2013a; Ricci 2013) and 'standard hospital surfaces' evaluated in seven studies (2386 participants; Andersen 1982; Ewing 1964; IRCT2015110619919N3; Jolley 2004; McGowan 2000; Mistiaen 2010; Nixon 1998). We used the term 'standard hospital surfaces' to cover 'usual care', 'standard mattress', 'standard operating table mattress', and 'any other pressure‐relieving devices' which were the terms used by the authors of these seven studies.
Full details of these interventions and comparators are listed in Effects of interventions below.
Nine studies specified co‐interventions they applied (e.g. repositioning, cushions). All but two of these stated or indicated that the same co‐interventions were applied in all study groups. The two exceptions applied heel protectors or usual care in participants allocated to experimental arms but this was not specified in the control arms (McGowan 2000; Mistiaen 2010). We assumed such co‐interventions were also applied for control participants.
Funding sources
Of the 20 studies, 16 specified the details of funding sources. Ten studies were completely or partly funded by industry or received the mattresses under evaluation from industries (Aronovitch 1999; Bliss 1995a; Cassino 2013a; Daechsel 1985; Jolley 2004; Lazzara 1991; McGowan 2000; Ricci 2013; Russell 2000; Van Leen 2018). Vermette 2012 noted no funding support. Public or charity funding supported the four remaining studies (Conine 1990; Mistiaen 2010; Nixon 1998; Stapleton 1986).
Excluded studies
We excluded 151 studies (with 186 records). The main reasons for exclusion were: irrelevant and ineligible interventions (64 studies); ineligible study design (e.g. non‐RCT, reviews, commentary articles; 52 studies); studies focused on the treatment rather than prevention of pressure ulcers (20 studies); non‐randomised methods (eight studies); studies with ineligible outcomes (four studies); clinical trials that were withdrawn (two studies; NCT02634892; NCT02735135); and ineligible participants (healthy subjects; one study). We also identified eight duplicates in screening the full‐texts (see Figure 1).
Ongoing studies
We did not identify any ongoing studies.
Studies awaiting classification
We were unable to make eligibility decisions for six studies (six records). We were unable to determine whether Gardner 2008 measured one or more outcomes relevant to this review. We could not obtain the full‐text of five studies ‐ in part due to more limited access to intra‐library loans during the COVID‐19 period ‐ despite extensive efforts made (Chaloner 2000; Henn 2004; Knight 1999; Mastrangelo 2010a; Melland 1998).
Risk of bias in included studies
We summarise 'Risk of bias' assessments for the primary outcome of this review in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
We judged four of the 20 studies as having unclear overall risk of bias for the primary outcome (Lazzara 1991; Nixon 1998; Ricci 2013; Sideranko 1992). We judged all the remaining 16 studies as having findings at high overall risk of bias, of which three had unclear risk of bias judgements for all domains (Ewing 1964; IRCT2015110619919N3; Stapleton 1986), and 13 had one or more domains with high risk of bias judgement (Andersen 1982; Aronovitch 1999; Bliss 1995a; Cassino 2013a; Conine 1990; Daechsel 1985; Hoshowsky 1994; Jolley 2004; McGowan 2000; Mistiaen 2010; Russell 2000; Van Leen 2018; Vermette 2012).
Of these 16 studies, nine had a high risk of bias judgement for the primary outcome in the domains of blinding of participants and personnel, blinding of outcome assessment, or both (Andersen 1982; Cassino 2013a; Daechsel 1985; Hoshowsky 1994; Jolley 2004; McGowan 2000; Mistiaen 2010; Russell 2000; Vermette 2012).
Publication bias
We ran a comprehensive search and consider the risk of having missed published reports to be low. We were able to locate one trial registry report (IRCT2015110619919N3). We were unable to assess for the risk of non‐publication of studies with negative findings as we could not present funnel plots given the small number of included studies in each analysis.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings 1. Reactive water surfaces compared with alternating pressure (active) air surfaces for preventing pressure ulcers.
| Reactive water surfaces compared with alternating pressure (active) air surfaces for preventing pressure ulcers | ||||||
| Patient or population: preventing pressure ulcers Setting: acute care setting and intensive care unit Intervention: reactive water surfaces Comparison: alternating pressure (active) air surfaces | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with alternating pressure (active) air surfaces | Risk with reactive water surfaces | |||||
| Proportion of participants developing a new pressure ulcer Follow‐up: median 10 days | Study population | RR 0.83 (0.35 to 1.93) | 358 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is any difference between reactive water surfaces and alternating pressure (active) air surfaces in the proportion of participants developing a new pressure ulcer. | |
| 65 per 1,000 | 54 per 1,000 (23 to 125) | |||||
| Time to pressure ulcer incidence | Included studies did not report this outcome. | |||||
| Support surface‐associated patient comfort | Included studies did not report this outcome. | |||||
| All reported adverse events | Included studies did not report this outcome. | |||||
| Health‐related quality of life | Included studies did not report this outcome. | |||||
| Cost effectiveness | Included studies did not report this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice for high risk of detection bias in 1 study contributing over 60% weight in the meta‐analysis and unclear overall risk of bias in another study. bDowngraded twice for substantial imprecision as the optimal information size (OIS) was not met and the very wide confidence interval crossed RR = 0.75 and 1.25.
Summary of findings 2. Reactive water surfaces compared with reactive air surfaces for preventing pressure ulcers.
| Reactive water surfaces compared with reactive air surfaces for preventing pressure ulcers | ||||||
| Patient or population: preventing pressure ulcers Setting: intensive care unit Intervention: reactive water surfaces Comparison: reactive air surfaces | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with reactive air surfaces | Risk with reactive water surfaces | |||||
| Proportion of participants developing a new pressure ulcer Follow‐up: 9.5 days | Study population | RR 2.35 (0.23 to 23.75) | 37 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is a difference in the proportion of participants developing a new ulcer between reactive water surfaces and reactive air surfaces. | |
| 50 per 1,000 | 118 per 1,000 (12 to 1,000) | |||||
| Time to pressure ulcer incidence | The included study did not report this outcome. | |||||
| Support surface‐associated patient comfort | The included study did not report this outcome. | |||||
| All reported adverse events | The included study did not report this outcome. | |||||
| Health‐related quality of life | The included study did not report this outcome. | |||||
| Cost effectiveness | The included study did not report this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded once for unclear overall risk of bias. bDowngraded twice for substantial imprecision because the OIS was not met and the very wide confidence interval crossed RRs = 0.75 and 1.25.
Summary of findings 3. Reactive fibre surfaces compared with alternating pressure (active) air surfaces for preventing pressure ulcers.
| Reactive fibre surfaces compared with alternating pressure (active) air surfaces for preventing pressure ulcers | ||||||
| Patient or population: preventing pressure ulcers Setting: acute care and long‐term care settings Intervention: reactive fibre surfaces Comparison: alternating pressure (active) air surfaces | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with alternating pressure (active) air surfaces | Risk with reactive fibre surfaces | |||||
| Proportion of participants developing a new pressure ulcer Follow‐up: range 17.7 days to 3 months | Study population | RR 1.11 (0.84 to 1.47) | 285 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain whether there is a difference in the proportion of participants developing a new pressure ulcer between reactive fibre surfaces and alternating pressure (active) air surfaces. | |
| 383 per 1,000 | 425 per 1,000 (322 to 563) | |||||
| Time to pressure ulcer incidence | The included studies did not report this outcome. | |||||
| Support surface associated patient comfort Follow‐up: 3 months | Conine 1990 reported 19 dropouts among 93 people using alternating pressure (active) air surfaces; and 17 of 94 using reactive fibre surfaces; the reason for dropout was given as discomfort. | ‐ | 187 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d,e | It is uncertain if there is any difference between reactive fibre surfaces and alternating pressure (active) air surfaces in support surface associated patient comfort. | |
| All reported adverse events | The included studies did not report this outcome. | |||||
| Health‐related quality of life | The included studies did not report this outcome. | |||||
| Cost effectiveness | The included studies did not report this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice for high risk of bias in domains other than performance bias in 2 studies contributing over 80% weight to the meta‐analysis. bDowngraded once for imprecision. cDowngraded once for unclear overall risk of bias for this outcome. dDowngraded once for indirectness. eDowngraded once for imprecision.
Summary of findings 4. Reactive fibre surfaces compared with foam surfaces for preventing pressure ulcers.
| Reactive fibre surfaces compared with foam surfaces for preventing pressure ulcers | ||||||
| Patient or population: preventing pressure ulcers Setting: acute care setting Intervention: reactive fibre surfaces Comparison: foam surfaces | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with foam surfaces | Risk with reactive fibre surfaces | |||||
| Proportion of participants developing a new pressure ulcer Follow‐up: unspecified | Study population | RR 0.86 (0.47 to 1.57) | 68 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between reactive fibre surfaces and foam surfaces. | |
| 412 per 1,000 | 354 per 1,000 (194 to 647) | |||||
| Time to pressure ulcer incidence | The included study did not report this outcome. | |||||
| Support surface‐associated patient comfort | The included study did not report this outcome. | |||||
| All reported adverse events | The included study did not report this outcome. | |||||
| Health‐related quality of life | The included study did not report this outcome. | |||||
| Cost effectiveness | The included study did not report this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice for unclear risk of bias in all domains. bDowngraded twice for imprecision as the OIS was not met and the wide confidence interval crossed RRs = 0.75 and 1.25.
Summary of findings 5. Reactive gel surfaces on operating tables followed by foam surfaces on ward beds compared with alternating pressure (active) air surfaces on operating tables and subsequently on ward beds for preventing pressure ulcers.
| Reactive gel surfaces on operating tables followed by foam surfaces on ward beds compared with alternating pressure (active) air surfaces on operating tables and subsequently on ward beds for preventing pressure ulcers | ||||||
| Patient or population: preventing pressure ulcers Setting: operating room Intervention: reactive gel surfaces used on operation tables followed by foam surfaces applied on ward beds Comparison: alternating pressure (active) air surfaces | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with alternating pressure (active) air surfaces | Risk with reactive gel surfaces used on operation tables followed by foam surfaces applied on ward beds | |||||
| Proportion of participants developing a new pressure ulcer Follow‐up: 7 days | Study population | RR 4.53 (1.31 to 15.65) | 415 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | Reactive gel surfaces used on operating tables followed by foam surfaces applied on hospital beds may increase the proportion of people developing a new pressure ulcer compared with alternating pressure (active) air surfaces applied on both operating tables and hospital beds. | |
| 14 per 1,000 | 65 per 1,000 (19 to 224) | |||||
| Time to pressure ulcer incidence | The included studies did not report this outcome. | |||||
| Support surface‐associated patient comfort | The included studies did not report this outcome. | |||||
| All reported adverse events Follow‐up: 7 days | Russell 2000 (198 participants) reported that approximately ½ of people in each group reported adverse events, with no difference between groups reported. No adverse events were related to the mattresses assigned. | ‐ | 198 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d | It is uncertain if there is a difference between the use of reactive gel surfaces followed by foam surfaces and alternating pressure (active) air surfaces in adverse events. | |
| Health‐related quality of life | The included studies did not report this outcome. | |||||
| Cost effectiveness | The included studies did not report this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded once for risk of bias (1 study contributing 36% of weight to the meta‐analysis was at high risk of attrition bias whilst the other study was at unclear risk of bias for more than 1 domain other than performance bias). bDowngraded once for imprecision as, despite the fact that the OIS was met, the confidence interval was very wide (imprecise). cDowngraded once for risk of bias in more than 1 domain other than performance bias. dDowngraded twice for imprecision due to small sample size.
Summary of findings 6. Reactive gel surfaces compared with reactive air surfaces for preventing pressure ulcers.
| Reactive gel surfaces compared with reactive air surfaces for preventing pressure ulcers | ||||||
| Patient or population: preventing pressure ulcers Setting: nursing home Intervention: reactive gel surfaces Comparison: reactive air surfaces | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with reactive air surfaces | Risk with reactive gel surfaces | |||||
| Proportion of participants developing a new pressure ulcer Follow‐up: 6 months | Study population | RR 0.80 (0.36 to 1.77) | 66 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is a difference in the proportion of participants developing a new ulcer between reactive gel surfaces and reactive air surfaces. | |
| 303 per 1,000 | 242 per 1,000 (109 to 536) | |||||
| Time to pressure ulcer incidence | The included study did not report this outcome. | |||||
| Support surface‐associated patient comfort | The included study did not report this outcome. | |||||
| All reported adverse events | The included study did not report this outcome. | |||||
| Health‐related quality of life | The included study did not report this outcome. | |||||
| Cost effectiveness | The included study did not report this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded once for unclear overall risk of bias. bDowngraded twice for imprecision because the OIS was not met and the very wide confidence interval crossed RRs = 0.75 and 1.25.
See Table 1; Table 2; Table 3; Table 4; Table 5; Table 6.
Unless otherwise stated, random‐effects analysis was used throughout. Each pooled result presented is an average effect, rather than a common effect and should be interpreted as such.
We did not pool data involving undefined non‐foam and non‐air‐filled surfaces or undefined control surfaces in the main body of the results (10 studies noted above). For completeness, we summarise the results of these studies in Appendix 4.
We performed data analyses for the following comparisons and outcomes. Where applicable, we performed pre‐specified sensitivity analyses as noted in Sensitivity analysis.
Comparison 1: Reactive water surfaces versus alternating pressure (active) air surfaces (three studies, 414 participants)
Three studies compared reactive water surfaces with alternating pressure (active) air surfaces (Andersen 1982; Bliss 1995a; Sideranko 1992). Bliss 1995a (56 participants) reported the outcome of the numbers of treatment sessions in which pressure ulcers developed or worsened, which we considered not directly relevant to this review.
Primary outcomes
Proportion of participants developing a new pressure ulcer (median follow‐up duration 10.0 days, minimum 10.0 days, maximum 17.7 days)
We pooled available data from two studies (358 participants; Andersen 1982; Sideranko 1992). It is uncertain whether there is a difference in the proportion of participants developing a new pressure ulcer between reactive water surfaces (9/172 (5.2%)) and alternating pressure (active) air surfaces (12/186 (6.5%)). The RR is 0.83 (95% CI 0.35 to 1.93; I2 = 0%; Analysis 1.1). Evidence is of very low certainty, downgraded twice for high risk of detection bias in one study contributing over 60% weight in the meta‐analysis and unclear overall risk of bias in another study, and twice for substantial imprecision as the optimal information size (OIS) was not met and the very wide confidence interval crossed RR = 0.75 and 1.25, which includes the possibility of both harm and benefit as well as no effect.
1.1. Analysis.

Comparison 1: Reactive water surfaces compared with alternating pressure (active) air surfaces, Outcome 1: Proportion of participants developing a new pressure ulcer
The included studies did not report data on time to pressure ulcer incidence.
Subgroup analysis
We considered the studies heterogeneous in terms of care setting, and overall risk of bias. However, we did not perform any pre‐specified subgroup analysis because, as noted in Subgroup analysis and investigation of heterogeneity, the number of included studies was fewer than 10, meaning it would be difficult to meaningfully interpret the results.
Sensitivity analyses
Sensitivity analysis with fixed‐effect (rather than random‐effects) model . The use of a fixed‐effect model resulted in a RR of 0.83 (95% CI 0.36 to 1.90; I2 = 0%). The result remained consistent with the main analysis (Appendix 5).
Secondary outcomes
None reported.
Comparison 2: Reactive water surfaces versus foam surfaces (one study, 117 participants)
Bliss 1995a compared reactive water surfaces with foam surfaces but reported no outcomes directly relevant to this review and so none of the data were analysable.
Comparison 3: Reactive water surfaces versus reactive air surfaces (one study, 37 participants)
Sideranko 1992 compared reactive water surfaces with reactive air surfaces.
Primary outcomes
Proportion of participants developing a new pressure ulcer (follow‐up duration 9.5 days)
Sideranko 1992 (37 participants) reported this outcome. It is uncertain if there is a difference in the proportion of participants developing a new ulcer between reactive water surfaces (2/17 (11.8%)) and reactive air surfaces (1/20 (5%)). The RR is 2.35 (95% CI 0.23 to 23.75; Analysis 2.1). Evidence is of very low certainty, downgraded once for unclear overall risk of bias and twice for substantial imprecision because the OIS was not met and the very wide confidence interval crossed RRs = 0.75 and 1.25, which failed to exclude important benefits or harms as well as no effect.
2.1. Analysis.

Comparison 2: Reactive water surfaces compared with reactive air surfaces, Outcome 1: Proportion of participants developing a new pressure ulcer
The included study did not report data on time to pressure ulcer incidence.
Secondary outcomes
None reported.
Comparison 4: Reactive water surfaces versus reactive fibre surfaces (one study, 87 participants)
Bliss 1995a compared reactive water surfaces with reactive fibre surfaces but reported no outcomes directly relevant to this review and so none of the data were analysable.
Comparison 5: Reactive fibre surfaces versus alternating pressure (active) air surfaces (four studies, 384 participants)
Four studies made this comparison (Bliss 1995a; Conine 1990; Daechsel 1985; Stapleton 1986), of which Bliss 1995a randomised participants into two types of fibre surfaces (in two individual study arms) that we combined into a single study arm. Bliss 1995a reported the outcome of the numbers of treatment sessions in which pressure ulcers developed or worsened, which we considered not directly relevant to this review.
Primary outcomes
Proportion of participants developing a new pressure ulcer (minimum follow‐up duration 17.7 days, maximum three months or unspecified)
We pooled the data from three studies (285 participants) for this outcome (Conine 1990; Daechsel 1985; Stapleton 1986). It is uncertain whether there is a difference in the proportion of participants developing a new pressure ulcer between reactive fibre surfaces (61/144 (42.4%)) and alternating pressure (active) air surfaces (54/141 (38.3%)). The RR is 1.11 (95% CI 0.84 to 1.47; I2 = 0%; Analysis 3.1). Evidence is very low certainty, downgraded twice for high risk of bias in domains other than performance bias in two studies contributing over 80% weight to the meta‐analysis, and once for imprecision as the 95% CI crossed RR = 1.25.
3.1. Analysis.

Comparison 3: Reactive fibre surfaces compared with alternating pressure (active) air surfaces, Outcome 1: Proportion of participants developing a new pressure ulcer
The included studies did not report data on time to pressure ulcer incidence.
Subgroup analysis
We considered these studies heterogeneous in terms of care settings, participants' average age and skin status at baseline. However, we did not perform any pre‐specified subgroup analysis because, as noted in Subgroup analysis and investigation of heterogeneity, the number of included studies was fewer than 10, meaning it would be difficult to meaningfully interpret the results.
Sensitivity analyses
Sensitivity analysis using complete case data . This resulted in a RR of 1.08 (95% CI 0.84 to 1.39; I2 = 0%). The result was consistent with the main analysis (Appendix 5).
Sensitivity analysis with fixed‐effect (rather than random‐effects) model . The use of a fixed‐effect model resulted in a RR of 1.11 (95% CI 0.84 to 1.47; I2 = 0%) and the result remained consistent with the main analysis (Appendix 5).
Secondary outcomes
Support‐surface‐associated patient comfort (follow‐up duration three months)
Only Conine 1990 (187 participants) reported this outcome. We are uncertain about any difference between reactive fibre surfaces and alternating pressure (active) air surfaces in patient comfort responses. Conine 1990 reported 17 dropouts among 94 people using reactive fibre surfaces and 19 of 93 using alternating pressure (active) air surfaces. The reason for dropout was given as discomfort. This was very low certainty evidence, downgraded once for unclear overall risk of bias for this outcome, once for indirectness as the reported outcome was indirectly relevant to this review, and once for imprecision.
All reported adverse events
Not reported.
Health‐related quality of life
Not reported.
Cost‐effectiveness
Not reported.
Comparison 6: Reactive fibre surfaces versus foam surfaces (two studies, 228 participants)
Bliss 1995a and Stapleton 1986 compared foam surfaces with reactive fibre surfaces, of which Bliss 1995a reported no outcomes directly relevant to this review and so none of the data were analysable.
Primary outcomes
Proportion of participants developing a new pressure ulcer (follow‐up duration unspecified)
Stapleton 1986 (68 participants) reported data for this outcome. It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between reactive fibre surfaces (12/34 (35.3%)) and foam surfaces (14/34 (41.2%)). The RR is 0.86 (95% CI 0.47 to 1.57; Analysis 4.1). The evidence is of very low certainty, downgraded twice for unclear risk of bias in all domains, and twice for imprecision as the OIS was not met and the wide confidence interval crossed RRs = 0.75 and 1.25.
4.1. Analysis.

Comparison 4: Reactive fibre surfaces compared with foam surfaces, Outcome 1: Proportion of participants developing a new pressure ulcer
The included study did not report data on time to pressure ulcer incidence.
Secondary outcomes
None reported.
Comparison 7: Reactive gel surfaces used on operating tables followed by foam surfaces on ward beds versus alternating pressure (active) air surfaces on operating tables and subsequently on ward beds (two studies, 415 participants)
Two studies (415 participants) were included in this comparison (Aronovitch 1999; Russell 2000).
Primary outcomes
Proportion of participants developing a new pressure ulcer (follow‐up duration of seven days)
Both studies (415 participants) reported this outcome (Aronovitch 1999; Russell 2000) and these data were pooled. Reactive gel surfaces used on operating tables followed by foam surfaces applied on hospital beds (14/205 (6.8%)) may increase the proportion of people developing a new pressure ulcer compared with alternating pressure (active) air surfaces applied on both operating tables and hospital beds (3/210 (1.4%)). However, the evidence is of low certainty. The RR is 4.53 (95% CI 1.31 to 15.65; I2 = 0%; Analysis 5.1). Evidence certainty was downgraded once for risk of bias (one study contributing 36% of weight to the meta‐analysis was at high risk of attrition bias whilst the other study was at unclear risk of bias for more than one domain other than performance bias) and once for imprecision as, despite the fact that the OIS was met, the confidence interval was very wide (imprecise).
5.1. Analysis.

Comparison 5: Reactive gel surfaces followed by foam surfaces compared with alternating pressure (active) air surfaces, Outcome 1: Proportion of participants developing a new pressure ulcer
The included studies did not report data on time to pressure ulcer incidence.
Subgroup analysis
We considered both studies similar in terms of care settings, follow‐up duration, overall risk of bias, participant characteristics and interventions: statistical heterogeneity was low (Chi2 test P value = 0.55; Tau2 = 0.00; I2 = 0%). Because the number of included studies was fewer than 10, we did not undertake a subgroup analysis.
Sensitivity analyses
Sensitivity analysis with fixed‐effect (rather than random‐effects) model . The use of a fixed‐effect model resulted in a RR of 4.74 (95% CI 1.39 to 16.16; I2 = 0%) and the result remained consistent with the main analysis (Appendix 5).
Secondary outcomes
Support‐surface‐associated patient comfort
None reported.
All reported adverse events (follow‐up duration seven days)
Only Russell 2000 (198 participants) reported this outcome. It is uncertain if there is a difference in adverse events between reactive gel surfaces followed by foam surfaces and alternating pressure (active) air surfaces. The study authors claimed that approximately one half of people in each group reported one or more types of adverse events, with no difference between groups reported. The study authors also noted that no adverse events were considered to be related to the mattresses assigned. Evidence is very low certainty, downgraded once for risk of bias in more than one domain other than performance bias, and twice for imprecision due to small sample size.
Health‐related quality of life
Not reported.
Cost‐effectiveness
Not reported.
Comparison 8: Reactive gel surfaces versus reactive air surfaces (one study, 74 participants)
Lazzara 1991 compared reactive gel surfaces with reactive air surfaces.
Primary outcomes
Proportion of participants developing a new pressure ulcer (follow‐up duration six months)
Lazzara 1991 (74 participants) reported this outcome and had analysable data for 66 participants. It is uncertain if there is a difference in the proportion of participants developing a new ulcer between reactive gel surfaces (8/33 (24.2%)) and reactive air surfaces (10/33 (30.3%)). The RR is 0.80 (95% CI 0.36 to 1.77; Analysis 6.1). Evidence is of very low certainty, downgraded once for unclear overall risk of bias and twice for imprecision because the OIS was not met and the very wide confidence interval crossed RRs = 0.75 and 1.25.
6.1. Analysis.

Comparison 6: Reactive gel surfaces compared with reactive air surfaces, Outcome 1: Proportion of participants developing a new pressure ulcer
The included study did not report data on time to pressure ulcer incidence.
Secondary outcomes
None reported.
Comparison 9: Reactive gel surfaces versus foam surfaces (one study, 135 participants)
Hoshowsky 1994 was a study with a split body design. Two of its six arms compared reactive gel surfaces on top of another type of surface. These were combined into a single study arm for this comparison and compared with the foam surfaces.
Primary outcomes
Proportion of participants developing a new pressure ulcer (follow‐up duration unspecified)
Hoshowsky 1994 (135 participants) reported this outcome but indicated that no pressure ulcers developed in the trial. It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between reactive gel surfaces and foam surfaces. The evidence is of very low certainty, downgraded twice for high risk of bias in domains other than performance bias, and twice for imprecision due to the small sample size and the low event rate.
The included study did not report data on time to pressure ulcer incidence.
Secondary outcomes
None reported.
Comparison 10: Comparison between two types of reactive gel surfaces (one study, 113 participants)
Using a split body design, Hoshowsky 1994 compared reactive gel surfaces (on top of reactive foam and gel surfaces) with reactive gel surfaces (on top of reactive foam surfaces).
Primary outcomes
Proportion of participants developing a new pressure ulcer (follow‐up duration unspecified)
Hoshowsky 1994 (113 participants) reported this outcome but indicated that no pressure ulcers developed. It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between these two types of use of reactive gel surfaces. Evidence is of very low certainty, downgraded twice for high risk of bias in domains other than performance bias, and twice for imprecision due to the small sample size and the low event rate.
The included study did not report data on time to pressure ulcer incidence.
Secondary outcomes
None reported.
Comparison 11: Reactive foam and gel surfaces versus reactive gel surfaces (one study, 166 participants)
Using a split body design, Hoshowsky 1994 made this comparison. We combined two arms receiving a reactive foam and gel surface and compared that combination with the combined study arms receiving reactive gel surfaces on top of other foam surfaces.
Primary outcomes
Proportion of participants developing a new pressure ulcer (follow‐up duration unspecified)
Hoshowsky 1994 (166 participants) reported this outcome but indicated that no pressure ulcers developed. It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between reactive foam and gel surfaces and reactive gel surfaces. Evidence is of very low certainty, downgraded twice for high risk of bias in domains other than performance bias, and once for imprecision due to the low event rate.
The included study did not report data on time to pressure ulcer incidence.
Secondary outcomes
None reported.
Comparison 12: Reactive foam and gel surfaces versus foam surfaces (one study, 91 participants)
Using a split body comparison design, Hoshowsky 1994 compared reactive foam and gel surfaces with foam surfaces.
Primary outcomes
Proportion of participants developing a new pressure ulcer (follow‐up duration unspecified)
Hoshowsky 1994 (91 participants) reported this outcome but indicated that no pressure ulcers developed. It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between reactive foam and gel surfaces and foam surfaces. Evidence is of very low certainty, downgraded twice for high risk of bias in domains other than performance bias, and twice for imprecision due to the small sample size and the low event rate.
The included study did not report data on time to pressure ulcer incidence.
Secondary outcomes
None reported.
Discussion
Summary of main results
We report evidence from 20 RCTs on the effects of many types of non‐foam and non‐air‐filled reactive surfaces compared with other types of beds, mattresses or overlays, on the incidence of pressure ulcers in any population in any setting. These non‐foam and non‐air‐filled reactive surfaces include: reactive water surfaces, reactive fibre surfaces, reactive gel surfaces, reactive foam and gel surfaces, reactive sheepskin surfaces, and three types of reactive surfaces that could not be defined using the NPIAP S3I terms: Bedcare Sense Textile multilayer mattress system (Van Leen 2018), microfluid static overlays (Vermette 2012), and Aiartex mattress overlays (Cassino 2013a; Ricci 2013). We did not analyse data reported in the 11 studies using intervention or control surfaces that could not be classified. For comparisons with available data, almost all evidence was uncertain in terms of effects on ulcer incidence or any other outcome such as patient comfort or adverse events. There is only low‐certainty evidence that reactive gel surfaces used on operating tables followed by foam surfaces applied on hospital beds may increase the proportion of people developing a new pressure ulcer compared with alternating pressure (active) air surfaces applied on both operating tables and hospital beds.
Overall completeness and applicability of evidence
As detailed in Search methods for identification of studies, we ran a comprehensive set of literature searches to maximise the relevant research included here.
Whilst the current pressure ulcer guidelines often recommend using an air‐filled or foam surface for people at risk for developing pressure ulcers (NICE 2014; EPUAP/NPIAP/PPPIA 2019), we found a range of non‐foam and non‐air‐filled reactive surfaces had been evaluated. These included reactive water surfaces, reactive fibre surfaces, reactive gel surfaces, reactive foam and gel surfaces, and reactive sheepskin surfaces.
Current guidelines seldom limit the appropriateness of any specific support surfaces to adults or children. All participants in included studies were adults (with the reported average age ranging from 37.2 to 85.4 years, median of 72.5 years). Across the included studies, more than half (57.0%) of enrolled participants were female. Almost all of the enrolled participants (4040/4653; 86.8%) were at (high) risk of pressure ulceration, with risk assessed using a risk assessment tool (e.g. the Braden scale), and most of the 3087 participants (76.4%) were ulcer‐free at the time of being recruited. Three included studies (with 953 participants) did include participants with superficial pressure ulcers at baseline.
Most of the included studies were small (half had fewer than 198 participants) whilst nine studies enrolled more than 200 participants. These nine studies together accounted for 80.3% (3737/4653) of the participants in this review.
The geographical scope of included studies was limited. Almost all the studies were from high‐income and upper‐middle‐income economies ‐ mostly from Europe and North America ‐ and one study was from Iran (IRCT2015110619919N3).
Included studies recruited participants from a variety of care settings including: acute care settings (10 studies); community and long‐term care settings (seven studies), operating rooms (two studies), and intensive care units (one study). Two of the 12 comparisons included studies from a variety of care settings (reactive water surfaces versus alternating pressure (active) air surfaces, and reactive fibre surfaces versus alternating pressure (active) air surfaces). However, due to a limited number of included studies for most comparisons, we could not perform pre‐specified subgroup analysis by different care settings. Thus, for these two comparisons, we are unable to draw conclusions about potential modification of treatment effects in different care settings. The remaining 10 comparisons included data that were only from either intensive care units, nursing home settings, acute care settings or operating rooms, and almost all of these 10 comparisons only included one study. Therefore, their evidence is very limited.
We note that some non‐foam and non‐air‐filled surfaces might not be clinically appropriate for some people who need a support surface (e.g. sheepskin surfaces). There was no analysable data for some comparisons, including the comparison involving reactive sheepskin surfaces. Further review work using network meta‐analysis adds to the findings reported here (Shi 2021).
Another limitation in the included studies was the large variation in terms of follow‐up durations (with a range from seven days to six months, median of four weeks ‐ longer than the median of 14 days' follow‐up in other related reviews). This is partly because different follow‐up durations are appropriate in different care settings. For example, participants staying at acute care settings are more likely to be discharged after a short‐term hospital stay whilst those staying at community and long‐term care settings can have long‐term follow‐up. We note that, for most comparisons in this review, the median duration of follow‐up for the pressure ulcer incidence outcome is shorter than the overall median of four weeks. The short median duration of follow‐up may contribute to an under‐estimation of pressure ulcer incidence across study groups of the included studies because most pressure ulcers would occur in the first two to four weeks after hospital admission (Schoonhoven 2007), and some incident pressure ulcers may have been missed in these studies.
Quality of the evidence
We implemented the GRADE approach for assessing the certainty of the evidence and found that most included evidence from our 12 meta‐analyses or syntheses across 10 comparisons was of very low certainty and only one piece of evidence was of low certainty. Downgrading of evidence was largely due to the unclear or high risk of bias of findings, and imprecision due to the small numbers of participants, events, wide confidence interval that failed to exclude important benefits or harms, or all of these. There was also some indirectness for one comparison.
We did not assess the certainty of the evidence for two comparisons: reactive water surfaces versus foam surfaces and reactive water surfaces versus reactive fibre surfaces. This is because the studies included in these two comparisons could not contribute to any synthesis.
Limitations in study design
We downgraded once or twice for study limitations for all of the 12 analyses. We assessed risk of bias according to seven domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, selective outcome reporting, incomplete follow‐up, and other potential biases. Of the 20 studies, we judged four as being at unclear overall risk of bias, and 16 at high overall risk of bias. The prevalence of high overall risk of bias is partly due to the non‐blinding of participants and personnel for most of the comparisons. We acknowledged that such blinding of participants and personnel is impractical for most comparisons. Therefore, we did not downgrade the certainty of evidence for studies at high overall risk of bias solely due to the possible presence of performance bias.
Nine studies were also at high risk of bias due to unblinded outcome assessment. Unblinded assessment has been found to exaggerate odds ratios (from subjective binary outcomes) by, on average, 36% (Hróbjartsson 2012). The outcome assessment of pressure ulcer incidence is subjective and blinded assessment, whilst operationally challenging, can be undertaken (for example, through masked adjudication of photographs of pressure areas (Baumgarten 2009)). Therefore, we considered unblinded pressure ulcer incidence assessment could substantially bias effect estimates in the included studies and downgraded the certainty of evidence for detection bias on a study‐by‐study basis.
Indirectness of evidence
We downgraded once for indirectness for the support‐surface‐associated patient comfort outcome in the comparison of reactive fibre surfaces versus alternating pressure (active) air surfaces. This was because we considered that the comfort outcome measure used in the only study (dropouts due to the discomfort of using the support surfaces) was an indirect measure of the comfort outcome for this review.
Inconsistency of results and unexplained heterogeneity
Statistical heterogeneity was low for all of the evidence syntheses we performed and we did not downgrade for inconsistency for this evidence. The low statistical heterogeneity was partly because all these syntheses included no more than four studies and nine of the 12 syntheses included only one study.
We have to note that although we planned to calculate prediction intervals to understand the implications of heterogeneity, all analyses included a small number (up to four) of included studies which was fewer than the 10 needed for this calculation.
Imprecision of results
We downgraded once or twice for imprecision for all of the 12 evidence syntheses. Study sample sizes were small in most cases (median sample size: 192.5) with often small numbers of events and wide associated confidence intervals around effect estimates. Confidence intervals often crossed the line of null effect or RRs = 0.75 and 1.25, or both, thus meaning we could not discern whether the true population effect was likely to be beneficial or harmful.
Publication bias
We did not downgrade the certainty of evidence for publication bias in all meta‐analyses. This is because (1) we have confidence in the comprehensiveness of our literature searches; and (2) we did not find any clear evidence of non‐reporting bias of study results. Although we planned to perform funnel plots for meta‐analysis to visually inspect publication bias, there was no analysis including more than ten studies.
Potential biases in the review process
We followed pre‐specified methods to review evidence in order to prevent potential bias in the review process. For example, we ran comprehensive electronic searches, searched trials registries, and checked references of systematic reviews identified in electronic searches.
This review also has limitations. Firstly, some included studies may have considered co‐interventions as 'usual care' but did not fully describe them. We assumed that all studies had provided co‐interventions equally to participants in their study groups if there was nothing to indicate that this was not the case. Secondly, we did not implement pre‐specified subgroup analysis as we mentioned above, mainly because no analysis included more than ten studies. Thirdly, of the 11 studies with surfaces that could not be classified, seven used controls that were described as 'standard hospital surfaces' but did not specify construction materials of these surfaces. Although we made efforts to collect information on these surfaces, we were not able to classify them. Traditionally, ‘standard hospital surfaces' meant foam surfaces, but we felt adopting that assumption was unwarranted. Accurate classification of these surfaces in the future could add evidence – for example, on reactive sheepskin surfaces – to this review. Finally, we were not able to pre‐specify the comparisons included in this review. This is because specific support surfaces applied could only be known and defined once eligible studies were included. However, we pre‐planned to use the NPIAP S3I 2007 support surface terms and definitions to define specific support surfaces in order to avoid any potential bias.
Agreements and disagreements with other studies or reviews
To our knowledge, among the 14 systematic reviews or meta‐analyses we identified in electronic searches of this review (Chou 2013; Huang 2013; McGinnis 2011; McInnes 2015; McInnes 2018; Mistiaen 2010a; De Oliveira 2017; Rae 2018; Reddy 2006; Reddy 2008; Serraes 2018; Shi 2018a; Smith 2013; Yao 2018), two recent comprehensive reviews include non‐foam and non‐air‐filled surfaces: Shi 2018a, and the Cochrane Review 'Support surfaces for pressure ulcer prevention' (McInnes 2015).
This review is different from Shi 2018a and McInnes 2015 in how specific non‐foam and non‐air‐filled support surfaces were classified and labelled. For example, Shi 2018a classified Aiartex mattresses used in Ricci 2013 and microfluid static overlays used in Vermette 2012 as foam surfaces. However, we considered their materials as undefined using the revised NPIAP S3I support surface terms and definitions. McInnes 2015 classified support surfaces into 'low‐tech' and 'high‐tech' groups in general and covered a range of reactive surfaces (the 'Silicore overlay', a 'water mattress', and a 'foam pad') ' using low‐tech 'constant low‐pressure devices'.
Shi 2018a grouped some interventions under the term 'standard hospital surfaces' but concluded that the types of surfaces labelled in this way varied over time, and by setting. We noted that the NPIAP S3I 2007 recommends specifying what 'standard hospital surfaces' are. In this review, we made great efforts to define surfaces where these surfaces were described as a 'standard hospital surface' in the included studies to ensure they were placed in the correct comparisons. We considered those 'standard hospital surfaces' that had no characteristic details and which we could not map to the NPIAP S3I 2007 classification as undefined surfaces.
These above re‐definitions and re‐classifications of specific support surfaces can explain some of the inconsistency between these reviews. For example, because 'standard hospital surfaces' were re‐defined as surfaces that could not be classified, we did not perform analysis for the relevant comparison involving reactive sheepskin surfaces. Additionally, Shi 2018a was a network meta‐analysis.
Shi 2018a considered pressure ulcer incidence and support‐surface‐associated patient comfort outcomes only, whilst this review added adverse effect evidence to the evidence base.
Authors' conclusions
Implications for practice.
Current NICE 2014 and EPUAP/NPIAP/PPPIA 2019 pressure ulcer guidelines primarily focus on foam and air‐filled surfaces in their recommendations. We found evaluations for a range of non‐foam and non‐air‐filled reactive surfaces. Comparative evidence is almost all uncertain about the relative effects of these types of non‐foam and non‐air‐filled reactive surfaces compared with alternatives explored in randomised controlled trials on ulcer incidence, health‐related quality of life, adverse events and patient comfort. However, reactive gel surfaces used on operating tables followed by foam surfaces applied on hospital beds may increase the risk of having new pressure ulcers compared with alternating pressure (active) air surfaces applied on both operating tables and hospital beds.
Implications for research.
Given the large number of different support surfaces available, future studies should prioritise which support surfaces to evaluate on the basis of the priorities of decision‐makers. For example, reactive gel surfaces versus foam surfaces or reactive air surfaces could be a high priority for further evaluation. All interventions used should be clearly described using the current classification system. Researchers should avoid use of terms such as 'standard hospital mattress' without further detail about the specific nature of the support surfaces being evaluated. Limitations in included studies are largely due to small sample size and sub‐optimal RCT design. The incidence of pressure ulcers can be low in certain settings and this needs to be considered in sample size calculations and when considering the feasibility of trial conduct. Under‐recruitment or over‐estimation of event rates that then fail to occur, or both, can lead to imprecision and less robust effect estimates.
Future studies should also consider carefully the choice of outcomes they report. Time‐to‐event data for pressure ulcer incidence should be used in studies. Careful and consistent assessment and reporting of adverse events needs to be undertaken to generate meaningful data that can be compared between studies. Likewise, patient comfort is an important outcome but poorly defined and reported, and this needs to be considered in future research studies. Further studies should aim to collect and report health‐related quality of life using validated measures. Finally, future studies should nest cost‐effectiveness analysis in their conduct where possible.
Any future studies must be undertaken to the highest standards possible. Whilst it is challenging to avoid the risk of performance bias in trials of support surfaces as blinding of participants and personnel is seldom possible, stringent protocols ‐ for example, in terms of encouraging consistent care and blinded decision‐making ‐ can help to minimise risk. It is also important to fully describe co‐interventions (e.g. repositioning) and ensure protocols mandate balanced use of these across trial arms. The risk of detection bias can also be minimised with the use of digital photography and adjudicators of the photographs being masked to support surfaces (Baumgarten 2009). Follow‐up periods should be for as long as possible and clinically relevant in different settings. Where possible and useful, data collection after discharge from acute settings may be considered.
What's new
| Date | Event | Description |
|---|---|---|
| 18 August 2021 | Amended | Minor amendment to include link to overview and network meta‐analysis. |
History
Protocol first published: Issue 5, 2020 Review first published: Issue 5, 2021
Acknowledgements
The authors are grateful to the following peer reviewers who provided feedback on both the protocol and the review: Julie Bruce and Zena Moore. Thanks are also due to Jessica Sharp for copy‐editing the protocol of this review, to Denise Mitchell for additional copy‐edit feedback, to Faith Armitage for copy‐editing the review and to Nicole Pitcher for writing the Plain Language Summary.
We also would like to thank Asmara Jammali‐Blasi for screening search records; and to thank Zhenmi Liu, Gill Norman, and Melanie Stephens for double‐checking data extraction and risk of bias assessment for this review. Thanks also to Cochrane Musculoskeletal, Oral, Skin and Sensory Network Editors Peter Tugwell and Jennifer Hilgart for feedback and final approval of the review for publication.
Appendices
Appendix 1. Full details of support surfaces classifications
| Overarching class of support surface (as used in this review) | Corresponding subclasses of support surfaces used inShi 2018a | Descriptions of support surfaces | Selected examples (with support surface brands if possible) |
| Reactive air surfaces | Powered/non‐powered reactive air surfaces | A group of support surfaces constructed of air cells, which redistribute body weight over a maximum surface area (i.e. has reactive pressure redistribution mode), with or without the requirement for electrical power. | Static air mattress overlay, dry flotation mattress (e.g. Roho, Sofflex), static air mattress (e.g. EHOB), and static mode of Duo 2 mattress. |
| Powered/non‐powered reactive low‐air‐loss air surfaces | A group of support surfaces made of air cells, which have reactive pressure redistribution modes and a low‐air‐loss function, with or without the requirement for electrical power. | Low‐air‐loss hydrotherapy. | |
| Powered reactive air‐fluidised surfaces | A group of support surfaces made of air cells, which have reactive pressure redistribution modes and an air‐fluidised function, with the requirement for electrical power. | Air‐fluidised bed (e.g. Clinitron). | |
| Foam surfaces | Non‐powered reactive foam surfaces | A group of support surfaces made of foam materials, which have a reactive pressure redistribution function, without the requirement for electrical power. | Convoluted foam overlay (or pad), elastic foam overlay (e.g. Aiartex, microfluid static overlay), polyether foam pad, foam mattress replacement (e.g. MAXIFLOAT), solid foam overlay, viscoelastic foam mattress/overlay (e.g. Tempur, CONFOR‐Med, Akton, Thermo). |
| Alternative reactive support surfaces (non‐foam or air‐filled): reactive fibre surfaces | Non‐powered reactive fibre surfaces | A group of support surfaces made of fibre materials, which have a reactive pressure redistribution function, without the requirement for electrical power. | Silicore (e.g. Spenco) overlay/pad. |
| Alternative reactive support surfaces (non‐foam or air‐filled): reactive gel surfaces | Non‐powered reactive gel surfaces | A group of support surfaces made of gel materials, which have a reactive pressure redistribution function, without the requirement for electrical power. | Gel mattress, gel pad used in operating theatre. |
| Alternative reactive support surfaces (non‐foam or air‐filled): reactive sheepskin surfaces | Non‐powered reactive sheepskin surfaces | A group of support surfaces made of sheepskin, which have a reactive pressure redistribution function, without the requirement for electrical power. | Australian Medical Sheepskins overlay. |
| Alternative reactive support surfaces (non‐foam or air‐filled): reactive water surfaces | Non‐powered reactive water surfaces | A group of support surfaces based on water, which has the capability of a reactive pressure redistribution function, without the requirement for electrical power. | Water mattress. |
| Alternating pressure (active) air surfaces | Powered active air surfaces | A group of support surfaces made of air cells, which mechanically alternate the pressure beneath the body to reduce the duration of the applied pressure (mainly via inflating and deflating to alternately change the contact area between support surfaces and the body; i.e. alternating pressure, or active, mode), with the requirement for electrical power. | Alternating pressure‐relieving air mattress (e.g. Nimbus II, Cairwave, Airwave, MicroPulse), large‐celled ripple. |
| Powered active low‐air‐loss air surfaces | A group of support surfaces made of air cells, which have the capability of alternating pressure redistribution as well as low‐air‐loss for drying local skin, with the requirement for electrical power. | Alternating pressure low‐air‐loss air mattress. | |
| Powered hybrid system air surfaces | A group of support surfaces made of air cells, which offer both reactive and active pressure redistribution modes, with the requirement for electrical power. | Foam mattress with dynamic and static modes (e.g. Softform Premier Active). | |
| Powered hybrid system low‐air‐loss air surfaces | A group of support surfaces made of air cells, which offer both reactive and active pressure redistribution modes as well as a low‐air‐loss function, with the requirement for electrical power. | Stand‐alone bed unit with alternating pressure, static modes and low‐air‐loss (e.g. TheraPulse). | |
| Standard hospital surfaces | Standard hospital surfaces | A group of support surfaces made of any materials, used as‐usual in a hospital and without reactive or active pressure redistribution capabilities, nor any other functions (e.g. low‐air‐loss, or air‐fluidised). | Standard hospital (foam) mattress, National Health Service Contract hospital mattress, standard operating theatre surface configuration, standard bed unit and usual care. |
Appendix 2. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR beds EXPLODE ALL AND INREGISTER
2 mattress* AND INREGISTER
3 (foam or transfoam) AND INREGISTER
4 overlay* AND INREGISTER
5 (pad or pads) AND INREGISTER
6 gel AND INREGISTER
7 (pressure NEXT relie*) AND INREGISTER
8 (pressure NEXT reduc*) AND INREGISTER
9 (pressure NEXT alleviat*) AND INREGISTER
10 ("low pressure" near2 device*) AND INREGISTER
11 ("low pressure" near2 support) AND INREGISTER
12 (constant near2 pressure) AND INREGISTER
13 "static air" AND INREGISTER
14 (alternat* next pressure) AND INREGISTER
15 (air next suspension*) AND INREGISTER
16 (air next bag*) AND INREGISTER
17 (water next suspension*) AND INREGISTER
18 sheepskin AND INREGISTER
19 (turn* or tilt*) next (bed* or frame*) AND INREGISTER
20 kinetic next (therapy or table*) AND INREGISTER
21 (net next bed*) AND INREGISTER
22 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 AND INREGISTER
23 MESH DESCRIPTOR Pressure Ulcer EXPLODE ALL AND INREGISTER
24 (pressure next (ulcer* or sore* or injur*)) AND INREGISTER
25 (decubitus next (ulcer* or sore*)) AND INREGISTER
26 ((bed next sore*) or bedsore*) AND INREGISTER
27 #23 OR #24 OR #25 OR #26 AND INREGISTER
28 #22 AND #27 AND INREGISTER
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Beds] explode all trees
#2 mattress*:ti,ab,kw
#3 (foam or transfoam):ti,ab,kw
#4 overlay*:ti,ab,kw
#5 "pad" or "pads":ti,ab,kw
#6 "gel":ti,ab,kw
#7 (pressure next relie*):ti,ab,kw
#8 (pressure next reduc*):ti,ab,kw
#9 (pressure next alleviat*):ti,ab,kw
#10 ("low pressure" near/2 device*):ti,ab,kw
#11 ("low pressure" near/2 support):ti,ab,kw
#12 (constant near/2 pressure):ti,ab,kw
#13 "static air":ti,ab,kw
#14 (alternat* next pressure):ti,ab,kw
#15 (air next suspension*):ti,ab,kw
#16 (air next bag*):ti,ab,kw
#17 (water next suspension*):ti,ab,kw
#18 sheepskin:ti,ab,kw
#19 (turn* or tilt*) next (bed* or frame*):ti,ab,kw
#20 kinetic next (therapy or table*):ti,ab,kw
#21 (net next bed*):ti,ab,kw
#22 {or #1‐#21}
#23 MeSH descriptor: [Pressure Ulcer] explode all trees
#24 (pressure next (ulcer* or sore* or injur*)):ti,ab,kw
#25 (decubitus next (ulcer* or sore*)):ti,ab,kw
#26 ((bed next sore*) or bedsore*):ti,ab,kw
#27 {or #23‐#26}
#28 (#22 and #27) in Trials
Ovid MEDLINE
1 exp Beds/
2 mattress*.mp.
3 (foam or transfoam).mp.
4 overlay*.mp.
5 (pad or pads).ti,ab.
6 gel.ti,ab.
7 pressure relie*.mp.
8 pressure reduc*.mp.
9 pressure alleviat*.mp.
10 (low pressure adj2 device*).mp.
11 (low pressure adj2 support).mp.
12 (constant adj2 pressure).mp.
13 static air.mp.
14 (alternat* adj pressure).mp.
15 air suspension*.mp.
16 air bag*.mp.
17 water suspension*.mp.
18 sheepskin.mp.
19 ((turn* or tilt*) adj (bed* or frame*)).mp.
20 (kinetic adj (therapy or table*)).mp.
21 net bed*.mp.
22 or/1‐21
23 exp Pressure Ulcer/
24 (pressure adj (ulcer* or sore*)).mp.
25 (decubitus adj (ulcer* or sore*)).mp.
26 (bed adj (ulcer* or sore*)).mp.
27 or/23‐26
28 and/22,27
29 randomized controlled trial.pt.
30 controlled clinical trial.pt.
31 randomi?ed.ab.
32 placebo.ab.
33 clinical trials as topic.sh.
34 randomly.ab.
35 trial.ti.
36 or/29‐35
37 exp animals/ not humans.sh.
38 36 not 37
39 28 and 38
Ovid Embase
1 exp Bed/
2 mattress*.mp.
3 (foam or transfoam).mp.
4 overlay*.mp.
5 (pad or pads).ti,ab.
6 gel.ti,ab.
7 pressure relie*.mp.
8 pressure reduc*.mp.
9 pressure alleviat*.mp.
10 (low pressure adj2 device*).mp.
11 (low pressure adj2 support).mp.
12 (constant adj2 pressure).mp.
13 static air.mp.
14 (alternat* adj pressure).mp.
15 air suspension*.mp.
16 air bag*.mp.
17 water suspension*.mp.
18 sheepskin.mp.
19 ((turn* or tilt*) adj (bed* or frame*)).mp.
20 (kinetic adj (therapy or table*)).mp.
21 net bed*.mp.
22 or/1‐21
23 exp Decubitus/
24 (pressure adj (ulcer* or sore*)).mp.
25 (decubitus adj (ulcer* or sore*)).mp.
26 (bed adj (ulcer* or sore*)).mp.
27 or/23‐26
28 and/22,27
29 Randomized controlled trials/
30 Controlled clinical study/
31 Single‐Blind Method/
32 Double‐Blind Method/
33 Crossover Procedure/
34 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab.
35 (doubl* adj blind*).ti,ab.
36 (singl* adj blind*).ti,ab.
37 or/29‐36
38 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
39 human/ or human cell/
40 and/38‐39
41 38 not 40
42 37 not 41
43 28 and 42
EBSCO CINAHL Plus
S50 S26 AND S49
S49 S48 NOT S47
S48 S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41
S47 S45 NOT S46
S46 MH (human)
S45 S42 OR S43 OR S44
S44 TI (animal model*)
S43 MH (animal studies)
S42 MH animals+
S41 AB (cluster W3 RCT)
S40 MH (crossover design) OR MH (comparative studies)
S39 AB (control W5 group)
S38 PT (randomized controlled trial)
S37 MH (placebos)
S36 MH (sample size) AND AB (assigned OR allocated OR control)
S35 TI (trial)
S34 AB (random*)
S33 TI (randomised OR randomized)
S32 MH cluster sample
S31 MH pretest‐posttest design
S30 MH random assignment
S29 MH single‐blind studies
S28 MH double‐blind studies
S27 MH randomized controlled trials
S26 S20 AND S25
S25 S21 OR S22 OR S23 OR S24
S24 TI decubitus or AB decubitus
S23 TI ( bed sore* or bedsore* ) or AB ( bed sore* or bedsore* )
S22 TI ( pressure ulcer* or pressure sore* ) or AB ( pressure ulcer* or pressure sore* )
S21 (MH "Pressure Ulcer")
S20 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19
S19 TI net bed* or AB net bed*
S18 TI ( kinetic therapy or kinetic table* ) or AB ( kinetic therapy or kinetic table* )
S17 TI ( turn* bed* or tilt* bed* ) or AB ( turn* frame* or tilt* frame* )
S16 TI sheepskin OR AB sheepskin
S15 TI water suspension or AB water suspension
S14 TI air bag* or AB air bag*
S13 TI air suspension or AB air suspension
S12 TI alternat* pressure or AB alternat* pressure
S11 TI static air or AB static air
S10 TI constant N2 pressure or AB constant N2 pressure
S9 TI low pressure N2 support or AB low pressure N2 support
S8 TI low pressure N2 device* or AB low pressure N2 device*
S7 TI pressure alleviat* or AB pressure alleviat*
S6 TI pressure reduc* or AB pressure reduc*
S5 TI pressure relie* or AB pressure relie*
S4 TI ( overlay* or pad or pads or gel ) or AB ( overlay* or pad or pads or gel )
S3 TI ( foam or transfoam ) or AB ( foam or transfoam )
S2 TI mattress* or AB mattress*
S1 (MH "Beds and Mattresses+")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air | Pressure Ulcer
bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air | Pressure Injury
bed OR mattress OR sheepskin OR gel OR pad OR foam OR pressure OR support OR air | Pressure Ulcers buttock
bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air | Ulcer, Pressure
bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air | Pressure Ulcer Stage 1
bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air | Pressure Ulcers Stage II
bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air | Pressure Ulcers Stage III
World Health Organization International Clinical Trials Registry Platform
pressure ulcer [title] and bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air [intervention]
pressure ulcer [condition] and bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air [intervention]
pressure injury [title] and bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air [intervention]
pressure injury [condition] and bed OR mattress OR sheepskin OR gel OR pad OR foam OR support OR air [intervention]
Appendix 3. Risk of bias
1 'Risk of bias' assessment in individually randomised controlled trials
1. Was the allocation sequence randomly generated?
Low risk of bias
The study authors describe a random component in the sequence generation process, such as referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots.
High risk of bias
The study authors describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example, sequence generated by odd or even date of birth, sequence generated by some rule based on date (or day) of admission, sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and study authors enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or study authors enrolling participants could possibly foresee assignments and thus introduce selection bias, e.g. allocation was based on: using an open random allocation schedule (e.g. a list of random numbers); or assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered), alternation or rotation, date of birth, case record number, any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding: was knowledge of the allocated interventions by participants and personnel adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others is likely to introduce bias.
Unclear
Any one of the following.
Insufficient information to permit a judgement of low or high risk of bias.
The study did not address this outcome.
4. Blinding: was knowledge of the allocated interventions by outcome assessors adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding.
Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome measurement is likely to be influenced by lack of blinding.
Blinding of outcome assessment attempted, but likely that the blinding could have been broken.
Unclear
Any one of the following.
Insufficient information to permit a judgement of low or high risk of bias.
The study did not address this outcome.
5. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is not sufficient to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes is not sufficient to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data is likely to be related to the true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is sufficient to induce clinically relevant bias in the intervention effect estimate.
For continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes is sufficient to induce clinically relevant bias in the observed effect size.
‘As‐treated’ analysis done, with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated; no reasons for missing data provided).
The study did not address this outcome.
6. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following.
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
7. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
2 'Risk of bias' assessment in cluster‐randomised controlled trials (cluster‐RCTs)
1. Recruitment bias
Recruitment bias (or identification bias) is the bias that occurs in cluster‐RCTs if the personnel recruiting participants know individuals’ allocation, even when the allocation of clusters has been concealed appropriately. The knowledge of the allocation of clusters may lead to bias because the individuals' recruitment in cluster trials is often behind the clusters' allocation to different interventions; and the knowledge of allocation can determine whether individuals are recruited selectively.
This bias can be judged through considering the following questions.
Were all the individual participants identified/recruited before randomisation of clusters?
Is it likely that selection of participants was affected by knowledge of the intervention?
Were there baseline imbalances that suggest differential identification or recruitment of individual participants between arms?
2. Baseline imbalance
Baseline imbalance between intervention groups can occur due to chance, problems with randomisation, or identification/recruitment bias. The issue of recruitment bias has been considered above.
In terms of study design, the risk of chance baseline imbalance can be reduced by the use of stratified or pair‐matched randomisation. Minimisation — an equivalent technique to randomisation — can be used to achieve better balance in cluster characteristics between intervention groups if there is a small number of clusters.
Concern about the influence of baseline imbalance can be reduced if studies report the baseline comparability of clusters, or statistical adjustment for baseline characteristics.
3. Loss of clusters
Similar to missing outcome data in individually randomised trials, bias can occur if clusters are completely lost from a cluster‐RCT, and are omitted from the analysis.
The amount of missing data, the reasons for missingness and the way of analysing data given the missingness should be considered in assessing the possibility of bias.
4. Incorrect analysis
Data analyses, which do not take the clustering into account, in cluster‐RCTs will be incorrect. Such analyses lead to a 'unit of analysis error' and over‐precise results (overly small standard error) and overly small P values. Though these analyses will not result in biased estimates of effect, they (if not correctly adjusted) will lead to too much weight allocated to cluster trials in a meta‐analysis.
Note that the issue of analysis may not lead to concern any more and will not be considered substantial if approximate methods are used by review authors to address clustering in data analysis.
5. Comparability with individually randomised trials
In the case that a meta‐analysis includes, for example, both cluster‐randomised and individually randomised trials, potential differences in the intervention effects between different trial designs should be considered. This is because the 'contamination' of intervention effects may occur in cluster‐RCTs, which would lead to underestimates of effect. The contamination could be known as a 'herd effect'; that is, within clusters, individuals' compliance with using an intervention may be enhanced, which in return affects the estimation of effect.
Appendix 4. Results of studies that involved undefined surfaces
| Outcomes | Results |
| Comparison: reactive water surfaces compared with undefined 'standard hospital surfaces' | |
| Proportion of participants developing a new pressure ulcer (follow‐up duration 10 days) | Andersen 1982 (316 participants) reported that 4.5% (7/155) of people using reactive water surfaces developed new pressure ulcers and the proportion was 13.0% (21/161) for those using standard hospital surfaces. The RR is 0.35 (95% CI 0.15 to 0.79). |
| Comparison: reactive gel surfaces compared with undefined 'standard hospital surfaces' | |
| Proportion of participants developing a new pressure ulcer (follow‐up duration eight days or unspecified) |
IRCT2015110619919N3 reported that reactive gel surfaces significantly reduced the incidence rates of sacral pressure ulcers compared with standard hospital surfaces (P = 0.01). Nixon 1998 (446 participants) reported 10.7% (22/205) of people using reactive gel surfaces developed new pressure ulcers and the proportion was 20.4% (43/211) for those using standard hospital surfaces. The RR is 0.53 (95% CI 0.33 to 0.85). |
| Comparison: reactive gel surfaces compared with undefined surfaces | |
| Proportion of participants developing a new pressure ulcer (follow‐up duration 4 and 12 weeks) | Two studies (122 participants) reported this outcome: Cassino 2013a reported 1 of 37 participants using reactive gel surfaces developed new pressure ulcers whilst none of participants developed new ulcers when using undefined surfaces; Ricci 2013 reported none of 25 participants in each study arm developed new ulcers. |
| Support‐surface‐associated patient comfort (follow‐up duration 12 weeks) | Ricci 2013 (50 participants) reported comfort that was measured by the study investigators using a non‐validated 4‐point scale (1 = poor, 2 = fair, 3 = good, 4 = excellent). They suggested no difference between reactive gel surfaces and undefined reactive surfaces in support surface associated patient comfort: Ricci 2013 reported 20 people using undefined reactive surfaces responded with 'good' and 5 with 'excellent'; and 24 people using reactive gel surfaces responded with 'good' and 1 with 'excellent'. |
| All reported adverse events (follow‐up duration 12 weeks) | Ricci 2013 (50 participants) reported this outcome but indicated no adverse events. |
| Comparison: reactive sheepskin surfaces versus undefined 'standard hospital surfaces' | |
| Proportion of participants developing a new pressure ulcer (follow‐up duration 30 days and six months or unspecified) | Three studies (1424 participants) reported data for this outcome (Jolley 2004; McGowan 2000; Mistiaen 2010). These 3 studies all suggested that reactive sheepskin surfaces were associated with lower proportions of participants developing a new pressure ulcer than 'standard hospital surfaces'. |
| Time to pressure ulcer incidence (follow‐up duration 30 days and six months or unspecified) | Three studies (1424 participants) reported this outcome (Jolley 2004; McGowan 2000; Mistiaen 2010) and these studies all suggested that the use of reactive sheepskin surfaces was associated with a lower hazard of having new ulcers than using standard hospital surfaces at any particular time up to 6 months. |
| Support‐surface‐associated patient comfort (follow‐up duration unspecified) | Only McGowan 2000 (297 participants) reported this outcome, measured using a 10‐point scale (10 = "very comfortable"). McGowan 2000 reported that patients using reactive sheepskin surfaces rated comfort significantly higher than those using standard hospital surfaces (Z value of the Mann‐Whitney U test = ‐7.74, P < 0.0001). |
| Health‐related quality of life (follow‐up duration 30 days) | Only Mistiaen 2010 (588 participants) reported this outcome, measured at 30 days using a 100‐point visual analogue scale (100 = the best health status that could be imagined). Mistiaen 2010 reported that the quality of life for those with ulcers using reactive sheepskin surfaces had a mean of 62.1 compared with 61.3 for those using standard hospital surfaces (Student’s t‐test P = 0.71). |
| Comparison: undefined surfaces compared with reactive air surfaces | |
| Proportion of participants developing a new pressure ulcer (follow‐up duration 14 days) | Vermette 2012 (110 participants) compared reactive air surfaces with alternating pressure (active) air surfaces or RIK® microfluid static overlay (MSO). Reported that 6 of 55 in MSO or low‐air‐loss dynamic mattress (LALDM); 2 of 55 in ISO (3.6%) using reactive air surfaces developed a new pressure ulcer and 6 of 55 (10.9%) people using undefined reactive surfaces developed new ulcers. The RR is 0.33 (95% CI 0.07 to 1.58). |
| Support‐surface‐associated patient comfort (follow‐up duration 14 days) | Vermette 2012 (110 participants) compared reactive air surfaces with alternating pressure (active) air surfaces or RIK® microfluid static overlay; defined this outcome as participants self‐rated comfort on a scale of 1 to 5 with 1 indicating very comfortable and 5 indicating not comfortable. In total, 68 participants rated comfort: 27 of 30 participants using undefined reactive surfaces and 29 of 34 using reactive air surfaces responded that they were comfortable or very comfortable. |
| Comparison: undefined surfaces compared with foam surfaces | |
| Proportion of participants developing a new pressure ulcer (follow‐up duration minimum 5 days maximum 7 months) | Van Leen 2018 (206 participants) compared foam surfaces with the Bedcare surface. Reported that 5 of 103 (4.9%) people using foam surfaces developed a new pressure ulcer and 9 of 103 (8.7%) people using undefined reactive surfaces developed new ulcers. The RR is 0.56 (95% CI 0.19 to 1.60). |
| All reported adverse events (follow‐up duration 12 weeks) | Van Leen 2018 (206 participants) compared foam surfaces with Bedcare surfaces. Reported this outcome but stated that there was no reported adverse events in either study group. It is uncertain if there is a difference in the adverse effects between foam surfaces and the undefined reactive surfaces. Evidence certainty was very low, downgraded twice for high risk of bias in a domain other than performance bias, and once for imprecision, as the sample size was small and the number of events was relatively low. |
Appendix 5. Sensitivity analyses
| Sensitivity analysis | Studies | Participants | Statistical Method | Effect Estimate |
| Comparison: reactive water surfaces compared with alternating pressure (active) air surfaces | ||||
| Outcome: proportion of participants developing a new pressure ulcer | ||||
|
2 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.36, 1.90] |
| Comparison: reactive fibre surfaces compared with alternating pressure (active) air surfaces | ||||
| Outcome: proportion of participants developing a new pressure ulcer | ||||
|
3 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.84, 1.39] |
|
3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.84, 1.47] |
| Comparison: reactive gel surfaces used on operating tables followed by foam surfaces on ward beds versus alternating pressure (active) air surfaces in operating tables and subsequently on ward beds | ||||
| Outcome: proportion of participants developing a new pressure ulcer | ||||
|
2 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.74 [1.39, 16.16] |
Data and analyses
Comparison 1. Reactive water surfaces compared with alternating pressure (active) air surfaces.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Proportion of participants developing a new pressure ulcer | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.35, 1.93] |
Comparison 2. Reactive water surfaces compared with reactive air surfaces.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Proportion of participants developing a new pressure ulcer | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 2.35 [0.23, 23.75] |
Comparison 3. Reactive fibre surfaces compared with alternating pressure (active) air surfaces.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Proportion of participants developing a new pressure ulcer | 3 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.84, 1.47] |
Comparison 4. Reactive fibre surfaces compared with foam surfaces.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Proportion of participants developing a new pressure ulcer | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.57] |
Comparison 5. Reactive gel surfaces followed by foam surfaces compared with alternating pressure (active) air surfaces.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Proportion of participants developing a new pressure ulcer | 2 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 4.53 [1.31, 15.65] |
Comparison 6. Reactive gel surfaces compared with reactive air surfaces.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Proportion of participants developing a new pressure ulcer | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.36, 1.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersen 1982.
| Study characteristics | ||
| Methods |
Study objective: to observe "the development of pressure sores in risk‐patients nursed on these mattresses [water‐mattresses and alternating pressure air‐mattresses] and compare the results with a similar group of patients nursed on ordinary hospital mattresses" Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: 10 days Number of arms: 3 Single centre or multi‐sites: single centre Study start date and end date: not described Setting: hospital |
|
| Participants |
Baseline characteristics Inclusion criteria: patients with acute conditions and a risk score of 2 or more (i.e. at risk) Exclusion criteria: "those who already had pressure sores" Sex (M:F): 60:101 in control; 73:93 in air; 73:82 in water Age (years): distribution of patients' ages described Baseline skin status: all at risk according to the risk score used by the authors, free of ulcers Group difference: no difference between groups according to age, sex, body weight, or risk score Total number of participants: not described; n = 482 available Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Alternating‐pressure air‐mattress
Water mattress
Ordinary hospital mattresses
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Six hundred patients at risk for pressure sores were randomised in either a control group or one of two experimental groups ... They were allotted to one of the three groups ..." Comment: method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Outcome group: primary outcome (i.e. the only outcome) Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Outcome group: primary outcome (i.e. the only outcome) Quote: "One of us [note: study's authors] assessed the condition of the skin ..." Comment: appears to have no blinding, and the pressure ulcer incidence outcome measurement is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Outcome group: primary outcome (i.e. the only outcome) Quote: "Six hundred patients at risk for pressure sores were randomised ..." Quote: "Among the 600 risk‐patients ... 118 dropped out during the first 24 hours before the first dermatologic inspection. This did not impair randomization." Quote: "The groups remained comparable throughout the 10‐day study period" Comment: unclear risk of bias was judged because authors claimed that randomisation was not impaired though the proportion of missing data was high and no reasons for missing data were provided. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Aronovitch 1999.
| Study characteristics | ||
| Methods |
Study objective: "... to determine the efficacy and safety of the experimental system (study group), in comparison with conventional management (control group), for the prevention of pressure ulcers in the operative and postoperative settings" Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: 7 days Number of arms: 2 Single centre or multi‐sites: single centre Study start date and end date: March 1997 to February 1998 Setting: tertiary care facility (operation theatre and wards) |
|
| Participants |
Baseline characteristics Inclusion criteria: "18 years of age or older undergoing a scheduled surgery with general anesthesia for at least 4 hours (actual operative time of 3 hours or more)" Exclusion criteria: patients "participated in a clinical trial within 30 days of the baseline visit ... or had a pressure ulcer at the baseline visit" Sex (M:F): 79:31 in experimental system; 77:27 in conventional management Age (years): mean 63.5 (SD 11.9) in experimental system; 64.7 (11.8) in conventional management Baseline skin status: Modified Knoll scale score ‐ on average less than 4 (range 0 to 13; a score of 12 or higher = at risk of pressure ulcer development) in both groups; and those with pressure ulcers at baseline excluded Group difference: no difference Total number of participants: 217 patients Unit of analysis: individuals Unit of randomisation (per patient): groups of participants by weeks |
|
| Interventions |
Intervention characteristics Experimental management
Conventional management
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed by week rather than by patient to decrease protocol error." Comment: unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Outcome group: primary outcome Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcome group: primary outcome Quote: "Patients were examined following surgery and daily for pressure ulcers, including number, stage (I‐IV), size (area), location, and appearance." Comment: insufficient information to permit judgement of low or high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Outcome group: primary outcome Quote: "Seven patients (8.75%) in the control group developed a total of 11 pressure ulcers ..." Comment: high risk of bias because 7 (8.75%) in control group implied 80 of 105 individuals were considered in data analysis, meaning a large proportion of missing data in the control group alone. However, the number of available cases in experimental group is not given. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Bliss 1995a.
| Study characteristics | ||
| Methods |
Study objective: to identify inexpensive and, if possible, non‐mechanical constant low pressure overlays effective for patients at long‐term risk in continuing‐care wards for elderly people Study design: randomised controlled trial (a poorly designed multi‐arm multi‐stage trial, with re‐randomisation) Study grouping: parallel group Duration of follow‐up: not given; assessment with a mean of 17.7 days Number of arms: 7 (the trial had a Vaperm as control arm but its participants were not randomised. Vaperm data were not extracted for this review) Single centre or multi‐sites: not specified Study start date and end date: not described Setting: hospital |
|
| Participants |
Baseline characteristics Inclusion criteria: patients liable to pressure sores; including those who already had superficial breaks in the skin of the pressure areas Exclusion criteria: patients with superficial sores > 5 cm and discoloured areas > 2 cm diameter Sex (M:F): overall 62:296 (treatment sessions rather than individuals) Age (years): mean 84.4 (range 67 to 97) Large cell Ripple bed (n = 71 treatment sessions of 34 patients); 85.2 (67 to 97) Preventix (n = 25 sessions of 20 patients); 85.6 (68 to 98) Groove (n = 66 sessions of 36 patients); 86.1 (68 to 98) Modular Propad (n = 60 sessions of 39 patients); 84.4 (68 to 93) Ardo Watersoft (n = 32 sessions of 22 patients); 85.6 (68 to 94) Spenco (n = 63 sessions of 35 patients); 84.3 (67 to 97) Surgicgoods Hollowcore (n = 41 sessions of 30 patients) Baseline skin status: not given; allowed inclusion of those with superficial ulcers Group difference: not given Total number of participants: n = 358 sessions of 216 patients Unit of analysis: treatment sessions of patients Unit of randomisation (per patient): treatment sessions of patients |
|
| Interventions |
Intervention characteristics Groove
Spenco
Propad
Preventix
Surgicgoods
Watersoft
Large cell Ripple bed
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patient was randomly allocated to an experimental overlay by the researcher writing the names of all those available at the time on slips of paper which were folded and offered to the nurse to choose one blind" Comment: low risk of bias because drawing of lots is applied to generate random sequence. |
| Allocation concealment (selection bias) | High risk | Quote: "the patient was randomly allocated to an experimental overlay by the researcher writing the names of all those available at the time on slips of paper which were folded and offered to the nurse to choose one blind. The designated overlay was then placed on the bed" Comment: high risk of bias because it appears difficult to conceal the allocation process as the authors. described. The nurse would have knowledge of which overlays were available at the time of consent. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information provided. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | High risk | Comment: high risk of bias because some individuals may be repeatedly observed and included in analysis (i.e. correlation issue in analysis). For example, Bliss stated "there were no written criteria determining the decision to stop a trial [i.e. using an overlay as the experimental intervention]. This depended mainly on these experienced nurses' unwillingness to allow it to continue because of enlargement of an existing sore, a new blister, discolouration, oedema ... Patients who developed pressure damage between assessments might also be taken off their overlay ... if they later improved ... they were re‐randomized for another trial period [i.e. comparisons of new overlays]". Additionally, overlays were observed for unequal periods of time. Treatments were discontinued or introduced without pre‐specified stopping rules. Some comparisons are not parallel. |
Cassino 2013a.
| Study characteristics | ||
| Methods |
Study objective: to evaluate the performance and effectiveness of an anti‐bedsore, three‐dimensional overlay (Aiartex®, Herniamesh) compared with a commonly‐used gel overlay (Akton® Overlay) Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: 12 weeks Number of arms: 2 Single centre or multi‐sites: multi‐sites Study start date and end date: 2012 Setting: 8 long‐term care centres |
|
| Participants |
Baseline characteristics Inclusion criteria: patients with pressure ulcers from I to IV degree Exclusion criteria: see above Sex (M:F): overall 17:55 Age (years): mean 85.4 (SD 9.1) Baseline skin status: all with ulcers; mean Norton score 9.8 (SD 1.8) Group difference: no significant difference; the group treated with Aiartex© showed a greater number of lesions in the advanced stage Total number of participants: 72 patients Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Aiartex
Akton
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Assignment to one aid or the other was randomised using closed envelopes which were opened at the moment of assignment. In the randomization lists the two aids were balanced at a ratio of 1:1." Comment: unclear risk of bias because the method of generating random sequence unspecified. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Assignment to one aid or the other was randomised using closed envelopes which were opened at the moment of assignment. In the randomization lists the two aids were balanced at a ratio of 1:1." Comment: unclear risk of bias because the method of concealing allocation unspecified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Outcome group: ulcer incidence Quote: "Open randomised multicenter study ..." Comment: high risk of bias because it is an open trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Outcome group: ulcer incidence Quote: "Open randomised multicenter study ..." Comment: high risk of bias because it is an open trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: ulcer incidence Comment: it appears to include all patients in analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Conine 1990.
| Study characteristics | ||
| Methods |
Study objective: to determine the efficacy of the alternating air mattress overlay and the silicone mattress overlay in preventing pressure ulcers Study design: sequential randomised controlled trial Study grouping: parallel group Duration of follow‐up: 3 months Number of arms: 2 Single centre or multi‐sites: single centre Study start date and end date: study took place between 1985 and 1988 Setting: extended care facility for neurological conditions |
|
| Participants |
Baseline characteristics Inclusion criteria: patients in extended care facility for neurological conditions; 18 to 55 years old; with no evidence of skin breakdown for at least 2 weeks prior to the study; and who were at high risk of developing ulcers according to the Norton's Scale (i.e. less than a score of 14). Exclusion criteria: the status of high risk changed during the study Sex (M:F): 31:41 in alternating air mattress; 29:47 in Silicore Age (years): mean 38.8 (SD 13.0) in alternating air mattress; 35.6 (13.0) in Silicore Baseline skin status: mean Norton score 12.9 (SD 2.1) in alternating air mattress; 12.4 (2.3) in Silicore Group difference: no difference Total number of participants: 187 randomised; 148 analysed Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Alternating air mattress
Silicore mattress overlay
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A modified sequential clinical trial ... was used to assign subjects randomly to one of the two mattresses in groups of 20" Comment: the method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Outcome group: primary outcome Comment: no information provided but understandably difficult to blind participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcome group: primary outcome Quote: "The research assistant ... was responsible for the assessment of all outcome measures. She ... was not informed about the study" Comment: low risk of bias because blinding is likely applied. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Outcome group: primary outcome Quote: "Thirty‐nine subjects did not complete the trial for reasons shown in Table 1" Comment: high risk of bias because over 20% of 187 randomised individuals missed and most of the dropouts were due to discomfort. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Daechsel 1985.
| Study characteristics | ||
| Methods |
Study objective: to assess 2 commonly used special mattresses in a randomised trial involving adult non‐geriatric chronic neurologic patients Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: 3 months Number of arms: 2 Single centre or multi‐sites: single centre Study start date and end date: not described Setting: long‐term care hospital for chronic neurologic conditions |
|
| Participants |
Baseline characteristics Inclusion criteria: consenting patients in a long‐term care hospital for chronic neurologic conditions ... a) between 19 and 60 years of age, b) free of any evidence of skin breakdown two weeks prior to the study, and c) considered to be at high risk of developing pressure ulcers based on assessments conducted by the ward team [Norton scale score of 14 or less; and clinical judgement] Exclusion criteria: none Sex (M:F): 10:6 in alternating air mattress; 6:10 in Silicore mattress Age (years): mean 42.6 (SD 13.7) in alternating air mattress; 38.5 (13.82) in Silicore mattress Baseline skin status: mean Norton score 13.35 (SD 1.86) in alternating air mattress; 12.97 (2.28) in Silicore mattress. Group difference: no difference Total number of participants: 32 Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Alternating air mattress
Silicore mattress
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All were randomly assigned to one of the two types of mattresses" Comment: the method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Outcome group: primary outcome Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Outcome group: primary outcome Quote: "one of the investigators (DD) conducted weekly skin checks of the subjects" Comment: high risk of bias for pressure ulcer incidence outcome because it is unlikely that the investigator who assessed skin conditions was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: primary outcome Quote: "Thirty‐two patients met the criteria for this study ... all admitted to the trial and completed it" Comment: no missing data. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Ewing 1964.
| Study characteristics | ||
| Methods |
Study objective: not described Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: 6 months Number of arms: 2 Single centre or multi‐sites: single centre Study start date and end date: not described Setting: hospital |
|
| Participants |
Baseline characteristics Inclusion criteria: criteria not clearly described, but authors mentioned "all inmates of the geriatric unit of a convalescent hospital ... suffering from diseases which (i) confined them to bed for the greater part of the day, or (ii) caused immobilization of their lower limbs by reason of a neurological disorder or by fixation of joints as a sequel of arthritis, or (iii) resulted in impairment of the circulation in the foot and leg" Exclusion criteria: not described Sex (M:F): not described Age (years): on average 72.5 Baseline skin status: not described Group difference: not described Total number of participants: 36 individuals Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Sheepskin
Control
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were ... allotted to a treated or a control group by random selection" Comment: the method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information provided. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no information provided. |
| Other bias | Unclear risk | Comment: no information provided. |
Hoshowsky 1994.
| Study characteristics | ||
| Methods |
Study objective: to examine the effects of 2 OR table mattresses and 1 mattress overlay on intraoperative pressure sore formation Study design: randomised controlled trial Study grouping: parallel group (within‐person comparison) Duration of follow‐up: not given Number of arms: 4 different treatment protocols (made up from 3 types of mattresses) tested in 6 different pairings Single centre or multi‐sites: single centre Study start date and end date: not described Setting: university teaching hospital |
|
| Participants |
Baseline characteristics Inclusion criteria: patients in the study were placement in the supine or prone positions while undergoing surgery; older than 12 years of age; and possession of symmetrical lower limbs Exclusion criteria: not given Sex (M:F): overall 184:321 (across all 6 comparisons) Age (years): overall mean 47 years (SD 17.1) and range 13 to 86 (across all 6 comparisons) Baseline skin status: not given Group difference: no difference within each comparison (due to within‐person comparison made) Total number of participants: standard foam mattress (SFM) vs. foam and gel mattress (FGM), n = 91; VEO‐Action above SFM vs. FGM, n = 92; SFM versus VEO above FGM, n = 62; VEO above SFM versus VEO above FGM, n = 113; SFM versus VEO above SFM, n = 73; and FGM versus VEO above FGM, n = 74. Overall: 505 across 6 comparisons Unit of analysis: treatment sessions of individuals Unit of randomisation (per patient): treatment sessions of individuals |
|
| Interventions |
Intervention characteristics Standard foam mattress
Foam and gel mattress (FGM)
VEO‐Action®
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: unclear risk of bias because each patient served as their own control but within the patient, the allocation of interventions was unspecified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Outcome group: ulcer incidence Quote: "Use of the overlay in this manner prevented the investigators from being blinded at the time of postoperative assessment whenever the overlay was used." Comment: high risk of bias because unblinding is clearly stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Outcome group: ulcer incidence Quote: "Use of the overlay in this manner prevented the investigators from being blinded at the time of postoperative assessment whenever the overlay was used." Comment: high risk of bias because unblinding is clearly stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information provided. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes. No data are reported on the number or rate of pressure ulcers by group and this would be expected. Only statistically significant odds were reported. |
| Other bias | High risk | Comment: the study appears to consider parts of a person's body as unit of analysis. However, the logistic regression as described does not appear to take into account the multiple measures per person. |
IRCT2015110619919N3.
| Study characteristics | ||
| Methods |
Study objective: to investigate the effectiveness of a silicon protective pad on pressure ulcers among patients undergoing coronary artery bypass graft (CABG) surgery Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: discharge Number of arms: 2 Single centre or multi‐sites: not given Study start date and end date: not described Setting: operating room |
|
| Participants |
Baseline characteristics Inclusion criteria: willingness to participate in the study and sign an informed consent form; age 30 to 75 years; undergoing bypass surgery for first time; no history of blood disorders; having a body mass index (BMI) of 18.5 to 24.9; connecting to pump circulation outside the body; no history of bedsores Exclusion criteria: long operation time ‐ more than 5 hours; emergency surgery; having skin problems such as hives, swelling, redness and sensitivity to drugs and environmental factors; having sensorimotor disability Sex (M:F): not given Age (years): not given Baseline skin status: not given Group difference: not given Total number of participants: not described Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Silicone protective pad
Standard mattress
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... 164 patients with coronary artery diseases and candidate for CABG surgery were randomly assigned ..." Comment: unclear risk of bias because the sequence generation process is not specified in this abstract. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided in this abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided in this abstract. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided in this abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information provided in this abstract. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no information provided in this abstract. |
| Other bias | Unclear risk | Comment: no information provided in this abstract. |
Jolley 2004.
| Study characteristics | ||
| Methods |
Study objective: to estimate the effectiveness of a new high‐performance Australian Medical Sheepskin (meeting Australian Standard 4480.1‐1998) in preventing pressure ulcers in a general hospital population at low to moderate risk of these ulcers Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: not specified Number of arms: 2 Single centre or multi‐sites: single centre Study start date and end date: June and November 2000 Setting: teaching hospital |
|
| Participants |
Baseline characteristics Inclusion criteria: all patients who were admitted to the hospital if they were at low to moderate risk of developing a pressure ulcer on the Braden Pressure Ulcer Risk Assessment Scale Exclusion criteria: assessed as at "no risk", or "high risk"; with any pre‐existing pressure ulcer; less than 18 years of age; with an expected length of stay less than 48 hours; or had darkly pigmented skin, making a Stage 1 ulcer difficult to detect Sex (M:F): 107:111 in sheepskin group; 116:107 in referent group Age (years): mean 63.2 (range 18 to 97) in sheepskin group; 61.1 (18 to 99) in referent group Baseline skin status: mean Braden score 15.7 (range 13 to 18) in sheepskin group; 15.9 (13 to 18) in referent group Group difference: no difference Total number of participants: 539 randomised; 441 analysed Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Australian Medical Sheepskin overlay
Referent group
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated to receive either the sheepskin or standard treatment, using numbered cards in individually sealed opaque envelopes; blocks of 16 envelopes (eight of each group) were shuffled before use" Comment: low risk of bias because investigators describe shuffled envelope method of randomisation. |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomly allocated to receive either the sheepskin or standard treatment, using numbered cards in individually sealed opaque envelopes; blocks of 16 envelopes (eight of each group) were shuffled before use" Comment: low risk of bias because researchers could not foresee next assignment because serially numbered, sealed opaque envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Outcome group: primary outcome Quote: "As it was logistically impossible to blind patients, ward staff and research nurses to the treatment group, this was an open label, unblinded trial" Comment: high risk of bias because clearly reported that there was no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Outcome group: primary outcome Quote: "As it was logistically impossible to blind patients, ward staff and research nurses to the treatment group ... Research nurses assessed each participant daily for pressure ulcer risk as described previously, and for skin integrity" Comment: high risk of bias because clearly reported that there was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Outcome group: primary outcome Comment: high risk of bias because 52 of 270 and 46 of 269 who were randomised were excluded from data analysis and of these exclusions 9 had pressure ulcers on day 1. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Lazzara 1991.
| Study characteristics | ||
| Methods |
Study objective: to compare the effectiveness of 2 pressure‐reducing devices [an air‐filled overlay and a gel mattress] in a group of elderly nursing home residents Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: 6 months Number of arms: 2 Single centre or multi‐sites: single centre Study start date and end date: not described Setting: a nursing home |
|
| Participants |
Baseline characteristics Inclusion criteria: all residents determined to be at risk for pressure ulcer development (defined by Norton scale, with a score of greater than 15 as high risk) Exclusion criteria: not specified Sex (M:F): 4:11 in SofCare overlay group; 2:10 in gel mattress group (sex was specified for only some of the participants) Age (years): mean 83.7 (SD 6.87) in SofCare overlay group; mean 83.5 (SD 9.22) in gel mattress group Baseline skin status: mean Norton score 18.06 (SD 3.94) in SofCare overlay group; 17.88 (3.80) in gel mattress group Group difference: no difference Total number of participants: 74 (those followed‐up for 4 to 6 months were analysed) Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics SofCare overlay
Gel mattress
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a table of random numbers, each subjected was placed into ..." Comment: low risk of bias because a proper randomisation was done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcome group: primary outcome Quote: "Patients in both study groups were assessed by the same researcher for the presence of pressure ulcer development over areas of bony prominence" Comment: unclear risk of bias because no information on blinding was reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Outcome group: primary outcome Quote: "... the initial study population was 76 subjects ..." Quote: "A total of 74 subjects were in the study ... Two subjects were excluded from the study ... Those subjects who participated in the study for four to six months were included in the data analysis. Eighteen residents developed pressure ulcers during the course of the study, nine residents had preexisting pressure ulcers, and 36 residents did not develop a pressure ulcer" Comment: unclear risk of bias because the patient flow is insufficiently clear and the proportion of missing data is probably between 10/74 (13.5%) and 13/74 (17.6%). |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
McGowan 2000.
| Study characteristics | ||
| Methods |
Study objective: to estimate the relative incidence of hospital‐acquired pressure ulcers among elderly orthopaedic patients nursed on a standard hospital mattress plus an Australian Medical Sheepskin overlay, compared to those nursed on either a standard mattress alone or a standard mattress with other low technology constant pressure supports. Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: not reported Number of arms: 2 Single centre or multi‐sites: multi‐sites Study start date and end date: not described Setting: acute care settings (hospitals) |
|
| Participants |
Baseline characteristics Inclusion criteria: orthopaedic patients aged ≥ 60; assessed as being at low or moderate risk of pressure ulcer development by Braden scale; intact skin; anticipated length of stay > 48 hours Exclusion criteria: no risk (requiring no intervention) or high risk (requiring more complex interventions) for developing pressure ulcers; patients with a pre‐existing pressure ulcer; non‐English speaking patients (unless an interpreter was available); patients with an anticipated stay of less than 48 hours; coloured skin patients where stage 1 ulcer detection is difficult Sex (M:F): 72:83 in sheepskin group; 55:87 in control group Age (years): mean 73.6 (SD 8.08) in sheepskin group; 74 (7.65) in control without sheepskin group Baseline skin status: mean Braden score 13.9 (1.08) in sheepskin group; 14.01 (1.4) in control group. All at risk but with intact skin Group difference: no difference Total number of participants: n = 297 Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Australian Medical Sheepskin overlay
Control (standard hospital mattress)
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated (using sealed envelopes) by research nurses to receive one of two interventions" Comment: unclear risk of bias because random sequence generation method unclear. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated (using sealed envelopes) by research nurses to receive one of two interventions" Comment: unclear risk of bias because the method of concealing allocation is not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Outcome group: all outcomes Quote: “Blinded outcome assessments were not possible because the support surfaces could not be disguised and patients could not be moved off the bed for assessment of their pressure ulcers” Comment: high risk of bias because this implies blinding of participants and personnel is not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Outcome group: all outcomes Quote: “Blinded outcome assessments were not possible because the support surfaces could not be disguised and patients could not be moved off the bed for assessment of their pressure ulcers” Comment: high risk of bias because it is clearly stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: all outcomes Quote: “data collected for patients up until the time of withdrawal has been included in the analysis with the exception of five controls and two patients from the experimental group for whom study participation time was not available” Quote: "A total of 268 patients (124 control and 144 experimental) were able to complete the rating scale on the level of comfort of the bed surface." Comment: low risk of bias because ITT analysis was conducted for pressure ulcer outcome and low rate of missing data for comfort outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Mistiaen 2010.
| Study characteristics | ||
| Methods |
Study objective: to investigate the effectiveness of the Australian Medical Sheepskin (AMS) in the prevention of sacral pressure ulcers in somatic nursing home patients Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: 30 days Number of arms: 2 Single centre or multi‐sites: multi‐sites Study start date and end date: not described Setting: nursing home |
|
| Participants |
Baseline characteristics Inclusion criteria: admitted for a primarily somatic reason, adult (aged 18 years and older), expected stay > 1 week, free of pressure ulcers on the sacrum at admission, not having darkly pigmented skin (because of difficulty in diagnosing grade 1 pressure ulcer), and no known allergy to wool Exclusion criteria: admitted for a primarily psycho‐geriatric reason Sex (M:F): 86:209 in sheepskin group; 97:196 in usual care group Age (years): mean 78 (range 26 to 97) in sheepskin group; 78 (27 to 98) in usual care group Baseline skin status: 47% with Braden score ≤ 18 in both sheepskin (n = 295) and usual care (n = 293) groups; no pre‐existing ulcer Group difference: no difference Total number of participants: 588 Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Australian Medical Sheepskins (AMS)
Usual care
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To ensure concealment of allocation, a randomization scheme was created in SPSS by assigning the intervention to a random sample of circa 50% in a list of 1,500 numbers and assigning the control group to the rest" Comment: low risk of bias due to the use of a proper randomisation method (computer randomisation). |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure concealment of allocation, a randomization scheme was created in SPSS by assigning the intervention to ... This allocation of the group (sheepskin, usual care) was then blinded on a paper list numbered 1 through 1,500 by a secretary not further involved in the project ... the admitting nurse called the principal investigator who then disclosed the allocation from that blinded list to the nurse and she, in turn, to the patient" Comment: low risk of bias due to the use of a proper method to conceal the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Outcome group: all outcomes Quote: "it is impossible to blind health professionals or patients to whether someone is in the experimental group or not, only the patient allocation itself was blinded to all parties involved" Comment: high risk of bias since it was clearly reported there was no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Outcome group: all outcomes Quote: "it is impossible to blind health professionals or patients to whether someone is in the experimental group or not, only the patient allocation itself was blinded to all parties involved ... assessed daily by the nurse caring for that patient that day" Comment: high risk of bias since it was clearly reported there was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: all outcomes Quote: "According to the intention‐to‐treat principle, all patients were analyzed in the groups they were randomised to" Comment: low risk of bias because low rates of missing data in both groups (ITT analysis is claimed but is not actually done). |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Nixon 1998.
| Study characteristics | ||
| Methods |
Study objective: to compare the postoperative pressure sore incidence in patients positioned on the standard operating table mattress with those positioned on the dry visco‐elastic polymer pad Study design: randomised controlled trial Study grouping: parallel group (sequential design) Duration of follow‐up: 8 days Number of arms: 2 Single centre or multi‐sites: multi‐sites Study start date and end date: not described; recruited from November 1994 to June 1996 Setting: operating rooms of hospitals |
|
| Participants |
Baseline characteristics Inclusion criteria: patients aged ≥ 55 years, admitted for elective major general, gynaecological or vascular surgery in supine or lithotomy position and free of preoperative pressure damage greater than grade 1 Exclusion criteria: liver, urology and breast surgery; pressure damage of Grade 1a or above observed preoperatively; ward staff provision of preoperative alternating pressure mattress; dark skin pigmentation which precludes reliable identification of Grade 0 and Grade 1a skin assessments; skin conditions over the sacrum, buttocks or heels which preclude reliable identification of Grade 0 and Grade 1a skin assessments Sex (M:F): 119:101 in dry visco‐elastic polymer pad; 116:107 in standard operating theatre table mattress Age (years): 124 participants between 55‐69 years and 98 participants 70+ years in dry visco‐elastic polymer pad group; 128 participants between 55‐69 years and 96 participants 70+ years in standard operating theatre table mattress group Baseline skin status: categories of risk scores reported; free of pressure ulcers greater than grade 1 Group difference: no difference Total number of participants: n = 446 Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Dry visco‐elastic polymer pad on operating table
Standard operating theatre table mattress
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a telephone randomisation schedule was developed within random permuted blocks of 6, with a run‐in of 8" Comment: low risk of bias because study likely used a proper randomisation method. |
| Allocation concealment (selection bias) | Low risk | Quote: "a telephone randomisation schedule was developed, and managed by the Northern and Yorkshire Clinical Trials and Research Unit" Comment: low risk of bias because study likely concealed allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Outcome group: pressure ulcer outcome Quote: "All pre and intra‐operative data were recorded by the research nurse, and post‐operative data recorded by recovery and ward staff who were blind to the intraoperative mattress allocation. The record pertaining to the intra‐operative randomised mattress allocation remained separate from the main data collection proforma to maintain the blind" Comment: unclear risk of bias because there is attempt to blind outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: pressure ulcer outcome Comment: low risk of bias because although intention‐to‐treat (ITT) analyses claimed by authors, low proportions of missing data (17 of 222 vs 13 of 224) occurred in analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Ricci 2013.
| Study characteristics | ||
| Methods |
Study objective: to assess the efficacy of Aiartex® compared with Akton® for the prevention of pressure ulcers development in aged patients at moderate/high risk Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: 4 weeks Number of arms: 2 Single centre or multi‐sites: multi‐sites Study start date and end date: May to September 2011 Setting: 2 long‐term care units |
|
| Participants |
Baseline characteristics Inclusion criteria: patients of both genders aged 65 years old or more, who had an anticipated hospitalisation period in the same unit lasting at least 28 days after assignment to the study groups; Braden score > 8 to < 14; Norton score > 6 to < 12; patients with pressure sores stage 1 eligible Exclusion criteria: those with ulcers of stage 2 or above; terminal or severely compromising illness, AIDS or hepatitis C; ongoing systemic corticosteroid therapy, immuno‐suppressant therapy or chemotherapy; enrolment within the past 3 months in any study related to wound healing; allergy to mattress overlay components Sex (M:F): 6:19 in Aiartex; 2:23 in Akton Age (years): mean 83.6 (SD 6.9) in Aiartex; 85.8 (6.9) in Akton Baseline skin status: mean Braden score 9.6 (SD 1.4) in Aiartex; 10.4 (1.3) in Akton Group difference: no difference Total number of participants: 50 Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Aiartex
Akton®
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | info@herniamesh.it and the contact author were contacted to clarify Aiartex but they did not add useful information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised according to a computer generated pre‐defined assignment list in sealed envelopes to use a standard mattress plus either three‐dimensional or viscoelastic overlay" Comment: low risk of bias due to the use of a proper randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomised according to a computer generated pre‐defined assignment list in sealed envelopes ..." Comment: unclear risk of bias because it is unclear if envelopes are opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Outcome group: all outcomes Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcome group: all outcomes Quote: "Patient’s conditions (any presence of skin lesions, pressure ulcers, erythema, area of skin maceration) were then re‐assessed at days 7, 14, 21, and day 28 (the last visit)" Quote: "The occurrence of any adverse event or allergic reaction was evaluated at each visit" Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: all outcomes Comment: low risk of bias because no missing data. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Russell 2000.
| Study characteristics | ||
| Methods |
Study objective: to determine the efficacy and safety of a multi‐cell pulsating dynamic mattress system in comparison with conventional management for the prevention of pressure ulcers in the operative and postoperative period in patients having cardiovascular surgery Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: 7 days Number of arms: 2 Single centre or multi‐sites: single centre Study start date and end date: not described Setting: hospital |
|
| Participants |
Baseline characteristics Inclusion criteria: 18 years of age or older and scheduled for cardiovascular surgery with general anaesthesia for at least 4 hours with an actual operative time of 3 hours or more Exclusion criteria: had a pressure ulcer at the baseline visit Sex (M:F): 75:23 in multi‐cell pulsating dynamic mattress group; 75:25 in conventional management group Age (years): mean 65.2 (SD 10.9) in multi‐cell pulsating dynamic mattress group; 65.2 (10.6) in conventional management group Baseline skin status: mean Knoll score 3.6 (SD 1) in multi‐cell pulsating dynamic mattress group; 3.8 (1) in conventional management group; no pressure ulcers Group difference: no difference Total number of participants: n = 198 Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Multi‐cell pulsating dynamic mattress
Conventional management
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Before surgery, patients were randomly assigned to either the multi‐cell pulsating dynamic mattress system or conventional management. Randomization was done blindly by using a sealed opaque envelope that contained the randomization information (i.e. multi‐cell pulsating dynamic mattress system vs. conventional management)" Comment: unclear risk of bias because randomisation method is not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was done blindly by using a sealed opaque envelope that contained the randomization information (i.e. multi‐cell pulsating dynamic mattress system vs. conventional management)" Comment: unclear risk of bias because randomisation method is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Outcome group: primary outcome Comment: high risk of bias because it is unlikely that participants were blinded though no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Outcome group: primary outcome Quote: "Patients were examined immediately post‐surgery for pressure ulcers, including number, stage (I to IV), size (area), location, and appearance. Patients were assessed daily for ... presence of pressure ulcers. A skin risk assessment was performed on days 1, 4, and 7 and on other days if a change in status was noted. Adverse events and concomitant medications were recorded daily" Comment: unclear risk of bias because information on outcome assessment is insufficient for a proper judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: primary outcome Quote: "Baseline characteristics and safety were evaluated for all randomised patients (i.e. intent‐to‐treat sample) ... The intent‐to‐treat sample included all patients who signed consent forms and who were placed either on a multi‐cell pulsating dynamic mattress system or on a conventional mattress and had at least 1 day of observation post‐surgery ... An evaluable sample of patients was defined as patients who signed consent forms, had a surgery length of at least 3 hours, and had a minimum of 3 days of observation post‐surgery ... One analysis included the intent‐to‐treat sample (multi‐cell pulsating dynamic mattress system, n = 89; conventional management, n = 96)" Comment: low risk of bias because of the use of intention‐to‐treat (ITT) analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Sideranko 1992.
| Study characteristics | ||
| Methods |
Study objective: to compare the pressure‐reducing properties of 3 types of mattress overlays (water, alternating air and static air mattress surfaces) as used with bed‐bound patients in a clinical setting Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: mean 10.0 (SD 10.9) days of surgical intensive care unit (SICU) stay in alternating air group; 9.4 (8.8) in static air group; 8.9 (7.1) in water group Number of arms: 3 Single centre or multi‐sites: single centre Study start date and end date: not described Setting: 2 surgical ICUs of a hospital |
|
| Participants |
Baseline characteristics Inclusion criteria: a minimum SICU stay of 48 hr; presence of ventilatory support, or some form of haemodynamic support on admission Exclusion criteria: those with any evidence of existing skin breakdown upon admission to the SICUs Sex (M:F): 33:24 across groups Age (years): mean 67.9 (SD 11.1) in alternating air group; 63.6 (18.6) in static air group; 66.1 (15.6) in water group. Baseline skin status: free of existing skin breakdown Group difference: no difference Total number of participants: n = 57 Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Alternating air
Static air
Water
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... subjects were randomly assigned to be placed on one of the three surfaces studied" Comment: unclear risk of bias because the method of randomisation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: all outcomes (primary outcome) Comment: no missing assumed. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Stapleton 1986.
| Study characteristics | ||
| Methods |
Study objective: not provided Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: not described Number of arms: 3 Single centre or multi‐sites: single centre Study start date and end date: not described Setting: acute care setting |
|
| Participants |
Baseline characteristics Inclusion criteria: female elderly patients with fractured neck of femur, without existing pressure ulcers, Norton score 14 or less Exclusion criteria: patients not meet the criteria, or admitted with existing pressure sores Sex (M:F): all female patients (0:32 in Large Cell Ripple group; 0:34 in polyether foam pad group; 0:34 in Spenco pad group). Age (years): mean 81 across groups Baseline skin status: mean Norton score 12.0 in Large Cell Ripple group; 12.8 in polyether foam pad group; 12.9 in Spenco pad group; no existing pressure ulcers Group difference: no difference Total number of participants: n = 100 Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Large Cell Ripple (Talley)
Polyether foam pad
Spenco pad
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients for the first two groups were selected by lottery, and thereafter patients were allocated to each group systematically, in rotation” Comment: unclear risk of bias because it is unclear if a proper randomisation method was applied. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information provided. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no information provided. |
| Other bias | Unclear risk | Comment: no information provided. |
Van Leen 2018.
| Study characteristics | ||
| Methods |
Study objective: to test the pressure ulcer preventive effect of this system [a pressure‐relieving, shear stress‐diminishing, and microclimate‐controlling skin interface multilayer support system (Bedcare; Sense Textile, ‘s‐Hertogenbosch, the Netherlands)] compared with a visco‐elastic foam mattress alone Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: 12 weeks of study period Number of arms: 2 Single centre or multi‐sites: multi‐sites Study start date and end date: not described Setting: nursing homes |
|
| Participants |
Baseline characteristics Inclusion criteria: all residents at medium/high risk (Braden score < 16) of pressure ulcers ... age older than 60 years, life expectancy greater than 3 months, and informed consent Exclusion criteria: a pressure ulcer in the last 3 months, participation in a comparable trial, or a physical and/or mental condition that could interfere with participation (such as sepsis, immune disease, palliative status) Sex (M:F): 71.8% of 103 females in multilayer mattress group; 69.9% of 103 females in visco‐elastic foam group Age (years): 83.1 in multilayer mattress group; 81.7 in visco‐elastic foam group Baseline skin status: Braden score 13.1 in multilayer mattress group; 13.3 in visco‐elastic foam group; at risk but no existing ulcers Group difference: no difference Total number of participants: n = 206 Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Multilayer mattress system
Visco‐elastic foam mattress
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization into 2 groups was performed by using the Castor randomization software (version 1.44; Mionix, Malmö, Sweden)." Comment: low risk of bias because of the use of a proper randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Data were collected weekly, controlled by an independent research nurse." Comment: unclear risk of bias because of the lack of sufficient information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low risk of bias because it appears to include all 206 patients in analysis. |
| Selective reporting (reporting bias) | High risk | Comment: high risk of bias because the study protocol is available from https://www.trialregister.nl/trial/4435 and it is clear that the pre‐specified costs outcome is not presented. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Vermette 2012.
| Study characteristics | ||
| Methods |
Study objective: to compare the efficacy of different surfaces in the prevention of pressure ulcers; to compare costs associated with the use of an inflated static overlay (ISO) with the standard treatment, which in the first author’s facility consists of renting a microfluid static overlay (MSO) or a low‐air‐loss dynamic mattress (LALDM) with pulsation for moderate to very high‐risk patients; to evaluate patient comfort Study design: randomised controlled trial Study grouping: parallel group Duration of follow‐up: maximum 14 days Number of arms: 2 Single centre or multi‐sites: single centre Study start date and end date: recruited from September 2009 to mid‐April 2010 Setting: acute care setting (a medical, surgical, active geriatric, or an intensive care unit (ICU) ward of a hospital) |
|
| Participants |
Baseline characteristics Inclusion criteria: had a Braden score of ≤ 14; had no skin lesion(s); were ≥ 18 years; weighed < 300lb; and submitted signed consent Exclusion criteria: not described Sex (M:F): 21:34 in MSO or LALDM group; 23:32 in ISO group Age (years): mean 77.7 (SD 10.6) in MSO or LALDM group, 77.9 (14.6) in ISO group Baseline skin status: mean Braden 11.8 (SD 1.6) in MSO or LALDM group; 12.3 (1.3) in ISO group; at risk and no skin lesions Group difference: no difference Total number of participants: n = 110 Unit of analysis: individuals Unit of randomisation (per patient): individuals |
|
| Interventions |
Intervention characteristics Microfluid static overlay or low‐air‐loss dynamic mattress
Inflated static overlay
|
|
| Outcomes |
Proportion of participants developing a new pressure ulcer
Time to pressure ulcer incidence
Support‐surface‐associated patient comfort
All reported adverse events using allocated support surfaces
Health‐related quality of life (HRQOL)
Cost‐effectiveness
Outcomes that are not considered in this review but reported in trials:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned a rented surface (MSO or LALDM) or an ISO. Once subject consent was obtained and signed, the allocation sequence for mattress type was done by draw by the research nurse using an opaque envelope and the subject witnessing the draw" Comment: low risk of bias because it is likely a proper randomisation method was used. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The allocation sequence was concealed from the research nurse enrolling and assessing the participants" Comment: unclear risk of bias because concealment approach is not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Outcome group: all outcomes Quote: "The purpose of this unblinded, randomised, prospective study ..." Quote: "Blinding was not obtained for the patient, the clinical staff, or the research evaluator because the surfaces were visible" Comment: high risk of bias because unblinding is clearly stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Outcome group: all outcomes Quote: "The purpose of this unblinded, randomised, prospective study ..." Quote: "Blinding was not obtained for the patient, the clinical staff, or the research evaluator because the surfaces were visible" Comment: high risk of bias because unblinding is clearly stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Outcome group: primary outcome Quote: "Analyses were performed in intention‐to‐treat involving all 110 randomly assigned patients" Comment: intention‐to‐treat (ITT) analysis conducted. Outcome group: comfort outcome Quote: "Of the 110 participants, 68 expressed opinions regarding comfort" Comment: high risk of bias because 42 of 110 missed. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12618000319279 | Treatment study |
| Allman 1987 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Andrews 1988 | Ineligible study design ‐ not a RCT |
| Anonymous 2006 | Ineligible study design ‐ review article |
| Ballard 1997 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Beeckman 2019 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Bell 1993 | Ineligible study design ‐ not a RCT |
| Bennett 1998 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Berthe 2007 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Bliss 1966 | Ineligible study design ‐ not a RCT |
| Bliss 1967 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Bliss 1993 | Ineligible study design ‐ review article |
| Bliss 1995b | Ineligible study design ‐ review article |
| Bliss 2003 | Reproduction of previous work |
| Bliss 2004 | Commentary on a trial |
| Branom 1999 | Treatment study |
| Branom 2001 | Treatment study |
| Brown 2001 | Summary of the Cochrane Review McInnes 2015 |
| Bueno de Camargo 2018 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Cadue 2008 | This RCT compared heel‐suspending device with the package of interventions |
| Caley 1994 | Treatment study |
| Cassino 2013b | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Cavicchioli 2007 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Chaloner 2000a | Incorrect randomisation method (quasi‐randomisation) |
| ChiCTR1800017466 | Ineligible interventions |
| Chou 2013 | Review articles |
| Cobb 1997 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Collier 1996 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Cooper 1998 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Cummins 2019 | Ineligible study design ‐ quality improvement project without RCT design |
| Day 1993 | Treatment study |
| Defloor 2005 | Ineligible interventions ‐ different combinations of turning and support surfaces under evaluations |
| Demarre 2012 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| De Oliveira 2017 | Review article |
| Devine 1995 | Treatment study |
| Economides 1995 | This RCT was to observe the breakdown of flaps after operations rather than the incidence of new ulcers |
| Evans 2000 | Treatment study |
| Exton‐Smith 1982 | This trial used alternation to allocate patients into groups. Proper randomisation not completed. |
| Ferrell 1993 | Treatment study |
| Ferrell 1995 | Treatment study |
| Feuchtinger 2006 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Finnegan 2008 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Fleischer 1997 | Ineligible study design |
| García Fernández 2004 | Commentary on a RCT |
| Gazzerro 2008 | Ineligible outcome (wound healing of flap surgery) |
| Gebhardt 1994a | Incorrect randomisation method (randomisation based on participants' hospital numbers) |
| Gebhardt 1994b | Incorrect randomisation method (randomisation based on participants' hospital numbers) |
| Gebhardt 1996 | Incorrect randomisation method |
| Geelkerken 1994 | Commentary |
| Goldstone 1982 | Incorrect randomisation method |
| Gray 1994 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Gray 2000 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Gray 2008 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Greer 1988 | Treatment study |
| Grindley 1996 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Groen 1999 | Treatment study |
| Gunningberg 2000 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Gunningberg 2001 | Ineligible study design (cross‐sectional design) |
| Haalboom 1994 | Commentary |
| Hale 1990 | Ineligible study design (cost analysis without RCT data) |
| Hampton 1997 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Hampton 1998 | Ineligible study design (not a RCT) |
| Hampton 1999 | Ineligible study design (not a RCT) |
| Hawkins 1997 | Ineligible study design (not a RCT) |
| Hofman 1994 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Holzgreve 1993 | Ineligible study design (not a RCT) |
| Hommel 2008 | Ineligible study design (not a RCT) |
| Hoskins 2007a | Summary of findings of Nixon 2006 |
| Hoskins 2007b | Summary of findings of Nixon 2006 |
| Huang 2013 | Review article |
| Huang 2018 | Ineligible interventions (head pad rather than beds or mattresses) |
| Hungerford 1998 | Commentary on a RCT |
| Iglesias 2006 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Inman 1993 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| IRCT2016091129781N1 | Ineligible interventions (cushions rather than beds or mattresses) |
| Ismail 2001 | Support surfaces used were not clearly specified. We do not know if the interventions were eligible for this review. |
| Jiang 2014 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| JPRN‐UMIN000029680 | Treatment study |
| Kemp 1993 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Keogh 2001 | Ineligible interventions (profiling bed rather than beds or mattresses) |
| Klein 1989 | Review article |
| Laurent 1998 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Lee 1974 | Ineligible study design (not a RCT) |
| Maklebust 1988 | Ineligible interventions (cushions rather than beds or mattresses) |
| Malbrain 2010 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Marutani 2019 | Incorrect randomisation method |
| Mastrangelo 2010a | Treatment study |
| McGinnis 2011 | Review article |
| McInnes 2015 | Review article |
| McInnes 2018 | Review article |
| Mendoza 2019 | Ineligible participants and outcome (flap closure) |
| Mistiaen 2010a | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Nakahara 2012 | Ineligible study design (not a RCT) |
| NCT01402765 | Ineligible outcome (interface pressure) |
| NCT02565797 | Ineligible study design (case control design) |
| NCT02634892 | RCT with the comparison of reactive air surfaces versus standard hospital surfaces withdrawn due to funding issue |
| NCT02735135 | Withdrew trial record, giving 'methodological difficulties' as the reason |
| NCT03048357 | Ineligible interventions (rotation therapy versus turning) |
| NCT03211910 | Ineligible interventions (not beds or mattresses) |
| NCT03351049 | Ineligible interventions (reactive air surfaces versus reactive surfaces) |
| Nixon 2006 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Nixon 2019 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Ooka 1995 | Ineligible study design (not a RCT) |
| Osterbrink 2005 | Treatment study |
| Ozyurek 2015 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Park 2017 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Phillips 1999 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Price 1999 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Pring 1998 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Rae 2018 | Review article |
| Rafter 2011 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Reddy 2006 | Review article |
| Reddy 2008 | Review article |
| Ricci 2013a | Treatment study |
| Rithalia 1995 | Ineligible participants (healthy people) |
| Rosenthal 2003 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Russell 1999 | Treatment study |
| Russell 2000b | Treatment study |
| Russell 2000c | Treatment study |
| Russell 2003a | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Russell 2003b | Treatment study |
| Sanada 2003 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Santy 1994 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Santy 1995 | Review article |
| Sauvage 2017 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Scheffel 2011 | Summary of a review |
| Schultz 1999 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Scott 2000 | Ineligible interventions |
| Scott‐Williams 2006 | Ineligible study design (not a RCT) |
| Serraes 2018 | Review article |
| Shakibamehr 2019 | Ineligible interventions (cushions rather than beds or mattresses) |
| Sharp 2007 | Ineligible study design |
| Shi 2018a | Review article |
| Smith 2013 | Review article |
| Stannard 1993 | Commentary on a RCT |
| Sterzi 2003 | Ineligible study design (not a RCT) |
| Strauss 1991 | Treatment study |
| Takala 1994 | Ineligible study design (not a RCT) |
| Takala 1996 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Taylor 1999 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Tewes 1993 | Review article |
| Theaker 2005 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Vanderwee 2005 | Ineligible intervention (imbalanced use of co‐interventions between study arms) |
| Van Leen 2011 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Van Leen 2013 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Van Rijswijk 1994 | Commentary |
| Vyhlidal 1997 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Wallace 2009 | Review article |
| Whitney 1984 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Whittingham 1999 | Ineligible interventions (i.e. comparisons of interventions that are ineligible for inclusion in this review) |
| Yao 2018 | Review article |
Characteristics of studies awaiting classification [ordered by study ID]
Chaloner 2000.
| Methods | Not available |
| Participants | Not available |
| Interventions | Two types of alternating pressure air surfaces |
| Outcomes | Not available |
| Notes | Unable to obtain full‐text |
Gardner 2008.
| Methods | Randomised controlled trial (2 arm) |
| Participants |
Inclusioncriteria: patients at risk of pressure injury (Waterlow score > 9) Exclusioncriteria: under 16 years, unable to tolerate extended time lying supine and with sacral pressure injury of Stage 2 or above Number of participants: 66 Age: on average 68 years Gender (M:F): 34:25 Baseline skin status: at risk of ulcer (Waterlow score > 9), without existing severe ulcers |
| Interventions | Airflotation and Ruby mattress
ComfortPlus mattress
|
| Outcomes |
Outcomes of the interest of this review
Outcomes unrelated to this review
|
| Notes |
Henn 2004.
| Methods | Not available |
| Participants | Not available |
| Interventions | Alternating pressure air surfaces and a type of surface that cannot be defined |
| Outcomes | Not available |
| Notes | Unable to obtain full‐text |
Knight 1999.
| Methods | Not available |
| Participants | Not available |
| Interventions | Pressure‐relieving surfaces that cannot be defined |
| Outcomes | Not available |
| Notes | Unable to obtain full‐text |
Mastrangelo 2010b.
| Methods | Not available |
| Participants | Not available |
| Interventions | 'Anti‐decubitis lesion mattress cover' that cannot be defined |
| Outcomes | Not available |
| Notes | Unable to obtain full‐text |
Melland 1998.
| Methods | Not available |
| Participants | Not available |
| Interventions | 'Freedom bed' that cannot be defined |
| Outcomes | Not available |
| Notes | Unable to obtain full‐text |
Differences between protocol and review
We changed the title of this review to 'Alternative reactive support surfaces (non‐foam and non‐air‐filled) for preventing pressure ulcers' whilst the title of the published protocol was 'Alternative reactive support surfaces (non‐foam or air‐filled) for preventing pressure ulcers' (Shi 2020).
Two review authors independently assessed the titles and abstracts of the new search results for relevance using Rayyan rather than using Covidence.
For new included studies, one review author independently extracted data and another review author checked all data, rather than two review authors carrying out independent data extraction.
When a study only had complete case data, we considered complete case data in the related main analysis (i.e. assuming no missing data issue). This was not pre‐planned.
We presented separate 'Summary of findings' tables for six of the 12 comparisons evaluated in this review. We did not present the tables for the following six comparisons: reactive water surfaces versus foam surfaces, reactive water surfaces versus reactive fibre surfaces, reactive gel surfaces versus reactive gel surfaces, reactive gel surfaces versus foam surfaces, reactive foam and gel surfaces versus reactive gel surfaces, and reactive foam and gel surfaces versus foam surfaces.
Where we did not pool data, we conducted a GRADE assessment and presented these assessments in a narrative format in 'Summary of findings' tables. This was not pre‐planned.
Contributions of authors
Chunhu Shi: conceived the review; designed the review; coordinated the review; extracted data; analysed or interpreted data; undertook quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing or editing the review; wrote to study authors/experts/companies; approved the final review prior to publication; is guarantor of the review.
Jo C Dumville: conceived the review; designed the review; coordinated the review; checked quality of data extraction; analysed or interpreted data; checked quality assessment; checked quality of statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; secured funding; performed previous work that was the foundation of the current review; approved the final review prior to publication.
Nicky Cullum: conceived the review; designed the review; checked quality of data extraction; analysed or interpreted data; contributed to writing or editing the review; advised on the review; secured funding; performed previous work that was the foundation of the current review; approved the final review prior to publication.
Sarah Rhodes: conceived the review; designed the review; checked quality of data extraction; checked quality assessment; checked quality of statistical analysis; contributed to writing or editing the review; advised on the review; approved the final review prior to publication.
Elizabeth McInnes: conceived the review; designed the review; coordinated the review; checked quality of data extraction; checked quality assessment; contributed to writing or editing the review; advised on the review; performed previous work that was the foundation of the current review; approved the final review prior to publication.
Contributions of the editorial base
Gill Norman (Editor): edited the protocol; advised on methodology, interpretation and content; approved the final protocol prior to publication.
Gill Rizzello (Managing Editor): coordinated the editorial process; advised on content; edited the protocol and the review.
Sophie Bishop (Information Specialist): designed the search strategy and edited the search methods section.
Tom Patterson (Editorial Assistant): edited the reference sections of the protocol and the review.
Sources of support
Internal sources
Division of Nursing, Midwifery and Social Work, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, UK
External sources
-
National Institute for Health Research, UK
This project is funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐1217‐20006). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
-
NIHR Manchester Biomedical Research Centre (BRC), UK
This research was co‐funded by the NIHR Manchester BRC. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
-
National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
-
National Institute for Health Research Applied Research Collaboration Greater Manchester, UK
Nicky Cullum and Jo Dumville’s work on this project was partially funded by the National Institute for Health Research Applied Research Collaboration Greater Manchester. The views expressed in this publication are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Declarations of interest
Chunhu Shi: I received research funding from the National Institute for Health Research (NIHR) (Research for Patient Benefit, Evidence synthesis for pressure ulcer prevention and treatment, PB‐PG‐1217‐20006). I received support from the Tissue Viability Society to attend conferences unrelated to this work. The Doctoral Scholar Awards Scholarship and Doctoral Academy Conference Support Fund (University of Manchester) also supported a PhD and conference attendance respectively, both were unrelated to this work.
Jo Dumville: I am Chief Investigator on a National Institute for Health Research grant that funded the conduct of this review (Research for Patient Benefit, Evidence synthesis for pressure ulcer prevention and treatment, PB‐PG‐1217‐20006). This research was co‐funded by the National Institute for Health Research Manchester Biomedical Research Centre and partly funded by the National Institute for Health Research Applied Research Collaboration Greater Manchester.
Nicky Cullum: I am Co‐investigator on a National Institute for Health Research grant that funded the conduct of this review (Research for Patient Benefit, Evidence synthesis for pressure ulcer prevention and treatment, PB‐PG‐1217‐20006). This research was co‐funded by the National Institute for Health Research Manchester Biomedical Research Centre, and partly funded by the National Institute for Health Research Applied Research Collaboration Greater Manchester.
My previous and current employers received research grant funding from the NHS Research and Development programme and subsequently the NIHR for previous versions of this review. The funders had no role in the conduct of the review. My previous employer received research grant funding from the NIHR for an RCT comparing different alternating pressure air surfaces for pressure ulcer prevention. This RCT (for which I was the Chief Investigator) was not eligible for inclusion in this review.
Sarah Rhodes: my salary is funded from three NIHR grants and a grant from Greater Manchester Cancer.
Elizabeth McInnes: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Andersen 1982 {published data only}
- Andersen KE, Jensen O, Kvorning SA, Bach E. Decubitus prophylaxis: a prospective trial on the efficacy of alternating-pressure air-mattresses and water-mattresses. Acta Dermatovener (Stockholm) 1982;63:227-30. [PubMed] [Google Scholar]
Aronovitch 1999 {published data only}
- Aronovitch SA, Wilber M, Slezak S, Martin T, Utter D. A comparative study of an alternating air mattress for the prevention of pressure ulcers in surgical patients. Ostomy/Wound Management 1999;45(3):34-44. [PubMed] [Google Scholar]
- Aronovitch SA. A comparative, randomized, controlled study to determine safety and efficacy of preventive pressure ulcer systems: preliminary analysis. Advances in Wound Care 1998;11 Suppl 3:15-6. [PubMed] [Google Scholar]
Bliss 1995a {published data only}
- Bliss MR. Preventing pressure sores in elderly patients: a comparison of seven mattress overlays. Age and Ageing 1995;24:297-302. [DOI] [PubMed] [Google Scholar]
- Bliss MR. Randomised controlled trial of seven pressure relieving mattress overlays for preventing pressure sores in elderly patients. In: Conference of the Tissue Viability Society; 1994; Harrogate (UK). 1994:5.
Cassino 2013a {published data only}
- Cassino R, Ippolito AM, Ricci E. Comparison of two mattress overlays in the prevention of pressure ulcers. Acta Vulnologica 2013;11(1):15-21. [Google Scholar]
Conine 1990 {published data only}
- Conine TA, Daechsel D, Choi AK, Lau MS. Costs and acceptability of two special overlays for the prevention of pressure sores. Rehabilitation Nursing 1990;15(3):133-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Conine TA, Daechsel D, Lau MS. The role of alternating air and Silicore overlays in preventing decubitus ulcers. International Journal of Rehabilitation Research 1990;13(1):133-7. [DOI] [PubMed] [Google Scholar]
Daechsel 1985 {published data only}
- Daechsel D, Conine TA. Special mattresses: effectiveness in preventing decubitus ulcers in chronic neurologic patients. Archives of Physical Medicine and Rehabilitation 1985;66(4):246-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ewing 1964 {published data only}
- Ewing MR, Garrow C, Presley TA, Ashley C, Kisella NM. Further experiences in the use of sheep skins as an aid in nursing. Australian Nurses' Journal 1964;1964 September:215-9. [Google Scholar]
Hoshowsky 1994 {published data only}
- Hoshowsky VM, Schramm CA. Intraoperative pressure sore prevention: an analysis of bedding materials. Research in Nursing & Health 1994;17(5):333-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
IRCT2015110619919N3 {published data only}
- IRCT2015110619919N3. The effect of silicone protective pad on pressure ulcer. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015110619919N3 2016.
Jolley 2004 {published data only}
- Jolley DJ, Wright R, McGowan S, Hickey MB, Campbell DA, Sinclair RD, et al. Preventing pressure ulcers with the Australian Medical Sheepskin: an open-label randomised controlled trial. Medical Journal of Australia 2004;180(7):324-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Jolley DJ. A multilevel analysis of three randomised controlled trials of the Australian Medical Sheepskin in the prevention of sacral pressure ulcers. Medical Journal of Australia 2011;194(2):104. [DOI] [PubMed] [Google Scholar]
Lazzara 1991 {published data only}
- Lazzara DJ, Buschmann MT. Prevention of pressure ulcers in elderly nursing home residents: are special support surfaces the answer? Decubitus 1991;4(4):42-4, 46, 48. [PMID: ] [PubMed] [Google Scholar]
McGowan 2000 {published data only}
- McGowan S, Montgomery K, Jolley D, Wright R. The role of sheepskins in preventing pressure ulcers in elderly orthopaedic patients. Primary Intention 2000:127-34.
Mistiaen 2010 {published data only}
- Mistiaen P, Achterberg W, Ament A, Halfens R, Huizinga J, Montgomery K, et al. Cost-effectiveness of the Australian Medical Sheepskin for the prevention of pressure ulcers in somatic nursing home patients: study protocol for a prospective multi-centre randomised controlled trial (ISRCTN17553857). BMC Health Services Research 2008;8:4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistiaen P, Achterberg W, Ament A, Halfens R, Huizinga J, Montgomery K, et al. The effectiveness of the Australian Medical Sheepskin for the prevention of pressure ulcers in somatic nursing home patients: a prospective multicenter randomized-controlled trial (ISRCTN17553857). Wound Repair and Regeneration 2010;18(6):572-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mistiaen P, Ament A, Francke AL, Achterberg W, Halfens R, Huizinga J, et al. An economic appraisal of the Australian Medical Sheepskin for the prevention of sacral pressure ulcers from a nursing home perspective. BMC Health Services Research 2010;10:226. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistiaen P, Francke A, Achterberg W, Ament A, Halfens R, Huizinga J. Australian Medical Sheepskin is effective for the prevention of pressure ulcers. Tijdschrift voor Ouderengeneeskunde 2009;5:186-90. [Google Scholar]
Nixon 1998 {published data only}
- Bridel-Nixon J, McElvenny D, Brown J, Mason S. A randomized controlled trial using a double-triangular sequential design: methodology and management issues. In: European Wound Management Association Conference; 1997 April 27-29; Milan (Italy). 1997:65-6.
- Bridel-Nixon J, McElvenny D, Brown J, Mason S. Findings from a double-triangular sequential-design randomized clinical trial of a dry polymer gel pad. In: European Wound Management Association Conference; 1997 April 27-29; Milan (Italy). 1997:20-1.
- Brown J, McElvenny D, Nixon J, Bainbridge J, Mason S. Some practical issues in the design, monitoring and analysis of a sequential randomized trial in pressure sore prevention. Statistics in Medicine 2000;19(24):3389-400. [PMID: ] [DOI] [PubMed] [Google Scholar]
- ISRCTN43076542. Pressure sore risk in the operating department. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN43076542.
- Nixon J, McElvenny D, Mason S, Brown J, Bond S. A sequential randomised controlled trial comparing a dry visco-elastic polymer pad and standard operating table mattress in the prevention of post-operative pressure sores. International Journal of Nursing Studies 1998;35(4):193-203. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ricci 2013 {published data only}
- Ricci E, Roberto C, Ippolito A, Bianco A, Scalise MT. A randomized study on the effectiveness of a new pressure-relieving mattress overlay for the prevention of pressure ulcers in elderly patients at risk. EWMA Journal 2013;13(1):27-32. [Google Scholar]
Russell 2000 {published data only}
- Dunlop V. Preliminary results of a randomized, controlled study of a pressure ulcer prevention system. Advances in Wound Care 1998;11(3 Suppl):14. [PMID: ] [PubMed] [Google Scholar]
- Lichtenstein S. A 7 day comparative randomized parallel single centre study to determine the safety and efficacy of the Micropulse system for the prevention of pressure ulcers. Micropulse 1997.
- Russell JA, Lichtenstein SL. Randomized controlled trial to determine the safety and efficacy of a multi-cell pulsating dynamic mattress system in the prevention of pressure ulcers in patients undergoing cardiovascular surgery. Ostomy/Wound Management 2000;46(2):46-51, 54-5. [PMID: ] [PubMed] [Google Scholar]
Sideranko 1992 {published data only}
- Sideranko S, Quinn A, Burns K, Froman RD. Effects of position and mattress overlay on sacral and heel pressures in a clinical population. Research in Nursing & Health 1992;15(4):245-51. [DOI] [PubMed] [Google Scholar]
Stapleton 1986 {published data only}
- Stapleton M. Preventing pressure sores--an evaluation of three products. Geriatric Nursing (London, England) 1986;6(2):23-5. [PMID: ] [PubMed] [Google Scholar]
Van Leen 2018 {published data only}
- NTR4557. Is lowering of shear and friction forces (cost)effective for prevention of pressure ulcers? trialregister.nl/trial/4435.
- Van Leen M, Halfens R, Schols J. Preventive effect of a microclimate-regulating system on pressure ulcer development: a prospective, randomized controlled trial in Dutch nursing homes. Advances in Skin & Wound Care 2018;31(1):1-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vermette 2012 {published data only}
- Vermette S, Reeves I, Lemaire J. Cost effectiveness of an air-inflated static overlay for pressure ulcer prevention: a randomized, controlled trial. Wounds 2012;24(8):207-14. [PMID: ] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12618000319279 {published data only}
- ACTRN12618000319279. Testing the effectiveness of pressure mattresses for people over 65 years residing in the community. www.anzctr.org.au/AnzctrAttachments/374599-Research%20Proposal%20Ethics%20V2%20(FINAL).pdf 2018.
Allman 1987 {published data only}
- Allman RM, Walker JM, Hart MK, Laprade CA, Noel LB, Smith CR. Air-fluidized beds or conventional therapy for pressure sores. A randomized trial. Annals of Internal Medicine 1987;107(5):641-8. [DOI] [PubMed] [Google Scholar]
Andrews 1988 {published data only}
- Andrews J, Balai R. The prevention and treatment of pressure sores by use of pressure distributing mattresses. Care Science and Practice 1989;7(3):72-6. [PubMed] [Google Scholar]
- Andrews J, Balai R. The prevention and treatment of pressure sores by use of pressure distributing mattresses. Decubitus 1988;1(4):14-21. [PubMed] [Google Scholar]
Anonymous 2006 {published data only}
- Anonymous. Effective methods for preventing pressure ulcers. Journal of Family Practice 2006;55(11):942. [PMID: ] [PubMed] [Google Scholar]
Ballard 1997 {published data only}
- Ballard K. Pressure-relief mattresses and patient comfort. Professional Nurse 1997;13(1):27-32. [PubMed] [Google Scholar]
Beeckman 2019 {published data only}
- Anrys C, Van Tiggelen H, Verhaeghe S, Van Hecke A, Beeckman D. Independent risk factors for pressure ulcer development in a high-risk nursing home population receiving evidence-based pressure ulcer prevention: results from a study in 26 nursing homes in Belgium. International Wound Journal 2019;16(2):325-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckman D, Serraes B, Anrys C, Van Tiggelen H, Van Hecke A, Verhaeghe S. A multicentre prospective randomised controlled clinical trial comparing the effectiveness and cost of a static air mattress and alternating air pressure mattress to prevent pressure ulcers in nursing home residents. International Journal of Nursing Studies 2019;97:105-13. [DOI] [PubMed] [Google Scholar]
- NCT03597750. Comparison of static air support devices (Repose®) and alternating-pressure devices in the prevention of pressure ulcers. ClinicalTrials.gov/show/NCT03597750.
Bell 1993 {published data only}
- Bell JC, Matthews SD. Results of a clinical investigation of four pressure-reduction replacement mattresses. Journal of ET Nursing 1993;20(5):204-10. [PMID: ] [PubMed] [Google Scholar]
Bennett 1998 {published data only}
- Bennett RG, Baran PJ, DeVone LV, Bacetti H, Kristo B, Tayback M, et al. Low airloss hydrotherapy versus standard care for incontinent hospitalized patients. Journal of the American Geriatrics Society 1998;46(5):569-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Berthe 2007 {published data only}
- Berthe JV, Bustillo A, Mélot C, De Fontaine D. Does a foamy-block mattress system prevent pressure sores? A prospective randomised clinical trial in 1729 patients. Acta Chirurgica Belgica 2007;107(2):155-61. [PubMed] [Google Scholar]
Bliss 1966 {published data only}
- Bliss MR, McLaren R, Exton-Smith AN. Mattresses for preventing pressure sores in geriatric patients. Monthly Bulletin of the Ministry of Health and the Public Health Laboratory Service 1966;25:238-68. [PMID: ] [PubMed] [Google Scholar]
Bliss 1967 {published data only}
- Bliss MR, McLaren R, Exton-Smith AN. Preventing pressure sores in hospital: controlled trial of a large-celled ripple mattress. BMJ 1967;1(5537):394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bliss 1993 {published data only}
- Bliss MR, Thomas JM. An investigative approach. An overview of randomised controlled trials of alternating pressure supports. Professional Nurse (London, England) 1993;8(7):437-44. [PMID: ] [PubMed] [Google Scholar]
Bliss 1995b {published data only}
- Bliss MR. Managing pressure ulcers. Advances in Wound Care 1995;8(5):6, 8. [PMID: ] [PubMed] [Google Scholar]
Bliss 2003 {published data only}
- Bliss MR. Clinical research in patient support systems. Journal of Tissue Viability 2003;13(4):154-6, 158, 160. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bliss 2004 {published data only}
Branom 1999 {published data only}
- Branom R, Knox L. Low-air-loss vs non-powered/dynamic surfaces in wound management. In: 31st Annual Wound, Ostomy and Continence Conference; Minneapolis (MN). 1999:483.
Branom 2001 {published data only}
- Branom R, Rappl LM. "Constant force technology" versus low-air-loss therapy in the treatment of pressure ulcers. Ostomy/Wound Management 2001;47(9):38-46. [PMID: ] [PubMed] [Google Scholar]
Brown 2001 {published data only}
- Brown SJ. Bed surfaces and pressure sore prevention: an abridged report. Orthopedic Nursing 2001;20(4):38-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bueno de Camargo 2018 {published data only}
- Bueno de Camargo WH, Pereira RD, Tanita MT, Heko L, Grion IC, Festti J, et al. The effect of support surfaces on the incidence of pressure injuries in critically ill patients: a randomized clinical trial. Critical Care Research and Practice 2018;2018:Article ID 3712067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02844166. Support surfaces to prevent pressure injuries. clinicaltrials.gov/show/NCT02844166.
Cadue 2008 {published data only}
- Cadue JF, Karolewicz S, Tardy C, Barrault C, Robert R, Pourrat O. Prevention of heel pressure sores with a foam body-support device. A randomized controlled trial in a medical intensive care unit [Efficacite de supports anatomiques en mousse pour la prevention des escarres de talons. Etude controlee randomisee en reanimation medicale]. Presse Medicale (Paris, France: 1983) 2008;37(1 Pt 1):30-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Caley 1994 {published data only}
- Caley L, Jones S, Freer J, Muller JS. Randomized prospective trial: treatment outcomes and cost-effectiveness of two types of low-air-loss therapy. Proceedings of the 9th Annual Clinical Symposium on Pressure Ulcer and Wound Management 1994:1-2.
Cassino 2013b {published data only}
- Cassino R, Ippolito AM, Cuffaro C, Corsi A, Ricci E. A controlled, randomized study on the effectiveness of two overlays in the treatment of decubitus ulcers. Minerva Chirurgica 2013;68(1):105-16. [PubMed] [Google Scholar]
Cavicchioli 2007 {published data only}
- Cavicchioli A, Carella G. Clinical effectiveness of a low-tech versus high-tech pressure-redistributing mattress. Journal of Wound Care 2007;16(7):285-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chaloner 2000a {published data only}
- Chaloner D, Cave J. Should weaker study designs ever be preferred over randomised controlled trials. Journal of Tissue Viability 2000;10(3 su):7-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
ChiCTR1800017466 {published data only}
- ChiCTR1800017466. The study on the application of fluidized positioner in preventing intraoperative pressure injury. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800017466 2018.
Chou 2013 {published data only}
- Chou R, Dana T, Bougatsos C, Blazina I, Starmer AJ, Reitel K, et al. Pressure ulcer risk assessment and prevention: a systematic comparative effectiveness review. Annals of Internal Medicine 2013;159(1):28-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cobb 1997 {unpublished data only}
- Cobb GA, Yoder LH, Warren JB. Pressure ulcers: patient outcomes on a KinAir bed or EHOB waffle mattress. TriService Nursing Research Program (TSNRP) 1997.
Collier 1996 {published data only}
- Collier ME. Pressure-reducing mattresses. Journal of Wound Care 1996;5(5):207-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cooper 1998 {published data only}
- Cooper PJ, Gray DG, Mollison J. A randomised controlled trial of two pressure-reducing surfaces. Journal of Wound Care 1998;7(8):374-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cummins 2019 {published data only}
- Cummins KA, Watters R, Leming-Lee T. Reducing pressure injuries in the pediatric intensive care unit. Nursing Clinics of North America 2019;54(1):127-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Day 1993 {published data only}
- Day A, Leonard F. Seeking quality care for patients with pressure ulcers. Decubitus 1993;6(1):32-43. [PMID: ] [PubMed] [Google Scholar]
Defloor 2005 {published data only}
- Defloor T, De Bacquer D, Grypdonck MH. The effect of various combinations of turning and pressure reducing devices on the incidence of pressure ulcers. International Journal of Nursing Studies 2005;42(1):37-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
Demarre 2012 {published data only}
- Demarre L, Beeckman D, Vanderwee K, Defloor T, Grypdonck M, Verhaeghe S. Multi-stage versus single-stage inflation and deflation cycle for alternating low pressure air mattresses to prevent pressure ulcers in hospitalised patients: a randomised-controlled clinical trial. International Journal of Nursing Studies 2012;49(4):416-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Demarre L, Vanderwee K, Beeckman D, Defloor T. Pressure ulcer prevention: randomized controlled trail comparing the effect of a standard alternating pressure air mattress and a alternating low pressure air mattress with gradual inflation and deflation. EWMA Journal 2010;10(2):44. [Google Scholar]
- Demarre L, Vanderwee K, Beeckman D, Defloor T. The effectiveness of a multistage low pressure air mattress in pressure ulcer prevention: an RCT Fourth European Nursing Congress. Journal of Clinical Nursing 2010;19:41.
- Demarre L, Verhaeghe S, Van Hecke A, Grypdonck M, Clays E, Vanderwee K, et al. The effectiveness of three types of alternating pressure air mattresses in the prevention of pressure ulcers in Belgian hospitals. Research in Nursing & Health 2013;36(5):439-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
De Oliveira 2017 {published data only}
- De Oliveira KF, Nascimento KG, Nicolussi AC, Chavaglia SR, De Araujo CA, Barbosa MH. Support surfaces in the prevention of pressure ulcers in surgical patients: an integrative review. International Journal of Nursing Practice (John Wiley & Sons, Inc.) 2017;23(4):doi: 10.1111/ijn.12553. [DOI] [PubMed] [Google Scholar]
Devine 1995 {published data only}
- Devine B. Alternating pressure air mattresses in the management of established pressure sores. Journal of Tissue Viability 1995;5(3):94-8. [Google Scholar]
Economides 1995 {published data only}
- Economides NG, Skoutakis VA, Carter CA, Smith VH. Evaluation of the effectiveness of two support surfaces following myocutaneous flap surgery. Advances in Wound Care 1995;8(1):49-53. [PMID: ] [PubMed] [Google Scholar]
Evans 2000 {published data only}
- Evans D, Land L, Geary A. A clinical evaluation of the Nimbus 3 alternating pressure mattress replacement system. Journal of Wound Care 2000;9(4):181-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Exton‐Smith 1982 {published data only}
- Exton-Smith AN, Overstall PW, Wedgwood J, Wallace G. Use of the 'air wave system' to prevent pressure sores in hospital. Lancet (London, England) 1982;1(8284):1288-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ferrell 1993 {published data only}
- Ferrell BA, Osterweil D, Christenson P. A randomized trial of low-air-loss beds for treatment of pressure ulcers. JAMA 1993;269(4):494-7. [PMID: ] [PubMed] [Google Scholar]
Ferrell 1995 {published data only}
- Ferrell BA, Keeler E, Siu AL, Ahn SH, Osterweil D. Cost-effectiveness of low-air-loss beds for treatment of pressure ulcers. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 1995;50(3):M141-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Feuchtinger 2006 {published data only}
- Feuchtinger J, De Bie R, Dassen T, Halfens R. A 4-cm thermoactive viscoelastic foam pad on the operating room table to prevent pressure ulcer during cardiac surgery. Journal of Clinical Nursing 2006;15(2):162-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Feuchtinger J. Preventing decubitus ulcer in heart surgery interventions: visco-elastic foam layer on the operating room table--a study [Dekubituspravention wahrend kardiochirurgischer Eingriffe: Viskoelastische Schaumstoffauflage auf dem Operationstisch--eine Studie]. Pflege Zeitschrift 2006;59(8):498-501. [PMID: ] [PubMed] [Google Scholar]
Finnegan 2008 {published data only}
- Finnegan MJ, Gazzerro L, Finnegan JO, Lo P. Comparing the effectiveness of a specialized alternating air pressure mattress replacement system and an air-fluidized integrated bed in the management of post-operative flap patients: a randomized controlled pilot study. Journal of Tissue Viability 2008;17(1):2-9. [DOI] [PubMed] [Google Scholar]
Fleischer 1997 {published data only}
- Fleischer I, Bryant D. Evaluating replacement mattresses. Nursing Management 1997;28(8):38-41. [PMID: ] [PubMed] [Google Scholar]
García Fernández 2004 {published data only}
- García Fernández FP, Pancorbo Hidalgo PL, Rodríguez Torres M. Utility and cost-effectiveness of air suspension bed in the prevention of pressure ulcers. Gerokomos 2004;15(3):162-7. [Google Scholar]
Gazzerro 2008 {published data only}
- Gazzerro L, Finnegan M. Comparing the effectiveness of alternating air pressure mattress replacement systems and air fluidized integrated beds in the management of flap and graft patients: ten case studies. Scientific and Clinical Abstracts from the 40th Annual Wound, Ostomy and Continence Nurses Annual Conference. Journal of Wound, Ostomy, and Continence Nursing 2008;35(3):S63. [Google Scholar]
Gebhardt 1994a {published data only}
- Gebhardt K, Bliss MR. A controlled study to compare the efficacy, practicability and cost of pressure relieving supports to prevent and heal pressure sores. In: 2nd European Conference on Advances in Wound Management; 1992 October 20-23; Harrogate (UK). 1993:166.
- Gebhardt K. A randomized trial of alternating pressure (AP) and constant low pressure (CLP) supports for the prevention of pressure sores. Journal of Tissue Viability 1994;4(3):93. [Google Scholar]
Gebhardt 1994b {published data only}
- Gebhardt KS, Bliss MR, Winwright PL. A randomised controlled trial to compare the efficacy of alternating and constant low pressure supports for preventing pressure sores in an intensive care unit (ICU). Clinical Science 1994;86(S30):39P.
Gebhardt 1996 {published data only}
- Gebhardt KS, Bliss MR, Winwright PL, Thomas J. Pressure-relieving supports in an ICU. Journal of Wound Care 1996;5(3):116-21. [DOI] [PubMed] [Google Scholar]
Geelkerken 1994 {published data only}
- Geelkerken RH, Breslau PJ, Hermans J, Wille J, Hofman A, Hamming JJ. Anti-decubitus mattress [Anti-decubitusmatrassen]. Nederlands Tijdschrift voor Geneeskunde 1994;138(36):1834; author reply 1834-5. [PMID: ] [PubMed]
Goldstone 1982 {published data only}
- Goldstone LA, Norris M, O'Reilly M, White J. A clinical trial of a bead bed system for the prevention of pressure sores in elderly orthopaedic patients. Journal of Advanced Nursing 1982;7(6):545-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gray 1994 {published data only}
- Gray D. A randomised clinical trial of two foam mattresses. Aberdeen Royal Hospitals NHS Trust. Medical Support System 1994.
- Gray D. A randomised controlled trial of two foam mattresses. Journal of Tissue Viability 1994;4(3):92. [Google Scholar]
- Gray DG, Campbell M. A randomised clinical trial of two types of foam mattresses. Journal of Tissue Viability 1994;4(4):128-32. [Google Scholar]
- Gray DG, Cooper PJ, Campbell M. A study of the performance of a pressure reducing foam mattress after three years of use. Journal of Tissue Viability 1998;8(3):9-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gray 2000 {published data only}
- Gray D, Smith M. A randomized controlled trial of two pressure-reducing foam mattresses. In: European Wound Management Association Conference; 1998 November; Harrogate (UK). 1998:4.
- Gray DG, Smith M. Comparison of a new foam mattress with the standard hospital mattress. Journal of Wound Care 2000;9(1):29-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gray 2008 {published data only}
- Gray D, Cooper P, Bertram M, Duguid K, Pirie G. A clinical audit of the Softform Premier Active™ mattress in two acute care of the elderly wards. Wounds UK 2008;4(4):124-8. [Google Scholar]
Greer 1988 {published data only}
- Greer DM, Morris J, Walsh NE, Glenn AM, Keppler J. Cost-effectiveness and efficacy of air-fluidized therapy in the treatment of pressure ulcers. Journal of Enterostomal Therapy 1988;15(6):247-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Grindley 1996 {published data only}
- Grindley A, Acres J. Alternating pressure mattresses: comfort and quality of sleep. British Journal of Nursing (Mark Allen Publishing) 1996;5(21):1303-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Groen 1999 {published data only}
- Groen HW, Groenier KH, Schuling J. Comparative study of a foam mattress and a water mattress. Journal of Wound Care 1999;8(7):333-5. [DOI] [PubMed] [Google Scholar]
Gunningberg 2000 {published data only}
- Gunningberg L, Lindholm C, Carlsson M, Sjoden PO. Effect of visco-elastic foam mattresses on the development of pressure ulcers in patients with hip fractures. Journal of Wound Care 2000;9(10):455-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gunningberg 2001 {published data only}
- Gunningberg L, Lindholm C, Carlsson M, Sjoden PO. Reduced incidence of pressure ulcers in patients with hip fractures: a 2-year follow-up of quality indicators. International Journal for Quality in Health Care 2001;13(5):399-407. [PMID: ] [DOI] [PubMed] [Google Scholar]
Haalboom 1994 {published data only}
- Haalboom JR. Anti-decubitus mattresses [Anti-decubitusmatrassen.]. Nederlands Tijdschrift voor Geneeskunde 1994;138(26):1309-10. [PMID: ] [PubMed] [Google Scholar]
Hale 1990 {published data only}
- Hale JS, Smith SH. Pressure reduction mattresses versus pressure reduction overlays: a cost analysis. Journal of Enterostomal Therapy 1990;17(6):241-3. [PMID: ] [PubMed] [Google Scholar]
Hampton 1997 {published data only}
- Hampton S. Evaluation of the new Cairwave Therapy System in one hospital trust. British Journal of Nursing (Mark Allen Publishing) 1997;6(3):167-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hampton 1998 {published data only}
- Hampton S. Can electric beds aid pressure sore prevention in hospitals? British Journal of Nursing (Mark Allen Publishing) 1998;7(17):1010-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hampton 1999 {published data only}
- Hampton S. Efficacy and cost-effectiveness of the Thermo contour mattress. British Journal of Nursing (Mark Allen Publishing) 1999;8(15):990-6. [DOI] [PubMed] [Google Scholar]
Hawkins 1997 {published data only}
- Hawkins JE. The effectiveness of pressure-reducing table pads as an intervention to reduce the risk of intraoperatively acquired pressure sores. Military Medicine 1997;162(11):759-61. [PMID: ] [PubMed] [Google Scholar]
Hofman 1994 {published data only}
- Hofman A, Geelkerken RH, Wille J, Hamming JJ, Hermans J, Breslau PJ. Pressure sores and pressure-decreasing mattresses: controlled clinical trial. Lancet (London, England) 1994;343(8897):568-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Holzgreve 1993 {published data only}
- Holzgreve A, Waldner M, Waldner PW, Hohlbach G. Bed sore prophylaxis in patients with chronic arterial occlusive disease with a new thermoactive pressure alternating mattress. Langenbecks Archiv fur Chirurgie 1993:1117.
Hommel 2008 {published data only}
- Hommel A, Thorngre KG, Ulander K. How to prevent patients with a hip fracture from developing pressure ulcers. EWMA Journal 2008;8(2):157. [Google Scholar]
Hoskins 2007a {published data only}
- Hoskins A. Alternating pressure mattresses were more cost effective than alternating pressure overlays for preventing pressure ulcers. Evidence-Based Nursing 2007;10(1):23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hoskins 2007b {published data only}
- Hoskins A. Similar proportions of patients developed pressure ulcers on alternating pressure overlays and alternating pressure mattresses. Evidence-Based Nursing 2007;10(1):22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Huang 2013 {published data only}
- Huang HY, Chen HL, Xu XJ. Pressure-redistribution surfaces for prevention of surgery-related pressure ulcers: a meta-analysis. Ostomy/Wound Management 2013;59(4):36-8, 42, 44, 46, 48. [PMID: ] [PubMed] [Google Scholar]
Huang 2018 {published data only}
- Huang W, Zhu Y, Qu H. Use of an alternating inflatable head pad in patients undergoing open heart surgery. Medical Science Monitor 2018;24:970-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hungerford 1998 {published data only}
- Hungerford K. A specially designed foam mattress replacement reduced pressure ulcers in nursing home residents. Evidence-Based Nursing 1998;1(2):51. [Google Scholar]
Iglesias 2006 {published data only}
- Iglesias C, Nixon J, Cranny G, Nelson EA, Hawkins K, Phillips A, et al. Pressure relieving support surfaces (PRESSURE) trial: cost effectiveness analysis. BMJ 2006;332(7555):1416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias CP, Nixon J, Cranny G, Nelson A, Hawkins K, Philips A, et al. The NHS Health Technology Assessment Programme. Pressure trial: cost effectiveness analysis of two alternating pressure surfaces for the prevention of pressure ulcers. EWMA Journal 2006;6(1):38. [Google Scholar]
- Iglesias CP, Nixon J, Cranny G. Pressure trial: cost effectiveness analysis of two alternating pressure surfaces for the prevention of pressure ulcers. In: European Wound Management Association Conference; 2005 September 15-17; Stuttgart (Germany). 2005:156.
Inman 1993 {published data only}
- Inman KJ, Sibbald WJ, Rutledge FS, Clark BJ. Clinical utility and cost-effectiveness of an air suspension bed in the prevention of pressure ulcers. JAMA 1993;269(9):1139-43. [PMID: ] [PubMed] [Google Scholar]
IRCT2016091129781N1 {published data only}
- IRCT2016091129781N1. Comparison gum gel and foam cushions to prevent bedsores. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016091129781N1.
Ismail 2001 {published data only}
- Ismail ZBM. Comparative study between the use of a pressure relieving overlay mattress and other mattresses commonly used by homebound patients in the community. Singapore Nursing Journal 2001;28(2):13-6. [Google Scholar]
Jiang 2014 {published data only}
- Jiang Q, Li X, Zhang A, Guo Y, Liu Y, Liu H, et al. Multicenter comparison of the efficacy on prevention of pressure ulcer in postoperative patients between two types of pressure-relieving mattresses in China. International Journal of Clinical and Experimental Medicine 2014;7(9):2820-7. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
JPRN‐UMIN000029680 {published data only}
- JPRN-UMIN000029680. Effects of robotic mattress on pressure ulcer healing, comfort level among pressure ulcer patients, and nursing work load: a randomized controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000029680.
Kemp 1993 {published data only}
- Kemp MG, Kopanke D, Tordecilla L, Fogg L, Shott S, Matthiesen V, et al. The role of support surfaces and patient attributes in preventing pressure ulcers in elderly patients. Research in Nursing & Health 1993;16(2):89-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Keogh 2001 {published data only}
- Keogh A, Dealey C. Profiling beds versus standard hospital beds: effects on pressure ulcer incidence outcomes. Journal of Wound Care 2001;10(2):15-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Klein 1989 {published data only}
- Klein L, Gilroy K. Evaluating mattress overlays and pressure relieving systems: a question of perception or reality? Journal of Enterostomal Therapy 1989;16(2):58-60. [PMID: ] [PubMed] [Google Scholar]
Laurent 1998 {published data only}
- Laurent S. Effectiveness of pressure decreasing mattresses in cardiovascular surgery patients: a controlled clinical trial. In: 3rd European Conference for Nurse Managers; 1997 Oct; Brussels (Belgium). 1998.
Lee 1974 {published data only}
- Lee EOK, Kim MJ. A comparative study on the effect of gel pad, sheepskin and sponge on prevention and treatment of decubitus ulcers. Journal of Nurses Academic Society 1974;4(3):93-104. [Google Scholar]
Maklebust 1988 {published data only}
- Maklebust J, Brunckhorst L, Cracchiolo-Caraway A, Ducharme MA, Dundon R, Panfilli R, et al. Pressure ulcer incidence in high-risk patients managed on a special three-layered air cushion. Decubitus 1988;1(4):30-40. [PMID: ] [PubMed] [Google Scholar]
Malbrain 2010 {published data only}
- Malbrain M, Hendriks B, Wijnands P, Denie D, Jans A, Vanpellicom J, et al. A pilot randomised controlled trial comparing reactive air and active alternating pressure mattresses in the prevention and treatment of pressure ulcers among medical ICU patients. Journal of Tissue Viability 2010;19(1):7-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Marutani 2019 {published data only}
- JPRN-UMIN000035568. Evaluation of pressure ulcer prevention and QOL for using dual-fit-air-cell-mattresses. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000035568.
- Marutani A, Okuwa M, Sugama J. Use of 2 types of air-cell mattresses for pressure ulcer prevention and comfort among patients with advanced-stage cancer receiving palliative care: an interventional study. Ostomy Wound Management 2019;65(5):24-32. [PubMed] [Google Scholar]
Mastrangelo 2010a {published data only}
- Mastrangelo D, Farina E, Gallicchio V, De Anna D, Bresadola F. Observational study of the use of antidecubitus mattress covers in the prevention and care of pressure ulcers. Acta Vulnologica 2010;8(2):87-92. [Google Scholar]
McGinnis 2011 {published data only}
- McGinnis E, Stubbs N. Pressure-relieving devices for treating heel pressure ulcers. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD005485. [DOI: 10.1002/14651858.CD005485.pub2] [DOI] [PubMed] [Google Scholar]
McInnes 2015 {published data only}
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Dumville JC, Middleton V, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD001735. [DOI: 10.1002/14651858.CD001735.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
McInnes 2018 {published data only}
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Leung V. Support surfaces for treating pressure ulcers. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD009490. [DOI: 10.1002/14651858.CD009490.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendoza 2019 {published data only}
- Mendoza RA, Lorusso GA, Ferrer DA, Helenowski IB, Liu J, Soriano RH, et al. A prospective, randomised controlled trial evaluating the effectiveness of the fluid immersion simulation system vs an air-fluidised bed system in the acute postoperative management of pressure ulcers: a midpoint study analysis. International Wound Journal 2019;16(4):989-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mistiaen 2010a {published data only}
- Mistiaen P, Jolley D, McGowan S, Hickey M, Spreeuwenberg P, Francke A. The Australian Medical Sheepskin prevents pressure ulcers: a combined multilevel analysis of three RCTs. Fourth European Nursing Congress. Journal of Clinical Nursing 2010;19:20.
- Mistiaen P, Jolley DJ, McGowan S, Hickey MB, Spreeuwenberg P, Francke AL. 'Australian Medical Sheepskin' in the prevention of decubitus: meta-analysis with individual patient data shows efficacy ['Australische Medische Schapenvacht' ter preventie van decubitus: meta-analyse met individuele patientendata toont effectiviteit]. Nederlands Tijdschrift voor Geneeskunde 2011;155(15):686-91. [PMID: ] [PubMed] [Google Scholar]
- Mistiaen P, Jolley DJ, McGowan S, Hickey MB, Spreeuwenberg P, Francke AL. Australian Medical Sheepskin for prevention of pressure ulcers: individual patient data meta-analysis shows effectiveness ['Australische Medische Schapenvacht' ter preventie van decubitus: meta-analyse met individuele patientendata toont effectiviteit]. Nederlands Tijdschrift voor Geneeskunde 2011;155(18):A3034. [PMID: ] [PubMed] [Google Scholar]
- Mistiaen PJ, Jolley DJ, McGowan S, Hickey MB, Spreeuwenberg P, Francke AL. A multilevel analysis of three randomised controlled trials of the Australian Medical Sheepskin in the prevention of sacral pressure ulcers. Medical Journal of Australia 2010;193(11-12):638-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NTR878. Cost-effectiveness of the Australian Medical Sheepskin for the prevention of pressure ulcers in somatic nursing home clients. trialregister.nl/trial/864.
Nakahara 2012 {published data only}
- Nakahara R, Ohto S, Kato H. Treating pressure ulcers using alternating pressure replacement mattresses. In: 4th Congress of the World Union of Wound Healing Societies; 2012 September 2-6; Yokohama (Japan). 2012.
NCT01402765 {published data only}
- NCT01402765. Interface pressure measures for mattresses: Nimbus 3 versus Summit. clinicaltrials.gov/show/NCT01402765.
NCT02565797 {published data only}
- NCT02565797. Comparative prevention-effectiveness trial of DabirAIR overlay system. ClinicalTrials.gov/show/NCT02565797.
NCT02634892 {published data only}
- NCT02634892. Pressure redistributing overlay with targeted cooling technology (PRO-TECT) for pressure ulcer prevention. ClinicalTrials.gov/show/NCT02634892.
NCT02735135 {published data only}
- NCT02735135. Comparison of 2 mattresses for the prevention of bedsores by measuring skin pressure in the sacral area. clinicaltrials.gov/show/NCT02735135.
NCT03048357 {published data only}
- NCT03048357. Effectiveness of Freedom Bed compared to manual turning in prevention of pressure injuries in persons with limited mobility due to traumatic brain injury and/or spinal cord injury. clinicaltrials.gov/show/NCT03048357.
NCT03211910 {published data only}
- NCT03211910. Sacral Savers: study of prevention and enhanced healing of sacral and trochenteric ulcers. ClinicalTrials.gov/show/NCT03211910.
NCT03351049 {published data only}
- NCT03351049. An RCT on support surfaces for pressure ulcer prevention. ClinicalTrials.gov/show/NCT03351049.
Nixon 2006 {published data only}
- ISRCTN78646179. Randomised controlled trial comparing alternating pressure overlays with alternating pressure mattresses for pressure sore prevention and treatment. isrctn.com/ISRCTN78646179.
- Nelson EA, Nixon J, Mason S, Barrow H, Phillips A, Cullum N. A nurse-led randomised trial of pressure-relieving support surfaces. Professional Nurse (London, England) 2003;18(9):513-6. [PMID: ] [PubMed] [Google Scholar]
- Nixon J, Cranny G, Iglesias C, Nelson EA, Hawkins K, Phillips A, et al. Randomised, controlled trial of alternating pressure mattresses compared with alternating pressure overlays for the prevention of pressure ulcers: PRESSURE (pressure relieving support surfaces) trial. BMJ 2006;332(7555):1413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon J, Cranny G, Nelson A, Iglesias C, Phillips A, Hawkins K, et al. Pressure trial clinical and patient outcomes. In: European Wound Management Association; 1998 November; Harrogate (UK). 2005:157.
- Nixon J, Cranny G, Nelson A, Iglesias C, Phillips A, Hawkins K, et al. The NHS Health Technology Assessment Programme. Pressure trial clinical and patient outcomes. EWMA Journal 2006;6(1):38. [Google Scholar]
- Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al. Pressure relieving support surfaces: a randomised evaluation. Health Technology Assessment (Winchester, England) 2006;10(22):1-163. [DOI] [PubMed] [Google Scholar]
- Nixon J, Thorpe H, Barrow H, Phillips A, Nelson EA, Mason SA, et al. Reliability of pressure ulcer classification and diagnosis. Journal of Advanced Nursing 2005;50(6):613-23. [DOI] [PubMed] [Google Scholar]
- Nixon J. Randomised, controlled trial of alternating pressure mattresses compared with alternating pressure overlays for the prevention of pressure ulcers: PRESSURE (pressure relieving support surfaces) trial. BMJ 2006;333:Erratum in: BMJ 2006; 333:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nixon 2019 {published data only}
- Brown S, Smith I, Brown J, Hulme C, Nixon J. Pressure relieving support surfaces: a randomised evaluation 2 (PRESSURE 2). Trials 2013;14(Suppl 1):P68. [Google Scholar]
- Brown S, Smith IL, Brown JM, Hulme C, McGinnis E, Stubbs N, et al. Pressure RElieving Support SUrfaces: a Randomised Evaluation 2 (PRESSURE 2): study protocol for a randomised controlled trial. Trials 2016;17(1):604. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN01151335. Pressure RElieving Support SUrfaces: a Randomised Evaluation 2. isrctn.com/ISRCTN01151335.
- McGinnis E, Brown S, Collier H, Faulks P, Gilberts R, Greenwood C, et al. Pressure RElieving Support SUrfaces: a Randomised Evaluation 2 (PRESSURE 2) photographic validation sub-study: study protocol for a randomised controlled trial. Trials 2017;18(1):132. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon J, Brown S, Smith IL, McGinnis E, Vargas-Palacios A, Nelson EA, et al. Comparing alternating pressure mattresses and high-specification foam mattresses to prevent pressure ulcers in high-risk patients: the PRESSURE 2 RCT. Health Technology Assessment (Winchester, England) 2019;23(52):1-176. [PMID: ] [DOI] [PMC free article] [PubMed]
- Nixon J, Smith IL, Brown S, McGinnis E, Vargas-Palacios A, Nelson EA, et al. Pressure relieving support surfaces for pressure ulcer prevention (PRESSURE 2): clinical and health economic results of a randomised controlled trial. EClinicalMedicine 2019;14:42-52. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ooka 1995 {published data only}
- Ooka M, Kemp MG, McMyn R, Shott S. Evaluation of three types of support surfaces for preventing pressure ulcers in patients in a surgical intensive care unit. Journal of Wound, Ostomy, and Continence Nursing 1995;22(6):271-9. [DOI] [PubMed] [Google Scholar]
Osterbrink 2005 {published data only}
- Osterbrink J, Mayer H, Schroder G. Clinical evaluation of the effectiveness of a multimodal static pressure relieving device. In: 8th European Pressure Ulcer Advisory Panel Open Meeting; 2005 May 5-7; Aberdeen (Scotland). 2005:73.
Ozyurek 2015 {published data only}
- Ozyurek P, Yavuz M. Prevention of pressure ulcers in the intensive care unit: a randomized trial of 2 viscoelastic foam support surfaces. Clinical Nurse Specialist CNS 2015;29(4):210-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Park 2017 {published data only}
- Park KH, Park J. The efficacy of a viscoelastic foam overlay on prevention of pressure injury in acutely ill patients: a prospective randomized controlled trial. Journal of Wound, Ostomy, and Continence Nursing 2017;44(5):440-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Phillips 1999 {published data only}
- Phillips L. Providing correct pressure-relieving devices for optimum outcome. British Journal of Nursing (Mark Allen Publishing) 1999;8(21):1447-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Price 1999 {published data only}
- Price P, Bale S, Newcombe R, Harding K. Challenging the pressure sore paradigm. Journal of Wound Care 1999;8(4):187-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pring 1998 {published data only}
- Pring J, Millman P. Evaluating pressure-relieving mattresses. Journal of Wound Care 1998;7(4):177-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rae 2018 {published data only}
- Rae KE, Isbel S, Upton D. Support surfaces for the treatment and prevention of pressure ulcers: a systematic literature review. Journal of Wound Care 2018;27(8):467-74. [DOI] [PubMed] [Google Scholar]
Rafter 2011 {published data only}
- Rafter L. Evaluation of patient outcomes: pressure ulcer prevention mattresses. British Journal of Nursing 2011;20(11):32. [DOI] [PubMed] [Google Scholar]
Reddy 2006 {published data only}
- Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA 2006;296(8):974-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Reddy 2008 {published data only}
- Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA 2008;300(22):2647-62. [DOI] [PubMed] [Google Scholar]
Ricci 2013a {published data only}
- Ricci E, Cassino R, Ippolito A. A randomized study on efficacy on 2 overlays in pressure sores treatment. EWMA Journal 2013;13(1):302.
Rithalia 1995 {published data only}
- Rithalia SV. Comparison of performance characteristics of the Nimbus and Airwave mattresses. International Journal of Rehabilitation Research 1995;18(2):182-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosenthal 2003 {published data only}
- Rosenthal MJ, Felton RM, Nastasi AE, Naliboff BD, Harker J, Navach JH. Healing of advanced pressure ulcers by a generic total contact seat: 2 randomized comparisons with low air loss bed treatments. Archives of Physical Medicine and Rehabilitation 2003;84(12):1733-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Russell 1999 {published data only}
- Russell L. Randomized comparative clinical trial of Pegasus Cairwave mattress and Proactive seating cushion and Huntleigh Nimbus 3 & Aura seating cushion. Journal of Tissue Viability 1999;9(3):103-4. [Google Scholar]
- Russell L. Randomized comparative clinical trial of the Pegasus Cairwave mattress and Proactive seating cushion and the Huntleigh Nimbus III mattress and Alpha Transcell seating cushion. In: European Wound Management Association and Journal of Wound Care Autumn Conference; 1998 November; Harrogate (UK). 1998:4.
Russell 2000b {published data only}
- Russell L, Reynolds T, Carr J, Evans A, Holmes M. A comparison of healing rates on two pressure-relieving systems. British Journal of Nursing (Mark Allen Publishing) 2000;9(22):2270-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Russell 2000c {published data only}
- Russell L, Reynolds TM, Carr J, Evans A, Holmes M. Randomised controlled trial of two pressure-relieving systems. Journal of Wound Care 2000;9(2):52-5. [DOI] [PubMed] [Google Scholar]
Russell 2003a {published data only}
- Russell LJ, Reynolds TM, Park C, Rithalia S, Gonsalkorale M, Birch J, et al. Randomized clinical trial comparing 2 support surfaces: results of the prevention of pressure ulcers study. Advances in Skin & Wound Care 2003;16(6):317-27. [PMID: ] [DOI] [PubMed] [Google Scholar]
Russell 2003b {published data only}
- Russell L, Reynolds TM, Towns A, Worth W, Greenman A, Turner R. Randomized comparison trial of the RIK and the Nimbus 3 mattresses. British Journal of Nursing (Mark Allen Publishing) 2003;12(4):254, 256-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sanada 2003 {published data only}
- Matsui Y, Miyake S, Kawasaki T, Konya C, Sugama J, Sanada H. Randomized controlled trial of a two layer type air cell mattress in the prevention of pressure ulcers. Japanese Journal of Pressure Ulcers 2001;3(3):331-7. [Google Scholar]
- Sanada H, Sugama J, Matsui Y, Konya C, Kitagawa A, Okuwa M, et al. Randomised controlled trial to evaluate a new double-layer air-cell overlay for elderly patients requiring head elevation. Journal of Tissue Viability 2003;13(3):112-4, 116, 118. [PMID: ] [DOI] [PubMed] [Google Scholar]
Santy 1994 {published data only}
- Santy JE, Butler MK, Whyman JD. A comparison study of 6 types of hospital mattress to determine which most effectively reduces the incidence of pressure sores in elderly patients with hip fractures in a District General Hospital. Report to Northern & Yorkshire Regional Health Authority 1994.
Santy 1995 {published data only}
- Santy J. Hospital mattresses and pressure sore prevention. Journal of Wound Care 1995;4(7):329-32. [DOI] [PubMed] [Google Scholar]
Sauvage 2017 {published data only}
- Sauvage P, Touflet M, Pradere C, Portalier F, Michel JM, Charru P, et al. Pressure ulcers prevention efficacy of an alternating pressure air mattress in elderly patients: E(2)MAO a randomised study. Journal of Wound Care 2017;26(6):304-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Scheffel 2011 {published data only}
- Scheffel S, Panfil EM. Mattresses and Co. for prevention of decubitus ulcer. What measures are effective? [Matratzen und Co. zur Dekubituspravention. Welche Massnahmen sind wirksam?]. Pflege Zeitschrift 2011;64(3):162-3. [PubMed] [Google Scholar]
Schultz 1999 {published data only}
- Schultz A, Bien M, Dumond K, Brown K, Myers A. Etiology and incidence of pressure ulcers in surgical patients. AORN Journal 1999;70(3):434, 437-40, 443-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Schultz AA. Study results: prediction and prevention of pressure ulcers in surgical patients. Advances in Wound Care 1998;11(3):11. [PubMed] [Google Scholar]
Scott 2000 {published data only}
- Scott EM. The prevention of pressure ulcers in the operating department. Journal of Wound Care 2000;9(1):18-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Scott‐Williams 2006 {published data only}
- Scott-Williams S, Lummas AC. Perioperative pressure ulcer assessment and prevention efficacy study of a multilayered pad for the operating room. Ostomy/Wound Management 2006;52(4):110-1. [Google Scholar]
Serraes 2018 {published data only}
- Serraes B, Van Leen M, Schols J, Van Hecke A, Verhaeghe S, Beeckman D. Prevention of pressure ulcers with a static air support surface: a systematic review. International Wound Journal 2018;15(3):333-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shakibamehr 2019 {published data only}
- Shakibamehr J, Rad M, Akrami R, Rad M. Effectiveness of Tragacanth gel cushions in prevention of pressure ulcer in traumatic patients: a randomized controlled trial. Journal of Caring Sciences 2019;8(1):45-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharp 2007 {published data only}
- Sharp A. Pilot study to compare the incidence of pressure ulceration on two therapeutic support surfaces in elderly care. In: 17th Conference of the European Wound Managment Association; 2007 May 2-4; Glasgow (Scotland). 2007:Oral presentation 114, 73.
Shi 2018a {published data only}
- Shi C, Dumville JC, Cullum N. Support surfaces for pressure ulcer prevention: a network meta-analysis. PLOS One 2018;13(2):e0192707. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013 {published data only}
- Smith ME, Totten A, Hickam DH, Fu R, Wasson N, Rahman B, et al. Pressure ulcer treatment strategies: a systematic comparative effectiveness review. Annals of Internal Medicine 2013;159(1):39-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stannard 1993 {published data only}
- Stannard D. Commentary on the role of support surfaces and patient attributes in preventing pressure ulcers in elderly patients. AACN Nursing Scan In Critical Care 1993;3(6):2-3. [DOI] [PubMed] [Google Scholar]
Sterzi 2003 {published data only}
- Sterzi S, Selvaggi G, Romanelli A, Valente P, Bertolini C. Evaluation of prevalence and incidence of pressure ulcers and their relationship with mattresses used in a general hospital intensive care unit. European Journal of Plastic Surgery 2003;25(7):401-4. [Google Scholar]
Strauss 1991 {published data only}
- Strauss MJ, Gong J, Gary BD, Kalsbeek WD, Spear S. The cost of home air-fluidized therapy for pressure sores. A randomized controlled trial. Journal of Family Practice 1991;33(1):52-9. [PMID: ] [PubMed] [Google Scholar]
Takala 1994 {published data only}
- Takala J, Soini HO, Soppi E, Kataja M, Olkkonen K. Can risk factors for pressure sores be decreased with a special mattress? Duodecim; Laaketieteellinen Aikakauskirja 1994;110(4):407-14. [PubMed] [Google Scholar]
Takala 1996 {published data only}
- Takala J, Varmavuo S, Soppi E. Prevention of pressure sores in acute respiratory failure: a randomised controlled trial. Clinical Intensive Care 1996;7(5):228-35. [Google Scholar]
Taylor 1999 {published data only}
- Taylor L. Evaluating the Pegasus Trinova: a data hierarchy approach. British Journal of Nursing (Mark Allen Publishing) 1999;8(12):771-4, 776-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tewes 1993 {published data only}
- Tewes M. Prevention and treatment of pressure sores--a neglected research subject? An overview of clinically controlled studies in the period 1987-91 [Tryksarforebyggelse og -behandling--et forsomt forskningsomrade? En gennemgang af klinisk kontrollerede undersogelser i perioden 1987-91]. Vard i Norden 1993;13(2):4-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Theaker 2005 {published data only}
- Theaker C, Kuper M, Soni N. Pressure ulcer prevention in intensive care - a randomised control trial of two pressure-relieving devices. Anaesthesia 2005;60(4):395-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vanderwee 2005 {published data only}
- Vanderwee K, Grypdonck MH, Defloor T. Effectiveness of an alternating pressure air mattress for the prevention of pressure ulcers. Age and Ageing 2005;34(3):261-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Leen 2011 {published data only}
- Van Leen M, Hovius S, Neyens J, Halfens R, Schols J. Pressure relief, cold foam or static air? A single center, prospective, controlled randomized clinical trial in a Dutch nursing home. Journal of Tissue Viability 2011;20(1):30-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Leen 2013 {published data only}
- Van Leen M, Hovius S, Halfens R, Neyens J, Schols J. Pressure relief with visco-elastic foam or with combined static air overlay? A prospective, crossover randomized clinical trial in a Dutch nursing home. Wounds 2013;25(10):287-92. [PMID: ] [PubMed] [Google Scholar]
Van Rijswijk 1994 {published data only}
- Van Rijswijk L. Pressure sores and pressure-decreasing mattresses: controlled clinical trial. Ostomy Wound Management 1994;40(6):12. [Google Scholar]
Vyhlidal 1997 {published data only}
- Vyhlidal SK, Moxness D, Bosak KS, Van Meter FG, Bergstrom N. Mattress replacement or foam overlay? A prospective study on the incidence of pressure ulcers. Applied Nursing Research 1997;10(3):111-20. [DOI] [PubMed] [Google Scholar]
Wallace 2009 {published data only}
- Wallace M. Review: alternative-foam mattresses and some operating-table overlays reduce pressure ulcers more than standard surfaces. Evidence-Based Nursing 2009;12(3):81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Whitney 1984 {published data only}
- Whitney JD, Fellows BJ, Larson E. Do mattresses make a difference? Journal of Gerontological Nursing 1984;10(9):20-1, 24-5. [DOI] [PubMed] [Google Scholar]
Whittingham 1999 {published data only}
- Whittingham K. Randomized control trial of six pressure-redistributing foam mattresses. Journal of Tissue Viability 1999;9(3):104. [Google Scholar]
Yao 2018 {published data only}
- Yao L, Ding N, Yang L, Han C, Jiang L, Jiang B, et al. Effects of different decompression device in the prevention of pressure sore: a network meta-analysis. Chinese Journal of Evidence-Based Medicine 2018;18(10):1086-92. [Google Scholar]
References to studies awaiting assessment
Chaloner 2000 {published data only}
- Chaloner D. A prospective, controlled comparison between two dynamic alternating mattress replacement systems within a community setting. In: 9th European Conference on Advances in Wound Management; 1999 November 9-11; Harrogate (UK). 2000.
Gardner 2008 {published data only}
- Gardner A, Dunk AM, Gardner G. Which mattress works best? A clinical trial of the comparative effectiveness of constant low pressure and alternating pressure devices in hospital patients deemed at risk of pressure injury. In: Australian Wound Management Association 7th National Conference. 2008:52.
Henn 2004 {published data only}
- Henn G, Russell L, Towns A, Taylor H. A two-centre prospective study to determine the utility of a dynamic mattress and mattress overlay. In: 2nd World Union of Wound Healing Societies Meeting; 2004 July 8-13; Paris (France). 2004:54.
Knight 1999 {published data only}
- Knight M, Simpson J, Fear-Price M. An evaluation of pressure-reducing overlay mattresses in the community situation. In: 9th European Conference on Advances in Wound Management; November 9-11; Harrogate (UK). 1999:25.
Mastrangelo 2010b {published data only}
- Mastrangelo D, Farina E, Gallicchio V, De Anna D, Bresadola F, Farina MA. Initial analyses of the prospective study randomized on the anti-decubitis lesion mattress cover. In: SAWC Spring: the Symposium on Advanced Wounds Care and the Wound Healing Society; 2010 April 17-20; Orlando (FL). 2010:S32.
Melland 1998 {published data only}
- Melland HI, Langemo D, Hanson D, Olson B, Hunter S. Clinical trial of the Freedom Bed. Prairie Rose 1998;67(2):11-2a. [PubMed] [Google Scholar]
Additional references
Baumgarten 2009
- Baumgarten M, Margolis DJ, Selekof JL, Moye N, Jones PS, Shardell M. Validity of pressure ulcer diagnosis using digital photography. Wound Repair and Regeneration 2009;17:287-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. West Sussex (UK): John Wiley & Sons, Ltd, 2009. [Google Scholar]
Borenstein 2017
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5-18. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. [DOI] [PubMed] [Google Scholar]
Clark 2011
- Clark M. Technology update: understanding support surfaces. Wounds International 2011;2(3):17-21. [Google Scholar]
Cullum 2016
- Cullum N, Buckley H, Dumville J, Hall J, Lamb K, Madden M, et al. Wounds Research for Patient Benefit: A 5-year Programme of Research. Southampton (UK): NIHR Journals Library, 2016. [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Demarré 2015
- Demarré L, Van Lancker A, Van Hecke A, Verhaeghe S, Grypdonck M, Lemey J, et al. The cost of prevention and treatment of pressure ulcers: a systematic review. International Journal of Nursing Studies 2015;52(11):1754-74. [DOI] [PubMed] [Google Scholar]
Eldridge 2019
- Eldridge S, Campbell M, Campbell M, Dahota A, Giraudeau B, Higgins J, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): additional considerations for cluster-randomized trials. www.bristol.ac.uk/media-library/sites/social-community-medicine/images/centres/cresyda/RoB2-0_cluster_parallel_guidance.pdf (accessed 01 October 2019).
EPUAP/NPIAP/PPPIA 2019
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel, and Pan Pacific Pressure Injury Alliance (EPUAP/NPIAP/PPPIA). Prevention and Treatment of Pressure Ulcers/Injuries: Quick Reference Guide. EPUAP/NPIAP/PPPIA, 2019. [Google Scholar]
Espejo 2018
- Espejo E, Andrés M, Borrallo RM, Padilla E, Garcia-Restoy E, Bella F, Complex Wounds Working Group. Bacteremia associated with pressure ulcers: a prospective cohort study. European Journal of Clinical Microbiology & Infectious Diseases 2018;37(5):969-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Essex 2009
- Essex HN, Clark M, Sims J, Warriner A, Cullum N. Health-related quality of life in hospital inpatients with pressure ulceration: assessment using generic health-related quality of life measures. Wound Repair and Regeneration 2009;17(6):797-805. [DOI] [PubMed] [Google Scholar]
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [DOI] [PubMed] [Google Scholar]
Gorecki 2009
- Gorecki C, Brown JM, Nelson EA, Briggs M, Schoonhoven L, Dealey C, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. Journal of the American Geriatrics Society 2009;57(7):1175-83. [DOI] [PubMed] [Google Scholar]
Gorecki 2013
- Gorecki C, Brown JM, Cano S, Lamping DL, Briggs M, Coleman S, et al. Development and validation of a new patient-reported outcome measure for patients with pressure ulcers: the PU-QOL instrument. Health and Quality of Life Outcomes 2013;11:95. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 30 September 2019. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Gray 2018
- Gray TA, Rhodes S, Atkinson RA, Rothwell K, Wilson P, Dumville JC, et al. Opportunities for better value wound care: a multiservice, cross-sectional survey of complex wounds and their care in a UK community population. BMJ Open 2018;8(3):e019440. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guest 2018
- Guest JF, Fuller GW, Vowden P, Vowden KR. Cohort study evaluating pressure ulcer management in clinical practice in the UK following initial presentation in the community: costs and outcomes. BMJ Open 2018;8(7):e021769. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence - study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64:407-15. [DOI] [PubMed] [Google Scholar]
Herdman 2011
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research 2011;21(10):1727-36. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2019
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Available from www.training.cochrane.org/handbook.
Hróbjartsson 2012
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non-blinded outcome assessors. BMJ 2012;344:e1119. [DOI] [PubMed] [Google Scholar]
Kirkham 2018
- Kirkham JJ, Altman DG, Chan AW, Gamble C, Dwan KM, Williamson PR. Outcome reporting bias in trials: a methodological approach for assessment and adjustment in systematic reviews. BMJ 2018;362:k3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Li 2019
- Li T, Higgins JP, Deeks JJ (editors). Chapter 5: Collecting data. In Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Nguyen 2015
- Nguyen KH, Chaboyer W, Whitty JA. Pressure injury in Australian public hospitals: a cost-of-illness study. Australian Health Review 2015;39(3):329-36. [DOI] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence (NICE). Pressure ulcers: prevention and management. www.nice.org.uk/guidance/cg179 (accessed 08 October 2019). [PubMed]
NPIAP 2016
- National Pressure Injury Advisory Panel (NPIAP). NPUAP Pressure Injury Stages. Available at cdn.ymaws.com/npuap.site-ym.com/resource/resmgr/npuap_pressure_injury_stages.pdf (accessed 18 February 2020).
NPIAP S3I 2007
- National Pressure Injury Advisory Panel (NPIAP) Support Surface Standards Initiative (S3I). Terms and Definitions Related to Support Surfaces. Available at cdn.ymaws.com/npiap.com/resource/resmgr/website_version_terms_and_de.pdf (accessed 18 February 2020).
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan — a web and mobile app for systematic reviews. Systematic Reviews 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2019
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Peryer 2019
- Peryer G, Golder S, Junqueira DR, Vohra S, Loke YK, (editors). Chapter 19: Adverse effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Available from www.training.cochrane.org/handbook.
Peters 2008
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991-6. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [PMID: ] [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80-97. [PMID: ] [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Giovane CD, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLOS One 2014;9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoonhoven 2007
- Schoonhoven L, Bousema Mente T, Buskens E, on behalf of the prePURSE-study group. The prevalence and incidence of pressure ulcers in hospitalised patients in the Netherlands: a prospective inception cohort study. International Journal of Nursing Studies 2007;44(6):927-35. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Shi 2018b
- Shi C, Westby M, Norman G, Dumville J, Cullum N. Node-making processes in network meta-analysis of non-pharmacological interventions should be well planned and reported. Journal of Clinical Epidemiology 2018;101:124-5. [DOI] [PubMed] [Google Scholar]
Shi 2018c
- Shi C, Dumville JC, Cullum N. Skin status for predicting pressure ulcer development: a systematic review and meta-analyses. International Journal of Nursing Studies 2018;87:14-25. [DOI] [PubMed] [Google Scholar]
Shi 2021
- Shi C, Dumville JC, Cullum N, Rhodes S, McInnes E, Goh EL, et al. Beds, overlays and mattresses for preventing and treating pressure ulcers: an overview of Cochrane Reviews and network meta-analysis. Cochrane Database of Systematic Reviews 2021, Issue 8. Art. No: CD013761. [DOI: 10.1002/14651858.CD013761.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Theisen 2012
- Theisen S, Drabik A, Stock S. Pressure ulcers in older hospitalised patients and its impact on length of stay: a retrospective observational study. Journal of Clinical Nursing 2012;21(3-4):380-7. [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693-708. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):472-83. [PMID: ] [PubMed] [Google Scholar]
World Health Organization 2019
- World Health Organization. EH90 Pressure ulceration. ICD-11 for Mortality and Morbidity Statistics (Version: 04/2019). Available at icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f45533017 (accessed 17 February 2020).
Wounds International 2010
- Wounds International. International Review. Pressure Ulcer Prevention: Pressure, Shear, Friction and Microclimate in Context. A Consensus Document. London (UK): Wounds International, 2010. [Google Scholar]
References to other published versions of this review
Shi 2020
- Shi C, Dumville JC, Cullum N, Rhodes S, McInnes E. Alternative reactive support surfaces (non-foam or air-filled) for preventing pressure ulcers. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD013623. [DOI: 10.1002/14651858.CD013623] [DOI] [PMC free article] [PubMed] [Google Scholar]


